Abstract
We estimated the effectiveness of Comirnaty and Vaxzevria vaccines among 371,423 residents in Lazio Region (Italy) vaccinated since 27/12/2020, and followed until diagnosis of SARS-CoV-2 infection or 25/4/2021, whichever came first. By the end of follow-up most of the Comirnaty-cohort (60%) had received the second dose at recommended time of 21 days (98%), while the Vaxzevria-cohort had received only one dose. Adjusted hazard ratios of SARS-CoV-2 infection at weekly intervals since the first dose were estimated through a Cox regression model using 0–13 days as reference time-interval. An increase in effectiveness with increasing time since administration was observed for Comirnaty (five-weeks = 81%, 95 %CI 71–88%; three-months = 94%, 95 %CI 84–98%). One dose of Vaxzevria showed an effectiveness of 63% (95 %CI 25–82%) after 7 weeks, although further analyses are needed after complete vaccination with two doses. These results could support the ongoing vaccination campaign by reinforcing evidence-based communication aimed at reducing vaccine hesitancy.
Keywords: Covid-19, SARS-CoV-2 infection, Vaccine effectiveness, Comirnaty, Vaxzevria, Cohort study
1. Introduction
By February 2022, since the beginning of the pandemic, the coronavirus disease (Covid-19) has caused nearly 12 million cases and over 150,000 deaths in Italy [1]. Lazio region (central Italy) was one of the most hit areas with 977.804 Covid-19 diagnosis and over 10,000 deaths, most of which occurred in the city of Rome [2].
In less than a year four vaccines to prevent Covid-19 were authorized in the European Union and gradually introduced into the market by the European Medicines Agency: Comirnaty, Moderna, Vaxzevria, and Janssen [3].
An increasing number of studies have attempted to evaluate the vaccination campaign on the general population in real-world settings, showing high vaccine effectiveness (VE) either against asymptomatic and symptomatic infections with severe adverse respiratory syndrome coronavirus 2 (SARS-CoV-2) or severe Covid-19 (infections leading to hospitalization or death) in the general population [4], [5]. Published studies are focused mostly on the Comirnaty vaccine and on selected populations such as health care workers [6], while very few evaluated the VE of the Vaxevria brand [7], [8].
The estimated effectiveness of the first dose of the Comirnaty vaccine for documented infections was 46% in a general population study [5] and 84% in a health care workers population [6], both studies referred to a period of 14–21 days since the first dose administration; another study conducted on health care workers in England [9] showed an effectiveness of 70% after 21 days since the first dose administration. These studies reported high estimated effectiveness of 85% [9], 92% [5], and 95% [6] seven days after the second dose. A recent meta-analysis on real-world based studies reported a VE of 85% after two doses in preventing SARSCoV-2 infections [10].
Among older adults in England, Vaxzevria vaccine was found to reduce the odds of Covid-19 disease by 60% from 28 to 34 days since the first dose administration, and by 73% from day 35 onwards [11]. In Sweden, it was reported 68% VE from two to four weeks after the second dose of Vaxzevria against SARS-CoV-2 infection of any severity [7].
In the Lazio region (over 5,7 million residents), as elsewhere in Italy, the vaccination campaign started on December 27, 2020. The Comirnaty vaccine was the first vaccine available for the administration. The selected strategy aimed at rapidly protecting the front-line healthcare workers, who were the most exposed to the risk of infection, and targeted individuals at high risk of contracting severe Covid-19, such as nursing home patients and elderly people ≥ 80 years. Thanks to rapid vaccine stocking implementation, it was soon possible to extend the offer to other vulnerable groups, such as: diabetic patients, patients with cancer, cardiovascular or respiratory diseases and other frail individuals. The Moderna vaccine was introduced on January 7, 2021 and was offered to the same target patients as the Comirnaty vaccine due to their similar characteristics. With the introduction of the Vaxzevria vaccine in mid-February, public force workers, school personnel and people aged 65–79 were also targeted for vaccination. Finally, the Janssen single-dose vaccine has been introduced since late April 2021 and recommended to people aged 60 years and over.
All residents in the Lazio region meeting the vaccination campaign enrollment criteria and in accordance with the established vaccination schedule by category, can be vaccinated anywhere in the region. The Local Health Authority Rome 2 (hereafter defined as RM2; 1,234,355 inhabitants on December 31, 2020; 470 km2) is the largest Local Health Authority of the Municipality of Rome (4,253,314 inhabitants on December 31, 2020) with 12 vaccination hubs in its territory.
This study aims at estimating the effectiveness of Comirnaty and Vaxzevria vaccines in preventing SARS-CoV-2 infection of any severity (both symptomatic or asymptomatic) among residents in the Lazio region who received at least one dose of vaccine from any RM2′s vaccination hubs.
2. Methods
A retrospective cohort study was conducted on all residents in the Lazio region who have received at least one dose of vaccine Comirnaty or Vaxzevria from one of the RM2 vaccination hubs since the beginning of the vaccination campaign on December 27, 2020 and up to April 11, 2021. Patients who received the Moderna vaccine were not included in the study population, due to the limited number of doses administered and the discontinuous utilisation during the study period.
Demographic information, vaccination priority group, dates of vaccine administration, and vaccine brand were retrieved from the regional vaccine database. Date of diagnoses of SARS-CoV-2 infection, detected using a RT-PCR test or an antigenic test and occurred between December 27, 2020 and April 25, 2021, were obtained through record linkage with the regional Covid-19 monitoring database updated on May 10, 2021, thus allowing for 15 days of possible notification delay. The two sources were matched on with a deterministic linkage procedure using the tax code as a unique identifier.
Vaccinated patients with a documented SARS-CoV-2 infection occurring before the administration of the first vaccine dose were excluded. A minimum of 14 days of follow-up after vaccination was required as an inclusion criterium, in order to allow a possible SARS-CoV-2 infection to be ascertained, considering the incubation period and the time elapsing from the prescription to the execution of the antigenic or PCR test.
The analyses were conducted separately for the cohorts receiving Comirnaty or Vaxzevria vaccine, as the two brands were offered to different target populations and at different calendar periods. For each cohort, the incidence rate (IR) of SARS-CoV-2 infection were computed for the time interval 0–13 days since the first dose administration and thereafter at weekly intervals. IRs were also computed by 15-day calendar intervals starting from the beginning of the vaccination campaign (December 27, 2020 for Comirnaty and February 11, 2021 for Vaxzevria vaccine).
A time-to-event analysis was conducted by means of a multivariable Cox regression model. Subjects were followed from the date of administration of the first dose until April 25, 2021, or the date of SARS-CoV-2 infection if it occurred before. The incidence of SARS-CoV2 infection in the first 14 days after the first dose was considered comparable to that of the unvaccinated population, assuming that the immunity in this time frame is negligible. The hazard of SARS-Cov2 infection in each weekly interval, starting from the 15th day since vaccine administration, were compared with the hazard in the first 14 days. The calendar time was specified as the time axis to account for variation in the epidemics intensity during the study period. Hazard ratios (HR) were adjusted for gender, age group (0–44, 45–64, 65–79, 80 + ), calendar week of first dose administration, and vaccination priority group based on age, working exposure and health status (see Table 1 ).
Table 1.
Distribution of the study population by vaccine brand, demographic characteristics, vaccination priority group, and calendar period of first dose administration. Person-days of follow-up and incidence rates of SARS-CoV-2 infection per 10,000 person-days.
| Vaccine brand |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total |
Comirnaty |
Vaxzevria |
|||||||||||||
| n | % | n | % | Person time (days) |
COVID-19 cases |
Incidence rate x 10,000 |
n | % | person time (days) |
COVID-19 cases |
Incidence rate x 10,000 |
||||
| Total | 371,423 | 100.0 | 221,000 | 59.5 | 1,189.6 | 1,616 | 1.4 | 150,423 | 40.5 | 579.3 | 1,017 | 1.8 | |||
| Gender | |||||||||||||||
| Males | 156,430 | 42.1 | 92,320 | 41.8 | 492.6 | 639 | 1.3 | 64,110 | 42.6 | 240.0 | 397 | 1.7 | |||
| Females | 214,993 | 57.9 | 128,680 | 58.2 | 697.1 | 977 | 1.4 | 86,313 | 57.4 | 339.3 | 620 | 1.8 | |||
| Age class (years) | |||||||||||||||
| 0–17 | 183 | 0.0 | 183 | 0.1 | 0.7 | 3 | 4.1 | 0 | 0.0 | 0.0 | 0 | 0.0 | |||
| 18–24 | 4,882 | 1.3 | 3,213 | 1.5 | 24.2 | 27 | 1.1 | 1,669 | 1.1 | 7.1 | 20 | 2.8 | |||
| 25–44 | 57,466 | 15.5 | 23,993 | 10.9 | 197.2 | 324 | 1.6 | 33,473 | 22.3 | 151.5 | 310 | 2.0 | |||
| 45–64 | 105,028 | 28.3 | 42,469 | 19.2 | 313.9 | 424 | 1.4 | 62,559 | 41.6 | 279.1 | 523 | 1.9 | |||
| 65–79 | 114,751 | 30.9 | 62,196 | 28.1 | 236.5 | 258 | 1.1 | 52,555 | 34.9 | 140.5 | 164 | 1.2 | |||
| 80+ | 89,113 | 24.0 | 88,946 | 40.2 | 417.2 | 580 | 1.4 | 167 | 0.1 | 0.7 | 0 | 0.0 | |||
| Vaccination priority group | |||||||||||||||
| age 80+ | 81,131 | 21.8 | 81,078 | 36.7 | 375.4 | 408 | 1.1 | 53 | 0.0 | 0.2 | 0 | 0.0 | |||
| nursing home patients | 6,532 | 1.8 | 6,529 | 3.0 | 50.1 | 179 | 3.6 | 3 | 0.0 | 0.0 | 0 | 0.0 | |||
| health care workers | 64,014 | 17.2 | 53,427 | 24.2 | 493.1 | 679 | 1.4 | 10,587 | 7.0 | 53.0 | 112 | 2.1 | |||
| frail/disabled patients | 14,588 | 3.9 | 14,430 | 6.5 | 48.4 | 82 | 1.7 | 158 | 0.1 | 0.6 | 0 | 0.0 | |||
| police/public officer | 46,637 | 12.6 | 584 | 0.3 | 2.8 | 3 | 1.1 | 46,053 | 30.6 | 207.2 | 388 | 1.9 | |||
| school personnel | 23,557 | 6.3 | 127 | 0.1 | 0.4 | 2 | 4.6 | 23,430 | 15.6 | 108.5 | 233 | 2.1 | |||
| age 65–79 | 88,958 | 24.0 | 41,170 | 18.6 | 128.6 | 118 | 0.9 | 47,788 | 31.8 | 124.3 | 138 | 1.1 | |||
| other | 23,644 | 6.4 | 12,524 | 5.7 | 62.8 | 107 | 1.7 | 11,120 | 7.4 | 43.6 | 84 | 1.9 | |||
| missing | 22,362 | 6.0 | 11,131 | 5.0 | 28.0 | 38 | 1.4 | 11,231 | 7.5 | 42.1 | 62 | 1.5 | |||
| Calendar period | |||||||||||||||
| <jan15 | 36,855 | 9.9 | 36,855 | 16.7 | 392.2 | 647 | 1.6 | 0 | 0.0 | 0.0 | 0 | 0.0 | |||
| jan 15 - jan 30 | 9,404 | 2.5 | 9,404 | 4.3 | 89.7 | 116 | 1.3 | 0 | 0.0 | 0.0 | 0 | 0.0 | |||
| jan 31 - feb 14 | 14,873 | 4.0 | 13,503 | 6.1 | 99.4 | 98 | 1.0 | 1,370 | 0.9 | 9.7 | 20 | 2.1 | |||
| feb 15 - mar 1 | 53,469 | 14.4 | 32,778 | 14.8 | 202.4 | 207 | 1.0 | 20,691 | 13.8 | 122.4 | 232 | 1.9 | |||
| mar 2 - mar 16 | 87,802 | 23.6 | 36,278 | 16.4 | 169.6 | 219 | 1.3 | 51,524 | 34.3 | 242.2 | 476 | 2.0 | |||
| mar 17 - mar 28 | 76,043 | 20.5 | 37,123 | 16.8 | 122.9 | 199 | 1.6 | 38,920 | 25.9 | 124.8 | 199 | 1.6 | |||
| mar 29 -apr 11 | 92,977 | 25.0 | 55,059 | 24.9 | 113.6 | 130 | 1.1 | 37,918 | 25.2 | 80.5 | 90 | 1.1 | |||
Vaccine Effectiveness (VE) were estimated from the adjusted HRs as follows: VE = (1 - HR) × 100.
All analyses were conducted using the Statistical Package Stata/MP 17 (StataCorp LP, College Station, TX, USA; STATA code for the effectiveness estimates is available in the Supplementary material). The dissemination of Covid-19 surveillance data was authorised by the Italian Presidency of the Council of Ministers on 27 February 2020 (Ordinance n. 640).
3. Results
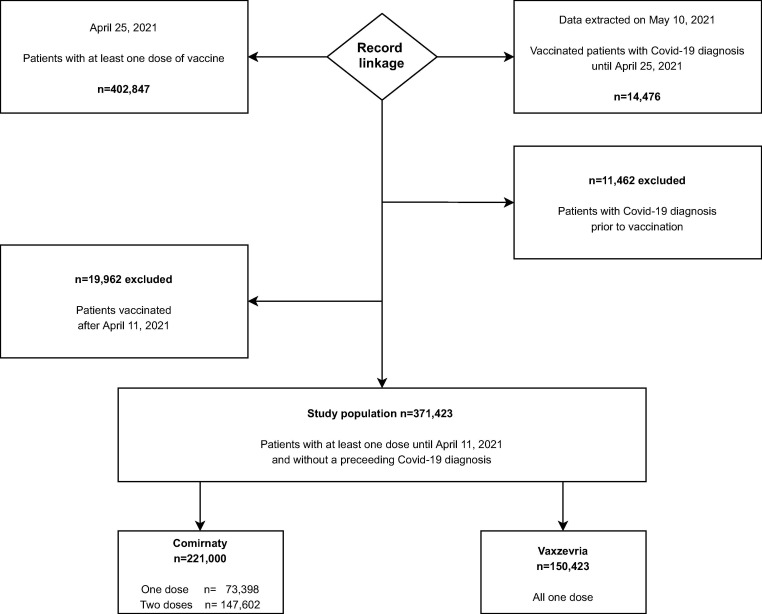
As of April 25, 2021 a total of 402,847 residents in the Lazio Region had received at least one dose of a vaccine from one of the RM2 vaccination hubs. Of these, a total of 11,462 (2,8%) individuals with a documented SARS-CoV-2 infection prior to the date of their first dose administration were excluded, as well as individuals receiving the first dose after April 11, 2021 (n = 19,962, 5.0%) (Fig. 1 ).
Fig. 1.
Flow chart of the selection criteria of the population under study.
A total of 371,423 individuals were included in the study. The distributions by demographic characteristics, vaccination priority group, and calendar period of administration, both overall and separately for each vaccine cohort, are described in Table 1. At the end of the follow-up, the majority of patients vaccinated with Comirnaty vaccine (60.0%) had received the second dose (67%), mostly at the recommended time of 21 days (98%); only 5% of individuals who received only the first dose of Comirnaty had a follow-up ≥ 21 days. All patients vaccinated with Vaxzevria vaccine (40.0%) had received only the first dose by the end of the study period. In both cohorts the percentage of vaccinated individuals was higher in women (58.2% Comirnaty, 57.4% Vaxzevria) than in men. Sixty-eight percent of individuals vaccinated with Comirnaty were aged 65 years and over, while 65% of those receiving Vaxzevria were younger than 65 years. The distribution by vaccination priority group reflects the different target populations to which the two brands were offered: Comirnaty was administered mainly to elderly population aged 80+ (36.7%), health care workers (24.2%) and elderly individuals aged 65–79 (18.6%); Vaxzevria vaccine was first administered to the police/public officer personnel (30.6%) and school personnel (15.6%), before being extended to people aged 65–79 (31.8%).
A decrease in Comirnaty administration is observed after the second week since the start of the vaccination campaign (January 15 to February 14, 2021), reflecting the sudden reduction in the distribution of Comirnaty vaccines in Europe. The pace/speed rose back again in mid-February, reaching a peak of 55,059 administrations in the latest two weeks. The calendar period of Vaxzevria administration shows a slow start in the first two weeks of administration (January 31 - February 14), and then a peak in the 5th and 6th week (March 2 – March 16).
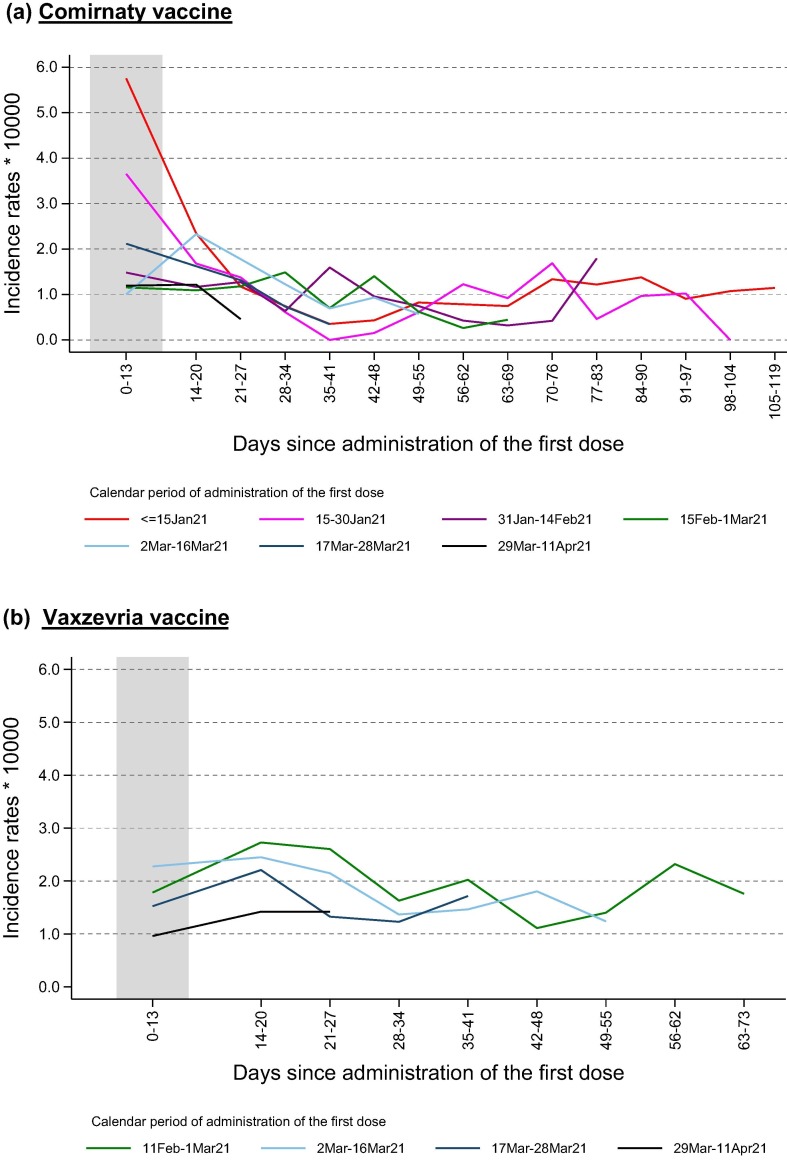
Fig. 2 shows the IRs of Covid-19 by time elapsed since the first dose and by calendar period of administration, separately for Comirnaty (a) and Vaxzevria (b) cohorts. In general, for both brands, the IRs decrease with the increase of days since vaccination, especially among those who received the Comirnaty vaccine in the early stage of the vaccination campaign, probably reflecting the fact that patients at higher risk of infection were prioritized during this initial phase.
Fig. 2.
Incidence rates of SARS-CoV-2 infection by number of days elapsed since the first dose and calendar period of administration. Patients vaccinated with at least one dose of Comirnaty vaccine (a) or Vaxzevria vaccine (b).
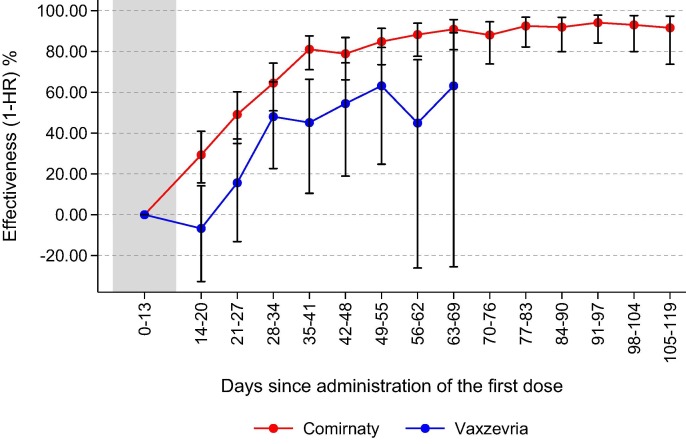
Fig. 3 shows the adjusted vaccine effectiveness of Comirnaty and Vaxzevria vaccines by days since the first dose administration. The effectiveness increased steeply until 35–41 days for Comirnaty (VE = 81.1%; 95% Confidence Interval, CI 71.1–87.7%) and until 49–55 days for Vaxzevria (VE = 63.2%; 95% CI 24.8–82.0%), and then slightly increased (up to 94.2% after three months) and stabilized, respectively (see also Table 1S available in the Supplementary material).
Fig. 3.
Adjusted* vaccine effectiveness against SARS-CoV-2 infection by week since first dose administration and vaccine brand. *percent Vaccine Effectiveness (VE) = (1 - HR) × 100; HR are the Hazard Ratios of Covid-19 infection for weekly intervals since first dose obtained from a Cox regression model adjusted for gender, age group (0–44, 45–64, 65–79, 80 + ), vaccination priority group and weekly calendar period of first dose administration.
4. Discussion
This is one of the first studies estimating the effectiveness of both Comirnaty and Vaxzevria in preventing SARS-CoV-2 infection in a large resident population in Italy.
We were able to provide weekly estimates of Comirnaty effectiveness up to three months from the first dose administration. We found an effectiveness of Comirnaty vaccine above 80% at 35–41 days, that is after five weeks from the first dose, or – given that the recommended time of 21 days for the second dose administration was respected - two weeks after the second dose. We observed an increasing effectiveness thereafter, reaching 93% at the end of follow-up (105–119 days after the first dose).
Assessment on the Vaxzevria cohort also showed a growing effectiveness with increasing time since vaccination. An effectiveness of 63% was observed after about two months from the first dose administration, lower compared to Comirnaty effectiveness at the same time point. However, it is important to note that almost all individuals vaccinated with Comirnaty had already received the second dose after two months from the first one, while none of those vaccinated with Vaxzevria had. This makes the comparison between the two vaccine brands largely incomplete with the data available at the time of the analyses.
Our estimates of Comirnaty vaccine effectiveness are comparable with those from other studies conducted in Israel [5], England [9], and Italy [4], [6] reporting a high effectiveness for documented infections after seven days from the second dose administration. Similar results were also reported by Liu et al. [10] when restricting the meta-analysis to the epidemic phase with prevalent circulation of the Alpha variant, which was predominant variant at the time of our study [12].
As for Vaxzevria, an efficacy of 64% (95% CI 51–74) from 21 days since the first dose and before the second dose is reported in a pooled analysis of four clinical trials [13]. An observational study conducted in England among older adults aged 70 years or more showed a 60% effectiveness in preventing symptomatic infections from 28 to 34 days since the first dose administration, and 73% from day 35 onwards [11].
Our study has several limitations. First, as the vaccination campaign for Vaxzevria had a later roll-out, the follow-up time was not long enough to estimate vaccine effectiveness for the latest time intervals and therefore to assess effectiveness of complete vaccination with two doses. Second, based on available data, it was not possible to estimate the effectiveness of vaccines in preventing other Covid-19 associated events (e.g. development of symptoms, hospitalization, and death), nor to account for virus variants. Third, vaccine effectiveness could have been underestimated due to the undetected asymptomatic individuals who did not undergo testing. Finally, vaccine effectiveness could have also been underestimated due to the assumption that the control group of individuals vaccinated since less than 15 days were completely unprotected from vaccine. However, several studies, where detailed information on unvaccinated people were unavailable, have used the time interval 0–14 days post first dose as reference to calculate vaccine effectiveness, showing estimates that were in line with those from other studies using the exposure time in unvaccinated people as reference [4], [14], [15], [16]. Moreover, using data from only vaccinated individuals might have contributed to reduce the possible bias due to different behaviors between vaccinated and unvaccinated persons (e.g., probability of testing and risk exposure due to participation to social activities).
5. Conclusion
The mass vaccination campaign conducted in Lazio region showed a high effectiveness in preventing SARS-CoV-2 infection. Vaxzevria vaccine showed a lower effectiveness compared to Comirnaty at the early stage of the vaccination campaign. These results contribute to the scientific knowledge on vaccine effectiveness and support the ongoing vaccination campaign by reinforcing evidence-based communication aimed at reducing vaccine hesitancy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Special thanks go to all the health care workers of RM2’s vaccination hubs for their dedication and hard work that is making this mass vaccination campaign possible. We would also like to thank Dario Consonni for his helpful suggestions in the statistical analyses, and Leyton Nightingale for revising the English language.
Authors’ contributions
VF conceived the study. All authors contributed to design of the study. VF, AA and IM performed data preparation. VF, EC and MF performed the statistical analyses. VF, EC, AC and MC wrote the manuscript. All authors critically revised the manuscript and approved its final version.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.02.063.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.https://lab.gedidigital.it/gedi-visual/2020/coronavirus-i-contagi-in-italia/mondo.php [Last accessed: February 11, 2022].
- 2.https://lab24.ilsole24ore.com/coronavirus/ [Last accessed: February 11, 2022].
- 3.European Medicines Agency website. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-Covid-19/treatments-vaccines/Covid-19-vaccines [February 11, 2022].
- 4.Fabiani M, Puopolo M, Morciano C, Spuri M, Spila Alegiani S, Filia A, D'Ancona F, Del Manso M, Riccardo F, Tallon M, Proietti V, Sacco C, Massari M, Da Cas R, Mateo-Urdiales A, Siddu A, Battilomo S, Bella A, Palamara AT, Popoli P, Brusaferro S, Rezza G, Menniti Ippolito F, Pezzotti P. Effectiveness of mRNA vaccines and waning of protection against SARS-CoV-2 infection and severe covid-19 during predominant circulation of the delta variant in Italy: retrospective cohort study. BMJ 2022;376:e069052 | doi: 10.1136/bmj-2021-069052. [DOI] [PMC free article] [PubMed]
- 5.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabiani M., Ramigni M., Gobbetto V., Mateo-Urdiales A., Pezzotti P., Piovesan C. Effectiveness of the Comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in preventing SARS-CoV-2 infection among healthcare workers, Treviso province, Veneto region, Italy, 27 December 2020 to 24 March 2021. Eurosurveillance. 2021;26(17):2100420. doi: 10.2807/1560-7917.ES.2021.26.17.2100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordström P., Ballin M., Nordström A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden [published online ahead of print, 2022 Feb 4] Lancet. 2022 doi: 10.1016/S0140-6736(22)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katikireddi S.V., Cerqueira-Silva T., Vasileiou E., Robertson C., Amele S., Pan J., et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil. Lancet. 2022;399(10319):25–35. doi: 10.1016/S0140-6736(21)02754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall V.J., Foulkes S., Saei A., Andrews N., Oguti B., Charlett A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q., Qin C., Liu M., Liu J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10(1):132. doi: 10.1186/s40249-021-00915-3. PMID: 34776011; PMCID: PMC8590867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O'Doherty M, Brown K, Cameron C, Stockton D, McMenamin J, Ramsay M. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed]
- 12.Istituto Superiore di Sanità. Prevalenza e distribuzione delle varianti di SARS-CoV-2 di interesse per la sanità pubblica in Italia . Rapporto n. 16 del 19 gennaio 2022. Available from: https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-rapporti-periodici-19-gennaio-2022.pdf [Last accessed on January 31, 2022].
- 13.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chodick G., Tene L., Patalon T., Gazit S., Ben Tov A., Cohen D., et al. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMA Netw Open. 2021;4(6):e2115985. doi: 10.1001/jamanetworkopen.2021.15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chodick G., Tene L., Rotem R.S., Patalon T., Gazit S., Ben-Tov A., et al. The effectiveness of the TWODOSE BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2022;74(3):472–478. doi: 10.1093/cid/ciab438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mateo-Urdiales A, Spila Alegiani S, Fabiani M, Pezzotti P, Filia A, Massari M, Riccardo F, Tallon M, Proietti V, Del Manso M, Puopolo M, Spuri M, Morciano C, D'Ancona FP, Da Cas R, Battilomo S, Bella A, Menniti-Ippolito F; Italian Integrated Surveillance of COVID-19 study group; on behalf of the Italian COVID-19 vaccines registry. Risk of SARS-CoV-2 infection and subsequent hospital admission and death at different time intervals since first dose of COVID-19 vaccine administration, Italy, 27 December 2020 to mid-April 2021. Euro Surveill 2021;26(25):2100507. doi: 10.2807/1560-7917.ES.2021.26.25.2100507. PMID: 34169819; PMCID: PMC8229378. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.