Abstract
Introduction
COPD is a heterogeneous disorder with varied phenotypes. We aimed to determine the prevalence of asthma history, peripheral eosinophilia and elevated FeNO levels along with the diagnostic utility of peripheral eosinophilia in identifying airway eosinophilic inflammation.
Methods
National Health and Nutrition Examination Survey data were analysed for the study period 2007–2010. Subjects aged ≥40 years with postbronchodilator FEV1/FVC ratio <0.70 were included. Receiver operator curve analysis was performed for sensitivity analysis. A p value of <0.001 is considered statistically significant.
Results
A total of 3 110 617 weighted COPD cases were identified; predominantly male (64.4%) and non-Hispanic whites (86.1%). Among our COPD subjects, 14.6% had a history of doctor diagnosed asthma, highest among females and other race Americans. The overall prevalence of peripheral eosinophilia is 36%, 38.3% among COPD subjects with asthma history, and 35.6% among COPD without asthma history. The overall prevalence of elevated FeNO ≥25 ppb is 14.3%; 28.7% among COPD subjects with asthma history and 13.0% among COPD without asthma history.
Discussion
The prevalence of FeNO levels ≥25 ppb and peripheral eosinophilia was significantly higher among COPD subjects with asthma compared with COPD without asthma history. Not all COPD subjects with peripheral eosinophilia and elevated FeNO levels have a reported history of asthma. Our study supports clinically phenotyping COPD subjects with eosinophilic inflammation be independent of their asthma history and peripheral eosinophilia can be used as a surrogate marker in resource-limited settings.
Keywords: asthma epidemiology, COPD epidemiology, exhaled airway markers, eosinophil biology
Key messages.
What is the key question?
Determine the prevalence of eosinophilic inflammation among COPD subjects by analysing elevated FeNO levels and peripheral eosinophil counts.
What is the bottom line?
Elevated peripheral eosinophil counts have a good sensitivity and specificity in identifying COPD subjects with eosinophilic inflammation with or without a reported history of asthma.
Why read on?
This study represents a comprehensive analysis of a nationwide representative sample evaluating the prevalence of eosinophilic inflammation among COPD subjects, with insights into the role of asthma history and peripheral eosinophilia in identifying this COPD subtype.
Introduction
COPD is the fourth-leading cause of death in the USA and an estimated 15.7 million US adults are currently diagnosed with the disease.1 2 Despite the historic notion of predominant neutrophil mediated airway inflammation, studies suggested the role of eosinophils in COPD, both during stable disease state and also during exacerbations.3–5 When current COPD treatment guidelines were largely based on the severity of airflow obstruction, the intensity of symptoms, and exacerbation risk, there has been increasing evidence suggesting the role of tailored therapy based on the phenotypic variants in COPD. Asthma-COPD overlap (ACO) is one such phenotype and unlike COPD, inhaled corticosteroids were recommended as first line therapy for such individuals. While the presence of asthma history COPD is one of the many proposed characteristics to identify the ACO subtype, not all of these subjects will have a documented asthma history and thereby not diagnosed as having ACO.5–7 Irrespective of asthma history, identifying COPD subjects with eosinophilic inflammation is clinically important as these are also shown to respond better with corticosteroid therapy when compared with non-eosinophilic COPD group.8 FeNO is an acceptable non-invasive marker for eosinophilic airway inflammation, thereby aide in identifying eosinophilic COPD phenotype. However, not all clinical settings are equipped with this testing forcing clinicians to rely on more readily available surrogate markers like peripheral eosinophilia to identify such phenotype especially among cases without asthma history.9 10
We sought to identify the role peripheral eosinophilia in identifying eosinophilic COPD phenotype using a population-based National Health and Nutrition Examination Survey database (NHANES).11 We also report the prevalence of peripheral eosinophilia and airway eosinophilic inflammation among COPD subjects stratified by asthma history status and demographics.
Materials and methods
NHANES was designed to assess the health and nutritional status of adults and children in the USA. Spirometry is one of the two NHANES sponsored components on respiratory health for years 2007–2010 to provide an estimate of the current prevalence of asthma and COPD and produce updated spirometry reference data for the US NHANES study population aged 40 years and above with spirometry studies that met or exceeded American Thoracic Society (ATS) spirometry quality standards were initially selected (spirometry studies with at least three reproducible curves with the two largest FVC or FEV1 values taken from two acceptable curves should agree within 100–150 mL).12 All eligible NHANES subjects underwent baseline spirometry and ones with the evidence of airflow obstruction in the baseline spirometry were selected for a follow-up bronchodilator spirometry test that was performed using two puffs of the β2-adrenergic bronchodilator albuterol with spacer.13 The quality of blood specimens were analysed for white blood cell count and differential as per the NHANES procedure manual.14 The self-reported responses to the medical conditions questionnaire were collected by trained personnel using the computer-assisted personal interviewing system. Subjects who have smoked within 1 hour before analysis/have exercised strenuously/have eaten nitric oxide rich meats and/or vegetables within 3 hours/have used inhaled, oral corticosteroids/have had a cough, cold or respiratory illness within 7 days were excluded for further analysis as the presence of these variables was shown to influence FeNO levels.15 Subjects with missing FeNO levels and peripheral eosinophil counts were also excluded. Spirometry confirmed COPD in our analysis was defined by the presence of FEV1/FVC ration of <0.07 on a postbronchodilator spirometry testing in accordance with Global Initiative for Chronic Obstructive Lung Disease (GOLD) document.16 Peripheral eosinophilia is defined by blood eosinophil counts ≥300 cells/µL.
COPD subjects were stratified in to ‘COPD with asthma history ’ group and ‘COPD without asthma history’ group based on their asthma history, identified using the question ‘Has a doctor or healthcare professional ever told that you have asthma?’. Subjects were further stratified based on gender, race, age groups, FeNO levels, peripheral eosinophilia and smoking status for subgroup analysis. Races included were non-Hispanic white, non-Hispanic black, Hispanic (which includes Mexican American and other Hispanic) and other race (which includes multiracial and other races). Age groups included were 40–49 years, 50–59 years, 60–69 years and 70–79 years. Based on the FeNO levels, subjects were classified into three groups: (1) low range group with FeNO ≤24 ppb, (2) intermediate range group with FeNO 25–50 ppb and (3) high range group with FeNO ≥51 ppb aligning with the ATS classification.9 Current smokers and current non-smokers were identified using the NHANES question ‘Do you now smoke cigarettes?’. Survey participants responded as ‘not at all’ were labelled as non-smokers and those who responded as ‘every day or someday’ were labelled as current smokers. As current smoking status influences the exhaled FeNO levels, subjects that were not current smokers were labelled as non-smokers in our analysis. However, the possibility of current non-smokers being former smokers cannot completely rule out and the precise information on the duration and smoking quantification is not provided in the NHANES data base. All our analysed questionnaire variables have <5% of subjects with missing/not reported responses, except for smoking status where responses were not available in 34.6% of the study subjects.
SPSS V.27 was used for analysis. Sample weights are created for each of the NHANES survey participant to account for the complex survey design, survey non-response and poststratification to ensure that calculated estimates are representative of the US population. NHANES variable WTMEC2YR was used for weighted analysis and sample weights were adjusted to account for combining two survey cycles following NHANES analytic guidelines.17 Statistical significance between descriptive proportions was analysed using the Pearson χ2 test and significance between the calculated means using analysis of variance testing. A receiver operating characteristic curve analysis was implemented to assess the performance of the peripheral eosinophil counts in distinguishing COPD subjects with FeNO levels ≥25 ppb from subjects with FeNO levels <25 ppb.
Results
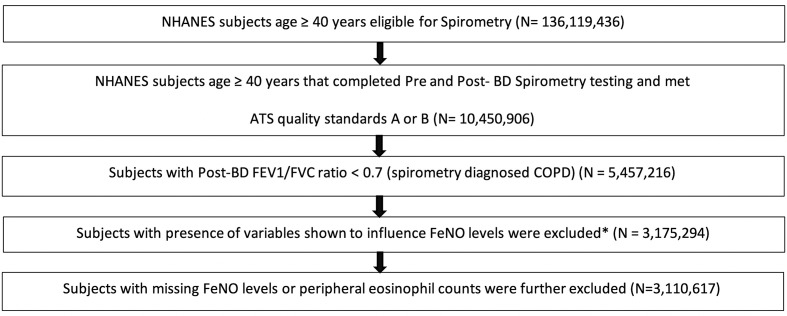
A total of 3 110 617 COPD subjects were identified as per the above-mentioned inclusion and exclusion criteria (figure 1). COPD subjects were elderly (mean age 59.3 years, SD 9.2 years), predominantly male (64.4%) and non-Hispanic whites (86.1%). The overall prevalence of asthma history among our COPD subjects was 14.6% (table 1).
Figure 1.
Flow diagram of the study population (number of weighted subjects remained for further analysis after applying the inclusion criteria). *Subjects who have smoked within 1 hour before analysis/have exercised strenously/have eaten nitric oxide rich meats and/or vegetables within 3 hours/have used inhaled, oral corticosteroids within 2 days/have had cough, cold or respiratory illness within 7days were excluded from further analysis as these were shown to influence FeNO levels. ATS, American Thoracic Society; NHANES, National Health and Nutrition Examination Survey.
Table 1.
Demographic characteristics of the study population
| Demographic variable | Total study population | COPD without asthma history | COPD with asthma history | P value* |
| Total study population | 3 110 617 (100) | 2 655 739 (85.4) | 454 878 (14.6) | <0.001 |
| Mean age in years (SD) | 59.3 (9.2) | 59.2 (9.4) | 59.9 (8.4) | <0.001 |
| Male, N (%) | 2 002 486 (64.4) | 1 812 842 (68.3) | 189 644 (41.7) | <0.001 |
| Female, N (%) | 1 108 131 (35.6) | 842 897 (31.7) | 265 234 (58.3) | <0.001 |
| Non-Hispanic white, N (%) | 2 678 509 (86.1) | 2 322 923 (87.5) | 355 586 (78.2) | <0.001 |
| Non-Hispanic black, N (%) | 145 384 (4.7) | 117 210 (4.4) | 28 175 (6.2) | <0.001 |
| Hispanic, N (%) | 91 785 (3.0) | 72 687 (2.7) | 19 098 (4.2) | <0.001 |
| Other race, N (%) | 194 938 (6.3) | 142 919 (5.4) | 52 019 (11.4) | <0.001 |
| 40–49 years, N (%) | 426 272 (13.7) | 373 492 (14.1) | 52 780 (11.6) | <0.001 |
| 50–59 years, N (%) | 1 148 202 (36.9) | 1 043 646 (39.3) | 104 556 (23.0) | <0.001 |
| 60–69 years, N (%) | 1 092 273 (35.1) | 832 106 (31.3) | 260 167 (57.2) | <0.001 |
| 70–79 years, N (%) | 443 870 (14.3) | 142 919 (15.3) | 37 376 (8.2) | <0.001 |
| Current smoker, N (%) | 833 824 (26.8) | 792 915 (29.9) | 40 909 (9.0) | <0.001 |
| Non-smoker, N (%) | 1 201 597 (38.6) | 167 286 (40.2) | 134 310 (29.5) | <0.001 |
| Unknown smoking status, N (%) | 1 075 197 (34.6) | 795 538 (30.0) | 279 659 (61.5) | <0.001 |
*P value is comparing the statistical difference between COPD without asthma history group and COPD with asthma history group.
The prevalence of peripheral eosinophilia among our COPD subjects is 36%, significantly higher in COPD with asthma history group compared with COPD without asthma history group (38.3% vs 35.6%, p<0.001) (table 2). On subgroup analysis, higher eosinophilia prevalence among COPD with asthma history group was only noted among females (45.3% vs 27.6; p<0.001), non-Hispanic blacks (36.4% vs 18.7%; p<0.001), other race Americans (81.6% vs 54.2%; p<0.001), age groups 50–59 years (50.4% vs 30.0%; p<0.001), 70–79 years (33.8% vs 29.8%; p<0.001), non-smokers (52.6% vs 20.5%; p<0.001) and subjects with intermediate range FeNO levels (84.4% vs 30.4%; p<0.001). For the remainder of the demographic subgroups analysed, the prevalence of peripheral eosinophilia was higher among the COPD without asthma history group (table 2).
Table 2.
Prevalence of peripheral eosinophilia among subjects with COPD without asthma history and COPD with asthma history, stratified by gender, race, age groups, smoking status and exhaled FeNO levels
| Demographic variable | COPD without asthma history (N=2 655 739) |
COPD with asthma history (N=454 878) |
P value |
| Total | 35.5% (35.5% to 35.6%) | 38.3% (38.1% to 38.5%) | <0.001 |
| Gender (95% CI) | |||
| Male | 39.2% (39.1% to 39.3%) | 28.5% (28.3% to 28.7%) | <0.001 |
| Female | 27.6% (27.5% to 27.7%) | 45.3% (45.1% to 45.5%) | <0.001 |
| Race (95% CI) | |||
| Non-Hispanic white | 35.4% (35.3% to 35.4%) | 34.2% (34.0% to 34.3%) | <0.001 |
| Non-Hispanic black | 18.7% (18.4% to 18.9%) | 36.4% (35.9% to 37.0%) | <0.001 |
| Hispanic | 30.9% (30.6% to 31.3%) | 0% | NA |
| Other race | 54.2% (54.0% to 54.4%) | 81.6% (81.3% to 82.0%) | <0.001 |
| Age groups (95% CI) | |||
| 40–49 years | 37.1% (37.0% to 37.3%) | 33.5% (33.1% to 33.9%) | <0.001 |
| 50–59 years | 30.0% (30.0% to 30.1%) | 50.4% (50.1% to 50.7%) | <0.001 |
| 60–69 years | 44.6% (44.5% to 44.7%) | 35.1% (34.9% to 35.3%) | <0.001 |
| 70–79 years | 29.7% (29.6% to 29.9%) | 33.8% (33.4% to 34.3%) | <0.001 |
| Smoking status (95% CI) | |||
| Current smoker | 50.7% (50.6% to 50.8%) | 43.2% (42.3% to 43.7%) | <0.001 |
| Non-smoker | 20.5% (20.4% to 20.6%) | 52.6% (52.3% to 52.9%) | <0.001 |
| Unknown smoking status | 40.6% (40.5% to 40.7%) | 30.7% (30.5% to 30.9%) | <0.001 |
| FeNO levels (ppb) (95% CI) | |||
| 0–24 | 34.6% (34.6% to 34.7%) | 27.6% (27.5% to 27.8%) | <0.001 |
| 25–50 | 30.4% (30.3% to 30.6%) | 84.4% (84.2% to 84.7%) | <0.001 |
| 51 and above | 42.3% (42.0% to 42.5%) | 0% | NA |
NA, not available.
Intermediate range FeNO level prevalence among our study population is 14.3%, significantly higher among COPD with asthma history group compared with COPD without asthma history group (22% vs 13.0%; p<0.001) (table 3). On subgroup analysis, higher prevalence among COPD with asthma history group was again only noted among females (28.0% vs 6.1%; p<0.001), non-Hispanic black (57.9% vs 14.4%; p<0.001), other race Americans (100% vs 14.9%; p<0.001), age groups 50–59 years (50.4% vs 5.0%; p<0.001), 60–69 years (18.2% vs 9.7%; p<0.001), non-smokers (35.3% vs 14.9%; p<0.001) and subjects with peripheral eosinophilia (48.5% vs 11.1%; p<0.001). For the remainder of the demographic subgroups, the prevalence of intermediate range FeNO levels was higher among COPD without asthma history group (table 3).
Table 3.
Prevalence of exhaled nitric oxide levels stratified by gender, race, age groups, smoking status, peripheral eosinophil count and clinical status
| Demographic variable | Intermediate level FeNO group (FeNO=25–50 ppb) (N=445 085) |
High level FeNO group (FeNO ≥51 ppb) (N=89 623) |
||||
| COPD without asthma history | COPD with asthma history | P value | COPD without asthma history | COPD with asthma history | P value | |
| Total | 13.0%(13.0% to 13.1%) | 22.0%(21.9% to 22.2%) | <0.001 | 2.2%(2.2% to 2.3%) | 6.7%(6.6% to 6.7%) | <0.001 |
| Gender (95% CI) | ||||||
| Male | 16.2%(16.2% to 16.3%) | 13.6%(13.5% to 13.8%) | <0.001 | 2.0%(2.0% to 2.0%) | 1.8%(1.7% to 1.8%) | <0.001 |
| Female | 6.1%(6.0% to 6.1%) | 28.0%(27.9% to 28.2%) | <0.001 | 2.8%(2.7% to 2.8%) | 10.2%(10.0% to 10.3%) | <0.001 |
| Race (95% CI) | ||||||
| Non-Hispanic white | 12.5%(12.4% to 12.5%) | 9.0%(8.9% to 9.1%) | <0.001 | 2.2%(2.2% to 2.2%) | 7.6%(7.5% to 7.7%) | <0.001 |
| Non-Hispanic black | 14.4%(14.2% to 14.6%) | 57.9%(57.3% to 58.4%) | <0.001 | 0% | 0% | NA |
| Hispanic | 23.0%(22.7% to 23.3%) | 0% | NA | 11.5%(11.2% to 11.7%) | 17.4%(16.9% to 18.0%) | <0.001 |
| Other race | 14.9% (14.7% to 15.1%) | 100%(99.9% to 100.0%) | <0.001 | 0% | 0% | NA |
| Age groups (95% CI) | ||||||
| 40–49 years | 20.9%(20.8% to 21.0%) | 0% | NA | 2.2%(2.2% to 2.3%) | 0% | NA |
| 50–59 years | 5.0%(4.9% to 5.1%) | 50.4%(50.1% to 50.7%) | <0.001 | 0% | 0% | NA |
| 60–69 years | 9.7%(9.7% to 9.8%) | 18.2%(18.1% to 18.4%) | <0.001 | 0% | 11.6%(11.5% to 11.8%) | NA |
| 70–79 years | 32.9%(32.8% to 33.1%) | 0% | NA | 0% | 0% | NA |
| Smoking status (95% CI) | ||||||
| Current smoker | 2%(1.9% to 2.0%) | 0% | NA | 0% | 0% | NA |
| Non-smoker | 14.9%(14.8% to 14.9%) | 35.3%(35.1% to 35.6%) | <0.001 | 4.8%(4.7% to 4.8%) | 2.5%(23.9% to 25.6%) | <0.001 |
| Unknown smoking status | 21.5%(21.4% to 21.6%) | 18.9%(18.7% to 19.0%) | <0.001 | 1.0%(1.0% to 1.1%) | 9.6%(9.5% to 9.7%) | <0.001 |
| Eosinophil count (cells/µL) (95% CI) | ||||||
| 300andabove | 11.1%(11.1% to 11.2%) | 48.5% (48.3% to 48.8%) | <0.001 | 6.3%(6.2% to 6.3%) | 0% | NA |
| 299and below | 14.0% (14.0% to 14.1%) | 5.6%(5.5% to 5.6%) | <0.001 | 0% | 10.8% (10.7% to 10.9%) | NA |
NA, not available.
Similarly, the prevalence of high range FeNO levels among our study population is 2.9%, significantly higher among COPD with asthma history group (6.7% vs 2.2.0%; p<0.001) (table 3). On subgroup analysis, higher prevalence among COPD with asthma history group was only noted among females (10.2 vs 2.8%; p<0.001), non-Hispanic whites (7.6 vs 2.2%; p<0.001), Hispanics (17.4 vs 11.5%; p<0.001), age groups 50–59 years (50.4% vs 5.0%; p<0.001), 60–69 years (18.2% vs 9.7%; p<0.001) and among subjects with unknown smoking status (9.6% vs 1.0%; p<0.001). For the remainder of the demographic sub-groups, the prevalence of high range FeNO levels was higher either among COPD without asthma history group or comparative analysis could not be performed secondary to insufficient cases (table 3).
Mean FeNO level for the study population was 16.4 (SD ±13.6), significantly higher among COPD with asthma history group compared with COPD without asthma history group (19.6 vs 15.8; p<0.001) (table 4). This trend of higher mean FeNO levels among COPD with asthma subjects remained significant among all subgroups compared except in males where no difference was noted (16.6 vs 16.6; p=0.7) and in age groups 40–49 years (17.2 vs 10.8; p<0.001) and 70–79 years (19.3 vs 11.7; p<0.001) where the mean FeNO level was higher in the COPD without asthma history group (table 4).
Table 4.
Mean exhaled nitric oxide levels, stratified by clinical status and demographics
| Demographic variable | Total study population | COPD without asthma history | COPD with asthma history | P value |
| Mean FeNO level (SD) | Mean FeNO level (SD) | Mean FeNO level (SD) | ||
| Total study population | 16.4 (13.6) | 15.8 (13.6) | 19.6 (13.2) | <0.001 |
| Gender | ||||
| Male | 16.6 (14.4) | 16.6 (14.6) | 16.6 (11.7) | 0.7 |
| Female | 16.0 (12.1) | 14.2 (11.0) | 21.7 (13.7) | <0.001 |
| Race | ||||
| Non-Hispanic white | 15.6 (13.1) | 15.4 (13.2) | 16.9 (11.7) | <0.001 |
| Non-Hispanic black | 16.1 (10.1) | 15.0 (8.9) | 20.6 (13.1) | <0.001 |
| Hispanic | 26.9 (26.0) | 26.6 (26.7) | 28.2 (23.1) | <0.001 |
| Other race | 22.1 (10.1) | 17.7 (8.2) | 34.2 (0.6) | <0.001 |
| Age groups | ||||
| 40–49 years | 16.4 (15.2) | 17.2 (16.0) | 10.8 (4.4) | <0.001 |
| 50–59 years | 13.3 (8.1) | 12.6 (7.0) | 19.8 (13.8) | <0.001 |
| 60–69 years | 18.7 (17.8) | 17.5 (18.8) | 22.4 (13.8) | <0.001 |
| 70–79 years | 18.6 (9.2) | 19.3 (9.3) | 11.7 (2.5) | <0.001 |
| Smoking status | ||||
| Current smoker | 9.0 (5.0) | 8.9 (5.0) | 12.4 (3.8) | <0.001 |
| Non-smoker | 19.2 (16.5) | 18.8 (16.9) | 21.8 (12.6) | <0.001 |
| Unknown smoking status | 18.9 (12.4) | 18.7 (11.8) | 19.6 (13.9) | <0.001 |
| Peripheral eosinophil count | ||||
| <300 cells/µL | 14.6 (9.6) | 13.9 (8.7) | 18.2 (14.6) | <0.001 |
| ≥300 cells/µL | 19.6 (18.1) | 19.2 (19.2) | 21.7 (10.1) | <0.001 |
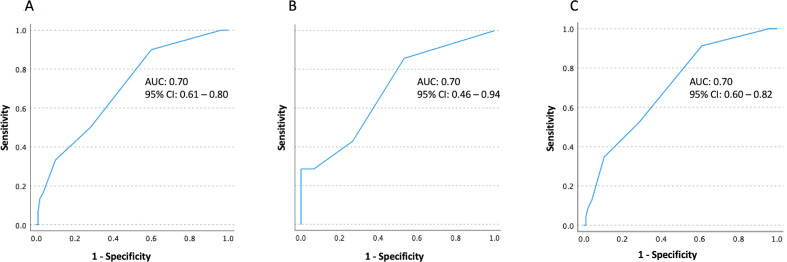
For distinguishing COPD subjects with FeNO levels ≥25 ppb from those with FeNO levels <25 ppb, peripheral eosinophil count thresholds of 100 cells/µL, 200 cells/µL and 300 cells/µL yielded sensitivities of 90%, 50% and 33%, respectively, and specificities of 40%, 72% and 90%, respectively, with area under the curve of 0.70 (95% CI 0.61 to 0.80) (figure 2A). Similarly, among COPD subjects with asthma history, thresholds of 100 cells/µL, 200 cells/µL and 300 cells/µL yielded sensitivities of 86%, 43% and 29%, and specificities of 47%, 73% and 93%, respectively, were with area under the curve of 0.70 (95% CI 0.46 to 0.94) (figure 2B). Similarly, among COPD subjects without asthma history, thresholds of 100 cells/µL, 200 cells/µL and 300 cells/µL yielded sensitivities of 91%, 52% and 35%, and specificities of 59%, 72% and 86%, respectively, were with area under the curve of 0.70 (95% CI 0.60 to 0.82) (figure 2C).
Figure 2.
(A) Receiver operating characteristic curve (ROC) analysis of peripheral eosinophil counts in distinguishing subjects with FeNO levels ≥25 ppb from subjects with FeNO levels <25 ppb among entire study population; (B) among COPD subjects with asthma history; (C) among COPD subjects without asthma history.
Discussion
COPD is a heterogeneous disease with different clinical phenotypes and identifying such phenotypes will enable tailoring therapeutic interventions. COPD subjects with coexisting asthma features were shown to have a lower health-related quality of life, increased exacerbation risk, prolonged hospital stays and higher healthcare cost utilisation compared with those with either asthma or COPD without asthma history.18–24 The reported prevalence of asthma history among COPD subjects varied from 15% to 50% in individual studies, with an overall pooled prevalence of 27%.25 The 14.6% prevalence of asthma history in our analysis was comparable to 13.6% reported by Hardin et al who used a similar definition of ‘self-reported physician diagnosis of asthma’ to identify asthma.24
Predominant airway eosinophilic inflammation subtype is one of many distinct COPD phenotypes that showed a greater response in lung function to corticosteroid treatment along with a reduction in exacerbation rates.26–32 Sputum analysis is considered the cost-effective, non-invasive and most reliable method of assessing airway eosinophilic inflammation. However, due to lack of availability in clinical settings, reliance on FeNO levels as a surrogate marker has evolved.33–35 Mean FeNO level in our study population was 16.4 (SD ±13.6). Significant differences in the mean FeNO levels were noted among our subgroups analysed. Healthy males were previously reported to have either comparable or higher FeNO levels compared with healthy females.36–39 Similar gender differences in mean FeNO were noted in our study population except for COPD with asthma history group where females were noted to have higher mean levels. Racial differences in mean FeNO levels both in healthy US adults and those with comorbidities has been reported.40 In our study subjects, Hispanics and other race Americans were noted to have higher mean FeNO levels compared with other races irrespective of their asthma history. In concordance with previous studies, mean FeNO levels in our analysis were higher in elderly age groups compared with younger age groups.38 Age-group 60–69 years has a higher mean FeNO level in the COPD with asthma history group and 70–79 years has the highest mean in COPD without asthma history group. Active smoking and environmental tobacco exposure were previously shown to significantly decrease FeNO levels in both atopic and non-atopic subjects, independent of age, sex and height.41–43 In our analysis, irrespective of their asthma history, COPD subjects who are current smokers were found to have lower mean FeNO levels compared with non-smokers.
COPD subjects with asthma history can be classified as having ACO and benefit from early initiation of inhaled steroids.44–47 However, eosinophilic inflammation among COPD subjects can exist even in the absence of asthma history and were also shown to benefit from early ICS initiation.27 28 While elevated FeNO levels aide in identifying eosinophilic COPD phenotype irrespective of their asthma history, it is not as readily available as peripheral eosinophil counts in the clinical practice. Peripheral eosinophil cut-off of value 300 cells/µL can identify eosinophilic COPD phenotype as in our analysis this cut-off yielded a specificity of 90% in distinguishing COPD subjects with FeNO ≥25 ppb from ones with FeNO <25 ppb, slightly higher at 93% among COPD subjects with asthma history compared with 89% among subjects without asthma history. Similarly, cut-off value of 100 cells/µL can be used to rule out eosinophilic COPD phenotype as this yielded a sensitivity of 90% in ruling out FeNO >25 ppd among COPD subjects, slightly higher at 91% among COPD subjects without asthma history compared with 86% among COPD subjects with asthma history.
Limitations
Despite high standards for data collection, NHANES examination and laboratory data are also subject to sampling and non-sampling errors. Interview (questionnaire) data used to identify subjects with asthma and smoking status was based on self-reports and are therefore subject to recall bias. Subjects with asthma in our analysis were identified using the question ‘Has a doctor or healthcare professional ever told that you have asthma’. Even though the response to this question is the most frequently used definition in previous studies to define asthma, reliance on this as definitive confirmation might lead to over or underestimation of the prevalence of clinically confirmed asthma.48 NHANES question ‘Do you smoke now?’ used to identify current smokers has 30% of responses missing in our included subjects.
Conclusion
The overall prevalence of asthma history among our COPD subjects was 14.6%. Mean FeNO level were significantly higher in COPD with asthma history group compared with COPD without asthma history group and so is the case with the prevalence of peripheral eosinophilia. Approximately 35.6% and 15.2% of COPD subjects without asthma history were found to have peripheral eosinophil and elevated FeNO, respectively. Peripheral eosinophil count cut-off of 100 cells/µL and 300 cells/µL yielded 90% sensitivity and 90% specificity, respectively, in identifying COPD subjects with FeNO levels≥25 ppb. Our study supports clinically phenotyping COPD subjects with eosinophilic inflammation be independent of their asthma history and peripheral eosinophilia can be used as a surrogate marker in resource-limited settings.
Footnotes
Twitter: @drsturge
Contributors: SA and SN contributed to conception and design, data analysis and interpretation, manuscript writing and final approval. JS contributed to manuscript writing and final approval. EF and MF contributed to conception and design, manuscript writing and final approval.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethics committee approval was not sought given publicly available deidentified nature of the NHANES database.
References
- 1. NCHS . Health, United States, 2015: with special feature on racial and ethnic health disparities, 2016. [PubMed]
- 2. Kochanek K, Murphy S, Xu J. Mortality in the United States, 2016. NCHS data brief, no 293. National Center for Health Statistics, 2017. [PubMed] [Google Scholar]
- 3. Hargreave FE, Leigh R. Induced sputum, eosinophilic bronchitis, and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:S53–7. 10.1164/ajrccm.160.supplement_1.14 [DOI] [PubMed] [Google Scholar]
- 4. Rutgers SR, Postma DS, ten Hacken NH, et al. Ongoing airway inflammation in patients with COPD who do not currently smoke. Thorax 2000;55:12–18. 10.1136/thorax.55.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tashkin DP, Wechsler ME. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2018;13:335–49. 10.2147/COPD.S152291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. From asthma, COPD, and Asthma-COPD overlap syndrome. Available: http://www.goldcopd.org/andhttp://www.ginasthma.org/
- 7. Kolsum U, Ravi A, Hitchen P, et al. Clinical characteristics of eosinophilic COPD versus COPD patients with a history of asthma. Respir Res 2017;18:73. 10.1186/s12931-017-0559-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;192:523–5. 10.1164/rccm.201502-0235LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dweik RA, Boggs PB, Erzurum SC, et al. An official ats clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602–15. 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karrasch S, Linde K, Rücker G, et al. Accuracy of FENO for diagnosing asthma: a systematic review. Thorax 2017;72:109–16. 10.1136/thoraxjnl-2016-208704 [DOI] [PubMed] [Google Scholar]
- 11. National health and nutrition examination survey data. Hyattsville, MD: U.S. department of health and human services, centers for disease control and prevention. 2007 to 2010. Centers for disease control and prevention (CDC). National center for health statistics (NCHS). Available: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm
- 12. Miller M, Hankinson J, Brusasco V, et al. ATS/ERS Task force: standardisation of spirometry. Eur Respir J 2005;26:319–38. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 13. Center for disease control and prevention. NHANES analytic guidelines. Available: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/bronchodilator.pdf
- 14. Center for Disease Control and Prevention . NHANES laboratory/medical technologists procedures manual. Atlanta, GA: Centers for Disease Control and Prevention, 2001. [Google Scholar]
- 15. American Thoracic Society, European Respiratory Society . ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912–30. 10.1164/rccm.200406-710ST [DOI] [PubMed] [Google Scholar]
- 16. Global initiative for chronic obstructive lung disease (GOLD) . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2019 update. Available: https://goldcopd.org [Accessed 01 Mar 2019].
- 17. Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat 2 2013;161:1–24. [PubMed] [Google Scholar]
- 18. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma 2011;48:279–85. 10.3109/02770903.2011.555576 [DOI] [PubMed] [Google Scholar]
- 19. Hardin M, Silverman EK, Barr RG, et al. The clinical features of the overlap between COPD and asthma. Respir Res 2011;12:1. 10.1186/1465-9921-12-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miravitlles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPD-asthma phenotype. focus on physical activity and health status. Respir Med 2013;107:1053–60. 10.1016/j.rmed.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 21. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest 2008;134:14–19. 10.1378/chest.07-2317 [DOI] [PubMed] [Google Scholar]
- 22. Rhee CK, Yoon HK, Yoo KH, et al. Medical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthma. COPD 2014;11:163–70. 10.3109/15412555.2013.831061 [DOI] [PubMed] [Google Scholar]
- 23. Andersén H, Lampela P, Nevanlinna A, et al. High Hospital burden in overlap syndrome of asthma and COPD. Clin Respir J 2013;7:342–6. 10.1111/crj.12013 [DOI] [PubMed] [Google Scholar]
- 24. Hardin M, Silverman EK, Barr RG, et al. The clinical features of the overlap between COPD and asthma. Respir Res 2011;12:127. 10.1186/1465-9921-12-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alshabanat A, Zafari Z, Albanyan O, et al. Asthma and COPD overlap syndrome (ACOs): a systematic review and meta analysis. PLoS One 2015;10:e0136065. 10.1371/journal.pone.0136065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brightling CE, Monteiro W, Ward R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2000;356:1480–5. 10.1016/S0140-6736(00)02872-5 [DOI] [PubMed] [Google Scholar]
- 27. Brightling CE, McKenna S, Hargadon B, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 2005;60:193–8. 10.1136/thx.2004.032516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leigh R, Pizzichini MMM, Morris MM, et al. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J 2006;27:964–71. 10.1183/09031936.06.00072105 [DOI] [PubMed] [Google Scholar]
- 29. Pizzichini E, Pizzichini MM, Gibson P, et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med 1998;158:1511–7. 10.1164/ajrccm.158.5.9804028 [DOI] [PubMed] [Google Scholar]
- 30. Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011;184:662–71. 10.1164/rccm.201104-0597OC [DOI] [PubMed] [Google Scholar]
- 31. Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J 2007;29:906–13. 10.1183/09031936.00146306 [DOI] [PubMed] [Google Scholar]
- 32. Papi A, Luppi F, Franco F, et al. Pathophysiology of exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:245–51. 10.1513/pats.200512-125SF [DOI] [PubMed] [Google Scholar]
- 33. Berry MA, Shaw DE, Green RH, et al. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy 2005;35:1175–9. 10.1111/j.1365-2222.2005.02314.x [DOI] [PubMed] [Google Scholar]
- 34. Warke TJ, Fitch PS, Brown V, et al. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax 2002;57:383–7. 10.1136/thorax.57.5.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Payne DN, Adcock IM, Wilson NM, et al. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med 2001;164:1376–81. 10.1164/ajrccm.164.8.2101145 [DOI] [PubMed] [Google Scholar]
- 36. Taylor DR, Mandhane P, Greene JM, et al. Factors affecting exhaled nitric oxide measurements: the effect of sex. Respir Res 2007;8:1. 10.1186/1465-9921-8-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Travers J, Marsh S, Aldington S, et al. Reference ranges for exhaled nitric oxide derived from a random community survey of adults. Am J Respir Crit Care Med 2007;176:238–42. 10.1164/rccm.200609-1346OC [DOI] [PubMed] [Google Scholar]
- 38. Brody DJ, Zhang X, Kit BK, et al. Reference values and factors associated with exhaled nitric oxide: U.S. youth and adults. Respir Med 2013;107:1682–91. 10.1016/j.rmed.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 39. Olin A-C, Bake B, Torén K. Fraction of exhaled nitric oxide at 50 mL/s: reference values for adult lifelong never-smokers. Chest 2007;131:1852–6. 10.1378/chest.06-2928 [DOI] [PubMed] [Google Scholar]
- 40. Wang D, Wang Y, Liang H, et al. Race and ethnicity have significant influence on fractional exhaled nitric oxide. Ann Allergy Asthma Immunol 2018;120:e1:272–7. 10.1016/j.anai.2017.11.021 [DOI] [PubMed] [Google Scholar]
- 41. Habib SS, Ahmed SM, Al Drees AM, et al. Effect of cigarette smoking on fractional exhaled nitric oxide in Saudi medical college students. J Pak Med Assoc 2011;61:120–3. [PubMed] [Google Scholar]
- 42. Nadif R, Matran R, Maccario J, et al. Passive and active smoking and exhaled nitric oxide levels according to asthma and atopy in adults. Ann Allergy Asthma Immunol 2010;104:385–93. 10.1016/j.anai.2010.03.013 [DOI] [PubMed] [Google Scholar]
- 43. Kharitonov SA, Robbins RA, Yates D, et al. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med 1995;152:609–12. 10.1164/ajrccm.152.2.7543345 [DOI] [PubMed] [Google Scholar]
- 44. Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? towards a consensus definition from a round table discussion. Eur Respir J 2016;48:664–73. 10.1183/13993003.00436-2016 [DOI] [PubMed] [Google Scholar]
- 45. Soler-Cataluña JJ, Cosío B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD–Asthma in COPD. Archivos de Bronconeumología 2012;48:331–7. 10.1016/j.arbr.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 46. Lee S-Y, Park HY, Kim EK, et al. Combination therapy of inhaled steroids and long-acting beta2-agonists in asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis 2016;11:2797–803. 10.2147/COPD.S114964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. GOLD . Diagnosis of diseases of chronic airflow limitation: asthma, COPD and Asthma-COPD overlap syndrome (ACOs), 2015. Available: https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf [Accessed 01 Mar 2020].
- 48. Sá‐Sousa A, Jacinto T, Azevedo LF, et al. Operational definitions of asthma in recent epidemiological studies are inconsistent. Clin Transl Allergy 2014;4:1. 10.1186/2045-7022-4-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.