Abstract
The rise in antibiotic-resistant bacteria, including strains that are resistant to last-resort antibiotics, and the limited ability of antibiotics to eradicate biofilms, have necessitated the development of alternative antibacterial therapeutics. Antibacterial biomaterials, such as polycationic polymers, and biomaterial-assisted delivery of non-antibiotic therapeutics, such as bacteriophages, antimicrobial peptides and antimicrobial enzymes, have improved our ability to treat antibiotic-resistant and recurring infections. Biomaterials not only allow targeted delivery of multiple agents, but also sustained release at the infection site, thereby reducing potential systemic adverse effects. In this Review, we discuss biomaterial-based non-antibiotic antibacterial therapies for the treatment of community- and hospital-acquired infectious diseases, with a focus in in vivo results. We highlight the translational potential of different biomaterial-based strategies, and provide a perspective on the challenges associated with their clinical translation. Finally, we discuss the future scope of biomaterial-assisted antibacterial therapies.
Web summary
The development of antibiotic tolerance and resistance has demanded the search for alternative antibacterial therapies. This Review discusses antibacterial biomaterials and biomaterial-assisted delivery of non-antibiotic therapeutics for the treatment of bacterial infectious diseases, with a focus on clinical translation.
[H1] Introduction
The discovery of penicillin in the 20th century provided a transformative advantage in the fight against bacterial infections. However, non-judicious use of antibiotics, requirements of high antibiotic doses to kill bacteria within biofilms, and evolution of bacteria have led to the development of antibiotic tolerance and resistance (Box 1).1–3 Antibiotic-resistant strains of bacteria are responsible for tens of thousands of global fatalities every year.4 In particular, the rise in multi-drug resistant strains of Gram-negative bacteria, such as Pseudomonas aeruginosa and carbapenemase-producing enterobacteriaceae (CPE), is alarming. These bacteria are not responsive to any antibiotic, except colistin, a last resort peptide-based antibiotic.5,6 Resistant strains of Gram-positive and Gram-negative bacteria are responsible for diverse community- and hospital-acquired infectious diseases, including osteomyelitis, respiratory infections, surgery- and implant-associated infections, and wound infections that are challenging to treat. TABLE 1 summaries the top pathogens that have resulted in healthcare-associated infections over a 7-year period (2011–2017), classified by the US Centers for Disease Control and Prevention (CDC).7–9
Box 1| Antibiotic resistance and tolerance.
Successful mitigation of bacterial infections by antibiotics depends on the concentration of the antibiotic and the time of exposure. 1,10,155 The minimum inhibitory concentration (MIC) is the minimum concentration required to prevent visible growth of a microorganism. The development of bacterial resistance is a result of genetic mutation or horizontal gene transfer in bacteria, ultimately preventing the antibiotic from binding to the bacteria.195 Therefore, an antibiotic dose significantly higher than the MIC is required to achieve a therapeutic effect. Antibiotic-tolerant bacteria usually remain susceptible to the antibiotic at the MIC; however, longer exposure time is required for infection mitigation.155
The development of antibiotic-tolerant bacteria is a result of a genetic mutation that leads to the development of phenotypes with a longer lag phase – the phase, in which bacteria are not rapidly growing.155 Such slow-growing bacteria can survive treatment with antibiotics, which require active bacteria to function. Initially, tolerance and resistance were considered to be unrelated; however, recent studies have shown that establishment of a tolerant phenotype often rapidly promotes the evolution of resistance has been reported in bacteria that are tolerant to antibiotics, in particular, following a treatment regimen, in which a high dose of antibiotic is initially given, followed by subsequent lower dosing of antiobiotics.1 Moreover, the presence of tolerant phenotypes in biofilms leads to challenging chronic infections.10 Thus, a constant dose of antibiotic, higher than the MIC, is required for an extended period of time to interrupt the tolerance/resistance cycle and to mitigate infection, highlighting the need for biomaterial-based delivery strategies.
Table 1.
| Pathogen | Year (% of total infection) | ||
|---|---|---|---|
| 2015–2017 | 2015–2017 Pediatric infection (patients <18 years old) | 2011– 2014 | |
| Escherichia coli | 17.5 | 12.3 | 15.4 |
| Staphylococcus aureus | 11.8 | 15.4 | 11.8 |
| Selected Klebsiella | 8.8 | 9.3 | 7.7 |
| Pseudomonas aeruginosa | 8 | 5.8 | 7.3 |
| Enterococcus faecalis | 7.9 | 8.7 | 7.4 |
| Coagulase-negative staphylococci | 6.8 | 12.1 | 7.7 |
| Enterobacter | 4.6 | 6.5 | 4.2 |
| Enterococcus faecium | 3.8 | 1.8 | 3.7 |
| Proteus | 3.2 | - | 2.8 |
Another major challenge in the treatment of bacterial infections is the killing of bacteria within a biofilm.2,3 A biofilm is a densely packed community of bacterial cells attached to a surface and embedded within an extracellular matrix (FIG. 1a). Biofilms shield bacteria from antibiotics, antibodies and immune cells, and result in localized, chronic infections that are difficult to eradicate. In addition, bacteria within a biofilm remain dormant and can cause recurring infections. Notably, low local concentrations of antibiotics within a biofilm promote the development of tolerance and resistance to host defense mechanisms and drug recalcitrance.10 Importantly, high doses of antibiotics often need to be used to treat infections with persistent biofilms, which can lead to adverse side effects, including allergic reactions, off-target toxicity, disruption of intestinal and vaginal microflora, and overgrowth of opportunistic pathogens, such as Clostridium difficile.11 To limit non-judicious use of antibiotics, the US CDC has developed a Standardized Antimicrobial Administration Ratio (SAAR).12 SAAR provides a standardized, risk-adjusted benchmark of antibiotic use and aims to reduce adverse drug events and unnecessary healthcare costs.
Figure 1. Biomaterial-based antibacterial therapies.
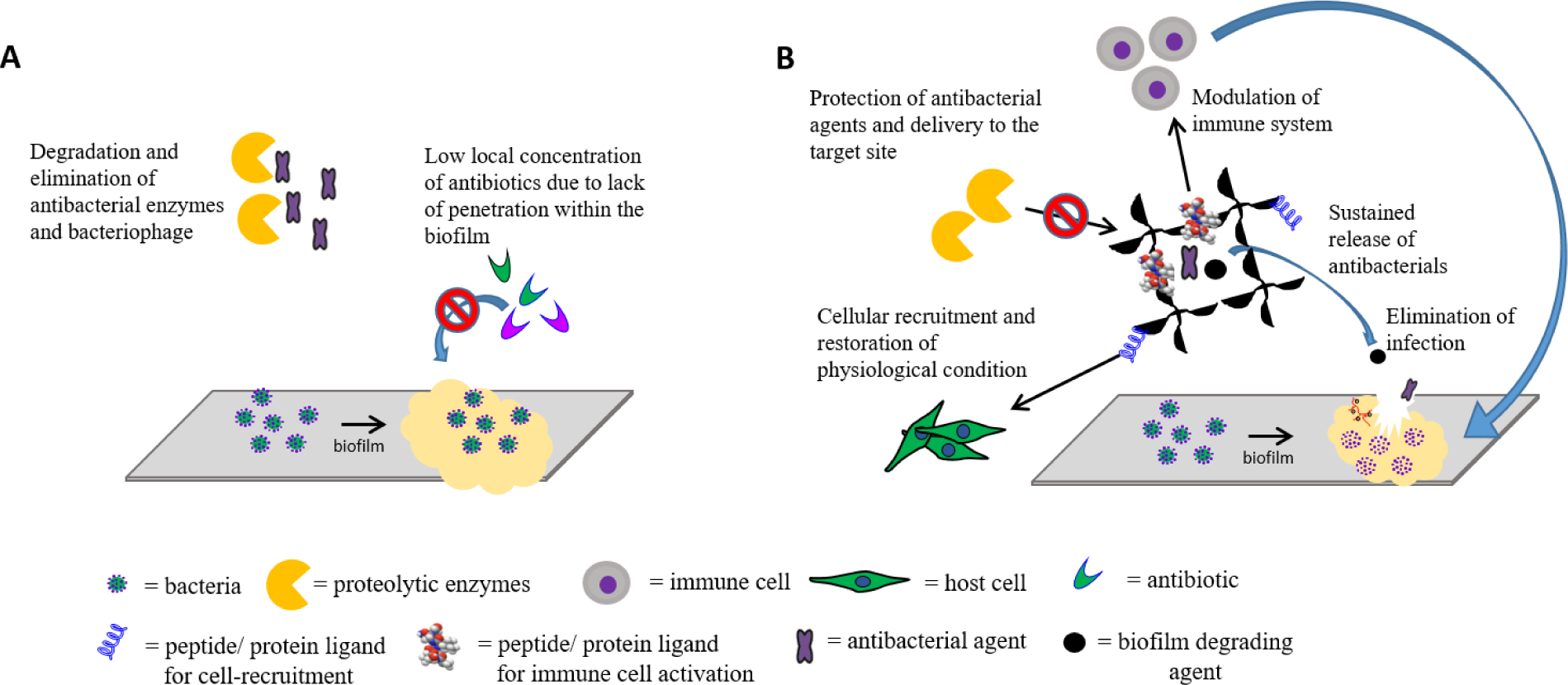
A) Challenges in the treatment of bacterial biofilm infections. Limited penetration of antibiotics into the biofilm owing to the presence of extracellular polymeric substances (EPS) limits the local concentration of the antibiotic at the site of infection, leading to inefficient mitigation of infection and to the development of tolerance and resistance. B) Optimal properties of biomaterial-assisted antibacterial therapies to treat challenging bacterial infections. The biomaterial can protect antibacterial agents from proteolytic enzymes in the body and facilitate the simultaneous delivery of multiple agents to treat infections, modulate immune responses and restore physiological conditions.
Antibacterial agents, such as bacteriophages, antimicrobial peptides (AMPs) and antimicrobial enzymes, have been explored as alternatives to, or in conjunction with, antibiotic therapy to treat bacterial infections. These alternative therapies have shown promise in the treatment of antibiotic-resistant infections (TABLE 2). For example, treatment with an engineered phage mixture led to clinical improvements in a 15-year-old patient with cystic fibrosis (homozygous for ΔF508 mutation in the CFTR gene) suffering from a drug-resistant Mycobacterium abscessus infection.13 Similarly, antibacterial enzymes, such as lysostaphin, and the bacteriophage lysin, have generated encouraging results in the treatment of bacterial infections.14,15 AMPs are cationic peptides that display antibacterial, antiviral and antifungal activity. Several peptide-like antibiotics are currently US Food and Drug Administration (FDA)-approved: vancomycin, colistin (N050356), telavancin (N022110), oritvacncin (N206334) and dalbavancin (N021883).16 The Therapeutic Proteins Database (THPdb, http://crdd.osdd.net/raghava/thpdb/),17 a subset of the FDA database, lists two additional peptide-based antibacterials, gramicidin D (Neosporin® and Sofradex®) and daptomycin (Cubicin®), which exhibit promising antibacterial properties. However, limited stability of some of these agents under physiological conditions, challenges in their delivery to the infected site and difficulties in engineering a formulation while maintaining the activity of these agents have resulted in limited clinical translation beyond topical use (for example, Neosporin®), with the exception of vancomycin, which has been approved for oral and intravenous administration to treat infections caused by S. aureus resistant to penicillin.
Table 2.
Non-antibiotic antibacterial therapies in clinical trials.
| NCT number/agent | Status | Bacteria | Infection/Disease | Study Director(s)/Principal Investigator/Reference |
|---|---|---|---|---|
| Bacteriophages | ||||
| NCT00945087 | Recruiting (last update 2013) | Various (Staphylococcus, Enterococcus, Pseudomonas, Escherichia, Klebsiella, Proteus, Citrobacter, Acinetobacter, Serratia, Morganella, Shigella, Salmonella, Enterobacter, Stenotrophomonas, or Burkholderia) | Various (non-healing postoperative wounds or bone, upper respiratory tract, genital or urinary tract infections) | Górski |
| NCT02116010 | Terminated; Prematurely terminated due to low number of eligible patients and low efficacy of phage cocktail compared |
E. coli
P. aeruginosa |
Wound infection | 171 |
| NCT03140085 | Completed; Phage therapy was not superior to the placebo; however, it was not inferior to standard-of-care antibiotics either |
Enterococcus spp., Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus spp., and Streptococcus spp. | Urinary tract infection | 172,173 |
| NCT04287478 | Not yet recruiting | E. coli and K. pneumonia | Urinary Tract Infection | R.J. Hopkins |
| NCT02664740 | Not yet recruiting | S. aureus (MRSA or MSSA) | Wound infection (Diabetic foot ulcers) | A. Sotto |
|
NCT04323475 (PGX-0100) |
Not yet recruiting | S. aureus, P. aeruginosa, or K. pneumoniae | Wound infection (Burn wound) | J. Brown |
|
NCT01640886 (MicroPhage diagnostic) |
Terminated 2012; Results did not meet the study requirement |
S. aureus (MRSA or MSSA) | Various (Blood infection identification) | D. Manna |
|
NCT01184339 (MicroPhage diagnostic) |
Completed No results posted |
S. aureus (MRSA or MSSA) | Various (Blood infection identification) | T. Kirn; C. Qi; B. Reller; C. Savor-Price |
| Antimicrobial peptides and enzyme | ||||
|
NCT03163446 Phage lysin (CF-301) |
Completed phase II; Demonstrated robust safety and tolerability profile |
S. aureus | Bacteremia and endocarditis | ContraFect149 |
|
NCT00027248 Omiganan (CLS001/MBI226) |
Phase II/III; No results posted |
- | Venous catheter-related infections | Pankovich, USA |
|
NCT01746654 P128 (peptide) |
Phase I & II Completed 2016; No results posted |
S. aureus | Various (targeting nasal colonization) | D.A. Fisher; S. Kher |
|
NCT02407106 BLIS (Bacteriocin-like Inhibitory Substance) K12 |
Not yet recruiting 2015 | S. Salivarius | Rheumatic Fever | Y. Garty |
|
NCT01855048 N-Rephasin® SAL200 |
Completed; Evaluation of pharmacokinetics, pharmacodynamics and tolerance. No severe adverse effect reported |
174 | ||
|
*NCT00800930 LL-37 and beta-defensin (using sodium butyrate to upregulate LL-37) |
Completed; Therapy resulted in reduced inflammation, increased expression and release of LL-37 |
Shigella flexneri | Shigellosis | R. Raqib. 175,176 |
|
*NCT01580007 LL-37 (using vitamin D and sodium phenylbutyrate to upregulate LL-37) |
Completed; Phenylbutarate and Vitamin D promoted favorable immunomodulation to treat tuberculosis in conjunction with antibiotics |
M. tuberculosis | Tuberculosis | 177 |
Database: clinicaltrials.gov and dramp.cpu-bioinfor.orgl Search criteria: Condition or disease: “Infection, Bacterial”; Other terms: “Bacteriophage”, “LL-37”, “Cathelicidin”, “K12”, “P128”, “Lysostaphin”, “Bacteriocin”, “Antimicrobial Peptide”, “Antimicrobial enzyme”, “KR-12”, “Lysin”, “Phage.”
Clinical trials, in which a therapeutic was used to upregulate the production of antimicrobial peptides or enzymes.
MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
In this Review, we discuss biomaterials-assisted non-antibiotic therapies for the treatment of bacterial infectious diseases, with a focus on studies that have shown promising results in vivo and translational potential (TABLE 3). In particular, we investigate strategies based on bacteriophages, antimicrobial peptides, enzymes and polycationic biomaterials. Alternative strategies, such as antibodies and vaccines, are not covered in this Review, because of their prophylactic (non-therapeutic) mechanism of action.
Table 3.
Biomaterial-assisted delivery of non-antibiotic antibacterial therapies
| Infection Type | Bacteria | Biomaterial | Reference |
|---|---|---|---|
| Bacteriophage | |||
| Bone | E. coli | Hydroxyapatite and β-tricalcium phosphate ceramics | 25 |
| E. faecalis | Alginate-nanohydroxyapatite hydrogel | 26 | |
| P. aeruginosa | PEG-4MAL hydrogel | 24 | |
| S. aureus | HPMC-coated wire and/or linezolid coated K-wire | 27 | |
| Lung | P. aeruginosa | PLGA microparticle | 32 |
| Lactose/lactoferrin 60 : 40 w/w matrix and deagglomerated respirable powder | 30 | ||
| M. tuberculosis | Nebulized particles | 28 | |
| S. aureus | 29 | ||
| K. pneumoniae | Liposomes | 31 | |
| Gastrointestinal | E. coli | Transdermal microemulsion | 36 |
| pH-responsive microparticles (Eudragit® S100 and alginate) | 33 | ||
| Chitosan nanoparticles | 38 | ||
| Salmonella | Alginate/CaCO3 microencapsulation | 37 | |
| S. aureus | Calcium carbonate microparticles | 36 | |
| Clostridium difficile | pH-responsive microparticles (Eudragit® S100 with and without alginate) | 34 | |
| Wound | P. aeruginosa, E. coli, and S. aureus | Nanofibers (honey, polyvinyl alcohol, chitosan) | 41 |
| E. coli | Nanofibers (polycaprolactone/collagen I (PCL-ColI)) | 42 | |
| S. aureus | Liposomes (phosphatidylcholine, cholesterol, tween 80, stearyl amine) | 43 | |
| K. pneumoniae | 44 | ||
| P. aeruginosa | Fibrin glue | 45 | |
| Urinary tract | P. mirabilis | Silicone-coated catheter | 178 |
| pH-sensitive catheter (PVA hydrogel layer and pH-responsive polymer poly(methyl methacrylate-co-methacrylic acid) (EUDRAGIT®S 100) layer) | 49 | ||
| P. aeruginosa and P. mirabilis | Hydrogel-silicone-coated catheter | 47 | |
| P. aeruginosa | Silicone catheters | 48 | |
| Catheter-related bloodstream infection | S. aureus | Catheters | 50 |
| S. epidermidis | Hydrogel-silicone-coated catheters | 51 | |
| P. aeruginosa | 52 | ||
| Various | E. coli | Paper (functionalized with carboxylic acid or chitosan) | 179 |
| Salmonella Rissen | Biomimetic hydroxyapatite nanocrystals | 180 | |
| Antimicrobial peptides | |||
| Bone | S. aureus | Titania nanopores | 68 |
| RADA16 hydrogel | 73 | ||
| S. aureus and P. aeruginosa | Coated titanium | 67 | |
| Calcium-phosphate-coated titanium | 72 | ||
| Polyetheretherketone coating | 74 | ||
| S. aureus and P. gingivalis | Coated titanium | 71 | |
| Lung Infection | P. aeruginosa | Porous silicon nanoparticles | 60 |
| Wound | P. aeruginosa | Modified hyaluronic acid nanogels | 181 |
| Agarose hydrogels | 85 | ||
| S. aureus and P. aeruginosa | Non-charged hydrophilic hydrogels | 87 | |
| S. aureus | pH-switchable antimicrobial hydrogel with nanofiber networks | 86 | |
| Crosslinked DNA nanostructures | 82 | ||
| Hydrogel (biodegradable poly(ethylene glycol) maleate citrate and poly(ethylene glycol) diacrylate) | 80 | ||
| Plasma surface activation-based practical β-peptide polymer modification to prepare antimicrobial surfaces | 62 | ||
| S. aureus and E. coli | Biocompatible composite membrane (biomimetic polydopamine-modified eggshell membrane nano- or microfibres, hyaluronic acid) | 81 | |
| E. coli, P. aeruginosa, S. aureus, and B. cereus | Tac, PhaP, and PHBHV surface coating | 182 | |
| P. aeruginosa and A. baumannii | CM11 peptide and 1% silver-doped bioactive glass | 183 | |
| E. coli | Glycopeptide hydrogel | 79 | |
| Urinary tract | P. aeruginosa and S. aureus S. saprophyticus | Anti-adhesive hydrophilic polymer catheter coating | 65 |
| S. aureus and P. aeruginosa | Anhydrous polycaprolactone (PCL) polymer-based dual layer catheter coating | 61 | |
| Various | S. aureus, E. coli, and P. aeruginosa | Bottlebrush-like surface coatings | 184 |
| S. aureus, P. aeruginosa | Transformable polymer-peptide (composed of a chitosan backbone and two functional peptides) | 59 | |
| S. aureus | ZnO Quantum Dots | 185 | |
| S. aureus, E. coli, P. aeruginosa and K. pneumoniae | Gold nanoparticles | 58 | |
| S. aureus and E. coli | Cross-linked waterborne polyurethanes containing gemini quaternary ammonium salts | 186 | |
| E. coli | Star-shaped polypeptides | 187 | |
| Eye infection | S. aureus and P. aeruginosa | Contact lenses | 89 |
| 91 | |||
| P. aeruginosa | 90 | ||
| Antimicrobial enzymes and proteins | |||
| Bone | S. aureus | Hydroxyapatite/chitosan composite cement | 95 |
| Injectable PEG hydrogel | 99 | ||
| PEG-4MAL hydrogel | 100 | ||
| Coated titan implants | 94 | ||
| S. mutans and E. faecali | SrF2 nanoparticles, YSZ nanoparticles, and poly-ε-l-lysin in combination | 188 | |
| Lung | S. Pneumoniae | Chitosan nanoparticle | 103 |
| Subcutaneous/dermal wound | S. aureus | Chitosan gel | 96 |
| Chitosan/collagen hydrogel | 93 | ||
| Alginate-(poly-L-lysine) microcapsule encapsulated with Mammalian cells engineered to respond to bacterial infection | 189 | ||
| P. aeruginosa | Chitosan- and an aldehyde-modified PEG hydrogel | 190 | |
| B. subtilis | Gelatin-chondroitin sulphate hydrogels | 191 | |
| Various | S. aureus | P128 as a chimeric protein (combines the muralytic enzyme of Phage K and the cell-wall-targeting-domain (SH3b) of lysostaphin) | 192 |
| Modified E. coli releasing bacteriocin | 98 | ||
| Nanocomposites | 193 | ||
| Injectable polysaccharide hydrogels | 194 | ||
| pH-sensitive liposomes | 102 | ||
PEG-4MAL, 4-armed poly(ethylene glycol) macromer with terminal maleimide groups. HPMC, hydroxypropyl methylcellulose. PLGA, poly(lactic-co-glycolic acid). PVA, polyvinyl acetate. Tac, tachyplesin I. Phap, polyhydroxyalkanoate-granule-associated protein. PHBHV, poly(3-hydroxybutyrate-co-3-hydroxyvalerate). PEG, poly(ethylene glycol). YSZ, Yttria-stabilized Zirconia.
[H1] Biomaterial-based antibacterial therapies
(Bio)materials have been engineered to assist the interactions between a living system and a therapeutic, with the aim to accelerate the restoration of homeostasis. Biomaterials, such as hydrogels and nano- or microparticles, facilitate the delivery of antibiotics and other antibacterial agents to the site of infection, and can be designed to provide release profiles suitable for maintaining effective local drug concentrations at the site of infection (FIG. 1b). In addition to serving as a delivery vehicle for antibacterial agents, scaffolds and films of polycationic and zwitterionic polymers can also have bactericidal and bacterial-resistant properties, respectively. Thus, physical coating and covalent grafting of polymeric brushes enables the tuning of the properties of biomedical devices to render them resistant to infections. Biomaterial-assisted delivery can further protect the antibacterial agent from degrading enzymes and inactivating factors in the body. Moreover, biomaterials allow simultaneous delivery of two or more agents, potentially improving the efficacy of the therapy. Biomaterials can also improve the therapeutic efficiency of antibiotics and reduce adverse side effects, typically by decreasing the required systemic dose and therefore, off-target effects. Importantly, the choice of biomaterial is crucial to achieve the desired delivery outcomes and therapies (TABLE 4).
Table 4.
Key considerations for biomaterials
| Biomaterial | Parameters | Comments |
|---|---|---|
| Delivery vehicles | ||
| Hydrogel | Mesh size; Degradability; Mechanical properties; Injectability; Loading capacity | Local delivery of antibacterial agent to treat bone and wound infections. Parameters should be tailored to facilitate the desired release and to allow recruitment of immune cells and other physiological agents that promote restoration of physiological conditions. Injectable hydrogels should be considered to achieve non-invasive delivery. |
| Particles | Particle size; Surface properties; Degradability; Loading capacity; Injectability | Local and systemic delivery of agents. Particle size should be optimized based on destination. For example, alveolar macrophages can rapidly eliminate particles with a diameter of 1–5 μm. Microparticles larger than 5 μm have shown acceptable lung delivery. Particles with a hydrophobic surface are not ideal for systemic delivery because they can undergo opsonization, which results in accelerated elimination. By contrast, amphiphilic nanoparticles reside longer in serum. pH-responsive particles may be ideal for targeted delivery to the gastrointestinal tract. |
| Modification of properties of an existing biomedical device | ||
| Polymeric coatings or brushes | Physical adsorption; Covalent conjugation | Preventing device-related infections. The duration of device application determines the type of biomaterial. Metal implants (long duration) benefit from covalent grafting of biomaterials, whereas single-use devices (e.g., catheters) benefit from physically adsorbed polymer coatings. |
| Antimicrobial biomaterials | ||
| Scaffolds or films of polycationic materials | Ratio of hydrophobicity to positive charge | Treatment of topical or external infections; new materials show promise for invasive infection mitigation. |
[H1] Delivery of bacteriophages
Bacteriophages (or phages) are viruses that infect and in many cases are lytic against a specific strain of bacteria. Treatment of bacterial infections with phages has many advantages, because phages infect and lyse specific bacteria and can degrade the biofilm matrix.18 Upon infecting the bacterium, phages hijack the bacterial machinery to produce multiple copies of itself, which ultimately leads to bacterial death (FIG 2).19 This mechanism of action, which is different than that of antibiotics, renders phage therapy effective against multidrug-resistant bacteria. In addition, phage therapy results in less endotoxin release, compared to antibiotics, and does not affect the commensal microbiota of the host.20,21 Phage therapy has been clinically used as a last resort to treat two patients infected with antibiotic-resistant bacteria in Europe and the USA, demonstrating the translational potential, efficacy and safety of phage therapy.22,23 Biomaterial-assisted phage therapy has shown promising preclinical results for the treatment of infectious diseases, including bone infections, lung infections, gastrointestinal infections, wound infections and catheter-related infections.
Figure 2. Proposed mechanism-of-action of different non-antibiotic antibacterial agents.
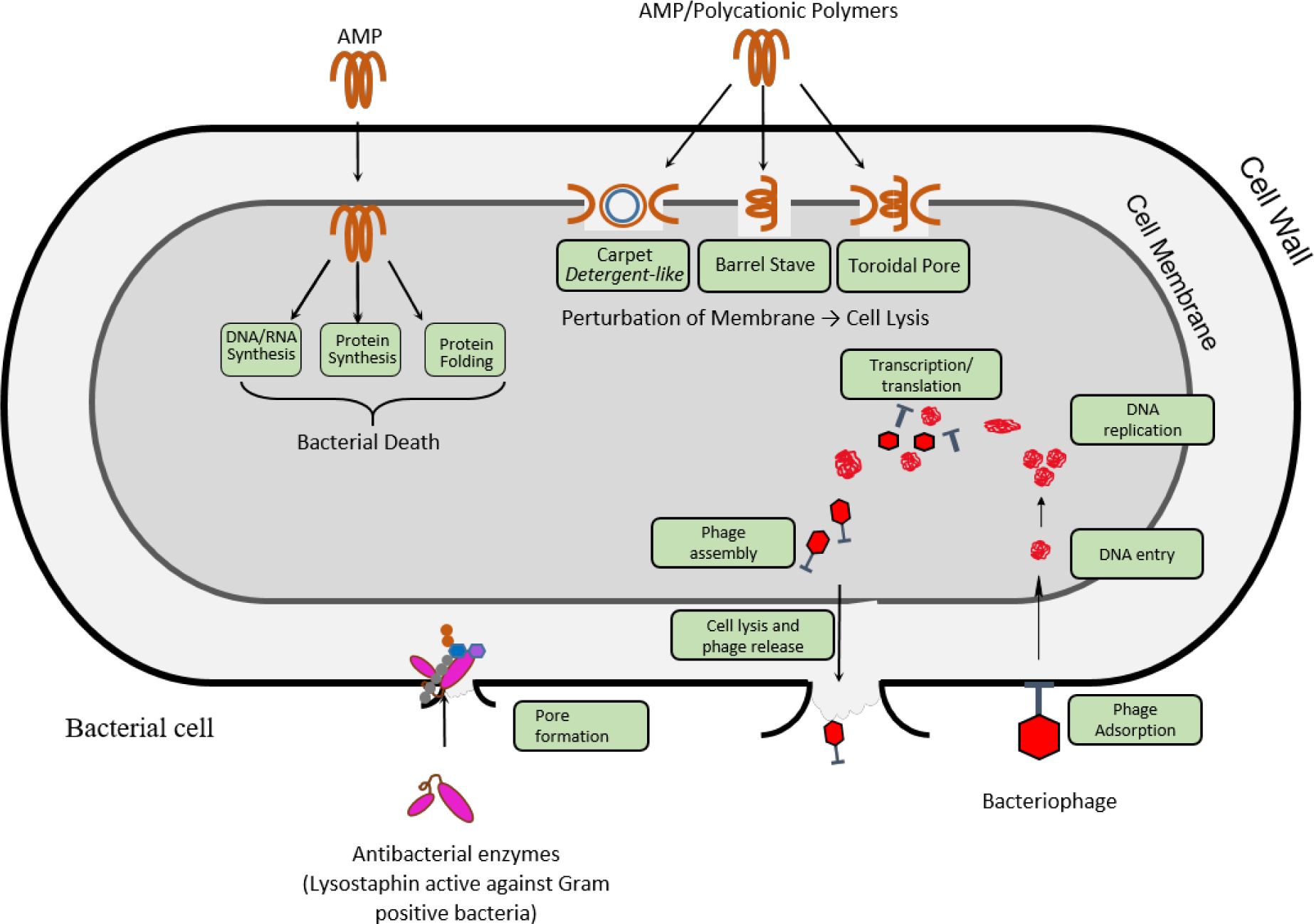
Cationic antimicrobial peptides and polymers can cause bacterial cell lysis through membrane perturbation. Proposed models for the underlying mechanism are the barrel stave model, the toroidal pore model and the carpet model. Alternatively, some peptides are proposed to cause bacterial death through membrane translocation followed by disruption of natural processes, such as DNA and RNA synthesis, protein synthesis and protein folding, which leads to cell death. Lysostaphin binds to the peptidoglycan layer of the bacterial cell wall in Gram-positive bacteria, such as S. aureus, and cleaves the pentaglycin bridges, leading to lysis and cell death. AMP, antimicrobial peptides. Adapted from ref. 19,53
[H2] Bone infection
Biomaterial-assisted delivery of bacteriophages has been explored to combat orthopaedic infections, which are often complicated by exposed wounds and trauma.24–27 For example, a synthetic hydrogel engineered from a 4-arm poly(ethylene glycol) (PEG) macromer can efficiently deliver bacteriophages that infect and kill the PsAer-9 strain of P. aeruginosa.24 Phages encapsulated within the hydrogel achieved a 5-fold reduction in counts of P. aeruginosa compared to unleaded hydrogels in a radial segmental bone defect mouse model.24 Similarly, a metal wire containing phages and linezolid, coated with hydroxypropyl methylcellulose (HPMC), could mitigate methicillin-resistant Staphylococcus aureus (MRSA) in a femur fracture murine model.27 Thus, these engineered devices show potent antimicrobial activity against S. aureus and P. aeruginosa, the two bacteria responsible for most device-related orthopaedic infections.25,27
[H2] Lung infection
Bacteriophages have also shown promise for the treatment of bacterial lung infections. For example, a nebulizer has been tested for the aerosolized delivery of bacteriophages to the lungs.28,29 Such nebulizer-based delivery of phages is facile; however, the use of nebulizers or intranasal administration can affect phage stability and is limited by low patient compliance. Biomaterial-assisted delivery of bacteriophages has shown efficacy in treating various forms of respiratory infections.29–31 Intranasal delivery of liposome-entrapped phages proved effective as a prophylactic against Klebsiella pneumoniae.31 Porous poly(lactic-co-glycolic acid) (PLGA) microparticles loaded with phages can be formulated as an inhalable dry powder and delivered to infected murine lungs via the endotracheal route (FIG. 3a)32. These phage-loaded microparticles significantly reduced bacterial counts in infected wild-type and transgenic mice with cystic fibrosis, whereas delivery of free phages (that is, no microparticles) did not reduce bacteria counts and had equivalent bacteria levels as animals treated with bacteria alone.32 These PLGA microparticles, formulated as dry powder, maintain phage activity and can kill clinical strains of P. aeruginosa in the lungs.
Figure 3. Nano- and microparticle-based antibacterial therapies.

A) Poly(lactic-co-glycolic acid) porous microparticles can be used for bacteriophage delivery and can be formulated as dry powder. B) Chitosan-peptide composite nanoparticles prolong the retention time at the site of infection. C) Magnetic nanorobots enable biofilm degradation. EPS, extracellular polymeric substances; PEG, poly(ethylene glycol). Reproduced from ref.32,59,123
[H2] Gastrointestinal infection
Bacteriophage-mediated treatment of gastrointestinal (GI) bacterial infections is challenging because of the susceptibility of phages to the extremely low pH levels in the stomach, as well as the presence of bile and intestinal tract enzymes. Therefore, biomaterials need to be used to deliver active phages to the GI tract. pH-responsive microparticles have been developed that can encapsulate and release phages at specific pH values.33,34 For example, Eudragit® S100, a poly(methyl methacrylate)-based polymer,35 and alginate particles have been shown to effectively deliver phage to treat Escherichia coli in the GI tract33 and Clostridium difficile in the colon, respectively34. Calcium carbonate microparticles preserve phage efficacy in simulated gastric fluid.36 Salmonella-targeting phages can be encapsulated in alginate/CaCO3 microparticles, which can be orally delivered to broiler chickens infected with Salmonella.37 In this case, encapsulation protects phages against the acidic pH of gastric juice, and thus, improves treatment efficacy, compared to free phage delivery.37 Chitosan nanoparticles can also maintain phage efficacy, following oral delivery for the treatment of E. coli infections in broiler chickens.38 Alternatively, a microemulsion-based transdermal system has been designed for the systemic delivery of phages to treat gastrointestinal E. coli infections, bypassing the need to protect phages from the pH conditions in the GI tract.39,40 Here, phages are delivered to the bloodstream through the skin, and the transdermal system showed promising results in vivo in rodents, significantly improving the survival rate of treated mice.39
[H2] Wound infection
Biomaterials, such as fibrin glue, nanofibres or liposomes, can also facilitate bacteriophage delivery to treat dermal wound infections.41–45 For example, fibrin glue containing bacteriophages exhibit sustained release of high titers of phages and reduced the counts of P. aeruginosa in vitro.45 Phage-coated nanofibres can further be applied to treat E. coli and P. aeruginosa infections, improving wound healing in murine41 and rabbit42 models. In vitro antibacterial assays demonstrated that phage- and bee venom-loaded nanofibres composed of honey, polyvinyl alcohol and chitosan can almost entirely eradicate a multidrug-resistant P. aeruginosa wound infection,41 and polycaprolactone/collagen I (PCL-ColI) nanofibres loaded with phages inhibit E. coli growth by >90%.42 These studies have established the potential of phage-loaded antibacterial wound dressings to control the development of antibiotic resistance.
Liposomes have also been explored as phage delivery vehicle to treat wound infections. For example, two phages lytic against S. aureus, can be encapsulated in liposomes for the treatment of dermal wound infections in a diabetic mouse model.43 Encapsulation of phages increases phage persistence at the infection site, leading to improved wound healing and infection mitigation, as compared to free phage treatment.43 Similarly, a phage cocktail loaded into liposomes can target Klebsiella pneumoniae, a bacterium common to burn wound infections.44 The liposome-entrapped phage therapy mitigates infection and improves wound healing at a greater rate than treatment with free phages.44 Entrapment of phages in liposomes increases their retention time in vivo by enabling sustained release, which increases wound treatment efficacy in a murine model.43,44
[H2] Catheter-associated infection
Catheter-associated urinary tract infections (CAUTIs) constitute 75% of reported urinary tract infections.46 CAUTIs can be prevented by using antibacterial catheters; in particular, bacteriophage-loaded or -coated catheters have garnered considerable interest owing to the rise of antibiotic resistance. Treatment of catheters with phages reduces the formation of P. aeruginosa and Pseudomonas mirabilis biofilms, both clinically relevant bacterial infections.47,48 Infection-responsive catheters can be designed with pH-triggered bacteriophage release.49 These coated catheters release bacteriophages in response to an increase in pH triggered by the expression of bacterial urease. The bacteriophages control the infection and lower the pH, which reduces the precipitation of struvite and apatite crystals. This strategy proved to be effective in infection treatment and reduction of catheter blockage caused by the precipitation of these crystals.
Intravascular catheter-related bloodstream infections occur in approximately 250,000 cases per year in the US.50 In addition, biofilms that form around intravascular catheters are more prone to the development of antimicrobial resistance.50 Bacteriophage-loaded or -coated catheters can mitigate and prevent bloodstream infections. For example, hydrogel-coated catheters, pre-treated with phages, significantly reduce biofilm formation by Staphylococcus epidermidis and P. aeruginosa.51,52 Similarly, the treatment of infected central venous catheters with phages significantly decreases bacterial activity and biofilm formation in a rabbit model.50 These results support the impetus for bacteriophage treatment as a prophylactic therapy to combat catheter-related bloodstream infections.
[H1] Antimicrobial peptide-containing materials
Antimicrobial peptides (AMPs) have been explored as antibacterial agents for infection treatment.53–55 Typically, AMPs are polycationic amphiphilic peptides with less than 50 amino acids. Based on their secondary structure, AMPs can be classified into α-helix, β-sheet, cyclic or extended-loop peptides. These cationic AMPs display bactericidal action by disrupting the bacterial cell membrane and by binding to intercellular targets (FIG. 2). AMPs are active against a broad spectrum of bacteria; however, high manufacturing costs, short half-lives (~hours) and cytotoxicity represent challenges for clinical translation.56,57
Advances in biomaterial engineering have promoted the use of AMPs to treat systemic58 and local59,60 infections by reducing side effects associated with antibacterial treatments. Surface coating with AMPs has been investigated to prevent device-associated infections,61–63 including catheter-related infections.64–66 Biomaterials facilitate co-delivery of an AMP and an adjuvant, improving the efficacy of AMP treatment. For example, transformable chitosan–AMP nanoparticles enable accumulation and long-term retention of AMPs at the site of infection after intravenous delivery, resulting in enhanced binding and killing of bacteria (FIG. 3b).59 These nanoparticles consist of chitosan conjugated with an AMP and a peptide that can be cleaved by gelatinase, an enzyme secreted by a broad spectrum of bacteria. Upon cleavage of the peptide by gelatinase, the nanoparticle assembly collapses and the chitosan-AMP is reoriented into nanofibres, which accumulate at the site of infection and provide sustained release of AMPs.59 Similarly, a tandem peptide cargo consisting of a bacterial membrane-targeting peptide and an AMP can be loaded onto porous silicon nanoparticles. The nanoparticles efficiently deliver the two peptides into the lungs, where they can act synergistically to reduce P. aeruginosa infections. Importantly, this treatment strategy showed an improved safety profile, compared to free peptide treatment, when delivered via tracheal instillation in a mouse lung infection model.60
[H2] Bone infection
Modification of titanium surfaces with AMPs can prevent or eradicate bacterial infections at the site of bone injury treated with biomedical devices. Titanium can be functionalized with AMPs using various approaches, including covalent tethering of AMPs,67 nano-topological treatment of titanium followed by adsorption of an AMP within the nanopores,68,69 and physical adsorption of an AMP-containing coating made of materials such as collagen, calcium phosphate, chitosan or fibrin.70,71 For example, AMPs, such as HHC36 (KRWWKWWRR) and its derivate with a terminal cysteine Tet213 (KRWWKWWRRC), can be loaded into calcium phosphate and coated onto titanium. The coating eradicates S. aureus and P. aeruginosa infections in vitro without impairing bone growth in a rabbit femur implant model.72 However, physical adsorption of single-layered coatings only provides antimicrobial activity for a limited time, which may not be sufficient to achieve complete clearance of infections in vivo. Alternatively, a layer-by-layer technique can be applied to coat a collagen-functionalized AMP on titanium, which achieves antimicrobial activity for more than a month against S. aureus and prevents biofilm formation in an in vitro assay.71 Similarly, covalent grafting of the AMP melimine on titanium surfaces reduces biofilm formation, achieves 100-fold more efficient clearance of P. aeruginosa and S. aureus, compared to unmodified surfaces, and allows the fabrication of robust antimicrobial implants that are stable upon ethylene oxide sterilization.67
Hydrogels and 3D scaffolds have been engineered to deliver AMPs for the treatment of osteomyelitis.73,74 Hydrogels based on RADA16 (COCH3-RADARADARADARADA-CONH2) 75 can deliver AMPs over a period of 28 days.73 RADA16 is composed of hydrophilic and hydrophobic domains, arranged in a defined order that facilitates self-assembly of the peptide.75,76 RADA16 hydrogels complexed with an AMP form an interwoven nanofibre assembly, which allows sustained release of AMPs, reducing bacterial load and improving bone formation in a rabbit osteomyelitis model. Similarly, porous poly(ether ketone) scaffolds provide sustained delivery of mouse beta-defensin-14 (MBD-14) to clear periprosthetic joint infections caused by S. aureus and P. aeruginosa in rodents.74
[H2] Wound infection
Hydrogels are the material of choice for the delivery of AMPs to wound infections.77–79 For example, hydrogels based on citric acid, egg-shell membranes or DNA nanostructures have been used in the treatment of acute wound infections.80–82 This DNA hydrogel, encapsulating an L12 peptide (LKKL)3, resulted in a 3-orders-of-magnitude reduction in S. aureus infection, compared to empty (no AMP) hydrogels, in an ex vivo porcine skin infection model. The treatment of refractory wounds, which are characterized by chronic bacterial infections, is difficult owing to the presence of biofilms.83,84 Using luciferase-expressing bacteria to track infection, a combination therapy of melittin, an AMP derived from honey bee venon, and tobramycin, loaded in an agarose hydrogel, achieves a 4-fold reduction of P. aeruginosa bioluminescence compared to mice treated with empty hydrogel or tobramycin treatment alone.85 A pH-switchable hydrogel has been designed based on a de novo synthesized AMP with alternating hydrophobic and hydrophilic amino acid sequences, and opposite charges at each end (FIG. 4a).86 At neutral pH, the AMP self-assembles into a supramolecular hydrogel matrix by non-covalent electrostatic interactions and hydrogen bonding. Lowering of the pH (pH = 5.5) leads to loss of electrostatic and hydrogen-bonding forces within the hydrogel and disassembly of the hydrogel network, and subsequently, the release of the AMP. Encapsulation of cypate, a photothermal agent, in this hydrogel, enables biofilm degradation upon heat activation, followed by mitigation of the infection by the AMP. This hydrogel system eradicated MRSA biofilms in vivo, and co-delivery of proline within the hydrogel improved wound healing in a diabetic mouse model.86
Hydrogel-based antibacterial therapies.

A) A pH-switchable hydrogel can be assembled by an antimicrobial peptide (AMP) with alternating hydrophobic and hydrophilic amino acid sequences, and opposite charges at each end. At neutral pH, the AMP self-assembles into a supramolecular hydrogel matrix by non-covalent electrostatic interactions and hydrogen bonding. At low pH (pH = 5.5), the hydrogel disassembles and releases the AMP. B) Schematic of a 4-arm polyethylene glycol (4-arm PEG) hydrogel that can be used for the delivery of lysostaphin. A 10 kDa 4-arm PEG macromer with maleimide functional groups can be cross-linked with a bi-thiol substituted degradable cross-linker and thiol-bearing, collagen-mimetic peptides (GFOGER). Lysostaphin is physically encapsulated within the hydrogel. Reproduced from ref.86,99
In addition to the treatment of chronic bacterial infections, treatments that prevent excessive inflammation in refractory wounds are needed. A hydroxypropyl cellulose hydrogel has been designed to deliver a dual-function thrombin-derived peptide (TCP-25), which is an antibacterial peptide and a scavenger of pathogen-associated molecular patterns, for example, lipopolysaccharide (LPS). The hydrogel allows the peptide to undergo conformational changes and LPS binding to simultaneously modulate bacterial infection and inflammation. The TCP-25-loaded hydrogel significantly reduces bacterial counts and inflammation in murine subcutaneous wound and porcine partial thickness wound models, and improves wound healing.87
[H2] Ophthalmic infection
AMPs have also been explored as antibacterial agents to treat eye infections. For example, silicone hydrogel lenses covalently coated with melimine can reduce the incidence of microbial keratitis, contact lens-induced acute red eye, and contact lens-induced peripheral ulcers.88–90 Melimine-coated contact lenses reduced the incidence of non-infectious keratitis in a guinea pig model and P. aeruginosa–driven microbial keratitis in a rabbit model, by reducing the number of adherent viable bacteria on the lens.89,90 A randomized, double-masked, one-day human clinical trial with follow-up visits after 1 and 4 weeks showed that melimine-coated contact lenses can be safely worn without any major adverse effects and that the lenses retained antimicrobial activity after wear.88 However, they were associated with increased corneal staining. Silicone hydrogel coated with Mel-4, a peptide derived from melamine, did not show signs of ocular irritation in a rabbit model.91
[H1] Delivery of antibacterial enzymes and proteins
Bacteriolytic enzymes produced by bacteria or bacteriophages have shown promise in the treatment of infections caused by pathogenic bacteria. Lysostaphin is a metalloendopeptidase produced by Staphylococcus simulans that is specific against Staphylococcal species. Lysostaphin is active against planktonic bacteria, sessile bacteria and MRSA, and it kills bacteria within a biofilm by cleaving the pentaglycine bridges in the peptidoglycan layer of Staphylococci (FIG 2).92 Therefore, lysostaphin is an ideal enzyme for the treatment of infections caused by Staphylococci. More than 66% of orthopaedic infections are caused by S. aureus and S. epidermidis, and thus, lysostaphin represents a potentially powerful therapeutic agent to combat orthopaedic infections.
Natural polymers, metals, bone cements and collagen matrices have been explored as carriers for lysostaphin.93–96 These materials can directly deliver lysostaphin to the infected site or deliver designer cells programmed to produce lysostaphin.97,98 Natural polymers, such as polysaccharides and collagen, are limited by batch-to-batch variability in structure and composition, which leads to inconsistencies in the physical and mechanical properties of the biomaterial, and limited control over polymerization kinetics. To overcome these limitations, synthetic hydrogels can be used; for example, hydrogels can be formed from 4-arm PEG, with each arm terminating in a maleimide group (PEG-4MAL) (FIG. 4b).99 The PEG-4MAL hydrogel system polymerizes through mild thiol-ene Michael addition chemistry, which can be carried out at physiological conditions. This synthetic hydrogel enables the stoichiometric incorporation of bioactive peptides and can serve a carrier with well-defined mesh size and mechanical properties. Moreover, the small degradation products of the hydrogel are excreted via urine, which makes it minimally toxic. The efficacy of PEG-4MAL hydrogel-based delivery of lysostaphin was demonstrated in two clinically relevant infection murine models – a femur fracture model and a non-union radial segmental bone defect model.99,100 In both models, hydrogel-based lysostaphin delivery completely cleared the infection, outperforming standard-of-care antibiotic therapy, and restored complete bone healing. In the non-union segmental bone defect model, the synthetic hydrogel enabled co-delivery of lysostaphin and bone morphogenetic protein-2 (BMP-2) to simultaneously eliminate S. aureus infection and promote bone regeneration to bridge the segmental bone defect. Notably, the lysostaphin-delivering hydrogel restored the inflammatory environment at the site of infection to a healthy (non-infected) microenvironment, as determined by inflammatory cytokine and immune cell levels, without causing local or systemic toxicity.
Lysins are a family of phage-encoded enzymes that bind and lyse the cell wall of bacteria with high specificity (FIG. 2).101 Lysins specific against Gram-positive and Gram-negative strains have been identified. Lysins can also kill antibiotic-resistant Gram-positive bacteria, such MRSA, and thus, have been explored in vivo and in clinical trials for the treatment of different types of infections. However, maintaining lysin stability and concentration at the site of infection remains a challenge, which can be addressed by biomaterial-assisted delivery. For example, encapsulation of LysRODI, an endolysin active against S. aureus, in pH-sensitive liposomes improves its stability.102 Loading Cpl-1, an antipneumococcal lysin derived from Cp-1 phage, into mucoadhesive chitosan nanoparticles increases its bioavailability, while it remains active against pneumococcal infection.102,103
[H1] Polycationic polymers
Polycationic polymers have broad-spectrum antimicrobial properties, anti-biofilm properties and bactericidal activity against multi-drug resistant bacteria.104–109 Although cationic polymers, such as polyethylene imine and polylysine, exhibit antimicrobial properties,105,110 their cytotoxicity towards mammalian cells has been a major barrier to their use for the treatment of systemic infections. Nanoparticles prepared from quaternary ammonium substituted polymer show potent bactericidal activity without deleterious effects on mammalian red blood cells.111 Polycationic antimicrobial polymers have the advantage that their properties can be tailored to the type of infection. For example, nanoparticles made of a dextran-based cationic block-copolymer (DA95B5) kill bacteria within biofilms by a unique mechanism-of-action.112 These nanoparticles adhere to bacteria within the biofilm and promote their dissipation. Once the bacteria are removed from the biofilm, they are killed by the quaternary ammonium group present on the block copolymer. The nanoparticles are bactericidal against antibiotic-resistant bacteria within biofilms, and a hydrogel pad dressing containing DA95B5 nanoparticles outperformed vancomycin in a murine dermal wound model.112 Cationic polymers have also been used to modify the properties of implantable devices. For example, medical-grade catheters can be made antibacterial by coating with a biodegradable paint designed from quaternary chitin polymers, significantly reducing MRSA infection in mice.106
Polycationic block copolymers display antimicrobial properties by virtue of their positive charge, which interacts with the negatively charged membrane of the bacterial cell membrane. The hydrophobic domain of the polymer then creates pores in the bacterial cell wall. Although the lack of selective toxicity toward bacteria has challenged clinical translation, an increased understanding of the mechanism(s) of action of these materials, as well as the development of chemistries, such as click chemistry and controlled radical polymerization, have allowed the precise modification of the structure of polycationic block copolymers, which has resulted in their re-emergence as valid candidates for treating bacterial infections.111,113 By tuning the ratio of hydrophobicity to positive charge, more selective antibacterial materials could be prepared, which have low toxicity against mammalian cells.114–116 Moreover, this new generation of materials has demonstrated lower propensity to trigger the development of bacterial resistance as compared to non-polymeric antibacterial therapies.117,118 The ability of these materials to treat antibiotic-resistant infections, including colistin-resistant infection in a mouse peritonitis model, has definitely expanded our arsenal to fight infectious diseases.118
[H1] Alternative antibacterial biomaterials
Alternative antibacterial therapies are being investigated in early stages of basic research.119–122 For example, magnetic robots and magnetically targeted composites are being explored for drug delivery to specific sites in the body. Magnetic catalytic antimicrobial robots (CARs) consisting of iron oxide nanoparticles can be engineered in a buffer containing hydrogen peroxide and extracellular polymeric substance (EPS)-degrading enzymes. The generation of bactericidal free radicals and the presence of degrading enzymes can mitigate bacterial infection and causes disruption of biofilms (FIG. 3c).123 Subsequent application of a magnetic field then leads to the assembly of the nanoparticles into a plow-like structure, which removes the biofilm. The magnetic CARs eliminated biofilms in a controlled manner and prevented biofilm regrowth in an ex vivo human tooth infection model. Alternatively, microwave-assisted magnetic carbon nanotube composites can penetrate into tissue and deliver gentamicin, which can eradicate osteomyelitis caused by MRSA in a rabbit model.124
Inhibiting the transmission of infectious diseases is paramount to curb the spread of an epidemic or pandemic. To restrict transmission of emerging infections caused by Gram-positive and Gram-negative bacteria, a reactive oxygen species (ROS)-releasing nanofibrous membrane has been developed, with a release mechanism sensitive to sunlight.125 These membranes are made of conjugated materials from benzophenones and polyphenols that allow electron transfer upon solar activation, which ultimately results in the release of ROS. The membrane showed 6 orders of magnitude higher killing efficiency against E. coli and Listeria innocua in <1h of day light irradiation compared to control poly(vinyl alcohol-co-ethylene) nanofibrous membranes. Good breathability, the capacity to filter fine particles, and high (>99.999%) bacterial and viral killing efficiency highlight the potential of these membranes to be used in masks and personal protective equipment.
[H1] Choosing a biomaterial
The year 2020 marked the hundredth year of research in polymer science.126 One century-long research has provided us with a variety of polymer materials, which can be used for antibacterial therapies. The choice of biomaterial is ultimately dictated by its desired role (TABLE 4); that is, delivery vehicle (nanoparticles, hydrogels); a material to modify the properties of a biomedical device (polymeric coatings and brushes); or a material with inherent antimicrobial properties (scaffolds or membranes of polycationic materials). In addition, the intended mode of delivery (systemic versus local), the site of infection, compatibility of the antimicrobial agent with the biomaterial, and desired rate of release should be considered. For example, systemic delivery requires materials that are stable in serum conditions, such as amphiphilic nanoparticles or liposomes.127 Similarly, for the treatment of local infections, the physiological environment at the site of infection needs to be considered. Biomaterials responsive to the local environment (pH, temperature, light, enzymes) can be used to achieve targeted delivery of antibacterial agents.37,125 Ideally, the goal should be to develop a formulation that not only treats the infection but that also restores the local physiological conditions to the pre-infection state. For example, hydrogel-based therapies can be used to treat bone infection, because they not only allow efficient release of the bactericidal agent but also be engineered to recruit osteogenic cells and inflammatory cells to promote bone regeneration 99,100
The compatibility of the antibacterial agent with the biomaterial is important to achieve sufficient loading efficiency without detrimental effects to the agent. Similarly, the biomaterial should be tunable so that the rate of release can be controlled by engineering its physical properties, mesh size and degradability.127 If delayed or extended release of the agent is desired, biomaterials with complementary chemical functional groups should be chosen, allowing the agent to be covalently conjugated to the biomaterial. Finally, injectable biomaterials should be considered for treatments, for which invasive surgeries are not desirable.
[H1] Outlook
[H2] Clinical translation of antimicrobial therapies
[H3] Development of chronic infection animal models
Most biomaterial-based therapies discussed in this Review are currently at different stages of preclinical evaluation in small animal models32,87,99,100. To achieve successful translation to the clinic, several important steps must be completed. First, antibacterial efficacy and safety need to be assessed in relevant infection models, especially in large animal models. The majority of biomaterial-based therapies have mainly been evaluated for antibacterial efficacy in vitro, and in vivo studies are often limited to the evaluation of biocompatibility or performed in models that do not consider important aspects of clinical settings, such as trauma, co-morbidities or established infections.72,73 Many preclinical studies evaluating antibacterial efficacy in vivo are limited to acute infection animal models, in which bacteria and treatment are delivered together or in close succession.99,100 Such studies are effective in evaluating the prophylactic efficacy of the antibacterial therapy, but do not encompass challenges associated with an established, chronic infection, such as an altered inflammatory environment or the presence of a biofilm.128 To date, only few models exist with consistent chronic bacterial infections.129,130 Therefore, small and large animal models need to be developed with established infections and presence of a biofilm for the robust evaluation of these therapies in more relevant settings. In addition, bacterial strains are often used that have adapted to lab culture and have lost expression of virulence and other important factors.131 Thus, studies with clinical isolates of relevant species are required. Moreover, such clinical isolates should be grown in relevant media that mimics the diseased environment, before inoculation in animals, to recapitulate the genetic make-up and evolution of bacteria from a clinical setting132,133.
[H3] Evaluation of host immune response
A majority of preclinical studies have mainly evaluated antibacterial efficacy, not considering the overall physiological effect of a therapy.62,85 Evaluation of the immune cell response to the therapy, the biomaterial carrier and tissue and bacteria debris should also be performed. This consideration is especially important for therapies that employ antibacterial agents with a bacterial or viral origin, because such therapies have the intrinsic potential to trigger immunogenic responses in the host.134–136 For example, treatment with lysostaphin can result in the production of anti-lysostaphin antibodies, which can neutralize its efficacy.134,135 Although mutants of lysostaphin have been engineered to lower its immunogenicity,137,138 the presence of anti-lysostaphin antibodies needs to be assessed in conjunction with antibacterial studies to demonstrate clinical potential of the therapy.
Host immune responses to the biomaterial carrier and therapeutic agent should also be evaluated. An ideal biomaterial should be non-toxic to mammalian cells and should not induce immunogenic responses at a therapeutically relevant concentration. The immune responses and toxicity profile of a biomaterial carrier can be influenced by the presence of a therapeutic agent and by the material formulation. For example, PEG is generally considered as a well-tolerated, safe material.139 However, the development of antibodies against PEG has been reported, and these antibodies can accelerate the clearance of a PEG formulation and reduce the efficacy of the therapy.140 The binding affinity of anti-PEG antibodies depends on the hydrophobicity of the terminal groups and on the length of the PEG backbone.141 Indeed, the PEG derivative oligo(ethylene glycol) (OEGMA), which has a hydrophilic PEG chain-end and multiple short PEG units attached to a methacrylate backbone, does not induce immunogenic responses when used to subcutaneously deliver a therapeutic peptide in a non-infection, diabetic mouse model.142 The potential significance of the presence of anti-PEG antibodies needs to be considered; however, it should be noted that these studies have focused on the systemic delivery of PEG chains covalently conjugated to the therapeutic. Additional research is necessary to ascertain whether anti-PEG antibodies are also generated against other PEG formulations (for example, hydrogel networks) that deliver therapeutics, and importantly, whether these antibodies have adverse consequences.
In addition to these scientific challenges, technological and financial hurdles need to be overcome for these technologies to progress to the clinic. Biomaterial-based antibacterial therapies are currently produced on a lab scale and are relatively expensive compared to antibiotic therapies.53 The scale-up of biomaterial-based technologies will require the large-scale production of all individual components, including the antibacterial agent, biomaterial and the fabrication of the antibacterial formulation. Advances in the high-throughput production of bacteriophages143 and antibacterial peptides144, and the reduction in the production cost of lysostaphin with the development of advanced techniques145–147 provide encouraging results for the scale-up and clinical use of these technologies, in particular, for materials that are commercially available.148 However, additional studies need to be performed to develop efficient strategies to formulate these technologies on a large scale and at low cost.
The recent success of antibacterial agents, such as the phage lysin, in clinical trials149, and the use of bacteriophages as a last-resort drug13,22,23, provide optimism for their clinical translation. Lysin, in particular, showed superior activity in combination with antibiotics against S. aureus in patients with bacteremia and endocarditis, without any additional adverse side effects compared to standard-of-care antibiotics used in the study.149 Start-up companies, such as ContraFect, GangaGen and Lysando GmbH, have also developed promising antibacterial technologies,150,151 and several encouraging antibacterial therapies are currently in clinical trials (TABLE 2).152–154
[H3] Approaches to reduce the development of resistance
A major rationale for the development of non-antibiotic therapies is the targeting of infections that are resistant to antibiotics. In response to non-antibiotic therapeutics, bacteria can undergo evolutionary changes and also develop mechanisms for resistance.129 Therefore, the potential development of resistance to these therapies should be investigated in preclinical studies. Moreover, combinatorial therapies could be applied to reduce the development of resistance. An advantage of biomaterial carriers is their ability to co-deliver two or more agents to the site of infection. This advantage could be exploited to minimize resistance development. The development of bacterial tolerance can be linked to bacterial resistance, and strategies have been explored to arrest this tolerance-resistance sequence by sustained delivery of the antibacterial at a dose above the minimum inhibitory concentration (BOX 1) 1,10,155. Biomaterials are ideal candidates for sustained and targeted delivery of antibacterial agents, allowing maintenance of the required antibacterial dose for a long duration of time. In addition to the antibacterial agents discussed in this Review, antibodies156,157, vaccines158,159, immune-stimulating agents,160 and adjuvant peptides, such as host and innate defense peptides160,161 as well as anti-biofilm peptides162 are emerging as promising antibacterial candidates. Although some of these agents do not have antibacterial properties per se, they play an indirect role in fighting infection by modulating the levels of anti-inflammatory chemokines and cytokines and/or by inhibiting pro-inflammatory cytokines, or by limiting biofilm formation in the case of anti-biofilm peptides.160–162 Currently, research on alternative antibacterial agents is mainly focused on designing therapies that are more efficacious than antibiotics, because this a key criterion to secure regulatory approval.53 Importance should also be given to the development of therapies that may not be as efficacious as antibiotics, but that may have less propensity to triggering the development of resistance.
[H2] Beyond conventional antibacterial therapies
With the development of next generation antibiotics and antibacterial therapies, an in-depth understanding of the host immune response and the dual action of antibacterial and anti-inflammatory formulations will pave way for the identification of new targets and strategies to combat infections.87,163–165 A better understanding of the mechanisms and factors that render some patients predisposed to certain infections remains an important area of research. For example, sepsis-induced inflammation has been linked to critical illness-related immune suppression.166,167 The underlying mechanism causing such immunosuppression has been identified; the inactivation of dendritic cells and alveolar macrophages leads to enhanced susceptibility of recovering patients to infections, such as hospital-acquired pneumonia.168 Interestingly, the epigenetic inactivation of immune cells is not triggered by the pathogen that causes the primary infection, but by signaling chemicals secreted by the host after clearance of the primary infection. Thus, the identification of pathways reveals new targets, which can be explored for the development of innovative and effective therapies. Similarly, the role of mast cells in modulating the immune response to microbial infiltration has been investigated, and agents have been reported that activate mast cells to promote clearance of skin infections caused by S. aureus.121 We envision future therapies employing a combinatorial strategy by using an antimicrobial agent and an immune-modulating agent to combat bacterial infections.
In summary, the development of new antibacterial therapies is increasingly challenging, in particular, against Gram-negative bacteria, with only a few new therapies in the pipeline.169,170 Biomaterial-based therapies can improve the efficacy of existing antibacterial agents by enabling sustained release to provide local, effective concentrations with reduced off-target effects and toxicities, by increasing the stability and activity of the therapeutic agent, by facilitating co-delivery of other agents and adjuvants, and by modulating the local microenvironment. With the development of advanced materials, coupled with the promising bactericidal properties of new antibacterial agents, translation of biomaterials-based therapies will be attained in the near future. Advances in biomaterial engineering will further allow the co-delivery of different classes of antibacterial agents to maximize the therapeutic index and minimize the development of resistance and other adverse effects.
Acknowledgements:
The authors acknowledge support from the Cystic Fibrosis Foundation (CFF GARCIA17G0) and the National Institutes of Health (R01 AR062920).
Footnotes
Competing interests: AJG is an inventor in a patent application on the lysostaphin-delivering hydrogel filed by the Georgia Tech Research Corporation (no. 16/191,685, filed on 15 November 2018). The authors declare no other competing interests.
References
- 1.Liu J, Gefen O, Ronin I, Bar-Meir M & Balaban NQ Effect of tolerance on the evolution of antibiotic resistance under drug combinations. Science 367, 200–204, doi: 10.1126/science.aay3041 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Costerton JW, Stewart PS & Greenberg EP Bacterial Biofilms: A Common Cause of Persistent Infections. Science 284, 1318–1322, doi: 10.1126/science.284.5418.1318 (1999). [DOI] [PubMed] [Google Scholar]
- 3.Koo H, Allan RN, Howlin RP, Stoodley P & Hall-Stoodley L Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 15, 740–755, doi: 10.1038/nrmicro.2017.99 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pumart P et al. Health and economic impacts of antimicrobial resistance in Thailand. J. Health Serv. Res. Policy 6, 352–360 (2012). [Google Scholar]
- 5.Sprenger M & Fukuda K New mechanisms, new worries. Science 351, 1263–1264, doi: 10.1126/science.aad9450 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Edelstein MV et al. Spread of extensively resistant VIM-2-positive ST235 Pseudomonas aeruginosa in Belarus, Kazakhstan, and Russia: a longitudinal epidemiological and clinical study. Lancet Infect. Dis. 13, 867–876, doi: 10.1016/s1473-3099(13)70168-3 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Weiner-Lastinger LM et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 41, 1–18, doi: 10.1017/ice.2019.296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiner-Lastinger LM et al. Antimicrobial-resistant pathogens associated with pediatric healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 41, 19–30, doi: 10.1017/ice.2019.297 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiner LM et al. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 37, 1288–1301, doi: 10.1017/ice.2016.174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin-Reisman I et al. Antibiotic tolerance facilitates the evolution of resistance. Science 355, 826–830, doi: 10.1126/science.aaj2191 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Langdon A, Crook N & Dantas G The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 8, 39–39, doi: 10.1186/s13073-016-0294-z (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Santen KL et al. The Standardized Antimicrobial Administration Ratio: A New Metric for Measuring and Comparing Antibiotic Use. Clin. Infect. Dis. 67, 179–185, doi: 10.1093/cid/ciy075 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Dedrick RM et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 25, 730–733, doi: 10.1038/s41591-019-0437-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuch R, Nelson D & Fischetti VA A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418, 884–889, doi: 10.1038/nature01026 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Loeffler JM & Fischetti VA Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob. Agents Chemother. 47, 375–377, doi: 10.1128/AAC.47.1.375-377.2003 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CH & Lu TK Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics (Basel) 9, doi: 10.3390/antibiotics9010024 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Usmani SS et al. THPdb: Database of FDA-approved peptide and protein therapeutics. PLoS One 12, e0181748, doi: 10.1371/journal.pone.0181748 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donlan RM Preventing biofilms of clinically relevant organisms using bacteriophage. Trends Microbiol. 17, 66–72, doi: 10.1016/j.tim.2008.11.002 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Labrie SJ, Samson JE & Moineau S Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317–327, doi: 10.1038/nrmicro2315 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Dufour N, Delattre R, Ricard JD & Debarbieux L The Lysis of Pathogenic Escherichia coli by Bacteriophages Releases Less Endotoxin Than by β-Lactams. Clin. Infect. Dis. 64, 1582–1588, doi: 10.1093/cid/cix184 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho I & Blaser MJ The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 13, 260–270, doi: 10.1038/nrg3182 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schooley RT et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 61, doi: 10.1128/aac.00954-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jennes S et al. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury—a case report. Critical Care 21, 129, doi: 10.1186/s13054-017-1709-y (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wroe JA, Johnson CT & García AJ Bacteriophage delivering hydrogels reduce biofilm formation in vitro and infection in vivo. J. Biomed. Mater. Res. A 108, 39–49, doi: 10.1002/jbm.a.36790 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meurice E et al. New antibacterial microporous CaP materials loaded with phages for prophylactic treatment in bone surgery. J. Mater. Sci. Mater. Med. 23, 2445–2452, doi: 10.1007/s10856-012-4711-6 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Barros JAR et al. Encapsulated bacteriophages in alginate-nanohydroxyapatite hydrogel as a novel delivery system to prevent orthopedic implant-associated infections. Nanomedicine 24, 102145, doi: 10.1016/j.nano.2019.102145 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Kaur S, Harjai K & Chhibber S In Vivo Assessment of Phage and Linezolid Based Implant Coatings for Treatment of Methicillin Resistant S. aureus (MRSA) Mediated Orthopaedic Device Related Infections. PLoS One 11, e0157626, doi: 10.1371/journal.pone.0157626 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrigy NB et al. Prophylaxis of Mycobacterium tuberculosis H37Rv Infection in a Preclinical Mouse Model via Inhalation of Nebulized Bacteriophage D29. Antimicrob. Agents Chemother. 63, doi: 10.1128/aac.00871-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prazak J et al. Nebulized Bacteriophages for Prophylaxis of Experimental Ventilator-Associated Pneumonia Due to Methicillin-Resistant Staphylococcus aureus. Crit. Care Med, doi: 10.1097/ccm.0000000000004352 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Golshahi L, Lynch KH, Dennis JJ & Finlay WH In vitro lung delivery of bacteriophages KS4-M and ΦKZ using dry powder inhalers for treatment of Burkholderia cepacia complex and Pseudomonas aeruginosa infections in cystic fibrosis. J. Appl. Microbiol. 110, 106–117, doi: 10.1111/j.1365-2672.2010.04863.x (2011). [DOI] [PubMed] [Google Scholar]
- 31.Singla S, Harjai K, Katare OP & Chhibber S Bacteriophage-loaded nanostructured lipid carrier: improved pharmacokinetics mediates effective resolution of Klebsiella pneumoniae-induced lobar pneumonia. J. Infect. Dis. 212, 325–334, doi: 10.1093/infdis/jiv029 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Agarwal R et al. Inhaled bacteriophage-loaded polymeric microparticles ameliorate acute lung infections. Nat Biomed Eng 2, 841–849, doi: 10.1038/s41551-018-0263-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinner GK, Richards K, Leppanen M, Sagona AP & Malik DJ Microencapsulation of Enteric Bacteriophages in a pH-Responsive Solid Oral Dosage Formulation Using a Scalable Membrane Emulsification Process. Pharmaceutics 11, doi: 10.3390/pharmaceutics11090475 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinner GK, Vladisavljević GT, Clokie MRJ & Malik DJ Microencapsulation of Clostridium difficile specific bacteriophages using microfluidic glass capillary devices for colon delivery using pH triggered release. PLoS One 12, e0186239, doi: 10.1371/journal.pone.0186239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thakral S, Thakral NK & Majumdar DK Eudragit: a technology evaluation. Expert Opin Drug Deliv 10, 131–149, doi: 10.1517/17425247.2013.736962 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Ma YP, Jennifer C; Wang Qi; Sabour Parviz M.; Huang Xiaoqing; Xu Yongping. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food Hydrocolloids 26, 434–440, doi: 10.1016/j.foodhyd.2010.11.017. (2012). [DOI] [Google Scholar]
- 37.Colom J et al. Microencapsulation with alginate/CaCO(3): A strategy for improved phage therapy. Sci. Rep. 7, 41441, doi: 10.1038/srep41441 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adamu Ahmad K, Sabo Mohammed A & Abas F Chitosan Nanoparticles as Carriers for the Delivery of ΦKAZ14 Bacteriophage for Oral Biological Control of Colibacillosis in Chickens. Molecules 21, 256, doi: 10.3390/molecules21030256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rastogi V, Yadav P, Verma A & Pandit JK Ex vivo and in vivo evaluation of microemulsion based transdermal delivery of E. coli specific T4 bacteriophage: A rationale approach to treat bacterial infection. Eur. J. Pharm. Sci. 107, 168–182, doi: 10.1016/j.ejps.2017.07.014 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Jain S, Chaudhari BH & Swarnakar NK Preparation and characterization of niosomal gel for iontophoresis mediated transdermal delivery of isosorbide dinitrate. Drug Deliv Transl Res 1, 309–321, doi: 10.1007/s13346-011-0035-1 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Sarhan WA & Azzazy HM Apitherapeutics and phage-loaded nanofibers as wound dressings with enhanced wound healing and antibacterial activity. Nanomedicine (Lond) 12, 2055–2067, doi: 10.2217/nnm-2017-0151 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Cheng W et al. Incorporation of bacteriophages in polycaprolactone/collagen fibers for antibacterial hemostatic dual-function. J. Biomed. Mater. Res. B Appl. Biomater. 106, 2588–2595, doi: 10.1002/jbm.b.34075 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Chhibber S, Kaur J & Kaur S Liposome Entrapment of Bacteriophages Improves Wound Healing in a Diabetic Mouse MRSA Infection. Front. Microbiol. 9, 561, doi: 10.3389/fmicb.2018.00561 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chadha P, Katare OP & Chhibber S Liposome loaded phage cocktail: Enhanced therapeutic potential in resolving Klebsiella pneumoniae mediated burn wound infections. Burns 43, 1532–1543, doi: 10.1016/j.burns.2017.03.029 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Rubalskii E et al. Fibrin glue as a local drug-delivery system for bacteriophage PA5. Sci. Rep. 9, 2091, doi: 10.1038/s41598-018-38318-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centers for Disease, C. Catheter-associated Urinary Tract Infections (CAUTI), <https://www.cdc.gov/hai/ca_uti/uti.html> (2015).
- 47.Lehman SM & Donlan RM Bacteriophage-mediated control of a two-species biofilm formed by microorganisms causing catheter-associated urinary tract infections in an in vitro urinary catheter model. Antimicrob. Agents Chemother. 59, 1127–1137, doi: 10.1128/aac.03786-14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao KS, Lehman SM, Tweardy DJ, Donlan RM & Trautner BW Bacteriophages are synergistic with bacterial interference for the prevention of Pseudomonas aeruginosa biofilm formation on urinary catheters. J. Appl. Microbiol. 113, 1530–1539, doi: 10.1111/j.1365-2672.2012.05432.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milo S et al. Prevention of encrustation and blockage of urinary catheters by Proteus mirabilis via pH-triggered release of bacteriophage. J Mater Chem B 5, 5403–5411, doi: 10.1039/c7tb01302g (2017). [DOI] [PubMed] [Google Scholar]
- 50.Lungren MP et al. Bacteriophage K antimicrobial-lock technique for treatment of Staphylococcus aureus central venous catheter-related infection: a leporine model efficacy analysis. J. Vasc. Interv. Radiol. 25, 1627–1632, doi: 10.1016/j.jvir.2014.06.009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curtin JJ & Donlan RM Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 50, 1268–1275, doi: 10.1128/aac.50.4.1268-1275.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu W et al. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob. Agents Chemother. 54, 397–404, doi: 10.1128/aac.00669-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mookherjee N, Anderson MA, Haagsman HP & Davidson DJ Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov 19, 311–332, doi: 10.1038/s41573-019-0058-8 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Di YP et al. Enhanced therapeutic index of an antimicrobial peptide in mice by increasing safety and activity against multidrug-resistant bacteria. Sci. Adv. 6, eaay6817, doi: 10.1126/sciadv.aay6817 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lazzaro BP, Zasloff M & Rolff J Antimicrobial peptides: Application informed by evolution. Science 368, eaau5480, doi: 10.1126/science.aau5480 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon YJ, Romanowski EG & McDermott AM A Review of Antimicrobial Peptides and Their Therapeutic Potential as Anti-Infective Drugs. Curr. Eye Res. 30, 505–515, doi: 10.1080/02713680590968637 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bacalum M & Radu M Cationic Antimicrobial Peptides Cytotoxicity on Mammalian Cells: An Analysis Using Therapeutic Index Integrative Concept. Int. J. Pept. Res. Ther. 21, 47–55, doi: 10.1007/s10989-014-9430-z (2015). [DOI] [Google Scholar]
- 58.Rai A et al. One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials 85, 99–110, doi: 10.1016/j.biomaterials.2016.01.051 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Qi GB, Zhang D, Liu FH, Qiao ZY & Wang H An “On-Site Transformation” Strategy for Treatment of Bacterial Infection. Adv. Mater. 29, doi: 10.1002/adma.201703461 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Kwon EJ et al. Porous Silicon Nanoparticle Delivery of Tandem Peptide Anti-Infectives for the Treatment of Pseudomonas aeruginosa Lung Infections. Adv. Mater. 29, doi: 10.1002/adma.201701527 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lim K et al. Anhydrous polymer-based coating with sustainable controlled release functionality for facile, efficacious impregnation, and delivery of antimicrobial peptides. Biotechnol. Bioeng. 115, 2000–2012, doi: 10.1002/bit.26713 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Qi F et al. Practical Preparation of Infection-Resistant Biomedical Surfaces from Antimicrobial β-Peptide Polymers. ACS Appl. Mater. Interfaces 11, 18907–18913, doi: 10.1021/acsami.9b02915 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Zhuk I et al. Self-Defensive Layer-by-Layer Films with Bacteria-Triggered Antibiotic Release. ACS Nano 8, 7733–7745, doi: 10.1021/nn500674g (2014). [DOI] [PubMed] [Google Scholar]
- 64.Zhang X-Y et al. Antimicrobial Peptide-Conjugated Hierarchical Antifouling Polymer Brushes for Functionalized Catheter Surfaces. Biomacromolecules 20, 4171–4179, doi: 10.1021/acs.biomac.9b01060 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Yu K et al. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 116, 69–81, doi: 10.1016/j.biomaterials.2016.11.047 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Gao Q et al. Rationally designed dual functional block copolymers for bottlebrush-like coatings: In vitro and in vivo antimicrobial, antibiofilm, and antifouling properties. Acta Biomater. 51, 112–124, doi: 10.1016/j.actbio.2017.01.061 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Chen R, Willcox MD, Ho KK, Smyth D & Kumar N Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 85, 142–151, doi: 10.1016/j.biomaterials.2016.01.063 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Shen X et al. Antibacterial and osteogenesis performances of LL37-loaded titania nanopores in vitro and in vivo. Int J Nanomedicine 14, 3043–3054, doi: 10.2147/IJN.S198583 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song Y-Y, Schmidt-Stein F, Bauer S & Schmuki P Amphiphilic TiO2 Nanotube Arrays: An Actively Controllable Drug Delivery System. J. Am. Chem. Soc. 131, 4230–4232, doi: 10.1021/ja810130h (2009). [DOI] [PubMed] [Google Scholar]
- 70.Kazemzadeh-Narbat M et al. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials 34, 5969–5977, doi: 10.1016/j.biomaterials.2013.04.036 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Shi J et al. Biological and immunotoxicity evaluation of antimicrobial peptide-loaded coatings using a layer-by-layer process on titanium. Sci. Rep. 5, 16336, doi: 10.1038/srep16336 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kazemzadeh-Narbat M et al. Drug release and bone growth studies of antimicrobial peptide-loaded calcium phosphate coating on titanium. J. Biomed. Mater. Res. B Appl. Biomater. 100, 1344–1352, doi: 10.1002/jbm.b.32701 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Yang G et al. Sustained Release of Antimicrobial Peptide from Self-Assembling Hydrogel Enhanced Osteogenesis. J. Biomater. Sci. Polym. Ed. 29, 1812–1824, doi: 10.1080/09205063.2018.1504191 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Yuan X et al. Multifunctional sulfonated polyetheretherketone coating with beta-defensin-14 for yielding durable and broad-spectrum antibacterial activity and osseointegration. Acta Biomater. 86, 323–337, doi: 10.1016/j.actbio.2019.01.016 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Cormier AR, Pang X, Zimmerman MI, Zhou H-X & Paravastu AK Molecular Structure of RADA16-I Designer Self-Assembling Peptide Nanofibers. ACS Nano 7, 7562–7572, doi: 10.1021/nn401562f (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Briuglia ML, Urquhart AJ & Lamprou DA Sustained and controlled release of lipophilic drugs from a self-assembling amphiphilic peptide hydrogel. Int. J. Pharm. 474, 103–111, doi: 10.1016/j.ijpharm.2014.08.025 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Irwansyah I et al. Gram-Positive Antimicrobial Activity of Amino Acid-Based Hydrogels. Adv. Mater. 27, 648–654, doi: 10.1002/adma.201403339 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Lohmann N et al. Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci. Transl. Med. 9, doi: 10.1126/scitranslmed.aai9044 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Li J, Liang S, Yan Y, Tian X & Li X O-Mannosylation Affords a Glycopeptide Hydrogel with Inherent Antibacterial Activities against E. coli via Multivalent Interactions between Lectins and Supramolecular Assemblies. Macromol. Biosci. 19, e1900124, doi: 10.1002/mabi.201900124 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Xie Z et al. Design of antimicrobial peptides conjugated biodegradable citric acid derived hydrogels for wound healing. J. Biomed. Mater. Res. A 103, 3907–3918, doi: 10.1002/jbm.a.35512 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu M et al. Fabrication of KR-12 peptide-containing hyaluronic acid immobilized fibrous eggshell membrane effectively kills multi-drug-resistant bacteria, promotes angiogenesis and accelerates re-epithelialization. Int J Nanomedicine 14, 3345–3360, doi: 10.2147/IJN.S199618 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Obuobi S et al. Facile and efficient encapsulation of antimicrobial peptides via crosslinked DNA nanostructures and their application in wound therapy. J. Control. Release 313, 120–130, doi: 10.1016/j.jconrel.2019.10.013 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Ch’ng J-H, Chong KKL, Lam LN, Wong JJ & Kline KA Biofilm-associated infection by enterococci. Nat. Rev. Microbiol. 17, 82–94, doi: 10.1038/s41579-018-0107-z (2019). [DOI] [PubMed] [Google Scholar]
- 84.Wolcott RD, Rhoads DD & Dowd SE Biofilms and chronic wound inflammation. J. Wound Care 17, 333–341, doi: 10.12968/jowc.2008.17.8.30796 (2008). [DOI] [PubMed] [Google Scholar]
- 85.Maiden MM, Zachos MP & Waters CM Hydrogels Embedded With Melittin and Tobramycin Are Effective Against Pseudomonas aeruginosa Biofilms in an Animal Wound Model. Front. Microbiol. 10, doi: 10.3389/fmicb.2019.01348 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J et al. pH-Switchable Antimicrobial Nanofiber Networks of Hydrogel Eradicate Biofilm and Rescue Stalled Healing in Chronic Wounds. ACS Nano 13, 11686–11697, doi: 10.1021/acsnano.9b05608 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Puthia M et al. A dual-action peptide-containing hydrogel targets wound infection and inflammation. Sci. Transl. Med. 12, eaax6601, doi: 10.1126/scitranslmed.aax6601 (2020). [DOI] [PubMed] [Google Scholar]
- 88.Dutta D, Ozkan J & Willcox MDP Biocompatibility of Antimicrobial Melimine Lenses: Rabbit and Human Studies. Optom. Vis. Sci. 91 (2014). [DOI] [PubMed] [Google Scholar]
- 89.Cole N et al. In vivo performance of melimine as an antimicrobial coating for contact lenses in models of CLARE and CLPU. Invest. Ophthalmol. Vis. Sci. 51, 390–395, doi: 10.1167/iovs.09-4068 (2010). [DOI] [PubMed] [Google Scholar]
- 90.Dutta D, Vijay AK, Kumar N & Willcox MD Melimine-Coated Antimicrobial Contact Lenses Reduce Microbial Keratitis in an Animal Model. Invest. Ophthalmol. Vis. Sci. 57, 5616–5624, doi: 10.1167/iovs.16-19882 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Dutta D et al. Development of Silicone Hydrogel Antimicrobial Contact Lenses with Mel4 Peptide Coating. Optom. Vis. Sci. 95, 937–946, doi: 10.1097/opx.0000000000001282 (2018). [DOI] [PubMed] [Google Scholar]
- 92.Gonzalez-Delgado LS et al. Two-site recognition of Staphylococcus aureus peptidoglycan by lysostaphin SH3b. Nat. Chem. Biol. 16, 24–30, doi: 10.1038/s41589-019-0393-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cui F et al. Development of chitosan-collagen hydrogel incorporated with lysostaphin (CCHL) burn dressing with anti-methicillin-resistant Staphylococcus aureus and promotion wound healing properties. Drug Deliv. 18, 173–180, doi: 10.3109/10717544.2010.509363 (2011). [DOI] [PubMed] [Google Scholar]
- 94.Windolf CD, Lögters T, Scholz M, Windolf J & Flohé S Lysostaphin-Coated Titan-Implants Preventing Localized Osteitis by Staphylococcus aureus in a Mouse Model. PLoS One 9, e115940, doi: 10.1371/journal.pone.0115940 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xue B et al. A Novel Controlled-Release System for Antibacterial Enzyme Lysostaphin Delivery Using Hydroxyapatite/Chitosan Composite Bone Cement. PLoS One 9, e113797, doi: 10.1371/journal.pone.0113797 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nithya S et al. Preparation, characterization and efficacy of lysostaphin-chitosan gel against Staphylococcus aureus. Int. J. Biol. Macromol. 110, 157–166, doi: 10.1016/j.ijbiomac.2018.01.083 (2018). [DOI] [PubMed] [Google Scholar]
- 97.Abulateefeh SR et al. Facile synthesis of responsive nanoparticles with reversible, tunable and rapid thermal transitions from biocompatible constituents. Chem. Commun, 6068–6070, doi: 10.1039/B911986H (2009). [DOI] [PubMed] [Google Scholar]
- 98.Guo S et al. Engineered Living Materials Based on Adhesin-Mediated Trapping of Programmable Cells. ACS Synth. Biol 9, 475–485, doi: 10.1021/acssynbio.9b00404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnson CT et al. Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing. Proceedings of the National Academy of Sciences 115, E4960–E4969, doi: 10.1073/pnas.1801013115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnson CT et al. Lysostaphin and BMP-2 co-delivery reduces S. aureus infection and regenerates critical-sized segmental bone defects. Sci. Adv. 5, eaaw1228, doi: 10.1126/sciadv.aaw1228 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nelson D, Loomis L & Fischetti VA Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. U. S. A. 98, 4107–4112, doi: 10.1073/pnas.061038398 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Portilla S, Fernández L, Gutiérrez D, Rodríguez A & García P Encapsulation of the Antistaphylococcal Endolysin LysRODI in pH-Sensitive Liposomes. Antibiotics (Basel) 9, doi: 10.3390/antibiotics9050242 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gondil VS et al. Comprehensive evaluation of chitosan nanoparticle based phage lysin delivery system; a novel approach to counter S. pneumoniae infections. Int. J. Pharm. 573, 118850, doi: 10.1016/j.ijpharm.2019.118850 (2020). [DOI] [PubMed] [Google Scholar]
- 104.Liu S. y. et al. Antimicrobial activity of a quaternary ammonium methacryloxy silicate-containing acrylic resin: a randomised clinical trial. Sci. Rep. 6, 21882, doi: 10.1038/srep21882 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Atar-Froyman L et al. Anti-biofilm properties of wound dressing incorporating nonrelease polycationic antimicrobials. Biomaterials 46, 141–148, doi: 10.1016/j.biomaterials.2014.12.047 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Hoque J, Akkapeddi P, Ghosh C, Uppu DSSM & Haldar J A Biodegradable Polycationic Paint that Kills Bacteria in Vitro and in Vivo. ACS Appl. Mater. Interfaces 8, 29298–29309, doi: 10.1021/acsami.6b09804 (2016). [DOI] [PubMed] [Google Scholar]
- 107.Liu L et al. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat. Nanotechnol. 4, 457–463, doi: 10.1038/nnano.2009.153 (2009). [DOI] [PubMed] [Google Scholar]
- 108.Chen Y et al. Design and Synthesis of Biocompatible, Hemocompatible, and Highly Selective Antimicrobial Cationic Peptidopolysaccharides via Click Chemistry. Biomacromolecules 20, 2230–2240, doi: 10.1021/acs.biomac.9b00179 (2019). [DOI] [PubMed] [Google Scholar]
- 109.Andrén OCJ et al. Antibiotic-Free Cationic Dendritic Hydrogels as Surgical-Site-Infection-Inhibiting Coatings. Adv. Healthc. Mater 8, e1801619, doi: 10.1002/adhm.201801619 (2019). [DOI] [PubMed] [Google Scholar]
- 110.Venkatesh M et al. Antimicrobial Activity and Cell Selectivity of Synthetic and Biosynthetic Cationic Polymers. Antimicrob. Agents Chemother. 61, e00469–00417, doi: 10.1128/aac.00469-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nederberg F et al. Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. 3, 409–414, doi: 10.1038/nchem.1012 (2011). [DOI] [PubMed] [Google Scholar]
- 112.Li J et al. Block Copolymer Nanoparticles Remove Biofilms of Drug-Resistant Gram-Positive Bacteria by Nanoscale Bacterial Debridement. Nano Lett. 18, 4180–4187, doi: 10.1021/acs.nanolett.8b01000 (2018). [DOI] [PubMed] [Google Scholar]
- 113.Rahman MA et al. Macromolecular-clustered facial amphiphilic antimicrobials. Nature Communications 9, 5231, doi: 10.1038/s41467-018-07651-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lienkamp K et al. Antimicrobial Polymers Prepared by ROMP with Unprecedented Selectivity: A Molecular Construction Kit Approach. J. Am. Chem. Soc. 130, 9836–9843, doi: 10.1021/ja801662y (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ilker MF, Nüsslein K, Tew GN & Coughlin EB Tuning the Hemolytic and Antibacterial Activities of Amphiphilic Polynorbornene Derivatives. J. Am. Chem. Soc. 126, 15870–15875, doi: 10.1021/ja045664d (2004). [DOI] [PubMed] [Google Scholar]
- 116.Engler AC et al. Antimicrobial Polycarbonates: Investigating the Impact of Balancing Charge and Hydrophobicity Using a Same-Centered Polymer Approach. Biomacromolecules 14, 4331–4339, doi: 10.1021/bm401248t (2013). [DOI] [PubMed] [Google Scholar]
- 117.Chin W et al. A macromolecular approach to eradicate multidrug resistant bacterial infections while mitigating drug resistance onset. Nature Communication 9, 917, doi: 10.1038/s41467-018-03325-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lam SJ et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nature Microbiology 1, 16162, doi: 10.1038/nmicrobiol.2016.162 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, Yang Y, Shi Y, Song H & Yu C Antibiotic-Free Antibacterial Strategies Enabled by Nanomaterials: Progress and Perspectives. Adv. Mater. 32, 1904106, doi: 10.1002/adma.201904106 (2020). [DOI] [PubMed] [Google Scholar]
- 120.Kirk JA et al. New class of precision antimicrobials redefines role of Clostridium difficile S-layer in virulence and viability. Sci. Transl. Med. 9, doi: 10.1126/scitranslmed.aah6813 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arifuzzaman M et al. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 5, eaav0216, doi: 10.1126/sciadv.aav0216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ram G, Ross HF, Novick RP, Rodriguez-Pagan I & Jiang D Conversion of staphylococcal pathogenicity islands to CRISPR-carrying antibacterial agents that cure infections in mice. Nat. Biotechnol. 36, 971–976, doi: 10.1038/nbt.4203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hwang G et al. Catalytic antimicrobial robots for biofilm eradication. Sci. Robot. 4, eaaw2388, doi: 10.1126/scirobotics.aaw2388 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qiao Y et al. Treatment of MRSA-infected osteomyelitis using bacterial capturing, magnetically targeted composites with microwave-assisted bacterial killing. Nat. Commun. 11, 4446, doi: 10.1038/s41467-020-18268-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Si Y et al. Daylight-driven rechargeable antibacterial and antiviral nanofibrous membranes for bioprotective applications. Sci. Adv. 4, eaar5931, doi: 10.1126/sciadv.aar5931 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Berry GC, Bockstaller MR & Matyjaszewski K Celebrating 100 years of polymer science. Prog. Polym. Sci. 100, 101193, doi: 10.1016/j.progpolymsci.2019.101193 (2020). [DOI] [Google Scholar]
- 127.Zhang L et al. Self-assembled lipid--polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano 2, 1696–1702, doi: 10.1021/nn800275r (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brady RA, Mocca CP, Plaut RD, Takeda K & Burns DL Comparison of the immune response during acute and chronic Staphylococcus aureus infection. PLoS One 13, e0195342–e0195342, doi: 10.1371/journal.pone.0195342 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hampton HG, Watson BNJ & Fineran PC The arms race between bacteria and their phage foes. Nature 577, 327–336, doi: 10.1038/s41586-019-1894-8 (2020). [DOI] [PubMed] [Google Scholar]
- 130.Fothergill JL, Neill DR, Loman N, Winstanley C & Kadioglu A Pseudomonas aeruginosa adaptation in the nasopharyngeal reservoir leads to migration and persistence in the lungs. Nat. Commun. 5, 4780, doi: 10.1038/ncomms5780 (2014). [DOI] [PubMed] [Google Scholar]
- 131.Fux CA, Shirtliff M, Stoodley P & Costerton JW Can laboratory reference strains mirror “real-world” pathogenesis? Trends Microbiol. 13, 58–63, doi: 10.1016/j.tim.2004.11.001 (2005). [DOI] [PubMed] [Google Scholar]
- 132.Palmer KL, Aye LM & Whiteley M Nutritional Cues Control Pseudomonas aeruginosa Multicellular Behavior in Cystic Fibrosis Sputum. J. Bacteriol. 189, 8079–8087, doi: 10.1128/jb.01138-07 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Turner KH, Wessel AK, Palmer GC, Murray JL & Whiteley M Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proc. Natl. Acad. Sci. 112, 4110–4115, doi: 10.1073/pnas.1419677112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Quickel KE Jr., Selden R, Caldwell JR, Nora NF & Schaffner W Efficacy and safety of topical lysostaphin treatment of persistent nasal carriage of Staphylococcus aureus. Appl. Microbiol. 22, 446–450 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Walsh S, Shah A & Mond J Improved Pharmacokinetics and Reduced Antibody Reactivity of Lysostaphin Conjugated to Polyethylene Glycol. Antimicrob. Agents Chemother. 47, 554–558, doi: 10.1128/AAC.47.2.554-558.2003 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kaur T et al. Immunocompatibility of Bacteriophages as Nanomedicines. Journal of Nanotechnology 2012, 247427, doi: 10.1155/2012/247427 (2012). [DOI] [Google Scholar]
- 137.Blazanovic K et al. Structure-based redesign of lysostaphin yields potent antistaphylococcal enzymes that evade immune cell surveillance. Mol. Ther. Methods Clin. Dev. 2, 15021, doi: 10.1038/mtm.2015.21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao H et al. Depletion of T cell epitopes in lysostaphin mitigates anti-drug antibody response and enhances antibacterial efficacy in vivo. Chem. Biol. 22, 629–639, doi: 10.1016/j.chembiol.2015.04.017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alcantar NA, Aydil ES & Israelachvili JN Polyethylene glycol–coated biocompatible surfaces. J. Biomed. Mater. Res. 51, 343–351, doi: (2000). [DOI] [PubMed] [Google Scholar]
- 140.Zhang P, Sun F, Liu S & Jiang S Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Release 244, 184–193, doi: 10.1016/j.jconrel.2016.06.040 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saifer MGP, Williams LD, Sobczyk MA, Michaels SJ & Sherman MR Selectivity of binding of PEGs and PEG-like oligomers to anti-PEG antibodies induced by methoxyPEG-proteins. Mol. Immunol. 57, 236–246, doi: 10.1016/j.molimm.2013.07.014 (2014). [DOI] [PubMed] [Google Scholar]
- 142.Qi Y et al. A brush-polymer/exendin-4 conjugate reduces blood glucose levels for up to five days and eliminates poly(ethylene glycol) antigenicity. Nat. Biomed. Eng 1, 0002, doi: 10.1038/s41551-016-0002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mancuso F, Shi J & Malik DJ High Throughput Manufacturing of Bacteriophages Using Continuous Stirred Tank Bioreactors Connected in Series to Ensure Optimum Host Bacteria Physiology for Phage Production. Viruses 10, 537, doi: 10.3390/v10100537 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wibowo D & Zhao C-X Recent achievements and perspectives for large-scale recombinant production of antimicrobial peptides. Appl. Microbiol. Biotechnol. 103, 659–671, doi: 10.1007/s00253-018-9524-1 (2019). [DOI] [PubMed] [Google Scholar]
- 145.Nour El-Din HT et al. A Rapid Lysostaphin Production Approach and a Convenient Novel Lysostaphin Loaded Nano-emulgel; As a Sustainable Low-Cost Methicillin-Resistant Staphylococcus aureus Combating Platform. Biomolecules 10, doi: 10.3390/biom10030435 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Szweda P, Gorczyca G, Filipkowski P, Zalewska M & Milewski S EFFICIENT PRODUCTION OF Staphylococcus simulans LYSOSTAPHIN IN A BENCHTOP BIOREACTOR BY RECOMBINANT Escherichia coli. Prep. Biochem. Biotechnol. 44, 370–381, doi: 10.1080/10826068.2013.829499 (2014). [DOI] [PubMed] [Google Scholar]
- 147.Mierau I et al. Industrial-scale production and purification of a heterologous protein in Lactococcus lactis using the nisin-controlled gene expression system NICE: The case of lysostaphin. Microbial Cell Factories 4, 15, doi: 10.1186/1475-2859-4-15 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hu C et al. Industrialization of lipid nanoparticles: From laboratory-scale to large-scale production line. Eur. J. Pharm. Biopharm. 109, 206–213, doi: 10.1016/j.ejpb.2016.10.018 (2016). [DOI] [PubMed] [Google Scholar]
- 149.Fowler VG Jr. et al. Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J. Clin. Invest. 130, 3750–3760, doi: 10.1172/jci136577 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Raymond Schuch RCN, Michael Wittekind, Han Lee, Brent Schneider. Bacteriophage lysin and antibiotic combinations against gram positive bacteria. United States patent 9889181 (2018). [Google Scholar]
- 151.Stefan M New Antimicrobial Agents. (2014). [Google Scholar]
- 152.Czaplewski L et al. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect. Dis. 16, 239–251, doi: 10.1016/s1473-3099(15)00466-1 (2016). [DOI] [PubMed] [Google Scholar]
- 153.Ting DSJ, Beuerman RW, Dua HS, Lakshminarayanan R & Mohammed I Strategies in Translating the Therapeutic Potentials of Host Defense Peptides. Front. Immunol. 11, doi: 10.3389/fimmu.2020.00983 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Abdelkader K, Gerstmans H, Saafan A, Dishisha T & Briers Y The Preclinical and Clinical Progress of Bacteriophages and Their Lytic Enzymes: The Parts are Easier than the Whole. Viruses 11, doi: 10.3390/v11020096 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fridman O, Goldberg A, Ronin I, Shoresh N & Balaban NQ Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 513, 418–421, doi: 10.1038/nature13469 (2014). [DOI] [PubMed] [Google Scholar]
- 156.DiGiandomenico A et al. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl. Med. 6, 262ra155–262ra155, doi: 10.1126/scitranslmed.3009655 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Secher T et al. The anti-Pseudomonas aeruginosa antibody Panobacumab is efficacious on acute pneumonia in neutropenic mice and has additive effects with meropenem. PLoS One 8, e73396, doi: 10.1371/journal.pone.0073396 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Palmu AA et al. Effect of pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) on outpatient antimicrobial purchases: a double-blind, cluster randomised phase 3–4 trial. Lancet Infect. Dis. 14, 205–212, doi: 10.1016/s1473-3099(13)70338-4 (2014). [DOI] [PubMed] [Google Scholar]
- 159.Nuccitelli A et al. Structure-based approach to rationally design a chimeric protein for an effective vaccine against Group B Streptococcus infections. Proc. Natl. Acad. Sci. U. S. A. 108, 10278–10283, doi: 10.1073/pnas.1106590108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hancock RE, Nijnik A & Philpott DJ Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 10, 243–254, doi: 10.1038/nrmicro2745 (2012). [DOI] [PubMed] [Google Scholar]
- 161.Scott MG et al. An anti-infective peptide that selectively modulates the innate immune response. Nat. Biotechnol. 25, 465–472, doi: 10.1038/nbt1288 (2007). [DOI] [PubMed] [Google Scholar]
- 162.de la Fuente-Núñez C, Reffuveille F, Haney EF, Straus SK & Hancock RE Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 10, e1004152, doi: 10.1371/journal.ppat.1004152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Krausgruber T et al. Structural cells are key regulators of organ-specific immune responses. Nature 583, 296–302, doi: 10.1038/s41586-020-2424-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Todd EM, Ramani R, Szasz TP & Morley SC Inhaled GM-CSF in neonatal mice provides durable protection against bacterial pneumonia. Sci. Adv. 5, eaax3387, doi: 10.1126/sciadv.aax3387 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Z, Nong J & Zhong Y Antibacterial, anti-inflammatory and neuroprotective layer-by-layer coatings for neural implants. J Neural Eng 12, 046015, doi: 10.1088/1741-2560/12/4/046015 (2015). [DOI] [PubMed] [Google Scholar]
- 166.Bouras M, Asehnoune K & Roquilly A Contribution of Dendritic Cell Responses to Sepsis-Induced Immunosuppression and to Susceptibility to Secondary Pneumonia. Front. Immunol. 9, 2590, doi: 10.3389/fimmu.2018.02590 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hotchkiss RS, Monneret G & Payen D Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 13, 862–874, doi: 10.1038/nri3552 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Roquilly A et al. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat. Immunol. 21, 636–648, doi: 10.1038/s41590-020-0673-x (2020). [DOI] [PubMed] [Google Scholar]
- 169.Lee JH, Jeong SH, Cha S-S & Lee SH A lack of drugs for antibiotic-resistant Gram-negative bacteria. Nat. Rev. Drug Discov. 6, 938–938, doi: 10.1038/nrd2201-c1 (2007). [DOI] [Google Scholar]
- 170.York A New drugs for the antibacterial pipeline? Nature Reviews Microbiology 18, 61–61, doi: 10.1038/s41579-019-0310-6 (2020). [DOI] [PubMed] [Google Scholar]
- 171.Jault P et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 19, 35–45, doi: 10.1016/s1473-3099(18)30482-1 (2019). [DOI] [PubMed] [Google Scholar]
- 172.Leitner L et al. Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomized, placebo-controlled, double-blind clinical trial. BMC Urol. 17, 90, doi: 10.1186/s12894-017-0283-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Leitner L et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomised, placebo-controlled, double-blind clinical trial. Lancet Infect. Dis., doi: 10.1016/s1473-3099(20)30330-3 (2020). [DOI] [PubMed] [Google Scholar]
- 174.Jun SY et al. Pharmacokinetics and Tolerance of the Phage Endolysin-Based Candidate Drug SAL200 after a Single Intravenous Administration among Healthy Volunteers. Antimicrob. Agents Chemother. 61, doi: 10.1128/aac.02629-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Raqib R et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl. Acad. Sci. U. S. A. 103, 9178–9183, doi: 10.1073/pnas.0602888103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Raqib R et al. Efficacy of sodium butyrate adjunct therapy in shigellosis: a randomized, double-blind, placebo-controlled clinical trial. BMC Infect. Dis. 12, 111, doi: 10.1186/1471-2334-12-111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rekha RS et al. Immune responses in the treatment of drug-sensitive pulmonary tuberculosis with phenylbutyrate and vitamin D(3) as host directed therapy. BMC Infect. Dis. 18, 303, doi: 10.1186/s12879-018-3203-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Melo LDVP, Cerca N, Kropinski AM, Almeida C, Azeredo J, Sillankorva S. Development of a Phage Cocktail to Control Proteus mirabilis Catheter-associated Urinary Tract Infections. Front. Microbiol. 7, doi: 10.3389/fmicb.2016.01024 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Meyer A, Greene M, Kimmelshue C & Cademartiri R Stabilization of T4 bacteriophage at acidic and basic pH by adsorption on paper. Colloids Surf B Biointerfaces 160, 169–176, doi: 10.1016/j.colsurfb.2017.09.002 (2017). [DOI] [PubMed] [Google Scholar]
- 180.Fulgione A et al. Biomimetic hydroxyapatite nanocrystals are an active carrier for Salmonella bacteriophages. Int J Nanomedicine 14, 2219–2232, doi: 10.2147/ijn.S190188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kłodzińska SN et al. Hyaluronic acid-based nanogels improve in vivo compatibility of the anti-biofilm peptide DJK-5. Nanomedicine 20, 102022, doi: 10.1016/j.nano.2019.102022 (2019). [DOI] [PubMed] [Google Scholar]
- 182.Xue Q et al. Anti-infective biomaterials with surface-decorated tachyplesin I. Biomaterials 178, 351–362, doi: 10.1016/j.biomaterials.2018.05.008 (2018). [DOI] [PubMed] [Google Scholar]
- 183.Moosazadeh Moghaddam M et al. Comparison of the antibacterial effects of a short cationic peptide and 1% silver bioactive glass against extensively drug-resistant bacteria, Pseudomonas aeruginosa and Acinetobacter baumannii, isolated from burn patients. Amino Acids 50, 1617–1628, doi: 10.1007/s00726-018-2638-z (2018). [DOI] [PubMed] [Google Scholar]
- 184.Gao Q et al. Rationally designed dual functional block copolymers for bottlebrush-like coatings: In vitro and in vivo antimicrobial, antibiofilm, and antifouling properties. Acta Biomater. 51, 112–124, doi: 10.1016/j.actbio.2017.01.061 (2017). [DOI] [PubMed] [Google Scholar]
- 185.Chen H et al. Versatile antimicrobial peptide-based ZnO quantum dots for in vivo bacteria diagnosis and treatment with high specificity. Biomaterials 53, 532–544, doi: 10.1016/j.biomaterials.2015.02.105 (2015). [DOI] [PubMed] [Google Scholar]
- 186.Zhang Y et al. Antibacterial and Biocompatible Cross-Linked Waterborne Polyurethanes Containing Gemini Quaternary Ammonium Salts. Biomacromolecules 19, 279–287, doi: 10.1021/acs.biomac.7b01016 (2018). [DOI] [PubMed] [Google Scholar]
- 187.Chen YF et al. Star-shaped polypeptides exhibit potent antibacterial activities. Nanoscale 11, 11696–11708, doi: 10.1039/c9nr02012h (2019). [DOI] [PubMed] [Google Scholar]
- 188.Hesaraki S, Karimi M & Nezafati N The synergistic effects of SrF(2) nanoparticles, YSZ nanoparticles, and poly-ε-l-lysin on physicomechanical, ion release, and antibacterial-cellular behavior of the flowable dental composites. Mater. Sci. Eng. C Mater. Biol. Appl. 109, 110592, doi: 10.1016/j.msec.2019.110592 (2020). [DOI] [PubMed] [Google Scholar]
- 189.Liu Y et al. Immunomimetic Designer Cells Protect Mice from MRSA Infection. Cell 174, 259–270.e211, doi: 10.1016/j.cell.2018.05.039 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhu C et al. A Hydrogel-Based Localized Release of Colistin for Antimicrobial Treatment of Burn Wound Infection. Macromol. Biosci. 17, doi: 10.1002/mabi.201600320 (2017). [DOI] [PubMed] [Google Scholar]
- 191.Kuijpers AJ et al. In vitro and in vivo evaluation of gelatin-chondroitin sulphate hydrogels for controlled release of antibacterial proteins. Biomaterials 21, 1763–1772, doi: 10.1016/s0142-9612(00)00064-8 (2000). [DOI] [PubMed] [Google Scholar]
- 192.Vipra AA et al. Antistaphylococcal activity of bacteriophage derived chimeric protein P128. BMC Microbiol. 12, 41, doi: 10.1186/1471-2180-12-41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pangule RC et al. Antistaphylococcal nanocomposite films based on enzyme-nanotube conjugates. ACS Nano 4, 3993–4000, doi: 10.1021/nn100932t (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Flynn J, Durack E, Collins MN & Hudson SP Tuning the strength and swelling of an injectable polysaccharide hydrogel and the subsequent release of a broad spectrum bacteriocin, nisin A. J Mater Chem B 8, 4029–4038, doi: 10.1039/d0tb00169d (2020). [DOI] [PubMed] [Google Scholar]
- 195.Blair JMA, Webber MA, Baylay AJ, Ogbolu DO & Piddock LJV Molecular mechanisms of antibiotic resistance. Nature Reviews Microbiology 13, 42–51, doi: 10.1038/nrmicro3380 (2015). [DOI] [PubMed] [Google Scholar]


