Abstract
The sodium–glucose cotransporter 2 inhibitor empagliflozin reduces the risk of cardiovascular death or heart failure hospitalization in patients with chronic heart failure, but whether empagliflozin also improves clinical outcomes when initiated in patients who are hospitalized for acute heart failure is unknown. In this double-blind trial (EMPULSE; NCT04157751), 530 patients with a primary diagnosis of acute de novo or decompensated chronic heart failure regardless of left ventricular ejection fraction were randomly assigned to receive empagliflozin 10 mg once daily or placebo. Patients were randomized in-hospital when clinically stable (median time from hospital admission to randomization, 3 days) and were treated for up to 90 days. The primary outcome of the trial was clinical benefit, defined as a hierarchical composite of death from any cause, number of heart failure events and time to first heart failure event, or a 5 point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days, as assessed using a win ratio. More patients treated with empagliflozin had clinical benefit compared with placebo (stratified win ratio, 1.36; 95% confidence interval, 1.09–1.68; P = 0.0054), meeting the primary endpoint. Clinical benefit was observed for both acute de novo and decompensated chronic heart failure and was observed regardless of ejection fraction or the presence or absence of diabetes. Empagliflozin was well tolerated; serious adverse events were reported in 32.3% and 43.6% of the empagliflozin- and placebo-treated patients, respectively. These findings indicate that initiation of empagliflozin in patients hospitalized for acute heart failure is well tolerated and results in significant clinical benefit in the 90 days after starting treatment.
Subject terms: Heart failure, Drug therapy
In a multinational trial, empagliflozin has clinical benefit when administered to hospitalized patients with acute heart failure, extending the reach of SGLT2 inhibitor therapy to this patient population.
Main
Acute heart failure is the most common cause of hospitalization in people older than 65 years and is associated with significant morbidity, mortality and poor quality of life1–3. Multiple randomized controlled trials testing pharmacological interventions in patients hospitalized for acute heart failure did not find improved post-discharge outcomes, highlighting a critical unmet need4–8.
The sodium–glucose cotransporter 2 (SGLT2) inhibitors empagliflozin and dapagliflozin significantly reduce the risk of cardiovascular death or hospitalization for heart failure in patients with chronic heart failure with a reduced left ventricular ejection fraction (LVEF)9,10. Empagliflozin additionally significantly reduces the risk of cardiovascular death or hospitalization for heart failure in patients with chronic heart failure with a preserved LVEF11. The combined SGLT1/2 inhibitor sotagliflozin has been shown to improve clinical outcomes in patients with diabetes and a recent worsening heart failure event (HFE)12. Whether the SGLT2 inhibitor empagliflozin provides clinical benefit in patients hospitalized for acute heart failure was unknown. In the early phase of hospital admission for heart failure, substantial fluid and electrolyte shifts as well as hemodynamic changes regularly occur. It remained uncertain whether it is safe to initiate treatment with an SGLT2 inhibitor in this phase. In addition, de novo heart failure patients were not eligible for inclusion in previous trials with SGLT2 inhibitors. Whether empagliflozin is effective and safe when started in patients with de novo hospitalization for acute heart failure who are not yet treated with background heart failure therapies remained to be established as well. We designed the present study to evaluate the effects of empagliflozin on three fundamental goals of care in patients hospitalized for acute heart failure: improvement of survival, reduction of HFEs, and improvement of symptoms.
Results
Patient characteristics
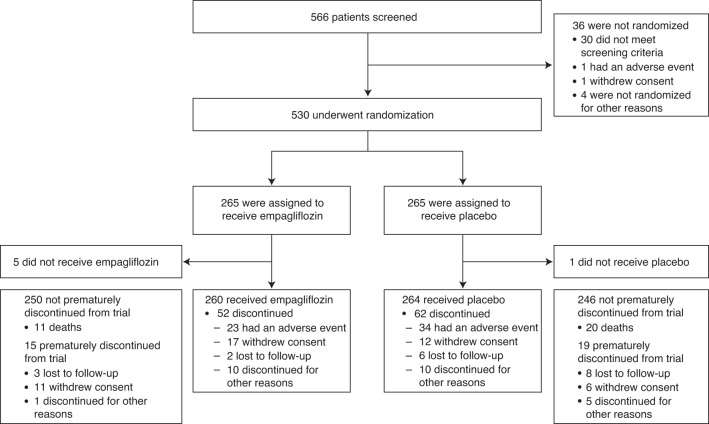
From June 2020 to February 2021 a total of 566 patients were screened and 530 patients were randomly assigned to empagliflozin (n = 265) or placebo (n = 265) at 118 centers in 15 countries (Fig. 1 and Supplementary Table 1). Table 1 lists the baseline characteristics of the randomized patients. The median age was 71 years (interquartile range, 61–78 years), 34% were women, and 78% were white. The median time from hospital admission to randomization was 3 days (interquartile range, 2–4 days). Other patient characteristics and medications at baseline were balanced between treatment groups (Table 1).
Fig. 1. Screening, randomization, and follow-up.
Flowchart of the double-blind EMPULSE trial (NCT04157751), in which 530 patients with a primary diagnosis of acute de novo or decompensated chronic heart failure, regardless of left ventricular ejection fraction, were randomly assigned to receive empagliflozin 10 mg once daily or placebo. This study was carried out at 118 centers in 15 countries.
Table 1.
Characteristics of the patients at baseline
| Empagliflozin (n = 265) Median (IQR) or n (%) |
Placebo (n = 265) Median (IQR) or n (%) |
|
|---|---|---|
| Age (years) | 71 (62–78) | 70 (59–78) |
| Sex | ||
| Men | 179 (67.5) | 172 (64.9) |
| Women | 86 (32.5) | 93 (35.1) |
| Race or ethnic group | ||
| White | 211 (79.6) | 202 (76.2) |
| Black | 21 (7.9) | 33 (12.5) |
| Asian | 32 (12.1) | 25 (9.4) |
| Other/mixed race | 1 (0.4) | 4 (1.5) |
| Missing | 0 | 1 (0.4) |
| Geographic region | ||
| Europe | 168 (63.4) | 171 (64.5) |
| North America | 66 (24.9) | 69 (26.0) |
| Asia | 31 (11.7) | 25 (9.4) |
| NYHA class | ||
| I | 8 (3.0) | 6 (2.3) |
| II | 95 (35.8) | 91 (34.3) |
| III | 134 (50.6) | 145 (54.7) |
| IV | 26 (9.8) | 23 (8.7) |
| Missing | 2 (0.8) | 0 |
| KCCQ-TSS | 37.5 (20.8–58.3) | 39.6 (22.4–58.3) |
| NT-proBNP (pg ml−1) | 3,299 (1,843–6,130) | 3,106 (1,588–6,013) |
| Blood pressure (mmHg) | ||
| Systolic | 120 (109.0–135.0) | 122 (110.0–138.0) |
| Diastolic | 72.0 (64.0–82.0) | 74.0 (67.0–80.0) |
| Body mass index (kg m−2) | 28.35 (24.54–32.46) | 29.08 (24.69–33.60) |
| Left ventricular ejection fraction (%) | 31.0 (23.0-45.0) | 32.0 (22.5–49.0) |
| ≤40% | 182 (68.7) | 172 (64.9) |
| >40% | 76 (28.7) | 93 (35.1) |
| Missing | 7 (2.6) | 0 |
| Estimated GFR (ml min−1 1.73 m−2) | 50.0 (36.0–65.0) | 54.0 (39.0–70.0) |
| <30 ml min−1 1.73 m−2 | 27 (10.2) | 24 (9.1) |
| Missing | 16 (6.0) | 14 (5.3) |
| Hemoglobin (g dl−1) | 13.2 (11.8–14.8) | 13.4 (11.8–14.8) |
| Medical history | ||
| Diabetes | 124 (46.8) | 116 (43.8) |
| Hypertension | 205 (77.4) | 221 (83.4) |
| Myocardial infarction | 66 (24.9) | 62 (23.4) |
| Atrial fibrillation | 134 (50.6) | 128 (48.3) |
| CABG or PCI | 78 (29.4) | 78 (29.4) |
| Valvular heart disease | 173 (65.3) | 167 (63.0) |
| Heart failure status | ||
| Decompensated CHF | 177 (66.8) | 178 (67.2) |
| Acute de novo | 88 (33.2) | 87 (32.8) |
| Medication | ||
| ACE inhibitor and/or ARB and/or ARNi | 186 (70.2) | 185 (69.8) |
| ACE inhibitor | 88 (33.2) | 89 (33.6) |
| ARB | 64 (24.2) | 52 (19.6) |
| ARNi | 36 (13.6) | 45 (17.0) |
| MRA | 151 (57.0) | 125 (47.2) |
| Beta-blocker | 213 (80.4) | 208 (78.5) |
| Loop diuretic | 233 (87.9) | 204 (77.0) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor neprilysin inhibitor; CABG, coronary artery bypass graft; CHF, chronic heart failure; GFR, glomerular filtration rate; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Follow-up
A total of 530 patients were included in the efficacy analyses using the intention-to-treat principle. Five hundred and twenty-four patients received at least one dose of the trial drug (260 in the empagliflozin group and 264 in the placebo group, Fig. 1). These patients were subsequently included in the safety analyses. Early discontinuation of the trial drug occurred in 114 patients (21.8%): 52 (20.0%) in the empagliflozin group and 62 patients (23.5%) in the placebo group. Eleven patients (2.1%) were lost to follow-up.
Primary outcome
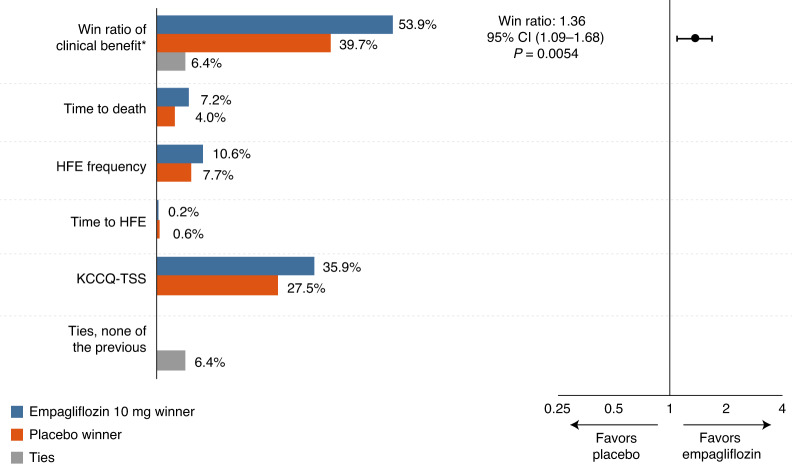
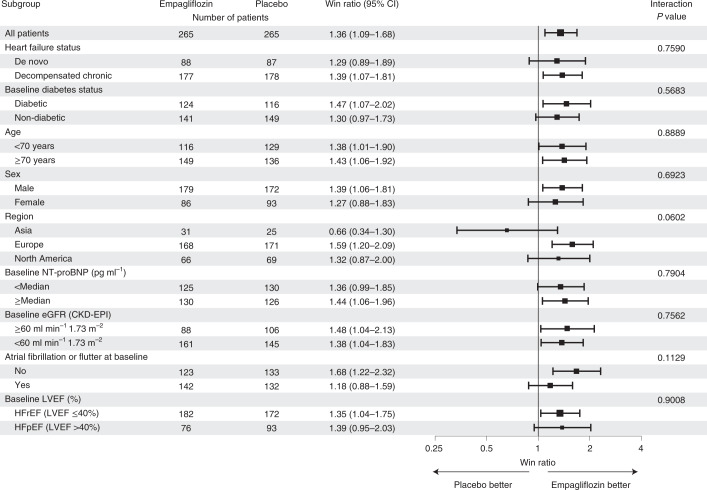
A total of 33 patients (6.2%) died, 11 patients (4.2%) in the empagliflozin group and 22 (8.3%) in the placebo group. Sixty-seven patients (12.6%) had at least one HFE (empagliflozin, 28 patients, 10.6%; placebo, 39 patients, 14.7%). The adjusted mean change in Kansas City Cardiomyopathy Questionnaire Total Symptom Score (KCCQ-TSS) from baseline to 90 days was 36.2 (95% confidence interval (CI): 33.3–39.1) in the empagliflozin group and 31.7 (95% CI: 28.8–34.7) in the placebo group. Figure 2 shows the primary efficacy analysis of the hierarchical assessment of all-cause mortality, number and time to first HFEs, and change in KCCQ-TSS using the stratified win ratio. Empagliflozin was superior in 53.9% of paired comparisons and placebo was superior in 39.7%, whereas 6.4% of comparisons were tied, yielding a win ratio of 1.36 in favor of empagliflozin (95% CI: 1.09–1.68, P = 0.0054). Table 2 lists the proportion of wins and win ratios for all components of the primary outcome. The effect of empagliflozin on the primary efficacy outcome was generally consistent across prespecified subgroups, including acute heart failure status (de novo versus decompensated chronic heart failure), diabetes status, age, sex, geographic region, baseline N-terminal pro-brain natriuretic peptide (NT-proBNP), kidney function, atrial fibrillation status and LVEF subgroup (Fig. 3).
Fig. 2. Primary efficacy outcome and components.
The stratified win ratio was calculated using a non-parametric generalized pairwise comparison within heart failure status strata; data are presented as the point estimate and 95% CI with a two-sided P value. For the components of the win ratio, the percentages do not reflect randomized comparisons. Please refer to Table 2 for the overall number of events and KCCQ-TSS data. *Hierarchical composite of death, number of HFEs, time to first HFE and change from baseline in KCCQ-TSS after 90 days of treatment.
Table 2.
Primary and secondary outcomes
| Empagliflozin (n = 265) | Placebo (n = 265) | P value | ||
|---|---|---|---|---|
| Primary endpoint | Win ratio (95% CI)a | |||
| Primary endpoint (% wins)b | 53.89 | 39.71 | 1.36 (1.09–1.68) | 0.0054 |
| Time to death (% wins) | 7.15 | 4.01 | ||
| HFE frequency (% wins)c | 10.59 | 7.65 | ||
| Time to first HFE (% wins) | 0.24 | 0.57 | ||
| ≥5 point difference in the KCCQ-TSS change from baseline to day 90 (% wins)d | 35.91 | 27.48 | ||
| Percentage of ties | 6.41 | 6.41 | ||
| Components described separately in the whole study population | ||||
| Deaths, n (%) | 11 (4.2) | 22 (8.3) | ||
| Patients with HFE, n (%) | 28 (10.6) | 39 (14.7) | ||
| Total HFEs, n | 36 | 52 | ||
| Change from baseline in KCCQ-TSS at day 90d | See secondary endpoint | |||
| Secondary endpoints | Hazard ratio (95% CI) | |||
| CVD or HFE until end-of-trial visit, n (%), events per 100 patient years (95% CI) | 34 (12.8), 55.01 (38.10–74.99) | 49 (18.5), 80.45 (59.52–104.49) | 0.69 (0.45–1.08) | |
| Odds ratio (95% CI) | ||||
| KCCQ-TSS improvement ≥10 points at day 90, n (%) | 220.1 (83.1) | 202.1 (76.3) | 1.522 (0.927–2.501) | |
| Adjusted mean difference (95% CI) | ||||
| KCCQ-TSS change from baseline to day 90, adjusted mean (95% CI) | 36.19 (33.28–39.09) | 31.73 (28.80–34.67) | 4.45 (0.32–8.59) | |
| Diuretic response (kg weight loss per mean daily loop diuretic dose)e, adjusted mean (95% CI) | ||||
| At day 15 | −3.33 (−4.38 to −2.29) | −1.02 (−2.04 to 0.00) | −2.31 (−3.77 to −0.85) | |
| At day 30 | −3.80 (−5.39 to −2.20) | −1.01 (−2.59 to 0.57) | −2.79 (−5.03 to −0.54) | |
| Adjusted geometric mean ratio (95% CI) | ||||
| AUC of change from baseline in NT-proBNP at day 30, adjusted geometric mean (95% CI)d | 24.07 (22.61–25.62) | 26.77 (25.15–28.48) | 0.90 (0.82–0.98) | |
| Adjusted mean difference (95% CI) | ||||
| Percentage of days alive and out of hospital from study drug initiation until 30 days after initial hospital discharge, mean (s.d.) | 81.37 (18.62) | 80.90 (21.25) | 0.47 (−2.97 to 3.91) | |
| Days alive and out of hospital from study drug initiation until 30 days after initial hospital discharge, mean (s.d.) | 28.00 (6.15) | 27.47 (6.63) | NA | |
| Percentage of days alive and out of hospital from study drug initiation until 90 days after randomization, mean (s.d.) | 87.55 (19.54) | 85.79 (22.76) | 1.76 (−1.91 to 5.43) | |
| Days alive and out of hospital from study drug initiation until 90 days after randomization, mean (s.d.) | 78.29 (20.17) | 76.13 (22.85) | NA | |
| Odds ratio (95% CI) | ||||
| Hospitalizations for heart failure until 30 days after initial hospital discharge, n (%) | 14 (5.3) | 12 (4.5) | 1.179 (0.534–2.601) | |
| Occurrence of chronic dialysis or renal transplant or sustained reduction of ≥40% eGFRCKD-EPIcr, or sustained eGFRCKD-EPIcr <15 ml min−1 1.73 m−2 for patients with baseline eGFR ≥30 ml min−1 1.73 m−2, sustained eGFRCKD-EPIcr <10 ml min−1 1.73 m−2 for patients with baseline eGFR <30 ml min−1 1.73 m−2, n (%) | 0 | 2 (0.8) | Not possible to fit a model | |
AUC, area under the curve; CKD-EPIcr, Chronic Kidney Disease Epidemiology Collaboration equation using serum creatinine concentration; CVD, cardiovascular death; eGFR, estimated glomerular filtration rate; NA, not applicable.
The stratified win ratio for the primary endpoint was calculated using a non-parametric generalized pairwise comparison within heart failure status strata. For the secondary endpoints, the hazard ratio was calculated using a Cox proportional hazards model, the odds ratios were calculated using logistic regression models, the adjusted geometric mean ratio was calculated with analysis of covariance (ANCOVA), and the adjusted mean differences were calculated with either ANCOVA or mixed effects models for repeated measures, as appropriate. No adjustments for multiple testing were made. Data are given as point estimates and 95% CI, with two-sided P values, where appropriate. Full details are provided in Supplementary Note 2.
aVariance calculated using the asymptotic normal U statistics approach.
bPairs are analyzed within strata for a stratified win ratio, applying weights that are analogous to a Mantel–Haenszel approach.
cFrequency based on events up to the earlier of the two censoring times.
dBased on multiple imputation with 100 iterations.
eExcluding patients not taking diuretics for more than 1 day during the time period; the units are kg per 40 mg i.v. furosemide (or 80 mg oral furosemide). The equivalent loop diuretic dose to a single dose of 40 mg furosemide is defined as 20 mg torasemide or 1 mg bumetanide.
Fig. 3. Primary efficacy outcome in all prespecified subgroups.
Win ratios were calculated using a non-parametric generalized pairwise comparison within subgroup strata; data are presented as point estimates and 95% CIs with two-sided interaction P values. No adjustments for multiple testing were made. HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Secondary outcomes
Table 2 lists the prespecified secondary outcomes. The incidence of cardiovascular death or HFE until the end-of-trial visit was 12.8% in the empagliflozin group and 18.5% in the placebo group (hazard ratio, 0.69; 95% CI: 0.45–1.08). There was no significant difference in the proportion of patients with a KCCQ-TSS improvement of 10 points or greater at day 90 between the treatment groups. Patients in the empagliflozin group had a greater absolute change in KCCQ-TSS from baseline to day 90 (adjusted mean difference, 4.45 points; 95% CI: 0.32–8.59) than patients in the placebo group.
Patients in the empagliflozin group had a greater reduction in NT-proBNP concentration (as measured using the area under the curve of the change from baseline) at day 30 (adjusted geometric mean ratio, 0.90; 95% CI: 0.82–0.98) than patients in the placebo group. Other secondary endpoints are listed in Table 2.
Safety analyses
A summary of adverse events is provided in Supplementary Table 2. Adverse events leading to discontinuation of empagliflozin or placebo occurred in 8.5% and 12.9% of patients, respectively. No ketoacidosis occurred in the empagliflozin or placebo groups. Rates of volume depletion were 12.7% in the empagliflozin group and 10.2% in the placebo group. Investigator-defined serious symptomatic hypotension occurred in 1.2% of the patients in the empagliflozin group and in 1.5% in the placebo group. Investigator-defined hypoglycemia occurred in 1.9% of patients treated with empagliflozin and in 1.5% of patients treated with placebo. Details of renal and urinary adverse events are given in Supplementary Table 3. Acute renal failure occurred in 7.7% of patients in the empagliflozin group and in 12.1% of patients in the placebo group. Urinary tract infection occurred in 4.2% of the patients in the empagliflozin group and in 6.4% in the placebo group. Adjusted mean changes in hematocrit, hemoglobin, alanine aminotransferase, aspartate aminotransferase, uric acid, and estimated glomerular filtration rate between baseline and day 90 are listed in Supplementary Table 4 and do not indicate any safety concerns for empagliflozin. The creatinine change between baseline and the last value on treatment was similar between the empagliflozin and placebo groups. There was a greater increase in hematocrit and hemoglobin in the empagliflozin group.
Regarding systolic blood pressure, the adjusted mean change from baseline to 90 days was 0.1 mmHg (95% CI: −2.5 to 2.7) in the empagliflozin group and 1.0 mmHg (95% CI: −1.6 to 3.6) in the placebo group. For diastolic blood pressure, the adjusted mean change from baseline to 90 days was −0.3 mmHg (95% CI: −1.8 to 1.3) in the empagliflozin group and −0.7 mmHg (95% CI: −2.3 to 0.8) in the placebo group.
Discussion
Initiation of the SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure resulted in a statistically significant and clinically meaningful benefit in the 90 days after randomization. Both a reduction in all-cause death and HFEs as well as an improvement in quality of life contributed to the increased number of wins in the empagliflozin group. We believe that the primary endpoint is meaningful because it allows the hierarchical assessment of benefit across three fundamental goals of care: improvement of survival, reduction of HFEs, and improvement of symptoms.
The effect was consistent across a broad spectrum of patients with reduced and preserved LVEF, acute de novo or decompensated chronic heart failure, and those with or without diabetes. Empagliflozin was well tolerated without safety concerns. The results of EMPULSE add to the accumulating evidence on the benefits of SGLT2 inhibitors in heart failure9–14.
EMPULSE is distinct from previous trials with SGLT2 inhibitors for several reasons. In particular, patients in EMPULSE were randomized early in the course of hospitalization for acute heart failure, at a median of 3 days after hospital admission. In the Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) trial, more than half of the patients were enrolled after hospital discharge and that trial included only patients with diabetes12. In addition, EMPULSE evaluated patients over the first 90 days after hospital admission, often considered the vulnerable phase of heart failure15. Also, EMPULSE included patients without a previous history of heart failure (that is, acute de novo), who were not yet treated for heart failure. The data support that adding empagliflozin to standard therapy was well tolerated and produced similar clinical benefit as in those with chronic decompensated heart failure. Thus, empagliflozin should be considered as an efficacious treatment in patients hospitalized for both de novo and decompensated acute heart failure.
The results of EMPULSE extend those of the pilot EMPA-RESPONSE-AHF trial (Randomized, Double Blind, Placebo Controlled, Multicenter Pilot Study on the Effects of Empagliflozin on Clinical Outcomes in Patients with Acute Decompensated Heart Failure), which suggested a clinical benefit of empagliflozin in patients hospitalized for acute heart failure13. Multiple large-scale drug trials in patients who were hospitalized for acute heart failure have failed to demonstrate compelling beneficial effects on their primary clinical outcome, although in several of these studies the therapy was given only for 24–48 hours and was not continued after hospital discharge4–8. PIONEER-HF (Comparison of Sacubitril/Valsartan Versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode) had a similar design as EMPULSE, but primarily assessed the improvement in the concentration of NT-proBNP rather than clinical outcomes16. In addition, concerns have been raised about the safety of initiating chronic heart failure therapies early after a hospital admission for acute heart failure17. Because patients hospitalized for acute heart failure are often aggressively treated with diuretics and other vasoactive agents, it was previously unclear whether an SGLT2 inhibitor would increase the risk for worsening renal function, volume depletion and ketoacidosis. In the present study we show that empagliflozin was well tolerated when initiated in-hospital with fewer serious adverse events than placebo and with a clinical benefit that was readily apparent by 90 days. Reassuringly, no events of ketoacidosis were reported. Changes in blood pressure were minor and comparable between the groups. Empagliflozin significantly increased hemoglobin and hematocrit concentrations, which might be related to its diuretic effects. This is supported by a greater diuretic response both at day 15 and day 90 in the empagliflozin-treated patients.
The clinical benefit of empagliflozin was generally consistent across all prespecified subgroups, including patients with acute de novo and decompensated chronic heart failure, and those with or without type 2 diabetes. Notably, empagliflozin showed a clinical benefit in patients with both normal and reduced LVEF. The Empagliflozin Outcome Trial in Patients With Chronic Heart Failure with Reduced Ejection Fraction (EMPEROR-Reduced) and EMPEROR-Preserved recently showed that empagliflozin reduced the combined risk of cardiovascular death or hospitalization for heart failure in ambulant patients with chronic heart failure with reduced and preserved ejection fraction, respectively, regardless of diabetes status and ejection fraction, but these trials excluded hospitalized patients9,11. The results of EMPULSE therefore extend and complement those of EMPEROR-Reduced and EMPEROR-Preserved by focusing on patients hospitalized for acute heart failure across the range of ejection fraction.
This trial has some limitations. The short enrollment window and the requirement for patient stabilization might have excluded older, frailer and more severely diseased patients. The use of sacubitril–valsartan in EMPULSE, similar to earlier SGLT2 trials9,10, was modest but similar to contemporary background therapy in routine practice.
In conclusion, the results suggest that the initiation of empagliflozin as part of usual care in patients who are hospitalized for acute heart failure will result in a clinically meaningful benefit in 90 days without safety concerns.
Methods
Trial design and oversight
We conducted a multicenter, randomized, double-blind, 90 day superiority trial to evaluate the effect on clinical benefit, safety and tolerability of once daily oral EMPagliflozin 10 mg compared with placebo, initiated in patients hospitalized for acUte heart faiLure who have been StabilisEd (EMPULSE; ClinicalTrials.gov identifier NCT04157751). The Ethics Committee of each of the 118 sites in 15 countries approved the protocol and all patients gave written informed consent18. Boehringer Ingelheim and Eli Lilly sponsored the trial.
The executive committee of EMPULSE (Supplementary Note 1), consisting of academic members and representatives of Boehringer Ingelheim, designed the protocol and provided oversight of the trial’s conduct together with the trial sponsor. The sponsor performed statistical analyses of the trial according to a prespecified statistical analysis plan with oversight by the executive committee (Supplementary Note 1). Jonathan Blatchford is the statistician who coordinated all analyses. An independent data and safety monitoring committee reviewed the safety data.
Patients
The trial design has been reported previously18. Participants were men or women aged at least 18 years (at least 21 years in Japan, being the age of legal consent) who were hospitalized with a primary diagnosis of acute heart failure with dyspnea on exertion or at rest, and at least two of the following: congestion on chest radiograph, rales on chest auscultation, clinically relevant edema (for example, at least 1+ on a 0–3+ scale), or an elevated jugular venous pressure. Patients were randomized after at least 24 h and no later than 5 days after admission, as early as possible after stabilization and while still in hospital. Patients were required to have a systolic blood pressure of at least 100 mmHg, no inotropic support for at least 24 h, no symptoms of hypotension, and in the 6 h prior to randomization no increase in the i.v. diuretic dose and no i.v. vasodilators including nitrates. Patients were required to have an NT-proBNP concentration of at least 1,600 pg ml−1 or a B-type natriuretic peptide (BNP) concentration of at least 400 pg ml−1. Patients in atrial fibrillation were required to have an NT-proBNP concentration of at least 2,400 pg ml−1 or a BNP concentration of at least 600 pg ml−1. Patients had to be treated with a minimum dose of 40 mg (20 mg for Japanese patients) i.v. furosemide or equivalent. Key exclusion criteria included cardiogenic shock; pulmonary embolism, cerebrovascular accident or acute myocardial infarction as the primary trigger for the current hospitalization or in the preceding 90 days before randomization; current or expected cardiac transplantation, left ventricular assist device, or inotropic support; an estimated glomerular filtration rate (eGFR) less than 20 ml min−1 per 1.73 m2 or requiring dialysis; and prior ketoacidosis. Key inclusion and exclusion criteria are listed in Supplementary Note 118
Trial visits and follow-up
Efficacy and safety parameters were assessed during follow-up visits at 3, 5, 15, 30 and 90 days after randomization. During the onsite visits at 15, 30 and 90 days, eGFR, natriuretic peptides, New York Heart Association class, and health status using the Kansas City Cardiomyopathy Questionnaire were assessed. Because of the ongoing coronavirus disease 2019 (COVID-19) pandemic, several adjustments to the study protocol were made, outlined in Supplementary Note 1. In brief, if patients were unable to come to the study site due to COVID-19 restrictions or safety concerns, a phone or home visit was allowed instead of an in-person site visit; if the collection of blood samples for the central laboratory was not possible, a local laboratory could be used and trial medication could be shipped if participants were unable to collect it. Overall, only two patients missed a visit due to COVID-19 disruption. In addition, 23 patients had a remote visit due to COVID-19 disruption. Compliance was assessed by tablet count at visits 3, 4 and 5.
Primary and secondary outcomes
The primary outcome was clinical benefit at 90 days, defined as a hierarchical composite outcome of time to all-cause death, the number of HFEs, time to first HFE, and a 5 point or greater difference in change from baseline in KCCQ-TSS after 90 days of treatment. HFEs included heart failure hospitalizations, urgent heart failure visits and unplanned outpatient heart failure visits. An event was considered a HFE only if worsening signs and symptoms of heart failure were present and an intensification of therapy (defined as an increase of oral or i.v. diuretics, augmentation of a vasoactive agent, or starting a mechanical or surgical intervention) was performed. The complete definition is provided in the study protocol (Supplementary Note 2). Secondary outcomes included time to first occurrence of cardiovascular death or hospitalization for heart failure, change in KCCQ-TSS, diuretic response after 15 and 30 days of treatment, change in NT-proBNP concentration over 30 days of treatment, days alive and out of hospital, occurrence of a heart failure hospitalization until 30 days after initial hospital discharge and occurrence of chronic dialysis or renal transplant or significant and sustained reduction of eGFR (definitions are provided in the study protocol; see Supplementary Notes 1 and 2). Safety parameters included markers of volume depletion, hypotension and acute renal failure (Supplementary Notes 1 and 2).
Statistical analysis
A sample size of 500 participants (250 per treatment arm) was estimated to provide a power of 87% at a one-sided alpha level of 0.025 under a set of assumptions previously published18 and listed in the study protocol (Supplementary Note 2). The primary analyses were performed according to the intention-to-treat principle and included all available data after randomization. The primary outcome analysis was performed using a stratified win ratio, which compares all patients randomized to empagliflozin with all patients randomized to placebo, in their heart failure status (acute de novo or decompensated chronic heart failure). Each comparison of two patients followed the hierarchy of comparing time to death, number of HFEs, time to HFE or a 5 point or greater difference in change from baseline in the KCCQ-TSS at day 90 until conclusion of a win or loss or otherwise concluding by a tie. We calculated the stratified win ratio19 as the number of wins in the empagliflozin group divided by the number of losses, which was then combined across both strata. A multiple imputation approach, according to whether patients were on treatment or off treatment, was used to impute missing data for the KCCQ-TSS. For more details on the win ratio and the imputation methods see Supplementary Note 1. For the secondary outcomes, a Cox proportional hazards model was used to analyze time to cardiovascular death or HFE. Comparison between treatment groups regarding improvement in KCCQ-TSS of 10 points or greater after 90 days of treatment was performed using a logistic regression model adjusting for heart failure status and baseline score. KCCQ-TSS at day 90 was evaluated using a mixed effects model for repeated measures adjusting for heart failure status and baseline score by visit interaction. The area under the curve of change from baseline in log-transformed NT-proBNP concentration, calculated using the linear trapezoidal rule, was assessed using an analysis of covariance model adjusting for heart failure status and the log of baseline NT-proBNP. Other secondary outcomes were analyzed using similar methods, as appropriate. The incidence of adverse events are shown descriptively. P values or confidence intervals are not adjusted for multiple comparisons.
All analyses were performed with SAS version 9.3 or higher (SAS Institute).
The Ethics Committee of each of the 118 sites in 15 countries approved the protocol and all patients gave written informed consent.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41591-021-01659-1.
Supplementary information
Supplementary Tables 1–4.
Acknowledgements
The sponsors of the trial were Boehringer Ingelheim and Eli Lilly and Company. Boehringer Ingelheim had the organizational oversight over the EMPULSE trial, which included trial conduct, supervision and monitoring of the enrolling study centers, data collection and storage as well as data analysis. The trial design was developed by the academic members of the executive committee in cooperation with representatives from Boehringer Ingelheim, who were also represented in the executive committee of the trial. The executive committee of EMPULSE, consisting of academic members and representatives of Boehringer Ingelheim, designed the protocol and provided oversight of the trial’s conduct together with the trial sponsor. The sponsor performed statistical analyses of the trial according to a prespecified statistical analysis plan with oversight by the executive committee. An independent data and safety monitoring committee reviewed the safety data.
Author contributions
A.A.V., C.E.A., J.R.T., S.P.C., M.K., J.B., J.P.F., M.E.N., M.A.P., J.T., M.B., A.S. and P.P. conceived and/or designed the study. A.A.V., S.P.C., M.E.N., C.J.W.B., C.M., J.C.-C., M.F., S.P.J., R.G.K., R.J.M., Y.S., H.S., U.Z., S.Z., M.B. and A.S. collected the data. A.A.V., M.B., A.S. and P.P. analysed or interpreted the data. J.B.P. performed the statistical analyses. A.A.V. prepared the first draft of the manuscript, which was reviewed and edited by all of the authors. All authors approved the final version.
Peer review
Peer review information
Nature Medicine thanks Gianluigi Savarese and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Michael Basson was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Data availability
To ensure independent interpretation of clinical study results, Boehringer Ingelheim grants all external authors access to relevant material, including participant-level clinical study data, as needed by them to fulfill their role and obligations as authors under the International Committee of Medical Journal Editors criteria. Clinical study documents and participant clinical study data are available to be shared on request after publication of the primary manuscript in a peer-reviewed journal, and if regulatory activities are complete and other criteria met as per the BI Policy on Transparency and Publication of Clinical Study Data (see https://www.mystudywindow.com). Bona fide, qualified scientific and medical researchers are eligible to request access to the clinical study data with corresponding documentation describing the structure and content of the datasets. Upon approval, and governed by a legal agreement, data are shared in a secure data-access system for a limited period of 1 year, which may be extended upon request. Prior to providing access, clinical study documents and data will be examined, and, if necessary, redacted and de-identified to protect the personal data of the study participants and personnel, and to respect the boundaries of the informed consent of the study participants. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com for further information.
Competing interests
A.A.V. has received research support and/or has been a consultant for Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim, Cytokinetics, Merck, Myokardia, Novo Nordisk, Novartis, and Roche Diagnostics. C.E.A. has received research/grant support and/or has been a consultant for Abbott, Boehringer Ingelheim, Medtronic, Novartis, ResMed, Thermo Fisher, Vifor and German Federal Ministry of Education and Research. J.R.T. has received research support and/or has been a consultant for Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Medtronic, Merck, Novartis, Servier, and Windtree Therapeutics. S.P.C. is a consultant for Aiphia, Siemens, Bristol Myers Squibb, Boehringer Ingelheim and Vixiar and receives research support from the NIH, PCORI, AstraZeneca and Beckman Coulter. M.K. has received research grants from AstraZeneca and Boehringer Ingelheim, and has served as a consultant for AstraZeneca, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Eli Lilly, Esperion Therapeutics, Janssen, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi and Vifor. J.P.F. is a consultant for Boehringer Ingelheim and receives research support from AstraZeneca. M.E.N. has received speaking honoraria from Abbott, and is a consultant for Vifor, Roche and Amgen. J.T. is supported by the National University of Singapore Start-Up grant and has been a consultant and holds minor stocks for Us2.ai, and has received personal fees from Roche Diagnostics, Daiichi Sankyo, Boehringer Ingelheim and has a patent awarded for an ‘Automatic clinical workflow’ that recognizes and analyzes 2D and Doppler modality echocardiogram images for automated cardiac measurements. C.J.W.B. has received personal fees from AstraZeneca, Boehringer Ingelheim and Novartis. C.M. has received personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Johnson & Johnson and Pfizer. J.C.-C. has received unrestricted grants from Vifor and Novartis paid directly to his institute, and consulting fees from AstraZeneca, Bayer, and Boehringer Ingelheim. M.F. has received research grants from AstraZeneca, Novartis and Vifor Pharma; and fees as a speaker or consultant for AstraZeneca, Bayer AG, Boehringer Ingelheim, Novo Nordisk, Novartis and Pharmacosmos. R.J.M. reports research support and personal fees from Boehringer Ingelheim, Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Medtronic, Merck, Novartis, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Y.S. reports consulting fees and honoraria from Boehringer Ingelheim. H.S. has received unrestricted grants from AstraZeneca paid directly to his institute, and consulting fees from Novartis and speaking fees from MSD, Pfizer, Sanofi and Amgen. M.S. has received personal payments from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Novartis. P.C.S. received honoraria and travel support from Bayer, AstraZeneca, Daiichi Sankyo, Novartis, Actelion, Roche, Sanofi Aventis, Pharmacosmos, Medtronic, Thoratec, Boehringer Ingelheim, Heartware, Coronus, Abbott, Edwards Inc., Boston Scientific, St. Jude Medical, Abiomed, and the German Cardiac Society. P.C.S. also received research support from the National Institute of Health (USA), the German Research Foundation, the Else Kröner Fresenius Foundation, German Heart Foundation, the European Society of Cardiology, Actelion, Medtronic, BMBF, Abiomed, Boehringer Ingelheim and Boston Scientific. P.C.S. served on advisory boards for the German Research Council, Eurotransplant, Novartis, Bayer, Pharmacosmos, AstraZeneca, Boehringer Ingelheim, the German Cardiac Society and the European Society of Cardiology. U.Z. received personal payments from AstraZeneca, Boehringer Ingelheim and Novartis. S.Z. has received consulting and or personal fees from Abbott, Akcea, AstraZeneca, Amgen, Alnylam, Bayer, Boehringer Ingelheim, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Otsuka, Pfizer, Servier and Vifor. M.B. and A.S. are employees of Boehringer Ingelheim. J.P.B. is an employee of Elderbrook Solutions GmbH. P.P. reports personal fees from Boehringer Ingelheim, AstraZeneca, Servier, Bristol Myers Squibb, Amgen, Novartis, Merck, Pfizer, Berlin Chemie, and grants and personal fees from Vifor Pharma. J.B., M.A.P., S.P.J., R.G.K., L.S., M.V. and J.K.W. declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41591-021-01659-1.
References
- 1.Writing Group Members, Mozaffarian, D. et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation133, e38–360 (2016). [DOI] [PubMed]
- 2.GBD Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tromp J, et al. Post-discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT-HF): a cohort study. Lancet Glob. Health. 2020;8:e411–e422. doi: 10.1016/S2214-109X(20)30004-8. [DOI] [PubMed] [Google Scholar]
- 4.Metra M, et al. Effects of serelaxin in patients with acute heart failure. N. Engl. J. Med. 2019;381:716–726. doi: 10.1056/NEJMoa1801291. [DOI] [PubMed] [Google Scholar]
- 5.Massie BM, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N. Engl. J. Med. 2010;363:1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 6.Konstam MA, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 7.Packer M, et al. Effect of ularitide on cardiovascular mortality in acute heart failure. N. Engl. J. Med. 2017;376:1956–1964. doi: 10.1056/NEJMoa1601895. [DOI] [PubMed] [Google Scholar]
- 8.Kozhuharov N, et al. Effect of a strategy of comprehensive vasodilation vs usual care on mortality and heart failure rehospitalization among patients with acute heart failure: the GALACTIC randomized clinical trial. JAMA. 2019;322:2292–2302. doi: 10.1001/jama.2019.18598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Packer M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 10.McMurray JJV, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 11.Anker SD, et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N. Engl. J. Med. 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 13.Damman K, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF) Eur. J. Heart Fail. 2020;22:713–722. doi: 10.1002/ejhf.1713. [DOI] [PubMed] [Google Scholar]
- 14.Zannad F, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819–829. doi: 10.1016/S0140-6736(20)31824-9. [DOI] [PubMed] [Google Scholar]
- 15.Greene SJ, et al. The vulnerable phase after hospitalization for heart failure. Nat. Rev. Cardiol. 2015;12:220–229. doi: 10.1038/nrcardio.2015.14. [DOI] [PubMed] [Google Scholar]
- 16.Velazquez EJ, et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N. Engl. J. Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 17.Swedberg K, et al. Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction. Results of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II) N. Engl. J. Med. 1992;327:678–684. doi: 10.1056/NEJM199209033271002. [DOI] [PubMed] [Google Scholar]
- 18.Tromp J, et al. Sodium-glucose co-transporter 2 inhibition in patients hospitalized for acute decompensated heart failure: rationale for and design of the EMPULSE trial. Eur. J. Heart Fail. 2021;23:826–834. doi: 10.1002/ejhf.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong G, Qiu J, Wang D, Vandemeulebroecke M. The stratified win ratio. J. Biopharm. Stat. 2018;28:778–796. doi: 10.1080/10543406.2017.1397007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1–4.
Data Availability Statement
To ensure independent interpretation of clinical study results, Boehringer Ingelheim grants all external authors access to relevant material, including participant-level clinical study data, as needed by them to fulfill their role and obligations as authors under the International Committee of Medical Journal Editors criteria. Clinical study documents and participant clinical study data are available to be shared on request after publication of the primary manuscript in a peer-reviewed journal, and if regulatory activities are complete and other criteria met as per the BI Policy on Transparency and Publication of Clinical Study Data (see https://www.mystudywindow.com). Bona fide, qualified scientific and medical researchers are eligible to request access to the clinical study data with corresponding documentation describing the structure and content of the datasets. Upon approval, and governed by a legal agreement, data are shared in a secure data-access system for a limited period of 1 year, which may be extended upon request. Prior to providing access, clinical study documents and data will be examined, and, if necessary, redacted and de-identified to protect the personal data of the study participants and personnel, and to respect the boundaries of the informed consent of the study participants. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com for further information.