Abstract
Extramedullary involvement (or extramedullary disease, EMD) represents an aggressive form of multiple myeloma (MM), characterized by the ability of a clone and/or subclone to thrive and grow independent of the bone marrow microenvironment. Several different definitions of EMD have been used in the published literature. We advocate that true EMD is restricted to soft-tissue plasmacytomas that arise due to hematogenous spread and have no contact with bony structures. Typical sites of EMD vary according to the phase of MM. At diagnosis, EMD is typically found in skin and soft tissues; at relapse, typical sites involved include liver, kidneys, lymph nodes, central nervous system (CNS), breast, pleura, and pericardium. The reported incidence of EMD varies considerably, and differences in diagnostic approach between studies are likely to contribute to this variability. In patients with newly diagnosed MM, the reported incidence ranges from 0.5% to 4.8%, while in relapsed/refractory MM the reported incidence is 3.4 to 14%. Available data demonstrate that the prognosis is poor, and considerably worse than for MM without soft-tissue plasmacytomas. Among patients with plasmacytomas, those with EMD have poorer outcomes than those with paraskeletal involvement. CNS involvement is rare, but prognosis is even more dismal than for EMD in other locations, particularly if there is leptomeningeal involvement. Available data on treatment outcomes for EMD are derived almost entirely from retrospective studies. Some agents and combinations have shown a degree of efficacy but, as would be expected, this is less than in MM patients with no extramedullary involvement. The paucity of prospective studies makes it difficult to justify strong recommendations for any treatment approach. Prospective data from patients with clearly defined EMD are important for the optimal evaluation of treatment outcomes.
Subject terms: Myeloma, Myeloma
Introduction
Multiple myeloma (MM) is a mature B-cell neoplasm defined by the presence of ≥10% of clonal plasma cells (PCs) in the bone marrow (or plasmacytoma confirmed by biopsy) and by evidence of end-organ damage (hypercalcemia, renal insufficiency, anemia, bone lesions) caused by the PC disorder [1]. While the PC proliferation is restricted to bone marrow in most patients with MM, a subset develops soft-tissue plasmacytomas, whereby clonal PCs escape and are found outside the bone marrow.
The presence of soft-tissue plasmacytomas represents an aggressive form of MM, characterized by the ability of a clone and/or subclone to thrive and grow independent of the bone marrow microenvironment. This is linked to high-risk genetic features, increased proliferation, evasion of apoptosis, and resistance to therapies [2, 3]. There are three principal ways that soft-tissue plasmacytomas can develop in patients with MM: (a) direct growth from skeletal tumors following cortical bone disruption; (b) growth in organs or soft tissue following hematogenous spread without contact with bony structures; and (c) rarely, growth triggered by invasive procedures [4–6].
It is important to better understand soft-tissue plasmacytomas in order to successfully direct research that will improve outcomes for affected patients. This task is complicated by the fact that several different definitions of extramedullary involvement in MM, or extramedullary disease (EMD), have been used in the published literature. A 2013 study proposed that it should comprise only of purely extramedullary plasmacytomas, and exclude bone-related plasmacytomas arising from adjacent bone marrow [7]. Other authors have contended that bone-related (or paraskeletal) plasmacytomas should be included under the definition of EMD, albeit as a distinct sub-type from extramedullary plasmacytomas [8, 9]. The former definition and distinction has been endorsed by a recent expert consensus review [4]. Solitary plasmacytomas (SP) should not be considered as EMD, since they occur in the absence of an MM diagnosis [9, 10]. Plasma cell leukemia (PCL) is also typically excluded from the definition of EMD, on the basis that it is a well-characterized pathologic entity with distinct prognostic implications and treatment recommendations [9, 11–13]. Definitions of these different clinical entities are shown in Table 1.
Table 1.
Definitions of plasma cell neoplasms.
| Plasma cell neoplasm | Definition |
|---|---|
| Extramedullary disease | An aggressive form of multiple myeloma characterized by the presence of soft-tissue plasmacytomas that result from hematogenous spread |
| Paraskeletal plasmacytoma | A form of multiple myeloma characterized by the presence of soft-tissue plasmacytomas that occur due to direct growth from skeletal tumors following cortical bone disruption |
| Solitary plasmacytoma | A single mass of clonal plasma cells (bone or extramedullary) with no or minimal BM plasmacytosis and with no other symptoms than those derived from the primary lesion |
| Plasma cell leukemia | A rare and aggressive variant of myeloma characterized by the presence of circulating plasma cells; diagnosis is based upon the percentage (≥20%) and absolute number (≥2 × 109/L) of plasma cells in peripheral blood |
Several genetic features have been linked to extramedullary involvement in MM. These include 17p deletion [14, 15], nuclear expression of p53 [16], a higher incidence of t(4;14) [17], and p53 deletion [18]. Other possible risk factors include loss of CD56 expression [19], MAFB overexpression [20], and MYC overexpression [21]. Treatment with novel agents has also been suggested as a risk factor for the emergence of EMD, although the data to support this are equivocal [4].
This review is based on a systematic literature search, and will focus on hematogenous EMD, including that with central nervous system (CNS) involvement. Areas covered are incidence, sites of occurrence, prognosis, and treatment outcomes.
Methods
The PubMed database was searched for publications of interest from January 1, 2010 to 31 December, 2020, using the following search terms: [Extramedullary AND (myeloma OR plasmacytoma)] OR [myeloma AND (bone-related OR soft tissue OR extraosseous)] OR [central nervous system AND (myeloma OR plasmacytoma OR extramedullary)]. Supplemental literature searches (for treatment outcomes) of proceedings from the following relevant conferences that took place in 2019 and 2020 were also conducted: European Haematology Association 2019 and 2020; American Society of Clinical Oncology 2019 and 2020; American Society of Hematology 2019 and 2020; International Myeloma Working Group 2019.
Following a check for duplicates, retrieved records (titles and abstracts) were screened to exclude those clearly not relevant for inclusion. At this stage, records were excluded on the basis of the following criteria: non-English language, review articles, preclinical studies, or individual case studies/case series involving fewer than 10 patients. Full-text articles for records deemed to be possibly relevant were then obtained and reviewed for inclusion in the qualitative synthesis (given the nature of the data it was not possible or appropriate to conduct a quantitative analysis). To be included, an article needed to clearly report data for hematogenous EMD and to clearly define the phase of MM (NDMM or RRMM); it also needed to include data for one or more of the areas of interest (sites of occurrence, incidence, prognosis, treatment outcomes, CNS EMD).
Results
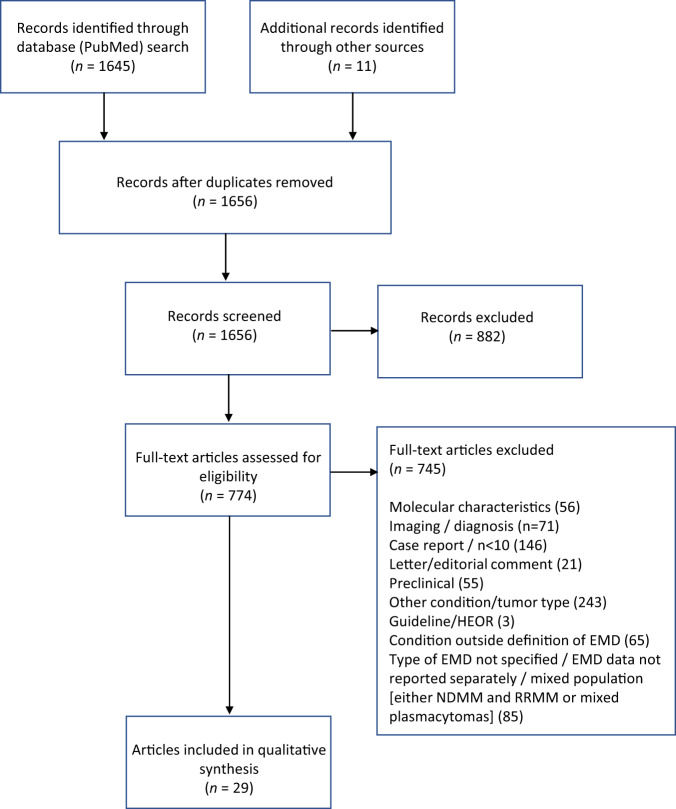
A total of 29 articles were included in the qualitative synthesis. The PRISMA flow diagram is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram for qualitative synthesis.
Incidence
In some patients, EMD can be identified as visible or palpable masses; however, imaging techniques are usually needed. Both paraskeletal plasmacytomas and EMD can be found at diagnosis of MM (sometimes referred to as primary), or at relapse (secondary) [20, 22, 23]. The reported incidence varies considerably across studies, most likely due to differences in the definitions applied and the methods of evaluation. Magnetic resonance (MRI) is the best imaging approach for spinal and central nervous system (CNS) involvement. However, functional whole-body techniques should ideally be used to detect EMD. A consensus statement by the International Myeloma Working Group (IMWG) specifically recommends 18F-FDG PET/CT for this purpose [24]. Hopefully, the previous lack of standardization is solved with the recent proposal of PET/CT standardization score according to the Deauville criteria based on the analysis of two prospective studies [25]. The reported incidence of EMD at diagnosis before the PET/CT era was low, ranging from 1.7 to 4.5% [4]. With the use of PET/CT, the incidence of EMD at diagnosis is between 6 and 10% [23, 24, 26, 27], which should help to stimulate sub-studies for EMD in prospective clinical trials (such as the CASSIOPET study of the CASSIOPEIA trial). Finally, PET/CT should be performed in clinical practice for all patients with a suspicion of EMD, such as those with high LDH serum levels or revised stage III [4].
A number of studies have reported incidence data specifically for EMD (Table 2). A meta-analysis of eight clinical trials in patients with NDMM, involving 2332 patients, found an overall incidence of soft-tissue plasmacytomas of 11.4%, and an incidence of EMD of 0.5% [26]. The authors acknowledge that their reported incidence of EMD is likely to be an underestimation due to the imaging techniques used in the study. A study of 3744 patients with NDMM reported an overall incidence of soft-tissue plasmacytomas of 18.2%, with paraskeletal involvement in 14.5% and extramedullary involvement in 3.7% [28]. Deng et al. [18] assessed EMD in 834 consecutive MM patients from a single center in China and found incidence rates of 4.8% and 3.4% in patients with NDMM and RRMM, respectively. Another single-center study in Boston, USA, found EMP in 55/663 consecutive patients (8.3%) undergoing stem-cell transplantation [29]. Among these 55 patients, EMD was present at diagnosis in eight, at relapse in 42, and at both diagnosis and relapse in five. An analysis of 226 patients with RRMM found soft-tissue plasmacytomas in 24% of patients; this was adjacent to bone (paraskeletal) in 10% and not adjacent in 14% [23]. Rasche et al. [21] screened their myeloma registry for patients who developed EMD relapse, and reported an incidence rate of 6.7% (24/357 patients). In a Mayo Clinic including 174 patients with RRMM enrolled in a phase 2 clinical trial [30], 16 patients (9.2%) were found to have EMD (3 patients [1.7%] at the time of diagnosis, 13 [7.5%] during the course of treatment).
Table 2.
Incidence rates of EMD in studies of patients with MM.
| Reference | No. of patients | Time period covered | Diagnostic approach | Incidence |
|---|---|---|---|---|
| Montefusco et al. [26] | 2322 | 2010–2018 (across all studies) | Skeletal survey, MRI, or CT; and/or physical examination | NDMM: 0.5% |
| Gagelmann et al. [28] | 3744 | 2005–2014 | NR | NDMM: 3.7% |
| Deng et al. [18] | 834 | 1993–2013 | X-ray, US, CT, physical examination; histologically confirmed where possible | NDMM: 4.8% RRMM: 3.4% |
| Weinstock et al. [29] | 663 | 2005–2011 | Pathological or radiological evidence of EMD at any time following the initial diagnosis of MM | RRMM: 8.3% |
| Pour et al. [23] | 226 | 2005–2008 | US, CT, or MRI | RRMM: 14% |
| Rasche et al. [21] | 357 | 2007–2010 | Cytology, biopsy of clinical/radiological lesions | RRMM: 6.7% |
| Short et al. [30] | 174 | 2007–2011 | PET/CT, MRI | NDMM: 1.7% RRMM: 7.5% |
CT computed tomography, MRI magnetic resonance imaging, NR not reported, PET positron emission tomography, US ultrasound.
Typical sites of occurrence
Plasmacytomas can be found in virtually any area of any tissue in the body. A recent real-world retrospective study of 226 MM patients presenting with plasmacytomas included 176 patients with EMD [31]. The most common sites of EMD occurrence were skin/muscle (24%), pleura (12%), lymph nodes (10%), liver (9%), and CNS (6%). Different sites appear to be involved in EMD identified at MM diagnosis and at relapse, with skin the most commonly involved tissue in NDMM [2, 20, 28, 29]. In RRMM, sites involved include liver, kidneys, lymph nodes, CNS, breast, pleura, and pericardium [2, 28, 29].
Prognosis
A retrospective study of transplant-eligible patients with MM compared survival outcomes in patients who had EMD (n = 17), bone-related plasmacytomas (n = 22), and no plasmacytoma involvement (n = 141) at diagnosis [32]. Five-year overall survival (OS) was 63% in patients who had bone-related plasmacytomas, 63% in patients who had EMD, and 80% in patients without plasmacytomas at diagnosis (p = 0.02). Five-year disease-free survival was 47% in patients who had bone-related plasmacytomas, 35% in patients with EMD, and 54% in MM patients who did not have plasmacytomas (p = 0.15). Moreau et al. [33] found that absence of EMD at diagnosis was an independent prognostic factor for longer progression-free survival (PFS; p < 0.001) and OS (p = 0.004). By multivariate analysis, the hazard ratios (HRs) for PFS and OS were 3.394 (95% CI 2.055–5.606) and 3.894 (95% CI 1.540–9.851), respectively. Another study assessed predictors of inferior clinical outcome in patients (n = 51) with standard-risk MM [34]; on univariate analysis, EMD was associated with significantly shorter OS (HR 3.05; 95% CI 0.57–16.29; p = 0.02).
Treatment outcomes
EMD in patients with NDMM
Currently available data for treatment outcomes in patients with NDMM and EMD are summarized in Table 3.
Table 3.
Treatment outcomes in patients with NDMM and EMD.
| Reference Study type | Total number of patients/ number with EMD | Time period covered | Treatments | PFS | OS |
|---|---|---|---|---|---|
|
Montefusco et al. [26] Meta-analysis of 8 trials |
267 EMD, n = 12 |
2010–2018 (across all studies) | IMiD-based therapy (n = 166), PI-based (n = 66), IMiD+ PI-based (n = 35) | Median (95% CI): 26.1 months (8.0–NR) |
2-year: 35% Median (95% CI): 70.1 months (16.9–NR) |
|
Batsukh et al. [35] Retrospective |
64 EMD, n = 22 |
2009–2016 |
Bortezomib/dexamethasone (n = 7) Thalidomide/dexamethasone (n = 23) Bortezomib/thalidomide/dexamethasone (n = 11) Bortezomib/melphalan/prednisone (n = 23) Lenalidomide/dexamethasone (n = 1) Mlphalan/prednisolone or dexamethasone (n = 6) ASCT (n = 28) |
Median (95% CI): 16.0 months (5.8–26.2) |
Median (95% CI): 27.8 months (5.8–26.5) |
|
Beksac et al. [31] Retrospective |
130 EMD, n = 92 |
2010–2017 | Median two lines of treatment and ASCT (44%) | Median (95% CI): 38.9 months (23.6–54.2) | Median (95% CI): 46.5 months (25.5–67.5) |
|
Gagelmann et al. [36] EBMT registry analysis |
488 EMD, n = 87 |
2003–2014 |
Induction with bortezomib (n = 355) vs non-bortezomib (n = 133) Transplants: autologous (n = 373) or tandem autologous (n = 84) or autologous-allogeneic transplant (n = 31) |
3-year (range): 39% (27–52) | 3-year (range): 60% (48–73) |
|
Gagelmann et al. [28] EBMT registry analysis |
682 EMD, n = 139 |
2005–2014 | Upfront single ASCT within 12 months of diagnosis or a tandem ASCT within 6 months from first ASCT as first-line therapy | 3-year (range): 39.9% (30.3–49.5) | 3-year (range): 58.0% (48.1–67.9) |
ASCT autologous stem-cell transplantation, EBMT European Society for Blood and Marrow Transplantation, EMD extramedullary disease, IMiD immunomodulatory drugs, OS overall survival, PFS progression-free survival, PI proteasome inhibitor.
The recent meta-analysis of eight trials of IMiD- or PI-based therapy in patients (n = 2332) with NDMM [26] included 267 patients with soft-tissue plasmacytomas (paraskeletal, n = 243; EMD, n = 12; not classified, n = 12). Median PFS was 26.1 months and 25.2 months in patients with EMD and patients without plasmacytomas, respectively; median OS was 70.1 months (EMD) and 79.9 months (no plasmacytomas). Batsukh et al. [35] retrospectively assessed outcomes in 64 NDMM patients with soft-tissue plasmacytomas who were treated with various regimens containing novel agents. Median PFS was similar (approximately 16 months) for patients with bone-related plasmacytomas and EMD, while median OS was significantly shorter (27.8 vs 54.2 months, p = 0.033) in the EMD group (mainly due to shorter PFS after first relapse).
Beksac et al. [31] conducted a multicenter, multinational retrospective study of 226 MM patients with plasmacytoma involvement; 176 had EMD (n = 92 at MM diagnosis, n = 84 at relapse) and 50 had paraskeletal plasmacytomas (n = 38 at MM diagnosis, n = 12 at relapse). The entire group received a median two lines of treatment and autologous SCT (44%) following their plasmacytoma diagnosis. PFS and OS were 38.9 months and 46.5 months for EMD at diagnosis and 51.7 months (p = 0.034) and not reached (p = 0.002) for paraskeletal plasmacytomas.
Gagelmann et al. [36] analyzed clinical and cytogenetic data from 488 patients with NDMM and soft-tissue plasmacytomas (paraskeletal, n = 374; EMD, n = 87; both, n = 27) who underwent SCT. High-risk cytogenetics were identified in 41% of the patients and found more frequently in those with EMD. Outcomes following single autologous SCT were significantly worsened in the presence of high-risk cytogenetic abnormalities, whereas a tandem autologous transplant strategy was shown to offset the poor prognosis. An analysis of 3744 patients with NDMM found no difference in 3-year PFS following first-line autologous SCT between those with single-site plasmacytomas (any location) and patients without plasmacytomas [28]. However, single EMD involvement was associated with worse 3-year OS compared with no plasmacytomas, which worsened still further when multiple sites of organs were involved. Note that there is some overlap in the patients included in these two studies [28, 36].
EMD in patients with RRMM
A summary of currently available data for treatment outcomes in patients with RRMM and EMD is provided in Table 4.
Table 4.
Treatment outcomes in patients with RRMM and EMD.
| Reference Study type | Total number of patients/ number with EMD | Time period covered | Treatments | PFS | OS | Other |
|---|---|---|---|---|---|---|
| Novel agents (various) and/or SCT | ||||||
|
Avivi et al. [37] Retrospective |
127 (all EMD) | 2010–2018 | First treatment included PIs (50%), IMiDs (39%), monoclonal antibodies (10%), and chemotherapy (53%) | – | – | 57% ORR ( ≥ PR) across all treatments |
|
Rasche et al. [21] Single-center registry |
24 (all EMD) | 2007–2010 |
Radiotherapy (n = 16) ASCT + intense dose chemotherapy (n = 6) Bortezomib (n = 16) Lenalidomide (n = 12) Thalidomide (n = 8) |
Median (95% CI): 2 months (0.08–3.92) | Median (95% CI): 7 months (3.56–10.43) | – |
|
Retrospective |
96 EMD, n = 84 |
2010–2017 | Median two lines of treatment and ASCT (44%) | Median (95% CI): 9.1 months (11.6–15.6) | Median (95% CI): 11.4 months (0.6–16.2) | Complete remission rate: 9% (vs 54.5% in patients with paraskeletal, p < 0.001) |
| IMiDs | ||||||
|
Short et al. [30] Phase 2 trial |
16 (all EMD) | 2007–2010 | Pomalidomide + low-dose dexamethasone | – | Median: 16 months | – |
| PIs | ||||||
|
Zhou et al. [39] Retrospective |
45 EMD, n = 25 |
Carfilzomib + dexamethasone-based | EMD significantly inferior PFS vs paraskeletal (p = 0.004) | EMD significantly inferior OS vs paraskeletal (p = 0.04) | – | |
|
Papanikolaou et al. [40] Retrospective |
28 (all EMD) | 1998–2011 |
At relapse:Bortezomib-containing regimens (32%) Platinum-containing (21%) Lenalidomide (21%) VAD (8%) |
– |
Median following relapse: 5 months Median from MM diagnosis: 38 months |
– |
| Chemotherapy/radiotherapy | ||||||
|
Rasche et al. [41] Retrospective |
11 (all EMD) | 2007–2012 | Dexa-BEAM (including dexamethasone, carmustine, cytarabine, etoposide, and melphalan) | Median: 4 months | – | Objective response (≥PR) achieved in 6/11 patients |
| Recently approved and investigational agents | ||||||
|
Richardson et al. [42] Phase 2 |
55 EMD, n = 27 |
Patients enrolled between Dec 2016 and Oct 2019 | Melflufen + dexamethasone | – | – |
ORR: Non-EMD, 32% Paraskeletal, 25% EMD, 22% |
|
Wang et al. [43] Single-center |
57 EMD, n = 17 |
2016–2018 | LCAR-B38M | Median: 8.1 months (vs 25 months in non-EMD; p < 0.001) | Median: 13.9 months (vs NR in non-EMD; p = 0.0019) | 82% ORR (≥ PR) vs 90% for non-EMD |
ASCT autologous stem cell transplantation, EMD extramedullary disease, MM multiple myeloma, NC not calculable, NR not reached, ORR overall response rate, OS overall survival, PFS progression-free survival, PR partial response, VAD vincristine/adriamycin/dexamethasone.
In a retrospective study of 127 consecutive patients with hematogenous EM relapse [37], first treatments for EMD included PIs (50%), IMiDs (39%), monoclonal antibodies (10%), and chemotherapy (53%). ORR (≥ partial response) was 57% across all treatments and IMiDs were associated with higher ORR compared with PIs (HR 2.2, 95% CI 1.02–4.7; p = 0.04). A single-center analysis of 24 patients with EMD on relapse reported very poor prognosis (median PFS of 2 months and median OS of 7 months) despite treatment with novel agents in 22 of the 24 patients [21].
In the multinational retrospective study conducted by Beksac et al. [31, 38], patients with EMD at relapse had a PFS and OS of 9.1 months and 11.4 months, respectively. The complete remission rate was significantly lower than the rate in patients with bone-related plasmacytomas (9% vs 54.4%, p = 0.001).
Short et al. [30] studied the detailed medical records of 174 consecutive patients with RRMM who enrolled in a phase 2 clinical trial of pomalidomide plus low-dose dexamethasone. Sixteen patients had EMD, and these patients had significantly worse OS from trial entry compared with non-EMD patients (median 16 months vs not reached; p = 0.002).
Zhou et al. [39] retrospectively assessed carfilzomib-containing therapies in 45 patients with RRMM and invovlement of plasmacytomas. PFS and OS are not reported separately for bone-related plasmacytomas and EMD, although EMD without adjacency to bone was associated with a significantly shorter PFS (p = 0.004) and OS (p = 0.04) compared with paraosseous lesions. In a study of 303 patients with MM, including 28 cases of EMD relapse, prior treatment with bortezomib was associated with a decreased hazard of EMD relapse (p = 0.041) [40]. Median OS from MM diagnosis was significantly shorter in the group with EMD relapse than in the non-EMD group (38 months vs 59 months; p = 0.006).
Rasche et al. [41] retrospectively analyzed the polychemotherapy regimen, Dexa-BEAM, in 18 patients with advanced MM (11 had EMD). Objective response (≥ partial response) to Dexa-BEAM was achieved in more than half (6/11) of the patients; subsequent high-dose consolidation strategy with autologous or allogeneic SCT improved upon the depth of remission in two-thirds of EMD patients (4/6) with ongoing remissions in three patients.
A phase 2 study (HORIZON) has assessed melflufen (plus dexamethasone) in patients with heavily pretreated RRMM, and a subgroup analysis of patients with involvement of plasmacytomas has been reported [42]. The study enrolled 157 patients, including 55 with plasmacytomas (bone-related, n = 28; EMD, n = 27). The ORR was 32% in the group without plasmacytoma involvement, 25% in the group with bone-related plasmacytomas, and 22% in the EMD group.
The CAR-T therapy LCAR-B38M has been assessed in a single-center study involving 57 patients (17 with EMD) [43]. At a median follow-up of 25.1 months, median PFS was 8.1 months in the EMD group and 25 months in those without EMD (p < 0.001); median OS was 13.9 months in the EMD group and not reached in those without EMD (p = 0.0019). Published trials of other CAR-T therapies have reported responses in patients with soft-tissue plasmacytomas, but do not report data specifically for patients with EMD [44, 45]. Very recently, data have been presented from a phase 1b/2 study of ciltacabtagene autoleucel [46]; 13 patients with EMD were included, and the authors report similar response rates in the EMD subgroup to those of the overall study population (ORR 94.7%).
Deng et al. [47] have recently reported on the safety and efficacy of humanized anti-B-cell maturation antigen (BCMA) CAR-T therapy in 7 patients with EMD compared with 13 with no EMD involvement. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxic syndrome (ICANS) were higher in patients with EMD, most likely related to the higher tumor burden in this population. While the response rate was similar in both groups, the 1-year PFS and OS rates were significantly shorter in patients with EMD. The unsatisfactory long-term efficacy of anti-BCMA CAR-T therapy in EMD, also highlighted by Wang et al. [43], is worrisome and needs to be further explored in forthcoming trials.
CNS involvement
Available data for treatment outcomes in patients with CNS soft-tissue involvement are summarized in Table 5. In a large (N = 172), retrospective, multi-institutional study, overall median OS after onset of CNS involvement was 6.7 months [48]. Median OS was 2 months for those untreated and 8 months for patients who received treatment for CNS disease. Another study of 16 patients treated with different combinations of systemic therapy, intrathecal chemotherapy, and radiotherapy highlighted a dismal outcome for patients with leptomeningeal involvement (median OS of 82 days) [49].
Table 5.
Treatment outcomes in studies of patients with CNS involvement.
| Reference Study type | No. of patients | Time period covered | Treatments | PFS | OS |
|---|---|---|---|---|---|
|
Bommer et al. [49] Retrospective |
16 (all LMM) | 2005–2016 | Intrathecal chemotherapy, radiotherapy | – |
Median OS: 82 days (after LMM diagnosis) |
|
Jurczyszyn et al. [48] Retrospective |
172 (38 at initial MM diagnosis, 134 at relapse/progression) |
1995–2014 |
Systemic therapy (n = 117) Radiotherapy (n = 56) Intrathecal therapy (n = 49) Steroids only (n = 5) Mass resection (n = 1) SCT (n = 32) |
– |
Median OS: 6.7 months (all patients) 2 months (untreated patients) 8 months (treated patients) |
|
Paludo et al. [50] Retrospective |
29 (7 at initial diagnosis of MM, 22 at relapse) | 1998–2014 |
Radiation therapy (n = 22) + adjuvant intrathecal chemotherapy (n = 6) Intrathecal + systemic therapy (n = 1) Systemic therapy (n = 2) Novel agents, including bortezomib (28%), thalidomide (14%), lenalidomide (10%), and pomalidomide (3%), administered after CNS involvement. ASCT after the diagnosis of CNS disease (24%) |
Median OS (95% CI): CNS involvement, 40 months (24–56) Control (no CNS involvement), 93 months (67–129) Patients with ASCT after CNS involvement, 19 months (10–67) |
|
|
Katodritou et al. [51] Retrospective |
31 | 2000–2013 |
Bortezomib-based (n = 12) IMiD-based (n = 5) Chemotherapy alone (n = 8) Intrathecal infusions (n = 3) Additional radiotherapy (n = 9) |
Median (95% CI) CNS involvement, 16 months (2–30.6) Control (no CNS involvement), 36 months (12–60) p = 0.004 |
Median (95% CI) CNS involvement, 47 months (32–62) Control (no CNS involvement), 84 months (31–137) p = 0.01 |
|
Abdallah et al. [52] Retrospective |
35 | 1996–2012 |
Chemotherapy, including intrathecal (n = 28) Intrathecal alone (n = 3) |
– | Median (range): 4 months (1–13) |
|
Chen et al. [53] Retrospective |
37 | 1999–2010 |
Intrathecal chemotherapy (81%) Cranial and/or spinal irradiation (78%) IMiDs (51%) Cisplatin-based (27%) Bortezomib (19%) Alkylators (11%) Dexamethasone alone (8%) ASCT (5%) |
Median (95% CI) after CNS involvement: 3.1 months (2.0–6.0) | Median (95% CI) after CNS involvement: 4.6 months (2.8–6.7) |
|
Lee et al. [54] Retrospective |
17 | 2000–2011 |
Systemic pharmacotherapy Intrathecal chemotherapy and/or radiotherapy |
– | Median (range) after CNS involvement: 4 months (1–23) |
| Gozzetti et al. [55] | 12 | 2000–2010 |
Systemic treatment Systemic treatment + radiotherapy Systemic + radiotherapy + intrathecal Radiotherapy + intrathecal Intrathecal (note: specific treatments received by patients with CNS-MM not specified) |
– | Median (range): 6 months (1–23) |
ASCT autologous stem cell transplantation, CNS central nervous system, IMiD immunomodulatory drug, LMM leptomeningeal myelomatosis, MM multiple myeloma.
Paludo et al. [50] compared a cohort of MM patients with CNS involvement (n = 29) with a control population of patients without CNS involvement. OS from diagnosis of MM was shorter in the CNS-MM group than in the control group (median 40 months vs 93 months); OS from detection of CNS involvement was 3.4 months. In patients who underwent ASCT after CNS involvement (n = 7), median OS was 19 months (95% CI 10–67 months) from the detection of CNS involvement.
Katoditrou et al. [51] retrospectively reviewed medical records of 31 patients with CNS-MM treated in centers from Greece. Both PFS and OS were significantly shorter in the patients treated for CNS-MM (n = 29) compared with the control group (MM with no CNS involvement), and treatment with novel agents did not confer a survival advantage. Another study retrospectively reviewed patients with CNS-MM (n = 35) identified from the University of Arkansas MM database. [52] Treatment comprised mainly systemic and/or intrathecal chemotherapy, and median OS was 4 months.
Yet another retrospective study, from a single center in Canada, identified 37 patients with CNS-MM [53]. Median OS was only 4.6 months, although nine patients had prolonged survival (median 17.1 months); these longer-term survivors were treated with radiotherapy, intrathecal chemotherapy, and IMiD-based regimens. Lee et al. [54] retrospectively analyzed 17 patients with CNS-MM, reporting a median OS of 4 months from time of CNS involvement. OS was significantly better in patients who received intrathecal chemotherapy than those who did not (20 months vs 2 months, respectively; p < 0.02). Another retrospective survey of 50 patients with intracranial involvement in MM included 12 patients with CNS-MM [55]; within the subgroup of patients with CNS involvement, the median survival was 6 months.
Discussion
The presence of soft-tissue plasmacytomas represents an aggressive form of MM, which can be found at the time of MM diagnosis or at relapse. Several different definitions of extramedullary involvement in MM or EMD have been proposed in the literature. We and others advocate that true EMD is restricted to plasmacytomas that arise due to hematogenous spread and have no contact with bony structures. Typical sites of EMD may vary according to the stage of MM. At diagnosis, EMD is typically found in skin; at relapse, typical sites involved include the liver, kidneys, lymph nodes, breast, pleura and pericardium, and the CNS.
In addition to the variation in definition, the published literature on soft-tissue plasmacytomas is difficult to navigate for several reasons: data may be reported for ‘mixed’ populations of patients with NDMM and RRMM; data may be reported for bone-related plasmacytomas and EMD combined; or a clear definition is lacking the type of plasmacytoma being studied. By including, as far as possible, only studies that clearly define the phase of MM and clearly specify EMD, this review differs from much of the previously published literature in this area.
The reported incidence of EMD varies considerably, and differences in diagnostic approach between studies are likely to contribute to this variability. In patients with NDMM, the reported incidence ranges from 0.5% to 4.8%, while in RRMM the reported incidence is 3.4–14%. Available data demonstrate that the prognosis is poor, and considerably worse than for MM without EMD. For patients with soft-tissue plasmacytomas, those with EMD typically have poorer outcomes than those with paraskeletal involvement. CNS involvement is rare, but prognosis is even more dismal than for EMD in other locations, particularly if there is leptomeningeal involvement.
The outcome of patients with EMD should be reported as a predefined subgroup in clinical trials. In this regard, the prospective IMAJEM study from the French group showed the prognostic impact of the presence of EMD [33]. In the CASSIOPEIA study, from the same group comparing bortezomib/thalidomide and dexamethasone with or without daratumumab as pretransplant induction regimen, a PET/CT substudy (CASSIOPET) is ongoing, which aims to investigate the prognostic impact and response to therapy, including MRD assessments, in patients with EMD [27]. Hopefully, the design of future trials will investigate the prognostic impact and treatment efficacy in patients with EMD.
Available data on treatment outcomes for EMD are almost entirely derived from retrospective studies. Some agents and combinations have shown a degree of efficacy but, as would be expected based on known prognoses, this is typically less than in MM patients with no extramedullary involvement. The paucity of prospective studies makes it difficult to justify strong recommendations for any treatment approach. The recent expert consensus review provided some possible treatment approaches for consideration [4]. For upfront treatment of EMD in transplant-ineligible patients, the addition of daratumumab to VMP or RVD was suggested. In transplant-eligible patients, intensive anti-myeloma/anti-lymphoma regimens (e.g. VTD/ or VRD/PACE combined with SCT) are proposed as a theoretical option [4]. Suggested treatments at relapse are also based on lymphoma-like regimens such as PACE, DCEP, or Dexa-BEAM, although duration of response is typically ≤4 months. Beyond this, novel-agent combinations (e.g. carfilzomib-, selinexor-, or isatuximab-based) or newer/investigational agents (e.g. CAR-T, BiTEs, melflufen) may be considered [4]. For CNS involvement, some combination of IMiD-based systemic therapy, intrathecal and radiation therapy appears to provide the best treatment outcome.
Current response criteria, including MRD assessment, should be applied in patients with EMD. As per the recent consensus on extramedullary involvement in MM, the first assessment of EMD identified by PET/CT (considering size and metabolic uptake) and/or MRI should be done at three months after treatment initiation and at physician discretion thereafter [4]. It is recommended that baseline and follow-up assessments should use the same imaging technique in order to minimize the inter-technique variability. To declare complete remission (CR), all evidence of EMD must have disappeared according to the standardization for metabolic CR definition recently proposed [25], as well as the disappearance of the serum and urine M-protein by immunofixation [56]. In addition, a bone marrow negative for MRD by flow cytometry (FC) will define not only CR, but also MRD negativity (so-called double negative at EM involvement PET/CT and at bone marrow FC) [27, 33]. There is an expectation that liquid biopsy with MRD assessment in peripheral blood may help to further ensure disease eradication in all compartments; however, data on this remains limited.
Prospective data from patients with clearly defined EMD are important for the optimal evaluation of treatment outcomes. Conducting trials that are adequately powered to assess outcomes is a challenge in this uncommon group of patients. In such cases, the inclusion of these patients in trials to allow subgroup assessment of treatment effects with a priori hypotheses may provide the best attainable evidence. There are signs from trials of the next wave of MM treatments that patients with EMD are at last being studied in greater detail, and this is a trend that should be encouraged. Widespread adoption of specific response criteria based on both morphological and functional evaluation, such as those proposed recently by Zamagni et al. [25], will also be important for understanding and comparing the impact of different treatments on EMD.
Acknowledgements
Medical writing support was provided by Tony Reardon of Aura, and Jaya Shumoogam of Ascend, both divisions of Spirit Medical Communications Group Limited, funded by Oncopeptides. Medical writing support was funded by Oncopeptides.
Author contributions
J.B. wrote the first draft and incorporated the comments by the co-authors in all subsequent drafts. M.B., J.C., A.J., M.v.L.-T., P.M., Leo Rasche, Laura Rosiñol, S.Z.U., E.Z., and P.R. made a number of comments on the first and subsequent drafts. All authors approved the final version of the manuscript.
Competing interests
J.B. has received speaker/lecture honoraria from Janssen, Celgene/BMS, Amgen, Takeda, and Oncopeptides. M.B. has received speaker honoraria from Amgen, Oncopeptides, Janssen, Sanofi, and Takeda. Jo Caers has received research funding from Takeda and Janssen; and honoraria from Janssen, Amgen, BMS-Celgene, and Sanofi. A.J. has received honoraria from Janssen, BMS, Takeda, Sanofi, GSK, Karyopharm, Oncopeptides, and Amgen. M.v.L.-T. has received research funding from Celgene and Oncopeptides; and honoraria from BMS/Celgene, Oncopeptides, Janssen, GSK, and Takeda. Philippe Moreau has received honoraria from Janssen, Celgene, Amgen, AbbVie, Oncopeptides, and Sanofi. Leo Rasche has received honoraria from BMS/Celgene, Sanofi, GSK, Oncopeptides, and Janssen. Laura Rosiñol has received speaker honoraria from Janssen, Celgene, Amgen, Takeda, GSK, and Sanofi. S.Z.U. has received research funding and/or honoraria from Amgen, Celgene, Janssen, Takeda, BMS, Oncopeptides, and Sanofi. E.Z. has received honoraria from, and been an advisory board member for, Janssen, BMS, Takeda, Sanofi, GSK, Karyopharm, Oncopeptides, and Amgen. P.R. has received institutional research support from Oncopeptides, Celgene/BMS, Takeda, and Karyopharm. He has received honoraria for his role as an Advisory Committee member from Karyopharm, Oncopeptides, Celgene/BMS, Takeda, Janssen, Sanofi, Secura Bio, GSK, Regeneron, AstraZeneca, and Protocol Intelligence.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 2.Bladé J, Fernández de Larrea C, Rosiñol L, Cibeira MT, Jiménez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29:3805–12. doi: 10.1200/JCO.2011.34.9290. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Jelloul F, Zhang Y, Bhavsar T, Ho C, Rao M, et al. Genetic basis of extramedullary plasmablastic transformation of multiple myeloma. Am J Surg Pathol. 2020;44:838–48. doi: 10.1097/PAS.0000000000001459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosiñol L, Beksac M, Zamagni E, Van de Donk NWCJ, Anderson KC, Badros A, et al. Expert review of soft-tissue plasmacytomas in multiple myeloma: definition, disease assessment and treatment considerations. Br. J. Haematol. 2021; 10.1111/bjh.17338. [DOI] [PubMed]
- 5.Rosiñol L, Fernández de Larrea C, Bladé J. Extramedullary myeloma spread triggered by surgical procedures: an emerging entity? Acta Haematol. 2014;132:36–8. doi: 10.1159/000354833. [DOI] [PubMed] [Google Scholar]
- 6.Muchtar E, Raanani P, Yeshurun M, Shpilberg O, Magen-Nativ H. Myeloma in scar tissue—an underreported phenomenon or an emerging entity in the novel agents’ era? A single center series. Acta Haematol. 2014;132:39–44. doi: 10.1159/000354830. [DOI] [PubMed] [Google Scholar]
- 7.Weinstock M, Ghobrial IM. Extramedullary multiple myeloma. Leuk Lymphoma. 2013;54:1135–41. doi: 10.3109/10428194.2012.740562. [DOI] [PubMed] [Google Scholar]
- 8.Sevcikova S, Minarik J, Stork M, Jelinek T, Pour L, Hajek R. Extramedullary disease in multiple myeloma - controversies and future directions. Blood Rev. 2019;36:32–9. doi: 10.1016/j.blre.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Jagosky MH, Usmani SZ. Extramedullary disease in multiple myeloma. Curr Hematol Malig Rep. 2020;15:62–71. doi: 10.1007/s11899-020-00568-3. [DOI] [PubMed] [Google Scholar]
- 10.Caers J, Paiva B, Zamagni E, Leleu X, Bladé J, Kristinsson SY, et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel. J Hematol Oncol. 2018;11:10. doi: 10.1186/s13045-017-0549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández de Larrea C, Kyle RA, Durie BGM, Ludwig H, Usmani S, Vesole DH, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27:780–91. doi: 10.1038/leu.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood. 2016;127:971–6. doi: 10.1182/blood-2015-07-635383. [DOI] [PubMed] [Google Scholar]
- 13.Bhutani M, Foureau DM, Atrash S, Voorhees PM, Usmani SZ. Extramedullary multiple myeloma. Leukemia. 2020;34:1–20. doi: 10.1038/s41375-019-0660-0. [DOI] [PubMed] [Google Scholar]
- 14.Chang H, Sloan S, Li D, Stewart AK. Multiple myeloma invoving central nervous system: high frequency of 17p13 (p53) deletions. Br J Haematol. 2014;127:280–4. doi: 10.1111/j.1365-2141.2004.05199.x. [DOI] [PubMed] [Google Scholar]
- 15.López-Anglada L, Gutiérrez NC, García JL, Mateos MV, Flores T, San Miguel JF. P53 deletion may drive the clinical evolution and treatment response in multiple myeloma. Eur J Haematol. 2010;84:359–61. doi: 10.1111/j.1600-0609.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- 16.Sheth N, Yeung J, Chang H. P53 nuclear accumulation is associated with extramedullary progression of multiple myeloma. Leuk Res. 2009;33:1357–60. doi: 10.1016/j.leukres.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Billecke L, Murga Penas EM, May AM, Engelhardt M, Nagler A, Leiba M, et al. Cytogenetics in multiple myeloma patients progressing into extramedullary disease. Br J Haematol. 2013;161:87–94. doi: 10.1111/bjh.12223. [DOI] [PubMed] [Google Scholar]
- 18.Deng S, Xu Y, An G, Sui W, Zou D, Zhao Y, et al. Features of extramedullary disease of multiple myeloma: high frequency of p53 deletion and poor survival: a retrospective single-center study of 834 cases. Clin Lymphoma Myeloma Leuk. 2015;15:286–91. doi: 10.1016/j.clml.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Katodritou E, Gastari V, Verrou E, Hadjiaggelidou C, Varthaliti M, Georgiadou S, et al. Extramedullary (EMP) relapse in unusual locations in multiple myeloma: is there an association with precedent thalidomide administration and a correlation of special biological features with treatment and outcome? Leuk Res. 2009;33:137–40. doi: 10.1016/j.leukres.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 20.Usmani SZ, Heuck C, Mitchell A, Szymonifka J, Nair B, Hoering A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica. 2012;97:1761–7. doi: 10.3324/haematol.2012.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasche L, Bernard C, Topp MS, Kapp M, Duell J, Wesemeier C, et al. Features of extramedullary myeloma relapse: high proliferation, minimal marrow involvement, adverse cytogenetics: a retrospective single-center study of 24 cases. Ann Hematol. 2012;91:1031–7. doi: 10.1007/s00277-012-1414-5. [DOI] [PubMed] [Google Scholar]
- 22.Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21:325–30. doi: 10.1093/annonc/mdp329. [DOI] [PubMed] [Google Scholar]
- 23.Pour L, Sevcikova S, Greslikova H, Kupska R, Majkova P, Zahradova L, et al. Role of dynamic contrast-enhanced sonography for characterization and monitoring of extramedullary myeloma. Haematologica. 2014;99:360–4. doi: 10.3324/haematol.2013.094409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavo M, Terpos E, Nanni C, Moreau P, Lentzsch S, Zweegman S, et al. Role of 18 F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017;18:e206–e217. doi: 10.1016/S1470-2045(17)30189-4. [DOI] [PubMed] [Google Scholar]
- 25.Zamagni E, Nanni C, Dozza L, Carlier T, Bailly C, Tacchetti P, et al. Standardization of (18)F-FDG-PET/CT according to Deauville criteria for metabolic complete response definition in newly diagnosed multiple myeloma. J Clin Oncol. 2021;39:116–25. doi: 10.1200/JCO.20.00386. [DOI] [PubMed] [Google Scholar]
- 26.Montefusco V, Gay F, Spada S, De Paoli L, Di Raimondo F, Ribolla R, et al. Outcome of paraosseous extra-medullary disease in newly diagnosed multiple myeloma patients treated with new drugs. Haematologica. 2020;105:193–200. doi: 10.3324/haematol.2019.219139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreau P, Zweegman S, Perrot A, Hulin C, Caillot D, Facon T, et al. Evaluation of the prognostic value of positron emission tomography-computed tomography (PET-CT) at diagnosis and follow-up in transplant-eligible newly diagnosed multiple myeloma (TE NDMM) patients treated in the phase 3 Cassiopeia Study: Results of the Cassiopet companion study. Blood. 2019;134(Supplement_1):692. [Google Scholar]
- 28.Gagelmann N, Eikema DJ, Iacobelli S, Koster L, Nahi H, Stoppa AM, et al. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: a study from the Chronic Malignancies Working Party of the EBMT. Haematologica. 2018;103:890–7. doi: 10.3324/haematol.2017.178434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstock M, Aljawai Y, Morgan EA, Laubach J, Gannon M, Roccaro AM, et al. Incidence and clinical features of extramedullary multiple myeloma in patients who underwent stem cell transplantation. Br J Haematol. 2015;169:851–8. doi: 10.1111/bjh.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Short KD, Rajkumar SV, Larson D, Buadi F, Hayman S, Dispenzieri A, et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25:906–8. doi: 10.1038/leu.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beksac M, Seval GC, Kanellias N, Coriu D, Rosiñol L, Ozet G, et al. A real world multicenter retrospective study on extramedullary disease from Balkan Myeloma Study Group and Barcelona University: analysis of parameters that improve outcome. Haematologica. 2020;105:201–8. doi: 10.3324/haematol.2019.219295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciftciler R, et al. Evaluation of the survival outcomes of multiple myeloma patients according to their plasmacytoma presentation at diagnosis. Turk J Haematol. 2019;37:256–62. doi: 10.4274/tjh.galenos.2019.2019.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreau P, Göker H, Demiroğlu H, Aksu S, Sayınalp N, Haznedaroğlu IC, et al. Prospective evaluation of magnetic resonance imaging and [18F]fluorodeoxyglucose positron emission tomography-computed tomography at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma included in the IFM/DFCI 2009 trial: results of the IMAJEM study. J Clin Oncol. 2017;35:2911–8. doi: 10.1200/JCO.2017.72.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badar T, Srour S, Bashir Q, Shah N, Al-Atrash G, Hosing C, et al. Predictors of inferior clinical outcome in patients with standard-risk multiple myeloma. Eur J Haematol. 2017;98:263–8. doi: 10.1111/ejh.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batsukh K, Lee SE, Min GJ, Park SS, Jeon YW, Yoon JH, et al. Distinct clinical outcomes between paramedullary and extramedullary lesions in newly diagnosed multiple myeloma. Immune Netw. 2017;17:250–60. doi: 10.4110/in.2017.17.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gagelmann N, Eikema DJ, Koster L, Caillot D, Pioltelli P, Lleonart JB, et al. Tandem autologous stem cell transplantation improves outcomes in newly diagnosed multiple myeloma with extramedullary disease and high-risk cytogenetics: a study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2019;25:2134–42. doi: 10.1016/j.bbmt.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Avivi I, Cohen YC, Suska A, Shragai T, Mikala G, Garderet L, et al. Hematogenous extramedullary relapse in multiple myeloma - a multicenter retrospective study in 127 patients. Am J Hematol. 2019;94:1132–40. doi: 10.1002/ajh.25579. [DOI] [PubMed] [Google Scholar]
- 38.Beksac M, Seval GC, Kanellias N, Coriu D, Rosiñol L, Ozet G, et al. A real world multicenter retrospective study on extramedullary disease from Balkan Myeloma Study Group and Barcelona University: analysis of parameters that improve outcome. Haematologica. 2020;106:1228. doi: 10.3324/haematol.2020.278272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, Flüchter P, Nickel K, Meckel K, Messerschmidt J, Böckle D, et al. Carfilzomib based treatment strategies in the management of relapsed/refractory multiple myeloma with extramedullary disease. Cancers (Basel) 2020;12:1035. doi: 10.3390/cancers12041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papanikolaou X, Repousis P, Tzenou T, Maltezas D, Kotsopoulou M, Megalakaki K, et al. Incidence, clinical features, laboratory findings and outcome of patients with multiple myeloma presenting with extramedullary relapse. Leuk Lymphoma. 2013;54:1459–64. doi: 10.3109/10428194.2012.746683. [DOI] [PubMed] [Google Scholar]
- 41.Rasche L, Strifler S, Duell J, Rosenwald A, Buck A, Maeder U, et al. The lymphoma-like polychemotherapy regimen “Dexa-BEAM” in advanced and extramedullary multiple myeloma. Ann Hematol. 2014;93:1207–14. doi: 10.1007/s00277-014-2023-2. [DOI] [PubMed] [Google Scholar]
- 42.Richardson PG, Mateos MV, Oriol A, Larocca A, Cavo M, Rodríguez-Otero P, et al. HORIZON (OP-106): melflufen plus dexamethasone (dex) in 55 patients (pts) with relapsed/refractory multiple myeloma with extramedullary disease (EMD)—subgroup analysis. Blood. 2020;136(Supplement 1):15–17. [Google Scholar]
- 43.Wang B, Liu J, Zhao WH, Chen YX, Cao XM, Yang Y, et al. Chimeric antigen receptor T cell therapy in the relapsed or refractory multiple myeloma with extramedullary disease-a single institution observation in China. Blood. 2020;136(Suppl 1):6. [Google Scholar]
- 44.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl J Med. 2019;380:1726–37. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munshi NC, Anderson LD, Jr, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl J Med. 2021;384:705–16. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 46.Usmani SZ, Berdeja JG, Madduri D, Jakubowiak AJ, Agha ME, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen–directed chimeric antigen receptor T-cell therapy, in relapsed/refractory multiple myeloma: updated results from CARTITUDE-1. Presented at ASCO 2021. Available from: https://www.oncologysciencehub.com/OncologyAM2021/cilta-cel/Usmani/ (Accessed June 2021).
- 47.Deng H, Liu M, Yuan T, Zhang H, Cui R, Li J, et al. Efficacy of humanized Anti-BCMA CAR T Cell therapy in relapsed/refractory multiple myeloma patients with and without extramedullary disease. Front Immunol. 2021;12:720571. doi: 10.3389/fimmu.2021.720571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jurczyszyn A, Grzasko N, Gozzetti A, Czepiel J, Cerase A, Hungria V, et al. Central nervous system involvement by multiple myeloma: a multi-institutional retrospective study of 172 patients in daily clinical practice. Am J Hematol. 2016;91:575–80. doi: 10.1002/ajh.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bommer M, Kull M, Teleanu V, Schwarzwälder P, Feuring-Buske M, Kroenke J, et al. Leptomeningeal myelomatosis: a rare but devastating manifestation of multiple myeloma diagnosed using cytology, flow cytometry, and fluorescent in situ hybridization. Acta Haematol. 2018;139:247–54. doi: 10.1159/000489484. [DOI] [PubMed] [Google Scholar]
- 50.Paludo J, Painuly U, Kumar S, Gonsalves WI, Rajkumar V, Buadi F, et al. Myelomatous involvement of the central nervous system. Clin Lymphoma Myeloma Leuk. 2016;16:644–54. doi: 10.1016/j.clml.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Katodritou E, Terpos E, Kastritis E, Delimpasis S, Symeonidis AS, Repousis P, et al. Lack of survival improvement with novel anti-myeloma agents for patients with multiple myeloma and central nervous system involvement: the Greek Myeloma Study Group Experience. Ann Hematol. 2015;94:2033–42. doi: 10.1007/s00277-015-2484-y. [DOI] [PubMed] [Google Scholar]
- 52.Abdallah AO, Atrash S, Shahid Z, Jameel M, Grazziutti M, Apewokin S, et al. Patterns of central nervous system involvement in relapsed and refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2014;14:211–4. doi: 10.1016/j.clml.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Chen CI, Masih-Khan E, Jiang H, Rabea A, Cserti-Gazdewich C, Jimenez-Zepeda VH, et al. Central nervous system involvement with multiple myeloma: long term survival can be achieved with radiation, intrathecal chemotherapy, and immunomodulatory agents. Br J Haematol. 2013;162:483–8. doi: 10.1111/bjh.12414. [DOI] [PubMed] [Google Scholar]
- 54.Lee D, Kalff A, Low M, Gangatharan S, Ho P, Bajel A, et al. Central nervous system multiple myeloma-potential roles for intrathecal therapy and measurement of cerebrospinal fluid light chains. Br J Haematol. 2013;162:371–5. doi: 10.1111/bjh.12404. [DOI] [PubMed] [Google Scholar]
- 55.Gozzetti A, Cerase A, Lotti F, Rossi D, Palumbo A, Petrucci MT, et al. Extramedullary intracranial localization of multiple myeloma and treatment with novel agents: a retrospective survey of 50 patients. Cancer. 2012;118:1575–84. doi: 10.1002/cncr.26447. [DOI] [PubMed] [Google Scholar]
- 56.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]