Graphical abstract
Abbreviations: 3-OH-Cot, trans-3′-hydroxycotinine; CI, 95%-confidence interval; Cot-gluc, cotinine-N-glucuronide; 3-OH-Cot-gluc, trans-3′-hydroxycotinine-N,O-glucuronide; B, blood; CC, combustible cigarettes; CNO, cotinine-N-1-oxide; Cot, cotinine; EC, electronic cigarettes; HTP, heated tobacco product; Hypybut, 4-OH-4-(3-pyridyl)-butanoic acid; IQR, inter-quartile range (25th–75th percentile); LC-MS/MS, liquid chromatography with tandem mass spectrometry; NCot, norcotinine; NNic, nornicotine; Nequ, nicotine equivalents; Nic, nicotine; Nic-gluc, nicotine-N’-glucuronide; Nic+10, nicotine and its 10 major metabolites Cot, 3-OH-Cot, Nic-gluc, Cot-gluc, 3-OH-Cot-gluc, NNic, NCot, NNO, CNO, Hypybut; NNO, nicotine-N-1′-oxide; NRT, nicotine replacement therapy; NU, non-user of any tobacco/nicotine product; OT, oral tobacco; P, plasma; S, saliva; SD, standard deviation of the mean; SEM, standard error of the mean; U, urine
Keywords: Nicotine, Combustible cigarettes, Electronic cigarettes, Heated tobacco products, Nicotine gum, Snus
Highlights
-
•
Nicotine uptake doses from various products show a large overlap between each other.
-
•
Combustible cigarettes and oral tobacco products are at the high end of the nicotine uptake doses.
-
•
Simple models for daily amounts of nicotine intake can predict the nicotine doses for most product users.
-
•
Larger deviations were observed for smokers and users of heated tobacco products.
-
•
The most probable reason for these deviations are differences in parameters for puffing and inhalation and actual behavior of the users.
Abstract
Today various tobacco and nicotine products are available, many of them can be regarded as potentially risk-reduced products when compared to the most frequently used product, combustible cigarettes (CCs). A commonality of these products is that they deliver nicotine, although in quite different amounts and uptake routes, the most common of which are inhalation through the lung and absorption through the oral mucosa. Product-specific nicotine delivery as well as the subject-related use patterns are important factors which determine the pharmacokinetics and achieved internal dose levels of the alkaloid. The latter two parameters are highly relevant for the long-term product loyalty and, consequently, for the implicated health risks, since the risk-reduced products will replace CCs in the long-term only when users will experience a similar level of satisfaction. We measured nicotine and its major metabolites in plasma, saliva and urine samples collected in a controlled clinical study with habitual users (10 per group) of CCs, electronic cigarettes (ECs), heated tobacco products (HTP), oral tobacco (OT), and nicotine replacement therapy (NRT). Non-users (NU) of any tobacco/nicotine products served as (negative) control group. Moderate to strong correlations were observed between the daily consumption and the urinary nicotine equivalents (comprising nicotine and its 10 major metabolites, Nic + 10) or plasma and saliva cotinine concentrations. The average daily nicotine dose as measured by the urinary excretion of Nic + 10 (reflecting approximately 95 % of the absorbed nicotine) amounted to 17 and 22 mg/24 h for smokers (CC) and OT users, respectively, while it was in the range of 6–12 mg/24 h for users of ECs, HTP and NRT products, with high inter-individual variations in each user group. The individual daily nicotine intake, which was calculated by applying product-specific models, showed none to good agreement with the corresponding internal nicotine dose measured by Nic + 10 excretion. Possible reasons for the observed deviations between calculated and objectively measured nicotine doses are discussed.
1. Introduction
Tobacco use, particularly smoking of combustible cigarettes (CCs), is associated with an increased risk for diseases such as cancer, cardiovascular (CVD) and chronic obstructive pulmonary diseases (COPD) (US Department of Health and Human Services, 1986, US Department of Health and Human Services, 2010). Efforts of the tobacco industry to reduce the smoking-related health risks, which include the introduction of cigarette filter tips, application of various filter materials (cellulose acetate, char coal), filter ventilation, reconstituted and expanded tobacco, application of additives, have been of only limited success, as long as tobacco is combusted prior to inhalation (Hoffmann et al., 2001, Parascandola, 2005). Reductions of the incriminated toxicants (Fowles and Dybing, 2003, Rodgman and Green, 2003) have to be substantial in order to result in potentially reduced risk products, acceptable by the public health community (Institute of Medicine (IOM), 2001). There is ample evidence that nicotine is probably not the only, but a major factor for indulging in the tobacco habit (Jarvis, 2004, US Department of Health and Human Services, 1988). Neurological effects of nicotine cover a broad spectrum, including pleasure, appetite suppression, arousal, cognitive, learning and memory enhancement, mood modulation, reduction of anxiety and tension (Benowitz, 2008). All these effects are presumably evoked by the release of a variety of neurotransmitters after activation of nicotinic cholinergic receptors by nicotine (Benowitz, 2008). On the other hand, there is no scientific evidence that, for healthy adults, nicotine is involved in the major smoking and tobacco use-related diseases such as cancer, CVD and COPD (Adlkofer, 1995, Benowitz, 1997).
For centuries (or even for millennia, if the origin of tobacco in South America is considered), humans use tobacco in all conceivable forms of application (Gately, 2007), all of which allow the systemic uptake of nicotine, although via various routes and in quite different velocities. The rate of nicotine delivery to the consumer was found to be important for the rewarding effects of the alkaloid, e.g. reduction of urge to smoke (Jensen et al., 2020). The puff-wise nicotine flux for CC users was estimated to amount to 100 µg/s (World Health Organization, 2021). It can be assumed that modern tobacco/nicotine inhalation products such as HTPs and ECs can deliver nicotine at similar rates. Oral nicotine products such as snus, nicotine gum or tobacco-free nicotine delivery pouches exhibit much slower nicotine fluxes in the range of a few µg/s (Lunell et al., 2020). The modern market of recreational tobacco and nicotine products in essence comprises the uptake routes via the lung (after either combustion or heating of a tobacco or a nicotine-containing matrix) or the oral mucosa (after depositing a tobacco or nicotine-containing matrix in the oral cavity for a time period ranging from minutes to hours). It is interesting to note that oral tobacco/nicotine products such as snus can replace cigarettes to a large extent even in the long run, as the prevalence of the two habits in Sweden impressively show, despite the differences in nicotine flux (Ramstrom et al., 2016). Taken together, these observations may lead to the conclusion that nicotine uptake is important for picking up and maintaining the habit, but the product-related rate of delivery and the uptake route appears of less importance. This observation is in agreement with the results of an internet poll, in which the dependence on cigarettes and snus was rated about similar, while that on NRT was rated significantly lower (Fagerstrom, 2018).
The objectives of this investigation were to compare the internal nicotine doses measured by suitable biomarkers in a controlled clinical study over 3 days in habitual users of CCs, ECs, HTPs, OT (primarily snus) and NRTs (primarily nicotine gum). In addition, the estimated daily nicotine intake was compared to the daily urinary nicotine excretion assessed by measuring nicotine and 10 of its major metabolites (termed Nic + 10 or nicotine equivalents, Nequ). The results are discussed with respect to suitable daily use frequency and consumption indicators. In particular, the following questions were addressed: (i) Are the time courses for the users of the various products comparable, or are there characteristic differences? (ii) Are there statistically different levels in the nicotine biomarkers between the groups and do the levels compare to those reported in the literature? (iii) Are there significant correlations between nicotine biomarker levels and commonly used daily consumption measures? (iv) Is there an acceptable agreement between the estimated daily intake of nicotine (by applying adjusted intake models (Scherer et al., 2021)) and the excretion of Nequ? If not, what are plausible reasons for the observed deviations?
2. Materials and methods
2.1. Study design, products and biological samples
The presented investigation is part of a research project on biomarkers of exposure to be analyzed by targeted and untargeted methods in various biological matrices in users of five tobacco/nicotine products (Sibul et al., 2021). The project aims to distinguish different nicotine user groups by either specific biomarkers or biomarker patterns. As a first step towards achieving this aim, this paper describes the results of the nicotine biomarkers of the 6 study groups. A controlled, single-center, open label trial was conducted comparing five nicotine product user groups, namely exclusive users (by definition, sole use of a respective nicotine product, no dual or multiple use) of CC, EC, HTP, OT, NRT with a control group of non-users (NU). Detailed information regarding the study design and the study population is provided by Sibul et al. (Sibul et al., 2021). The study protocol was approved by the ethics committee of the Medical Association Hamburg, Germany. The study was primarily aimed to identify specific biomarkers or biomarker patterns for the different nicotine user groups by means of targeted and untargeted analytical methods. Briefly, 10 subjects per group stating that they were exclusive users of the respective products since at least 6 months (or at least 3 months in case of NRT) prior to the study start, were confined for 76 h to a clinic, during which free use of the products (own brands) was allowed. Users were handed out their product prior to each single use by the clinic staff. In addition, user groups were spatially separated upon product use.
The 10 smokers (CC) smoked manufactured cigarettes with ISO nicotine yields of 0.8 (N = 8), 0.7 (N = 1) and 0.5 (N = 1) mg/cigarette. In the EC group, 9 subjects used refillable tank systems, one vaper used a pod-type e-cigarette. A broad range of flavors were applied in this group. Nicotine concentrations in e-liquid were in the range 3–12 (mean: 5.1 mg/ml). All 10 users of heated tobacco products (HTP) used the device of the main supplier with sticks varying in the flavor type. Nominal nicotine release was 0.5 mg/stick for all users in this group. In the NRT group, two subjects used nicotine spray (also in the confinement period) with 1 mg nicotine per stroke. The other group members applied nicotine gums with 2 (N = 4) and 4 mg nicotine (N = 4) per piece. Eight of the 10 OT users utilized bagged snus, two participants applied loose snus products. The nicotine content was in the range of 14–43 mg/g (mean: 18.3, N = 9, one unknown).
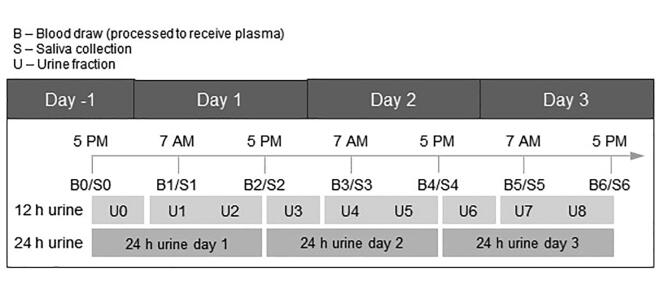
Blood samples were collected at 7 am and 5 pm on each day starting in the evening of Day −1, when the subjects were admitted to the clinic (Fig. 1). Tobacco/nicotine products were not allowed to be used at least 20 min prior to blood or saliva collection. In total, 420 plasma and saliva samples were analyzed for nicotine (Nic, in plasma only), cotinine (Cot) and trans-3′-hydroxycotinine (3-OH-Cot). All urine voids were collected separately throughout the course of the study. The total volume of each void was determined gravimetrically together with the time of void. Urine fractions were pooled, yielding six 12 h urines (U0, U1/2, U3, U4/5, U6, U7/8; pooled according to Fig. 1) and analyzed for total nicotine equivalents (Nequ). For the 24-h-urine of Day 3, consisting of U6, and U7/8 (Fig. 1), Nequ was calculated (mg/24 h) and compared with the estimated daily nicotine intake on that day.
Fig. 1.
Time schedule for sample collection.
2.2. Analytical methods
Urinary Nequ, comprising the molar sum of Nic and its 10 major metabolites (Nic + 10), namely Cot, 3-OH-Cot, nicotine-N-glucuronide (Nic-gluc), Cot-gluc, 3-OH-Cot-gluc, 4-OH-4-(3-pyridyl)-butanoic acid (Hypybut), nornicotine (NNic), norcotinine (NCot), nicotine N-oxide (NNO), and cotinine N-oxide (CNO) were determined by liquid chromatography with tandem mass spectrometry (LC-MS/MS) according to Piller et al. after major modifications (Piller et al., 2014). To 50 µL urine, 10 µL of deuterated internal standard (IS) mix (containing 7.5 ng Nic-gluc-D3, 10 ng of each Nic-D7, Cot-D3, 3-OH-Cot-gluc-D3, 12.5 ng NNic-D4, 20 ng NCot-D4, 35 ng NNO-D3, 50 ng 3-OH-Cot-D3, 50 ng CNO-D3, 100 ng Hypybut-D3, 250 ng Cot-gluc-D3) and 600 µL 0.5 % formic acid were added. Nic-D7, 3-OH-Cot-D3, Nic-gluc-D3, Cot-gluc-D3 were obtained from Aptochem, Montreal, QC, Canada; Cot-D3, NNic-D4, NCot-D4, CNO-D3, Hypybut-D3 were obtained from TRC, Toronto, ON, Canada; 3-OH-Cot-gluc-D3 was obtained from Dr. Mark, Worms, Germany and NNO-D3 was obtained from Santa Cruz Biotechnology, Dallas, Texas, USA. The mixture was loaded on a StrataTM-X-C 96-SPE-Well-Plate (30 mg sorbent) which was pre-conditioned sequentially with 1 mL methanol followed by 1 mL water and 1 mL ammonium formate buffer (20 mM, pH 2.5). The SPE-Well-Plate was washed with 1 mL ammonium formate buffer (20 mM, pH 2.5), 1 mL water, 1 mL methanol and 1 mL acetonitrile/methanol (6:4, vol/vol). After drying the plate with a vaccum manifold, the analytes were eluted with 0.5 mL 10 % ammonium hydroxide in methanol into a 96-Well-Collection Plate (1 mL) (96-Well-SPE-Plate, Vacuum Manifold and Collection Plate were purchased from Phenomenex, Aschaffenburg, Germany). The eluates were evaporated in a SpeedVac (Jouan RV10.22, Thermo Scientific, Dreieich, Germany) to complete dryness and reconstituted in 100 µL 10% water in acetonitrile. Ten µL of the solution was injected into an UPLC-MS/MS instrument (Waters GmbH, Eschborn, Germany: Acquity UPLC Class-I coupled to a Xevo TQ-S) equipped with an Acquity BEH HILIC column (150 × 2.1 mm, 1.7 µm). Chromatographic separation was achieved by gradient elution with 10 mM ammonium formate buffer in water (pH 4.5, solvent A) and 10 mm ammonium formate buffer in acetonitrile (solvent B). Gradient elution started with 4 % A for 2 min, followed by a linear increase to 35 % A until 11 min and held until 12 min. Re-equilibration to 4 % A was ensued from 12.1 to 23 min. The flow rate was 600 µL/min from 0 to 12 min, 700 µL/min from 12.1 to 22.91 min and 600 µL/min from 22.91 to 23 min. MS/MS analysis was conducted in the ESI positive mode, using the following m/z transitions: Nic (quantifier, qualifier): 163/130, 163/132; Nic-D7: 170/135 (IS); Cot: 177/98, 177/80; Cot-D3: 180/101 (IS); 3-OH-Cot: 193/80, 193/134; OH-Cot-D3: 196/80 (IS); Nic-gluc: 339/163, 339/132; Nic-gluc-D3: 342/166 (IS); Cot-gluc: 353/177, 353/146; Cot-gluc-D3: 356/180 (IS); 3-OH-Cot-gluc: 369/193, 369/118; 3-OH-Cot-gluc-D3: 372/196 (IS); NNic: 149/130, 149/132; NNor-D4: 153/130 (IS); NCot: 163/80, 163/84; NCot-D4: 167/84 (IS); NNO: 179/132, 179/130; NNO-D3: 182/132 (IS); CNO: 193/96, 196/79; CNO-D3: 196/96 (IS); Hypybut: 182/108, no qualifier; Hypybut-D3: 185/109 (IS). Limits of quantification (LOQs) in urine were 5 ng/ml for all analytes. For simplification, it is assumed that Nic + 10 in urine represent virtually 100 % of the nicotine dose taken up (Tricker, 2006).
Nic, Cot and 3-OH-Cot in plasma (obtained after centrifugation of the fresh EDTA blood samples at 2500 × g for 10 min) as well as Cot and 3-OH-Cot in saliva were determined by applying the LC-MS/MS method for Cot and 3-OH-Cot as described for saliva earlier (Landmesser et al., 2019, Scherer et al., 2007), with modifications. Briefly, to 100 µL plasma or saliva, 20 µL of internal standard (IS) mix (containing 0.4 ng Nic-D7, 1 ng of each Cot-D3 and 3-OH-Cot-D3 in acetonitrile; and 1 mL ethyl acetate were added. After vortexing, the organic phase was evaporated to almost dryness and the residue was dissolved in 200 µL acetonitrile. Five µL of the solution was injected into an UPLC-MS/MS instrument (Waters GmbH, Eschborn, Germany: Acquity UPLC Class-I coupled to a Xevo TQ-S) equipped with an Acquity BEH HILIC column (150 × 2.1 mm, 1.7 µm). Isocratic chromatography was performed using 20 % of 100 mM ammonium formate buffer, pH 3.5 and 80 % acetonitrile at a flow of 0.4 mL/min at 40 °C over 4.0 min. MS/MS analysis was conducted in the ESI positive mode, using the following m/z transitions: Nic: 163/84 (quantifier), 163/132 (qualifier); Nic-D7: 170/135 (IS); Cot: 177/80 (quantifier), 177/98 (qualifier); Cot-D3: 180/80 (IS); 3-OH-Cot: 193/80 (quantifier), 193/134 (qualifier); 3-OH-Cot-D3: 196/80 (IS). Limits of quantification (LOQs) in plasma and saliva were 0.1, 0.5 and 0.5 ng/mL for Nic, Cot and 3-OH-Cot, respectively. Due to the possibility of external contamination of saliva by product use, Nic was not determined in this matrix.
Usually single measurements were performed for each sample. Re-analysis was conducted, if the tolerance (±15 %) of the corresponding quality control (QC) sample was exceeded.
2.3. Estimations of the product use-related daily nicotine intake doses
The nicotine intake on Day 3 of this study was estimated by applying product-specific models as described in a recent review (Scherer et al., 2021) with modifications as indicated below. Instead of using ranges of values for the variables in the models, individual values for each subject were plugged in the equations. If available, actual consumption data for Day 3 were used for the estimation. Since for vapers (EC), e-liquid consumption was only assessed as the sum of Day 1 to 3, the average of 3 days was used for this group.
The calculation of the daily amount of nicotine taken in by smoking (CC use) was based on the approach described by Logue et al. (Logue et al., 2017) (Eq. (1)):
| (1) |
with: Icc = Intake (mg nicotine/d) by smoking conventional cigarettes (CC); Ycc = yield (mg nicotine/cig), values on the cigarette package were used (i.e. yields determined according to the ISO smoking regime (International Standard Organisation (ISO, 1991)); CPD = cigarettes smoked on Day 3 of the study (cig/d); MSp = mouth spill (fraction of smoke immediately released from the mouth, not taken into the lung); due to a general underestimation of ICC, MSp of 0 % was used; R = respiratory retention (fraction of nicotine retained in the lung, not exhaled immediately after inhalation), a value of 0.965 was used for the calculation (Baker and Dixon, 2006, Feng et al., 2007, Logue et al., 2017).
The basic model of Logue et al. (Logue et al., 2017) was also used for vapers (users of ECs). However, as will be shown in the results section, the daily consumption was based on the consumption of e-liquid (measured over 3 days), instead of using the recorded number of puffs taken per day, which turned out to be a very poor indicator for actual daily consumption. The model for estimating the individual daily nicotine intake is, therefore, represented by Eq. (2):
| (2) |
with: Iec = Intake (mg nicotine/d) by using electronic cigarettes (EC) based on the amount of e-liquid consumed per day; NCec = nicotine content in e-liquid (mg nicotine/mL); LPD = amount of e-liquid consumed per day (mL), the amount measured in g for 3 days was divided by 3, for conversion to volume a density of 1.11485 g/mL was assumed (theoretical density of an e-liquid consisting of 1:1 mixture of 1,2-propylene glycol and glycerol); MSp = mouth spill, a value of 0.6 was assumed. This assumption is presently not based on experimental data, but rather on visual impression (vapers spill out large clouds of vapor); R = respiratory retention, as for smokers, a value of 0.965 was used (Baker and Dixon, 2006, Feng et al., 2007, Logue et al., 2017). We are well aware of the fact that the assumed mouth spill for vapers has to be verified by future studies.
For estimating the daily nicotine take for the group of HTP product users, the same model as for smokers was used (Eq. (3)):
| (3) |
with: I = Intake (mg nicotine/d) by using heated tobacco products (HTP); Yhtp = yield (mg nicotine/stick), for all users in the study, a value of 0.5 mg/stick was used (label on the stick package); StPD = sticks consumed on Day 3; MSp = mouth spill, for the same reasons as for smokers, a value of MSp = 0.0 was used; R = respiratory retention, as for CCs and ECs, a value of 0.965 was used.
The estimation of the daily intake of nicotine by using snus or snus-like oral tobacco (OT) products was performed by applying the following relationship (Eq. (4)):
| (4) |
with: Iot = Intake (mg/d) of a nicotine by using OT on Day 3; CNICot = nicotine content in the OT (mg/g); PORot = amount of tobacco (g) per portion used; PEXot = percentage of nicotine extracted from the OT, a median extraction rate of 0.218 for tobacco-containing pouches derived from various reports (Bishop et al., 2020, Digard et al., 2013, Lunell and Curvall, 2011) was used; DCOT = consumption of OT on Day 3 (portions of 1 g per day).
The estimation of the daily intake of nicotine by NRT users was limited to those using nicotine gums (NGs). Eight of the 10 NRT users consumed NGs, 2 used nasal nicotine sprays. The following relationship was used for the nicotine intake by chewing NGs (Equation (5)):.
| (5) |
with: Ing = Intake (mg/d) of nicotine by using NGs on Day 3; CNICng = content of nicotine in the NGs used (mg/piece), subjects used either 2 mg or 4 mg products; PEXng = percentage of nicotine extracted from NG, a median value of 0.70 was deduced from four literature reports (Benowitz et al., 1987, Bishop et al., 2020, Digard et al., 2013, Lunell et al., 2020); DCng = consumption of NG on Day 3 (pieces/d).
2.4. Statistical analysis
Sample sizes were estimated on the basis of known differences in biomarker levels of various toxicants between the groups of interest. Normal distribution was tested using Shapiro-Wilk and D’Agostino K-squared test. Since the nicotine biomarker levels in most of the investigated 6 groups (NU, CC, EC, HTP, NRT, OT) were not normally distributed, the non-parametric Mann-Whitney U test (comparison of two groups) and Kruskal-Wallis ANOVA (comparison of multiple groups) was used to assess statistical significances between groups. For correlations, the non-parametric Spearman rank test was utilized. The significance level was set to p < 0.05. For statistical analysis and generations of graphs, GraphPad Prism Software (San Diego, CA, USA), Version 9.2.0 was applied.
3. Results
Subjects’ characteristics by group are summarized in Table 1. With the exception of OT users, an about equal gender distribution was achieved in all groups. Mean ages of groups ranged from 28.1 to 36.1 years and were not statistically different between the groups. Reported average consumption rates were similar to those recorded during the confined study phase for all groups.
Table 1.
Characteristics of the study subjects by group, means (min–max).
| All | NU | CC | EC | HTP | NRT | OT | |
|---|---|---|---|---|---|---|---|
| N | 60 | 10 | 10 | 10 | 10 | 10 | 10 |
| Males/Females | 38/22 | 6/4 | 6/4 | 6/4 | 6/4 | 5/5 | 9/1 |
| Age (years) | 34.3 (21–63) | 32.9 (21–47) | 35.1 (27–53) | 36.1 (24–61) | 35.3 (22–56) | 35.3 (22–63) | 28.1 (21–45) |
| Reported consumption 1 | – | – | 16.1 cig/d (10–25) | 9.75 mL/d (2–20) | 15.5 st/d (12–20) | 8.3p/d (4–16) | 6.0 g/d (2.5–11) |
| Recorded consumption on Day 1 2 | – | – | 13.7 cig/d (9–19) | 162.5 puffs/d 3 (111–250) | 14.3 st/d (5–25) | 6.5p/d (3–17) | 6.9 g/d (2–10) |
| Recorded consumption on Day 2 2 | – | – | 14.8 cig/d (11–21) | 156.1 puffs/d 3 (105–224) | 16.5 st/d (3–23) | 7.0p/d (3–22) | 7.4 g/d (3–14) |
| Recorded consumption on Day 3 2 | – | – | 11.8 cig/d (6–19) | 137.7 puffs/d 3 (108–176) | 13.3 st/d (1–22) | 5.3p/d (2–16) | 6.7 g/d (2–10) |
: Extracted from a general questionnaire completed by the subjects.
: Recorded by the clinical staff in the daily use protocols during the confined period of the study.
: Consumption of e-liquid over 3 days was: 15.4 (3.1–36.2) mL.
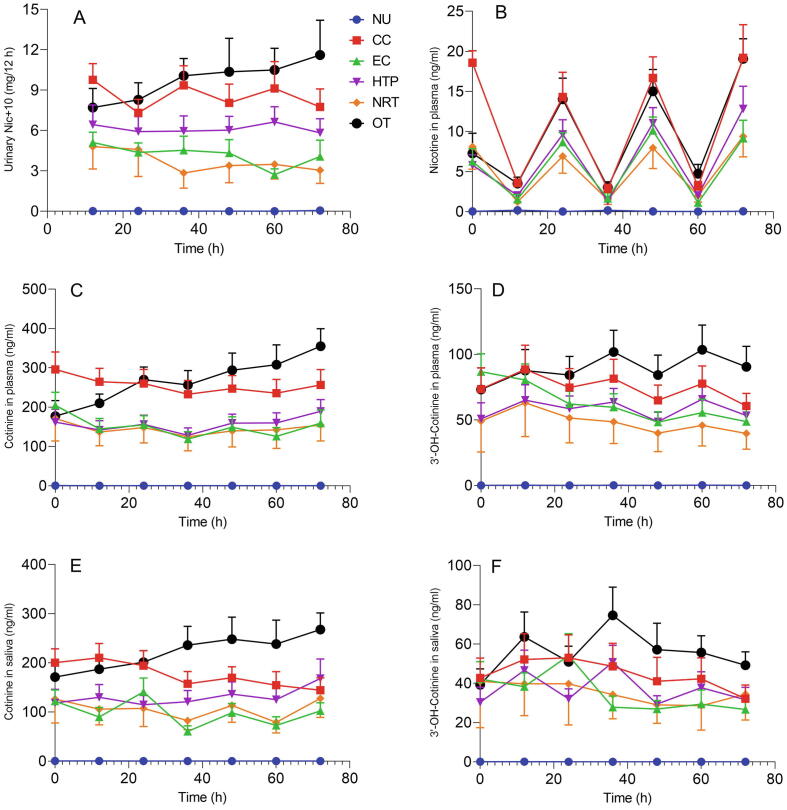
The time courses of urinary Nequ, plasma Nic, Cot and 3-OH-Cot as well as salivary Cot and 3-OH-Cot for the 6 study groups during the confinement period of the study (74 h) are shown in Fig. 2(A–F). The nicotine-related biomarkers can be assigned to three segments in the graph: CC and OT users were at the highest end; HTP, EC and NRT users were in the middle segment; NU were at the low end, showing biomarker levels at or below the LOQ. The Nic plasma levels reflect the short half-life of the compound (∼2h) by showing the peak levels in the evening and the trough level in the morning (after overnight abstinence from using nicotine products) of each study day in all user groups. The other nicotine biomarkers feature no typical circadian changes, except for the urinary Nequ of smokers (CC). Smokers exhibited elevated Nequ levels in the 12-h-urine collected over-night until about 7 am of each study day compared to the 12-h-urine collected during the day. OT users’ Nequ amount in the 12-h-urine samples increased over the complete confined study period. This trend is also observable for nicotine and cotinine in plasma as well as cotinine in saliva. Interestingly, 3-OH-Cot in plasma and saliva of OT/HTP users and CC smokers tended to be higher in the morning samples compared to the evening samples. These were only trends which are not statistically significant.
Fig. 2.
Time courses of Nequ (A) in urine, Nic (B), Cot (C) and 3-OH-Cot (D) in plasma as well as Cot (E) and 3-OH-Cot (F) in saliva for all groups over the confined study period of 74 h. Error bars represent SEMs.
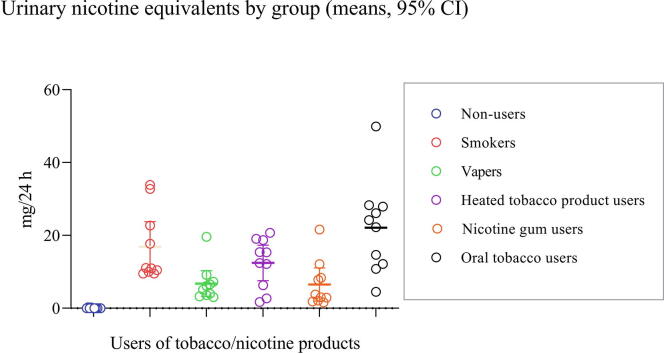
Since the biomarker data for Day 3 of the study can be regarded as those obtained under the most controlled conditions in terms of product use and other factors such as food, results for Day 3 were used for further evaluations. Descriptive statistics for the 6 nicotine-related biomarkers analyzed (urinary Nequ (Nic + 10), plasma Nic, Cot and 3-OH-Cot, salivary Cot and 3-OH-Cot) for all 6 study groups (NU, CC, EC, HTP, NRT, OT) are shown in Table 2. As expected, nicotine biomarkers in NU were significantly different from all other groups. As was seen with the time courses of these biomarkers, users of CC and OT exhibited the highest levels (Fig. 2), which is confirmed by the data in Table 2. These two groups were not found to be significantly different in any of the 6 biomarkers. Significant differences to these two groups (CC and OT) were only occasionally observed, namely for vapers (EC) and NRT users, but not for HTP users (Table 2).
Table 2.
Descriptive statistics of nicotine biomarkers on Day 3 by group (N = 10 subjects in each group).
| Biomarker | NU | CC | EC | HTP | NRT | OT | |
|---|---|---|---|---|---|---|---|
| Nequ (Nic + 10) (mg/24 h) | Mean ± SD | 0.06 ± 0.09 | 16.87 ± 9.66 | 6.79 ± 4.92 | 12.47 ± 6.80 | 6.53 ± 6.35 | 22.10 ± 12.73 |
| Median (IQR)3 | 0.02 (0.01–0.07) | 11.03 (9.92–25.24) | 5.62 (3.55–7.75) | 13.89 (5.41–18.80) | 3.41 (2.07–9.31) | 23.26 (11.82–28.03) | |
| Min–Max | 0.01–0.27 | 9.51–33.86 | 3.09–19.64 | 1.73–20.74 | 1.64–21.63 | 4.53–49.94 | |
| Different from | CC,EC,HTP,NRT,OT | NU,EC,NRT | NU,CC,OT | NU | NU,CC,OT | NU,EC,NRT | |
| Plasma nicotine1(ng/mL) | Mean ± SD | 0.05 ± 0.07 | 19.2 ± 13.1 | 9.1 ± 7.2 | 12.8 ± 8.9 | 9.4 ± 8.1 | 19.1 ± 7.9 |
| Median (IQR)3 | 0.00 (0.00–0.10) | 21.0 (4.3–28.4) | 7.4 (3.6–12.5) | 13.9 (5.3–19.5) | 6.5 (2.3–16.4) | 19.6 (11.6–27.4) | |
| Min–Max | 0.00–0.20 | 2.8–39.0 | 2.5–26.7 | 0.02–27.4 | 0.3–21.9 | 7.3–30.9 | |
| Different from | CC,EC,HTP,NRT,OT | NU | NU | NU | NU | NU | |
| Plasma cotinine1(ng/mL) | Mean ± SD | 0.09 ± 0.03 | 256.3 ± 124.2 | 159.1 ± 106.9 | 189.4 ± 94.3 | 154.5 ± 128.8 | 354.8 ± 141.1 |
| Median (IQR)3 | 0.10 (0.10–0.10) | 257.5 (116.1–367.6) | 125.0 (87.1–203.1) | 217.2 (106.4–252.1) | 121.3 (46.5–283.7) | 371.2 (225.0–578.3) | |
| Min–Max | 0.00–0.10 | 114.7–466.7 | 73.2–430.9 | 16.1–301.8 | 133.2–385.3 | 136.1–578.3 | |
| Different from | CC, EC, HTP, NRT, OT | NU | NU,OT | NU | NU,OT | NU,EC,NRT | |
| Plasma 3-OH-Cot1(ng/mL) | Mean ± SD | 0.07 ± 0.12 | 60.6 ± 30.3 | 48.7 ± 34.5 | 53.4 ± 26.4 | 39.4 ± 37.81 | 90.5 ± 49.3 |
| Median (IQR)3 | 0.00 (0.00–0.20) | 56.4 (39.4–119.1) | 43.2 (20.6–128.4) | 56.5 (38.3–80.4) | 34.3 (14.5–49.5) | 75.7 (43.9–139.9) | |
| Min–Max | 0.00–0.30 | 12.4–119.1 | 16.8–128.4 | 8.8–82.2 | 1.0–134.5 | 38.3–158.2 | |
| Different from | CC,EC,HTP,NRT,OT | NU | NU | NU | NU,OT | NU,NRT | |
| Saliva cotinine2(ng/mL) | Mean ± SD | 0.17 ± 0.13 | 144.2 ± 79.7 | 102.1 ± 51.9 | 168.1 ± 125.3 | 127.5 ± 122.1 | 267.7 ± 106.8 |
| Median (IQR)3 | 0.21 (0.04–0.26) | 127.0 (83.0–210.5) | 84.9 (60.7–136.2) | 174.2 (43.7–292.1) | 108.8 (35.2–167.0) | 264.9 (191.0–350.3) | |
| Min–Max | 0.00–0.37 | 41.3–271.8 | 51.6–217.8 | 1.76–353.4 | 27.3–388.8 | 97.7–431.0 | |
| Different from | CC,EC,HTP,NRT,OT | NU | NU,OT | NU | NU,OT | NU,EC,NRT | |
| Saliva 3-OH-Cot2(ng/mL) | Mean ± SD | 0.05 ± 0.12 | 32.1 ± 22.0 | 26.7 ± 14.1 | 31.6 ± 19.8 | 34.4 ± 41.5 | 49.2 ± 21.5 |
| Median (IQR)3 | 0.00 (0.00–0.02) | 26.7 (15.8–45.5) | 33.0 (10.2–39.0) | 33.2 (11.8–46.9) | 14.8 (8.9–54.3) | 46.6 (27.6–67.1) | |
| Min–Max | 0.00–0.37 | 9.5–83.2 | 5.6–41.3 | 1.0–61.8 | 0.7–134.4 | 21.9–82.1 | |
| Different from | CC,EC,HTP,NRT,OT | NU | NU | NU | NU | NU | |
| Estimated nicotine intake4(mg/d) | Mean ± SD | 6.17 ± 0.76 | 9.38 ± 5.63 | 4.49 ± 2.30 | 7.70 ± 4.67 | 17.31 ± 10.28 | |
| Median (IQR)3 | 6.22 (4.18–7.84) | 8.39 (5.12–11.51) | 4.56 (2.62–7.09) | 7.00 (3.50–10.50) | 15.57 (10.27–20.54) | ||
| Min–Max | 2.03–10.27 | 4.41–23.31 | 0.34–7.43 | 2.80–16.80 | 3.66–39.37 | ||
| Different from | OT | HTP |
Plasma sample from Day 3 at 5 pm (B6, Fig. 1).
Saliva sample from Day 3 at 5 pm (S6).
IQR: Inter quartile range (25th −75th percentile).
Calculated for Day 3 by applying the models described in Section 2.3.
The correlation between the nicotine biomarkers Nequ (Nic + 10) on Day 3, plasma and saliva cotinine at 5 pm of Day 3 and the recorded consumption data for this day were also analyzed. The non-parametric Spearman correlation coefficients and the significance levels for each of the 5 user groups are shown in Table 3. Urinary Nequ was found to significantly correlate with the daily consumption parameters for all user groups, except for vapers (EC) when the number of recorded puffs per day was used as a consumption measure. Amount of e-liquid consumed was found to be a more suitable indicator for EC consumption. Similar results were observed for the corresponding correlations with plasma and saliva cotinine. Leaving out the puff numbers as consumption marker of EC users, 12 of 15 correlation coefficients were > 0.5. However, only those for smokers (CC) and HTP users were found to be statistically significant for all 3 nicotine biomarkers.
Table 3.
Spearman correlation coefficients between three selected nicotine biomarker levels and the recorded consumption on Day 3 for ten users of 5 different nicotine products.
| User group (consumption variable) | N | Nequ (mg/24 h) for Day 3 | Plasma cotinine (ng/mL) in B6 | Saliva cotinine (ng/mL) in S6 |
|---|---|---|---|---|
| CC (cigarettes/Day 3) | 10 | 0.845** | 0.802** | 0.754* |
| EC (puffs on Day 3) | 10 | −0.236 | 0.007 | −0.042 |
| EC (mL e-liquid/d) | 10 | 0.661* | 0.236 | 0.624(*) |
| HTP (sticks/Day 3) | 10 | 0.683* | 0.841** | 0.854** |
| NRT (pieces NG/Day 3) | 8 | 0.714(*) | 0.605 | 0.457 |
| OT (g/Day 3) | 9 | 0.809* | 0.514 | 0.174 |
Statistical significance levels: *: p < 0.05; **: p < 0.01; (*): borderline significance, p < 0.06.
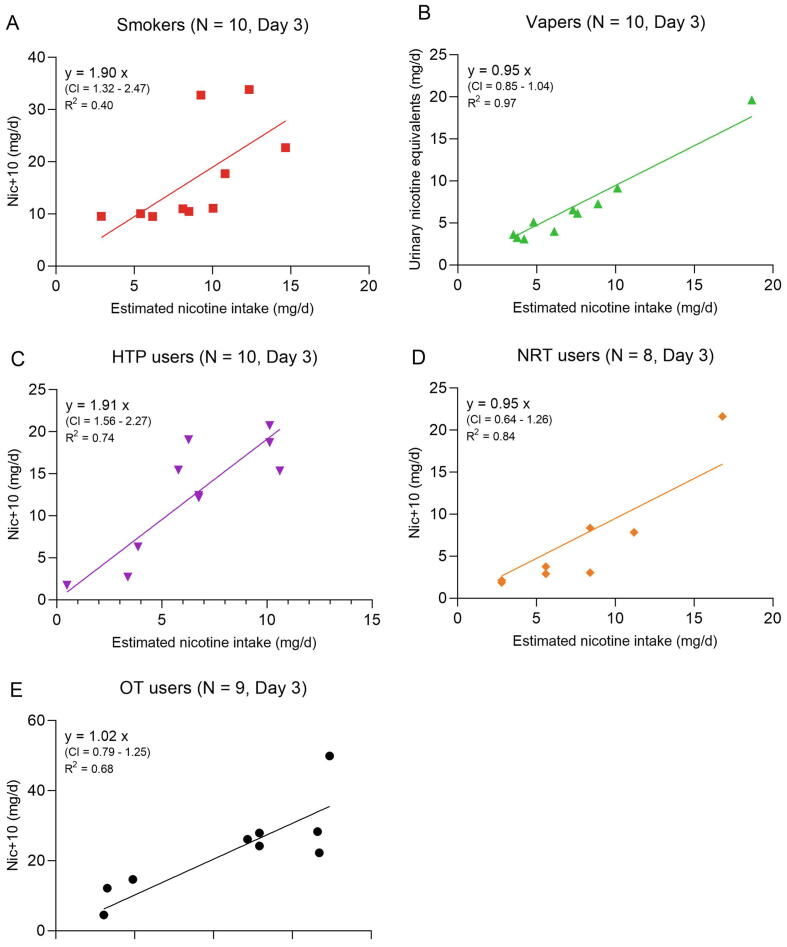
The urinary Nequ comprising nicotine and its 10 major metabolites (Nic + 10) represent virtually the complete nicotine dose taken up by any route. This allows a direct comparison of the daily nicotine intake doses estimated by the models described in Section 2.3 with the Nic + 10 data (mg/24 h) for Day 3. A simple linear regression model of the form y = ax was applied for this purpose (with y = individual Nequ on Day 3; x = estimated individual nicotine intake on Day 3 using Eqs. (1), (2), (3), (4), (5)). The slopes are measures of the agreement between the intake estimates and the measured actual uptake dose of nicotine. It is assumed that the lines for the linear regressions run through the origin which is regarded as biologically plausible in terms of nicotine absorption. The slopes for EC users (with the amount of consumed e-liquid used as consumption parameter), NRT users (limited to the 8 nicotine gum users) and OT users (with one user excluded due to unclear nicotine content of the product used) were found to be close to 1.0, indicating that the applied models for the intake calculation work fairly well (Fig. 3). Linear regressions for smokers (CC) and HTP users showed more scattering with slopes approaching almost 2. Possible reasons for this finding are discussed in the Discussion section.
Fig. 3.
Linear regressions between estimated nicotine intake and urinary excretion of Nequ (Nic + 10) for individual smokers (A), vapers (B) and users of HTP (C), NRT (D) and OT (E) on Day 3. Note that for vapers the intake was calculated with the daily average of consumed e-liquid from Day 1 to 3. CI is the 95 % confidence interval for the slope. R2 (square of the Pearson coefficient of correlation) is used as an indicator for the goodness of fit.
4. Discussion
The first question to be answered by the study results is that of the comparability of the time courses of the nicotine biomarker levels between the 5 different user groups. The time courses of urinary Nequ, plasma Nic, Cot and 3-OH-Cot, as well as salivary Cot and 3-OH-Cot for the 6 study groups during the confined period of the study (74 h) show the expected patterns. Relatively stable levels over time were achieved for urinary Nequ as well as plasma and saliva Cot and 3-OH-Cot in all groups except for smokers (CC); plasma Nic levels were highest in the samples collected at 5 pm on each day (Fig. 2). The observed time courses in plasma and saliva are in accordance with the half-life of Nic (1–3 h, (Rosenberg et al., 1980, Scherer et al., 1988), Cot (16–18 h, (Benowitz et al., 1983, Curvall et al., 1990, Scherer et al., 1988)) and 3-OH-Cot (∼6h, (Scherer et al., 1988)). Somewhat surprising is the finding that the nicotine plasma concentrations at about 5 pm in smokers and OT users are almost identical (Fig. 2B). This, however, is due to the fact that blood sampling took place at least 20 min after product use, which lead to missing the smoking-related plasma nicotine peak. The putative circadian rhythm for Nequ in smokers (higher levels in the 12-h-urine collected over-night compared to that collected during the day, Fig. 2A) could best be explained by the fact that more cigarettes were consumed in the time period which is reflected in the 12-h-urine finalized in the morning than during the period reflected in the 12-h-urine until the early evening. Hourly records for the cigarette consumption show 15 % higher consumption in the period from 3 pm of to midnight than between 7 am and 3 pm. All nicotine biomarkers showed a separation into two groupings: users of CCs and OTs consistently exhibited the highest levels, whereas users of ECs, HTPs and NRTs showed lower but similar levels. Concentrations of nicotine biomarkers in NU were at or below the LOQ throughout the study. It is interesting to note that in all groups the levels at admission to the clinic (reflecting the product use behavior under field conditions a few days prior to the study) were similar to that observed during the study. However, this does not imply that all subjects are compliant to the reported nicotine/tobacco product in the days prior to the study, since all those product categories contain nicotine. For example, by evaluating a biomarker of a typical combustion chemical such as acrylonitrile, we found evidence that some self-reported NRT only users probably also smoked cigarettes (Rögner et al., 2021). This issue is presently investigated systematically with the biomarker data from this study. Furthermore, nicotine biomarker levels for OT users were observed to consistently increase during the course of the study (Fig. 2). Average OT consumption was relative stable during the study (6.9, 7.4 and 6.7 pouches/d on Day 1, 2 and 3, respectively, Table 1). Perhaps other use parameters (e.g. the time period the products were kept in the mouth) increased from Day 1 to Day 3. This parameter, however, was not assessed in this study. The observed time courses (Fig. 2) allow the conclusion that, although only a rough timely resolution was applied (blood and saliva samples were only collected in the morning and late afternoon), Cot in plasma and saliva as well as urinary Nequ are suitable biomarkers for nicotine uptake in users of these products with sampling time being uncritical, provided the product use pattern has achieved about steady-state. In answering the first question, the time courses for the various nicotine biomarker levels were found to be similar between the 5 product user groups with levels somewhat high in smokers (CC) and OT users.
The second question to be answered was whether there were statistically significant differences in the nicotine biomarker levels between the groups and how the observed levels compare to those reported in the literature. We assume that the study volunteers had reached steady-state conditions in terms of product use on Day 3. Therefore, quantitative comparisons between groups as well as reported levels in the literature were performed with the results for Day 3. A remarkable finding of the present study was that the levels of the nicotine biomarkers show a high variation within each group, indicated by the high relative coefficients of variation (SD/mean: 50–100 %) and the large min–max ranges for each biomarker in each group (Table 2). A high variation was also observed in the daily consumption values in each product group during the study (Table 1). This finding may already suggest that nicotine uptake by product use is primarily determined by the use pattern and less so by the class of tobacco/nicotine product consumed. Of course, all nicotine biomarker levels of all user groups were significantly different from the non-user group (NU, Table 2). Furthermore, smokers (CC), users of HTP and OT were not found to be significantly different for any of the 6 measured nicotine biomarkers on Day 3. Vapers (EC) and NRT users were found to exhibit significantly lower plasma and saliva cotinine levels than OT users (who showed the highest biomarker levels). In terms of urinary Nequ excretion, EC and NRT users showed significantly lower levels than CC and OT users. Of particular interest is the finding that the plasma nicotine concentrations, which indicate the bioavailability of the alkaloid, were found to be in the mean range of 9–19 ng/mL and were not significantly different between the 5 user groups (Table 2). It was expected that user of the inhalation products (CC, EC, HTP) exhibit significantly higher nicotine plasma concentration than users of the oral products (NRT, OT). Two reasons might be responsible for this finding, (i) the small sample size could have prevented to reach statistical significance and (ii) the study design required at least 20 min between last product use and blood sampling, which definitely resulted in missing the plasma nicotine peaks in the users of the inhalation products.
An evaluation of literature data for urinary nicotine equivalents as well as cotinine in plasma and saliva of users of CC, EC, HTP, nicotine gum (NG, as a representative of NRT products), OT and of NU of any tobacco/nicotine products has recently been published (Scherer et al., 2021). A comparison of the reported averages for Nequ (which have been adjusted in the review to 100 % of the excreted nicotine dose) and those for cotinine in plasma and saliva shows that the measured mean or median levels of the groups in our study (Table 2) almost completely fall into the ranges of the published values for the corresponding groups (relevant literature cited in the review (Scherer et al., 2021)).
Except for smokers (CC) and non-users (NU), we found no suitable reports for plasma or saliva 3-OH-Cot concentrations in users of the tobacco/nicotine products of interest (EC, HTP, NRT, OT). IQR of salivary OH-Cot for 100 NUs and 260 smokers (CC) were reported to be 0.2–0.6 and 28–92 ng/mL for NU and CC, respectively (Scherer et al., 2007), which is in good agreement with the 3-OH-Cot concentrations in saliva and plasma of NU and CC in our study (Table 2).
With the limitations addressed above, plasma nicotine levels in samples collected at 5 pm of Day 3 can be compared to published data for the products of interest. The major restriction is due to the fact that time since last product use prior to the blood drawing was not stipulated, other than it had to be > 20 min. In smokers (CC), we found an average plasma Nic concentration of 19.2 (range: 2.8–39.0) ng/mL (Table 2). In some older publications, mean plasma Nic levels in the range of 8.5–40 ng/mL were obtained under similar conditions (blood sample drawn in the late afternoon of a smoking day) were reported (Oates et al., 1988, Benowitz et al., 1987, Gupta et al., 1995, Russell et al., 1976, Russell et al., 1975). Despite the mentioned uncertainties, there is a good agreement between literature data and our results. Vapers (EC) in our study exhibited mean plasma Nic concentrations (ranges) of 9.1 (2.5–26.7) ng/mL (Table 2). Corresponding values for HTP users were 12.8 (0.02–27.4) ng/mL. No published reports suitable for comparison with our data could be identified. NRT users (mainly users of 4 mg nicotine gums) in our study exhibited mean plasma Nic concentrations (ranges) of 9.4 (0.3–21.9) ng/mL (Table 2). Comparable averages from the literature were in the range of 10–27 ng/mL (Benowitz et al., 1987, Hansson et al., 2017, Lunell and Lunell, 2005, Russell et al., 1976), which is higher than in our study. Lower consumption of gums as well as lower frequency of use per unit of time can be reasons for this difference. With respect to the latter factor, it was found that using 6 mg nicotine gums over 12 h at a frequency of 1/h led to significantly higher plasma Nic concentrations than at 1/1.5 h (37 versus 25 ng/mL) (Hansson et al., 2017). Average plasma Nic levels (ranges) of OT users in our study were 19.1 (7.3–30.9) ng/mL (Table 2). Lunell et al. (Lunell and Lunell, 2005) reported a range of 10–30 ng/mL for users of 4 different brands of snus consumed at an hourly basis over 11 h, which is in good agreement with our findings. Taken together, comparisons of our nicotine biomarker data of the various users groups are in good accordance with corresponding data reported in the literature. In answering the second question, it can be stated that smokers (CC) and OT users showed statistically higher nicotine biomarker levels compared to the other user groups only for some of the biomarkers. In general, nicotine biomarker levels exhibited a high variability in all user groups and show good agreement to data reported in the literature.
The third question to be answered by the study results was how well the nicotine biomarker levels correlated with commonly used daily consumption measures in the various user groups. During the 3 study days, the subjects’ product use pattern was recorded in an hourly structured timetable by the clinic staff. For vapers (EC) each puff they drew from their device was recorded. It is assumed that this procedure provided rather reliable daily consumption data (Table 1). Despite of that, not all correlations between the 3 nicotine biomarkers (Nequ, plasma Cot and saliva Cot) and the daily consumption on Day 3 were found to be statistically significant (Table 3), indicating that other product use factors, in addition to daily consumption, are pivotal for the nicotine uptake. For the inhalation products (CC, EC, HTP), puffing topography as well as extent of inhalation (depth and duration) are assumed to be important determinants for the uptake of smoke/aerosol constituents (Appleton et al., 2015, Jones et al., 2020, Robinson et al., 2020, Vansickel et al., 2018). A striking finding was that no correlation at all was observed between the nicotine biomarkers and number of puffs in vapers (Table 3). This underlines that in addition to mere number of puffs taken, also topographic parameters such as puff volume and duration as well as mouth spill and depth of inhalation would be required as nicotine dose determinants. A moderate to strong correlation was, however, found for vapers when the amount of consumed e-liquid was used as a consumption variable (Table 3). Similar results were obtained, when 1,2-propylene glycol in plasma and urine of vapers was correlated with various dose markers (Burkhardt et al., 2021). For the oral products (nicotine gum, OT), the usage time of the product in the mouth is regarded to be crucial for nicotine exposure dose per unit used (Gale et al., 2012). The determination of these product use parameters were, however, beyond the scope of this study. In answering the third question, it can be stated that not all correlations between the nicotine biomarker levels and product consumption were found to be statistically significant (Table 3). This appears to be dependent on the nicotine biomarker applied, the product under investigation and the consumption measure used. Furthermore, limited number of users per group (8–10) certainly have prevented to reach statistical significance. A clear finding was that number of puffs taken by vapers is not a suitable measure of EC dose. On the other hand, number of cigarettes (CC) or sticks (HTP) as well consumed amount of e-liquid (EC) were observed to be well-suited consumption markers.
Finally (forth question), the predictability of the actually absorbed nicotine dose (measured by the determination of urinary Nic + 10 (Piller et al., 2014)) by the product use-related nicotine intake (modelled by the equations described in Section 2.3) was investigated. Accordance between measurement and model calculations can be directly deduced from the slopes of the linear regressions. Fig. 3 shows that slopes of approximately 1.0 were obtained for use of ECs (applying the amount of e-liquid consumed as consumption variable), NRT (limited to the 8 nicotine gum users) and OT (with one user excluded for an unclear nicotine content of the used product). For vapers, a mouth spill of 60 % was assumed in Equation (3). It has to be stated that this mouth spill rate is not based on experimental data, but best fits with the measured nicotine uptake and also appears to be plausible (vapers spill out large clouds of aerosol). Interestingly, a 60 % mouth spill was observed at the upper end (90th–95th percentile) in a study with 139 smokers (St Charles et al., 2013). Despite of that, the assumed mouth spill for vapers has to be verified by suitable studies. Significant deviations from slope values of 1.0 were found for smokers (CC) and HTP users (slopes of 1.9 in both cases). As a possible reason for the deviations between model and measurement, it could be speculated that the applied nicotine yields in the models (CC: 0.5–0.8 mg/cig according to ISO; HTP: 0.5 mg/stick for all 10 users) markedly underestimate the actual, subject-derived nicotine yields. In a similar estimate of daily nicotine doses with slightly different models and application of data from the literature for consumption and products’ nicotine yields, median intakes of 11.7 and 9.0 mg/d for smokers (CC) and HTP users, respectively were reported (Scherer et al., 2021), approximately twice as high as the estimated intakes in this study (Table 2). When comparing the values of the variables in the two approaches, it becomes evident that for the main part the higher nicotine yields (CC: 0.6–1.7 mg/cig, HTP: 0.4–1.2 mg/stick (Scherer et al., 2021)) were responsible for the higher intake estimates based on literature data compared to this study. More investigations are certainly required to substantiate this hypothesis. In answering the forth question, it can be stated that the applied models lead to daily amounts of nicotine intake that were in acceptable agreement with the daily excretion of Nequ for users of EC, NRT (nicotine gum) and OT. Nicotine intakes for smokers (CC) and HTP users were underestimated by almost a factor of 2, which is probably due to the too low nominal nicotine yields.
Our study has a number of limitations, including: (a) small sample size (10 subjects per group); (b) user groups are certainly not representative, this is due, on the one hand, to the small sample size and, on the other hand, to the heterogeneity of the products within a group; (c) rapid advancement in product development (particularly in ECs and oral nicotine products) may have led to the fact that products used in this study do not reflect the actual market situation; (d) a more detailed characterization of the product use (e.g. puffing topography and mouth spill for vapers or nicotine extraction efficiency in OT users) could have improved the nicotine intake models.
5. Conclusions
The results of this study lead us to the following conclusions:.
-
(1)
Users of five nicotine/tobacco products, although rather limited in number per group (8–10), showed a broad range of estimated nicotine intakes and measurable levels of nicotine biomarkers during the confined phase of the study with ad libitum product use.
-
(2)
Although absorbed nicotine doses largely overlap between the users of the different product classes, two clusters in terms of nicotine biomarker levels can be differentiated: (a) smokers (CC) and users of OT at the high end, (b) users of EC, HTP and NRT in the center field.
-
(3)
For all user groups significant correlation between the nicotine biomarker levels and the recorded daily consumption data were observed. In vapers, the nicotine biomarker levels significantly correlated with the amount of e-liquid consumed, but not with the number of recorded puffs, indicating that puffing topography (e.g. puff volume and duration) as well as extent of inhalation are probably important additional determinants for estimating the nicotine uptake.
-
(4)
Urinary Nequ and cotinine in plasma and saliva are suitable biomarkers of exposure to nicotine. Time to last using a nicotine product is uncritical, as long as almost steady-state conditions are achieved.
-
(5)
Model calculations for the individual daily intake were found to be in good agreement with the amount of excreted Nequ in the urine for users of EC, NRT and OT, but underestimated the nicotine intake by almost a factor of 2 for users of CC and HTP. The deviation is probably attributable to too low machine-derived nicotine yields applied in the models.
Funding
This study was funded with a grant from the Foundation for a Smoke-Free World (FSFW), a US nonprofit 501(c)(3) private foundation. FSFW had no role in the planning and execution of this study, data analysis and publication of the results. The Foundation accepts charitable gifts from PMI Global Services Inc. (PMI); under the Foundation’s Bylaws and Pledge Agreement with PMI, the Foundation is independent from PMI and the tobacco industry.
CRediT authorship contribution statement
Gerhard Scherer: Conceptualization, Writing – original draft. Janina Mütze: Formal analysis, Writing – review & editing. Nikola Pluym: Conceptualization, Writing – review & editing, Funding acquisition. Max Scherer: Conceptualization, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Max Scherer reports financial support was provided by Foundation for a Smoke-Free World (FSFW).
Contributor Information
Gerhard Scherer, Email: gerhard.scherer@abf-lab.com, max.scherer@abf-lab.com.
Janina Mütze, Email: janina.muetze@abf-lab.com.
Nikola Pluym, Email: nikola.pluym@abf-lab.com.
Max Scherer, Email: max.scherer@abf-lab.com.
References
- Adlkofer F.X. In: Effects of Nicotine on Biological Systems II. Clarke P.B.S., Quik M., Adlkofer F., Thurau K., editors. Birkhäuser Basel; Basel: 1995. Involvement of nicotine and its metabolites in the pathology of smoking-related diseases: Facts and hypotheses; pp. 17–25. [Google Scholar]
- Appleton S., Liu J., Lipowicz P.J., Sarkar M. Effect of cigarette design on biomarkers of exposure, puffing topography and respiratory parameters. Environ. Health Perspect. 2015;123:A97. doi: 10.1289/ehp.123-A97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R.R., Dixon M. The retention of tobacco smoke constituents in the human respiratory tract. Inhal. Toxicol. 2006;18(4):255–294. doi: 10.1080/08958370500444163. [DOI] [PubMed] [Google Scholar]
- Benowitz N.L. Clinical Pharmacology of Nicotine: Implications for Understanding, Preventing, and Treating Tobacco Addiction. Clin. Pharmacol. Ther. 2008;83(4):531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Oates J.A., Wood A.J.J., Benowitz N.L. Pharmacologic aspects of cigarette smoking and nicotine addiction. New Engl. J. Med. 1988;319(20):1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- Benowitz N.L. The role of nicotine in smoking-related cardiovascular disease. Prev. Med. 1997;26(4):412–417. doi: 10.1006/pmed.1997.0175. [DOI] [PubMed] [Google Scholar]
- Benowitz N.L., Jacob P., III, Savanapridi C. Determinants of nicotine intake while chewing nicotine polacrilex gum. Clin. Pharm. Ther. 1987;41:467–473. doi: 10.1038/clpt.1987.58. [DOI] [PubMed] [Google Scholar]
- Benowitz N.L., Kuyt F., Jacob P., Jones R.T., Osman A.-L. Cotinine disposition and effects. Clin. Pharm. Ther. 1983;34(5):604–611. doi: 10.1038/clpt.1983.222. [DOI] [PubMed] [Google Scholar]
- Bishop E., East N., Bozhilova S., Santopietro S., Smart D., Taylor M., Meredith S., Baxter A., Breheny D., Thorne D., Gaca M. An approach for the extract generation and toxicological assessment of tobacco-free 'modern' oral nicotine pouches. Food Chem. Toxicol. 2020;145:111713. doi: 10.1016/j.fct.2020.111713. [DOI] [PubMed] [Google Scholar]
- Burkhardt T., Pluym N., Scherer G., Scherer M. 1,2-Propylene Glycol: A Biomarker of Exposure Specific to e-Cigarette Consumption. Separations. 2021;8:180. doi: 10.3390/separations8100180. [DOI] [Google Scholar]
- Curvall M., Elwin C.-E., Kazemi-Vala E., Warholm C., Enzell C.R. The pharmacokinetics of cotinine in plasma and saliva from non-smoking healthy volunteers. Eur. J. Clin. Pharmacol. 1990;38(3):281–287. doi: 10.1007/BF00315031. [DOI] [PubMed] [Google Scholar]
- Digard H., Proctor C., Kulasekaran A., Malmqvist U., Richter A. Determination of nicotine absorption from multiple tobacco products and nicotine gum. Nicotine Tob. Res. 2013;15(1):255–261. doi: 10.1093/ntr/nts123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom K. A Comparison of Dependence across Different Types of Nicotine Containing Products and Coffee. Int. J. Environ. Res. Public Health. 2018;15:1609. doi: 10.3390/ijerph15081609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Plunkett S.E., Lam K., Kapur S., Muhammad R., Jin Y., Zimmermann M., Mendes P., Kinser R., Roethig H.J. A new method for estimating the retention of selected smoke constituents in the respiratory tract of smokers during cigarette smoking. Inhal. Toxicol. 2007;19(2):169–179. doi: 10.1080/08958370601052022. [DOI] [PubMed] [Google Scholar]
- Fowles J., Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob. Control. 2003;12:424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale, N., Digard, H., McAdam, K., Williamson, J., 2012. Influence of usage time on exposure of snus users to nicotine, NNN and NNK from snus pouches. In: Poster at the 18th Annual SRNT Meeting 2012, Houston, Texas, USA.
- Gately I. Open Road+ Grove/Atlantic; New York: 2007. Tobacco: a cultural history of how an exotic plant seduced civilization. [Google Scholar]
- Gupta S.K., Hwang S.S., Causey D., Rolf C.N., Gorsline J. Comparison of the nicotine pharmacokinetics of Nicoderm (nicotine transdermal system) and half-hourly cigarette smoking. J. Clin. Pharmacol. 1995;35:985–989. doi: 10.1002/j.1552-4604.1995.tb04014.x. [DOI] [PubMed] [Google Scholar]
- Hansson A., Rasmussen T., Kraiczi H. Single-dose and multiple-dose pharmacokinetics of nicotine 6 mg gum. Nicotine Tob. Res. 2017;19:477–483. doi: 10.1093/ntr/ntw211. [DOI] [PubMed] [Google Scholar]
- Hoffmann D., Hoffmann I., El Bayoumy K. The less harmful cigarette: A controversial issue. A tribute to Ernst L. Wynder. Chem. Res. Toxicol. 2001;14:767–790. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM), 2001. Clearing the smoke: assessing the science base for tobacco harm reduction. In: Stratton, K., Shetty, P., Wallace, R., Bondurant, S., (Eds.). National Academy Press, Washington, D.C. [PubMed]
- International Standard Organisation (ISO), 1991. ISO 4387: Cigarettes; Determination of total and nicointe free dry particulate matter using a routine analytical smoking machine.
- Jarvis M.J. Why people smoke. Br. Med. J. 2004;328(7434):277–279. doi: 10.1136/bmj.328.7434.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K.P., Valentine G., Gueorguieva R., Sofuoglu M. Differential effects of nicotine delivery rate on subjective drug effects, urges to smoke, heart rate and blood pressure in tobacco smokers. Psychopharmacol. (Berl). 2020;237(5):1359–1369. doi: 10.1007/s00213-020-05463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J., Slayford S., Gray A., Brick K., Prasad K., Proctor C. A cross-category puffing topography, mouth level exposure and consumption study among Italian users of tobacco and nicotine products. Sci. Rep. 2020;10 doi: 10.1038/s41598-019-55410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser A., Scherer M., Pluym N., Sarkar M., Edmiston J., Niessner R., Scherer G. Biomarkers of Exposure Specific to E-vapor Products Based on Stable-Isotope Labeled Ingredients. Nicotine Tob. Res. 2019;21:314–322. doi: 10.1093/ntr/nty204. [DOI] [PubMed] [Google Scholar]
- Logue J.M., Sleiman M., Montesinos V.N., Russell M.L., Litter M.I., Benowitz N.L., Gundel L.A., Destaillats H. Emissions from electronic cigarettes: Assessing vapers' intake of toxic compounds, secondhand exposures, and the associated health impacts. Environ. Sci. Technol. 2017;51(16):9271–9279. doi: 10.1021/acs.est.7b00710. [DOI] [PubMed] [Google Scholar]
- Lunell E., Curvall M. Nicotine delivery and subjective effects of Swedish portion snus compared with 4 mg nicotine polacrilex chewing gum. Nicotine Tob. Res. 2011;13(7):573–578. doi: 10.1093/ntr/ntr044. [DOI] [PubMed] [Google Scholar]
- Lunell E., Fagerström K., Hughes J., Pendrill R. Pharmacokinetic comparison of a novel non-tobacco-based nicotine pouch (ZYN®) with conventional, tobacco-based Swedish snus and American moist snuff. Nicotine Tob. Res. 2020;22:1757–1763. doi: 10.1093/ntr/ntaa068. [DOI] [PubMed] [Google Scholar]
- Lunell E., Lunell M. Steady-state nicotine plasma levels following use of four different types of Swedish snus compared with 2-mg Nicorette chewing gum: a crossover study. Nicotine Tob. Res. 2005;7(3):397–403. doi: 10.1080/14622200500125468. [DOI] [PubMed] [Google Scholar]
- Parascandola M. Lessons from the history of tobacco harm reduction: the National Cancer Institute's Smoking and Health Program and the “less hazardous cigarette”. Nicotine Tob. Res. 2005;7(5):779–789. doi: 10.1080/14622200500262584. [DOI] [PubMed] [Google Scholar]
- Piller M., Gilch G., Scherer G., Scherer M. Simple, fast and sensitive LC-MS/MS analysis for the simultaneous quantification of nicotine and 10 of its major metabolites. J. Chromatogr. B. 2014;951–952:7–15. doi: 10.1016/j.jchromb.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Ramstrom L., Borland R., Wikmans T. Patterns of Smoking and Snus Use in Sweden: Implications for Public Health. Int. J. Environ. Res. Public Health. 2016;13:1110. doi: 10.3390/ijerph13111110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R.J., Sarles S.E., Jayasekera S., Al Olayan A., Difrancesco A.G., Eddingsaas N.C., Hensel E.C. A Comparison between Cigarette Topography from a One-Week Natural Environment Study to FTC/ISO, Health Canada, and Massachusetts Department of Public Health Puff Profile Standards. Int. J. Environ. Res. Public Health. 2020;17:3444. doi: 10.3390/ijerph17103444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgman A., Green C.R. Toxic Chemicals in Cigarette Mainstream Smoke – Hazard and Hoopla. Beitr. Tabakforsch. Int. 2003;20:481–545. [Google Scholar]
- Rögner N., Hagedorn H.-W., Scherer G., Scherer M., Pluym N. A Sensitive LC–MS/MS Method for the Quantification of 3-Hydroxybenzo[a]pyrene in Urine-Exposure Assessment in Smokers and Users of Potentially Reduced-Risk Products. Separations. 2021;8:171. doi: 10.3390/separations8100171. [DOI] [Google Scholar]
- Rosenberg J., Benowitz N.L., Jacob P.I.I.I., Wilson K.M. Disposition kinetics and effects of intravenous nicotine. Clin. Pharmacol. Therapeut. 1980;28:517–522. doi: 10.1038/clpt.1980.196. [DOI] [PubMed] [Google Scholar]
- Russell M.A.H., Feyerabend C., Cole P.V. Plasma nicotine levels after cigarette smoking and chewing nicotine gum. Br. Med. J. 1976;1:1043–1046. doi: 10.1136/bmj.1.6017.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M.A.H., Wilson C., Patel U.A., Feyerabend C., Cole P.V. Plasma nicotine levels after smoking cigarettes with high, medium, and low nicotine yields. Br. Med. J. 1975;2:414–416. doi: 10.1136/bmj.2.5968.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G., Engl J., Urban M., Gilch G., Janket D., Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul. Toxicol. Pharmacol. 2007;47:171–183. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Scherer G., Jarczyk L., Heller W.D., Biber A., Neurath G.B., Adlkofer F. Pharmacokinetics of nicotine, cotinine, and 3'-hydroxycotinine in cigarette smokers. Klin. Wochenschr. 1988;66(Suppl 11):5–11. [PubMed] [Google Scholar]
- Scherer G., Pluym N., Scherer M. Intake and uptake of chemicals upon use of various nicotine/tobacco products: Can users be differentiated by single or combinations of biomarkers? Contr. Tob. Nicotine Res. 2021;30:167–198. [Google Scholar]
- Sibul F., Burkhardt T., Kachhadia A., Pilz F., Scherer G., Scherer M., Pluym N. Identification of biomarkers specific to five different nicotine product user groups: Study protocol of a controlled clinical trial. Contemp. Clin. Trials Commun. 2021;22:100794. doi: 10.1016/j.conctc.2021.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Charles F.K., McAughey J., Shepperd C.J. Methodologies for the quantitative estimation of toxicant dose to cigarette smokers using physical, chemical and bioanalytical data. Inhal. Toxicol. 2013;25:383–397. doi: 10.3109/08958378.2013.794177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricker A.R. Biomarkers derived from nicotine and its metabolites: a review. Beitr. Tabakforsch. Int. 2006;22:147–175. [Google Scholar]
- US Department of Health and Human Services, 1986. The Healh Consequences of Using Smokeless Tobacco. A Report of the Surgeon General. http://resource.nlm.nih.gov/101584932X65.
- US Department of Health and Human Services, 1988. The health consequences of smoking. Nicotine addiction. A report of the Surgeon General. Public Health Service, Rockville, Maryland.
- US Department of Health and Human Services, 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. A Report of the Surgeon General. https://www.ncbi.nlm.nih.gov/books/NBK53017/. [PubMed]
- Vansickel A.R., Edmiston J.S., Liang Q., Duhon C., Connell C., Bennett D., Sarkar M. Characterization of puff topography of a prototype electronic cigarette in adult exclusive cigarette smokers and adult exclusive electronic cigarette users. Regul. Toxicol. Pharmacol. 2018;98:250–256. doi: 10.1016/j.yrtph.2018.07.019. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2021. WHO study group on tobacco product regulation: report on the scientific basis of tobacco product regulation: eighth report of a WHO study group. [PubMed]