Abstract
Introduction
Tracheal neoplasms account for less than 0.1% of all malignancies. The majority of tracheal malignancies are secondary neoplasms from direct tracheal invasion from adjacent structures and less commonly from hematogenous or lymphatic spread from distal malignancies. Evidence-based guidelines for management are lacking. Less invasive bronchoscopic ablation modalities are an option for non-operable patients. We report a case of endotracheal oligometastatic adenocarcinoma successfully treated with photodynamic therapy.
Case
A 59-year-old man with past medical history of stage 1 right upper and left upper lobe neoplasm treated with right lobectomy 2 years ago and wedge resection of the lingula ten months ago presented with cough and hemoptysis. Flexible bronchoscopy revealed a 10 mm endoluminal exophytic lesion of the mid-trachea confirmed as lung adenocarcinoma on biopsy. Considering the lack of extra-cartilaginous spread and the small size, a multidisciplinary team recommended local treatment using photodynamic therapy. Surveillance biopsies out to 2 years confirmed lack of disease recurrence. The patient did not experience any adverse effects.
Conclusion
PDT as first line therapy for a 10 mm oligometastatic endotracheal adenocarcinoma from recurrent pulmonary malignancy with no extra-cartilaginous spread is an effective modality that is well tolerated and produces a durable response, which in our patient led to complete response without recurrence at 24 months and with no adverse effects or complications. Determining depth of tumor penetration is paramount if the treatment is with curative intent. PDT should be considered as part of a multidisciplinary approach for endotracheal malignant disease.
Keywords: Tracheobronchial malignancies, Photodynamic therapy, Bronchoscopic ablation, Tracheal adenocarcinoma
Abbreviations
- CR
complete response
- CT
computed tomography
- EBUS
endobronchial ultrasound
- HRCT
high resolution computed tomography
- PDT
photodynamic therapy
- PET
positron emission tomography
- RML
right middle lobe
1. Introduction
Tracheal neoplasms account for less than 0.1% of all malignancies [1]. Secondary tracheal neoplasms are more common than primary tracheal tumors [1] and are usually from direct tracheal invasion from primary malignancies of adjacent structures such as the thyroid gland, lungs, esophagus, head and neck, or larynx [2,[7], [8], [9]]. Although less common, hematogenous or lymphatic spread from non-pulmonary malignancies such as breast, prostate, ovary, colorectal, renal, soft tissue (sarcoma) and skin (melanoma) account for 18% of secondary tracheal neoplasms [[3], [4], [5], [6],9].
Evidence-based guidelines for management of tracheal malignancies are lacking. Based on expert opinion, complete surgical resection with primary reconstruction offers the best chance of cure for pre-invasive or minimally invasive tracheal tumors [21]. Bronchoscopy offers a minimally invasive technique for palliation of visible tracheobronchial malignancies and for treatment of microinvasive tumors of the tracheobronchial tree. Bronchoscopic methods include brachytherapy, photodynamic therapy (PDT), cryotherapy, and thermocoagulation (laser, electrocautery, and argon plasma coagulation).
PDT is an established modality to manage early-stage micro invasive non-small cell lung cancer (NSCLC) of the central airways with curative intent. Squamous cell NSCLC is the most common subtype involved [[10], [11], [12], [13], [14]]. We report a unique case of a tracheal oligometastatic lesion from recurrent lung adenocarcinoma successfully treated with PDT with a durable response extending out to two years at the time of publication.
2. Case
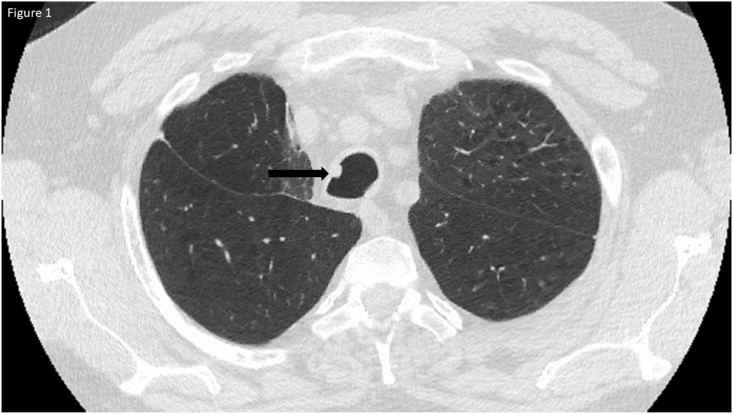
A 59-year-old man presented with cough and hemoptysis. He was a former smoker with a 140 pack-year history and had a history of moderately severe chronic obstructive pulmonary disease, adenocarcinoma (pT1bN0M0) of the right upper lobe treated with lobectomy two years prior, and adenocarcinoma (pT1cN0M0) of the lingula treated with wedge resection ten months prior. No endobronchial lesions were present on CT imaging from before either of the resections. This was confirmed with intra-operative bronchoscopy prior to each of the surgical procedures. Physical examination was remarkable for decreased breath sounds bilaterally without any stridor and well healed surgical scars. Computed tomography (CT) of the chest demonstrated a right middle lobe (RML) infiltrate and a 10 mm right mid-tracheal lesion (Fig. 1).
Fig. 1.
CT chest showing 6 mm right mid-tracheal lesion at the 10 o'clock position.
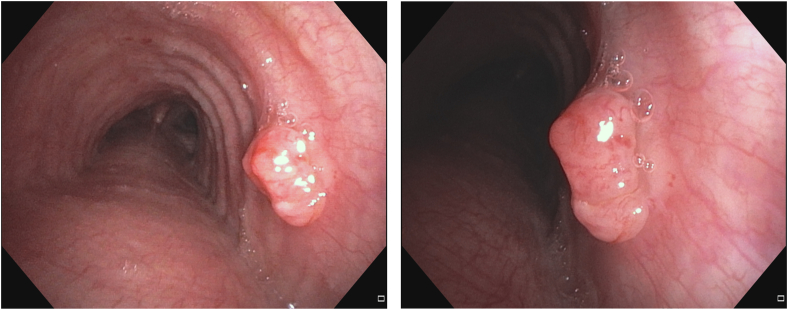
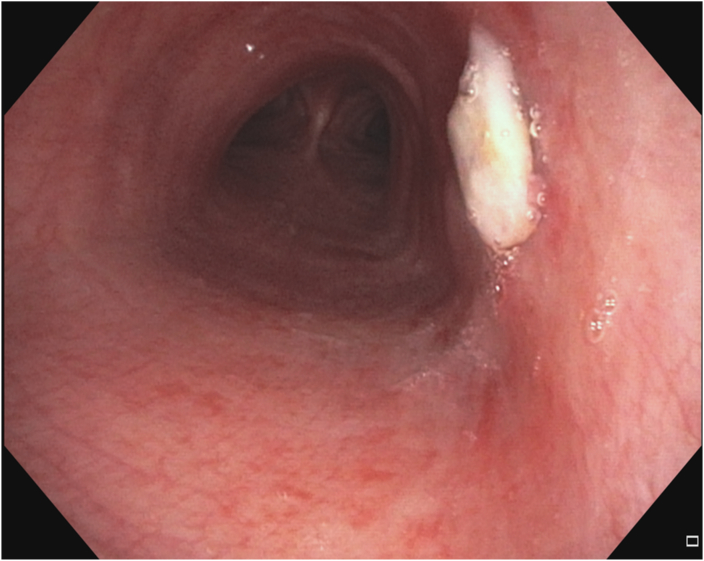
Flexible bronchoscopy revealed a 10 mm endoluminal exophytic lesion on the right lateral wall of the mid-trachea (Fig. 2A and B). Biopsy of the lesion confirmed lung adenocarcinoma. The RML infiltrate was benign (likely infectious) based on negative transbronchial biopsies, lack of hypermetabolic activity on positron emission tomography (PET) scan after completion of antibiotic treatment, and complete resolution on surveillance imaging. The case was discussed at a multidisciplinary conference with representatives from thoracic surgery, medical oncology, radiation oncology, and interventional pulmonology. The consensus was to proceed with local treatment using PDT. Depth was determined based on high resolution CT chest imaging. Depth assessment and mediastinal staging with linear EBUS was contemplated but would have necessitated another procedure and anesthetic event. The possibility of occult nodal disease was discussed at the multidisciplinary thoracic oncology conference but was felt to be unlikely based on his imaging characteristics (no enlarged or hypermetabolic nodes on PET/CT). After discussion with the patient and the multidisciplinary team, the consensus was to proceed directly to PDT.
Fig. 2.
A&B: Right mid-tracheal lesion before photodynamic therapy treatment.
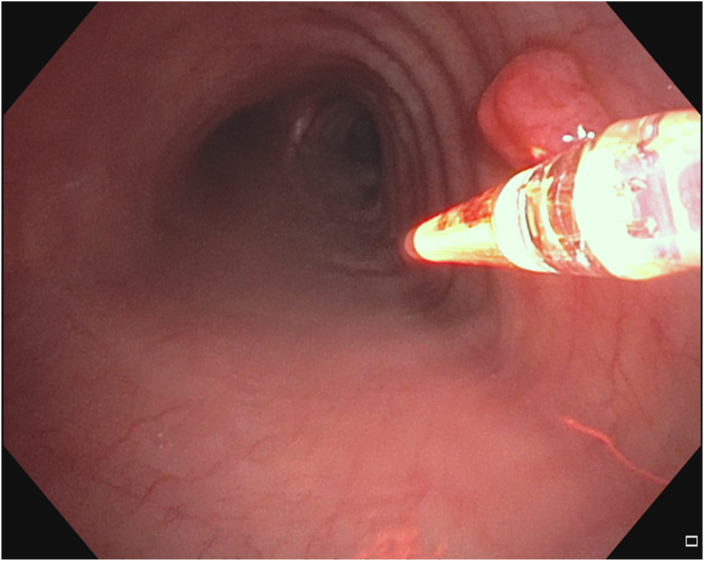
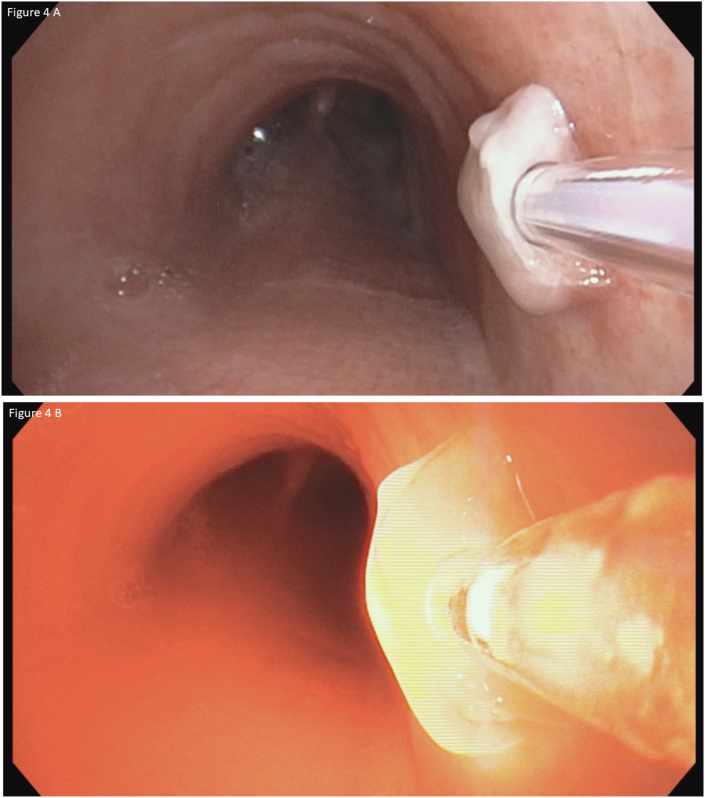
The patient received a 2mg/kg intravenous infusion of porfimer sodium (Photofrin®, Pinnacle Biologics, Inc, Bannockburn, IL) followed by the initial illumination 72 hours later. Flexible bronchoscopy was performed via a supraglottic airway device. The procedure was performed under local anesthesia (10 ml of topical lidocaine 1%) and moderate sedation (propofol 30 mg and fentanyl 100 μg intravenous injection). Dexamethasone (10 mg intravenous) was administered to prevent the central airways edema. There were no immediate postoperative complications. A 1.0 cm rigid diffuser fiber was positioned adjacent to the lesion for the first light treatment in which 200J/cm was delivered over 500 seconds (Fig. 3). Two days later, a second PDT treatment was performed using a 1.5 cm diffuser, which was positioned interstitially (Fig. 4). On post-operative day five, necrotic changes of the lesion were noted (Fig. 5). There was no evidence of residual tumor on airway examination at 2, 12, and 24 months (Fig. 6A, Fig. 6BA&B). Surveillance biopsies confirmed the absence of microscopic disease at 2,12, and 24 months. Surveillance chest CT imaging performed at 6-month intervals out to 2 years at the time of publication did not reveal any lesions suggestive of recurrent malignancy. Surveillance bronchoscopy with endobronchial tracheal biopsies out to 2 years did not demonstrate any evidence of even microscopic recurrence. The patient avoided direct sunlight for 4 weeks after receiving the porfimer sodium infusion and had no adverse effects from the medication or treatment.
Fig. 3.
Tracheal lesion during first Photodynamic Therapy light activation.
Fig. 4.
A&B: Interstitial fiber placement during the second photodynamic therapy light activation.
Fig. 5.
Necrotic changes of the tumor on post-operative day 5.
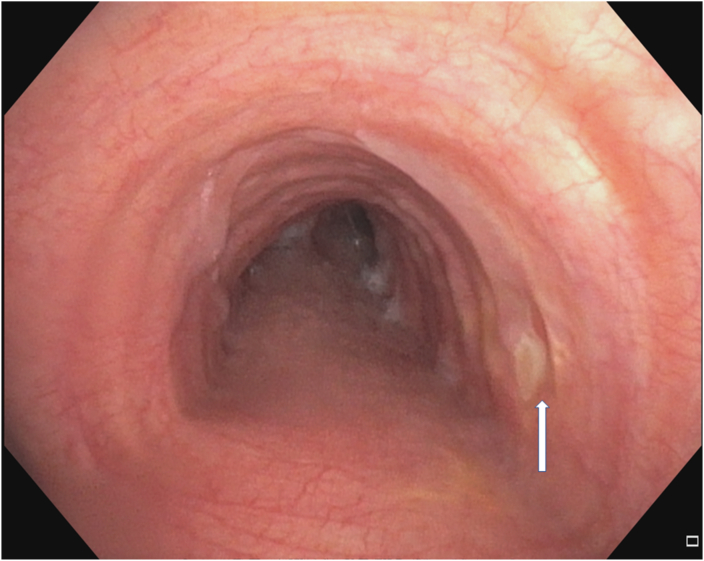
Fig. 6A.
Follow up bronchoscopy 2 months after photodynamic therapy.
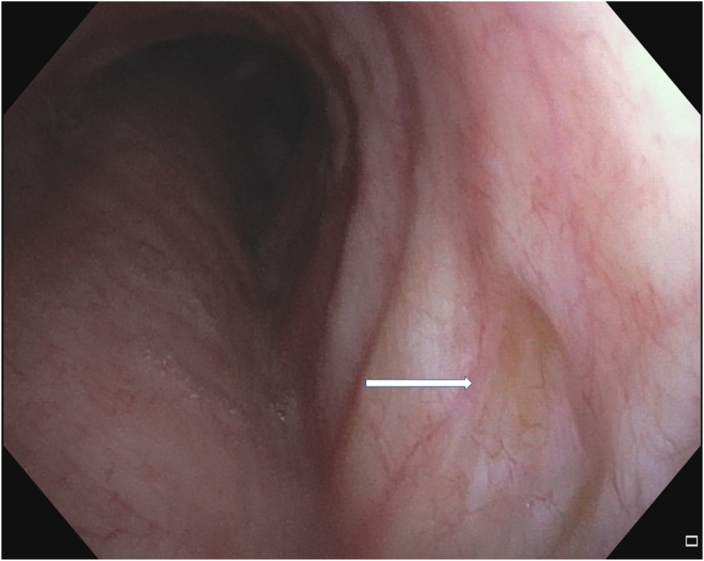
Fig. 6B.
Follow up bronchoscopy 12 months after photodynamic therapy.
3. Discussion
PDT with porfimer sodium, a first-generation photosensitizer, is approved by the United States Food and Drug Administration for management of microinvasive non-small cell lung cancer of the tracheobronchial tree and for palliation in advanced stage disease when surgical resection is not a viable option. It is a minimally invasive non-thermal evidence-based modality that acts as a light activated localized chemotherapy [22].
Porfimer sodium is given intravenously at a dose of 2 mg/kg and enters malignant cells by endocytosis. Malignant cells retain a higher concentration of photosensitizer for a prolonged period due to their increased permeability, acidic pH and impaired lymphatic drainage as compared to healthy cells. After a 48–72-h washout period, the tracheobronchial tumor is bronchoscopically exposed to a specific wavelength light (630 nm). The light is delivered circumferentially by a cylindrical quartz fiber inserted through the working channel of a standard bronchoscope. The fiber can be positioned either adjacent to or within the tumor. Penetration of light is limited to 1cm from the tissue surface but can be overcome by interstitial placement of the rigid diffuser. The light initiates an intracellular photo-oxidative reaction, which converts molecular oxygen into singlet oxygen and free radicals that lead to tumor vessel thrombosis, cell membrane damage, and consequently cell death [23]. The higher concentration of porfimer sodium in malignant cells and targeted light exposure to the lesion make it a relatively selective intervention for malignant lesions providing an advantage over other non-selective ablative modalities [10].
With our patient, a multidisciplinary team recommended local bronchoscopic ablative therapy. Given his two prior surgical lung resections, the patient was not a good candidate for a tracheal sleeve resection. PDT was selected because of its favorable safety profile, ease of delivery, and durability of response. Additionally, tumor vascularity, clinical stability, and lack of submucosal invasion on CT (Fig. 1) made PDT an appropriate option.
Based on our patient's tumor characteristics, PDT was performed with curative intent. This approach is guided in part after determining the depth of invasion into the tracheobronchial wall (intra-cartilaginous vs. extra-cartilaginous), which requires meticulous assessment to ensure an optimal outcome. Endobronchial ultrasound (EBUS) can be used to make this determination accurately. Miyazu et al. reported that nine patients with tracheobronchial malignancies who were without any extra-cartilaginous spread based on EBUS achieved complete remission with PDT with a median follow up of 32 months [14]. While CT scanning is one of the most commonly used imaging modalities for radiological assessment of tracheobronchial malignancies, conventional CT in these patients was only 63–85% sensitive in detecting airway abnormalities. High resolution computed tomography (HRCT) provides a better delineation of margins for tracheobronchial malignancies. Sutedja et al. demonstrated that complete response (CR) was achieved in 91% of patients on extended follow up in which there was no extra cartilaginous tumor spread based on HRCT assessment [15].
In our patient, formal assessment of tumor depth and mediastinal staging could have both been accomplished with EBUS. This would have increased our confidence that this was isolated oligometastatic disease. It would have also necessitated another procedure with anesthesia. Based on historic CT imaging and bronchoscopies, we knew the tracheal lesion was not present at the time of his prior surgeries. Current PET/CT imaging was not concerning for mediastinal nodal involvement or distant metastatic disease. After careful deliberation and a shared decision-making discussion with the patient, the consensus was to proceed directly to PDT. We recommend using EBUS for depth assessment and mediastinal staging (when indicated) prior to considering PDT if possible.
The CR rate was studied in a large series of 264 patients diagnosed with early-stage central tracheobronchial tumors. CR was achieved in 95% of cases for lesions <1 cm in size and decreased to 80% and 44% for lesions of 1–2 cm and >2 cm, respectively [16]. In a systematic review of 15 studies by Moghissi et al., a total of 626 patients with 715 central airway early-stage malignancies had a CR rate ranging from 30 to 100% [18]. In another study of 48 patients, the CR rate was 94%, but 20% had subsequent local recurrence [19]. Methods for assessing extra-cartilaginous spread in these studies were often ambiguous.
Bronchoscopic PDT has been used as the sole intervention in palliative cases, as neo-adjuvant therapy before surgical intervention, or as combination therapy with other ablative bronchoscopic interventions. It is effective at palliating respiratory symptoms due to primary or secondary tracheobronchial malignancies. Litle et al. in a case series of 27 patients with secondary airway malignancies reported that relief of hemoptysis and dyspnea was achieved in 85% of patients, and follow-up bronchoscopy two days after initial treatment revealed tumor necrosis in all the patients [11]. Similarly, Taber et al. and Minnich et al. reported an 80% relief of symptoms in patients with primary and secondary tracheobronchial malignancy [12,13]. Few studies reported duel bronchoscopic intervention (cryoablation followed by PDT) to treat endotracheal primary or secondary malignancies with curative intent leading to CR without any complication. Authors used PDT (tumor selective modality) after cryoablation to treat the minimal residual lesions in which margins cannot be appreciated with naked eye [23,24]. Recently, Mehta and colleagues evaluated the role of PDT for positive margins after resection of NSCLC in individuals who are not candidates for repeat surgical intervention or radiotherapy. 10/11 (91%) of participants did not have any local recurrence at the PDT site at 2 years follow up [25].
Complications from PDT may also occur. Taber et al. also compared PDT with neodymium-doped yttrium aluminum garnet (Nd:YAG) laser and found that morbidity rates were comparable in both groups (31% vs 22%; p > 0.05) [12]. Respiratory failure and hypoxemia were the most common complications and were equally common in both groups and were as common as reported by Litle et al. in his study at 5–6% [11]. McCaughan et al. reported a decrease in the mean percentage obstruction from secondary metastatic non-pulmonary tracheobronchial tumors by 72% post-PDT [16]. Minnich et al., in his retrospective study that included 133 patients, reported that PDT is safe and effective in obstructive, highly vascular, and bleeding tracheobronchial lesions in severely debilitated and profoundly symptomatic patients who failed core out or laser bronchoscopic ablations [13]. PDT ablation should be avoided in an acutely decompensating patient secondary to malignant central airway obstruction as it does not have an immediate effect.
Studies have demonstrated the durability of response from PDT and the longer time to follow up treatment as compared to non-PDT ablation [20]. Skin photosensitivity is the most common side effect (8–28%) due to delayed clearance of the drug from the skin, liver, and spleen. Avoidance of direct sunlight is recommended for 4 weeks after treatment [13]. Dyspnea may be caused by airway obstruction from necrotic debris and local airway edema, but these complications are less common in the context of early-stage disease than when used in a palliative scenario. Tracheobronchial stenosis can develop as a delayed complication following PDT, especially with early-stage disease, as that is when there is increased exposure of the normal airway wall to the activating light. Fortunately, no stenotic lesion was noticed in our patient during surveillance bronchoscopy up to 24 months post-operatively.
4. Conclusion
Our case demonstrates the utility of PDT as a first line bronchoscopic treatment for endotracheal tumors. PDT is an effective modality that is well tolerated and produces a durable response, which in our patient has led to complete response without recurrence at 24 months and with no adverse effects or complications. Determining depth of tumor penetration is paramount if the treatment is with curative intent. PDT should be considered as part of a multidisciplinary approach to manage endotracheal malignant lesions.
Disclosures statement
The authors report no disclosures.
Declaration of competing interest
The authors report no conflicts of interest.
Acknowledgment section
Each of the authors significantly contributed to this manuscript. Drs. Singh, Jani, Abdalla were greatly involved in drafting manuscript. Dr Kurman and Dr Benn were involved in critical revision of the manuscript and contributed vital intellectual content. Dr Harpreet Singh (corresponding author, guarantor) takes responsibility for the content of the manuscript, including the data and analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2022.101620.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Flexible bronchoscopy showing second photodynamic therapy treatment.
References
- 1.Gaissert H.A., Mark E.J. Tracheobronchial gland tumors. Cancer Control. 2006 Oct;13(4):286–294. doi: 10.1177/107327480601300406. PMID: 17075566. [DOI] [PubMed] [Google Scholar]
- 2.Kameyama K., Huang C.L., Liu D., Okamoto T., Hayashi E., Yamamoto Y., Yokomise H. Problems related to TNM staging: patients with stage III non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2002 Sep;124(3):503–510. doi: 10.1067/mtc.2002.123810. PMID: 12202867. [DOI] [PubMed] [Google Scholar]
- 3.Albertini R.E., Ekberg N.L. Endobronchial metastasis in breast cancer. Thorax. 1980 Jun;35(6):435–440. doi: 10.1136/thx.35.6.435. PMID: 7434298; PMCID: PMC471305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe S., Oda M., Ohta Y., Watanabe G. Endotracheal metastasis of rectal cancer. Eur. J. Cardio. Thorac. Surg. 2002 May;21(5):924. doi: 10.1016/s1010-7940(02)00098-2. PMID: 12062288. [DOI] [PubMed] [Google Scholar]
- 5.MacMahon H., O'Connell D.J., Cimochowski G.E. Pedunculated endotracheal metastasis. AJR Am. J. Roentgenol. 1978 Oct;131(4):713–714. doi: 10.2214/ajr.131.4.713. PMID: 102169. [DOI] [PubMed] [Google Scholar]
- 6.Koyi H., Brandén E. Intratracheal metastasis from malignant melanoma. J. Eur. Acad. Dermatol. Venereol. 2000 Sep;14(5):407–408. doi: 10.1046/j.1468-3083.2000.00100.x. PMID: 11305386. [DOI] [PubMed] [Google Scholar]
- 7.Chong S., Kim T.S., Han J. Tracheal metastasis of lung cancer: CT findings in six patients. AJR Am. J. Roentgenol. 2006 Jan;186(1):220–224. doi: 10.2214/AJR.04.1711. PMID: 16357405. [DOI] [PubMed] [Google Scholar]
- 8.Maki Y., Kimizuka Y., Sasaki H., Yamamoto T., Watanabe C., Sano T., Tagami Y., Misawa K., Miyata J., Fujikura Y., Shimazaki H., Kawana A. Lung adenocarcinoma with repetitive endotracheal/endobronchial metastasis 20 years after surgery: a case report. Thorac. Cancer. 2021 Jan;12(1):133–136. doi: 10.1111/1759-7714.13730. Epub 2020 Nov 14. PMID: 33188565; PMCID: PMC7779200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madariaga M.L., Gaissert H.A. Secondary tracheal tumors: a systematic review. Ann. Cardiothorac. Surg. 2018 Mar;7(2):183–196. doi: 10.21037/acs.2018.02.01. PMID: 29707496; PMCID: PMC5900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loewen G.M., Pandey R., Bellnier D., Henderson B., Dougherty T. Endobronchial photodynamic therapy for lung cancer. Laser Surg. Med. 2006 Jun;38(5):364–370. doi: 10.1002/lsm.20354. PMID: 16788932. [DOI] [PubMed] [Google Scholar]
- 11.Litle V.R., Christie N.A., Fernando H.C., Buenaventura P.O., Ferson P.F., Luketich J.D. Photodynamic therapy for endobronchial metastases from nonbronchogenic primaries. Ann. Thorac. Surg. 2003 Aug;76(2):370–375. doi: 10.1016/s0003-4975(03)00345-x. discussion 375. PMID: 12902066. [DOI] [PubMed] [Google Scholar]
- 12.Taber S.W., Buschemeyer W.C., 3rd, Fingar V.H., Wieman T.J. The treatment of malignant endobronchial obstruction with laser ablation. Surgery. 1999 Oct;126(4):730–733. discussion 733-5. PMID: 10520922. [PubMed] [Google Scholar]
- 13.Minnich D.J., Bryant A.S., Dooley A., Cerfolio R.J. Photodynamic laser therapy for lesions in the airway. Ann. Thorac. Surg. 2010 Jun;89(6):1744–1748. doi: 10.1016/j.athoracsur.2010.02.025. discussion 1748-9. PMID: 20494021. [DOI] [PubMed] [Google Scholar]
- 14.Miyazu Y., Miyazawa T., Kurimoto N., Iwamoto Y., Kanoh K., Kohno N. Endobronchial ultrasonography in the assessment of centrally located early-stage lung cancer before photodynamic therapy. Am. J. Respir. Crit. Care Med. 2002 Mar 15;165(6):832–837. doi: 10.1164/ajrccm.165.6.2108092. PMID: 11897652. [DOI] [PubMed] [Google Scholar]
- 15.Sutedja G., Golding R.P., Postmus P.E. High resolution computed tomography in patients referred for intraluminal bronchoscopic therapy with curative intent. Eur. Respir. J. 1996 May;9(5):1020–1023. doi: 10.1183/09031936.96.09051020. PMID: 8793466. [DOI] [PubMed] [Google Scholar]
- 16.McCaughan J.S., Jr. Survival after photodynamic therapy to non-pulmonary metastatic endobronchial tumors. Laser Surg. Med. 1999;24(3):194–201. doi: 10.1002/(sici)1096-9101(1999)24:3<194::aid-lsm4>3.0.co;2-#. PMID: 10229150. [DOI] [PubMed] [Google Scholar]
- 18.Moghissi K., Dixon K. Update on the current indications, practice and results of photodynamic therapy (PDT) in early central lung cancer (ECLC) Photodiagnosis Photodyn. Ther. 2008 Mar;5(1):10–18. doi: 10.1016/j.pdpdt.2007.11.001. Epub 2008 Feb 13. PMID: 19356631. [DOI] [PubMed] [Google Scholar]
- 19.Endo C., Miyamoto A., Sakurada A., Aikawa H., Sagawa M., Sato M., Saito Y., Kondo T. Results of long-term follow-up of photodynamic therapy for roentgenographically occult bronchogenic squamous cell carcinoma. Chest. 2009 Aug;136(2):369–375. doi: 10.1378/chest.08-2237. Epub 2009 Mar 24. PMID: 19318660. [DOI] [PubMed] [Google Scholar]
- 20.Jayadevappa R., Chhatre S., Soukiasian H.J., Murgu S. Outcomes of patients with advanced non-small cell lung cancer and airway obstruction treated with photodynamic therapy and non-photodynamic therapy ablation modalities. J. Thorac. Dis. 2019 Oct;11(10):4389–4399. doi: 10.21037/jtd.2019.04.60. PMID: 31737325; PMCID: PMC6837945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Refaely Y., Weissberg D. Surgical management of tracheal tumors. Ann. Thorac. Surg. 1997 Nov;64(5):1429–1432. doi: 10.1016/S0003-4975(97)00818-7. discussion 1432-3. PMID: 9386715. [DOI] [PubMed] [Google Scholar]
- 22.https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020451s020lbl.pdf.
- 23.Kurman J.S., Pastis N.J., Murgu S.D. Photodynamic therapy and its use in lung disease. Curr. Pulmonol. Rep. 2019;8:215–221. doi: 10.1007/s13665-019-00241-y. [DOI] [Google Scholar]
- 24.Nam S.J., Oak C.H., Jang T.W., Jung M.H., Chun B.K. Successful treatment of a tracheal squamous cell carcinoma with a combination of cryoablation and photodynamic therapy. Thorac. Cancer. 2013 May;4(2):191–194. doi: 10.1111/j.1759-7714.2012.00126.x. PMID: 28920198. [DOI] [PubMed] [Google Scholar]
- 25.Mehta H.J., Biswas A., Fernandez-Bussy S., Pipkin M., Machuca T., Jantz M.A. Photodynamic therapy for bronchial microscopic residual disease after resection in lung cancer. J. Bronchol. Interv. Pulmonol. 2019 Jan;26(1):49–54. doi: 10.1097/LBR.0000000000000510. PMID: 29771775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flexible bronchoscopy showing second photodynamic therapy treatment.