Abstract
Objectives
We aimed to investigate and compare waning vaccine effectiveness (VE) against COVID-19 infection, COVID-19 related ICU admission, and COVID-19-related death for BNT162b2 and CoronaVac vaccines.
Methods
We consolidated national data on COVID-19 vaccination and outcomes, and used cases from September 1st–30th, 2021 to compare VE between the ‘early’ (fully vaccinated in April–June 2021) and ‘late’ (July–August 2021) groups. We estimated VE against COVID-19 infection with a negative binomial regression and VE against ICU admission and death among confirmed COVID-19 cases with a logistic regression.
Results
For BNT162b2, VE against COVID-19 infections declined from 90.8% (95% CI 89.4, 92.1) in the ‘late’ group to 79.3% (95% CI 76.1, 82.1) in the ‘early’ group. VE for BNT162b2 against ICU admission and death were stable. For CoronaVac, VE waned against COVID-19 infections from 74.5% (95% CI 70.6, 78.0) to 30.4% (95% CI 18.8, 40.3). Effectiveness against ICU admission waned from 56.0% (95% CI 51.2, 60.2) to 28.7% (95% CI 12.2, 42.1). CoronaVac's effectiveness against death remained stable.
Conclusion
VE against COVID-19 infection waned after 3–5 months of full vaccination for both BNT162b2 and CoronaVac vaccines in Malaysia. For CoronaVac, protection against ICU admission also declined.
Keywords: COVID-19 Vaccines, Waning, Effectiveness, SARS-CoV-2, Malaysia
INTRODUCTION
Since early 2021, clinical trials (Falsey et al., 2021; Tanriover et al., 2021; Thomas et al., 2021) and real-world studies on vaccine effectiveness (Jara et al., 2021; Lopez Bernal et al., 2021; Suah et al., 2021) have demonstrated efficacy and effectiveness measures well above the World Health Organization (WHO)’s benchmark of 50% (WHO 2020). This led to regulatory approval and widespread global use of an increasing number of vaccines. Vaccination also appeared to be substantially protective against severe disease from all main variants, including the Delta (B.1.617.2) variant (Krause et al., 2021; Lopez Bernal et al., 2021). However, although booster doses are being rolled out globally, uncertainty over the scale and pace in the waning of immunity underscores consideration over the timing for booster doses in the future.
Concerns over possible waning of the protective immunity conferred by the vaccines emerged when breakthrough infections and disease were increasingly documented among fully vaccinated persons. In the United States, although still largely protective against hospital admissions, the effectiveness of the BNT162b2 vaccine against SARS-CoV-2 infections declined from 88% during the first month after full vaccination to 47% after 5 months (Tartof et al., 2021). A similar pattern of waning in the effectiveness of the BNT162b2 vaccine against SARS-CoV-2 infections was observed in Qatar (Chemaitelly et al., 2021) and Israel (Goldberg et al., 2021). In the United Kingdom, vaccine effectiveness against symptomatic disease fell after 20 weeks against the Delta variant among both BNT162b2 and AZD1222 vaccine recipients (Andrews et al., 2021). Evidence on the protection of COVID-19 vaccines against severe outcomes over time is mixed (Andrews et al., 2021; Chemaitelly et al., 2021; Goldberg et al., 2021; Tartof et al., 2021). Although immunogenicity studies showed that adaptive immune responses, such as memory B cells, remain in the circulation over time after full vaccination, findings were limited to 6–8 weeks of follow-up (Chen et al., 2021). Moreover, evidence on the association between these adaptive responses and protection against severe disease and fatality in the real-world remains scarce, especially for the CoronaVac vaccine, despite its dominant use in many low to middle-income countries (LMICs) (VIPER Group COVID19 Vaccine Tracker Team, 2021)
Malaysia's nationwide mass COVID-19 immunization rollout, the National COVID-19 Immunisation Programme (Program Imunisasi COVID-19 Kebangsaan; PICK), began on February 24th, 2021. As of August 31st, 2021, 65.4% of the adult population has been fully vaccinated (COVID-19 Immunisation Task Force Malaysia, 2021). PICK used a diverse set-up of predominantly BNT162b2, CoronaVac, and AZD1222 vaccines, owing to global vaccine inequity and broadly lower supply to LMICs. This led to widespread use of CoronaVac, then approved for usage among individuals aged 18 years and older, including the elderly. CoronaVac accounted for 49.9% of all completed vaccinations as of August 31st, 2021. The usage breakdowns of the 3 vaccines by phases of PICK are provided in Table S1 of the Supplementary Appendix. The chronology of PICK's phases and the effectiveness of the vaccines were described previously (Suah et al., 2021). However, breakthrough COVID-19 cases started to increase since late July 2021, with a cumulative rate of 1.09% up to August 31st, 2021. Additionally, breakthrough ICU admission and death rates were both close to 0.01% (Figures S1, S2, and S3 of the Supplementary Appendix). Hence, there is an imminent need to investigate whether the protective immunity wanes over time, which underpins policy decisions on the need, and timing for booster doses of COVID-19 vaccination. Given that Malaysia and other LMICs are faced with limited supply and potentially increased hesitancy, research on waning effectiveness will directly impact the dynamic planning and redistribution of boosters.
Given the lack of immunogenicity studies in Malaysia, our study is instead grounded on real-world data consolidated from nationally representative data on COVID-19 vaccination and patient outcomes. We investigated the presence and scale of waning vaccine effectiveness against COVID-19 infection, COVID-19-related ICU admission, and COVID-19-related death for BNT162b2 and CoronaVac vaccines among adults in Malaysia.
METHODS
Data Environment
In Malaysia, the notification of COVID-19 cases and deaths are legally mandated under the provision of the Prevention and Control of Infectious Diseases Act 1988 (Act 342). At the time of writing, aggregated and granular data on COVID-19 cases, deaths, and vaccination are available and updated continuously on the Ministry of Health's GitHub repository (COVID-19 Immunisation Task Force Malasia, 2021; Ministry of Health Malaysia, 2021)
Data of all confirmed COVID-19 cases were extracted from the Malaysia national electronic COVID-19 cases register, which is the national COVID-19 surveillance system. Using case and personal identification numbers, all confirmed COVID-19 cases were linked deterministically with their vaccination date and status (using the COVID-19 vaccine recipients line listing) and clinical outcomes of interest: admission into ICU (using the ICU admissions register) and deaths (using the COVID-19 deaths line listing). Details on the definition of outcomes and data sources have been described previously (Suah et al., 2021). Additionally, 3 administrative data sets were used to measure exposure risk, testing behaviour, and occupational risk, respectively: register of all ‘contacts’ identified by Malaysia's check-in-based automated contact tracing system, register of all supervised and approved reverse transcription polymerase chain reaction (RT-PCR) and rapid antigen (RTK-Ag) tests taken at all testing facilities and register of all healthcare ‘frontliners’.
Study Design, and Comparator Groups
The study period spanned September 1st, 2021–September 30th, 2021, during which the Delta variant was predominant in Malaysia (Hodcroft 2021). All individuals with confirmed SARS-CoV-2 infections occurring outside of this period or have received vaccines other than homologous CoronaVac and BNT162b2 were excluded. Individuals with confirmed SARS-CoV-2 infections before September 1st, 2021 were excluded to avert potential biases in the effectiveness estimates due to infection-acquired immunity. Although AZD1222 was the third most predominant vaccine in PICK, the first individual was fully vaccinated with AZD1222 on July 21st, 2021, resulting in an insufficient follow-up period to evaluate the waning of vaccine effectiveness at the point of analysis. Full vaccination status is defined as ≥14 days after the receipt of the second dose of any of the 2 vaccines (BNT162b2 and CoronaVac) in this study.
Our study compared 3 groups: (i) those vaccinated in April–June 2021 (‘early’ group), corresponding to Phases 1 and 2 of PICK; (ii) those vaccinated in July–August 2021 (‘late’ group), corresponding to Phase 3 of PICK; and (iii) those unvaccinated in September 2021. This grouping enabled estimation of vaccine effectiveness at 1–2 months, and 3–5 months after vaccination. Due to insufficient granularity on individual-level data for those who tested negative, we could not infer waning effectiveness as in a traditional cohort or case-control study, which includes uninfected individuals. Hence, we investigated whether the period of vaccination affected the rate of confirmed SARS-CoV-2 infection using a retrospective population cohort approach. We subsequently investigated the rates of COVID-19-related ICU admission and death using a retrospective cohort of confirmed COVID-19 cases, which exploited granular data to adjust for confounders.
Retrospective Population Cohort using Census Data: Study Design, Methodology, and Statistical Analysis
We constructed a unified population cohort by merging data on vaccination, confirmed COVID-19 cases, and the Department of Statistics Malaysia's Current Population Estimates (Department of Statistics Malaysia, 2021) for year 2021 to compare the rates of infection for individuals vaccinated in different periods relative to those who are unvaccinated. This analysis included individuals ≥aged 15, which reflects the age groups for whom census-based population estimates were available; whereas estimates for the cutoff of ≥18 years were unavailable.
We calculated the number of events for outcomes in the study period, excluding those with confirmed SARS-CoV-2 infections before September 1st, 2021 and reinfections and number of individuals vaccinated, indexed to the timing of vaccination (for those who were vaccinated), age groups, sex, and states of vaccination. We calculated the size of the unvaccinated population over time from the number of vaccinated individuals and the census data. Vaccine effectiveness by vaccine types for both ‘early’ and ‘late’ groups are estimated with a negative binomial regression, adjusting for (i) age groups, (ii) state or region, and (iii) sex, with the cumulative person-days as the offset and the unvaccinated group as the baseline. Further details on the negative binomial regression model were described in the Supplementary Appendix.
Retrospective Cohort of Confirmed COVID-19 Cases: Study Design, Methodology, and Statistical Analysis
Waning vaccine effectiveness against severe outcomes (ICU admission and death) over time was investigated by comparing the odds between individuals with confirmed COVID-19 who were vaccinated in different periods relative to those who are unvaccinated.
The study population includes all confirmed COVID-19 cases aged ≥18, with confirmatory test dates of between September 1st, 2021 and September 30th, 2021, and excludes those with confirmed SARS-CoV-2 infections before September 1st, 2021 (reinfected in September 2021). To account for delays in the onset of severe outcomes after the confirmation of infection, the cohort records all deaths and ICU admissions occurring in September 2021 and up to November 2021. Vaccine effectiveness over time is estimated using a logistic regression, adjusting for (i) age as a continuous variable, (ii) presence of comorbidities, (iii) sex, (iv) nationality, (v) baseline (before 1 September 2021) number of tests taken, (vi) baseline number of times flagged as a ‘contact’, (vii) frontliner status (general population, public healthcare, or private healthcare), and (viii) state of residence. Further details on the logistic regression model are in the Supplementary Appendix.
Sensitivity Analysis
We conducted 3 sensitivity analyses. First, we redefined ICU admissions and deaths as 1 outcome (‘severe’). Second, we stratified by age groups to evaluate age-specific waning of effectiveness. Third, we inspected robustness to the inclusion and exclusion of all combinations of confounders in the logistic regression. All sensitivity analyses are reported in the Supplementary Appendix.
All analyses were conducted with Python, version 3.9 and R, version 4.1.2.
RECoVaM study and Ethical Consideration
This study, commissioned by the Ministry of Health, Malaysia, is part of The Real-World Evaluation of COVID-19 Vaccines under the Malaysia National COVID-19 Immunisation Programme (RECoVaM) study registered in the National Medical Research Register (NMRR-21-1660-60697). This study was conducted according to guidelines of the Declaration of Helsinki and was granted ethnical approval by the Medical Research and Ethics Committee (MREC), Ministry of Health, Malaysia.
RESULTS
Retrospective Population Cohort using Census Data
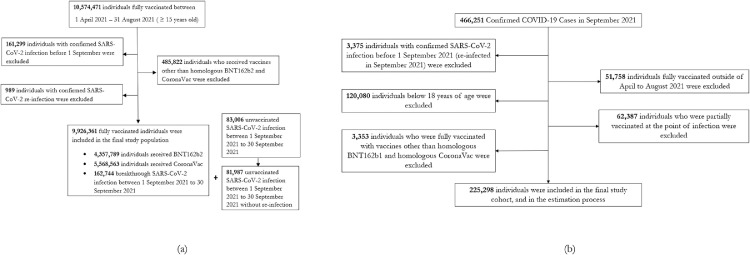
Figure 1(a) depicts the eligible cohort of fully vaccinated individuals. After excluding individuals with infections outside of the study period and recipients of other vaccines, 9,926,361 individuals were fully vaccinated with homologous BNT162b2 or CoronaVac vaccines. There were 244,731 confirmed COVID-19 cases throughout the outcome observation period in September 2021, of whom 162,744 were vaccinated, and 81,987 were unvaccinated.
Figure 1.
Study participants and cohort eligibility for the retrospective population cohort using census data, and the retrospective cohort of confirmed COVID-19 cases.
(a) Individuals who were fully vaccinated with homologous CoronaVac or BNT162b2 between April 1st, 2021 and August 31st, 2021, aged ≥15, had no confirmed SARS-CoV-2 infections prior to the outcomes observation period (September 1st, 2021 to September 30th, 2021). (b) Confirmed COVID-19 cases between September 1st, 2021 and September 30th, 2021, aged ≥18, with no prior confirmed SARS-CoV-2 infections, and fully vaccinated with homologous BNT162b2 and CoronaVac fully vaccinated (14 days after dose 2) between April 1st, 2021 and August 31st, 2021, or unvaccinated.
Table 1 presents the baseline characteristics by type of vaccine received and the timing of vaccination (‘early’ and ‘late’ groups) for the fully vaccinated individuals included in the cohort. Overall, there were more individuals who were fully vaccinated with BNT162b2 in the early group, whereas the reverse is observed in the late group. This corresponds to the composition of vaccine platforms used across the various phases of PICK, as described previously (Suah et al., 2021).
Table 1.
Baseline characteristics of the study population by duration since full vaccination
|
Vaccination period |
p-value (Chi-Squared Test of Independence) |
||
|---|---|---|---|
|
‘Late’ (Fully Vaccinated in July to August) |
‘Early’ (Fully Vaccinated in April to June) |
||
| Participants | 8,570,680 | 1,355,681 | |
| Vaccine Type | <0.001 | ||
| BNT162b2 | 3,250,182 (37.9%) | 1,107,616 (81.7%) | |
| CoronaVac | 5,320,498 (62.1%) | 248,065 (18.3%) | |
| Age Groups (in years) | <0.001 | ||
| 15 to 39 | 3,611,111 (42.1%) | 535,385 (39.5%) | |
| 40 to 59 | 3,077,536 (35.9%) | 360,542 (26.5%) | |
| ≥60 | 1,882,033 (22.0%) | 459,754 (33.9%) | |
| Sex | <0.001 | ||
| Male | 4,221,281 (49.3%) | 669,731 (49.4%) | |
| Female | 4,349,399 (50.7%) | 685,950 (50.5%) | |
Abbreviations: COVID-19, coronavirus disease; BNT162b2, Pfizer-BioNTech; CoronaVac, Sinovac
The ‘Late’ group are individuals vaccinated in July to August 2021 (1 to 2 months after vaccination during the outcomes observation period in September 2021), ‘Early’ group are individuals vaccinated in April to June 2021 (3 to 5 months after vaccination)
Table 2 presents the unadjusted incidence rates for COVID-19 infection and vaccine effectiveness for both early and late groups among recipients of both vaccines (BNT162b2 and CoronaVac). Suppose there is no waning of effectiveness, one would expect to observe the similar incidence rates in both groups. However, for fully vaccinated individuals with either vaccine (BNT162b2 and CoronaVac), the incidence rates in the early group are higher than in the late group, indicating possible waning protection offered by the vaccines.
Table 2.
Vaccine effectiveness against COVID-19 infection by vaccine type and duration since full vaccination
|
‘Late' (Fully Vaccinated in July to August) |
‘Early' (Fully Vaccinated in April to June) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Person-days at risk | Incidence rates/1,000 persons | VE | CI (95%) | Total | Person-days at risk | Incidence rates/1,000 persons | VE | CI (95%) | ||
| COVID-19 Infection | |||||||||||
| Overall | Unvaccinated | 81,987 | 46,811,305 | 1.751 | ref | ref | 81,987 | 46,811,305 | 1.751 | ref | ref |
| BNT162b2 | 30,070 | 136,056,979 | 0.221 | 90.8 | 89.4, 92.1 | 21,888 | 41,756,536 | 0.524 | 79.3 | 76.1, 82.1 | |
| CoronaVac | 104,747 | 141,155,066 | 0.742 | 74.5 | 70.6, 78.0 | 6,039 | 3,843,839 | 1.571 | 30.4 | 18.8, 40.3 | |
|
Age Group (in years) |
|||||||||||
| 15 to 39 | Unvaccinated | . . | . . | . . | ref | ref | . . | . . | . . | ref | ref |
| BNT162b2 | . . | . . | . . | 91.9 | 90.2, 93.3 | . . | . . | . . | 86.1 | 83.2, 88.5 | |
| CoronaVac | . . | . . | . . | 73.9 | 68.4, 78.5 | . . | . . | . . | 67.3 | 60.1, 73.3 | |
| 40 to 59 | Unvaccinated | . . | . . | . . | ref | ref | . . | . . | . . | ref | ref |
| BNT162b2 | . . | . . | . . | 88.9 | 86.5, 90.9 | . . | . . | . . | 77.3 | 72.3, 81.3 | |
| CoronaVac | . . | . . | . . | 70.5 | 64.1, 75.7 | . . | . . | . . | 32.4 | 16.7, 45.2 | |
| ≥60 | Unvaccinated | . . | . . | . . | ref | ref | . . | .. | .. | ref | ref |
| BNT162b2 | . . | . . | . . | 90.9 | 89.1, 92.4 | . . | . . | . . | 69.8 | 63.7, 74.9 | |
| CoronaVac | . . | . . | . . | 78.6 | 74.4, 82.2 | . . | . . | . . | -38.6 | -68.4, -14.1 | |
Abbreviations: CI, confidence intervals; COVID-19, coronavirus disease; VE, vaccine effectiveness; BNT162b2, Pfizer-BioNTech; CoronaVac, Sinovac.
The estimates were adjusted for age, gender and states of residence
The analysis compared between the ‘Late’ group, individuals vaccinated in July–August 2021 (1 to 2 months after vaccination during the outcomes observation period in September 2021), and the ‘Early’ group, individuals vaccinated in April–June 2021 (3–5 months after vaccination), and unvaccinated individuals by August 31st, 2021, who act as the baseline
For BNT162b2, vaccine effectiveness against COVID-19 infection declined from 90.8% (95% CI 89.4, 92.1) among individuals vaccinated in the early group to 79.3% (95% CI 76.1, 82.1) in the late group. The degree of waning was observed to be greater among individuals in older age groups (aged ≥60 years, and 40–59 years old) than their younger counterparts.
For CoronaVac, vaccine effectiveness against COVID-19 infection declined from 74.5% in the late group (95% CI 70.6, 78.0) to 30.4% (95% CI 18.8, 40.3) in the early group. Similar to the findings for BNT162b2, CoronaVac exhibits greater waning of effectiveness against COVID-19 infections among the older age groups.
Retrospective Cohort of Confirmed COVID-19 Cases
Vaccine effectiveness over time against ICU admission and death were measured using the adjusted odds ratio between different period of vaccination, relative to the unvaccinated, among confirmed COVID-19 cases in September 2021 without previously confirmed infections. Figure 1(b) shows the selection criteria for the study cohort, which includes 225,298 confirmed COVID-19 cases.
Table 3 presents the baseline characteristics for the 225,298 confirmed COVID-19 cases according to the status of vaccination, type of vaccination received (BNT162b2, CoronaVac) and the timing of vaccination. The crude numbers here, however, need to be enumerated into incidence rates and adjusted for potential confounders before vaccine effectiveness can be inferred.
Table 3.
Baseline characteristics of the retrospective cohort of confirmed COVID-19 cases
| Unvaccinated |
BNT162b2 |
CoronaVac |
p-value (Chi-Squared Test of Independence) |
|||
|---|---|---|---|---|---|---|
|
‘Late’ (Fully Vaccinated in July to August) |
‘Early’ (Fully Vaccinated in April to June) |
‘Late’ (Fully Vaccinated in July to August) |
‘Early’ (Fully Vaccinated in April to June) |
|||
| Participants | 62,506 | 30,088 | 21,906 | 104,746 | 6,052 | |
| Admitted to ICU | 1,238 (2.0%) | 101 (0.3%) | 91 (0.4%) | 830 (0.8%) | 110 (1.8%) | <0.001 |
| Death | 2,550 (4.1%) | 175 (0.6%) | 98 (0.4%) | 762 (0.7%) | 85 (1.4%) | <0.001 |
| Age Groups | <0.001 | |||||
| 18–39 | 39,846 (63.7%) | 12,598 (41.9%) | 11,784 (53.8%) | 52,174 (49.8%) | 2,197 (36.3%) | |
| 40–59 | 14,998 (24%) | 9,440 (31.4%) | 6,616 (30.2%) | 34,904 (33.3%) | 1,771 (29.3%) | |
| ≥60 | 7,662 (12.3%) | 8,050 (26.8%) | 3,506 (16%) | 17,668 (16.9%) | 2,084 (34.4%) | |
| Nationality | <0.001 | |||||
| Malaysian | 45,519 (72.8%) | 29,596 (98.4%) | 21,872 (99.8%) | 101,669 (97.1%) | 6,027 (99.6%) | |
| Non-Malaysian | 16,987 (27.2%) | 492 (1.6%) | 34 (0.2%) | 3077 (2.9%) | 25 (0.4%) | |
| Sex | <0.001 | |||||
| Male | 37,921 (60.7%) | 14,255 (47.4%) | 11,315 (51.7%) | 53,977 (51.5%) | 3,369 (55.7%) | |
| Female | 24,585 (39.3%) | 15,833 (52.6%) | 10,591 (48.3%) | 50,769 (48.5%) | 2,683 (44.3%) | |
| Presence of Comorbidities | <0.001 | |||||
| Yes | 19,315 (30.9%) | 6,618 (22.0%) | 4,618 (21.1%) | 11,310 (10.8%) | 1,223 (20.2%) | |
| No | 43,191 (69.1%) | 23,470 (78.0%) | 17,288 (78.9%) | 93,436 (89.2%) | 4,829 (79.8%) | |
| Frontliner Status | <0.001 | |||||
| General Population | 62,318 (99.7%) | 29,411 (97.7%) | 14,764 (67.4%) | 103,836 (99.1%) | 5,546 (91.6%) | |
| Public Healthcare | 83 (0.1%) | 234 (0.8%) | 5,169 (23.6%) | 40 (0.0%) | 68 (1.1%) | |
| Private Healthcare | 105 (0.2%) | 443 (1.5%) | 1,973 (9.0%) | 870 (0.8%) | 438 (7.2%) | |
| Baseline Number of Times Flagged as ‘Contact’ | <0.001 | |||||
| 0 | 59,152 (94.6%) | 25,775 (85.7%) | 18,038 (82.3%) | 87,255 (83.3%) | 5,286 (87.3%) | |
| 1 | 2,490 (4.0%) | 3,257 (10.8%) | 2,919 (13.3%) | 12,803 (12.2%) | 578 (9.6%) | |
| 2–4 | 781 (1.2%) | 1,001 (3.3%) | 903 (4.1%) | 4,359 (4.2%) | 173 (2.9%) | |
| 5–9 | 73 (0.1%) | 53 (0.2%) | 45 (0.2%) | 296 (0.3%) | 15 (0.2%) | |
| ≥10 | 10 (0.0%) | 2 (0.0%) | 1 (0.0%) | 33 (0.0%) | 0 (0.0%) | |
| Baseline Number of Supervised Tests Taken | <0.001 | |||||
| 0 | 48,385 (77.4%) | 21,581 (71.7%) | 13,549 (61.9%) | 68,853 (65.7%) | 4,181 (69.1%) | |
| 1 | 8,910 (14.3%) | 5,228 (17.4%) | 4,491 (20.5%) | 20,719 (19.8%) | 1,113 (18.4%) | |
| 2–4 | 4,781 (7.6%) | 2,987 (9.9%) | 3,615 (16.5%) | 13,706 (13.1%) | 710 (11.7%) | |
| 5–9 | 417 (0.7%) | 252 (0.8%) | 237 (1.1%) | 1,305 (1.2%) | 43 (0.7%) | |
| ≥10 | 13 (0.0%) | 40 (0.1%) | 14 (0.1%) | 163 (0.2%) | 5 (0.1%) | |
Abbreviations: COVID-19, coronavirus disease; ICU, intensive care unit; VE, vaccine effectiveness; BNT162b2, Pfizer-BioNTech; CoronaVac, Sinovac.
Breakdown by states of residence have been omitted for brevity.
Percentages in parentheses are by vaccination type and timing.
The ‘Late’ group are individuals vaccinated in July to August 2021 (1 to 2 months after vaccination during the outcomes observation period in September 2021), ‘Early’ group are individuals vaccinated in April to June 2021 (3 to 5 months after vaccination).
Table 4 shows the incidence rates for the outcomes of interest (ICU admission and deaths) and the corresponding vaccine effectiveness estimates by vaccination status, type, and timing of vaccination. The incidence rates and effectiveness estimates are further stratified by age groups (18–39 years, 40–59 years, and ≥60 years).
Table 4.
Vaccine effectiveness against COVID-19-related ICU admission and death by vaccine type and duration since full vaccination
|
‘Late’ (Fully Vaccinated in July to August) |
‘Early’ (Fully Vaccinated in April to June) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Event | per 1,000 | VE | CI (95%) | Total | Event | per 1,000 | VE | CI (95%) | ||||
| Age Group (in years) | ICU Admission | ||||||||||||
| Overall | Unvaccinated | 62,506 | 1,238 | 20 | ref | ref | 62,506 | 1,238 | 20 | ref | ref | ||
| BNT162b2 | 30,088 | 101 | 3 | 86.0 | (82.8, 88.6) | 21,906 | 91 | 4 | 77.5 | (71.7, 82.1) | |||
| CoronaVac | 104,746 | 830 | 8 | 56.0 | (51.2, 60.2) | 6,052 | 110 | 18 | 28.7 | (12.2, 42.1) | |||
| 18–39 | Unvaccinated | 39,846 | 267 | 7 | ref | ref | 39,846 | 267 | 7 | ref | ref | ||
| BNT162b2 | 12,598 | 6 | 0 | 94.9 | (88.3, 97.7) | 11,784 | 7 | 1 | 92.3 | (82.6, 96.6) | |||
| CoronaVac | 52,174 | 64 | 1 | 81.9 | (75.1, 86.8) | 2,197 | 11 | 5 | 43.5 | (-5.1, 69.6) | |||
| 40–59 | Unvaccinated | 14,998 | 536 | 36 | ref | ref | 14,998 | 536 | 36 | ref | ref | ||
| BNT162b2 | 9,440 | 37 | 4 | 90.2 | (86.2, 93.0) | 6,616 | 22 | 3 | 89.9 | (83.3, 93.9) | |||
| CoronaVac | 34,904 | 323 | 9 | 71.2 | (66.1, 75.5) | 1,771 | 34 | 19 | 38.3 | (10.9, 57.2) | |||
| ≥60 | Unvaccinated | 7,662 | 435 | 57 | ref | ref | 7,662 | 435 | 57 | ref | ref | ||
| BNT162b2 | 8,050 | 58 | 7 | 83.8 | (78.5, 87.8) | 3,506 | 62 | 18 | 57.1 | (43.2, 67.6) | |||
| CoronaVac | 17,668 | 443 | 25 | 46.1 | (37.1, 53.8) | 2,084 | 65 | 31 | 30.3 | (7.7, 47.4) | |||
| Age Group (in years) | Death | ||||||||||||
| Overall | Unvaccinated | 62,506 | 2,550 | 41 | ref | ref | 62,506 | 2,550 | 41 | ref | ref | ||
| BNT162b2 | 30,088 | 175 | 6 | 91.5 | (89.8, 92.9) | 21,906 | 98 | 4 | 91.2 | (88.6, 93.1) | |||
| CoronaVac | 104,746 | 762 | 7 | 79.2 | (76.8, 81.4) | 6,052 | 85 | 14 | 76.2 | (68.8, 81.9) | |||
| 18–39 | Unvaccinated | 39,846 | 243 | 6 | ref | ref | 39,846 | 243 | 6 | ref | ref | ||
| BNT162b2 | 12,598 | 4 | 0 | 94.3 | (84.3, 97.9) | 11,784 | 3 | 0 | 94.4 | (80.3, 98.4) | |||
| CoronaVac | 52,174 | 25 | 0 | 88.3 | (81.1, 92.7) | 2,197 | 4 | 2 | 59.5 | (-11.5, 85.3) | |||
| 40–59 | Unvaccinated | 14,998 | 740 | 49 | ref | ref | 14,998 | 740 | 49 | ref | ref | ||
| BNT162b2 | 9,440 | 28 | 3 | 94.5 | (91.7, 96.3) | 6,616 | 9 | 1 | 94.6 | (89.0, 97.4) | |||
| CoronaVac | 34,904 | 162 | 5 | 85.5 | (82.1, 88.3) | 1,771 | 8 | 5 | 82.6 | (63.6, 91.7) | |||
| ≥60 | Unvaccinated | 7,662 | 1,567 | 205 | ref | ref | 7,662 | 1,567 | 205 | ref | ref | ||
| BNT162b2 | 8,050 | 143 | 18 | 90.4 | (88.2, 92.3) | 3,506 | 86 | 25 | 89.4 | (86.0, 92.0) | |||
| CoronaVac | 17,668 | 575 | 33 | 76.3 | (72.7, 79.4) | 2,084 | 73 | 35 | 75.4 | (66.7, 81.9) | |||
Abbreviations: CI, confidence intervals; COVID-19, coronavirus disease; ICU, intensive care unit; VE, vaccine effectiveness; BNT162b2, Pfizer-BioNTech; CoronaVac, Sinovac.
The estimates were adjusted for age, gender, presence of comorbidities, nationality, and states of residence.
The analysis compares between the ‘Late’ group, individuals vaccinated in July–August 2021 (1–2 months after vaccination during the outcomes observation period in September 2021), and the ‘Early’ group, individuals vaccinated in April–June 2021 (3–5 months after vaccination), and unvaccinated individuals who act as the baseline
For BNT162b2, overall vaccine effectiveness against ICU admission and death were largely similar between the late and early groups. Overall vaccine effectiveness against ICU admissions dropped slightly from 86.0% (95% CI 82.8, 88.6) in the late group to 77.5% (95% CI 71.7, 82.1) in the early group. When stratified by age groups, the decline was stronger among cases aged ≥60 years, for whom effectiveness fell to 57.1% (95% CI 43.2, 67.6). For deaths, vaccine effectiveness among both early and late groups remained at around 90%, suggesting no waning of effectiveness. When stratified by age, effectiveness against death among cases aged ≥60 years was lower than the other age groups, but similarly did not wane.
For CoronaVac, vaccine effectiveness waned for ICU admissions but remained stable against deaths. Moreover, CoronaVac's effectiveness estimates were generally lower than BNT162b2. For ICU admissions, vaccine effectiveness declined from 56.0% (95% CI 51.2, 60.2) in the late group to 28.7% (95% CI 12.2, 42.1) in the early group. The waning of effectiveness against ICU admissions was observed in all age groups. In contrast, vaccine effectiveness against death remained at above 75%. The age-stratified analysis shows that CoronaVac's effectiveness against death remained stable for the older age groups (40–59 years old, ≥60 years); whereas for those aged 18–39 years old, the number of deaths were too small to estimate reliably.
Table S2 in the supplementary appendix shows that the broad findings are robust to redefining ICU admission and death as a single outcome. BNT162b2, consistent with the main analysis, showed no waning of effectiveness. CoronaVac showed waning effectiveness and lies between that of ICU admission and death. Figure S4 further shows the distribution of vaccine effectiveness estimated, by cycling through all combinations of covariates, against COVID-19-related ICU admission and death by timing of vaccination for both vaccine types. Although the unadjusted and partially adjusted effectiveness estimates vary in quantum from the fully adjusted estimates presented in Table 2, the broad findings are robust to the inclusion and exclusion of covariates.
DISCUSSION
Overall, our study findings suggest significant waning of vaccine effectiveness against COVID-19 infections for both BNT162b2 and CoronaVac. Moreover, for CoronaVac, we found evidence of waning vaccine effectiveness against COVID-19-related ICU admissions but not for COVID-19-related deaths. The decline in vaccine effectiveness against COVID-19 infections was in accordance with trends observed in previous studies (Andrews et al., 2021; Chemaitelly et al., 2021; Goldberg et al., 2021; Tartof et al., 2021), but the decline in effectiveness against ICU admission warrants critical deliberation and, to the best of our knowledge, is among the first evidence of its kind for CoronaVac.
The magnitude of waning vaccine effectiveness against infection was observed to be greater among older recipients, particularly those aged ≥60 years old, in this retrospective population cohort. For BNT162b2, although effectiveness declined, it remained above WHO's 50% primary efficacy endpoint (WHO, 2020). In contrast, our study observed significant declines in CoronaVac's vaccine effectiveness against COVID-19 infections, particularly in the older age groups (40–59 years old, ≥60 years), among whom the COVID-19 infection incidence rates after 3–5 months of being fully vaccinated were similar to that of the unvaccinated.
We then estimated the vaccine effectiveness for severe outcomes in a retrospective cohort of confirmed COVID-19 cases. Overall, we found that BNT162b2 vaccine remained highly protective against ICU admission and deaths over time. Studies elsewhere also demonstrated that BNT162b2 retained high effectiveness rates against severe outcomes for up to 6 months (Chemaitelly et al., 2021; Tartof et al., 2021). There is, however, a small decline in effectiveness over time against ICU admission among individuals ≥60 years in our study. This is in line with findings in the United States where effectiveness of BNT162b2 against hospitalisation declined slightly after 120 days for the elderly (Tenforde et al., 2021).
For CoronaVac vaccine recipients, our study observed significant declines in vaccine effectiveness against ICU admission. Older age groups also registered lower effectiveness. This could be partly due to greater use of CoronaVac than BNT162b2 vaccines in the older and comorbid population who are at the greatest risk of developing severe illness, and hence were prioritised in the early phases of PICK amid vaccine supply constraints (Sim et al., 2020), which our analysis attempted to control for. The lower effectiveness observed among older age groups is consistent with emerging evidence that older persons mount a lower immune response after a standard primary series of the CoronaVac vaccine than younger individuals and BNT162b2 recipients (Li et al., 2021; (WHO, 2021). This lower immune response was similarly observed among recipients who were immunocompromised compared to healthy adult recipients (Medeiros-Ribeiro et al., 2021). Findings from a study in Hong Kong SAR concluded that vaccination with BNT162b2 induced stronger humoral responses than CoronaVac (Mok et al., 2021). CoronaVac's neutralizing antibody levels have also been reported to fall by 7-fold within 6 months of vaccination (Mok et al., 2021; Pan et al., 2021). In addition, low effectiveness may also be attributed to the immunologic escape in the CoronaVac recipients against the Delta variant as the neutralisation property of this inactivated vaccine reduced by 31.6-fold compared to ancestral lineage, whereas the BNT162b2 was reduced by only 3- to 5-fold (Planas et al., 2021; Vacharathit et al., 2021).
Although CoronaVac's effectiveness against ICU admissions declined over time, our study showed that it retained substantial protection against death. Early reception of ICU care may have contributed towards better survival. In Malaysia, daily COVID-19-related ICU occupancy eased from an average of 1400 in July–August 2021 to an average of 1166 in September 2021 (Ministry of Health, Malaysia 2021)). This allowed more COVID-19 cases to receive ICU care when necessary. Nevertheless, the waning of effectiveness against ICU admissions after 3–5 months of full vaccination with the CoronaVac vaccine warrants concern and motivates consideration for the timing and need for booster doses among its recipients. The lower vaccine effectiveness and subsequent waning suggests that CoronaVac primary vaccination series may require 3 doses, as the protection from the current 2-dose regime appears inadequate, as per WHO recommendation (WHO, 2021). In Singapore, recipients of the CoronaVac vaccine are now required to complete a 3-dose primary vaccination series (Ministry of Health Singapore, 2021).
Our study has important policy implications. In Malaysia, at the point of writing, boosters are offered to all CoronaVac vaccine recipients who had been fully vaccinated for at least 3 months before, consistent with our findings that CoronaVac's vaccine effectiveness significantly waned after at least 3 months. On the other hand, recipients of the BNT162b2 vaccine were initially offered boosters after 6 months of being fully vaccinated, before being reduced to 3 months beginning December 28th, 2021. The rollout of booster doses prioritised the elderly (≥60 years) before younger age groups in age de-escalation manner. This follows our finding that waning of effectiveness was observed more strongly among older age groups. The narrative here highlights the rollout of booster doses in Malaysia as an example of evidence-based policymaking.
Our findings on waning effectiveness for CoronaVac may guide policies in LMICs that used CoronaVac in respective rollouts. The decline in effectiveness against ICU admissions, especially among individuals aged ≥60 years, signals caution towards its continued usage contingent on country-specific context. In Malaysia, because of vaccine inequity and supply challenges, CoronaVac was used in all individuals aged ≥18 years, including the elderly, in the early phases of PICK. When data emerged that the effectiveness of CoronaVac against ICU admissions waned, booster doses were offered promptly. Nevertheless, due to its continued protection against deaths, CoronaVac was retained in PICK. For policymakers in LMICs, future use of the CoronaVac vaccine should consider carefully its waning effectiveness over time, especially against ICU admission. Any targeted rollouts of CoronaVac should further consider its age-specific protection over time.
Our study has 4 strengths. First, we used a rich, consolidated database from multiple official and granular data sources, which are nationally representative of Malaysia. Second, by restricting the outcome observation period to September 2021 when lockdown measures were harmonised nationwide and when testing remained high, we were able to estimate vaccine effectiveness and compare the different timing of vaccination with minimal potential unobserved confounders. Third, the effectiveness estimates on COVID-19-related ICU admissions and deaths were able to account for prior testing behaviour, exposure risk, as well as occupational risk. Finally, our findings on CoronaVac's effectiveness over time, to our best knowledge, is among the first investigated and made available. This may have important implications on the future monitoring of this vaccine, as it is one of the most widely used vaccine worldwide (VIPER Group COVID-19 Vaccine Tracker Team)
However, our study is not without limitations. First, in our estimation of VE against SARS-CoV-2 infection, aggregated population census data were used; thus we were unable to adjust for covariates other than age groups, state (region), and sex. Second, in the absence of a national testing strategy during the study period (September 2021), the capturing of COVID-19 infections may not be standardised nationally, although testing remained high. Third, the lack of adequate genomic surveillance in Malaysia rendered impossible to ascertain variant-specific vaccine effectiveness and their variation, if any, over time. Our study period also preceded the detection of the Omicron variant of concern.
Moving forward, we recommend that the monitoring of vaccine effectiveness over time remains prioritised to guide policy decisions on the rollout of booster doses. To build on our study findings, monitoring of effectiveness for other vaccine platforms used in Malaysia and among adolescents, which were first offered the COVID-19 vaccines in September 2021, should be initiated. Genomic surveillance efforts should also be strengthened to enable investigation into variant-specific vaccine effectiveness. Finally, as booster doses are rolled out, the performance and safety of the booster doses should be assessed to furnish data needs for policy calibration. In several studies conducted elsewhere, early findings on booster doses have been encouraging (Barda et al., 2021; Bar-On et al., 2021).
Authors Contribution
JLS, MH, PSKT, BHT, TT, EVL, KMP, and SS designed the study. MRA, HY, SMZ, and FMZ collected the data. JLS, MH, and TT performed the data curation, analysis, and visualization. The data was interpreted by all authors. PSKT wrote the original draft of the manuscript. All authors reviewed and edited the manuscript. JLS, MH, and TT accessed and verified the data. The study was supervised by BHT, KMP, and SS. All authors had full access to all data in the studies and had final responsibility for the decision to submit for publication.
Conflict of interests
The authors have no conflict of interests to declare.
Data Availability
The data collected for the study, including deidentified participant data will be available upon request.
Funding
None.
Acknowledgements
We would like to thank the Director-General of Health Malaysia for his permission to publish this article, as well as Dr Chee Peng Hor from the Ministry of Health Malaysia for his valuable feedback and comments on the article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.03.028.
Appendix. Supplementary materials
REFERENCES
- Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. medRxiv [Preprint]. 2021. [posted 2021, Oct 6, cited 2021, Dec 12]. Available from: https://www.medrxiv.org/content/10.1101/2021.09.15.21263583v2.
- Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. http://doi.org/10.1016/S0140-6736(21)02249-2. Epub 2021 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. New Eng J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. http://doi.org/10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yin S, Tong X, Tao Y, Ni J, Pan J, et al. Dynamic SARS-CoV-2 specific B cell and T cell responses following immunization of an inactivated COVID-19 vaccine. Clin Microbiol Infect. 2021;26 doi: 10.1016/j.cmi.2021.10.006. S1198-743X(21)00605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. New Eng J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CoVariants: SARS-CoV-2 Mutations and Variants of Interest [Internet]. Hodcroft EB. 2021. [ cited 2021, Dec 12]. Available from: https://covariants.org/.
- Current Population Estimates, Malaysia, 2021 [Internet]. Department of Statistics Malaysia. 2021. [ cited 2021, Dec 12]. Available from: https://www.dosm.gov.my/v1/index.php.
- Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. New Eng J Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. http://doi.org/10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, et al. Waning immunity after the BNT162b2 vaccine in Israel. New Eng J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. http://doi.org/10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. New Eng J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. http://doi.org/10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PR, Fleming TR, Peto R, Longini IM, Figueroa JP, Sterne JA, et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet. 2021;398:1377–1380. doi: 10.1016/S0140-6736(21)02046-8. http://doi.org/10.1016/S0140-6736(21)02046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Yang J, Wang L, Wu Q, Wu Z, Zheng W, et al. A booster dose is immunogenic and will be needed for older adults who have completed two doses vaccination with CoronaVac: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. medRxiv [preprint]. 2021. [posted 2021, Aug 8, cited 2021, Dec 12]. Available from: https://www.medrxiv.org/content/10.1101/2021.08.03.21261544v1.
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B. 1.617. 2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros-Ribeiro AC, Aikawa NE, Saad CG, Yuki EF, Pedrosa T, Fusco SR, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27:1744–1751. doi: 10.1038/s41591-021-01469-5. http://doi.org/10.1038/s41591-021-01469-5. [DOI] [PubMed] [Google Scholar]
- Ministry of Health Singapore. Expert Committee on COVID-19 Vaccination: Updated Recommendation on Use of COVID-19 Vaccines [Internet]. 2021. [cited 2021, Dec 12]. Available from: https://www.moh.gov.sg/news-highlights/details/expert-committee-on-covid-19-vaccination-updated-recommendation-on-use-of-covid-19-vaccines/.
- Mok CKP, Cohen CA, Cheng S, et al. Comparison of the Immunogenicity of BNT162b2 and CoronaVac COVID-19 Vaccines in Hong Kong: An Observational Cohort Study. Respirology. 2021 doi: 10.1111/resp.14191. https://doi.org/10.1111/resp.14191. Epub 2021, Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Open data on Malaysia's National COVID-19 Immunisation Programme [Internet]. COVID-19 Immunisation Task Force Malaysia (CITF-Malaysia), Ministry of Health Malaysia. 2021. [ cited 2021, Dec 12]. Available from: https://github.com/CITF-Malaysia/citf-public/.
- Open Data on COVID-19 in Malaysia [Internet]. Ministry of Health Malaysia. 2021. [cited 2021, Dec 12]. Available from: https://github.com/MoH-Malaysia/covid19-public/.
- Pan H, Wu Q, Zeng G, et al. Immunogenicity and safety of a third dose, and immune persistence of CoronaVac vaccine in healthy adults aged 18-59 years: interim results from a double-blind, randomized, placebo-controlled phase 2 clinical trial. MedRxiv. [preprint]. 2021. [posted 2021, Jul 25, cited 2021, Dec 12].Available from: https://www.medrxiv.org/content/10.1101/2021.07.23.21261026v1.
- Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. http://doi.org/10.1038/s41586-021-03777-9. Epub 2021 Jul 8. [DOI] [PubMed] [Google Scholar]
- Sim BLH, Chidambaram SK, Wong XC, Pathmanathan MD, Peariasamy KM, Hor CP, et al. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: A nationwide observational study. Lancet Reg Health West Pac. 2020;4 doi: 10.1016/j.lanwpc.2020.100055. http://doi.org/10.1016/j.lanwpc.2020.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suah JL, Tok PSK, Ong SM, Husin M, Tng BH, Sivasampu S, et al. PICK-ing Malaysia's Epidemic Apart: Effectiveness of a Diverse COVID-19 Vaccine Portfolio. Vaccines. 2021;9:1381. doi: 10.3390/vaccines9121381. http://doi.org/10.3390/vaccines9121381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. http://doi.org/10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. http://doi.org/10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenforde MW, Self WH, Adams K, Gaglani M, Ginde AA, McNeal T, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326:2043–2054. doi: 10.1001/jama.2021.19499. http://doi.org/10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SJ, Moreira ED, Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. New Eng J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. http://doi.org/10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacharathit V, Aiewsakun P, Manopwisedjaroen S, Srisaowakarn C, Laopanupong T, Ludowyke N, et al. CoronaVac induces lower neutralising activity against variants of concern than natural infection. Lancet Infect Dis. 2021;21:1352–1354. doi: 10.1016/S1473-3099(21)00568-5. http://doi.org/10.1016/S1473-3099(21)00568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIPER Group COVID19 Vaccine Tracker Team [Internet] COVID-19 Vaccine Tracker. Sinovac: CoronaVac. 2021 https://covid19.trackvaccines.org/vaccines/7/ [cited 2021, Dec 12]. Available from: [Google Scholar]
- World Health Organization. Considerations for evaluation of Covid-19 vaccines. [Internet]. 2020. [ cited 2021, Dec 12]. Available from: https://www.who.int/medicines/regulation/prequalification/prequal-vaccines/WHO_Evaluation_Covid_Vaccine.pdf.
- World Health Organization. Interim recommendations for use of the inactivated COVID-19 vaccine, CoronaVac, developed by Sinovac [Internet]. 2021. [cited 2021, Dec 22]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-Sinovac-CoronaVac-2021.1/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data collected for the study, including deidentified participant data will be available upon request.