Abstract
Introduction:
Evidence strongly suggests that soluble oligomers of amyloid β-protein (oAβ) help initiate the pathogenic cascade of Alzheimer’s disease. To date, there have been no validated assays specific for detecting and quantifying oAβ in human blood.
Methods:
We developed an ultrasensitive oAβ immunoassay using a novel capture antibody (71A1) with N-terminal antibody 3D6 for detection that specifically quantifies soluble oAβ in human brain, CSF and plasma.
Results:
Two new antibodies (71A1; 1G5) are oAβ-selective, label Aβ plaques in non-fixed AD brain sections, and potently neutralize the synaptotoxicity of AD brain-derived oAβ. The 71A1/3D6 assay showed excellent dilution linearity in CSF and plasma without matrix effects, good spike-recovery, and specific immunodepletion.
Discussion:
We have created a sensitive, high throughput and inexpensive method to quantify synaptotoxic oAβ in human plasma for analyzing large cohorts of aged and AD subjects to assess the dynamics of this key pathogenic species and response to therapy.
Keywords: Alzheimer’s disease, amyloid β-protein, oligomeric Aβ, plasma biomarkers
Introduction
In the last two decades, the recognition that soluble oligomers of the amyloid β-protein (Aβ) can confer synaptic and neuritic injury [1–4] and microgliosis [5–7] and that soluble Aβ levels in Alzheimer’s disease (AD) brains correlate with synaptic changes and cognitive impairment [8, 9] have inspired extensive investigation of aqueously soluble Aβ in the oligomeric state (oAβ). There is growing evidence that oAβ 1) causes defects in synaptic transmission primarily in electrically active neurons [10]; 2) decreases long-term potentiation and induces long-term depression in mouse hippocampus in vitro and in vivo [1, 2, 11–14]; and 3) can distinguish demented from non-demented aged humans when brain oAβ levels are ratioed to amyloid plaque levels [15]. These and numerous other studies indicate that diffusible oAβ assemblies correlate strongly with, and potentially even induce, the neuropathological and clinical phenotypes of AD patients.
Aβ exists in a dynamic equilibrium between various monomeric, oligomeric, and higher-order filamentous forms, and these have diverse synaptotoxic properties. The complex equilibrium between various sizes of Aβ oligomers/polymers poses an inherent challenge in isolating and studying naturally occurring oAβ species. Many antibodies targeting oAβ have been developed (e.g., [16–29]); yet the technical challenges of quantifying naturally occurring oAβ in cerebrospinal fluid (CSF) and especially plasma still remain. Previously, we reported a sensitive sandwich immunoassay using a conformation-specific antibody (1C22) for capture and an antibody to the N-terminus (3D6) as detector to quantify low levels of oAβ in human CSF [30]. In a follow-up study [31], we reported the first evidence of successful target engagement of Aβ oligomers in humans by a therapeutic antibody [Crenezumab (Roche)], suggesting that quantifying oAβ in CSF could become a useful pharmacodynamic readout of anti-amyloid approaches in AD clinical trials.
Despite the mounting evidence that soluble oAβ plays a central role in early AD pathogenesis, there exists a major unmet need for a sensitive and specific method to detect and quantify oAβ in human plasma. Here we report 2 monoclonal antibodies, 1G5 and 71A1, generated by using a synthetic Aβ1–40 cyclic peptide as immunogen, which we have extensively characterized and used to develop oligomer-specific immunoassays. We show that 1) 1G5 and 71A1 recognize soluble oAβ from human brain, CSF and plasma without reactivity to Aβ monomers; 2) 71A1 neutralizes the synaptotoxicity of oAβ-rich AD brain extracts in electrophysiological assays; and 3) an ultra-sensitive immunoassay using 71A1 as capture paired with 3D6 as detector achieves a lower LLoQ of 0.6 pg/mL and reliably quantifies oAβ in human brain, CSF and plasma with low coefficients of variation (CVs). The availability of a sensitive and specific immunoassay that quantifies endogenous human oAβ in blood should enable future studies of the dynamic process of Aβ oligomerization and disassembly in brain and biofluids and establish correlations between oAβ and other known AD biomarkers including Aβ monomers [32–34], Tau fragments [35, 36] and pTau [37–43] in the blood. Moreover, our findings support the potential therapeutic benefit of neutralizing 71A1-immunoreactive oAβ. Together, these advances will deepen our understanding of the pathobiological role of oAβ in AD and pave the way to monitoring this key pathogenic form of amyloid β-protein in Alzheimer’s disease and age-related β-amyloidosis.
Methods
Generation of Aβ oligomer specific antibodies 71A1 and 1G5
Monoclonal antibodies 71A1 (a subclone of parent clone 7A1a) and 1G5 were raised against a synthetic conformational peptide immunogen designed to potentially mimic the three-dimensional structure formed upon dimerization of the monomeric Aβ. The peptide immunogen was synthesized containing amino acid residues 9–18 of Aβ1–40 and post-synthetically modified to cyclize and allow folding into a stable dimer formation (patent pending). The rationale behind the selection of residues 9–18 of the Aβ peptide was that the amino acids in this region would likely re-associate into a unique three-dimensional structure only present in aggregated forms of Aβ. Balb/c mice were immunized with this conformational peptide immunogen using a maleimide-activated keyhole-limpet-hemocyanin (KLH) carrier complex for primary and secondary immunizations and a proprietary T helper 2 cell epitope for subsequent boosts prior to the splenectomy procedures. Fusions were performed using immunized mouse splenocytes and mouse myeloma F0 cells as the fusion partner. After primary screening with the immunogen and subcloning, two monoclonal candidates, 71A1 and 1G5, in particular, showed high specificity to cyclized peptide and no specificity to linear peptide. Initial characterization of these antibodies had demonstrated high specificity for synthetic and endogenous oligomeric forms of Aβ and no binding to monomeric Aβ [44].
Reagents
Synthetic β-amyloid peptides 1–40 and 1–42 were purchased from Anaspec. Aβ 1–40 in which serine 26 was substituted with cysteine (Aβ S26C) were purchased from the Keck Biotechnology Center (Yale University, New Haven, CT). Amyloid-β derived diffusible ligands (ADDLs) [1] and S26C-dimer were prepared as per previous reports [45].
Mice
Both male and female C57BL/6J mice were used. All procedures involving mice were in accordance with the animal welfare guidelines of Harvard Medical School and Brigham and Women’s hospital.
Preparation of soluble brain “soaking” extracts
Extraction of soluble protein from postmortem brain tissue using the “soaking” method was performed as described previously[11]. Briefly, cortical grey matter was dissected from freshly thawed coronal slices, then chopped at 0.5-mm-wide intervals on a McIlwain tissue chopper. Chopped tissue bits were weighed and added at 1:5 w:v to extraction buffer (25 mM Tris, 150 mM NaCl, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 2 μg/ml pepstatin, 120 μg/ml Pefabloc, 5 mM EDTA, 5 mM NaF, pH 7.2). Tissue bits were soaked by nutating at 4°C in 50-ml Eppendorf protein LoBind tubes for 30 min. Tissue bits were removed by spinning for 10 min at 4°C at 2,000 g in a Fiberlite F14–14 × 50cy rotor in a Sorvall Lynx 6000 superspeed centrifuge (Thermo Fisher). The upper ~90% of supernatant was removed and then spun in an SW41Ti rotor in an Optima L90K ultracentrifuge (Beckman Coulter) at 40,000 rpm for 110 min at 4°C. The upper ~90% of supernatant was retained as the “soaking extract” for further studies. Brain tris-buffer saline (TBS) soaking extracts were aliquoted into 1.5-ml Eppendorf Protein LoBind tubes and stored at −80°C.
Human biofluids and brain tissue
Human CSF and plasma were obtained from two clinical cohorts, one from the BWH Division of Cognitive and Behavioral Neurology, and one from the Mayo Clinic Study of Aging. Table 1 shows demographic characteristics, and further details of both cohorts. The BWH patients referred for diagnostic lumbar puncture were consented for donation of a plasma sample and extra CSF and access to their medical records under BWH IRB approval. Blood was collected into EDTA tubes, centrifuged at 1500 g for 15 min, and the plasma aliquoted and frozen at −80°C, all within 3 h of collection. CSF was drawn directly into polypropylene tubes (Sarstedt). A portion of CSF was sent to Athena Diagnostics for the ADmark Panel (consisting of Aβ1–42, T-tau, and P-tau) and the remainder frozen immediately on dry ice, then thawed and aliquoted at a later date. Clinical diagnostic information was obtained through chart review by a board-certified behavioral neurologist prior to the ADmark or research ELISA results being known.
Table. 1.
Patient demographics in current study
| Cohort | Age (mean +/− SD) | Sex (% male) | Functional category (N, %) † | Most clinically suspected neuropathology (N, %) |
|---|---|---|---|---|
| BWH (CSF) | 65.9 +/− 8.3 | 38.9 | Unimpaired (2, 5.6); Subjective cognitive decline (1, 2.8); MCI (19, 52.8); Dementia (14, 38.9); Unknown (2, 5.6) | AD (23, 63.9); LBD (2, 5.6); FTLD (2, 5.6); None (6, 16.7); Unknown (3, 8.3) |
| Mayo Clinic (Plasma) | 74.4 +/− 8.9 | 53.4 | Cognitively normal (73, 100) | Normal (73, 100) |
Totals exceed 100% because some subjects were on the cusp of the MCI or dementia category and were counted twice, once per category.
The Mayo Clinic Study of Aging (MCSA) is a population-based, prospective study of residents living in Olmsted County, MN. Details of study design and participant recruitment are published [46, 47]. In 2004, the Rochester Epidemiology Project (REP) medical records linkage system was used to enumerate Olmsted County residents between the ages of 70 and 89, as described [48]. In 2012, the MCSA was extended to include those aged 50 and older. The present analyses included 73 Mayo participants in whom we measured plasma Aβ oligomers, as described herein. Subject enrollment, sample collection and sharing of samples across sites was approved by the Mayo Clinic and the Olmsted Medical Center. The institutional review boards of the Mayo Clinic and the Olmsted Medical Center approved study protocols and written informed consent was obtained from all participants.
Human brain tissue was obtained at BWH or MGH from deceased donors with probable AD undergoing diagnostic autopsy. One hemisphere was fixed for diagnostic purposes and one hemisphere was sliced coronally and frozen at −80°C. All human subjects research received prior approval by the Mass General Brigham Institutional Review Board, and informed consent was obtained for all human subjects.
Electrophysiological extracellular Field recordings
Experiments were performed as previously described [13]. Briefly, mice (1–3 mo) were deeply anesthetized with halothane and decapitated. Transverse acute hippocampal slices (350 μm) were cut in ice-cold oxygenated sucrose-enhanced artificial cerebrospinal fluid (aCSF) containing 206 mM sucrose, 2 mM KCl, 2 mM MgSO4, 1.25 mM NaH2PO4, 1 mM CaCl2, 1 mM MgCl2, 26 mM NaHCO3, 10 mM D-glucose, pH 7.4. After dissection, slices were incubated in aCSF that contained the following (in mM): 124 NaCl, 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 2.5 CaCl2, 26 NaHCO3, 10 D-glucose saturated with 95% O2 and 5% CO2 (pH 7.4), in which they were allowed to recover for at least 90 min before recording. Recordings were performed in the same solution at room temperature in a chamber submerged in aCSF. To record field EPSPs (fEPSPs) in the CA1 region of the hippocampus, standard procedures were used. Test stimuli were applied at low frequency (0.05 Hz) at a stimulus intensity that elicited a fEPSP amplitude that was 40–50% of maximum, and the test responses were recorded for 10 min before the experiment was begun to ensure stability of the response. Once a stable test response was attained, for experimental treatments, 0.5 mL AD brain TBS soaking extracts were added to the 9.5 mL aCSF perfusate, and a baseline was recorded for an additional 30 min. For the anti-Aβ antibody experiments, the 71A1 antibody was added to an aliquot of AD brain extract and incubated with mixing for 30 min, then the mixture was added to the brain slice perfusion buffer. To induce LTP, two consecutive trains (1 s) of stimuli at 100 Hz separated by 20 s were applied to the slices. Traces were obtained by pClamp 11 and analyzed using Clampfit 11. Data analysis was as follows. The fEPSP magnitude was measured using the initial fEPSP slope, and 3 consecutive slopes (1 min) were averaged and normalized to the mean value recorded 10 min before the conditioning stimulus. Data are presented as means ± SEMs. Significant differences were determined using an unpaired multiple t-test.
Electrophoresis and WB
Samples were loaded onto 4–12% or 12% Bis-Tris gels (SurePAGE, Genscript) using MES-SDS running buffer (Invitrogen), transferred to nitrocellulose membranes, and probed for various proteins using standard WB. The resultant blots were detected by ECL and signals were captured by film.
Immunoprecipitation
800 μL human brain TBS soaking extracts or CSF samples were incubated with 10 μg antibodies for 1 hr at 4°C. The immunoprecipitates (IPs) were incubated with Protein G Magnetic Beads (Bio-Rad) at 4°C overnight and then washed three times in TBS. Post-IP solutions were also saved for analysis. The immunoprecipitated proteins were then eluted by 8M guanidine hydrochloride (GnCL) (Thermo-Fisher).
Size-exclusion chromatography (SEC)
Brain TBS soaking extracts or CSF (350 μL total volume) were injected onto a Superdex 200 increase and run on a fast protein liquid chromatography (FPLC) system (AKTA; GE Healthcare) in TBS, pH 7.4. 500 μL fractions were collected for downstream experiments. Columns were calibrated with Gel Filtration Standards (Bio-Rad), which range from 1,350 to 670,000 Da.
Affinity purification using 71A1
Brain TBS soaking extracts (2 mL) were mixed with pre-conjugated High-Capacity Streptavidin Agarose resin (Pierce) with 50 ug biotinylated 71A1 for 2 hr at room temperature (RT) under nutation. Beads were extensively washed with 10 column volumes of SMCxPRO wash buffer and eluted with 0.2 M Glycine (pH 3) followed by neutralization with 1 M Tris-HCl (pH 8.5). Affinity purified material was desalted with PD MidiTrap G-25 into PBS, pH 7.4, before application on hippocampal slices.
MSD ELISA
Human brain extracts, CSF, immunoprecipitated samples and SEC fractions were eeeach diluted with 1% BSA in wash buffer (TBS supplemented with 0.05% Tween). For our home-made Meso Scale Discovery assay (MSD) electrochemiluminescence platform, each well of an uncoated 96-well multi-array plate (Meso Scale Discovery, #L15XA-3) was coated with 30 μL of a PBS solution containing capture antibody (3 μg/mL 266, a monoclonal antibody recognizes mid-region of Aβ [murine analog of Solanezumab], for all human Aβ ELISAs) and incubated at RT overnight followed by blocking with 5% BSA in wash buffer for 1h at RT with shaking at >300 rpm. A detection antibody solution was prepared with biotinylated detection antibody, 100 ng/mL Streptavidin Sulfo-TAG (Meso Scale Discovery, #R32AD-5), and 1% BSA diluted in wash buffer. Following the blocking step, 50 μL/well of the sample, followed by 25 μL/well of detection antibody solution were incubated for 2 h at room temperature with shaking at >300 rpm, washing wells with 150 μL wash buffer between incubations. The plate was read and analyzed according to the manufacturer’s protocol. The antibodies used to detect specific antigens were for hAβ (l-40 specific), 139–5, (rabbit recombinant, Biolegend); and for hAβ (1–42 specific), D3E10 (rabbit recombinant, Cell Signaling Technology).
SMCxPRO Immunoassay
The SMCxPRO (platform Sigma Millipore) is based on single-molecule-counting technology and typically allows a 20- to 100-fold increase in sensitivity compared with traditional detection systems. Biotinylated capture mAbs (1C22, 1G5 and 71A1) were conjugated to streptavidin magnetic particles (MPs) (Dynabeads MyOne, Thermo Fisher Scientific) at a ratio of 12.5 μg biotinylated antibody per milligram of MPs using a kit from Sigma Millipore. MPs with bound capture mAb were diluted to 50 μg/mL in the Aβ Oligomer Assay Buffer (Tris buffer; 50 mM Tris, 150 mM NaCl, pH 7.6), with 1% Triton X-100, 0.0005% (w/v) d-des-thio-biotin and 0.1% bovine serum albumin), and 50 μL of this suspension was added to 150 μL of sample, standard or blank and incubated at 600 rpm on a shaking incubator at 25°C for 2 h. MPs were isolated using a magnet and unbound material was removed by washing with 1x SMC wash buffer using a HydroFlex plate washer (Tecan Group AG, Männedorf, Switzerland). Fluorescently labeled (Alexa-647 dye) detection antibody 3D6 (20 μL, 200 ng/mL) was added to each well. MPs bearing the antibody–oligomer Aβ sandwich were then incubated with agitation using a Jitterbug shaker (Boekel, Feasterville, PA, USA) for 1 h at 25°C. Unbound detection reagent was removed by washing 4 times with the wash buffer. The wash buffer was removed by aspiration, and fluorescently labeled 3D6 detection antibody was released by shaking in an Elution Buffer B (11.5 μL/well) for 10 min at 25°C. 11 μL of the eluates were then transferred to the wells of a clean 96-well plate containing the Neutralization Buffer D (11 μL/well). The neutralized sample (20 μL/well) was then transferred to a black 384-well-read plate (Aurora) and read by the SMCxPRO instrument. When fluorescently labeled antibodies are excited by a 642 nm laser and pass through the interrogation space, they emit light that is measured using a confocal microscope lens and a photon detector. The output from the detector is a train of pulses, with each pulse representing one photon that was detected. The lower limit of reliable quantification (LLoQ) was defined as the lowest back interpolated standard that provides a signal two-fold the background with a percentage of recovery calculated between 80% and 100% and coefficient of variance (CV) ≤20%.
Immunohistochemistry
Fresh or thawed (from frozen stock) human brain blocks were embedded in Tissue-Tek® O.C.T. Compound and frozen at −80oC overnight. Before sectioning at 20–30 μm thickness using a cryostat (Leica), frozen blocks were changed to −20oC for 2 h to soften tissue for sectioning. Cryo-sections were then directly mounted on MAS-GP™ Adhesion Microscope Slides (Matsunami) and stored at 4oC before staining. For 3,30-diaminobenzidine (DAB) staining, cryo-sections were equilibrated in PBS containing 0.3% Triton-X100 (PBST) for 30 min followed by blocking endogenous peroxidase activity and antibody non-specific binding, ffor 1 h respectively. Primary antibodies were diluted in PBST and incubated with sections at 4°C overnight. After 3 washes with PBST, sections were then incubated with biotinylated secondary antibodies for 1 h. The immunoreactive products were visualized by incubating with 1) DAB containing nickel ammonium sulfate as an enhancing reagent or 2) Vina Green chrome (Biocare Medical). Stained sections were observed using an Axioskop 2 (Zeiss).
Quantification and statistical analysis
All statistical analysis was performed using GraphPad Prism 9 software. Statistical details of experiments are described in the text and/or figure legends.
Results
Immunogen design and monoclonal antibody generation of 71A1 and 1G5
Monoclonal antibodies 71A1 (a subclone of parent clone 7A1a) and 1G5 were raised against a synthetic conformational peptide immunogen designed to mimic the potential three-dimensional structure formed upon the dimerization of the monomeric amyloid β-protein (Aβ). The peptide immunogen was synthesized to contain amino acid residues 9–18 of Aβ1–40 and post-synthetically modified to cyclize and allow folding into a stable dimer-like formation (patent pending). The rationale behind the selection of residues 9–18 of the Aβ peptide was that the amino acids in this region would likely re-associate into a unique three-dimensional structure only present in aggregated forms of Aβ.
Balb/c mice were immunized with this cyclized conformational peptide using a maleimide-activated keyhole-limpet-hemocyanin (KLH) carrier complex for primary and secondary immunizations and a proprietary T helper 2 cell epitope for subsequent boosts prior to the splenectomy procedures. Fusions were performed using immunized mouse splenocytes and mouse myeloma F0 cells as the fusion partner. After primary screening with the immunogen and subcloning, two monoclonal antibody candidates in particular (71A1 and 1G5) showed high specificity to cyclized peptide and no specificity to linear synthetic peptide. Initial characterization of these antibodies had demonstrated high specificity for synthetic and endogenous oligomeric forms of Aβ and no binding to monomeric Aβ [44].
71A1 and 1G5 recognize Aβ in human brain biochemically and histologically
We tested these two novel antibodies, 1G5 and 71A1, on natural sources of human Aβ. First, we used a recently developed method [11] called “soaking extraction” to obtain highly diffusible Aβ species from minced brain bits of neuropathologically typical AD cortex incubated for just 30 minutes in Tris-buffered saline (TBS) without homogenization. These diffusible aqueous extracts have been shown to retain most of the synaptotoxic activity of AD cortical samples; subsequent homogenization of the brain bits post-soaking yields much more Aβ, but this has little synaptotoxic activity [11]. The soaking procedure is illustrated in Figure 1A. We used 1G5 and 71A1 to conduct immunoaffinity pulldown from AD brain soaking extracts, alongside positive control antibodies 4G8 (principally anti-monomer) and 1C22 (anti-oligomer) and as a negative control normal murine IgG. Compared to 4G8 and 1C22, both 1G5 and 71A1 pulled down far less Aβ from the AD brain soaking extracts but consistently more than murine IgG which pulled down no Aβ, as judged by immunoblots probed with 2 rabbit monoclonal recombinant antibodies (mAb) raised against different Aβ epitopes (Figure 1B). Because in denaturing SDS-PAGE, soluble oAβ are disassembled into monomers and some covalently bonded dimers [49], we observed immunoblot signals around and below ~6–7 kDa [Figure 1B, lower panel (longer exposure)]. To confirm this finding, we then used our highly specific home-brew Aβ x-40 and x-42 monomer immunoassays [50, 51] to quantify the Aβ contents pulled down from three individual AD brains by 1G5, 71A1, 1C22 (positive control) and murine IgG (negative control). As shown in Figure 1C [Aβ pulled down with protein G beads, then denatured by guanidine hydrochloride (GnCl)] and Figure 1D (Aβ remaining in the post-IP supernatant, then denatured by GnCl), both 1G5 and 71A1 pulled down small but consistent amounts of Aβ from AD brain soaking extracts, always more than the negative-control murine IgG but far less than did 1C22, a result consistent with the immunoblot data (Figure 1B). Also, to demonstrate the consistency of the pulldowns and immunodepletion by the 4 antibodies, we calculated the Aβ mass (in ng, measured against Aβ monomer 1–40 or 1–42 standard) obtained in the IPs and post-IPs by the 4 antibodies from 3 AD brains (Figure S1).
Figure 1. 1G5 and 71A1 pull down Aβ from human brain soaking extract.
(A) Schematic illustration of preparation of human brain soaking extracts. (B) immunoblots of immunoprecipitation with protein G by different antibodies from human brain soaking extract, detected by 2 antibodies against middle region and N-terminal of Aβ. 7.5 μL of the extract was used for the input lane and IP was from 800 μL of extract. (C-D) Aβ x-40 and x-42 measured by ELISA of immunoprecipitation or post-immunoprecipitation supernatant (all denatured in 8M GnCl) by the specified antibodies from human brain soaking extracts, n = 3, means ± SD.
After thus establishing that 1G5 and 71A1 can bind Aβ from highly soluble human brain extracts, we asked whether 1G5 and 71A1 can label Aβ plaques in the human brain using immunohistochemistry. When we first tested 1C22 for its use in immunohistochemistry, we found that it could not stain typical PFA-fixed AD brain sections but could readily stain unfixed cryo-sections (Figure 2A). Moreover, 4% PFA treatment of the latter sections for 20 minutes diminished most of the 1C22 immunoreactivity (Figure 2A). This phenomenon is common among antibodies that recognize a conformational epitope. For 1G5 and 71A1, we found that 1) both antibodies labeled extracellular amyloid plaques (i.e., deposited Aβ) in unfixed cryosections with similar patterns; and 2) 20-minute 4% PFA treatment substantially diminished the labeling (Figure 2A).
Figure 2. 1G5 and 71A1 recognize Aβ in human brain in situ.
(A) Immunohistochemistry using 1C22, 1G5, and 71A1 on human brain cryo-sections; scale bar = 200 μm. (B) Immunohistochemistry using 1C22 or 71A1 on human brain cryo-sections, with double labeling by Aβ monomer antibody D54D2; bar = 200 μm for left two panels and 100 μm for right panel. (C) LTP induction after treatment with aCSF (n = 6), human brain soaking extract (n = 5), 2.12 ug/mL 71A1 (n = 4), or human brain soaking extract premixed with 2.12 ug/mL 71A1 (n = 4), means ± SD. (D) LTP induction after treatment with aCSF (n = 6) or 71A1 affinity purified oAβ (n=4), mean ± SD.
To establish further that these antibodies labeled parenchymal Aβ, we utilized double immunostaining: sequentially labeling cryo-sections with D54D2 (to the Aβ N-terminal region, representing total Aβ visualized by DAB) and then either 1C22 or 71A1 (visualized by Vina Green). In Figure 2B, the left panels show that D54D2 alone labeled amyloid plaques; the middle panels show amyloid plaques double-labeled by D54D2 for total Aβ (DAB) and 1C22 or 71A1 (vina Green); and the right panel shows the double-labeled plaques at higher magnification. These findings confirm that 71A1 and 1G5 recognize Aβ brain deposits in situ. We also tested 1C22, 1G5 and 71A1 on a control brain devoid of plaques to show their specificity towards Aβ, and all 3 antibodies failed to detect any signals (data not shown).
As the immunoreactivity of 1G5 and 71A1 were similar to the previously demonstrated oligomer-preferring 1C22 [52, 53], we next asked if the two new antibodies could protect against Aβ-induced synaptic toxicity of soluble AD brain extracts. Using electrophysiology of wild-type (wt) mouse brain hippocampal slices [54], we found that 71A1 added to the slice perfusate at 2.12 μg/mL fully prevented the inhibition of hippocampal LTP by AD soaking extract while having no effect by itself on LTP (Figure 2C). Specifically, fEPSP slope in artificial cerebrospinal fluid (aCSF) vehicle alone was 152.6 ± 5.3% (N=6); in aCSF + AD extract: 115.7 ± 4.9% (N=5); in aCSF + 71A1 alone: 149 ± 8.3% (N=4); and in aCSF + AD extract pre-mixed with 71A1: 154.9 ± 9.8% (N=4). This effect was closely similar to the oAβ-neutralizing benefits of mAbs 3D6 and 82E1 we previously reported [13]. Furthermore, we used 71A1 to affinity purify oAβ from the same AD brain soaking extract and tested the synaptotoxicity of the purified oAβ on wt mouse brain hippocampal slices. The affinity-purified oAβ also significantly suppressed hippocampal LTP (Figure 2D). Statistical analyses of the differences 1) between AD brain extract alone and AD brain extract pre-mixed with 71A1 confirmed that 71A1 significantly rescued the LTP deficits caused by the extract (Figure S2A), and 2) between 71A1-purified oAβ and aCSF control showed that 71A1-purified oAβ significantly inhibited LTP, similar to the input soaking extract (Figure S2B). Collectively, the data indicate that the 71A1-reactive Aβ species, although a minor population of oAβ in AD brain as shown by immunoprecipitation with protein G (Figure 1B-D), confers synaptotoxicity that can be neutralized by 71A1.
71A1 and 1G5 bind Aβ species preferably in CSF over brain, the converse of 1C22
Next, we asked to what extent 1G5 and 71A1 could recognize Aβ from another natural source, human CSF. Using a similar experimental setup as that employed affinity pulldown from brain soaking extracts (Fig. 1C, D), we used 1G5, 71A1, 1C22 and murine normal IgG (control) to pull down from three individual CSF samples from AD patients collected at the Memory Disorders Clinic at Brigham and Women’s Hospital. 1G5 and 71A1 each immunoprecipitated surprisingly high amounts of Aβ, as measured by Aβ x-40 and x-42 monomer immunoassays after GnCL denaturation (Figure 3A, upper panel), with corresponding decreases in the post-IP supernatants (Figure 3A, lower panel). Moreover, in contrast to the soluble AD brain extracts studied above, both 1G5 and 71A1 pulled down higher relative amounts of Aβ x-42 from CSF than did 1C22, suggesting a possible difference in the native Aβ populations between human brain and CSF. In all three CSF samples, we also found that 71A1 showed a greater Aβ pull-down efficiency than 1G5 (Figure 3A). Therefore, we decided to use 71A1 to perform IP-ELISAs on 19 different CSFs from the same BWH clinic (see Table 1 for patient demographics and diagnoses).71A1 immunoprecipitated Aβ from all of the CSF samples (Figure 3B). Next, we asked if this IP-ELISA data correlated with the commercial ADmark Aβ 1–42 levels obtained in the same CSFs by our clinic. The Aβ x-42 signal (post-GnCl denaturation) from our 71A1 immunoprecipitations (IPs) correlated significantly with ADmark CSF Aβ 1–42 levels across our 19 CSFs (R2=0.43, p=0.0046) (Figure 3C), supporting the specificity and reliability of our 71A1 affinity purification. Here we also calculated the Aβ mass in ng from IPs and post-IPs by 4 antibodies from the 3 CSFs in Figure 3A, as shown in Figure S3. We found that IP + post IP are not perfectly matched for the 4 different antibodies (Figure S3C), unlike the brain soaking extracts (Figure S1), although they shared the same trend across the 3 individual CSF samples, a finding which precluded our using IP-ELISA further to quantify the oAβ accurately from biofluids samples.
Figure 3. 1G5 and 71A1 recognize Aβ from human CSF.
(A) Aβ x-40 and x-42 measured by ELISA of immunoprecipitates or post-immunoprecipitation supernatants (all denatured by 8M GnCl) by different antibodies from 3 human CSFs, with technical replicates = 3, means ± SD. (B) Aβ x-40 and x-42 measured by ELISA of immunoprecipitatees or post-immunoprecipitation supernatants (all denatured by 8M GnCl) by 71A1 from 19 individual human CSFs, technical replicates = 3, means ± SD. (C) correlation between Aβ x-40 and x-42 measured by ELISA of immunoprecipiates (all denatured by 8M GnCl) by 71A1 and the ADmark Aβ 1–42 measurements from the same CSFs. Pearson correlation is used.
The above result highlights the technical limitations of thee Aβ IP-ELISA approach for quantifying the relative levels of 71A1-reactive Aβ species in large numbers of brain, CSF and potentially plasma samples. Therefore, we proceeded to develop sandwich immunoassays to more accurately quantify apparent Aβ oligomers in biofluids. In this context, we had previously quantified 1C22-reactive Aβ oligomers in human CSF, but 1C22 pulled down small amounts of CSF Aβ oligomers (Fig. 3A), forcing us to use CSF without dilution (neat) to get sufficient levels from all samples, even with the high-sensitivity Erenna Immunoassay System (Millipore). Taking advantage of the unique property (above) of 71A1 having a higher binding capability towards Aβ oligomers in CSF than in brain extracts (in direct contrast to 1C22), we designed a sandwich immunoassay using 71A1 as capture to better quantify oAβ levels in biofluids such as CSF and ultimately plasma.
The 71A1/3D6 assay sensitively and specifically quantifies Aβ oligomers
We developed sensitive immunoassays to quantify 1G5- and 71A1-reactive Aβ species for detection in human CSF and plasma. Similar to our established 1C22/3D6 oAβ sandwich ELISA on the Erenna (Millipore) platform [30, 31], we used biotinylated 1G5 or 71A1 as the capture antibody conjugated to magnetic streptavidin-coated beads, and 3D6 (labeled with Alexa-647 dye) as the detector antibody on the bead-based immunoassay platform SMCxPRO (an upgraded version of Erenna from Millipore) (Figure 4A). As shown in Figures 4B-D, when we used a conventional calibrator of synthetic ADDLs [1], both 71A1/3D6 and 1G5/3D6 assays gave 1) a LLoQ of 0.6 pg/mL, the same as our previously developed 1C22/3D6 oligomer assay [30]; 2) recovery calculated between 80 & 120%; and 3) intra-assay CV well below 20% over the ADDL concentration range from 0.6 to 80 pg/mL, which readily covers the entire range of detectable analyte concentrations at appropriate dilution factors used in CSF and plasma (see below). As observed in our IP-ELISA assay in which 71A1 outperformed 1G5 in terms of pull-down efficacy and consistency (Figure 3A), we focused on validating the 71A1/3D6 assay for its specificity towards oligomeric Aβ found in human biofluids.
Figure 4. Development of 71A1/3D6 immunoassay specific for oAβ.
(A) Schematic illustration of the SMCxPRO beads-based immunoassay. (B-C) 1G5/3D6 and 71A1/3D6 assay performance on ADDLs as calibrator; (D) average CV and recovery of ADDLs signals using 1G5/3D6 and 71A1/3D6 assays. (E) 1C22/3D6 and 71A1/3D6 assay performance on ADDLs as calibrator. (F) CBB staining of PAGE analysis of Aβ1–40 S26 dimer without (−) or with (+) reduction by DTT. (G-H) 1C22/3D6 and 71A1/3D6 assay performance on Aβ1–40 S26 dimer without (native) or with reduction by DTT. (I) 71A1/3D6 assay performance on human brain soaking extract: left panel: raw value of 71A1/3D6 signals from serial diluted brain extract calibrated by ADDLs; middle panel: calculated value adjusted by dilution factors; right panel; recovery of each dilution normalized to 1:1K; n = 3, mean ± SD.
To assess whether 71A1 preferentially recognizes oAβ over monomeric Aβ, we utilized a synthetic S26C Aβ40 dimer [45] covalently linked via a disulfide bond, which can be reversed by a reducing agent such as DTT into its monomeric form. Before we compared the new 71A1/3D6 and earlier 1C22/3D6 assays on this Aβ40 dimer, we first demonstrated that the two assays have the same sensitivity towards ADDLs (Figure 4E). Both the 1C22/3D6 oAβ assay and the 71A1/3D6 assay showed a strong and significant binding preference for the non-reduced S26C Aβ40 dimer (confirmed by SDS-PAGE in Figure 4F) over its reduced monomeric counterpart (Figure 4G, H). This result suggests that both 1C22 and 71A1 antibodies preferentially bind to Aβ with higher order structures, while binding monomeric Aβ more than 100-fold less. Moreover, the 71A1/3D6 assay showed much greater sensitivity toward the S26C Aβ40 dimer than did the 1C22/3D6 assay: 8 pg/mL S26C Aβ40 dimer in the 71A1/3D6 assay had similar signal as 1.5 ng/mL S26C Aβ40 dimer in the 1C22/3D6 assay (Figure 4G vs. H; note difference in units on the abscissas), which may help explain the difference observed between 71A1 and 1C22 in terms of their respective binding preferences toward AD brain derived oAβ versus oAβ in CSF (compare Figures 1C and 3A).
Having assessed the sensitivity and specificity of the 71A1/3D6 assay on synthetic oligomeric vs. monomeric Aβ, we proceeded to test whether the 71A1/3D6 assay can readily and accurately measure oAβ from natural sources, such as human brain soaking extracts. We serially diluted a human brain soaking extract (tissue:soaking buffer = 1:5 w/v) to 1-, 2-, 4- and 20-thousand-fold and measured the diluted samples with the 71A1/3D6 assay (Figure 4I). We found that the signals from the diluted brain soaking extract samples were perfectly dilutable, with the average % recovery at 20,000-fold being 93.3% (Figure 4I, right panel). This dilution linearity test of the 71A1/3D6 assay demonstrated its capacity to measure oAβ from natural biological materials over a wide concentration range.
The 71A1/3D6 assay recognizes high molecular weight Aβ oligomers in brain
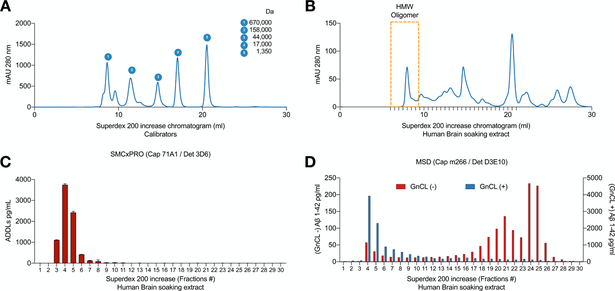
Next, we explored what kind of natural oAβ species are detected by the 71A1/3D6 immunoassay. Using non-denaturing size exclusion chromatography (SEC), we fractionated human AD brain soaking extracts on a Superdex 200 Increase high resolution column, as calibrated by molecular weight (MW) standards (Figure 5A). We performed the 71A1/3D6 assay on 30 SEC fractions starting from the void volume of the column (UV280 chromatogram of the fractions in Figure 5B). The 71A1/3D6 signal (measured in pg/mL units of the ADDL calibrator) peaked at fractions #3–6, which are within the void volume range, indicating that the estimated analyte size is at least 670 kDa (Figure 5C). To confirm that this 71A1/3D6 signal in fractions #3–6 indeed represents Aβ, we used 8M GnCl to denature the 30 fractions and then measured them with a well-established monomeric Aβ42 immunoassay (capture: m266, detector: D3E10, on the MSD platform) [11, 50]. We found that the GnCl-released monomeric Aβ species peaked at the same fractions #3–6 as the 71A1/3D6 assay (compare Figure 5C to Figure 5D [GnCl (+) = blue bars]). With the same Aβ monomer assay, we found that the natively monomeric species (Figure 5D: GnCl (−) = red bars) primarily eluted into fractions #19–26. Together, the data suggest that the abundant 71A1/3D6 signals observed in fractions #3–6 of AD brain soaking extracts represent higher order, high MW soluble Aβ oligomers which can be denatured by GnCl into Aβ monomers, and the assay detects monomers very poorly, similar to the pattern that the 1C22/3D6 oAβ assay detected previously [30, 53].
Figure 5. 71A1/3D6 immunoassay recognizes high molecular weight oAβ from human brain.
(A-B) Chromatogram (UV280nm absorbance) of molecular weight calibrators and human brain soluble extract fractioned by Superdex 200 increase SEC column. (C) oAβ measured by 71A1/3D6 immunoassay from SEC fractions natively; n = 3, mean ± SD. (D) monomeric Aβ x-42 measured by MSD ELISA from SEC fractions natively (red bars) or denatured (blue bars), n = 2, mean ± SD.
The 71A1/3D6 assay quantifies low molecular weight Aβ oligomers in human CSF
To validate the 71A1/3D6 assay on human CSF we performed dilution linearity testing, similar to the steps used on brain soaking extracts (Figure 6A). We observed an average recovery of 98.94% for 1:2 and 1:4 dilutions, indicating that the measurements were accurate and stable in that range, but the dilution recovery was suboptimal at 1:8 dilution (Fig. 6A) for unknown reasons. We proceeded to measure CSF from a cohort of 36 subjects with varied neurological diagnoses collected in our Memory Disorders Clinic (Brigham and Women’s Hospital) using the 71A1/3D6 assay (Table 1: patient demographic and diagnostic information). All 36 samples were quantified by the 71A1/3D6 assay, with the average level being 5.47 ng/mL and a range of 1.75 to 20.38 ng/mL (Figure 6B-D). We asked whether these CSF 71A1/3D6 levels correlated with other biomarkers of AD pathology in CSF, as measured by the widely used commercial ADmark clinical assay (Athena Diagnostics). As ADmark data was available for 29 out of 36 samples, the correlations were performed on these 29 samples. There was no statistically significant correlation between 71A1/3D6 oAβ signals and ADmark Aβ1–42 monomer levels in these CSFs (Figure 6B), but there were strongly significant correlations between 71A1/3D6 signals and both the ADmark total tau (R2 = 0.41, p=0.0002) and phosho-tau (pT181) (R2 = 0.42, p = 0.0001; Pearson correlation coefficients) (Figure 6C-D). Similar to our analysis of the brain soaking extract SEC fractions (Figure 5), we performed the 71A1/3D6 assay on 30 SEC fractions of human CSF starting from the void volume of the column (UV280 chromatogram shown in Figure 6E). The 71A1/3D6 signal (measured in pg/mL units of the ADDL calibrator) had two peaks: a smaller one at #3–6 and a larger one at #24–28 (Figure 6F), which was a different distribution than the brain soaking extract (Figure 5C). These data together demonstrate that the 71A1/3D6 assay can quantify the low molecular weight Aβ oligomers found in human CSF in addition to the high molecular weight oligomers found in the brain soaking extract.
Figure 6. 71A1/3D6 immunoreactive oAβ in CSF correlates with t-Tau and p-Tau levels.
(A) 71A1/3D6 assay performance on human CSF: left panel: raw values of 71A1/3D6 signals from serially diluted CSF (against ADDLs calibrator); middle panel: calculated value adjusted by dilution factors; right panel: recovery of each dilution normalized to 1:2; n = 3, means ± SD. (B-D) Correlations between 71A1/3D6 reactive oAβ levels in CSF and ADmark measurements of Aβ 1–42, t-Tau and p-Tau; n = 29. Pearson correlation is used. (E) Chromatogram (UV280nm absorbance) of human CSF fractioned on a Superdex 200 Increase SEC column. (F) oAβ measured by the 71A1/3D6 immunoassay in SEC fractions natively; n = 3, mean ± SD.
The 71A1/3D6 assay quantifies Aβ oligomers in human plasma
CSF is far less attractive for biomarker development because its collection is viewed as too invasive by many people and is relatively cumbersome and expensive. Moreover, serial lumbar puncture measurements are very rarely done. In contrast, blood collection is routinely performed, minimally invasive and inexpensive. We therefore sought to establish the accuracy of the 71A1/3D6 assay in plasma, a highly complex matrix posing technical challenges for accurate quantification by immunoassay. We tested for the degree of matrix interference in the plasma via 1) plasma dilution and recovery; 2) analyte spike-and-recovery; and 3) immunodepletion. Relative to a 4-fold dilution, the 71A1/3D6 assay showed near 100% dilution recovery (mean of 96.6%) at up to 16-fold dilution of each of six individual plasmas (from the Mayo Clinic Alzheimer’s Disease Research Center) (Figure 7A). We also observed a matrix effect in plasma on this assay only if using neat plasma (without dilution) (Figure S4A, B). Therefore, we chose to use the 1:8 dilution for the following experiments. We conducted analyte spike-and-recovery experiments on 8-fold diluted plasma samples using three different sources of natural oAβ from AD subjects, brain homogenate, brain soaking extract and CSF, which gave excellent mean recovery values of 97.5%, 92.9%, and 99.7%, respectively (Figure 7B). Then, we attempted immunodepletion experiments on 8-fold diluted individual plasmas using biotinylated antibodies and streptavidin-coated plates. 71A1 immunodepleted an average of 61.5% of the 71A1/3D6 signal whereas 1C22 immuno-depleted only 15.6% of the 71A1/3D6 signal (Figure 7C), demonstrating the specificity of the 71A1/3D6 assay for oAβ detection in plasma. Further, we examined the specificity of the 71A1/3D6 assay toward Aβ oligomers vs. monomers in brain homogenate, CSF and plasma. We measured these samples with or without 8M GnCl oligomer-denaturing treatment and found that GnCl decreased the 71A1/3D6 signal from brain homogenate by >70% and from plasma by >84%, while abolishing the signal from CSF (>99%) (Figure 7D). All the samples were extensively diluted before assaying to eliminate the negative effects of GnCl on the immunoassay (final concentration of GnCl <0.25 M).
Figure 7. 71A1/3D6 immunoassay accurately quantifies oAβ from human plasma.
(A) 71A1/3D6 assay performance on human plasma: left panel: raw value of 71A1/3D6 signals from serially diluted plasma against ADDLs calibrator; middle panel: calculated values adjusted by dilution factors; right panel: recovery of each dilution normalized to 1:4; n = 3, means ± SD. (B) spike-in recovery test by spiking human brain homogenate (Brain H), human brain soaking extract (Brain S) or human CSF into 8-fold diluted individual plasma; n = 3, means ± SD. (C) 71A1/3D6 assay of oAβ from 8-fold diluted individual plasmas immunodepleted by different antibodies, compared to the input plasma; n = 4. (D) 71A1/3D6 assay of oAβ from human brain soaking extract, CSF and plasma denatured by 8M GnCl (or not); n = 3, means ± SD. (E) 71A1/3D6 assay of oAβ from 73 human plasmas (Mayo Aging cohort), n = 4.
In summary, data from plasma dilution-recovery and spike-and-recovery experiments with three different natural sources of oAβ from AD subjects all demonstrated no significant matrix interference in the plasma 71A1/3D6 assay. Further, the immunodepletion experiments highlight the abilityof 71A1 to bind its target in individual human plasmas. We therefore performed the 71A1/3D6 immunoassay on 8-fold diluted plasma samples on a cohort of 73 cognitively normal individuals (Mayo Clinic Alzheimer’s Disease Research Center). The mean dilution-adjusted concentration based on the synthetic oAβ (ADDL) standard curve was 43.34 ± 29.09 pg/mL (Figure 7E, left). The CVs of these quadruplicate plasma samples were ≤20% except for five subjects (Figure 7E, right), demonstrating the consistency of the plasma assay.
Discussion
To better understand the potentially key pathogenic role of Aβ oligomers in the mechanism of AD, rigorous characterization and quantification of oAβ from human brains and biofluids are critical. Given the dynamic equilibrium in which Aβ exists between monomeric, oligomeric, and higher-order aggregated forms, determining the nature of certain oligomeric assemblies in the AD brain and human biofluids has been challenging, as the metastable oAβ assemblies could quickly become further aggregated and/or dissociate into other, more stable structural forms during experimental manipulations.
Since the detection of synthetic and natural Aβ oligomers modified the amyloid hypothesis some two decades ago [1, 2], there have been continuous efforts to generate better tools to visualize and distinguish oAβ from Aβ monomers and fibrils, the structure of which could overlap in part with smaller oligomeric species. Monoclonal antibodies have been developed in an attempt to recognize and even neutralize soluble oAβs, which are believed to be the more synaptotoxic forms of Aβ relative to insoluble Aβ plaques in the brain [3, 4, 14]. Among them, monoclonal antibody 1C22 [26] has been shown to be highly specific to oAβ in both the brain and CSF [30, 53]. We previously quantified 1C22-positive oligomers in CSF from 104 AD subjects participating in the ABBY and BLAZE phase 2 trials of the anti-Aβ antibody Crenezumab from Roche and found that oligomer Aβ levels measured by the 1C22/3D6 assay were significantly decreased in a high proportion of Crenezumab-treated patients, whereas no systematic change occurred in the placebo group [31]. These data encouraged us to engage further in the testing of novel antibodies to oAβ with potentially greater avidity and specificity. Through the design of a cyclized Aβ peptide immunogen to potentially mimic a dimeric conformation, positive clones 1G5 and 71A1 demonstrated high binding capability and specificity to the immunogen and to synthetic Aβ oligomers. In this study, we have validated these two novel antibodies with regard to their capacity to a) specifically recognize oAβ; b) quantify oAβ in human brain extracts, CSF and plasma; and c) neutralize the synaptotoxicity of diffusible Aβ oligomers derived from AD cerebral cortex.
We initially examined the ability of 1G5 and 71A1 to bind Aβ assemblies in AD brain soaking extracts, which have been validated to contain highly diffusible and synaptotoxic oAβ species [11]. We found that both 1G5 and 71A1 successfully pulled down Aβ as examined by IP-immunoblots probed with both mid-region and N-terminal (Asp-1) specific antibodies, and by IP-ELISA using ELISAs detecting Aβ x-40 and x-42. Interestingly, compared to our oligomer-specific benchmark antibody 1C22 [30], both 1G5 and 71A1 showed much lower binding capacity toward soluble Aβ species in the human brain soaking extracts. Similarly, when we compared these three antibodies by immunohistochemistry, 1G5 and 71A1 recognized plaques in unfixed cryostat sections, as confirmed through double labeling with a standard Aβ antibody D54D2. All three oligomer-preferring antibodies stained Aβ deposits well in native cryo-sections, but the staining intensities were substantially diminished by fixation of the sections with 4% PFA, suggesting that these antibodies recognize conformational epitopes sensitive to fixative crosslinking. 71A1 and 1G5 recognized a smaller portion of Aβ deposits in the brain than did 1C22. Nonetheless, 71A1 showed a consistent ability to protect against the inhibition of hippocampal LTP induced by AD brain soaking extracts, corroborating the previous finding that the small portion of brain Aβ (~12%) obtained by simple diffusion out of cortical pieces over just 30 minutes confers much of the recoverable synaptotoxicity [11]. Moreover, oAβ affinity-purified with 71A1 from AD soaking extracts confers potent synaptotoxicity, and in turn, 71A1 can neutralize the toxicity of AD brain-derived soluble oAβ, demonstrating the attractive potentially therapeutic properties of this new antibody.
In contrast to these data on soluble Aβ from brain (Figure 1C), our IP-ELISA showed that the rank order of Aβ binding capability in CSF was reversed: 71A1 >1G5 >>1C22 (Figure 3A). This difference between the brain soaking extracts and CSF is striking and can be interpreted to suggest a difference in soluble oAβ assemblies between brain and CSF. Furthermore, all three of these oAβ-preferring antibodies immunoprecipitated Aβ40 and Aβ42 equally, suggesting oAβ in CSF contains diverse Aβ monomer variants. To more accurately quantify low levels of oAβ from biofluids such as plasma beyond immunoprecipitation, we developed an ultra-sensitive sandwich immunoassay on the Erenna SMCxPRO platform (Millipore) using 71A1 as capture antibody and 3D6 (to the Aβ Asp-1 N-terminus) as detector antibody, achieving a LLoQ of 0.6 pg/mL and high reproducibility as demonstrated by low intra-assay %CVs (<10%) and excellent (~100%) recovery ratios (Fig. 4D). The specificity of the 71A1/3D6 immunoassay toward oAβ was demonstrated by 71A1’s strong preference for synthetic ADDLs [1] and S26C Aβ1–40 dimers [45] over their monomeric components (Fig. 4).
After thus validating the stability and relative oligomer specificity of the 71A1/3D6 immunoassay on oAβ prepared from synthetic Aβ peptides, we performed the assay on native SEC fractions of AD brain soaking extracts and observed that the assay recognized the high MW Aβ oligomers without recognizing Aβ monomers (Fig. 5C, D), similar to the oligomer-specific 1C22/3D6 assay previously reported [53]. After confirming the oligomer-specificity of the 71A1/3D6 assay, we proceeded to quantify oAβ levels in human CSFs and plasmas. Importantly, we found that the 71A1-immunoreactive oAβ levels in CSF (n = 29) weree significantly correlated with total tau and pT181 tau in the same samples, as measured by the CLIA-approved ADmark assay. However, we did not observe a correlation between 71A1/3D6 signals and Aβ 1–42 monomer levels.
We then proceeded to characterize the 71A1/3D6 immunoassay on human plasma, the major goal of this study. To demonstrate the validity of quantifying oAβ in plasma without the effects of plasma matrix interference, we investigated: 1) dilution recovery; 2) spike-and-recovery with 3 different natural sources of Aβ (human brain soluble homogenate, human brain soaking extract and CSF); 3) immunodepletion by 71A1, 1C22, and negative-control antibody 4–64 (raised against HIV glycoprotein 120); and 4) brain homogenate, CSF, and plasma treated with GnCl (a potent chaotropic salt that rapidly disassembles oAβ into monomers). The 71A1/3D6 immunoassay passed all these tests, showing 1) optimal recovery in both plasma dilution and spike-in experiments; 2) immunodepletable signals by 71A1 but not by 1C22 or control antibody; and 3) markedly reduced or abolished 71A1 signals after GnCl treatment of all 3 sample types. Finally, we conducted the 71A1/3D6 assay on a cohort of plasmas from 73 cognitively normal human subjects (demographic data in Table 1) and obtained an average (+/−SD) concentration of 71A1-positive oligomers in plasma of 43.34 ± 29.09 pg/mL.
Our study provides several salient findings. First, we report the design and detailed characterization of new antibodies which recognize conformational epitopes in natural human oligomeric Aβ. Second, we show that 1G5 and 71A1 have higher binding capability to oAβ in biofluids than a reference oligomer-preferring antibody, 1C22. Third, 71A1 neutralizes the synaptic toxicity of highly diffusible oligomers in AD brain soaking extracts. And fourth, the novel 71A1/3D6 sandwich ELISA sensitively and reliably quantifies oAβ in human plasma. To our knowledge, this is the first report of quantifying oAβ in human plasma using an oligomer-selective conformational antibody. This assay will now become a unique tool to probe the biology of endogenous oAβ, including its structural properties, its dynamics in human plasma and CSF, and its functional cytotoxicity. We found three reports of oAβ plasma immunoassays [55–57], all of which involve using identical capture and detector antibodies, thus requiring at least two identical exposed epitopes on the surface for quantification. One such assay using 82E1 as both capture and detector antibody is commercially available from Immuno-Biological Laboratories (IBL). Those assays would recognize a broad range of Aβs from dimer to protofibril and even fibril, as long as the N-terminal epitopes are available and exposed. Also, all the reported assays lack detailed characterizations, such as dilution linearity, spike-in recovery and immunodepletion to test the assay specificity. Our 71A1/3D6 assay exhibits improved specificity by using a novel oligomer-specific antibody that does not detect monomers as capture and improved sensitivity via the SMCxPRO system.
Now that it has been established and technically validated, the new assay can be used by us and others to systematically examine large, well-defined cross-sectional and longitudinal human cohorts and correlate plasma oAβ levels with other established and emerging AD biomarkers, such as Aβ monomer assays that detect all six C-terminal variants from Aβ37 to Aβ43 [32], the NT-1 tau assay to detect N-terminal tau fragments which correlate with multiple AD phenotypes [35, 36], and highly promising recent assays that detect tau phosphorylated at Thr181, Thr217 and Thr231 (see Introduction). Such detailed correlative analyses on multiple cohorts should reveal further insights into the involvement of oAβ dynamics in the early pathogenesis and course of AD, including to monitor the effects of emerging anti-amyloid treatments in CSF and plasma. The creation, analytical validation and initial application of this sensitive and specific assay for endogenous oAβ in human plasma provides a long-sought method to detect and follow the species that extensive evidence from many laboratories suggests is the key bioactive from of Aβ in AD and thus a major target for disease-modifying therapeutics.
Supplementary Material
Figure S3 Adjusted Aβ amounts of the IPs and the Post-IP supernatants of CSFs. (A) Aβ x-40 and x-42 amounts adjusted from Figure 3A (upper panel) by the volumes. (B) Aβ x-40 and x-42 amount adjusted from Figure 3A (lower panel) by the volumes of the Post-IP supernatants. (C) Aβ x-40 and x-42 amount of (A) and (B) combined.
Figure S1 Adjusted Aβ amounts of the IPs and the Post-IP supernatants of AD brain soaking extracts. (A) Aβ x-40 and x-42 amounts adjusted from Figure 1C by volume of the elutes. (B) Aβ x-40 and x-42 amounts adjusted from Figure 1D by volume of the Post-IP supernatant. (C) Aβ x-40 and x-42 amounts of (A) and (B) combined.
Figure S2 Statistical test of electrophysiological recordings on hippocampal slices. (A) Multiple t-test analysis of (Figure 2C) human brain soaking extracts treatment vs. the same extract premixed with 2.12 ug/mL of 71A1 treatment: q value distribution throughout the 60 min recording. (B) multiple t-test analysis of (Figure 2D) between aCSF treatment vs. 71A1-purified oAβ treatment; q value distribution throughout the 60 min recording.
Figure S4 Test of plasma matrix effects in the 71A1/3D6 assay. (A) Raw values of 71A1/3D6 signals from serially diluted plasma (against ADDLs calibrator); n = 3, mean ± S.; (B) Recovery of each dilution normalized to the average of all dilutions; n = 3, mean ± SD.
Acknowledgments
We thank Drs. Michael B. Miller and Mel B. Feany for providing human brain tissue samples. We thank Amirah K. Anderson for aliquoting the plasma and CSF samples. We are grateful to Dr. Ronald C. Petersen and colleagues, Mayo Clinic, Rochester, MN for generously providing plasma samples. We thank the NeuroTechnology Studio at Brigham and Women’s Hospital for providing instrument access to Zeiss Axioskop 2 microscope and consultation on data acquisition and data analysis. We are grateful to members of the Selkoe laboratory for helpful discussions.
Funding:
This work was funded by National Institutes of Health grants R01 AG006173 (DJS) and P01 AG015379 (DJS), and the Davis APP program at BWH (DJS). LL was supported by National Institutes of Health grant R03AG063046 and MADRC development project grant. AMS was supported by National Institutes of Health fellowship R25 NS 065743. The funders had no role in data collection, analysis, or decision to publish.
Abbreviations:
- AD
Alzheimer’s disease
- oAβ
oligomeric amyloid β-protein
- LLoQ
Lower Limit of Quantification
- MSD
Meso Scale Discovery Assay
- SMCxPRO
Single Molecule Counting platform
- CLIA
Clinical Laboratory Improvement Amendments.
- ADDLs
synthetic amyloid-β derived diffusible ligands.
Footnotes
Conflicts of interest: T.L.L is the technical founder of Abyssinia Biologics, LLC and is responsible for the original immunogen design and production of monoclonal antibodies 71A1 and 1G5. D.J.S. is a director and consultant of Prothena Biosciences. T.L.L and D.J.S. are planning a patent application on uses of anti-oligomeric amyloid β monoclonal antibodies for Alzheimer’s disease and related diseases. All other authors have nothing to disclose.
References
- [1].Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M et al. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A 1998; 95: 6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002; 416: 535–539. [DOI] [PubMed] [Google Scholar]
- [3].Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M et al. Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. Journal of Neuroscience 2007; 27: 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A 2009; 106: 4012–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sondag CM, Dhawan G, Combs CK. Beta amyloid oligomers and fibrils stimulate differential activation of primary microglia. Journal of neuroinflammation 2009; 6: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dhawan G, Floden AM, Combs CK. Amyloid-β oligomers stimulate microglia through a tyrosine kinase dependent mechanism. Neurobiology of aging 2012; 33: 2247–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Xu H, Rajsombath MM, Weikop P, Selkoe DJ. Enriched environment enhances β-adrenergic signaling to prevent microglia inflammation by amyloid-β. EMBO molecular medicine 2018; 10: e8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol 1999; 155: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 1999; 46: 860–866. [DOI] [PubMed] [Google Scholar]
- [10].Zott B, Simon MM, Hong W, Unger F, Chen-Engerer HJ, Frosch MP et al. A vicious cycle of β amyloid-dependent neuronal hyperactivation. Science 2019; 365: 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hong W, Wang Z, Liu W, O’Malley TT, Jin M, Willem M et al. Diffusible, highly bioactive oligomers represent a critical minority of soluble Aβ in Alzheimer’s disease brain. Acta Neuropathol 2018; 136: 19–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 2009; 62: 788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li S, Jin M, Liu L, Dang Y, Ostaszewski BL, Selkoe DJ. Decoding the synaptic dysfunction of bioactive human AD brain soluble Aβ to inspire novel therapeutic avenues for Alzheimer’s disease. Acta Neuropathol Commun 2018; 6: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 2008; 14: 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Esparza TJ, Zhao H, Cirrito JR, Cairns NJ, Bateman RJ, Holtzman DM et al. Amyloid-β oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol 2013; 73: 104–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wong CW, Quaranta V, Glenner GG. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A 1985; 82: 8729–8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Selkoe DJ, Abraham CR, Podlisny MB, Duffy LK. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer’s disease. J Neurochem 1986; 46: 1820–1834. [DOI] [PubMed] [Google Scholar]
- [18].Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 2000; 6: 916–919. [DOI] [PubMed] [Google Scholar]
- [19].Lambert MP, Viola KL, Chromy BA, Chang L, Morgan TE, Yu J et al. Vaccination with soluble Aβ oligomers generates toxicity-neutralizing antibodies. Journal of neurochemistry 2001; 79: 595–605. [DOI] [PubMed] [Google Scholar]
- [20].O’Nuallain B, Wetzel R. Conformational Abs recognizing a generic amyloid fibril epitope. Proc Natl Acad Sci U S A 2002; 99: 1485–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lambert MP, Velasco PT, Chang L, Viola KL, Fernandez S, Lacor PN et al. Monoclonal antibodies that target pathological assemblies of Abeta. J Neurochem 2007; 100: 23–35. [DOI] [PubMed] [Google Scholar]
- [22].O’Nuallain B, Klyubin I, Mc Donald JM, Foster JS, Welzel A, Barry A et al. A monoclonal antibody against synthetic Aβ dimer assemblies neutralizes brain-derived synaptic plasticity-disrupting Aβ. Journal of neurochemistry 2011; 119: 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Morgado I, Wieligmann K, Bereza M, Rönicke R, Meinhardt K, Annamalai K et al. Molecular basis of β-amyloid oligomer recognition with a conformational antibody fragment. Proceedings of the National Academy of Sciences 2012; 109: 12503–12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Savage MJ, Kalinina J, Wolfe A, Tugusheva K, Korn R, Cash-Mason T et al. A sensitive aβ oligomer assay discriminates Alzheimer’s and aged control cerebrospinal fluid. Journal of Neuroscience 2014; 34: 2884–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Phay M, Welzel AT, Williams AD, McWilliams-Koeppen HP, Blinder V, O’Malley TT et al. IgG Conformer’s Binding to Amyloidogenic Aggregates. PLoS One 2015; 10: e0137344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mably AJ, Liu W, Mc Donald JM, Dodart JC, Bard F, Lemere CA et al. Anti-Aβ antibodies incapable of reducing cerebral Aβ oligomers fail to attenuate spatial reference memory deficits in J20 mice. Neurobiol Dis 2015; 82: 372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Murakami K, Tokuda M, Suzuki T, Irie Y, Hanaki M, Izuo N et al. Monoclonal antibody with conformational specificity for a toxic conformer of amyloid β42 and its application toward the Alzheimer’s disease diagnosis. Sci Rep 2016; 6: 29038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ochiishi T, Itakura A, Liu L, Akatsu H, Kohno H, Nishimura M et al. Immunohistochemical detection of the delayed formation of nonfibrillar large amyloid-β aggregates. Genes Cells 2016; 21: 200–211. [DOI] [PubMed] [Google Scholar]
- [29].Gibbs E, Silverman JM, Zhao B, Peng X, Wang J, Wellington CL et al. A rationally designed humanized antibody selective for amyloid beta oligomers in Alzheimer’s disease. Scientific reports 2019; 9: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang T,T O’Malley T, Kanmert D, Jerecic J, Zieske LR, Zetterberg H et al. A highly sensitive novel immunoassay specifically detects low levels of soluble Aβ oligomers in human cerebrospinal fluid. Alzheimer’s research & therapy 2015; 7: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yang T, Dang Y, Ostaszewski B, Mengel D, Steffen V, Rabe C et al. Target engagement in an Alzheimer trial: Crenezumab lowers amyloid β oligomers in cerebrospinal fluid. Annals of neurology 2019; 86: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu L, Stern A, Dang Y, Ostaszewski B, Chhatwal JP, Selkoe DJ. Predicting development of AD clinical symptoms and their progression through a collection of novel plasma Aβ immunoassays: Biomarkers (non-neuroimaging): Blood based biomarkers. Alzheimer’s & Dementia 2020; 16: e043670. [Google Scholar]
- [33].Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018; 554: 249–254. [DOI] [PubMed] [Google Scholar]
- [34].Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019; 93: e1647–e1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen Z, Mengel D, Keshavan A, Rissman RA, Billinton A, Perkinton M et al. Learnings about the complexity of extracellular tau aid development of a blood-based screen for Alzheimer’s disease. Alzheimer’s & Dementia 2019; 15: 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chhatwal JP, Schultz AP, Dang Y, Ostaszewski B, Liu L, Yang H-S et al. Plasma N-terminal tau fragment levels predict future cognitive decline and neurodegeneration in healthy elderly individuals. Nature Communications 2020; 11: 6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement 2018; 14: 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. The Lancet Neurology 2020; 19: 422–433. [DOI] [PubMed] [Google Scholar]
- [39].Janelidze S, Stomrud E, Smith R, Palmqvist S, Mattsson N, Airey DC et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat Commun 2020; 11: 1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Barthélemy NR, Bateman RJ, Hirtz C, Marin P, Becher F, Sato C et al. Cerebrospinal fluid phospho-tau T217 outperforms T181 as a biomarker for the differential diagnosis of Alzheimer’s disease and PET amyloid-positive patient identification. Alzheimers Res Ther 2020; 12: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med 2020; 26: 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. Jama 2020; 324: 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med 2020; 26: 379–386. [DOI] [PubMed] [Google Scholar]
- [44].van Helmond Z, Heesom K, Love S. Characterisation of two antibodies to oligomeric Abeta and their use in ELISAs on human brain tissue homogenates. J Neurosci Methods 2009; 176: 206–212. [DOI] [PubMed] [Google Scholar]
- [45].O’Nuallain B, Freir DB, Nicoll AJ, Risse E, Ferguson N, Herron CE et al. Amyloid beta-protein dimers rapidly form stable synaptotoxic protofibrils. J Neurosci 2010; 30: 14411–14419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30: 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS et al. Prevalence of mild cognitive impairment is higher in men: The Mayo Clinic Study of Aging. Neurology 2010; 75: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].St Sauver JL, Grossardt BR, Yawn BP, Melton LJ III, Pankratz JJ, Brue SM et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. International journal of epidemiology 2012; 41: 1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Brinkmalm G, Hong W, Wang Z, Liu W, O’Malley TT, Sun X et al. Identification of neurotoxic cross-linked amyloid-β dimers in the Alzheimer’s brain. Brain 2019; 142: 1441–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu L, Ding L, Rovere M, Wolfe MS, Selkoe DJ. A cellular complex of BACE1 and γ-secretase sequentially generates Aβ from its full-length precursor. Journal of Cell Biology 2019; 218: 644–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu L, Lauro BM, Wolfe MS, Selkoe DJ. Hydrophilic loop 1 of Presenilin-1 and the APP GxxxG transmembrane motif regulate γ-secretase function in generating Alzheimer-causing Aβ peptides. Journal of Biological Chemistry 2021; 100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jin M, O’Nuallain B, Hong W, Boyd J, Lagomarsino VN, O’Malley TT et al. An in vitro paradigm to assess potential anti-Aβ antibodies for Alzheimer’s disease. Nature communications 2018; 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yang T, Li S, Xu H, Walsh DM, Selkoe DJ. Large soluble oligomers of amyloid β-protein from Alzheimer brain are far less neuroactive than the smaller oligomers to which they dissociate. Journal of Neuroscience 2017; 37: 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. Journal of Neuroscience 2011; 31: 6627–6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang MJ, Yi S, Han J-y, Park SY, Jang J-W, Chun IK et al. Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimer’s research & therapy 2017; 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Meng X, Li T, Wang X, Lv X, Sun Z, Zhang J et al. Association between increased levels of amyloid-β oligomers in plasma and episodic memory loss in Alzheimer’s disease. Alzheimer’s research & therapy 2019; 11: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Youn YC, Lee BS, Kim GJ, Ryu JS, Lim K, Lee R et al. Blood amyloid-β oligomerization as a biomarker of Alzheimer’s disease: A blinded validation study. Journal of Alzheimer’s Disease 2020; 1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S3 Adjusted Aβ amounts of the IPs and the Post-IP supernatants of CSFs. (A) Aβ x-40 and x-42 amounts adjusted from Figure 3A (upper panel) by the volumes. (B) Aβ x-40 and x-42 amount adjusted from Figure 3A (lower panel) by the volumes of the Post-IP supernatants. (C) Aβ x-40 and x-42 amount of (A) and (B) combined.
Figure S1 Adjusted Aβ amounts of the IPs and the Post-IP supernatants of AD brain soaking extracts. (A) Aβ x-40 and x-42 amounts adjusted from Figure 1C by volume of the elutes. (B) Aβ x-40 and x-42 amounts adjusted from Figure 1D by volume of the Post-IP supernatant. (C) Aβ x-40 and x-42 amounts of (A) and (B) combined.
Figure S2 Statistical test of electrophysiological recordings on hippocampal slices. (A) Multiple t-test analysis of (Figure 2C) human brain soaking extracts treatment vs. the same extract premixed with 2.12 ug/mL of 71A1 treatment: q value distribution throughout the 60 min recording. (B) multiple t-test analysis of (Figure 2D) between aCSF treatment vs. 71A1-purified oAβ treatment; q value distribution throughout the 60 min recording.
Figure S4 Test of plasma matrix effects in the 71A1/3D6 assay. (A) Raw values of 71A1/3D6 signals from serially diluted plasma (against ADDLs calibrator); n = 3, mean ± S.; (B) Recovery of each dilution normalized to the average of all dilutions; n = 3, mean ± SD.