Abstract
Chaperonins are nanomachines that harness ATP hydrolysis to power and catalyze protein folding, a chemical action that is directly linked to the maintenance of cell function through protein folding/refolding and assembly. GroEL and the GroEL–GroES complex are archetypal examples of such protein folding machines. Here, variable-temperature electrospray ionization (vT-ESI) native mass spectrometry is used to delineate the effects of solution temperature and ATP concentrations on the stabilities of GroEL and GroEL–GroES complexes. The results show clear evidence for destabilization of both GroEL14 and GroES7 at temperatures of 50 and 45 °C, respectively, substantially below the previously reported melting temperature (Tm ~ 70 °C). This destabilization is accompanied by temperature-dependent reaction products that have previously unreported stoichiometries, viz. GroEL14–GroESy–ATPn, where y = 1, 2, 8 and n = 0, 1, 2, 8, that are also dependent on Mg2+ and ATP concentrations. Variable-temperature native mass spectrometry reveals new insights about the stability of GroEL in response to temperature effects: (i) temperature-dependent ATP binding to GroEL; (ii) effects of temperature as well as Mg2+ and ATP concentrations on the stoichiometry of the GroEL–GroES complex, with Mg2+ showing greater effects compared to ATP; and (iii) a change in the temperature-dependent stoichiometries of the GroEL–GroES complex (GroEL14–GroES7 vs GroEL14–GroES8) between 24 and 40 °C. The similarities between results obtained by using native MS and cryo-EM [Clare et al. An expanded protein folding cage in the GroEL–gp31 complex. J. Mol. Biol. 2006, 358, 905–911; Ranson et al. Allosteric signaling of ATP hydrolysis in GroEL–GroES complexes.Nat. Struct. Mol. Biol. 2006, 13, 147–152] underscore the utility of native MS for investigations of molecular machines as well as identification of key intermediates involved in the chaperonin-assisted protein folding cycle.
Graphical Abstract
INTRODUCTION
Chaperonins are a class of protein complexes found in all living systems that recognize and bind non-native proteins and assist in the folding of the protein to more stable, native state(s), thereby inhibiting misfolding and aggregation. GroEL, an E. coli chaperone, is a model system for understanding molecular chaperones. The GroEL tetradecameric complex (GroEL14) is composed of two heptameric rings stacked back to back.1 When bound to its co-chaperonin, GroES, a heptameric complex that binds to the apical domain of GroEL, this complex promotes proper folding of the non-native substrate protein. While detailed mechanisms for the assembly of the GroEL–GroES complex have been described,2 largely based on the structural analysis of final products of the processes,3,4 many details about the dynamics, stabilities,5,6 and assembly of the individual units that comprise the GroEL tetradecamer and the GroEL–GroES complex are still not fully understood. Most notably, GroEL stability, structure and function(s) are ATP-dependent, the conformational dynamics of GroEL are directly linked to ATP binding and hydrolysis, and specific domains of GroEL subunits, denoted as apical, intermediate, and equatorial,7 are known to exist as three distinct conformations that are linked to specific orientations of the apical domain.8 The N-terminal apical domain of each GroEL subunit is directly involved in binding substrate protein and the GroES co-chaperone. The C-terminal equatorial domains are involved in subunit–subunit interactions both within and between the heptameric GroEL rings as well as binding of nucleotide (ATP), Mg2+, and K+ ions.9 Communication between the apical and equatorial domains is largely achieved by changes in the conformation through a hinge-like motion of the intermediate domain. Collectively, the dynamics of the individual domains within these complexes act as a two-stroke folding machine, wherein encapsulation of substrate by the one ring, denoted the cis ring, followed by GroES binding, forming the bullet-shaped (BS) structure, initiates the folding reaction.4,10-12 ATP binding drives a structural change and formation of the hydrophilic chamber that triggers protein folding, while ATP hydrolysis acts as a timer, controlling the lifetime of the GroEL–GroES folding chamber.13 Rye et al. proposed that binding of ATP to the trans ring, following hydrolysis of ATP in the cis ring, triggers disassembly of the GroEL–GroES cis ring complex and subsequent release of GroES and the substrate protein into free solution.13 Yang et al. proposed an alternative model whereby a second GroES binds to the trans ring of the GroEL14–GroES7 complex to form the symmetric football-shaped (FS) complex, in which ATP hydrolysis and asymmetric nucleotide distributions are essential but can happen in either ring in an stochastic manner.14 Potential roles of bullet- and football-shaped GroEL–GroES complexes (BS- and FB-conformations, respectively) remain somewhat controversial.15,16 Bigman suggests that BS and FB structures may coexist and that their relative activities may be linked to ATP concentrations, which have been shown to be widely variable.17 A more complete summary of bullet- vs football-shaped GroEL–GroES complexes can be found in a recent review by Horovitz et al.18
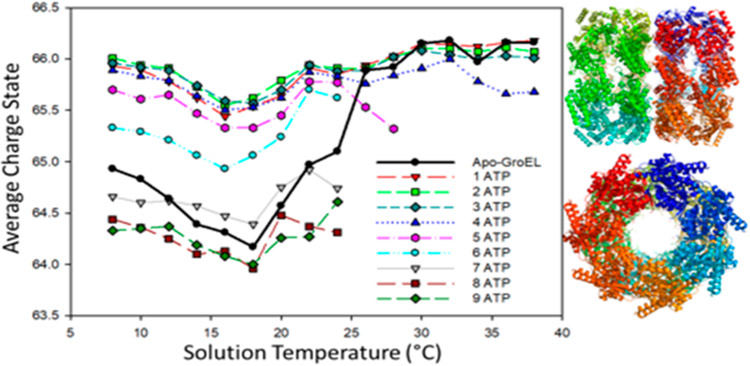
Early mass spectrometry (MS) studies of GroEL–GroES demonstrated the utility of MS for the analysis of large protein complexes19,20 and that these measurements are complementary to other structural biology techniques, for example, electron microscopy, X-ray crystallography, and NMR spectroscopy. MS analyses provide unparalleled sensitivity and dynamic range for studies of conformationally heterogeneous systems, including direct analysis of reaction products formed by interactions with ligands, cofactors, and intermediates formed on the pathway from initial reactants to final products.21,22 Specific examples that are directly related to GroEL include work by Dyachenko et al., where native MS was used to investigate the allostery associated with Mg2+/ATP binding to GroEL,23 and van Duijin et al., who used native MS to study folding and assembly of gp23, the major capsid protein of bacteriophage T4, by the GroEL–gp31 complex (a 912 kDa complex).24 These studies were performed at different temperatures, 25 and 20 °C, and different native MS buffers, ethylenediamonium diacetate (EDDA) and ammonium acetate (AmA), respectively. Dyachenko specifically noted differences for ATP binding and charge state distributions for GroEL using AmA buffers compared to solutions of EDDA.23 Here, we report data obtained by using variable-temperature ESI (vT-ESI) native mass spectrometry which provides additional evidence on the stability and dynamics of the GroEL and GroEL–GroES complex. An earlier report of results from vT-ESI native mass spectrometry was interpreted as evidence for conformational drift associated with ATP binding to GroEL.25 The vT-ESI-MS experiments report on changes in the protein–protein complex that occur in the solution contained within the ESI emitter vis-à-vis processes that occur prior to the transition from solution to the gas phase. Figure 1 contains a plot of the average charge (Zavg) for GroEL14 (solid black trace) and GroEL14–ATPn (n = 1–9) bound states of the GroEL tetradecamer complex versus temperature of the ESI solution; under these solution conditions complexes with n >9 are not observed. For the apo-GroEL tetradecamer (solid black trace), Zavg shifts from 64.8 to 64.3 as temperature is increased from 8 to 18 °C, followed by an increase in Zavg to 65.8 between 18 and 26 °C. At temperatures greater than 26 °C, Zavg values for GroEL14 are centered around 66. Note that Zavg for the GroEL14–ATPn (n = 1–9) products are also temperature-dependent. For n = 1–4, Zavg decreases slightly in the 8–18 °C range, but changes for n = 5 and 6 are larger as are the changes for n = 7–9. Note also that Zavg for n = 5 and 6 are lower relative to those for n = 1 −4. At temperatures greater than 18 °C changes in Zavg are relatively small. The observed changes in Zavg and apparent binding affinities for ATP were attributed to changes in the solvent accessible surface area (SASA) for the apo-GroEL and GroEL–ATPn complexes because of temperature- and ATP-dependent conformation changes.25 The temperature-dependent Zavg for the GroEL14 and GroEL14–ATPn are consistent with changes in SASA of the complexes that have been noted for other systems26-28 and with well-known cold- and heat-induced folding/refolding reactions of proteins and protein complexes.29
Figure 1.
Plot showing temperature-dependent changes in average charge (Zavg) of GroEL14 and GroEL14–ATPn (n = 1–9) (adapted from ref 25). Solution conditions are 1 μM GroEL, 200 mM ammonium acetate, and 125 μM ATP. Note that Zavg for n = 1–4 are similar, but larger changes in Zavg are observed for n = 5–9. At T >24 °C the ion abundances for n = 6–9 are below the detection threshold. Also, the abundance of n = 5 begins to decline at T >24 °C. This behavior is very different from that obtained by using ethylenediammonium diacetate buffer (vide infra).
In the work presented below, the effects of temperature and ATP binding on the stability and conformation of GroEL, GroES, and GroEL–GroES complexes are investigated by using vT-ESI native mass spectrometry.25,30,31 Conformational changes induced by ATP binding as well as cold- and heat-induced changes in stabilities are directly linked to changes in Gibbs energy.26,27,32,33 The vT-ESI source is used to control the temperature of the protein-containing solution in the ESI emitter; consequently, the observed changes in stability/conformation are occurring in the solution prior to ESI ionization. While we cannot completely rule out that some reactions may occur in the confined environment of the nanodroplets formed by ESI, evaporative cooling of the nanodroplets has been shown to be an effective mechanism for kinetically trapping (freeze-drying)34 ions during the transition from solution to the gas phase.34,35 The relationships between ESI freeze-drying and cryo-EM have been noted previously22 and is supported by detection of intermediates and final products that are similar to those previously reported by Ranson et al. using cryo-EM.3 The similarities between results obtained by using native MS and cryo-EM,3,36 underscore the potential utility of native MS for investigations of molecular machines as well as identification of key intermediates that may be involved in the chaperone-assisted protein folding cycle.
RESULTS
Thermal Stability of GroEL and GroES as Determined by VT-ESI Native MS.
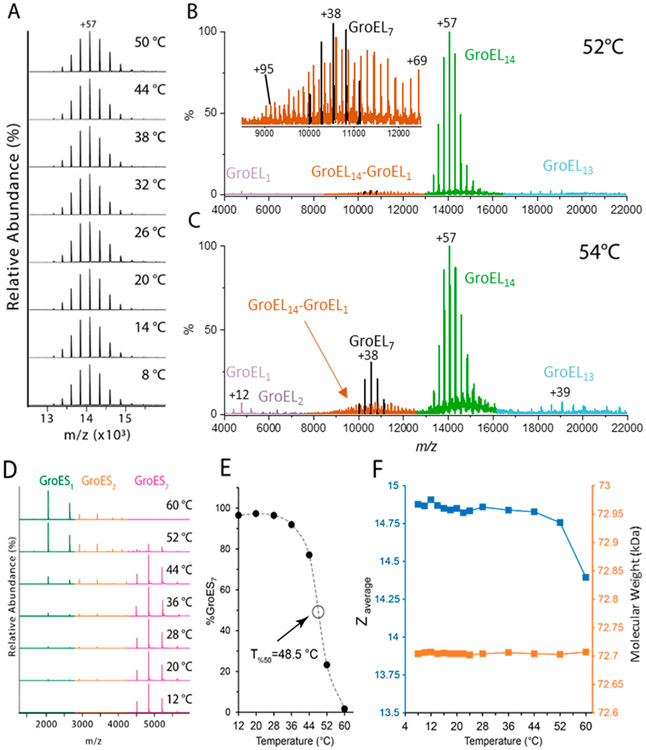
Cooling and heating EDDA buffered solutions of GroEL contained within the ESI emitter over a temperature range from 8 to 50 °C did not result in significant changes in the average charge state (Zavg), the charge state distribution (CSD), or the measured m/z values of GroEL14 ions (Figure 2A); however, evidence for T-dependent stabilities for GroEL14 are observed at T >52 °C. The region of the mass spectrum shown in the inset of Figure 2B contains ions that have m/z values that correspond to GroEL7 and GroEL14–GroEL1. Ions having similar m/z values are detected at 54 °C (Figure 2C); note the abundance of GroEL7 ions (Figure 2C) is increased relative to that for GroEL14–GroEL1. This spectrum also contains low abundance ions that are assigned as GroEL1 and GroEL2. It appears that these ions are formed by solution-phase thermal decomposition of GroEL14. The increased relative abundances for GroEL7 and GroEL14–GroEL1 at 54 °C compared to 52 °C are consistent with a large shift in stability of the GroEL14 (see Supporting Information Figure S1). While we cannot rule out the possibility that some fractions of these ions are formed by gas phase collision-induced dissociation (CID), the increase in abundances of GroEL1, GroEL2, and GroEL7 at 54 °C supports a solution-phase thermal decomposition mechanism.
Figure 2.
Effects of temperature on stability of GroEL14 and GroES7. (A) Charge state distribution (CSD) for intact GroEL tetradecamer (1.2 μM in 200 mM EDDA) at T = 8–50 °C and the high m/z range (4000–22200) of mass spectrum of GroEL14 acquired at (B) T = 52 °C and (C) at T = 54 °C; signals for GroEL7, GroEL14–GroEL1, and GroEl14 are color coded in black, orange, and green, respectively. See Figure S2 for deconvoluted spectra. (D) Mass spectra of GroES7 (7 μM, heptamer) in 200 mM EDDA at T = 12–60 °C. (E) A plot showing temperature-dependent changes in the relative abundance of GroES7; signal intensity for GroES as a percentage of the total ion signal in spectra shown in (D). (F) Plot of average charge state (Zavg) (blue) and measured molecular weight (orange) of GroES7 as a function of temperature.
The effects of heating and cooling the ESI solutions on the stability of GroES7 were also investigated (Figure 2D). The GroES7 complex is the most abundant species observed in the range of 12 < T <28 °C, but at T >44 °C there are notable increases in the abundances of GroES1 and GroES2 ions. Duckworth and co-workers assigned the fragment ions to gas phase collisional activation reactions;37 however, on the basis of a series of studies, we are confident that under these experimental conditions these product ions result from reactions that occur in the solution.30,38 The midpoint in the curve shown in Figure 2E denotes the temperature at which the relative abundance of the GroES7 ion has been reduced by 50%, i.e., T50% = 48.5 °C (see the Experimental section), which is significantly lower than the Tm of ~74 °C reported by Boudker et al.39 It is important to note, however, that their ΔCp vs T curve revealed evidence for a low-temperature endotherm at ~40 °C, which agrees well with our T50% for GroES7. Geels et al.40 and Dyachenko et al.41 have reported Tm data for GroES using mass spectrometry, and they report similar Tm values (~70 °C), but both of these studies were performed by using ammonium acetate buffer. Moreover, the differential scanning calorimetry (DSC) and vT-ESI results reveal strong evidence for T-dependent instability,39 and the vT-ESI results suggest that this transition may serve as the early steps leading to disassembly of GroES7 complex. In both experiments it appears that these are reversible even for solutions that have been heated to 60 °C. These results and the lower charge states (Zavg = 4.66 and 6.19, respectively) suggest that the GroES1 and GroES2 do not undergo irreversible unfolding upon heating in EDDA buffered solutions; consequently, Tm values determined by CD (circular dichroism) spectroscopy report on the 2° structure, whereas T-dependent measurements using native MS of protein complexes report changes in architecture, stoichiometry, and quaternary structure, i.e., changes in SASA that accompany changes in tertiary and quaternary structure.
Temperature-Dependent ATP Binding to the GroEL14 Complex.
Dyachenko et al. previously reported thermochemical data (Ka) for ATP binding to GroEL14.23 While their measurements were performed on samples incubated at 25 °C, the actual temperature of the solution in the ESI emitter was not specified. Owing to the observed temperature-dependent stabilities for GroEL14 in EDDA buffered solutions, we investigated the effects of temperature on ATP binding to GroEL for vT-ESI solutions with different Mg2+ and ATP concentrations. Galan et al. observed a low-temperature endotherm of GroEL by DSC that they proposed as evidence for conformation changes for the GroEL14 complex, specifically modifications of tertiary and quaternary structure and minor changes in secondary structure.42 Similar conformational changes were also observed by using fluorescence.43 VT-ESI native MS can potentially provide additional evidence for conformational changes as well as how such changes affect ATP binding.
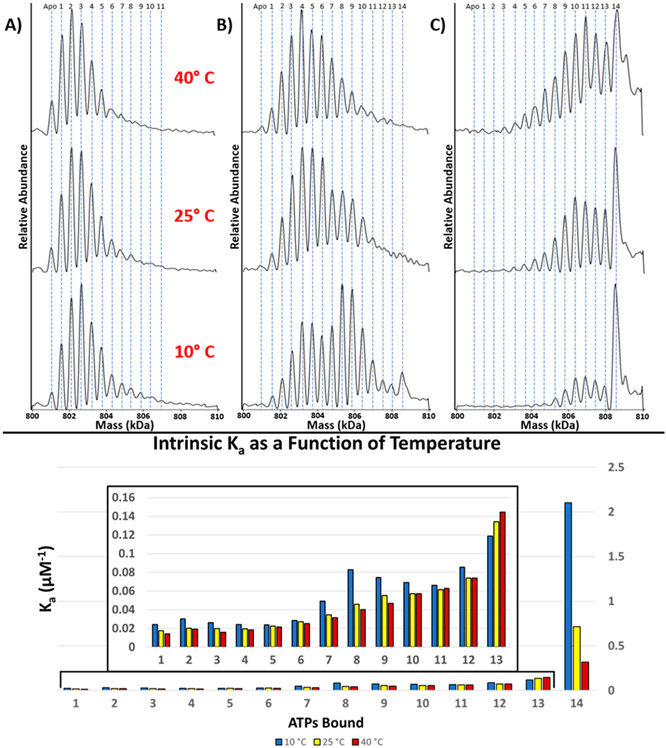
For GroEL14 solutions containing 10 μM ATP (solution “A”, see Table 1) the products observed in the spectra were GroEL14–ATP1–11 (Figure 3A) at low temperatures, but at higher solution temperatures, binding of ATP becomes less favorable (see Figure 3A). An increased concentration of ATP to 25 μM (Figure 3B) (solution “B”, Table 1) yields GroEL14–ATP1–14 at 10 °C, but as T is increased, the abundances of all these products decrease, especially for n > 7. Under these conditions ATP binding to the second GroEL ring is even more favored at 10 °C, whereas at 40 °C the more abundant signals correspond to GroEL–ATP3–6 (Figure 3B). Clearly lower solution temperatures are more favorable for ATP binding, and under these conditions the complexes also carry lower Zavg, which is consistent with an ATP-induced conformational change of GroEL14.42 Zavg trends as a function of temperature for GroEL–ATPn in EDDA appear to be similar to those observed in Figure 1; stepwise addition of ATP induces a small decrease in Zavg with each ATP bound. A similar temperature dependence is observed for solutions containing 50 μM ATP (“solution C”, Table 1). Most notably the binding of 14 ATPs to GroEL14 is highly favored compared to the previous binding events, but this effect diminishes at higher solution temperatures. While ATP and ADP are not distinguishable at the mass resolution used in this study, the affinity of GroEL for the binding of ATP is an order of magnitude greater than for the binding of ADP.44 The ATPase activity of GroEL will also be negligible without the presence of K+.45,46
Table 1.
Effects Mg2+ and ATP Concentrations on ATP Binding and Stoichiometry of GroEL–ES Complex at 24 °C in 200 mM EDDA
| solution “X” | GroEL (nM) | Mg2+ (μM) | ATP (μM) | GroES (μM) | ATP/GroEL | Mg2+/GroEL | GroELx–GroESy–ATPn |
|---|---|---|---|---|---|---|---|
| A | 500 | 1000 | 10 | 20 | 2000 | GroEL14–ATP1–4 | |
| B | 500 | 1000 | 25 | 50 | 2000 | GroEL14–ATP1–14 | |
| C | 500 | 1000 | 50 | 100 | 2000 | GroEL14–ATP3–14 | |
| D | 260 | 225 | 110 | 12 | 420 | 850 | GroEL14–GroES14–ATP14 |
| E | 120 | 25 | 50 | 12 | 420 | 200 | GroEL14–GroES7–8–ATP8 |
| F | 600 | 500 | 50 | 12 | 80 | 850 | GroEL14–GroES7–8–ATP8 |
| G | 600 | 25 | 50 | 12 | 80 | 40 | GroEL14–GroES0–2–ATP0–2 |
| H | 500 | 12 | not detected |
Figure 3.
ATP binding is favored at low temperatures. Mass spectra of deconvoluted charge state of GroEL (0.5 μM, tetradecamer) in 200 mM EDDA and 1 mM Mg2+ incubated with ATP (A) 10, (B) 25, and (C) 50 μM at different temperatures. (D) Calculated intrinsic association constants (Ka) for binding of ATP to GroEL 10 °C (blue), 25 °C (yellow), and 40 °C (red). Binding is more favorable at lower temperatures, and affinity decreases with increase in solution temperature. Macroscopic Ka data and the methods used to calculate intrinsic Ka values are provided as Supporting Information (Figure S2).
The higher relative abundance for ATP3–5 and ATP8–10 (Figure 3B, 10 °C) raises an interesting question: is the binding of up to three ATP molecules at each ring more favorable than the remaining ATPs? Interestingly, Chapman et al. previously suggested that binding of three ATP molecules is required for successful substrate binding and release.47 The intrinsic association constants (Ka) for temperature-dependent ATP–GroEL binding (10, 25, and 40 °C) are shown in Figure 3D. These Ka values show that ATP is bound with a higher affinity at lower temperatures and the affinities diminishes at elevated solution temperature. The intermediate (e.g., ATPs 7–10) and last (ATP 14) binding affinities are most affected by temperature (Figure 3D). The binding of the 14th ATP draws particular interest due to the relative binding affinity compared to the other affinities preceding it. These Ka values are consistent with positive cooperativity reported by Yifrach and Horovitz48 and Dyachenko23 for intra-ring ATP binding, as reflected by the increase in the Ka values for the GroEL14–ATP1–3 and GroEL14–ATP7–8.
Ligand- and Temperature-Dependent Stabilities of the GroEL–GroES Complexes.
Results for temperature-dependent GroEL–ATP binding prompted further investigations on how solution temperature influences the binding of GroES to GroEL14. As noted previously,23 mass spectra acquired from EDDA-buffered solutions give well-resolved peaks and are less congested, thereby greatly simplifying assignment of mass spectral signals. The mass spectra obtained from EDDA-buffered solutions containing a range of concentrations of GroEL14, GroES7, Mg2+, and ATP (Table 1) were examined. The mass spectrum obtained from solution “D” contain ions corresponding to GroEL14–GroES14–ATPn, where n = 12–14, presumably the FS structure.49,50 Lorimer suggested that K+ facilitates ATP turnover and promotes formation of the BS structure, GroEL14–GroES7–ADP, and serves as the acceptor state in the substrate refolding cycle.51 Here, we avoided the effects of K+ because this also further complicates assignment of the products observed in the mass spectrum. On the basis of preliminary data acquired from an EDDA solution containing K+ (800 μM), we can report that the presence of K+ significantly increases ATP binding, but the stoichiometry of the resulting GroEL–GroES complexes does not change.
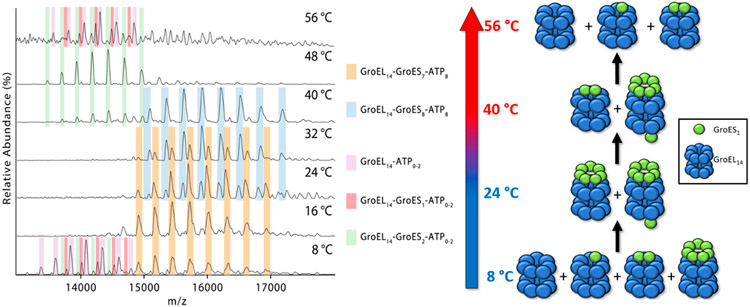
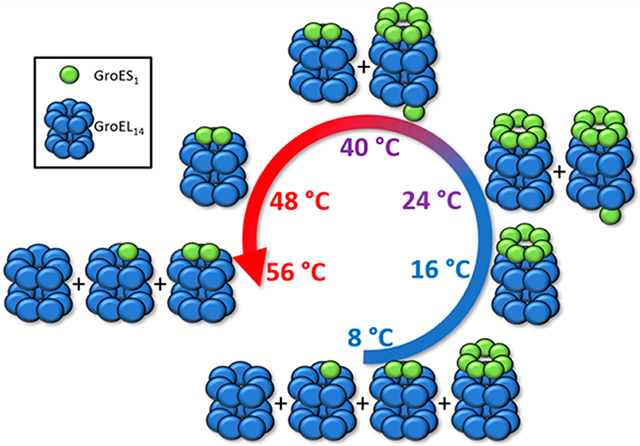
The mass spectra shown in Figure 4 were acquired from solution “E” (25 μM Mg2+ and 50 μM ATP) at temperatures between 8 and 56 °C. At the lowest temperature (8 °C) the spectra contain abundant ions corresponding to GroEL14–ATP0–2 (violet), GroEL14–GroES1–ATP0–2 (magenta), GroEL14–GroES2–ATP0–2 (green), and GroEL14–GroES7–ATP8 (orange). Increasing the temperature to 16 °C results in formation of GroEL14–GroES7–ATP8, and at 24 and 32 °C the major products correspond to GroEL14–GroES7–ATP8 and GroEL14–GroES8–ATP8 (blue); however, the ratios of these two products change in the range 24–32 °C. At T >40 °C the signal for these complexes is diminished, possibly a result of disassembly, first to form GroEL14–GroES2–ATP0–2 followed by formation of the initial products observed at 8 °C. The assignment of ATP8 is consistent with measured mass differences for GroEL14–GroES7 and GroEL14–GroES8 masses and the measured mass of the GroEL14–GroES7 complex.
Figure 4.
Mass spectra showing the region corresponding to the GroEL–GroES complexes formed in solution E (Table 1 solution “E” 120 nM GroEL, 12 μM GroES, 25 μM Mg2+, and 50 μM ATP in 200 mM EDDA). The accompanying illustration summarizes the observed products formed upon heating solution “E” at temperatures of 8–56 °C. Lower temperatures (8 °C) GroEL14–GroESy (y = 0–2) products are formed, whereas GroEL14–GroES7 and GroEL14–GroES8 products are formed at higher temperatures. At T >40 °C the observed species revert back to the lower mass complexes, which become the most abundant products at 48–56 °C.
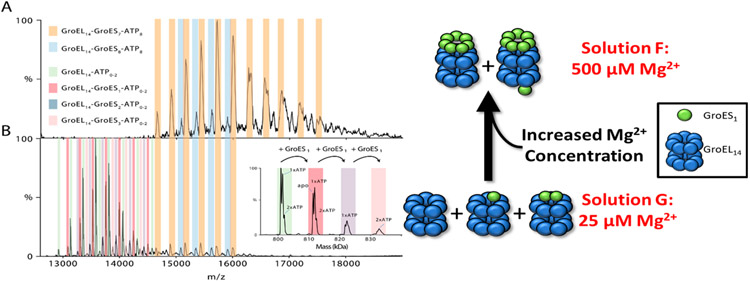
The mass spectrum obtained for solution “F” (Figure 5A), which has a higher concentration of ATP and Mg2+ than solution “D”, is most similar to the mass spectrum obtained (at 24 °C) by using solution “E”; however, the ratios of GroEL14–GroES7–ATP8 and GroEL14–GroES8–ATP8 are different. These results underscore the importance of both Mg2+ and ATP in determining the stoichiometry of GroEL–GroES complexes. The presence of GroEL14–GroES1 and GroEL14–GroES2 product ions (magenta and green, respectively) are very different from vT-ESI of solutions containing low Mg2+/GroEL14 and ATP/GroEL14 ratios (solutions “E” and “F”, respectively). Decreasing the ratio of Mg2+/GroEL14 and Mg2+/ATP (solution “G” relative to solution “F” (Table 1)) results in a mass spectrum (Figure 5B) containing signals for GroEL14–ATP0–2, GroEL14–GroES1–3–ATP0–2, and low abundances of GroEL14–GroES7–8–ATP8. The ions at higher m/z values are identical with ions observed from solution “F”, whereas the ions at lower m/z correspond to GroEL14–GroES1–2. These results suggest that binding of GroES7 to the GroEL–ATP complex is directly linked to the concentrations of both ATP and Mg2+ ions. The concentration of Mg2+ will also affect the effective concentration of ATP as Mg2+ is necessary for ATP to bind.52 The binding of individual GroES subunits to GroEL has not been previously reported and seems counterintuitive. The binding of individual GroES subunits could explain the formation of GroEL14–GroES8 ions in solution “E”. Presumably, the GroEL14–GroES8 complex has a single GroES subunit bound to the trans ring and an intact GroES7 bound to the cis ring. VT-ESI analysis of solution “F” is similar to solution “E” in terms of increased abundances of GroEL–ATP1–2 and GroEL14–GroES2–ATP2 at 8 and ~50 °C, respectively. Collectively, the results suggest that these experimental conditions alter the stability/dynamics of the GroES7 and/or the positioning of GroES with the apical domain of the GroEL complex.
Figure 5.
Mg2+ regulates the stoichiometry of the GroEL–ES complex. (A) Mass spectra shown in (A) and (B) illustrate the effects of Mg2+ concentration on the formation of GroEL14–GroES7 and GroEL14–GroES8 complexes. The solution conditions used (solutions F and G) only differ in concentrations of Mg2+, (A) 500 μM and (B) 25 μM collected at 12K resolution (HCD = 200 V) at 24 °C. The inset in (B) illustrates the resolving power available to assign the GroES bound state of the GroEL as well as the ATP bound state of each GroEL14–GroES7 state.
The role of Mg2+ and ATP concentration on the stoichiometry of GroEL–GroES complex, specifically formation of substoichiometric GroES complexes, is illustrated by the data shown in Figure 5. The spectrum shown in Figure 5A was obtained from solution “F” (500 μM Mg2+, 50 μM ATP, and 12 μM GroES7), and the spectrum shown in Figure 5B was obtained from solution “G” (25 μM Mg2+, 50 μM ATP and 12 μM GroES7). The solution containing higher Mg2+ concentration produces higher abundances of GroEL14–GroES7–ATP8 and GroEL14–GroES8–ATP8. Low abundance signals for the same complexes are formed at low Mg2+ concentrations (25 μM), but the dominant species correspond to GroEL14–ATP0–2, GroEL14–GroESn–ATP0–2, where n = 1–3. Moreover, GroEL–GroES complexes are not detected from solutions that do not contain ATP and Mg2+ (solution “H”). Collectively, these results corroborate the role of Mg2+ in driving the reaction. Boudker et al. previously reported Mg2+-dependent stability for GroES7 (Kd ~ 0.5 mM),39 and Sakane et al. reported reduced stabilities for GroES7 at low concentrations.53 Studies are currently underway, including vT-ESI, in an effort to better understand the underlying mechanisms that favor formation of substoichiometric GroEL–GroES complexes.
DISCUSSION
The vT-ESI GroEL studies were limited to a single native MS buffer EDDA because of greater stability and structural integrity when compared to that for the more commonly used native MS buffer ammonium acetate.21,25 The charge states produced from ammonium acetate solutions differ from those in EDDA, and the data shown in Figures 1 and 2 reveal differences in thermal stability of GroEL in the different buffers. The upregulation of heat shock proteins and chaperonins raises questions of how the GroEL–GroES system responds to both cold and hot conditions. Temperature has strong effects on the dielectric constant of water54 as well as the Gibbs free energy of the system, both of which have direct effects on protein structure. In general, cold denaturation favors more compact conformers because of solvent penetration of the protein, and heat denaturation favors elongated conformers.55 Both cold- and heat-induced conformational changes are evidenced by changes in the SASA (see Figure 1).55 Our results show that both GroEL and GroES are stable up to ~50 and 44 °C in EDDA, respectively, but this is limited to a relatively narrow range of concentrations, as evidenced by temperature-dependent ATP binding for GroEL14 and changes in SASA for both GroEL14 and GroES7. The discrepancies between the previously reported thermal stabilities of GroEL (Tm ~ 67 °C)5 and GroES, (Tm ~ 76.4 °C)39 and our results may be due to differences in solution conditions. Moreover, the previously reported Tm values were determined by using biased techniques, viz., CD spectroscopy, which measures changes in 2° structure; native MS reports changes in Zavg, related to changes in SASA, as well as T-dependent changes in ligand binding. As shown in Figure 2E,F, there are no significant changes in Zavg for GroES7 at T < 44 °C, but at T >44 °C signals for GroES1 and GroES2 ions appear. Note, however, that at T >44 °C the mass and charge state distributions (CSD) of the GroES heptamer are unchanged, which suggests that these ions are native-like. GroEL, GroES, and GroEL–GroES complexes are highly dynamic, and even small changes in the solution conditions, viz. chemical potential (Gibbs free energy) of the local environment (T, P, pH, osmolytes, chemical chaperones, and concentration of GroEL–GroES), strongly affect conformation, stability, and possibly the behavior of these nanomachines. This explanation is consistent with the previously reported low-temperature endotherm in the DSC profiles detected at ATP concentrations of 0.25 and 0.50 mM;42 these authors note the “existence of a conformational state of GroEL with modified 3°/4° structures having increased exposure of hydrophobic surfaces, but minor rearrangements of its 2° structure.”
We show that in EDDA solutions GroEL14 becomes unstable above 50 °C, and unfolding of the monomer resembles previously reported self-chaperonin activity of GroEL5 even though the size of GroEL monomer (GroEL1: ~57 kDa) is near the limit of substrate size reported previously, ~70 kDa.5 This self-protection mechanism in response to heat shock stress is evidenced by our observation of a highly stable octamer in ammonium acetate that most likely consisted of a single ring GroEL and a trapped unfolded subunit that cannot be dissociated in the gas phase (data not shown). We conjecture that such high stability plays a role in the capability of this chaperonin to regain its functionality after removal of heat stress. The self-assembly and monomer unfolding can be connected to a reversible, low-temperature endotherm reported from thermodynamics measurements of GroEL.42
Disassembly of GroES7 at elevated temperatures (T > 44 °C) can be attributed to the increased Coulombic repulsion of negatively charged residues, Glu50 and Glu53, localized on the roof of the GroES7 complex and near the subunit–subunit interface.56,57 The CSD and Zavg of unfolded subunits are not detected by native MS experiments upon heating to 60 °C, suggesting that dissociation of subunits precedes the backbone unfolding. Supporting evidence for this statement is the observed reassembly of GroES7 upon cooling the solution. The native MS studies performed at subambient temperature (T < 24 °C) do not reveal changes in quaternary structures of GroEL14 and GroES7 which might be expected for assembly mechanisms involving purely hydrophobic interactions; however, for GroEL, hydrogen bonds and salt bridges are known to provide stabilization of the complex.5,58-61
Impact of Ions and Small Molecules on the GroEL–GroES Function.
The physiological function of molecular chaperones such as GroEL is ATP-, Mg2+-, and K+-dependent,12,62 and nucleotide hydrolysis serves as the energy source to power specific conformational changes.13,63 Solution temperature can significantly influence the thermodynamics and kinetics of small molecule binding. High-resolution native MS and vT-ESI afford means to study these binding events with unprecedented detail at concentration levels that mimic physiological conditions, including experimental conditions that allow final products to be analyzed. On the other hand, native MS affords the ability to investigate chaperones over a range of experimental conditions that may reveal previously hidden details, for example, the presence and roles of intermediates that are either off- or on-pathway to final products. As a single example, the fact that ATP binding is diminished at elevated temperatures provides a clue regarding the contribution of ionic interactions, with a major role of the phosphate group compared to the hydrophobic moiety (adenosine).63 The formation of ADP, which is expected to increase the heterogeneity of the system, is inevitable in our experiments; however, because ADP is similar to ATP in thermochemistry, it does not interfere with the vT-ESI of the GroEL–GroES complex. In fact, we have previously shown that ADP binding to Gln K is enthalpically driven and disfavored at elevated temperatures.32 Moreover, we cannot rule out the contribution of conformational dynamics of GroEL, mainly local destabilization of nucleotide binding and the observed changes in ATP binding at elevated temperatures. Nevertheless, our data provide insight not only into the effect of temperature on ATP binding but also the on ATP-induced conformational change on GroEL. The observed decrease in the Zavg of the GroEL14–ATP complex compared to apo-GroEL14 is consistent with structural rearrangement or conformational drift that has been previously reported.64
T-Dependent GroEL–GroES Interactions.
Cold-induced disassembly of protein complexes is associated with a decreased entropic penalty for ordering water near hydrophobic residues.29 The disassembly of GroEL14–GroES7, the BS structure, at T <8 °C points to a major contribution of hydrophobic interactions, mainly engagement of GroES “mobile loop” with apical domain of GroEL.65 Previously, Todd et al.66 reported fast release of GroES7 from the GroEL14–ADP complex at 4 °C, corroborating the role of hydrophobic interactions in this binding process.
The higher stability of the FS structure (GroEL14–GroES14) compared to the BS complex at low and high temperatures suggests that the apo ring might compromise the stability of GroEL ring capped with GroES7. In other words, the structural changes in the free ring upon heating or cooling the solution destabilizes the subunit–subunit interactions in the GroES-bound ring to disrupt GroES binding. Additional evidence for compromised inter-ring stability of the BS complex is the observation of single ring GroEL (GroEL7) in the vT-ESI of GroEL14 (Figure 2). Our data suggest that heat-induced unfolding of GroEL subunits destabilizes the subunit–subunit interactions between two rings. In fact, our results show that subunit unfolding precedes the appearance of single ring and suggests that unfolding of GroEL subunits in one ring disfavors intra-ring interactions. Such ring splitting of GroEL14 has been previously reported under heat shock conditions,67 and the importance of such effects serves as the basis for planned future studies—the target being how GroES7 binding to both rings seems to inhibit such conformational changes and increases the stability of complex over a broader range of temperatures. The fact that the FS structure is fully loaded with ATP molecules might also enhance the stability of the GroEL14–GroES14 complex. Furthermore, the resistance of the FS structure against cold and hot treatment points out to the limited dynamics of this structure and in contrast to the GroEL biological function. On the other hand, previous studies have shown that the ADP-bound BS structure is the acceptor state of the GroEL–GroES system and is more physiologically relevant.68
The observation of intermediates, such as GroEL14–GroES1–2, upon both cooling and heating of the BS structure is an unexpected result as is the observation of GroEL14–GroES8 at temperatures between 24 and 40 °C. The increased abundances of these complexes at elevated temperatures can be justified based on temperature- and concentration-dependent stabilities of GroES7.39,53 The formation of GroEL14–GroES1–2 at both low (8 °C) and high temperatures (up to 56 °C) can be explained by temperature-dependent disassembly of the GroES7 complex, and this is supported by the 0.5 mM Kd reported by Sakane et al.53 and Boudker et al.,39 which raises yet another possible explanation. Specifically, that a destabilized GroES7 binds to the apical domain of GroEL through a limited number of GroES subunits, and the exposed subunits then reorganize and disassemble. Evidences for this hypothesis are the reported instabilities previously reported39,53 which are further supported by the effects of Mg2+ concentration shown in Figure 5. Lastly, these intermediates are only observed in Mg2+- or ATP-deprived solution, which corroborates their transient nature.
The number of bound ATPs required for GroEL and GroES interaction as well as substrate refolding has been previously investigated by Chapman et al.47 In their study, ATP binding to a GroEL mutant (I493C) was inhibited in the presence of the cyclopentane–carboxamide derivative molecule, EC3016. It was shown that only 50% of W2M5 complex (W: wild type; M: mutant) is capable of binding GroES in the presence of an inhibitor, and at least three ATP molecules are required for GroES binding. Here, we observed binding of 1–3 individual GroES subunits to GroEL in solutions where Mg2+ and ATP concentrations are below physiological concentration, which are highly variable.16 We discount the possibility that these products are formed by dissociation of a GroES7 that is bound to the GroEL apical domain; however, we also detected a correlation in the number of ATP molecules bound to GroEL and the stoichiometry of the GroEL14–GroESx–ATPn complex. For n = 1–3, up to two GroES subunits can interact with GroEL, and increasing Mg2+/ATP concentrations increases ATP binding, 6 < n < 9, as well as binding of intact GroES (GroES7). We propose that binding of ATP1–4 can allow for rotation of the apical domain of GroEL to recruit individual GroES subunits but not enough to allow for binding intact GroES.43 Specifically, binding of intact co-chaperone requires specific amounts of Mg2+, and in its absence binding of ATPn molecules (n > 5) is not sufficient to capture the native GroES complex. Previously, Azem et al. showed that Mg2+ not only increases ATP binding and hydrolysis but also increases the dynamics of GroEL.62
The X-ray structure of GroES7 provides more insight into observations of individual subunits of GroES binding to GroEL. Hunt et al. discovered an unexpected asymmetry in terms of subunit–subunit interactions in GroES7 structure as well as interfacial residues.56 In fact, R47 and K55 residues form salt bridges with neighboring residues in only two subunits, and these stabilizing interactions are missing in the other five subunits. This structural heterogeneity41 might also explain the observation of monomer and dimer ions in native mass spectrum of GroES7 (Figure 3) and previous studies.37,41 The GroEL14–GroES1–3 complexes may be formed by reactions with GroES1 and GroES2 binding to the hydrophobic (apical) domain similar to that for unfolded substrates. Alternatively, we cannot rule out the possibility that the energy requirement for disassembly of the GroES complex can be compensated by the binding energy to GroEL14. Previous studies have shown that both ATP and GroES binding are necessary for apical domain movement and for transition from substrate-binding mode (hydrophobic surface exposed) to the folding-active state (hydrophilic chamber).69,70 The role of GroES binding in this transition suggests an alternative mechanism under extreme conditions wherein sequential binding of GroES subunits to GroEL may indeed become favorable. These factors may also have implications in refolding of multidomain proteins where GroESy binding (y < 7) could allow for partial or full release of a single GroES subunit, providing the gap necessary for a piece of polypeptide to extrude to the exterior of the GroEL–GroES complex.71,72
The vT-ESI data offer insight into thermal stability of GroEL14–GroES1–3 complexes. The higher abundance of GroES1 and GroES8 at 32 °C < T < 40 °C (Figure 4) indicates that binding of a single GroES subunit to a GroEL ring is thermodynamically stable, whereas increasing temperature favors binding of two subunits (~44 °C). Above this temperature both GroEL and GroES become unstable and GroEL–GroES interactions are lost. At low temperatures, also due to the hydrophobic nature of the GroEL–GroES interaction, these intermediates are no longer formed. Overall, these results showcase the power of native high-resolution MS and vT-ESI to investigate the role of nucleotide and small molecules in chaperonins and their interaction with cochaperonins. Future studies with substrate can shed light on the thermal dependency of protein chaperonins and substrate interactions and cofactors roles in driving the refolding reaction.
EXPERIMENTAL DATA AND MATERIALS
Materials.
All reagents including magnesium acetate and EDDA were purchased from Sigma-Aldrich (St. Louis, MO). GroEL and GroES were overexpressed in E. coli as described previously.73 Adenosine triphosphate sodium (ATP) was purchased from Jena Bioscience and dissolved in deionized water (Barnstead Easy Pure II, Thermo Scientific). Sample aliquots were stored at −20 °C and freshly diluted with water then added to protein prior to analysis. Protein concentration was measured by using Bradford assay and are for tetradecameric and heptameric proteins unless otherwise mentioned. Fresh GroEL (6 μM, 10 μL) and GroES (60 μM, 10 μL) were diluted 3-fold with EDDA and buffer exchanged into buffer by using Micro Biospin P-6 gel column (BioRad).
Native Mass Spectrometry.
Mass spectra of proteins were collected on a Q Exactive UHMR (ultrahigh mass range) Hybrid Quadrupole Orbitrap mass spectrometer (Thermo Scientific, CA) with the mass range of m/z 350–80000. The instrument was calibrated by using CsI (2 mg/mL in 50:50 IPA: H2O) in positive mode. The capillary voltage varied from 1.0 to 1.6 kV for the highest signal and better desolvation. To obtain better resolved peaks, insource trapping and activation (150–250 V) was used; higher collision energies are important for solution with higher Mg2+/ ATP concentration. To increase the mass accuracy and ion abundances, HCD activation was also used (150–250 V) for ATP binding studies. The temperature of the instrument ion source was maintained at 100 °C for all the vT-ESI experiments. The temperature of the instrument ion source was varied to promote better desolvation and more abundance ion signals. The temperature of the vT-ESI source was controlled as described previously.25 For melting experiments the response factors for multiple oligomeric states of the complexes in question were assumed to be identical, meaning that the detection of all signals for all m/z values were assumed to be unbiased. Mass spectra analyses were performed by using UniDec74 and/or Protein Metrics Intact Mass to determine the average charge state and experimental molecular weights. The Zavg for GroEL14–ATPn was calculated by averaging the Zavg for individual ATP bindings.
ATP Association Constants.
The Ka values for the binding of ATPs 1–14 by GroEL14 were calculated by using an ATP titration study. The solution conditions were 500 nM GroEL14 in 200 mM EDDA buffer with 1 mM MgAc. ATP concentrations used in the study were 0, 0.2, 0.4, 0.6, 0.8, 1, 2.5, 5, 10, 15, 20, 25, 30, 40, and 50 μM. Each concentration was analyzed at three different temperatures (10, 25, and 40 °C). The solution temperature was allowed to equilibrate for a minimum of 3 min before the measurement was conducted. Mass spectra were deconvoluted by using UniDec and incorporated the four most abundant charge states of the GroEL14–ATPn distributions in each spectrum for the deconvolution. Macroscopic association constants (Ka) for ATP binding to GroEL14 were calculated by using a sequential ligand binding model. Macroscopic Ka values were then statistically corrected to render intrinsic Ka values; more information about this process is available in the Supporting Information and the referenced literature therein.
VT-ESI-MS Experiments.
The temperature of the solution contained in the ESI emitter is controlled by using a vT-ESI apparatus that has been described previously.25 Briefly, the ESI emitter is positioned within an aluminum block, and the temperature of the Al block is heated/cooled by using a three-tier Peltier chip controlled by a thermoelectric cooler (Meanwell LRS-100 24). A ceramic rod also transferred heat efficiently from emitter. Borosilicate glass capillary tips were pulled in house by using Sutter 1000, and ions were generated via a platinum wire inserted into the capillary. The temperature of solution in vT-ESI was calibrated by using a T-Type thermocouple (Physitemp, Clifton, NJ) inserted into the capillary.
CONCLUSION
Variable-temperature ESI native mass spectrometry was used to investigate the effects of ATP and GroES binding on the stability of the GroEL complex. This approach complements similar studies performed by using differential scanning calorimetry (DSC), the traditional method used for studies of protein heat capacities (ΔCp), and determinations of melting temperature (Tm).75 The sensitivity and dynamic range of native MS afford the ability to detect previously hidden products, non-native states as well as reaction intermediates, including changes in the structure/conformation of the protein complex.75 Temperature-dependent changes in structure/conformation originate from changes of both secondary (2°), tertiary (3°), and/or quaternary (4°) structure of protein complexes. Tm values for a given protein or protein complex may vary depending on solution conditions, solvent, pH, pressure, ionic strength, and presence/absence of other solutes, all of which contribute to the Gibbs energy of the system. Oftentimes low-energy endotherms, changes in ΔCp that occur at temperatures below the Tm, are observed because of formation of misfolded states and/or subtle changes in conformation, changes in 3° structure (“conformational drift”) under conditions whereby 2° structure is retained. The changes in Zavg obtained by using vT-ESI native MS also reflect temperature-dependent and ATP-dependent conformation changes of GroEL and the GroEL–GroES complexes that arise from changes in the solvent accessible surface area (SASA) of the proteins that accompany ATP binding. Galán et al.42 reported low-temperature endotherms associated with ATP binding to GroEL by using DSC; however, as noted above, the origin(s) of the ΔCp signals are difficult to assign from such measurements.
In summary, this work demonstrates the utility of vT-ESI and high-resolution native mass spectrometry to investigate the role of cofactor and co-chaperone molecules in stability of chaperonins by using a broad range of experimental conditions. Our results specifically shed light on the thermal stability of GroEL, GroES, and GroEL–GroES intermediates resulting from cooling and heating the solution. Although evidence for thermal instabilities of GroEL and GroEL–GroES complexes has been observed previously,42 the specific effects have not been fully characterized. Thermodynamic studies, inclusive of ΔG, ΔH, and TΔS that are currently underway, can reveal even greater insights into the formation of these complexes and for ATP binding that may provide greater details and may provide even further understanding of the GroEL–GroES operational mechanism. Nonetheless, this study clearly illustrates the utility vT-ESI native mass spectrometry for analysis of biologically complex systems. The presence of intermediates and their role/impact on the operation of the GroEL molecular machine are still open to debate, especially, the potential role of the effects noted in this study might impact our views of folding/refolding of non-native substrate proteins.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the NIH for funding this work. The authors also thank a reviewer for their careful reading of the manuscript, especially for their thoughtful critique and guidance to key papers that have greatly improved the content of this manuscript. We also acknowledge Dr. Christopher Mallis for data processing and preparation of supplemental material used for Figure S1.
Funding
Funding for this work was provided by DHHS-National Institutes of Health-National Institute of General Medical Sciences R01 GM121751 (D.E.C., A.L., D.H.R.), R01 GM138863 (A.L., D.H.R.), P41 GM128577 (D.H.R.), R01 GM134063 (H.R.), and the National Institutes of Health R44 GM133239 (A.L., D.H.R.).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c11341.
Mass spectral data showing effect of temperature on GroEL14–GroEL1 and GroEL7; calculated macroscopic association constants (Ka) for binding of ATP to GroEL (PDF)
The authors declare no competing financial interest.
Contributor Information
Thomas E. Walker, Department of Chemistry, Texas A&M University, College Station, Texas 77843, United States
Mehdi Shirzadeh, Department of Chemistry, Texas A&M University, College Station, Texas 77843, United States.
He Mirabel Sun, Department of Chemistry, Texas A&M University, College Station, Texas 77843, United States.
Jacob W. McCabe, Department of Chemistry, Texas A&M University, College Station, Texas 77843, United States.
Andrew Roth, Department of Biochemistry and Biophysics, Texas A&M University, College Station, Texas 77843, United States.
Zahra Moghadamchargari, Department of Chemistry, Texas A&M University, College Station, Texas 77843, United States.
David E. Clemmer, Department of Chemistry, Indiana University, Bloomington, Indiana 47401, United States.
Arthur Laganowsky, Department of Chemistry, Texas A&M University, College Station, Texas 77843, United States.
Hays Rye, Department of Biochemistry and Biophysics, Texas A&M University, College Station, Texas 77843, United States.
David H. Russell, Department of Chemistry, Texas A&M University, College Station, Texas 77843, United States.
REFERENCES
- (1).Boisvert DC; Wang J; Otwinowski Z; Norwich AL; Sigler PB The 2.4 Å crystal structure of the bacterial chaperonin GroEL complexed with ATPγS. Nat. Struct. Biol 1996, 3 (2), 170–177. [DOI] [PubMed] [Google Scholar]
- (2).Horwich AL; Farr GW; Fenton WA GroEL–GroES-Mediated Protein Folding. Chem. Rev 2006, 106 (5), 1917–1930. [DOI] [PubMed] [Google Scholar]
- (3).Ranson NA; Clare DK; Farr GW; Houldershaw D; Horwich AL; Saibil HR Allosteric signaling of ATP hydrolysis in GroEL–GroES complexes. Nature Structural & Molecular Biology 2006, 13 (2), 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Saibil HR; Fenton WA; Clare DK; Horwich AL Structure and Allostery of the Chaperonin GroEL. J. Mol. Biol 2013, 425 (9), 1476–1487. [DOI] [PubMed] [Google Scholar]
- (5).Sot B; Bañuelos S; Valpuesta JM; Muga A GroEL stability and function. Contribution of the ionic interactions at the inter-ring contact sites. J. Biol. Chem 2003, 278 (34), 32083–90. [DOI] [PubMed] [Google Scholar]
- (6).Skjaerven L; Grant B; Muga A; Teigen K; McCammon JA; Reuter N; Martinez A Conformational Sampling and Nucleotide-Dependent Transitions of the GroEL Subunit Probed by Unbiased Molecular Dynamics Simulations. PLoS Comput. Biol 2011, 7 (3), e1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Xu Z; Horwich AL; Sigler PB The crystal structure of the asymmetric GroEL–GroES–(ADP)7 chaperonin complex. Nature 1997, 388 (6644), 741–750. [DOI] [PubMed] [Google Scholar]
- (8).Roh S-H; Hryc CF; Jeong H-H; Fei X; Jakana J; Lorimer GH; Chiu W Subunit conformational variation within individual GroEL oligomers resolved by Cryo-EM. Proc. Natl. Acad. Sci. U. S. A 2017, 114, 201704725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Viitanen PV; Lubben TH; Reed J; Goloubinoff P; O’Keefe DP; Lorimer GH Chaperonin-facilitated refolding of ribulose bisphosphate carboxylase and ATP hydrolysis by chaperonin 60 (groEL) are potassium dependent. Biochemistry 1990, 29 (24), 5665–5671. [DOI] [PubMed] [Google Scholar]
- (10).Lin Z; Rye HS GroEL-mediated protein folding: making the impossible, possible. Crit. Rev. Biochem. Mol. Biol 2006, 41 (4), 211–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Lorimer G Protein folding Folding with a two-stroke motor. Nature 1997, 388 (6644), 720–721. [DOI] [PubMed] [Google Scholar]
- (12).Grason JP; Gresham JS; Lorimer GH Setting the chaperonin timer: A two-stroke, two-speed, protein machine. Proc. Natl. Acad. Sci. U. S. A 2008, 105 (45), 17339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Rye HS; Burston SG; Fenton WA; Beechem JM; Xu Z; Sigler PB; Horwich AL Distinct actions of cis and trans ATP within the double ring of the chaperonin GroEL. Nature 1997, 388 (6644), 792–798. [DOI] [PubMed] [Google Scholar]
- (14).Yang D; Ye X; Lorimer GH Symmetric GroEL:GroES2 complexes are the protein-folding functional form of the chaperonin nanomachine. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (46), E4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Bigman LS; Horovitz A Reconciling the controversy regarding the functional importance of bullet- and football-shaped GroE complexes. J. Biol. Chem 2019, 294 (37), 13527–13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Taguchi H Reaction Cycle of Chaperonin GroEL via Symmetric “Football” Intermediate. J. Mol. Biol 2015, 427 (18), 2912–2918. [DOI] [PubMed] [Google Scholar]
- (17).Yaginuma H; Kawai S; Tabata KV; Tomiyama K; Kakizuka A; Komatsuzaki T; Noji H; Imamura H Diversity in ATP concentrations in a single bacterial cell population revealed by quantitative single-cell imaging. Sci. Rep 2015, 4 (1), 6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Horovitz A; Reingewertz TH; Cuéllar J; Valpuesta JM Chaperonin Mechanisms: Multiple and (Mis)Understood? Annu. Rev. Biophys 2022, 51, 115–33. [DOI] [PubMed] [Google Scholar]
- (19).Rostom AA; Robinson CV Detection of the Intact GroEL Chaperonin Assembly by Mass Spectrometry. J. Am. Chem. Soc 1999, 121 (19), 4718–4719. [Google Scholar]
- (20).Robinson CV; Groß M; Eyles SJ; Ewbank JJ; Mayhew M; Hartl FU; Dobson CM; Radford SE Conformation of GroEL-bound α-lactalbumin probed by mass spectrometry. Nature 1994, 372 (6507), 646–651. [DOI] [PubMed] [Google Scholar]
- (21).Clemmer DE; Russell DH; Williams ER Characterizing the Conformationome: Toward a Structural Understanding of the Proteome. Acc. Chem. Res 2017, 50 (3), 556–560. [DOI] [PubMed] [Google Scholar]
- (22).McCabe JW; Hebert MJ; Shirzadeh M; Mallis CS; Denton JK; Walker TE; Russell DH THE IMS PARADOX: A PERSPECTIVE ON STRUCTURAL ION MOBILITY-MASS SPECTROMETRY. Mass Spectrom. Rev 2021, 40 (3), 280–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Dyachenko A; Gruber R; Shimon L; Horovitz A; Sharon M Allosteric mechanisms can be distinguished using structural mass spectrometry. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (18), 7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).van Duijn E; Bakkes PJ; Heeren RMA; van den Heuvel RHH; van Heerikhuizen H; van der Vies SM; Heck AJR Monitoring macromolecular complexes involved in the chaperonin-assisted protein folding cycle by mass spectrometry. Nat. Methods 2005, 2 (5), 371–376. [DOI] [PubMed] [Google Scholar]
- (25).McCabe JW; Shirzadeh M; Walker TE; Lin C-W; Jones BJ; Wysocki VH; Barondeau DP; Clemmer DE; Laganowsky A; Russell DH Variable-Temperature Electrospray Ionization for Temperature-Dependent Folding/Refolding Reactions of Proteins and Ligand Binding. Anal. Chem 2021, 93 (18), 6924–6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Konermann L; Silva EA; Sogbein OF Electrochemically induced pH changes resulting in protein unfolding in the ion source of an electrospray mass spectrometer. Anal. Chem 2001, 73 (20), 4836–44. [DOI] [PubMed] [Google Scholar]
- (27).Shirzadeh M; Poltash ML; Laganowsky A; Russell DH Structural Analysis of the Effect of a Dual-FLAG Tag on Transthyretin. Biochemistry 2020, 59 (9), 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Benesch JLP; Sobott F; Robinson CV Thermal Dissociation of Multimeric Protein Complexes by Using Nano-electrospray Mass Spectrometry. Anal. Chem 2003, 75 (10), 2208–2214. [DOI] [PubMed] [Google Scholar]
- (29).Privalov PL Cold Denaturation of Protein. Crit. Rev. Biochem. Mol. Biol 1990, 25 (4), 281–306. [DOI] [PubMed] [Google Scholar]
- (30).El-Baba TJ; Woodall DW; Raab SA; Fuller DR; Laganowsky A; Russell DH; Clemmer DE Melting Proteins: Evidence for Multiple Stable Structures upon Thermal Denaturation of Native Ubiquitin from Ion Mobility Spectrometry-Mass Spectrometry Measurements. J. Am. Chem. Soc 2017, 139 (18), 6306–6309. [DOI] [PubMed] [Google Scholar]
- (31).Woodall DW; Brown CJ; Raab SA; El-Baba TJ; Laganowsky A; Russell DH; Clemmer DE Melting of Hemoglobin in Native Solutions as measured by IMS-MS. Anal. Chem 2020, 92 (4), 3440–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Cong X; Liu Y; Liu W; Liang X; Russell DH; Laganowsky A Determining Membrane Protein-Lipid Binding Thermodynamics Using Native Mass Spectrometry. J. Am. Chem. Soc 2016, 138 (13), 4346–9. [DOI] [PubMed] [Google Scholar]
- (33).Marchand A; Czar MF; Eggel EN; Kaeslin J; Zenobi R Studying biomolecular folding and binding using temperature-jump mass spectrometry. Nat. Commun 2020, 11 (1), 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Lee S-W; Freivogel P; Schindler T; Beauchamp JL Freeze-Dried Biomolecules: FT-ICR Studies of the Specific Solvation of Functional Groups and Clathrate Formation Observed by the Slow Evaporation of Water from Hydrated Peptides and Model Compounds in the Gas Phase. J. Am. Chem. Soc 1998, 120 (45), 11758–11765. [Google Scholar]
- (35).Silveira JA; Fort KL; Kim D; Servage KA; Pierson NA; Clemmer DE; Russell DH From solution to the gas phase: stepwise dehydration and kinetic trapping of substance P reveals the origin of peptide conformations. J. Am. Chem. Soc 2013, 135 (51), 19147–53. [DOI] [PubMed] [Google Scholar]
- (36).Clare DK; Bakkes PJ; van Heerikhuizen H; van der Vies SM; Saibil HR An expanded protein folding cage in the GroEL-gp31 complex. J. Mol. Biol 2006, 358 (3), 905–11. [DOI] [PubMed] [Google Scholar]
- (37).Donald LJ; Stokell DJ; Holliday NJ; Ens W; Standing KG; Duckworth HW Multiple equilibria of the Escherichia coli chaperonin GroES revealed by mass spectrometry. Protein Sci. 2005, 14 (5), 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Raab SA; El-Baba TJ; Woodall DW; Liu W; Liu Y; Baird Z; Hales DA; Laganowsky A; Russell DH; Clemmer DE Evidence for Many Unique Solution Structures for Chymotrypsin Inhibitor 2: A Thermodynamic Perspective Derived from vT-ESI-IMS-MS Measurements. J. Am. Chem. Soc 2020, 142 (41), 17372–17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Boudker O; Todd MJ; Freire E The structural stability of the co-chaperonin GroES. J. Biol. Chem 1997, 272 (5), 770–779. [DOI] [PubMed] [Google Scholar]
- (40).Geels RBJ; Calmat S; Heck AJR; van der Vies SM; Heeren RMA Thermal activation of the co-chaperonins GroES and gp31 probed by mass spectrometry. Rapid Commun. Mass Spectrom 2008, 22 (22), 3633–3641. [DOI] [PubMed] [Google Scholar]
- (41).Dyachenko A; Tamara S; Heck AJR Distinct Stabilities of the Structurally Homologous Heptameric Co-Chaperonins GroES and gp31. Journal of The American Society for Mass Spectrometry 2019, 30 (1), 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Galán A; Llorca O; Valpuesta JM; Pérez-Pérez J; Carrascosa JL; Menéndez M; Bañuelos S; Muga A ATP hydrolysis induces an intermediate conformational state in GroEL. Eur. J. Biochem 1999, 259 (1–2), 347–355. [DOI] [PubMed] [Google Scholar]
- (43).Hansen JE; Gafni A Fluorescence detection of conformational changes in GroEL induced by thermal switching and nucleotide binding. J. Biol. Chem 1994, 269 (9), 6286–9. [PubMed] [Google Scholar]
- (44).Terada TP; Kuwajima K Thermodynamics of nucleotide binding to the chaperonin GroEL studied by isothermal titration calorimetry: evidence for noncooperative nucleotide binding. Biochim. Biophys. Acta 1999, 1431 (2), 269–81. [DOI] [PubMed] [Google Scholar]
- (45).Inobe T; Makio T; Takasu-Ishikawa E; Terada TP; Kuwajima K Nucleotide binding to the chaperonin GroEL: noncooperative binding of ATP analogs and ADP, and cooperative effect of ATP. Biochim. Biophys. Acta 2001, 1545 (1–2), 160–73. [DOI] [PubMed] [Google Scholar]
- (46).Todd MJ; Viitanen PV; Lorimer GH Hydrolysis of Adenosine 5′-Triphosphate by Escherichia-Coli Groel - Effects of Groes and Potassium-Ion. Biochemistry 1993, 32 (33), 8560–8567. [DOI] [PubMed] [Google Scholar]
- (47).Chapman E; Farr GW; Fenton WA; Johnson SM; Horwich AL Requirement for binding multiple ATPs to convert a GroEL ring to the folding-active state. Proc. Natl. Acad. Sci. U. S. A 2008, 105 (49), 19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Yifrach O; Horovitz A Nested cooperativity in the ATPase activity of the oligomeric chaperonin GroEL. Biochemistry 1995, 34 (16), 5303–5308. [DOI] [PubMed] [Google Scholar]
- (49).Schmidt M; Rutkat K; Rachel R; Pfeifer G; Jaenicke R; Viitanen P; Lorimer G; Buchner J Symmetric complexes of GroE chaperonins as part of the functional cycle. Science 1994, 265 (5172), 656. [DOI] [PubMed] [Google Scholar]
- (50).Koike-Takeshita A; Arakawa T; Taguchi H; Shimamura T Crystal Structure of a Symmetric Football-Shaped GroEL:GroES2-ATP14 Complex Determined at 3.8Å Reveals Rearrangement between Two GroEL Rings. J. Mol. Biol 2014, 426 (21), 3634–3641. [DOI] [PubMed] [Google Scholar]
- (51).Ye X; Lorimer GH Substrate protein switches GroE chaperonins from asymmetric to symmetric cycling by catalyzing nucleotide exchange. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (46), E4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Wilson JE; Chin A Chelation of divalent cations by ATP, studied by titration calorimetry. Anal. Biochem 1991, 193 (1), 16–9. [DOI] [PubMed] [Google Scholar]
- (53).Sakane I; Hongo K; Motojima F; Murayama S; Mizobata T; Kawata Y Structural stability of covalently linked GroES heptamer: advantages in the formation of oligomeric structure. J. Mol. Biol 2007, 367 (4), 1171–85. [DOI] [PubMed] [Google Scholar]
- (54).Owen BB; Miller RC; Milner CE; Cogan HL The Dielectric Constant of Water as a Function of Temperature and Pressure. J. Phys. Chem 1961, 65 (11), 2065–2070. [Google Scholar]
- (55).Matysiak S; Debenedetti PG; Rossky PJ Role of Hydrophobic Hydration in Protein Stability: A 3D Water-Explicit Protein Model Exhibiting Cold and Heat Denaturation. J. Phys. Chem. B 2012, 116 (28), 8095–8104. [DOI] [PubMed] [Google Scholar]
- (56).Hunt JF; Weaver AJ; Landry SJ; Gierasch L; Deisenhofer J The crystal structure of the GroES co-chaperonin at 2.8 Å resolution. Nature 1996, 379 (6560), 37–45. [DOI] [PubMed] [Google Scholar]
- (57).Zondlo J; Fisher KE; Lin Z; Ducote KR; Eisenstein E Monomer-heptamer equilibrium of the Escherichia coli chaperonin GroES. Biochemistry 1995, 34 (33), 10334–9. [DOI] [PubMed] [Google Scholar]
- (58).Bartolucci C; Lamba D; Grazulis S; Manakova E; Heumann H Crystal Structure of Wild-type Chaperonin GroEL. J. Mol. Biol 2005, 354 (4), 940–951. [DOI] [PubMed] [Google Scholar]
- (59).Yifrach O; Horovitz A Two lines of allosteric communication in the oligomeric chaperonin GroEL are revealed by the single mutation Arg196–>Ala. J. Mol. Biol 1994, 243 (3), 397–401. [DOI] [PubMed] [Google Scholar]
- (60).Fei X; Yang D; LaRonde-LeBlanc N; Lorimer GH Crystal structure of a GroEL-ADP complex in the relaxed allosteric state at 2.7 A resolution. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (32), E2958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Sewell BT; Best RB; Chen S; Roseman AM; Farr GW; Horwich AL; Saibil HR A mutant chaperonin with rearranged inter-ring electrostatic contacts and temperature-sensitive dissociation. Nat. Struct. Mol. Biol 2004, 11 (11), 1128–33. [DOI] [PubMed] [Google Scholar]
- (62).Azem A; Diamant S; Goloubinoff P Effect of Divalent Cations on the Molecular Structure of the GroEL Oligomer. Biochemistry 1994, 33 (21), 6671–6675. [DOI] [PubMed] [Google Scholar]
- (63).Chaudhry C; Farr GW; Todd MJ; Rye HS; Brunger AT; Adams PD; Horwich AL; Sigler PB Role of the γ-phosphate of ATP in triggering protein folding by GroEL–GroES: function, structure and energetics. EMBO Journal 2003, 22 (19), 4877–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Clare DK; Vasishtan D; Stagg S; Quispe J; Farr GW; Topf M; Horwich AL; Saibil HR ATP-Triggered Conformational Changes Delineate Substrate-Binding and -Folding Mechanics of the GroEL Chaperonin. Cell 2012, 149 (1), 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Landry SJ; Zeilstra-Ryalls J; Fayet O; Georgopoulos C; Gierasch LM Characterization of a functionally important mobile domain of GroES. Nature 1993, 364 (6434), 255–258. [DOI] [PubMed] [Google Scholar]
- (66).Todd MJ; Viitanen PV; Lorimer GH Dynamics of the chaperonin ATPase cycle: implications for facilitated protein folding. Science 1994, 265 (5172), 659. [DOI] [PubMed] [Google Scholar]
- (67).Llorca O; Galán A; Carrascosa JL; Muga A; Valpuesta JM GroEL under heat-shock. Switching from a folding to a storing function. J. Biol. Chem 1998, 273 (49), 32587–94. [DOI] [PubMed] [Google Scholar]
- (68).Lin Z; Puchalla J; Shoup D; Rye HS Repetitive Protein Unfolding by the trans Ring of the GroEL-GroES Chaperonin Complex Stimulates Folding. J. Biol. Chem 2013, 288 (43), 30944–30955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Roseman AM; Chen S; White H; Braig K; Saibil HR The Chaperonin ATPase Cycle: Mechanism of Allosteric Switching and Movements of Substrate-Binding Domains in GroEL. Cell 1996, 87 (2), 241–251. [DOI] [PubMed] [Google Scholar]
- (70).Rye HS; Roseman AM; Chen S; Furtak K; Fenton WA; Saibil HR; Horwich AL GroEL-GroES Cycling: ATP and Nonnative Polypeptide Direct Alternation of Folding-Active Rings. Cell 1999, 97 (3), 325–338. [DOI] [PubMed] [Google Scholar]
- (71).Sakikawa C; Taguchi H; Makino Y; Yoshida M On the Maximum Size of Proteins to Stay and Fold in the Cavity of GroEL underneath GroES *. J. Biol. Chem 1999, 274 (30), 21251–21256. [DOI] [PubMed] [Google Scholar]
- (72).Motojima F; Yoshida M Polypeptide in the chaperonin cage partly protrudes out and then folds inside or escapes outside. EMBO Journal 2010, 29 (23), 4008–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Weaver J; Jiang M; Roth A; Puchalla J; Zhang J; Rye HS GroEL actively stimulates folding of the endogenous substrate protein PepQ. Nat. Commun 2017, 8 (1), 15934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Marty MT; Baldwin AJ; Marklund EG; Hochberg GKA; Benesch JLP; Robinson CV Bayesian Deconvolution of Mass and Ion Mobility Spectra: From Binary Interactions to Polydisperse Ensembles. Anal. Chem 2015, 87 (8), 4370–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Cooper A Protein Heat Capacity: An Anomaly that Maybe Never Was. J. Phys. Chem. Lett 2010, 1 (22), 3298–3304. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.