Abstract
Cryptococcus neoformans isolates that exhibited unusual patterns of resistance to fluconazole and voriconazole were isolated from seven isolates from two different geographical regions: one isolate from an Israeli non-AIDS patient and six serial isolates from an Italian AIDS patient who had suffered six recurrent episodes of cryptococcal meningitis. Each isolate produced cultures with heterogeneous compositions in which most of the cells were susceptible, but cells highly resistant to fluconazole (MICs, ≥64 μg/ml) were recovered at a variable frequency (7 × 10−3 to 4.6 × 10−2). Evidence showed that this type of resistance is innate and is unrelated to drug exposure since the Israeli patient had never been treated with azoles or any other antimycotic agents. Analysis of clonal subpopulations of these two strains showed that they exhibited heterogeneous patterns of resistance. The number of subpopulations which grew on fluconazole or voriconazole agar declined progressively with increasing azole concentration without a sharp cutoff point. For the Italian serial isolates, the number of clonal populations resistant to fluconazole (64 μg/ml) and voriconazole (1 μg/ml) increased steadily, yielding the highest number for the isolate from the last episode. Attempts to purify a sensitive subpopulation failed, but clones highly resistant to fluconazole (100 μg/ml) and moderately resistant to voriconazole (1 μg/ml) always produced a homogeneous population of resistant cells. Upon maintenance on drug-free medium, however, the majority of the homogeneously resistant cells of these subclones lost their resistance and returned to the stable initial heteroresistant phenotype. The pattern of heteroresistance was not affected by the pH or osmolarity of the medium but was influenced by temperature. The resistance appeared to be suppressed at 35°C and was completely abolished at 40°C. Although heterogeneity in azole resistance among subpopulations of single isolates has been reported for Candida species, the transient changes in expression of resistance under different growth conditions reported here have not been observed in fungal pathogens.
Cryptococcosis, which is caused by the basidiomycetous yeast Cryptococcus neoformans, is one of the most serious mycoses that primarily affects immunocompromised patients, in particular, those with AIDS (14). Following induction therapy for the treatment of cryptococcosis, the infection is managed by lifelong maintenance therapy to prevent recurrences. Fluconazole has been the most widely used antifungal agent for maintenance therapy for this disease. Recurrences of cryptococcal meningitis during maintenance therapy have not been uncommon in AIDS patients, and most of these recurrences were known to be due to persistence of the original infecting strains rather than reinfection with a new cryptococcal strain (4, 6, 28). The MICs for serial isolates from AIDS patients experiencing persistent cryptococcosis generally did not change significantly, and strains resistant to antimycotic agents were infrequently isolated from these patients during episodes of recurrent cryptococcal meningitis (4, 6, 35). In vitro assays have shown that voriconazole (UK-109,496; a fluconazole-derived new triazole) is more potent than fluconazole against C. neoformans and that strains resistant to fluconazole are generally susceptible to voriconazole (20). Recent biochemical studies have suggested that there may be at least two mechanisms of triazole tolerance in clinical isolates of C. neoformans: alterations in the susceptibility of the target enzyme (cytochrome P-450-dependent 14-α-sterol demethylase) to fluconazole and decreases in the cellular content of fluconazole (32). The genetic basis of these mechanisms, however, is unknown in C. neoformans.
We have investigated the resistance to fluconazole and voriconazole of clonal subpopulations of seven C. neoformans isolates from two geographical regions: six isolates from an Italian AIDS patient who suffered from six recurrent episodes of meningitis and one isolate from a non-AIDS patient who was living in Israel and who had never been exposed to any antimycotic agents. The patterns of fluconazole and voriconazole resistance in these isolates are reminiscent of the pattern of methicillin heteroresistance reported in Staphylococcus aureus and have not been reported in fungal pathogens.
MATERIALS AND METHODS
Microorganisms.
Seven clinical isolates of C. neoformans were obtained from two different geographical locations (Table 1): six serial isolates (isolates B-4539, B-4540, B-4541, B-4542, B-4543, and B-4544) were from a 35-year-old male AIDS patient in Italy (patient A) and one (isolate B-4548) was from a 32-year-old human immunodeficiency virus-negative patient in Israel (patient B) who had never been treated with antimycotic agents (21). The antimycotic therapy received by these patients at the time of C. neoformans isolation is summarized in Table 1. A susceptible laboratory strain, strain B-4476, was used as the control.
TABLE 1.
Antimycotic therapy at the time of isolation of seven isolates of C. neoformans from two patients
| Patient | Isolate | Sample | Time (wk) | Total amphotericin B dose (mg/kg of body wt) | Itraconazole dosage (mg/daily) | Fluconazole dosage (mg/daily) |
|---|---|---|---|---|---|---|
| Aa | −12 | —b | — | 200 (1 wk) | ||
| −4 | — | 400 (10 days) | — | |||
| B-4539 | CSFc | 0d | 15 | — | — | |
| B-4540 | CSF | 13 | 10 | 400 (13 wk) | — | |
| B-4541 | CSF | 23 | 15 | — | 400 (10 wk) | |
| B-4542 | CSF | 30 | 17 | 400 (7 wk) | — | |
| B-4543 | CSF | 38 | 35 | 400 (8 wk) | — | |
| B-4544 | CSF | 47 | 35 | 400 (9 wk) | — | |
| B | B-4548 | Brain tissue | 0 | — | — | — |
The patient was treated with fluconazole for oral candidasis 3 months prior to the first episode of cryptococcosis.
—, no treatment.
CSF, cerebrospinal fluid.
Diagnosis of cryptococcosis (the first episode).
Antifungal agents.
Fluconazole, itraconazole, and voriconazole (UK-109,496) were provided as powders by Pfizer Central Research (Groton, Conn.). Stock azole solutions were prepared in the solvent dimethyl sulfoxide at concentrations of 50, 20, and 2.5 mg/ml for fluconazole, itraconazole, and voriconazole, respectively.
Strain typing.
An Iatron Crypto-check test kit was used to identify the serotypes of the isolates. Isolates were crossed with the reference strains B-4476 (MATa) and B-4500 (MATα) on V8 juice agar and were incubated at 30°C to determine their mating types. Strain genotyping was performed by contour-clamped homogeneous electric field analysis (30) as well as randomly amplified polymorphic DNA (RAPD) analysis (29). Genomic DNA was isolated from cells grown overnight at 30°C in 5 ml of yeast extract-peptone-dextrose (YEPD) broth. The cells were lyophilized for 2 h and were pulverized with 1 ml of glass beads (diameter, 3 mm) in a disposable 15-ml tube. Two milliliters of extraction buffer (100 mM Tris-HCl [pH 7.5], 0.7 M NaCl, 10 mM EDTA, 1% [wt/vol], CTAB [mixed alkyltrimethylammonium bromide; Sigma], 1% [vol/vol] β-mercaptoethanol, 0.3 mg of proteinase K per ml) was added, and the suspension was incubated at 65°C for 30 min. The DNA was extracted with 2 ml of chloroform and was precipitated with 2 ml of 2-propanol. After incubation with 50 μg of RNase A (Boehringer) per ml at 37°C for 30 min, the DNA was finally extracted with phenol and chloroform and was reprecipitated in ethanol. The primers used for RAPD analysis were primer 1 (5′-GCGATCCCCA-3′) and primer 2 (5′-AACGCGCAAC-3′) (29).
Susceptibility testing.
The susceptibilities of the strains to amphotericin B, fluconazole, and itraconazole were initially compared by the E test (AB BIODISK, Solna, Sweden) with solidified (1.5% agar) RPMI 1640–2% glucose medium, and the strains were incubated at 35°C for 48 h. Susceptibility to itraconazole and fluconazole was tested by the M27-A microdilution method as recommended by the National Committee for Clinical Laboratory Standards (19). Briefly, 104 cells were inoculated in yeast nitrogen base (YNB; Difco, Detroit, Mich.) with glucose (5 g/liter) broth buffered to pH 7.0 with 0.05 M morpholinepropanesulfonic acid (MOPS) and were incubated at 35°C for 72 h (11). Agar dilution screening was also performed as described by Kirkpatrick et al. (13) to determine susceptibility to fluconazole.
Resistance of clonal populations.
A single colony from the growth of each isolate was suspended in phosphate-buffered saline, and the suspension was plated on Sabouraud modified antibiotic medium 13 agar medium (BBL, Cockeysville, Md.) with various concentrations of fluconazole or voriconazole. For each concentration, about 900 cells were inoculated onto three plates. The number of colonies that grew on plates containing fluconazole or voriconazole at 30°C for 72 h was compared with the number that grew on plates without the drug.
Environmental influence.
The environmental influence on expression of resistance was analyzed by using Sabouraud modified antibiotic medium 13 agar medium containing fluconazole (64 μg/ml) or voriconazole (1 μg/ml) with various pHs (4.5 to 8) and osmolarities (NaCl, 0.5 to 5%). The influence of temperature was studied with temperatures of 30, 35, 37, and 40°C.
Stability of fluconazole resistance in vitro.
Isolates B-4539, B-4544, and B-4548 and their highly resistant subclones B-4539R, B-4544R, and B-4548R, respectively, were transferred daily (0.05 ml of culture) into 5 ml of fresh drug-free Sabouraud modified antibiotic medium 13 (BBL), at 30 and 37°C. The proportion of subpopulations resistant to fluconazole (64 μg/ml) was determined periodically.
RESULTS
In vitro susceptibility.
The susceptibilities of the isolates were first determined against the various antifungal agents that have been used for therapy. The first three isolates (isolates B-4539, B-4540, and B-4541) from patient A were determined to be susceptible to amphotericin B and itraconazole by the E test (Table 2). Although the halos that formed around the fluconazole E-test strips were not clear-cut and a number of colonies appeared throughout the zone of inhibition, growth in the majority of the cells was inhibited by fluconazole at 6 to 16 μg/ml (Table 2). The isolates obtained from subsequent episodes (fourth to sixth episodes) showed increased levels of resistance to fluconazole (MICs, ≥64 μg/ml) but not to amphotericin B or itraconazole, despite the absence of fluconazole from the therapeutic regimen beyond the third episode (Tables 1 and 2). When fluconazole susceptibility was assayed by the microdilution method or agar dilution screening, the cutoff points showed no correlation with the E-test results, but a trend for the stepwise reduction of susceptibility to be inversely related to the concentration of the drug was evident by each method (Table 2). The strain isolated from patient B exhibited resistance to fluconazole (48 μg/ml), despite the absence of any azole therapy (Table 1 and 2). The zone of inhibition for this isolate was also not clearly demarcated, with clonal populations of the isolate exhibiting heterogeneous expression of resistance.
TABLE 2.
Antifungal susceptibilities of seven isolates of C. neoformans from two patients
| Patient | Isolate | E-test MIC (μg/ml)
|
Microdilution MIC80 (μg/ml) of fluconazole | Agar dilution MIC100 (μg/ml) of fluconazole | % of cells resistant to the followinga:
|
|||
|---|---|---|---|---|---|---|---|---|
| Fluconazole | Amphotericin B | Itraconazole | Fluconazole (64 μg/ml) | Voriconazole (1 μg/ml) | ||||
| Control | B-4476 | 0.5 | 0.008 | 0.032 | 2 | 16 | 0 | 0 |
| A | B-4539 | 6 | 0.125 | 0.094 | 6 | 80 | 0.7 | 0.3 |
| B-4540 | 16 | 0.19 | 0.064 | 6 | 100 | 0.8 | 0.13 | |
| B-4541 | 12 | 0.19 | 0.064 | 8 | 100 | 1.4 | 1 | |
| B-4542 | 64 | 0.125 | 0.094 | 18 | 100 | 1.9 | 0.5 | |
| B-4543 | 64 | 0.064 | 0.064 | 12 | >100 | 3.5 | 2.5 | |
| B-4544 | 128 | 0.19 | 0.064 | 23 | >100 | 4.6 | 3.4 | |
| B | B-4548 | 48 | 0.25 | 4 | 16 | >100 | 0.8 | 0.3 |
Number of CFU on drug (fluconazole or voriconazole)-containing plate/number of CFU on drug-free plates × 100.
Unlike the isolates from patient A, the isolate from patient B was resistant to itraconazole (Table 2), with no evidence of heterogeneity. The E test showed sharp MIC endpoints.
Strain typing.
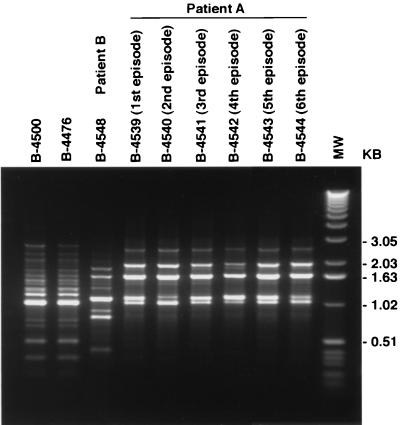
All six isolates from patient A were determined to be of serotype D and of the α mating type, and they had identical karyotypes (data not shown). The identities of these isolates were further confirmed by RAPD analysis with two different primers. DNAs from the six isolates from patient A obtained by amplification with primer 1 had identical RAPD patterns which were different from the pattern produced with DNA from the isolate from patient B as well as two isogenic laboratory strains, strains B-4500 and B-4476 (Fig. 1). The RAPD profiles generated with primer 2 also confirmed the identities of these six isolates from patient A (data not shown). The isolate from patient B was of serotype A and of the α mating type.
FIG. 1.
Amplified RAPD products obtained with primer 1 from DNAs of isolates from patient B (isolate B-4548), patient A (isolates B-4539, B-4540, B-4541, B-4542, B-4543, and B-4544), and an isogenic set of isolates (isolates B-4500 and B-4476) as controls. Lane MW, molecular size marker (1-kb DNA ladder; Life Technologies, GIBCO BRL).
Heteroresistant phenotype.
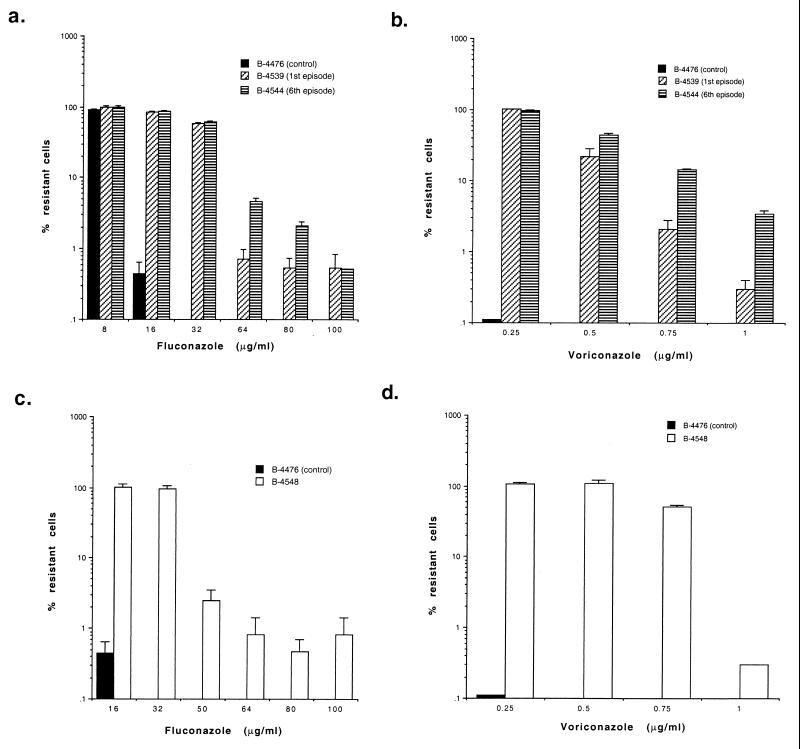
Since heterogeneity in fluconazole resistance among clonal subpopulations of the isolates from patients A and B was evident by the E test, the viabilities of such clonal populations grown in the presence of different concentrations of fluconazole (0 to 100 μg/ml; Fig. 2a and c) were determined by using Sabouraud modified antibiotic medium 13 (BBL) at 30°C. Although the patients were not treated with voriconazole, the presence of heteroresistance to this triazole was also studied with different concentrations (0 to 1 μg/ml; Fig. 2b and d).
FIG. 2.
Percentage of clonal populations resistant to drugs. Isolates retrieved from patient A during the first and the last episodes (a and b) and the isolate retrieved from patient B (c and d) were grown on antibiotic medium 13 agar with different concentrations of fluconazole or voriconazole.
A single clone of each of the six isolates from patient A, selected at random from among colonies of isolates grown on nonselective medium, showed a heterogeneous composition in which most of the cells were susceptible but in which some resistant cells were observed with ≥64 μg of fluconazole per ml (Fig. 2a) and 1 μg of voriconazole per ml (Fig. 2b). The frequency of such resistant clonal populations was highest for the last of the serial isolates. Subpopulations resistant to fluconazole (selected on 64 μg/ml) accounted for less than 1% of isolates from the first episode, while the proportion of such populations increased to about 4.6% among isolates from the last episode (Table 2). The proportion of populations of these serial isolates resistant to voriconazole (1 μg/ml) also increased (Table 2). Additional subpopulations of clones randomly selected from isolates from each episode also showed similar proportions of resistance.
While patient B had never been exposed to fluconazole, 0.8 and 0.3% of the clonal populations were able to grow on fluconazole (100 μg/ml) and voriconazole (1 μg/ml), respectively (Fig. 2c and d, respectively).
A serotype D reference strain, strain B-4476, which was used as a control, showed clear cutoff points: no growth in the presence of 16 μg of fluconazole per ml or 0.25 μg of voriconazole per ml (Fig. 2).
Clonal subpopulations inoculated onto different media such as YEPD, YNB, and Sabouraud agar showed no differences in the patterns or frequencies of resistance.
Resistant clones.
Single colonies grown on medium with 64 μg of fluconazole per ml or 1 μg of voriconazole per ml contained a homogeneous population of resistant cells. Regardless of whether they were isolated from the first or the last episode from patient A or patient B, nearly 100% were able to grow on 64 μg of fluconazole per ml or 1 μg of voriconazole per ml at 30°C (Table 3). These clones could tolerate concentrations of up to 400 μg of fluconazole per ml and 2 μg of voriconazole per ml.
TABLE 3.
Percentage of subpopulation of resistant clones which grew on agar plates supplemented with 64 μg of fluconazole per ml or 1 μg of voriconazole per ml at different temperatures
| Resistant clonea | % Growth
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 30°C
|
35°C
|
37°C
|
40°C
|
|||||
| Fluconazole (64 μg/ml) | Voriconazole (1 μg/ml) | Fluconazole (64 μg/ml) | Voriconazole (1 μg/ml) | Fluconazole (64 μg/ml) | Voriconazole (1 μg/ml) | Fluconazole (64 μg/ml) | Voriconazole (1 μg/ml) | |
| B-4539R | 97 | 100 | 96b | 1.3b | 95b | 0 | 0 | 0 |
| B-4544R | 96 | 89 | 98b | 0.9b | 96b | 0 | 0 | 0 |
| B-4548R | 95 | 103 | 9b | 8.3b | 0 | 0 | 0 | 0 |
Resistant clones were selected on a Sabouraud agar plate containing 64 μg of fluconazole per ml.
The sizes of the colonies were considerably smaller than those of colonies grown on drug-free medium.
Stability of resistance in vitro.
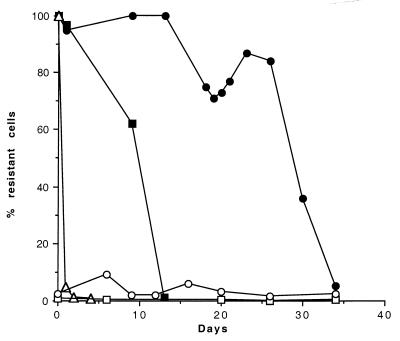
The initial heteroresistant phenotype was regenerated when the clonal cultures homogeneously resistant to ≥64 μg of fluconazole per ml were transferred daily on drug-free medium for a prolonged period (Fig. 3). The homogeneous resistance was lost at rates that were characteristic for each isolate and that were also dependent on the growth temperature. The highly resistant clones from patient A, B-4539R (from the first episode) and B-4544R (from the sixth episode), showed an abrupt loss of resistance by the 10th and 27th transfers on drug-free medium at 30°C (Fig. 3) and by the 2nd and 14th transfers at 37°C (data not shown), respectively. Isolate B-4548R (patient B) reverted to the initial heteroresistance phenotype after only one transfer at 30°C (Fig. 3).
FIG. 3.
Stability of fluconazole resistance of isolates B-4539 (□) and B-4544 (○) and their highly resistant clones B-4539R (■), B-4544R (●), and B-4548R (▵) (selected on 64 μg of fluconazole per ml) after daily transfers in drug-free medium.
The original basal level of resistant subpopulations, however, persisted even after 35 transfers. Daily transfers (total 35 days) of the isolates from the first and sixth episodes on drug-free medium also showed no significant changes in the maintenance of the initial proportion of the subpopulations that were able to form colonies on 64 μg of fluconazole per ml (0.72 to 4.7%) (Fig. 3).
Environmental influence.
Expression of resistance was found to be unaffected by other environmental factors such as the pH (4.5 to 8) or osmolarity (NaCl, 0.5 to 5%) of the medium (data not shown) but was found to be influenced by incubation temperature (Table 3). The growth rates of resistant clones B-4539R and B-4544R were substantially slower on medium with fluconazole (64 μg/ml) at 37°C, and the clones became completely susceptible when they were incubated at 40°C. The effect of temperature on the expression of fluconazole resistance was more profound for resistant clone B-4548R derived from the isolate from patient B. Only 9% of the cells of B-4548R grew at 35°C on fluconazole-containing medium, while no growth was seen at 37°C. On voriconazole-containing agar, the resistant clones were more thermosensitive. The proportions of resistant subpopulations of B-4539R, B-4544R, and B-4548R decreased significantly at 35°C, and all became sensitive when they were grown at 37°C (Table 3).
DISCUSSION
Drug resistance in eukaryotic cells apparently evolved gradually over time via several mechanisms such as reduction in uptake, degradation of imported drug, changes in the interaction with the target enzyme, and increased efflux of the drug (17, 33). Although the mechanisms of azole resistance have not been clearly defined in C. neoformans, it is thought that exposure to the drug triggers the development of resistance. The emergence of fluconazole resistance during long-term therapy has been reported in a few isolated cases of AIDS-associated cryptococcal meningitis (1–4, 22). Moreover, in a murine model of cryptococcal meningitis, Velez et al. (31) demonstrated a correlation between the in vitro susceptibility of a strain and the in vivo response to fluconazole. Other studies based on serial C. neoformans isolates from 124 AIDS patients, however, found no evidence of such emerging resistance (4, 6, 35). The development of heteroresistance to fluconazole described here appears to be unrelated to drug exposure, since the proportion of highly resistant subpopulations beyond the third episode (patient A) steadily increased, even though the patients had not been on fluconazole maintenance therapy. Although the itraconazole maintenance therapy in patient A could have been involved in the occurrence of the fluconazole-heteroresistant strain, it is unlikely since resistance to itraconazole was not observed in these isolates. The possibility that a resistant clone would be selected during fluconazole treatment prior to the onset of cryptococcosis in patient A does not explain the isolation of a highly resistant strain from patient B who has never been exposed to fluconazole.
What distinguishes the pattern observed in our strains from the patterns of other resistant strains of C. neoformans is that a single cell produces progeny with a wide range of fluconazole or voriconazole susceptibilities and purification of a sensitive subpopulation is not possible by repeated subcloning. Furthermore, clones consisting of homogeneous resistant cells are selectable on medium with a high drug concentration after a single passage, while the original heterogeneous phenotype reemerges by subsequent repeated culturing on drug-free medium. No association between heteroresistance and serotype was suggested since the phenomenon was observed in both serotype A (patient B) and D (patient A) isolates.
This phenomenon is different from the epigenic resistance recently described in Candida albicans (5, 17). The cells of C. albicans isolates exposed to fluconazole became resistant to the drug within 15 to 20 days in vitro and 15 days in vivo. The resistance was unstable in both cases; the susceptible phenotype was restored in 100% of cells once the drug was removed from the growth medium (5, 17). It is also different from the high variability in azole resistance observed between different subclones of single strains for Candida spp. (26). Schoofs et al. (26) isolated strains of Candida glabrata, Candida kruzei, and C. albicans which were composed of subpopulations that were highly variable while growing in the presence of specific concentrations of itraconazole and fluconazole. Unlike our isolates, however, the variations in the relative growth phenotype were stable in subcultures regardless of the presence or absence of drugs in the culture medium. Furthermore, the passage of a clone through a medium containing fluconazole increased the proportion of the population that was resistant but did not select clones that consisted of homogeneously resistant cells.
The patterns of heteroresistance to fluconazole and voriconazole were similar for the isolates from both patients A and B. In contrast to the isolates from patient A, however, the isolate from patient B showed cross-resistance to itraconazole, suggesting differences in mechanisms of resistance. Unlike with fluconazole, no heterogeneous expression of resistance to itraconazole was observed for any of the isolates, and the drug inhibition level was clearly demarcated.
Voriconazole was described to be more active than fluconazole in blocking ergosterol synthesis in C. albicans (25) and more effective at inhibiting the growth of C. neoformans (20, 23). In vitro tests with voriconazole against 566 clinical isolates of C. neoformans, MICs at which 90% of isolates are inhibited (MIC90s) were 0.12 to 0.25 μg/ml, which indicates that it has a higher efficiency than fluconazole (MIC90s, 8 to 16 μg/ml) (23). Growth of our isolates was similarly inhibited on Sabouraud agar supplemented with 64 μg of fluconazole per ml and on Sabouraud agar supplemented with 1 μg of voriconazole per ml, indicating cross-resistance between these two azoles. Voriconazole, however, was about 70-fold more active against our isolates than fluconazole in vitro at 30°C, and the fluconazole-resistant isolates showed no resistance to voriconazole at 37°C.
A heteroresistance pattern similar to that of our isolates has been reported for methicillin resistance in S. aureus (7, 27). The methicillin-heteroresistant S. aureus strains produced cultures in which the MIC for most of the bacteria (99.9%) was about 5 μg/ml, but isolates for which MICs were 250 μg/ml or higher were also recovered at a low frequency (10−6 to 10−7). The degree of heterogeneity and stability of the resistant clones was shown to be influenced by environmental factors such as temperature, osmolarity, pH, light, anaerobiosis, chelating agents, and metal ions (18). Although the mechanisms for the intriguing features of methicillin heteroresistance in S. aureus are far from explained, complex genetic controls have been suggested (7–9, 15, 24, 34). To date, several genes associated with methicillin resistance have been identified, and their molecular regulation is of research interest (7, 10, 16). A plausible explanation for heteroresistance could be that all cells carry the genetic marker(s) for resistance, but phenotypic expression of such resistance occurs only in a very small fraction of the population (12). Such an explanation may apply to our isolates of C. neoformans since all the subpopulations of the highly resistant clones tolerated high concentrations of the drug, their resistance was influenced by temperature, and their phenotypes could be reverted to the initial phenotype. Genetic mutations generally occur at a low frequency (10−6 to 10−8/gene) and do not explain this type of heteroresistance. Moreover, the basal level of heterogeneity in our isolates appears to be stable and strain dependent, as is the case in methicillin resistance of S. aureus (12).
ACKNOWLEDGMENTS
We thank Dan Sheehan at Pfizer Inc., Roerig Division, U.S. Pharmaceuticals Group, for providing us with fluconazole and voriconazole.
REFERENCES
- 1.Armengou A, Porcar C, Mascaro J, Garcia-Bragaro F. Possible development of resistance to fluconazole during suppressive therapy for AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1996;23:1337–1338. doi: 10.1093/clinids/23.6.1337-a. [DOI] [PubMed] [Google Scholar]
- 2.Berg J, Clancy C J, Nguyen M H. The hidden danger of primary fluconazole prophylaxis for patients with AIDS. Clin Infect Dis. 1998;26:186–187. doi: 10.1086/517056. [DOI] [PubMed] [Google Scholar]
- 3.Birley H D L, Johnson E M, McDonald P, Parry C, Carey P B, Warnock D W. Azole drug resistance as a cause of clinical relapse in AIDS patients with cryptococcal meningitis. Int J STD AIDS. 1995;6:353–355. doi: 10.1177/095646249500600510. [DOI] [PubMed] [Google Scholar]
- 4.Brandt M E, Pfaller M A, Hajjeh R A, Graviss E A, Rees J, Spitzer E D, Pinner R W, Mayer L W Cryptococcal Disease Active Surveillance Group. Molecular subtypes and antifungal susceptibilities of serial Cryptococcus neoformans isolates in human immunodeficiency virus-associated cryptococcosis. J Infect Dis. 1996;174:812–820. doi: 10.1093/infdis/174.4.812. [DOI] [PubMed] [Google Scholar]
- 5.Calvet H M, Yeaman M R, Filler S G. Reversible fluconazole resistance in Candida albicans: a potential in vitro model. Antimicrob Agents Chemother. 1997;41:535–539. doi: 10.1128/aac.41.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall A, Spitzer E D, Webb D, Rinaldi M G. Susceptibilities of serial Cryptococcus neoformans isolates from patients with recurrent cryptococcal meningitis to amphotericin B and fluconazole. Antimicrob Agents Chemother. 1993;37:1383–1386. doi: 10.1128/aac.37.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers H F. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev. 1997;10:781–791. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLencastre H, Figuiredo A M S, Tomasz A. Genetic control of population structure in heterogeneous strains of methicillin resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1993;12:S13–S17. doi: 10.1007/BF02389872. [DOI] [PubMed] [Google Scholar]
- 9.Duran S P, Kayser F H, Berger-Bachi B. Impact of sar and arg on methicillin-resistance in Staphylococcus aureus. FEMS Microbiol Lett. 1996;141:255–260. doi: 10.1111/j.1574-6968.1996.tb08394.x. [DOI] [PubMed] [Google Scholar]
- 10.Fujimura T, Murakami K. Increase of methicillin resistance in Staphylococcus aureus caused by deletion of a gene whose product is homologous to lytic enzymes. J Bacteriol. 1997;179:6294–6301. doi: 10.1128/jb.179.20.6294-6301.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghannoum M A, Ibrahim A S, Fu Y, Shafiq M C, Edwards J E, Jr, Criddle R S. Susceptibility testing of Cryptococcus neoformans: a microdilution technique. J Clin Microbiol. 1992;30:2881–2886. doi: 10.1128/jcm.30.11.2881-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartman B J, Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986;29:85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirkpatrick W R, McAtee R K, Revankar S G, Forthergill A W, McCarthy D I, Rinaldi M G, Patterson T F. Comparative evaluation of NCCLS broth macrodilution and agar dilution screening methods for testing fluconazole susceptibility of Cryptococcus neoformans. J Clin Microbiol. 1998;36:1330–1332. doi: 10.1128/jcm.36.5.1330-1332.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon-Chung K J, Bennett J E. Medical mycology. Malvern, Pa: Lea & Febiger; 1992. Cryptococcosis; pp. 397–446. [Google Scholar]
- 15.Maki H, Yamaguchi T, Murakami K. Cloning and characterization of a gene affecting the methicillin resistance level and the autolysis rate in Staphylococcus aureus. J Bacteriol. 1994;176:4993–5000. doi: 10.1128/jb.176.16.4993-5000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maki H, Murakami K. Formation of potent hybrid promoters of the mutant llm gene by IS256 transposition in methicillin-resistant in Staphylococcus aureus. J Bacteriol. 1997;179:6944–6948. doi: 10.1128/jb.179.22.6944-6948.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marr K A, Lyons C N, Rustad T, Bowden R A, White T C. Rapid, transient fluconazole resistance in Candida albicans is associated with increased mRNA levels of CDR. Antimicrob Agents Chemother. 1998;42:2584–2589. doi: 10.1128/aac.42.10.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews P R, Stewart P R. Resistance heterogeneity in methicillin-resistant Staphylococcus aureus. FEMS Microbiol Lett. 1984;22:161–166. [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard. NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 20.Nguyen M H, Yu C Y. In vitro comparative efficacy of voriconazole and itraconazole against fluconazole-susceptible and -resistant Cryptococcus neoformans isolates. Antimicrob Agents Chemother. 1998;42:471–472. doi: 10.1128/aac.42.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orni-Wasserlauf R, Siegman-Igra Y, Izkhakov E, Bash E, Polacheck I, Giladi M. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Fluconazole-resistant Cryptococcus neoformans isolated from an immunocompetent patient without prior exposure to fluconazole, abstr. J-24; p. 458. [DOI] [PubMed] [Google Scholar]
- 22.Paugam A, Dupouy-Camet J, Blanche P, Gangneux J P, Tourte-Schaefer C, Sicard D. Increased fluconazole resistance of Cryptococcus neoformans isolated from a patient with AIDS and recurrent meningitis. Clin Infect Dis. 1993;17:431–436. doi: 10.1093/clinids/19.5.975-a. [DOI] [PubMed] [Google Scholar]
- 23.Pfaller M A, Zhang J, Messer S A, Brandt M E, Hajjeh R A, Jessup C J, Tumberland M, Mbidde E K, Ghannoum M A. In vitro activities of voriconazole, fluconazole, and itraconazole against 566 clinical isolates of Cryptococcus neoformans from the United States and Africa. Antimicrob Agents Chemother. 1999;43:169–171. doi: 10.1093/oxfordjournals.jac.a020873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryffel C, Strassle A, Kayser F H, Berger-Bachi B. Mechanisms of heteroresistance in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:724–728. doi: 10.1128/aac.38.4.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanati H, Belanger P, Fratti R, Ghannoum M. A new triazole, voriconazole (UK-109,496), blocks sterol biosynthesis in Candida albicans and Candida krusei. Antimicrob Agents Chemother. 1997;41:2492–2496. doi: 10.1128/aac.41.11.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoofs A, Odds F C, Colebunders R, Ieven M, Wouters L, Goossens H. Isolation of Candida species on media with and without added fluconazole reveals high variability in relative growth susceptibility phenotypes. Antimicrob Agents Chemother. 1997;41:1625–1635. doi: 10.1128/aac.41.8.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seligman S J. Methicillin-resistant staphylococci: genetics of the minority population. J Gen Microbiol. 1966;42:315–322. doi: 10.1099/00221287-42-2-315. [DOI] [PubMed] [Google Scholar]
- 28.Spitzer E D, Spitzer S G, Freundlich L F, Casadevall A. Persistence of initial infection in recurrent Cryptococcus neoformans meningitis. Lancet. 1993;341:595–596. doi: 10.1016/0140-6736(93)90354-j. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan D, Haynes K, Moran G, Shanley D, Coleman D. Persistence, replacement, and microevolution of Cryptococcus neoformans strains in recurrent meningitis in AIDS patients. J Clin Microbiol. 1996;34:1739–1744. doi: 10.1128/jcm.34.7.1739-1744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varma A, Kwon-Chung K J. Formation of a minichromosome in Cryptococcus neoformans as a result of electroporative transformation. Curr Genet. 1994;26:54–61. doi: 10.1007/BF00326305. [DOI] [PubMed] [Google Scholar]
- 31.Velez J D, Allendoerfer R, Luther M, Rinaldi M G, Graybill J R. Correlation of in vitro azole susceptibility with in vivo response in a murine model of cryptococcal meningitis. J Infect Dis. 1993;168:508–510. doi: 10.1093/infdis/168.2.508. [DOI] [PubMed] [Google Scholar]
- 32.Venkateswarlu K, Taylor M, Manning N J, Rinaldi M G, Kelly S L. Fluconazole tolerance in clinical isolates of Cryptococcus neoformans. Antimicrob Agents Chemother. 1997;41:748–751. doi: 10.1128/aac.41.4.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White T C, Marr K A, Bowden R A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whu S, DeLencastre H, Thomasz A. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J Bacteriol. 1996;178:6036–6042. doi: 10.1128/jb.178.20.6036-6042.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witt M D, Lewis R J, Larsen R A, Milefchik E N, Leal M A E, Haubrich R H, Richie J A, Edwards J E, Jr, Ghannoum M A. Identification of patients with acute AIDS-associated cryptococcal meningitis who can be effectively treated with fluconazole: the role of antifungal susceptibility testing. Clin Infect Dis. 1996;22:322–328. doi: 10.1093/clinids/22.2.322. [DOI] [PubMed] [Google Scholar]