Abstract
Despite the development of several agents, new classes of antimicrobials with activity against the Mycobacterium avium complex (MAC) are needed. Based on a broad screening of compounds, we found that mefloquine has MICs of 8 to 16 μg/ml by the BACTEC system and 16 μg/ml by broth microdilution for five MAC strains tested. An expansion of the screening with broth microdilution to 24 macrolide-susceptible strains and 6 macrolide-resistant strains determined that the MIC for all strains was 16 μg/ml. To determine the intracellular activity of mefloquine, U937 macrophage monolayers infected with MAC strain 101, 100, or 109 (serovars 1, 8, and 4) were treated with mefloquine daily, and the number of intracellular bacteria was quantitated after 4 days. Significant growth inhibition against the three MAC strains at concentrations greater than or equal to 10 μg/ml (P < 0.05) was obtained. Due to the encouraging anti-MAC activity, in vivo efficacy in beige mice infected with MAC 101 was evaluated. Animals were treated with 5, 10, 20, or 40 mg/kg of body weight daily, three times a week, twice a week, or once a week for 4 weeks, and bacteria were quantitated in blood, liver, and spleen. No toxicity was observed with any of the treatment regimens. Mefloquine had borderline bactericidal activity at a dosage of 40 mg/kg daily (100% inhibition compared with a 1-week control), and significant inhibition was obtained at dosages of 40 mg/kg three times a week, as well as 20 mg/kg daily. Mefloquine had no significant effect on bacteremia. A combination of mefloquine and ethambutol showed significantly more activity than did either drug alone in liver, spleen, and blood; the combination was also bactericidal against M. avium. Although safety is a potential concern, mefloquine and related compounds deserve further investigation as anti-MAC therapies.
Organisms of the Mycobacterium avium complex (MAC) are a common cause of bacteremia and disseminated disease in patients in the advanced stages of AIDS (13, 14). Currently, only a few compounds such as macrolides, ethambutol, and rifabutin have activity against MAC organisms in vivo. The emergence of macrolide resistance and drug interactions between rifamycins and protease inhibitors emphasize the need for additional compounds with anti-MAC activity.
Mefloquine (a derivative of 4-quinolinemethanol) is an antimicrobial agent widely used for the prophylaxis of chloroquine-resistant Plasmodium falciparum malaria (19, 22). As part of a comprehensive screening program by the National Cooperative Drug Discovery Groups for Opportunistic Pathogens to identify new antimycobacterial agents, we screened numerous compounds and found mefloquine to be active in vitro against organisms of the MAC. Due to the pharmacological characteristics of mefloquine, which has been shown to reach concentrations in tissues 80 times greater than the concentration achieved in serum and has a long half-life (16), it was recognized that the compound had desirable properties for treating an intracellular pathogen. We describe the results of the evaluation of the activity of mefloquine against MAC strains in vitro, in the macrophage test system, and in vivo.
MATERIALS AND METHODS
Mycobacteria.
The MAC strains used in this study (100 to 105, 107 to 109, 110, 111, 113, 116, 117, 128, 500 to 508, 511 to 513, J5L, and JWL) were isolated from the blood of AIDS patients with disseminated MAC disease (each strain was isolated from a different patient). Each isolate was identified as M. avium by using a commercially available DNA probe (Gen-Probe, Inc., San Diego, Calif.). MAC 101 CLA-R is a clarithromycin-resistant strain isolated from mice (3). MAC strains 511 to 513, JJL, and JWL are clarithromycin-resistant strains isolated from patients. MAC strains 101 (serovar 1), 109 (serovar 4), and 100 (serovar 8) were used for all macrophage assays. MAC strain 101 was used in the studies with mice. MAC 101 is a virulent strain in the mouse test system and causes reproducible levels of infection and mortality in beige mice (1). MAC organisms were cultured in Middlebrook agar 7H10 medium (Difco Laboratories, Detroit, Mich.) supplemented with oleic acid, albumin, dextrose, and catalase (Difco) for 10 days at 37°C. Only transparent colony types were used in the studies. For the macrophage assays and mouse studies, colonies were harvested and suspended in Hanks’ buffered salt solution (HBSS) to concentrations of 4 × 108 or 3 × 108 CFU/ml, respectively, by comparison with a McFarland no. 1 turbidity standard; samples were plated onto 7H10 agar to confirm the concentrations of the inocula.
Prior to the infection of macrophages, the suspension was vortex agitated for 2 min and passed through a 23-gauge needle five times to disperse clumps. Microscopic observation confirmed the dispersion of the inoculum. Beige mice were infected with 100 μl of the original suspension (3 × 107 bacteria).
Drug.
Mefloquine used in the reported experiments was from two sources: (i) for use in vitro, mefloquine powder was kindly provided by Roche, Inc., and (ii) for use in vivo, the commercial preparation of mefloquine was utilized. For the in vitro assays, mefloquine was dissolved in dimethyl sulfoxide and subsequently diluted in phosphate buffer to the desired concentration. For the in vivo studies, the powdered drug was suspended in 0.2% Tween 80 plus 2.5% gum arabic (Sigma Chemical Co.) as previously described (3). Ethambutol was purchased from Sigma and diluted in water to prepare the desired concentration.
In vitro susceptibility testing.
MICs were determined by a radiometric broth macrodilution method, the T100 method of data analysis, and the broth microdilution method (15). The inoculum for susceptibility testing was prepared by placing 5 to 10 colonies from a 7H11 agar plate into 7H9 broth and was either tested directly or frozen at −70°C. The inoculum was adjusted to approximately 5 × 104 CFU/ml by comparison with a McFarland no. 1 turbidity standard. Isolates that clumped and could not be easily dispersed were shaken with glass beads. Controls included undiluted inoculum with no drug added (no-drug control), inoculum diluted to 1:100 (99% control), and inoculum diluted 1:1,000 (99.9% control). In addition, one vial was inoculated with a suspension of mycobacteria which were boiled for 5 min prior to inoculation in order to monitor the non-growth-related release of carbon dioxide in the BACTEC system. The period of observation and the end points were determined by daily monitoring of the control and text cultures, but a period of 7 days was sufficient for most isolates. MAC 101 was tested against amikacin as a control for overall performance.
Minimum bactericidal concentration was determined as described previously (2) by exposing bacteria to mefloquine in 7H9 broth at concentrations ranging from 1 to 256 μg/ml, and then, after 7 days, plating the bacterial suspension to establish the number of live bacteria.
Macrophage test system.
The source of macrophages was the human monocyte cell line U937 cultured in RPMI 1640 medium (pH 7.2) (Gibco, Chicago, Ill.) supplemented with 5% fetal bovine serum (Sigma Chemical Co.) and 2 mM l-glutamine. The assays were performed as previously described (2). Briefly, cells were grown to a density of 5 × 108 cells per ml and then centrifuged, washed, and resuspended in supplemented RPMI 1640 medium. The concentration of cells was adjusted to 106 cells per ml, and 1 ml of the cell suspension was added to each well of a 24-well tissue culture plate (Costar, Cambridge, Mass.). Monolayers were treated with 1 μg of phorbol myristate acetate per ml for 24 h to stimulate maturation of the monocytes. The monolayers were monitored for the number of cells, and no difference was observed in the extent of cell detachment among the treatment and control groups.
MAC strains were cultured for 10 days in Middlebrook agar 7H10 medium (Difco Laboratories). On the day of the experiment, bacteria were harvested, washed twice in HBSS, and suspended in HBSS, and a dispersed inoculum was prepared as described above. The turbidity of the suspension was adjusted to that of a McFarland no. 1 turbidity standard, and the suspension was diluted to a final concentration of approximately 5 × 107 CFU/ml. Each monolayer was infected with 100 μl of the final suspension, and the actual number of CFU per milliliter in the final suspension was determined by quantitative plate counts. Four hours after infection, the number CFU of mycobacteria per well of macrophage monolayer was determined by lysing the macrophages and performing quantitative plate counts to establish the initial inoculum (baseline), as previously reported (2). Infected monolayers were then treated with mefloquine at different concentrations. Drug and medium were replenished daily for 4 days. After the treatment period (4 days), the medium was removed and the monolayers were lysed as previously described (5). Briefly, ice-cold sterile water (0.5 ml) was added to each monolayer well, and the mixture was allowed to stand for 10 min at room temperature. Then, 0.5 ml of a second lysing solution (1.1 ml of Middlebrook 7H9 broth plus 0.4 ml of 0.25% sodium dodecyl sulfate in phosphate buffer) was added to each well, and the mixture was allowed to stand for an additional 10 min. The wells were vigorously scraped with a rubber policeman, and the macrophage lysates were resuspended in 0.5 ml of 20% bovine serum in sterile water to neutralize the sodium dodecyl sulfate. The suspension was vortex agitated for 2 min to ensure the complete lysis of the macrophages. Finally, the lysate was sonicated for 5 s to disrupt clumps of bacilli. To control for the osmotic stability of the mycobacteria, a suspension of mycobacteria alone was plated for quantitation before and after being subjected to the lysis procedure as described above, and in each instance there was no change in the number of CFU per milliliter before or after the lysis treatment procedure.
The final macrophage lysate suspension was serially diluted, and 0.1 ml was plated on 7H10 agar. The plates were allowed to dry at room temperature for 15 min and incubated at 37°C in 5% CO2 for 2 weeks. Duplicate plates were prepared for each well, and the results were reported as mean numbers of CFU per milliliter of macrophage lysate. Each assay was performed in triplicate, and each experiment was repeated six times.
Animal test system.
The potential therapeutic efficacy of mefloquine was determined by using the beige mouse test system as previously described (1–3). This system employs 8- to 10-week-old female C57BL/6 bg+ bg+ mice (Jackson Laboratories, Bar Harbor, Maine). Briefly, each mouse was infected through the caudal vein with 3 × 107 bacteria of MAC 101; after 7 days, treatment was initiated with mefloquine at 5, 10, 20, or 40 mg/kg of body weight/day once a week, twice a week, three times a week, or once daily. The drug was administered by daily gavage for 28 days. Animals were harvested 72 h after the end of therapy to prevent a carryover effect of the drug. A control group of mice was infected but received a drug vehicle in place of the antibiotic. An additional group of mice was examined 7 days after infection in order to establish the level of infection in the liver and spleen before the initiation of therapy. A total of 15 mice were used for each of the control and experimental groups. At the termination of therapy, the livers and spleens of the control and treated mice were aseptically removed, weighed, and then homogenized in 5 ml of 7H9 broth (Difco) with a tissue homogenizer. The tissue suspensions were serially diluted in 7H9 broth and plated onto 7H11 agar plates supplemented with oleic acid, albumin, dextrose, and catalase for the quantitation of viable bacteria. The number of mycobacteria in the blood was determined by collecting 0.05 ml of blood at day 7 and 28, inoculating a measured volume of blood into 4 ml of BACTEC 12 B medium (Johnston Laboratories, Sparks, Nev.) and by using the T100 method of data analysis as previously reported (2, 15).
Statistical analysis.
The differences between results in untreated control and experimental groups in macrophage experiments at identical time points were determined by a Student’s t test. The statistical significance of the differences between the number of organisms recovered from internal organs was evaluated by a one- or two-variable analysis of variance. Differences between results for experimental groups and between results for experimental groups and control groups were considered statistically significant if P values were <0.05.
RESULTS
MIC studies.
The MICs at which 50% and 90% of the 24 isolates were inhibited mefloquine were 16 μg/ml and 16 μg/ml, respectively, by both the radiometric system and broth microdilution. Bacteria were tested against mefloquine at concentrations ranging from 0.25 to 65 μg/ml at pH 6.8 (pH of the MAC environment within a macrophage vacuole [20]). We also tested the activity of mefloquine against six macrolide-resistant MAC strains (101-R, 511 to 513, JJL, and JWT). All the macrolide-resistant strains had their growth inhibited with 16 μg of mefloquine per ml. The minimum bactericidal concentration of mefloquine for five MAC strains was 64 μg/ml (strains 100, 101, 109, 108) and 16 μg/ml (strain 116).
Human macrophage studies.
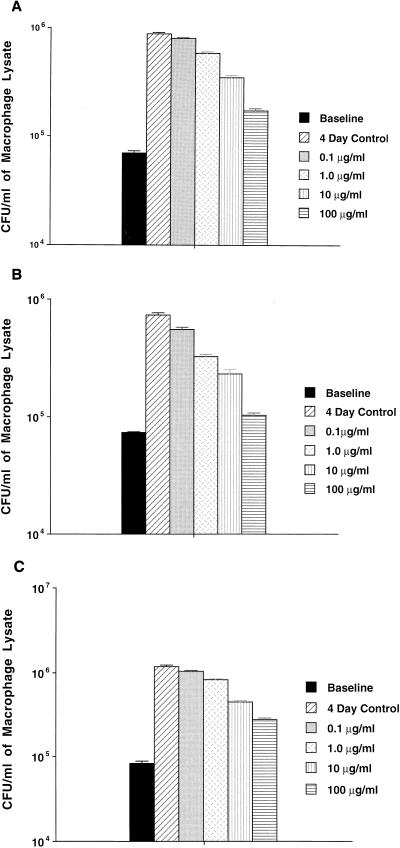
As shown in Fig. 1, mefloquine was active against MAC strains 100, 101, and 109 within macrophages at concentrations equal to or greater than 10 μg/ml. It was bacteriostatic after 4 days of treatment, even at concentrations of 100 μg/ml, probably an achievable tissue concentration in the host (in tissue, mefloquine can achieve a concentration 80 times greater than the concentration in serum [16]).
FIG. 1.
Activity of mefloquine against MAC strains 100 (A), 101 (B), and 109 (C). U937 macrophages were infected with MAC organisms, and then the monolayers were treated with different concentrations of mefloquine for 4 days. The cells were subsequently lysed, and the mycobacteria were plated for quantitation, as described in Materials and Methods.
Animal studies.
Mefloquine was administered at a dose of 5, 10, 20, or 40 mg/kg at a frequency of once a week, twice a week, three times a week, or daily to 5 mice per experimental group in three experiments (15 mice/group). Mefloquine was not toxic, and its administration was not associated with increased mortality. Tables 1, 2, and 3 show the effects of treatment with mefloquine on the number of bacteria in liver, spleen, and blood. Concentrations of 20 mg/kg administered daily and 40 mg/kg given either 3 days a week or daily were very effective in decreasing the bacterial burden in liver and spleen. Mefloquine had no significant antimycobacterial activity in the blood (Table 3).
TABLE 1.
Effect of treatment with mefloquine on the number of MAC organisms in liver
| Dose (mg/kg) or regimen | No. of MAC organisms (mean ± SE) with frequency of administrationa
|
|||
|---|---|---|---|---|
| QW | BIW | TIW | QD | |
| 5 | (7.0 ± 1.0) × 108 | (9.2 ± 1.2) × 108 | (1.5 ± 0.9) × 109 | (8.1 ± 1.7) × 108 |
| 10 | (6.4 ± 1.4) × 108 | (7.5 ± 1.0) × 108 | (6.0 ± 0.8) × 108 | (6.8 ± 1.4) × 108 |
| 20 | (6.5 ± 1.1) × 108 | (6.3 ± 1.2) × 108 | (6.5 ± 0.8) × 108 | (3.9 ± 1.2) × 108**,*** |
| 40 | (6.6 ± 1.1) × 108 | (5.3 ± 0.9) × 108 | (3.6 ± 0.5) × 108**,*** | (1.1 ± 0.3) × 108**,*** |
| Control at 4 wk | (1.1 ± 0.2) × 109 | |||
| Control at 1 wk | (1.1 ± 0.2) × 108 | |||
Mice infected with MAC strain 101 were treated for 4 weeks, and then the bacterial load was determined as described in Materials and Methods. Values are CFU per gram of tissue. QW, once a week; BIW, twice a week; TIW, three times a week; QD, daily. **, P < 0.05 compared with control at 4 weeks; ***, P > 0.05 compared with control at 1 week.
TABLE 2.
Effect of treatment with mefloquine on the number of MAC organisms in spleen
| Dose (mg/kg) or regimen | No. of MAC organisms (mean ± SE) with frequency of administrationa
|
|||
|---|---|---|---|---|
| QW | BIW | TIW | QD | |
| 5 | (2.3 ± 0.4) × 109 | (2.4 ± 0.8) × 109 | (1.6 ± 0.9) × 109 | (2.0 ± 0.5) × 109 |
| 10 | (2.1 ± 0.7) × 109 | (2.0 ± 0.3) × 109 | (2.0 ± 0.9) × 109 | (1.3 ± 0.3) × 109 |
| 20 | (1.5 ± 0.2) × 108 | (1.1 ± 0.3) × 109 | (1.6 ± 0.3) × 109 | (8.0 ± 1.6) × 108**,*** |
| 40 | (1.5 ± 0.3) × 109 | (1.5 ± 0.3) × 109 | (7.8 ± 1.6) × 108**,*** | (2.3 ± 0.6) × 108**,*** |
| Control at 4 wk | (2.3 ± 0.3) × 109 | |||
| Control at 1 wk | (2.0 ± 1.8) × 109 | |||
Mice infected with MAC strain 101 were treated for 4 weeks, and then the bacterial load was determined as described in Materials and Methods. Values are CFU per gram of tissue. QW, once a week; BIW, twice a week; TIW, three times a week; QD, daily. **, P < 0.05 compared with control at 4 weeks; ***, P < 0.05 compared with control at 1 week.
TABLE 3.
Effect of treatment with mefloquine on the bacteremia of MAC-infected mice
| Dose (mg/kg) or regimen | Change in CFU/ml (mean ± SE) with frequency of administrationa
|
|||
|---|---|---|---|---|
| QW | BIW | TIW | QD | |
| 5 | +0.64 ± 0.29** | +0.55 ± 0.14 | −0.26 ± 0.71 | +0.39 ± 0.21 |
| 10 | +0.92 ± 0.28 | +0.49 ± 0.23 | +0.42 ± 0.26 | +1.02 ± 0.16 |
| 20 | +0.56 ± 0.17 | +0.66 ± 0.2 | +0.45 ± 0.2 | +0.41 ± 0.2 |
| 40 | +1.0 ± 0.3 | +0.26 ± 0.4 | +0.30 ± 0.2 | +0.09 ± 0.3 |
| Control at 4 wk | +0.44 ± 0.13 | |||
Values are in Δlog CFU per milliliter between 0 and 4 weeks of treatment. QW, once a week; BIW, twice a week; TIW, three times a week; QD, daily. **, P > 0.05 for the results with all the doses compared with control.
As shown in Table 4, the combination of mefloquine at 40 mg/kg/day and ethambutol at 100 mg/kg/day showed significantly greater activity than either compound alone. No toxicity was observed during the period of therapy. The combination of the two drugs was bactericidal in the liver and spleen (Table 4) and was active in the blood at a higher level than ethambutol alone.
TABLE 4.
Activity of mefloquine in combination with ethambutol against MAC organisms in mice
| Therapeutic regimen | Dosea | No. of mice | CFU/g (mean ± SEM) in:
|
Mean (SEM) Δlog CFU/ml in blood | |
|---|---|---|---|---|---|
| Liver | Spleen | ||||
| Ethambutol | 100 | 19 | (3.7 ± 0.9) × 108b | (1.3 ± 0.3) × 109b | +0.23 (0.23) |
| Mefloquine | 40 | 21 | (9.4 ± 1.2) × 107b,d,e | (3.6 ± 0.6) × 108b,d | +0.08 (0.2) |
| Mefloquine + ethambutol | 40 + 100 | 21 | (2.1 ± 0.3) × 107b,c | (5.9 ± 1.2) × 107b,c | −0.65 (0.26) |
| Untreated control at 4 wk | 21 | (6.7 ± 1.1) × 108 | (2.8 ± 0.6) × 109 | +0.64 (0.14) | |
| Untreated control at 1 wk | 11 | (1.8 ± 0.2) × 108 | (2.5 ± 0.3) × 108 | ||
Mice were given, orally, either 100 mg of ethambutol or 40 mg of mefloquine per kg per day or both.
P < 0.05 compared with untreated control at 4 weeks.
P < 0.05 compared with either ethambutol or mefloquine alone.
P < 0.05 compared with ethambutol alone.
P < 0.05 compared with 1-week control.
DISCUSSION
Infection caused by organisms of the MAC has been a common cause of morbidity and mortality in patients with AIDS (13, 14). MAC organisms are intracellular bacteria that are characteristically resistant to the majority of the antituberculosis antimicrobials (12, 17).
Since the onset of the AIDS pandemic, a limited number of antimicrobials, such as the new macrolides and azalides, rifabutin, ethambutol, and amikacin, have proved clinically effective against MAC infections. Rifabutin and ethambutol, however, have only marginal activity against at least 50% of the clinical isolates; resistance to macrolides frequently occurs only a few months after the initiation of therapy (6, 21).
We demonstrated that mefloquine is active against MAC strains in vitro, in cultured macrophages against three MAC strains belonging to the most common serovars found infecting AIDS patients (9), and in vivo in the beige mouse test system. Although the MIC was 16 μg/ml, a significant inhibition of intracellular bacteria was achieved at extracellular concentrations greater than or equal to 10 μg/ml. A number of studies have shown that mefloquine can achieve 60 to 80 times greater intracellular concentrations than extracellular concentrations (16), which probably explains our observations in the macrophage test system. In vitro bactericidal activity is observed with mefloquine concentrations of 64 μg/ml. When the agent is administered to mice daily at a concentration of 40 mg/kg, borderline activity against MAC 101 was observed.
Mefloquine is a 4-quininemethanol that has been proven efficacious in treating both chloroquine-susceptible and multidrug-resistant strains of P. falciparum. Like other quinoline antimalarials, such as chloroquine, mefloquine is assumed to interfere with parasite feeding (10). The mechanism of action of mefloquine, however, is not completely understood but may differ from that of chloroquine. Chloroquine is a dibasic drug that has been shown to accumulate about 1,000-fold in the acidic vacuole of Plasmodium. High intravacuole concentrations of chloroquine are postulated to inhibit the polymerization of heme. As a result, heme released during hemoglobin breakdown builds up to toxic levels, thereby killing the parasite. However, the more lipophilic mefloquine is not concentrated extensively in the food vacuole of the parasite and likely has alternative sites of action (18).
The unexpected activity of mefloquine against MAC suggests the possibility of a novel target within mycobacteria. Among the MAC organisms we tested were strains resistant to the new macrolides, isoniazid, streptomycin, pyrazinamide, and rifampin, but these were all found to be susceptible to mefloquine.
Another advantage of mefloquine for the treatment of mycobacterial infections is the long half-life of the drug, making it possible to administer the drug once or twice a week. While our studies with mice showed that doses of 20 or 40 mg/kg were efficacious against MAC organisms only when administered daily or three times a week, the fact that mice have a very efficient (rapid) metabolic system suggests that, in humans, mefloquine could be given at a lower frequency.
With the exception of clarithromycin and azithromycin, mefloquine at 40 mg/kg/day is more effective than any other drug previously examined for the treatment of disseminated MAC infection in beige mice (4). We did, however, fail to observe mefloquine activity in blood, independent of dose or schedule of administration. The reason for this lack of activity in the blood is presently unknown, but it is possible that the rapid uptake of mefloquine by erythrocytes and in deep tissue leaves little compound to maintain the concentration needed for anti-MAC activity in the blood (11).
The combination of mefloquine with ethambutol was remarkably more active against MAC organisms than each drug alone, suggesting that synergism between the compounds exists. Ethambutol and mefloquine together were also active in the blood at a greater level than ethambutol alone. This finding suggests that mefloquine has some effect on the membranes of MAC organisms, as suggested by the anti-Plasmodium effect (8).
Dizziness and impaired hearing are well-described side effects of mefloquine; these side effects limit the drug’s utility for some patients (7). This clearly remains a concern when mefloquine is considered for the treatment of mycobacterial diseases. Nonetheless, our identification of mefloquine’s activity should spur efforts to identify the target of this agent in mycobacteria and to develop less toxic and more active analogues for clinical use.
ACKNOWLEDGMENTS
We thank Chris Lambros for providing us with mefloquine in the first place and for continuous support of this work. We also thank Karen Allen for preparing the manuscript.
This work was supported by NIH contract NO1-AI-25140.
REFERENCES
- 1.Bermudez L E, Inderlied C B, Kolonoski P, Petrofsky M, Young L S. Clarithromycin, dapsone, and a combination of both used to treat or prevent disseminated Mycobacterium avium infection in beige mice. Antimicrob Agents Chemother. 1994;38:2717–2722. doi: 10.1128/aac.38.12.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez L E, Inderlied C B, Kolonoski P, Wu M, Barbara-Burnham L, Young L S. Activity of Bay Y 3118, levofloxacin, and ofloxacin alone or in combination with ethambutol against Mycobacterium avium complex in vitro, in human macrophages, and in beige mice. Antimicrob Agents Chemother. 1996;40:546–551. doi: 10.1128/aac.40.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez L E, Petrofsky M, Kolonoski P, Young L S. Emergence of Mycobacterium avium populations resistant to macrolides during experimental chemotherapy. Antimicrob Agents Chemother. 1998;42:180–183. doi: 10.1128/aac.42.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez L E, Young L S. New drugs for the therapy of mycobacterial infections. Curr Opin Infect Dis. 1995;8:428–437. [Google Scholar]
- 5.Bermudez L E, Young L S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988;9:3006–3013. [PubMed] [Google Scholar]
- 6.Chaisson R E, Benson C A, Dube M P, Heifets L B, Korvick J A, Elkin S, Smith T, Craft J C, Sattler F R the AIDS Clinical Trials Group Protocol 157 Study Team. Clarithromycin therapy for bacteremic Mycobacterium avium complex. Ann Intern Med. 1994;121:905–911. doi: 10.7326/0003-4819-121-12-199412150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Croft A M, Clayton T C, World M J. Side effects of mefloquine prophylaxis for malaria: an independent randomized controlled trial. Trans R Soc Trop Med Hyg. 1997;91:199–203. doi: 10.1016/s0035-9203(97)90223-6. [DOI] [PubMed] [Google Scholar]
- 8.Desneves J, Thorn G, Berman A, Galatis D, LaGreca N, Sinding J, Foley M, Deady L W, Cownian A F, Tilley L. Photoaffinity labeling of mefloquine-binding proteins in human serum, uninfected erythrocytes and Plasmodium falciparum-infected erythrocytes. Mol Biochem Parasitol. 1996;82:182–194. doi: 10.1016/0166-6851(96)02732-6. [DOI] [PubMed] [Google Scholar]
- 9.Falkinham J O., III Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996;9:177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foley M, Tilley L. Quinolone antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol Ther. 1998;79:55–87. doi: 10.1016/s0163-7258(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 11.Gilman A G, et al., editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 8th ed. New York, N.Y: McGraw-Hill; 1993. [Google Scholar]
- 12.Havlir D V, Dube M P, Sattler F R, Fortal D N, Demper C A, Dunne M W, Parenti D M, Lavelle J P, White A C, Jr, Witt M D, Bozzette S A, McCutchan J A the California Collaborative Treatment Group. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. N Engl J Med. 1996;335:392–398. doi: 10.1056/NEJM199608083350604. [DOI] [PubMed] [Google Scholar]
- 13.Horsburgh C R., Jr Mycobacterium avium complex in the acquired immunodeficiency syndrome (AIDS) N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 14.Inderlied C B, Kemper C A, Bermudez L E M. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inderlied C B, Young L S, Yamada J K. Determination of in vitro susceptibility of Mycobacterium avium complex isolates to antimycobacterial agents by various methods. Antimicrob Agents Chemother. 1987;31:1697–1702. doi: 10.1128/aac.31.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karbwang J, Bunnag D, Breckenridge A M, Back D J. The pharmacokinetics of mefloquine when given alone or in combination with sulphadoxine and pyrimethamine in Thai male and female subjects. Eur J Clin Pharmacol. 1987;32:173–177. doi: 10.1007/BF00542191. [DOI] [PubMed] [Google Scholar]
- 17.Korvick J, Benson C. Advances in the treatment and prophylaxis of Mycobacterium avium complex in individuals infected with human immunodeficiency virus. In: Korvick J, Benson C, editors. Mycobacterium avium-complex infection: progress in research and treatment. New York, N.Y: Marcel Dekker, Inc.; 1996. pp. 241–262. [Google Scholar]
- 18.Schlesinger P H, Krogstad D J, Herwaldt B L. Antimicrobial agents: mechanisms of action. Antimicrob Agents Chemother. 1988;32:793–798. doi: 10.1128/aac.32.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt L H, Crosby R, Rasco J, Vaughan D. Antimalarial activities of various 4-quinolinemethanols with special attention to WR-142,490 (mefloquine) Antimicrob Agents Chemother. 1978;13:1011–1030. doi: 10.1128/aac.13.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 21.Sullam P M, Gordin F M, Wynne B A The Rifabutin Treatment Group. Efficacy of rifabutin in the treatment of disseminated infection due to Mycobacterium avium complex. Clin Infect Dis. 1994;19:84–86. doi: 10.1093/clinids/19.1.84. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Report of the Steering Committee of the Scientific Working Groups on Malaria. Geneva, Switzerland: World Health Organization; 1984. [Google Scholar]