ABSTRACT
Objectives:
This study investigated the effect of early mobilization [EM; physical rehabilitation with the intensity needed to sit on the edge of the bed started within 5 days of intensive care unit (ICU) admission] in relation to improvements in gait independence and other clinical outcomes.
Methods:
This retrospective single-center study evaluated patients aged at least 18 years who stayed in the ICU for at least 48 h and were categorized into EM and late mobilization (LM; physical rehabilitation started more than 5 days after ICU admission) groups. Outcomes were compared after adjusting for 20 background factors by propensity score matching and inverse probability of treatment weighting. The primary outcome was independent gait at discharge. The secondary outcomes were medical costs, 90-day survival, and durations of ICU and hospital stays.
Results:
Of 177 patients, 85 and 92 were enrolled in the EM and LM groups, respectively. Propensity score matching created 37 patient pairs. There was no significant difference in the 90-day survival rate (P=0.308) or medical costs (P=0.054), whereas independent gait at discharge (P=0.025) and duration of hospital stay (P=0.013) differed significantly. Multivariate logistic regression analysis showed that EM was independently associated with independent gait at discharge (P=0.011) and duration of hospital stay (P=0.010) but was not associated with 90-day survival (odds ratio: 2.64, 95% confidence interval: 0.67–13.12, P=0.169).
Conclusions:
Early mobilization in the ICU did not affect 90-day survival and did not lower medical costs but was associated with independent gait at discharge and shorter hospital stays.
Keywords: early mobilization, gait independence, medical cost, survival
INTRODUCTION
Patients who undergo mechanical ventilation or sedation in the intensive care unit (ICU) may require long-term rehabilitation because they develop post-intensive care syndrome (PICS).1) PICS is a collection of symptoms that persist even after successful discharge from the ICU. Patients with PICS may have new or worsening cognitive, emotional, and physical symptoms.1,2,3)
Patients receiving mechanical ventilation in the ICU may require long-term rehabilitation if they develop severe physical impairments such as abnormal gait even after they have recovered from their acute illness and are discharged from the ICU.1,2) Gait disability after discharge from the ICU occurs in 40%–70% of ICU survivors1,2,3,4) and may last for several months or years after hospital discharge.5)
Early mobilization (EM) is recommended to address these issues and improve functional prognosis.6) Previous studies have indicated that initiating EM soon after ICU admission could reduce the incidences of ICU-acquired weakness (ICU-AW) and delirium,7,8) lengths of ICU and hospital stay,8,9) duration of mechanical ventilation,8) and medical costs.10,11) Although many reports have indicated that EM improves functional outcomes among ICU patients, there is no clear information regarding the optimal timing and targets for EM. Furthermore, the definition of “early” in the literature varies from 2 to 7 days after admission. Moreover, consensus and clarification from Japanese experts are lacking,12) making inter-study comparisons difficult.13,14) Morris et al.9) reported that mobilization within 5 days of admission was associated with shorter ICU and hospital stays, although this study did not adequately evaluate physical function such as gait independence. Schweickert et al. applied EM and interruption of sedation within 72 h of ICU admission and reported higher independent functionality at hospital discharge.8) In contrast, initiating mobilization later, such as after 1 week of ICU admission, exhibited no beneficial effect.15) The Extra Physiotherapy in Critical Care (EPICC) trial conducted on ICU patients reported that EM in the ICU showed no difference in physical outcomes after 6 months relative to standard care.16) In contrast, some of the studies conducted in recent years have not reported positive outcomes in relation to EM.17,18) This is because the subjects, content of the intervention, intervention duration, start time, and initiation criteria are different in each study. Whereas previous studies have recommended out-of-bed mobilization as soon as possible, there are few reports with a clear start time.19) Similarly, few reports have described the intensity of rehabilitation that should be achieved in the ICU. The purpose of early rehabilitation in ICU patients is to promote physical activity and improve muscle strength and walking ability as soon as possible. As such, a unified early rehabilitation program should be established to allow the efficacy of mobilization to be determined.20) To optimize the outcomes of ICU patients, there is significant need for a defined rehabilitation start time and intensity that could be used as indicators of the efficacy of EM in the ICU, thereby allowing the collection of a body of evidence for the Japanese population.
In this study, it was hypothesized that EM in the ICU would improve outcomes. Here, the term “mobilization” indicates restoring strength (rehabilitation) by sitting on the edge of the bed, and “EM” indicates that the time to achieve mobilization is within 5 days of ICU admission. The Intensive Care Rehabilitation Expert Consensus in Japan recommends that mobilization be started within 5 days of ICU admission.12) These definitions are based on previous studies.13,14,20,21,22,23) This single-center retrospective cohort study aimed to investigate the association between EM in the ICU and clinical outcomes.
MATERIALS AND METHODS
Study Design
This study was a secondary analysis of the dataset collected in a previous study that investigated daily differences in the barriers to, and implementation status of, EM in the first week of ICU stay.21) All patients admitted to the ICU from January 2016 to March 2019 were screened. Patients were excluded if they were discharged within 48 h, were less than 18 years old, were unable to walk independently before hospitalization, were neurologically impaired, had difficulty communicating, had mobility-limiting conditions (e.g., unstable pelvic fractures), were considered terminal or at the end of life, or died during the ICU stay; all other patients were included. The included patients were categorized into two groups: the EM group, which was composed of patients who improved their strength by sitting on the bedside within the first 5 days of ICU admission, and the late mobilization (LM) group, which was composed of patients who could not achieve EM.
The EM Protocol
The following items were assessed based on the existing literature: problems in the ICU related to implementation of the EM strategy, the availability of effective evaluation systems and protocols, the presence of discontinuation criteria, and the occurrence of potential adverse events.8,9,10,16) The EM protocol was an early goal-directed protocol that included five levels and was not revised during the study period. Tables 1 and 2 show the EM protocol, including the initiation and discontinuation criteria.20) The final decision on whether mobilization could be provided was made by the clinician, depending on each patient’s condition. Table 2 shows the important routine care parameters monitored in the ICU, which remained the same during the study period: these parameters included pain management, sedation, delirium management, and weaning from mechanical ventilation. In addition, each doctor and physiotherapist involved in implementing the EM protocol in this study had more than 10 years of experience in the ICU; therefore, there was no change in the person-in-charge during the study period.
Table 1. Early mobilization protocol of the Nagoya Medical Center.
|
Level 1 Respiratory
RASS −5 to −3 |
Level 2 HOB
RASS ≥ −3 |
Level 3 Sitting
RASS ≥ −1 |
Level 4 Standing
RASS ≥0 |
Level 5 Walking
RASS ≥0 |
|
Physical therapy
□Passive ROM exercise □Respiratory physical therapy |
Physical therapy
□Positioning □Passive ROM exercise □Active ROM exercise □Respiratory physical therapy □Continuous lateral rotation therapy |
Physical therapy
□Positioning □Passive ROM exercise □Active ROM exercise □Sitting at side of bed □Rising from the supine position |
Physical therapy
□Positioning □Passive ROM exercise □Active ROM exercise □Standing at side of bed □Stand and pivot to a chair |
Physical therapy
□Positioning □Passive ROM exercise □Active ROM exercise □Walk with assistance □Walk independently |
|
Positioning
□Posture change □HOB ≤45° |
Positioning
□Posture change □HOB ≥60° |
Positioning
□Posture change □HOB ≥60° |
Positioning
□Posture change □HOB ≥60° |
Positioning
□Posture change □HOB ≥60° |
|
Step up criteria
□Oxygenation/ hemodynamic stability □Can withstand posture change □Can withstand HOB ≤45° |
Step up criteria
□Can withstand supplementary motion of physical therapy □Can withstand HOB ≤60° □Anti-gravity movement possible |
Step up criteria
□Can endure the active movement of physical therapy □Can withstand HOB ≤60° □Can withstand sitting at side of bed |
Step up criteria
□All exercise can be carried out □Can withstand partial weight standing |
Step up criterion
□Increase walking distance gradually |
|
Step up criterion to level 3 or higher
are defined as
□RASS: −2 to +1, □BPS ≤3 or NRS ≤5, □SpO2 ≥90%, □FIO2 <0.6, □PEEP <10 cmH2O, □Respiratory rate: <35 times/min, □Mean blood pressure ≥65 mmHg, □Heart rate: 50 to 120 beats/min, □There were no new arrhythmias, □No additional administration of vasopressors, □No bleeding, no wound with the possibility of separation, □No unstable fracture. | ||||
ROM, range of motion; HOB, head of bed; BPS, behavioral pain scale; NRS, numeric rating scale; PEEP, positive end expiratory pressure.
The EM protocol includes 5 levels: Level 1: head of bed elevation ≤45° and passive ROM; Level 2: head of bed elevation ≥60°, active ROM, and continuous lateral rotation therapy; Level 3: sitting on the side of the bed and rising from the supine position; Level 4: standing at the side of the bed, and standing and pivoting to a chair; and Level 5: walking with assistance and walking independently.
From Watanabe et al.21)
Table 2. Daily care and background of research in the Nagoya Medical Center ICU.
| ICU staff | |
| Nurses | Nurse-to-patient ratio is 1:2 |
| Doctors | Doctor-to-patient ratio is 1:2 (1–3) |
| Rehabilitation therapists | One full-time physiotherapist (15 years of experience) and one half-time speech therapist (10 years of experience) |
| Analgesia | ICU doctors use NRS and BPS to assess pain and adjust the dose of analgesics |
| Sedation | ICU doctors assess RASS and prescribe sedatives and analgesics based on the assessment |
| Agitation and delirium | ICU doctors prescribe or adjust sedatives or antipsychotics based on the assessment of delirium |
| Mechanical ventilation | No specific ventilation protocols. ICU physicians adjust the mode or settings based on patient condition |
ICDSC, intensive care delirium screening checklist.
Nagoya Medical Center is a tertiary care hospital with a 740-bed general hospital and a 6-bed mixed ICU in which the role of ICU physicians is to provide mandatory consultation at all ICU admissions. The admission route to the ICU is from the emergency room and hospital wards. All admissions to the ICU from the emergency room are the result of unscheduled urgent serious illness, and admission from the hospital ward is the result of a postoperative or unscheduled emergency that occurs in the ward. On the dayshift, ICU staff includes ICU physicians (three intensivists, one junior resident) and nurses (including one nurse certified in critical care), a physical therapist, a speech therapist, a pharmacist, and a dietitian.
After ICU discharge or in the general ward, all patients completed personalized rehabilitation protocols defined by physical or occupational therapists. Rehabilitation protocols in the general ward included muscle strengthening exercises, balance exercises, walking exercises, and stair training for 20 min/day on weekdays.
Data Collection
Patients’ electronic medical records were searched to collect relevant data. Baseline characteristics recorded on ICU admission included age; sex; body mass index; Charlson Comorbidity Index24); admission source; diagnosis at ICU admission; Acute Physiology and Chronic Health Evaluation II (APACHE II) score; Sequential Organ Failure Assessment (SOFA) score; and the use of mechanical ventilation, vasopressors, continuous sedation, continuous analgesia, corticosteroids, neuromuscular blocking agents, and/or dialysis. The average Richmond Agitation Sedation Scale (RASS) score was recorded by the nurse every 2 h during days 1–5 in the ICU. Data regarding medical costs and discharge destination were collected from the medical affairs department. Medical costs were calculated based on the diagnosis procedure combination/per-diem payment system and were converted from Japanese yen to US dollars at an exchange rate of 108 yen/dollar.25)
Study Outcomes
The primary outcome was gait independence at hospital discharge. The secondary outcomes included medical costs, the survival rate at 90 days after ICU discharge, duration of mechanical ventilation, duration of ICU and hospital stays, discharge destination (home, rehabilitation center, another hospital, nursing home, or death), delirium during the ICU stay, nosocomial pneumonia during the hospital stay, and ICU-AW status at ICU discharge. Other outcomes included ICU rehabilitation parameters (time to first rehabilitation and mobilization, number of total and daily rehabilitation exercises, and highest mobility score in the ICU) and ward rehabilitation parameters (number of total and daily rehabilitation exercises). The ICU mobility scale (IMS) is a sensitive 11-point ordinal scale with scores ranging from 0 (no mobilization) to 10 (independent ambulation).26) At the time of ICU discharge, physiotherapists assessed the patient’s muscle strength by using the Medical Research Council sum score to determine whether ICU-AW was present (full strength: 60/60 points, ICU-AW: <48/60 points).19)
Statistical Analysis
The EM and LM groups were compared to identify differences in baseline characteristics, clinical and economic outcomes, and mobilization outcomes. Continuous variables were compared using the Mann–Whitney U test, and categorical variables were compared using the χ2 test. Propensity score matching was applied to reduce the influence of 20 potential confounding factors11,21,27,28) that were expected to influence the outcomes, as per the current literature (Table 3). Logistic regression analysis was used to calculate the propensity scores, and 1:1 pair-matching (nearest-neighbor matching) was performed using a greedy matching technique and a caliper width of 0.2. Standardized differences were used to measure covariate balances, and a meaningful imbalance was considered at values of >10%. In sensitivity analysis, the inverse probability of treatment weighting (IPTW) method based on propensity scores was applied to the outcome factors that were associated with EM.29) By using the estimated propensity scores to construct data weights, we were able to adjust for confounding factors between the binary groups; this approach facilitated an evaluation of causal effects without reducing the sample size. Variables were modeled as continuous data when appropriate or were dichotomized using clinically relevant cutoff values. Univariate logistic regression analysis was performed based on the propensity score matching and IPTW odds ratios (ORs) and confidence intervals (CIs).
Table 3. Comparison of items related to baseline data.
| Total population | Matched population | |||||||
| Early mobilization |
Late mobilization |
SD | P | Early mobilization |
Late mobilization |
SD | P | |
| Baseline characteristics | n=85 | n=92 | n=37 | n=37 | ||||
| Age (years) | 69 [60–78] | 70 [63–79] | 0.109 | 0.680 | 71 [64–80] | 69 [64–79] | 0.042 | 0.492 |
| Male, n (%) | 57 (67) | 64 (70) | 0.054 | 0.720 | 25 (68) | 26 (70) | 0.058 | 0.802 |
| Body mass index (kg/m2) | 21 [18–24] | 21 [17–24] | 0.070 | 0.458 | 21 [18–24] | 20 [18–25] | 0.006 | 0.721 |
| Charlson comorbidity index | 2 [1–4] | 2 [1–4] | 0.038 | 0.684 | 2 [1–3] | 2 [1–3] | 0.071 | 0.745 |
| Admission source, n (%) | ||||||||
| Emergency department | 61 (72) | 68 (74) | 0.024 | 0.748 | 30 (81) | 31 (84) | 0.071 | 1.000 |
| Hospital ward | 24 (28) | 24 (26) | 7 (19) | 6 (16) | ||||
| ICU admission diagnosis, n (%) | ||||||||
| Respiratory, including pneumonia | 25 (29) | 20 (22) | 0.177 | 0.242 | 10 (27) | 10 (27) | 0.000 | 1.000 |
| Cardiovascular | 18 (21) | 19 (21) | 0.013 | 0.932 | 9 (24) | 12 (32) | 0.181 | 0.439 |
| Gastrointestinal | 20 (24) | 14 (15) | 0.211 | 0.161 | 5 (14) | 4 (11) | 0.083 | 1.000 |
| Trauma | 4 (5) | 3 (3) | 0.074 | 0.712 | 2 (5) | 2 (5) | 0.000 | 1.000 |
| Sepsis, non-pulmonary | 7 (8) | 19 (21) | 0.359 | 0.020 | 6 (16) | 5 (14) | 0.076 | 1.000 |
| Others | 11 (13) | 17 (18) | 0.153 | 0.313 | 5 (14) | 4 (11) | 0.083 | 1.000 |
| APACHE II score | 19 [15–26] | 22 [17–30] | 0.481 | 0.008 | 23 [18–26] | 21 [15–27] | 0.007 | 0.478 |
| SOFA score at ICU admission | 6 [4–8] | 8 [6–10] | 0.831 | <0.0001 | 7 [6–9] | 7 [5–10] | 0.027 | 0.952 |
| Patients receiving mechanical ventilation (%) | 55 (65) | 73 (79) | 0.331 | 0.030 | 26 (70) | 26 (70) | 0.000 | 1.000 |
| Patients receiving continuous vasopressor (%) | 46 (54) | 68 (74) | 0.421 | 0.006 | 24 (65) | 24 (65) | 0.000 | 1.000 |
| Patients receiving continuous sedation (%) | 63 (74) | 80 (87) | 0.329 | 0.030 | 28 (76) | 29 (78) | 0.064 | 0.782 |
| RASS score from day 1 to 5 | −1 [−2 to 0] | −3 [−4 to 1] | 0.122 | <0.0001 | −2[−3 to 0] | −1 [−3 to 0] | 0.013 | 0.987 |
| Patients receiving continuous analgesia (fentanyl), n (%) | 57 (67) | 74 (80) | 0.307 | 0.043 | 28 (76) | 28 (76) | 0.000 | 1.000 |
| Patients receiving steroids, n (%) | 27 (32) | 32 (35) | 0.064 | 0.671 | 11 (30) | 9 (24) | 0.122 | 0.794 |
| Patients receiving neuromuscular blocking agent, n (%) | 10 (12) | 16 (17) | 0.160 | 0.291 | 3 (8) | 6 (16) | 0.250 | 0.479 |
| Patients receiving dialysis (%) | 15 (18) | 33 (36) | 0.421 | 0.006 | 7 (19) | 7 (19) | 0.067 | 1.000 |
Data are presented as median [interquartile range] or number (%).
Analysis by independent-sample Mann–Whitney U-test or χ2 test.
Furthermore, a sub-analysis was performed to investigate the effectiveness of EM in patients who stayed in the ICU for 5 days and more. Multiple logistic regression analysis was performed to determine the primary outcome with covariates, including admission source, APACHE II score, SOFA score, continuous vasopressor use, and RASS score from days 1 to 5, which were considered factors related to the primary and secondary outcome in previous reports.11,27,28,30,31,32) For the sub-analysis of secondary outcomes and other outcomes, multiple linear and logistic regression analyses were performed for log-transformed continuous and categorical variables, respectively; the covariates used for the secondary and other outcomes were the same as those used in analyzing the primary outcome. The sub-analysis was conducted to compare patients who fulfilled the EM initiation criteria but had delayed mobilization with those who achieved EM. To analyze only patients who satisfied the EM initiation criteria, multiple linear and logistic regression analyses, adjusted for the same covariates, were performed after excluding patients in the LM group who did not meet the criteria for mobilization by day 5 of admission to the ICU.
By changing the definition of “early mobilization” into mobilization within 3, 4, 6, or 7 days of ICU admission, we employed the same method (univariate logistic regression analysis after propensity score matching) as the primary analysis to investigate the impact on outcomes of the differences in the definition of “early mobilization”.
All analyses were performed using JMP (version 13.0; SAS Institute, Cary, NC, USA) and IBM SPSS software (version 23.0; IBM, Armonk, NY, USA), and the differences were considered statistically significant at two-sided P-values of <0.05.
Ethics and Consent
The study was conducted after receiving approval from the Institutional Review Board (IRB) at Nagoya Medical Center Hospital (IRB approval number 2019–78). All data were de-identified to protect the confidentiality of the personal information. The study qualified for exempt status according to the IRB because the data were collected from existing patient records. Therefore, the need for patient consent was waived.
RESULTS
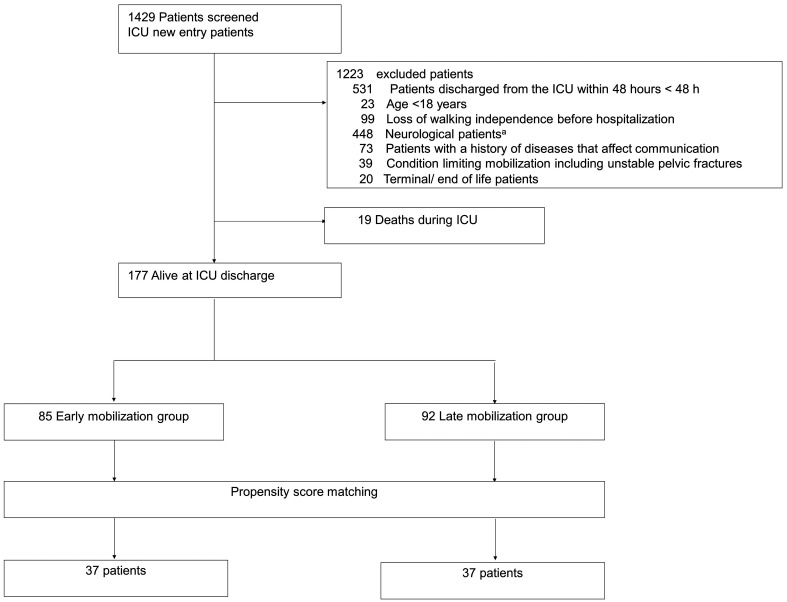
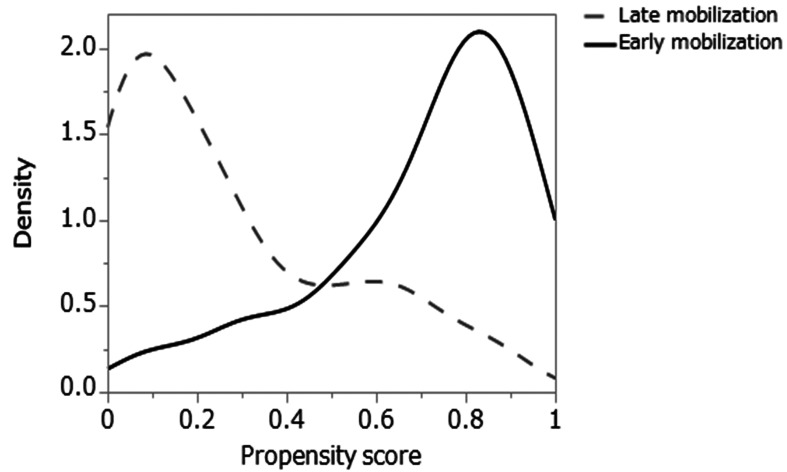
During the study period (January 2016 to March 2019), the ICU admitted 1429 patients, of which 177 patients were eligible for this study (Fig. 1). However, considering the exclusion criteria, 19 patients who died during the ICU stay were excluded. Before the propensity score matching, the LM group had significantly lower values for the ICU admission diagnosis (sepsis) (P=0.020), APACHE II score (P=0.008), SOFA score (P <0.001), mechanical ventilation use (P=0.030), continuous vasopressor use (P=0.006), continuous sedation (P=0.030), analgesia use (P=0.043), and median RASS score during days 1–5 (P <0.001) (Table 3). The propensity scores were calculated using logistic regression analysis that was adjusted for 20 background factors; this produced 37 pairs of patients from the EM and LM groups (Fig. 2).
Fig. 1.
Flowchart of patient selection process. aNeurological diseases (in excluded patients) included cerebral infarction, cerebral hemorrhage, acute subdural hematoma, acute epidural hematoma, traumatic subarachnoid hemorrhage, and encephalitis.
Fig. 2.
Data distribution before and after propensity score matching for early mobilization (EM) and late mobilization (LM). The propensity scores before matching: EM, 0.701 ± 0.237; LM, 0.276 ± 0.268; propensity scores after matching: EM, 0.515 ± 0.237; LM, 0.512 ± 0237.
After the matching, the two groups had very similar propensity scores (EM: 0.515 ± 0.237, LM: 0.512 ± 0.237), and no significant difference was observed in the baseline characteristics (Table 3). Patient background factors generally had a standard deviation (SD) of <0.1; however, intraoperative admission diagnosis, cardiovascular complications, and continuous administration of steroids and neuromuscular blocking agents had an SD >0.1.
Gait independence at discharge was significantly different between the EM (89%) and LM (65%) groups (P=0.025). Medical costs in the EM group were approximately 30% lower than those in the LM group; however, no significant difference was observed [median: 25th–75th percentile; USD 16,773 (range: USD 10,769–27,852) vs. USD 23,895 (range: USD 15,100–29,277); P=0.054]. The EM group had significantly shorter hospital stays (P=0.013) and significantly lower incidence of nosocomial pneumonia (P=0.024). There were no significant differences in terms of the 90-day survival rate (P=0.308), the duration of mechanical ventilation (P=0.708), length of ICU stay (P=0.584), discharge destination (P=0.277), or delirium (P=0.469) during the ICU stay or in ICU-AW at ICU discharge (P=0.309) (Table 4).
Table 4. Comparison of clinical and economic primary outcomes.
| Total population | Matched population | |||||
| Early mobilization |
Late mobilization |
P | Early mobilization |
Late mobilization |
P | |
| Baseline characteristics | n=85 | n=92 | n=37 | n=37 | ||
| Primary outcome | ||||||
| Gait independence at discharge, n (%) | 77 (91) | 48 (52) | <0.0001 | 33 (89) | 24 (65) | 0.025 |
| Secondary outcome | ||||||
| 90-day survival, n (%) | 80 (94) | 70 (76) | <0.0001 | 34 (92) | 30 (81) | 0.308 |
| Total medical costs (USD) | 19,210 [11,107–26,620] | 28,789 [20,969–41,853] | <0.0001 | 16,773 [10,769–27,852] | 23,895 [15,100–29,277] | 0.054 |
| Duration of mechanical ventilation, days | 2 [0–4] | 6 [2–9] | <0.0001 | 4 [0–5] | 3 [0–6] | 0.708 |
| ICU length of stay, days | 4 [3–5] | 7 [4–10] | <0.0001 | 5 [3–6] | 4 [3–8] | 0.584 |
| Hospital length of stay, days | 23 [17–39] | 38 [27–62] | <0.0001 | 22 [17–38] | 37 [23–49] | 0.013 |
| Discharge destination, n (%) | ||||||
| Home | 69 (82) | 44 (48) | <0.0001 | 26 (7) | 22 (59) | 0.277 |
| Rehabilitation center | 4 (5) | 9 (10) | 2 (5) | 3 (8) | ||
| Another hospital | 7 (8) | 16 (17) | 5 (14) | 5 (14) | ||
| Nursing home | 3 (3) | 3 (3) | 3 (8) | 1 (3) | ||
| Death | 2 (2) | 20 (22) | 1 (3) | 6 (16) | ||
| Complications | ||||||
| Delirium during ICU stay, n (%) | 21 (25) | 45 (49) | 0.001 | 12 (32) | 15 (41) | 0.469 |
| Nosocomial pneumonia, n (%) | 7 (8) | 32 (35) | <0.0001 | 2 (5) | 10 (27) | 0.024 |
| ICU-AW at ICU discharge, n (%) | 23 (27) | 44 (48) | 0.004 | 13 (35) | 9 (24) | 0.309 |
Data are presented as median [interquartile range] or number (%).
Analysis by independent-sample Mann–Whitney U-test or χ2 test.
Table 5 shows the rehabilitation parameters in the ICU and ward. Compared with the LM group, the EM group had significantly shorter times to first rehabilitation (P=0.009) and first out-of-bed mobilization (P <0.001), as well as the highest IMS score in the ICU (P <0.001). There was a significant intergroup difference in the number of daily rehabilitation exercises per person (P=0.005) (Table 5). In addition, the frequency of rehabilitation exercises of intensity levels 3 and 4 was significantly higher in the EM group than in the LM group (P=0.024).
Table 5. Comparison of clinical outcomes between study groups.
| Total population | Matched population | |||||
| Early mobilization |
Late mobilization |
P | Early mobilization |
Late mobilization |
P | |
| Baseline characteristics | n=85 | n=92 | n=37 | n=37 | ||
| ICU rehabilitation | ||||||
| Time to first rehabilitation | 2 [2–4] | 5 [2–7] | <0.0001 | 2 [2–4] | 4 [2–6] | 0.009 |
| Time to first out-of-bed mobilization, days | 4 [3–5] | 9 [7–14] | <0.0001 | 4 [3–5] | 7 [6–8] | <0.001 |
| Number of total rehabilitations per person, days | 2 [0–3] | 4 [1–6] | <0.0001 | 3 [1–4] | 2 [0–5] | 0.791 |
| Number of total rehabilitations per person, times | 3 [0–7] | 6 [1–10] | 0.014 | 6 [1–10] | 3 [0–9] | 0.302 |
| Number of daily rehabilitations per person, times/day | 2 [2–3] | 2 [1–2] | <0.0001 | 2 [2–2] | 2 [1–2] | 0.005 |
| Highest IMS at ICU entry | 3 [3–5] | 1 [1–3] | <0.0001 | 5 [3–6] | 1 [1–3] | <0.001 |
| EM protocol level, sessions | ||||||
| Level 1 | 2 [1–2] | 4 [2–6] | <0.0001 | 2 [1–3] | 2 [1–5] | 0.174 |
| Level 2 | 1 [0–2] | 2 [1–3] | <0.0001 | 1 [0–2] | 2 [1–3] | 0.069 |
| Level 3 | 1 [0–1] | 0 [0–1] | <0.0001 | 1 [0–1] | 0 [0–0] | 0.001 |
| Level 4 | 0 [0–1] | 0 [0–0] | <0.0001 | 0 [0–1] | 0 [0–0] | 0.009 |
| Level 5 | 0 [0–0] | 0 [0–0] | <0.0001 | 0 [0–1] | 0 [0–0] | 0.040 |
| Ward rehabilitation | ||||||
| Number of total rehabilitations per person, days | 7 [1–16] | 16 [8–26] | <0.0001 | 7 [2–14] | 15 [8–21] | 0.024 |
| Number of total rehabilitations per person, times | 9 [1–16] | 18 [9–34] | <0.0001 | 11 [3–16] | 18 [10–30] | 0.014 |
| Number of daily rehabilitations per person, times/day | 1 [1–1] | 1 [1–1] | 0.008 | 1 [1–1] | 1 [1–2] | 0.112 |
Data are presented as median [interquartile range] or number (%).
Analysis by independent-sample Mann–Whitney U-test or χ2 test.
Univariate logistic regression analysis based on propensity score matching revealed significant associations between EM and independent gait at discharge (OR: 4.47, 95% CI: 1.39–17.43, P=0.011), length of hospital stay (<28 days) (OR: 0.29, 95% CI: 0.11–0.75, P=0.010), and the presence of pneumonia (P=0.009) (Table 6). Sensitivity analysis performed using the IPTW method showed a similar trend, with significant association between EM and independent gait at discharge (OR: 4.26, 95% CI: 1.29–14.04, P=0.017). Similarly, multivariate logistic regression analysis of patients who stayed in the ICU for more than 5 days revealed significant association between independent gait at discharge and EM (adjusted P=0.014) (Table 7). Notably, multivariate logistic regression analysis, which excluded patients in the LM group (who did not meet the criteria for mobilization by day 5 after admission to the ICU) from propensity score matching showed a similar trend (Table 7).
Table 6. Propensity score matched and weighted odds ratios for achievement of early mobilization within 5 days.
| After PS matching | IPTW | |||
| Baseline characteristics | OR (95% CI) | P | OR (95% CI) | P |
| Physical function | ||||
| Gait independence at discharge | 4.47 (1.39–17.43) | 0.011 | 4.26 (1.29–14.04) | 0.017 |
| Survival | ||||
| <90 days | 2.64 (0.67–13.12) | 0.169 | 5.53 (0.78–17.20) | 0.103 |
| Total hospital costs | ||||
| <2500 USD | 0.51 (0.19–1.29) | 0.154 | 0.57 (0.21–1.55) | 0.268 |
| Outcome | ||||
| Home | 1.61 (0.62–4.30) | 0.329 | 0.96 (0.34–2.69) | 0.937 |
| Duration of mechanical ventilation | ||||
| >2 days | 0.89 (0.35–2.26) | 0.814 | 0.80 (0.33–1.97) | 0.626 |
| ICU length of stay | ||||
| >7 days | 0.51 (0.18–1.43) | 0.201 | 0.79 (0.24–2.54) | 0.688 |
| Length of hospital stay | ||||
| >28 days | 0.29 (0.11–0.75) | 0.010 | 0.47 (0.18–1.25) | 0.129 |
| Complications | ||||
| Delirium | 0.70 (0.27–1.82) | 0.468 | 0.54 (0.21–1.41) | 0.209 |
| Pneumonia | 0.15 (0.02–0.65) | 0.009 | 0.66 (0.15–2.82) | 0.571 |
| ICU-AW | 0.59 (0.21–1.61) | 0.308 | 0.89 (0.35–2.27) | 0.799 |
PS, propensity score.
Table 7. Comparison of EM and LM groups in patients who stayed in the ICU for 5 days or more or excluding patients in the LM group who did not meet the mobilization criteria by day 5 of admission to the ICU.
| Patients who stayed in ICU for 5 days or more |
Patients excluding LM group members who did not meet mobilization criteria by day 5 of admission to ICU | |||||
| Early mobilization |
Late mobilization |
Adjusted P a | Early mobilization |
Late mobilization |
Adjusted P a | |
| Baseline characteristics | n=29 | n=64 | n=85 | n=46 | ||
| Primary outcome | ||||||
| Gait independence at discharge, n (%) | 25 (86) | 32 (50) | 0.014 | 77 (91) | 27 (59) | 0.002 |
| Secondary outcome | ||||||
| 90-day survival, n (%) | 27 (93) | 48 (75) | 0.110 | 80 (94) | 39 (85) | 0.162 |
| Total medical costs (USD) | 23,193 [16,206–31,737] |
34,783 [25,660–49,303] |
0.744 | 19,210 [11,107–26,620] |
27,885 [19,459–40,857] |
0.073 |
| Duration of mechanical ventilation, days | 5 [3–6] | 8 [6–11] | 0.623 | 2 [0–4] | 4 [2–7] | 0.334 |
| ICU length of stay, days | 5 [5–7] | 9 [7–12] | 0.134 | 4 [3–5] | 6 [4–9] | 0.950 |
| Hospital length of stay, days | 31 [22–49] | 42 [30–68] | 0.567 | 23 [17–39] | 43 [29–66] | 0.014 |
| Discharge destination, n (%) | ||||||
| Home | 19 (66) | 27 (42) | 0.371 | 69 (82) | 27 (59) | 0.464 |
| Complications | ||||||
| Delirium during ICU stay, n (%) | 12 (41) | 34 (53) | 0.217 | 21 (25) | 22 (48) | 0.168 |
| Nosocomial pneumonia, n (%) | 4 (14) | 26 (41) | 0.049 | 7 (8) | 16 (35) | 0.015 |
| ICU-AW at ICU discharge, n (%) | 12 (41) | 35 (55) | 0.919 | 23 (27) | 18 (39) | 0.751 |
Data are presented as median [interquartile range] or number (%).
a The covariates were selected from patient characteristics reported as significant independent factors associated with the primary outcome, i.e., admission source, APACHE II score, SOFA score, continuous vasopressor use, RASS score from day 1 to day 5.
The results of univariate logistic regression analysis when using different definitions of EM—within 3, 4, 6, or 7 days—are shown in Table 8. No significant correlation was observed between 90-day survival and gait independence at discharge; however, shorter times to EM (5 days or less) showed stronger correlations of EM with total medical costs, duration of mechanical ventilation, and length of hospital stay (Table 8).
Table 8. Propensity score matched for different definition of early mobilization.
| Variable | Definition of early mobilization in ICU days | ||||
| Within 3 days | Within 4 days | Within 5 days | Within 6 days | Within 7 days | |
| Physical function | |||||
| Gait independence at discharge | 2.22 (0.53–15.22) | 3.46 (0.85–23.45) | 4.47 (1.39–17.43) | 8.62 (2.67–30.67) | 7.56 (2.16–28.47) |
| Survival | |||||
| <90 days | 3.80 (0.65–72.53) | 2.64 (0.63–11.15) | 3.00 (0.75–12.14) | 3.60 (0.80–15.10) | |
| Total hospital costs | |||||
| <2500 USD | 0.30 (0.06–1.05) | 0.27 (0.07–0.85) | 0.51 (0.19–1.29) | 0.51 (0.18–1.41) | 0.43 (0.13–1.41) |
| Outcome | |||||
| Home | 2.56 (0.72–12.10) | 2.75 (0.87–10.59) | 1.61 (0.62–4.30) | 2.79 (0.98–8.08) | 2.16 (0.65–7.18) |
| Duration of mechanical ventilation | |||||
| >2 days | 0.12 (0.02–0.42) | 0.27 (0.09–0.77) | 0.89 (0.35–2.26) | 1.06 (0.37–2.93) | 1.50 (0.46–4.92) |
| ICU length of stay | |||||
| >7 days | 0.32 (0.04–1.33) | 0.35 (0.08–1.23) | 0.51 (0.18–1.41) | 0.39 (0.13–1.16) | 0.30 (0.09–1.03) |
| Length of hospital stay | |||||
| >28 days | 0.29 (0.07–0.94) | 0.23 (0.07–0.69) | 0.29 (0.11–0.75) | 0.09 (0.02–0.32) | 0.12 (0.02–0.49) |
| Complications | |||||
| Delirium | 0.57 (0.14–1.89) | 0.34 (0.08–1.06) | 0.70 (0.27–1.82) | 0.69 (0.24–1.96) | 0.72 (0.22–2.44) |
| Pneumonia | 0.31 (0.02–1.82) | 0.21 (0.01–1.18) | 0.15 (0.02–0.65) | 0.21 (0.05–0.75) | 0.15 (0.04–0.57) |
| ICU-AW | 0.83 (0.21–2.80) | 0.73 (0.21–2.23) | 0.59 (0.21–1.61) | 0.58 (0.20–1.74) | 0.23 (0.06–0.76) |
Data presented as odds ratio (95% confidence interval).
DISCUSSION
This study focused on associations between EM (within 5 days) and clinical outcomes, especially survival. The results revealed that EM within 5 days showed significant association with decreased length of hospital stay and independent gait at discharge. However, no significant difference in survival after 90 days or in medical costs were found. The results were no different even after adjusting for previously reported prognostic factors among ICU patients, which included the most common barriers to achieving mobilization, circulatory status on ICU days 1 and 2, and consciousness level on days 3–5.21) A similar trend was observed with the exclusion of patients who stayed in the ICU for more than 5 days or those in the LM group who did not meet the mobilization criteria by day 5 of admission to the ICU. In addition, reducing the number of days to EM seemed to further shorten the length of hospital stay and the duration of mechanical ventilation and to reduce medical costs. A recent systematic review indicated that ambitious targets toward achieving EM did not affect the risk of mortality.33) The current study showed that there was no difference in survival even after adjusting for the prognostic factors that were reported as independent factors associated with survival in previous studies.34,35,36) Furthermore, continuous vasopressor use and RASS scores were recorded from days 1–5 as covariates based on the authors’ previous studies on barriers to achieving mobilization. This study suggests that EM-based interventions alone might be insufficient to improve survival outcomes.
The post-matching IPTW analyses identified significant relationships between EM and independent gait at discharge. However, some recent randomized studies have failed to detect significant improvements in the EM group,37) which may have been related to the mobilization initiation being delayed for approximately 1 week after ICU admission. A 10% reduction in muscle mass has been observed between days 1 and 7 among ICU patients, with a 17.7% reduction observed on day 10.38) Therefore, delaying the start of measures designed to achieve EM may mitigate any improvements in functional outcomes. In the present study, the median time to mobilization was 4 days in the EM group and 7 days in the LM group, and the EM group had better clinical outcomes (stronger likelihood of independent gait at discharge and shorter hospital stays).9) Previous reports have also indicated that achieving EM within 1 week does not affect survival but is effective in improving functional outcomes and shortening hospital stays.39,40) Liu et al.11) also reported a cost reduction of approximately USD 6500/patient in cases with EM, which is slightly higher than the reduction of USD 5500/patient observed in our EM group. In this study, there was a decreasing trend in medical costs of the EM group when compared with the LM group. A previous study indicated that this reduction in medical costs is related to several factors, including improvements in the patient’s critical condition, improvement to independent gait at discharge, and shorter hospital stays.41) Similarly, these results indicated that a higher rate of gait independence at discharge and shortened length of hospital stay could have contributed to the lower medical costs in the EM group. Consequently, achieving EM in the ICU, as shown in this study, might help prevent disuse syndrome, achieve independent gait, and potentially decrease medical costs.
For this study, EM was defined as initiating rehabilitation by achieving a seated position on the edge of the bed within 5 days of ICU admission. The literature includes various mobilization timings13,14); however, recent studies have indicated that the initiation of mobilization within 48–72 h might be optimal.22) In a previous study, EM also included passive range-of-motion exercises on the bed.42,43) Previous studies have described improvements, not only in physical function but also in respiratory function and the level of consciousness,44) as an effect of sitting on the edge of the bed. The results of the current study suggest that it is adequate to achieve mobilization of sitting on the edge of a bed or higher within 5 days of ICU admission. However, comparing the number of times the EM protocol was performed in the ICU and the protocol intensities, the frequency of rehabilitation exercises of intensity level 3 or higher was significantly higher in the EM group than in the LM group. In this study, we focused on the timing of EM initiation; future studies should also investigate the relationships between outcomes and the maximum intensities of mobilization in the ICU.
The present study’s major limitations are a lack of complete data, a small sample size, and a single-center design. In addition, a comparison of patients after propensity score matching showed that intraoperative inpatient diagnosis, cardiovascular complications, and continuous administration of steroids and neuromuscular blockers did not have an SD of <0.1 because of the small sample size. Furthermore, only relatively short-term survival and functional outcomes were evaluated. However, the prevention of physical dysfunction has become a new challenge in the field of emergency and intensive care, with greater emphasis being placed on the long-term post-discharge quality of life and functional outcomes.2,45) Furthermore, in this study, unmeasured confounders influenced the relationships observed between EM and independent gait at discharge, length of hospital stay, and medical costs. For example, data regarding medications, sedation dose, pain, infection, ventilator settings, and weaning were not collected despite having the potential to influence the findings.12,46,47,48) Therefore, further prospective studies are needed to investigate whether these factors influenced our findings. Furthermore, a multicenter, prospective, randomized controlled trial that includes all ICU patients is needed to validate and correlate the unanswered questions of this study.
CONCLUSION
The present study revealed that EM was not significantly associated with 90-day survival or medical costs but was associated with a higher likelihood of independent gait at discharge and shorter hospital stays. EM, which refers to achieving the strength to sit on the edge of the bed within the first 5 days of the ICU stay, might be an adequate target to improve clinical outcomes. Further validations of the results are necessary.
ACKNOWLEDGMENTS
The authors thank study coordinators Yuichiro Hayami and Akiko Kada. The authors also thank the entire ICU staff at Nagoya Medical Center Hospital.
Footnotes
CONFLICTS OF INTEREST: The authors report no conflicts of interest.
REFERENCES
- 1.Brahmbhatt N,Murugan R,Milbrandt EB: Early mobilization improves functional outcomes in critically ill patients. Crit Care 2010;14:321. 10.1186/cc9262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Needham DM,Davidson J,Cohen H,Hopkins RO,Weinert C,Wunsch H,Zawistowski C,Bemis-Dougherty A,Berney SC,Bienvenu OJ,Brady SL,Brodsky MB,Denehy L,Elliott D,Flatley C,Harabin AL,Jones C,Louis D,Meltzer W,Muldoon SR,Palmer JB,Perme C,Robinson M,Schmidt DM,Scruth E,Spill GR,Storey CP,Render M,Votto J,Harvey MA: Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012;40:502–509. 10.1097/CCM.0b013e318232da75 [DOI] [PubMed] [Google Scholar]
- 3.Ehlenbach WJ,Hough CL,Crane PK,Haneuse SJ,Carson SS,Curtis JR,Larson EB: Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA 2010;303:763–770. 10.1001/jama.2010.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee M,Kang J,Jeong YJ: Risk factors for post-intensive care syndrome: a systematic review and meta-analysis. Aust Crit Care 2020;33:287–294. 10.1016/j.aucc.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 5.Marra A,Pandharipande PP,Girard TD,Patel MB,Hughes CG,Jackson JC,Thompson JL,Chandrasekhar R,Ely EW,Brummel NE: Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med 2018;46:1393–1401. 10.1097/CCM.0000000000003218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishida O,Ogura H,Egi M,Fujishima S,Hayashi Y,Iba T,Imaizumi H,Inoue S,Kakihana Y,Kotani J,Kushimoto S,Masuda Y,Matsuda N,Matsushima A,Nakada T,Nakagawa S,Nunomiya S,Sadahiro T,Shime N,Yatabe T,Hara Y,Hayashida K,Kondo Y,Sumi Y,Yasuda H,Aoyama K,Azuhata T,Doi K,Doi M,Fujimura N,Fuke R,Fukuda T,Goto K,Hasegawa R,Hashimoto S,Hatakeyama J,Hayakawa M,Hifumi T,Higashibeppu N,Hirai K,Hirose T,Ide K,Kaizuka Y,Kan’o T,Kawasaki T,Kuroda H,Matsuda A,Matsumoto S,Nagae M,Onodera M,Ohnuma T,Oshima K,Saito N,Sakamoto S,Sakuraya M,Sasano M,Sato N,Sawamura A,Shimizu K,Shirai K,Takei T,Takeuchi M,Takimoto K,Taniguchi T,Tatsumi H,Tsuruta R,Yama N,Yamakawa K,Yamashita C,Yamashita K,Yoshida T,Tanaka H,Oda S: The Japanese clinical practice guidelines for management of sepsis and septic shock 2016 (J-SSCG 2016). Acute Med Surg 2018;5:3–89. 10.1002/ams2.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe S,Iida Y,Ito T,Mizutani M,Morita Y,Suzuki S,Nishida O: Effect of early rehabilitation activity time on critically ill patients with intensive care unit-acquired weakness: a Japanese retrospective multicenter study. Prog Rehabil Med 2018;3:20180003. 10.2490/prm.20180003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweickert WD,Pohlman MC,Pohlman AS,Nigos C,Pawlik AJ,Esbrook CL,Spears L,Miller M,Franczyk M,Deprizio D,Schmidt GA,Bowman A,Barr R,McCallister KE,Hall JB,Kress JP: Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874–1882. 10.1016/S0140-6736(09)60658-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris PE,Goad A,Thompson C,Taylor K,Harry B,Passmore L,Ross A,Anderson L,Baker S,Sanchez M,Penley L,Howard A,Dixon L,Leach S,Small R,Hite RD,Haponik E: Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med 2008;36:2238–2243. 10.1097/CCM.0b013e318180b90e [DOI] [PubMed] [Google Scholar]
- 10.Watanabe S,Morita Y,Suzuki S,Someya F: Association between early rehabilitation for mechanically ventilated intensive care unit patients and oral ingestion. Prog Rehabil Med 2018;3:20180003. 10.2490/prm.20180009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu K,Ogura T,Takahashi K,Nakamura M,Ohtake H,Fujiduka K,Abe E,Oosaki H,Miyazaki D,Suzuki H,Nishikimi M,Komatsu M,Lefor AK,Mato T: A progressive early mobilization program is significantly associated with clinical and economic improvement: a single-center quality comparison study. Crit Care Med 2019;47:e744–e752. 10.1097/CCM.0000000000003850 [DOI] [PubMed] [Google Scholar]
- 12.Ad Hoc Committee for Early Rehabilitation, The Japanese Society of Intensive Care Medicine: Evidence based expert consensus for early rehabilitation in the intensive care unit [in Japanese]. Jpn J Int Care Med 2017;24:255–303. [Google Scholar]
- 13.Burtin C,Clerckx B,Robbeets C,Ferdinande P,Langer D,Troosters T,Hermans G,Decramer M,Gosselink R: Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med 2009;37:2499–2505. 10.1097/CCM.0b013e3181a38937 [DOI] [PubMed] [Google Scholar]
- 14.Hodgson CL,Berney S,Harrold M,Saxena M,Bellomo R: Clinical review: early patient mobilization in the ICU. Crit Care 2013;17:207. [DOI] [PMC free article] [PubMed]
- 15.Fuest K,Schaller SJ: Recent evidence on early mobilization in critical-ill patients. Curr Opin Anaesthesiol 2018;31:144–150. 10.1097/ACO.0000000000000568 [DOI] [PubMed] [Google Scholar]
- 16.Wright SE,Thomas K,Watson G,Baker C,Bryant A,Chadwick TJ,Shen J,Wood R,Wilkinson J,Mansfield L,Stafford V,Wade C,Furneval J,Henderson A,Hugill K,Howard P,Roy A,Bonner S,Baudouin S: Intensive versus standard physical rehabilitation therapy in the critically ill (EPICC): a multicentre, parallel-group, randomised controlled trial. Thorax 2018;73:213–221. 10.1136/thoraxjnl-2016-209858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denehy L,Skinner EH,Edbrooke L,Haines K,Warrillow S,Hawthorne G,Gough K,Hoorn S,Morris ME,Berney S: Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care 2013;17:R156. 10.1186/cc12835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss M,Nordon-Craft A,Malone D,Van Pelt D,Frankel SK,Warner ML,Kriekels W,McNulty M,Fairclough DL,Schenkman M: A randomized trial of an intensive physical therapy program for patients with acute respiratory failure. Am J Respir Crit Care Med 2016;193:1101–1110. 10.1164/rccm.201505-1039OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denehy L,Lanphere J,Needham DM: Ten reasons why ICU patients should be mobilized early. Intensive Care Med 2017;43:86–90. 10.1007/s00134-016-4513-2 [DOI] [PubMed] [Google Scholar]
- 20.Watanabe S,Kotani T,Taito S,Ota K,Ishii K,Ono M,Katsukawa H,Kozu R,Morita Y,Arakawa R,Suzuki S: Determinants of gait independence after mechanical ventilation in the intensive care unit: a Japanese multicenter retrospective exploratory cohort study. J Intensive Care 2019;7:53. 10.1186/s40560-019-0404-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe S,Keibun L,Morita Y,Kanaya T,Naito Y,Arakawa R,Suzuki S,Katsukawa H,Lefor AK,Kotani T:. Changes in barriers to implementing early mobilization in the intensive care unit: a single center retrospective cohort study. J Nagoya Med Sci 2021;83:443–464. 10.1186/s40560-019-0404-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhn KF,Schaller SJ: Comment on Early versus delayed mobilization for in-hospital mortality and health-related quality of life among critically ill patients: a systematic review and meta-analysis (Okada et al., Journal of Intensive Care 2019). J Intensive Care 2020;8:21. 10.1186/s40560-020-0436-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrold ME,Salisbury LG,Webb SA,Allison GT, Australia and Scotland ICU Physiotherapy Collaboration: Early mobilisation in intensive care units in Australia and Scotland: a prospective, observational cohort study examining mobilisation practises and barriers. Crit Care 2015;19:336. 10.1186/s13054-015-1033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME,Pompei P,Ales KL,MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa Y,Takemura T,Yoshihara H,Nakagawa Y: A new accounting system for financial balance based on personnel cost after the introduction of a DPC/DRG system. J Med Syst 2011;35:251–264. 10.1007/s10916-009-9361-y [DOI] [PubMed] [Google Scholar]
- 26.Hodgson C,Needham D,Haines K,Bailey M,Ward A,Harrold M,Young P,Zanni J,Buhr H,Higgins A,Presneill J,Berney S: Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung 2014;43:19–24. 10.1016/j.hrtlng.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 27.Conlon N,O’Brien B,Herbison GP,Marsh B: Long-term functional outcome and performance status after intensive care unit re-admission: a prospective survey. Br J Anaesth 2008;100:219–223. 10.1093/bja/aem372 [DOI] [PubMed] [Google Scholar]
- 28.de Grooth HJ,Geenen IL,Girbes AR,Vincent JL,Parienti JJ,Oudemans-van Straaten HM: SOFA and mortality endpoints in randomized controlled trials: a systematic review and meta-regression analysis. Crit Care 2017;21:38. 10.1186/s13054-017-1609-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukunaga S,Nagami Y,Shiba M,Ominami M,Tanigawa T,Yamagami H,Tanaka H,Muguruma K,Watanabe T,Tominaga K,Fujiwara Y,Ohira M,Hirakawa K,Arakawa T: Long-term prognosis of expanded-indication differentiated-type early gastric cancer treated with endoscopic submucosal dissection or surgery using propensity score analysis. Gastrointest Endosc 2017;85:143–152. 10.1016/j.gie.2016.06.049 [DOI] [PubMed] [Google Scholar]
- 30.Pun BT,Balas MC,Barnes-Daly MA,Thompson JL,Aldrich JM,Barr J,Byrum D,Carson SS,Devlin JW,Engel HJ,Esbrook CL,Hargett KD,Harmon L,Hielsberg C,Jackson JC,Kelly TL,Kumar V,Millner L,Morse A,Perme CS,Posa PJ,Puntillo KA,Schweickert WD,Stollings JL,Tan A,D’Agostino McGowan L,Ely EW: Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 2019;47:3–14. 10.1097/CCM.0000000000003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkatesh B,Finfer S,Cohen J,Rajbhandari D,Arabi Y,Bellomo R,Billot L,Correa M,Glass P,Harward M,Joyce C,Li Q,McArthur C,Perner A,Rhodes A,Thompson K,Webb S,Myburgh J, ADRENAL Trial Investigators and the Australian–New Zealand Intensive Care Society Clinical Trials Group: Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 2018;378:797–808. 10.1056/NEJMoa1705835 [DOI] [PubMed] [Google Scholar]
- 32.Azoulay E,Adrie C,De Lassence A,Pochard F,Moreau D,Thiery G,Cheval C,Moine P,Garrouste-Orgeas M,Alberti C,Cohen Y,Timsit JF: Determinants of postintensive care unit mortality: a prospective multicenter study. Crit Care Med 2003;31:428–432. 10.1097/01.CCM.0000048622.01013.88 [DOI] [PubMed] [Google Scholar]
- 33.Tipping CJ,Harrold M,Holland A,Romero L,Nisbet T,Hodgson CL: The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med 2017;43:171–183. 10.1007/s00134-016-4612-0 [DOI] [PubMed] [Google Scholar]
- 34.Wilcox MG,Howard TJ,Plaskon LA,Unthank JL,Madura JA: Current theories of pathogenesis and treatment of nonocclusive mesenteric ischemia. Dig Dis Sci 1995;40:709–716. 10.1007/BF02064966 [DOI] [PubMed] [Google Scholar]
- 35.Daviaud F,Grimaldi D,Dechartres A,Charpentier J,Geri G,Marin N,Chiche JD,Cariou A,Mira JP,Pène F: Timing and causes of death in septic shock. Ann Intensive Care 2015;5:16. 10.1186/s13613-015-0058-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leone M,Bechis C,Baumstarck K,Ouattara A,Collange O,Augustin P,Annane D,Arbelot C,Asehnoune K,Baldési O,Bourcier S,Delapierre L,Demory D,Hengy B,Ichai C,Kipnis E,Brasdefer E,Lasocki S,Legrand M,Mimoz O,Rimmelé T,Aliane J,Bertrand PM,Bruder N,Klasen F,Friou E,Lévy B,Martinez O,Peytel E,Piton A,Richter E,Toufik K,Vogler MC,Wallet F,Boufi M,Allaouchiche B,Constantin JM,Martin C,Jaber S,Lefrant JY: Outcome of acute mesenteric ischemia in the intensive care unit: a retrospective, multicenter study of 780 cases. Intensive Care Med 2015;41:667–676. 10.1007/s00134-015-3690-8 [DOI] [PubMed] [Google Scholar]
- 37.Morris PE,Berry MJ,Files DC,Thompson JC,Hauser J,Flores L,Dhar S,Chmelo E,Lovato J,Case LD,Bakhru RN,Sarwal A,Parry SM,Campbell P,Mote A,Winkelman C,Hite RD,Nicklas B,Chatterjee A,Young MP: Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: a randomized clinical trial. JAMA 2016;315:2694–2702. 10.1001/jama.2016.7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puthucheary ZA,Rawal J,McPhail M,Connolly B,Ratnayake G,Chan P,Hopkinson NS,Padhke R,Dew T,Sidhu PS,Velloso C,Seymour J,Agley CC,Selby A,Limb M,Edwards LM,Smith K,Rowlerson A,Rennie MJ,Moxham J,Harridge SD,Hart N,Montgomery HE: Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591–1600. 10.1001/jama.2013.278481 [DOI] [PubMed] [Google Scholar]
- 39.Ding N,Zhang Z,Zhang C,Yao L,Yang L,Jiang B,Wu Y,Jiang L,Tian J: What is the optimum time for initiation of early mobilization in mechanically ventilated patients? A network meta-analysis. PLoS One 2019;14:e0223151. 10.1371/journal.pone.0223151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okada Y,Unoki T,Matsuishi Y,Egawa Y,Hayashida K,Inoue S: Early versus delayed mobilization for in-hospital mortality and health-related quality of life among critically ill patients: a systematic review and meta-analysis. J Intensive Care 2019;7:57. 10.1186/s40560-019-0413-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corcoran JR,Herbsman JM,Bushnik T,Van Lew S,Stolfi A,Parkin K,McKenzie A,Hall GW,Joseph W,Whiteson J,Flanagan SR: Early rehabilitation in the medical and surgical intensive care units for patients with and without mechanical ventilation: an interprofessional performance improvement project. PM R 2017;9:113–119. 10.1016/j.pmrj.2016.06.015 [DOI] [PubMed] [Google Scholar]
- 42.Bakhru RN,McWilliams DJ,Wiebe DJ,Spuhler VJ,Schweickert WD: Intensive care unit structure variation and implications for early mobilization practices. an international survey. Ann Am Thorac Soc 2016;13:1527–1537. 10.1513/AnnalsATS.201601-078OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fontela PC,Forgiarini LA Jr,Friedman G: Clinical attitudes and perceived barriers to early mobilization of critically ill patients in adult intensive care units. Rev Bras Ter Intensiva 2018;30:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okubo N: Effectiveness of the sitting position without back support. Australas J Neurosci 2015;25:31–39. 10.21307/ajon-2017-111 [DOI] [Google Scholar]
- 45.Winters BD,Eberlein M,Leung J,Needham DM,Pronovost PJ,Sevransky JE: Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med 2010;38:1276–1283. 10.1097/CCM.0b013e3181d8cc1d [DOI] [PubMed] [Google Scholar]
- 46.Devlin JW,Skrobik Y,Gélinas C,Needham DM,Slooter AJ,Pandharipande PP,Watson PL,Weinhouse GL,Nunnally ME,Rochwerg B,Balas MC,van den Boogaard M,Bosma KJ,Brummel NE,Chanques G,Denehy L,Drouot X,Fraser GL,Harris JE,Joffe AM,Kho ME,Kress JP,Lanphere JA,McKinley S,Neufeld KJ,Pisani MA,Payen JF,Pun BT,Puntillo KA,Riker RR,Robinson BR,Shehabi Y,Szumita PM,Winkelman C,Centofanti JE,Price C,Nikayin S,Misak CJ,Flood PD,Kiedrowski K,Alhazzani W: Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018;46:e825–e873. 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 47.Routsi C,Gerovasili V,Vasileiadis I,Karatzanos E,Pitsolis T,Tripodaki ES,Markaki V,Zervakis D,Nanas S: Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care 2010;14:R74. 10.1186/cc8987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leditschke AI,Green M,Irvine J,Bissett B,Mitchell IA: What are the barriers to mobilizing intensive care patients? Cardiopulm Phys Ther J 2012;23:26–29. 10.1097/01823246-201223010-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]