Highlights
-
•
Prenatal exposure to stress predicts amygdala degree centrality in young adulthood.
-
•
High (vs. low) stress group showed lower structural covariance degree of amygdala.
-
•
These effects were particularly significant in men.
-
•
Global network parameters did not drive these effects.
Keywords: Prenatal stress, Structural covariance, Amygdala, Degree centrality, ELSPAC, ALSPAC
Abstract
Background
Prenatal stress influences brain development and mood disorder vulnerability. Brain structural covariance network (SCN) properties based on inter-regional volumetric correlations may reflect developmentally-mediated shared plasticity among regions. Childhood trauma is associated with amygdala-centric SCN reorganization patterns, however, the impact of prenatal stress on SCN properties remains unknown.
Methods
The study included participants from the European Longitudinal Study of Pregnancy and Childhood (ELSPAC) with archival prenatal stress data and structural MRI acquired in young adulthood (age 23–24). SCNs were constructed based on Freesurfer-extracted volumes of 7 subcortical and 34 cortical regions. We compared amygdala degree centrality, a measure of hubness, between those exposed to high vs. low (median split) prenatal stress, defined by maternal reports of stressful life events during the first (n = 93, 57% female) and second (n = 125, 54% female) half of pregnancy. Group differences were tested across network density thresholds (5–40%) using 10,000 permutations, with sex and intracranial volume as covariates, followed by sex-specific analyses. Finally, we sought to replicate our results in an independent all-male sample (n = 450, age 18–20) from the Avon Longitudinal Study of Parents and Children (ALSPAC).
Results
The high-stress during the first half of pregnancy ELSPAC group showed lower amygdala degree particularly in men, who demonstrated this difference at 10 consecutive thresholds, with no significant differences in global network properties. At the lowest significant density threshold, amygdala volume was positively correlated with hippocampus, putamen, rostral anterior and posterior cingulate, transverse temporal, and pericalcarine cortex in the low-stress (p(FDR) < 0.027), but not the high-stress (p(FDR) > 0.882) group. Although amygdala degree was nominally lower across thresholds in the high-stress ALSPAC group, these results were not significant.
Conclusion
Unlike childhood trauma, prenatal stress may shift SCN towards a less amygdala-centric SCN pattern, particularly in men. These findings did not replicate in an all-male ALSPAC sample, possibly due to the sample’s younger age and lower prenatal stress exposure.
1. Introduction
Stress experienced early in life is a major risk factor for psychiatric illnesses, including major depressive disorder (Kessler, 1997) and anxiety (APA, 2013). Greater maternal stress during pregnancy has also been linked with more mood disturbance among young adults without a psychiatric diagnosis (Mareckova et al., 2019). Adversity-related neuroanatomical alterations may begin as early as the prenatal period, as a consequence of maternal distress in pregnancy, disrupting tightly programmed, highly orchestrated biological processes, such as neuronal proliferation, migration, and differentiation, synaptogenesis and pruning, or myelination (Knuesel et al., 2014). Associations between maternal stress during pregnancy and offspring brain structure and function have been reported in fetuses (Wu and Limperopoulos, 2020), newborns (Qiu et al., 2015, Qiu et al., 2017, Qiu et al., 2013, Scheinost et al., 2016), children (Lebel et al., 2016, Wen et al., 2017, Soe et al., 2018, McQuaid et al., 2019), adolescents and young adults (Mareckova et al., 2019, McQuaid et al., 2019, Favaro et al., 2015, Mareckova et al., 2020). Research in fetuses showed associations between maternal stress and altered cortical gyrification and impaired brain metabolism (Wu and Limperopoulos, 2020). Research in newborns has reported decreased functional connectivity between amygdala and other subcortical regions in preterm neonates (vs. controls) and showed that prenatal stress exposure amplified these decreases (Scheinost et al., 2016). Further research in newborns also demonstrated that the relationships between maternal anxiety and brain structure are time-specific (Qiu et al., 2013) and moderated by genetic variation in the COMT gene (Qiu et al., 2015). Research in children then specifically pointed out links between maternal depression during pregnancy and larger amygdala volume (Wen et al., 2017) and functional connectivity between the amygdala and cortico-striatal circuitry (Soe et al., 2018), which is essential for emotion regulation.
The amygdala is a limbic subcortical brain structure critical for emotion processing (Whalen and Phelps, 2009), stress responsiveness and mood pathophysiology (LeDoux, 2000, Sierra-Mercado et al., 2011, McEwen, 2007, Janak and Tye, 2015), that is considered to be particularly sensitive to adversity during development (Buss et al., 2012, Lupien et al., 2011, Tottenham and Sheridan, 2009). The amygdala develops during the early embryonic stage (Humphrey, 1968) and might be selectively responsive to elevated glucocorticoids (Teicher et al., 2003), which can alter its developmental trajectory (Graham et al., 2019). Consistent with this notion, research in rodents demonstrated a link between prenatal exposure to stress and larger amygdala volume in offspring (Salm et al., 2004). In humans, all the major amygdala nuclei are fully formed by the 15th week of gestational development (Nikolić and Kostović, 1986) and prior work, including our own, has observed associations between prenatal stress and amygdala volume (Buss et al., 2012, Lupien et al., 2011, Mareckova et al., 2022, Jones et al., 2019). Larger amygdala volume has been observed in the offspring of mothers exposed to stress stemming from an ice storm during pregnancy (Jones et al., 2019), mothers with chronic depression (Lupien et al., 2011), as well as mothers with higher levels of cortisol over the course of pregnancy (Buss et al., 2012). Moreover, Buss et al. (Buss et al., 2012) demonstrated that amygdala volume in girls mediated the relationship between maternal cortisol and affective symptoms in the offspring.
Neuroimaging approaches that model patterns of structural covariance among brain regions have the potential to reveal more about the etiology of neuropathology than a focal approach since they consider coordinated changes among pairs and networks of regions, whose joint impact is much more likely to reflect the complexity of neurodevelopmental impacts and emergent disease (Evans, 2013, Bullmore and Sporns, 2009). The use of structural covariance network analysis in the search for biomarkers of disease in connectivity or neurodevelopmental disorders was also recommended by Yee et al. (Yee et al., 2018), who demonstrated that structural covariance is explained by transcriptomic similarity, distance of brain regions, and structural connectivity.
Structural covariance is defined as the statistical association of pairs of brain regions based on their anatomical properties, usually cortical thickness or volume (Yee et al., 2018). Structural covariance networks (SCNs) can be analyzed using graph theory that describes the centrality or “hubness” of a particular brain region, or node, as well as measures of network-wide properties such as segregation (e.g., modularity – capturing the degree to which the network tends to segregate into relatively independent modules or subnetworks) or integration (e.g., mean path length – capturing the average shortest path between nodes or the degree to which nodes tend to be connected).
Variations in structural covariance can be, at least in part, explained by variations in functional (Kelly et al., 2012) and structural (Gong et al., 2012) connectivity. It has also been demonstrated that SCNs agree with networks of synchronized neurodevelopment, determined by the expression of common genetic cues during early development (Raznahan et al., 2011). Therefore, aberrant gene expression (Pezawas et al., 2008, Schmitt et al., 2016, Gilmore et al., 2010) or early adversity (Voss and Zatorre, 2015) can both result in altered structural covariance. Gray-matter network studies have additionally supported this view and showed that exposure to maltreatment in childhood was associated with altered degree centrality in anterior cingulate cortex, anterior insula and precuneus, and suggested these alterations might represent a vulnerability factor and provide a potential mechanism for how maltreatment increases risk for psychopathology (Sun et al., 2018, Teicher et al., 2014).
In addition, our group recently identified variations in structural covariance associated with the impact of stress across species (Nikolova et al., 2018). Specifically, we demonstrated that unpredictable chronic mild stress in eight-week-old mice and early life stress in humans, assessed by the Childhood Trauma Questionnaire, were associated with higher structural covariance degree of the amygdala in 13-week-old mice and 18–22-year-old humans, specifically involving regions implicated in motivation and socioemotional processing, occurring against the background of globally reduced network clustering and modularity (Nikolova et al., 2018). These alterations in structural covariance suggest a shift towards an amygdala-centric SCN pattern that may represent a mechanistic pathway linking stress to risk for depression and related psychopathology. A recent study demonstrated a link between maternal depressive symptoms during pregnancy and altered pair-wise structural coupling between offspring amygdala volume and thickness in several cortical areas in early life (Lee et al., 2019). However, the impact of prenatal adversity on amygdala structural covariance computed on the whole-brain network level remains unknown. It is also unknown whether any impact of prenatal adversity on amygdala-related SCN properties remains detectable in young adulthood, when vulnerability to first depressive episodes is generally elevated (Kessler et al., 2005).
To answer these questions, in the current study we sought to evaluate the associations between prenatal stress experienced in the first and second half of pregnancy, and SCN properties of the amygdala in young adulthood. We tested group differences in amygdala degree centrality, which indicates the amygdala’s relative importance and influence on the rest of the network (Sporns, 2011), between young adults exposed to high vs. low prenatal stress, defined by stressful life events experienced by the mother during pregnancy. The impact of prenatal stress on whole-brain SCN properties was further assessed by network modularity and transitivity (reflecting network segregation) and global mean distance (reflecting network integration). Based on our prior cross-species findings (Nikolova et al., 2018), we hypothesized that higher exposure to stress prenatally will be associated with higher degree of the amygdala with lower or unchanged global structural covariance parameters. Based on prior work showing sex-specificity in the effects of prenatal stress on offspring brain structure (Mareckova et al., 2020, Lee et al., 2019, Sutherland and Brunwasser, 2018) and well-documented sex differences in amygdala volume (Ruigrok et al., 2014), we also hypothesized that the relationship between prenatal stress and structural covariance might differ by sex. Finally, given the findings from our group (Mareckova et al., 2020, Mareckova et al., 2022) as well as others (Class et al., 2011) on the importance of timing of prenatal stress exposure on brain development, we also hypothesized that the associations between prenatal stress and structural covariance might differ based on the timing of the exposure. Last but not least, we sought to replicate any emerging findings in an independent sample of young adults.
2. Methods
2.1. Study 1 – Young adults from ELSPAC/VULDE cohort
2.1.1. Participants
Participants included young adults (Tottenham and Sheridan, 2009, Humphrey, 1968), recruited from the European Longitudinal Study of Pregnancy and Childhood (ELSPAC; (Piler et al., 2017), a prenatal cohort from Czech Republic, to undergo a neuroimaging follow-up Biomarkers and underlying mechanisms of vulnerability to depression (VULDE; FP7-IEF-2013) at Central European Institute of Technology, Masaryk University. All of them were white Caucasians and of normal birth weight (M = 3347 g, SD = 527 g). Ethical approval was obtained by the ELSPAC Ethics Committee and written informed consent was obtained from all participants. These participants thus had both historic prenatal stress data as well as structural MRI acquired in young adulthood.
2.1.2. Assessment of prenatal stress and definition of the groups
Prenatal exposure to stress was quantified based on a 40-item self-report questionnaire, filled-in by participants’ mothers (Kessler, 1997) at mid-pregnancy and (APA, 2013) about 2 weeks after birth, regarding the number and impact of stressful life events the mothers experienced during first and second half of pregnancy, respectively (see Supplementary Methods). Offspring groups exposed to high vs. low stress during the first and second half of pregnancy were defined based on median split. For the first half of pregnancy, both structural MRI and prenatal stress data were available for 93 participants (57% women). For the second half of pregnancy, both structural MRI and prenatal stress data were available for 125 participants (54% women). This total of 125 participants included the 93 participants whose mothers were recruited at the beginning of pregnancy and an additional 32 participants whose mothers joined the study in second half of pregnancy.
2.1.3. Assessment of mood disturbance in young adulthood
Mood disturbance was measured in young adulthood using the long version of the Profile of Mood States questionnaire (POMS; (McNair et al., 1971). The POMS questionnaire measures the following components of current mood state: depression/dejection, tension/anxiety, fatigue/inertia, anger/hostility, confusion/bewilderment, and vigor/activity.
2.1.4. Acquisition of MRI data
All participants were scanned using a 3 Tesla Siemens Prisma MRI scanner. T1-weighted (T1w) MPRAGE images of the whole brain were acquired with 64 channel head/neck coil using the following acquisition parameters: voxel size 1 mm3, repetition time (TR) 2300 ms, echo time (TE) 2.34 ms, inversion time (TI) 900 ms, flip angle 8 degrees.
2.1.5. Analyses
T1w images were processed through an automated cortical reconstruction pipeline by FreeSurfer v6.0.0 (‘recon-all’). Quality control was based on successful registration of MR scans to T1w and completion of cortical reconstruction by Freesurfer without any reported errors. To ensure accuracy, all segmented volumes and reconstructed surfaces were assessed with Freesurfer quality assurance tools and visually inspected by a trained examiner, A.M. Regional volumes were calculated for 41 bilateral network nodes − 34 cortical regions, amygdala, and 6 additional subcortical regions (thalamus, caudate nucleus, putamen, pallidum, hippocampus, nucleus accumbens), all defined in accordance with the Desikan-Killiany atlas and adjusted for sex and total intracranial volume. Volumes from corresponding regions in both hemispheres were summed prior to analysis. SCN construction was based on Pearson correlation and a range of group-specific correlation coefficient thresholds, corresponding to network densities ranging from 5% to 40% in 1% increments. At each density threshold, negative correlations were discarded (i.e., replaced with 0) and “connections” between regions were retained if their corresponding correlation coefficient fell within the specified range of observed values.
Group differences in global and regional network parameters, namely network transitivity, defined as the fraction of existing triangles to all possible triangles; network modularity, defined as the degree to which the network tends to segregate into relatively independent modules or subnetworks; global mean distance, defined as the mean path length between the nodes; and amygdala degree centrality, defined as the number of links connected to the amygdala, were calculated in R using the igraph tools and tested across all density thresholds (5–40%). For each network parameter and at each density threshold, significance of observed between-group differences was determined using non-parametric permutation testing (n = 10,000). Consistent with prior work (Nikolova et al., 2018), between-group differences for a particular network parameter were considered significant if they reached p < 0.05 (over 10,000 permutations) at five consecutive density thresholds and were not any further corrected for multiple comparisons. Finally, posthoc analyses in the high-stress and low-stress groups assessed the strength of the correlations between amygdala volume and the volume of other nodes “connected” to it at the lowest significant density threshold, using false discovery rate (FDR) to correct for multiple comparisons across all possible connections (n = 40). Therefore, significance is reported using q values.
Additional exploratory analyses then tested group differences in degree for each of the 9 amygdala nuclei, segmented using the Freesurfer automated pipeline. Methodological details of these analyses are provided in Supplementary Information.
2.2. Study 2 – Young adults from ALSPAC cohort
2.2.1. Participants
Participants included young adult men (Sierra-Mercado et al., 2011), recruited from the Avon Longitudinal Study of Parents and Children (ALSPAC (Boyd et al., 2013, Fraser et al., 2013, Northstone et al., 2019); http: //www.alspac.bris.ac.uk), a prenatal cohort from United Kingdom, which includes data on 15 454 pregnant mothers and their children. Please note that the study website contains details of all the data that is available through a fully searchable data dictionary and variable search tool (http://www.bristol.ac.uk/alspac/researchers/our-data/). A total of 450 participants had both historic prenatal stress data as well as good quality structural MRI in young adulthood and thus were included in the current project Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time.
2.2.2. Assessment of prenatal stress and definition of the groups
Prenatal exposure to stress was quantified based on the same self-report questionnaire, filled-in by their mothers at mid-pregnancy regarding the number and impact of stressful life events the mothers experienced during first half of pregnancy (see Supplementary Methods). Maternal stress in the ALSPAC cohort was slightly lower than in the ELSPAC cohort (median score of 6 vs. 8). For consistency across cohorts, groups of offspring exposed to high vs. low stress during the first half of pregnancy were defined based on the median split approach used in Study 1 (i.e., using the median value defined by the ELSPAC/VULDE cohort). There were 193 participants in the high-stress group and 257 participants in the low-stress group.
2.2.3. Acquisition of MRI data
Magnetic resonance imaging (MRI) scans were acquired on a General Electric 3 T HDx scanner using an 8-channel head coil. High-resolution T1w images were acquired using 3D FSPGR (fast spoiled gradient-echo) sequences with the following parameters: voxel size 1 mm3, repetition time (TR) 7.9 ms, echo time (TE) 3 ms, inversion time (TI) 450 ms, flip angle 20 degrees.
2.2.4. Analyses
Similarly to Study 1, T1w scans were processed through an automated cortical reconstruction pipeline by FreeSurfer v6.0.0 (‘recon-all’). We excluded three participants who failed to pass quality control of the image-analysis pipeline. The remaining steps were conducted using the same methodology and scripts.
3. Results
3.1. Study 1 – Young adults from ELSPAC/VULDE cohort
3.1.1. Characteristics of the low and high prenatal stress groups
The low-stress and high-stress groups did not differ by sex, birth weight, or amygdala volume (corrected for ICV). The high-stress (vs. low-stress) group experienced more mood disturbance, as measured with POMS, in young adulthood, regardless of time of exposure (Table 1).
Table 1.
Group differences in sex, birth weight, and symptoms of depression and anxiety in young adults from the ELSPAC/VULDE cohort (LS – low prenatal stress group, HS – high prenatal stress group).
| First half of pregnancy |
Second half of pregnancy |
|||||
|---|---|---|---|---|---|---|
| LS group (n = 46) | HS group (n = 47) | Group difference | LS group (n = 61) | HS group (n = 64) | Group difference | |
| Sex | 18 M, 28F | 22 M, 25F | X2 = 0.56, p = 0.45 | 24 M, 37F | 34 M, 30F | X2 = 2.39, p = 0.12 |
| Birth weight (M, SE) | M = 3320,65, SE = 80.96 | M = 3368.09, SE = 80.09 | t(91) = -0.42, p = 0.68 | M = 3388.52, SE = 67.90 | M = 3305.83, SE = 68.46 | t(1 1 9) = 0.86, p = 0.39 |
| Mood disturbance (POMS) in young adulthood (M, SE) | M = 3.33, SE = 0.11 | M = 3.65, SE = 0.11 | t(91) = 2.08, p = 0.04 | M = 3.34, SE = 0.10 | M = 3.67, SE = 0.11 | t(1 2 3) = 2.14, p = 0.04 |
| Left amygdala volume (corrected for ICV) | M = 1797.09, SE = 29.05 | M = 1749.09, SE = 28.43 | t(91) = -1.18, p = 0.24 | M = 1791.66, SE = 27.90 | M = 1750.81, SE = 29.78 | t(1 2 3) = -1.00, p = 0.32 |
| Right amygdala volume (corrected for ICV) | M = 1882.41, SE = 30.82 | M = 1852.90, SE = 30.15 | t(91) = -0.68, p = 0.50 | M = 1884.61, SE = 29.49 | M = 1847.64, SE = 31.48 | t(1 2 3) = -0.86, p = 0.39 |
3.1.2. Prenatal exposure to maternal stress and amygdala degree in the young adult offspring
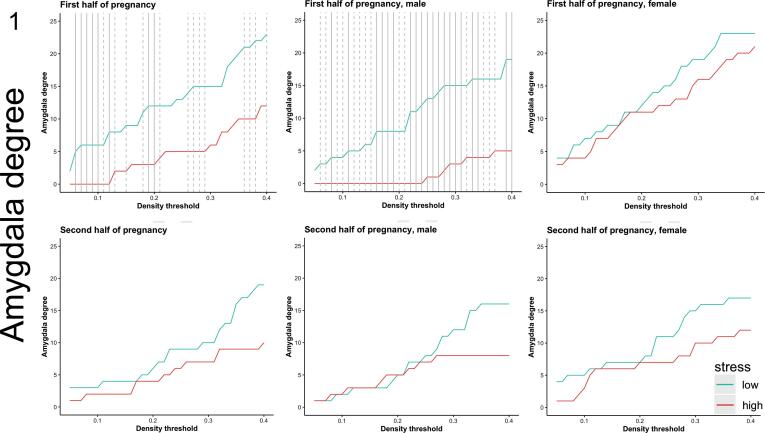
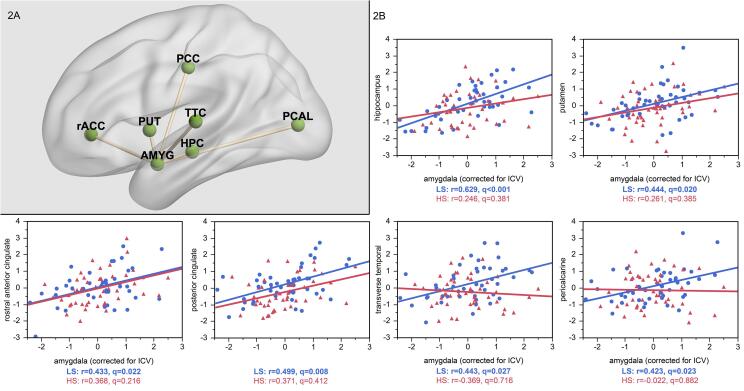
Individuals with high (vs. low) exposure to maternal stress experienced during the first half of pregnancy, but not during the second half of pregnancy, showed significantly lower amygdala degree (Fig. 1). At the lowest network density threshold at which this effect was significant (6%), the high-stress group showed no significant correlations (all q values > 0.22) but the low-stress group showed strong positive correlations between the amygdala volume and volumes of the hippocampus (r = 0.629, q < 0.001), putamen (r = 0.444, q = 0.020), rostral anterior cingulate cortex (r = 0.433, q = 0.022), posterior cingulate cortex (r = 0.499, q = 0.008), pericalcarine cortex (r = 0.423, q = 0.023), and transverse temporal cortex (r = 0.443, q = 0.027; Fig. 2), suggesting a stress-related loss of structural coupling between the amygdala and these regions. All correlations, p values and q values for each group are reported in Supplementary Table 1. Importantly, since our significant between-group SCN results are based on overall amygdala degree computed across multiple density thresholds, rather than targeted pair-wise structural covariance testing, between-group differences in pair-wise correlations at this illustrative threshold should be regarded as descriptive and exploratory. Similarly, since the SCN analytic framework we adopted can only conduct between group-comparisons, stress-by-amygdala interaction effects in predicting the volume of additional brain regions were not explicitly tested in these post hoc analyses.
Fig. 1.
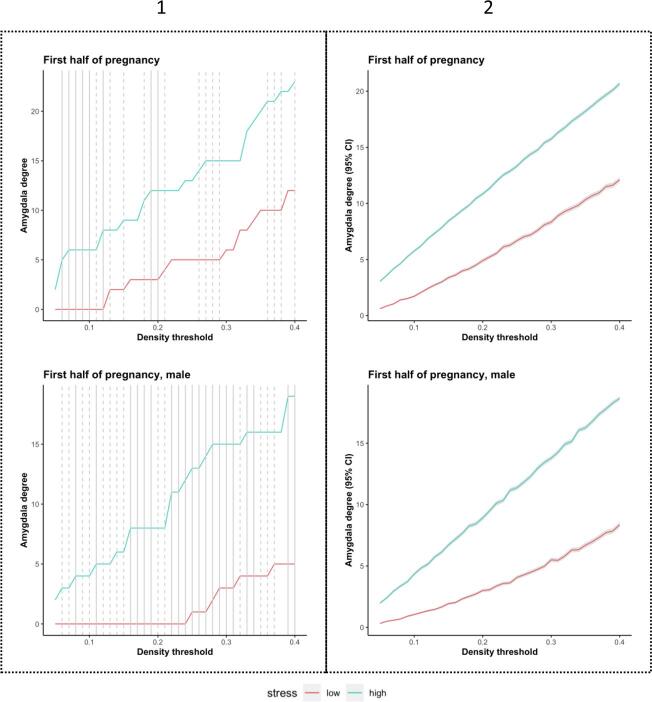
Prenatal exposure to maternal stress and amygdala degree in the young adult offspring from ELSPAC/VULDE cohort. Amygdala degree was significantly higher in the low-stress group (blue) than in the high-stress group (red) during the first half of pregnancy but not during the second half of pregnancy. These effects were driven by male subjects. Solid line indicates p ≤ 0.05, dashed line indicates p ≤ 0.10. Please note these results were not corrected for multiple comparisons.
Fig. 2.
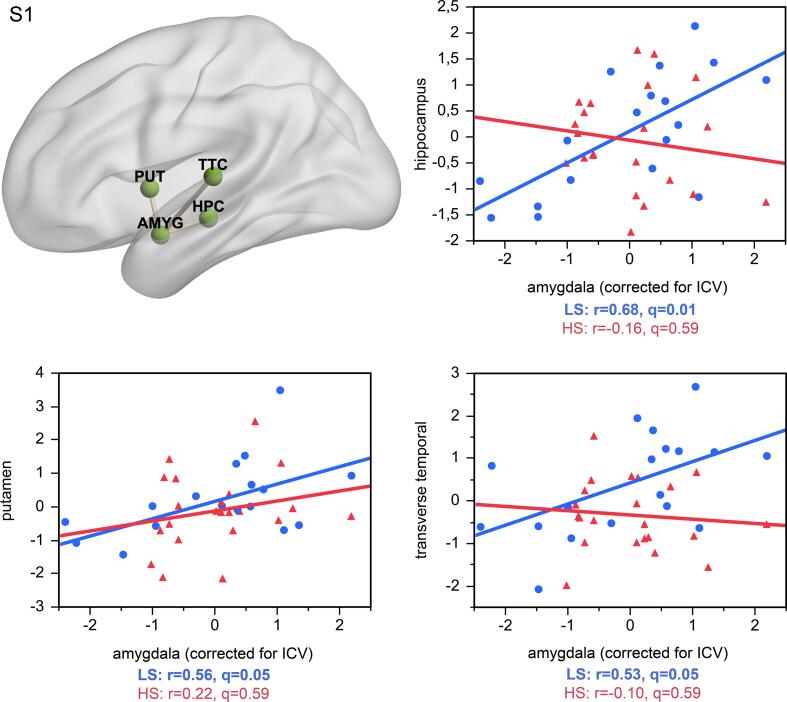
At the lowest significant network density threshold (6%), amygdala volume was positively correlated with volumes in the hippocampus, putamen, rostral anterior cingulate, posterior cingulate, transverse temporal, and pericalcarine cortex in the low stress (LS) group from ELSPAC/VULDE cohort (blue; all r>0.42, p(FDR)<0.027), but no significant associations emerged in the high stress (HS) group from ELSPAC/VULDE cohort (red; all r>-0.02, p(FDR)<0.882). AMYG = amygdala; HPC = hippocampus; PUT = putamen; PCAL = pericalcarine cortex; PCC = posterior cingulate cortex; rACC = rostral anterior cingulate cortex; TTC = transverse temporal cortex.
Sex-specific analyses revealed that the lower values of the amygdala degree, observed in the high-stress group relative to the low-stress group in the sex-pooled sample, were driven by group differences in men, and were directionally consistent and significant across a wide range of density thresholds. In other words, men exposed to higher levels of maternal stress during the first half of pregnancy had significantly fewer amygdala connections, as indicated by the absence of positive correlations between nodal volumes, than men exposed to lower levels of maternal stress during that same period. No such differences were detected in women or when stress exposure was based on reports about the second half of pregnancy (Fig. 1). At the lowest network density threshold at which this effect was significant in men (8%), the high-stress group showed no significant correlations (p > 0.31), while the low-stress group showed a positive correlation between volume in the amygdala and the hippocampus (r = 0.68, q = 0.01; Supplementary Fig. 1) and trends for positive correlations between volume of the amygdala and putamen (r = 0.56, q = 0.05) and volume of the amygdala and transverse temporal (r = 0.53, q = 0.05). The density-specific permutation-based p-values for both whole-sample as well as sex-specific analyses are provided in Supplementary Table 2.
Stability and reliability of within-group network parameter estimates and between-group differences therein were confirmed with bootstrapping and split-half cross validation, as described in the Supplementary Methods section. Results of these analyses are reported in Supplementary Table 5 and Supplementary Table 6, and they are depicted in Supplementary Fig. 2.
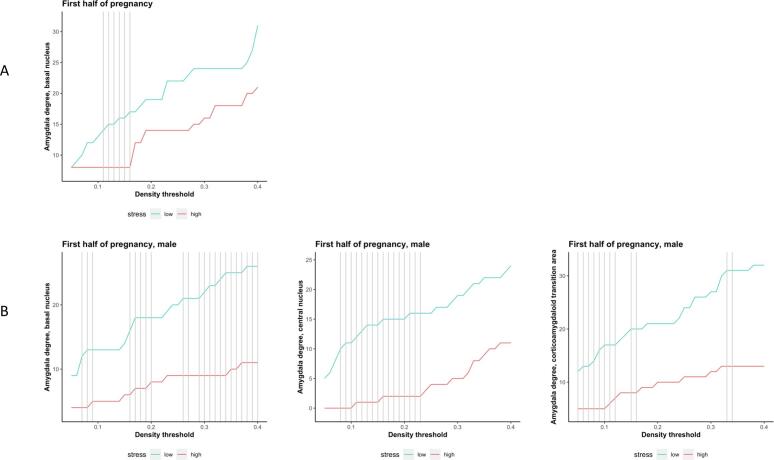
Finally, the exploratory analyses testing group differences in degree for each of the 9 amygdala nuclei in the whole sample detected a significant between-groups difference in degree (HS < LS) for the basal nucleus (Supplementary Table 7, Supplementary Fig. 3A). When tested in the males-only subsample, we detected significant between-groups differences in degree (HS < LS) for the basal nucleus, central nucleus, and corticoamygdaloid transition area (Supplementary Table 8, Supplementary Fig. 3B). Detailed results of these analyses are provided in the Supplementary Information.
3.1.3. Prenatal exposure to maternal stress and global SCN network properties (modularity, transitivity, and mean distance) in the young adult offspring
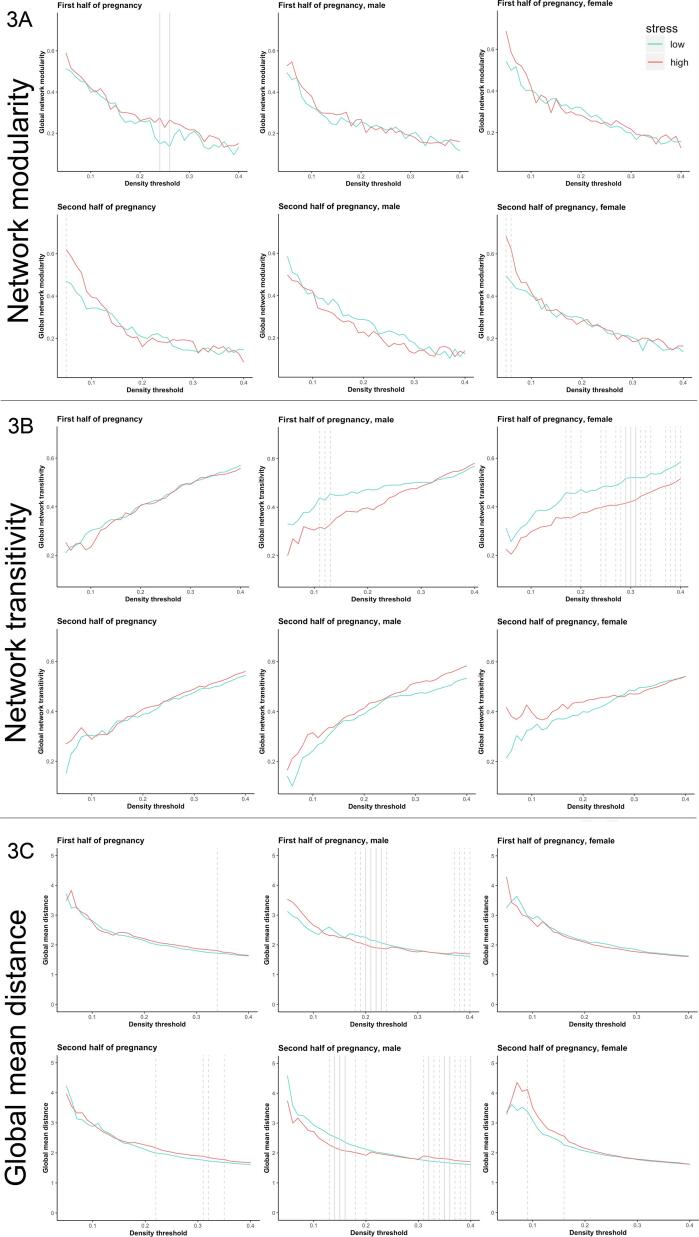
Global network parameters did not vary significantly as a function of maternal stress exposure during either the first or second half of pregnancy (Fig. 3). However, sex-specific analyses revealed trend-level effects (p < 0.10 at five consecutive density thresholds) of prenatal stress exposure on global network transitivity and global mean distance. These trend-level group differences indicated that women with higher (vs. lower) exposure to maternal stress during the first half of pregnancy, but not during the second half of pregnancy, showed lower global network transitivity (Fig. 3). They also indicated that men with higher (vs. lower) exposure to maternal stress during the first half of pregnancy showed lower global mean distance (Fig. 3) while men with higher (vs. lower) exposure to maternal stress during the second half of pregnancy showed higher global mean distance (Fig. 3). No other sex-specific analyses showed such trends for group differences in global network parameters. The density-specific permutation-based p-values for both whole-sample as well as sex-specific analyses are provided in Supplementary Table 3.
Fig. 3.
Prenatal exposure to maternal stress and overall structural covariance (modularity, transitivity and mean distance) in the young adult offspring from ELSPAC/VULDE cohort. Global network parameters did not vary significantly as a function of maternal stress. However, when trend-level values were considered as part of the five consecutive density thresholds, sex-specific group differences in global network transitivity and global mean distance were observed. Females with higher maternal stress exposure during the first half of pregnancy showed lower global network transitivity (density thresholds = 27–34%). Males with higher maternal stress exposure during the first half of pregnancy showed lower global mean distance (density thresholds = 18–24%) while males with higher maternal stress exposure during the second half of pregnancy shower higher global mean distance (density thresholds = 31–40%). High stress group is depicted in red, low stress group is depicted in blue, solid line indicates p ≤ 0.05, dashed line indicates p ≤ 0.10.
3.2. Study 2 – Young adult men from ALSPAC cohort
3.2.1. Characteristics of the low and high prenatal stress groups
The low-stress and high-stress groups did not differ in birth weight or amygdala volume (corrected for ICV; see Table 2).
Table 2.
Group differences in birth weight and amygdala volume in young men from the ALSPAC cohort (LS – low prenatal stress group, HS – high prenatal stress group).
| First half of pregnancy |
|||
|---|---|---|---|
| LS group (n = 257) | HS group (n = 193) | Group difference | |
| Birth weight(M, SE) | M = 3517.49, SE = 591.91 | M = 3526.74, SE = 544.50 | t(445*) = 0.17, p = 0.86 |
| Left amygdala volume (corrected for ICV) | M = 1646.05, SE = 159.66 | M = 1630.23, SE = 175.93 | t(4 5 0) = −0.98, p = 0.33 |
| Right amygdala volume (corrected for ICV) | M = 1835.99, SE = 164.79 | M = 1839.35, SE = 149.30 | t(4 5 0) = 0.23, p = 0.82 |
*4 participants in HS group and 1 participant in LS group did not have birth weight data.
3.2.2. Prenatal exposure to maternal stress during first half of pregnancy, amygdala degree and global network measures in the young adult men
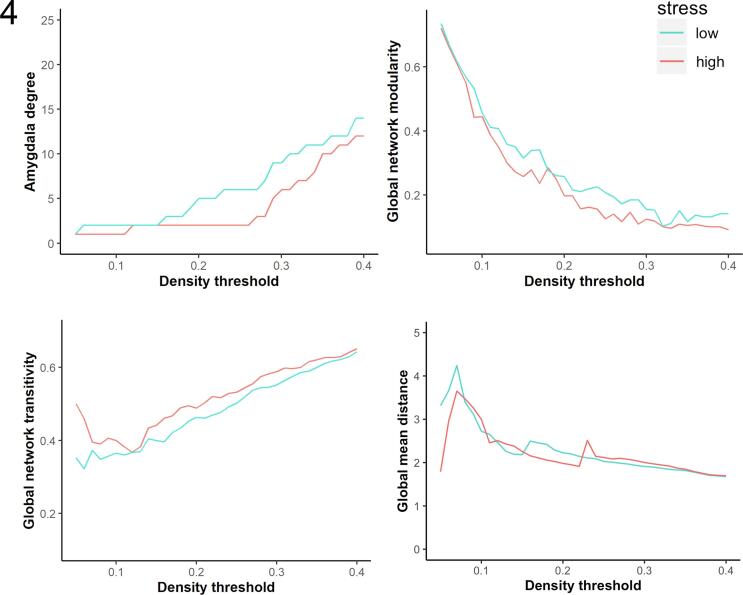
Calculating group differences in global and regional network parameters across density thresholds (5–40%) revealed that amygdala degree in the high-stress group was nominally lower across all thresholds except 5% and 12–15%, where it was equivalent across groups. These effects are directionally consistent with the patterns observed in Study 1. However, unlike Study 1 between-group differences in amygdala degree did not reach significance at any individual threshold. Similarly, no significant differences emerged in global network modularity, transitivity and mean distance (Fig. 4). The density-specific permutation-based p-values are provided in Supplementary Table 4.
Fig. 4.
Prenatal exposure to maternal stress, amygdala degree and overall structural covariance (modularity, transitivity and mean distance) in the young adult men from ALSPAC. Neither amygdala degree, nor the global network parameters (modularity, transitivity and mean distance) significantly varied as a function of maternal stress during pregnancy. High stress group is depicted in red, low stress group is depicted in blue.
4. Discussion
We demonstrated that the offspring of mothers exposed to higher vs. lower levels of stress during the first half of pregnancy have lower amygdala degree centrality and particularly lower structural covariance between the amygdala and the hippocampus, anterior and posterior cingulate cortex, putamen, pericalcarine cortex and transverse temporal cortex. The differences in the amygdala degree centrality between the high vs. low prenatal stress group were particularly pronounced in men, where they were evident at 10 consecutive thresholds (p ≤ 0.05). Men in the high-stress vs. low-stress group showed lower structural covariance between the amygdala and the hippocampus and trends for lower structural covariance between the amygdala and putamen as well as transverse temporal cortex. The global network parameters (modularity, transitivity and global mean distance) showed only trends for associations between higher maternal stress exposure during the first half of pregnancy and lower global network transitivity in women and lower global mean distance in men and between higher maternal stress exposure during the second half of pregnancy and higher global mean distance in men. Thus, our findings suggest that stress-related differences in amygdala degree centrality are unlikely to be driven by group differences in overall network properties.
Given the emerging view of brain disorders as network diseases (Sporns, 2011), amygdala degree might serve as an early marker of disease risk. The degree of a node determines whether it may play a peripheral or more central role in a network and thus indicates its relative importance and influence on the rest of the network (Sporns, 2011). Our findings demonstrating lower amygdala degree in the high (vs. low) prenatal stress group thus suggest stress may result in brain network reorganization patterns that place the amygdala in a more isolated, peripheral role and decouple it from regulatory structures. This may in turn reflect a diminished ability to regulate fear and anxiety in young adults exposed to more prenatal stress. This interpretation is consistent with studies of hub connectivity which have shown that structural hubs are present from a very early developmental stage (Oldham and Fornito, 2019). Binary topology of hub connectivity is established prior to birth and while spatial topography undergoes protracted period of consolidation spanning into late adolescence, the location of hubs is consistent throughout development (Oldham and Fornito, 2019).
The lower structural covariance of amygdala in individuals exposed to higher levels of stress during the first half of pregnancy supports the possibility that stress may trigger brain network reorganization possibly reflective of reallocation of neural network resources associated with a shift in behavioral priorities (Nikolova et al., 2018). However, we previously reported lower amygdala structural covariance in individuals exposed to lower (not higher) levels of childhood stress, as assessed with Childhood Trauma Questionnaire (CTQ) in a non-overlapping sample (Nikolova et al., 2018). This discrepancy suggests that amygdala-related SCN adaptations may be critically sensitive to the timing of the stress exposure; not only in terms of directionality but also in terms of location of the regions showing altered structural synchrony with the amygdala. Specifically, amygdala networks may contract after greater exposure to prenatal stress but expand after greater exposure to childhood stress. In addition, while both prenatal and childhood stress affect the amygdala’s structural covariance with the hippocampus and putamen, only prenatal stress affects the amygdala’s covariance with the pericalcarine cortex, cingulate cortex and transverse temporal cortex. In contrast, childhood but not prenatal stress affects the amygdala’s structural covariance with the pallidum, parahippocampal gyrus, temporal fusiform cortex, and temporal pole. Experimental studies in animal models should study these effects of stress timing with more precision and probe potential molecular and cellular mechanisms that may account for the distinct patterns of SCN reorganization around the amygdala.
Our findings are also partially consistent with other studies on the relationships between prenatal development and structural covariance, such as Nosarti et al. (Nosarti et al., 2011) who demonstrated complex alterations of structural covariance in adolescents born very preterm vs. healthy controls, and Lee et al (Lee et al., 2019) who showed that higher maternal depression during pregnancy was positively associated with structural covariance of the amygdala and insula at birth but negatively associated with structural covariance of the amygdala and prefrontal cortex during early childhood. These findings suggest that the effects of early life adversity on structural covariance may change over development, which may in turn also partially account for the diverse findings linking early exposure to stress to amygdala degree centrality in the current study and in prior work (Nikolova et al., 2018).
In our study, the low prenatal stress group showed largest correlations between the volumes of the amygdala and the hippocampus (r = 0.629), consistent with prior studies showing that closer structures might show greater structural covariance than more distant regions (Boyd et al., 2013). High structural covariance might also be found in functionally related regions (Paus et al., 2008). This is in agreement with a mouse brain study by Yee et al (Yee et al., 2018) who identified distance of the regions as the most important predictor of structural covariance and showed that structural covariance can be significantly explained by distance (17% of the variation), connectivity (15% of the variation), and transcriptomic similarity (13% of the variation) of the covarying structures (Yee et al., 2018). Together, these three factors explained 37% of structural covariance (Yee et al., 2018). The fact that the high prenatal stress group did not show any such significant correlations with the amygdala suggests that prenatal stress exposure may interfere with the typical development of the amygdala’s SCN properties to disrupt normal patterns of structural synchrony within the temporal lobe and surrounding structures. Importantly, however, our analytic framework was geared towards identifying between-group differences in overall amygdala degree centrality across a range of network density thresholds, rather than pair-wise structural coupling relationships. Hence, any between-group differences in pairwise correlation between the amygdala and other regions reported illustratively at the lowest significant density threshold should be regarded as preliminary until confirmed in future work.
Our findings suggest that the male brain may be particularly sensitive to amygdala-related SCN changes early in development. Prenatal stress and the associated maternal cortisol (Barbazanges et al., 1996) acts on the fetus via the placenta (Harris and Seckl, 2011, Cottrell and Seckl, 2009). While placental 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) serves as a functional barrier to protect the fetus from excessive exposure to high levels of maternal cortisol, this enzyme is less effective in anxious women Entringer et al., 2015; Buss et al., 2012;5 (Entringer et al., 2015, Buss et al., 2012.) and thus their offspring might be more affected by prenatal stress. Elevated levels of maternal cortisol affect the neurogenesis, synaptogenesis, and growth of axons and dendrites in the fetal brain (Moisiadis and Matthews, 2014, Matthews, 2000). The sex-specific response to prenatal stress might then be related to greater vulnerability of the male fetus to changes in maternal environment and particularly to greater vulnerability of the male fetus to stress due to sex differences in epigenetic placental gene regulation (Mueller and Bale, 2008). Mueller and Bale demonstrated that male placenta exhibited lower expression of DNMT1, the enzyme responsible for methylation maintenance, than female placenta, possibly indicating that males are less able to circumvent the effects of stress by strengthening the maintenance of normal DNA methylation patterns (Mueller and Bale, 2008). An independent line of research also reported sex differences in placental glucocorticoid receptor functioning (Saif et al., 2015, Teicher et al., 2006) and sex differences in timing of glucocorticoid receptor expression in the fetal brain (Owen and Matthews, 2003). These mechanisms might lead to sex differences in fetal glucocorticoid exposure and associated phenotypic alterations such as the amygdala’s structural coupling patterns.
The effects of high stress on lower amygdala degree in all participants, and in men in particular, as well as the consistent trends for the effect of high stress on lower global network transitivity in women appeared during the first but not the second half of pregnancy. These findings suggest that structural covariance patterns of the brain are particularly vulnerable to stress during the first half of pregnancy. This is consistent with literature pointing out that the timing of early life stress exposure is critical (Teicher et al., 2006, Teicher et al., 2006) and further research demonstrating that stress experienced during the first half of pregnancy has particularly strong and long-lasting impact (Yong Ping et al., 2015). Buss et al (Buss et al., 2012) demonstrated that variations in maternal cortisol in early gestation may produce larger variations in fetal cortisol then the same degree of variation in maternal cortisol in later gestation, when the fetal adrenal is active in terms of de novo cortisol production and feedback regulation. Further research also demonstrated that the expression level of genes supporting cell proliferation and neuron differentiation is higher during the early fetal developmental stage than at any other period in prenatal or postnatal life, and genes sustaining gliogenesis are also being robustly expressed in the amygdala (Kang et al., 2011).
Consistent with our previous research in this cohort, which reported a relationship between exposure to prenatal stress and experience of more mood disturbance in young adulthood (Teicher et al., 2006), the current study also showed that the high stress group, which had lower amygdala network degree, experienced more mood disturbance in young adulthood. Although SCN analysis provides a single value per group of participants, hence precluding parametric association between SCN properties and symptoms on the individual level, the group-level differences we observed are in line with research in clinical populations which described dysregulated circuit including amygdala and hippocampus in major depressive disorder (Hariri and Holmes, 2015).
Our findings regarding the effect of group on amygdala degree were also partially mirrored by the group-specific covariance patterns observed in three amygdala nuclei – basal nucleus, central nucleus and corticoamygdaloid transition area. Similarly to the amygdala degree analyses, the amygdala nuclei analyses showed stress-related loss of structural coupling between amygdala subregions and the hippocampus, putamen, and transverse temporal cortex. But novel patterns of covariance, indicating additional alterations in “connectivity”, were also observed. Taken together, these results suggest a stress-related loss of structural coupling within the amygdala itself and between amygdala subregions and various cortical and subcortical structures, as observed in the main analyses.
Even though the independent sample of young adult men from the Avon Longitudinal Study of Parents and Children (ALSPAC) also showed nominally lower values of amygdala degree in the high (vs. low) stress group at most of the density thresholds tested, these effects did not reach significance at any single threshold. Given the literature on changes in structural covariance in young adulthood (e.g. increases until 21 years followed by decreases until 30 years) (Aboud et al., 2019), one possible reason for this discrepancy may be the fact that participants from the ALSPAC cohort were younger at the time of scan (Sierra-Mercado et al., 2011, McEwen, 2007, Janak and Tye, 2015) than the young adults from ELSPAC (Tottenham and Sheridan, 2009, Humphrey, 1968) and thus the amygdala networks might not have been fully mature yet. Another possible reason might be the fact that the prenatal stress experienced by the ALSPAC cohort was lower than the prenatal stress experienced by the ELSPAC/VULDE cohort. Even though we attempted to account for this by splitting the ALSPAC groups based on the ELSPAC/VULDE-based median value, it is still possible that stress-related changes in SCN properties were less detectable in ALSPAC due to overall lower stress exposure of the cohort.
Our study has several limitations. First, the lack of network metrics for individuals does not allow us to calculate a standard measure of effect size or to determine whether amygdala degree might mediate the relationship between prenatal stress and more mood disturbance in young adulthood. Second, the structural covariance analysis does not allow us to calculate interactions with the group or sex and thus we can provide only group-specific analyses in the whole-sample and sex-specific analyses. Third, the sample size for the sex-specific analyses was relatively small and sex-specific findings should be replicated in future studies with larger sample size. Such future research should also correct for variables which might have influenced structural covariance during childhood and adolescence, such as parental behavior. Future studies might also extend the structural covariance findings to other neuroimaging modalities and clarify whether the sex- and time-specific effects of prenatal stress on structural covariance might also manifest in differences in functional connectivity and behavioral outcomes. Similarly, while we did not find any significant relationships between maternal stress during pregnancy and global network parameters, future research might explore the associations between prenatal stress and structural covariance network properties of other stress-related regions.
Overall, we demonstrated that prenatal stress exposure during the first half of pregnancy is associated with lower amygdala structural covariance degree in young adulthood. As our non-clinical data likely capture early stages of mood and anxiety pathology or risk thereof, our findings might facilitate the development of methods for early identification of vulnerable individuals, as well as targeted early intervention and prevention.
CRediT authorship contribution statement
Klara Mareckova: Investigation, Formal analysis, Visualization, Funding acquisition, Writing – original draft. Amy Miles: Formal analysis, Methodology, Software, Visualization. Zhijie Liao: Formal analysis, Software. Lenka Andryskova: Resources. Milan Brazdil: Funding acquisition. Tomas Paus: Resources, Supervision. Yuliya S. Nikolova: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the European Union (Marie Curie Intra-European Fellowship for Career Development, FP7-PEOPLE-IEF-2013, grant #6485124), the Czech Health Research Council (NU20J-04-00022), and the Czech Ministry of Education, Youth and Sports/MEYS CR (CZ.02.1.01/0.0/0.0/18_046/0015975; CEITEC 2020, LQ1601, LM2018121). We also acknowledge the core facility MAFIL of CEITEC MU supported by the Czech-BioImaging large RI project (LM2018129 funded by MEYS CR) for their support with obtaining scientific data presented in this paper. YSN is supported by a Koerner New Scientist Award and a Paul Garfinkel New Investigator Catalyst Award administered by the CAMH Foundation. The UK Medical Research Council and Wellcome Trust (Grant ref: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf). This research was specifically funded by Wellcome Trust and MRC (076467/Z/05/Z). This research was supported by a grant from the National Institutes of Health (R01MH085772 to T. Paus). This publication is the work of the authors and T. Paus will serve as guarantors for the contents of this paper and does not necessarily represent the official views of the National Institutes of Health. Z. Liao is supported by China Scholarship Council Award (201806380177). The UK Medical Research Council and Wellcome (Grant ref: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and Klara Mareckova, Amy Miles, Zhijie Liao, Lenka Andryskova, Milan Brazdil, Tomas Paus and Yuliya Nikolova will serve as guarantors for the contents of this paper. We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.102976.
Contributor Information
Klara Mareckova, Email: klara.mareckova@ceitec.muni.cz.
Yuliya S. Nikolova, Email: yuliya.nikolova@camh.ca.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
References
- Kessler R.C. The effects of stressful life events on depression. Annu. Rev. Psychol. 1997;48(1):191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM–5). Arlington, VA2013.
- Mareckova K., Klasnja A., Bencurova P., Andryskova L., Brazdil M., Paus T. Prenatal stress, mood, and gray matter volume in young adulthood. Cereb. Cortex. 2019;29(3):1244–1250. doi: 10.1093/cercor/bhy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I., Chicha L., Britschgi M., Schobel S.A., Bodmer M., Hellings J.A., Toovey S., Prinssen E.P. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014;10(11):643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- Wu Y., Limperopoulos C. Pregnancy stress, anxiety, and depression sequela on neonatal brain development-reply. JAMA Pediatr. 2020;174(9):908–909. doi: 10.1001/jamapediatrics.2020.1079. [DOI] [PubMed] [Google Scholar]
- Qiu A., Tuan T.A., Ong M.L., Li Y., Chen H., Rifkin-Graboi A., Broekman B.F.P., Kwek K., Saw S.-M., Chong Y.-S., Gluckman P.D., Fortier M.V., Holbrook J.D., Meaney M.J. COMT haplotypes modulate associations of antenatal maternal anxiety and neonatal cortical morphology. Am. J. Psychiatry. 2015;172(2):163–172. doi: 10.1176/appi.ajp.2014.14030313. [DOI] [PubMed] [Google Scholar]
- Qiu A., Shen M., Buss C., Chong Y.-S., Kwek K., Saw S.-M., Gluckman P.D., Wadhwa P.D., Entringer S., Styner M., Karnani N., Heim C.M., O'Donnell K.J., Holbrook J.D., Fortier M.V., Meaney M.J. Effects of antenatal maternal depressive symptoms and socio-economic status on neonatal brain development are modulated by genetic risk. Cereb. Cortex. 2017;27(5):3080–3092. doi: 10.1093/cercor/bhx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, A., Rifkin-Graboi, A., Chen, H., Chong, Y.S., Kwek, K., Gluckman, P.D., et al. 2013. Maternal anxiety and infants' hippocampal development: timing matters. Transl. Psychiatry 3, e306. [DOI] [PMC free article] [PubMed]
- Scheinost D., Kwon S.H., Lacadie C., Sze G., Sinha R., Constable R.T., Ment L.R. Prenatal stress alters amygdala functional connectivity in preterm neonates. Neuroimage Clin. 2016;12:381–388. doi: 10.1016/j.nicl.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walton M., Letourneau N., Giesbrecht G.F., Kaplan B.J., Dewey D. Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biol. Psychiatry. 2016;80(11):859–868. doi: 10.1016/j.biopsych.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Wen, D.J., Poh, J.S., Ni, S.N., Chong, Y.S., Chen, H., Kwek, K., et al. 2017. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl. Psychiatry. 7(4), e1103. [DOI] [PMC free article] [PubMed]
- Soe N.N., Wen D.J., Poh J.S., Chong Y.-S., Broekman B.F., Chen H., Shek L.P., Tan K.H., Gluckman P.D., Fortier M.V., Meaney M.J., Qiu A. Perinatal maternal depressive symptoms alter amygdala functional connectivity in girls. Hum. Brain Mapp. 2018;39(2):680–690. doi: 10.1002/hbm.23873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid G.A., Darcey V.L., Avalos M.F., Fishbein D.H., VanMeter J.W. Altered cortical structure and psychiatric symptom risk in adolescents exposed to maternal stress in utero: a retrospective investigation. Behav. Brain Res. 2019;375:112145. doi: 10.1016/j.bbr.2019.112145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro A., Tenconi E., Degortes D., Manara R., Santonastaso P. Neural correlates of prenatal stress in young women. Psychol. Med. 2015;45(12):2533–2543. doi: 10.1017/S003329171500046X. [DOI] [PubMed] [Google Scholar]
- Mareckova K., Miles A., Andryskova L., Brazdil M., Nikolova Y.S. Temporally and sex-specific effects of maternal perinatal stress on offspring cortical gyrification and mood in young adulthood. Hum. Brain Mapp. 2020;41(17):4866–4875. doi: 10.1002/hbm.25163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen P.J., Phelps E.A., editors. The human amygdala. The Guilford Press; 2009. [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23(1):155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D., Padilla-Coreano N., Quirk G.J. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36(2):529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Janak P.H., Tye K.M. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C., Davis E.P., Shahbaba B., Pruessner J.C., Head K., Sandman C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. USA. 2012;109(20):E1312–E1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., Parent S., Evans A.C., Tremblay R.E., Zelazo P.D., Corbo V., Pruessner J.C., Seguin J.R. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc. Natl. Acad. Sci. USA. 2011;108(34):14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Sheridan M.A. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey T. The development of the human amygdala during early embryonic life. J. Comp. Neurol. 1968;132(1):135–165. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Andersen S.L., Polcari A., Anderson C.M., Navalta C.P., Kim D.M. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003;27(1-2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Graham A.M., Rasmussen J.M., Entringer S., Ben Ward E., Rudolph M.D., Gilmore J.H., Styner M., Wadhwa P.D., Fair D.A., Buss C. Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol. Psychiatry. 2019;85(2):172–181. doi: 10.1016/j.biopsych.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salm A.K., Pavelko M., Krouse E.M., Webster W., Kraszpulski M., Birkle D.L. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Brain Res. Dev. Brain Res. 2004;148(2):159–167. doi: 10.1016/j.devbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Nikolić I., Kostović I. Development of the lateral amygdaloid nucleus in the human fetus: transient presence of discrete cytoarchitectonic units. Anat. Embryol. 1986;174(3):355–360. doi: 10.1007/BF00698785. [DOI] [PubMed] [Google Scholar]
- Mareckova K., Marecek R., Andryskova L., Brazdil M., Nikolova Y.S. Impact of prenatal stress on amygdala anatomy in young adulthood: timing and location matter. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2022;7(2):231–238. doi: 10.1016/j.bpsc.2021.07.009. [DOI] [PubMed] [Google Scholar]
- Jones S.L., Dufoix R., Laplante D.P., Elgbeili G., Patel R., Chakravarty M.M., King S., Pruessner J.C. Larger amygdala volume mediates the association between prenatal maternal stress and higher levels of externalizing behaviors: sex specific effects in project ice storm. Front. Hum. Neurosci. 2019;13 doi: 10.3389/fnhum.2019.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A.C. Networks of anatomical covariance. Neuroimage. 2013;80:489–504. doi: 10.1016/j.neuroimage.2013.05.054. [DOI] [PubMed] [Google Scholar]
- Bullmore E.d., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev.Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Yee Y., Fernandes D.J., French L., Ellegood J., Cahill L.S., Vousden D.A., Spencer Noakes L., Scholz J., van Eede M.C., Nieman B.J., Sled J.G., Lerch J.P. Structural covariance of brain region volumes is associated with both structural connectivity and transcriptomic similarity. Neuroimage. 2018;179:357–372. doi: 10.1016/j.neuroimage.2018.05.028. [DOI] [PubMed] [Google Scholar]
- Kelly C., Toro R., Di Martino A., Cox C.L., Bellec P., Castellanos F.X., Milham M.P. A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage. 2012;61(4):1129–1142. doi: 10.1016/j.neuroimage.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G., He Y., Chen Z.J., Evans A.C. Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex. Neuroimage. 2012;59(2):1239–1248. doi: 10.1016/j.neuroimage.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Lerch J., Lee N., Greenstein D., Wallace G., Stockman M., Clasen L., Shaw P., Giedd J. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72(5):873–884. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L., Meyer-Lindenberg A., Goldman A.L., Verchinski B.A., Chen G., Kolachana B.S., Egan M.F., Mattay V.S., Hariri A.R., Weinberger D.R. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Mol. Psychiatry. 2008;13(7):709–716. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- Schmitt J.E., Yi J., Calkins M.E., Ruparel K., Roalf D.R., Cassidy A., Souders M.C., Satterthwaite T.D., McDonald-McGinn D.M., Zackai E.H., Gur R.C., Emanuel B.S., Gur R.E. Disrupted anatomic networks in the 22q11.2 deletion syndrome. Neuroimage Clin. 2016;12:420–428. doi: 10.1016/j.nicl.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Schmitt J.E., Knickmeyer R.C., Smith J.K., Lin W., Styner M., Gerig G., Neale M.C. Genetic and environmental contributions to neonatal brain structure: a twin study. Hum. Brain Mapp. 2010;(8):1174–1182. doi: 10.1002/hbm.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss P., Zatorre R.J. Early visual deprivation changes cortical anatomical covariance in dorsal-stream structures. Neuroimage. 2015;108:194–202. doi: 10.1016/j.neuroimage.2014.12.063. [DOI] [PubMed] [Google Scholar]
- Sun D., Peverill M.R., Swanson C.S., McLaughlin K.A., Morey R.A. Structural covariance network centrality in maltreated youth with posttraumatic stress disorder. J. Psychiatr. Res. 2018;98:70–77. doi: 10.1016/j.jpsychires.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Anderson C.M., Ohashi K., Polcari A. Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biol. Psychiatry. 2014;76(4):297–305. doi: 10.1016/j.biopsych.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova Y.S., Misquitta K.A., Rocco B.R., Prevot T.D., Knodt A.R., Ellegood J., Voineskos A.N., Lerch J.P., Hariri A.R., Sibille E., Banasr M. Shifting priorities: highly conserved behavioral and brain network adaptations to chronic stress across species. Transl. Psychiatry. 2018;8(1) doi: 10.1038/s41398-017-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Poh J.S., Wen D.J., Guillaume B., Chong Y.-S., Shek L.P., Fortier M.V., Qiu A. Long-term influences of prenatal maternal depressive symptoms on the amygdala-prefrontal circuitry of the offspring from birth to early childhood. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2019;4(11):940–947. doi: 10.1016/j.bpsc.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Sporns O. The non-random brain: efficiency, economy, and complex dynamics. Front. Comput. Neurosci. 2011;5:5. doi: 10.3389/fncom.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland S., Brunwasser S.M. Sex differences in vulnerability to prenatal stress: a review of the recent literature. Curr. Psychiatry Rep. 2018;20(11):102. doi: 10.1007/s11920-018-0961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok A.N.V., Salimi-Khorshidi G., Lai M.-C., Baron-Cohen S., Lombardo M.V., Tait R.J., Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class Q.A., Lichtenstein P., Långström N., D'Onofrio B.M. Timing of prenatal maternal exposure to severe life events and adverse pregnancy outcomes: a population study of 2.6 million pregnancies. Psychosom. Med. 2011;73(3):234–241. doi: 10.1097/PSY.0b013e31820a62ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piler, P., Kandrnal, V., Kukla, L., Andryskova, L., Svancara, J., Jarkovsky, J., et al. 2017. Cohort profile: the European longitudinal study of pregnancy and childhood (ELSPAC) in the Czech Republic. Int. J. Epidemiol. 46(5), 1379-f. [DOI] [PMC free article] [PubMed]
- McNair D.M., Lorr M., Droppleman L.F. Educational and Industrial Testing Services; San Diego, CA: 1971. Manual for the Profile of Mood States. [Google Scholar]
- Boyd, A., Golding, J., Macleod, J., Lawlor, D.A., Fraser, A., Henderson, J., et al. Cohort profile: the ‘Children of the 90s’—the index offspring of the Avon longitudinal study of parents and children. Int. J. Epidemiol. 422013. p. 111-27. [DOI] [PMC free article] [PubMed]
- Fraser A., Macdonald-Wallis C., Tilling K., Boyd A., Golding J., Davey Smith G., Henderson J., Macleod J., Molloy L., Ness A., Ring S., Nelson S.M., Lawlor D.A. Cohort profile: the avon longitudinal study of parents and children: ALSPAC mothers cohort. Int. J. Epidemiol. 2013;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northstone K., Lewcock M., Groom A., Boyd A., Macleod J., Timpson N., Wells N. The Avon Longitudinal Study of Parents and Children (ALSPAC): an update on the enrolled sample of index children in 2019. Wellcome Open Res. 2019;4:51. doi: 10.12688/wellcomeopenres.15132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S., Fornito A. The development of brain network hubs. Dev Cogn Neurosci. 2019;36:100607. doi: 10.1016/j.dcn.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C., Mechelli A., Herrera A., Walshe M., Shergill S.S., Murray R.M., Rifkin L., Allin M.P.G. Structural covariance in the cortex of very preterm adolescents: a voxel-based morphometry study. Hum. Brain Mapp. 2011;32(10):1615–1625. doi: 10.1002/hbm.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Toro R., Leonard G., Lerner J.V., Lerner R.M., Perron M., Pike G.B., Richer L., Steinberg L. Morphological properties of the action-observation cortical network in adolescents with low and high resistance to peer influence. Soc. Neurosci. 2008;3(3-4):303–316. doi: 10.1080/17470910701563558. [DOI] [PubMed] [Google Scholar]
- Barbazanges A., Piazza P.V., Le Moal M., Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J. Neurosci. 1996;16(12):3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A., Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm. Behav. 2011;59(3):279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Cottrell E.C., Seckl J.R. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S., Buss C., Wadhwa P.D. Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology. 2015;62:366–375. doi: 10.1016/j.psyneuen.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C., Entringer S., Wadhwa P.D. Fetal programming of brain development: intrauterine stress and susceptibility to psychopathology. Sci Signal. 2012;5(245):pt7. doi: 10.1126/scisignal.2003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisiadis V.G., Matthews S.G. Glucocorticoids and fetal programming part 2: mechanisms. Nat. Rev. Endocrinol. 2014;10(7):403–411. doi: 10.1038/nrendo.2014.74. [DOI] [PubMed] [Google Scholar]
- Matthews S.G. Antenatal glucocorticoids and programming of the developing CNS. Pediatr Res. 2000;47(3):291–300. doi: 10.1203/00006450-200003000-00003. [DOI] [PubMed] [Google Scholar]
- Mueller B.R., Bale T.L. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 2008;28(36):9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif Z., Hodyl N.A., Stark M.J., Fuller P.J., Cole T., Lu N., Clifton V.L. Expression of eight glucocorticoid receptor isoforms in the human preterm placenta vary with fetal sex and birthweight. Placenta. 2015;36(7):723–730. doi: 10.1016/j.placenta.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Polcari A., McGreenery C.E. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am. J. Psychiatry. 2006;163(6):993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- Owen D., Matthews S.G. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144(7):2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Tomoda A., Andersen S.L. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann. N. Y. Acad. Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- Yong Ping E., Laplante D.P., Elgbeili G., Hillerer K.M., Brunet A., O'Hara M.W., et al. Prenatal maternal stress predicts stress reactivity at 2(1/2) years of age: the Iowa Flood Study. Psychoneuroendocrinology. 2015;56:62–78. doi: 10.1016/j.psyneuen.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Kang H.J., Kawasawa Y.I., Cheng F., Zhu Y., Xu X., Li M., Sousa A.M.M., Pletikos M., Meyer K.A., Sedmak G., Guennel T., Shin Y., Johnson M.B., Krsnik Ž., Mayer S., Fertuzinhos S., Umlauf S., Lisgo S.N., Vortmeyer A., Weinberger D.R., Mane S., Hyde T.M., Huttner A., Reimers M., Kleinman J.E., Šestan N. Spatio-temporal transcriptome of the human brain. Nature. 2011;478(7370):483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Holmes A. Finding translation in stress research. Nat. Neurosci. 2015;18(10):1347–1352. doi: 10.1038/nn.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboud K.S., Huo Y., Kang H., Ealey A., Resnick S.M., Landman B.A., Cutting L.E. Structural covariance across the lifespan: Brain development and aging through the lens of inter-network relationships. Hum. Brain Mapp. 2019;40(1):125–136. doi: 10.1002/hbm.24359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.