Abstract
Coccidiosis is a devastating worldwide disease and is considered a dreadful disease in lovebirds. Indeed, a problem has been appeared cocktail lovebirds kept in a private pet birdhouse in Sheikh Zayed City, Giza, Egypt, in the shape of blood-tinged diarrhea, birds huddled together and showing signs of inappetence, ruffled feathers, unable to fly, general weakness and emaciation associated with high mortalities. Therefore, this study aimed to diagnose and find a suitable treatment to overcome such problems. To achieve this aim, blood and droppings samples were collected from infected and healthy birds for parasitological and hematological examinations, and tissue samples were collected from freshly dead birds for postmortem and histopathological examinations. A treatment trial was adopted on 50 infected birds and 25 healthy and parasitological negative birds and groups were classified as follows: group 1) 25 infected birds treated with Diclazuril, group 2) infected birds treated with Coccicure, and group 3) 25 birds kept as control negative reference birds. The parasitological identification revealed the presence of Eimeria aratinga (E. aratinga) oocysts in the infected bird intestine. Finally, we concluded that E. aratinga is a serious protozoon parasite infesting lovebirds revealing severe clinical signs, high mortalities, histopathological changes in the intestine and alteration in blood parameters. Diclazuril is an effective drug in treating E. aratinga in cocktail lovebirds.
Key words: cocktail lovebirds; blood parameters, diclazuril, Eimeria aratinga; histopathology; parasitology, sulfonamide
INTRODUCTION
The global pets trade has served as the primary means of introducing non-native bird species worldwide, allowing the emergence of uncommon diseases and their spread among different bird species in many countries (Abd El-Hack et al., 2018). The Cocktail lovebird is related to small parrots named “Agapornis”. These birds are common pets and brought individually or in couples. They are also used in business for pet shops or local breeders due to their high genetic or exotic value, which is profitable as these birds are bought at high prices. Moreover, these birds are reservoirs to many diseases, including parasitic infections that cause severe adverse effects on the bird, including inappetence, weight loss, low egg production, ruffled feathers, varying degrees of diarrhea, increased susceptibility to other infections, and even high mortalities (Adams et al., 2021; Platonova et al., 2021; Soliman et al., 2021). Parasitism is a devastating problem that results in severe economic losses (Salem and Attia, 2021). Birds can be infested by external and internal parasites (Attia et al., 2021; Salem et al., 2022). There are different species of endoparasites, including nematodes, trematodes, cestodes, acanthocephalans, and protozoa, that can parasitize lovebirds (Olsen and Orosz, 2000).
Coccidiosis is a major parasitic disease affecting lovebirds worldwide, causing diarrhea, poor growth, decreased feed utilization, and resulting in high mortalities, especially in young birds. Moreover, the birds become more susceptible to other pathogenic microorganisms (Abd El-Hack et al., 2022; El-Shall et al., 2022). Coccidiosis is an intracellular protozoan parasite affecting the intestinal tract of birds caused by different Eimeria species (spp.) and is considered a life-threatening problem in unhygienic cages. Infection with coccidia parasites seriously impairs growth and reveals severe economic losses (Yun et al., 2000). Eimeria aratinga (E. aratinga) is the main cause of coccidiosis in the Cocktail lovebirds, causing severe bloody diarrhea and weight loss associated with a high mortality rate, especially in young birds. On the other hand, the hygienic condition plays a vital role in spreading coccidiosis as direct contact with humans, captivity conditions, and the physical environment, for example, rainfall, humidity, and ambient temperature. In addition, the control programs of coccidiosis, depending on chemotherapy or vaccination, require great attention to hygienic measures (Chapman 2014; 2018). Many treatment strategies are abundant among avian species, including antibiotics, chemical preparations, and anthelmintics. Still, recently the world has been directed to find new safe and environmentally friend products as herbal extract (Abd El-Hack et al., 2022a), bioactive plant compounds (El-Saadony et al., 2021), natural pigments (Ashour et al., 2021), polyphenols (Ashour et al., 2020), organic acids (Abd El-Hack et al., 2022b), amino acids (Abou-Kassem et al., 2022; Alagawany et al., 2021a; Arif et al., 2022), probiotics, essential oils (El-Tarabily et al., 2021; Alagawany et al., 2021b; Abd El-Hack et al., 2022c), nanomedicine (Yousry et al., 2020; Salem et al., 2021), and vaccination strategies (El-Naggar et al., 2022) to control such infections, and enhance birds’ performance. Therefore, this study aimed to identify the possible cause of such problem with the demonstration of postmortem and histopathological alterations in the affected tissues as well as, check the blood parameters of the infected birds. Moreover, comparative chemotherapeutic control of coccidiosis caused by E. aratinga using Diclazuril and Coccicure.
MATERIALS AND METHODS
Sampling
Samples were collected from 150 cocktail birds kept in a private pet birdhouse at Sheikh Zayed City, Giza, Egypt. The birds were in cages, forming couples, or bigger cages according to their age from May to August 2021. During our investigation, freshly voided droppings samples were collected from various places of the trays of the cages and subjected to direct microscopic examination under a light microscope (Olympus, Japan).
Then freshly dead birds were exposed to postmortem examination and tissue samples were collected from the intestine and fixed in formalin saline 10% for further examinations. All viscera were examined for gross pathological changes and the mucus of the duodenum, jejunum, ileum, and the ceca were examined for the presence of Eimeria spp. (Mattiellio, 1990).
Treatment Trials Using Diclazuril and Coccicure
On the investigated flock, 50 birds showing clinical signs plus 25 healthy birds were divided into 3 groups (25 birds each) as follows; group 1 (G1) treated with Diclazuril 2.5% 0.3 mg per kg body weight per day in drinking water for 3 successive days. At the same time, group 2 (G2) was treated with Coccicure in which each gram contains sodium sulphaquinoxaline (150 mg), sodium sulphadimidine (70 mg), sodium sulphadiazine (70 mg), Vitamin K3 (2 mg). and Vitamin A (8,000 IU) in a dose of 0. 5 gm Coccicure / 1 liter of drinking water for 3 successive days. The third group (G3) was the negative healthy group used to analyze the reference value. All birds were observed for 2 wk post-treatment. Cages hygien have been adjusted during the observation period in all experimental groups.
Blood samples were collected from the wing vein of diseased birds on anticoagulant-coated test tubes to detect blood parameters. Oocyst counts were performed in 1 gm freshly voided dropping using McMaster technique before and after treatment at 3; 7; 9 and 12 d post-treatment.
Parasitological Examination
Wet smears of mucosal scrapings were prepared from intestinal and cecal tissues for microscopic examination of Eimeria spp., and identified according to the site of infection and oocysts morphology, including size, color, presence, or absence of micropyle and micropyle cap and time of sporulation using a light microscope (Olympus). Eimeria spp. oocysts were isolated from cecal and lower intestinal mucosa using saturated sodium chloride solution by floatation technique following the procedures (Permin and Hansen, 1998). Intestinal contents were collected from freshly dead birds and were performed in a 2.5% aqueous solution of potassium dichromate (K2Cr2O7) for sporulation of oocyst. A modification of McMaster's oocyst-counting technique was used (Soulsby, 1982).
Hematological Examination
Blood samples were collected twice from the wing vein of infected birds before and two weeks after treatments and then samples were transferred immediately into a sterile tube containing ethylenediaminetetraacetic acid (EDTA). The total red blood cells (TRBC) were performed in a 1:200 dilution of blood in Hayem's solution. The differential leukocyte counts were determined by preparing blood smears stained with Wright's stain. The hemoglobin (Hb) concentration and packed cell volume (PCV) were evaluated. Red blood indices, mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentrations (MCHC) were evaluated (Ashour et al., 2020).
Histopathological Examination
Representative tissue samples from the intestines were collected in 10% Neutral buffer formalin and then processed for paraffin embedding using alcohol as a drying agent and xylene as a clearing agent for histopathological evaluation. The sections were cut at a thickness of 4 to 5 mL and stained with Harris hematoxylin and eosin as described by Bancroft and Gamble (2008).
Statistical Analysis
The data were recorded as mean ± standard deviation, analyzed using SPSS Version 18.0 software (Inc., Chicago, IL). After studying the data normality, the blood parameters were compared using an independent t test. All data were considered significant when the P-value was less than 0.05.
RESULTS
Clinical Signs
The investigation of the infected birds during 2 wk from the onset of the clinical signs revealed that the morbidity rate, cumulative mortality and case-fatality were evaluated as 26.6% (40 of 150), 20% (30 of 150), and 75% (30 of 40), respectively.
The observed clinical manifestations include off-food, ruffled feathers, inability to fly, lethargy-sleepy appearance, dark brown, or blood-tinged droppings, as seen in Figure 1. The postmortem examination of freshly dead birds revealed; protrusion of keel bone with slight paleness of the breast muscle, liver congestion and severe intestinal congestion with variable degrees of hemorrhagic enteritis, as seen in Figure 2, Figure 3, Figure 4.
Figure 1.
A: Cocktail love bird showing signs of depression, ruffled feathers and unable to fly. (B) Freshly dead cocktail love bird 24 h after the onset of the clinical signs.
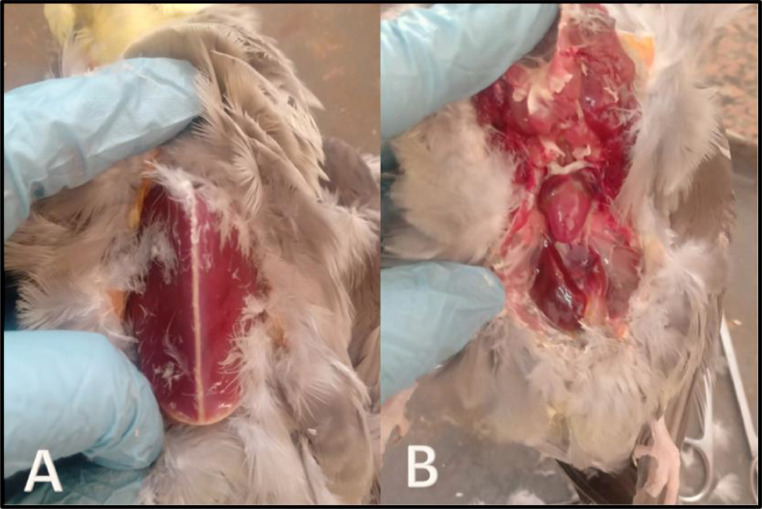
Figure 2.
(A) Postmortem examination of cocktail love bird showing protrusion of keel bone with slight paleness of the breast muscle. (B) Postmortem examination of cocktail love bird showing severe liver congestion.
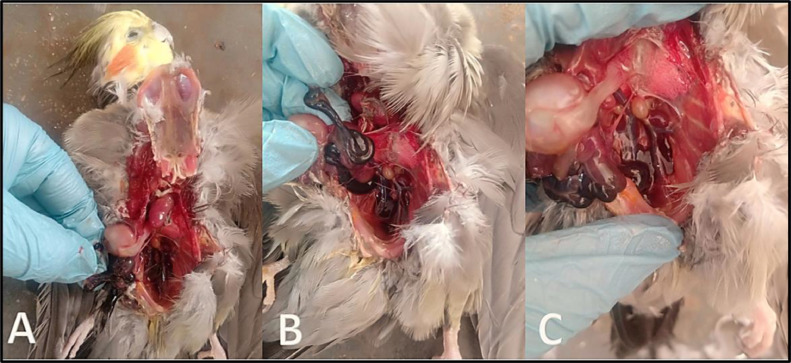
Figure 3.
(A–C) Postmortem examination of cocktail love bird showing hemorrhagic enteritis.
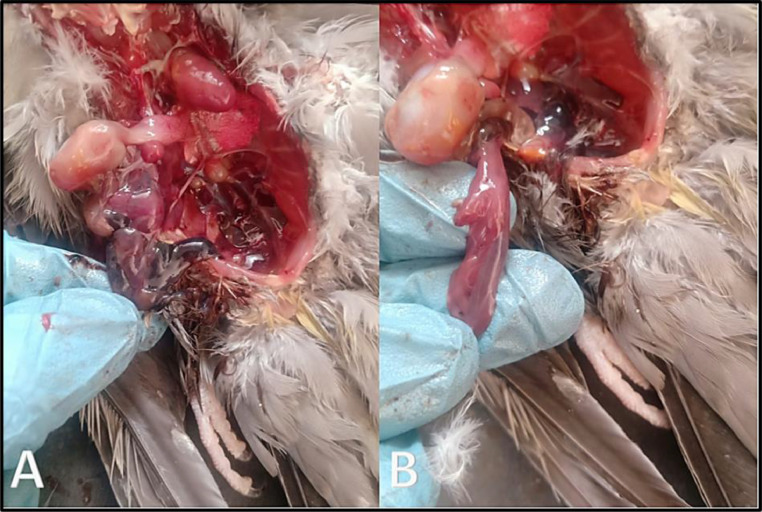
Figure 4.
(A, B) Postmortem examination of cocktail love bird showing severs intestinal congestion with variable degrees of hemorrhagic enteritis.
One week post-treatment, clinical signs and mortalities were stopped in group 1 and fecal examination revealed complete cessation of oocyst shedding while birds in group 2 continued in mortalities for 2 d post-treatment (2 birds of 5) 40%, clinical signs were improved, and fecal examination was negative for Eimeria oocyst.
Blood parameters
The hematological examination revealed that infected birds showed severe anemic pictures that appeared in the shape of a marked reduction in red blood cells (RBC) and PCV. At the same time, differential leukocyte counts resulted in lymphocytosis, monocytosis, eosinophilia, and heterophilia. While blood analyzes of recovered birds 2 wk post-treatments showed a significant improvement in blood parameters, as summarized in Table 1.
Table 1.
Blood parameters of birds before and after treatment.
| Items | Before treatment (Means ± E) | After treatment in G1 (Means ± SE) | After treatment in G2 (Means ± SE) | Reference G3 |
|---|---|---|---|---|
| RBCs (1 × 106 cells per microliter) | 1.8 ± 0.082 | 2 ± 0.082 | 1.9 ± 0.082 | 2.5–3.5 |
| PCV (%) | 21.226 ± 1.32 | 33.33 ± 3.091 | 28 ± 2.944 | 35–55 |
| MCV (fL) | 142 ± 6.683 | 92 ± 9.534 | 85 ± 4.922 | 90–140 |
| MCH (pg) | 57 ± 2.16 | 39.333 ± 3.3 | 37 ± 1.247 | 33–47 |
| MCHC (g%) | 34.667 ± 4.784 | 28.333 ± 1.247 | 27 ± 1.247 | 26–35 |
| Hemoglobin (g.dL−1) | 7.433 ± 1.034 | 9.333 ± 1.247 | 9.667 ± 1.247 | 7–13 |
| Heterophils (%) | 22.667 ± 2.055 | 6.333 ± 1.247 | 5.833 ± 0.624 | 0–1 |
| Eosinophils (%) | 8 ± 0.816 | 3.333 ± 1.247 | 2.9 ± 0.262 | 0.3 |
| Lymphocytes (%) | 62.032 ± 6.128 | 41.667 ± 8.498 | 41 ± 0.816 | 34 |
| Monocytes (%) | 4.567 ± 0.544 | 3.5 ± 0.408 | 4 ± 0.408 | 2.8 |
Data indicated as mean ± SE.
Abbreviations: MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentrations; MCV, mean corpuscular volume; PCV, packed cell volume.
Oocyst Count
On 3, 7, 9, and 12 d post-treatment, the mean oocyst count was significantly lower after both treatments (Coccicure and Diclazuril) with different used chemicals but with variation (P < 0.01) as presented in Table 2. The oocyst per gram was significantly lower in Diclazuril (P < 0.01) treated birds than in Coccicure treated group.
Table 2.
Oocyst count using McMaster technique before and after treatment.
| Items | Before treatment | 3 DPT | 7 DPT | 9 DPT | 12 DPT |
|---|---|---|---|---|---|
| G1 | 1255.5 ± 20.55 | 828.59 ± 19.78 | 655.89 ± 30.00 | 300.00 ± 10.94 | 159.33 ± 22.25 |
| G2 | 1359.6 ± 19.98 | 989.99 ± 15.67 | 758.35 ± 18.45 | 374.00 ± 10.65 | 182.56 ± 15.36 |
| G3 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
The negative control healthy group (G3) had no Eimeria oocysts over the observation period.
Identification of the Eimeria Species
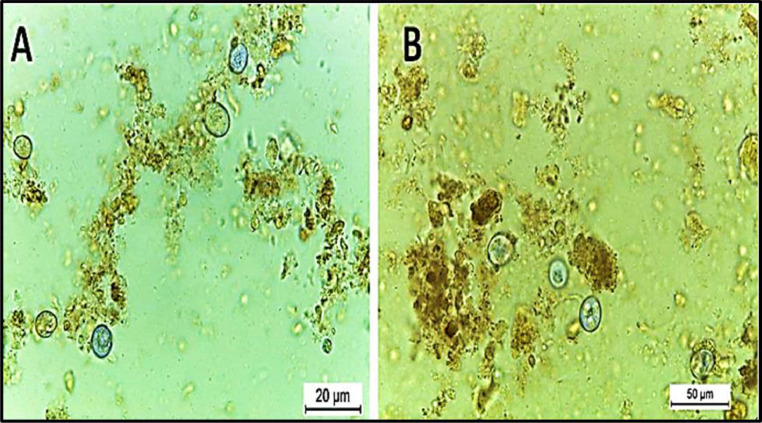
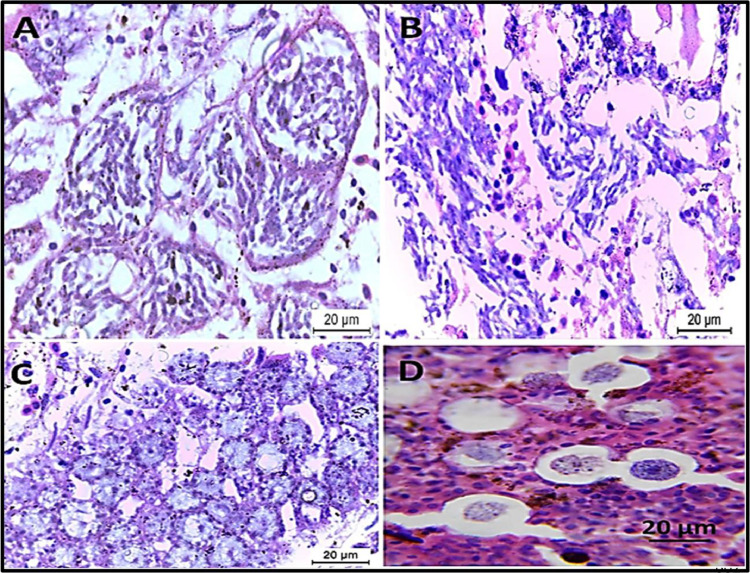
The present Eimeria spp. Belonging to E. aratinga, which had sporulated oocysts that were subspherical, with a smooth bi-layered oocyst wall. Oocysts measured 15.5- 25.2 (23.0 × 17.5 - 14.9 × 19.5 mm). The micropyle was present. Sporocysts were elongated to the oval while the sporocyst residuum was present. Each sporocyst contained 2 bananas-shaped sporozoites, as seen in Figure 5.
Figure 5.
Eimeria aratinga in Cocktail love birds’ feces; (A) un-sporulated oocyst; (B)sporulated oocyst of E. aratinga after sporulation with Potassium dichromate.
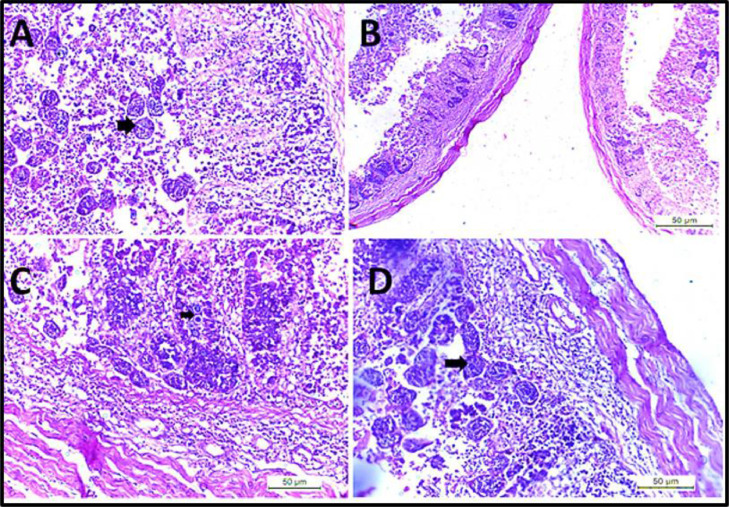
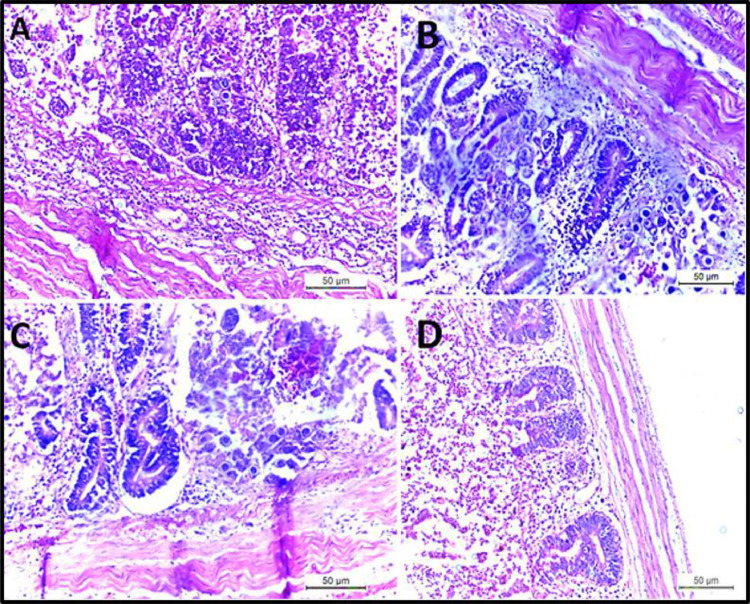
Histopathological Findings
The histopathological examination of the intestine revealed desquamation of enterocytes epithelial tissue, congestion of blood vessels indicating disruption followed by hemorrhage, severe muscular edema, necrosis of the submucosa, loss of villi, disruption of the intestinal mucosa, presence of different Eimeria spp., developmental stages as schizont; microgametocyte and macrogametocyte, marked hemorrhage with necrosis of the intestinal mucosa-associated with heterophils and mononuclear cells infiltrated in large numbers as seen in Figure 6, Figure 7, Figure 8.
Figure 6.
Eimeria aratinga infected intestine stained with H&E; (A, B) intestine of birds heavily infected with schizont; (C) macrogametes of Eimeria aratinga in the infected intestine that round with large eosinophilic plastic granules on the periphery. (D) microgametes of the Eimeria aratinga with multiple nuclei.
Figure 7.
(A) Intestine showing developing stages of Eimeria schizonts (arrow) in lamina propria of villi. (B) Intestine showing desquamation of enterocytes epithelium, mononuclear cell infiltration in muscularis mucosa. (C and D) Intestine showing loss of epithelial tissue, severe muscular edema, necrosis of submucosa, loss of villi, disruption of the intestinal mucosa, clusters of Eimeria different developmental stage (arrow) with mononuclear cells infiltrations.
Figure 8.
(A–C) Intestine showing the presence of different developing stages of Eimeria in lamina propria of villi with necrosis of submucosa and severe muscular edema. (D) Intestine showing desquamation of enterocytes epithelium with mononuclear cells infiltrations with muscular edema.
DISCUSSION
Birds are an integral part of virtually every ecosystem, and it is not surprising that they are commonly found in households and zoos worldwide (Attia and Salem, 2022; Cocumelli et al., 2021). Many serious pathogens can infect avian spp., including bacteria (Abd El Hamid et al., 2019; Marouf et al., 2020, Marouf et al., 2022), viruses (Setta et al., 2018), parasites, and fungi, as well as environmental and management stressors, revealing in severe economic losses (Abd El-Hack et al., 2021a,b; 2022b). However, there is much literature on avian medicine, including parasitic diseases (Olsen and Orosz, 2000). Due to an increased risk of exposure, parasites can lead to severe problems or even to death in birds recently brought into captivity, kept for prolonged periods in confined housings, and stressed by injuries, illnesses, or adaptation to new environments (Krone and Cooper, 2002; Salem et al., 2021a). It is important to identify and control parasite species capable of producing diseases in captive birds. There is a clear need for parasitological studies on avian species.
Several pieces of literature denoted the parasitic infection in pet birds and another investigation determines the coccidian intestinal parasites' high risk of infection in professional workers, for example, veterinarians' biologists, workers, tradesmen, and farm owners (Krone and Cooper, 2002). A first point to note is that our study confirms the findings of other studies that show coccidiosis is one of the most frequently reported diseases of birds worldwide (Cocumelli et al., 2021). This protozoan disease is responsible for significant worldwide economic losses to the poultry industry, with an estimated world annual loss of more than 3 billion USD (Dkhil, 2013).
Coccidiosis remains the most critical economically intestinal disease in the poultry industry. Control of coccidiosis has been focused on prophylaxis with anticoccidial drugs in food (Awaad et al., 2003). Resistance problem has been reported for all drugs currently used against coccidia infection in birds. Therefore, using drugs with unique modes of action is imperative as chemotherapy remains the principal means to control this disease (Meireles et al., 2003).
In this study, bloody diarrhea of all treated and infected groups was seen 4 to 6 d after infection with E. aratinga. But the extent of bloody diarrhea in the groups treated with Diclazuril was milder than that of other groups treated with Coccicure. Many oocysts were produced in all the infected groups. However, the oocysts count of birds treated with Diclazuril was significantly lower than the treated group with Coccicure active ingredient and the infected nontreated group.
Water-soluble Diclazuril induced significant (P ≤ 0.05) reduction in the mean oocyst shedding, dropping score, lesion score and mortality rate when compared with infected nonmedicated control positive group. Moreover, the efficacy of Diclazuril administration in drinking water was also proved by El-Banna et al. (2005), who investigated that Diclazuril in water or feed were of similar efficacy in the elimination of experimental infection with E. tenella, E. acervulina, E. necatrix, E. maxima, and E. Brunetti. These results agree with those reported by El-Dakhly et al. (2006), who noted that Diclazuril in the drinking water was appropriate for use in the prevention and treatment of Eimeria infection in chickens as indicated by decreased oocyst number and lesion score in the treated groups and stated that Diclazuril breaks down all intracellular developmental stages of asexual and sexual cycles of Eimeria spp.
Due to Diclazuril water solubility, short duration of treatment (2 d), small dosage, rapid anticoccidial action against all intracellular developmental stages of the coccidial infections in all poultry species make Diclazuril more effective in the prevention and control of coccidiosis (Amer et al., 2007). Moreover, several successful trials had been done to discuss the effect of using Diclazuril alone or in comparison with other anticoccidial drugs in the prevention of coccidial infection of birds (Kiaei et al., 2001; Conway et al., 2001a,b, 2002a,b; Awaad et al., 2003; Meireles et al., 2003) and the drug not only proved high efficacy but also superseded the others in the prevention of the disease El-Dakhly et al. (2006). and Amer et al. (2007) confirmed that the Diclazuril solution was important in stopping the cycle of coccidial development inside the medicated birds, mainly when applied on the first day of the blood appearance of birds’ droppings. The present study confirmed that adding a water-soluble formulation of Diclazuril in the drinking water is very efficacious for preventing and controlling the naturally infected lovebirds with E. aratinga.
On the other hand, sulfonamides (sulfadimethoxine and sulfaquinoxaline) have broad-spectrum activity against Gram-negative, Gram-positive bacteria and Eimeria spp. and they act on developing schizonts and sexual stages. It has been proved that the accidental human consumption of sulfonamide-contaminated products can cause central nervous system effects, gastrointestinal disturbances, and hypersensitivity reactions (Lebkowska-Wieruszewska and Kowalski, 2010). Sulfonamides are only used very rarely in US broiler production because of the high potential for residues, it harms the kidney, and it affects the egg production in layer chickens as well as, in Europe, sulfonamides are not approved for the prevention of coccidiosis in poultry (Meireles et al., 2003).
On the other hand, Diclazuril should be considered superior to any sulphonamide drugs in studies of the health impact of coccidiosis because it specifically reacts on the apicoplast. The apicoplast is an organelle of botanical origin, which is unique to the apicomplexan parasites. Treatment of coccidiosis by Diclazuril is thus devoid of any problems accompanying the use of broad-spectrum antibiotics, such as incidentally affecting some bacterial infections that the experimenters cannot monitor.
Moreover, the infection with E. aratinga causes macrocytic hypochromic anemia. These results agreed with (Youssef et al., 2015), who stated that Eimeria infection caused severe anemia due to severe hemorrhage caused by the second generation schizonts development. Furthermore, the total leucocytic count, heterophil, monocyte, and eosinophil showed a marked increase in the infected nontreated and treated groups. That accompanied the stress condition of infection then gradually changed to normal value (Youssef et al., 2008). Histopathological examination of the intestine revealed desquamation of enterocytes epithelial tissue, congestion of blood vessels indicating disruption followed by hemorrhage, severe muscular edema, necrosis of the submucosa, loss of villi, disruption of the intestinal mucosa, presence of different Eimeria developmental stages, marked hemorrhage with necrosis of the intestinal mucosa-associated with heterophils and mononuclear cells infiltrated in large numbers. Our findings concur with Marquardt et al. (2015), Fanatico (2006) and Sharma et al. (2015) as they record similar histopathological findings in bird's intestines infected with different Eimeria spp.
CONCLUSIONS
Reviewing the available literature, there are no previously reported cases of E. aratinga infection in cocktail lovebirds in any Egyptian study. So, this study is considered an endeavor to extend the understanding of the clincopathological picture of E. aratinga in cocktail lovebirds and shed light on the role of Diclazuril in its treatment and alleviation of the subsequent economic losses.
ACKNOWLEDGMENTS
We appreciate and thank Taif University for the financial support for Taif University Researchers Supporting Project (TURSP-2020/09), Taif University, Taif, Saudi Arabia.
Author contributions: H.M.S., M.A.S. and M.M.A. conceived, designed the experiments and carried out the methodology section. A.F.K. and M.T.E.-S. organized, interpreted and analyzed the data using the statistical program software. H.M.S. and M.A.S. validated the results. M.M.A. and A.F.K. wrote the original draft. M.M.S., S.A.A. and M.T.E.-S. wrote, reviewed and edited the final version of the manuscript. All authors have read and approved the final manuscript.
Ethics statement: The work was carried out according to the IACUC guidelines and was approved by the Ethical Committee, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt.
DISCLOSURES
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Abd El Hamid M.I., Abd El-Moaty D.A.M., El-Sergany E.F., Salem H.M., El-Sawy H., Abbas A.M. Utility of molecular biology tools for identification and characterization of Egyptian Riemerella anatipestifer duck isolates. Int. J. Vet. Sci. 2019;8:335–341. [Google Scholar]
- Abd El-Hack M.E., Alagawany M., Elrys A.S., Desoky E.S.M., Tolba H.M.N., Elnahal A.S.M., Elnesr S.S., Swelum A.A. Effect of forage moringa oleifera l. (moringa) on animal health and nutrition and its beneficial applications in soil, plants and water purification. Agriculture (Switzerland) 2018;8:145. [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., El-Shall N.A., Saad A.M., Salem H.M., El-Tahan A.M., Khafaga A.F., Taha A.E., AbuQamar S.F., El-Tarabily K.A. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives – a comprehensive review. Poult. Sci. 2022;101:101590. doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., Gado A.R., Nader M.M., Saad A.M., El-Tahan A.M., Taha A.E., Salem H.M., El-Tarabily K.A. Hot red pepper powder as a safe alternative to antibiotics in organic poultry feed: an updated overview. Poult. Sci. 2022;101:101684. doi: 10.1016/j.psj.2021.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Saad A.M., Salem H.M., Ashry N.M., Abo Ghanima M.M., Shukry M., Swelum A.A., Taha A.E., El-Tahan A.M., AbuQamar S.F., El Tarabily K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B.A., Taha A.E., Soliman S.M., Ahmed A.E., El-kott A.F., Al Syaad K.M., Swelum A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult. Sci. 2022;101:101696. doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S.…Abdel-Moneim A.M.E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021:1–10. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. Int. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abou-Kassem D.E., El-Abasy M.M., Al-Harbi M.S., Abol-Ela S., Salem H.M., El-Tahan A.M., El-Saadony M.T., Abd El-Hack M.E., Ashour E.A. Influences of total sulfur amino acids and photoperiod on growth, carcass traits, blood parameters, meat quality and cecal microbial load of broilers. Saudi J. Biol. Sci. 2022;29:1683–1693. doi: 10.1016/j.sjbs.2021.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D.R., Golnar A.J., Hamer S.A., Slotman M.A., Hamer G.L. Culex quinquefasciatus (Diptera: Culicidae) survivorship following the ingestion of bird blood infected with Haemoproteus sp. Parasites. Parasitol. Res. 2021;120:2343–2350. doi: 10.1007/s00436-021-07196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R., El-Naggar K., Taha A.E., Khafaga A.F., Madkour M., Salem H.M., Eltahan A., El-Saadony M.T., Abd El-Hack M. Betaine and related compounds: chemistry, metabolism, and role in mitigating heat stress in poultry. J. Therm. Biol. 2021;104:103168. doi: 10.1016/j.jtherbio.2021.103168. [DOI] [PubMed] [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer M., Abd El-Ghany W.A., Amer A.M., Hanafei A.E.A., Zohair G.A. The efficacy of Diclazuril (liquid formulation) in the prevention and control of coccidiosis in broiler chicken. BS. Vet. Med. J. 2007:96–101. November 2007, 5 th Scientific conference. [Google Scholar]
- Arif M., Baty R.S., Althubaiti E.H., Ijaz M.T., Fayyaz M., Shafi M.E., Albaqami N.M., Alagawany M., Abd El-Hack M.E., Taha A.E., Salem H.M., El-Tahan A.M., Elnesr S.S. The impact of betaine supplementation in quail diet on growth performance, blood chemistry, and carcass traits. Saudi J. Biol. Sci. 2022;29:1604–1610. doi: 10.1016/j.sjbs.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour E.A., Ab El-Hack M.E., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A.…El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., Tufarelli V., Mulla Z.S., El-Ghareeb W.R., El-Saadony M.T. Vol. 10. Agriculture; Switzerland: 2020. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers; pp. 1–19. [Google Scholar]
- Ashour E.A., Farsi R.M., Alaidaroos B.A., Abdel-Moneim A.M.E., El-Saadony M.T., Osman A.O., Abou Sayed-Ahmed E.T., Albaqami N.M., Shafi M.E., Taha A.E., Abd El-Hack M.E. Impacts of dietary supplementation of pyocyanin powder on growth performance, carcase traits, blood chemistry, meat quality and gut microbial activity of broilers. Ital. J. Anim. Sci. 2021;20:1357–1372. [Google Scholar]
- Attia M.M., Salem H.M. Morphological and molecular characterization of Pseudolynchia canariensis (Diptera: Hippoboscidae) infesting domestic pigeons. Int. J. Trop. Insect Sci. 2022;101 [Google Scholar]
- Attia M.M., Yehia N., Soliman M.M., Shukry M., El-Saadony M.T., Salem H.M. Evaluation of the antiparasitic activity of the chitosan-silver nanocomposites in the treatment of experimentally infested pigeons with Pseudolynchia canariensis. Saudi J. Biol. Sci. 2021;29:1644–1652. doi: 10.1016/j.sjbs.2021.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awaad M.H., Afify M.A., Zouelfakar S.A., Hilali M.A. Anticoccidial efficacy of steroidal sapogenins (organic coccidiostate) in broiler chickens (semi-field and field trials) Egypt. Vet. Med. Soci. Parasitol. J. 2003;1:123–136. [Google Scholar]
- Bancroft J.D., Gamble M., editors. Theory and Practice of Histological Techniques. Elsevier Health Sciences; China: 2008. [Google Scholar]
- Chapman H.D. Applied strategies for the control of coccidiosis in poultry. CAB Rev. 2018;13:1–11. [Google Scholar]
- Chapman H.D., Jeffers T.K. Vaccination of chickens against coccidiosis ameliorates drug resistance in commercial poultry production. Int. J. Parasitol. Drugs Drug Resist. 2014;4:214–217. doi: 10.1016/j.ijpddr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocumelli C., Iurescia M., Diaconu E.L., Galietta V., Raso C., Buccella C., Stravino F., Grande F., Fiorucci L., De Liberato C., Caprioli A., Battisti A. Plasmodium matutinum causing avian malaria in lovebirds (Agapornis roseicollis) hosted in an Italian zoo. Microorganisms. 2021;9:1356. doi: 10.3390/microorganisms9071356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway D.P., Mathis G.F., Johnson J., Baldwin C. The use of Diclazuril in extended withdrawal anticoccidial programmes: 1. Efficacy against Eimeria species in broiler chickens in floor pens. Poult. Sci. 2001;81:349–352. doi: 10.1093/ps/81.3.349. [DOI] [PubMed] [Google Scholar]
- Conway D.P., Mathis G.F., Johnson J., Schwartz M., Baldwin C. Efficacy of Diclazuril in comparison with chemical ionophorous anticoccidials against Eimeria spp. in broiler chickens in floor pens. Poult. Sci. 2001;80:426–430. doi: 10.1093/ps/80.4.426. [DOI] [PubMed] [Google Scholar]
- Conway D.P., Mathis G.F., Lang M. The use of Diclazuril in extended withdrawal anticoccidial programmes: 1. Efficacy against Eimeria spp. in broiler chickens in floor pens. Poult. Sci. 2002;81:349–352. doi: 10.1093/ps/81.3.349. [DOI] [PubMed] [Google Scholar]
- Conway D.P., Mathis G.F., Lang M. The use of Diclazuril in extended withdrawal anticoccidial programmes: 2. Immunity to Eimeria tenella challenge after drug withdrawal. Poult. Sci. 2002;81:353–355. doi: 10.1093/ps/81.3.353. [DOI] [PubMed] [Google Scholar]
- Dkhil M.A.( Anti-coccidial, anthelmintic and antioxidant activities of pomegranate (Punica granatum) peel extract. Parasitol. Res. 2013;112:2639–2646. doi: 10.1007/s00436-013-3430-3. [DOI] [PubMed] [Google Scholar]
- El-Banna H.A., El-Bahy M.M., M M., El-Zorba H.Y., ElHady M. Anticoccidial efficacy of drinking water soluble diclazuril on experimental and field coccidiosis in broiler chickens. J. Vet. Med. (series A) 2005;52:287–291. doi: 10.1111/j.1439-0442.2005.00727.x. BS. VET. MED. 5 TH scientific conference, P. 96-101. [DOI] [PubMed] [Google Scholar]
- El-Dakhly K.M., Azza A., El-Sawah A.A.Shalaby, El-Nesr K.A. The efficacy of Lactobacillus acidophilus and/or Diclazuril for inhibition and control of Eimeria tenella infection in balady chicks. Kafr El-Sheikh Vet. Med. J. 2006;4:1–18. [Google Scholar]
- El-Naggar M.S., Ibrahim H.M., Salem H.M., Marouf S. A novel locally prepared inactivated bivalent mycoplasma vaccine for chicken flocks in Egypt. Adv. Anim. Vet. Sci. 2022;10:55–61. [Google Scholar]
- El-Saadony M.T., Zabermawi N.M., Burollus M.A., Shafi M.E., Alagawany M., Yehia N., Askar A.M., Alsafy S.A., Noreldin A.E., Khafaga A.F., Dhama K., Elnesr S.S., Elwan H.A.M., Di Cerbo A., El-Tarabily K.A., Abd El-Hack M.E. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev. Int. 2021;37:1–23. [Google Scholar]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F.…Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28:5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanatico, A. (2006). Parasite management for natural and organic poultry Coccidiosis. Accessed Sept. 2021. http://attra.ncat.Org/attar-pub/PDF/coccidiosis.pdf.www.Saxonet.De/coccido2.htm.
- Kiaei M.M., Rahbari S., Modirsanei M., Rahimi R., Aref-Pazhouhi P. The effect of Artemisia sieberi and a chemical anticoccidial drug on control of coccidiosis and broiler chicks performance. J. Fac. Vet. Med., Univ. Tehran. 2001;56:53–57. [Google Scholar]
- Krone, O., and Cooper, J. E. (2002). Pages 105–120 in Parasitic diseases in Birds of Prey: Health and Diseases (3rd ed) J. E. Cooper, Ed., Oxford, UK: Blackwell Science,
- Lebkowska-Wieruszewska B.I., Kowalski C.J. Sulfachlorpyrazine residues depletion in turkey edible tissues. J Vet Pharmacol Ther. 2010;33:389–395. doi: 10.1111/j.1365-2885.2009.01147.x. [DOI] [PubMed] [Google Scholar]
- Marouf S., Khalf M.A., Alorabi M., El-Shehawi A.M., El-Tahan A.M., Abd El-Hack M.E., El-Saadony M.T., Salem H.M. Mycoplasma gallisepticum: a devastating organism for the poultry industry in Egypt. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marouf S., Moussa I.M., Salem H., Sedeik M., Elbestawy A., Hemeg H.A., Dawoud A. S. Mubarakb T.M., Mahmouda H., Alsubki R.A., Bahkali Ali H. A picture of Mycoplasma gallisepticum and Mycoplasma synoviae in poultry in Egypt: phenotypic and genotypic characterization. J. King Saud Univ. Sci. 2020;32:2263–2268. [Google Scholar]
- Marquardt, C. W., S. R. Demaree, and B. R. Grieve. 2015. Page 152 in Parasitology and vector biology. 2nd ed: San Diego, London, Boston, New York, Tokyo, Tornto. Clinicopathological Studies on the Effect of Artemisia cina (Sheih Baladi) on Coccidiosis in Chickens Egypt. J. Vet. Sci. 45-46:11-24.
- Mattiello R. Detect subclinical coccidiosis. Misset’s World Poult. Misset Oct./Nov. 1990;1:82–83. [Google Scholar]
- Meireles M.V., Kavavata G.M., Almeida S.M., Hisano M. Santos Evaluation of resistance to anticoccidial drugs in field isolates of Eimeria oocysts from broiler farms. Rev. Bras. Cienc. 2003;10:72–77. [Google Scholar]
- Olsen G.H., Orosz S.E. Mosby, Inc.; St. Louis, MO: 2000. Manual of Avian Medicine. [Google Scholar]
- Permin A., Hansen J.W. FAO Animal Health Manuals; Rome, Italy: 1998. Page 155 in The Epidemiology, Diagnosis and Control of Poultry Parasites. [Google Scholar]
- Platonova E., Aželytė J., Iezhova T., Ilgūnas M., Mukhin A., Palinauskas V. Experimental study of newly described avian malaria parasite Plasmodium (Novyella) collidatum n. sp., genetic lineage pFANTAIL01 obtained from South Asian migrant bird. Malar. J. 2021;20:82. doi: 10.1186/s12936-021-03588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Attia M.M. Accidental intestinal myiasis caused by Musca domestica L. (Diptera: Muscidae) larvae in broiler chickens: a field study. Int. J. Trop. Insect Sci. 2021;41:2549–2554. [Google Scholar]
- Salem H.M., Ismael E., Shaalan M. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomed. 2021;16:6783–6796. doi: 10.2147/IJN.S319708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Khattab M.S., Yehia N., Abd El-Hack M.E., El-Saadony M.T., Alhimaidi A.R., Swelum A.A., Attia M.M. Morphological and molecular characterization of Ascaridia columbae in the domestic pigeon (Columba livia domestica) and the assessment of its immunological responses. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Yehia N., Al-Otaibi S., El-Shehawi A.M., Elrys A.A.M.E., El-Saadony M.T., Attia M.M. The prevalence and intensity of external parasites in domestic pigeons (Columba livia domestica) in Egypt with special reference to the role of deltamethrin as insecticidal agent. Saudi J. Biol. Sci. 2021;29:1825–1831. doi: 10.1016/j.sjbs.2021.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setta A., Salem H.M., Elhady M., El-Hussieny A., Arafa A.A. Molecular and genetic characterization of infectious bronchitis viruses isolated from commercial chicken flocks in Egypt between 2014 and 2016. J. World Poult. Res. 2018;8:01–08. [Google Scholar]
- Sharma S., Azmi Sh., Iqbal A., Nasirudullah N., Mushtaq I. Pathomorphological alterations associated with chicken coccidiosis in Jammu division of India. J. Parasit. Dis. 2015;39:147–151. doi: 10.1007/s12639-013-0302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman S.M., Attia M.M., Al-Harbi M.S., Saad A.M., El-Saadony M.T., Salem H.M. Low host specificity of Hippobosca equina infestation in different domestic animals and pigeon. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulsby E.J.L. Academic press; London, UK: 1982. Pages 630–639 in Helminths, Arthropods and Protozoa Of Domesticated Animals. [Google Scholar]
- Yousry C., Zikry P.M., Salem H.M., Basalious E.B., El-Gazayerly O.N. Integrated nano vesicular/selfnanoemulsifying system (INV/SNES) for enhanced dual ocular drug delivery: statistical optimization, in vitro and in vivo evaluation. Drug Deliv. Transl. Res. 2020;1:801–814. doi: 10.1007/s13346-020-00716-5. [DOI] [PubMed] [Google Scholar]
- Youssef F.M., Abd El-Hamid H.A., El Sheshtawy E.A. Clinicopathological studies on the effect of Artemisia cina (Sheih Baladi) on coccidiosis in chickens. Egyptian J Vet Sci. 2015;46:11–24. [Google Scholar]
- Youssef F.M., Mansour D.H., Ramadan M.T., Dessouki A.A. Pages 197–208 in 8th Scientific Conference of the Egyptian Veterinary Poultry Association. 2008. Comparative clinicopathological studies on the effect of some herbal plants as anticoccidial agents in broiler chickens. [Google Scholar]
- Yun C.H., Lillehoj H.S., Lillehoj E.P. Intestinal immune responses to coccidiosis. Dev. Comp. Immunol. 2000;24:303–332. doi: 10.1016/s0145-305x(99)00080-4. [DOI] [PubMed] [Google Scholar]