Abstract
Objectives
To evaluate the cost-effectiveness of a booster strategy in the United States.
Methods
We developed a decision-analytic Markov model of COVID-19 to evaluate the cost-effectiveness of a booster strategy of the Pfizer-BioNTech BNT162b2 (administered 6 months after the second dose) among older adults from a healthcare system perspective.
Results
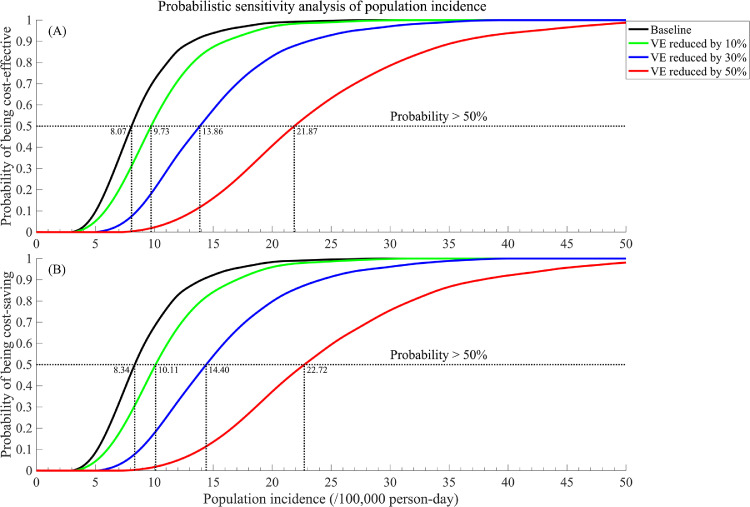
Compared with 2 doses of BNT162b2 without a booster, the booster strategy in a 100,000 cohort of older adults would incur an additional cost of $3.4 million in vaccination cost but save $6.7 million in direct medical cost and gain 3.7 quality-adjusted life-years in 180 days. This corresponds to a benefit-cost ratio of 1.95 and a net monetary benefit of $3.4 million. Probabilistic sensitivity analysis indicates that a booster strategy has a high chance (67%) of being cost-effective. Notably, the cost-effectiveness of the booster strategy is highly sensitive to the population incidence of COVID-19, with a cost-effectiveness threshold of 8.1/100,000 person-day. If vaccine efficacies reduce by 10%, 30%, and 50%, this threshold will increase to 9.7/100,000, 13.9/100,000, and 21.9/100,000 person-day, respectively.
Conclusion
Offering the BNT162b2 booster to older adults aged ≥65 years in the United States is likely to be cost-effective. Less efficacious vaccines and boosters may still be cost-effective in settings of high SARS-CoV-2 transmission.
Keywords: COVID-19, Booster, Cost-effective analysis, Markov model, BNT162b2
1. Introduction
As of December 15, 2021, the COVID-19 pandemic has claimed more than 5 million lives around the globe (JHU, 2021). The economic costs of the COVID-19 pandemic are enormous in many countries (Amewu et al., 2020; Andam et al., 2020, McKibbin and Fernando, 2020). In the United States, nearly 50 million people have been infected by SARS-CoV-2, resulting in more than 800,000 deaths. At the same time, over 70% of the US population aged ≥12 years has been fully vaccinated, and the percentage has reached 86% among older adults aged ≥65 years (CDC, 2021b). Although the rapid development and distribution of COVID-19 vaccines brought about hopes of curbing the pandemic (Burgos et al., 2021; Corey et al., 2020), recent reports of a growing number of breakthrough infections have raised serious concerns from the public (CDC 2021; Bergwerk et al., 2021; Zhang et al., 2020; Zhang et al., 2020). Both the rampant transmission of the SARS-CoV-2 Delta variant and the waning protection of the existing vaccines likely contribute to the increasing number of breakthrough infections. New vaccination strategies such as providing vaccine boosters to those who have been fully vaccinated are currently being discussed (Burki, 2021; Shen et al., 2021).
The US Food and Drug Administration (FDA) approved the use of a booster shot for those who received the 2-dose Pfizer-BioNTech COVID-19 vaccine on September 22, 2021. Although the FDA approved the use of booster shots, the debate continues as whether booster shots should be offered and, if so, to which populations (Burki, 2021). On the one hand, real-world data from countries such as Israel showed that booster shots significantly increased the vaccine's effectiveness, which provides additional protection against COVID-19, especially for older adults and individuals with compromised immunity (Bar-On et al., 2021). In addition, recent serological studies have demonstrated a substantial waning of vaccine-elicited immunity against SARS-CoV-2 infection 6-11 months after administering its second dose (Chemaitelly et al., 2021; Goldberg et al., 2021; Thomas et al., 2021). On the other hand, the wide dissemination of COVID-19 booster shots may further increase the already skyrocketing healthcare costs and exacerbate the health equity issue exposed by the pandemic (Shadmi et al., 2020; Wang and Tang, 2020). Thus, there is an urgent need to analyze the potential impact of widely administering booster shots in the United States.
In this study, we aimed to assess the cost-effectiveness of the COVID-19 booster strategy in the United States. On the basis of a well-designed decision-analytic Markov model, our findings will aid public health practitioners and policymakers to determine whether universally administering booster shots among those aged ≥65 years who have been fully vaccinated would be a cost-effective strategy. Our study also explored the key factors that may affect its cost-effectiveness.
2. Methods
2.1. Study design
We conducted an economic evaluation on the cost-effectiveness of booster vaccination of Pfizer-BioNTech (6 months after second dose) in those aged ≥65 years on the basis of a decision-analytic Markov model. The evaluation was conducted from a healthcare system perspective. The model was constructed using TreeAge Pro 2021 R1.1, and the analysis was conducted according to the Consolidated Health Economic Evaluation Reporting Standards statement (Husereau et al., 2013).
2.2. Modelling
A decision-analytic Markov model was constructed to simulate the disease progression of SARS-CoV-2 infection in a designated initial cohort of 100,000 individuals aged ≥65 years over a period of 180 days. As the Delta variant of SARS-CoV-2 has become dominant in the United States, our study mainly focused on modelling the transmission of the Delta variant. Existing evidence indicated that the vaccine efficacy (VE) of Pfizer-BioNTech/BNT162b2 would gradually wane after 6 months (Chemaitelly et al., 2021; Goldberg et al., 2021; Thomas et al., 2021). Thus, we defined the vaccine efficacy from 2 weeks to 6 months after the second dose of vaccines as a “short-term VE”, whereas the vaccine efficacy 6 months beyond the second dose was defined as a “long-term VE”.
The model consisted of 9 health states depicting varied disease progression of COVID-19 (Figure S1). A fully vaccinated individual may be infected by SARS-CoV-2 and enter a “latent infection state”. After a mean incubation period of 5.2 (4.1–7.0) days (Li et al., 2020), about 83% of infected individuals developed symptoms (Reddy et al., 2021), and the remaining asymptomatic infections would spontaneously recover. A symptomatic infection might first exhibit “mild/moderate” symptoms. It might “recover” or deteriorate to a “severe” state. A patient in the “severe” state might “recover” or progress to the “critical” state. Similarly, a patient in the “critical” state might “recover” or “die.” Transition probabilities between states were estimated using the formula , where denoted the daily transition rate (Reddy et al., 2021). Model cycle length was 1 day, with a half-cycle correction applied. We defined the approach of 2-dose BNT162b2 without booster shot as the “baseline” and evaluated the health and cost benefits of implementing the booster strategy in older adults aged ≥65 years in the United States.
Table 1.
The results of cost-effectiveness analysis of BNT162b2 booster vaccination for COVID-19 in older adults aged ≥65 years in United States. (Reference scenario: older adults fully vaccinated with BNT162b2 but not booster; a cohort of 100,000 individuals over an evaluation period of 180 days).
| Full vaccination with BNT162b2 | Full vaccination with BNT162b2 + booster | Incremental benefits* | |
| QALY | 48,908.4 | 48,912.1 | 3.7 |
| Uninfected individuals | 48,526.1 | 48,796.9 | – |
| Infected individuals | 383.3 | 115.2 | – |
| Costs, $ | 8,901,608 | 5,637,280 | -$3,264,328 |
| Vaccination cost | 0 | 3,427,607 | $3,427,607 |
| Direct medical cost | 8,901,608 | 2,209,673 | -$6,691,935 |
| Death cases | 4.66 | 0.87 | 3.79 |
| ICER | – | – | Cost saving |
| Benefit-cost ratio | – | – | 1.95 |
| Cost/death prevented, $ | – | – | $904,382 |
| Net monetary benefit, $ | – | – | $3,449,328 |
| * Incremental benefits = difference between the booster and the reference scenarios.Benefit-cost ratio: each dollar invested in vaccination will save 1.95 dollars of direct medical cost.Cost/death prevented: every 904,382 dollar invested in vaccination will prevent one death.Net monetary benefit (NMB) is calculated as (incremental benefit x threshold) – incremental cost.QALY = quality-adjusted life year. | |||
2.3. Data collection
We collected information on the vaccine efficacy of BNT162b2 for SARS-CoV-2 (Delta variant) infection in older adults aged ≥65 years on the basis of an ongoing systematic review conducted by the International Vaccine Access Center (IVAC, 2021). We included 10 eligible studies to estimate the pooled short-term and long-term VE of the 2-dose vaccination and also the VE of the booster shot (Appendix 1.2). On the basis of the varied VE for preventing COVID-19 infection and severe progression, we developed a mathematical model to estimate the distributions of clinical disease stages after being infected by SARS-CoV-2 in vaccinated individuals compared with that in unvaccinated individuals (Appendix 1.3). We estimated the population incidence of COVID-19 in older adults in the United States to be 9.1/100,000 person-day by averaging out the published data (of US Centers for Disease Control and Prevention [CDC]) over the last 180 days before the approval of a booster shot on September 22, 2021 (CDC, 2021a) (Appendix 1.4).
The costs of booster vaccination included the cost of BNT162b2 vaccine ($19.5/dose) (MHE, 2021) and the vaccination administration ($17.1/dose) (Kohli et al., 2021; Services CfMaM 2020). The cost of PCR testing for SARS-CoV-2 infection was estimated to be $51.0/person according to the COVID-19 testing pricing from the medical insurance administrative contractor (CDC, 2021c). We collected the total direct medical costs from hospitalization for each COVID-19 clinical stage based on the Projected Economic Impact Report of the US Healthcare System and Health System Tracker (Cox et al., 2020, FAIR, 2020). We calculated the corresponding daily cost by dividing the total cost by the duration of the clinical stages (Table S1, Appendix 1.5).
Health utility scores for patients with COVID-19 were derived from the disutility weights of severe lower respiratory tract infection (Global 2018; Cleary et al., 2021) and the estimates of pricing models for COVID-19 treatments published by the Institute for Clinical and Economic Review (Kohli et al., 2021) (Appendix 1.6).
We assumed a discount rate of 3% (0–6%) annually for both cost and quality-adjusted life-years (QALYs). We calculated the incremental costs and incremental QALYs for booster vaccination strategy compared with no booster (baseline). The incremental cost-effectiveness ratio (ICER) was defined as the incremental cost per QALY gained. We used a cost-effectiveness threshold of ICER < $50,000 (McDougall et al., 2020; Neumann et al., 2014). We conducted additional economic evaluations by calculating the benefit-cost ratio, cost/death saved, and net monetary benefit.
2.4. Sensitivity analysis
We performed a univariable sensitivity analysis to examine the impact of model parameters within their respective ranges on the ICER to identify the most sensitive parameters and visualized the results using tornado diagrams. In addition, we conducted a probabilistic sensitivity analysis (PSA) based on 100,000 simulations to determine the probability of the booster strategy being cost-effective (Figure 1). The distributions of all model parameters were provided in Appendix 1.7. We conducted a 2-way sensitivity analysis to examine the impact of various combinations of vaccine efficacies, vaccination, and direct medical cost on booster cost-effectiveness (Figure 2). By varying the population incidences between 0–50/100,000 person-day, we conducted the additional PSA (1,000 simulations for each population incidence) and presented the probabilities of the booster strategy being cost-effective and cost-saving (Figure 3). We further investigated the probabilities of being cost-effective and cost-saving under scenarios of less efficacious vaccines and boosters, where their efficacies for protection against infection and severe COVID-19 were reduced by 10%, 30%, and 50%, respectively. We estimated the threshold of population incidence for cost-effectiveness when the probabilities passed 50% for each scenario.
Figure 1.
The cost-effectiveness analysis of Pfizer booster vaccination strategy. (A) The result of probabilistic sensitivity analysis based on 100,000 simulations (67.37% of being cost-effective, including 64.95% of being cost-saving); (B) Tornado plot of one-way sensitivity analyses. A horizontal bar was generated for each parameter analysis. The width of the bar indicates the potential effect of the associated parameter on the ICER when the parameter is changed within its range (as shown in Table S1). The red part of each bar indicates high values of input parameter ranges, whereas the blue part indicates low values. The dotted vertical line represents the threshold of willingness-to-pay (WTP) of the baseline.
Figure 2.
The result of 2-way sensitivity analysis of Pfizer booster vaccination strategy. (A) Additional vaccine efficacy (VE) of booster for preventing a SARS-CoV-2 infection and for preventing a severe COVID-19 case; (B) Additional VE of booster and long-term VE of 2-dose BNT162b2 for preventing a SARS-CoV-2 infection; (C) Additional VE of booster and long-term VE of 2-dose BNT162b2 for preventing a severe COVID-19 case; (D) Vaccination and direct medical cost.
Figure 3.
Probability and population incidence threshold of the Pfizer booster strategy being (a) cost-effective; (b) cost-saving with various vaccine (and corresponding booster) efficacies.
Existing evidence demonstrated that another mRNA COVID-19 vaccine, Moderna mRNA-1273, had comparable or even higher efficacy than BNT162b2 (Self et al., 2021; Tenforde et al., 2021, Tenforde et al., 2021), and the vaccine cost of Moderna mRNA-1273 was lower than that of BNT162b2 (MHE, 2021). Intuitively, Moderna mRNA-1273 would be more cost-effective than BNT162b2. In this study, we also conducted similar analyses for Moderna mRNA-1273 as a part of the sensitivity analysis (Appendix 1.8).
3. Results
3.1. Current BNT162b2 booster strategy is cost-saving in the US
We identified decremental costs and incremental QALYs for the BNT162b2 booster vaccination compared with full-vaccination without boosters in a designated cohort of 100,000 older adults aged ≥65 years for 180 days. Overall, the booster strategy would incur an additional cost of $3,427,607 but save $6,691,935 owing to reduced direct medical care, corresponding to a benefit-cost ratio of 1.95. This suggested that the booster strategy is a cost-saving strategy. Furthermore, the strategy would result in a gain of 3.7 QALYs during the 180 days, and together with the monetary gain, it would amount to a net monetary benefit of $3,449,328. The strategy would prevent 3.8 COVID-19 deaths, indicating a requirement of $904,382 to prevent 1 COVID-19 death.
The probabilistic sensitivity analysis (PSA) based on 100,000 simulations demonstrated that the probability of being cost-effective (including being cost-saving) with the current booster strategy was 67.37%, indicating a high chance of cost-effectiveness (Figure 1A). In contrast, the tornado diagram of univariate sensitivity analysis showed that varying any individual model parameter except population incidence of COVID-19 at one time would not change the conclusion of cost-effectiveness of the booster strategy (Figure 1B). The population incidence of COVID-19 was the only factor that may alone alter the conclusion of cost-effectiveness of the booster strategy. We also noted that both the increase of vaccination cost and decrease in direct medical cost for COVID-19 treatment would reduce the cost-effectiveness of the booster strategy but not sufficient to alter the conclusion individually.
3.2. Impact of vaccine efficacies on booster cost-effectiveness
The 2-way sensitivity analysis showed that the booster strategy remained cost-effective at various combinations of vaccine efficacies. Figure 2A shows that if the booster provided 62% additional protection against the infection to fully vaccinated older adults, it would be cost-effective even if the booster did not provide additional protection against the development of severe COVID-19 disease. Similarly, a combination of 50% additional protection against a SARS-CoV-2 infection and 39% additional protection against severe COVID-19 would render the strategy cost-effective.
Comparing the protective efficacies of 2-dose vaccination against infection, with and without a booster, only when the long-term efficacy of a 2-dose vaccine program remained above 93% would a booster not confer sufficient additional protection to be cost-effective (Figure 2B). If the long-term efficacy against infection of a typical 2-dose vaccine only lay in the range of 30%–40%, then a booster would only be required to provide 18%–20% additional protection to enable it to be cost-effective in those aged ≥65. On the contrary, comparing the protective efficacies of a 2-dose vaccine against severe COVID-19 disease, the booster strategy would always be cost-effective (Figure 2C).
3.3. Impact of vaccine and medical cost on booster cost-effectiveness
Doubling the vaccination cost or halving the direct medical cost for COVID-19 treatment alone would not change the cost-effectiveness status of the booster strategy (Figure 2D). However, certain combinations in the simultaneous changes of vaccination and medical cost, such as a 50% increase in vaccination cost and 26% reduction in direct medical cost for COVID-19 treatment, would render the booster strategy not cost-effective.
3.4. Impact of population incidence and vaccine efficacies on booster cost-effectiveness
Figure 3 investigates the impact of varying population incidence (from 0 to 50/100,000 person-day) and declining vaccine and booster efficacies on the cost-effectiveness of the booster strategy. Our findings showed that for the current booster shots to be cost-effective (>50% chance), the population incidence of COVID-19 in the United States among those aged ≥65 years needed to be ≥8.1/100,000 person-day. This threshold would increase with a decreasing vaccine and booster efficacy. For example, if the proactive efficacies against infection and severe COVID-19 disease by both the 2-dose vaccine and booster would reduce by 10% (as of BNT162b2), the population incidence threshold needed to be 9.7/100,000 person-day for the booster to be cost-effective. Furthermore, if the vaccine and booster efficacies were reduced by 30%–-50% (a weak vaccine), the corresponding population incidence threshold would increase to 13.9 and 21.9/100,000 person-day. We also demonstrated similar results for the probability of being cost-saving (Figure 3B).
We also demonstrated similar key findings and conclusions regarding the cost-effectiveness of the Moderna mRNA-1273 booster as part of sensitivity analysis (details in Appendix 1.8).
4. Discussion
The study extensively evaluated the cost-effectiveness of a BNT162b2 booster strategy among older adults aged ≥65 years in the United States. With an average population incidence of COVID-19 in older adults of 9.1/100,000 person-day, the probability that a booster strategy would be cost-effective is high (67%). In fact, our findings demonstrated that with every dollar of investment in the booster vaccination, the US government might save nearly $2 because of fewer COVID-19 hospitalizations. Implementing the booster strategy in a cohort of 100,000 older adults would result in a net momentary benefit of $3.8 million in 180 days. Notably, the cost-effectiveness of the booster strategy is highly sensitive to the population incidence of COVID-19, with a threshold of 8.1/100,000 person-day being required to ensure the booster strategy to be cost-effective. This threshold will increase with a decrease in vaccine and booster efficacies.
Our study indicated that a COVID-19 booster strategy is likely to be cost-effective for older adults in the United States. We estimated that the booster strategy is cost-saving because the benefit of preventing 1 patient from being hospitalized and the subsequent needs of ICU and ventilation would outweigh the cost of delivering boosters to a large population of older adults. However, a combination of an increase in vaccine price (∼50%) and a decrease in direct medical cost (∼30%) may make boosters less cost-effective. As the demand for COVID-19 vaccines continue to surge worldwide and the increasing pressure faced by the US government to provide more vaccines to middle- and low-income countries, the vaccine price in the United States may increase at some point. Furthermore, the latest studies have documented significant development of antiviral drugs for COVID-19 treatment. Molnupiravir, the first oral medicine for the antiviral treatment of COVID-19, is highly effective in reducing viral loads in infected patients (Fischer et al., 2021). Similarly, Paxlovid, another antiviral drug, has been shown to be 89% effective in patients at risk of serious illness (Mahase, 2021). Because a novel and effective antiviral drug may potentially reduce the medical cost for treating patients with COVID-19, the booster strategy may become no longer cost-effective or even necessary in the future.
Our study indicates that the potential cost-effectiveness of the booster will reduce when the population incidence rate falls. In fact, the booster strategy will no longer be cost-effective if the population incidence in older adults reduces below 8.1/100,000 person-day. In a setting with an already high 2-dose vaccination coverage, the booster may further reduce the population incidence to below the threshold value in the elderly population and render it not cost-effective. However, a complete termination of the booster strategy may see a waning population immunity and subsequent rebound of the population incidence (Li et al., 2021, Shen et al., 2021, Zou et al., 2022). If immunity falls after each booster, a regular yearly vaccination program with further rounds of booster may be necessary to contain the epidemic to a low level.
Results from our sensitivity analyses suggest even in countries where vaccines with various efficacies are used, a booster strategy may be cost-effective for older adults. For example, for vaccines whose 2-dose and booster efficacies were 10%–50% lower than that of BNT162b2, a booster strategy would still be cost-effective as long as the COVID-19 incidence is greater than 21.7/100,000 person-day. Nevertheless, for countries with a low percentage of fully vaccinated population, it is essential to first achieve a high level of vaccination coverage before the booster strategy can be rolled-out.
Despite its cost-effectiveness, the booster strategy needs to be considered in light of vaccine equity. So far, 14% of those aged ≥65 years in the United States remain unvaccinated; therefore, efforts should be taken to help those unvaccinated or partially vaccinated receive their full vaccines in addition to providing boosters to those who are fully vaccinated. In addition, there exist stark racial and ethnic disparities in vaccination rate and COVID-19 disease outcomes in the United States (CDC, 2021b). African-Americans reportedly have a lower vaccination rate and a greater disease burden of COVID-19 compared with White populations (Mackey et al., 2021). Targeted interventions such as culture-sensitive education and mass media campaigns that can improve the acceptance of COVID-19 vaccines among racial and ethnic minorities (Feifer et al., 2021; Momplaisir et al., 2021) should be implemented to reduce racial and ethnic disparities.
Our study has several limitations. First, our study used a decision-analytic Markov model and did not account for the dynamic changes of population incidence of COVID-19. Second, we estimated the efficacy of the BNT162b2 booster against the Delta variant based on a synthesis of evidence from real-world data rather than randomized controlled trials. We conducted various sensitivity analyses to account for uncertainty in real-world data and parameter biases. Third, we did not consider the other COVID-19 vaccines (eg, Moderna or J&J/Janssen) in the United States. Although the other vaccines, particularly the Moderna vaccine, represent a substantial proportion of COVID-19 vaccines administered in the United States, they differ from the BNT162b2 vaccines in efficacy and price. It may not be reasonable to combine different vaccines together. Nevertheless, the BNT162b2 vaccine comprises the largest proportion of all COVID-19 vaccines in the United States; thus, the cost-effectiveness result of the BNT162b2 vaccines and boosters would be most policy-relevant vaccines. As the latest data showed that the Moderna vaccine could be more effective than the BNT162b2 vaccine in preventing hospitalizations (Self et al., 2021), the cost-effectiveness of a booster strategy, in reality, is likely to be more favorable than what we estimated. Fourth, we assumed that the efficacy of COVID-19 vaccines begins to wane in 6 months after full vaccination. In reality, the efficacy of vaccines is more likely to gradually decline without a clear cutoff. This assumption may have led to an overestimate of vaccine efficacy in the short term and an underestimate in the long term. Fifth, we only evaluated the booster strategy for older adults. It is unclear whether the conclusion would be applicable to younger adults and children who have a lower vaccination rate but, at the same time, a lower risk of hospitalization if diagnosed with COVID-19. This research question warrants further investigation. Finally, we did not consider recent emergence of the Omicron variant—a “highly mutated” variant—in our model as well as its impact on vaccine and booster effectiveness. Early data suggest that the current COVID-19 vaccines might be less effective in preventing infections caused by the Omicron variant, but the data and evidence are far from conclusive (Karim and Karim, 2021; Mohiuddin and Kasahara, 2022).
In conclusion, offering Pfizer-BioNTech booster shots to older adults aged ≥65 years in the United States is likely to be cost-effective, but its cost-effectiveness is highly sensitive to the population incidence of COVID-19. Less efficacious vaccines and boosters may still be cost-effective in settings of high SARS-CoV-2 transmission. Given limited public health resources and escalating health inequity during the pandemic, there is a need for more targeted, local-based vaccine and booster distribution strategies that can achieve a tradeoff between cost-effectiveness and equity. Further research is needed to inform the design of such strategies to alleviate the burden of COVID-19, reducing health care costs, and achieving equity.
Ethics approval and consent to participate
Not applicable.
Disclosures
The authors declare that they have no competing interests.
Funding source
The work is supported by the Bill & Melinda Gates Foundation (INV-006104). LZ is supported by the National Natural Science Foundation of China (Grant number: 81950410639), Outstanding Young Scholars Funding (Grant number: 3111500001), Xi'an Jiaotong University Basic Research and Profession Grant (Grant number: xtr022019003 and xzy032020032), and Xi'an Jiaotong University Young Talent Support Grant (Grant number: YX6J004). MS was supported by the National Natural Science Foundation of China (Grant number: 12171387, 11801435), China Postdoctoral Science Foundation (Grant number: 2018M631134, 2020T130095ZX), the Fundamental Research Funds for the Central Universities (Grant number: xjh012019055), Natural Science Basic Research Program of Shaanxi Province (Grant number: 2019JQ-187), and Young Talent Support Program of Shaanxi University Association for Science and Technology (Grant number: 20210307). XL was supported by the Special emergency public health safety project of Shaanxi Provincial Education Department (Grant number: 20JG007).
Authors’ contributions
LZ conceived the study. LZ, YL, MS, and RL designed and constructed the model. RL performed the modelled analyses and graphed and interpreted the results. RL, HL, ZZ, LX, and XL contributed to the collection of data and model parameters. RL, LZ, YL, and HL drafted the manuscript. LZ, YL, and MS critically revised the manuscript. All authors reviewed the manuscript and approved the final version.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.03.029.
Contributor Information
Mingwang Shen, Email: mingwangshen521@xjtu.edu.cn.
Yan Li, Email: yan.li1@mountsinai.org.
Lei Zhang, Email: lei.zhang1@monash.edu.
Appendix. Supplementary materials
References
- Amewu S, Asante S, Pauw K, Thurlow J. The Economic Costs of COVID-19 in Sub-Saharan Africa: Insights from a Simulation Exercise for Ghana. Eur J Dev Res. 2020:1–26. doi: 10.1057/s41287-020-00332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos RM, Badowski ME, Drwiega E, Ghassemi S, Griffith N, Herald F, et al. The race to a COVID-19 vaccine: opportunities and challenges in development and distribution. Drugs Context. 2021;10 doi: 10.7573/dic.2020-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki T. Booster shots for COVID-19-the debate continues. Lancet Infect Dis. 2021;21(10):1359–1360. doi: 10.1016/S1473-3099(21)00574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. COVID-19 Weekly Cases and Deaths per 100,000 Population by Age; 2021a. Available from: https://covid.cdc.gov/covid-data-tracker/#demographicsovertime. [Accessed Oct 28 2021].
- CDC. Demographic Trends of People Receiving COVID-19 Vaccinations in the United States; 2021b. Available from: https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends. [Accessed Dec 15, 2021].
- CDC. Medicare Administrative Contractor (MAC) COVID-19 Test Pricing; 2021c. Available from: https://www.cms.gov/files/document/mac-covid-19-test-pricing.pdf. [Accessed Oct 07 2021].
- Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N Engl J Med. 2021 doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary SM, Wilkinson T, Tamandjou Tchuem CR, Docrat S, Solanki GC. Cost-effectiveness of intensive care for hospitalized COVID-19 patients: experience from South Africa. BMC Health Serv Res. 2021;21(1):82. doi: 10.1186/s12913-021-06081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L, Mascola JR, Fauci AS, Collins FS. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368(6494):948–950. doi: 10.1126/science.abc5312. [DOI] [PubMed] [Google Scholar]
- COVID-19 Vaccine Breakthrough Infections Reported to CDC - United States January 1-April 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(21):792–793. doi: 10.15585/mmwr.mm7021e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C RR, Neuman T, Cubanski J, Rae M. How health costs might change with COVID-19; 2020. Available from: https://www.healthsystemtracker.org/brief/how-health-costs-might-change-with-covid-19/. [Accessed Oct 08 2021].
- FAIR. COVID-19: the projected economic impact of the COVID-19 pandemic on the US healthcare system; 2020. Available from: https://s3.amazonaws.com/media2.fairhealth.org/brief/asset/COVID-19%20-%20The%20Projected%20Economic%20Impact%20of%20the%20COVID-19%20Pandemic%20on%20the%20US%20Healthcare%20System.pdf. [Accessed Oct 08 2021].
- Feifer RA, Bethea L, White EM. Racial Disparities in COVID-19 Vaccine Acceptance: Building Trust to Protect Nursing Home Staff and Residents. J Am Med Dir Assoc. 2021;22(9):1853–1855. doi: 10.1016/j.jamda.2021.07.006. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Eron JJ, Holman W, Cohen MS, Fang L, Szewczyk LJ, et al. Molnupiravir, an Oral Antiviral Treatment for COVID-19. medRxiv 2021.
- Global regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, et al. Waning Immunity after the BNT162b2 Vaccine in Israel. N Engl J Med. 2021 doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Andam KSE, Oboh Victor, Pauw Karl, Thurlow James. International Food Policy Research Institute (IFPRI); Washington, DC: 2020. Estimating the economic costs of COVID-19 in Nigeria. Hyacinth. NSSP Working Paper 63Available from: [DOI] [Google Scholar]
- IVAC. Results of COVID-19 Vaccine Effectiveness Studies: An Ongoing Systematic Review; 2021. Available from: https://view-hub.org/resources. [Accessed Oct 21 2021].
- JHU. Coronavirus Resource Center: COVID-19 Tracking; 2021. Available from: https://coronavirus.jhu.edu. [Accessed Dec 15, 2021.
- Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398(10317):2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli M, Maschio M, Becker D, Weinstein MC. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: Use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine. 2021;39(7):1157–1164. doi: 10.1016/j.vaccine.2020.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Rui, Li Yan, Zou Zhuoru, et al. Evaluating the Impact of SARS-CoV-2 Variants on the COVID-19 Epidemic and Social Restoration in the United States: A Mathematical Modelling Study. Front Public Health. 2021;9:801763. doi: 10.3389/fpubh.2021.801763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey K, Ayers CK, Kondo KK, Saha S, Advani SM, Young S, et al. Racial and Ethnic Disparities in COVID-19-Related Infections, Hospitalizations, and Deaths: A Systematic Review. Ann Intern Med. 2021;174(3):362–373. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. doi: 10.1136/bmj.n2713. [DOI] [PubMed] [Google Scholar]
- McDougall JA, Furnback WE, Wang BCM, Mahlich J. Understanding the global measurement of willingness to pay in health. J Mark Access Health Policy. 2020;8(1) doi: 10.1080/20016689.2020.1717030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibbin W, Fernando R. The economic impact of COVID-19. Econ Time COVID-19. 2020;45 (10.1162) [Google Scholar]
- MHE. The Price Tags on the COVID-19 Vaccines; 2021. Available from: https://www.managedhealthcareexecutive.com/view/the-price-tags-on-the-covid-19-vaccines. [Accessed Sep 17 2021].
- Mohiuddin M, Kasahara K. Investigating the aggressiveness of the COVID-19 Omicron variant and suggestions for possible treatment options. Respiratory Medicine. 2022;191 doi: 10.1016/j.rmed.2021.106716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momplaisir FM, Kuter BJ, Ghadimi F, Browne S, Nkwihoreze H, Feemster KA, et al. Racial/Ethnic Differences in COVID-19 Vaccine Hesitancy Among Health Care Workers in 2 Large Academic Hospitals. JAMA Netw Open. 2021;4(8) doi: 10.1001/jamanetworkopen.2021.21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- Reddy KP, Shebl FM, Foote JHA, Harling G, Scott JA, Panella C, et al. Cost-effectiveness of public health strategies for COVID-19 epidemic control in South Africa: a microsimulation modelling study. Lancet Glob Health. 2021;9(2):e120–e1e9. doi: 10.1016/S2214-109X(20)30452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions - United States, March-August 2021. MMWR Morbidity and mortality weekly report. 2021;70(38):1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Services CfMaM. Physician fee schedule; 2020. Available from: https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.asp. [Accessed Oct 25 2021].
- Shadmi E, Chen Y, Dourado I, Faran-Perach I, Furler J, Hangoma P, et al. Health equity and COVID-19: global perspectives. Int J Equity Health. 2020;19(1):104. doi: 10.1186/s12939-020-01218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Mingwang, Zu Jian, Fairley Christopher K, et al. Projected COVID-19 epidemic in the United States in the context of the effectiveness of a potential vaccine and implications for social distancing and face mask use. Vaccine. 2021;39(16):2295–2302. doi: 10.1016/j.vaccine.2021.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Mingwang, Xiao Yanni, Zhuang Guihua, et al. Mass testing-An underexplored strategy for COVID-19 control. Innovation (N Y) 2021;2(2):100114. doi: 10.1016/j.xinn.2021.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenforde MW, Olson SM, Self WH, Talbot HK, Lindsell CJ, Steingrub JS, et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years - United States, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):674–679. doi: 10.15585/mmwr.mm7018e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenforde MW, Self WH, Naioti EA, Ginde AA, Douin DJ, Olson SM, et al. Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults - United States, March-July 2021. MMWR Morbidity and mortality weekly report. 2021;70(34):1156–1162. doi: 10.15585/mmwr.mm7034e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SJ, Moreira ED, Jr., Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. 2021 doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Tang K. Combating COVID-19: health equity matters. Nat Med. 2020;26(4):458. doi: 10.1038/s41591-020-0823-6. [DOI] [PubMed] [Google Scholar]
- Zhang Lei, Tao Yusha, Shen Mingwang, et al. Early characteristics of the COVID-19 outbreak predict the subsequent size. International Journal of Infectious Diseases. 2020;97:219–224. doi: 10.1016/j.ijid.2020.05.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Lei, Shen Mingwang, Ma Xiaomeng, et al. What Is Required to Prevent a Second Major Outbreak of SARS-CoV-2 upon Lifting Quarantine in Wuhan City, China. Innovation (N Y) 2020;1(1):100006. doi: 10.1016/j.xinn.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Zhuoru, Fairley Christopher K, Shen Mingwang, et al. Critical timing and extent of public health interventions to control outbreaks dominated by SARS-CoV-2 variants in Australia: a mathematical modelling study. International Journal of Infectious Diseases. 2022;115:154–165. doi: 10.1016/j.ijid.2021.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.