Abstract
Tumor lysis syndrome (TLS) is a life-threatening oncological emergency. Only one TLS case has been reported in patients with esophageal cancer. We report the case of a 61-year-old man with recurrent spontaneous TLS caused by esophageal cancer. He was admitted to our hospital to investigate low back pain and dysphagia. Endoscopy and computed tomography revealed esophageal cancer with multiple liver and bone metastases. He was diagnosed with laboratory TLS based on high serum uric acid and phosphorus. After intravenous fluids and allopurinol were administrated, chemotherapy with 5-fluorouracil and cisplatin was started the next day. Although he transiently developed clinical TLS, it was resolved with conservative treatment. However, mild renal dysfunction was prolonged and cisplatin was reduced in the second course. As a consequence, recurrence of spontaous TLS (sTLS) was induced at the end of the course. In the third course, docetaxel was added to the regimen, and since then the patient have not develop sTLS. To the best of our knowledge, this is the first report regarding recurrent sTLS developed on the basis of solid tumors and was successfully controlled by chemotherapy. Although TLS complications are rare in esophageal cancer, early diagnosis and the adjustment of regimen resulted in stable chemotherapy.
Keywords: Tumor lysis syndrome, Esophageal cancer, Spontaneous tumor lysis syndrome, Laboratory tumor lysis syndrome, Chemotherapy
Introduction
Tumor lysis syndrome (TLS) occurs when cancer cells release their contents into the bloodstream, either spontaneously or in response to chemotherapy, resulting in metabolic abnormalities that include hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia [1, 2]. These metabolic complications can progress to clinical toxic states, including renal insufficiency, cardiac arrhythmias, seizures, and death due to multiorgan failure [1, 2]. TLS is more common in hematologic cancers than solid cancers, including gastrointestinal cancers. Notably, only one TLS case has been reported in patients with esophageal cancer [3]. Moreover, to the best of our knowledge, there is no previous report about recurrent spontaneous TLS (sTLS) in patients with solid cancer. In this study, we report a case of esophageal cancer with recurrent sTLS that was improved by adjusting the chemotherapy regimen.
Case presentation
A 61-year-old man was admitted to our hospital because of low back pain and dysphagia. He had been suffering from low back pain for a month. The pain gradually worsened and was accompanied by hypoalgesia of his left leg and movement disorders. Additionally, he had dysphagia for solids in the past month. His latest medical examination was 2 years ago and was normal. He had a history of a hemorrhagic gastric ulcer 4 years ago, treated using endoscopic intervention at another hospital, and a history of smoking and alcohol consumption of 1 L of beer a day.
On admission, his vital signs were normal. Physical examination revealed swelling of left supraclavicular lymph nodes, lower back pain on palpation, decreased sensation, and weakness from the left buttock to the posterior left lower limb. Blood tests revealed an elevated C-reactive protein (CRP) of 9.11 mg/dL, a high lactate dehydrogenase (LDH) of 1141 U/L, a high AST, 44 U/L, creatine phosphatase (CPK) of 514 U/L, and normal potassium (K), phosphorus (P), and calcium (Ca). Serum squamous cell carcinoma antigen (SCC Ag), carcinoembryonic antigen, and carbohydrate antigen 19-9 were normal. Computed tomography (CT) revealed a wall thickening of the esophagus with multiple metastases in the liver, right pubis, and supraclavicular and paraesophageal lymph nodes (Fig. 1). Magnetic resonance imaging showed metastatic bone tumors in the thoracic and sacral vertebrae with infiltration into the spinal canal (Fig. 2). Endoscopy revealed an advanced semicircular cancer in the lower thoracic esophagus (Fig. 3). Pathological analysis revealed atypical cells with a high nuclear–cytoplasmic (N/C) ratio proliferating under the epithelium in an irregular honeycomb pattern (Fig. 4A, B). These atypical cells were positive for P40 and negative for neuroendocrine markers (Synaptophysin, Chromogranin A, and CD56) (Fig. 4C–F), suggesting poorly differentiated SCC. He was diagnosed with stage IVb (T3, N3, M1) esophageal cancer according to the Japanese classification of esophageal cancer.
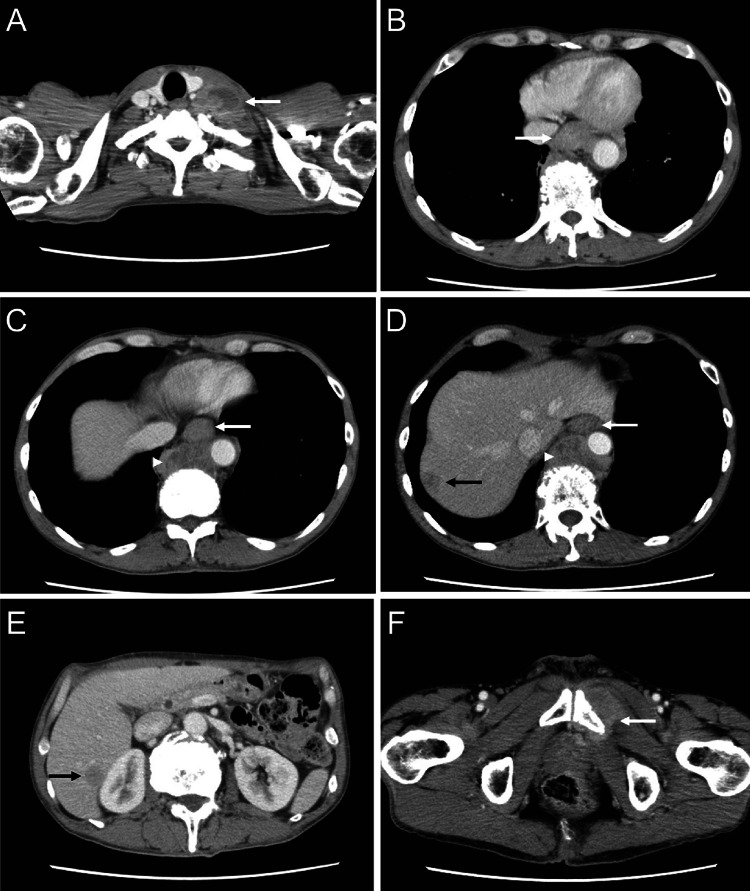
Fig. 1.
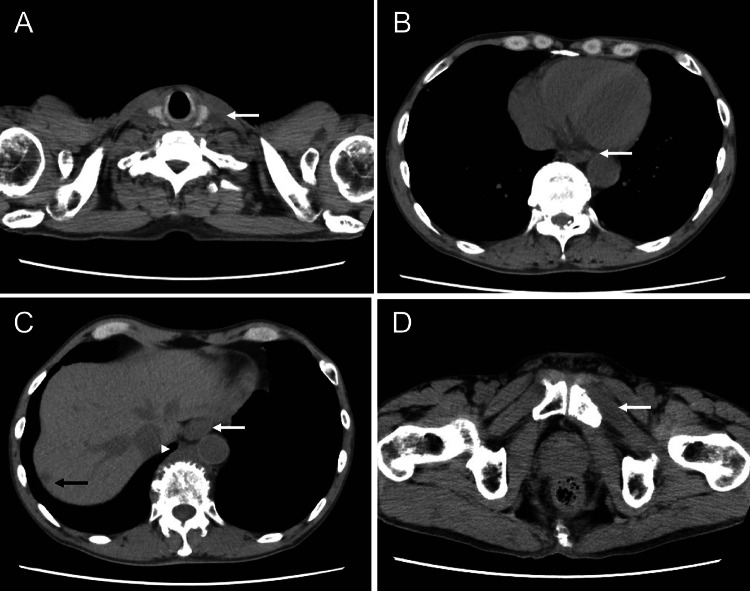
CT at first admission suggesting lower thoracic esophageal cancer with multiple metastases in the liver, lymph nodes, and bones. A Swollen left supraclavicular lymph nodes (31 mm, arrow) with poor internal enhancement, suggesting metastasis. B–E Wall thickening of the lower thoracic esophagus (white arrow), swelling of the paraesophageal lymph nodes (34 mm, white arrowhead), and multiple hypovascular tumors in the liver (black arrow). F Tumor infiltrating the left pubis (white arrow)
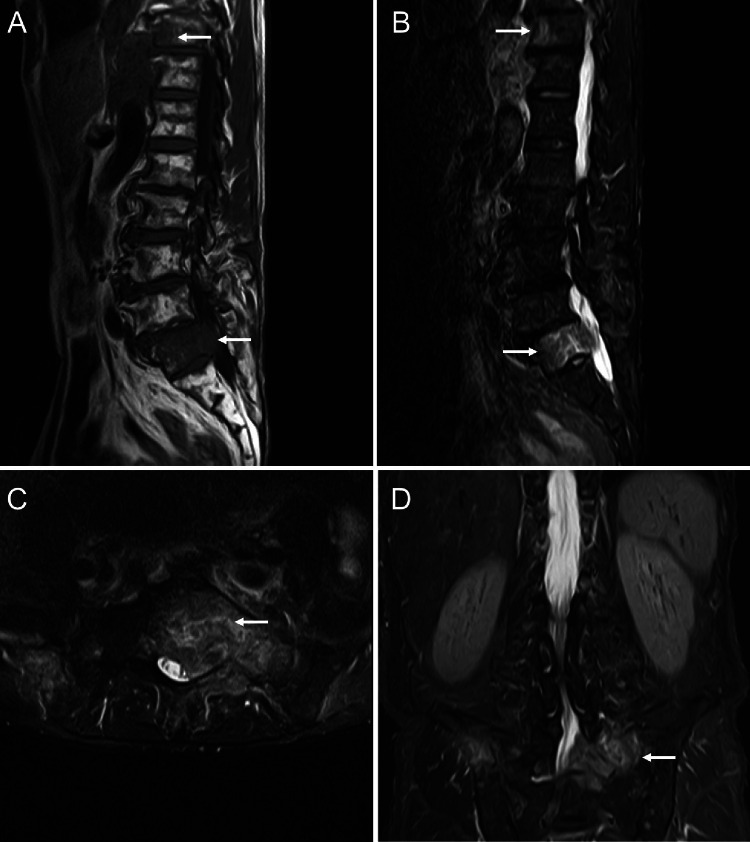
Fig. 2.
Magnetic resonance imaging on the first admission suggests bone metastasis with infiltration in the spinal canal. A T1-weighted image shows low signal intensity in the thoracic and sacral vertebra (arrow). B T2-weighted short TI inversion recovery (T2W-STIR) image shows high signal intensity in thoracic and sacral vertebra (arrow), suggesting bone metastasis. C, D T2W-STIR image (C axial), (D coronal) showing tumor infiltration in the spinal canal
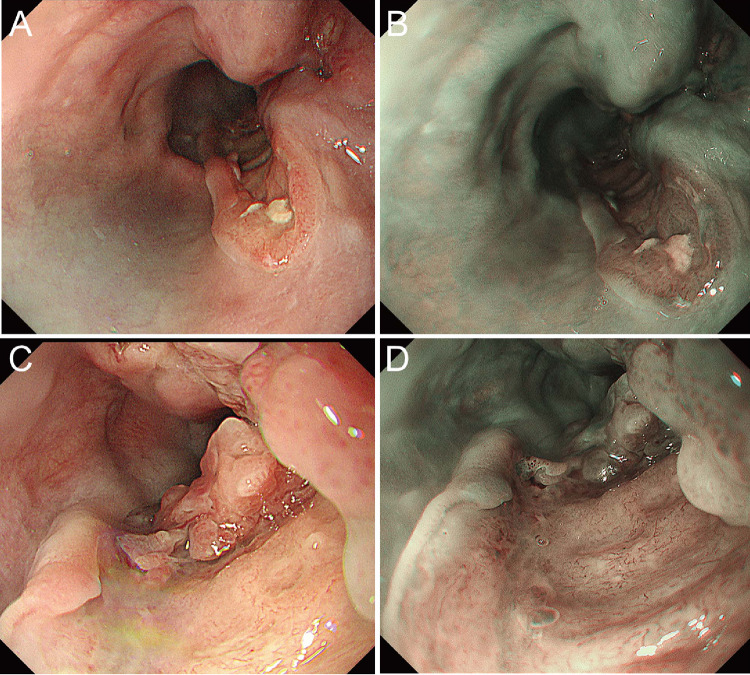
Fig. 3.
Endoscopy revealed semicircular advanced esophageal cancer showing SMT-like growth. A, B Distant view in the lower thoracic esophagus. A White light imaging (WLI) revealed a semiperipheral advanced esophageal cancer showing SMT-like growth with severe wall deformity. B Narrowband imaging (NBI) revealed a brownish area in the recess, not in the periphery. C, D A close-up of advanced esophageal cancer. C WLI revealed mucosa with an irregular structure in the recess, but no ulcer was found. D NBI revealed irregular microvascularity in the recess
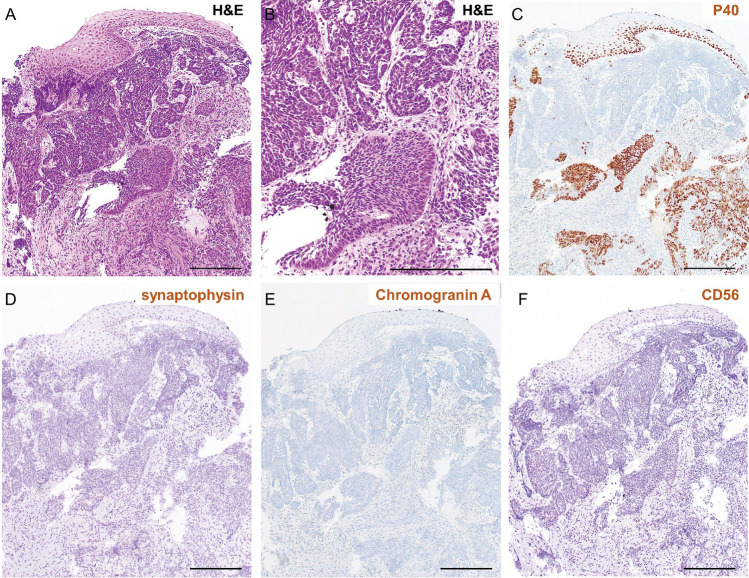
Fig. 4.
Poorly differentiated squamous cell carcinoma diagnosed by pathological analysis. A, B Hematoxylin and eosin (H&E) staining in low (A) and high (B) magnification. Atypical cells with a high nuclear–cytoplasmic (N/C) ratio proliferate under the epithelium in an irregular honeycomb pattern. Scale bar: 100 μm. C P40 immunostaining revealed many atypical P40 positive cells and some P40 negative cells. D–F Neuroendocrine marker immunostaining is shown. D Synaptophysin and E Chromogranin A and F CD56 immunostaining revealed that almost all the atypical cells were negative for these markers
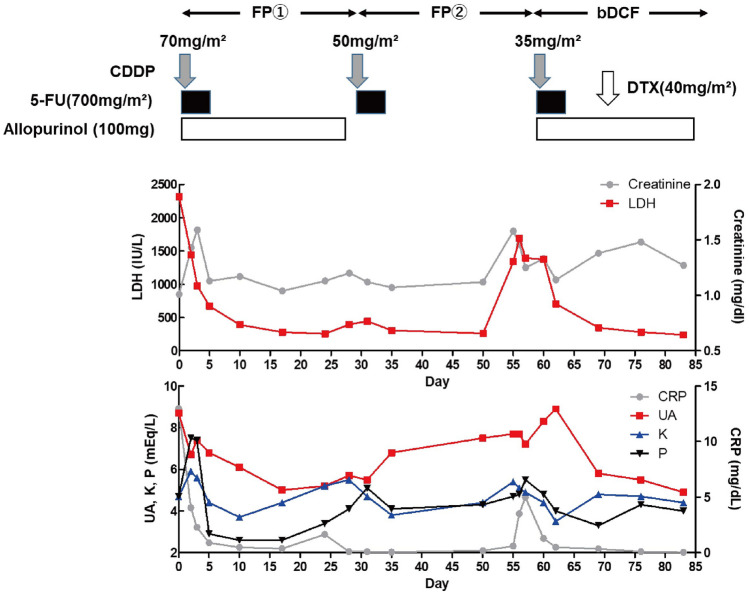
Twelve days after admission, he had a fever of 37.2 °C and general malaise. Additionally, his CRP, LDH, and CPK levels increased along with uric acid (UA) 8.7 mg/dL, P 4.7 mg/dL, K 4.7 mEq/L, and creatinine (Cr) 1.01 mg/dL (Table 1), suggesting laboratory tumor lysis syndrome according to TLS panel consensus criteria [1]. After the administration of intravenous fluids and allopurinol, chemotherapy with 700 mg/m2 of 5-fluorouracil (5-FU) and 70 mg/m2 of cisplatin was started the next day (Day 1, Fig. 5). After chemotherapy, Cr as well as K and P levels increased (Day 1–Day 4, Fig. 5), suggesting clinical TLS according to TLS panel consensus criteria. Conversely, CRP and LDH decreased (Day 1–Day 4, Fig. 5), suggesting effective chemotherapy. By increasing the fluid infusion and administering diuretics, K, P, and Cr levels as well as CRP and LDH decreased (Day 6–Day 11, Fig. 5). Consistently, the patient’s fever and general malaise gradually improved. No blood test alterations were observed after discharge (Day 18–Day 28, Fig. 5). As the second course of chemotherapy, cisplatin was reduced to 50 mg/m2 because of the prolonged renal dysfunction (Day 29, Fig. 5). Although the patient had almost no symptoms during the second course (Day 29–Day 51, Fig. 5), he suddenly showed the temperature 37.3 °C and general malaise again as well as increased K, P, Cr, CRP and LDH levels (Day 56, Fig. 5), suggesting the relapse of spontaneous TLS (sTLS). Suprisingly, CT performed after two course of chemotherapy showed a reduction in the tumor diameter (Fig. 6). Although chemotherapy was considered to be effective for the tumor in CT evaluation, reduced cisplatin dose would weaken the suppression of tumor cell proliferation at the end of the second course. After the administration of intravenous fluids and allopurinol again, the third course of chemotherapy with the reduction of cisplatin to 35 mg/m2 (due to renal dysfunction) was started (Day 57, Fig. 5). After chemotherapy, his symptoms as well as K, P, CRP and LDH levels gradually improved (Day 57–Day 70, Fig. 5). In this course, 40 mg/m2 of docetaxel was added to the regimen to avoid the relapse of sTLS (Day 71, Fig. 5). As the results, the patient did not relapse sTLS in this course (Day 71–Day 84, Fig. 5) and he will receive a forth course of chemotherapy.
Table 1.
Laboratory data 12 days after the first admission
| WBC | 6200/μL | GGT | 87 IU/L |
| RBC | 349 10^4/μL | CPK | 909 IU/L |
| Hemoglobin | 12.1 g/dL | BUN | 13.6 mg/dL |
| Platelets | 39.3 10^4/μL | Creatinine | 1.01 mg/dL |
| PT | 90% | UA | 8.7 mEq/L |
| APTT | 28.7 s | Na | 131 mEq/L |
| CRP | 12.97 mg/dL | K | 4.7 mEq/L |
| Albumin | 3.7 g/dL | Ca | 9.0 mEq/L |
| Total bilirubin | 0.4 mg/dL | P | 4.7 mEq/L |
| AST | 67 IU/L | CEA | 2.4 ng/mL |
| ALT | 34 IU/L | CA19-9 | 2.7 U/mL |
| LDH | 2318 IU/L | SCC | 1.1 ng/mL |
WBC white blood cell, RBC red blood cell, PT prothrombin time, APTT activated partial thromboplastin time, CRP C-reactive protein, AST aspartate aminotransferase, ALT alanine aminotransferase, LDH lactate dehydrogenase, GGT gamma-glutamyl transferase, CPK creatine phosphokinase, BUN urea nitrogen, UA uric acid, Na natrium, K kalium, Ca calcium, P phosphorus, CEA carcinoembryonic antigen, CA19-9 carbohydrate antigen 19-9, SCC squamous-cell-carcinoma-antigen
Fig. 5.
Schema of the treatment and laboratory data after chemotherapy. Elevated serum lactate dehydrogenase (LDH), C-reactive protein (CRP), uric acid (UA) and phosphorus (P) levels in day 0 indicated laboratory TLS according to TLS panel consensus criteria. As chemotherapy started, creatinin, kalium (K) and P levels increased (Day 1–Day 4), suggesting clinical TLS. These laboratory abnormalities except for mild renal dysfunction were recovered by conservative therapy (Day 6–Day 28). Increase in LDH, CRP, UA, K, P and creatinin levels observed at the end of second course (Day 56) suggested the relapse of spontaneous TLS (sTLS), which was gradually improved after further chemotherapy with additional administration of docetaxel. FP chemotherapy with 5-fluorouracil (5-FU) and cisplatin (CDDP), bDCF chemotherapy with 5-FU, CDDP and docetaxel (DTX), Day 0 one day before the administration of chemotherapy, Day X X days from the administration of chemotherapy
Fig. 6.
CT performed after two course of chemotherapy showed a decrease in tumor diameter. A Swollen left supraclavicular lymph nodes (9 mm, arrow) decreased when compared with before chemothrapy. B, C Wall thickening of the lower thoracic esophagus (white arrow) along with decreasing paraesophageal lymph nodes (20 mm, white arrowhead) and liver metastasis (9 mm, black arrow). D Decreasing tumor infiltrating the left pubis (white arrow)
Discussion
TLS is a life-threatening oncological emergency characterized by metabolic abnormalities. It can occur rapidly after chemotherapy [1]. Complications resulting from TLS can disturb continuous chemotherapy and influence morbidity and mortality [1, 4]. TLS often occurs in hematologic cancers after chemotherapy; however, it can also occur in solid tumors with high proliferative activity or large tumor size or burden [1, 5]. Other risks of developing TLS in solid tumors include liver metastasis, a high LDH or UA level, high sensitivity to chemotherapy, pretreatment renal dysfunction, treatment with nephrotoxic drugs, infection, and dehydration [1]. Spontaneous TLS in solid tumors is a rare phenomenon [6]; thus, the risk of developing spontaneous TLS has not been well defined.
Only one TLS case has been reported in a patient with esophageal cancer [3]. In that case, the patient was diagnosed with a highly differentiated squamous cell esophageal cancer with multiple bone and lung metastases. The patient developed TLS after chemotherapy with docetaxel and nedaplatin [3]. Although the patient survived with treatment with allopurinol and a large fluid infusion, his renal function was irreversibly impaired (approximately 5 mg/dL) [3].
In our case, the patient was considered as high risk for TLS at the first admission because of high LDH, liver metastasis, and rapidly progressing symptoms as a sign of TLS [7]; however, solid tumors are classified as low risk for TLS (incidence < 1%) [1]. Expectedly, the patient developed laboratory TLS when esophageal cancer was pathologically diagnosed (12 days after the first admission). After the administration of intravenous fluids and allopurinol, chemotherapy was started the next day. Consequently, the patient transiently developed clinical TLS because of chemotherapy cytotoxicity and the nephrotoxic effect of cisplatin. In the previous review of spontaneous TLS associated with solid tumors, the mortality rate was 69%, higher than spontaneous TLS associated with hematologic malignancies [8]. In our case, however, TLS was resolved within 6 days owing to conservative treatment, including fluids, diuretics and allopurinol. One possible explanation for this outcome is that chemotherapy affects tumors by cytotoxicity and cell cycle suppression [9, 10]. Consistently, the previous review of spontaneous TLS revealed that patients receiving chemotherapy had a higher survival rate (86%) than patients with conservative therapy (14%) [8], although patient’s general condition at the time of the diagnosis of TLS may have affected the prognosis. Due to mild renal dysfunction caused by TLS, cisplatin was reduced in the second course, but it caused the recurrence of sTLS at the end of that course. In the third course, additional administration of docetaxel prevented the recurrence of sTLS, although cisplatin was further reduced due to renal dysfunction. There is no previous report regarding recurrent sTLS developed on the basis of solid tumors and was successfully controlled by chemotherapy. Serum calcium level is known to decrease due to the hyperphosphatemia in the TLS patients. However, calcium level was always normal in our case, suggesting early detection and intervention of TLS would have prevented secondary hypocalcemia. As our case shows, in solid tumors with sTLS, adjustment of the regimen according to the symptoms and laboratory data can benefit the continuation of chemotherapy. As mentioned above, chemotherapy in sTLS may be beneficial, whereas it risks exacerbating TLS and can lead to the patient’s death. Nevertheless, early detection and early chemotherapy could be beneficial for patients with spontaneous TLS if an adequate explanation about the choices and the risk of treatment is provided. Moreover, a more objective and prospective cohort study is expected in the future.
In conclusion, we report a case of a patient with recurrent sTLS caused by esophageal cancer and was successfully controlled by adjusting chemotherapy. This case suggests the importance of early consideration and detection of TLS, even in tumors rarely associated with TLS.
Acknowledgements
The authors would like to thank Enago (http://www.enago.jp) for the English language review.
Declarations
Conflict of interest
The authors state that they have no conflict of interest (COI).
Informed consent and confidentiality
Written informed consent was given by the patient for the publication of this manuscript. Identifying information, aside from age and sex, was removed and the images provided were anonymized to protect patient confidentiality.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cairo MS, Coiffier B, Reiter A, et al. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578–586. doi: 10.1111/j.1365-2141.2010.08143.x. [DOI] [PubMed] [Google Scholar]
- 2.Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tokunou K, Takeda S, Yoshino S, et al. A case of esophageal cancer patient who developed tumor lysis syndrome after chemotherapy. Gan To Kagaku Ryoho. 2008;35:2030–2032. [PubMed] [Google Scholar]
- 4.Hsu HH, Chan YL, Huang CC. Acute spontaneous tumor lysis presenting with hyperuricemic acute renal failure: clinical features and therapeutic approach. J Nephrol. 2004;17:50–56. [PubMed] [Google Scholar]
- 5.Coiffier B, Altman A, Pui CH, et al. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26:2767–2778. doi: 10.1200/JCO.2007.15.0177. [DOI] [PubMed] [Google Scholar]
- 6.Kearney MR, Chen EY, Stenzel P, et al. Colorectal cancer-associated spontaneous tumor lysis syndrome: a case report and review of the current literature. J Gastrointest Cancer. 2019;50:668–673. doi: 10.1007/s12029-018-0102-7. [DOI] [PubMed] [Google Scholar]
- 7.Gemici C. Tumour lysis syndrome in solid tumours. Clin Oncol R Coll Radiol. 2006;18:773–780. doi: 10.1016/j.clon.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Sommerhalder D, Takalkar AM, Shackelford R, et al. Spontaneous tumor lysis syndrome in colon cancer: a case report and literature review. Clin Case Rep. 2017;5:2121–2126. doi: 10.1002/ccr3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe S, Tsuchiya K, Nishimura R, et al. TP53 mutation by CRISPR system enhances the malignant potential of colon cancer. Mol Cancer Res. 2019;17:1459–1467. doi: 10.1158/1541-7786.MCR-18-1195. [DOI] [PubMed] [Google Scholar]
- 10.Liu HC, Chen GG, Vlantis AC, et al. Induction of cell cycle arrest and apoptosis by 5-fluorouracil in laryngeal cancer cells containing HPV16 E6 and E7 oncoproteins. Clin Biochem. 2008;41:1117–1125. doi: 10.1016/j.clinbiochem.2008.06.007. [DOI] [PubMed] [Google Scholar]