Abstract
Invasive thymomas with intraluminal tumor thrombi are rare. Removal of the thymoma and infiltration of the superior vena cava (SVC) is a curative alternative. We report an autopsy case of invasive thymoma with intraluminal growth into the intracardiac right atrium extension. Furthermore, the patient died of massive intracardiac thrombosis 5 days after the start of chemotherapy. A 66-year-old man with SVC syndrome was referred to our hospital. He had been aware of swelling of the face for 6 months. The patient was diagnosed with invasive thymoma by a CT-guided needle biopsy of the anterior mediastinal mass. Contrast-enhanced chest computed tomography showed a mass in the anterior mediastinum extending to the SVC and right atrium. As a result of discussion with surgeons and radiotherapists, we planned a multidisciplinary treatment in which neoadjuvant chemotherapy would reduce the tumor size, and surgery and postoperative radiotherapy were followed by chemotherapy. He was administered neo-adjuvant chemotherapy with CBDCA + PTX (carboplatin, area under the curve = 6, and paclitaxel, 200 mg/m2). On the 4th day of chemotherapy, he suddenly developed obstructive shock due to intracardiac thrombosis in the right ventricle. We believe that chemotherapy may trigger rapid thrombus formation. If an invasive thymoma spreads into a large vessel or the right atrium, surgical treatment should be considered if possible. However, if surgery is impossible, administration of anticoagulants should be considered to prevent thrombus formation before chemotherapy.
Keywords: Invasive thymoma, Tumor thrombus, SVC syndrome, Chemotherapy
Introduction
Invasive thymoma rarely involves great vessels in the mediastinum with intraluminal tumor thrombus and causes superior vena cava syndrome, which may cause death [1, 2]. No randomized clinical trials have provided definitive guidance for the management of invasive thymomas with tumor thrombosis. Surgical resection is the first choice for rescue if resectable based on case reports [3–6]. In an unresectable case, we have no choice but to administer neoadjuvant chemotherapy or chemoradiotherapy before surgery; however, this may increase the risk of further thrombus formation.
We present an autopsy case of an invasive thymoma patient who died because of thrombosis in the right ventricle immediately after the start of chemotherapy.
Case report
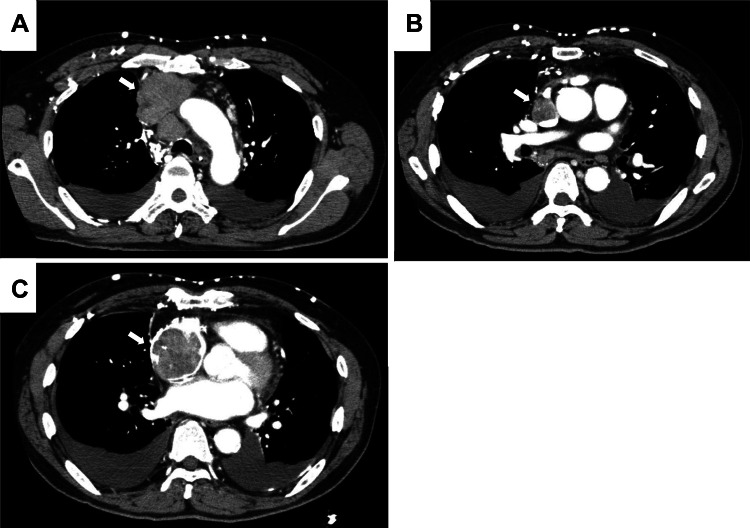
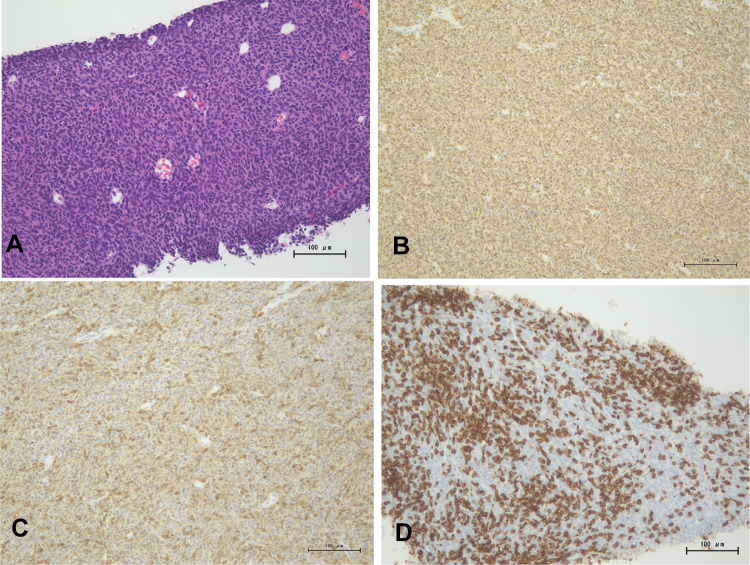
A 66-year-old man was admitted to our hospital for examination and treatment of a mediastinal tumor. He had complained of facial edema in the previous 6 months. He visited a clinic owing to productive cough and progressive dyspnea on exertion for 2 weeks. Chest radiography showed enlargement of the upper mediastinum and bilateral pleural effusion (Fig. 1) and he was referred to our hospital. He had hypertension and type 2 diabetes melitus. He was an ex-smoker (25 pack-years). His vital signs on admission were as follows: blood pressure, 120/90 mmHg; pulse rate, 142 beats/min; respiratory rate, 20 breaths/min; and oxygen saturation, 94% on 4 L/min of oxygen via a nasal cannula. He had edema over the face, neck and both upper limbs with visible dilated veins. Levels of carcinoembryonic antigen, cytokeratin 19 fragment, and neuron-specific enolase were slightly elevated, at 6.4 ng/mL (normal: 0.0–6.0 ng/mL), 8.98 ng/mL (normal: 0.00–2.08 ng/mL), and 20.0 ng/mL (normal: 0.00–16.3 ng/mL), respectively. Prothrombin time (PT), and activated partial thromboplastin time (aPTT) were within the normal range. The D-dimer level was slightly elevated, at 1.7 mg/L (normal: 0–1.0 mg/L). Contrast-enhanced chest computed tomography (CT) showed a mass (measuring 6 cm × 5 cm) in the anterior mediastinum extending to the superior vena cava (SVC) and the right atrium (RA) with heterogenous enhancement (Fig. 2). Transthoracic echocardiography (TTE) showed an RA intracavitary mass which appeared to be tumor rather than thrombus extending up to the SVC and decreased left ventricular ejection fraction (LVEF) of 40%. When evaluated by cardiac MRI to determine the nature of the tumor, the lesion with heterogenous contrast effects extended from the right anterior mediastinum to the right internal jugular vein, bilateral innominate veins, superior vena cava, and right atrium, forming a mass in the right atrium, with no evidence of thrombus formation. A CT-guided needle biopsy of the anterior mediastinal mass was performed. Pathological examination of the mediastinal mass revealed that spindle-shaped cells were proliferated with sparsely dispersed lymphocytes. Immunohistochemical analysis revealed positive pan-cytokeratin (AE1/AE3), CK5/6, and CD3 (Fig. 3). At the time of biopsy, thymoma was suspected; however, the detailed histological type could not be determined. He was suspected to have WHO type A thymoma, Masaoka stage III. According to the National Comprehensive Cancer Network Guidelines and the Japanese Lung Cancer Society Guidelines for Thymus Tumors, radiation therapy and chemotherapy are appropriate if unresectable. In this case, surgery could not be performed as electrical conduction to the RA might have been impaired.
Fig. 1.
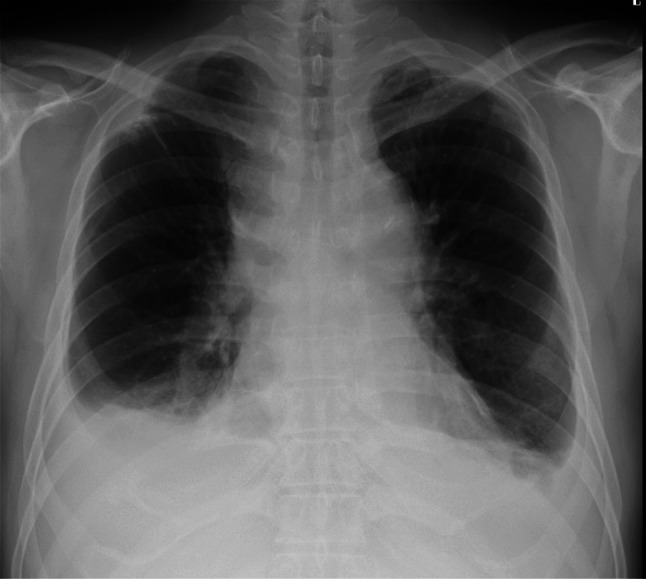
Chest radiography on admission. Chest radiography reveals enlargement of the upper mediastinum and bilateral pleural effusion
Fig. 2.
A contrast-enhanced chest computed tomography (CT) on admission. CT reveals a mass (measuring 6 cm × 5 cm) in the anterior mediastinum (A) extending to the superior vena cava (B) and the right atrium (C) with heterogenous enhancement (arrow)
Fig. 3.
A A specimen obtained by computed tomography (CT)-guided needle biopsy in the anterior mediastinal mass. Pathologically, spindle cells are proliferating with sparsely dispersed lymphocytes (hematoxylin and eosin staining). Immunohistochemical analysis of the tumor. B Positive reaction for CK AE1/AE3 in spindle cells; C Positive reaction for CK5/6 in spindle-shaped cells; D CD3 positive lymphocytes infiltrating the tumor
Additionally, since the radiation field was thought to be wide, we planned a multidisciplinary treatment in which neoadjuvant chemotherapy would reduce the tumor size, and surgery and postoperative radiotherapy were followed by chemotherapy. He was treated with CBDCA + PTX (carboplatin, area under the curve = 6, and paclitaxel, 200 mg/m2). On the 3rd day of chemotherapy, chest discomfort was transiently observed. However, no abnormalities were observed on chest radiography or ECG. On the 4th day, he suddenly experienced chest pain and his vital signs showed severe hypotension and respiratory failure (blood pressure of 99/70 mmHg, oxygen saturation of 90% on 10 L/min of oxygen mask) with cold and clammy skin in the extremities. Emergency contrast-enhanced computed tomography (CT) was performed. CT showed a tumor in the RA extending to the right ventricle (RV) without pulmonary embolism (Fig. 4). Since rapid tumor growth into the RV was observed, we considered that thrombus in contact with the tumor was formed in a short period rather than the growth of the thymoma itself. The D-dimer level was significantly elevated to 11.0 mg/L. He underwent intensive care management and anticoagulant therapy with 15,000 UI per day unfractionated heparin was started to achieve an APTT prolongation of 1.5–2.5 the normal range; however, he died the next day. He and his family did not provide consent for cardiopulmonary resuscitation. A pathological autopsy was performed with the approval of his family.
Fig. 4.
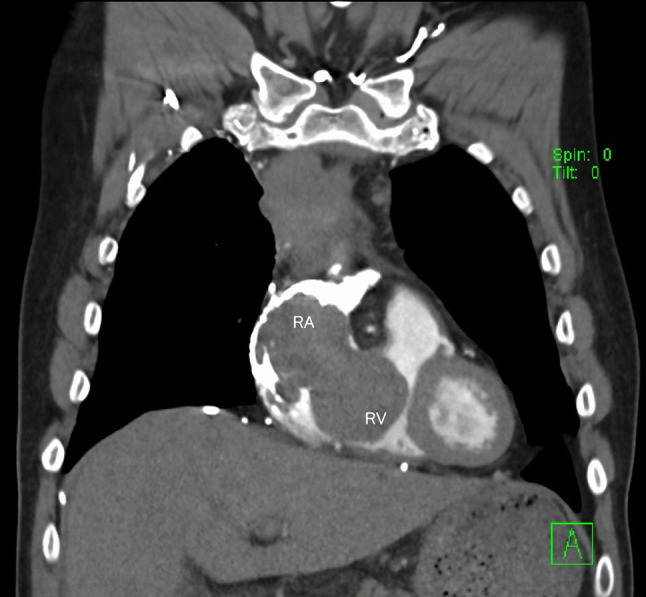
Contrast-enhanced computed tomography (CT) reveals a tumor from the right atrium extending to the right ventricle. RA: the right atrium, RV: the right ventricle
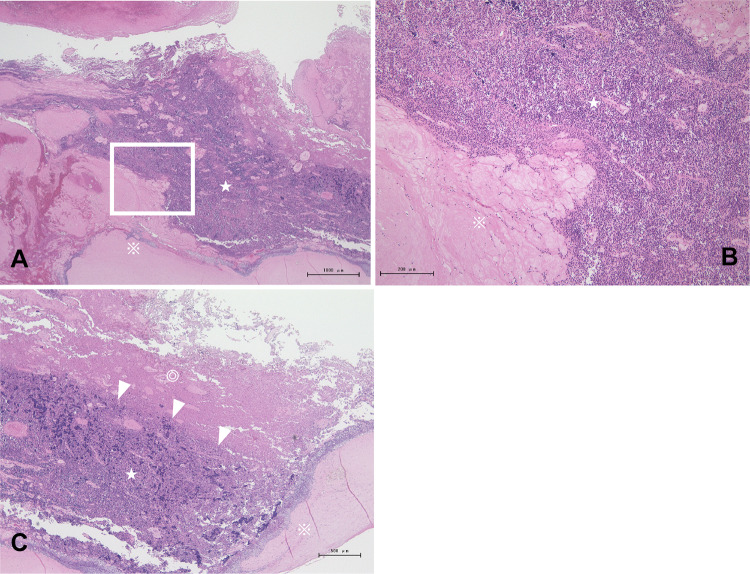
An autopsy revealed a 6 cm × 10 cm tumor in the right upper mediastinum, which had grown into the SVC. The tumor invaded the SVC into the RA. RA was filled with tumor (Fig. 5A) and a large thrombus attached to the tumor spread to the RV (Fig. 5B). The tumor also grossly invaded the upper lobe of the right lung. No pulmonary thromboembolism was observed. Pathological findings showed that the tumor was adjacent to the blood clot, and some parts of the tumor were necrotic (Fig. 6). The spindle cells with mild atypia proliferated in a fascicular pattern. Immunostaining was positive for pan-cytokeratin (AE1/AE3). The nuclei of tumor cells were slightly swollen and mitotic figures were rare. Lymphocytes infiltrating the tumor were positive for CD3 and CD5. The patient was diagnosed with WHO type A thymoma, Masaoka stage III. Metastasis was observed in the hilar lymph nodes and the lower right lobe. A thymoma (type A) was diagnosed, although it showed extracapsular and invasion to other organs and metastases. The liver and spleen were slightly congested. From the above results, we concluded that thrombus formation involving the tumor rapidly progressed after chemotherapy from RA to RV, resulting in right heart failure and death.
Fig. 5.
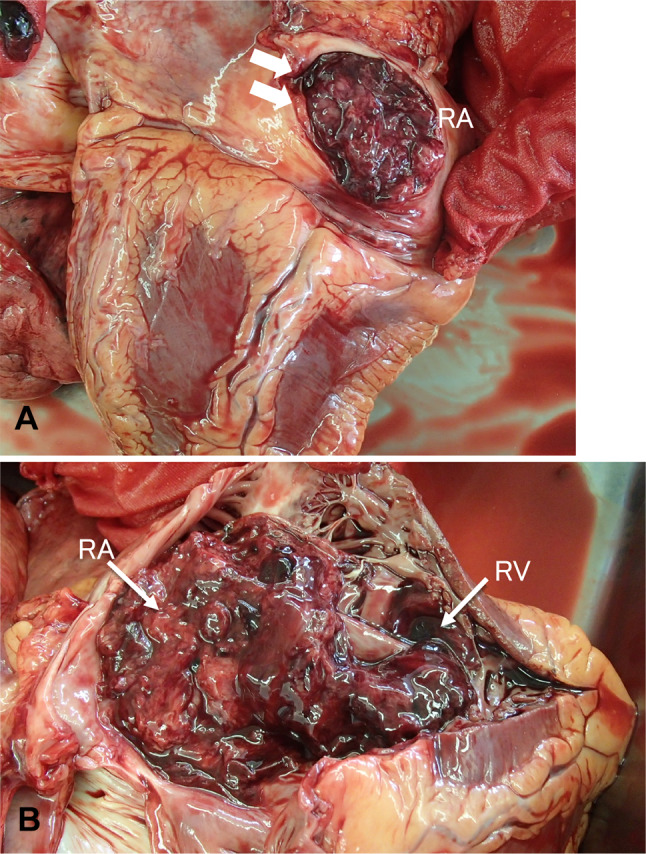
Macroscopic findings at autopsy. A Tumor thrombus in the right atrium (arrow). B Tumor thrombus reaching into the right ventricle. RA: the right atrium, RV: the right ventricle
Fig. 6.
Histopathological findings of tumor thrombosis at autopsy. A, B Histology revealed that the tumor (☆) was adjacent to the blood clot (※). C Necrotic tissue (◎) is detected inside the tumor adjacent to the thrombus (arrowheads) (hematoxylin and eosin staining)
Discussion
Invasive thymoma is typically an anterior mediastinal tumor that frequently invades neighboring organs, including the lung, pleura, large vessels, trachea, pericardium, and heart. Invasive thymoma sometimes causes SVC syndrome by extrinsic encasement and compression of the SVC; however, rarely by the tumoral intraluminal invasion of the great vessels [1]. Some patients are asymptomatic, while others present with chest pain, cough, or dyspnea [7]. The present patient had been symptomatic for 6 months and was considered to have progressed slowly.
In principle, surgical resection is the most favorable treatment for resectable tumors. Surgical resection is the mainstay of thymoma treatment as most of these tumors are localized [8]. If complete resection is not possible, neoadjuvant chemotherapy or chemoradiotherapy is administered before surgery. For advanced-stage of thymoma, chemotherapy with (or without) radiation therapy (RT) is recommended. In this case, since the tumor had spread to the RA, which was assumed to damage the cardiac conduction system, surgery was not possible. In addition, the irradiation range was thought to be wide. We adopted the following policy after thorough discussions with the departments of respiratory surgery and radiotherapy: chemotherapy was applied first, the tumor size was reduced, and then surgical treatment was performed, followed by radiation therapy.
As mentioned above, this patient’s tumor was considered to have progressed slowly as he had been aware of swelling in his face for 6 months. In contrast, symptoms due to thrombosis, such as chest discomfort, were observed on the 3rd day after administration of the anticancer drugs, and the condition rapidly deteriorated on the 4th day. There is a case report of invasive thymoma that infiltrated the lumen of the SVC extending into the RA, and the patient survived 50 months without treatment [9]. No thrombosis was observed on contrast-enhanced CT before the administration of the anticancer drug. Although there have been no similar reports, the above findings suggest that chemotherapy might trigger rapid thrombus formation.
Several possible etiologies can cause thrombus formation. Since thrombus formation was observed immediately after the start of chemotherapy, chemotherapy was considered to be related to the mechanism of thrombus formation. Anticancer drugs that may cause treatment-related thrombosis include platinum compounds, taxane-based drugs, angiogenesis inhibitors, multi-targeted tyrosine kinase inhibitors, and immunomodulatory drugs [10]. It has been reported that cisplatin-based chemotherapy increases the risk of venous thromboembolic events (VTE) [11]. Another report demonstrated that there was no difference in the frequency of VTE between cisplatin and carboplatin in patients with lung cancer treated with the respective drugs [12]. In this case, administration of carboplatin (platinum preparation) and paclitaxel (taxane-based drug) may induce thrombus formation.
Since necrotic tumor tissue was found near the thrombus at autopsy, it was assumed that the thrombus was formed based on chemotherapy-induced necrosis. Chemotherapy-related necrosis (inflammation) can contribute to the overall activation of blood coagulation in patients with cancer [13]; in this case, chemotherapy-induced tumor necrosis may have led to the formation of a thrombus by the same mechanism.
Strictly speaking, thymoma is not a malignant tumor, but we investigated whether there was any hypercoagulability associated with malignancy, the so-called Trousseau syndrome. We could not find any case reports of Trousseau syndrome associated with thymoma in the literature, and since no thromboemboli were found in other organs at autopsy, Trousseau syndrome was not considered to be associated.
There is no clear consensus regarding anticoagulant therapy for intracardiac tumors. The choice of therapy primarily depends on the clinical condition of the patient and the underlying etiology. In the absence of bleeding, anticoagulation is the mainstay of therapy to prevent clot progression and thrombus shedding [14].
A case of sudden death due to the hemorrhagic expansion of the intra-arterial component of Wilms’ tumor immediately after commencement chemotherapy, which had some similarities to the present case was reported by Vaideeswar [15]. Although the sensitivity to chemotherapy differs between thymomas and Wilms’ tumors, the possibility of serious incidents due to intra-tumor hemorrhage or thrombus formation, as in this case, due to responsiveness to chemotherapy should be considered.
If a huge invasive thymoma, as in this case, is spreading into a large vessel or the right atrium, surgical treatment should be considered if possible. If surgery is challenging, anticoagulants might be considered before chemotherapy to prevent thrombus formation, although evidence is lacking, and further research is needed.
Funding
None.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Motoori K, Shimura H, Funabashi N, et al. Thymoa presenting as an intraluminal growth into the superior vena cava and the cardiac cavity. Eur J Radiol Extra. 2008;68:13–15. doi: 10.1016/j.ejrex.2008.05.025. [DOI] [Google Scholar]
- 2.Kurata A, Saji H, Ikeda N, et al. Intracaval and intracardiac extension of invasive thymoma complicated by superior and inferior vena cava syndrome. Pathol Int. 2013;63:56–62. doi: 10.1111/pin.12023. [DOI] [PubMed] [Google Scholar]
- 3.Rizzardi G, Arena V, Passera E, et al. Radical surgery in an unusual case of thymoma with intraluminal growth into the superior vena cava and right atrium. Interact Cardiovasc Thorac Surg. 2013;17:1054–1055. doi: 10.1093/icvts/ivt397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afzal A, Wong I, Korniyenko A, et al. Superior vena cava syndrome from an invasive thymoma with transcaval invasion to the right atrium. J Surg Case Rep. 2016;4:1–3. doi: 10.1093/jscr/rjw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panda PK, Wig N, Kumar S, et al. Invasive thymoma presenting as classic superior vena cava syndrome: a case of venous spread metastasis. BMJ Case Rep. 2016 doi: 10.1136/bcr-2016-217695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong YQ, Liang JS, Zhang XM, et al. Surgical treatment of an invasive thymoma extending into the superior vena cava and right atrium. World J Surg Oncol. 2014 doi: 10.1186/1477-7819-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ettinger DS, Riely GJ, Akerley W, et al. Thymomas and thymic carcinomas: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2013;11:562–576. doi: 10.6004/jnccn.2013.0072. [DOI] [PubMed] [Google Scholar]
- 8.Kondo K. Optimal therapy for thymoma. J Med Invest. 2008;55:17–28. doi: 10.2152/jmi.55.17. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi S, Sogo Y, Hamaguchi R, et al. An autopsy case of invasive thymoma extending to the right atrium with superior vena cava syndrome as the initial manifestation. Nihon Kokyuki Gakkai Zasshi. 2007;45:997–1002. [PubMed] [Google Scholar]
- 10.Mukai M, Oka T. Mechanism and management of cancer-associated thrombosis. J Cardiol. 2018;72:89–93. doi: 10.1016/j.jjcc.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Moore RA, Adel N, Riedel E, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29:3466–3473. doi: 10.1200/JCO.2011.35.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellema WW, van der Hoek D, Postmus PE, et al. Retrospective evaluation of thromboembolic events in patients with non-small cell lung cancer treated with platinum-based chemotherapy. Lung Cancer. 2014;86:73–77. doi: 10.1016/j.lungcan.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Caine GJ, Stonelake PS, Lip GY, et al. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4:465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faiek S, Malik I, Farquhar R, et al. Pulmonary vein tumor thrombus with intracardiac extension secondary to poorly differentiated bronchogenic carcinoma. Cureus. 2020 doi: 10.7759/cureus.8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaideeswar P, Chaudhari JP. Wilms’ tumor with right heart extension: report of a post-chemotherapeutic fatality. Indian J Pathol Microbiol. 2012;55:381–383. doi: 10.4103/0377-4929.101752. [DOI] [PubMed] [Google Scholar]