Abstract
Introduction
My Dose Coach (MDC) is a US Food and Drug Administration-approved digital smartphone application designed to help users with type 2 diabetes (T2D) titrate their basal insulin (BI) according to a clinician-prescribed individualized titration plan. The aim of this analysis was to assess the impact of the frequency of MDC use on clinical outcomes.
Methods
This retrospective observational analysis included people with T2D who were registered for MDC (August 1st, 2018–April 30th, 2020) and received BI. Users with an activated care plan and ≥2 fasting blood glucose (FBG) observations spanning ≥2 weeks were defined as active. Outcomes included percentage achieving their individual FBG target, time to FBG target, change in FBG, change in insulin dose and hypoglycemia. Users were stratified into high (>3 days per week), moderate (>1– ≤3 days per week), and low (≤1 day per week) MDC usage groups.
Results
The analysis included 2517 active MDC users. Approximately 49% of users had high MDC usage. Overall, 44% of users across all usage frequencies achieved their individual FBG target. High MDC use was associated with significantly better FBG target achievement and less time to FBG target versus moderate- and low-usage groups (p≤0.01 for all). Insulin dose change was significantly greater in the high- versus moderate-usage group (p=0.01). There was no significant difference in hypoglycemia incidence among MDC usage groups (12%–16% of users in any usage group).
Conclusions
More frequent MDC usage was associated with better FBG outcomes without increased hypoglycemia risk.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-022-01245-9.
Keywords: Glycemic control, Mobile application, Self-management, Type 2 diabetes mellitus
Key Summary Points
| Why carry out this study? |
| People living with type 2 diabetes (T2D) may require basal insulin (BI) therapy. Optimization of insulin management is an important aspect of glycemic control |
| Technological innovations such as smartphone applications are increasingly being used to support the optimization of BI titration; the My Dose Coach (MDC) smartphone app is designed to help users with T2D titrate their BI according to an individualized plan defined by their healthcare provider (HCP) |
| This study assessed the impact of frequency of MDC app use on achievement of relevant clinical outcomes (e.g., achievement of fasting blood glucose target [FBG]) |
| What was learned from this study? |
| Almost half of MDC active users achieved their individualized FBG target, with greater frequency of MDC usage shown to be associated with positive FBG outcomes but not with any increase in hypoglycemia incidence |
| These results indicate that digital tools to support basal insulin titration could be useful to both people with diabetes and HCPs in diabetes management in terms of achieving better glycemic control with no increased risk of hypoglycemia |
Introduction
Glycemic control is essential for preventing microvascular complications in people with diabetes [1, 2], but despite the variety of efficacious therapies available many people with diabetes still experience difficulty in reaching their glycemic targets [3]. Key challenges to achieving glycemic control include fear of hypoglycemia (which may impact on patient adherence to insulin dosing regimen), clinical/therapeutic inertia, lack of patient education and limited availability/affordability of diabetes treatment [3–9]. In those people with type 2 diabetes (T2D) who require basal insulin (BI) therapy, such challenges may lead to delayed initiation of BI, inadequate titration and failure to adhere to prescribed BI treatments, which could negatively impact the achievement of glycemic targets [3, 10].
Optimization of BI titration is an important aspect of glycemic management and may include the use of individualized glucose targets and/or dose plans that are simple to follow (for both the person with diabetes and the healthcare provider) [11, 12]. However, the combination of more complex, individualized management approaches and the increasing numbers of people with T2D globally has placed an increasing burden on healthcare professionals (HCP) in terms of time pressure, as they are increasingly required to convey/consider a variety of issues relevant to treatment during a relatively short appointment time [13]. There is consequently a need for technological innovations that can support patients in initiating and/or titrating their BI and reduce the management burden for HCPs.
Contemporary diabetes technologies include flash/continuous glucose monitoring (CGM), closed-loop systems (involving integration of a CGM device and insulin pump), smart insulin pens and motivational smartphone applications [14]. The use of such technologies may be even more relevant in situations such as the COVID-19 pandemic, during which access to healthcare providers has been more challenging. In this unprecedented period, the need for and utility of virtual/teleconsultations and diabetes technology for disease management has become increasingly apparent [15, 16].
My Dose Coach (MDC) is a US Food and Drug Administration-approved digital smartphone application compatible with iOS 10.0 and above or Android version 5.0 and above designed to help users with T2D titrate their BI, based on individualized titration plans provided by their physician [17–20]. MDC combines a web portal where HCPs define a long-acting BI titration plan and a smartphone application that provides dose and titration recommendations to patients based on their fasting blood glucose (FBG) and hypoglycemia data [21]. Prior real-world data has indicated that use of the MDC application could help people with T2D to titrate their BI and achieve their fasting blood glucose (FBG) target [22]. The objective of this study was to assess the impact of frequency of MDC use during titration on achievement of relevant clinical outcomes, such as the percentage of people reaching their individual FBG target, time to achievement of FBG target, mean change in FBG/insulin dose and incidence of hypoglycemia.
Methods
Study design and population
This retrospective observational cohort analysis included people with T2D who registered for MDC between August 1st, 2018, and April 30th, 2020, and received BI; MDC users agreed to allow their anonymized data to be used retrospectively as part of the registration process. HCPs generated an individualized care plan for each user, which was uploaded to the MDC app. Users then recorded FBG in the app as well as receiving individualized BI dose recommendations from the app based on their care plan. Users with an activated care plan and ≥2 FBG observations spanning at least 2 weeks were defined as active users and subsequently included in analyses. The titration period was defined as beginning at initial care plan registration and ending when the user either reached their FBG target or finished their care plan. Multiple care plans could be included for a single individual and were viewed as a continuation of the same titration period, provided that the activation date of the following care plan was within 14 days after the end of the prior care plan. If a patient had multiple titration periods, only the first titration period was considered in the analysis.
Outcomes
Study outcomes included the percentage of users achieving FBG target and time to achieving FBG target. Change in FBG and insulin dose in all users were also recorded, as was occurrence of hypoglycemia (defined as FBG below the HCP-defined hypoglycemia cut-off as per the titration plan) in those users who achieved FBG target. Users who logged ≥3 consecutive FBG measurements within their prescribed FBG target range (pre-specified by the HCP during care plan creation) were defined as reaching titration target. Outcomes were considered by MDC usage group (i.e., high [>3 days per week], moderate [>1– ≤3 days per week], and low [≤1 day per week]).
Data analysis
Results were described using descriptive statistics, with number and percentage for categorical variables and means with standard deviation (SD)/medians with interquartile ranges for continuous variables. MDC usage was stratified by titration duration (≤4 weeks/>4 weeks). Duration of titration was defined as the period of time between activation of first care plan and end of titration (i.e., the earliest date at which the patient reached their FBG target or last reported care plan activation date/deactivation date of previous care plan). The percentage of users achieving FBG target was analyzed using the Chi-square test for trend (i.e., a linear trend in the percentage of users achieving FBG target across MDC usage groups), and Fisher’s exact test was used to analyze the association between MDC use and achieving FBG target. For those users who achieved FBG target, a two-sample t-test was used to analyze time to target according to MDC usage level. Mean change in FBG between different app usage groups was analyzed using a linear regression model and controlled for initial FBG value, while mean change in insulin dose between app usage groups was analyzed using a two-sample t-test. Hypoglycemia incidence for those achieving FBG target was examined using a Chi-square test for trend (i.e., a linear trend in hypoglycemia incidence across MDC usage groups) and a Fisher’s exact test for association between MDC use and hypoglycemia.
Results
Study participants
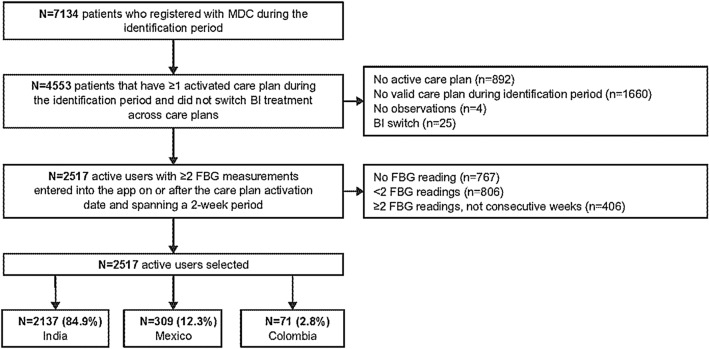
A total of 7134 people with T2D registered with MDC between August 1st, 2018, and April 30th, 2020 (Fig. 1). Of these, 4553 had ≥1 activated care plan during the analysis period and did not switch BI. A total of 2517 users had ≥2 FBG measurements that had been entered into the MDC app on or after the care plan activation date and spanned a 2-week period. Of the 2517 active users, 2137 (84.9%) were from India, 309 (12.3%) in Mexico and 71 (2.8%) in Colombia.
Fig. 1.
Study disposition. FBG fasting blood glucose, MDC My Dose Coach
Baseline Characteristics
Overall, the mean age of active users was 52.8 years; the majority (78.4%) were aged between 26 and 64 years (Table 1). Approximately half of all users (48.6%) were stratified into the high-usage group (>3 days per week), while 24.9% and 26.5% were stratified into the moderate- (>1– ≤3 days per week) and low- (≤1 day per week) usage groups, respectively. Age, weight and sex distribution were equally represented among users irrespective of usage level (Table 1). Overall, approximately 60% of users received Gla-100 while 40% received Gla-300. A larger percentage of users (46.7%) in the high-usage group received Gla-300 compared with the moderate- or low-usage groups (34.2% and 36.8% respectively). Shorter titration duration was observed for the high-usage group compared with the moderate- or low-usage groups.
Table 1.
Participant characteristics by MDC usage
| Characteristic | All users (N = 2517) | High usage (n = 1223) | Moderate usage (n = 626) | Low usage (n = 668) |
|---|---|---|---|---|
| Percentage of all users, % | 100 | 48.6 | 24.9 | 26.5 |
| Age in years | ||||
| Mean (SD) | 52.8 (13.4) | 53.1 (13.6) | 52.0 (13.7) | 53.3 (12.8) |
| Median (IQR) | 54 (18) | 54 (17) | 53 (19) | 54 (17) |
| Age groupa, n (%) | ||||
| ≤25 | 65 (2.6) | 29 (2.4) | 21 (3.4) | 15 (2.2) |
| 26–49 | 864 (34.3) | 424 (34.7) | 222 (35.5) | 218 (32.6) |
| 50–64 | 1109 (44.1) | 531 (43.4) | 267 (42.7) | 311 (46.6) |
| ≥65 | 478 (19.0) | 238 (19.5) | 116 (18.5) | 124 (18.6) |
| Male sex, n (%) | 1380 (54.8) | 679 (55.5) | 333 (53.2) | 368 (55.1) |
| Weight in kgb, n (%) | ||||
| ≤60 | 582 (23.1) | 252 (20.6) | 155 (24.8) | 175 (26.2) |
| 61–70 | 727 (28.9) | 333 (27.2) | 184 (29.4) | 210 (31.4) |
| 71–80 | 620 (24.6) | 313 (25.6) | 143 (22.8) | 164 (24.6) |
| 81–90 | 350 (13.9) | 176 (14.4) | 94 (15.0) | 80 (12.0) |
| 91–100 | 146 (5.8) | 81 (6.6) | 34 (5.4) | 31 (4.6) |
| >100 | 81 (3.2) | 59 (4.8) | 14 (2.2) | 8 (1.2) |
| Medication usec | ||||
| Insulin glargine 100 IU/ml | 1485 (59.0) | 651 (53.2) | 412 (65.8) | 422 (63.2) |
| Insulin glargine 300 IU/ml | 1031 (41.0) | 571 (46.7) | 214 (34.2) | 246 (36.8) |
| Titration duration, days | ||||
| Mean (SD) | 43.3 (49.9) | 19.7 (26.2) | 46.6 (47.7) | 83.4 (58.1) |
| Median (IQR) | 28 (46) | 11 (23) | 28 (38) | 72 (77) |
IU international units, IQR interquartile range, SD standard deviation
aAge data not available for one participant
bWeight data not available for 11 participants
cOne participant received insulin degludec 100 U/ml
FBG Target Achievement
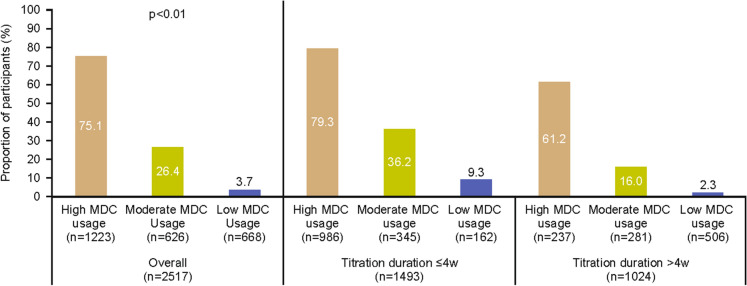
Overall, 1108 (44.0%) participants achieved their FBG target (Supplementary material; Table S1). Of the remainder, 652 (25.9%) were still titrating their BI and 757 (30.1%) had stopped using MDC at end of titration. When stratified by age group, approximately half of all users aged 26–49, 50–64 and ≥65 years (43.5%–46.2%) met their FBG target; conversely, only 21.9% of users aged ≤25 years met their FBG target by study end. Overall, MDC users achieved a mean (SD) FBG reduction of 40.4 (74.7) mg/dl. The extent of FBG reduction was greater for users who had achieved FBG target (−45.2 mg/dl) compared with those who were still titrating (−36.2 mg/dl) or had stopped using MDC altogether (−37.1 mg/dl; Table S2). More frequent MDC use was associated with significantly better FBG target achievement (75.1% in the high-usage group vs. 26.4% and 3.7% in the moderate- and low-usage groups, respectively [p<0.01]; Fig. 2). This trend was consistent regardless of titration duration (i.e., shorter duration [≤4 weeks] or longer duration [>4 weeks]).
Fig. 2.
FBG target achievement by MDC usage and stratified by titration duration. FBG fasting blood glucose, MDC My Dose Coach
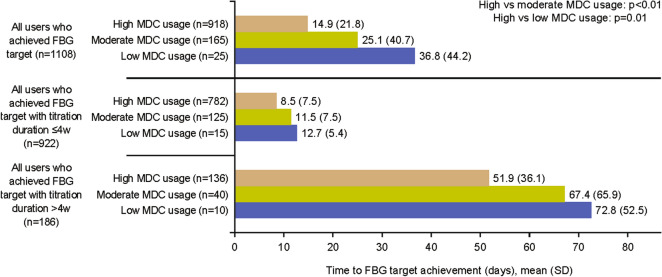
Time to FBG Target Achievement by Frequency of MDC Usage
Users in the high-usage group took the shortest time to achieve FBG target versus moderate- and low-usage groups (14.9 days vs. 25.1 days and 36.8 days respectively; p≤0.01 for both comparisons; Fig. 3). The observed association between MDC usage and time to FBG target achievement was consistent irrespective of titration duration (i.e., ≤4 weeks or >4 weeks). Mean time to FBG target achievement (all usage groups) ranged from 8.5 to 12.7 days for users with a titration duration of ≤4 weeks and from 51.9 to 72.8 days for users with a titration duration of >4 weeks.
Fig. 3.
Time to FBG achievement by MDC usage and stratified by titration duration. FBG fasting blood glucose, MDC My Dose Coach, SD standard deviation
Change in FBG by Frequency of MDC Usage
High-usage was associated with a greater reduction in FBG versus moderate and low usage (p<0.01 for both; Fig. 4). The mean treatment difference in FBG reduction was −4.7 mg/dl between the high and moderate groups and −14.4 mg/dl between the high- and low-usage groups (p=0.01 for both). As before, this association was consistent irrespective of titration duration (i.e., ≤4 weeks or >4 weeks).
Fig. 4.
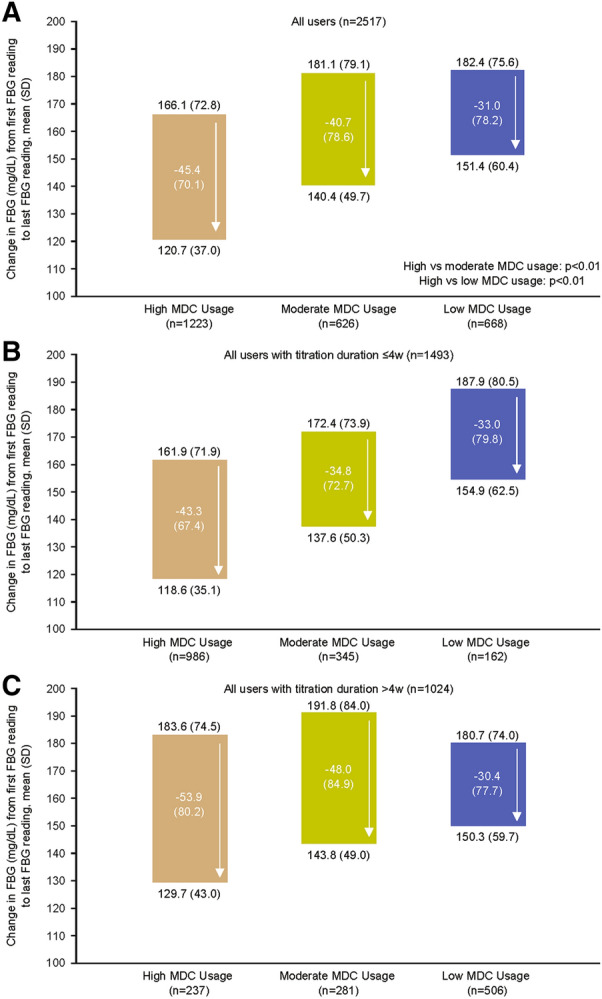
FBG change by MDC usage groups: A overall and stratified by titration duration (B ≤4 weeks; C >4 weeks). FBG fasting blood glucose, MDC My Dose Coach, SD standard deviation
Change in Insulin Dose by Frequency of MDC Usage
The majority of users reported at least one insulin dose during the study period (94.6% of the high-usage group, 86.9% of the moderate-usage group and 77.8% of the low-usage group). Among all users, the extent of insulin dose change was significantly greater in the high-usage group compared with the moderate-usage group (p=0.01) and did not achieve statistical significance versus the low-usage group (p=0.08; Figure S1). Insulin dose change was consistently statistically significantly higher in the high-usage group versus the moderate- and low-usage groups, respectively for titration duration ≤4 or >4 weeks (Figure S1).
Hypoglycemia
Of the 44.0% of users who achieved their FBG target (n=1108), 12.9% (n=143) experienced hypoglycemia events. There was no significant difference in hypoglycemia incidence among MDC usage groups (12.3%, 16.4% and 12.0% of users in high-, moderate- and low-usage groups, respectively; p>0.29 for all). There was no significant difference in hypoglycemia incidence among MDC usage groups in patients with titration duration ≤4 weeks (10%, 11% and 7% of users in high-, moderate- and low-usage groups, respectively; p=0.87) and >4 weeks (28%, 33% and 20% of users in high-, moderate- and low-usage groups, respectively p=0.73). No significant association was found between insulin dose and incidence of hypoglycemia.
Discussion
In this real-world study of 2517 people with T2D in India, Mexico and Colombia, almost half of MDC active users achieved their individualized FBG target. Frequency of MDC usage was a key predictor of positive FBG outcomes, including higher rates of target achievement, shorter time to target, greater reduction in FBG and greater change in insulin dose, without any observed increase in hypoglycemia.
The percentage of users in the high-usage group who achieved FBG target was almost three times higher than that observed in the moderate-usage group and almost 19 times higher than that observed in the low-usage group (75.1% vs. 26.4% and 3.7%, respectively), while time to FBG target achievement was 10 days shorter with high versus moderate usage and was almost 22 days shorter for high versus low usage (both p=0.01). A similar trend in time to achieve FBG target was observed when stratified by length of titration ≤4 weeks and >4 weeks, with the shortest time in the high-usage group; however, time to FBG target achievement was shorter for all usage groups in the shorter titration length cohort versus the longer titration length cohort for (8.5–12.7 days vs. 51.9–72.8 days, respectively).
As well as reducing time to FBG target, high MDC usage was also associated with greater reductions in FBG versus the other usage groups, both overall and stratified by titration duration. These improvements in FBG outcomes may reflect the observed changes in insulin dose, as a significantly greater increase in insulin dose was observed for high versus moderate usage in the overall population and for high- versus moderate- and low-usage groups when stratified by titration time. Reassuringly, none of these positive clinical outcomes (particularly change in insulin dose) were accompanied by any significant increase in the incidence of hypoglycemia, either overall or stratified by titration duration. As noted above, the impact of MDC usage frequency was consistent across outcomes, regardless of titration duration. However, it should be noted that the majority of the high-usage group had a titration duration less than 4 weeks and that users with a shorter titration duration were more likely to achieve their FBG goal. The results presented here suggest that use of the MDC application may allow users to feel more at ease with managing their own treatment and provide them with greater confidence in optimizing their BI titration with the ongoing support of their HCP. Use of this application may therefore equip users to overcome known challenges to achieving glycemic control, particularly since increased MDC use was not associated with any increased risk of hypoglycemia.
The data from this study are consistent with results from studies of other mobile applications supporting BI titration in T2D; improvements in HbA1c measurements and HbA1c target achievement were reported in a Chinese study that examined the use of a mobile app to support insulin self-management and titration in people with T2D [23]. The mobile app provided information on various aspects of diabetes self-management, including dose titration, blood glucose monitoring, hypoglycemia, diet and exercise, with titration recommendations based on the Chinese Diabetes Society Guidelines [23]. After 3 and 6 months of use, mean HbA1c was reduced by approximately 1.0% from a baseline value of 8.3%, and the percentage of patients achieving HbA1c targets increased from 24% at baseline to 67% at 6 months. Similarly, FBG was reduced by approximately 17 mg/dl after 3 and 6 months of use from a baseline of 144 mg/dl [23]. Interestingly, there appeared to be no increase in insulin dose from baseline to 6 months (mean 0.23 U/kg at both time points), suggesting that patients may have benefitted from the diet and exercise information provided by the application and thus avoided unnecessary insulin dose adjustments [23]. Another trial (the TeleDiab 2 study) compared the impact of two telemonitoring systems (a simple interactive voice response system [IVRS] that provided titration information and the Diabeo-BI application, which provided basal insulin calculation, telemonitoring and teleconsultations) versus standard care in optimization of BI initiation in people with inadequately controlled T2D [24]. Both the IVRS and mobile app approaches were shown to improve glycemic control versus standard care (HbA1c reduction of 0.5% with IVRS and 0.6% with Diabeo-BI; p ≤ 0.002 vs. standard care control for both), with no additional hypoglycemia risk [24]. While both of these studies support the clinical effectiveness of mobile apps to support BI dosing, another key goal of such technologies is to reduce the time burden on HCPs. This time-saving potential is supported by other studies, with one reporting a 55% decrease in time spent on insulin dose adjustments by certified diabetes educators when supported by a mobile application compared with conventional methods (i.e., weekly adjustment of insulin doses through telephone contact, based on ≥3 daily glucose readings) [25]. Therefore, such approaches may be of utility to people with diabetes and HCPs alike.
When interpreting the results of the current study, the following points should be considered. FBG goals were individualized according to the treating physician, so the percentage of participants achieving their FBG goal could be affected by differences in how strict or lenient HCPs were in setting personal FBG targets for MDC users. FBG baseline readings may influence titration duration, as users with a high FBG at baseline may take longer to achieve their FBG target; as shown, shorter titration length was associated with an improved likelihood of achieving FBG targets. Selection criteria for patient records did not specify whether patients could have prior experience of insulin or not; therefore, results shown likely include both previously insulin-naïve populations and insulin pre-treated individuals who switched to a different insulin. It is also worth noting that not all people with T2D will have access to smartphone devices in a real-life clinical setting, which may limit the number of patients who may be able to use MDC.
The strengths of this study include the high number of participants (n>2000) and the fact that it was conducted according to real-world clinical practice. These results are consequently readily applicable to countries that have a high burden of T2D and may benefit from technological solutions to relieve the burden on HCPs of actively managing patients’ insulin titration requirements. Study limitations include the retrospective study design, which limits the inferences that can be made, and the lack of data on baseline HbA1c, duration of diabetes and user compliance with physician recommendations. Also, one third of users stopped using MDC, but it is unclear whether these users stopped BI treatment or only ceased to use the MDC app. Finally, the positive results reported here for MDC use will need to be integrated with further data to better understand the impact of MDC on glucose control and lives of people with diabetes.
Conclusions
In summary, more frequent usage of the MDC app was associated with better FBG outcomes and no increased risk of hypoglycemia. The latter point (in combination with improved glycemic control) is particularly encouraging and should help to reassure patients who might be anxious about insulin initiation for this reason [10, 26]. These results indicate that digital tools to support basal insulin titration could be useful to both people with diabetes and HCPs in diabetes management in terms of achieving better glycemic control.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the MDC users and healthcare professionals who participated in the trial. All authors take complete responsibility for the interpretation of the data in this review.
Funding
This study and Rapid Service Fee was funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
LH and LZ performed the statistical analyses. All authors contributed to data interpretation, drafting of the manuscript and critical revision of the manuscript. All authors approved the final version before submission.
Medical Writing, Editorial and Other Assistance
The authors received editorial/writing support in the preparation of this manuscript provided by Hanna Mourad-Agha, PhD, of Fishawack Communications Ltd, part of Fishawack Health, funded by Sanofi.
Disclosures
AG Unnikrishnan has been a member of a speakers’ bureau and advisory board for Sanofi. Vijay Viswanathan has attended and received honoraria for Sanofi advisory board meetings, Leonardo Mancillas-Adame and Jose Fernando Botero have nothing to declare. Fang Liz Zhou, Lichen Hao, Prithvi Kamath and Monica Bertolini are employees of Sanofi and may hold shares and/or stock options in the company.
Compliance with Ethics Guidelines
This retrospective observational cohort analysis included people with T2D who registered for MDC between August 1, 2018 and April 30, 2020 and received BI; MDC users agreed to allow their anonymized data to be used retrospectively as part of the registration process.
Data Availability
The datasets generated during and/or analyzed during the current study are available on reasonable request.
Prior Presentation
Data included in this work has previously been presented at the following meetings: Advanced Technologies and Treatments for Diabetes (ATTD) February 17–20, 2021; Paris, France. Diabetes Technology Society (DTS); November 12–14, 2020; virtual event; data from Mexico only. Advanced Technologies and Treatments for Diabetes (ATTD); February 19–22, 2020; Madrid, Spain; data from India only
References
- 1.American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S73–S84. [DOI] [PubMed]
- 2.Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in Type 2 Diabetes: impact of early glycemic control on future complications (The Diabetes & Aging Study) Diabetes Care. 2019;42:416–426. doi: 10.2337/dc17-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20:427–437. doi: 10.1111/dom.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Diabetes Federation. IDF Diabetes Atlas, Ninth edition. 2019. https://www.diabetesatlas.org/en/. Accessed 19 May 2021
- 5.Mauricio D, Meneghini L, Seufert J, et al. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA. Diabetes Obes Metab. 2017;19:1155–1164. doi: 10.1111/dom.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moses CR, Seshiah V, Sahay BK, et al. Baseline results indicate poor glycemic control and delay in initiation and optimization of insulin therapy: results from the improving management practices and clinical outcomes in type 2 diabetes study. Indian J Endocrinol Metab. 2012;16:S432–S433. doi: 10.4103/2230-8210.104120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attridge M, Creamer J, Ramsden M, Cannings-John R, Hawthorne K. Culturally appropriate health education for people in ethnic minority groups with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD006424.pub3:CD006424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis SE, Speroff T, Dittus RS, et al. Diabetes patient education: a meta-analysis and meta-regression. Patient Educ Couns. 2004;52:97–105. doi: 10.1016/S0738-3991(03)00016-8. [DOI] [PubMed] [Google Scholar]
- 9.Essien O, Otu A, Umoh V, et al. Intensive patient education improves glycaemic control in Diabetes compared to conventional education: a randomised controlled trial in a Nigerian Tertiary Care Hospital. PLoS ONE. 2017;12:e0168835. doi: 10.1371/journal.pone.0168835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berard L, Bonnemaire M, Mical M, Edelman S. Insights into optimal basal insulin titration in type 2 diabetes: results of a quantitative survey. Diabetes Obes Metab. 2018;20:301–308. doi: 10.1111/dom.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun J, Strong J, Urquhart S. Insulin initiation and titration in patients with Type 2 Diabetes. Diabetes Spectr. 2019;32:104–111. doi: 10.2337/ds18-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forst T, Choudhary P, Schneider D, Linetzky B, Pozzilli P. A practical approach to the clinical challenges in initiation of basal insulin therapy in people with type 2 diabetes. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3418:e3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrivastav M, Gibson W, Jr, Shrivastav R, et al. Type 2 Diabetes management in primary care: the role of retrospective. Prof Contin Glucose Monit Diabetes Spectr. 2018;31:279–287. doi: 10.2337/ds17-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daly A, Hovorka R. Technology in the management of type 2 diabetes: present status and future prospects. Diabetes Obes Metab. 2021 doi: 10.1111/dom.14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wicaksana AL, Hertanti NS, Ferdiana A, Pramono RB. Diabetes management and specific considerations for patients with diabetes during coronavirus diseases pandemic: a scoping review. Diabetes Metab Syndr. 2020;14:1109–1120. doi: 10.1016/j.dsx.2020.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg S, Norman GJ. Impact of COVID-19 on health economics and technology of diabetes care: use cases of real-time continuous glucose monitoring to transform health care during a global pandemic. Diabetes Technol Ther. 2021;23:S15–S20. doi: 10.1089/dia.2020.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Food and Drug Administration. My Dose Coach Food and Drug Administration approval. 2017. https://www.accessdata.fda.gov/cdrh_docs/pdf16/K163099.pdf. Accessed 19 May 2021.
- 18.My Dose Coach app. https://play.google.com/store/apps/details?id=com.sanofi.us.MyDoseCoach.android&hl=en_GB&gl=US Accessed Jan 2022.
- 19.My Dose Coach. https://apps.apple.com/dz/app/my-dose-coach/id1120665335. Accessed Feb 2022.
- 20.My Dose Coach public registration. https://www.salute.gov.it/interrogazioneDispositivi/RicercaDispositiviServlet?action=ACTION_MASCHERA (product ID: MTD-MDCR). Accessed Feb 2022.
- 21.Sanofi. My Dose Coach. 2020. https://www.mystarsanofi.com/products/mydosecoach. Accessed May 2021.
- 22.Modi KD, Jha S, Panda M, Sr, et al. Digital-tool-supported basal insulin (BI) titration: real-world effectiveness of my dose coach In India. Diabetes. 2019;68:138-LB. doi: 10.2337/db19-138-LB. [DOI] [Google Scholar]
- 23.Cai X, Zhang F, Lin C, et al. Achieving effective and efficient basal insulin optimal management by using mobile health application (APP) for Type 2 Diabetes Patients in China. Diabetes Metab Syndr Obes. 2020;13:1327–1338. doi: 10.2147/DMSO.S244826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franc S, Joubert M, Daoudi A, et al. Efficacy of two telemonitoring systems to improve glycaemic control during basal insulin initiation in patients with type 2 diabetes: the TeleDiab-2 randomized controlled trial. Diabetes Obes Metab. 2019;21:2327–2332. doi: 10.1111/dom.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon A, Fatehi F, Ding H, et al. Outcomes of a feasibility trial using an innovative mobile health programme to assist in insulin dose adjustment. BMJ Health Care Inform. 2019;26. [DOI] [PMC free article] [PubMed]
- 26.Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20:488–496. doi: 10.1111/dom.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available on reasonable request.