Key Points
Question
Does prevalence of strongyloidiasis interact with the relative risk (RR) of mortality in ivermectin trials for the treatment of COVID-19?
Findings
In this meta-analysis of 12 randomized clinical trials involving 3901 patients, favorable mortality results were limited to trials in high-prevalence regions, with no evidence that ivermectin had a mortality benefit in low-prevalence regions. Meta-regression found an association between the regional prevalence of strongyloidiasis and risk of mortality, with a decrease in RR of 39% for each 5% increase in strongyloidiasis prevalence.
Meaning
Evidence supports that strongyloidiasis prevalence interacts with the RR of mortality in ivermectin trial results; no evidence was found to suggest ivermectin has any role in preventing mortality in patients with COVID-19 in regions where strongyloidiasis is not endemic.
This meta-analysis assesses the association between regional prevalence of strongyloidiasis and the finding of reduced COVID-19 mortality in ivermectin trials.
Abstract
Importance
A widely cited meta-analysis of randomized clinical trials has claimed ivermectin as an effective treatment for prevention of mortality in COVID-19. However, an unrecognized interaction variable with the relative risk (RR) of mortality may substantially change the appropriate interpretation of this analysis.
Objective
To evaluate the association between regional prevalence of strongyloidiasis and ivermectin trial results for the outcome of mortality by testing the hypothesis that strongyloidiasis prevalence interacts with the RR of mortality.
Data Sources
Original meta-analysis as well as a manual review of all references in a dedicated ivermectin trial database (c19ivermectin) from January 1, 2019, to November 6, 2021.
Study Selection
Randomized clinical trials using ivermectin as a treatment for COVID-19 and reporting the outcome of mortality. Studies were excluded in the event of publications revealing suspected trial fraud and/or randomization failure.
Data Extraction and Synthesis
Study characteristics and RR estimates were extracted from each source. Estimates were pooled using random-effects meta-analysis. Differences by strongyloidiasis prevalence were estimated using subgroup meta-analysis and meta-regression. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline was followed.
Main Outcomes and Measures
Relative risk of mortality in ivermectin trials in regions of high vs low strongyloidiasis prevalence and correlation coefficient of meta-regression analysis between RR of mortality and regional prevalence of strongyloidiasis.
Results
A total of 12 trials comprising 3901 patients were included in the analysis. Four trials (33%) took place in regions of high strongyloidiasis prevalence and 8 (67%) trials took place in regions of low strongyloidiasis prevalence. Ivermectin trials that took place in areas of low regional strongyloidiasis prevalence were not associated with a statistically significant decreased risk of mortality (RR, 0.84 [95% CI, 0.60-1.18]; P = .31). By contrast, ivermectin trials that took place in areas of high regional strongyloidiasis prevalence were associated with a significantly decreased risk of mortality (RR, 0.25 [95% CI, 0.09-0.70]; P = .008). Testing for subgroup differences revealed a significant difference between the results of groups with low and high strongyloidiasis prevalence (χ21 = 4.79; P = .03). The estimate for τ2 (the variance of the study effect sizes) was 0 (95% CI, 0.0000-0.2786), and the estimate for I2 (percentage of variability that is explained by between-study heterogeneity) was 0 (95% CI, 0-43.7%). The meta-regression analysis revealed an RR decrease of 38.83% (95% CI, 0.87%-62.25%) for each 5% increase in strongyloidiasis prevalence.
Conclusions and Relevance
In this meta-analysis of 12 trials including 3901 patients, strongyloidiasis prevalence was found to interact with the RR of mortality for ivermectin as a treatment for COVID-19. No evidence was found to suggest ivermectin has any role in preventing mortality among patients with COVID-19 in regions where strongyloidiasis was not endemic.
Introduction
Strongyloides stercoralis is an intestinal helminth endemic in Latin America,1 Southeast Asia, and sub-Saharan Africa.2 Strongyloides hyperinfection syndrome (SHS) is a severe manifestation that occurs when autoinfection accelerates, leading to increased numbers of the parasite in the tissues involved in the autoinfection cycle.3 The global mean prevalence of strongyloidiasis is estimated to be 8.1%, and prevalence is highly variable across different countries.4 Disseminated disease occurs when the parasite spreads to organs other than those involved in its life cycle.3,5 The wide range of presentations combined with lack of familiarity result in SHS and disseminated disease often being misdiagnosed,6 and therefore the prevalence of strongyloidiasis is currently unknown.
Although SHS can occur in immunocompetent hosts,7,8,9 it is associated with immunosuppression, particularly from corticosteroid use. Iatrogenic corticosteroid use is commonly noted in disseminated strongyloidiasis, with a disease onset as early as 5 days and a mortality rate as high as 90%.10 Strongyloides hyperinfection syndrome has been observed after initiation of corticosteroid therapy for COVID-19.11,12 Of note, corticosteroids do not need to be given for disseminated strongyloidiasis to occur. For example, eosinopenia is associated with COVID-19 infections, even in patients not receiving corticosteroids,13 and eosinopenia is associated with risk of poor prognosis from SHS.14 Various recommendations have suggested that clinicians empirically treat patients with COVID-19 from strongyloidiasis-endemic regions with ivermectin before initiating corticosteroid therapy to prevent hyperinfection.15
Strongyloides hyperinfection syndrome is a potentially concerning interaction in ivermectin trials for the treatment of COVID-19 because these trials overwhelmingly take place in strongyloidiasis-endemic regions, and corticosteroids are often given as part of the standard care to which patients in control groups are assigned. Under ideal circumstances, all these patients would be empirically treated with ivermectin before receiving corticosteroids; however, because these patients are control patients in an ivermectin trial, this concomitant medication is prohibited. This effectively creates a study design that systematically places the control group at an increased risk of mortality compared with the treatment group, artificially causing the mortality results of the ivermectin treatment group to look favorable for the treatment of COVID-19. First, any parasites present in the treatment group are effectively treated while the untreated patients remain in the control group. Second, administration of corticosteroids as standard of care without ivermectin further amplifies the risk of hyperinfection in the control group. Third, COVID-19 itself is associated with eosinopenia even in the absence of corticosteroid use.13 Eosinophils play an important role in modulating parasitic infections, and eosinopenia has been associated with an increased risk of poor prognosis from SHS.14 Therefore, even if trials did not give corticosteroids to patients in the control group, conducting trial designs of this nature in endemic regions may still interact with outcomes. Despite taking place in strongyloidiasis-endemic regions, ivermectin trials overwhelmingly have no mention of helminthic diagnostic tests or any mention of alternative antihelminthic treatments in the control group to account for this interaction. Therefore, results of ivermectin trials conducted in strongyloidiasis-endemic regions cannot be extrapolated to patients who are not at increased risk for Strongyloides species infection.
Methods
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. Based on a previously published meta-analysis,16 an updated subgroup analysis by regional strongyloidiasis prevalence (above and below global mean prevalence) for the primary outcome of mortality was performed. For the variable of strongyloidiasis prevalence, country-level prevalence by parasitological methods and more granular within-country regional prevalence estimates where possible were used. Data sources included the original meta-analysis as well as a manual review performed from September to November 2021, exhausting all references in a dedicated ivermectin trial database (c19ivermectin) from January 1, 2019, to November 6, 2021. Details of the search strategy and vetting process for database logging that c19ivermectin uses is detailed in eMethods in the Supplement. This manual database review was performed by 2 investigators independently (A.B. and C.P.M.). Trial characteristics and outcomes were also extracted in duplicate by 2 investigators (A.B and C.P.M.). One investigator (A.B.) assessed risk of bias of each trial. The previous meta-analysis was updated by including the results of the 3 trials17,18,19 released since its publication that reported mortality end points. Trials that have since come under scrutiny for trial fraud and/or randomization failure were excluded20 (Figure 1).
Figure 1. Study Flow Diagram.
RCT indicates randomized clinical trial.
aAt least 1 death in either the treatment group or the control group.
We performed Mantel-Haenszel random-effects subgroup analysis meta-analytic summation with 0.5 imputation as continuity correction for the outcome of relative risk (RR) of mortality and a mixed-effects meta-regression. Both models were performed in R, version 4.1.2,21 using the meta (version 5.1.1)22 and metafor (version 3.0.2)23 packages with tidyverse (version 1.3.1)24 for data preparation. For both the models, the data for each study’s control and intervention groups were used, including each group’s mortality events and total participants. The data were then visually inspected using box plots and scatterplots from the R package ggplot2 (version 3.3.5)25 to ensure that data collection was properly and accurately performed.
The random-effects subgroup analysis was performed using the studies with regional strongyloidiasis prevalence greater than or equal to the global mean (≥8.1%) in one subgroup, and below the global mean in the other subgroup (<8.1%). The model was configured with a restricted maximum likelihood estimator to estimate the τ2 parameter, a Q-profile method to estimate the 95% CIs for τ2 and τ, and a continuity correction of 0.5 in studies with zero cell frequencies. The model’s estimations for τ2 and I2 and a test for heterogeneity were used to assess the heterogeneity of the studies included. The τ2 and τ values represent the variance and standard deviation of the distribution from which the study effect sizes are drawn, respectively. The Q-profile method is a method for the estimation of the 95% CI for τ, whereas I2 represents the percentage of variability of the estimates that is accounted for by between-study heterogeneity.26 Finally, a test for subgroup differences was used to assess whether the subgroups’ pooled estimates for the risk ratio were statistically significantly different.
Mixed-effects meta-regression was performed, regressing the natural log RR for all-cause mortality on the regional Strongyloides prevalence (reported as a percentage). The model was specified with a restricted maximum likelihood estimator to estimate the τ2 parameter, a Q-profile method to estimate the 95% CIs for τ2 and τ, and a continuity correction of 0.5 in studies with zero cell frequencies. The model’s estimations for τ2 and I2 were used to assess the heterogeneity of the studies included. Similar to the random-effects subgroup analysis, τ2 and τ represent the variance and standard deviation of the distribution from which the study effect sizes are drawn, respectively. The Q-profile method is a method for the estimation of the 95% CI for τ, whereas I2 represents the percentage of variability of the estimates that is accounted for by between-study heterogeneity.26
Three sensitivity analyses were performed in addition to our main analysis. Two of the sensitivity analyses, which helped establish whether the model estimates and inferences were robust to the uncertainty in τ, included the use of the Knapp-Hartung estimator for the 95% CIs of the model coefficients (used when the number of studies is small to help account for the uncertainty in the estimate of τ). A permutation test on the meta-regression with 10 000 iterations was also performed to assess the robustness of model estimates and inferences in resampled data. The last test was used to assess whether our estimates and inferences were robust to inclusion only of trials using random number generators for their randomization protocols. The details of these sensitivity analyses can be found in eMethods in the Supplement.
Risk of publication bias was assessed using funnel plot analysis with the Harbord test, given the reported RR measures and the binary nature of the data.27 A risk-of-bias summary was performed using the Cochrane randomized clinical trial risk-of-bias tool.28 In addition, a test for residual heterogeneity was used to assess how much remaining heterogeneity there was after accounting for the predictor variable.
Results
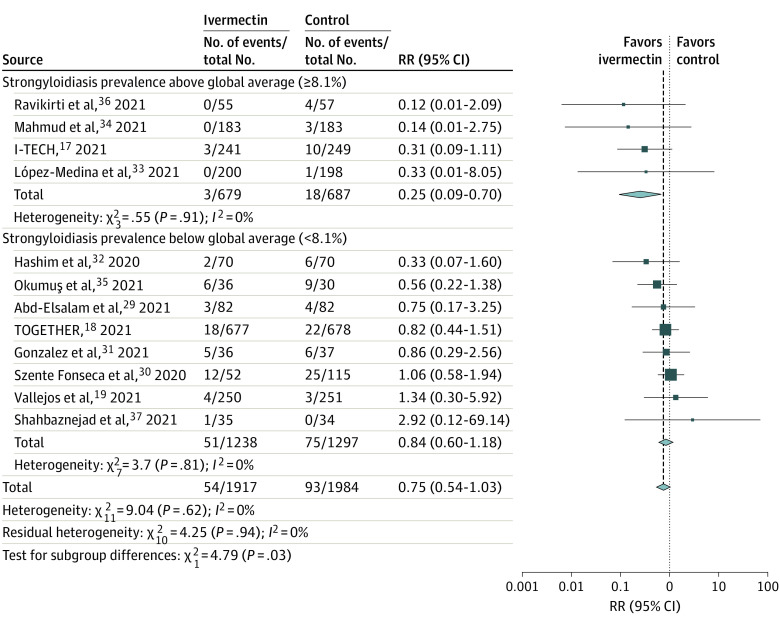
Twelve trials17,18,19,29,30,31,32,33,34,35,36,37 comprising 3901 patients were included in this analysis (Table 1). Ivermectin trials performed in areas of low regional strongyloidiasis prevalence18,19,29,30,31,32,35,37 were not associated with a statistically significant decreased risk of mortality (RR, 0.84 [95% CI, 0.60-1.18]; P = .31). By contrast, ivermectin trials that took place in areas of high regional strongyloidiasis prevalence17,33,34,36 were associated with a significant decreased risk of mortality (RR, 0.25 [95% CI, 0.09-0.70]; P = .008). Testing for subgroup differences revealed a significant difference between the results of groups with low and high strongyloidiasis prevalence (χ21 = 4.79; P = .03) (Figure 2). The estimate for τ2 (the variance of the study effect sizes) was 0 (95% CI, 0.000-0.9432), and the estimate for I2 (percentage of variability that is explained by between-study heterogeneity) was 0 (95% CI, 0-58.3%).
Table 1. Characteristics of Included Studies.
| Source (country or region) | Design | Sample size | Ivermectin dose | Comparator | Origin of data | Strongyloidiasis prevalence, % | Corticosteroid use |
|---|---|---|---|---|---|---|---|
| Abd-Elsalam et al,29 2021 (Egypt) | RCT | 164 | 12 mg/d for 3 d | SOC | Published in PR journal | 4.94 | As indicated per Egyptian Ministry of Health SOC |
| Szente Fonseca et al,30 2020 (North Brazil) | Double-blind | 167 | 14 mg/d for 3 d (plus placebo for 2 d) | Hydroxychloroquine, 400 mg BID, on day 0 then daily for 4 d; chloroquine, 450 mg BID on day 0 then daily for 4 d | Published in PR journal | 5.31 | 97% in experimental group, 98%-100% in control group |
| Gonzalez et al,31 2021 (Mexico) | Double-blind | 73 | 12 mg once | Placebo | medRxiv preprint | 7.04 | 58.3% in experimental group, 51.3% in control group |
| Hashim et al,32 2020 (Iran) | Quasi-RCT | 140 | 0.2 mg/kg for 2 d with or without third dose 1 wk later | SOC | Published in PR journal | 5.34 | Dexamethasone, 6 mg/d, or methylprednisolone, 40 mg BID, if indicated |
| I-TECH,17 2021 (Malaysia) | RCT | 490 | 0.4 mg/kg daily for 5 d | SOC | Preliminary report by Ministry of Health of Malaysia | 15.94 | 26.9% in experimental group, 26.5% in control group |
| López-Medina et al,33 2021 (Colombia) | Double-blind | 398 | 0.3 mg/kg for 5 d | Placebo | Published in PR journal | 18.44 | 3% in experimental group, 6.1% in control group |
| Mahmud et al,34 2021 (Bangladesh) | Double-blind | 366 | 12 mg in single dose | Placebo plus SOC | Published in PR journal | 17.34 | As indicated per local SOC guidelines |
| Okumuş et al,35 2021 (Turkey) | RCT | 66 | 0.2 mg/kg for 5 d | SOC | Published in PR journal | 5.64 | Unknown |
| Ravikirti et al,36 2021 (India) | Double-blind | 112 | 12 mg for 2 d plus SOC | Placebo plus SOC | Published in PR journal | 10.44 | All patients received at least 1 dose |
| Shahbaznejad et al,37 2021 (Iran) | Double-blind | 69 | 0.2 mg/kg for 1 dose | SOC | Published in PR journal | 4.84 | Unknown |
| TOGETHER,18 2021 (Southeast Brazil) | RCT | 1355 | 400 μg/kg to 90 kg of weight daily for 3 d | Placebo | Presentation published online | 3.91 | Unknown |
| Vallejos et al,19 2021 (Argentina) | RCT | 501 | Patients weighing ≤80 kg: 12 mg/d for 2 d; patients weighing 80-110 kg: 18 mg/d for 2 d; patients weighing >110 kg: 24 mg/d for 2 d | SOC | Published in PR journal | 5.14 | 4.8% in experimental group, 4.4% in control group |
Abbreviations: BID, twice daily; I-TECH, Ivermectin Treatment Efficacy in COVID-19 High-Risk Patients; PR, peer-reviewed; RCT, randomized clinical trial; SOC, standard of care; TOGETHER, Early Treatment of COVID-19 With Repurposed Therapies: The TOGETHER Adaptive Platform Trial.
Figure 2. Meta-analysis of Ivermectin Trials.
I-TECH indicates Ivermectin Treatment Efficacy in COVID-19 High Risk Patients; RR, relative risk; TOGETHER, Early Treatment of COVID-19 With Repurposed Therapies: The TOGETHER Adaptive Platform Trial.
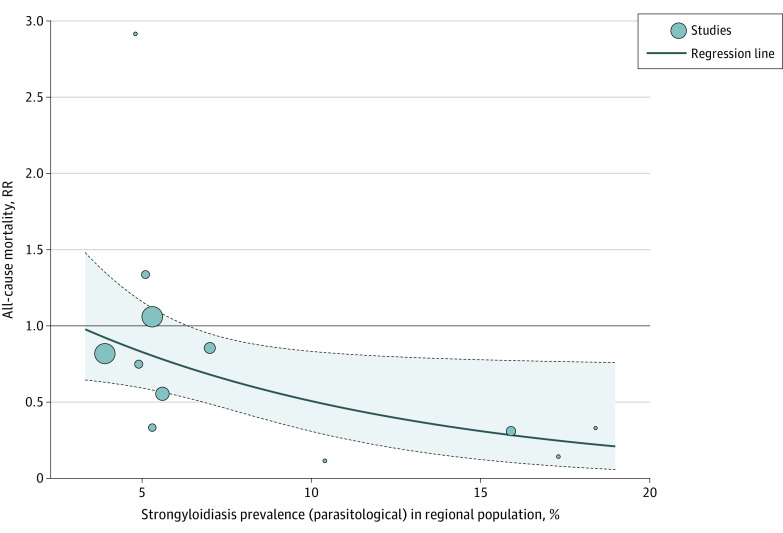
The meta-regression analysis revealed a linear coefficient of −0.0983 (P = .046) for the strongyloidiasis prevalence and the natural log RR for all-cause mortality. From this, the decrease in RR for each 5% increase in strongyloidiasis prevalence was calculated to be 38.83% (95% CI, 0.87%-62.25%) (Figure 3). The estimate for τ2 (the variance of the study effect sizes) was 0 (95% CI, 0.0000-0.2786), and the estimate for I2 (percentage of variability that is explained by between-study heterogeneity) was 0 (95% CI, 0-43.7%). Testing for residual heterogeneity returned a test statistic of QE10 = 5.06 (P = .89). Testing for assumptions of linearity and residual distribution checks are described in eFigures 1 and 2 in the Supplement.
Figure 3. Meta-regression Analysis of Ivermectin Trials.
The shaded region within the dashed lines represents the 95% CIs. RR indicates relative risk.
In addition, no qualitative differences were found in our estimates and inferences within the 3 sensitivity analyses, 2 of which (the Knapp-Hartung estimator and permutation analysis) were used to help account for uncertainty in our estimate of τ and 1 of which was used to assess the exclusion of trials not using random number generators. The results of the first 2 analyses suggested that our main model’s estimates and inferences were not qualitatively different with respect to uncertainty in the estimate of τ. The results of the third sensitivity analysis suggest that our model’s estimates and inferences did not change qualitatively, despite inclusion of trials using random number generators (eFigures 3 and 4 in the Supplement).
A risk-of-bias summary is provided in Table 2. Assessment of risk of publication bias using funnel plot analysis with the Harbord test did not show funnel plot asymmetry (P = .16) (eFigure 5 in the Supplement).
Table 2. Risk-of-Bias Summary.
| Source | Risk of bias by itema | ||||||
|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | |
| Abd-Elsalam et al,29 2021 | Low | High | High | High | Low | Low | Unclear |
| Szente Fonseca et al,30 2020 | Low | Low | Low | Low | Low | Low | Low |
| Gonzalez et al,31 2021 | High | High | High | Unclear | Unclear | Unclear | High |
| Hashim et al,32 2020 | High | High | High | High | Low | Low | Unclear |
| I-TECH et al,17 2021 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| López-Medina et al,33 2021 | Low | Low | Low | Low | Low | Low | Low |
| Mahmud et al,34 2021 | Low | Low | Low | Low | Low | Low | Low |
| Okumuş et al,35 2021 | High | Unclear | High | Unclear | Low | Low | Low |
| Ravikirti et al,36 2021 | Low | Low | Low | Unclear | Unclear | Low | Low |
| Shahbaznejad et al,37 2021 | Low | Low | Low | Unclear | Unclear | Unclear | Unclear |
| TOGETHER,18 2021 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Vallejos et al,19 2021 | Low | Low | Low | Low | Low | Low | Low |
Abbreviations: I-TECH, Ivermectin Treatment Efficacy in COVID-19 High-Risk Patients; TOGETHER, Early Treatment of COVID-19 With Repurposed Therapies: The TOGETHER Adaptive Platform Trial.
Determined by reviewer judgment for each trial.
Discussion
Consistent with the findings of the subgroup analyses and meta-regression, an association between the observed mortality benefits of ivermectin dependent on the regional prevalence of strongyloidiasis was found. This argues in favor of the hypothesis that strongyloidiasis prevalence interacts with the RR of mortality in ivermectin trials for the outcome of mortality, rather than having a treatment effect on COVID-19 per se.
In future research assessing potential mechanistic explanations for viral clearance, this interaction should also be kept in mind, given that helper T cell 2 (TH2) immune responses driven by helminth parasites may improve clinical outcomes at the cost of slower viral clearance, whereas treating such parasites alleviates the TH2 response, allowing for a more robust TH1 response to accelerate viral clearance at the cost of TH1 cytokine storm–related responses that may worsen clinical outcomes.38 Of course, the administration of corticosteroids in the presence of Strongyloides infection would be expected to supersede in terms of clinical risk. Thus, even if future trials indicate an increased viral clearance, it may be the case that ivermectin in and of itself has no inherent effect on viral clearance, resulting in another end point that will not extrapolate to nonendemic regions. Indeed, even without the use of corticosteroids, Strongyloides infection may still interact with the RR of mortality, because the treatment group is still receiving standard care for a given condition whereas the control group is not. This may be less likely to affect mortality without corticosteroids, but secondary outcomes may be impacted.
In line with prior recommendations, it is prudent that patients at risk for strongyloidiasis be empirically treated with ivermectin before the initiation of corticosteroid therapy.15,39 In the context of a trial wherein ivermectin is the treatment, there are several options to consider. One option is a trial design wherein an alternative antihelminthic other than ivermectin is used. However, this may not be ideal, because evidence suggests that alternatives such as albendazole are not as effective at treatment compared with ivermectin, and the strength of the evidence is of weaker certainty for thiabendazole efficacy.40 The ideal scenario to handle this interaction is to simply perform trials in nonendemic regions. Finally, institutional review boards should consider the ethical implications of trials designed with a control group resulting in substandard therapy.
Limitations
There are several limitations to our analysis. First, the state of ivermectin trial publications at large is tenuous, with several trials coming under heavy scrutiny for egregious violations, including fraud. For this reason, a conservative approach in including studies was taken, excluding studies under scrutiny of trial fraud, and a sensitivity analysis only including trials with appropriate randomization protocols was performed. Second, details on the proportion of patients given corticosteroids (which may serve as a confounder or an interaction with the RR of mortality in its own right) in each trial were not clear for all trials, precluding the inclusion of this variable in the regression model. Third, low event counts in the trials may make the results less reliable. Fourth, varying trial recruitment across urban and rural populations (where strongyloidiasis prevalences often differ) may diminish the reliability of strongyloidiasis trial prevalence estimates. Despite these limitations, the findings warrant concern for ivermectin trials for the treatment of COVID-19 that are not designed to address this interaction.
Conclusions
In this meta-analysis of 12 trials comprising 3901 patients, strongyloidiasis prevalence was found to interact with the RR of mortality when ivermectin was used as a treatment for COVID-19. No evidence was found to suggest that ivermectin has any role in preventing mortality in patients with COVID-19 in regions where strongyloidiasis is not endemic. Results of ivermectin trials in strongyloidiasis-endemic regions may not extrapolate to strongyloidiasis-nonendemic regions. Future trials in nonendemic regions may provide insight into the true effect of ivermectin in this context. In the interim, we strongly caution against extrapolation for patients not at increased risk for strongyloidiasis.
eMethods. Subgroup and Sensitivity Analyses and Database Search Details
eReferences
eFigure 1. Meta Regression Assumptions: Linearity Check
eFigure 2. Meta Regression Assumptions: Residual Distribution Check
eFigure 3. Sensitivity Analysis Excluding Trials With High Risk of Bias Due to Randomization
eFigure 4. Sensitivity Analysis Excluding Trials With High Risk of Bias Due to Randomization Protocols (Sensitivity Analysis Meta-regression)
eFigure 5. Funnel Plot Assessing Publication Bias
References
- 1.Paula FM, Costa-Cruz JM. Epidemiological aspects of strongyloidiasis in Brazil. Parasitology. 2011;138(11):1331-1340. doi: 10.1017/S003118201100120X [DOI] [PubMed] [Google Scholar]
- 2.Puthiyakunnon S, Boddu S, Li Y, et al. Strongyloidiasis—an insight into its global prevalence and management. PLoS Negl Trop Dis. 2014;8(8):e3018. doi: 10.1371/journal.pntd.0003018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasquez-Rios G, Pineda-Reyes R, Pineda-Reyes J, Marin R, Ruiz EF, Terashima A. Strongyloides stercoralis hyperinfection syndrome: a deeper understanding of a neglected disease. J Parasit Dis. 2019;43(2):167-175. doi: 10.1007/s12639-019-01090-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buonfrate D, Bisanzio D, Giorli G, et al. The global prevalence of Strongyloides stercoralis infection. Pathogens. 2020;9(6):E468. doi: 10.3390/pathogens9060468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassalik M, Mönkemüller K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol (N Y). 2011;7(11):766-768. [PMC free article] [PubMed] [Google Scholar]
- 6.Marcos LA, Terashima A, Dupont HL, Gotuzzo E. Strongyloides hyperinfection syndrome: an emerging global infectious disease. Trans R Soc Trop Med Hyg. 2008;102(4):314-318. doi: 10.1016/j.trstmh.2008.01.020 [DOI] [PubMed] [Google Scholar]
- 7.Chan FLY, Kennedy B, Nelson R. Fatal Strongyloides hyperinfection syndrome in an immunocompetent adult with review of the literature. Intern Med J. 2018;48(7):872-875. doi: 10.1111/imj.13940 [DOI] [PubMed] [Google Scholar]
- 8.Gopalakrishna H, Nair GB, Conti R. Parasitic necrotizing pneumonia in an immunocompetent patient in United States. J Community Hosp Intern Med Perspect. 2021;11(1):69-71. doi: 10.1080/20009666.2020.1824333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myint A, Chapman C, Almira-Suarez I, Mehta N. Strongyloides hyperinfection syndrome in an immunocompetent host resulting in bandemia and death. BMJ Case Rep. 2017;2017:bcr2016217911. doi: 10.1136/bcr-2016-217911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geri G, Rabbat A, Mayaux J, et al. Strongyloides stercoralis hyperinfection syndrome: a case series and a review of the literature. Infection. 2015;43(6):691-698. doi: 10.1007/s15010-015-0799-1 [DOI] [PubMed] [Google Scholar]
- 11.Lier AJ, Tuan JJ, Davis MW, et al. Case report: disseminated strongyloidiasis in a patient with COVID-19. Am J Trop Med Hyg. 2020;103(4):1590-1592. doi: 10.4269/ajtmh.20-0699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchese V, Crosato V, Gulletta M, et al. Strongyloides infection manifested during immunosuppressive therapy for SARS-CoV-2 pneumonia. Infection. 2021;49(3):539-542. doi: 10.1007/s15010-020-01522-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soni M. Evaluation of eosinopenia as a diagnostic and prognostic indicator in COVID-19 infection. Int J Lab Hematol. 2021;43(S1)(suppl 1):137-141. doi: 10.1111/ijlh.13425 [DOI] [PubMed] [Google Scholar]
- 14.Ganesh S, Cruz RJ Jr. Strongyloidiasis: a multifaceted disease. Gastroenterol Hepatol (N Y). 2011;7(3):194-196. [PMC free article] [PubMed] [Google Scholar]
- 15.Stauffer WM, Alpern JD, Walker PF. COVID-19 and dexamethasone: a potential strategy to avoid steroid-related Strongyloides hyperinfection. JAMA. 2020;324(7):623-624. doi: 10.1001/jama.2020.13170 [DOI] [PubMed] [Google Scholar]
- 16.Bryant A, Lawrie TA, Dowswell T, et al. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther. 2021;28(4):e434-e460. doi: 10.1097/MJT.0000000000001402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Director General of Health Malaysia. Kenyataan Akhbar KPK 3 November 2021—Hasil Dapatan Kajian Keberkesanan Rawatan Ivermectin Untuk Pesakit COVID-19 Berisiko Tinggi (I-TECH Study) [in Malaysian]. November 3, 2021. Accessed November 6, 2021. https://kpkesihatan.com/2021/11/03/kenyataan-akhbar-kpk-3-november-2021-hasil-dapatan-kajian-keberkesanan-rawatan-ivermectin-untuk-pesakit-covid-19-berisiko-tinggi-i-tech-study/
- 18.Mills E. Early treatment of COVID-19 with repurposed therapies: the TOGETHER adaptive platform trial. August 21, 2021. Accessed November 3, 2021. https://rethinkingclinicaltrials.org/news/august-6-2021-early-treatment-of-covid-19-with-repurposed-therapies-the-together-adaptive-platform-trial-edward-mills-phd-frcp/
- 19.Vallejos J, Zoni R, Bangher M, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis. 2021;21(1):635. doi: 10.1186/s12879-021-06348-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence JM, Meyerowitz-Katz G, Heathers JAJ, Brown NJL, Sheldrick KA. The lesson of ivermectin: meta-analyses based on summary data alone are inherently unreliable. Nat Med. 2021;27(11):1853-1854. doi: 10.1038/s41591-021-01535-y [DOI] [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistical Computing [statistical software]. R Foundation for Statistical Computing; 2021. [Google Scholar]
- 22.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153-160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 24.Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Source Software. 2019;4(43):1686. doi: 10.21105/joss.01686 [DOI] [Google Scholar]
- 25.Wickham H. ggplot2: elegant graphics for data analysis. In: Gentleman R, Hornik K, Parmigiani G, eds. Use R! Series. Springer-Verlag; 2016. [Google Scholar]
- 26.Harrer M, Cuijpers, P, Furukawa TA, Ebert DD. Doing Meta-Analysis With R: A Hands-On Guide. CRC Press; 2021. [Google Scholar]
- 27.StataCorp LLC. STATA Statistical Software: Release 17 [manual]. StataCorp LLC; 2021. [Google Scholar]
- 28.Higgins JP, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. [Google Scholar]
- 29.Abd-Elsalam S, Noor RA, Badawi R, et al. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: a randomized controlled study. J Med Virol. 2021;93(10):5833-5838. doi: 10.1002/jmv.27122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szente Fonseca SN, de Queiroz Sousa A, Wolkoff AG, et al. Risk of hospitalization for COVID-19 outpatients treated with various drug regimens in Brazil: comparative analysis. Travel Med Infect Dis. 2020;38:101906. doi: 10.1016/j.tmaid.2020.101906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez JLB, González Gámez M, Enciso EAM, et al. Efficacy and safety of ivermectin and hydroxychloroquine in patients with severe COVID-19: a randomized controlled trial. medRxiv. Preprint posted online February 23, 2021. doi: 10.1101/2021.02.18.21252037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashim A, Maulood MF, Rasheed AM, Fatak DF, Kabah KK, Abdulanir AS. Controlled randomized clinical trial on using ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv. Preprint posted online October 27, 2020. doi: 10.1101/2020.10.26.20219345 [DOI] [Google Scholar]
- 33.López-Medina E, López P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325(14):1426-1435. doi: 10.1001/jama.2021.3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahmud R, Rahman MM, Alam I, et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res. 2021;49(5):3000605211013550. doi: 10.1177/03000605211013550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okumuş N, Demirtürk N, Çetinkaya RA, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21(1):411. doi: 10.1186/s12879-021-06104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravikirti RR, Roy R, Pattadar C, et al. Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: a double-blind randomized placebo controlled trial in Eastern India. J Pharm Pharm Sci. 2021;24:343-350. doi: 10.18433/jpps32105 [DOI] [PubMed] [Google Scholar]
- 37.Shahbaznejad L, Davoudi A, Eslami G, et al. Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial. Clin Ther. 2021;43(6):1007-1019. doi: 10.1016/j.clinthera.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolday D, Gebrecherkos T, Arefaine ZG, et al. Effect of co-infection with intestinal parasites on COVID-19 severity: a prospective observational cohort study. EClinicalMedicine. 2021;39:101054. doi: 10.1016/j.eclinm.2021.101054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ontario COVID, Science Advisory Table, the Drugs, and Biologics Clinical Practice Guidelines Working Group . Ivermectin treatment for Strongyloides infection in patients with COVID-19. Can Commun Dis Rep. 2021;47(7-8):316-321. doi: 10.14745/ccdr.v47i78a04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henriquez-Camacho C, Gotuzzo E, Echevarria J, et al. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. 2016;(1):CD007745. doi: 10.1002/14651858.CD007745.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Subgroup and Sensitivity Analyses and Database Search Details
eReferences
eFigure 1. Meta Regression Assumptions: Linearity Check
eFigure 2. Meta Regression Assumptions: Residual Distribution Check
eFigure 3. Sensitivity Analysis Excluding Trials With High Risk of Bias Due to Randomization
eFigure 4. Sensitivity Analysis Excluding Trials With High Risk of Bias Due to Randomization Protocols (Sensitivity Analysis Meta-regression)
eFigure 5. Funnel Plot Assessing Publication Bias