Abstract
The current study evaluated the effects of dietary protein and fiber levels on growth performance, gout occurrence, intestinal microbial communities, and immunoregulation in the gut-kidney axis of goslings. A completely randomized 2 × 3 factorial design was adopted with 2 CP levels (180 [18CP] and 220 [22CP] g/kg) and 3 crude fiber (CF) levels (30 [low CF], 50 [mid CF], and 70 [high CF] g/kg). The high CP or low CF diets predisposed the goslings to gout. The high protein diets worsened renal function; serum concentrations of UA and Cr as well as XOD activity in 9-day-old goslings fed 22% CP diets were significantly increased. Although CF levels from 3 to 7% did not directly affect kidney health, increasing CF levels might accelerate the increase of probiotics in the cecum of goslings and withhold maleficent bacteria, alleviating the gut dysbiosis caused by high protein diets. An analysis of the cecal microbiota via 16Sr RNA sequencing revealed that the abundance of Enterococcus in the 22CP group was higher than that in the 18CP group but decreased with increasing CF levels on d 9. The abundance of Lactobacillus increased with increasing CF levels. Additionally, higher serum LPS and proinflammatory cytokine concentrations and upregulated mRNA expression levels in the cecal, tonsil, and kidney tissues indicated that high-protein diets could activate the TLR4/MyD88/NFκB pathway and induce both intestinal and renal inflammation in young goslings. Serum LPS concentrations on d 9 were found to decrease with increasing CF, although altering dietary CF levels did not directly affect the serum immune indices of goslings. In conclusion, the high CP diet exerted a negative effect on gout occurrence, microbial communities, and immunoregulation in the gut-kidney axis of goslings, while appropriately increased dietary fiber levels helped maintain intestinal balance and reduced serum LPS concentration. We propose a diet of 18% CP paired with a 5% CF as the optimal combination for gosling feed.
Key words: crude fiber, protein, gosling gout, intestinal microbial communities, gut-kidney axis
INTRODUCTION
Geese, which are herbivorous, depend on dietary fiber to perform normal activities. Moderate crude fiber (CF) levels in diets are known to enhance disease resistance and promote growth in poultry by modulating intestinal microecological balance and enhancing immune functions (Jha and Mishra, 2021) Wils-Plotz et al. (2013). reported that inclusion of dietary pectin upregulated IL-12 expression in the ileal mucosa and increased interferon-γ production in cecal tonsils. Several studies have indicated that a low CF diet reduces microbial diversity of as well as the relative abundance of beneficial microbiota in geese ceca on d 70, thereby negatively affecting growth performance, nutrient utilization, and intestinal mucosal immunity of these geese (Li et al., 2017; Li et al., 2018a). Dietary CF may help maintain intestinal barrier function by strengthening mucosal structure and function, as well as by increasing the population and diversity of commensal bacteria in the gastrointestinal tract. More importantly, young birds are reportedly more sensitive to changes in dietary nutrients and intestinal microbial communities than adults (Gao et al., 2017). However, studies on the effect of dietary CF levels on gut microbiota and disease resistance in goslings are scarce.
Growth performance in birds is less sensitive to changes in protein levels than to changes of dietary energy in feed to satisfy their original volitant nature (Shi et al., 2006). It has been reported that no significant difference in weight gain was observed in geese with dietary protein levels ranging from 16 to 22%, which seems overly general in scope (Summers et al., 1986). Conversely, the concentrations of dietary protein exert a strong effect on intestinal flora balance and disease resistance in poultry (Lee et al., 2020). Visceral gout, which occurs mainly in birds, is a metabolic disease caused by impaired kidney function, followed by an accumulation of urate crystals in various organs (Zhang et al., 2018). A previous study of ours demonstrated that high-protein diets were implicated in kidney injury and gut microbiota dysbiosis associated with gout in goslings (Xi et al., 2020a). The results of this study showed that, compared with goslings fed 16 and 18% crude protein (CP) diets, goslings fed 22% CP diets suffered intestinal epithelial cell injury and cecal microbiota dysbiosis, leading to kidney injury, or even gosling gout. Gastrointestinal tract microbiota and its metabolites appear to play a central role in strengthening the immunity of the gut-kidney axis in poultry (De Cesare et al., 2019; Xi et al., 2020b). Thus, identifying an appropriate nutritional program, which focuses on the effects of dietary CF and CP levels, may help minimize the incidence of gout and enhance immunity by maintaining intestinal microecological balance and improving relative immune regulation in goslings.
The intestinal microbiome exerts a profound effect on gut immune regulation, which affects systemic immunity and contributes to immune balance. Changes in dietary composition, including the CP and CF levels, affect the composition and metabolic activities of microbiota that adapt to the intestinal environment (Liu et al., 2014). In poultry, any disruption of the microbiome may induce an imbalance in systemic immunity, contributing to a decrease in disease resistance. In a previous study of ours, we found that gut microbiota dysbiosis increased the risk of visceral gout in goslings by increasing inflammation as well as the translocation of gut-derived lipopolysaccharide (LPS) in the gut-kidney axis. In this complex cascade, LPS ligation activates the nuclear factor, kappa-light-chain-enhancer of the activated B cell (NF-κB) pathway and the production of pro-inflammatory cytokines, such as interleukin 1β (IL1β) and tumor necrosis factor α (TNFα), thus promoting the development of immunological tolerance in both the gut and kidney of goslings (Xi et al., 2019). The present study focused on investigating the microbial communities inhabiting the cecum of goslings fed different levels of dietary CP and CF, and measured changes in gout occurrence by analyzing the immunoregulation and inflammatory responses induced in the gut-kidney axis.
MATERIALS AND METHODS
Ethical Approval
The study was approved by the Research Committee of Jiangsu Academy of Agricultural Sciences and conducted according to the Regulations of the Administration of Affairs Concerning Experimental Animals (Order No. 63 of the Jiangsu Academy of Agricultural Science on July 8, 2014). All experiments were conducted in accordance with ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Experimental Design
This study was conducted from November 2 to 17, 2020, at the Experimental Animal Center of the Jiangsu Academy of Agricultural Sciences (Nanjing, China). A total of 1,620 one-day-old Taizhou geese (Anser domestica, 100% ♀), supplied by the Tianzhijiao Breeding Geese Limited Company (Chuzhou, China), were randomly assigned to 6 experimental groups. Each treatment had 6 replicates, and each replicate contained 45 birds. A completely randomized 2 × 3 factorial design was used for the experiment. Six diets with 2 CP levels and three CF levels were prepared (Table 1). The 2 CP levels were 180 (18CP) and 220 (22CP) g/kg and the three CF levels were 30 (low CF), 50 (mid CF), and 70 (high CF) g/kg. All goslings were raised in a thermostatic house with stainless steel cages of identical size (1.20 × 1.00 × 0.50 m3, 9 goslings per cage, stocking density: 7.5 birds/m2), which were placed 0.50 m above the ground. The goslings were fed under standard management conditions with pellet feed and ad libitum water. The number of hanging feeders and nipple drinkers in each cage was sufficient throughout the study. Ambient temperature was maintained at 30°C from d 0 to 3, 29°C from d 4 to 6, 28°C from d 7 to 9, and 26°C from d 10 until the end of the experiment. Relative humidity throughout the 21-d experimental period was approximately 60%. The light sources (white light, 400–760 nm) were equalized to an illuminance of 15 ± 0.3 lux at the bird-head level, with a light schedule of 22 h of light from d 0 to 3, 18 h of light from d 4 to 14, and 16 h of light from d 15 to 21 on a 24-h cycle.
Table 1.
Composition of experimental diets.
| Item | 18CP |
22CP |
||||
|---|---|---|---|---|---|---|
| Low CF | Mid CF | High CF | Low CF | Mid CF | High CF | |
| Feed composition, % | ||||||
| Corn | 52.0 | 52.0 | 51.0 | 43.0 | 43.0 | 42.0 |
| Soybean meal | 25.0 | 22.0 | 14.5 | 25.0 | 22.0 | 14.50 |
| Corn gluten meal | - | 4.0 | 11.5 | 9.0 | 13.0 | 20.5 |
| Wheat bran | 7.0 | 4.0 | - | 7.0 | 4.0 | - |
| Rice bran | 7.0 | 4.0 | 3.0 | 7.0 | 4.0 | 3.0 |
| Rice hull powder | - | 5.0 | 11.0 | - | 5.0 | 11.0 |
| CaCO 3 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| CaHPO4 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| NaCl | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Premix1 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Fermented feed | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Nutritional ingredients2 | ||||||
| Poultry ME, kcal/kg | 2,629 | 2,652 | 2,629 | 2676 | 2,652 | 2,676 |
| CP, % | 18.06 | 18.21 | 18.17 | 22.41 | 22.29 | 22.55 |
| CF, % | 3.15 | 5.17 | 6.90 | 3.17 | 5.08 | 6.94 |
| Neutral detergent fiber, % | 9.24 | 11.16 | 14.36 | 9.56 | 11.49 | 14.21 |
| Acid detergent fiber, % | 5.03 | 5.24 | 5.28 | 5.10 | 5.20 | 5.86 |
| Lysine, % | 0.87 | 0.87 | 0.85 | 0.94 | 0.94 | 0.93 |
| Methionine, % | 0.30 | 0.31 | 0.32 | 0.41 | 0.42 | 0.42 |
| Calcium, % | 1.15 | 0.93 | 0.86 | 1.16 | 0.87 | 0.90 |
| Total Phosphorus, % | 0.60 | 0.50 | 0.46 | 0.59 | 0.56 | 0.53 |
| Available phosphorus, % | 0.30 | 0.29 | 0.29 | 0.30 | 0.30 | 0.29 |
Provided per kilogram of diet: Vitamin D, 1,000 IU; Vitamin A, 4,500 IU; Vitamin E, 30 IU; Vitamin K3, 1.3 mg; Vitamin B1, 2.2 mg; Vitamin B2, 10 mg; Vitamin B12, 1.013 mg; Vitamin B6, 4 mg; calcium, 7.5 mg; niacin, 20 mg; folic acid, 0.5 mg; biotin, 0.04 mg; Copper, 7.5 mg; Iron, 60 mg; Zinc, 65 mg; Manganese, 110 mg; Iodine, 1.1 mg; Selenium, 0.15 mg.
ME, metabolizable energy; CP, crude protein; CF, crude fiber. The Poultry ME, neutral detergent fiber, acid detergent fiber, Lysine, Methionine, and available phosphorus were calculated values; CP, CF, calcium, and phosphorus were analyzed values.
The basal diet was formulated to meet or exceed National Research Council (NRC, 1994) nutrient requirements for growing geese. The composition of experimental diets is presented (Table 1). Dietary samples (>1% of fresh feed) randomly acquired from 5 locations were mixed for the purpose of analysis. CP and CF were measured according to GB/T6432 (Chinese National Standard: Determination of crude protein in feed) and GB/T6434 (Chinese National Standard: Determination of crude fiber in feed).
The ADFI and the BW (live weight) of goslings were recorded using an electronic scale (YP60001, Hengji, Shanghai, China) before feeding during the test and ADG and feed conversion ratio (FCR) were calculated at the end of the experiment. In addition, the cumulative morbidity of gout of all replicates (each replicate contained 45 birds) among different treatments was summed after the experimental period. The selection standard for gout was defined as a gosling exhibiting gross kidney lesions and a serum uric acid (UA) concentration above the marginal value of urate supersaturation (male, 416 μmol/L and female, 357 μmol/L). The typical predominant gross lesions of the kidneys are pale, mottled, and swollen, and the renal tubules and ureters are distended with excess urates (Xi et al., 2020a).
Serum Metabolite Measurement
Thirty-six goslings (n = 6 goslings/treatment) were randomly chosen for immediate blood collection (5 mL/gosling), following decapitation, via blood collection tubes without anticoagulant on d 9 and 18, respectively. All blood samples were incubated at 37°C for 2 h after collection and centrifuged at 1,500 × g for 15 min. Obtained sera were stored in 0.6 mL Eppendorf tubes at −80°C until further analysis. Serum UA levels were determined using phosphotungstic acid colorimetry. Concentrations of creatinine (Cr) and urea nitrogen (UN), as well as xanthine oxidase (XOD) activity were determined via enzymatic colorimetry using a microplate spectrophotometer (Promega Corporation, Madison, WI). These kits were supplied by the Jiancheng Bioengineering Institute (Nanjing, China); the codes were C012 (UA), C011-2 (Cr), C013-2 (UN), and A002 (XOD). Serum IgM, IgA, and IgG concentrations in the experimental geese were measured using goose immunoglobulin ELISA kits purchased from Shanghai J&I Bio-Technology Co., Ltd (Shanghai, China). Serum circulating immune complexes (CIC), IL-1β, and TNF-α concentration were determined using a commercial goose ELISA kit (Jiancheng Bioengineering Institute, Nanjing, China). The activity of diamine oxidase (DAO) was measured via enzymatic colorimetry using an automatic biochemical analyzer (Hitachi, Tokyo, Japan). Serum LPS was measured using the traditional Limulus assay (Limulus Assay Biotechnology Company Ltd., Xiamen, China). The detection ranges of LPS in the Limulus assay ranged from 0.015 to 0.6 EU/mL. All materials used for collecting blood and measuring endotoxins were pyrogen-free. All assays were performed according to the instructions of the manufacturer. Serum samples were tested in triplicate. Intra- and interassay coefficients of variation for the assays were <10% and <15%, respectively.
Histomorphological Observation
The ceca (length = 1 cm) of 36 goslings (n = 6 goslings/treatment) were collected after blood sample collection on d 9 and 18 for histological analysis. Samples collected from goslings were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned (slice thickness: 3 μm; 4 slices per gosling). Pathological changes in the cecum (approximately 7 cm distal to the pyloric sphincter) were examined under a light microscope (OLYMPUS, Tokyo, Japan) after hematoxylin and eosin (HE) staining. Villus height and crypt depth of the cecum were measured using ImageJ software (version 1.8.0; National Institutes of Health, Bethesda, MD). Eight measurements of different intact villi per slice were recorded (8 measurements in 3 successive vision fields). Statistical analyses of histological measurements were performed based on an average of 32 measurements per gosling (4 slices per gosling and 8 measurements per slice). Goblet cell density was calculated as the goblet cell count divided by the corresponding villus length, which was then averaged and expressed as goblet cell number per 100 μm of villus length.
16S rRNA Sequencing of the Cecum Contents
The cecum contents of goslings (6 goslings/treatment × 6 groups × sampling twice = 72 goslings) were collected on d 9 and d 18 in 2 mL sterile, internally threaded cryogenic vials and immediately stored in liquid nitrogen for 16S rDNA analysis. DNA from cecum content samples was extracted using a MicroElute Genomic DNA Kit (D3096-01, Omega Biotek Inc., Norcross, GA) following the manufacturer's instructions. Sample blanks consisting of unused swabs were processed through DNA extraction, and they were checked to not produce 16S amplicon. Total DNA was eluted in 50 µL of elution buffer using a modified procedure described by the manufacturer (QIAGEN, Dusseldorf, Germany) and stored at −80°C.
Using the total DNA of samples as a template and 16S rDNA primers (343F - 5′- TACGGRAGGCAGCAG -3′; 798R - 5′- AGGGTATCTAATCCT-3′), we amplified the V3–V4 region of bacterial 16S rRNA. All reactions were carried out in a 25 µL (total volume) mixture containing 25 ng of genomic DNA extract, 12.5 µL of PCR Premix, 2.5 µL of each primer, and PCR-grade water to adjust the volume. PCR products were normalized using a AxyPrep Mag PCR Normalizer (Axygen Biosciences, Union City, CA), which enabled the quantification step to be skipped, regardless of the PCR volume submitted for sequencing. Amplicon pools were prepared for sequencing using AMPure XT beads (Beckman Coulter Genomics, Danvers, MA; performed by OebioTech Co., Ltd, Shanghai, China). The size and quantity of the amplicon library were assessed using LabChip GX (Perkin Elmer, Waltham, MA) and a Kapa Library Quantification Kit for Illumina (Kapa Biosciences, Woburn, MA), respectively. The PhiX Control library (v3) (Illumina) was combined with the amplicon library (expected at 30%). The library was clustered at a density of approximately 570 K/mm2. The libraries were sequenced on 300PE MiSeq runs, where one library was sequenced with both protocols using standard Illumina sequencing primers, thereby eliminating the need for a third (or fourth) index read. The reads were filtered using quantitative insights into microbial ecology (QIIME; http://qiime.org/tutorials/processing_illumina_data.html) quality filters. The CD-HIT pipeline was used to select operational taxonomic units (OTUs) by preparing an OTU table. Sequences with 97% similarity were assigned to OTUs. Representative sequences were chosen for each OTU, and taxonomic data were then assigned to each representative sequence using the Ribosomal Database Project (RDP) classifier. The GenBank accession number of these OTU nucleotide sequences is SAMN21014082. To estimate alpha diversity, the OTU table was rarified, and the following 4 metrics were calculated: the Chao1 metric to estimate richness; the observed species metric as a count of unique OTUs found in the sample; the Shannon index; and the Simpson index.
Real-Time PCR
Kidney cortex tissues and cecal tonsil tissues of 36 goslings (n = 6 goslings/treatment) were collected on d 9 and 18 for real-time PCR (RT-PCR) analysis. Total RNA was extracted from the tissues, using TRIzol reagent (Life Technologies, Grand Island, NY), and reverse transcribed using a Reverse Transcription Levels kit (TaKaRa, Dalian, China), according to the manufacturer's protocol. β-actin was used as an invariant control. Primers were designed using the Primer Premier 5.0 software and their sequences are listed (Table 2). RT-PCR was performed using SYBR Premix Ex Taq (Roche, Basel, Switzerland). All RT-PCRs were performed in triplicate. The relative expression levels of target genes were determined using the 2− ΔΔCt method.
Table 2.
Primer sequences for RT-PCR.
| Gene name1 | Forward primer (5′ to 3′) | Reversed primer (5′ to 3′) | Product length (bp) |
|---|---|---|---|
| β-actin | TGACGCAGATCATGTTTGAGA | GCAGAGCGTAGCCCTCATAG | 159 |
| TLR2A | CAGCACCTCCACATTCACG | CTTCCGGGCTCATACAGA | 199 |
| TLR4 | GGTGCCACATCCATACAAT | TAGGTCAGTCAGAGAGGATA | 173 |
| MyD88 | CCCTGGGGAAAGACTAAGAGC | AAGAAGGTGTCGGAGGATGGT | 100 |
| NFκB | GCCCAATGCCTCCAACTTAAA | ATATCATCTTTCTGAACCTTGTCAC | 108 |
| IL1β | ACTGGGCATCAAGGGCTA | GGTAGAAGATGAAGCGGGTC | 122 |
| TNFα | TGTGTATGTGCAGCAACCCGTAGT | GGCATTGCAATTTGGACAGAAGT | 229 |
Abbreviations: IL1β, interleukin-1β; MyD88,myeloid differentiation primary response gene 88; NFκB, nuclear factor-κB; TLR2/4, Toll-like receptor 2/4; TNFα, tumor necrosis factor-α.
Data Analysis
Experimental data derived from 6 replicates per treatment were analyzed using the IBM SPSS Statistics 16 program (IBM Corporation, Somers, NY). Each replicate was considered as an experimental unit and the statistical procedure used was a multivariate analysis of variance using the general linear model procedure (GLM). When significant differences were determined, treatment means were separated and compared using Duncan's multiple-range test. A two-tailed test was considered statistically significant at a probability level of less than 5% (P < 0.05).
RESULTS
The Effects of Dietary Protein and Fiber Levels on Growth Performance and Gout Occurrence in Goslings
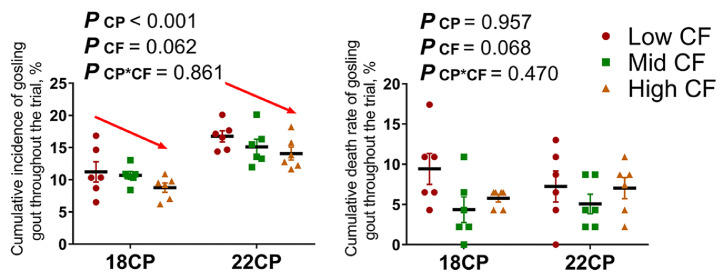
Gout emerged when the goslings were 7 to 9-day-old and peaked at 10 to 14 d, following which the mortality rate decreased gradually until the end of the third week. The morbidity of gosling gout in groups fed 22% CP diets was considerably higher than that in the 18CP groups throughout the experiment (P < 0.001), in addition to which gosling gout-related morbidity was observed to decrease slightly with increasing dietary CF levels (P = 0.062; Figure 1). The effects of dietary fiber on growth performance of goslings from 1 to 21 d of age are shown in Table 3. There was no significant difference among the groups in BW1, BW21, ADFI, and ADG from d 1 to 21. The goslings in the 22CP group had a lower FCR than those in the 18CP group (P < 0.05). Renal function parameters corresponding to the different CP and CF levels are shown (Table 4). On d 9, serum UA level in the 22CP group was higher than that in the 18CP group (P < 0.001), further to which it was observed to increase with increasing dietary CF levels (P < 0.001). In particular, goslings fed the 22% CP and 7% CF diets displayed dangerously high UA levels (483.28 μmol/L), which were above the upper limit for normal females (357 μmol/L). Similarly, serum concentrations of Cr (P = 0.041) and serum XOD activities (P < 0.001) were also higher in the 22CP group. No differences in the kidney function indices were observed among groups on d 18 (P > 0.05).
Figure 1.
The effects of dietary protein and fiber levels on gout occurrence in goslings. The cumulative incidence of gosling gout and related death rates throughout the trial; n = 6 replicates.
Table 3.
The effects of dietary protein and fiber levels on growth performance of goslings.
| Treatment |
BW1, g | BW21, g | ADFI, g | ADG, g | FCR | |
|---|---|---|---|---|---|---|
| Protein | Fiber | |||||
| 18CP | LowCF | 92.78 | 963.57 | 82.04 | 46.23 | 1.98 |
| MidCF | 93.40 | 1,030.22 | 81.63 | 48.81 | 1.83 | |
| HighCF | 92.61 | 992.45 | 81.82 | 48.17 | 1.91 | |
| 22CP | LowCF | 90.75 | 1,044.38 | 80.93 | 50.17 | 1.78 |
| MidCF | 92.14 | 980.57 | 79.92 | 46.29 | 1.89 | |
| HighCF | 95.36 | 1,020.41 | 80.62 | 48.99 | 1.83 | |
| SEM | 0.86 | 8.00 | 0.46 | 0.49 | 0.14 | |
| Mean effects1 | ||||||
| CP | 18CP | 92.93 | 995.54 | 81.83 | 42.98 | 1.91a |
| 22CP | 92.74 | 1,015.12 | 80.49 | 43.92 | 1.84b | |
| CF | LowCF | 91.75 | 1,003.98 | 81.48 | 43.44 | 1.88 |
| MidCF | 92.77 | 1,005.46 | 80.78 | 43.46 | 1.86 | |
| HighCF | 93.99 | 1,006.43 | 81.22 | 43.45 | 1.87 | |
| Significance (P-value) | ||||||
| CF | 0.603 | 0.989 | 0.833 | 0.996 | 0.846 | |
| CP | 0.920 | 0.164 | 0.177 | 0.158 | 0.016 | |
| CF × CP | 0.517 | 0.220 | 0.962 | 0.201 | 0.309 | |
Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; BW, body weight; FCR, feed conversion ratio.
Different lowercase letters on the shoulders indicate significant differences (P < 0.05), and different uppercase letters indicate extremely significant differences (P < 0.01).
Table 4.
The effects of dietary protein and fiber levels on renal function of goslings1.
| Treatment |
d 9 |
d 18 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | Fiber | UA, μmol/L | Cr, μmol/L | UN, mmol/L | XOD, U/L | UA, μmol/L | Cr, μmol/L | UN, mmol/L | XOD, U/L |
| 18CP | LowCF | 172.87 | 16.81 | 4.28 | 5.25 | 209.69 | 16.97 | 3.33 | 2.50 |
| MidCF | 229.64 | 22.00 | 5.28 | 7.83 | 149.09 | 17.99 | 4.01 | 1.81 | |
| HighCF | 321.70 | 26.55 | 4.91 | 7.55 | 210.16 | 21.40 | 2.08 | 2.14 | |
| 22CP | LowCF | 359.50 | 23.28 | 5.13 | 16.58 | 156.69 | 21.65 | 2.48 | 2.37 |
| MidCF | 370.85 | 30.57 | 5.29 | 17.1 | 226.11 | 25.43 | 2.47 | 2.75 | |
| HighCF | 483.28 | 40.03 | 4.50 | 18.79 | 157.02 | 20.17 | 2.39 | 3.80 | |
| SEM | 14.76 | 2.25 | 0.25 | 0.34 | 11.39 | 1.21 | 0.18 | 0.18 | |
| Mean effects2 | |||||||||
| CP | 18CP | 241.40a | 21.79a | 4.82 | 6.88a | 189.65 | 18.79 | 3.14 | 2.15 |
| 22CP | 404.54b | 31.29b | 4.97 | 17.49b | 179.94 | 22.42 | 2.45 | 2.97 | |
| CF | LowCF | 266.19a | 20.05 | 4.71 | 10.92 | 183.19 | 19.31 | 2.91 | 2.44 |
| MidCF | 300.25ab | 26.29 | 5.29 | 12.47 | 187.60 | 21.71 | 3.24 | 2.28 | |
| HighCF | 402.49b | 33.29 | 4.71 | 13.17 | 183.59 | 20.79 | 2.24 | 2.97 | |
| Significance (P-value) | |||||||||
| CF | <0.001 | 0.068 | 0.532 | 0.071 | 0.985 | 0.441 | 0.161 | 0.176 | |
| CP | <0.001 | 0.041 | 0.761 | <0.001 | 0.679 | 0.898 | 0.107 | 0.549 | |
| CF × CP | 0.938 | 0.975 | 0.574 | 0.179 | 0.056 | 0.081 | 0.213 | 0.920 | |
Abbreviations: Cr, creatinine; UA, uric acid; UN, urea nitrogen; XOD, xanthine oxidase; n = 6 replicates.
Different lowercase letters in each column indicate significant differences (P < 0.05), and different uppercase letters indicate extremely significant differences (P < 0.01).
The Effects of Dietary Protein and Fiber Levels on Intestinal Epithelial Morphology, Permeability, and Microbial Communities in Goslings
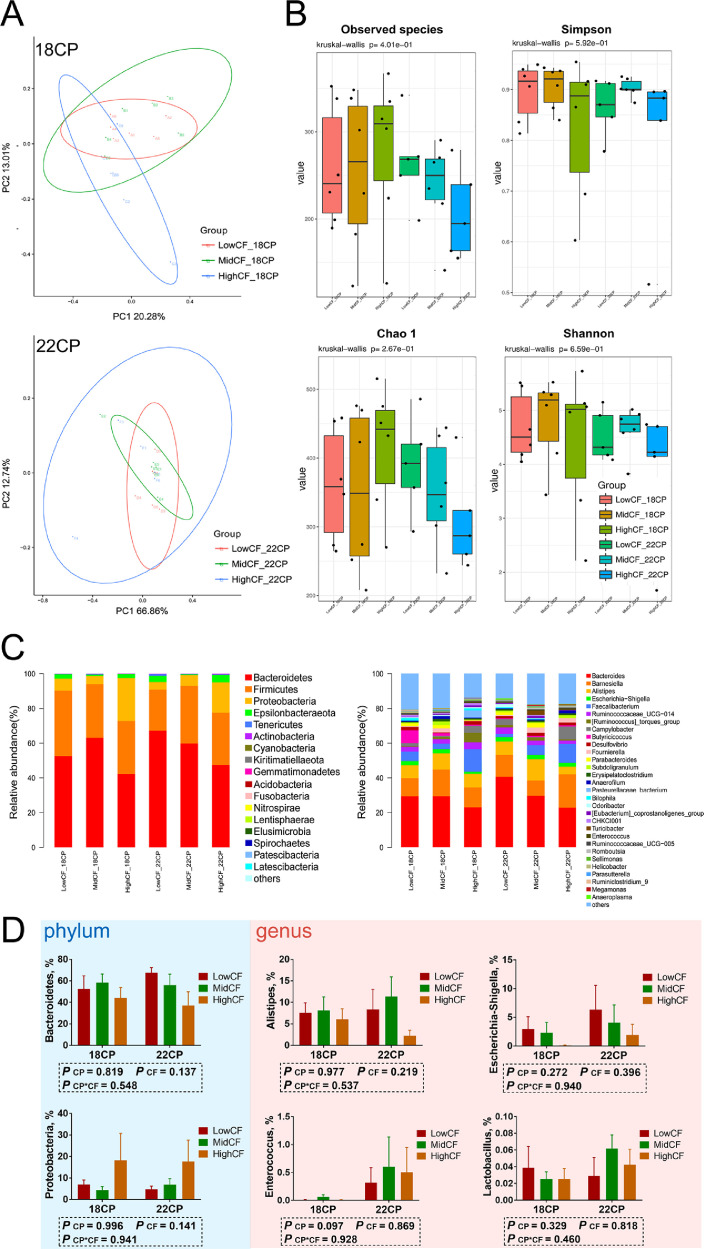
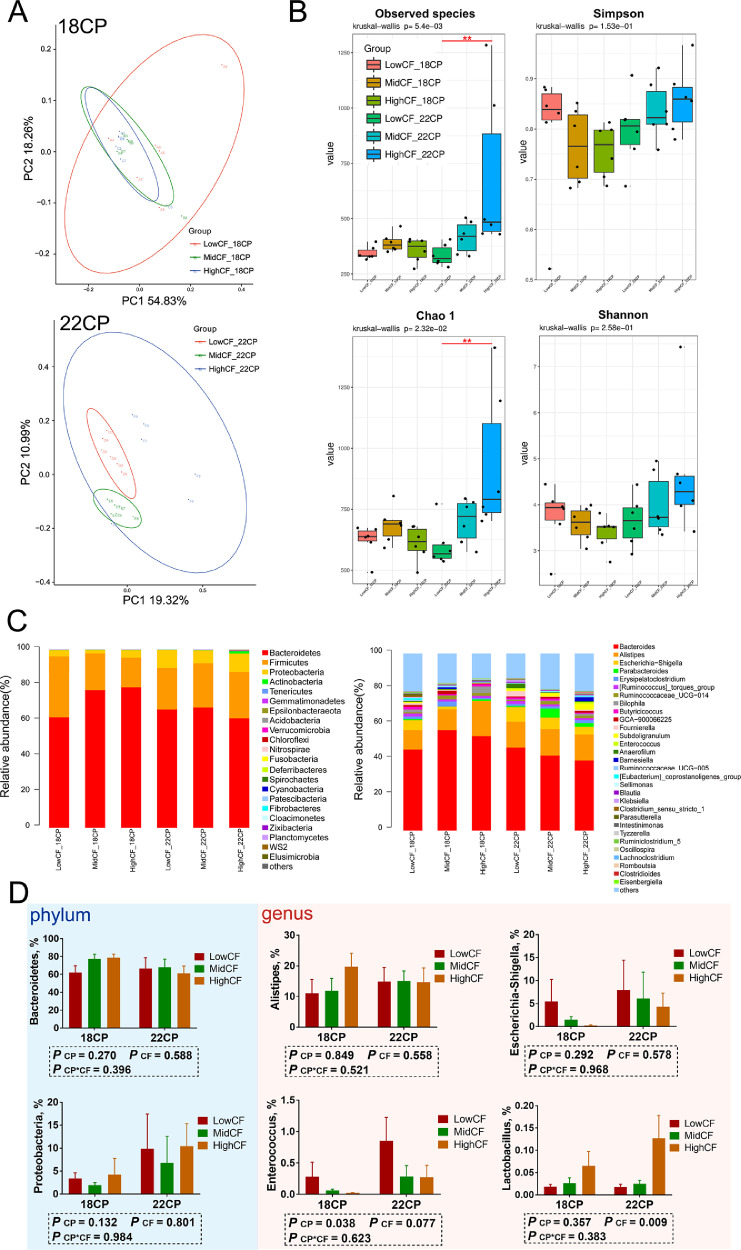
In the analysis of cecal microbiota by 16Sr RNA (V3–V4) gene sequencing, after quality control and chimera removal, 2,263,059 valid tags were retained, with an average of 31,431 tags per sample, and identified as of bacterial origin. These sequences were assigned to 2,459 OTUs of bacterial species based on a 97% similarity cut-off. A comparison of bacterial 16S rRNA datasets based on Principal Coordinate Analysis (PCoA) demonstrated that the microbiota of goslings corresponding to diets with different CF levels did not cluster separately from the others (Figures 2A and 3A). However, many subtle differences were observed among the microbial communities in goslings fed different dietary CP and CF levels. The key components of biodiversity, including species richness, evenness, and rarity, were measured by Shannon, Simpson, and Chao1 indices (Figures 2B and 3B). On d 9, the observed species and Chao1 indices for the high CF-22CP group were increased (P < 0.01) compared with those for the low CF-22CP group. The overall composition of the microbiota as well as average relative abundance at phylum and genus levels on d 9 and 18 are shown (Figures 2C and 3C, respectively). Among groups, the dominant phyla were Bacteroidetes, Firmicutes, and Proteobacteria, while the dominant genera were Bacteroides, Alistipes and Escherichia-Shigella, Ruminococcus, and an uncultured bacterium in Ruminococcaceae. On d 9, the abundance of Enterococcus in the low CF groups was increased (P < 0.05), further to which its average abundance in the 22CP groups was higher than in the 18CP groups (P = 0.077; Figure 2D). In addition, the average abundance of Escherichia-Shigella displayed a decreasing trend with increasing CF levels, whereas that of Lactobacillus showed an increase (P < 0.01). There were no significant differences between the average abundances of Alistipes, Escherichia-Shigella, and Lactobacillus in the groups on d 18 (Figure 3D). Only the average abundance of Enterococcus showed an increasing trend in the 22CP groups compared to the 18CP groups (P = 0.097).
Figure 2.
The effects of dietary protein and fiber levels on microbial communities in goslings on d 9 of age. (A) PCoA analysis; (B) alpha diversity indices (the observed species, Chao1, and Shannon indices); (C) the overall composition of the microbiota and average relative abundance in the groups at phylum and genus levels; (D) the average abundance of Bacteroidetes, Proteobacteria, Alistipes, Escherichia−Shigella, Enterococcus and Lactobacillus among groups. n = 6 replicates. Abbreviation: PCoA, principal coordinate analysis.
Figure 3.
The effects of dietary protein and fiber levels on microbial communities in goslings on d 18 of age. (A) PCoA analysis; (B) alpha diversity indices (the observed species, Chao1, and Shannon indices); (C) the overall composition of microbiota and average relative abundance in the experimental groups at phylum and genus levels; (D) the average abundance of bacteroidetes, proteobacteria, Alistipes, Escherichia−Shigella, Enterococcus and Lactobacillus among groups. n = 6 replicates. Abbreviation: PCoA, principal coordinate analysis.
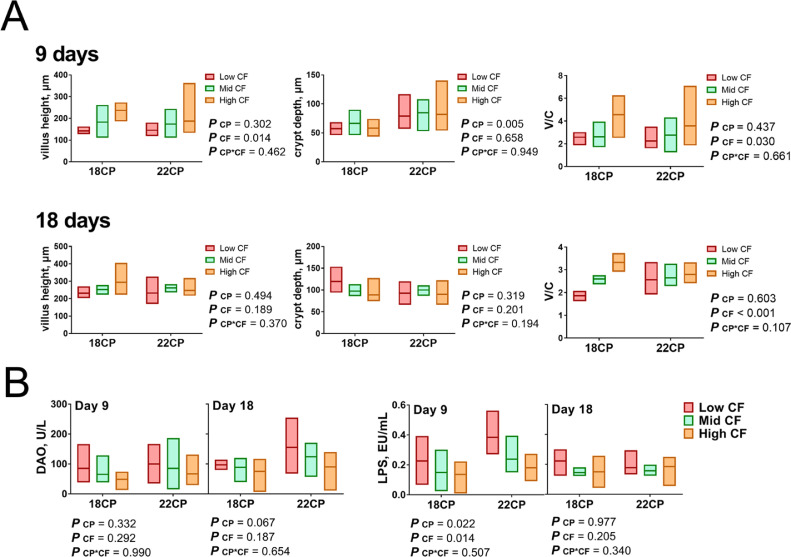
We also focused on morphological changes in the cecal tissues of goslings in different groups (Figure 4A). On d 9, the crypt depth of goslings fed high CP diets increased (P < 0.01), while no morphological differences were found among goslings with different CF levels (P > 0.05). The villus height and V/C ratio (villus height/crypt depth ratio) in goslings on d 9 increased with increasing CF levels (P < 0.05), but the CP level in the diets did not exert a significant effect on goslings in different groups (P > 0.05). On d 18, the V/C ratio of villi increased with increasing CF levels (P < 0.01). However, no differences in villus height and crypt depth on d 18 were observed among the experimental groups (P > 0.05). Moreover, DAO activities and serum LPS levels of groups were compared on d 9 and 18 (Figure 4B). DAO activity in the 22CP group was slightly higher than that in the 18CP group on d 9 (P = 0.067). On the same day, the serum LPS levels of goslings increased when the dietary CP reached 22% (P = 0.022) but decreased with increasing dietary CF levels (P = 0.014).
Figure 4.
The effects of dietary protein and fiber levels on intestinal epithelial morphology and permeability in goslings throughout the trial. (A) Comparison of villus height, crypt depth, and their ratio in the cecum among groups; (B) comparison of DAO activity and serum LPS levels among groups (n = 6 replicates). Abbreviation: DOA, diamine oxidase.
The Effects of Dietary Protein and Fiber Levels on Circulating Immune Function in Goslings
The effects of dietary protein and fiber levels on circulating immune function in goslings are shown (Table 5). Serum concentrations of IgA and IgG in the high CF groups were higher than those in the low CF groups on both d 9 and 18 (P < 0.05) or very close to P = 0.05. However, no differences were observed between the serum IgM concentrations of the experimental groups (P > 0.05). On d 9, goslings in the 22CP group had higher serum CIC concentrations than those in the 18CP group (P < 0.001), while no differences were found between groups on d 18 (P > 0.05). Compared with the 18CP groups, serum TNF-α and IL-1β concentrations in the 22CP groups were higher on d 9 (P < 0.05). No differences in serum inflammatory factors were found among the groups on d 18 (P > 0.05).
Table 5.
The effects of dietary protein and fiber levels on serum immunoglobulins, circulating immune complexes, and cytokines of goslings1.
| Treatment |
Serum immunoglobulins |
Immune complexes |
Serum cytokines |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d 9 |
d 18 |
d 9 | d 18 | d 9 |
d 18 |
||||||||
| Protein | Fiber | IgA, mg/mL | IgM, mg/mL | IgG, mg/mL | IgA, mg/mL | IgM, mg/mL | IgG, mg/mL | CIC, mU/L | CIC, mU/L | TNF-α, ng/L | IL-1β, ng/L | TNF-α, ng/L | IL-1β, ng/L |
| 18CP | LowCF | 1.38 | 1.01 | 3.84 | 1.59 | 1.46 | 5.69 | 565.75 | 702.82 | 111.25 | 94.76 | 121.48 | 205.32 |
| MidCF | 1.94 | 1.14 | 4.33 | 2.04 | 1.82 | 6.08 | 662.23 | 834.36 | 80.88 | 100.41 | 123.28 | 211.99 | |
| HighCF | 2.26 | 1.00 | 5.38 | 2.43 | 1.53 | 8.98 | 713.03 | 691.28 | 69.76 | 97.05 | 111.12 | 192.56 | |
| 22CP | LowCF | 1.73 | 1.13 | 4.37 | 0.97 | 1.77 | 6.28 | 809.62 | 814.10 | 104.21 | 117.86 | 122.12 | 193.94 |
| MidCF | 2.24 | 1.09 | 5.27 | 2.02 | 1.67 | 8.35 | 790.23 | 678.46 | 107.60 | 142.07 | 119.36 | 205.98 | |
| HighCF | 2.37 | 1.19 | 6.39 | 2.17 | 1.45 | 9.26 | 834.34 | 801.54 | 111.51 | 130.37 | 122.81 | 167.35 | |
| SEM | 0.42 | 0.30 | 0.36 | 0.49 | 0.05 | 0.41 | 17.64 | 21.54 | 3.74 | 5.82 | 3.26 | 5.74 | |
| Mean effects2 | |||||||||||||
| CP | 18CP | 1.86 | 1.05 | 4.52 | 2.02 | 1.60 | 6.92 | 647.00a | 742.82 | 87.30a | 97.41a | 118.63 | 203.29 |
| 22CP | 2.11 | 1.14 | 5.34 | 1.72 | 1.63 | 7.96 | 811.40b | 764.70 | 107.77b | 130.10b | 121.43 | 189.09 | |
| CF | LowCF | 1.56a | 1.07 | 4.11a | 1.28 | 1.62 | 5.99a | 687.69 | 758.46 | 107.73 | 106.31 | 121.80 | 199.63 |
| MidCF | 2.09ab | 1.12 | 4.80ab | 2.03 | 1.75 | 7.22b | 726.23 | 756.41 | 94.24 | 121.24 | 121.32 | 208.99 | |
| HighCF | 2.32b | 1.10 | 5.89b | 2.30 | 1.49 | 9.12c | 773.69 | 746.41 | 90.64 | 113.71 | 116.97 | 179.96 | |
| Significance (P-value) | |||||||||||||
| CF | 0.026 | 0.799 | 0.030 | 0.054 | 0.220 | 0.003 | 0.153 | 0.971 | 0.215 | 0.590 | 0.831 | 0.153 | |
| CP | 0.271 | 0.120 | 0.076 | 0.379 | 0.842 | 0.141 | <0.001 | 0.615 | 0.017 | 0.010 | 0.697 | 0.242 | |
| CF*CP | 0.901 | 0.216 | 0.821 | 0.774 | 0.235 | 0.473 | 0.324 | 0.124 | 0.058 | 0.813 | 0.660 | 0.800 | |
Abbreviations: CIC, circulating immune complexes; IgA, immunoglobulins A; IgM, immunoglobulins M; IgG, immunoglobulins G; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; n = 6 replicates.
Different lowercase letters in the columns indicate significant differences (P < 0.05), and different uppercase letters indicate extremely significant differences (P < 0.01).
The Effects of Dietary Protein and Fiber Levels on the Inflammatory Response of the Gut-Kidney Axis in Goslings
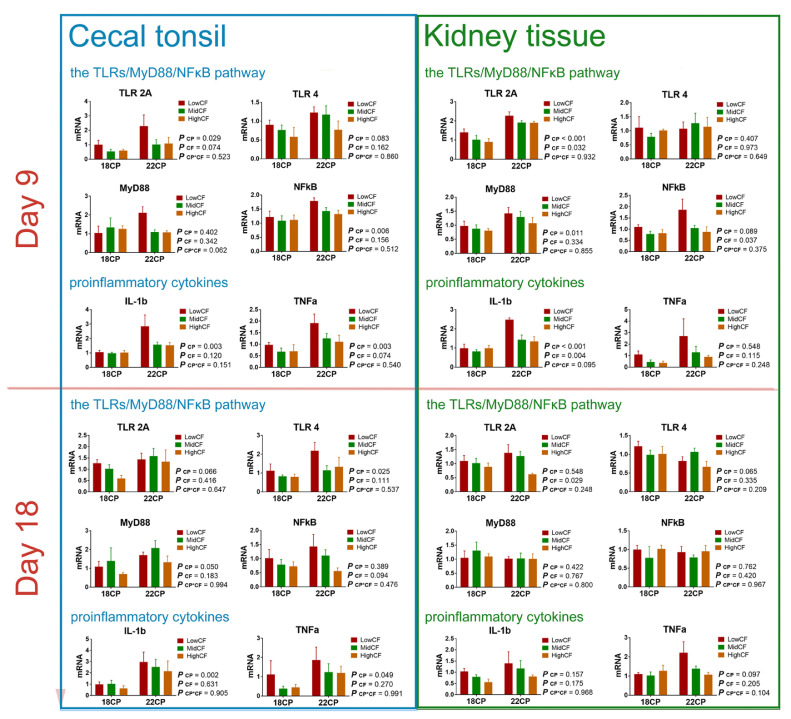
We also measured the mRNA levels of toll-like receptors (TLRs), the MyD88/NFκB inflammatory signaling pathway, and proinflammatory cytokines in the kidney tissues and cecal tonsil of goslings fed different diets (Figure 5). On d 9, the mRNA expression levels of TLR2A (P < 0.001), MyD88 (myeloid differentiation primary response gene 88; P = 0.011), NFκB (nuclear factor-κB) (P = 0.089), and IL1β (interleukin-1β) (P < 0.001) in the kidney tissue of the 22CP group was increased, compared to those in the 18CP group. The mRNA expression levels of TLR2A (P = 0.032), NFκB (P = 0.037), and IL1β (P = 0.004) in kidney tissue were downregulated with increasing CF levels. On d 18, the changes in dietary protein and fiber had relatively less impact on the mRNA expression of TLRs and the MyD88/NFκB pathway (P > 0.05). Only the mRNA expression of TLR2A in kidney tissue was downregulated with increasing dietary CF levels on d 18 (P < 0.05).
Figure 5.
The inflammatory response of the gut-kidney axis in goslings. The mRNA expression levels of proinflammatory cytokines and the TLRs/NFκB inflammatory signaling pathway in kidney tissue and cecal tonsil among groups on d 9 and 18. n = 6 replicates. Abbreviations: IL1β, interleukin-1β; MyD88, myeloid differentiation primary response gene 88; NFκB: Nuclear factor-κB; TLR2/4, toll-like receptor 2/4; TNFα, tumor necrosis factor-α.
In the cecal tonsil of goslings, on d 9, the mRNA expression levels of TLR2A (P < 0.05), TLR4 (P = 0.083), NF-κB (P < 0.01), IL1β (P < 0.01), and TNFα (P < 0.01) in the 22CP group were upregulated, compared to those in the 18CP group. The mRNA expression levels of TLR2A (P = 0.074) and TNFα (P = 0.074) in the cecal tonsil were slightly downregulated when the dietary CF levels increased. On d 18, the mRNA expression levels of TLR2A (P = 0.066), TLR4 (P < 0.05), MyD88 (P = 0.05), IL1β (P < 0.01), and TNFα (P < 0.05) in the cecal tonsils of goslings in the 22CP group were upregulated compared to those in the 18CP group. However, changes in dietary fiber had relatively less impact on the mRNA expression of TLRs and the MyD88/NFκB pathway on d 18 (P > 0.05).
DISCUSSION
In goose production, scientists are always focused on increasing the survival rate of newborn goslings; some reports have indicated that dietary fiber and protein levels exert significant effects on the development and disease resistance of geese (Li et al., 2017; Xi et al., 2020a). In this study, we analyzed the relationship between gout and the levels of dietary protein and fiber. We found that high CP or low CF diets predisposed goslings to gout. Gosling gout is, in most cases, closely associated with renal injury, wherein overall kidney function is assessed by measuring the serum concentrations of UA, Cr, UN, and XOD (Xi et al., 2019; Wu et al., 2020). The results of the present study indicate that serum concentrations of UA and Cr as well as serum XOD activity in goslings fed the 22% CP diet were increased on d 9 of age. These results concur with those of previous studies on chickens and geese (Guo et al., 2005; Xi et al., 2020a). It is known that high protein diets, which significantly increase UA production, induce kidney damage leading to gosling gout. Interestingly, although our high-CF diets also increased serum UA levels on d 9, no changes were observed in serum Cr concentrations or XOD activity. Categorization of young goslings as a high-risk group for hyperuricemia is attributed to a lack of urate oxidase, which oxidizes poorly soluble UA to water-soluble allantoin, resulting in the elevation of blood UA (Guo et al., 2005). However, it is noteworthy that kidney damage associated with hyperuricemia is not directly caused by UA, but by superoxide free radicals produced during the formation of UA by XOD (Lin et al., 2016). Thus, we contend that dietary fiber levels ranging from 3 to 7% may not influence kidney function, despite increased serum UA levels. In addition, kidney function indices among groups displayed no differences on d 18, which may possibly be attributed to the stepwise maturation of kidneys in goslings.
Regulating the nutritive value of poultry diets in a fitting manner may enhance gut tissue integrity, maintain the balance between microbial populations and low numbers of potentially pathogenic strains, support appropriate immune responses, and control inflammation, all of which are factors that affect disease resistance in young poultry (Jha and Mishra, 2021; Luo et al., 2021). In the present study, we used high-throughput sequencing of the V3–V4 region of the 16S rRNA genes to investigate the effects of dietary protein and fiber levels on cecal microbiota composition in goslings. The observed species index reflects the number of microbial species, while the Chao1 index reflects the abundance of microbiota. In general, a more diverse intestinal microbial community shows stronger homeostasis and resistance to pathogens (Li et al., 2018b). Our results showed that goslings in the high CF group fed 22% CP level diets had higher observed species and Chao1 values than those in the low CF group on d 9, indicating that increasing dietary CF levels may improve microbial diversity, and even disease resistance, in goslings fed a high CP diet (Wang et al., 2012). Moreover, the results of the present study, which indicated that Bacteroidetes, Firmicutes, and Proteobacteria were the main bacteria in the cecum of goslings, were substantiated by the results of Liu et al. (2018). A similar situation has been reported in chickens (Yang et al., 2017). Some previous data also suggest that adult geese (5–13 wk) fed on a high fiber diet may contain a higher abundance of Firmicutes and a decreased abundance of Bacteroides (Liu et al., 2018). In this regard, young birds differ considerably from adults and they have a very poor digestive system for fiber. In this study, changing dietary CF levels did not affect the average abundance of Firmicutes and Bacteroidetes, although the members of these 2 phyla are both capable of degrading fiber in a manner which benefits the host (Matsui et al., 2010). Hence, it is surmised that although changes in dietary CF only exert a limited effect on cellulose-decomposing microorganisms of young goslings, these changes may affect goslings by excluding potential pathogens from the gut (Baughn and Malamy, 2004). Interestingly, although Bacteroides is not a common pathogen, its proliferation appears to be closely related to gout occurrence in both humans and birds (Guo et al., 2005; Shao et al., 2017), and studies have shown that diets rich in proteins always favor the growth of Bacteroidetes (Wu et al., 2011). However, our results did not indicate that the average abundance of Bacteroidetes showed an increasing trend with increasing CP levels, possibly due to the use of fermented feed in gosling diets in this study, which may have alleviated the proliferation of Bacteroidetes caused by high-protein diets (Xi et al., 2020a). Proteobacteria were the third major group of bacteria in the gut microbiota of geese in this study. This phylum includes a wide variety of pathogens, such as Escherichia, Salmonella, Vibrio, Helicobacter, and many other notable genera (Rizzatti et al., 2017). Escherichia and Shigella are gram-negative, rod-shaped bacteria belonging to the family Enterobacteriaceae. Geese have a lower tolerance to Escherichia and Shigella infections. In this study, it is noteworthy that the average abundance of Escherichia-Shigella displayed a decreasing trend with increasing CF levels on both sampling days, indicating high-CF diets might have apparent inhibitory effect on harmful bacteria in goslings. Rice hulls were the main fibrous feed material used in our diets and the unique nanoporous silica layer on the surface of the rice hull powder enabled scanning electron microscopy to reveal colonization and strong attachment of bacterial cells to it, which may have led to the suppression of harmful intestinal bacteria (Nuñal et al., 2014). Additionally, previous data from our study revealed that goslings with gout usually suffer from gut dysbiosis associated with Escherichia-Shigella infection (Xi et al., 2019). Therefore, goslings fed high fiber diets may benefit from reduced Escherichia-Shigella infections and gout occurrence may be alleviated. Moreover, it was obvious that high-protein or low-fiber diets used in the present study may have facilitated increased Enterococcus proliferation, wherein the effect of high dietary protein continued until d 18. It has been proven that high CP diets always improve Enterococcus proliferation in the gosling gut (Xi et al., 2020a). However, a significant increase of Enterococcus in the low CF groups on d 9 was noted. Although some members of Enterococcus participate in fiber decomposition that does not explain the increase in the abundance of Enterococcus in goslings fed a low CF diet. Enterococcus is the natural flora emanating from the enteron of geese and is treated as an important indicator bacterium of the degree of imbalance in gut bacteria in gosling gout (Xi et al., 2019, 2020a). Thus, proliferation of Enterococcus may be associated with gut dysbiosis in goslings that ingest low fiber diets. In addition, we also found that the average abundance of Lactobacillus, a common probiotic, increased with increasing CF levels on d 9, suggesting that the level of dietary fiber, which benefits the gastrointestinal health of goslings, was also adequate to relieve the risk of gout (Li et al., 2014) and increase the survival of young goslings.
Dietary protein and fiber not only affect microbiota composition, but also influence intestinal epithelial morphology (Macfarlane and Macfarlane, 2011). The results of this study showed that appropriately increasing dietary fiber levels from 3 to 7% increased villus height as well as the V/C ratio, and improved the growth of gosling intestines, whereas increasing the dietary protein level from 18 to 22% increased crypt depth. Shortening of villi and the thickening of crypts may be caused by excessive apoptosis of villus cells due to gut dysbiosis (Li et al., 2018b). Interestingly, the prebiotic effect of appropriate fiber levels on intestinal epithelial morphology opposes that of high-protein levels. Previous studies have shown that an increase in dietary protein intake prompts an increase in the precursors of numerous bacterial metabolites, including ammonia, hydrogen sulfide, amines, indoles, phenols, and organic acids, which exert cytotoxic effects on intestinal epithelial cells in the distal intestinal tract (Lan et al., 2015). In contrast, the prebiotic effect of CF stimulates beneficial bacteria, such as Lactobacillus, that optimize gastrointestinal health by attaching to the intestinal mucosa and preventing the growth of pathogens in the distal part of the gut, thereby warding off gut infections (Jha et al., 2021). Thus, an appropriate nutritional program that balances the effects of dietary CF and protein levels may facilitate the development of the cecal lumen and wall in goslings.
Adult geese display highly efficient innate and acquired immune systems, whereas neonatal goslings exhibit transient susceptibility to infectious diseases during the first 10 d of life. Similar patterns have been observed in other poultry (Jha et al., 2021). Such short-term susceptibility in gosling may be closely associated with the occurrence of gout, caused by immune activation in the gut-kidney axis during the first week of life (Jin et al., 2018; Xi et al., 2020a). In the present study, we focused on the effects of dietary protein and fiber levels on immune activation in the gut-kidney axis. We measured immunoglobulin concentrations and found that serum IgA and IgG concentrations in the high CF groups were higher than those in the low CF groups on both d 9 and 18. In this study, we used rice hull powder (a by-product of rice milling) as the main fibrous feed material in the diet, which usually contains 35 to 45% CF, 74 to 87% neutral detergent fiber, 59 to 69% acid detergent fiber, 14 to 18% hemicellulose, 20 to 26% lignin, and other constituents (Li and Lei, 2019). These fibrous substances increase immune activity, possibly by acting as stressors themselves. The heterophil to lymphocyte ratio, a measure of stress, was reportedly increased in all birds fed a large amount of fibrous substances (Huff et al., 2011) Gao et al. (2008). reported that broiler chicks fed a corn-soybean based diet supplemented with 2.5 g/kg yeast cell wall exhibited higher serum lysozyme activity, better gut morphology, higher IgM concentrations and sIgA concentrations, compared with chicks in the control group. Macromolecular CICs consist of polymeric immunoglobulin, immunoglobulin-containing immune complexes, or both. CICs are found at a relatively high frequency in the sera of patients with kidney diseases (Van et al., 1988). The present study revealed that high-protein diets increased the concentrations of serum CICs in goslings on d 9, whereas dietary fiber levels had no effect. Previous studies have shown that high-protein diets are implicated in kidney injury and gut dysbiosis associated with the occurrence of gosling gout (Xi et al., 2020a), suggesting that immune activation in the circulatory system may act as a bridge between kidney injury and gut dysbiosis.
Additionally, we observed that the serum LPS concentration in goslings fed a high-protein diet was higher than that in goslings fed low-protein diets on d 9. LPS, from the gut microbiota, is specifically recognized by TLR4, a recognition receptor of the innate immune system. TLR4 activation upregulates the expression of pro-inflammatory cytokines, such as IL1β and TNFα, and also activates the TLR4/MyD88/NFκB signaling pathway in several epithelial and endothelial cell types (Desai et al., 2017). The results of the current study indicate that high-protein diets caused intestinal as well as renal inflammation in young goslings by activating the TLR4/MyD88/NFκB pathway, as evidenced by the upregulation of relevant mRNA expressions (TLR2A, TLR4, MyD88, NFκB, IL1β, and TNFα) in cecal tonsil and kidney tissues and increases in serum proinflammatory cytokine (TNF-α and IL-1β) concentrations. However, the effect exerted by dietary CF levels on immune activation in the gut-kidney axis is extremely complex. On one hand, the rational use of fibrous feed material in the diet may effectively inhibit the proliferation of harmful intestinal bacteria, and thereby decrease the inflammatory immune response to bacteria and their metabolites (Nuñal et al., 2014). In this study, we found that the serum LPS concentrations of goslings decreased with increasing dietary CF levels by d 9 of age. On the other hand, fibrous substances themselves act as stressors, increasing immune activity at both enteral and parenteral levels (Jha and Mishra, 2021). This may be viewed as a degree of counterbalance. Certainly, considering that excessive fiber levels in diets reduce digestibility and hinder the growth of poultry (Liu et al., 2018), goose farmers should strictly control the proportion of fiber in feed to a dietary CF level of approximately 5% in order to increase the survival rate of the goslings.
CONCLUSIONS
It is necessary to determine the inclusion levels of fiber and protein in geese diets to attain optimal survival rates and economic benefits. In this study, a diet of 18% CP paired with a 5% CF as an optimal combination in gosling feed was effective in preventing gosling gout. We found that the high CP diets exert a negative effect on gout occurrence, microbial communities, and immunoregulation in the gut-kidney axis of goslings, whereas appropriately increasing dietary fiber levels may help maintain intestinal balance, reduce serum LPS concentrations, and increase the survival rate.
ACKNOWLEDGMENTS
This study was supported by the National Science Foundation of China (grant no. 31902190), China Agriculture Research System of MOF and MARA (grant no. CARS-42-20) and Jiangsu Agriculture Science and Technology Innovation Fund (JASTIF) [grant no. CX(21)2016].
DISCLOSURES
The authors declare that they have no conflicts of interest.
REFERENCES
- Baughn A.D., Malamy M.H. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature. 2004;427:441–444. doi: 10.1038/nature02285. [DOI] [PubMed] [Google Scholar]
- De Cesare A., Faria do Valle Ì., Sala C., Sirri F., Astolfi A., Castellani G., Manfreda G. Effect of a low protein diet on chicken ceca microbiome and productive performances. Poult. Sci. 2019;98:3963–3976. doi: 10.3382/ps/pez132. [DOI] [PubMed] [Google Scholar]
- Desai J., Steiger S., Anders H.J. Molecular pathophysiology of gout. Trends Mol. Med. 2017;23:756–768. doi: 10.1016/j.molmed.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Gao J., Zhang H.J., Yu S.H., Wu S.G., Yoon I., Quigley J., Gao Y.P., Qi G.H. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 2008;87:1377–1384. doi: 10.3382/ps.2007-00418. [DOI] [PubMed] [Google Scholar]
- Gao P., Ma C., Sun Z., Wang L., Huang S., Su X., Xu J., Zhang H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome. 2017;5:91. doi: 10.1186/s40168-017-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Huang K., Tang J. Clinicopathology of gout in growing layers induced by high calcium and high protein diets. Br. Poult. Sci. 2005;46:641–646. doi: 10.1080/00071660500302661. [DOI] [PubMed] [Google Scholar]
- Huff G.R., Dutta V., Huff W.E., Rath N.C. Effects of dietary yeast extract on turkey stress response and heterophil oxidative burst activity. Br. Poult. Sci. 2011;52:446–455. doi: 10.1080/00071668.2011.600753. [DOI] [PubMed] [Google Scholar]
- Jha R., Mishra P. Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: a review. J. Anim. Sci. Biotechnol. 2021;12:51. doi: 10.1186/s40104-021-00576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M., Wang X., Ning K., Liu N., Zhang D. Genetic characterization of a new astrovirus in goslings suffering from gout. Arch. Virol. 2018;163:2865–2869. doi: 10.1007/s00705-018-3932-5. [DOI] [PubMed] [Google Scholar]
- Lan A., Andriamihaja M., Blouin J.M., Liu X., Descatoire V., Desclee de Maredsous C., Davila A.M., Walker F., Tome D., Blachier F. High-protein diet differently modifies intestinal goblet cell characteristics and mucosal cytokine expression in ileum and colon. J. Nutr. Biochem. 2015;26:91–98. doi: 10.1016/j.jnutbio.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Lee C.Y., Song A.A., Loh T.C., Abdul Rahim R. Effects of lysine and methionine in a low crude protein diet on the growth performance and gene expression of immunity genes in broilers. Poult. Sci. 2020;99:2916–2925. doi: 10.1016/j.psj.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.C., Lei Z.D. The functional fiber source in poultry diet: Rice husk meal and its application in the meat duck diet. Anim. Sci. Vet. Sci. 2019;6:20–22. In Chinese. [Google Scholar]
- Li M., Yang D., Mei L., Yuan L., Xie A., Yuan J. Screening and characterization of purine nucleoside degrading lactic acid bacteria isolated from Chinese sauerkraut and evaluation of the serum uric acid lowering effect in hyperuricemic rats. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.P., Wang Z.Y., Yang H.M., Xu L., Sheng D.F. Effects of dietary fiber on growth performance, slaughter performance, serum biochemical parameters, and nutrient utilization in geese. Poult. Sci. 2017;96:1250. doi: 10.3382/ps/pew385. [DOI] [PubMed] [Google Scholar]
- Li Y., Yang H., Lei X., Wang Z., Chen X. Effects of dietary fiber levels on cecal microbiota composition in geese. Asian-Australas. J. Anim. Sci. 2018;31:1285–1290. doi: 10.5713/ajas.17.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang H., Su W., Ying Z., Chen Y., Zhang L., Lu Z., Wang T. Effects of dietary Bacillus amyloliquefaciens supplementation on growth performance, intestinal morphology, inflammatory response, and microbiota of intra-uterine growth retarded weanling piglets. J. Anim. Scim Biotechnol. 2018;9:22. doi: 10.1186/s40104-018-0236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Chen Y., Huang Q., Guo X., Liu P., Liu W., Zhang C., Cao H., Hu G. Prokaryotic expression of the chicken xanthine oxidase (XOD) subunit and its localization in liver and kidney. Int. J. Biol. Macromo. 2016;87:341–347. doi: 10.1016/j.ijbiomac.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Liu G., Luo X., Zhao X., Zhang A., Jiang N., Yang L., Huang M., Xu L., Ding L., Li M., Guo Z., Li X., Sun J., Zhou J., Feng Y., He H., Wu H., Fu X., Meng H. Gut microbiota correlates with fiber and apparent nutrients digestion in goose. Poult. Sci. 2018;97:3899–3909. doi: 10.3382/ps/pey249. [DOI] [PubMed] [Google Scholar]
- Liu X., Blouin J.M., Santacruz A., Lan A., Andriamihaja M., Wilkanowicz S., Benetti P.H., Tomé D., Sanz Y., Blachier F., Davila A.M. High-protein diet modifies colonic microbiota and luminal environment but not colonocyte metabolism in the rat model: the increased luminal bulk connection. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307:G459–G470. doi: 10.1152/ajpgi.00400.2013. [DOI] [PubMed] [Google Scholar]
- Luo D., Yang N., Liu Z., Li T., Wang H., Ge M., Zhang R. Effects of astragalus polysaccharide on intestinal inflammatory damage in goslings infected with gosling plague. Br. Poult. Sci. 2021;62:353–360. doi: 10.1080/00071668.2020.1859094. [DOI] [PubMed] [Google Scholar]
- Macfarlane G.T., Macfarlane S. Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J. Clin. Gastroenterol. 2011;45:S120–S127. doi: 10.1097/MCG.0b013e31822fecfe. [DOI] [PubMed] [Google Scholar]
- Matsui H., Kato Y., Chikaraishi T., Moritani M., Ban-Tokuda T., Wakita M. Microbial diversity in ostrich ceca as revealed by 16S ribosomal RNA gene clone library and detection of novel Fibrobacter species. Anaerobe. 2010;16:83–93. doi: 10.1016/j.anaerobe.2009.07.005. [DOI] [PubMed] [Google Scholar]
- National Research Council . 9th rev. ed. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Nuñal S.N., Santander-DE Leon S.M., Bacolod E., Koyama J., Uno S., Hidaka M., Yoshikawa T., Maeda H. Bioremediation of heavily oil-polluted seawater by a bacterial consortium immobilized in cocopeat and rice hull powder. Biocontrol Sci. 2014;19:11–22. doi: 10.4265/bio.19.11. [DOI] [PubMed] [Google Scholar]
- Rizzatti G., Lopetuso L.R., Gibiino G., Binda C., Gasbarrini A. Proteobacteria: a common factor in human diseases. Biomed. Res. Int. 2017;2017 doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao T., Shao L., Li H., Xie Z., He Z., Wen C. Combined signature of the fecal microbiome and metabolome in patients with gout. Front. Microbiol. 2017;8:268. doi: 10.3389/fmicb.2017.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S.R., Wang Z.Y., Yang H.M., Zhang J. Effects of dietary metabolizable energy and crude protein levels on growth performance in 5-10 weeks goslings. Feed Industry (In Chinese). 2006;27:39–42. [Google Scholar]
- Summers J.D., Hurnik G., Leeson S. Carcass composition and protein utilization of embden geese fed varying levels of dietary protein supplemented with lysine and methionine. Can. J. Anim. Sci. 1986;67:159–164. [Google Scholar]
- Van Es L.A., van den Wall Bake A.W., Valentijn R.M., Daha M.R. Composition of IgA-containing circulating immune complexes in IgA nephropathy. Am. J. Kidney Dis. 1988;12:397–401. doi: 10.1016/s0272-6386(88)80033-7. [DOI] [PubMed] [Google Scholar]
- Wang Y., Sheng H.F., He Y., Wu J.Y., Jiang Y.X., Tam N.F., Zhou H.W. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 2012;78:8264–8271. doi: 10.1128/AEM.01821-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wils-Plotz E.L., Jenkins M.C., Dilger R.N. Modulation of the intestinal environment, innate immune response, and barrier function by dietary threonine and purified fiber during a coccidiosis challenge in broiler chicks. Poult. Sci. 2013;92:735–745. doi: 10.3382/ps.2012-02755. [DOI] [PubMed] [Google Scholar]
- Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A., Bewtra M., Knights D., Walters W.A., Knight R., Sinha R., Gilroy E., Gupta K., Baldassano R., Nessel L., Li H., Bushman F.D., Lewis J.D. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Xu R., Lv Y., Bao E. Goose astrovirus infection affects uric acid production and excretion in goslings. Poult. Sci. 2020;99:1967–1974. doi: 10.1016/j.psj.2019.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y.M., Huang Y.P., Li Y., Yan J.S., Shi Z.D. Fermented feed supplement relieves caecal microbiota dysbiosis and kidney injury caused by high-protein diet in the development of gosling gout. Animals (Basel). 2020;10:2139. doi: 10.3390/ani10112139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y.M., Yan J.S., Li M.Y., Ying S.J., Shi Z.D. Gut microbiota dysbiosis increases the risk of visceral gout in goslings through translocation of gut-derived lipopolysaccharide. Poult. Sci. 2019;98:5361–5373. doi: 10.3382/ps/pez357. [DOI] [PubMed] [Google Scholar]
- Xi Y.M., Ying S.J., Shao C.R., Zhu H.X., Yan J.S., Shi Z.D. Metabolomic profiling of goslings with visceral gout reveals a distinct metabolic signature. Br. Poult. Sci. 2020;61:258–265. doi: 10.1080/00071668.2020.1723790. [DOI] [PubMed] [Google Scholar]
- Yang L., Liu S., Ding J., Dai R., He C., Xu K., Honaker C.F., Zhang Y., Siegel P., Meng H. Gut microbiota co-microevolution with selection for host humoral immunity. Front. Microbiol. 2017;8:1243. doi: 10.3389/fmicb.2017.01243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Cao Y., Wang J., Fu G., Sun M., Zhang L., Meng L., Cui G., Huang Y., Hu X., Su J. Isolation and characterization of an astrovirus causing fatal visceral gout in domestic goslings. Emerg. Microbes Infect. 2018;7:71. doi: 10.1038/s41426-018-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]