Abstract
Background
Cutaneous small vessel vasculitis (CSVV) has been reported after exposure to direct oral anticoagulants (DOACs), such as dabigatran, rivaroxaban, apixaban, and edoxaban.
Objective
We used the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS) to describe clinical characteristics associated with CSVV among DOAC-exposed patients. Furthermore, we characterized this signal in the Sentinel System to relate the clinical data from the individual FAERS cases to population-based electronic healthcare data.
Methods
We queried FAERS for all cases of CSVV associated with DOACs from U.S. approval date of each DOAC through March 16, 2018. Within the Sentinel System, we identified incident CSVV cases using ICD-9 and ICD-10 diagnosis codes among adults aged ≥ 30 years who received a DOAC in the prior 90 days between January 1, 2010, and June 30, 2018. We excluded patients with evidence of select autoimmune diagnoses in the 183 days prior to their CSVV diagnoses and reported patient characteristics in the 183-day period prior to CSVV diagnoses.
Results
In FAERS, we identified 50 cases of CSVV reported with rivaroxaban (n=26), apixaban (n=14), dabigatran (n=9), and edoxaban (n=1). Approximately 50% of the cases reported time to onset within 10 days after DOAC exposure. When specified, the predominant type of CSVV reported was leukocytoclastic vasculitis (n=31), followed by Henoch-Schonlein purpura (n=4). Hospitalization occurred in most of the cases (n=37). Switching of the offending agent after the development of CSVV was reported (n=26). Three rivaroxaban (n=3) cases and one dabigatran case (n=1) reported positive rechallenge. In the Sentinel system, we identified 3659 CSVV cases with prior DOAC exposure, with 85% of events occurring within 10 days.
Conclusions
The assessment of FAERS cases, combined with the temporal clustering of the Sentinel System cases suggest a possible causal relationship of DOACs and CSVV. Future efforts should characterize the risk of CSVV among the various DOAC users.
Keywords: vitamin K antagonist oral anticoagulants, cutaneous small vessel vasculitis, leukocytoclastic vasculitis, Henoch-Schonlein purpura, adverse drug reaction
Cutaneous small vessel vasculitis (CSVV) is a single organ, skin-isolated small vessel vasculitis, often leukocytoclastic vasculitis (LCV), without apparent systemic vasculitis or extracutaneous involvement.1–3 CSVV can be triggered by infections, medications, autoimmune disease, or malignancy. It also can be idiopathic when there is no evidence of an inciting factor.1,4 Various terms are used interchangeably in the medical literature to describe CSVV, including drug-induced vasculitis, LCV, hypersensitivity vasculitis, hypersensitivity angiitis, cutaneous leukocytoclastic angiitis, and allergic vasculitis.3,5 The incidence of CSVV is not well known; however, available studies estimate it to be between 15 and 45 cases per million adults per year.6–8
Classically, CSVV presents as a symmetric palpable purpura of the lower extremities but can sometimes involve skin on the trunk and upper extremities.3,5 Clinical presentation such as urticarial lesions and ulcerative vesicles or nodules may be indicative of deeper or medium vessel involvement.3 Confirmation of CSVV diagnosis is by skin biopsy, ideally taken from a lesion present for 18 to 48 hours to avoid non-specific results.1,4 Histologically, CSVV presents with inflammatory infiltrate composed of neutrophils, primarily affecting postcapillary venules, with fibrin deposits around the vessel wall, endothelial swelling, and extravasation of red blood cells.1,4
By definition, CSVV primarily is limited to the skin; however, an organ-threatening systemic vasculitis can occur later in the disease course.1,2 Therefore, CSVV is considered a symptom that requires excluding potential systemic involvement that can affect management and prognosis.1,2 For example, Henoch-Schonlein purpura (HSP), a subset of CSVV is characterized by cutaneous, gastrointestinal, joint, and/or kidney involvement that has the same initial presentation as CSVV; however, its management and prognosis are different.1,2 Additionally, patients initially diagnosed with CSVV can later develop systemic forms of small vessel vasculitides (e.g., antineutrophil cytoplasmic antibody–associated vasculitis).2
The association of drugs with the development of CSVV is documented with various therapeutic agents.9 Symptoms usually occur 7 to 10 days after drug initiation; however, shorter and longer periods of drug exposure have been reported with various agents.9,10 In most cases, discontinuation of the offending agent can resolve drug-induced CSVV with good prognosis.4 Treatment with corticosteroids and immunosuppressive agents has been used in some cases.11 Although not completely understood, the mechanism of drug-induced CSVV is thought to be mediated by the deposition of immune complexes in small vessels.
According to current guidelines direct oral anticoagulants (DOACs), also known as non–vitamin K oral anticoagulants (NOACs), such as dabigatran, rivaroxaban, apixaban and edoxaban, are now the preferred treatment for reducing the risk of stroke in atrial fibrillation (AFib) and in the prevention and treatment of venous thromboembolism (VTE).12,13 Betrixaban, the newest approved DOAC, was not included in this study due to its limited market uptake. Mechanistically, DOACs directly target the enzymatic activity of thrombin or factor Xa. In the postmarket setting, DOACs appear to be most frequently associated with type III and type IV delayed drug hypersensitivity reactions both mild and severe in nature.14 Additionally, all DOACs are labeled for hypersensitivity reactions ranging from skin rash to anaphylactic reactions, suggesting that these drugs may induce an immune response in some patients. Research exploring the pathogenesis of DOAC-associated CSVV is lacking. However, CSVV associated with warfarin and heparin has been described and attributed to immune-complex deposition.15,16
In the pivotal DOAC trials, vasculitis adverse events including CSVV were reported in patients treated with DOACs. For example, in the ROCKET-AF trial (7111 patients treated with rivaroxaban) the incidence of cutaneous vasculitis in the rivaroxaban arm was low (0.01%).17,18 Similarly, vasculitis adverse events including CSVV were reported in all premarketing trial data supporting the approval of all DOACs, but the incidence of these adverse events was low19–21 and similar to the comparator (i.e., warfarin or placebo). Notably, warfarin already is labeled for vasculitis in the Adverse Reactions section of the prescribing information.22
During routine postmarketing surveillance, the U.S. Food and Drug Administration (FDA) Division of Pharmacovigilance identified postmarketing cases of CSVV reported after DOAC exposure.23–26 This prompted a review of all CSVV cases submitted to the U.S. FDA Adverse Event Reporting System (FAERS) database and published in the medical literature. Furthermore, we characterized this signal in the Sentinel System to relate the clinical data from the individual FAERS cases to population-based electronic healthcare data. This evaluation provides a complementary assessment of DOAC-associated CSVV using two different data sources available to the FDA in the postmarket setting.
Methods
FAERS and Literature Case Series Data
We conducted a postmarketing case series analysis to identify and describe all potential cases of CSVV reported with DOACs. We queried the FAERS database from the U.S. approval date of each DOAC for postmarketing cases of CSVV reported with DOACs received by FDA through March 16, 2018. FAERS is a computerized spontaneous reporting system that encompasses> 14 million adverse event reports submitted voluntarily by health care professionals, consumers, and mandatorily by manufacturers. The FAERS database is designed to support the FDA’s postmarking safety surveillance program for drug and therapeutic biologic products.27 Adverse events are coded using the Medical Dictionary for Regulatory Activities (MedDRA) terminology. MedDRA is the international medical terminology developed by the International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use.28
To identify cases of CSVV, we searched the FAERS database using the Standardized MedDRA Query (SMQ) “Vasculitis” (narrow search). SMQs are validated, pre-determined sets of MedDRA terms grouped together after extensive review, testing, analysis, and expert discussion.28 We included cases that met the following case definition: clinical diagnosis of CSVV with or without histological confirmation by skin biopsy. Cases that met the case definition were assessed for a causal association with DOACs using published FDA guidance.29 A case had a probable causal association if there was a plausible temporal sequence to a DOAC along with skin biopsy findings consistent with CSVV and absence of factors with a contributory or confounding role (i.e., unknown or negative dechallenge, concomitant drugs frequently associated with CSVV9 or active diseases associated with CSVV including malignancies, infectious, autoimmune disease). Possible cases had a plausible temporal sequence to a DOAC administration that included clinical descriptions of CSVV (i.e., palpable purpura) documented by a physician (e.g., dermatologist), but lacked specific skin biopsy findings. PubMed and Embase were searched for additional cases published in the literature but not submitted to FAERS. The search terms consisted of (“dabigatran” OR “ rivaroxaban” OR “apixaban” OR “edoxaban”) AND (“vasculitis” OR “leukocytoclastic vasculitis”) through September 20, 2019. All literature case reports obtained were reviewed using the same case definition and causal assessment used in the FAERS search.
The assessment of cases included patient demographics, DOAC reported reason for use, time to onset of CSVV, CSVV adverse event characteristics (e.g., biopsy confirmed, type of CSVV, dechallenge/rechallenge and treatment information, and serious adverse drug experiences). The regulatory definition of serious adverse drug experiences includes outcomes of death, life-threatening events, hospitalizations, disability, congenital anomalies, and other important medical events. Only descriptive statistics were used to characterize results.
Investigation of CSVV in Sentinel System
The Sentinel System is a distributed data network of electronic healthcare databases used by FDA for active surveillance of medical product safety. The Sentinel System includes large national insurers, integrated delivery care networks, and the 100% Medicare fee-for-service plan. Currently, the Sentinel System has a cumulative 310.8 million patient identifiers from 2000 to 2018. Each Data Partner maintains information on their enrollees and periodically formats quality checked data into a Common Data Model.30,31 This facilitates quick and systematic querying of the data in response to safety questions. The data include medical and pharmacy claims, including inpatient and outpatient diagnosis, procedures, retail, and outpatient dispensing. After the specifications are agreed on with FDA staff, the Sentinel Operation Center distributes an analytic program that can be executed against the Common Data Model to the various Data Partners. The analytic programs are run locally, and summary-level statistics are returned to the Sentinel Operation Center for aggregation.
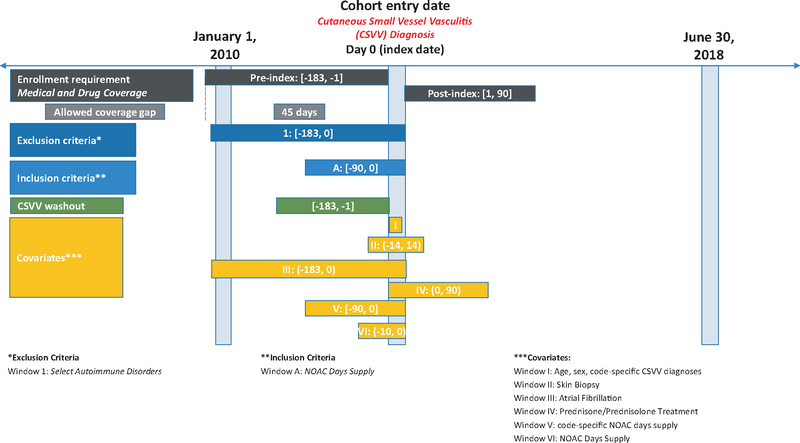
We used data from the Sentinel System, provided by 17 Data Partners from January 1, 2010, to June 30, 2018. The cohort included patients who received a DOAC (defined using National Drug Codes) (Appendix S1) in the 90 days prior to the index date (Figure 1). Index date was defined as first diagnosis of CSVV (defined using ICD-9 and ICD-10 codes) within 183 days (washout period) (Appendix S2). Because most of the CSVV cases identified in FAERS were in adults over ≥ 30 years, we limited the analysis to that population. We required continuous enrollment in health plans with both medical and pharmacy coverage of at least 183 days prior to CSVV diagnosis and 90 days post index date. Gaps in coverage of 45 days were allowed since they usually represent administrative gaps and not actual disenrollment. Given the association of CSVV with autoimmune disease, we excluded patients with autoimmune disease (defined using ICD-9 and ICD-10 codes) (Appendix S3) in the 183 days prior to index date. Among patients with diagnosis of CSVV, we examined the proportion of patients with a recorded history of skin biopsy and prednisone/prednisolone treatment within 14 and 90 days post index date, respectively. We reported patient characteristics in the 183-day period prior to CSVV diagnoses (e.g., patient demographics, any DOAC dispensing up to 10 days prior to CSVV diagnosis and CSVV coding on day of diagnosis). Only descriptive statistics were used to characterize the results.
Figure 1.
Sentinel system patient diagram for inclusion in the CSVV and prior DOAC exposure cohort.
Results
FAERS and Literature Case Series Data
The FAERS search retrieved 50 cases that met the case definition and plausible causal association to a DOAC. Thirteen of the fifty cases from FAERS were also reported in the literature. We did not identify any additional cases in the literature search. Rivaroxaban accounted for the largest number of cases (n=26), followed by apixaban (n=14), dabigatran (n=9), and edoxaban (n=1). The mean age was 70 years and the median age was 68 years (range 28–90 years); 26 (52%) were males and 24 (48%) were females. AFib was the most common indication for all DOACs (n=33, 66%), followed by VTE (n=15, 30%). All 50 cases reported a serious outcome per regulatory definition, mostly specifying hospitalization (n=37, 74%). The descriptive characteristics of all 50 cases are presented in Table 1.
Table 1.
Descriptive Characteristics of Cases Reporting CSVV Associated with DOACs in FAERS or Published Literature from Approval of each DOAC through March 16, 2018 (n=50)a,b
| Selected Characteristics | Dabigatran (n=9) | Rivaroxaban (n=26) | Apixaban (n=14) | Edoxaban (n=1) |
|---|---|---|---|---|
| FDA approval date | 10/19/2010 | 07/11/2011 | 12/28/2012 | 01/08/2015 |
| Age (years)c | n=8 | n=25 | n=14 | |
| Mean | 69 | 65 | 73 | - |
| Median age, years (range) | 70 (54–78) | 68 (28–87) | 76 (49–90) | - |
| Sex | ||||
| Male | 6 | 10 | 9 | 1 |
| Female | 3 | 16 | 5 | - |
| Reported reason for use | ||||
| Atrial fibrillation | 8 | 14 | 10 | 1 |
| Venous thromboembolism | 1 | 10 | 4 | - |
| Not reported | - | 2 | - | - |
| Time-to-onset (days) | ||||
| Median (range) | 10 (2–23) | 12 (1–120) | 10 (1–547) | 7 |
| Biopsy confirmed | 7 | 18 | 7 | 1 |
| Physician diagnosed | 2 | 8 | 7 | - |
| Dechallenge/Rechallenge | ||||
| Positive dechallenge | 9 | 26 | 13 | 1 |
| Rechallenge | 1 | 3 | - | - |
| Not reported | - | - | 1 | - |
| Type of CSVV | ||||
| Leukocytoclastic | 8 | 15 | 8 | - |
| Henoch-Schonlein | - | 4 | - | - |
| Not specifiedd | 1 | 7 | 6 | 1 |
| Clinical Interventione | ||||
| Discontinuation of offending DOAC | 9 | 25 | 13 | 1 |
| Treatment with corticosteroids | 6 | 17 | 3 | 1 |
| Offending anticoagulant substitutione | ||||
| Another DOAC | 4 | 3 | 2 | 1 |
| Vitamin K antagonist | 2 | 5 | 1 | - |
| Low molecular weight heparin | 1 | 5 | 2 | - |
| Not reported | 2 | 13 | 9 | - |
| Serious Outcomese,f | ||||
| Hospitalization | 8 | 19 | 9 | 1 |
| Life threatening | - | 1 | 1 | - |
| Other serious | 1 | 11 | 12 | - |
| Causality Assessment | ||||
| Probable | 7 | 18 | 7 | 1 |
| Possible | 2 | 8 | 7 | - |
Literature search was conducted through September 20, 2019.
Betrixaban the newest approved DOAC was not included in this study due to its limited market uptake.
Refers to the cases in which age information was reported.
Include non-specific types of CSVV (e.g., vasculitis/ulcerative/necrotic vasculitis).
More than one clinical intervention, outcome, or DOAC may have been reported per case.
Per 21 CFR 314.80, the regulatory definition of serious is any adverse drug experience occurring at any dose that results in any of the following outcomes: death, a life-threatening adverse drug experience, inpatient hospitalization or prolongation of existing hospitalization, a persistent or significant disability/incapacity, a congenital anomaly/birth defect, and other serious important medical events.
Approximately 50% of the cases reported time to onset within 10 days post DOAC exposure (range 1–547 days). When specified, the predominant type of CSVV reported was LCV (n=31, 62%), followed by HSP (n=4, 8%) with the remaining cases describing non-specific types of CSVV including necrotic and ulcerative vasculitis (n=15, 30%). A positive dechallenge was reported in all 50 cases. Additionally, three rivaroxaban cases and one dabigatran case reported a positive rechallenge. Switching of the offending DOAC after the development of CSVV was reported in 26 (52%) cases. This switch included 10 (20%) cases reporting a switch to another DOAC, followed by vitamin K antagonists (VKAs) (n=8, 16%) and low molecular weight heparins (LMWHs) (n=8, 16%). Corticosteroids were the most frequently reported treatment after the development of CSVV (n=26, 52%). The 50 cases included 33 (66%) skin biopsy confirmed cases and 17 (34%) physician and or dermatologist diagnosed cases.
We assessed 33 (66%) cases as probable from a causal association perspective considering the plausible temporal sequence to a DOAC, the histopathologic confirmation of CSVV via skin biopsy and the lack of known predisposing factors such as other concomitant medications and/or underlying diseases known to be associated with CSVV. We assessed the remaining 17 (34%) cases as having a possible causal association with a DOAC due to reasonable although less plausible temporal sequence, the lack of skin biopsy findings to confirm the diagnosis of CSVV as well as other predisposing factors.
Sentinel System Data
We identified 3659 CSVV cases with evidence of DOAC dispensing at least 90 days before the case date. Approximately 85% of patients had a DOAC dispensing at least 10 days before the CSVV diagnosis. Similar to the FAERS data, the demographic characteristics among patients with a CSVV diagnosis in the Sentinel System showed a 1:1 male:female ratio with the number of CSVV cases increasing with age. The mean age (75.2 years) of patients with CSVV diagnosis post DOAC exposure in the Sentinel System was approximately similar to those in FAERS (Table 2). Similar to the FAERS data, atrioventricular fibrillation diagnosis was present in a majority of patients (n=2876, 78.6%). Skin biopsy up to 14 days before or after the CSVV diagnosis occurred in 704 patients (19.2%). Corticosteroid treatment within 90 days after CSVV diagnosis occurred in 1123 patients (30.7%). Similar to the FAERS cases, the most common CSVV diagnosis in the Sentinel System was vascular disorders of the skin (1040 patients, 28.4%), followed by HSP (752 patients, 20.6%).
Table 2.
Clinical Characteristics of DOAC Cases Associated with CSVV in Sentinel System
| Patient Characteristic | N | % |
|---|---|---|
| Number of unique patients | 3659 | |
| Demographics | ||
| Mean Age, (±SD) | 75.2 (±10.3) | |
| Age | ||
| 30–39 | 33 | 0.9 |
| 40–49 | 71 | 1.9 |
| 50–59 | 223 | 6.1 |
| 60–69 | 642 | 17.5 |
| 70+ | 2690 | 73.5 |
| Sex | ||
| Male | 1793 | 49.0 |
| Female | 1865 | 51.0 |
| Any DOAC dispensing, up to 10 days prior to CSVV diagnosis | 3112 | 85.1 |
| Clinical Characteristics | ||
| Atrial Fibrillation, up to 183 days before CSVV diagnosis | 2876 | 78.6 |
| Skin biopsy, up to 14 days before or after CSVV diagnosis | 704 | 19.2 |
| Prednisone and/or Prednisolone treatment, up to 90 days after CSVV diagnosis | 1123 | 30.7 |
| Prednisone and/or Prednisolone treatment, up to 90 days after CSVV biopsy, up to 14 days before or after CSVV diagnosis | 244 | 6.7 |
| Cutaneous Small Vessel Vasculitis Coding on day of Diagnosisa | ||
| Vascular disorders of skin | 1040 | 28.4 |
| Henoch-Schonlein allergic purpura | 752 | 20.6 |
| Vasculitis limited to the skin, unspecified | 598 | 16.3 |
| Allergic purpura | 378 | 10.3 |
| Other specified hypersensitivity angiitis | 368 | 10.1 |
| Other vasculitis limited to the skin, specified NEC | 265 | 7.2 |
| Hypersensitivity angiitis | 225 | 6.1 |
| Hypersensitivity angiitis, unspecified | 138 | 3.8 |
Counts may sum to greater than the total number of unique patients due to patients with multiple valid index-defining codes on their index date.
Discussion
To our knowledge, the 50 cases identified in FAERS and the literature represents the largest published case series of DOAC-associated CSVV. Consistent with U.S. utilization patterns, rivaroxaban accounted for the largest number of cases followed by apixaban, dabigatran, and edoxaban.32 Approximately 50% and 85% of events occurred within 10 days of DOAC exposure in FAERS and Sentinel System, respectively. This finding in FAERS and the Sentinel System suggests that DOAC-associated CSVV may be an acute event. This is generally consistent with drug-induced CSVV that typically appears within 7 to 10 days after exposure to a drug but may range from 2 days to years.9 Moreover, this finding represents an adequate time to allow for a sufficient quantity of antibody to produce an antibody-antigen complex.9 Furthermore, in the FAERS case series and in the Sentinel System, CSVV cases had a similar mean age and were equally reported in both males and females, consistent with the epidemiology of CSVV2 and the patient population typically exposed to DOACs.
We identified three rivaroxaban-associated CSVV cases with a positive rechallenge. In two cases, rivaroxaban was restarted because rivaroxaban-associated CSVV was not frequently reported in the medical literature.33,34 Similarly, we identified a dabigatran rechallenge case where the DOAC was restarted because the physician was unaware of the patient’s previous history of dabigatran-associated CSVV and it recurred within 10 days.
The predominant reported type of CSVV in the case series was LCV, followed by HSP. The LCV cases reported a palpable purpura, characterized by small skin lesions suggesting only small vessel involvement. The HSP cases reported cutaneous and kidney involvement.35 Several cases reported urticarial lesions, ulcerative vesicles, or nodules suggestive of small and medium vessel involvement.1 In one HSP case, the initial diagnosis of rivaroxaban-associated LCV lesions began to resolve five days after switching to apixaban. However, on follow-up, persistent proteinuria prompted a kidney biopsy that showed HSP.36 This highlights the importance of follow-up post-CSVV diagnosis because the initial presentation of HSP can be indistinguishable from other types of CSVV; however, its management and prognosis are different.2 Similar to the FAERS cases, the most common CSVV diagnosis in the Sentinel System was vascular disorders of the skin followed by HSP. However, we are unable to determine which patients in the cohort had CSVV and then subsequently developed HSP.
The treatment of drug-induced CSVV involves discontinuation of the suspected agent, usually with good prognosis. Typically, corticosteroids and immunosuppressive agents are reserved for extensive disease.1,4 Fifty percent of the patients in the FAERS case series were treated with corticosteroids after discontinuation of the suspected DOAC. Furthermore, 31% of the Sentinel System cohort showed evidence of prednisone and/or prednisolone treatment, up to 90 days after CSVV diagnosis. This may suggest that some patients did not require treatments with corticosteroids, although topical and other injectable corticosteroids may have been used to treat these patients, which was not captured in the Sentinel System analysis. In FAERS, some patients improved after the suspected switching to other anticoagulants. Researchers reported a similar finding of switching patients that experienced hypersensitivity reactions with DOACs to VKAs and LMWH.14 The FAERS case series also highlights the ability to switch to other DOACs after experiencing CSVV. They reported a similar finding in which patients who developed a reaction to rivaroxaban tolerated other DOACs and vice versa.14 The successful switch to another DOAC after developing DOAC-associated CSVV in the case series supports a lack of cross-reactivity between these agents, although additional studies are necessary to elucidate this finding.14
Historically, numerous terms have been used interchangeably to describe CSVV. The American College of Rheumatology (ACR) criteria of 1990 refers to CSVV as “hypersensitivity vasculitis.”5 However, the Chapel Hill Consensus Conference (CHCC) revised 2012 nomenclature system refers to CSVV as “cutaneous leukocytoclastic angiitis.”3 The use of multiple terms to describe CSVV may account for the variability in MedDRA preferred terms used to identify vasculitis adverse events in FAERS and for the variability of diagnostic codes observed in the Sentinel System when attempting to capture CSVV events as an outcome.
The study is not without limitations. The limitations of the FAERS data are well-known and have been described elsewhere.29,37 In this feasibility assessment in the Sentinel System, we did not exclude alternative causes of CSVV except autoimmune disease; the history of active infection or active malignancy was difficult to ascertain. Additionally, we did not differentiate incident from prevalent DOAC exposure or adjust for potential confounders. Additional limitations include the lack of validation for the codes used to identify CSVV events and skin biopsy. Therefore, the positive predictive value of the procedures and outcome codes used in this analysis are unknown. Moreover, we did not account for the presence of other drugs that may be associated with CSVV in the Sentinel System analysis.
Given the high use of DOACs and the underreporting of adverse events in the postmarketing setting, we cannot calculate true incidence of CSVV as a denominator is not available.37 CSVV represents a relatively rare adverse event associated with DOACs in susceptible individuals that may result in systemic involvement and hospitalization.11 Additional observational studies should be planned to further characterize the risk of CSVV among DOAC users and to evaluate if there is differential risk by individual DOAC.
Supplementary Material
Appendix S1 National Drug Codes (NDCs) used to define DOACs.
Appendix S2 Codes used to define CSVV.
Appendix S3 Codes used to define select autoimmune diseases.
Acknowledgments
Data partners who provided data used in the analysis: Aetna, a CVS Health company, Blue Bell, PA; Blue Cross Blue Shield of Massachusetts, Boston, MA; Duke University School of Medicine, Department of Population Health Sciences, Durham, NC, through the Centers for Medicare and Medicaid Services which provided data; Harvard Pilgrim Health Care Institute, Boston, MA; HealthCore, Inc., Translational Research for Affordability and Quality, Alexandria, VA; HealthPartners Institute, Minneapolis, Minnesota; Humana, Inc., Healthcare Research, Miramar, FL; Kaiser Permanente Colorado Institute for Health Research, Aurora, CO; Kaiser Permanente Center for Integrated Health Care Research Hawai’i, Honolulu, HI; Kaiser Permanente Mid-Atlantic States, Mid-Atlantic Permanente Research Institute, Rockville, MD; Kaiser Permanente Northern California, Division of Research, Oakland, CA; Kaiser Permanente Northwest Center for Health Research, Portland, OR; Kaiser Permanente Washington Health Research Institute, Seattle, WA; Marshfield Clinic Research Institute, Marshfield, WI; Meyers Primary Care Institute, Worcester, MA; OptumInsight Life Sciences Inc., Boston, MA; Vanderbilt University Medical Center, Department of Health Policy, Nashville, TN, through the TennCare Division of the Tennessee Department of Finance & Administration which provided data.
Funding: This work was funded by the Food and Drug Administration’s Sentinel Initiative (HHSF223201400030I).
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose. LG was employed by the U.S. FDA at the time of conducting this study and developing this manuscript and has no conflicts of interest to disclose.
Disclaimer: The opinions expressed in this manuscript are those of the authors and not necessarily of the U.S. FDA.
Prior Presentation: Preliminary findings were presented at the International Conference on Pharmacoepidemiology and Therapeutic Risk Management (ICPE) in Philadelphia, PA August 24–28, 2019.
References
- 1.Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol 2014;15(4):299–306. [DOI] [PubMed] [Google Scholar]
- 2.Micheletti RG, Werth VP. Small vessel vasculitis of the skin. Rheumat Dis Clin North Am 2015;41(1):21–32, vii. [DOI] [PubMed] [Google Scholar]
- 3.Jennette JC, Falk RJ, Bacon PA, et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65(1):1–11. [DOI] [PubMed] [Google Scholar]
- 4.Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol 2006;45(1):3–13. [DOI] [PubMed] [Google Scholar]
- 5.Hunder GG, Arend WP, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Introduction. Arthrit Rheumat 1990;33(8):1065–7. [DOI] [PubMed] [Google Scholar]
- 6.Arora A, Wetter DA, Gonzalez-Santiago TM, Davis MD, Lohse CM. Incidence of leukocytoclastic vasculitis, 1996 to 2010: a population-based study in Olmsted county. Minnesota. Mayo Clinic Proc 2014;89(11):1515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watts RA, Jolliffe VA, Grattan CE, Elliott J, Lockwood M, Scott DG. Cutaneous vasculitis in a defined population-clinical and epidemiological associations. J Rheumatol 1998;25 (5):920–4. [PubMed] [Google Scholar]
- 8.García-Porrúa C, González-Gay MA. Comparative clinical and epidemiological study of hypersensitivity vasculitis versus Henoch-Schönlein purpura in Adults. Semin Arthritis Rheum 1999;28(6):404–12. [DOI] [PubMed] [Google Scholar]
- 9.ten Holder SM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug-induced vasculitis. Ann Pharmacoth 2002;36(1):130–47. [DOI] [PubMed] [Google Scholar]
- 10.Kearsley JH, Jeremy RW, Coates AS. Leucocytoclastic vasculitis and skin necrosis following subcutaneous heparin calcium. Aust N Z J Med 1982;12(3):288–9. [DOI] [PubMed] [Google Scholar]
- 11.Grau RG. Drug-induced vasculitis: new insights and a changing Lineup of suspects. Curr Rheumatol Rep 2015;17(12):71. [DOI] [PubMed] [Google Scholar]
- 12.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: chest guideline and expert panel report. Chest 2016;149(2):315–52. [DOI] [PubMed] [Google Scholar]
- 13.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation 2019;140(2):e125–e51. [DOI] [PubMed] [Google Scholar]
- 14.Carli G, Farsi A, Chiarini F, Lippolis D, Cortellini G. Hypersensitivity reactions to non-vitamin k oral anticoagulants - a review of literature and diagnostic work-up proposal. Eur Ann Allergy Clin Immunol. 2019;51(1):7–14. [DOI] [PubMed] [Google Scholar]
- 15.Hsu CY, Chen WS, Sung SH. Warfarin-induced leukocytoclastic vasculitis: a case report and review of literature. Inter Med 2012;51(6):601–6. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado Cid P, Alonso de Celada RM, Noguera Morel L, Feito-Rodríguez M, Gómez-Fernández C, Herranz Pinto P. Cutaneous adverse events associated with heparin. Clin Exp Dermatol 2012;37(7):707–11. [DOI] [PubMed] [Google Scholar]
- 17.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365(10):883–91. [DOI] [PubMed] [Google Scholar]
- 18.Hofmeier KS. Hypersensitivity reactions to modern antiplatelet and anticoagulant drugs. Allerg J Int 2015;24(2):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361(12):1139–51. [DOI] [PubMed] [Google Scholar]
- 20.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365(11):981–92. [DOI] [PubMed] [Google Scholar]
- 21.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369(22):2093–104. [DOI] [PubMed] [Google Scholar]
- 22.Coumadin (Warfarin). Prescribing Information. Bristol-Myers Squibb Pharma Company. Revised August 2017. [Google Scholar]
- 23.Cakmak MA, Sahin S, Cinar N, Karsidag S. Adverse skin reaction caused by dabigatran. Eur Rev Med Pharmacol Sci 2014;18(18):2595. [PubMed] [Google Scholar]
- 24.Potolidis E, Mandros C, Kotsa K, Mitsiou E, Potolidis D, Fanourgiakis P. Dabigatran associated leukocytoclastic vasculitis. Case Rep Med. 2015;2015:616109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaaya G, Jaller-Char J, Ghaffar E, Castiglioni A. Rivaroxaban-induced leukocytoclastic vasculitis: a challenging rash. Ann Allergy Asthma Immunol. 2016;116(6):577–8. [DOI] [PubMed] [Google Scholar]
- 26.Nasir UB, Kumar A, Easwar A. Apixaban Causing Leukocytoclastic Vasculitis. J Allergy Clin Immunol Pract 2018;6 (5):1744–45. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration. FDA Adverse Event Reporting System (FAERS) [Online]. Available At: www.Fda.Gov/Drugs/Guidancecomplianceregulatoryinformation/Surveillance/Adversedrugeffects/Default.Htm. Accessed November 16, 2017. [Google Scholar]
- 28.Maintenance and Support Services Organization [Homepage on the Internet]. Medical dictionary for regulatory activities. Mclean (Va): Meddra Msso; 2013. Available at: http://www.meddra.org. Accessed December 21, 2019. [Google Scholar]
- 29.US Food and Drug Administration. Guidance for Industry: Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment [Online]. 2005. Available At: www.fda.Gov/Downloads/Drugs/Guidancecomplianceregulatoryinformation/Guidances/Ucm071696.Pdf. Accessed December 10, 2019.
- 30.Behrman RE, Benner JS, Brown JS, McClellan M, Woodcock J, Platt R. Developing the sentinel system–a national resource for evidence development. N Engl J Med 2011;364(6):498–9. [DOI] [PubMed] [Google Scholar]
- 31.Ball R, Robb M, Anderson SA, Dal Pan G. The FDA’S sentinel initiative–a comprehensive approach to medical product surveillance. Clin Pharmacol Ther 2016;99(3):265–8. [DOI] [PubMed] [Google Scholar]
- 32.Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128(12):1300–5.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasbal NB, Basturk T, Koc Y, Sahutoglu T, Bayrakdar Caglayan F, Unsal A. Leukocytoclastic vasculitis associated with a new anticoagulant: rivaroxaban. Turkish J Haematol 2017;34 (1):116–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HL, Kim L, Kim CW, Kim JS, Nam HS, Ryu JS. Case of both rivaroxaban- and dabigatran-induced leukocytoclastic vasculitis, during management of pulmonary thromboembolism. Respir Med Case Rep 2019;26:219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calvo-Rio V, Loricera J, Mata C, et al. Henoch-Schonlein purpura in Northern Spain: clinical spectrum of the disease in 417 patients from a single center. Medicine 2014;93(2):106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung EY, Chen J, Roxburgh S. A case report of Henoch-Schonlein purpura and IGA nephropathy associated with rivaroxaban. Nephrology 2018;23(3):289–90. [DOI] [PubMed] [Google Scholar]
- 37.Goldman SA. Limitations and strengths of spontaneous reports data. Clin Ther 1998;20(Suppl C):C40–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 National Drug Codes (NDCs) used to define DOACs.
Appendix S2 Codes used to define CSVV.
Appendix S3 Codes used to define select autoimmune diseases.