Abstract
Objective:
To evaluate the cytotoxic effects of three different resin-modified orthodontic band adhesives.
Materials and Methods:
Three resin-modified orthodontic band adhesives (Bisco Ortho Band Paste LC™, Multi-Cure Glass Ionomer Band Cement™, and Transbond Plus Light Cure Band Adhesive™) were prepared and the samples were extracted in 3 mL of Basal Medium Eagle with 10% newborn calf serum for 24 hours. The L929 cells were plated (25,000 cells/mL) in wells of 96-well dishes and maintained in a humidified incubator for 24 hours at 37°C, 5% CO2, and 95% air. After 24-hour incubation of the cells, the incubation medium was replaced by the immersed medium in which the samples were stored. Then L929 cells were incubated in contact with eluates for 24 hours. The cell mitochondrial activity was evaluated by the methyltetrazolium test. Twelve wells were used for each specimen, and methyltetrazolium tests were applied two times. The data were statistically analyzed using one-way analysis of variance and Tukey Honestly Significantly Different tests.
Results:
Results with L929 fibroblasts demonstrated that all freshly prepared resin-modified orthodontic band adhesive materials reduced vital cell numbers (P > .05), in comparison to the control group. Our data demonstrate that all materials showed significant cytotoxicity compared to the control group.
Conclusions:
The results indicate that all materials showed significant cytotoxicity compared to the control group, and further studies using different test methods are needed for all resin-modified orthodontic band adhesives.
Keywords: Biocompatibility, Orthodontic band adhesive, Cytotoxicity
INTRODUCTION
Despite the advantage of bonded attachments, molar bands are generally used in fixed orthodontic treatment. Band cements are necessary for mechanical retention or adhesion. In addition, they also serve to seal and fill the gaps.1 Glass ionomer cements (GICs) offer considerable advantages in physical properties and provide superior clinical performance as a result of the reduced band failure they afford.2,3 Furthermore, GICs leach fluoride over prolonged periods.
A huge number of combinations of GIC and resin-based adhesives have been developed in recent years (hybrid, ionomers). Materials with similar composition are also applied as pit and fissure sealants and luting composites and are used in the bonding of brackets and orthodontic bands.4 These band adhesive materials range in composition from materials with a pronounced resin-based composite characteristic (compomers, polyacid-modified resin-based composites) and materials that are very similar to conventional GICs with an aqueous base (resin-modified GICs).4 The advantages they offer include the following: they result in improved handling characteristics, as a result of the command setting; they have a longer working time; and they display a greater tolerance to moisture. The bond strength of modified GICs is reported1,5 to be superior to that of traditional GICs.
Although development and improvements related to the resin-modified orthodontic band adhesive (RM-OBA) materials are very satisfying and amazing, the cytotoxicity of RM-OBA still is a question for orthodontists. In addition, there are no data available in the literature related to the cytotoxicity of the commercially available orthodontic band adhesives. Orthodontists are using a large variety of bonding and banding adhesives that, insofar as it is possible, must be harmless. Newer orthodontic adhesive materials present new challenges because of their potential for interaction.
No comprehensive data are available regarding the cytotoxicity of RM-OBAs. It should be critically emphasized that the manufacturers of these materials possess comprehensive test data. Therefore, the aim of the present study was to evaluate the cytotoxic effects of three different RM-OBAs that are commercially available.
MATERIALS AND METHODS
The RM-OBAs selected were Bisco Ortho Band Paste LC™ (Bisco Ortho Band; Bisco Inc, Schaumburg, Ill], 3M Unitek Multi-Cure Glass Ionomer Band Cement™ (Multi Cure GIC; 3M Unitek Ortho Prod, Monrovia, Calif), and Transbond Plus Light Cure Band Adhesive™ (Transbond Plus; 3M Unitek Ortho Products). Details related to these products and their components are listed in Table 1.
Table 1.
The Orthodontic Band Adhesives Included in the Study
Test specimens were prepared according to the manufacturers' instructions in standard Teflon discs measuring 5 mm in diameter, 1.5 mm in thickness, and 2 mm in height. All specimens were prepared and handled under aseptic conditions to limit the influence of biological contamination on the cell culture tests.
Specimens were prepared between Mylor and glass slabs to minimize the oxygen inhibition and to maximize the surface smoothness. Elipar FreeLight 2 (3M ESPE Dental Products, St Paul, Minn) (LED) was used as a light curing device, having a tip of 8 mm with a light intensity of 1200 mW/cm2 for curing light-polymerizing specimens.
Four samples were prepared for each group for cytotoxicity testing. The samples were immersed in 7 mL of culture medium for 24 hours at 37°C to extract residual monomer or cytotoxic substances. The culture medium containing material extracts was sterile and filtered to use on the cell cultures.
Cytotoxicity Testing
L929 cells (ATCC CCL 1, Şap Enstitüsü, Ankara, Turkey) were cultured in Basal Medium Eagle containing 10% newborn calf serum and 100 mg/mL penicillin/streptomycin at 37°C in a humidified atmosphere of 95% air/5% CO2. Cell cultures of between the 12 and 15 passages were used in this study. Confluent cells were detached with 0.25% trypsin and seeded at a density of 5 × 103 into each well of a 96-well plate for 24 hours at 37°C and 5% CO2. After 24 hours of incubation, the culture medium was replaced with 200 µL of culture medium containing material extracts of RM-OBA materials. The original culture medium served as a control in this study.
Cultures were incubated for 24 hours at 37°C and 5% CO2. The viability of cells exposed to material extracts was assessed using succinic dehydrogenase activity. The succinic dehydrogenase activity has been shown6 to be reasonably representative of mitochondrial activity in the cells and reflects both cell number and activity. The old medium was removed and the cell cultures were rinsed with phosphate-buffered saline, and 0.5-mL aliquots of freshly prepared 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) solution (0.5 mg/mL in Basal Medium Eagle) were added to each well.
After a 2-hour incubation period (37°C, 5% CO2) the supernatant was removed and the intracellularly stored MTT formazan was solubilized in 200 µL of dimethyl sulfoxide for 30 minutes at room temperature. The absorbance at 540 nm was spectrophotometrically measured.
The worksheets were incorporated into the software, Excel version XP, and then recalculated as follows:
where a is the OD value at 540 nm derived from a well added with a test chemical, b is the mean optical density value at 540 nm derived from blank wells, and c is the mean OD value at 540 nm derived from control wells (ie, added culture medium as a test chemical).
Twelve replicate cell cultures were exposed to each concentration of a single material in at least two independent experiments. The cell survival rates in treated groups compared to those of untreated controls. Differences between median values were statistically analyzed using the one-way analysis of variance and Tukey Honestly Significantly Different tests.
Cell survival of L929 cells in a MTT test after exposure to RM-OBA materials was measured. Data are expressed as a percentage of the control cultures. Cell survival rates were calculated from independent experimental cultures.
Cell Morphology Evaluation
Morphological alteration of L929 was observed directly using an inverted microscope (TS100 Nikon Eclipse, Tokyo, Japan) (10× magnification) and photographed by a Nikon camera.
RESULTS
Descriptive data of cell survival rates for each group are given in Table 2 and Figure 1. There were significant differences among RM-OBA in terms of cell survival percentage (P < .001).
Table 2.
The Cell Viability Percentages by 3-(4,5-Dimethyl-Thiazol-2-yl)-2,5-Diphenyl-Tetrazolium Bromide (MTT) Assay
Figure 1.
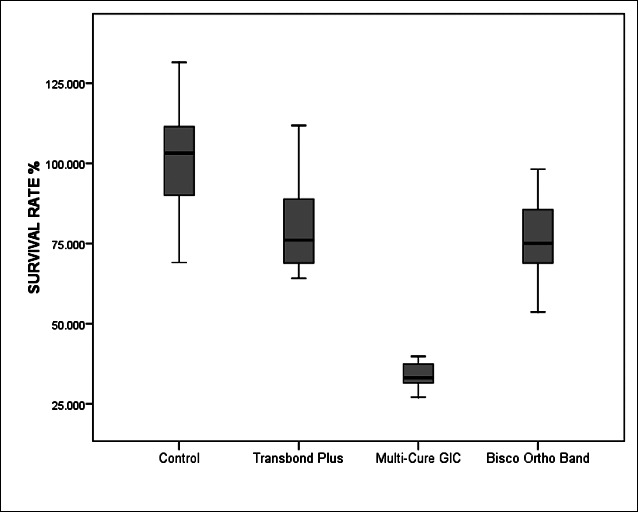
Descriptive values of cell viability by MTT assay.
All tested materials showed a significantly decreased cell survival percentage when compared to the control group (P < .001). In general, the rank order with respect to cytotoxicity was found to be as follows: Multi-Cure GIC (35.68% ± 12.47%) < Bisco Ortho Band (75.91% ± 12.47%) < Transbond Plus (78.86% ± 13.03%). Multi-Cure GIC had a statistically significantly smaller effect on cell survival and growth as compared to other adhesive systems and control groups (P < .001). Although there are no statistically significant differences between Bisco Ortho Band and Transbond Plus (P > .05), both of them were cytotoxic when compare to the control group (P < .001).
Morphological Assessment
Figures 2–5 show the characteristic appearance of the cell cultures with the use of media containing the materials tested, including normal, altered, and dead cells, as well as the individual cell density.
Figure 2.
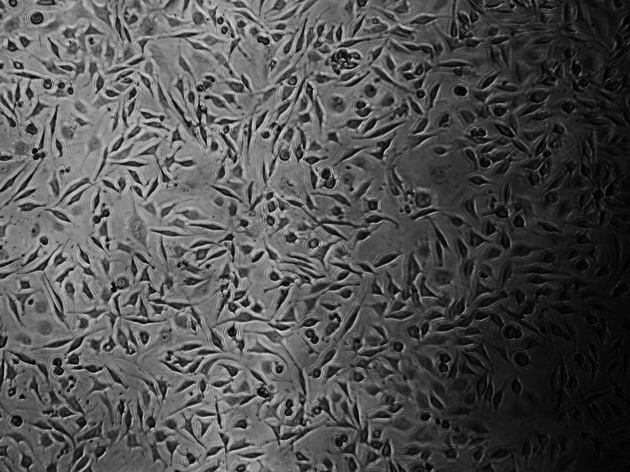
Cultured L929 cells for control group.
Figure 5.
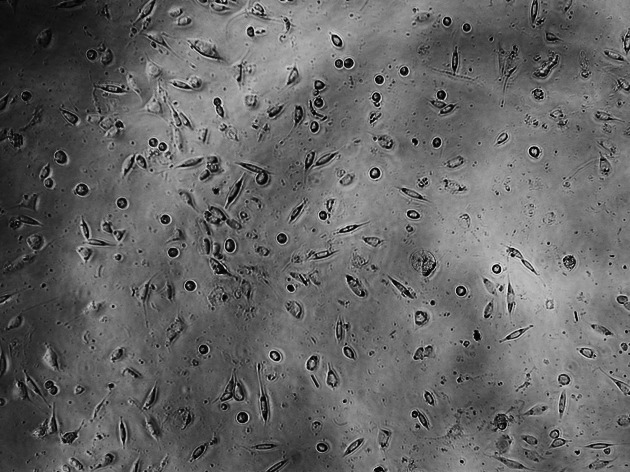
Cultured L929 cells for Multi-Cure GIC.
The control culture displays regular, dense L929 cells, which were elongated and spindle-shaped in appearance, with long slender cells and numerous mitoses (Figure 2). With the Transbond Plus, the L929 culture appears less dense than in the control culture, but normal cells were available. There are only a few rounded or dead fibroblasts. While Transbond Plus led to enlargement of the intercellular space, the cells kept their spindle shape. But the cell densities were decreased when compared with the control (Figure 3). The cell culture exposed to media containing Bisco Ortho Band appears less dense than the control. Degenerative effects such as retraction fibers and rounded cells are visible, and some L929 are in the process of dying. The cells were retracted and were rounded in appearance, which led to enlargement of the intercellular space, while the cells did not show any differences when compared with the control (Figure 4). However, all L929 cells exposed to media containing Multi-Cure GIC are in the process of dying; many cells are already rounded or float in the culture medium. The cells were significantly retracted, rounded, and also increased the intercellular space in the Multi-Cure GIC group (Figure 5).
Figure 3.
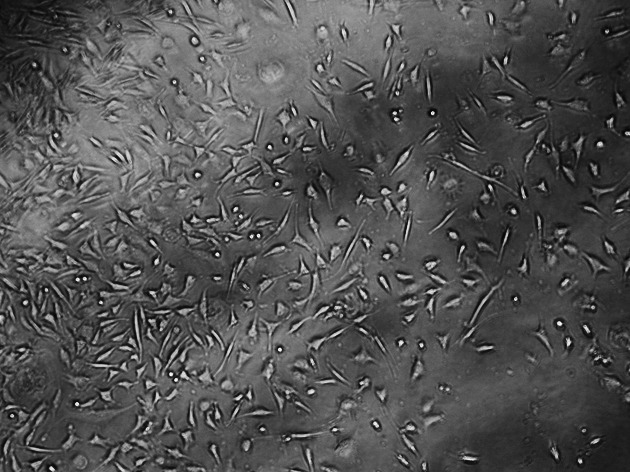
Cultured L929 cells for Transbond Plus.
Figure 4.
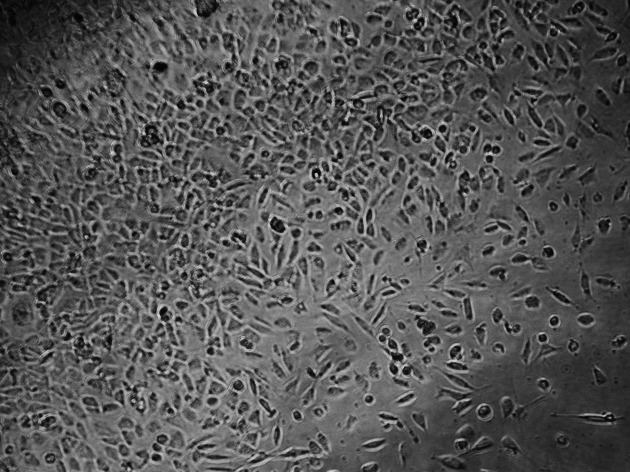
Cultured L929 cells for Bisco Ortho Band.
DISCUSSION
Recent data indicate that some RM-OBA materials produce toxic reactions in cell culture. In the present study the effects of three different RM-OBAs on L929 fibroblasts were investigated with the same standardized MTT assay test system. The investigation obviously showed that the Multi-Cure GIC was more toxic than other tested materials. In addition, Bisco Ortho Band and Transbond Plus were also cytotoxic materials, when compared to the control.
Generally these materials are the most recent generation of RM-OBA, and they are very commonly used in orthodontic clinics. The choice to “cure on demand” has led an increasing number of orthodontist to use RM-OBAs instead of the more traditional liquid-powder cements that require in-office mixing. By now the light-initiated resins have become the most popular adhesives for a majority of orthodontists.7
Photo-activated RM-OBAs are the bonding agent of choice for orthodontic banding because of their ease of use and the extended time they allow for band placement. The development of light-activated RM-OBAs is an active area in orthodontic research, and manufacturers are continuously focused on modifying their formulation for superior physical and mechanical properties. The more rapid setting reaction and the higher bond strength to enamel of RM-OBAs, compared with the conventional formulations, make them very attractive materials for orthodontic applications.8 But what about the biocompatibility or cytotoxicity of RM-OBAs? To our knowledge, no research in the orthodontic literature has investigated the biocompatibility or cytotoxicity associated with using RM-OBAs.
It is very important to evaluate the cytotoxicity of RM-OBAs, because these materials are located proximate to periodontal tissue and alveolar bone. Substances released from these adhesives may cause a reaction (inflammation or necrosis) in adjacent tissues, such as oral mucosa and gingiva, or alveolar bone. There are several ways in which these materials may influence the health of soft tissues, by delivering water-soluble components into the saliva and the oral cavity as well as by interaction directly with adjacent tissues.9 In addition, there are studies in conservative or operative dentistry about resin-based dental cements, and the authors10,11 of some of these reports have suggested that these materials are very cytotoxic on pulpal or gingival tissue. When considering the amount of material used clinically, RM-OBA is used much more frequently than other dental cements, and when placed in the oral cavity, these agents come into contact with the gingival or other oral mucosa. The extraction assay is one of the most frequently used methods to assay the mechanism of cytotoxicity in the study of RM-OBAs.
Another important topic—the toxicity of materials—is also relevant to the practitioner from the standpoint of the health of the dental team. In many cases, the risk of the adverse effects of biomaterials is much greater for the dental team than for the patients, because the dental team is chronically exposed to the materials during manipulation of the materials when they are being placed, set, or removed. Furthermore, there is information indicating that the dental materials used by orthodontists can pose some risk to the patient and dental team. It is the orthodontic clinicians' problem to decide whether this evidence is deserved and to estimate the risk of these materials in orthodontic practice.6
In traditional GIC formulations, vacuum-dried polyacrylic acid is also incorporated into the glass powder. Tap water or distilled water is used for the mixing of GICs. Important modifications have been made in the liquid component of the light-cured resin-based GICs. The most prominent changes are the replacement of water by a water-HEMA (Hyroxyethyl Methacrylate) mixture and the incorporation of photoinitiators and/or chemical initiators for free radical polymerization.8 In some materials, methacrylate-based monomers such as Bisphenol-A diglycidyl dimethacrylate, triethylene glycol dimethacrylate (TEGDMA), and UDMA (Urethane Dimethacrylate) are added to the polyacrylic acid solution.8 Polymerization in products used today is mainly initiated by light; the light-sensitive initiator camphorquinone acts together with an aliphatic amine-type catalyst. TEGDMA has an important function, because it decreases the viscosity of the matrix. More recent resin-based adhesives are based on a Si-O scaffold with methacrylic side chains, which are necessary for polymerization.12 In addition, resin-modified adhesives are light-curable, and the setting of resin-modified adhesives is a highly exothermic reaction. Some of these products generate more heat than resin-based composites, perhaps as a result of a high concentration of HEMA.13
Furthermore, various ions are leached out at different times and in different conditions. Recently, data from animal studies concerning biodegradation of HEMA/TEGDMA have been presented.14,15 Both “water-soluble” substances are used in a variety of resin-based adhesives (HEMA/TEGDMA) and thus are released from materials. Swallowed HEMA/TEGDMA were almost completely absorbed by the organism. These ions are released from orthodontic resin-based adhesives and their diffusion through oral tissues, and these components are cytotoxic. In addition, early Bisphenol-A exposure can influence several mechanisms important for body weight regulation, including adipocyte deposition, glucose uptake and homeostasis, and the development and maturation of pathways and circuits important for energy homeostasis.16
When evaluating the ingredient of tested RM-OBA in the present study, there are significant differences in resin matrixes. Bisco Ortho Band contains Bisphenol-A diglycidyl dimethacrylate, Multi-Cure GIC contains HEMA, and Transbond Plus contains 2-hydroxy-1,3-dimethacryloxypropane. In addition, the filler weights of these materials are different. Multi-Cure GIC showed minimal filler weight compare to other tested materials. Hence, high cytotoxicity of Multi-Cure GIC could be explained by the presence of HEMA in organic matrix and low filler weight.
The results of this study showed that light-cured orthodontic band adhesives do not have acceptable biocompatibility, when compared to the control group. This issue may be explained by considering the combination of orthodontic adhesives' monomers, content, and filler weight. Furthermore, clinically inadequate polymerization would be a problem, because of the difficulties associated with illuminating the RM-OBA evenly from each side of the band and of reaching everywhere in the oral cavity with the light source. Hence, considerable residual monomer may be found between tooth and band; thus, inadequate polymerization of resin matrix of the orthodontic band adhesive results in inferior physico-mechanical properties and also superior cytotoxicity.
In orthodontic practice, alternatives to RM-OBAs are traditional GICs and zinc phosphate cements. Different zinc phosphate cements have been revealed to be highly cytotoxic immediately after mixing.17 Completely set specimens that were eluted in 0.9% saline solution or in cell culture medium for 7 days were not cytotoxic (cell culture medium) or were only slightly cytotoxic (saline solution) in cultures of periodontal ligament fibroblasts. Toxic reactions were observed4 in a permanent growing cell line (3T3 mouse fibroblasts). Schmalz4 also documented that the toxicity of zinc phosphate cements in mouse fibroblast cultures and periodontal ligament fibroblast cell cultures was dependent on the setting time.
Kasten et al.18 compared the in vitro biocompatibility of an experimental fluoride composite resin with fluoride-releasing and non–fluoride-releasing materials. They used a human gingival epithelial cell line, and the biocompatibility was evaluated by counting the viable cells. According to the results of this study, a traditional GIC exhibited the least toxicity, and cell viability was approximately 90% in this GIC group. The cytotoxicity of conventional GICs has been investigated in different studies19–21 using different cell types. Interestingly, one specific GIC product, Ketac Cem™, caused no alterations in cell morphology, but inhibited RNA and protein synthesis of the treated gingival fibroblasts. Epithelial cells were unaffected.4,19 In contrast to zinc phosphate cement, there was no histological inflammatory response observed when GIC was brought into contact with the oral mucosa.4 Clinical studies also showed that GIC is not damaging to the oral mucosa. Optimally shaped GIC fillings that were in contact with the gingival mucosa caused no increased inflammation, compared with control teeth with no restorations.4,22
When one takes these facts into account, the clinical relevance of the results of the present in vitro study remain unclear, and further investigative further studies using different test methods are needed for Multi-Cure GIC, Bisco Ortho Band, and Transbond Plus. Research efforts should focus on assessing the long-term biological effects of orthodontic composites.
CONCLUSION
The tested orthodontic resin-based band adhesives all caused significant cellular alterations.
Acknowledgments
The authors are grateful to Pasa Dental, Medifarm, who donated the orthodontic band adhesive materials used in this study.
REFERENCES
- 1.Johnson N. Orthodontic banding cements. J Orthod. 2000;27:283–284. doi: 10.1179/ortho.27.3.283. [DOI] [PubMed] [Google Scholar]
- 2.Millett D. T, Hallgren A, McCluskey L. A, et al. A clinical retrospective evaluation of 2 orthodontic band cements. Angle Orthod. 2001;71:470–476. doi: 10.1043/0003-3219(2001)071<0470:ACREOO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Stirrups D. R. A comparative clinical trial of a glass ionomer and a zinc phosphate cement for securing orthodontic bands. Br J Orthod. 1991;18:15–20. doi: 10.1179/bjo.18.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Schmalz G. Cements and ceramics. In: Schmalz G, Arenholt-Bindlev D, editors. Biocompatibility of Dental Materials. Berlin, Germany: Springer; 2009. pp. 139–187. [Google Scholar]
- 5.Mennemeyer V. A, Neuman P, Powers J. M. Bonding of hybrid ionomers and resin cements to modified orthodontic band materials. Am J Orthod Dentofacial Orthop. 1999;115:143–147. doi: 10.1016/s0889-5406(99)70341-0. [DOI] [PubMed] [Google Scholar]
- 6.Wahata J. C. Principles of biocompability. In: Brantley W. A, Eliades T, editors. Orthodontic Materials: Scientific and Clinical Aspects. Stuttgart, Germany: Thieme; 2001. pp. 271–286. [Google Scholar]
- 7.Malkoc S, Uysal T, Usumez S, Isman E, Baysal A. In vitro assessment of temperature rise in the pulp during orthodontic bonding. Am J Orthod Dentofacial Orthop. 2010;137:379–383. doi: 10.1016/j.ajodo.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 8.Eliades T, Eliades G. Cements in orthodontics. In: Brantley W. A, Eliades T, editors. Orthodontic Materials: Scientific and Clinical Aspects. Stuttgart, Germany: Thieme; 2001. pp. 221–240. [Google Scholar]
- 9.Huang T. H, Tsai C. Y, Chen S. L, Kao C. T. An evaluation of the cytotoxic effects of orthodontic bonding adhesives upon a primary human oral gingival fibroblast culture and a permanent, human oral cancer-cell line. J Biomed Mater Res. 2002;63:814–821. doi: 10.1002/jbm.10412. [DOI] [PubMed] [Google Scholar]
- 10.Ulker H. E, Sengun A. Cytotoxicity evaluation of self adhesive composite resin cements by dentin barrier test on 3D pulp cells. Eur J Dent. 2009;3:120–126. [PMC free article] [PubMed] [Google Scholar]
- 11.Koulaouzidou E. A, Helvatjoglu-Antoniades M, Palaghias G, Karanika-Kouma A, Antoniades D. Cytotoxicity of dental adhesives in vitro. Eur J Dent. 2009;3:3–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Schmalz G. Resin-based composites. In: Schmalz G, editor. Biocompatibility of Dental Materials. Berlin, Germany: Springer; 2009. pp. 99–138. [Google Scholar]
- 13.Kanchanavasita W, Pearson G. J, Anstice H. M. Temperature rise in ion-leachable cements during setting reaction. Biomaterials. 1995;16:1261–1265. doi: 10.1016/0142-9612(95)98134-z. [DOI] [PubMed] [Google Scholar]
- 14.Reichl F. X, Durner J, Kunzelmann K. H, et al. Biological clearance of TEGDMA in guinea pigs. Arch Toxicol. 2001;75:22–27. doi: 10.1007/s002040000159. [DOI] [PubMed] [Google Scholar]
- 15.Reichl F. X, Durner J, Manhart J, et al. Biological clearance of HEMA in guinea pigs. Biomaterials. 2002;23:2135–2141. doi: 10.1016/s0142-9612(01)00344-1. [DOI] [PubMed] [Google Scholar]
- 16.Rubin B. S, Soto A. M. Bisphenol A: perinatal exposure and body weight. Mol Cell Endocrinol. 2009;304:55–62. doi: 10.1016/j.mce.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanks C. T, Anderson M, Craig R. G. Cytotoxic effects of dental cements on two cell culture systems. J Oral Pathol. 1981;10:101–112. doi: 10.1111/j.1600-0714.1981.tb01255.x. [DOI] [PubMed] [Google Scholar]
- 18.Kasten F. H, Pineda L. F, Schneider P. E, Rawls H. R, Foster T. A. Biocompatibility testing of an experimental fluoride-releasing resin using human gingival epithelial cells in vitro. In Vitro Cell Dev Biol. 1989;25:57–62. doi: 10.1007/BF02624411. [DOI] [PubMed] [Google Scholar]
- 19.Caughman W. F, Caughman G. B, Dominy W. T, Schuster G. S. Glass ionomer and composite resin cements: effects on oral cells. J Prosthet Dent. 1990;63:513–521. doi: 10.1016/0022-3913(90)90067-m. [DOI] [PubMed] [Google Scholar]
- 20.Muller J, Bruckner G, Kraft E, Horz W. Reaction of cultured pulp cells to eight different cements based on glass ionomers. Dent Mater. 1990;6:172–177. doi: 10.1016/0109-5641(90)90024-9. [DOI] [PubMed] [Google Scholar]
- 21.Schmalz G, Thonemann B, Riedel M, Elderton R. J. Biological and clinical investigations of a glass ionomer base material. Dent Mater. 1994;10:304–313. doi: 10.1016/0109-5641(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 22.van Dijken J. W, Sjostrom S. The effect of glass ionomer cement and composite resin fillings on marginal gingiva. J Clin Periodontol. 1991;18:200–203. doi: 10.1111/j.1600-051x.1991.tb01134.x. [DOI] [PubMed] [Google Scholar]