Abstract
Objectives
The association between visceral adiposity index (VAI) and the prevalence of non-alcoholic fatty liver disease (NAFLD) has not been fully determined. Here, we aimed to explore the association between VAI and NAFLD in the general US population, and further investigate whether the association involves population differences.
Design
Cross-sectional population-based study.
Setting
The National Health and Nutrition Examination Survey (2003–2018).
Participants
A total of 7522 participants aged 20 years or older who have complete information for NAFLD assessment test were included in this study.
Primary and secondary outcome measures
NAFLD was assessed by the modified fatty liver index for the US population (USFLI) using a cut-off point of 30. Correlation between VAI and NAFLD prediction scores was calculated using the partial correlation analysis. Logistic regression models were further used to estimate ORs and 95% CIs.
Results
Insulin resistance (IR), inflammation and waist circumference-adjusted partial correlation analysis indicated that VAI scores were positively correlated with USFLI (r=0.404 for men, and r=0.395 for women; p<0.001). In a comparison of the highest versus the lowest quartiles of VAI, multivariable logistic regression analysis demonstrated a positive association between VAI and NAFLD (OR (95% CI)=1.97 (1.12 to 3.47) for men, OR (95% CI)=4.03 (1.98 to 8.20) for women). The stratified analyses revealed that the positive association involves age/gender-specific and ethnic differences. As for the impact of metabolic disorders, our results revealed that the association was independent of IR and diabetes, but it would be confounded by other metabolic disorders. However, no significant association was found between VAI and hepatic fibrosis.
Conclusion
VAI is positively associated with the prevalence of NAFLD, but not hepatic fibrosis among US adults, and the association involves age/gender-specific and ethnic differences. The results reported here have important public health implications in NAFLD screening in the future.
Keywords: public health, epidemiology, hepatology
Strengths and limitations of this study.
The quality and scale of the National Health and Nutrition Examination Survey database and the rigour of its measures ensure the statistical power and reliability of our results.
The strict exclusion criteria ensure the homogeneity of the study population.
Multiple potential confounders were well controlled in the study.
Although we used well-validated non-alcoholic fatty liver disease and fibrosis models, there is a chance of misclassification in some cases due to lacking information on image and histology of the liver.
The cross-sectional nature of this study limits the assessment of causality.
Introduction
With an accelerated pace of nutrition transition, non-alcoholic fatty liver disease (NAFLD) has become an emerging public health issue with high prevalence worldwide, affecting up to one-third of the population,1 and its incidence is expected to rise rapidly in the future alongside increasing rates of obesity.2 According to epidemiological data, nearly a quarter of patients with NAFLD would progress to steatohepatitis with fibrosis, which could lead to serious liver-related complications and death.3 4 On the other hand, NAFLD is closely related to higher rates of several cardiometabolic disorders, including diabetes mellitus, metabolic syndrome (MetS) and cardiovascular diseases (CVDs).2 5 Thus, the increasing prevalence of NAFLD is particularly life-threatening. It is important to identify modifiable risk factors for NAFLD for reducing the disease burden.
Currently, the pathogenesis of NAFLD has not been completely understood, and increasing evidence suggests that visceral fat accumulation plays a crucial role in the pathogenesis of NAFLD.6 7 In recent years, the visceral adiposity index (VAI) based on waist circumference (WC), body mass index (BMI), plasma triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) has been proposed as a reliable marker to assess the content and function of visceral fat.8 Although VAI has been proven to be a powerful indicator of type 2 diabetes mellitus (T2DM),9 MetS10 and cardiovascular events,11 there are controversial data regarding the association between VAI and NAFLD. A prior study reported that VAI was related to significant fibrosis in patients with NAFLD,12 while other researchers revealed that the association did not exist in obese subjects or subjects without diabetes with NAFLD.13 14 In addition to the small sample size, variations in the participants with or without additional metabolic disorders largely contributed to these conflicting results. According to current evidence, insulin resistance (IR) is related to NAFLD,15 while VAI is a valuable indicator of IR.8 To date, it is unknown whether the status of insulin sensitivity is the result of the controversy among patients with NAFLD underlying different clinical statuses. Furthermore, variations in genetic background may be another explanation for these controversial results in NAFLD.16 Two recent cohort studies with large-scale population conducted in China and Japan implied that higher VAI levels are correlated with an increased incidence of NAFLD in Asian population,17 18 while a similar study has not been done or published in the USA. In this study, we intend to investigate whether VAI is significantly associated with the prevalence of NAFLD in the US general population, independent of IR and its related metabolic disorders, using the data from the National Health and Nutrition Examination Survey (NHANES).
Methods
The design, implementation, analysis and reporting of this study were conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement.19
Study population
The NHANES is a multistage, ongoing, complex cross-sectional health examination and survey designed to collect the health data of the US non-institutionalised civilian population. The survey was conducted by the Centers for Disease Control and Prevention. The data are freely available from the NHANES website public archive. Information regarding interview processes, examination protocols and sample collection can also be found on the website.20 Given that the information about fasting plasma glucose (FPG) and insulin was available since 2003, data from 2003 to 2018 were obtained for analysis.
Of all participants, we initially selected non-pregnant subjects aged 20 years or older. Then, we excluded individuals missing the information about anthropometric parameters (BMI and WC) and blood pressure. Subsequently, we excluded individuals with the following reasons: excessive alcohol consumption (defined by >1 drink/day for women or >2 drinks/day for men), viral hepatitis (defined by positive serum hepatitis B or C antibody and/or positive serum hepatitis B surface antigen), and missing laboratory data to rule in or rule out the presence of NAFLD. Given the unique condition of puerperium women, we also excluded women who were at 0–12 weeks post partum.21 Finally, 7522 subjects were included (figure 1).
Figure 1.
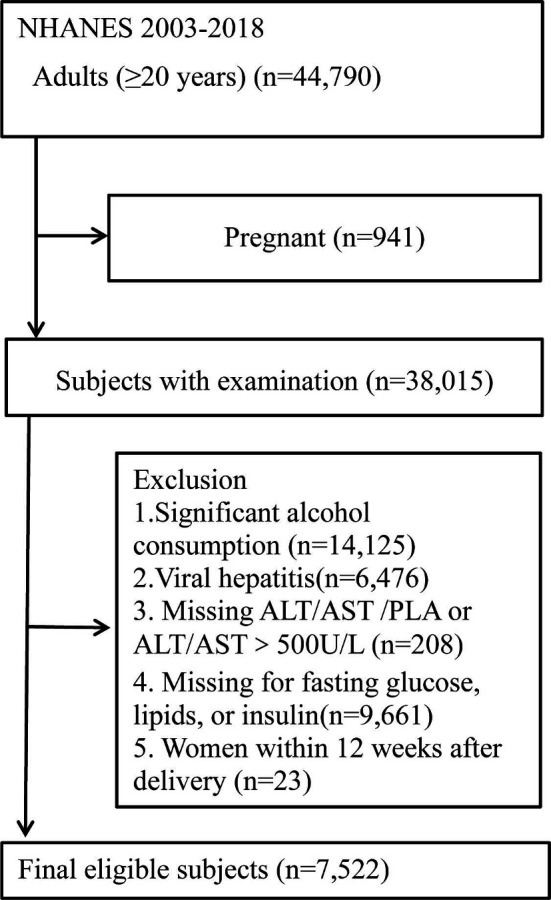
Flow chart of the participants’ inclusion and exclusion in this study. ALT, alanine aminotransferase; AST, aspartate aminotransferase; PLA, platelet. NHANES, National Health and Nutrition Examination Survey.
Assessment of VAI
We calculated VAI using the following formulas8:
| (1) |
| (2) |
Definitions of covariates
Ethnicity was categorised as non‐Hispanic white, non‐Hispanic black or Hispanic. High education was defined as completing a high school degree or above. Current smokers were defined as the participants who smoked at least 100 cigarettes in lifetime and now smoke cigarettes every day or some days. Similarly, current drinkers were defined as the participants who had at least 12 alcohol drinks their entire life and now drink alcohol every day or some days. Physical activity was defined as engaging in moderate or vigorous exercise regularly (≥20 min at a time and at least three times per week).22 The poverty-to-income ratio (PIR) was calculated by dividing family income by the poverty guidelines specific to the survey year.23
Regarding the influence of IR, inflammation and related metabolic complications on the relationship between visceral fat and NAFLD, the homeostasis model assessment of IR (HOMA-IR), inflammation (C reactive protein (CRP)) and several diseases were calculated and defined. HOMA-IR was defined as fasting glucose (mg/dL)×fasting insulin (μU/mL)/405.24 Overweight and obese participants were defined as those with BMI ≥25 kg/m2 for non-Asians.25 T2DM was defined based on fasting glucose (≥126 mg/dL) and/or receiving insulin or oral hypoglycaemic therapy. Hypertension was defined as systolic pressure ≥140 mm Hg and/or diastolic pressure ≥90 mm Hg, or antihypertensive therapy.26 MetS was defined according to the National Cholesterol Education Program Adult Treatment Panel III report, as an individual who has three or more of all criteria.27 CVD was defined as the composite of self-report history of stroke, myocardial infarction, coronary revascularisation procedure, angina and congestive heart failure.28
Definitions of NAFLD and liver fibrosis
The definition of NAFLD was based on the US fatty liver index (USFLI) which was calculated by gamma-glutamyl transferase (U/L), WC (cm), fasting glucose (mg/dL) and fasting insulin (pmol/L).29 The cut-off of 30 was used to define NAFLD. Furthermore, the presence of fibrosis among individuals with NAFLD was assessed using NAFLD fibrosis score (NFS), fibrosis-4 index and aspartate aminotransferase to platelet ratio index.30–32 The cut-offs of 0.676, 2.67 and 1.0 were used to define NAFLD significant fibrosis, respectively. All calculation formulas were described as follows:
| (3) |
| (4) |
| (5) |
| (6) |
Statistical analysis
We summarised the weighted median (IQR) for continuous variables, and weighted proportions for categorical variables in table 1. For the full dataset analysis, we created 16-year weights as one-eighth of the value of the fasting subsample weights (WTSAF2YR×1/8) since this represented the smallest subsample of the study.33 Given the calculation of VAI differed between gender, we divided the participants into men and women. The p value was analysed according to VAI quartiles using Kruskal-Wallis analysis and Χ2 tests, respectively. Partial correlation analysis was performed to investigate the relationship between VAI and NAFLD prediction models. Non-normally distributed data were transformed to Gaussian distribution before assessing the partial correlation analysis via Blom’s rank-based inverse normal transformations.34 In addition, we ran three logistic models to calculate variable-adjusted ORs (with 95% CIs) for NAFLD, taking the lowest category of VAI as the reference. The three models were as follows: model 1 was unadjusted. Model 2 was adjusted for age, ethnicity and the survey cycle year. Model 3 was adjusted for all the variables in model 2 plus education level, PIR, alcohol drinking, smoking, physical activity status, HOMA-IR, CRP, blood pressure and the same variable between VAI and NAFLD prediction scores. After testing for multicollinearity, we observed that all models presented were free from collinearity (the variance inflation factor <1.61). Moreover, to further investigate potential factors that influenced the association between VAI and the prevalence of NAFLD, we performed stratified analyses by age, ethnicity, smoking status, IR and presence of several metabolic disorders, using the fully adjusted model (excluding the stratification variable). All data were analysed with SPSS complex sample module V.21.0, and significance was accepted at a two-tailed p<0.05.
Table 1.
Characteristics of NHANES participants, 2003–2018, by VAI quartiles
| Men | Women | |||||||||
| Quartile 1 (≤0.87) |
Quartile 2 (0.87–1.45) |
Quartile 3 (1.46–2.49) |
Quartile 4 (>2.49) |
P value | Quartile 1 (≤0.99) |
Quartile 2 (1.00–1.63) |
Quartile 3 (1.63–2.65) |
Quartile 4 (>2.65) |
P value | |
| Age (years) | 51 (36–64) | 53 (41–65) | 53 (40–65) | 51 (41–64) | <0.001 | 51 (37–64) | 55 (41–67) | 56 (45–68) | 57 (43–67) | <0.001 |
| Ethnicity (%) | ||||||||||
| Hispanic | 10.4 | 12.4 | 12.1 | 11.5 | <0.001 | 10.0 | 14.7 | 15.3 | 13.5 | <0.001 |
| White | 66.6 | 72.4 | 74.6 | 78.8 | <0.001 | 64.5 | 69.0 | 68.4 | 75.2 | <0.001 |
| Black | 17.2 | 10.7 | 6.6 | 3.9 | <0.001 | 18.6 | 11.6 | 9.6 | 3.8 | <0.001 |
| High education (%) | 83.8 | 83.7 | 83.8 | 82.4 | <0.001 | 86.5 | 82.3 | 79.5 | 76.5 | <0.001 |
| Current smokers (%) | 12.8 | 16.5 | 17.0 | 17.7 | <0.001 | 7.1 | 10.0 | 14.1 | 19.2 | <0.001 |
| Current drinkers (%) | 17.7 | 13.6 | 11.4 | 13.2 | <0.001 | 16.7 | 13.2 | 15.0 | 14.8 | <0.001 |
| Physical activity (%) | 80.2 | 74.3 | 71.8 | 69.1 | <0.001 | 72.6 | 66.9 | 61.9 | 56.7 | <0.001 |
| Obese (%) | 21.6 | 26.8 | 44.0 | 53.1 | <0.001 | 20.3 | 34.0 | 49.3 | 61.0 | <0.001 |
| CVD (%) | 9.7 | 11.0 | 17.7 | 18.4 | <0.001 | 7.4 | 10.9 | 9.9 | 14.8 | <0.001 |
| T2DM (%) | 9.1 | 11.6 | 20.6 | 26.0 | <0.001 | 5.1 | 9.9 | 17.5 | 27.2 | <0.001 |
| Hypertension (%) | 33.4 | 43.4 | 49.0 | 54.6 | <0.001 | 30.8 | 42.9 | 49.0 | 57.1 | <0.001 |
| MetS (%) | 14.1 | 23.8 | 51.9 | 85.8 | <0.001 | 12.6 | 30.1 | 50.4 | 87.5 | <0.001 |
| NAFLD (%) | 14.9 | 28.8 | 50.3 | 68.2 | <0.001 | 5.3 | 18.6 | 35.0 | 62.3 | <0.001 |
| PIR (%) | 3.5 (1.9–5.0) | 3.6 (2.0–5.0) | 3.2 (1.8–5.0) | 3.3 (1.83–5.0) | <0.001 | 3.4 (1.7–5.0) | 3.0 (1.5–4.9) | 2.6 (1.3–4.6) | 2.3 (1.3–4.0) | <0.001 |
| BMI (kg/m2) | 24.9 (23.2–29.3) | 27.3 (24.8–30.3) | 29.4 (26.2–32.9) | 30.4 (27.7–34.4) | <0.001 | 24.6 (22.0–28.7) | 27.3 (24.2–32.1) | 29.9 (25.8–35.1) | 31.8 (27.7–36.6) | <0.001 |
| WC (cm) | 94.5 (85.8–104.6) | 100 (92.5–108.3) | 106.2 (97.2–115.8) | 107.6 (100.5–118.8) | <0.001 | 86.5 (79.0–95.5) | 94.0 (86.0–104.5) | 100.5 (91.3–111.6) | 106.1 (97.0–117) | <0.001 |
| SBP (mm Hg) | 120.7 (111–131) | 122.7 (112–132) | 121.3 (114.0–121) | 124.7 (116–134) | <0.001 | 114.7 (104–129) | 121 (109–135) | 123 (112–137) | 124 (114–138) | <0.001 |
| DBP (mm Hg) | 70 (63–76) | 71 (64–78) | 73 (66–80) | 74 (67–82) | <0.001 | 68 (61–74) | 69 (62–76) | 70 (62–76) | 70 (62–76) | <0.001 |
| ALT (U/L) | 21 (17–26) | 24 (19–30) | 26 (20–34) | 28 (22–38) | <0.001 | 17 (14–21) | 18 (14–21) | 19 (15–24) | 21 (16–27) | <0.001 |
| AST (U/L) | 23 (20–27) | 24 (20–27) | 24 (20–28) | 25 (21–30) | <0.001 | 21 (18–24) | 21 (18–24) | 21 (18–24) | 22 (19–27) | <0.001 |
| FPG (mg/dL) | 100 (94–108) | 102 (96–110) | 105 (96–117) | 106 (98–122) | <0.001 | 94 (89–100) | 98 (92–107) | 100 (94–109) | 105 (96–122) | <0.001 |
| Insulin (µU/mL) | 6.3 (4.2–9.7) | 8.1 (5.5–12.8) | 11.4 (7.2–17.2) | 14.2 (9.3–21.9) | <0.001 | 5.8 (3.9–8.5) | 8.9 (5.9–12.4) | 10.5 (6.9–16.8) | 14.8 (9.4–22.3) | <0.001 |
| HOMA-IR | 1.6 (1.0–2.5) | 2.1 (1.4–3.4) | 3.0 (1.8–4.8) | 3.9 (2.6–6.6) | <0.001 | 1.4 (0.9–2.1) | 2.2 (1.4–3.2) | 2.7 (1.7–4.3) | 4.0 (2.5–6.5) | <0.001 |
| CRP | 0.50 (0.09–1.58) | 0.40 (0.11–1.42) | 0.39 (0.12–1.31) | 0.40 (0.18–1.32) | <0.001 | 0.40 (0.08–1.20) | 0.50 (0.16–1.85) | 0.65 (0.22–2.16) | 0.74 (0.34–2.60) | <0.001 |
| HDL-C (mmol/L) | 1.5 (1.3–1.8) | 1.3 (1.2–1.4) | 1.1 (1.0–1.2) | 1.1 (1.0–1.3) | <0.001 | 1.9 (1.6–2.2) | 1.6 (1.4–1.7) | 1.4 (1.2–1.5) | 1.1 (1.0–1.3) | <0.001 |
| TC (mmol/L) | 4.6 (4.0–5.3) | 4.8 (4.2–5.5) | 4.9 (4.2–5.5) | 5.1 (4.5–5.9) | <0.001 | 5.0 (4.3–5.7) | 5.0 (4.3–5.7) | 5.1 (4.6–5.8) | 5.4 (4.6–6.3) | <0.001 |
| TG (mmol/L) | 0.7 (0.5–0.8) | 1.1 (0.9–1.2) | 1.5 (1.3–1.7) | 2.5 (2.1–3.2) | <0.001 | 0.7 (0.5–0.8) | 1.0 (0.9–1.1) | 1.4 (1.2–1.6) | 2.2 (1.8–2.7) | <0.001 |
| USFLI | 9.9 (5.1–21.7) | 18.8 (9.9–33.6) | 30.2 (15.8–50.0) | 43.0 (25.5–64.7) | <0.001 | 6.6 (3.1–12.1) | 14.7 (7.7–24.9) | 21.6 (11.4–39.4) | 37.9 (20.0–55.0) | <0.001 |
| NFS | −1.29 (−2.30 to –0.23) | −1.18 (−2.31 to 0.02) | −1.00 (−2.3 to –0.08) | −0.89 (−1.92 to,–0.16) | <0.001 | −1.73 (−2.78 to –0.60) | −1.34 (−2.36 to –0.24) | −1.08 (−2.21 to –0.12) | −1.02 (−2.02 to 0.19) | <0.001 |
| FIB-4 | 1.17 (0.74−1.69) | 1.10 (0.75−1.59) | 1.05 (0.74−1.49) | 1.05 (0.73−1.47) | <0.001 | 1.06 (0.72−1.55) | 1.06 (0.74−1.49) | 1.02 (0.71−1.40) | 0.99 (0.69−1.40) | <0.001 |
| APRI | 0.28 (0.23−0.36) | 0.27 (0.22−0.35) | 0.28 (0.22−0.35) | 0.29 (0.23−0.38) | <0.001 | 0.23 (0.18−0.30) | 0.22 (0.18−0.29) | 0.21 (0.17−0.27) | 0.22 (0.17−0.29) | <0.001 |
ALT, alanine aminotransferase; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; BMI, body mass index; CRP, C reactive protein; CVD, cardiovascular disease; DBP, diastolic blood pressure; FIB-4, fibrosis-4 index; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; MetS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; NFS, NAFLD fibrosis score; NHANES, National Health and Nutrition Examination Survey; PIR, poverty-to-income ratio; SBP, systolic blood pressure; TC, total cholesterol; T2DM, type 2 diabetes mellitus; TG, triglycerides; USFLI, US fatty liver index; VAI, visceral adiposity index; WC, waist circumference.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Characteristics of participants classified according to the VAI quartiles
The study analysed a total of 7522 participants including 3789 men and 3733 women from the NHANES 2003–2018. Among these participants, 2789 individuals (1551 men and 1238 women) with NAFLD were defined by USFLI (37.0%). The baseline characteristics stratified by VAI quartiles were summarised in table 1. VAI was categorised by quartiles using the values 0.87, 1.46 and 2.49 in men, and using the values 0.99, 1.63 and 2.65 in women. In both genders, subjects with higher VAI levels were more likely to be older and non-Hispanic white. Likewise, those in the higher quartile of VAI tended to have higher levels of BMI, WC, diastolic blood pressure, FPG, fasting insulin, HOMA-IR, total cholesterol and TG, and lower level of HDL-C. Regarding the clinical condition, the proportions of obesity, T2DM, hypertension, CVD and MetS were increased with the increase of VAI level. Similarly, subjects with the higher quartile of VAI had more NAFLD burden. The liver fibrosis burden had no significant difference among VAI quartiles.
Correlations between VAI and NAFLD-related prediction scores
By performing the Pearson correlation analysis, we found that the indices of IR (HOMA-IR) and inflammation (CRP) were positively correlated with VAI and NAFLD indices, respectively, which indicated that IR and inflammation might be the important factors connecting visceral fat and NAFLD (online supplemental table 1). In addition, the mediating effect of the same variable between VAI and NAFLD indices should also be taken into account (WC for USFLI; BMI for NFS). Therefore, we performed the partial correlation analysis which adjusts the influence of these variables to calculate the correlation coefficients between VAI and NAFLD indices. As shown in table 2, VAI was found to be significantly correlated with USFLI in both genders (r=0.404, p<0.001 for men, and r=0.395, p<0.001 for women), but not liver fibrosis indices.
Table 2.
Partial correlation coefficients between VAI and NAFLD indices
| Models | Men | Women | ||
| r | P value | r | P value | |
| NAFLD defined by | ||||
| USFLI score | 0.404* | <0.001 | 0.395* | <0.001 |
| Fibrosis defined by | ||||
| NFS score | −0.029 | 0.140 | 0.019 | 0.328 |
| FIB-4 | 0.026 | 0.185 | −0.013 | 0.514 |
| APRI score | −0.030 | 0.118 | −0.047 | 0.017 |
Partial correlation coefficients are calculated by adjusting for HOMA-IR, CRP and the same variable between VAI and NAFLD indices (WC for USFLI; BMI for NFS).
*P<0.001.
APRI, AST to platelet ratio index; AST, aspartate aminotransferase; BMI, body mass index; CRP, C reactive protein; FIB-4, fibrosis-4 index; HOMA-IR, homeostasis model assessment of insulin resistance; NAFLD, non-alcoholic fatty liver disease; NFS, NAFLD fibrosis score; USFLI, US fatty liver index; VAI, visceral adiposity index; WC, waist circumference.
bmjopen-2021-058517supp001.pdf (89.9KB, pdf)
The OR of NAFLD across quartiles of VAI
We further conducted logistic regression analyses to calculate the OR and 95% CI to assess the association between VAI and NAFLD, using the lowest VAI level as the reference. As the results shown in table 3, the positive association between VAI and NAFLD persisted in all VAI categories in unadjusted model and model adjusted for age, ethnicity and the survey cycle year. In the most multivariable-adjusted model, although the positive associations were weakened in both genders, the associations remained statistically significant in the top quartile of VAI (OR (95% CI)=1.97 (1.12 to 3.47) for men, OR (95% CI)=4.03 (1.98 to 8.20) for women). Regarding NAFLD-related fibrosis, we found no association between VAI and liver fibrosis in both genders.
Table 3.
The multivariable OR for NAFLD and fibrosis according to VAI levels
| Men | VAI levels | |||
| Quartile 1 (≤0.87) |
Quartile 2 (0.87–1.45) |
Quartile 3 (1.46–2.49) |
Quartile 4 (>2.49) |
|
| NAFLD | ||||
| Defined by USFLI | ||||
| Model 1 | 1.00 | 2.58 (2.06 to 3.25) | 5.70 (4.56 to 7.12) | 13.69 (10.88 to 17.22) |
| Model 2 | 1.00 | 2.55 (2.01 to 3.23) | 5.83 (4.61 to 7.36) | 14.97 (11.73 to 19.11) |
| Model 3 | 1.00 | 1.30 (0.75 to 2.27) | 1.16 (0.66 to 2.02) | 1.97 (1.12 to 3.47) |
| NAFLD-related fibrosis | ||||
| Defined by NFS | ||||
| Model 1 | 1.00 | 0.60 (0.38 to 0.95) | 0.67 (0.44 to 1.02) | 0.56 (0.37 to 0.84) |
| Model 2 | 1.00 | 0.84 (0.50 to 1.43) | 1.13 (0.69 to 1.86) | 1.22 (0.75 to 1.98) |
| Model 3 | 1.00 | 1.19 (0.77 to 1.83) | 1.96 (0.61 to 1.50) | 1.13 (0.72 to 1.79) |
| By FIB-4 | ||||
| Model 1 | 1.00 | 0.96 (0.67 to 1.40) | 0.74 (0.50 to 1.10) | 0.51 (0.33 to 0.79) |
| Model 2 | 1.00 | 0.98 (0.65 to 1.46) | 0.80 (0.52 to 1.24) | 0.66 (0.41 to 1.06) |
| Model 3 | 1.00 | 1.04 (0.62 to 1.73) | 0.61 (0.34 to 1.09) | 0.63 (0.34 to 1.16) |
| Defined by APRI | ||||
| Model 1 | 1.00 | 0.95 (0.51 to 1.77) | 1.00 (0.54 to 1.84) | 1.54 (0.88 to 2.69) |
| Model 2 | 1.00 | 0.91 (0.46 to 1.71) | 0.97 (0.52 to 1.81) | 1.53 (0.85 to 2.73) |
| Model 3 | 1.00 | 0.97 (0.46 to 2.04) | 0.71 (0.32 to 1.59) | 1.06 (0.50 to 2.22) |
| Women | Quartile 1 (≤0.99) |
Quartile 2 (1.00–1.63) |
Quartile 3 (1.63–2.65) |
Quartile 4 (>2.65) |
| NAFLD | ||||
| Defined by USFLI | ||||
| Model 1 | 1.00 | 3.45 (2.59 to 4.61) | 7.88 (5.98 to 10.38) | 19.98 (15.15 to 26.33) |
| Model 2 | 1.00 | 3.14 (2.34 to 4.21) | 7.31 (5.51 to 9.70) | 20.07 (15.06 to 26.74) |
| Model 3 | 1.00 | 1.73 (0.85 to 3.53) | 2.90 (1.43 to 5.88) | 4.03 (1.98 to 8.20) |
| NAFLD-related fibrosis | ||||
| Defined by NFS | ||||
| Model 1 | 1.00 | 0.95 (0.53 to 1.73) | 0.83 (0.47 to 1.45) | 0.69 (0.40 to 1.19) |
| Model 2 | 1.00 | 0.91 (0.48 to 1.74) | 0.83 (0.45 to 1.53) | 0.79 (0.43 to 1.45) |
| Model 3 | 1.00 | 1.26 (0.43 to 3.71) | 0.66 (0.23 to 1.86) | 0.70 (0.25 to 1.96) |
| By FIB-4 | ||||
| Model 1 | 1.00 | 1.12 (0.58 to 2.17) | 0.58 (0.27 to 1.28) | 0.64 (0.30 to 1.38) |
| Model 2 | 1.00 | 0.84 (0.42 to 1.67) | 0.41 (0.18 to 0.92) | 0.48 (0.22 to 1.07) |
| Model 3 | 1.00 | 0.69 (0.26 to 1.83) | 0.36 (0.11 to 1.11) | 0.33 (0.11 to 1.00) |
| Defined by APRI | ||||
| Model 1 | 1.00 | 1.20 (0.37 to 3.95) | 1.40 (0.44 to 4.42) | 1.20 (0.37 to 3.95) |
| Model 2 | 1.00 | 1.12 (0.33 to 3.73) | 1.23 (0.38 to 4.03) | 1.12 (0.33 to 3.83) |
| Model 3 | 1.00 | 0.83 (0.13 to 5.26) | 0.83 (0.13 to 5.18) | 0.54 (0.08 to 3.61) |
APRI, AST to platelet ratio index; AST, aspartate aminotransferase; FIB-4, fibrosis-4 index; NAFLD, non-alcoholic fatty liver disease; NFS, NAFLD fibrosis score; USFLI, US fatty liver index; VAI, visceral adiposity index.
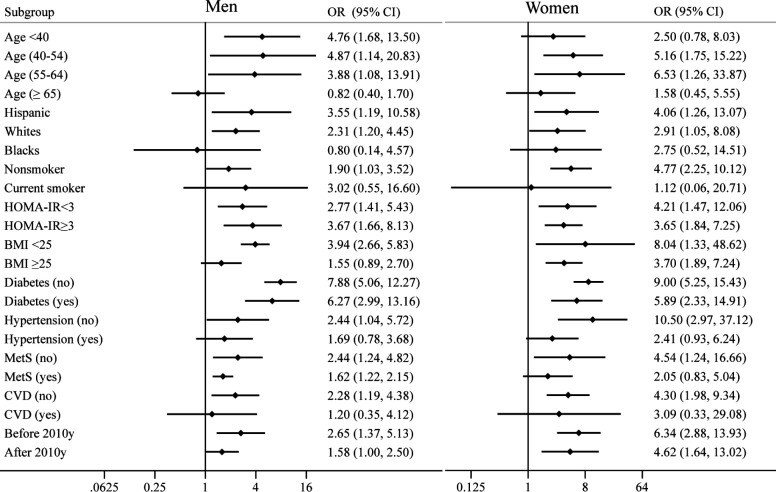
The stratified analyses of VAI and risk of NAFLD
To investigate the effect of confounding factors, the ORs comparing the highest versus the lowest quartile of VAI were calculated in all subgroups. As shown in figure 2, when stratified by age, we found a positive association between VAI and NAFLD in men aged <55 years, and women aged 40–64 years. With respect to ethnicity, we found a positive correlation between VAI and NAFLD only in Hispanic and non-Hispanic white population, but not in the non-Hispanic black population in both genders. In addition, the positive associations were consistently seen in all evaluated subgroups when stratified by the status of IR and diabetes. When stratified by the smoking status and other metabolic disorders (hypertension, CVD and MetS), the positive associations were only persisted in individuals without these conditions. Of note, the stronger positive associations were found in individuals with normal BMI, while the associations were weakened in overweight and obese people. Considering the different prevalence rates of obesity during the period of more than 10 years, we also tested the consistency of our results by stratifying the data release year (before 2010 and after 2010). Similar to the subgroup of obesity, the positive association was weakened in recent years.
Figure 2.
The association between VAI and the risk of NAFLD stratified by age, ethnicity, smoking, insulin resistance and other related metabolic diseases. BMI, body mass index; CVD, cardiovascular disease; HOMA-IR, homeostasis model assessment of insulin resistance; MetS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; VAI, visceral adiposity index.
Model 1 is unadjusted; model 2 is adjusted for age, ethnicity and the survey cycle year; model 3 is adjusted for all the variables in model 2 plus education level, PIR, alcohol drinking, smoking, physical activity status, HOMA-IR, CRP, blood pressure and the same variable between VAI and NAFLD indices (WC for USFLI; BMI for NFS).
Discussion
In this study, we found a positive association between VAI and NAFLD after controlling for several potential confounders, whereas no significant association was found between VAI and NAFLD-related fibrosis. The stratified analyses revealed that the positive association between VAI and NAFLD involves age/gender-specific and ethnic differences. In addition, as for the impact of metabolic disorders, our results revealed that the association was independent of IR and diabetes, but it would be confounded by other metabolic disorders, such as hypertension, CVD and MetS. To our best knowledge, this is the first large population-based study to report a strong association between VAI and the risk of NAFLD in the USA. Furthermore, this is also the first study that reveals the role of age, gender, ethnicity and multiple metabolic disorders in the association between VAI and NAFLD.
As far as we know, there are only two large-scale studies that investigated the VAI in subjects with NAFLD. Xu et al found that VAI was associated with NAFLD in 4809 Chinese participants after multivariate adjustment.17 However, these findings were limited to the population with normal weight from underdeveloped areas in China. Although Okamura et al confirmed the association in 8399 Japanese people by a nationally representative, population-based cohort study,18 this study still lacked the data of plasma insulin level and could not evaluate the impact of IR on the relationship between VAI and NAFLD. However, our study had the unique feature in examining whether the relationship was independent of IR and various metabolic diseases. Moreover, we also explored the association between VAI and the prevalence of NAFLD-related fibrosis. Although Petta et al concluded that VAI was independently associated with significant fibrosis,12 the previous study performed by Ercin et al concluded that VAI was not associated with hepatic fibrosis in patients without diabetes with NAFLD.14 We considered that the discrepant findings of these studies may be due to differences in the composition of participants. Different from the study performed by Ercin et al, some participants in the study conducted by Petta et al were patients with hypertension, diabetes or MetS. The correlation between VAI and liver fibrosis in the study done by Petta et al might be affected by these metabolic disorders. Overall, our results are consistent with previous studies and indicate that VAI is an independent risk factor for NAFLD, but not NAFLD-related fibrosis.
Since VAI is a surrogate marker of both visceral fat distribution and dysfunction, the relationship between VAI and NAFLD could be explained by some potential mechanisms. Among several hypotheses that have been formulated, the ‘portal theory’ describes the directly toxic properties of visceral fat on the liver.35 The theory proposes that visceral fat releases free fatty acids via its unique location and enhanced lipolysis, which travel through the portal vein to the liver, with consequently increased accumulation of TG in the liver, promoting the development of hepatic IR and liver steatosis. Thus, IR has been traditionally considered as a physiological connection between visceral fat and NAFLD. However, our findings demonstrated that VAI was still associated with a higher prevalence of NAFLD in subjects without IR. The results suggest that there are some other mechanisms that directly link the visceral fat to NAFLD along with IR. Of note, in addition to lipotoxicity, there is mounting evidence proposed that changes in adipokine expression and secretion also participate in the development of NAFLD, as well as the infiltration of macrophage and T cells in visceral fat.36 37 Similar to FFAs, these proinflammatory cytokines and adipokines are carried directly to the liver via the portal vein, causing ballooning degeneration of hepatocytes or promoting the transformation of hepatic cells to myofibroblastic phenotypes.38–40 Furthermore, other proposed pathways including endoplasmic reticulum stress, toll-like receptor activation and impaired oxygenation may be also involved in the connection between visceral fat and NAFLD.41 42
Although the present study showed that high VAI was an independent risk factor for the presence of NAFLD, some information in the subgroup analysis also should be worthy of note. First, in the subgroup analysis by ethnicity, we found a positive association between VAI and NAFLD in the Hispanic and non-Hispanic white population, but not in the non-Hispanic black population. On the one hand, the formula of VAI which was evaluated based on a Caucasian population might have limitations regarding the non-Hispanic black population. On the other hand, the ethnic difference might be attributed to lower VAI levels in non-Hispanic black population. As the results are shown in table 3, VAI was only positively associated with NAFLD in the top quartile in men and higher quartiles in women. In addition to ethnic disparities, we also found the age/gender-specific difference. We found a null association in women aged <40 years, which might be explained by sexual dimorphism and fat distribution. Oestrogens could enhance the sympathetic tone differentially to the adipose tissue, favouring lipid accumulation in the subcutaneous depot in premenopausal women, whereas women would shift to accrue more visceral fat after menopause.43 Different from women, men are susceptible to visceral fat deposition in any stage of life. However, we did not find a valid association between VAI and the prevalence of NAFLD in people aged ≥65 years. According to epidemiological evidence, the prevalence of obesity, hypertension and MetS increased with age.44 45 However, as shown in our subgroup analysis, the status of these metabolic diseases would weaken or abolish the association between VAI and the risk of NAFLD in both genders. Thus, the association of VAI and NAFLD in older people might be confounded by the status of metabolic disease. Moreover, we also found that the effect of VAI was highlighted in normal-weight people compared with the overweight or obese ones. As reported, non-obese NAFLD affects about one-third of the persons with NAFLD in the USA,46 and these individuals probably could not get as much attention from doctors as obese ones. The association reported here has important clinical and public health implications in NAFLD screening in the future.
This study has several strengths. First, this is a large population-based analysis using well-examined nationwide data, and the findings could be generalised for most US population. Second, it is valued because we provided solid evidence of an independent association between VAI and NAFLD by performing multiple logistic regression and the stratified analyses. However, we are also aware of several limitations in our study. First, the cross-sectional nature of the study design meant that we could not investigate the longitudinal dynamic association between the progression of NAFLD and changes in VAI levels across several therapeutic interventions, such as lifestyle modification, exercise and weight control. Second, although we used well-validated NAFLD and fibrosis models, there is a chance of misclassification in some cases due to lacking information on image and histology of the liver. Third, estimates across some subgroups should be interpreted with caution due to limited sample sizes, such as subjects with diabetes or CVD.
Conclusions
In conclusion, our study documents that VAI might be a useful indicator for NAFLD, but not for hepatic fibrosis among US adults, and there exists age/gender-specific and ethnic differences. The results reported here have important public health implications in NAFLD screening in the future.
Supplementary Material
Footnotes
Contributors: QL and XZ conceived and designed the study. QL and JW performed the database search and checked the results against the inclusion and exclusion criteria. QL and YW analysed the data. QL wrote the initial draft of the paper. LW, JW and XZ reviewed and edited the manuscript. QL is responsible for the overall content as the guarantor. All authors have read and approved the final version.
Funding: This research was funded by the 2021 Postgraduate Education Reform and Quality Improvement Project of Henan Province (YJS2021KC07), the National Key R&D Program of China (2018 YFC 0114501), and Soft Science Project of Science and Technology Department of Henan Province (202400410345).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. The NHANES is a multistage, ongoing, complex cross-sectional health examination and survey designed to collect the health data of the US non-institutionalised civilian population. The data supporting reported results can be freely available from the NHANES website public archive, accessible at NHANES questionnaires, data sets and related documentation repository (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants. The survey was approved by the National Center for Health Statistics (NCHS) ethics review board. The NCHS IRB/ERC protocol number in this survey covers 98-12, 2005-06, 2011-17 and 2018-01. Participants gave informed consent to participate in the study before taking part.
References
- 1. Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis 2009;13:511–31. 10.1016/j.cld.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Stepanova M, Younossi Y, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020;69:564–8. 10.1136/gutjnl-2019-318813 [DOI] [PubMed] [Google Scholar]
- 3. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547–55. 10.1053/j.gastro.2014.11.039 [DOI] [PubMed] [Google Scholar]
- 4. Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-Term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865–73. 10.1002/hep.21327 [DOI] [PubMed] [Google Scholar]
- 5. Loomba R, Sanyal AJ. The global NAFLD epidemic: NAT Rev Gastroenterol Hepatol 2013;10:686–90. [DOI] [PubMed] [Google Scholar]
- 6. Eguchi Y, Eguchi T, Mizuta T, et al. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J Gastroenterol 2006;41:462–9. 10.1007/s00535-006-1790-5 [DOI] [PubMed] [Google Scholar]
- 7. Koda M, Kawakami M, Murawaki Y, et al. The impact of visceral fat in nonalcoholic fatty liver disease: cross-sectional and longitudinal studies. J Gastroenterol 2007;42:897–903. 10.1007/s00535-007-2107-z [DOI] [PubMed] [Google Scholar]
- 8. Amato MC, Giordano C, Galia M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010;33:920–2. 10.2337/dc09-1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang M, Zheng L, Li P, et al. 4-Year trajectory of visceral adiposity index in the development of type 2 diabetes: a prospective cohort study. Ann Nutr Metab 2016;69:142–9. 10.1159/000450657 [DOI] [PubMed] [Google Scholar]
- 10. Baveicy K, Mostafaei S, Darbandi M, et al. Predicting metabolic syndrome by visceral adiposity index, body Roundness index and a body shape index in adults: a cross-sectional study from the Iranian RaNCD cohort data. Diabetes Metab Syndr Obes 2020;13:879–87. 10.2147/DMSO.S238153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kouli G-M, Panagiotakos DB, Kyrou I, et al. Visceral adiposity index and 10-year cardiovascular disease incidence: the Attica study. Nutr Metab Cardiovasc Dis 2017;27:881–9. 10.1016/j.numecd.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 12. Petta S, Amato MC, Di Marco V, et al. Visceral adiposity index is associated with significant fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2012;35:238–47. 10.1111/j.1365-2036.2011.04929.x [DOI] [PubMed] [Google Scholar]
- 13. Díez-Rodríguez R, Ballesteros-Pomar MD, Calleja-Fernández A, et al. Insulin resistance and metabolic syndrome are related to non-alcoholic fatty liver disease, but not visceral adiposity index, in severely obese patients. Rev Esp Enferm Dig 2014;106:522–8. [PubMed] [Google Scholar]
- 14. Ercin CN, Dogru T, Genc H, et al. Insulin resistance but not visceral adiposity index is associated with liver fibrosis in nondiabetic subjects with nonalcoholic fatty liver disease. Metab Syndr Relat Disord 2015;13:319–25. 10.1089/met.2015.0018 [DOI] [PubMed] [Google Scholar]
- 15. Kitade H, Chen G, Ni Y, et al. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients 2017;9. 10.3390/nu9040387. [Epub ahead of print: 14 Apr 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macaluso FS, Maida M, Petta S. Genetic background in nonalcoholic fatty liver disease: a comprehensive review. World J Gastroenterol 2015;21:11088–111. 10.3748/wjg.v21.i39.11088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu C, Ma Z, Wang Y, et al. Visceral adiposity index as a predictor of NAFLD: a prospective study with 4-year follow-up. Liver Int 2018;38:2294–300. 10.1111/liv.13941 [DOI] [PubMed] [Google Scholar]
- 18. Okamura T, Hashimoto Y, Hamaguchi M, et al. The visceral adiposity index is a predictor of incident nonalcoholic fatty liver disease: a population-based longitudinal study. Clin Res Hepatol Gastroenterol 2020;44:375–83. 10.1016/j.clinre.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 19. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 20. CDC . NHANES survey methods and analytic guidelines. Available: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx
- 21. Subhan FB, Shulman L, Yuan Y, et al. Association of pre-pregnancy BMI and gestational weight gain with fat mass distribution and accretion during pregnancy and early postpartum: a prospective study of Albertan women. BMJ Open 2019;9:e026908. 10.1136/bmjopen-2018-026908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee Y-ho, Kim JE, Roh YH, et al. The combination of vitamin D deficiency and mild to moderate chronic kidney disease is associated with low bone mineral density and deteriorated femoral microarchitecture: results from the KNHANES 2008-2011. J Clin Endocrinol Metab 2014;99:3879–88. 10.1210/jc.2013-3764 [DOI] [PubMed] [Google Scholar]
- 23. U.S. Department of Health & Human Services . Poverty guidelines, research, and measurement. Washington, DC: U.S: Department of Health & Human Services, 2022. http://aspe.hhs.gov/POVERTY/index.shtml [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 25. WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 26. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560–72. 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 27. Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: report of the National heart, lung, and blood Institute/American heart association conference on scientific issues related to definition. Circulation 2004;109:433–8. 10.1161/01.CIR.0000111245.75752.C6 [DOI] [PubMed] [Google Scholar]
- 28. Parikh NS, VanWagner LB, Elkind MSV, et al. Association between nonalcoholic fatty liver disease with advanced fibrosis and stroke. J Neurol Sci 2019;407:13. 10.1016/j.jns.2019.116524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National health and nutrition examination survey. Aliment Pharmacol Ther 2015;41:65–76. 10.1111/apt.13012 [DOI] [PubMed] [Google Scholar]
- 30. Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104–12. 10.1016/j.cgh.2009.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin Z-H, Xin Y-N, Dong Q-J, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011;53:726–36. 10.1002/hep.24105 [DOI] [PubMed] [Google Scholar]
- 32. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–54. 10.1002/hep.21496 [DOI] [PubMed] [Google Scholar]
- 33. CDC . NHANES - continuous nhanes web tutorial - specifying weighting parameters. Available: https://wwwn.cdc.gov/nchs/nhanes/tutorials/Module3.aspx
- 34. Bishara AJ, Hittner JB. Testing the significance of a correlation with nonnormal data: comparison of Pearson, Spearman, transformation, and resampling approaches. Psychol Methods 2012;17:399–417. 10.1037/a0028087 [DOI] [PubMed] [Google Scholar]
- 35. Item F, Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obes Rev 2012;13 Suppl 2:30–9. 10.1111/j.1467-789X.2012.01035.x [DOI] [PubMed] [Google Scholar]
- 36. Bence KK, Birnbaum MJ. Metabolic drivers of non-alcoholic fatty liver disease. Mol Metab 2021;50:101143. 10.1016/j.molmet.2020.101143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Doyle SL, Donohoe CL, Lysaght J, et al. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc Nutr Soc 2012;71:181–9. 10.1017/S002966511100320X [DOI] [PubMed] [Google Scholar]
- 38. Ahmed MH, Barakat S, Almobarak AO. Nonalcoholic fatty liver disease and cardiovascular disease: has the time come for cardiologists to be hepatologists? J Obes 2012;2012:1–9. 10.1155/2012/483135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuk JL, Katzmarzyk PT, Nichaman MZ, et al. Visceral fat is an independent predictor of all-cause mortality in men. Obesity 2006;14:336–41. 10.1038/oby.2006.43 [DOI] [PubMed] [Google Scholar]
- 40. Polyzos SA, Kountouras J, Mantzoros CS. Adipose tissue, obesity and non-alcoholic fatty liver disease. Minerva Endocrinol 2017;42:92–108. 10.23736/S0391-1977.16.02563-3 [DOI] [PubMed] [Google Scholar]
- 41. Fricker ZP, Pedley A, Massaro JM, et al. Liver fat is associated with markers of inflammation and oxidative stress in analysis of data from the Framingham heart study. Clin Gastroenterol Hepatol 2019;17:1157–64. 10.1016/j.cgh.2018.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Exley MA, Hand L, O'Shea D, et al. Interplay between the immune system and adipose tissue in obesity. J Endocrinol 2014;223:R41–8. 10.1530/JOE-13-0516 [DOI] [PubMed] [Google Scholar]
- 43. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol 2015;402:113–9. 10.1016/j.mce.2014.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015-2016. NCHS Data Brief 2017;289:1–8. [PubMed] [Google Scholar]
- 45. Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by Race/Ethnicity and sex in the United States, National health and nutrition examination survey. Prev Chronic Dis 2017;16:160287:1988–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zou B, Yeo YH, Nguyen VH, et al. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999-2016. J Intern Med 2020;288:139–51. 10.1111/joim.13069 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058517supp001.pdf (89.9KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. The NHANES is a multistage, ongoing, complex cross-sectional health examination and survey designed to collect the health data of the US non-institutionalised civilian population. The data supporting reported results can be freely available from the NHANES website public archive, accessible at NHANES questionnaires, data sets and related documentation repository (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).