Abstract
Objective:
To compare, in vitro, the shear bond strength (SBS) of two bond systems: Transbond XT/XT primer (TXT/XT) and Transbond Plus Color Change/Transbond Self Etching Primer (TPCC/TSEP).
Materials and Methods:
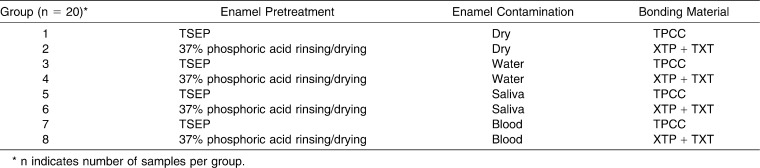
Each system was examined under four enamel surface conditions (dry, water, saliva, and blood), and 160 bovine teeth were divided into eight groups of 20 according to enamel surface condition. Group 1 used TPCC/TSEP and Group 2 used TXT/XT under dry conditions; Group 3 used TPCC/TSEP and Group 4 used TXT/XT with water; Group 5 used TPCC/TSEP and Group 6 used TXT/XT under saliva; and Group 7 used TPCC/TSEP and Group 8 used TXT/XT with blood. Brackets were bonded, and the samples were thermocycled 500 times between 5°C and 55°C; they were then submitted to a shear bond test with a universal testing machine with a 50 kgf load cell at 5 mm/min.
Results:
Although water and saliva affected TXT/XT more than they affected TPCC/TSEP, there were no significant differences among the groups (Groups 3 and 4: P = .940; Groups 3 and 5 and Groups 4 and 6: P = 1.000; Groups 3 and 6: P = .988; Groups 4 and 5: P = .690; and Groups 5 and 6: P = .861).
Conclusions:
The presence of blood resulted in the lowest SBS from both bond systems, but especially from TXT/XT. TPCC/TSEP resulted in a higher SBS than TXT/XT under all conditions except the dry enamel surface.
Keywords: Shear bond strength, Composite, Bonding, Contamination
INTRODUCTION
More than 50 years ago, Buonocore demonstrated that the adhesion of acrylic restorative materials increased significantly when it was preceded by an acid-etch technique on enamel.1 Since then, additional advances have been made that improve bond strength on tooth enamel. Although traditional bond materials must be applied in completely dry and isolated fields to produce clinically acceptable bond strengths,2–7 some manufacturers have recently started to introduce hydrophilic substances into their compositions. These substances allow for greater shear bond strength (SBS) on wet surfaces by enabling the composite to pass beyond the organic adhesive coating formed by moisture on the enamel's surface.
Hydrophilic bond systems have been an important development in orthodontic practice because many routine clinical procedures are not carried out under ideal conditions. In particular, it can be difficult to bond attachments to hard-to-reach places such as the gingival area, second molars, and partially erupted or impacted teeth.8–11 In these cases, bonding failure is common, and rebonding, which consumes chair time and is a burden for both orthodontist and patient, becomes necessary.11
In unsuccessful bonding procedures, most of the porosities produced by the enamel acid-etch procedure become plugged with moisture. As a result, resin penetration is impaired, and when insufficient numbers and lengths of resin tags form, bond strength is reduced.5,12,13 Contamination of the enamel surface therefore requires the etching procedure to be repeated to ensure the adequate bonding of composites.12 For this reason, several studies have evaluated the effects of contaminants like water, saliva, and blood on SBS.3,4,6,8,10,14
The aim of this research was to evaluate, after thermocycling, the SBS of hydrophobic Transbond XT/XT primer (TXT/XT; 3M/Unitek, Monrovia, Calif) and hydrophilic Transbond MR Plus Color Change/Transbond Self Etching Primer (TPCC/TSEP; 3M/Unitek) bonding systems exposed to four surface conditions (dry, water, saliva, and blood).
MATERIALS AND METHODS
One hundred and sixty freshly extracted bovine permanent incisors were obtained from a slaughterhouse and stored in a 0.5% thymol solution for 1 week at 4°C. Each crown was individually embedded in acrylic resin and its vestibular surface was polished8,15 with sandpaper under water until a 6-mm diameter flat area was obtained. A jig was used to align the vestibular surface of the tooth perpendicular to the base of a cylinder that was previously filled with acrylic resin; the root was fixed into the cylinder. The samples were kept in distilled water when they were not undergoing prophylactic, bonding, or testing procedures. All of the teeth were randomly assigned into eight groups of 20 specimens each and received a mask to standardize the bond area. The enamel area exposed within the mask was polished with a rubber cup and fluoride-free pumice for 10 seconds, sprayed with water, and dried with a compressed oil-free air stream.
Upper incisor brackets (1030101, edgewise standard, Morelli, Sorocaba, Brazil) were bonded according to the manufacturer's instructions. A thin layer of TSEP was brushed for 5 seconds onto the enamel surface of each incisor in the TPCC/TSEP groups (1, 3, 5, and 7). Samples of Groups 3, 5, and 7 were then exposed to 0.01 mL of water, saliva, and blood, respectively (Table 1). The incisors in the TXT/XT groups (2, 4, 6, and 8) underwent acid-etching with 37% phosphoric gel for 15 seconds followed by water rinsing and air drying. Groups 2, 4, 6, and 8 were coated with a thin layer of XT primer on the vestibular surface. The specimens of Groups 4, 6, and 8 were then exposed to 0.01 mL of water, saliva, and blood, respectively (Table 1). After primers and the contaminations were applied, a compressed oil-free air system was used for 1 second to eliminate excess material. The brackets were then oriented with their bases parallel to the floor, pressed firmly against the enamel surface, and excess adhesive was removed with an explorer. The sample was light-cured (Optilux, Demetron Research, Danbury, Conn) for 20 seconds (10 seconds mesially and 10 seconds distally). A power output of 850 mW/cm2 was determined with a Demetron radiometer (Model 100, Demetron).
Table 1.
Bonding Procedures for Different Enamel Surface Conditions
Water, saliva, and blood were collected immediately before the contamination procedure. Water was taken from a water distiller machine. Saliva and blood were collected from one of the researchers every five samples. The donor was instructed to brush her teeth and refrain from eating for 1 hour so that saliva could be collected. To collect the blood, her index finger was cleaned with alcohol and then punctured with a hypodermic needle.
Following ISO 1140516 recommendations, the specimens were thermocycled (500×) between 5°C and 55°C, with a dwell time in each bath of 30 seconds and a transfer time between baths of 15 seconds. Twenty-four hours after thermocycling, they were placed in a custom-made stabilizing stand and subjected to a shear load test in a Universal Testing Machine (model DL10.000, EMIC, São José dos Pinhais, Brazil). A knife-edged shearing rod was used for the test at a crosshead speed of 5 mm/min and a 50 kgf load cell was used for the SBS test. Force was applied parallel to the tooth's surface at the bracket base-enamel interface and the shear load at the point of failure was recorded in Newtons (N).
The debonded enamel surfaces were examined with a stereomicroscope (Swift M28, Zeiss, Germany) at 16× magnification to determine the amount of composite remaining. The composite remaining was classified according to the Adhesive Remnant Index (ARI).17
Statistical analyses were performed with SPSS 13.0 software (SPSS Inc, Chicago, Ill). Descriptive statistics of SBS (mean, standard deviation, median, minimum, maximum, and significance) were calculated for all groups. One-way analysis of variance (ANOVA) and Mann-Whitney tests were carried out for SBS and ARI, respectively, to determine significant differences among the groups. The statistical significance level was established at P < .05.
RESULTS
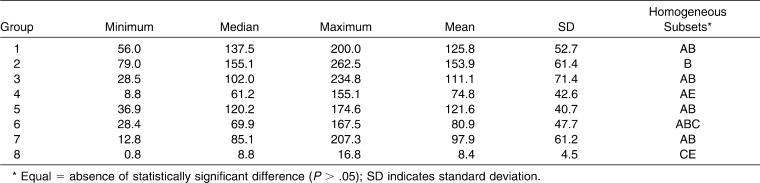
There were no significant differences among enamel surface conditions for TPCC/TSEP: for Groups 1, 3, and 5, P = 1.000; for Groups 1 and 7, P = .995; for Groups 3 and 7, P = 1.000; and for Groups 5 and 7, P = .999. Of the samples bonded with the TXT/XT bond system, those under dry conditions (Group 2) showed the highest SBS. This value was significantly higher than that of Group 8 (P = .000), which was exposed to blood.
When the mean values of all the groups were compared, no statistically significant differences were identified among Groups 1, 2, 3, and 5 (Table 2). Sixty-two percent of the samples bonded with the TXT/XT bond system had a score of 0. Among the samples bonded with TPCC/TSEP, score 2 was predominant.
Table 2.
Descriptive Statistics of Shear Bond Strengths (N)
DISCUSSION
In the present study, mean bond strengths of 125.8 N, 111.1 N, 121.6 N, and 97.8 N were found for TPCC/TSEP exposed to dry conditions, water, saliva, and blood, respectively. Mean bond strengths of 153.9 N, 74.7 N, 80.8 N, and 8.3 N were found for TXT/XT exposed to dry conditions, water, saliva, and blood, respectively. Water, saliva, and blood contamination of the enamel surface reduced the mean bond strengths of both TXT/XT and TPCC/TSEP. However, the mean bond strength of TXT/XT was lower than that of TPCC/TSEP in all of the wet enamel surface conditions (Table 2). This result may be explained by the properties of TSEP. Newer hydrophilic enamel primers have been formulated with acetone and/or alcohol, which displace moisture from the enamel surface isolated for bonding.3
Under water conditions, there were no statistically significant SBS differences (P = .940) between TPCC/TSEP (Group 3 = 111.1 N) and TXT/XT (Group 4 = 74.7 N). However, the SBS for TXT/XT was up to 50% less than that of TPCC/TSEP when exposed to water. This was more evident when comparing TPCC/TSEP (Groups 1 and 3: P = 1.000) and TXT/XT (Groups 2 and 4: P = .023) under dry and water conditions, respectively.
Saliva exposure had little influence on the mean bond strength of brackets bonded with TPCC/TSEP (Group 5 = 121.6 N) compared to Group 1 (TPCC/TSEP under dry conditions). However, samples bonded with TXT/XT (Group 6 = 80.8 N) had a 47% reduced SBS compared to TXT/XT under dry conditions. No significant statistical differences were found between hydrophobic and hydrophilic bond systems with exposure to saliva (P = .861). Previous studies of bond strength with hydrophilic bond systems and saliva contamination have produced controversial results; some have found an increase in bond strength,5–11 and others found either no significant decrease4 or a significant decrease.7 Differences in experimental design, such as the use of artificial or human saliva, and their quantity could explain such varying results. Furthermore, the composition of saliva can differ greatly depending on the conditions under which it is produced.12
When comparing saliva and water, water had the lowest SBS for both bond systems, but no statistically significant results were achieved between Groups 3 and 5, 3 and 6, 4 and 5, or 4 and 6 (Table 2). These results differ from those found by Grandhi et al.;8 however, it should be noted that their experiments used a different bond system (Transbond XT composite and moisture insensitive primer, MIP) and they applied two coats of saliva onto the etched surface.
The presence of blood (Groups 7 and 8) produced the worst conditions for both bond systems and reduced bond strength more than either water or saliva contamination. The bond strength of TXT/XT was reduced much more with blood (Group 8 = 8.3 N) than under dry conditions (P = .000) or with the presence of water (P = .132) or saliva (P = .061); the difference between the blood and dry conditions was statistically significant. The reduction in SBS for TPCC/TSEP in the presence of blood (Group 7 = 97.8 N) was not significantly different from the results of Groups 1, 2, 3, 4, 5, and 6; this result differs from the findings of Hobson et al.,9 Oonsombat et al.,10 and Faltermeier et al.,11 The variability of the results can be attributed to different self-etching primers and to the use of fresh blood in the present study, whereas Oonsombat et al.10 used blood mixed with anticoagulants.
The different degrees of interference caused by water, saliva, and blood on bonding procedures are the result of the differing compositions of the substances. Saliva is more complex than water,9 and the difference in type and amount of inorganic and organic substances in blood makes it a greater mechanical barrier than saliva,18 Thus, it is reasonable that even with a hydrophilic bond system, blood interfered the most with SBS and was followed (in interference intensity) by saliva and water. The specific components of blood that are involved in this process are still not clear, and it is therefore not possible to fully explain why blood moisture interferes with bonding more than saliva or water. The fact that no anticoagulants were used in this experiment could have made the blood a mechanical barrier interfering directly in bonding.
Shear bond experiments that tested similar materials under various enamel surface conditions have produced differing results; this may be the result of a number of other variables, such as thermocycling tests, shear bond machines, direction of the force used to debond the brackets, cross head speed, substrate, type of brackets, absence of standardization for applied moisture, the quantity and the application of different products, or other small variations in the materials and methods used.
In the orthodontic clinical routine it is more important to achieve adequate bond strength that allows for safe debonding than to obtain the greatest possible bond strength.19 Thus, ARI scores are used to define the site of bond failure between the enamel, the adhesive, and the bracket base through the remaining composite on the enamel surface. In orthodontic bond strength testing, cohesive fractures in the composite (ARI score 3) reflect the internal strength of the composite rather than the actual adhesion to the surface under study.20 In this experiment, TXT/XT and TPCC/TSEP produced similar ARI scores. Under dry conditions, most of the adhesive remained on the surface of the teeth after debonding, indicating failure at the bracket-adhesive interface; there was no difference (P = .052) between the bond systems tested. With water, saliva, and blood contamination, a score of 2 was predominant for TPCC/TSEP whereas a score of 0 was predominant for TXT/XT. Water, saliva, and blood affected the TXT/XT bonding pattern on the enamel more than they affected TPCC/TSEP. The ARI scores showed the absence of enamel fracture for all groups, which means that even the highest bond strength values were not sufficient to damage the enamel surface.
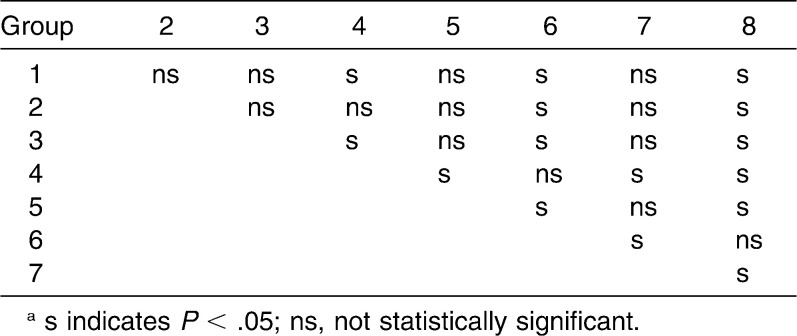
Although differences were found between the two bond systems when they were submitted to surface contaminations, only the results among Groups 1 and 8 (P = .000), 5 and 8 (P = .000), and 7 and 8 (P = .004) were statistically significant (Table 3). There was no difference (P = .994) between TXT/XT and TPCC/TSEP under dry conditions (Group 1: 125.8 N; Group 2: 153.9 N).
Table 3.
Statistical Comparison Through Mann-Whitney Test for ARI Scoresa
CONCLUSIONS
TPCC/TSEP showed higher SBS values than TXT/XT under all moisture conditions, but it showed the lowest SBS value under dry conditions.
In both systems, the weakest mean bond strength was achieved in the presence of blood; however, TPCC/TSEP showed higher mean bond strength.
The use of a hydrophilic bond system should be considered with blood exposure.
Acknowledgments
The authors wish to thank the FAPERJ for donation of the Universal Testing Machine model DL10.000 (EMIC, São José dos Pinhais, Brazil).
REFERENCES
- 1.Buonocore M. G. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955;34:849–853. doi: 10.1177/00220345550340060801. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds I. A review of direct orthodontic bonding. Br J Orthod. 1975;2:171–178. [Google Scholar]
- 3.Webster M. J, Nanda R. S, Duncanson M. G, Khajotia S. S, Sinha P. K. The effect of saliva on shear bond strengths of hydrophilic bonding systems. Am J Orthod Dentofacial Orthop. 2001;119:54–58. doi: 10.1067/mod.2001.109888. [DOI] [PubMed] [Google Scholar]
- 4.Cacciafesta V, Sfondrini M. F, De Angelis M, Scribante A, Klersy C. Effect of water and saliva contamination on shear bond strength of brackets bonded with conventional, hydrophilic, and self-etching primers. Am J Orthod Dentofacial Orthop. 2003;123:633–640. doi: 10.1016/s0889-5406(03)00198-7. [DOI] [PubMed] [Google Scholar]
- 5.Oztoprak M. O, Isik F, Sayinsu K, Arun T, Aydemir B. Effect of blood and saliva contamination on shear bond strength of brackets bonded with 4 adhesives. Am J Orthod Dentofacial Orthop. 2007;131:238–242. doi: 10.1016/j.ajodo.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Sayinsu K, Isik F, Sezen S, Aydemir B. Effect of blood and saliva contamination on bond strength of brackets bonded with a protective liquid polish and light-cure adhesive. Am J Orthod Dentofacial Orthop. 2007;131:391–394. doi: 10.1016/j.ajodo.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 7.Turk T, Elekdag-Turk S, Isci D, Cakmak F, Ozkalayci N. Saliva contamination effect on shear bond strength of self-etching primer with different debond times. Angle Orthod. 2007;77:901–906. doi: 10.2319/100906-415. [DOI] [PubMed] [Google Scholar]
- 8.Grandhi R. K, Combe E. C, Speidel T. M. Shear bond strength of stainless steel orthodontic brackets with a moisture-insensitive primer. Am J Orthod Dentofacial Orthop. 2001;119:251–255. doi: 10.1067/mod.2001.110988. [DOI] [PubMed] [Google Scholar]
- 9.Hobson R. S, Ledvinka J, Meechan J. G. The effect of moisture and blood contamination on bond strength of a new orthodontic bonding material. Am J Orthod Dentofacial Orthop. 2001;120:54–57. doi: 10.1067/mod.2001.115037. [DOI] [PubMed] [Google Scholar]
- 10.Oonsombat C, Bishara S. E, Ajlouni R. The effect of blood contamination on the shear bond strength of orthodontics brackets with the use of a new self-etch primer. Am J Orthod Dentofacial Orthop. 2003;123:547–550. doi: 10.1067/mod.2003.S0889540603000490. [DOI] [PubMed] [Google Scholar]
- 11.Faltermeier A, Behr M, Rosentritt M, Reicheneder C, Mussig D. An in vitro comparative assessment of different enamel contaminants during bracket bonding. Eur J Orthod. 2007;29:559–563. doi: 10.1093/ejo/cjm052. [DOI] [PubMed] [Google Scholar]
- 12.Littlewood S. J, Mitchell L, Greenwood D. C, Bubb N. L, Wood D. J. Investigation of a hydrophilic primer for orthodontic bonding in an in vitro study. J Orthod. 2000;27:181–186. doi: 10.1093/ortho/27.2.181. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopal R, Padmanabhan S, Gnanamani J. A comparison of shear bond strength and debonding characteristics of conventional, moisture-insensitive, and self-etching primers in vitro. Angle Orthod. 2004;74:264–268. doi: 10.1043/0003-3219(2004)074<0264:ACOSBS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Godoy-Bezerra J, Vieira S, Oliveira J. H, Lara F. Shear bond strength of resin-modified glass ionomer cement with saliva present and different enamel pretreatments. Angle Orthod. 2006;76:470–474. doi: 10.1043/0003-3219(2006)076[0470:SBSORG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Holzmeier M, Ernst C. P, Willershausen B, Hirschfelder U. In-vitro shear bond strength of self-etching versus traditional adhesives for orthodontic luting. J Orofac Orthop. 2006;67:244–259. doi: 10.1007/s00056-006-0546-4. [DOI] [PubMed] [Google Scholar]
- 16.Guidance on Testing of Adhesion to Tooth Structure. International Organization for Standardization (ISO), TR 11405. Geneva, Switzerland: International Organization for Standardization Dental Material; 1994. [Google Scholar]
- 17.Artun J. A. B. S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am J Orthod. 1984;85:333–339. doi: 10.1016/0002-9416(84)90190-8. [DOI] [PubMed] [Google Scholar]
- 18.Itoh T, Fukushima T, Inoue Y, Arita S, Miyazaki K. Effect of water, saliva and blood contamination on bonding of metal brackets with a 4-META/MMA/TBB resin to etched enamel. Am J Dent. 1999;12:229–304. [PubMed] [Google Scholar]
- 19.Saito K, Sirirungrojying S, Meguro D, Hayakawa T, Kasai K. Bonding durability of using self-etching primer with 4-META/MMA-TBB resin cement to bond orthodontic brackets. Angle Orthod. 2005;75:260–265. doi: 10.1043/0003-3219(2005)075<0256:BDOUSP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Zachrisson B. U, Buyukyilmaz T. Surface preparation for orthodontic bonding to porcelain. Am J Orthod Dentofacial Orthop. 1996;109:420–430. doi: 10.1016/s0889-5406(96)70124-5. [DOI] [PubMed] [Google Scholar]