Abstract
Background
We investigated the putative associations of alcohol, cigarette, e-cigarette and marijuana use with kidney function and proteinuria among adolescents and young adults (AYA) with pediatric-onset chronic kidney disease (CKD) enrolled in the Chronic Kidney Disease in Children (CKiD) study.
Methods
Participants responded to questions about past year and 30-day substance use. Associations between each substance and kidney function, proteinuria, nephrotic range proteinuria and high blood pressure were separately estimated using repeated measures regression models, adjusting for sociodemographic characteristics. Models controlled for covariates at the present visit (contemporaneous), and additionally controlled for disease severity at the year prior to reporting substance use (lagged).
Results
A total of 441 participants ≥16 years contributed 1,245 person-visits with 39% reporting alcohol and 16%, 17% and 15% reporting cigarette, e-cigarette and marijuana use, respectively, over the previous year. In adjusted lagged models, past year and 30-day cigarette use were significantly associated with higher levels of proteinuria (+18.6%, 95%CI: +2.8%, +36.9%; and +20.0%, 95%CI: +0.7%, +43.1%, respectively). Inferences were similar when controlling for secondhand smoke exposure.
Conclusions
In a cohort of AYA with pediatric kidney diseases, substance use was non-trivial, and cigarette use was associated with higher proteinuria, although the prevalence was low. Occasional alcohol, e-cigarette and marijuana use were not associated with proteinuria, disease progression, or elevated blood pressure. Pediatric nephrologists as specialty care providers are well-positioned to discuss substance use and should encourage tobacco prevention/treatment efforts among AYA at high risk for use in order to preserve kidney function and promote well-being.
Keywords: Kidney Diseases, Substance Use, Tobacco Use, Adolescent, Young Adult, Epidemiology
BACKGROUND
Identifying modifiable risk factors in an effort to delay disease progression to end stage kidney disease (ESKD) among those with pediatric chronic kidney disease (CKD) is a clinical priority, especially in adolescents and young adults (AYA). Secondhand cigarette smoke (SHS) has been identified as a risk factor for pediatric CKD progression [1–3], and substance use and its relationship to kidney injury suggests potential for long-term harm [4, 5]. However, an overview of the relationship of alcohol, cigarette, e-cigarette, and marijuana use with CKD progression in these patients has not been characterized. Using data from the Chronic Kidney Disease in Children (CKiD) study, we investigated the extent to which substance use (SU) was associated with CKD severity and elevated blood pressure (BP) in a population of AYA with CKD.
METHODS
The CKiD study is a prospective cohort of children and AYA with childhood-onset CKD in the US and Canada. Details on the study design and methods used in CKiD have been previously described [6]. All participants provided informed consent/assent prior to enrollment.
Participants answered questions on SU at annual visits starting at age 12. Participants were asked if they had used alcohol, tobacco products (cigarettes, cigars, cigarillos or little cigars), e-cigarettes, or marijuana over the past year. If yes, subsequent questions included how many days over the past 30 days (p30d) they consumed alcohol, if they currently smoke tobacco products and the average number of products smoked per week, the number of days over the p30d they used an e-cigarette, and the number of times over the p30d they used marijuana. Data were collected from 2005 to 2018, with the exception of information on e-cigarettes and p30d use of alcohol and marijuana, which began in September 2014.
Outcomes of interest were estimated glomerular filtration rate (serum creatinine and cystatin C-based eGFR [7]) in ml/min per 1.73m2, proteinuria (urine protein-to-creatinine ratio; uPCR) in mg/mgCr, nephrotic range proteinuria (uPCR ≥2.0 mg/mgCr), and uncontrolled high BP. Uncontrolled BP was defined as systolic BP ≥120 mmHg or diastolic BP ≥80 mmHg (incorporating elevated BP, and stages 1 and 2 hypertension based on 2017 American Academy of Pediatrics guidelines [8]).
Covariates were age (categorized as two-year bins from 12 to <22, and ≥22 years), primary CKD etiology (glomerular vs. non-glomerular), sex (male vs. female), race (Black vs. non-Black), ethnicity (Hispanic vs. non-Hispanic), household annual income (≤$36,000 vs. >$36,000 to ≤$75,000 vs. >$75,000), mother’s education level (college or more vs. less than college), and anemia (yes vs. no) defined as hemoglobin <5th percentile for sex, age and race [9].
Repeated measures regression models estimated the association of SU as the exposure (defined as previous year or p30d SU in separate models) with eGFR, uPCR, nephrotic range proteinuria and uncontrolled BP (as outcomes), with person-visits as the unit of analysis. Linear models were fit for eGFR and uPCR, and logistic models for nephrotic range proteinuria and uncontrolled BP. Models controlled for age, CKD etiology, sex, race, ethnicity, maternal education, and income at the present visit where the exposure and outcome were also measured. These models controlled only for sociodemographic variables measured at the same time as the outcomes (‘contemporaneous’ models). A second set of models were fit controlling for the same covariates as well as the previous year eGFR, uPCR, uncontrolled BP and anemia (‘lagged’ models). The purpose of this adjustment was to compare people with similar disease severity at the prior year. Generalized estimating equations were used to calculate 95% confidence intervals to account for within-person correlation. Log-transformed eGFR and uPCR were expressed as percent differences; odds ratios were the measures of association for uncontrolled BP and nephrotic range proteinuria.
SHS exposure was considered a confounder in the cigarette to uPCR relationship [1], and was included as a covariate (defined as self-reported presence of a household smoker) in additional models with cigarette use as the exposure and uPCR as the outcome.
RESULTS
A total of 441 CKiD participants 16 years of age or older provided SU data over 1,245 person-visits. Person-visits contributed by those 12 to <16 years of age were excluded because prevalence rates were less than 2% for p30d use of each substance, and less than 4% for the previous year, with the exception of previous year use of alcohol, which was 6.5%. Characteristics of the analytic population are presented in Supplemental Table 1. Briefly, nearly half of person-visits were contributed by those 16 to <18 years, 60% were male, 20% were Black, and 39% had glomerular CKD. Among all person-visits, median eGFR and uPCR were 51 (IQR: 36–68) and 0.47 (IQR: 0.14–1.28), respectively; 15% had nephrotic range proteinuria, and 36% had uncontrolled BP.
A total of 38.8% and 34.1% reported alcohol use over the previous year and p30d, respectively; 15.9% and 8.5% reported cigarette use over the previous year and p30d; 16.6% and 11.7% reported e-cigarette use over the previous year and p30d; and 14.8% and 10.8% reported marijuana use over the previous year and p30d. Among those who reported p30d use, lower frequency of SU was more common than higher frequency: for alcohol, 73.8% reported using ≤5 days and 8.7% reported >10 days for use over the p30d. For cigarettes, 45.1% reported using ≤5 cigarettes per week and 28.4% reported using >20 cigarettes per week on average. Among p30d e-cigarette users, 72.9% reported using ≤5 days and 15.3% reported using >10 days. However, for p30d marijuana users, 38.2% reported using ≤5 and 45.5% reported using >10 times.
Figure 1 presents the results from the contemporaneous and lagged models and the associations between past year and p30d SU. Most estimates from the lagged models were attenuated compared to contemporaneous. Additionally, most estimates were not statistically significant. All statistically significant estimates indicated that SU was associated with worse kidney health, although p30d marijuana use was associated with 4.7% higher GFR after adjusting for previous year disease severity (p = 0.04).
Figure 1.
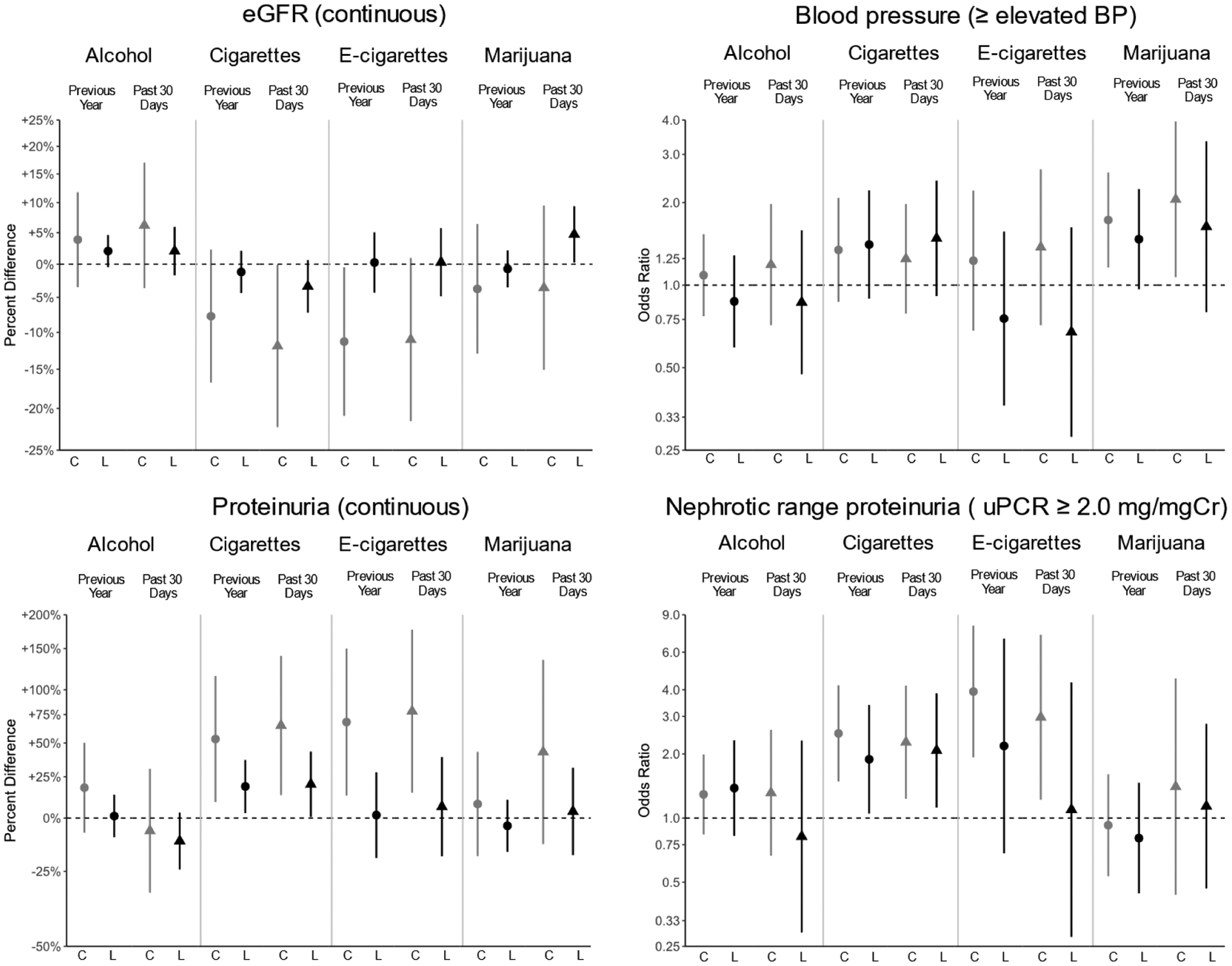
Contemporaneous (C, gray) and lagged (L, black) model associations between previous year (●) and past 30-day (▲) substance use as exposures and markers of kidney health as outcomes for those 16 years and older. Each substance and period of use was modeled separately. Estimates are presented as percent differences for eGFR and proteinuria, and relative odds for elevated blood pressure or higher (elevated BP, Stage 1 or Stage 2 hypertension) and nephrotic range proteinuria (≥ 2.0mg/mg of creatinine), with 95% confidence interval bars.
Cigarette smoking was consistently and significantly associated with uPCR and nephrotic range proteinuria. Those who smoked cigarettes over the past year, compared to those who did not, had, on average, higher proteinuria by 53.3% (95%CI: +9.0%, +115.5%) and 18.6% (95%CI: +2.8%, +36.9%) in the contemporaneous and lagged models, respectively. Similarly, those who smoked cigarettes over the p30d had 64.9% (95%CI: +13.2%, +140.3%) and 20.0% (95%CI: +0.7%, +43.1%) higher proteinuria in the contemporaneous and lagged models, respectively. Odds ratios for nephrotic range proteinuria were also significantly higher among those who reported cigarette smoking. In contemporaneous and lagged models for past year cigarette smoking, odds ratios for nephrotic range proteinuria were 2.50 (95%CI: 1.49, 4.19) and 1.89 (95%CI: 1.05, 3.39), respectively. For p30d use, odds ratios were 2.27 (95%CI: 1.23, 4.18) and 2.08 (95%CI: 1.12, 3.85) for contemporaneous and lagged, respectively.
Additionally, when controlling for the presence of a household smoker, the directionality and significance of continuous uPCR associations typically remained: past year cigarette smoking was associated with an increase in proteinuria of 54.1% (95%CI: +9.0%, +117.7%) and 17.4% (95%CI: +1.7%, +35.5%) in the contemporaneous and lagged models, respectively, and p30d cigarette smoking showed similar results: 66.8% (95%CI: +15.9%, +140.0%) and 15.3% (95%CI: −3.5%, +37.7%), respectively.
DISCUSSION
In this study, we described the association between SU and kidney function in AYA with CKD using data from a representative prospective cohort. Alcohol, e-cigarettes and marijuana use were not associated with lower eGFR, worse proteinuria or uncontrolled BP; however, cigarette use was associated with both higher uPCR and nephrotic range proteinuria.
Our results were consistent with a previous CKiD analysis indicating that urine cotinine levels were associated with nephrotic range proteinuria [1]. Our additional analysis controlling for living with a household smoker indicated that active cigarette smoking, beyond SHS exposure, was independently associated with worsening proteinuria.
The use of alcohol, e-cigarettes and marijuana in this population appeared to confer little to no additional risk of progression. While it is unlikely that these substances are benign, infrequent use may explain the lack of an observed association. This is consistent with analyses in adults: consumption of >2 alcoholic beverages per day was associated with progression to ESKD compared to those consuming ≤2 in adults with CKD [10]. Bundy et al. reported that persistent alcohol consumption was associated with disease progression, while marijuana use was not [11]. We additionally investigated multiple use of substances, and no consistent pattern was observed for any of the four outcomes.
We noted a modest, but significant, protective effect of p30d marijuana use on eGFR, while previous studies found no significant associations between marijuana use and kidney function [12, 13]. Our finding could be an artifact of the data, may reflect a healthy user effect (i.e., those who were healthier were more likely to use), may be due to social-desirability bias, or may have resulted from limitations in estimating intensity and actual exposure of marijuana use. Further investigation of marijuana and CKD progression in other AYA cohorts is needed.
An important strength of this study was the use of both contemporaneous and lagged models adjusting for previous disease severity. This capitalized on CKiD’s prospective design which provided valid comparisons of participants with similar disease severity prior to reporting SU. Solely utilizing contemporaneous models, which only used cross-sectional data, could lead to biased causal interpretations of SU on pediatric CKD outcomes. However, the contemporaneous models may provide insight and suggested that those with worse kidney health may be more likely to use, while not necessarily leading to accelerated progression (with the exception of cigarette smoking and proteinuria)
One limitation of these data is that CKiD participants are primarily recruited from clinical settings and may not be generalizable to AYA with pediatric CKD who are not receiving specialty care or participating in research. In addition, this analysis focused on the effects of relatively low levels of SU on kidney function and blood pressure. These results may not extend to other indicators of health (e.g., social and neurocognitive, or more comprehensive cardiovascular indicators), which are known to be affected by SU in AYA populations without CKD [14, 15]. Lastly, e-cigarette data collection in CKiD only began in 2014. Continued data collection will become more important as legislation, availability and types of devices evolve over time.
In conclusion, occasional alcohol, e-cigarette and marijuana use were not strong risk factors for kidney disease progression in AYA, while even low levels of cigarette use contributed to worsening proteinuria. Pediatric nephrologists should discuss and screen for SU in AYA patients, especially those living with a smoker, in order to provide support, prevent use and maintain kidney health.
Supplementary Material
ACKNOWLEDGEMENTS
Data in this manuscript were collected by the Chronic Kidney Disease in Children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri - Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD and Derek K. Ng, PhD) at the Johns Hopkins Bloomberg School of Public Health. CKiD is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01 DK066143, U01 DK066174, U24 DK082194, U24 DK066116). The CKiD website is located at https://statepi.jhsph.edu/ckid.
Footnotes
Conflicts of Interest/Competing Interest: The authors declare no relevant financial or non-financial conflicts of interest.
Code Availability: Code for this analysis will be available on the NIDDK Central Repository website.
CRediT Author Statement
Andrea R. Molino: Conceptualization, Methodology, Software, Formal Analysis, Data Curation, Writing – Original Draft, Writing – Reviewing & Editing, Visualization
Judith Jerry-Fluker: Conceptualization, Resources, Data Curation, Writing – Review & Editing, Project Administration
Meredith A. Atkinson: Investigation, Resources, Writing – Review & Editing, Supervision
Susan L. Furth: Conceptualization, Investigation, Resources, Writing – Review & Editing, Supervision, Funding Acquisition
Bradley A. Warady: Conceptualization, Investigation, Resources, Writing – Review & Editing, Supervision, Funding Acquisition
Derek K. Ng: Conceptualization, Methodology, Investigation, Resources, Writing – Review & Editing, Supervision
Availability of Data and Material:
Data for this analysis will be available on the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repository website.
REFERENCES
- 1.Omoloja A, Jerry-Fluker J, Ng DK, et al. (2013) Secondhand smoke exposure is associated with proteinuria in children with chronic kidney disease. Pediatr Nephrol 28:1243–1251. 10.1007/s00467-013-2456-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omoloja A, Tyc VL (2015) Tobacco and the pediatric chronic kidney disease population. Pediatr Nephrol 30:235–243. 10.1007/s00467-014-2804-9 [DOI] [PubMed] [Google Scholar]
- 3.Omoloja A, Stolfi A, Chand D, et al. (2014) Tobacco exposure in children and adolescents with chronic kidney disease: parental behavior and knowledge. A study from the Midwest Pediatric Nephrology Consortium. Clin Nephrol 81:307–312. 10.5414/CN108024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speeckaert MM, Delanghe JR, Vanholder RC (2013) Chronic nicotine exposure and acute kidney injury: new concepts and experimental evidence. Nephrology Dialysis Transplantation 28:1329–1331. 10.1093/ndt/gft019 [DOI] [PubMed] [Google Scholar]
- 5.Varga ZV, Matyas C, Paloczi J, Pacher P (2017) Alcohol Misuse and Kidney Injury: Epidemiological Evidence and Potential Mechanisms. Alcohol Res 38:283–288 [PMC free article] [PubMed] [Google Scholar]
- 6.Furth SL, Cole SR, Moxey-Mims M, et al. (2006) Design and Methods of the Chronic Kidney Disease in Children (CKiD) Prospective Cohort Study. Clin J Am Soc Nephrol 1:1006–1015. 10.2215/CJN.01941205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz GJ, Schneider MF, Maier PS, et al. (2012) Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney International 82:445–453. 10.1038/ki.2012.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn JT, Kaelber DC, Baker-Smith CM, et al. (2017) Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. 140:74. [DOI] [PubMed] [Google Scholar]
- 9.Hollowell JG, van Assendelft OW, Gunter EW, et al. (2005) Hematological and iron-related analytes--reference data for persons aged 1 year and over: United States, 1988–94. Vital Health Stat 11 1–156 [PubMed] [Google Scholar]
- 10.Perneger TV, Whelton PK, Puddey IB, Klag MJ (1999) Risk of end-stage renal disease associated with alcohol consumption. Am J Epidemiol 150:1275–1281. 10.1093/oxfordjournals.aje.a009958 [DOI] [PubMed] [Google Scholar]
- 11.Bundy JD, Bazzano LA, Xie D, et al. (2018) Self-Reported Tobacco, Alcohol, and Illicit Drug Use and Progression of Chronic Kidney Disease. Clin J Am Soc Nephrol 13:993–1001. 10.2215/CJN.11121017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rein JL, Wyatt CM (2018) Marijuana and Cannabinoids in ESRD and Earlier Stages of CKD. American Journal of Kidney Diseases 71:267–274. 10.1053/j.ajkd.2017.06.020 [DOI] [PubMed] [Google Scholar]
- 13.Lu C, Papatheodorou SI, Danziger J, Mittleman MA (2018) Marijuana Use and Renal Function Among US Adults. Am J Med 131:408–414. 10.1016/j.amjmed.2017.10.051 [DOI] [PubMed] [Google Scholar]
- 14.Yuan M, Cross SJ, Loughlin SE, Leslie FM (2015) Nicotine and the adolescent brain. J Physiol 593:3397–3412. 10.1113/JP270492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Squeglia LM, Jacobus J, Tapert SF (2009) The Influence of Substance Use on Adolescent Brain Development. Clin EEG Neurosci 40:31–38. 10.1177/155005940904000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this analysis will be available on the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repository website.


