Abstract
Background.
Reported outbreaks of invasive group A Streptococcus (iGAS) infections among people who inject drugs (PWID) and people experiencing homelessness (PEH) have increased, concurrent with rising US iGAS rates. We describe epidemiology among iGAS patients with these risk factors.
Methods.
We analyzed iGAS infections from population-based Active Bacterial Core surveillance (ABCs) at 10 US sites from 2010 to 2017. Cases were defined as GAS isolated from a normally sterile site or from a wound in patients with necrotizing fasciitis or streptococcal toxic shock syndrome. GAS isolates were emm typed. We categorized iGAS patients into four categories: injection drug use (IDU) only, homelessness only, both, and neither. We calculated annual change in prevalence of these risk factors using log binomial regression models. We estimated national iGAS infection rates among PWID and PEH.
Results.
We identified 12 386 iGAS cases; IDU, homelessness, or both were documented in ~13%. Skin infections and acute skin breakdown were common among iGAS patients with documented IDU or homelessness. Endocarditis was 10-fold more frequent among iGAS patients with documented IDU only versus those with neither risk factor. Average percentage yearly increase in prevalence of IDU and homelessness among iGAS patients was 17.5% and 20.0%, respectively. iGAS infection rates among people with documented IDU or homelessness were ~14-fold and 17- to 80-fold higher, respectively, than among people without those risks.
Conclusions.
IDU and homelessness likely contribute to increases in US incidence of iGAS infections. Improving management of skin breakdown and early recognition of skin infection could prevent iGAS infections in these patients.
Keywords: group A Streptococcus, epidemiology, surveillance, injection drug use, homelessness
Group A Streptococcus (GAS) causes a range of disease, from mild infections, such as pharyngitis, to life-threatening invasive disease, such as necrotizing fasciitis. Invasive GAS (iGAS) infections are monitored at 10 US sites by the Centers for Disease Control and Prevention’s (CDC) Active Bacterial Core surveillance (ABCs). The US rate of iGAS infections rose from 4.0/100 000 in 2010 to 7.26/100 000 in 2017 [1, 2]. CDC estimated that >23 000 cases and >1900 deaths occurred nationally in 2017. Outbreaks of iGAS infection have been well described in long-term care facilities [3–6]. Since 2015, reports of outbreaks of iGAS infection among people who inject drugs (PWID) and people experiencing homelessness (PEH) in the United States, United Kingdom, and Canada have increased [7–11].
Established risk factors for iGAS infections among adults include older age, exposure to children with pharyngitis, household crowding, injection drug use (IDU), alcohol abuse, and comorbid conditions such as human immunodeficiency virus (HIV) infection, diabetes, cancer, and chronic heart and liver diseases. Skin breakdown from surgery, wounds, and blunt or penetrating trauma also increase disease risk [3, 12].
We used ABCs population-based surveillance to characterize patients with iGAS infection and documentation of IDU, homelessness, or both during 2010–2017. We estimate prevalence of IDU, homelessness, or both among patients with iGAS infections during 2010–2017; describe GAS strain distribution among these patients; and estimate US rates of iGAS infections among PWID and PEH.
METHODS
Surveillance
ABCs is an active, population- and laboratory-based surveillance system that tracks iGAS infections at 10 US sites, covering approximately 34.2 million people as of 2017 [2, 13]. The ABCs sites consistent since 2010 are listed in Table 1. ABCs defines an iGAS case as infection in a surveillance area resident from whom GAS is isolated from a normally sterile site (eg, blood) or from a wound culture in a patient with necrotizing fasciitis or streptococcal toxic shock syndrome (STSS).
Table 1.
Invasive GAS Patients With Documented Injection Drug Use (IDU) Only, Homelessness Only, Both, or Neither by ABCs Site, 2010–2017
| Neither Risk Factor (N = 10 807) |
IDU Only (N = 683) |
Homelessness Only (N = 531) |
Both IDU and Homelessness (N = 365) |
|
|---|---|---|---|---|
| ABCs Site | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) |
| California (3 San Francisco Bay area counties) | 72.7 (70.4–75.0) | 7.0 (5.7–8.4) | 13.0 (11.3–14.8) | 7.3 (6.0–8.7) |
|
| ||||
| Colorado (5 Denver metropolitan area counties) | 85.6 (83.7–87.4) | 2.6 (1.9–3.6) | 8.7 (73–10.3) | 3.0 (2.2–4.0) |
|
| ||||
| Connecticut (entire state) | 94.0 (92.4–95.4) | 4.1 (3.0–5.5) | .6 (.2–1.2) | 1.3 (.7–2.2) |
|
| ||||
| Georgia (20 Atlanta counties) | 97.3 (96.3–98.1) | 1.4 (.8–2.2) | .9 (.5–1.5) | .4 (.2–1.0) |
|
| ||||
| Maryland (6 Baltimore counties) | 78.8 (76.2–81.3) | 12.7 (10.7–14.9) | 1.9 (1.1–2.9) | 6.6 (5.2–8.3) |
|
| ||||
| Minnesota (entire state) | 96.7 (95.8–97.5) | 1.8 (1.2–2.5) | 1.3 (.8–1.9) | .2 (0–.5) |
|
| ||||
| New Mexico (entire state) | 83.8 (81.8–85.7) | 9.7 (8.2–11.4) | 3.8 (2.9–4.9) | 2.7 (1.9–3.7) |
|
| ||||
| New York (15 counties in Rochester and Albany) | 94.4 (92.5–96.9) | 4.8 (3.4–6.6) | .1 (0–.7) | .7 (.2–1.6) |
|
| ||||
| Oregon (3 Portland counties) | 75.3 (72.1–78.2) | 8.1 (6.3–10.2) | 8.7 (6.8–10.9) | 7.9 (6.1–10.1) |
|
| ||||
| Tennessee (20 counties) | 90.1 (88.3–91.7) | 6.5 (5.2–8.1) | 2.1 (1.4–3.1) | 1.3 (.7–2.1) |
Abbreviations: ABCs, Active Bacterial Core surveillance; CI, confidence interval; GAS, group A Streptococcus infection; IDU, injection drug use.
GAS Strain Characterization
GAS isolates collected prior to 2015 were emm subtyped. Isolates collected in 2015–2017 were characterized through whole genome sequencing and an associated bioinformatics pipeline at the CDC’s Streptococcus laboratory, as previously described [14, 15].
Determination of IDU and Homelessness Status
We categorized patients with iGAS infection into four mutually exclusive groups: those with documented IDU only, those with documentation of experiencing homelessness only, those for whom both IDU and homelessness are documented, and those with neither risk factor. ABCs categorized iGAS patients as PWID if IDU was documented in the medical record within the past 12 months. In 2010, ABCs added “homeless” to a variable that captured patient residence at the time of positive GAS culture. From 2010 to 2015, ABCs categorized iGAS patients as PEH if they were documented as homeless or residing in a shelter. In 2016, ABCs’ definition of homeless was expanded to include patients who resided in a mission, medical respite, or church community center at the time of positive culture.
Statistical Analysis
We included all iGAS cases with culture dates 1 January 2010 to 31 December 2017. We calculated proportions and Clopper-Pearson confidence intervals for univariate analyses of demographic characteristics, clinical syndromes, and comorbidities among iGAS patients stratified by the 4 risk factor groups described above [16]. Case fatality ratios were calculated using outcome at the time of discharge from hospital, emergency department, or clinic. We used log binomial regression models to calculate average percentage yearly change in prevalence of IDU, homelessness, or both among all iGAS patients during 2010–2017 by site and for all sites combined.
We stratified emm types into the 17 most common types; remaining types were categorized as “other.” We compared emm type prevalence among risk groups to prevalence among iGAS patients with neither risk factor. We classified the 17 predominant emm types into 1 of 48 functional emm clusters, which share structural and binding properties [17, 18]. Analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA).
Rate Estimates
We calculated the national iGAS infection rate among people with documented IDU during 2011, the most recent year for which a US population estimate was available for this behavior [19]. Because this estimate was for the total US population aged ≥13 years in 2011, we limited analysis of GAS cases to the same age range. We estimated the national iGAS infection rate among PEH for 2010–2017 using iGAS patients for whom homelessness was documented as the numerator, and annual point-in-time estimates from the US Department of Housing and Urban Development (HUD) of PEH as the denominator [20]. We estimated the number of iGAS patients with IDU or who were homeless in the United States (numerators) for the same years that relevant denominator data were available. We calculated the percent of iGAS patients identified by ABCs with documented IDU or homelessness during the year and multiplied this by the national estimate of iGAS cases for that year. We compared estimated iGAS incidence among PWID and among PEH to iGAS incidence among iGAS patients without documented IDU or homelessness, respectively, in medical charts.
This project was reviewed in accordance with CDC human research protection procedures and determined to be nonresearch, public health surveillance.
RESULTS
Demographic Characteristics
During 2010–2017, 12 368 cases of iGAS infection were reported: 683 (5.5%) in PWID, 531 (4.3%) in PEH, and 365 (2.9%) in patients with both risk factors. The iGAS patients with documented homelessness were more often male (77%) compared to other risk groups (Table 2). Nearly half of iGAS patients with documented IDU only were aged 18–34 years; >50% of iGAS patients with documented homelessness only were 50–64 years of age. Most iGAS patients were non-Hispanic white, reflecting the underlying population. Patients with documented IDU, homelessness, or both were more likely to receive Medicaid (60.5%–70.9%) or lack health insurance (12.1%–16.7%) than those with no risk factor (28.9% and 5.3%, respectively). Most iGAS patients in every group were hospitalized for their infection (94.7%–96.6%). The case fatality ratio was highest (10.6%) among those with neither risk factor; 5.7% among those with documented IDU only; 5.3% among those with documented homelessness only; and lowest (0.8%) among those for whom both IDU and homelessness were documented (P-value for each risk group compared to those with neither risk factor: <.001).
Table 2.
Characteristics of Invasive GAS Patients With Documented IDU Only, Homelessness Only, Both, or Neither—Active Bacterial Core Surveillance, 2010–2017
| Neither Risk Factor (N = 10 807) |
IDU Only (N = 683) |
Homelessness Only (N = 531) |
Both IDU and Homelessness (N = 365) |
|
|---|---|---|---|---|
| Characteristic | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) |
| Age (years) | ||||
|
| ||||
| Mean (range) | 53 (0–106) | 38 (17–72) | 51 (18–85) | 38 (19–73) |
|
| ||||
| <18 | 10.3 (9.7–10.9) | .2 (.0–.8) | 0 (0–.7) | 0 (0–1.0) |
|
| ||||
| 18–34 | 11.2 (10.6–11.8) | 473 (43.5–51.1) | 10.0 (7.6–12.9) | 46.9 (41.6–52.1) |
|
| ||||
| 35–49 | 18.0 (17.3–18.7) | 31.8 (28.3–35.4) | 27.5 (23.7–31.5) | 37.0 (32.0–42.2) |
|
| ||||
| 50–64 | 26.2 (25.3–27.0) | 19.0 (16.2–22.2) | 51.8 (474–56.1) | 14.8 (11.3–18.9) |
|
| ||||
| ≥65 | 34.4 (33.5–35.3) | 1.8 (.9–3.0) | 10.7 (8.2–13.7) | 1.4 (.4–3.2) |
|
| ||||
| Sexa | ||||
|
| ||||
| Male | 53.9 (53.0–54.9) | 51.1 (473–54.9) | 77.0 (73.4–80.7) | 59.5 (54.2–64.5) |
|
| ||||
| Female | 46.1 (45.1–47.0) | 48.9 (45.1–52.7) | 22.8 (19.3–26.6) | 40.6 (35.5–45.8) |
|
| ||||
| Race | ||||
|
| ||||
| White | 64.6 (63.7–65.5) | 78.3 (75.0–81.4) | 62.2 (579–66.3) | 73.7 (68.9–78.1) |
|
| ||||
| Black | 15.3 (14.6–16.0) | 7.6 (5.7–9.9) | 16.4 (13.3–19.8) | 11.2 (8.2–14.9) |
|
| ||||
| American Indian/Alaska Native | 4.9 (4.5–5.3) | 2.5 (1.5–4.0) | 5.3 (3.5–7.5) | 2.2 (.10–4.3) |
|
| ||||
| Asian/Pacific Islander | 3.5 (3.2–3.9) | 1.3 (.6–2.5) | 2.3 (1.2–3.9) | 1.4 (.4–3.2) |
|
| ||||
| Other/Unknown | 11.7 (11.1–12.3) | 10.3 (8.1–12.8) | 13.9 (11.1–17.2) | 11.5 (8.4–15.2) |
|
| ||||
| Ethnicity | ||||
|
| ||||
| Hispanic/Latino | 11.1 (10.5–11.7) | 16.1 (13.4–19.1) | 13.9 (11.1–17.2) | 12.6 (9.4–16.5) |
|
| ||||
| Non-Hispanic/Latino | 65.5 (64.6–66.4) | 64.9 (61.1–68.4) | 71.6 (67.5–75.4) | 71.2 (66.3–75.8) |
|
| ||||
| Unknown | 23.4 (22.6–24.2) | 19.0 (16.2–22.2) | 14.5 (11.6–17.8) | 16.2 (12.5–20.3) |
|
| ||||
| Residence at time of initial cultureb | ||||
|
| ||||
| Private residence | 91.7 (91.5–92.5) | 94.6 (92.6–96.2) | N/A | N/A |
|
| ||||
| Long-term care facility | 6.5 (6.1–7.1) | 1.2 (.5–2.3) | N/A | N/A |
|
| ||||
| Long-term acute care facility | .2 (.1–.3) | .2 (0–.8) | N/A | N/A |
|
| ||||
| Incarcerated | .4 (.3–.5) | 1.5 (.7–2.7) | N/A | N/A |
|
| ||||
| Other | .9 (.7–1.1) | 2.6 (1.6–4.1) | N/A | N/A |
|
| ||||
| Insurance typec | ||||
|
| ||||
| Private | 45.4 (44.4–46.4) | 22.3 (19.2–25.8) | 6.8 (4.7–9.3) | 16.8 (13.0–21.1) |
|
| ||||
| Medicare | 37.6 (36.6–38.5) | 11.4 (9.0–14.1) | 19.3 (15.9–23.0) | 6.0 (3.7–9.0) |
|
| ||||
| Medicaid | 28.9 (28.0–29.8) | 60.5 (56.6–64.3) | 70.0 (65.8–74.0) | 70.9 (65.9–75.6) |
|
| ||||
| Otherd | 7.0 (6.5–7.5) | 5.0 (3.4–7.0) | 6.4 (4.4–8.9) | 3.7 (2.0–6.3) |
|
| ||||
| None | 5.3 (4.9–5.8) | 16.7 (13.9–19.8) | 12.1 (9.4–15.3) | 15.4 (11.8–19.6) |
|
| ||||
| Unknown | 5.2 (4.8–5.7) | 3.6 (2.3–5.3) | 3.8 (2.3–5.8) | 2.9 (1.4–5.2) |
Abbreviations: CI, confidence interval; GAS, group A Streptococcus infection; IDU, injection drug use; N/A, not applicable.
Data on sex were missing from 2 cases in an individual with GAS without documented risk factors and 1 case in an individual with documentation of homelessness in their medical record.
Data on residence were missing from 41 cases in individuals with GAS without risk factors.
Insurance variable added in 2011; n’s are accordingly lower than those for variables with data collected for a longer period (neither risk factor: N = 9666; IDU only: N = 640, homelessness only: N = 503: both IDU and homelessness: N = 351). Individuals could have more than 1 type of insurance coverage.
Other = military insurance, Indian Health Service, prison, and other. Prison insurance added in 2012.
ABCs sites with highest prevalence of documented IDU among iGAS patients included San Francisco Bay area, California (7.0%); the Baltimore, Maryland area (12.7%); New Mexico (9.7%); and the Portland, Oregon area (8.1%) (Table 1). Documented homelessness was most common in the San Francisco Bay area (13.0%); Denver, Colorado (8.7%); and Portland, Oregon (8.7%).
Clinical Syndromes and Comorbidities
Cellulitis was more prevalent among iGAS patients with documented IDU or homelessness (range, 48.2%–55.5%) than patients with neither risk factor (39.8%) (Table 3). Abscess was more frequent among those with both risk factors (16.7%) and with documented IDU only (13.8%) compared to those with neither risk factor (6.9%). Those with documented IDU only were 10 times more likely to be diagnosed with endocarditis than other groups (see Table 3). The iGAS patients with documented homelessness were approximately 2 times more likely to be diagnosed with osteomyelitis than other groups. Septic shock, meningitis, and pneumonia were most common among iGAS patients with neither risk factor.
Table 3.
Clinical Syndromes and Comorbidities Among Invasive GAS Patients With Documented IDU Only, Homelessness Only, Both, or Neither—ABCs Site, 2010–2017
| Neither Risk Factor (N = 10 807) |
IDU Only (N = 683) |
Homelessness Only (N = 531) |
Both IDU and Homelessness (N = 365) |
|
|---|---|---|---|---|
| Clinical Syndromea | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) |
| Cellulitis | 39.8 (38.9–40.7) | 55.5 (51.7–59.3) | 48.2 (43.9–52.6) | 53.2 (47.9–58.4) |
|
| ||||
| Bacteremia | 21.1 (20.3–21.9) | 11.6 (9.3–14.2) | 23.0 (19.5–26.8) | 18.1 (14.3–22.4) |
|
| ||||
| Septic shock | 18.5 (178–19.2) | 12.6 (10.2–15.3) | 11.1 (8.6–14.1) | 6.9 (4.5–9.9) |
|
| ||||
| Pneumonia | 15.0 (14.3–15.7) | 11.3 (9.0–13.9) | 7.9 (5.8–10.5) | 7.7 (5.2–10.9) |
|
| ||||
| Septic arthritis | 10.3 (9.7–10.8) | 13.2 (10.7–15.9) | 11.5 (8.9–14.5) | 15.3 (11.8–19.5) |
|
| ||||
| Necrotizing fasciitis | 6.7 (6.2–7.1) | 8.1 (6.1–10.4) | 6.4 (4.5–8.8) | 5.2 (3.2–8.0) |
|
| ||||
| Abscess | 6.9 (6.4–7.3) | 13.8 (11.3–16.6) | 7.0 (5.0–9.5) | 16.7 (13.0–20.9) |
|
| ||||
| Osteomyelitis | 5.5 (5.0–5.9) | 4.7 (3.2–6.6) | 9.6 (7.2–12.4) | 4.7 (2.7–7.4) |
|
| ||||
| Streptococcal toxic shock syndrome | 3.4 (3.1–3.8) | 1.2 (.5–2.3) | .9 (.3–2.2) | .3 (0–1.5) |
|
| ||||
| Empyema | 2.2 (1.9–2.5) | 3.1 (1.9–4.7) | .6 (.1–1.6) | 2.7 (1.3–5.0) |
|
| ||||
| Peritonitis | 1.7 (1.5–2.0) | .4 (.1–1.3) | 1.1 (.4–2.4) | 0 (0–1.0) |
|
| ||||
| Endometritis | 1.0 (.9–1.2) | 0 (0–.5) | 0 (0–.7) | .3 (0–1.5) |
|
| ||||
| Meningitis | .9 (.8–1.1) | .2 (0–.8) | .4 (0–1.4) | .6 (.1–2.0) |
|
| ||||
| Endocarditis | .7 (.6–0.9) | 7.2 (5.4–9.4) | 1.3 (.5–2.7) | 4.1 (2.3–6.7) |
|
| ||||
| Comorbidities | ||||
|
| ||||
| Chronic underlying conditions | ||||
|
| ||||
| Diabetes mellitus | 29.4 (28.5–30.3) | 8.9 (6.9–11.3) | 19.4 (16.1–23.0) | 8.0 (5.4–11.2) |
|
| ||||
| Obesity | 18.8 (18.1–19.6) | 4.4 (3.0–6.2) | 8.7 (6.4–11.4) | 1.9 (.8–3.9) |
|
| ||||
| Coronary artery disease | 13.1 (12.5–13.8) | 1.2 (.5–2.3) | 5.1 (3.4–7.3) | 1.1 (.3–2.8) |
|
| ||||
| Congestive heart failure | 12.8 (12.1–13.4) | 2.6 (1.6–4.1) | 10.6 (8.1–13.5) | .3 (0–1.5) |
|
| ||||
| Chronic renal disease | 12.3 (11.7–13.0) | 2.2 (1.2–3.6) | 5.1 (3.4–7.3) | 1.4 (.4–3.2) |
|
| ||||
| Chronic obstructive pulmonary disease | 10.4 (9.8–10.9) | 4.8 (3.3–6.7) | 11.1 (8.6–14.1) | 3.8 (2.1–6.4) |
|
| ||||
| Solid organ malignancy | 9.6 (9.0–10.1) | 2.9 (1.8–4.5) | 2.6 (1.4–4.4) | .6 (.1–2.0) |
|
| ||||
| Dialysis | 4.8 (4.4–5.3) | .2 (0–.8) | 0.8 (.2–1.9) | .6 (.1–2.0) |
|
| ||||
| Chronic liver disease | 4.2 (3.9–4.6) | 14.1 (11.5–16.9) | 16.0 (13.0–19.4) | 12.6 (9.4–16.5) |
|
| ||||
| HIV infectionb | 1.7 (1.5–2.0) | 5.7 (4.1–7.8) | 3.8 (2.3–5.8) | 8.6 (5.9–12.0) |
|
| ||||
| Behaviors | ||||
|
| ||||
| Current smoker | 15.6 (14.9–16.3) | 71.0 (67.4–74.4) | 44.8 (40.5–49.2) | 73.4 (68.6–77.9) |
|
| ||||
| Alcohol abuse | 6.8 (6.3–7.3) | 13.2 (10.7–15.9) | 42.6 (38.3–46.9) | 16.7 (13.0–20.9) |
|
| ||||
| Current other drug use | 4.8 (4.4–5.2) | 38.7 (35.0–42.4) | 30.1 (26.3–34.2) | 46.0 (40.8–51.3) |
|
| ||||
| Skin breakdown c | ||||
|
| ||||
| Acute skin breakdown | 19.2 (18.5–19.9) | 46.3 (42.5–50.1) | 28.4 (24.6–32.5) | 46.6 (41.4–51.8) |
|
| ||||
| Chronic skin breakdown | 11.3 (10.7–11.9) | 7.3 (5.5–9.5) | 10.7 (8.2–13.7) | 8.8 (6.1–12.2) |
|
| ||||
| No underlying conditions | ||||
|
| ||||
| None | 21.0 (20.2–21.8) | 0 (0–.5) | 4.3 (2.8–6.4) | 0 (0–1.0) |
Abbreviations: ABCs, Active Bacterial Core surveillance; CI, confidence interval; GAS, group A Streptococcus infection; HIV, human immunodeficiency virus; IDU, injection drug use.
Not included: epiglottitis, hemolytic uremic syndrome, pericarditis, septic abortion, chorioamnionitis, puerperal sepsis, otitis media, and unknown infection, because case counts for each of these conditions were <90.
Excluding New York, which is not allowed to report HIV status (no risk factors: N = 10 101; documented IDU: N = 647; documented homelessness N = 530; both IDU and homelessness documented: N = 360).
Acute skin breakdown defined as: recent surgery, varicella, penetrating trauma, blunt trauma, surgical wound or burns. Chronic skin breakdown defined as: chronic dermatological condition in which integrity of the skin is compromised such as psoriasis, eczema or other chronic skin ulcers, including decubitus ulcers.
Several chronic underlying conditions (eg, diabetes, obesity, coronary artery disease, chronic renal disease, solid malignancies) were most common among iGAS patients with neither risk factor. Acute skin breakdown, however, was twice as likely in iGAS patients with documented IDU only (46.3%) and both risk factors (46.6%) than among those with neither (19.2%). Alcohol abuse (42.6%) and chronic liver disease (16.0%) were more frequent among PEH only than the other 3 groups. HIV infection was 5 times more likely to be a comorbidity among those with both IDU and homelessness documented (8.6%) compared to those with neither risk factor (1.7%). Current smoking and other drug use were more common among those with documented IDU, homelessness, or both (44.8%–73.4%) than those with neither risk factor (15.6%).
Trends Over Time
IDU and homelessness prevalence among iGAS patients increased significantly during 2010–2017. Prevalence of IDU only among iGAS patients had an average annual increase of 17.5% (P < .001) (Figure 1). Prevalence of homelessness only among iGAS patients had an average annual increase of 20.0% (P < .001). Prevalence of both IDU and homelessness had the largest increase—35.3% (P < .001). In 2017, the prevalence of IDU only, homelessness only, and both risk factors among iGAS patients was 8.7%, 5.8%, and 6.1%, respectively. All sites except California and New York observed statistically significant yearly increases from 2010 to 2017 in prevalence of IDU, homelessness, and both (Supplementary Figure 1 and Table 1).
Figure 1.
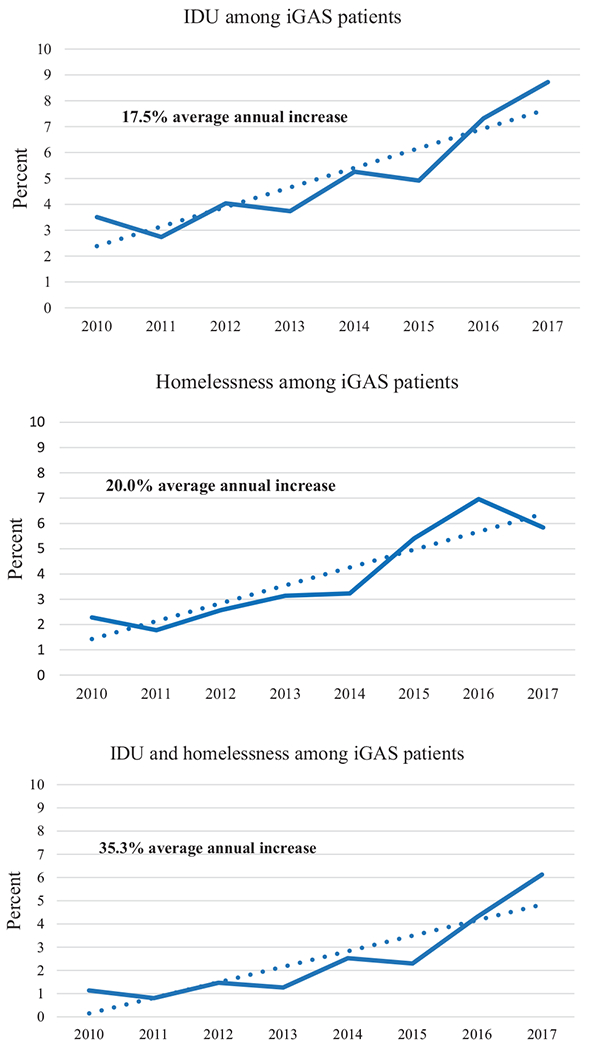
Prevalence of Injection Drug Use (IDU), Homelessness, or Both Among iGAS Patients--Active Bacterial Core surveillance, 2010–2017. Dotted lines indicate average annual increase in prevalence of the risk factor. Solid lines indicate percent of cases for which the risk factors was noted, by year.
Rate Estimates
The estimated US rate of iGAS infection among people aged ≥13 years who injected drugs in the previous year was approximately 52/100 000 in 2011, nearly 14 times the rate among people without documented IDU in the same year (3.8/100 000) (Supplementary Table 3). The estimated rate of iGAS infection among PEH in the United States was nearly 55/100 000 people in 2010, with a 9-fold increase to 511/100 000 people in 2017. Rates of iGAS infection among those without documented homelessness doubled (from 3.2 to 6.4/100 000 population from 2010 to 2017).
Emm Sequence Typing
GAS isolates were available for typing for 83% of cases. The 17 most common emm types accounted for 80.2%–83.6% of cases across all 4 groups. Distributions of specific types, however, varied by risk group (Figure 2). Among those with neither risk factor, the most common emm types were 1, 89, 12, and 28 (cumulatively 49% of isolates). Among those with documented IDU only, the most common emm types were 92, 89, 82, 49, and 1 (cumulatively 44% of isolates). Among iGAS patients with documented homelessness only, the most common emm types were 49, 82, 92, and 59 (cumulatively 57% of isolates). Emm82 comprised only 3.4% of isolates among iGAS patients with neither risk factor but was more frequent among iGAS patients with documented IDU only (9.2%) and iGAS patients with documented homelessness only (17.1%) (Supplementary Table 2). Similarly, emm92 comprised only 2.4% of isolates among iGAS patients with neither risk factor but was >5 times as common among iGAS patients with documented IDU only (14.2%) or homelessness only (13.4%), and among those with documentation of both risk factors (13.3%). Most emm types commonly found among iGAS patients with documented IDU only or homelessness only were part of the E emm cluster (E2, E3, E4, E6) [17, 18].
Figure 2.
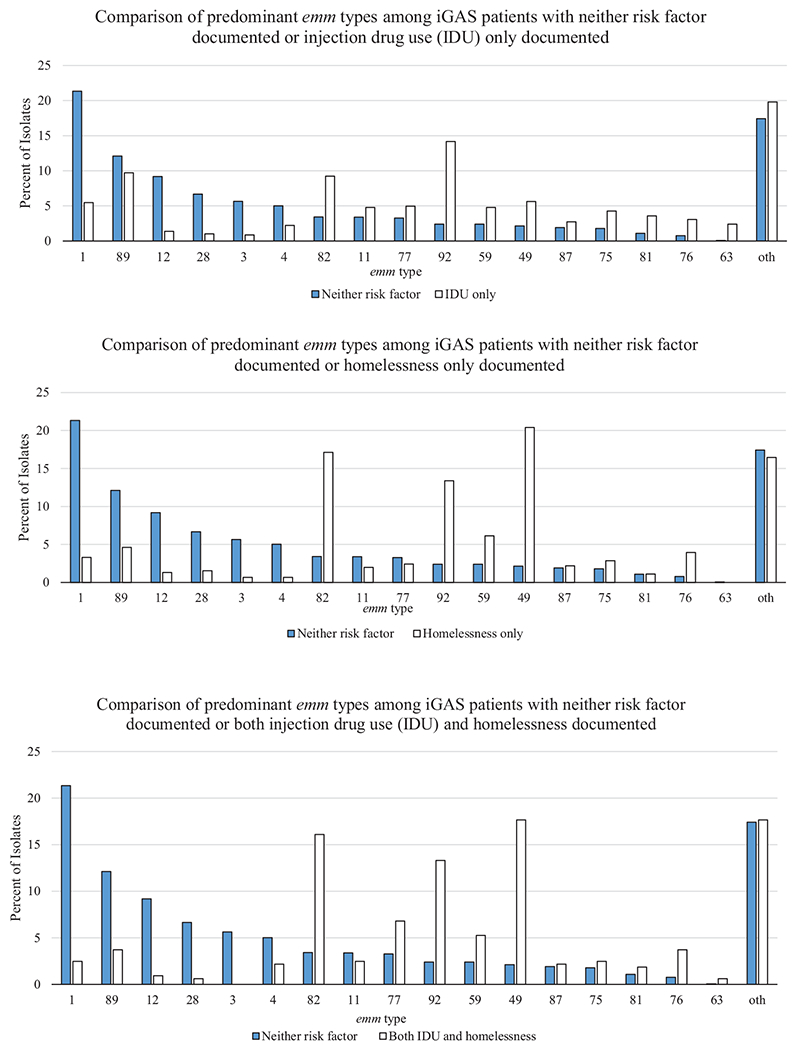
Comparison of predominant emm type distribution among iGAS patients--Active Bacterial Core surveillance, 2010–2017. Oth: other emm types.
DISCUSSION
Using robust population-based data, we documented recent increases in severe iGAS infections among 2 high-risk populations. Concurrent with an overall 81% increase in the rate of iGAS infections in the United States during 2010–2017, the prevalence of IDU, homelessness, and both among iGAS patients increased annually by 17.5%, 20.0%, and 35.3%, respectively [1, 2].In 2017, iGAS patients with documented IDU or homelessness accounted for approximately 21% of iGAS infections reported through ABCs. In 2011, the estimated rate of iGAS infection was approximately 14 times greater among PWID than those without documented IDU. In 2017, rates of iGAS infection were approximately 80 times greater among PEH than those without documented homelessness. In our study, GAS strains in patients with documented IDU or homelessness differed substantially from those among patients without such risk factors, suggesting expansion of selected GAS strains within these vulnerable populations.
The predominance of skin infections, such as cellulitis and abscesses, among patients with documented IDU or homelessness has also been noted in investigations of GAS outbreaks among these at-risk populations and through iGAS infection surveillance in Europe and Canada [8–11, 21–27]. These common manifestations and higher prevalence of acute skin breakdown (ie, breakdown from recent surgery, varicella, penetrating or blunt trauma, surgical wounds, or burns) suggest that broken skin is a prominent route of acquiring GAS infection in these populations. PEH have been shown to have increased skin breakdown due to poor hygiene, exposure to extreme temperatures, and trauma from fights or falls [28]. Approximately 1 in 5 PEH develop foot problems due to repeated trauma and standing for long periods, increasing risk for venous stasis, and lower extremity edema [28].
In our study, these high-risk iGAS patients were more likely to have chronic liver disease and HIV infection, and to smoke, use other drugs, and abuse alcohol. In a 2009 outbreak caused by a tetracycline-resistant GAS clone in France, most patients were young men who presented with infections secondary to skin injuries or abscesses and who had risk factors such as alcohol abuse, homelessness, drug use, hepatitis C infection, and HIV infection [23].
Other studies have also reported lower case fatality ratios (CFRs) among patients who reported IDU than those who did not report IDU [22, 27, 29]. This lower mortality observed both among homeless iGAS patients and those injecting drugs is likely a result of these patients being younger and presenting less frequently with disease manifestations associated with higher CFRs (eg, septic shock, meningitis, pneumonia) than patients with neither risk behavior [30]. Also, 2 emm types that are common in the United States (emm1, emm3) and are independently associated with higher fatality and severe disease (eg, STSS, necrotizing fasciitis) are disproportionately rare among patients with IDU or homelessness [27, 30]. Although these high-risk iGAS patients were found to have lower CFRs than patients with neither risk factor, the 10-fold increased frequency of endocarditis among iGAS patients with documented IDU and 2-fold increased risk of osteomyelitis among iGAS patients with documented homelessness suggest that a subset of these patients will need long-term antibiotic treatment and other interventions, including surgery.
The emm types that are substantially more common among iGAS patients with documented IDU or homelessness were all part of the E emm cluster (E2–E6) pattern, suggesting that these strains may have a predilection for skin infections [17, 18]. The predominance we observed of relatively uncommon emm types among PWID and/or PEH is consistent with findings from Canada and Europe. Published outbreaks among these risk groups have been caused by emergence of a specific emm type, including emm25.2 (PWID in Spain), emm83 (PWID in the United Kingdom) emm44 (PEH in France), emm32.2 (Liverpool, United Kingdom), emm66 (United Kingdom), and emm74 (Toronto, Ontario) [7, 10, 22–25, 29, 31, 32]. In emm59 outbreaks in Canada and Arizona, United States, homelessness and IDU were common [7, 10, 22–25, 29, 31, 32].
Increases in other infections associated with IDU have recently been reported in the United States. Methicillin-resistant Staphylococcus aureus (MRSA) surveillance identified a steady increase in the proportion of invasive MRSA cases among PWID, from 4.1% to 9.2% during 2011–2016 [33]. Surveillance of viral hepatitis identified a 3.5-fold increase in cases of acute hepatitis C infections from 2010 to 2016 associated with rising rates of IDU [34–36]. In 2015, an outbreak of HIV infection occurred among young, white men who were syringe-sharing partners, facilitated rapid transmission of HIV [37].
It is unclear what proportion of the overall increase in GAS incidence from 2010 to 2017 can be attributed to IDU and homelessness. We do not know if the increase in the proportion of patients with GAS infections with documentation of one or both of these risk factors represents a change in the rate of GAS infections among these groups. It could reflect an increase in the size of the population of PWID or PEH in ABCs sites, although HUD data suggest that homelessness in the United States decreased during this interval [20]. Annual estimates of the number of PWID in the United States using consistent methodology could help address this question [38].
The increase in prevalence of IDU among iGAS patients could be due to increases in predisposing factors (eg, unsafe injection practices, poor hygiene, skin breakdown). Studies in other countries have produced conflicting results. In Switzerland, no differences between cases and controls in sharing of paraphernalia or type of shelter were noted [21]. However, in Spain, PWID with GAS infection reported a higher number of injections per day (odds ratio [OR] 18.8), more frequently shared paraphernalia (OR 11.1), and were more likely to purchase substances at a specific location (OR 33.9) or from a specific dealer (OR 72.0) than PWID who did not have GAS infection [22]. Increases in prevalence of known risk factors (eg, skin breakdown, coinfection with HIV or hepatitis C) among PWID or PEH might have increased their susceptibility to iGAS infection, leading to an increased incidence of iGAS over time. For example, PWID might have increased skin breakdown from changes in injection practices. Alternatively, the increase might be partially related to the expansion of a limited number of relatively uncommon emm types among these iGAS patients, as noted in investigations in other countries [25, 27]. Similar to the general population, iGAS patients with documented IDU or homelessness may have developed long-lasting immunity to emm types most common in the United States (eg, emm 1, 3, 12) during childhood when their immune systems were healthy; relatively uncommon strains may now be spreading among these patients with generally poor immune status and no immunity from prior exposure.
There are several limitations to our analysis. First, in ABCs, patient information is obtained from medical record review; IDU and homelessness might not be accurately documented, likely resulting in underestimation of the proportion of iGAS patients with these risk factors. Second, the 2016 change in ABCs’ definition of homelessness may have contributed to the increase in prevalence of homelessness among patients with iGAS infection in that year. Third, we do not have concurrent estimates of the denominator of PWID and of PEH that exactly match the ABCs catchment populations, limiting our ability to precisely estimate iGAS infection rates among these populations.
Effective prevention measures that reduce morbidity associated with invasive GAS infection among patients with documentation of IDU and experiencing homelessness are greatly needed. A proposed 30-valent M-protein-based GAS vaccine that includes most emm types causing disease among these high-risk populations is still under development [39]. Healthcare providers can connect patients with iGAS infection who report IDU to harm reduction services, such as syringe service programs or medication-assisted treatment, and iGAS patients experiencing homelessness to local community outreach programs [40]. Educating patients at risk for iGAS infection on good skin care, general hygiene, safe injection practices, and early signs and symptoms of infection could also help to prevent invasive GAS infections.
Supplementary Material
Acknowledgments.
The authors thank all the ABCs team members, both at the sites and at CDC-Atlanta, for their contributions to data collection and management. They also thank CDC’s Streptococcus Laboratory for emm typing.
Financial support.
This work was supported by the CDC’s Emerging Infections Program.
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Potential conflicts of interest. L. H. reports personal fees from GSK, Sanofi-Pasteur, Merck, and Pfizer, outside the submitted work. W. S. reports DSMB fees from Pfizer and consulting fees from Roche Diagnostics, outside the submitted work. All other authors have no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Active Bacterial Core surveillance (ABCs) report, Emerging Infections Program Network, group A Streptococcus, 2010. Available at: https://www.cdc.gov/abcs/reports-findings/survreports/gas10.pdf. Accessed 29 April 2020.
- 2.Centers for Disease Control and Prevention. Active Bacterial Core surveillance (ABCs) report, Emerging Infections Program Network, group A Streptococcus, 2017. Available at https://www.cdc.gov/abcs/reports-findings/survreports/gas17.pdf. Accessed 29 April 2020.
- 3.Jordan HT, Richards CL Jr, Burton DC, Thigpen MC, Van Beneden CA. Group A streptococcal disease in long-term care facilities: descriptive epidemiology and potential control measures. Clin Infect Dis 2007; 45:742–52. [DOI] [PubMed] [Google Scholar]
- 4.Dooling KL, Crist MB, Nguyen DB, et al. Investigation of a prolonged group A streptococcal outbreak among residents of a skilled nursing facility, Georgia, 2009–2012. Clin Infect Dis 2013; 57:1562–7. [DOI] [PubMed] [Google Scholar]
- 5.Deutscher M, Schillie S, Gould C, et al. Investigation of a group A streptococcal outbreak among residents of a long-term acute care hospital. Clin Infect Dis 2011; 52:988–94. [DOI] [PubMed] [Google Scholar]
- 6.Harris AM, Yazzie D, Antone-Nez R, et al. Community-acquired invasive GAS disease among Native Americans, Arizona, USA, Winter 2013. Emerg Infect Dis 2015; 21:177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelthaler DM, Valentine M, Bowers J, et al. Hypervirulent emm59 clone in invasive group A Streptococcus outbreak, Southwestern United States. Emerg Infect Dis 2016; 22:734–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gittzus JWJ, Caine L, Hansen K, et al. An outbreak of invasive group A streptococcal infections in injection drug users. Open Forum Infect Dis 2017; 4:S241. [Google Scholar]
- 9.Mosites E, Frick A, Gounder P, et al. Outbreak of invasive infections from subtype emm26.3 group A Streptococcus among homeless adults-Anchorage, Alaska, 2016–2017. Clin Infect Dis 2018; 66:1068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bundle N, Bubba L, Coelho J, et al. Ongoing outbreak of invasive and non-invasive disease due to group A Streptococcus (GAS) type emm66 among homeless and people who inject drugs in England and Wales, January to December 2016. Euro Surveill 2017; 22:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickson C, Pham MT, Nguyen V, et al. Community outbreak of invasive group A Streptococcus infection in Ontario, Canada. Can Commun Dis Rep 2018; 44:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Factor SH, Levine OS, Schwartz B, et al. Invasive group A streptococcal disease: risk factors for adults. Emerg Infect Dis 2003; 9:970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Active bacterial core surveillance (ABCs) methodology. Available at: https://www.cdc.gov/abcs/methodology/index.html. Accessed 29 April 2020.
- 14.Centers for Disease Control and Prevention. Protocol for emm typing. Available at: https://www.cdc.gov/streplab/groupa-strep/emm-typing-protocol.html. Accessed 29 April 2020.
- 15.Chochua S, Metcalf BJ, Li Z, et al. Population and whole genome sequence based characterization of invasive group A streptococci recovered in the United States during 2015. MBio 2017; 8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clopper C, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26:404–13. [Google Scholar]
- 17.Sanderson-Smith M, De Oliveira DM, Guglielmini J, et al. ; M Protein Study Group. A systematic and functional classification of Streptococcus pyogenes that serves as a new tool for molecular typing and vaccine development. J Infect Dis 2014; 210:1325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smeesters PR, Laho D, Beall B, Steer AC, Van Beneden CA. Seasonal, geographic, and temporal trends of emm clusters associated with invasive group A streptococcal infections in US multistate surveillance. Clin Infect Dis 2017; 64:694–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lansky A, Finlayson T, Johnson C, et al. Estimating the number of persons who inject drugs in the united states by meta-analysis to calculate national rates of HIV and hepatitis C virus infections. PLoS One 2014; 9:e97596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The US Department of Housing and Urban Development. The 2017 Annual Homeless Assessment Report (AHAR): part 1-PIT estimates of homelessness in the U.S Available at: https://www.hudexchange.info/resource/5639/2017-ahar-part-1-pit-estimates-of-homelessness-in-the-us/. Accessed 29 April 2020.
- 21.Böhlen LM, Mühlemann K, Dubuis O, Aebi C, Täuber MG. Outbreak among drug users caused by a clonal strain of group A Streptococcus. Emerg Infect Dis 2000; 6:175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sierra JM, Sanchez F, Castro P, et al. Group A streptococcal infections in injection drug users in Barcelona, Spain: epidemiologic, clinical, and microbiologic analysis of 3 clusters of cases from 2000 to 2003. Medicine (Baltimore) 2006; 85:139–46. [DOI] [PubMed] [Google Scholar]
- 23.Cady A, Plainvert C, Donnio P, et al. Clonal spread of Streptococcus pyogenes emm44 among homeless persons, Rennes, France. Emerg Infect Dis 2011; 17:315–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyrrell GJ, Lovgren M, St Jean T, et al. Epidemic of group A Streptococcus M/emm59 causing invasive disease in Canada. Clin Infect Dis 2010; 51:1290–7. [DOI] [PubMed] [Google Scholar]
- 25.Athey TB, Teatero S, Sieswerda LE, et al. High incidence of invasive group A Streptococcus disease caused by strains of uncommon emm types in Thunder Bay, Ontario, Canada. J Clin Microbiol 2016; 54:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyrrell GJ, Fathima S, Kakulphimp J, Bell C. Increasing rates of invasive group A streptococcal disease in Alberta, Canada; 2003–2017. Open Forum Infect Dis 2018; 5:ofy177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamagni TL, Neal S, Keshishian C, et al. Epidemic of severe Streptococcus pyogenes infections in injecting drug users in the UK, 2003–2004. Clin Microbiol Infect 2008; 14:1002–9. [DOI] [PubMed] [Google Scholar]
- 28.Raoult D, Foucault C, Brouqui P. Infections in the homeless. Lancet Infect Dis 2001; 1:77–84. [DOI] [PubMed] [Google Scholar]
- 29.Cornick JE, Kiran AM, Vivancos R, et al. Epidemiological and molecular characterization of an invasive group A Streptococcus emm32.2 outbreak. J Clin Microbiol 2017; 55:1837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson GE, Pondo T, Toews KA, et al. Epidemiology of invasive group A streptococcal infections in the United States, 2005–2012. Clin Infect Dis 2016; 63:478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finkelstein MMA, Sachdeva H, Dohoo C, et al. Outbreak of group A Streptococcus (GAS) in a shelter for homeless men, Toronto, Canada. AMMI Canada-CACMID 2017 Annual Conference. Toronto, ON, Canada, 2017. [Google Scholar]
- 32.Teatero S, McGeer A, Tyrrell GJ, et al. Canada-wide epidemic of emm74 group A Streptococcus invasive disease. Open Forum Infect Dis 2018; 5:ofy085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson KA, Bohm MK, Brooks JT, et al. Invasive methicillin-resistant Staphylococcus aureus infections among persons who inject drugs: six sites, 2005–2016. MMWR Morb Mortal Wkly Rep 2018; 67:625–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Viral hepatitis surveillance–United States, 2016. Available at: https://www.cdc.gov/hepatitis/statistics/2016surveillance/pdfs/2016HepSurveillanceRpt.pdf. Accessed 29 April 2020.
- 35.Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health 2018; 108:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis 2014; 59:1411–9. [DOI] [PubMed] [Google Scholar]
- 37.Peters PJ, Pontones P, Hoover KW, et al. HIV infection linked to injection use of oxymorphone in Indiana, 2014–2015. N Engl J Med 2016; 375:229–39. [DOI] [PubMed] [Google Scholar]
- 38.Metraux S, Culhane D, Raphael S, et al. Assessing homeless population size through the use of emergency and transitional shelter services in 1998: results from the analysis of administrative data from nine US jurisdictions. Public Health Rep 2001; 116:344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dale JB, Smeesters PR, Courtney HS, et al. Structure-based design of broadly protective group A streptococcal M protein-based vaccines. Vaccine 2017; 35:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Prevent bacterial and fungal infections in patients who inject drugs. Available at: https://www.cdc.gov/vitalsigns/staph/pdf/vs-safe-drug-use_hcp.pdf. Accessed 29 April 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


