Stone et al explore the interconnectiveness of regulation of megakaryopoiesis and regulation of the bone marrow microenvironment in a timely and unique review. In particular, they focus on the now-recognized multiple and divergent pathways from hematopoietic stem cells to mature megakaryocytes and how this is influenced by, and influences, the stem cell niche.
Visual Abstract
Abstract
Megakaryocytes (MKs), the largest of the hematopoietic cells, are responsible for producing platelets by extending and depositing long proplatelet extensions into the bloodstream. The traditional view of megakaryopoiesis describes the cellular journey from hematopoietic stem cells (HSCs) along the myeloid branch of hematopoiesis. However, recent studies suggest that MKs can be generated from multiple pathways, some of which do not require transit through multipotent or bipotent MK-erythroid progenitor stages in steady-state and emergency conditions. Growing evidence suggests that these emergency conditions are due to stress-induced molecular changes in the bone marrow (BM) microenvironment, also called the BM niche. These changes can result from insults that affect the BM cellular composition, microenvironment, architecture, or a combination of these factors. In this review, we explore MK development, focusing on recent studies showing that MKs can be generated from multiple divergent pathways. We highlight how the BM niche may encourage and alter these processes using different mechanisms of communication, such as direct cell-to-cell contact, secreted molecules (autocrine and paracrine signaling), and the release of cellular components (eg, extracellular vesicles). We also explore how MKs can actively build and shape the surrounding BM niche.
Introduction
Platelets are small anucleate discoid cells that range from 1 to 3 µm in diameter. They are the most critical players in maintaining hemostasis and vascular integrity and have key roles in thrombosis and inflammatory responses. Platelets are formed from megakaryocytes (MKs), their precursor cells, which reside predominantly in the bone marrow (BM). MKs are large hematopoietic cells, making up ≈0.05% of the cell population in human BM. After MKs are terminally differentiated, they undergo a complex maturation process during which they increase in size, become full of platelet-specific granules, expand their cytoplasmic content of cytoskeletal proteins, and develop a highly tortuous invaginated (demarcation) membrane system.1
Megakaryopoiesis is the process by which MKs are derived from hematopoietic stem cells (HSCs). HSCs sit atop a hierarchy of progenitors that undergo consecutive commitment steps, becoming progressively restricted in their lineage potential and finally producing unipotent progenitors.2 According to the classical model of hematopoiesis, each mature MK is derived from an HSC that sequentially transitions through the multipotent progenitor (MPP), common myeloid progenitor (CMP), MK-erythroid progenitor (MEP), and MK progenitor (MkP) states.3 During the last decade, this developmental structure has been disputed, with the origin of MK precursors being one of the most actively debated subjects. Recent studies have suggested that MKs are generated from multiple pathways, some of which do not require transit through multipotent or bipotent MEP stages in steady-state and emergency conditions.4,5 Hematopoietic stem and progenitor cells (HSPCs) reside in specialized local microenvironments within the BM, known as BM niches,6 that provide crucial signals to regulate HSPC survival, quiescence, mobilization, and differentiation. Growing evidence suggests that stress-induced molecular changes in the BM niche directly and/or indirectly regulate HSC activation, expansion, and differentiation.7 In this review, we explore how the BM niche can encourage and alter MK development.
MKs, the largest of the hematopoietic cells, assemble and release platelets by extending long proplatelet structures into the bloodstream. Because of the inaccessibility of the BM, the cellular and molecular basis of how MKs give birth to platelets has historically been poorly understood. However, the purification and cloning of the glycoprotein hormone thrombopoietin (TPO) in 1994 allowed the first insights into the differentiation of these unique cells.8 TPO is the principal regulator of megakaryopoiesis, regulating all stages of MK differentiation, from the HSC through cytoplasmic maturation.9,10 TPO has also been linked to regulation of HSC survival and proliferation.11,12 Nonetheless, mice lacking TPO or its receptor c-Mpl have platelets and MKs, although at severely reduced levels (thrombocytopenia), suggesting that TPO signaling is not the sole regulator of HSC differentiation into MKs.11-13 In addition, although TPO directs the commitment of HSCs to the MK lineage and controls MK maturation, it does not regulate the production of platelets from MKs.2 During the final stages of maturation, the MK cytoplasm undergoes massive cytoskeletal remodeling to induce the formation of proplatelets, which are shed into the bloodstream, where they fracture into platelets.14,15 Of note, our understanding of what triggers mature MKs to begin the process of proplatelet formation is still very limited.
Models of megakaryocyte differentiation from HSCs
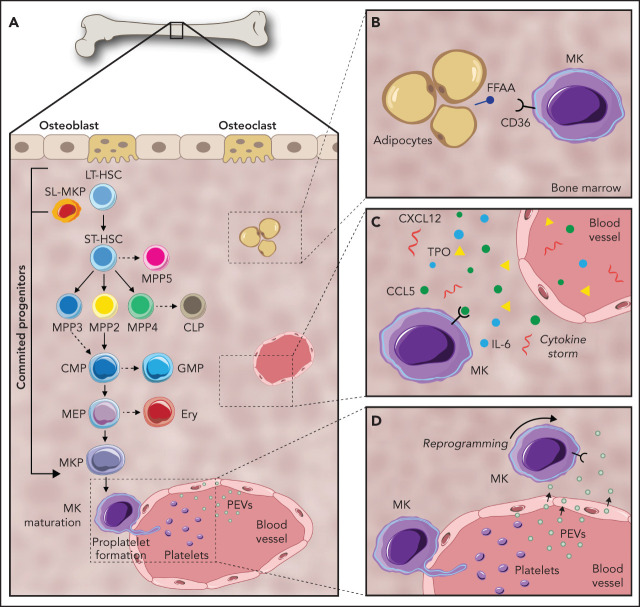
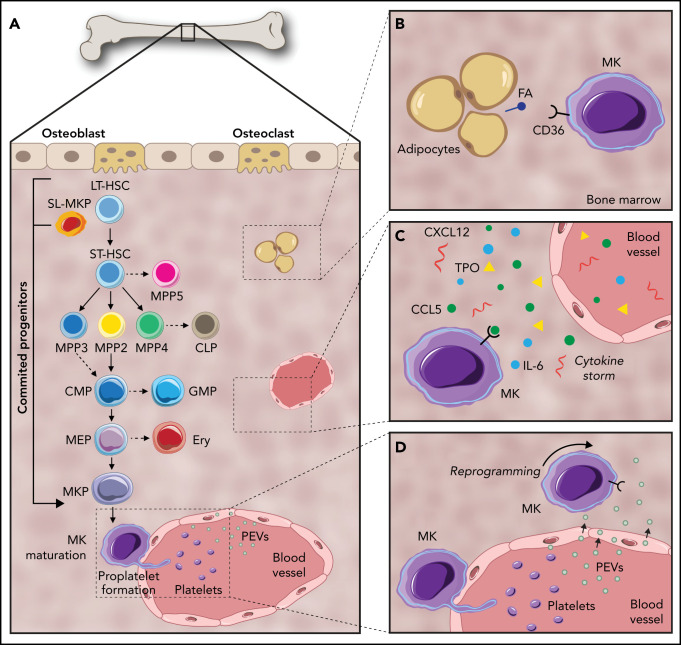
Despite tremendous diversity in cell morphology and function, blood and immune cells are all derived from multipotent HSCs.16 In the classical model of hematopoiesis, HSCs sit atop of a developmental hierarchy and give rise to increasingly restricted progenitors that eventually produce, and continuously replenish, ≈1 trillion mature blood cells per day.17 In this model, long-term repopulating HSCs (LT-HSCs) give rise to short-term repopulating HSCs (ST-HSCs). These ST-HSCs have a reconstitution ability of <1 month upon transplantation, after which they differentiate into MPPs with no self-renewal capacity.18 MPPs (MPP2-4) have been designated by researchers, such as Pietras et al, as a set of unique signature and lineage-biased progenitors independently generated by HSCs; MPP1-4 are unable to generate other MPPs in vivo after transplantation.19 MPP2 is biased toward the MK/erythroid lineage, MPP3 is largely granulocyte/macrophage biased, and MPP4 is largely lymphoid biased.19 Recently, an additional population, MPP5, has been described in mice; these cells can generate MPP1-4 but not HSCs.20 As such, MPP5 is located immediately downstream of HSCs but upstream of the more committed MPP2-4.20 MPPs differentiate into common lymphoid progenitors, which display lymphoid potential and differentiate into mature B and T cells, and CMPs that generate granulocyte-macrophage progenitors and MEPs. MEPs then give rise to unipotent MkPs, which mature into MKs (Figure 1A).2
Figure 1.
Pathways of megakaryopoiesis, highlighting key mechanisms of communication within the BM niche. (A) Canonical megakaryopoiesis involves differentiation through LT-HSCs, ST-HSCs, and MPPs (MPP2, MPP3, MPP4, and MPP5). In the first bifurcation, MPPs differentiate into CMPs or common lymphoid progenitors (CLP). Continuing down the myeloid lineage, CMPs can then differentiate into granulocyte-macrophage progenitors (GMP) as well as MEPs. MEPs can then differentiate to MkPs that terminally differentiate into MKs. The MK-biased pathway and stem-like MK-committed progenitors (SL-MkP), are highlighted here using an arrow. (B) MKs interact with their surrounding cells, such as adipocytes. MKs uptake fatty acids (FA) released by adipocytes via a CD36-dependent mechanism to facilitate their maturation. (C) Secreted molecules, such as growth factors, cytokines, and lipids, help to orchestrate and maintain the hematopoietic response. (D) PEVs can leave the circulation and infiltrate the BM, where they bind to resident BM cells, including HSCs and MKs, and affect their development.
Over the past decade, the concept of a hierarchical lineage tree and stable discrete HSC populations has been revised into a more dynamic model.21 These findings are now supported by advances in single-cell omics analyses, which are further unraveling novel HSC subpopulations.22 In particular, the origin of MKs has been under debate; recent findings revealed similarities between HSCs and MKs, pointing toward the possibility that the MK pathway is bifurcated earlier than assumed in the classical model.19,23 In 2007, Månsson et al described an MK-like subset of myeloid-biased HSCs using global and single-cell genomics tools.4 Under steady-state conditions, they found HSCs with high expression of MK-associated genes (Gata1, Epor, Mpl, and vWF). Further evidence for MK-primed HSCs was reported simultaneously by 2 groups. Gekas and Graf identified a CD41+ subset of myeloid-biased HSCs that becomes more prevalent with age,24 and Jacobsen and colleagues found that LT-HSCs expressing vWF are a TPO-dependent HSC population capable of short-term and stable long-term platelet-biased reconstitution after transplantation.25 Shortly after, Park and colleagues revealed that c-Kithi cells are also an MK-biased population.26 Intriguingly, Sanjuan-Pla et al showed coexpression of the MK bias markers vWF and CD41 on 67.9% of LSK/CD34−/CD150+/CD48− cells.25 Moving forward, another marker, GABRR1, was found to be expressed in subsets of HSCs and MkPs, but not in other progenitor populations.27 Collectively, these studies reveal a variety of “MK-biased” HSCs identifiable through distinct marker expression.
Efforts to examine the functionality of MK-biased populations found HSCs that differentiate directly into MK, MK-erythroid, or common myeloid lineages.21 Based on single-cell transplantation experiments, a myeloid bypass model of differentiation was proposed, directly branching from the most primitive HSCs. Later, Haas et al reported that the HSC compartment contains stem-like MK-committed progenitors (SL-MkPs) that remain quiescent during steady-state.5 Acute inflammatory signaling triggers cell cycle activation of these SL-MkPs, initiating the translation of MK transcripts. The investigators showed that 28 proteins typically expressed in mature MKs and platelets (eg, CD41, vWF, and CXCL4 (platelet factor 4) were upregulated in HSCs upon inflammation, resulting in a rapid maturation of SL-MkPs and other MkPs5 (Figure 1A). Similarly, HSCs have the capacity to differentiate directly into MKs in myelofibrosis,28 and the numbers of LT-HSCs, ST-HSCs, and MkPs also increase during aging.29,30 Grover et al revealed that HSC aging is accompanied by upregulation of platelet-lineage gene expression.30 Further, their study showed that the age-dependent increase in platelet bias correlates with a selective expansion of platelet-primed vWF+ HSCs, implicating MK-biased HSCs in the aging platelet phenotype. Taken together, these data suggest that, in contrast to the concept of stable and discrete HSPC populations, a more dynamic relationship exists with plasticity in the proliferation and differentiation capacity of HSCs. Furthermore, there is compelling evidence that HSC subsets can be partially or completely biased toward MK differentiation and platelet production during inflammation. In the context of these findings, it is evident that interactions within the BM niche impact regulation of hematopoiesis and megakaryopoiesis. In the remainder of this review, we uncover some ways in which the BM environment may shape and regulate megakaryopoiesis and how resulting MKs impact the niche.
The BM niche and megakaryopoiesis
Organs and tissues are organized to facilitate their function, and the BM is no different. The BM is a highly complex tissue comprising an intricate network of endothelial cells (including sinusoids, arterioles, and transition zone vessels), multipotent mesenchymal stromal cells (MSCs) and their progeny (osteoblasts, chondrocytes, and adipocytes), and HSPCs and their progeny, such as MKs and macrophages.31,32 The term “niche,” first coined by Schofield in 1978, refers to the environment in which a cell can be found that is fundamental to its survival and function.33 It has been well documented that the BM niche can be extensively remodeled in a variety of conditions, such as inflammation,5 infection,34 radiation therapy,35 and aging,36,37 which has a propagating effect on HSPC differentiation and development. Understanding the mechanisms by which the HSC niche is regulated can be crucial to improving treatment outcomes and eradicating disease.38 For example, Psaila et al used single-cell omics to demonstrate MK-biased hematopoiesis in myelofibrosis propagated by HSPCs with aberrant expression of G6B on their surface.28 This G6B expression can now be exploited as a selective immunotherapeutic target in the clinic.
BM niche changes result from insults that affect factors such as BM cellular composition, local microenvironment, architecture, or a combination of these factors. The effectors of these changes are broad and range from physical insults, such as radiation, to signaling induced by secreted factors in the blood. In this section, we expand on how 3 components, direct cell-to-cell contact, secreted molecules (autocrine and paracrine signaling), and the release of cellular components like extracellular vesicles (EVs), impact MKs and the BM niche.
HSCs rely on interactions in their niche with stroma, endothelium, and hematopoietic cells that facilitate their survival and regulate their function and differentiation.39-42 Building on this and other early observations, the HSC niche is now viewed as a complex assembly of multiple components existing in close proximity to each other that provide molecular and physical cues that regulate the quiescence, proliferation, and differentiation of HSCs.43 Within the field examining the BM microenvironment, considerable focus has been placed on the development of methods to identify the in situ localization of HSCs and other cellular components that contribute to their niche. For example, a study of myelopoietic organization in murine sternal marrow identified a predominantly sinusoidal localization for myeloid lineage cells and progenitors.44 This study demonstrated coordination of myelopoiesis in sinusoidal niches by colony-stimulating factor 1 expression in selected vessels.44 Better knowledge of the constituents of the MK niche and the cells with which they interact may improve our understanding of megakaryopoiesis during normal and disease states.
Interactions between MKs and their surrounding cells
Despite representing a minor proportion of hematopoietic cells in the BM, the large size of MKs and their ubiquitous distribution suggest the potential to interact with many cells. Using a whole-mount 3-dimensional immunofluorescence imaging technique, Bruns et al revealed that nearly 30% of HSCs are found within 5 μm of MKs, a higher ratio than would be expected from random simulations.45 This was corroborated by a recent study showing that a proportion of α-catulin+ HSCs were found within 5 μm of MKs, suggesting a preferential localization of some HSCs near MKs.46 Another study indicated that MKs play an important role in the regulation of HSC quiescence by production of transforming growth factor-β1 (TGF-β1) and recovery after stress by transient fibroblast growth factor (FGF) signaling.47 Additionally, when MKs are depleted from the BM niche, there is a significant increase in HSC numbers, pointing toward MKs as a potential regulator of HSC quiescence.45,47-49 Of note, MK depletion is also enough to reprogram vWF+ HSCs from myeloid-biased to balanced-lineage contributions.49 Mechanistically, because MKs inhibit HSC proliferation through secreted proteins, including TGF-β147 and CXCL4,45 direct contact between MKs and HSCs may not be required46 (Table 1). CXCL4 is also known to inhibit the proliferation of megakaryocytes and promotes maturation via autocrine signaling.50,51 Collectively, these data reveal an important functional role for MKs in the BM niche beyond the production of platelets.
Table 1.
Secreted molecules that have an effect on BM MKs
| Molecule | Type | Effect | References |
|---|---|---|---|
| CCL5 | Cytokine | Positive bias in myeloid cell production | 62-64 |
| CXCL12 | Cytokine | Regulates the spatial distribution of MKs in the BM | 69-70 |
| CXCL4 | Cytokine | Negatively promotes HSC proliferation | 45, 50, 51 |
| IL-1α | Cytokine | TPO-independent differentiator of MKs | 67-68 |
| PAF | Lipid | Affects the growth of human myeloid and erythroid progenitors | 81-82 |
| PtdIns3P | Lipid | Involved in DMS formation | 79 |
| S1P | Lipid | Involved in MK differentiation and proplatelet formation | 85 |
| TGF-β1 | Cytokine | Maintains HSC quiescence | 47 |
| TNF | Cytokine | Rapid maturation of SL-MkPs and MkPs | 5 |
| TPO | Cytokine | Regulates MK differentiation from HSCs | 8,10 |
DMS, demarcation membrane system; IL-1α, interleukin-1α; PtdIns3P, phosphatidylinositol 3-monophosphate; TNF, tumor necrosis factor.
Another unique cellular relationship is the engulfment and subsequent release of neutrophils by MKs in a process termed emperipolesis. Observations of neutrophils inside MKs were first reported from electron microscopy images taken in the 1980s.52 A more recent investigation highlighted the functional implications of this relationship; an exchange of membrane material between the MK and neutrophil can occur during emperipolesis, with the potential for exchange of other materials during this process.53 This interaction results in accelerated maturity to platelet production by MKs.53 However, the percentage of MKs that undergo this process and the frequency with which it occurs have yet to be reliably determined under normal and stress hematopoiesis, leaving unanswered questions as to the role of this phenomenon for normal and/or pathological MK development.
Given that proplatelet extension through the endothelium into the vascular lumen is required for platelet production, there are undoubtedly many key interactions between MKs and endothelial cells. Penetration into the vessels occurs through podosomes, actin-driven structures that facilitate a transendothelial extension.54 Little is known about how MKs determine the polarity of their proplatelet extensions toward endothelial cells and what interactions are necessary to facilitate podosome formation and the elongation of these extensions through the cytoplasm of the endothelial cells. Furthermore, endothelial cells are extremely sensitive and changeable during inflammation, upregulating production of cytokines and adhesion molecules.55 For example, Fernandez et al showed that BM endothelial cells promote expansion of HPSCs by a Notch-dependent mechanism upon inflammation.55 However, these interactions have been difficult to study, because they require complex coculture systems that recapitulate BM architecture. As such, further studies are needed to understand the complex relationships between endothelial cells and MKs.
BM adipocytes have been described as negative regulators of the hematopoietic environment.56 Recent studies have also introduced a potentially key role for lipids from adipocytes in cell fate decisions (Figure 1B). A study carried out by Severin and colleagues57 showed that MKs take up fatty acids released by adipocytes via a CD36-dependent mechanism to facilitate their maturation, suggesting that lipids have an essential role in MK development. Further supporting this, Kelly et al demonstrated that fatty acid incorporation through acetyl-CoA carboxylase in the de novo lipogenesis pathway is a key regulator of late-stage MK maturation and platelet formation.58 This interaction raises questions about the relationship between megakaryopoiesis and adipocytes in the context of metabolic diseases. For example, the established increase in BM adiposity with age59 may result in altered MK or platelet function, which could help explain the platelet hyperreactivity that contributes to the increased risk of heart attack and stroke.
The role of secreted molecules
BM-resident cells secrete growth factors, cytokines, and lipids that orchestrate and maintain the hematopoietic response by mediating short- and long-range communication (Table 1).60 Therefore, examining these secreted signals is essential in understanding the remodeling of BM niches. Cytokines play a pivotal role in HSC regulation and maintenance and are also required for megakaryopoiesis.9 However, little is known about the precise sources of these molecules; they may originate from BM cells, permeate from the blood stream, or derive from a combination of many origins (Figure 1C). The most well-studied is TPO, which is expressed mainly in the liver and accesses the BM via the bloodstream. There, it regulates MK differentiation from HSCs and the subsequent progenitor cells.10 In addition to MK differentiation, TPO has an important role in HSC maintenance. A study by Decker et al showed that following targeted deletion of TPO from hepatocytes, BM HSCs are depleted, pointing to hepatocyte-derived TPO as the major functional source of TPO for BM HSC maintenance under steady-state conditions.61
Although many investigators have explored how TPO acts on HSC differentiation and MK maturation,2 few studies have addressed the effect of other cytokines and their impact on megakaryopoiesis. Levels of CCL5, or RANTES, increase in the BM microenvironment with aging, which is associated with a positive bias in myeloid cell production.62 Accordingly, exposure to CCL5 or constitutive expression of CCL5 results in lineage skewing to favor production of myeloid cells and a corresponding decrease in lymphocyte production.62 Recent studies demonstrate that CCL5 could be used pharmacologically following therapeutic or accidental radiation injury as a result of its role in hematopoietic regeneration.63 These findings agree with previous studies from our laboratory that found CCL5 signaling through its receptor CCR5 enhanced MK ploidy and proplatelet formation through prosurvival signaling downstream of BCL2-associated agonist of cell death phosphorylation.64 Together, these studies suggest that CCL5 may bias HSCs toward the megakaryocytic lineage and subsequently enhance platelet production from mature MKs.
Members of the interleukin (IL) family can also directly influence HSCs, driving their proliferation and skewing them toward the myeloid lineage.65,66 IL-1β and IL-6 increase MK maturation and, consequently, platelet counts.37 However, their mechanisms of action were ultimately discovered to be predominantly through an indirect effect of increasing TPO production.2 Conversely, IL-1α, which is involved in inflammatory processes and hematopoiesis, has been reported to drive TPO-independent differentiation of MKs.67 Nishimura et al further reported that IL-1α induces enhanced platelet release through rupture of the mature MK membrane.68
Stromal cell derived factor-1/CXCL12 was the first chemokine to be implicated in MK maturational chemotaxis to the vascular niche,69 a concept that is now being challenged. CXCL12 is produced by several cell types in the BM, such as osteoblasts, endothelial cells, and perivascular MSCs. Niswander et al demonstrated that CXCL12 regulates the spatial distribution of MKs in the marrow vasculature,70 pointing toward the importance of CXCL12 in the organization of the BM niche. Moreover, a recent study found that β4GalT1, regulated by CXCL12, controls β1 integrin function in MKs and HSC homeostasis.71 Similar to CXCL12, administration of FGF4 increases platelet counts in vivo and partially rescues thrombopoiesis in Tpo−/− and Mpl−/− mice by directly augmenting cellular adhesion of MKs to endothelial cells.72
The capacity for cytokine signaling within the BM can be regulated to some degree by extracellular matrix (ECM) components distributed throughout the BM niche. ECM is composed of various proteins and glycoproteins that provide structure to the niche, as well as mechanical and biochemical signals to cells in the BM environment, including MKs.73 During megakaryopoiesis, ECM components, such as type-1 collagen, fibronectin, and fibrinogen, directly affect MK development and platelet production in the BM.74,75 In accordance, dysregulation of ECM production and remodeling is associated with BM pathologies. For example, during myelofibrosis, secretion of TGF-β1 by MKs results in overproduction of ECM by stroma and subsequent restriction of BM function.76
Lipids are another class of molecules that regulate many vital processes in hematopoiesis and homing of HSPCs in the BM niche. However, less is known about the effects of bioactive lipids and lipid mediators on regulating hematopoiesis and, by extension, megakaryopoiesis. During MK maturation, MKs develop a complex demarcation membrane system that becomes enriched with the plasma membrane lipids phosphoinositide and phosphatidylinositol 4,5-bisphosphate, which represent markers for internal membranes of mature MKs.77 Similarly, phosphatidylinositol 3-monophosphate is a key component in vesicular trafficking and autophagy. Of note, abnormal autophagy is associated with impaired MK differentiation and platelet production ( see Sun et al78 for additional information). Recently, phosphatidylinositol 3-monophosphate, produced by Vps34 (phosphatidylinositol 3-kinase), has emerged as an important regulator of MK/platelet structure and function79; inhibiting Vps34 at early stages of MK development leads to aberrant demarcation membrane system development.80 Another important lipid, platelet activation factor (PAF), is present in the BM and regulates the function of HSPCs. PAF diminishes the growth of human myeloid and erythroid progenitors, and PAF dysregulation in the BM may influence trafficking, activation, and gene expression patterns in neighboring cells.81,82 In addition, sphingosine-1-phosphate (S1P) regulates important biological functions, including cell migration, in the BM compartment.83 S1P also induces CXCL12 secretion from MSC populations in the BM niche.84 In the context of megakaryopoiesis, S1P and the MK S1P receptor (S1pr1) are indispensable for normal BM thrombopoiesis, and Niazi et al proposed that S1P1 serves as a critical directional cue guiding the elongation of proplatelet extensions from the interstitium into BM sinusoids.85 Moreover, the investigators also found that S1P restricts megakaryopoiesis through S1pr1 and can further suppress thrombopoiesis through S1pr2 when aberrantly secreted in the hematopoietic niche,85 suggesting a more complex role for this signaling pathway. In sum, there is emerging literature indicating an important role for secreted molecules in HSC differentiation and MK maturation. Further knowledge of the microenvironmental regulation of the MK lineage could lead to improved therapeutic strategies that can specifically enhance or repress MK differentiation from HSCs.
EVs as messengers within the niche
Interest in EVs has increased dramatically as a result of their implication in cell-to-cell communication, disease development and progression, and candidacy as potential therapeutics. EVs are membrane-enclosed structures of varying size (30-10 000 nm) that are released from cells and mediate local and distant intercellular communication.86 Indeed, EVs of various origins have been identified in every biofluid tested to date, with substantial variation in their size, structure, content, and function.87 EVs bind to and are internalized by cells to alter cell function.88-93 Membrane fusion and/or internalization of EVs allow EV membrane and cargo to be transferred into the recipient cell cytoplasm; lipids, RNA, and protein are delivered by these mechanisms to target cells.94,95 As such, EVs can be messengers of diverse cargo, reflecting the local environment from which they originate. Growing evidence supports the importance of EVs released from BM cells in communication within the BM microenvironment.96
EVs derived from MKs (MKEVs) and EVs derived from platelets (PEVs) are the most abundant in circulation, accounting for almost 80% of the total population.97-100 PEVs are released upon platelet stimulation and are generally characterized as CD41+/CD62P+, whereas MKEVs are constitutively released and are generally CD41+/CD62P−.101 Within the BM, MKEVs interact with HSPCs through receptors such as ICAM-1, CD43, CD18, and CD11b.91 Functionally, the internalization of MKEVs redirects the differentiation of HSPCs toward MKs, with limited effects on the phenotype of endothelial or stromal cells.91 The mechanism of this reprogramming was found to be through RNA in MKEVs, later suggested to be miR-1915-3p.91
In addition, our group recently demonstrated that PEVs leave the circulation and infiltrate the BM, where they bind to resident BM cells, including HSCs and MKs, and can affect their development (Figure 1D).101 This finding introduces the idea that EVs may be communicating from cells within the BM, as well as from cells at distant sites. Circulating platelets are known to endocytose many components of plasma; thus, during disease their content changes to reflect the disease state. These findings suggest a model whereby PEVs can penetrate the BM and relay pathologies from the plasma directly to the BM microenvironment.
Conclusions
The study of HSCs in the BM niche has traditionally been dominated by different concepts: (1) a static long-term HSC responsible for maintaining hematopoiesis, (2) a model of progenitor hierarchy in which HSCs differentiate into increasingly committed progenitors and, subsequently, mature lineages along nonoverlapping paths, and (3) an HSC niche, where HSCs reside in discrete anatomical microenvironments. However, during the past decade it has been shown that the BM niche may be more dynamic and complicated, especially once activated by conditions like inflammation.
Moving forward, studies continuing to explore the roles that MKs play in reshaping the BM niche will be key in understanding hematopoiesis during health and disease. For example, although committed MkPs have been reported by many investigators to expand during BM stress, the specific molecules and signaling pathways responsible for this are still unknown. In addition, most of the in vitro experiments discussed in this review were done in 2-dimensional culture systems, which do not take into account BM architecture. The use of biochips and 3-dimensional culture systems will be essential in helping to gain a better understanding of how the complex relationships of cells and matrix within the BM niche create and shape the local environment.
Acknowledgments
The authors thank Kellie R. Machlus for her support in this review and Kristin Johnson for producing the figures.
M.N.B. is supported by grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases K01DK111515 and R03DK124746 and National Heart, Lung, and Blood Institute R01HL151494.
Authorship
M.N.B designed, wrote, and edited the manuscript; A.P.S. wrote and revised the manuscript; and T.F.N. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maria N. Barrachina, Karp Family Research Building, 1 Blackfan Circle, Room 11.004.H, Boston, MA 02115; e-mail: maria.barrachina@childrens.harvard.edu.
REFERENCES
- 1.Behnke O. An electron microscope study of the rat megacaryocyte. II. Some aspects of platelet release and microtubules. J Ultrastruct Res. 1969;26(1):111-129. [DOI] [PubMed] [Google Scholar]
- 2.Noetzli LJ, French SL, Machlus KR. New insights into the differentiation of megakaryocytes from hematopoietic progenitors. Arterioscler Thromb Vasc Biol. 2019;39(7):1288-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machlus KR, Italiano JE Jr. The incredible journey: from megakaryocyte development to platelet formation. J Cell Biol. 2013; 201(6):785-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Månsson R, Hultquist A, Luc S, et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26(4):407-419. [DOI] [PubMed] [Google Scholar]
- 5.Haas S, Hansson J, Klimmeck D, et al. Inflammation-induced emergency megakaryopoiesis driven by hematopoietic stem cell-like megakaryocyte progenitors. Cell Stem Cell. 2015;17(4):422-434. [DOI] [PubMed] [Google Scholar]
- 6.Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20(5):303-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batsivari A, Haltalli MLR, Passaro D, Pospori C, Lo Celso C, Bonnet D. Dynamic responses of the haematopoietic stem cell niche to diverse stresses [published correction appears in Nat Rev Mol Cell Biol. 2020;22(2):1]. Nat Cell Biol. 2020;22(1):7-17. [DOI] [PubMed] [Google Scholar]
- 8.de Sauvage FJ, Hass PE, Spencer SD, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369(6481):533-538. [DOI] [PubMed] [Google Scholar]
- 9.Couldwell G, Machlus KR. Modulation of megakaryopoiesis and platelet production during inflammation. Thromb Res. 2019;179:114-120. [DOI] [PubMed] [Google Scholar]
- 10.Kaushansky K, Lok S, Holly RD, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994; 369(6481):568-571. [DOI] [PubMed] [Google Scholar]
- 11.Kimura S, Roberts AW, Metcalf D, Alexander WS. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci USA. 1998; 95(3):1195-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 1996;87(6):2162-2170. [PubMed] [Google Scholar]
- 13.Murone M, Carpenter DA, de Sauvage FJ. Hematopoietic deficiencies in c-mpl and TPO knockout mice. Stem Cells. 1998;16 (1):1-6. [DOI] [PubMed] [Google Scholar]
- 14.Thon JN, Montalvo A, Patel-Hett S, et al. Cytoskeletal mechanics of proplatelet maturation and platelet release. J Cell Biol. 2010;191(4):861-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thon JN, Italiano JE. Visualization and manipulation of the platelet and megakaryocyte cytoskeleton. Methods Mol Biol. 2012;788:109-125. [DOI] [PubMed] [Google Scholar]
- 16.Haas S, Trumpp A, Milsom MD. Causes and consequences of hematopoietic stem cell heterogeneity. Cell Stem Cell. 2018;22(5):627-638. [DOI] [PubMed] [Google Scholar]
- 17.Woolthuis CM, Park CY. Hematopoietic stem/progenitor cell commitment to the megakaryocyte lineage. Blood. 2016; 127(10):1242-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, Bryder D, Adolfsson J, et al. Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3− short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105(7):2717-2723. [DOI] [PubMed] [Google Scholar]
- 19.Pietras EM, Reynaud D, Kang YA, et al. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions [published correction appears in Cell Stem Cell. 2015;17(2):246]. Cell Stem Cell. 2015;17(1):35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sommerkamp P, Romero-Mulero MC, Narr A, et al. Mouse multipotent progenitor 5 cells are located at the interphase between hematopoietic stem and progenitor cells. Blood. 2021;137(23):3218-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto R, Morita Y, Ooehara J, et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154(5):1112-1126. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen SEW, Nerlov C. Haematopoiesis in the era of advanced single-cell technologies. Nat Cell Biol. 2019;21(1):2-8. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Fraticelli AE, Wolock SL, Weinreb CS, et al. Clonal analysis of lineage fate in native haematopoiesis. Nature. 2018;553(7687):212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gekas C, Graf T. CD41 expression marks myeloid-biased adult hematopoietic stem cells and increases with age. Blood. 2013;121(22):4463-4472. [DOI] [PubMed] [Google Scholar]
- 25.Sanjuan-Pla A, Macaulay IC, Jensen CT, et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502(7470):232-236. [DOI] [PubMed] [Google Scholar]
- 26.Shin JY, Hu W, Naramura M, Park CY. High c-Kit expression identifies hematopoietic stem cells with impaired self-renewal and megakaryocytic bias. J Exp Med. 2014; 211(2):217-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu F, Feng M, Sinha R, et al. The GABA receptor GABRR1 is expressed on and functional in hematopoietic stem cells and megakaryocyte progenitors. Proc Natl Acad Sci USA. 2019;116(37):18416-18422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Psaila B, Wang G, Rodriguez-Meira A, et al. ; NIH Intramural Sequencing Center . Single-cell analyses reveal megakaryocyte-biased hematopoiesis in myelofibrosis and identify mutant clone-specific targets. Mol Cell. 2020;78(3):477-492.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davizon-Castillo P, McMahon B, Aguila S, et al. TNF-α-driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood. 2019;134(9):727-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grover A, Sanjuan-Pla A, Thongjuea S, et al. Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nat Commun. 2016;7(1):11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comazzetto S, Shen B, Morrison SJ. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev Cell. 2021;56(13):1848-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1-2): 7-25. [PubMed] [Google Scholar]
- 34.Johnson CB, Zhang J, Lucas D. The role of the bone marrow microenvironment in the response to infection. Front Immunol. 2020;11:585402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowers E, Slaughter A, Frenette PS, Kuick R, Pello OM, Lucas D. Granulocyte-derived TNFα promotes vascular and hematopoietic regeneration in the bone marrow. Nat Med. 2018;24(1):95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maryanovich M, Zahalka AH, Pierce H, et al. Author correction: Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med. 2019;25(4):701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho YH, Del Toro R, Rivera-Torres J, et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell. 2019;25(3):407-418.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanoun M, Zhang D, Mizoguchi T, et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014;15(3):365-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches [published correction appears in Naturel. 2014;514:262]. Nature. 2013;495(7440): 231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinho S, Lacombe J, Hanoun M, et al. PDGFRα and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210(7): 1351-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977-988. [DOI] [PubMed] [Google Scholar]
- 43.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20(8):833-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Wu Q, Johnson CB, et al. In situ mapping identifies distinct vascular niches for myelopoiesis. Nature. 2021;590(7846):457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruns I, Lucas D, Pinho S, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20(11):1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kokkaliaris KD, Kunz L, Cabezas-Wallscheid N, et al. Adult blood stem cell localization reflects the abundance of reported bone marrow niche cell types and their combinations. Blood. 2020;136(20):2296-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao M, Perry JM, Marshall H, et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20(11):1321-1326. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura-Ishizu A, Takubo K, Kobayashi H, Suzuki-Inoue K, Suda T. CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow [published correction appears in J Exp Med. 2015;212(13):2323]. J Exp Med. 2015;212(12):2133-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinho S, Marchand T, Yang E, Wei Q, Nerlov C, Frenette PS. Lineage-biased hematopoietic stem cells are regulated by distinct niches. Dev Cell. 2018;44(5):634-641.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lambert MP, Wang Y, Bdeir KH, Nguyen Y, Kowalska MA, Poncz M. . Platelet factor 4 regulates megakaryopoiesis through low-density lipoprotein receptor-related protein 1 (LRP1) on megakaryocytes. Blood. 2009;114(11):2290-–2298.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lambert MP, Rauova L, Bailey M, Sola-Visner MC, Kowalska MA, Poncz M. . Platelet factor 4 is a negative autocrine in vivo regulator of megakaryopoiesis: clinical and therapeutic implications. Blood. 2007;110(4):1153-–1160.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thiele J, Krech R, Choritz H, Georgii A. Emperipolesis – a peculiar feature of megakaryocytes as evaluated in chronic myeloproliferative diseases by morphometry and ultrastructure. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;46(3):253-263. [DOI] [PubMed] [Google Scholar]
- 53.Cunin P, Bouslama R, Machlus KR, et al. Megakaryocyte emperipolesis mediates membrane transfer from intracytoplasmic neutrophils to platelets. eLife. 2019;8:e44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eckly A, Scandola C, Oprescu A, et al. Megakaryocytes use in vivo podosome-like structures working collectively to penetrate the endothelial barrier of bone marrow sinusoids. J Thromb Haemost. 2020;18(11):2987-3001. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez L, Rodriguez S, Huang H, et al. Tumor necrosis factor-alpha and endothelial cells modulate Notch signaling in the bone marrow microenvironment during inflammation. Exp Hematol. 2008;36(5):545-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valet C, Batut A, Vauclard A, et al. Adipocyte fatty acid transfer supports megakaryocyte maturation. Cell Rep. 2020;32(1):107875. [DOI] [PubMed] [Google Scholar]
- 58.Kelly KL, Reagan WJ, Sonnenberg GE, et al. De novo lipogenesis is essential for platelet production in humans. Nat Metab. 2020; 2(10):1163-1178. [DOI] [PubMed] [Google Scholar]
- 59.Ambrosi TH, Scialdone A, Graja A, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20(6):771-784.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Faict S, Maes K, et al. Extracellular vesicle cross-talk in the bone marrow microenvironment: implications in multiple myeloma. Oncotarget. 2016;7(25):38927-38945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Decker M, Leslie J, Liu Q, Ding L. Hepatic thrombopoietin is required for bone marrow hematopoietic stem cell maintenance. Science. 2018;360(6384):106-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ergen AV, Boles NC, Goodell MA. Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood. 2012;119(11):2500-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piryani SO, Kam AYF, Vu UT, Chao NJ, Doan PL. CCR5 signaling promotes murine and human hematopoietic regeneration following ionizing radiation. Stem Cell Reports. 2019;13(1):76-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Machlus KR, Johnson KE, Kulenthirarajan R, et al. CCL5 derived from platelets increases megakaryocyte proplatelet formation. Blood. 2016;127(7):921-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pietras EM, Mirantes-Barbeito C, Fong S, et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol. 2016;18(6):607-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao JL, Ma C, O’Connell RM, et al. Conversion of danger signals into cytokine signals by hematopoietic stem and progenitor cells for regulation of stress-induced hematopoiesis. Cell Stem Cell. 2014;14(4):445-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nieswandt B, Stritt S. Megakaryocyte rupture for acute platelet needs. J Cell Biol. 2015;209(3):327-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishimura S, Nagasaki M, Kunishima S, et al. IL-1α induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J Cell Biol. 2015;209(3): 453-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamada T, Möhle R, Hesselgesser J, et al. Transendothelial migration of megakaryocytes in response to stromal cell-derived factor 1 (SDF-1) enhances platelet formation. J Exp Med. 1998;188(3):539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niswander LM, Fegan KH, Kingsley PD, McGrath KE, Palis J. SDF-1 dynamically mediates megakaryocyte niche occupancy and thrombopoiesis at steady state and following radiation injury. Blood. 2014; 124(2):277-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giannini S, Lee-Sundlov MM, Rivadeneyra L, et al. β4GALT1 controls β1 integrin function to govern thrombopoiesis and hematopoietic stem cell homeostasis. Nat Commun. 2020;11(1):356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10(1):64-71. [DOI] [PubMed] [Google Scholar]
- 73.Abbonante V, Di Buduo CA, Malara A, Laurent PA, Balduini A. Mechanisms of platelet release: in vivo studies and in vitro modeling. Platelets. 2020;31(6):717-723. [DOI] [PubMed] [Google Scholar]
- 74.Leiva O, Leon C, Kah Ng S, Mangin P, Gachet C, Ravid K. The role of extracellular matrix stiffness in megakaryocyte and platelet development and function. Am J Hematol. 2018;93(3):430-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malara A, Currao M, Gruppi C, et al. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells. 2014;32(4):926-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ciurea SO, Merchant D, Mahmud N, et al. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood. 2007;110(3):986-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schulze H, Korpal M, Hurov J, et al. Characterization of the megakaryocyte demarcation membrane system and its role in thrombopoiesis. Blood. 2006;107(10):3868-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun RJ, Shan NN. Megakaryocytic dysfunction in immune thrombocytopenia is linked to autophagy. Cancer Cell Int. 2019;19(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valet C, Levade M, Bellio M, Caux M, Payrastre B, Severin S. Phosphatidylinositol 3-monophosphate: a novel actor in thrombopoiesis and thrombosis. Res Pract Thromb Haemost. 2020;4(4):491-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bertović I, Kurelić R, Milošević I, et al. Vps34 derived phosphatidylinositol 3-monophosphate modulates megakaryocyte maturation and proplatelet production through late endosomes/lysosomes. J Thromb Haemost. 2020;18(7):1756-1772. [DOI] [PubMed] [Google Scholar]
- 81.Dupuis F, Desplat V, Praloran V, Denizot Y. Effects of lipidic mediators on the growth of human myeloid and erythroid marrow progenitors. J Lipid Mediat Cell Signal. 1997;16(3):117-125. [DOI] [PubMed] [Google Scholar]
- 82.Foulks JM, Marathe GK, Michetti N, et al. PAF-acetylhydrolase expressed during megakaryocyte differentiation inactivates PAF-like lipids. Blood. 2009;113(26):6699-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allende ML, Tuymetova G, Lee BG, Bonifacino E, Wu YP, Proia RL. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J Exp Med. 2010;207(5):1113-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Golan K, Vagima Y, Ludin A, et al. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood. 2012;119(11):2478-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niazi H, Zoghdani N, Couty L, et al. Murine platelet production is suppressed by S1P release in the hematopoietic niche, not facilitated by blood S1P sensing. Blood Adv. 2019;3(11):1702-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barrachina MN, Calderón-Cruz B, Fernandez-Rocca L, García Á. Application of extracellular vesicles proteomics to cardiovascular disease: guidelines, data analysis, and future perspectives. Proteomics. 2019;19(1-2):e1800247. [DOI] [PubMed] [Google Scholar]
- 87.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Janowska-Wieczorek A, Majka M, Kijowski J, et al. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001; 98(10):3143-3149. [DOI] [PubMed] [Google Scholar]
- 89.Barry OP, FitzGerald GA. Mechanisms of cellular activation by platelet microparticles. Thromb Haemost. 1999;82(2):794-800. [PubMed] [Google Scholar]
- 90.Faille D, El-Assaad F, Mitchell AJ, et al. Endocytosis and intracellular processing of platelet microparticles by brain endothelial cells. J Cell Mol Med. 2012;16(8):1731-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang J, Kao CY, Papoutsakis ET. How do megakaryocytic microparticles target and deliver cargo to alter the fate of hematopoietic stem cells? J Control Release. 2017;247:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005;25(7):1512-1518. [DOI] [PubMed] [Google Scholar]
- 93.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107(9):1047-1057. [DOI] [PubMed] [Google Scholar]
- 94.Mause SF, Ritzel E, Liehn EA, et al. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation. 2010;122(5):495-506. [DOI] [PubMed] [Google Scholar]
- 95.Barry OP, Pratico D, Lawson JA, FitzGerald GA. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest. 1997;99(9):2118-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Butler JT, Abdelhamed S, Kurre P. Extracellular vesicles in the hematopoietic microenvironment. Haematologica. 2018;103(3):382-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20(9):1487-1495. [DOI] [PubMed] [Google Scholar]
- 98.Flaumenhaft R, Dilks JR, Richardson J, et al. Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113(5):1112-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604-1611. [DOI] [PubMed] [Google Scholar]
- 100.Horstman LL, Ahn YS. Platelet microparticles: a wide-angle perspective. Crit Rev Oncol Hematol. 1999;30(2): 111-142. [DOI] [PubMed] [Google Scholar]
- 101.French SL, Butov KR, Allaeys I, et al. Platelet-derived extracellular vesicles infiltrate and modify the bone marrow during inflammation. Blood Adv. 2020;4 (13):3011-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]