Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a disease of irreversible airways obstruction in which patients often suffer exacerbations. Sometimes these exacerbations need hospital care: telehealthcare has the potential to reduce admission to hospital when used to administer care to the pateint from within their own home.
Objectives
To review the effectiveness of telehealthcare for COPD compared with usual face‐to‐face care.
Search methods
We searched the Cochrane Airways Group Specialised Register, which is derived from systematic searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO; last searched January 2010.
Selection criteria
We selected randomised controlled trials which assessed telehealthcare, defined as follows: healthcare at a distance, involving the communication of data from the patient to the health carer, usually a doctor or nurse, who then processes the information and responds with feedback regarding the management of the illness. The primary outcomes considered were: number of exacerbations, quality of life as recorded by the St George's Respiratory Questionnaire, hospitalisations, emergency department visits and deaths.
Data collection and analysis
Two authors independently selected trials for inclusion and extracted data. We combined data into forest plots using fixed‐effects modelling as heterogeneity was low (I2 < 40%).
Main results
Ten trials met the inclusion criteria. Telehealthcare was assessed as part of a complex intervention, including nurse case management and other interventions. Telehealthcare was associated with a clinically significant increase in quality of life in two trials with 253 participants (mean difference ‐6.57 (95% confidence interval (CI) ‐13.62 to 0.48); minimum clinically significant difference is a change of ‐4.0), but the confidence interval was wide. Telehealthcare showed a significant reduction in the number of patients with one or more emergency department attendances over 12 months; odds ratio (OR) 0.27 (95% CI 0.11 to 0.66) in three trials with 449 participants, and the OR of having one or more admissions to hospital over 12 months was 0.46 (95% CI 0.33 to 0.65) in six trials with 604 participants. There was no significant difference in the OR for deaths over 12 months for the telehealthcare group as compared to the usual care group in three trials with 503 participants; OR 1.05 (95% CI 0.63 to 1.75).
Authors' conclusions
Telehealthcare in COPD appears to have a possible impact on the quality of life of patients and the number of times patients attend the emergency department and the hospital. However, further research is needed to clarify precisely its role since the trials included telehealthcare as part of more complex packages.
Plain language summary
Telehealthcare for COPD ‐ bronchitis and emphysema
The smoking related diseases of bronchitis and emphysema are now considered under the umbrella term of chronic obstructive pulmonary disease, COPD. This is because they are diseases which leave people breathless and often with a cough and increased phlegm. Such people often have times when their COPD worsens and they cannot "get their breath" and have to go into hospital for treatment. It is very expensive to look after people this way and often they do not want to spend time in hospital but there are few alternatives. Telehealthcare involves using technology such as telephones, video cameras and the Internet to allow people to stay at home and communicate with a nurse or doctor when they have a period of increased breathlessness. The professional can obtain information from the patient to allow them to prescribe treatments and monitor the patient closely without them having to go into hospital or to the emergency department. This study shows that people treated this way do manage to stay out of hospital longer than people treated by conventional systems of care. There are also some data showing that although these systems are expensive to start off with, if they are successful at keeping people out of hospital, then the cost saving from this means that they are cheaper in the long run.
Background
Description of the condition
The Global Initiative for Obstructive Lung Disease defines Chronic Obstructive Pulmonary Disease, COPD, as follows:
"COPD is a preventable and treatable disease with some significant extrapulmonary effects that may contribute to the severity in individual patients. Its pulmonary component is characterized by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gases." (GOLD 2007)
COPD is an increasingly important disease globally. It is a classical gene X environment disease, meaning that genetic susceptibility is influenced by environmental factors resulting in the disease. However, thinking about the pathogenesis of COPD has rapidly evolved recently. Traditionally COPD has been considered to be a disease of smokers. Long term lung damage results from exposure to toxins in cigarette smoke and there is a loss of the elasticity of the lung tissue. Recently there has been a broadening of the concept of COPD to include the influence of other environmental factors including exposure to industrial and biomass fuel toxins (Mannino 2007).
The major feature of COPD is airflow limitation, measured and classified using spirometry. As lung function declines from its peak in the 3rd or 4th decade, and it progresses with age, it often results in severe limitations to function once the patient is elderly. In people with underlying susceptibility and smoke or dust exposure the disease is frequently life‐limiting.
In addition, the extrapulmonary effects of COPD are increasingly being seen as part of a systemic disease (The Lancet 2007). These include muscle wasting, cardiovascular disease, depression, reduced fat free mass, osteopenia and chronic infections. There is increasing recognition that patients are more likely to die from these co‐morbidities than from the COPD alone.
The prevalence of COPD is very variable across different populations and global estimates of morbidity and mortality are often underestimates due to poor recognition of COPD as a contributor to the events (Mannino 2007).
In some countries, such as the UK (Pride 2002), there is good evidence that hospital admissions for exacerbations and home oxygen supplies account for significant healthcare costs.
The clinical course of COPD is that of an increasingly debilitated trajectory as a result of airflow limitation. This is punctuated by acute exacerbations of "shortness of breath" with increased sputum production and reduced exercise tolerance. Often during these exacerbations the patient is admitted to hospital for antibiotics and inhaled therapies including oxygen support. It may take a number of weeks for the patient to recover during which time they lose muscle mass as a result of reduced activity. The rehabilitation of the patient takes time and often does not achieve the level of functioning they had before they were admitted to hospital. The later years of COPD illness are often characterised by repeated increasing periods spent in hospital (GOLD 2007).
Description of the intervention
It is clear that the terminology of "telehealthcare", "telemedicine", and "telehealth" is growing rapidly and that there is significant overlap between these terms (Busey 2008; HRSA; Mahen 2006). For the purposes of this review we have chosen to describe the interventions undergoing study as "telehealthcare". This term most usefully encompasses a number of technologies previously described under various terms such as "telehealth", "telemedicine", "telecare" and "telenursing".
The American Nursing Association argues that when a nurse is providing care, "telenursing" is interchangeable with "telehealth" because nurses still follow nursing processes by formulating care plans and providing care using their nursing knowledge and skills. For example, in videoconferencing this includes communication skills such as picking up subtle clues from a patient's tone of voice and facial expression (Lorentz 2008).
We favour "telehealthcare" over the terms telemedicine and telenursing as these latter terms imply a specific professional delivering the healthcare. Such roles are increasingly blurred and politicised and trying to distinguish between them is therefore not particularly helpful. This intervention has as its focus the disease of COPD and the aim of the studies should be to improve the patient's COPD with the help of a doctor, nurse or allied healthcare professional.
"Telehealthcare" has the following elements (adapted from Miller 2007):
information from the patient whether voice, video, other audio, oxygen saturation, breath sounds or other;
electronic transfer of such information over a distance;
there is personalised feedback from a healthcare professional who exercises their skills and judgement in the giving of tailored advice to the patient.
Interventions captured within the terms telehealthcare include synchronous interactions, e.g. telephone and videoconferencing enabled consultations; and asynchronous care using store and forward technology, e.g. storing two weeks worth of spirometry results then sending them on to a nurse who replies by email or telephone.
We use telehealthcare to mean business to consumer or B2C communication: i.e. communication involving an interaction between a health professional and a patient with COPD. This is as opposed to business‐to‐business communication or "B2B communication" which might involve intraprofessional communication for second opinions, this is sometimes referred to as telemedicine and is beyond the scope of our definition.
Also beyond the scope of our definition is passive information provision, e.g. online education, where a healthcare professional is not involved in an exchange with the patient.
How the intervention might work
Telehealthcare is a complex intervention as defined by the MRC and therefore it can be difficult to pin down exactly what is the "active ingredient" of the intervention. Typically, in COPD, there are a number of ingredients including some education, some assisted planning, emotional support, pragmatic advice, monitoring with equipment, etc. which, taken together, may or may not benefit the patient. We see the purpose of this review as being to establish whether or not telehealthcare has a positive impact. Further, theoretical work, including qualitative studies will be required to unpick precisely how any effect is delivered. However, we have so far found the following potential mechanisms through which quality of care may be enhanced and cost savings achieved through the use of telehealthcare, as adapted from Finkelstein 2000:
providing patient education and counselling for primary prevention and early detection of COPD evolution;
improving adherence to medications and other treatment regimens;
collecting patient data remotely;
replacing face‐to‐face nursing/physician visits;
providing early detection of incipient disease exacerbation and timely intervention for early symptom management;
reducing unscheduled/unnecessary visits to the physician and emergency room;
preventing and reducing repeat hospitalisations.
Why it is important to do this review
In the economically‐developed world many electronic tools for remotely helping patients with COPD are now being implemented in the absence of an explicit evidence base. Exploration of the existing evidence of the values or risks of these interventions is therefore urgently required. A recent Cochrane assessment of teleconsultations compared with face‐to‐face consultations found little evidence of clinical benefit, variable and inconclusive results for other outcomes (psychological measures) and no analysable data of cost‐effectiveness. The authors concluded that further research is clearly required (Currell 2008). Mair 2000b performed a systematic review regarding patient satisfaction with telehealthcare and found that it was not clear precisely when telehealthcare might be a feasible alternative for consultation or how telehealthcare might impact on the patient's relationship with their healthcare professional. Thus, the benefits of telehealthcare interventions are unquantified.
In addition, it is necessary to consider that the world's rapidly aging population means that there will be a stretching of health and social care resources (Darzi 2008). Healthcare delivery needs to become much more efficient. It is hoped that telehealthcare will help meet such needs. Part of the rationale for telehealthcare is that long term running costs may be lower than in conventional care because early disease could be detected and treated, thereby preventing ensuing morbidity and hospitalisations. In the case of COPD, the potential exists to manage patients with exacerbations largely in their own homes, thereby saving on hospitalisation costs. Initial start‐up costs of telehealthcare are thought to be substantial. The number and quality of cost‐effectiveness studies regarding telehealthcare so far provide inadequate evidence of it as a cost‐effective means of delivering healthcare (Whitten 2002).
In addition, COPD can be an extremely debilitating disease and patients with COPD can have limited quality of life. Telecommunications such as those used in telehealthcare programmes hold some promise for releasing such people from illness‐imposed isolation.
Objectives
To review the effectiveness of telehealthcare for COPD compared with face‐to‐face usual care in improving quality of life and reducing accident and emergency department visits and hospitalisations.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials which compared a telehealthcare intervention with a control group. We did not expect studies to be blinded as participants were necessarily aware of the interventions.
Types of participants
We chose studies where the focus was participants with COPD, as diagnosed by a clinician, with no exclusions on the basis of age, gender, ethnicity or language spoken. We considered both primary and secondary care settings. We did not include studies of people with asthma only.
Types of interventions
Interventions included the following examples of where pathways had been set up using a telehealthcare mechanism. There was a focus on the proactive use of technology to provide the information the healthcare professional required to make their decisions. The technology is central and its use was sustained.
Video or telephone links with healthcare professionals in real time or using store and forward technologies.
Systems of care using Internet‐based telecommunication with healthcare professionals.
Systems of care using both wired and wireless telemetry for telemonitoring of spirometry (FEV1/FVC), respiratory rate, blood pressure and oxygen saturations involving feedback to the patient, which has been processed or authorised by a healthcare professional.
Other systems of remote healthcare.
Complex intervention studies if it is possible to tease out the individual telehealthcare elements.
Interventions in all settings and from all types of healthcare provider.
Excluded interventions would include:
Telehealthcare which is only educational without the input of a professional, e.g. in an emergency waiting room.
Decision support which functions without the input of a healthcare professional.
Types of outcome measures
Primary outcomes
Total exacerbations.
Quality of life (e.g. St George's Respiratory Questionnaire).
Emergency Department visits.
Hospitalisations.
Deaths.
Secondary outcomes
FEV1.
FVC.
Patient satisfaction.
Study withdrawal.
Costs.
Cost effectiveness.
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module for further details).
All records in the Specialised Register coded as 'COPD' were searched using the following terms:
telehealth* or tele‐health* or telemedicine* or tele‐medicine* or Internet* or computer* or web* or interactive* or telecommunication* or telephone or phone or SMS or tele‐monitor* or telemonitor* or telemanagement or tele‐management or teleconsultation or tele‐consultation or telecare* or tele‐care* or telematic* or telepharmacy or tele‐pharmacy or telenurs* or tele‐nurs* or video or email or e‐mail or "remote consult*" or wireless or bluetooth or tele‐homecare or telehomecare or "remote care" or tele‐support or telesupport or "mobile healthcare" or "computer mediated therapy" or ehealth or e‐health or mhealth or m‐health
The search has been conducted up to January 2010.
Searching other resources
In an attempt to uncover any additional relevant published data, grey literature, unpublished data, and research in progress, we:
• contacted authors of the identified articles and asked to identify other published and unpublished randomised controlled trials (see Table 1); • searched the references of all included articles for further randomised controlled trials; • searched the UK's National Research Register: https:// portal.nihr.ac.uk/ Pages/ NRRArchive.aspx ; • searched websites listing ongoing trials: http://clinicaltrials.gov/, http:// www.controlled‐trials.com/ and http://www.actr.org.au/.
1. Author contact table.
| Name of author | Date contacted | Reply |
| Bourbeau | 03‐Mar‐09 | ‐ |
| Casas Troosters | 03‐Mar‐09 | ‐ |
| Chandler | 14‐Jan‐10 | ‐ |
| de Toledo | 14‐Jan‐10 | 19‐Jan‐10 |
| Finkelstein | 03‐Mar‐09 | ‐ |
| Garcia‐Aymerich | 14‐Jan‐10 | ‐ |
| Johnston | 14‐Jan‐10 | ‐ |
| Nguyen | 03‐Mar‐09 | 18‐Mar‐09 |
| Whitten | 03‐Mar‐09 | ‐ |
| Wong | 03‐Mar‐09 | ‐ |
| Vitacca | 20‐May‐10 | ‐ |
Data collection and analysis
Selection of studies
The search strategy above was implemented by SM and JL with support from Liz Arnold (trials search co‐ordinator in the Cochrane Airways Group) and references imported to Endnote and duplicates deleted. SM and JL independently checked the titles and abstracts of potentially eligible studies. We obtained full text copies of potentially relevant studies and SM and JL assessed their eligibility for inclusion against the criteria outlined above. Disagreements were resolved through discussion. We set out reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
Data were extracted by two independent reviewers (SM and UN) from selected studies using a customised data extraction form. The following data were extracted:
country and setting;
design (description of randomisation, blinding if possible, number of study centres and location, number of study withdrawals);
participants (N, mean age, age range of the study gender ratio, baseline lung function);
system of telehealthcare being investigated, intervention and control description (provider, material delivered, control intervention (if any), duration, level of interactivity with professionals);
outcomes and definitions of outcomes used;
proportion of participants with follow‐up data;
any harms or adverse effects;
sources of funding.
Assessment of risk of bias in included studies
We assessed the quality of each trial following the Cochrane approach using the methods detailed in section six of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We concentrated on the following parameters to assess quality:
Was the allocation sequence adequately generated?
Was allocation adequately concealed?
Was knowledge of the allocated intervention adequately prevented during the study? (Blinding?)
Were incomplete outcome data adequately addressed?
Are reports of the study free of any suggestion of selective outcome reporting?
Was the study apparently free of other problems that could put it at a high risk of bias?
Each parameter was judged to be at high, low or unclear risk of bias.
Measures of treatment effect
Data were combined using Review Manager 5 software. We used a fixed‐effect standard mean difference for continuous data variables, such as scores from St Georges Respiratory Questionnaire and Chronic Respiratory Questionnaire. We used a fixed‐effect odds ratio for dichotomous variables in the absence of significant heterogeneity (I2< 40%; random‐effects meta‐analysis would have been undertaken if the I2was > 40%). We aimed to conduct an intention‐to‐treat analysis, i.e. including all those randomised to their original groups, whether or not they remained in the study.
For the primary outcome of exacerbations we calculated a number needed to treat to benefit (NNTB) for the different levels of risk, as represented by the usual care group event rates over a specified time period.
Unit of analysis issues
Odds ratios were used in calculations relating to dichotomous outcomes such as patients with one or more exacerbations. Standardised mean differences were used in relation to continuous outcomes such as score value on the St Georges Respiratory Questionnaire.
Dealing with missing data
We would have reported and investigated missing data, where possible. We would have contacted the study authors if necessary.
Assessment of heterogeneity
Statistical variation between the combined studies was measured using the I2 statistic (Higgins 2008). If this had exceeded 40% we would have investigated the potential causes of heterogeneity through subgroup analyses.
Assessment of reporting biases
We would have used funnel plots and associated statistical models to assess possible reporting bias, had there been a sufficiently large number of studies in the meta‐analysis for it to be meaningful. This was not the case as our largest meta‐analysis contained only three studies.
Data synthesis
Data were synthesised into Forest plots and where possible a meta‐analysis performed to provide the best summary estimates.
Subgroup analysis and investigation of heterogeneity
We planned to analyse subgroups according to age, socioeconomic status, ethnicity and type of intervention, had the data been available.
Sensitivity analysis
We planned to conduct sensitivity analysis on the basis of risk of bias in studies and methods of data analysis (fixed‐ and random‐effects models).
Results
Description of studies
See Characteristics of included studies table for details of included studies. The two largest studies which contributed to the results of this review and which featured most heavily in the Forest plots, i.e. Bourbeau 2003 and Casas 2006, both involved telehealthcare as part of a complex intervention, of which telehealthcare was only part, and so teasing out exactly which aspects of the complex intervention were responsible for the results remains to be done. In Bourbeau 2003, patients in the intervention group had access to a complete COPD management programme with skills taught by nursing and physiotherapy COPD case managers who saw the patients at regular intervals face‐to‐face as well as conducting telephone follow‐ups. Casas 2006 included an integrated care intervention which was supported and co‐ordinated by telecommunications. The patients saw case managers face‐to‐face for education before discharge, then following discharge co‐ordination of their care was enhanced by the use of a web‐based tool and the telephone. These are state‐of‐the‐art integrated chronic illness care plans and the contribution of telecommunications per se is very difficult to determine. It remains uncertain whether any improvements are due to the nature of the complex intervention regardless of the nature of the technology used to deliver it. The Nguyen 2008 study was the only one which appears to address the telecommunications technologies as independent factors.
Results of the search
We identified 10 randomised controlled trials reported in 12 papers which satisfied the inclusion criteria, involving 1004 patients (Bourbeau 2003; Casas 2006; Garcia‐Aymerich 2007; Chandler 1990; de Toledo 2006; Finkelstein 2004; Finkelstein 2006; Johnston 2000; Nguyen 2008; Vitacca 2009; Whitten 2007; Wong 2005; see Figure 1). Finkelstein 2004 was related to the Finkelstein 2006 paper in that the latter had an extra outcome measure but was the same study. Casas 2006 was an extended reporting of the Garcia‐Aymerich 2007 paper including extra data. . Of the 1004 participants involved, the vast majority had COPD except for in two studies. Where these studies did not specify the exact number of patients with COPD we wrote to the authors to request clarification. Authors who responded are acknowledged in Table 1.
1.

Included studies
The participants, interventions and outcomes are listed in the Characteristics of included studies table. Three studies (Bourbeau 2003; Chandler 1990; Wong 2005) used the telephone system. Casas 2006/Garcia‐Aymerich 2007; Nguyen 2008; Vitacca 2009 used the Internet. de Toledo 2006 used a specialist independent network with video and Johnston 2000; Finkelstein 2004/Finkelstein 2006;and Whitten 2007 used videoconferencing. Of the videoconferencing studies, Finkelstein 2004/Finkelstein 2006 also used other physiological telemonitoring systems alongside the videoconferencing. We found details of 21 ongoing or unpublished trials: these are listed in the description of ongoing studies and description of studies awaiting classification.
Excluded studies
We excluded studies if they were not randomised controlled trials and if they were not COPD‐focused or did not fit our inclusion criteria. These studies are listed in the Characteristics of excluded studies table.
Risk of bias in included studies
Allocation
Three of the studies were randomised using computer generated random numbers (Nguyen 2008; Vitacca 2009; Wong 2005), and a further six stated that they were randomised but did not describe the method (Bourbeau 2003; Chandler 1990; de Toledo 2006; Finkelstein 2004/Finkelstein 2006; Johnston 2000; Whitten 2007). The Casas 2006/Garcia‐Aymerich 2007 study used a 2:1 randomisation ratio during the first three months of the study at its Barcelona site. The effect of this difference is to make interpretation more difficult.
Blinding
It was not possible to blind the patients to treatment allocation due to the interactive nature of the intervention. However, some attempt to blind outcome assessors to the participants' allocation was made in Bourbeau 2003 and Chandler 1990. None of the other studies blinded outcome assessors or data analysers.
Incomplete outcome data
Incomplete outcome data was a feature in the following trials: in Casas 2006/ Garcia‐Aymerich 2007 only 57% of patients finished the intervention arm at 12 months and so an intention‐to‐treat analysis could not be performed. In Finkelstein 2006, a reduced number of patients finished the study protocol. Whitten 2007 had a problem with turnover of study nurses and nurses being reluctant to record study data and so complete data were only available for 37 intervention patients and 30 in the control group. In the Wong 2005 study, data for missing patients were replaced with the mean of the other participants' scores: this is not necessarily a valid method to replace data.
Selective reporting
Chandler 1990 did not report on several outcomes. Finkelstein 2004 selected which outcomes on which to report thus potentially biasing the reader to anticipate positive information. The majority of papers reported according to their methods section. No protocols were sought with which to compare the results sections.
Other potential sources of bias
There was a risk of selection bias in Bourbeau 2003. Casas 2006/Garcia‐Aymerich 2007 did not have other sources of bias. Participation bias was a risk in de Toledo 2006 as people in the intervention arm may have felt better cared for. In Finkelstein 2004/Finkelstein 2006 the early reporting of favourable outcomes was probably the most important source of bias. Interventions were made in both groups in the Nguyen 2008, i.e. one group was introduced to an electronic dyspnoea management program and one group was introduced to a face‐to‐face programme. This brings the validity of this face‐to‐face programme as a control into question because it is also a change to the normal procedure of care and so could be considered to be an intervention ‐ albeit a non‐telehealthcare intervention. Whitten 2007 and Wong 2005 were free of other apparent biases.
Effects of interventions
Primary outcomes
Total exacerbations
The only study to record the total number of exacerbations as a separate figure was the Bourbeau 2003 study. Bourbeau 2003 reported 362 acute exacerbations of COPD in the control group (N = 95) and 299 exacerbations in the intervention group (N = 96) over a 12 month period. The difference was of borderline statistical significance; P = 0.06.
Vitacca 2009 reported that the mean number of exacerbations per month was significantly higher in controls than in the telehealthcare group (0.78±0.77 and 0.23±0.38, respectively; p<0.0001). Time free from exacerbation days was reported as a Kaplan‐Meier chart: in the intervention group 30% of patients (17/57) were free of an exacerbation at one year while in the control group only 5% of patients (2/44) were free of an exacerbation at one year.
Quality of life
Bourbeau 2003 and Casas 2006/Garcia‐Aymerich 2007 reported health related quality of life scores using the validated St George Respiratory Questionnaire (SRGQ). This scale goes from 0 (better health) to 100 (worse health). Negative change means improvement, the minimal clinically significant difference in health status is a change in score by 4 points (Hajiro 2002). The meta‐analysis by random‐effects generated a mean difference of ‐6.57 (95% confidence interval (CI) ‐13.62 to 0.48), that is a minimally clinically significant change although the confidence intervals are very wide (see Figure 2).
2.

Forest plot of comparison: 1 Quality of Life, outcome: 1.1 Quality of Life over 12 months.
Nguyen 2008 compared face‐to‐face dyspnoea management for COPD patients with a dyspnoea management program delivered via an electronic web‐networked interface. The validated Chronic Respiratory Questionnaire, the CRQ, was used to assess health related quality of life. The minimal clinically important difference is 0.5 (Hajiro 2002).
A meta‐analysis combining CRQ and SRGQ score should not be used as Puhan 2006 has shown that the CRQ is a much more responsive tool than the SRGQ.
Emergency department visits
A meta‐analysis of the three studies (Bourbeau 2003; de Toledo 2006;Vitacca 2009) which reported data on emergency department visits during their 12 months was performed by random‐effects (see Figure 3). This showed that patients with telehealthcare were much less likely to attend the emergency department than patients in the control group: OR 0.27 (95% CI 0.11 to 0.66).
3.
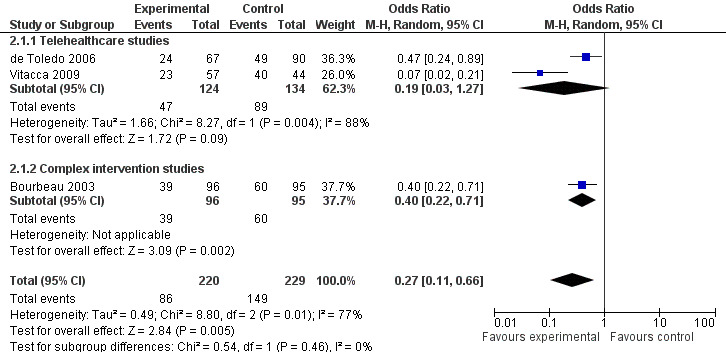
Forest plot of comparison: 2 Emergency Dept Visits, outcome: 2.1 Number of patients with one or more emergency dept attendance over 12 months.
One patient was sent to the emergency department in the Nguyen 2008 study, an insignificant result. Wong 2005 recorded the average number of visits per patient over a three month period as greater in the control group, OR 0.17 (95% CI 0.04 to 0.67). In contrast to these findings, Johnston 2000 reported that the average number of visits per patient was 1.79 (SD 1.48) for intervention patients and only 1.53 (SD 1.43) for control patients. No other studies reported on emergency department visits.
Hospitalisations
The following studies produced data on hospitalisation: Bourbeau 2003; de Toledo 2006; Casas 2006; Vitacca 2009. In order to pool all four trials the variable for number of patients with one or more hospital admissions during the 12 month period was entered into the Analysis 3.1. This generated an odds ratio of 0.46 (95% CI 0.33 to 0.65); P < 0.00001, see Figure 4.
3.1. Analysis.
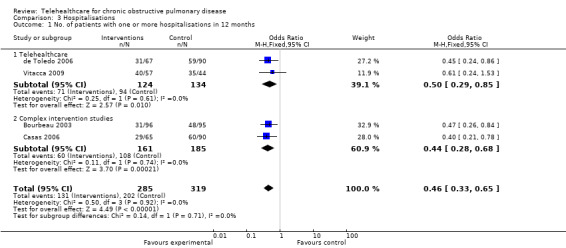
Comparison 3 Hospitalisations, Outcome 1 No. of patients with one or more hospitalisations in 12 months.
4.
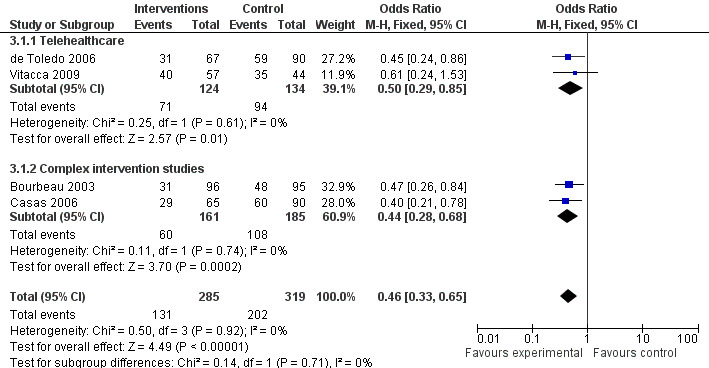
Forest plot of comparison: 3 Hospitalisations, outcome: 3.2 No. of patients with one or more hospitalisations in 12 months.
Finkelstein 2006 reported on the outcome measure of discharge to a higher level of care, either hospital or a nursing home, see Analysis 3.2; OR 0.29 (95% CI 0.08 to 1.05), i.e. telehealthcare patients have a lower odds of being discharged to a higher level of care than usual care patients.
3.2. Analysis.
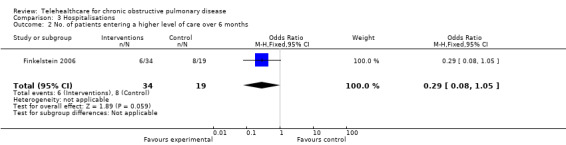
Comparison 3 Hospitalisations, Outcome 2 No. of patients entering a higher level of care over 6 months.
Wong 2005 reported that there was no significant difference between the telephone and the control group in hospitalisation rates at three months: P = 0.182.
Deaths
Four studies (Bourbeau 2003; Casas 2006; de Toledo 2006; Vitacca 2009) reported the number of deaths in each arm over the course of the 12 month study and so three of these were combined using the fixed‐effect model. Vitacca 2009 included data from patients who did not have COPD, however, when stratified for diagnosis mortality rate these did not differ between the intervention and control groups. Analysis 4.1 resulted in an odds ratio of 1.05 (95% CI 0.63 to 1.75) but this was non‐significant with P = 0.86; see Figure 5.
4.1. Analysis.
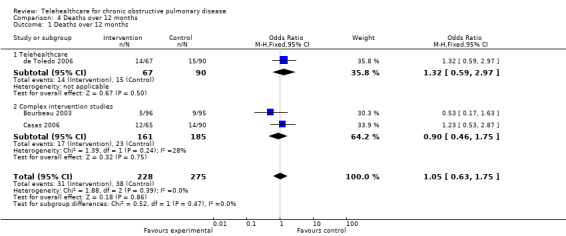
Comparison 4 Deaths over 12 months, Outcome 1 Deaths over 12 months.
5.

Forest plot of comparison: 1 Deaths over 12 months, outcome: 1.1 Deaths over 12 months.
In Finkelstein 2006, patients with congestive heart failure or COPD were allocated to either traditional skilled nursing at home ‐ the control group, or a video intervention involving virtual visits with videoconferencing technology, or a physiological monitoring group, including pulse oximetry, electronic spirometers and automatic blood pressure cuffs and virtual visits. There was no statistically significant difference in mortality between the three groups: (26.3% of control, 20.6% in the virtual and monitoring groups; P = 0.74). These figures are treated separately in Analysis 4.2 because the congestive heart failure patients cannot be separated from the COPD patients and the data are collected for six months only.
4.2. Analysis.
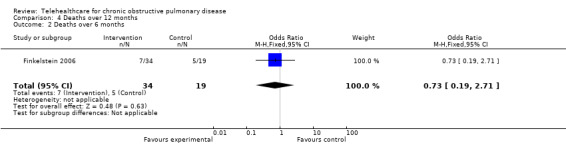
Comparison 4 Deaths over 12 months, Outcome 2 Deaths over 6 months.
There were no further deaths in any of the other studies.
Secondary outcomes
Forced Expiratory Volume in 1 minute in litres (FEV1)
In Bourbeau 2003 lung function did not change significantly from baseline to the end of the study. Mean ± SD at baseline was 0.98 ± 0.31 in the usual care group and 1.01 ± 0.36 at 12 months. In the intervention group, baseline FEV1 was 1.0 ± 0.33 and 0.96 ± 3.2 at 12 months.
Casas 2006/Garcia‐Aymerich 2007 reported mean change ± SD in FEV1 of 0.06 ± 0.35 in the usual care group and 0.01 ± 0.14 in the intervention group, i.e. the usual care group increased by more but not by a statistically significant difference, P = 0.6.
Forced Vital Capacity in litres (FVC)
In Bourbeau 2003 FVC mean ± SD was 2.24 ±0.69 at baseline and 2.3 ± 0.68 at 12 months in the control group and 2.27 ± 0.74 at baseline and 2.31 ± 0.77 at 12 months in the intervention group. These differences were not significant.
Patient satisfaction
Three studies reported on patient satisfaction using different and unvalidated scales and so their results are difficult to interpret.
Johnston 2000 reported that overall more than 95% of both groups agreed or strongly agreed with the following statements pertinent to in‐person visits:
appreciated having health provider visit home;
confidence in provider's ability to assess health condition during home health condition during home health care in‐person visits;
comfortable discussing personal problems or concerns with provider during home health care in‐person visits;
received appropriate level of personal care and attention from provider during home health care in‐person visits.
All between group differences were statistically non‐significant.
With respect to the survey of the intervention group's satisfaction with remote video visits, again over 90% of the group agreed or strongly agreed with the following statements:
appreciated having remote video visit system in my home;
confident in provider's ability to assess health condition by using remote video visit system;
comfortable discussing personal problems with my provider by using remote video visit system;
received appropriate level of personal care and attention from my provider when using remote video visit system;
remote video visits were convenient for me;
remote video visits allowed timely access to provider.
Nguyen 2008 reported satisfaction with dyspnoea self‐management programme according to a 3 point scale where 1 = not at all satisfied, 2 = quite satisfied and 3 = very satisfied. Satisfaction scores for both programmes were similar: 2.7 ± 0.47 for the face‐to‐face overall program and 2.6 ± 0.51 for the telehealthcare programme.
Whitten 2007 conducted interviews with 49 patients using Likert scales from 1 = strongly disagree to 5 = strongly agree with the following statements:
I found the home telehealth equipment easy to use;
I think telehealth is a good way to provide home health care for patients with heart/lung disease;
I think home telehealth is a good way to provide educational information on COPD/CHF management;
it was easy to communicate with the other person during the home telehealth consultation;
the care that I received via the home telehealth visit was as good as a regular in‐person visit;
I would rather be seen in person than over the home telehealth equipment;
home telehealth should only be used when a healthcare professional cannot be physically present;
overall, I am satisfied with the home telehealth service that I received.
Patients were in agreement with all of these statements. Overall this suggeststhat the patients were very satisfied with the telehealthcare programmes.
Study withdrawal
Details of study withdrawals are reported in the Risk of Bias tables in the 'Incomplete outcome data' section.
Costs
Three studies (de Toledo 2006; Finkelstein 2006; Johnston 2000) reported costs relevant to their studies. Here they are presented in US dollars (USD) as converted in June 2010.
de Toledo 2006 compared the costs of their clinical experiment of EUR 38,932, USD 47,849, with the cost of one day's admission for COPD, EUR 220, USD 270.34 a day. They calculated that the reduction of hospitalisation days would pay for the system before the end of the first year (mean duration of hospitalisation 2.8 days, no. of patients = 157).
Finkelstein 2006 calculated that the cost per visit of an actual visit by a nurse was USD 48.27 primarily due to the amount of additional nursing time required to conduct an actual visit and related travel costs. The cost of a virtual visit only was USD 22.11 per visit. The cost of a virtual visit with monitoring was USD 33.11 per visit.
Johnston 2000 found that outpatient costs for the two groups did not vary by much. However, hospital costs per patient were much greater for patients in the control group than for patients in the intervention group and that the total mean cost per patient in the control group was USD 2674 (SD = USD 6313) and in the intervention group was USD 1948 (SD = USD 3681). These large standard deviation figures mean that caution should be exercised when generalising from these results.
Bourbeau 2006 was a follow‐on paper reporting economic benefits of the Bourbeau 2003 study. Total per‐patient cost of the self‐management intervention was USD 3778, mostly accounted for by the case manager's salary (USD 3338). In the study each case manager supervised 14 patients and there was no significant difference in costs between the experimental and control groups.
Cost effectiveness
Bourbeau 2006 calculated cost effectiveness as USD 4214 per hospitalisation prevented with a caseload of 14 patients per case manager. With assumed increasing caseloads of 30, 50 and 70 patients per case manager the estimated incremental cost‐effectiveness ratios were USD 2053, USD 1326 and USD 1016 per hospitalisation prevented, respectively.
Discussion
Summary of main results
Published research suggests that telehealthcare has the capacity to reduce exacerbations and may improve the quality of life of the people using it in comparison to usual care. Emergency department visits are significantly reduced, as are hospital admissions in COPD patients, without clearly increasing morbidity or incurring excessive costs.
Overall completeness and applicability of evidence
These results seem to be very encouraging for the supporters of telehealthcare. There are clear advantages to patients and healthcare systems in the studies from which we were able to pool results: emergency department visits and hospital admission rates are reduced with minimal impact on mortality, morbidity and quality of life. This leaves considerable scope for cost savings and there is some evidence for this from the studies which have studied cost as an outcome.
One limitation of the evidence is whether or not it was valid to perform meta‐analysis with this type of complex intervention. Telehealthcare may be regarded as a complex healthcare intervention, as defined by the MRC framework for design and evaluation of complex interventions. The most extensive forest plot in this review contains only four studies. If we look more closely at these studies we find that we have not necessarily been comparing like with like and so the validity of any conclusion drawn from these figures must be interpreted with caution. Bourbeau 2003, Casas 2006 and de Toledo 2006 together seem to support the conclusion that telehealthcare does not increase deaths. However, Casas 2006 intervention was a form of post‐discharge integrated support involving multiple ingredients. Breathing exercises, physical exercises, education, psychological support, specialist nursing and other elements were all built into an individual care plan. Telehealthcare came in only after the patient was discharged to maintain access to these ingredients via a web‐based communication device. Bourbeau 2003 had even less emphasis on telehealthcare, in this case the intervention was delivered through the medium of home visits over a two month period and the telephone was only used for follow‐up. de Toledo 2006 has perhaps the most reliance on technology, but again the telehealthcare is only the delivery mechanism for an enhanced package of integrated chronic illness care.
It may indeed be valid criticism of this study to say that the improved outcomes demonstrated were entirely due to the provision of these integrated care programmes and the fact that the programmes were delivered from a distance by telehealthcare is entirely irrelevant. The answer to this is "maybe", we don't know for sure yet due to the design of the trials available. The small Nguyen 2008 study was probably the closest design to answering this as the dyspnoea management programme was made available to one arm by means of face‐to‐face interaction and to the other by Internet. Unfortunately, this study stopped early due to multiple technical challenges, at this stage the authors reported positive improvements in both arms of the study with no significant differences with respect to program modality. However, the study is small with a significant withdrawal rate and can be criticised for not having an additional usual care arm ‐ otherwise how are we to know that there was not simply a seasonal or other improvement across both arms within the study?
The ingredient which telehealthcare has the potential to offer that does not come from other forms of integrated programme is that of "access", improving the access to the programme's effective elements, be that education, other advice, verbal or written support, or potentially monitoring for reassurance. This concept of access could be better defined for future research. It may be that elements of current COPD care are already effective, they just need to be made more accessible.
If we return to the list adapted from Finkelstein of the mechanisms through which telehealthcare appears to mediate its success:
providing patient education and counselling for primary prevention and early detection of disease;
improving adherence to medications and other treatment regimens;
collecting patient data remotely;
replacing face‐to‐face nursing/physician visits;
providing early detection of incipient disease exacerbation and timely intervention for early symptom management;
reducing unscheduled/unnecessary visits to the physician and emergency room;
preventing repeat hospitalisations;
it can be seen that the idea of access is central to many of these mechanisms.
In order to apply telehealthcare successfully in the future, studies need to be carefully designed so as to consider the "access" as enabled by the telehealthcare. This will avoid the benefits of telehealthcare being lost in studies which cover new programmes of healthcare that introduce multiple elements only one of which is the fact that care can be delivered from a distance.
Also, in terms of the applicability of the evidence we need to consider the fact that several studies failed due to technological challenges. This was not only unreliability of the technology but difficulties of an older pre‐technological population struggling to use these systems. It is exactly this vulnerable population that we wish to target in terms of preventing increasing admissions to hospital with increasing morbidity, as they enter the severe "end‐stage" of their COPD trajectory (Murray 2006).
Quality of the evidence
The evidence is very heterogeneous, particularly in terms of its definition of COPD ‐ some patients were recruited on discharge from hospital, but not all. The interventions are heterogeneous ‐ not only in terms of the technology employed, whether telephone, video or Internet but in terms of the ingredients within the intervention. These would be better understood as "complex interventions". In fact it can be seen that, in terms of the MRC's framework for complex health interventions, phase 0 (theoretical work) and phase 1 (modelling how the intervention works) of the clinical trial agenda have been bypassed and researchers have launched into phase 2 (exploratory or pilot trials) and phase 3 (definitive randomised controlled trials), without sufficient preparation. The result of this is that the trials are not adequately designed to address sufficiently and specifically the question of the "access" ingredient because so many other factors are liable to influence the results of the experiments. In addition, the technical challenges from what is now regarded as old technology have resulted in a body of evidence of only moderate quality. In the future, further qualitative research will help to characterise what is valued about access and what is practicable within that. This would then set a basis for quantitative research looking at hospital admission, morbidity, mortality and cost‐effectiveness. The quantitative research would be designed along the lines of the Nygugen study in which the two arms of the trial differed only in the nature of the method of delivery of the intervention, i.e. telehealthcare versus standard, and not in the content of the intervention. The content of the intervention delivered would have to be current state‐of‐the‐art practice in terms of its clinical elements.
Potential biases in the review process
This review process should have minimum bias. We used a very broad search strategy in conjunction with The Cochrane Library in order to capture as many studies as possible. This methodology means that both completed and ongoing randomised controlled trials coded as COPD by the Cochrane Airways Group were searched for a broad range of telehealthcare terms. Two authors then independently chose relevant articles from this list and met to discuss a final list of included studies. Although all the trials included were published in English we would have been prepared to translate and include relevant trials in other languages, thereby helping to minimise bias.
Agreements and disagreements with other studies or reviews
Polisena 2010 is the only other systematic review of telehealthcare in COPD that we have come across. We agree with its findings that telehealthcare, whether home telemonitoring or telephone support, reduces rates of hospitalisation and emergency department visits. Unfortunately, their result showing an increase in the mortality due to telehealthcare appears to be due to a transposition of the results of the Borbeau study between the intervention and control groups. We have informed the authors of this. Polisena 2010 included a number of studies in which we considered that the main intervention did not fall into our definition of telehealthcare and its inclusion criteria were not limited to randomised controlled trials. This can introduce bias as about half of their studies were observational rather than randomised.
There are an extensive number of other studies ongoing or which have only reported in abstract form so far. It will be necessary to update this review over the next two years as these projects come to fruition.
Authors' conclusions
Implications for practice.
Overall, only a small evidence base has been uncovered to support the use of telehealthcare in COPD. However, medical manufacturers and policy makers are keen to introduce this technology widely due to a very optimistic view of its potential. We would advocate caution in this endeavour as our research shows that substantial aspects of the technology are inconclusive ‐ for example, its impact on quality of life. The limited evidence available suggests that telehealthcare does not significantly increase nor decrease mortality in COPD patients, but the confidence intervals are too wide to conclude that there is no impact on mortality.
Implications for research.
Most telehealthcare interventions for COPD have been introduced as part of a complex package of enhanced care. In future, research studies need to build up an increased body of qualitative work which will help to determine the precise contribution of telehealthcare to the package. A theoretical framework for telehealthcare will help to establish a consistent intervention. Detailed description of the precise nature of the intervention when reporting studies will help when comparing interventions. Separate classification of the form and then the function of the telehealthcare; e.g. videoconferencing (form) to keep people out of hospital (function) is required. In this way, different technologies may be compared where their function is the same ‐ drawing again from Finkelstein's mechanisms. An alternative to videoconferencing to treat an exacerbation and thereby avoid hospital admission, might be the use of an Internet package with oxygen saturation telemonitoring or remote spirometry with a telephone component, i.e. same function, different forms of intervention. This kind of theoretically based study will go further in telling us which functions of telehealthcare are most successful with which forms of delivery. These classified interventions should then be compared in larger trials with longer durations. In addition, more detail should be obtained regarding the outcomes of the studies so that precise comparisons may be made as to the relative advantage of telehealthcare over other types of intervention. Similarly, studies which stratify the COPD patients by their severity might find that a specific subgroup of patients stand to gain greatest independence from their illness through the use of telehealthcare technologies.
The deployment of telehealthcare for COPD is widely anticipated, however, more detailed research is needed in order to realise its potential fully.
What's new
| Date | Event | Description |
|---|---|---|
| 19 June 2012 | Amended | Author (JC) affiliations updated. |
Data and analyses
Comparison 1. Quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of Life over 12 months | 2 | 253 | Mean Difference (IV, Random, 95% CI) | ‐6.57 [‐13.62, 0.48] |
1.1. Analysis.

Comparison 1 Quality of life, Outcome 1 Quality of Life over 12 months.
Comparison 2. Emergency department visits.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with one or more emergency dept attendance over 12 months | 3 | 449 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.11, 0.66] |
| 1.1 Telehealthcare studies | 2 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.03, 1.27] |
| 1.2 Complex intervention studies | 1 | 191 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.22, 0.71] |
| 2 Number of patients with one or more emergency dept attendance over 3 months | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.67] |
2.1. Analysis.

Comparison 2 Emergency department visits, Outcome 1 Number of patients with one or more emergency dept attendance over 12 months.
2.2. Analysis.

Comparison 2 Emergency department visits, Outcome 2 Number of patients with one or more emergency dept attendance over 3 months.
Comparison 3. Hospitalisations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. of patients with one or more hospitalisations in 12 months | 4 | 604 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.33, 0.65] |
| 1.1 Telehealthcare | 2 | 258 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.29, 0.85] |
| 1.2 Complex intervention studies | 2 | 346 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.28, 0.68] |
| 2 No. of patients entering a higher level of care over 6 months | 1 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.05] |
Comparison 4. Deaths over 12 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deaths over 12 months | 3 | 503 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.63, 1.75] |
| 1.1 Telehealthcare | 1 | 157 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.59, 2.97] |
| 1.2 Complex intervention studies | 2 | 346 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.46, 1.75] |
| 2 Deaths over 6 months | 1 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.19, 2.71] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bourbeau 2003.
| Methods | A multicentre randomised clinical trial | |
| Participants | 191 patients were randomised. All patients in each participating hospital, of 3 in Quebec, who had been hospitalised for at least once in the preceding year for an acute exacerbation of COPD were screened for participation. The eligibility criteria included: be a current or previous smoker with FEV1 after bronchodilator between 25% and 70% of predicted normal value; have no evidence of asthma, left congestive heart failure, terminal disease, dementia or uncontrolled psychiatric illness. | |
| Interventions | Intervention: The intervention group received a COPD self management program, consisting of 1 hour a week teaching at home for 7 to 8 weeks, in English or French. Supervised by experienced nurses or respiratory therapists who acted as case managers. Follow‐up consisted of weekly telephone calls for 8 weeks during the educational period then monthly calls for the remainder of the study. The patients could also contact their case manager for advice during this time. Control: Both groups continued their usual care by their respective general practitioners and specialists and there was no restriction on their access to the regional universal health program. |
|
| Outcomes | 1. Medication profile. 2. Spirometry., 3. 6 minute walk test. 4. Dyspnoea measurements after exercise. 5. Health related quality of life as measured by the St George Respiratory Questionnaire (SGRQ). 6. Healthcare utilisation. 7. Costs. 8. Cost effectiveness. |
|
| Notes | There were 469 eligible patients; however, 251 refused to participate and 27 who agreed to participate were deemed to live too far away thus 191 patients were randomised. This level of exclusion risks introducing selection bias especially as certain socioeconomic groups are perhaps more likely to refuse to participate in studies. However, the authors do state that those who refused were similar to the study group with respect to sex, age and level of airflow obstruction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | “randomisation with the use of a central computer generated list of random numbers”, “The blocking factor was not known by the investigators or their staff in each participating centre. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "As a double blind design was impossible, an independent evaluator unaware of the patient assignment was responsible for the evaluation process in each centre. The evaluator was cautioned not to ask about the workbook modules and types of contact" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26 patients dropped out after randomisation, 1 was lost to follow up and 11 found that the burden of evaluation was too great. 14 patients in total died. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | Possible selection bias |
Casas 2006.
| Methods | Randomised controlled trial. | |
| Participants | Patients: Conducted in Barcelona (Spain) and Leuven (Belgium), 155 COPD patients were recruited from two tertiary hospitals immediately following hospital discharge. All patients had been previously admitted for a COPD exacerbation for more than 48 hours. | |
| Interventions | Intervention: A well‐defined integrated care intervention with the support of information and communication technologies. This was standardised across the two centres and included physical and social assessment, education and co‐ordination by a case manager between hospital and primary care teams. Co‐ordination was facilitated by a web‐based call centre. Weekly phone calls during the first month after discharge helped to embed the lessons learnt during the education sessions. Control: Both groups continued their usual care by their respective general practitioners and specialists and there was no restriction on their access to the regional universal health program. |
|
| Outcomes | 1. Hospital re‐admission. 2. Quality of life was assessed by St George Respiratory questionnaire. 3. Clinical features. 4. Co‐morbid conditions. 5. Healthcare utilisation. |
|
| Notes | Only 19% of the 850 patients screened were deemed appropriate for randomisation following the application of the inclusion criteria. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | All patients were blindly assigned (1:1 ratio) using computer generated random numbers to either intervention or control. A different randomisation ratio was used in one of the centres (Barcelona 1:2 ratio) during part of the study, resulting in the arms having different numbers of patients (65 intervention, 90 control) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient evidence |
| Blinding (performance bias and detection bias) All outcomes | High risk | Impossible to blind from intervention, no evidence of blinding outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomisation a total of 4 patients moved on to palliative care, there were 26 deaths, 2 new neoplasms and 3 changes of address, and so 35 patients were excluded from the trial. Of 155, 30% excluded. |
| Selective reporting (reporting bias) | Unclear risk | Data reported across two papers, Garcia paper does not make clear that it is part of a larger trial |
| Other bias | High risk | Risk of selection bias as only 19% of the 850 patients screened were deemed appropriate for randomisation following the application of the inclusion criteria. |
Chandler 1990.
| Methods | This was a randomised pilot clinical investigation. | |
| Participants | 13 adult patients with COPD, asthma or both who were receiving theophylline from an outpatient department of pulmonary medicine, Kentucky, United States of America. | |
| Interventions | The intervention group was taught how to measure their theophylline level at home using a specialised instrument which only required a small blood sample obtained by fingerstick and then they phoned the clinic for advice on drug dosage. Control: The control group attended the clinic for traditional therapeutic drug monitoring, i.e. blood testing and advice face‐to‐face. This continued for 6 months. |
|
| Outcomes | 1. Pulmonary function testing with an electronic spirometer at each clinic visit. 2. Patients were evaluated for degree of dyspnoea at each clinic visit using the baseline dyspnoea index and the transitional dyspnoea index. 3. Visual analogue scales for night time cough and daytime cough, wheezing and breathlessness were completed once a month. 4. Questionnaires on adverse effects were also completed once a month, addressing nausea, vomiting, diarrhoea, nervousness, headache, tremor, sleeplessness and heart palpitations. 5. Patients; health attitudes and beliefs were measured using the Krantz Health Opinion Survey and the Multidimensional Health Locus of Control to measure patient attitudes towards different healthcare approaches and the degree to which individuals believed their health to be affected by internal or external factors, respectively. |
|
| Notes | The major risk of bias in this study came from the fact that just 13 patients were recruited and only 11 patients finished the study: constituting a very small study group. In addition, only eight of the patients had COPD, the rest had asthma. Also, there were 4 male patients and 7 female patients. With these shortcomings in mind, it is unlikely that results from this study can be generalised despite the authors careful attempts to randomise and blind the participants appropriately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “patients were assigned by a random numbers table into one of two treatment groups” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “Spirograms were administered by a respiratory therapist blinded to treatment group”; “each patient was interviewed by a blinded investigator who did not know the group assignments or results of pulmonary function tests.” Although the patient was clearly not blinded from the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 11 patients completed the study, 2 patients were lost to follow up |
| Selective reporting (reporting bias) | High risk | Several outcome measures not fully reported, no reason given as to why not, potentially because the difference across groups was not significant. |
| Other bias | High risk | Only 8 patients with COPD were recruited, too small a sample for meaningful generalisation. |
de Toledo 2006.
| Methods | Randomised controlled trial | |
| Participants | 157 COPD patients recruited during a tertiary hospital admission for an acute episode, randomised to an intervention group: N = 67; age = 71 ± 8 years, 2.3 % women and control group: N = 90; age = 72 ± 9, 3.2% women | |
| Interventions | The care team shared a web‐based patient record which also featured mobile home visits units and fixed home units. There was also integrated support for education (of both professionals and patients) and videoconferencing with the patients. The intervention group patients also had 24 hour access to the multidisciplinary care team via a call centre. The call centre was not intended for dealing with emergency calls and stored out‐of‐hours calls until the next day. Control patients did not have access to the call centre but received education and home visits as well. Follow‐up for these patients was made without the mobile home visit unit and web‐based patient management module. |
|
| Outcomes | 1. Number of readmissions. 2. Number of visits to emergency department. 3. Mortality. 4. Acceptability to professionals. 5. Patterns of use and equipment and communication costs. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly distributed in an intervention and a control group" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Impossible to blind patients to intervention, no evidence of outcome assessor or data analyser blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information, no details of patients withdrawing or lost to follow up. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting, all outcomes in methods are reported. |
| Other bias | High risk | Patients in the intervention group may have experienced a feeling of being better cared for that may have improved their outcomes. Extreme sex ratio in study groups may limit generalisability |
Finkelstein 2004.
| Methods | Randomised controlled trial | |
| Participants | This study included patients with a mix of conditions: Congestive heart failure, chronic obstructive pulmonary disease and chronic wound‐care patients. 68 subjects were enrolled, there was a requirement that either the subject or a supportive care partner was able to physically and cognitively use the equipment within the home environment. | |
| Interventions | Intervention: there were two intervention groups for this study. Firstly, standard care plus videoconferencing/Internet access and, secondly, standard care plus videoconferencing plus physiological monitoring, e.g. spirometry in COPD. These technologies allowed Virtual Visits to be conducted between the nurse at the central site and the subject at home with audio and video interactions. Patients were trained to use the equipment. Control: the control group received standard home health care. |
|
| Outcomes | 1. Termination from home care or loss of eligibility for home care. 2. Time to discharge to a higher level of care such as a nursing home or hospital. 3. Mortality. 4. Morbidity. 5. Patient perception of telehealthcare (Telemedicine Perception Questionnaire TMPQ). 6. Patient satisfaction Home Care Client Satisfact Instrument (HCCSI). 7. Quality and clinical usefulness of virtual visits. 8. Patient utilisation of services. 9. Cost for both subjects and service providers. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient data |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data |
| Blinding (performance bias and detection bias) All outcomes | High risk | Impossible to blind participants to intervention, not stated whether outcome assessor was blinded or data analyser. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Out of 68 patients randomised 53 completed the study. 47 patients were interviewed after they concluded the study to gather patient satisfaction data. |
| Selective reporting (reporting bias) | High risk | There is a significant amount of data missing from this report including costs and clinical effectiveness. Publishing this favourable report on patient opinions may condition the reader to expect further favourable results. The authors state that the reason for not yet publishing hard clinical endpoint cost data is that the data were not yet available. |
| Other bias | High risk | Results for COPD cannot be separated from results for other conditions and so this limits interpretation |
Finkelstein 2006.
| Methods | Patients, Intervention, Control, Outcome are the same as the 2004 study. | |
| Participants | Same as for 2004 | |
| Interventions | Same as 2004 | |
| Outcomes | As 2004 plus this study reports the additional outcomes of discharge to a higher level of care, i.e. hospital or nursing home, mortality, morbidity as evaluated by changes in the knowledge behaviour and status scales of the Omaha assessment tool and data on costs. | |
| Notes | Control: same as for 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See 2004 report |
| Allocation concealment (selection bias) | Unclear risk | See 2004 report |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | See 2004 report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 53 subjects completed the study out of the 68 enrolled. |
| Selective reporting (reporting bias) | High risk | The age distributions in all three groups were similar. For several of the statistical analyses the virtual visit group and the monitoring group were combined into a single intervention group. This influences the results generated. |
| Other bias | Unclear risk | Insufficient data |
Garcia‐Aymerich 2007.
| Methods | This study is a duplicate report of the Barcelona part of the Casas paper above. This may introduce selective reporting bias as it is not clear why the Belgian results are not reported in this paper. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Casas paper |
Johnston 2000.
| Methods | "quasi experimental", "randomly assigned to an intervention or control group", not clear why this is not described as a randomised controlled trial | |
| Participants | Newly referred patients with COPD, congestive heart failure, cerebral vascular accident, cancer, diabetes, anxiety or need for wound care were randomly assigned to intervention (N = 102) or control (N = 110). The patients all had a projected need for two or more visits a week. The study took place in Sacramento California through a large health maintenance insurance organisation. 29 intervention group patients and 19 of the control group had COPD. | |
| Interventions | Intervention: Both groups received routine home health care with visits and access to telephone contact. However, the study group also used a remote video system allowing nurses and patients to undertake virtual visits in real time any time during 24 hours a day. There was also equipment attached to the video for testing cardiopulmonary status. Control: Controls used routine healthcare with home care visits but without a video system. They also had access to telephone advice and triage. |
|
| Outcomes | 1. Use of services. 2. Costs for inpatient and outpatient services. 3. Visits to emergency departments. 4. Costs for pharmacy services, laboratory, physician, emergency department visits, inpatient treatment, home healthcare costs and videoconferencing costs. 5. Patients' compliance with medication regimen. 6. Patients' knowledge about their disease. 7. Patients' ability to move towards self care. 8. Patient satisfaction survey. |
|
| Notes | Results and interventions for COPD patients are not presented separately from other illnesses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "quasi experimental", "randomly assigned to an intervention or control group" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | High risk | Total costs of items are reported rather than absolute numbers of emergency dept visits etc. However, this is in accordance to the methods implied reporting. |
| Other bias | High risk | Funded by Kaiser Permanente and so only collected data from plan members which may rule out balance of sociodemographic groups. COPD patients are not measured or analysed separately and so some data are of limited use. |
Nguyen 2008.
| Methods | A randomised controlled trial. | |
| Participants | Patients: 50 patients with moderate to severe COPD who were current Internet users were assigned to one of two dyspnoea management intervention programs, in California or Seattle. Patients were recruited from web and non‐web sources, including distribution lists, chest clinic referrals, and support groups both real and digital. | |
| Interventions | Intervention: The Internet based dyspnoea management intervention program (eDSMP) focused on education, skills training and ongoing support for dyspnoea self management and was delivered via a personal digital assistant or Internet. Control: The face to face dyspnoea management intervention program (fDSMP) delivered the same content via education sessions, reinforcement contacts and peer interactions: all face to face. |
|
| Outcomes | Evaluations were performed at 3 and 6 months. 1. Dyspnoea with activities of daily living,and quality of life as measured with the Chronic Respiratory Questionnaire. 2. Exercise behaviour exercise performance. 3. COPD exacerbations were also measured. 4. Self‐efficacy and social support were measured as mediators. 5. At the final visit a satisfaction survey and a semi‐structured interview were performed. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "an investigator who was not involved in the day‐to‐day study operations generated the randomisation sequence using the SPSS version 14.0 random sequence generator feature" |
| Allocation concealment (selection bias) | Low risk | "separate sealed opaque envelopes." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unable to blind study nurse to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 50 patients randomised, 39 remained after 6 months. Five control patients dropped out, 1 unable to schedule a visit and 4 discontinued due to schedule conflict, personal problems, losing interest or not eligible after baseline. Seven intervention patients discontinued. Four were unable or unwilling to access website, 1 schedule conflict, 1 patient had recurrent angina and one moved out of the area. |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods are reported |
| Other bias | Low risk | No evidence of further bias |
Vitacca 2009.
| Methods | Randomised clinical trial | |
| Participants | 240 chronic respiratory patients, all of whom require home oxygen, some of whom were on home mechanical ventilation, 101 with COPD were enrolled. Other reasons for respiratory failure were Amyotrophic lateral sclerosis (a type of motor neurone disease), restrictive chest disease or other neuromuscular disease. Inclusion criteria were also that the patient had had at least one hospitalisation for respiratory illness in the previous year. | |
| Interventions | Teleassistance program for patients and their families who have COPD. The teleassistance programme was based on a continuous 24 hour on‐call service and pulse oximetry available. Some patients received pulse oximetry with solid memory card and a modem system for transmission through the home telephone line to the teleassistance nurse who was available from 0800 to 1600 for 5 days a week to provide a real time teleconsultation. Out of hours the pulmonologist on duty was contacted if needed. Control: traditional face‐to‐face care, i.e. out‐patient follow‐up regimen, discharge plans did not include home nurse visits |
|
| Outcomes | Reduction in hospitalisations. Reduction in urgent GP calls. Acute emergency department admissions. Also costs after set‐up equipment had been paid for. |
|
| Notes | Some results were reported separately for COPD patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a set of computer‐generated random numbers in 1:1 ratio patients were assigned to the treatment or control group" |
| Allocation concealment (selection bias) | High risk | The study reports no allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither patients nor investigators were blinded to the allocation of patients, nor were outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals were reported and some reasons given ‐ the data from these patients were not included in the analyses |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | High risk | All patients on long term oxygen therapy (LTOT) were considered together regardless of whether the cause of their dependence was COPD, restrictive, amyotrophic lateral sclerosis, other neuromuscular or other disease. These causes were not analysed separately. |
Whitten 2007.
| Methods | Randomised controlled trial, where qualitative data is also collected in detail. | |
| Participants | Patients with a diagnosis of COPD and/or Congestive Heart Failure (CHF) who were prescribed home health care services by their health insurance group were recruited. Intervention group N = 83 and control group N = 78. | |
| Interventions | Intervention: This group received a combination of traditional face‐to‐face home healthcare and virtual telemedicine visits. Control: This group received only conventional home care without virtual visits |
|
| Outcomes | 1. The Short Form 36 (SF‐36), Outcome and Assessment information Set (OASIS) and patient charts were used to collect outcome data. 2. Patient perceptions of the home telecare services were collected via telephone interviews, from the intervention group, in a qualitative piece of work. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" but no details |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | As far as health outcomes were concerned only patients with complete pre‐ and post‐data sets were included, i.e. this wasn't an intention‐to‐treat analysis. The study notes problems with turnover of project nurses and unwillingness to comply with data collection protocols. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in methods were reported. |
| Other bias | High risk | The data of patients with COPD and CHF were analysed together |
Wong 2005.
| Methods | Randomised controlled study | |
| Participants | Patients: 60 participants with COPD were recruited from an acute care hospital in Hong Kong. | |
| Interventions | Intervention: This study aimed to determine whether a nurse‐led telephone follow‐up programme would increase patients’ self efficacy when it came to managing their COPD dyspnoea. A person with high self efficacy feels more confident about engaging in activities and makes more effort to overcome challenges. Two phone calls were made in the first four weeks after discharge from hospital as this is felt to be a particularly vulnerable time for patient re‐admission. Control: control patients had routine care with no additional telephone input from nurses. |
|
| Outcomes | 1. Self efficacy was measured by the Chinese Self Efficacy Scale. 2. Number of visits to accident and emergency department. 3. Number of hospitalisations. 4. Unscheduled visits by physicians. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised using the Research Randomizer software which generated 30 sets of numbers." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "data were collected by a research assistant who was blind to the grouping" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four patients who refused to answer second questions had their results replaced by the group mean. This may not have been statistically appropriate. |
| Selective reporting (reporting bias) | Low risk | All outcome measures stipulated in methods reported in findings of |
| Other bias | Low risk | No evidence of other bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aiken 2006 | Management in hospital and COPD figures not separable |
| Balas 1997 | Not asthma or COPD |
| Brooks 2002 | Rehabilitation programme |
| Carrieri 2005 | Not focusing on telehealthcare |
| Demiris 2003 | Visits reviewed for technical quality |
| Donesky‐Cuenco 2003 | Exercise focused, not telehealthcare focused |
| Egan 2002 | Not telehealthcare, hospital based case manager and discharge planner |
| Farrero 2001 | Not telehealthcare ‐ most visits were face‐to‐face |
| Gadoury 2005 | Face‐to‐face visits |
| Griffiths 2005 | Not telehealthcare ‐ expert patient intervention face‐to‐face |
| Hernandez 2003 | Not telehealthcare ‐ home visits |
| Hibbert 2003 | Not a randomised controlled trial ‐ qualitative study |
| Hopp 2006 | Patients had multiple conditions and COPD was not included |
| Jerant 2008 | Not COPD separable |
| Jimison 2008 | Includes studies of different chronic conditions and all types of literature. Not RCT. |
| Mair 1999 | Not a randomised controlled trial, small pilot study with 6 patients |
| Mair 2000a | Not limited to COPD and results not fully reported but summarised |
| Mair 2002 | Full text not available, despite extensive search and contact with author |
| Mair 2005 | Outcome measures of interest in this study were not reported |
| Maltais 2005 | Not telehealthcare ‐ all interventions were face‐to‐face |
| Martens 2007 | Not telehealthcare |
| Moore 2007 | Exercise focused |
| Moxam 2007 | The Effects of a Home Exercise Video Programme for Patients With Chronic Obstructive Pulmonary Disease, not telehealthcare |
| Oh 2003 | Not COPD |
| Pare 2006 | Non‐randomised case‐control study |
| Petty 2006 | Video library |
| Poels 2008 | GP practice‐based |
| Rebuck 1996 | The accuracy of a handheld portable spirometer, not telehealthcare |
| Ries 1995 | Not telehealthcare |
| Ries 2003 | Not telehealthcare |
| Sridhar 2008 | Not telehealthcare |
| Tierney 2005 | Not telehealthcare |
| Trappenburg 2008 | Non‐randomised controlled multicentre study |
| Wasson 1992 | Unable to see COPD data |
| Welch 2000 | Only 30% of patients were "mixed respiratory", COPD patients were not identified |
| Whitten 2002 | Covered multiple conditions |
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN 12608000112369.
| Methods | Interventional randomised controlled trial |
| Participants | Participants will have a minimum age of 45 with COPD FEV1/FVC < 0.7 post‐bronchodilator. Severity FEV1 30‐80% of predicted and greater than 10 pack year smoking history. |
| Interventions | Patients in the active arm will have an individual management plan developed by the research team. This will use behaviour change and motivational interviewing techniques. Some of this plan will be delivered by nurses using the telephone. |
| Outcomes | Quality of life, SF‐36 and St George's Respiratory Questionnaire, Depression scale, post‐traumatic stress disorder (PTSD) checklist, anxiety measure. Hospital Anxiety and Depression scale, Satisfaction with Life survey, Client Satisfaction Questionnaire, lung function, spirometry, healthcare utilisation prescriptions for oral steroids and antibiotics, physical activity as measured with accelerometer. |
| Notes | Setting: University of Tasmania, Australia |
ACTRN 12609000428268.
| Methods | Randomised controlled trial to compare hospital admissions and emergency department visits in home care clients with COPD undergoing telehealth home monitoring versus usual care. |
| Participants | Have a confirmed diagnosis of COPD and are currently receiving home oxygen services. Male and females over age 18 years. |
| Interventions | Remote home monitoring of client's vital signs by a nurse will be undertaken via monitoring equipment installed in the client's home, including blood pressure, weight, heart rate, oxygen saturation levels. In addition, client's responses to questions regarding their general state of health, coughing and sputum production will be collected and forwarded to the nurse who reviews them and gives advice including whether or not to involve a doctor. |
| Outcomes | Number of hospital admissions, number of emergency department visits and health related quality of life as measured by this chronic respiratory questionnaire. Cost savings of home monitoring equipment compared to usual care. |
| Notes | Setting: Silver Chain Nursing Association, commercial sector, Western Australia |
Alonso 2004.
| Methods | Awaiting a home‐care pilot in COPD |
| Participants | The study is still at the proposed stage |
| Interventions | An Integrated Chronic Care Platform that allows voice and data to be transmitted to the required healthcare professional regardless of his/her location |
| Outcomes | Not yet specified |
| Notes | This was very much a technical paper describing what was envisioned |
Battaglia 2007.
| Methods | Study still at the recruiting stage, only 77 patients enrolled so far |
| Participants | Plans to enrol 600 patients for 24 months, 300 in control group 300 in intervention |
| Interventions | Patients receive periodic nursing assistance and clinical monitoring by a general practitioner who is in contact with a respiratory specialist at a call centre. Intervention patients also have hospital visits and analysis every three months |
| Outcomes | Numbers of nursing accesses made, number of GP accesses and numbers of unscheduled visits and hospitalisations |
| Notes | Difficulties in enrolment blamed on bureaucratic ethics committees and difficult co‐ordination between hospital and local health administrations. |
Battaglia 2008.
| Methods | AIRTEM ‐ Integrated Assistance by Respiratory TeleMedicine is a multicentred study carried out in Bilan to evaluate the advantages of home telemonitoring patients with COPD and oxygen therapy. They plan to enrol 600 patients over 24 months |
| Participants | There are significant problems with slow recruitment due to problems with bureaucratic ethics committees and difficult co‐ordination |
| Interventions | Following an initial visit to a respiratory specialist and entry of clinical data onto an Internet database the patients will be seen by their GPs and by the specialists with have access to these records |
| Outcomes | 77 patients enrolled by May 19 2008 |
| Notes | Ongoing |
ISRCTN41424840.
| Methods | Randomised controlled trial |
| Participants | To be recruited for the study from the Prince Philip and West Wales General Hospital Pulmonary Rehabilitation Scheme. The standard inclusion criteria to be accepted through the scheme for pulmonary rehabilitation is patients who feel limited by their chest, have a primary physician, spirometric diagnosis of COPD, are on maximal respiratory medication, have no unstable cardiac disease and no cognitive impairments. |
| Interventions | Patients in two groups will either receive standard care or 6 months telehealthcare support with doc@home which is an integrated solution for the remote health management. |
| Outcomes | Are home electronic monitoring and electronic learning resources feasible and safe for patients with moderate to severe COPD? Measured at baseline, 1 month and 6 months only. Does home telehealthcare reduce respiratory hospital admissions? Reduce community specialist team visits? And improve quality of life and mood? And is home telehealthcare telemedicine cost‐effective? |
| Notes | Setting: UK |
ISRCTN96634935.
| Methods | Randomised controlled trial with nested qualitative study |
| Participants | Male and female participants with no age limits, registered to Lothian general practices admitted with an exacerbation of COPD as the primary diagnosis to one of the three acute hospitals in Lothian in the previous year. |
| Interventions | A modified touch screen computer with video capability which is linked by broadband to a secure N3 connection to the Internet. Patients will be given a written management plan and an emergency supply of antibiotics and steroids. Every morning the machine will monitor peak flow and oxygen saturation using validated instruments. This detail will be sent to clinicians in charge of the patients. The respiratory team routinely survey the online data and contact the patients if they have forgotten to send it or are unable to send it. If the information is outside the expected range the clinicians can contact the patient to repeat measurements and institute management. |
| Outcomes | Time until first hospital admission up to one year post‐randomisation, exacerbations and admissions including bed‐days, deaths, changes in medication, St Georges' Respiratory Questionnaire, hospital anxiety and depression scale, patient knowledge and self efficacy, lung function, patient engagement with procession. Cost effectiveness. |
| Notes | Trial setting in UK |
Kalter‐Leibovici 2009.
| Methods | Randomized open label active control parallel assignment efficacy study |
| Participants | Not yet open to participant recruitment |
| Interventions | Disease management and home telemonitoring in addition to best care according to clinical guidelines for COPD patients |
| Outcomes | Hospital admission for acute exacerbation of COPD or all‐cause mortality is the primary outcome. Secondary outcomes are total days of hospitalisation for acute exacerbations of COPD, quality of life, total number of acute exacerbations of COPD, depression, functional capacity, spirometry parameters. |
| Notes | Setting: Israel |
Koff 2006.
| Methods | Randomised pilot study |
| Participants | 40 GOLD stage III and stage IV patients with COPD |
| Interventions | An Internet‐based eHealth device 'the Health Buddy', spirometer, pedometer, oxygen saturation meter and other peripheral devices. |
| Outcomes | St George's Quality of Life Respiratory Questionnaire and costs of healthcare in US dollars, showed non significant improvement in health status and saving in costs for intervention group |
| Notes | Power calculations showed that a study in a larger group would be required to generate significant results |
NCT 00918905.
| Methods | Prevention randomized open label parallel assignment trial |
| Participants | Patients admitted acutely to the medical ward with an exacerbation of COPD |
| Interventions | Patients were discharged within 24 hours with a computer with web cam, microphone, spirometry and saturation monitoring equipment. A nurse used the equipment to observe the patient until their follow‐up appointment 4 weeks later. |
| Outcomes | Prevention of re‐admission to hospital within 4 weeks of discharge, duration of hospital re‐admission |
| Notes | Setting: OUH Svenborg Hospital, Svendborg, Denmark |
Prior 2009.
| Methods | Randomized open label parallel assignment safety/efficacy trial |
| Participants | Study not yet open for participant recruitment |
| Interventions | Integrated health monitoring, alarm handling and videoconferencing systems compared with usual care alone to delay transfer to nursing or elderly homes. |
| Outcomes | Health related quality of life as assessed by the SF 36 at 0, 15 and 30 months. Secondary outcomes include time to permanent transfer to elderly homes, total and average length of stay in hospital, number of consultations with GPs and medical specialists. No. of home visits by nurses and other secondary outcomes. |
| Notes | Setting: Belgium, patients with diabetes mellitus, chronic heart failure and chronic obstructive pulmonary disease. |
Troosters 2003.
| Methods | Structured education, supported discharge by telephone contacts and intensified interactions with primary were provided to the intervention group. |
| Participants | 42 patients admitted with acute exacerbations were randomly allocated to either usual care or intervention arm |
| Interventions | The intervention consisted of education during hospitalisation and supported discharge ‐ weekly contact with nurse specialist and contact with general practitioner |
| Outcomes | During the year of follow‐up, 48% of supported discharge patients were re‐admitted and 90% of usual care patients were re‐admitted. The median hospital free time was 388 days in telephone group and 86 days in the usual care group, P = 0.016. |
| Notes | The authors concluded that this low cost intervention merits implementation in clinical routine |
Vandivier 2010.
| Methods | Interventional treatment randomised open label, active control, parallel assignment, efficacy study |
| Participants | Male and female over 40 years old with airflow obstruction on spirometry defined as FEV1/FVC less than or equal to 70% and an FEV1 less than or equal to 50% predicted, or an FEV1 greater than 50% predicted with a history of a COPD exacerbation within the previous year. |
| Interventions | Integrated care involving COPD specific education, self management instruction, remote monitoring and enhanced communication with a co‐ordinator. |
| Outcomes | Healthcare utilization over 9 months, quality of life by St. Georges Respiratory Questionnaire, guideline‐based medical therapy, exercise capacity, oxygen utilization and pre‐ and post‐exercise oxygen saturations, BMI, obstruction, dyspnoea, exercise capacity and symptoms on MMRC Dyspnea scale |
| Notes | Undertaken in Colorado, USA in association with Kaiser Permanente and dept of veterans affairs. |
Characteristics of ongoing studies [ordered by study ID]
Brown 2009.
| Trial name or title | Evaluation of programs of coordinated care and disease management COCA |
| Methods | This is an extension of an original study in which 16 demonstration programs provided care coordination services to beneficiaries with chronic illness in medicare's fee for service program. A five‐year CMS‐funded study tested whether the programs can improve patients' use of medical services, improve outcomes and satisfaction with care and reduce Medicare costs. The study also assessed physicians' satisfaction with the programs. |
| Participants | Had a variety of conditions including congestive heart failure, diabetes, coronary artery disease, COPD, cancer, cerebrovascular disease, alzheimer's disease, psychotic disorder and major depression. |
| Interventions | Behavioural intervention: care co‐ordination consisting variously of nurse telephonic counselling nurse in‐patient home visits, home telemonitoring equipment and physician education and feedback. |
| Outcomes | Medicare program expenditures |
| Starting date | Feb 15 2008 |
| Contact information | Study directors: Randal S Brown, Mathematica Policy research and Carol A Magee, Centres from Medicare and Medicaid services. |
| Notes |
Carrieri‐Kohlman 2005.
| Trial name or title | Internet‐Based and Established Dyspnoea Self‐Management Programs in COPD Patients |
| Methods | Comparisons of electronic, Internet based dyspnoea self management program and face‐to‐face dyspnoea self management program |
| Participants | Age > 40 years, participants with mild COPD and ADL limited by dyspnoea, both genders |
| Interventions | The two programs have the same education exercise and monitoring components, however, one is administered via Internet the other is delivered face‐to‐face |
| Outcomes | Dyspnoea, exercise adherence and performance, pulmonary exacerbations |
| Starting date | Sept 2003 |
| Contact information | Carrieri‐Kohoman at the University of California and Nguyen at the University of Washington |
| Notes | This appears to be a record of the study which is published and covered in our "included studies" section under Nyguyen |
Finkelstein 2009.
| Trial name or title | Effectiveness of Home Automated telemanagement in Chronic Obstructive Pulmonary disorder |
| Methods | Randomized single blind (outcomes assessor) active control, parallel assignment efficacy study |
| Participants | 280 participants, 21 years and older and understands spoken English, physician diagnosis of COPD moderate to severe COPD according to NHLB/WHO global initiative for COPD (GOLD) classification stages II to III |
| Interventions | Creation of a computer program that can help people learn about their COPD and how to manage it themselves, then determination of whether the computer program, called Home Automated Telemanagement (HAT) helps patients with COPD in managing their disease and following their treatment plans. |
| Outcomes | Clinical health including lung function and respiratory symptoms, measured and baseline and every 3 months for 18 months. Disease specific quality of life, exercise tolerance, urgent health care utilization, self efficacy for COPD patients and activities of daily living. |
| Starting date | December 2003 |
| Contact information | Joseph Finkelstein, John Hopkins University, Baltimore, Maryland, USA |
| Notes |
ISRCTN 18443546.
| Trial name or title | Home telemonitoring for patients with COPD |
| Methods | Randomised controlled cross‐over trial |
| Participants | 240 subjects living with a primary diagnosis of COPD identified from hospital admissions database. If they have had two or more admissions to any of the following hospitals in the last two years: Prince Philip, West Wales General, Withybush or Bronglais, participants must be at least 40 years old. |
| Interventions | 120 will be randomised to receive telemonitoring for 1 year whilst the other 120 receive standard care. After 1 year the telemonitoring will be swapped into the homes of the second group in a cross‐over trial for 1 more year of monitoring. |
| Outcomes | Number of hospital admissions, quality of life measures healthcare contacts (GP visits, nurse contacts etc.) a cost evaluation will also be undertaken after the 2 years. |
| Starting date | ISRCTN date assigned 08/1/2010 |
| Contact information | De Keir Lewis Respiratory Centre, 01554‐783133 or k.e.lewis@swansear.ac.uk |
| Notes | Llanelli Wales |
Jerant 2005.
| Trial name or title | Homing in on Health, Study of a Home Delivered Chronic Disease Self Management Programme; A randomised Trial of Home Self‐Efficacy Enhancement |
| Methods | There will be 3 groups comparing the effectiveness and cost effectiveness of two models of home care with usual care to improve chronic illness outcomes. These will be in‐person visits and telephone calls compared with clinic visits. |
| Participants | Participants will suffer from diabetes mellitus, congestive heart failure, COPD, asthma, arthritis and depression. |
| Interventions | Home care will be delivered as above and will explore the mechanisms of effectiveness through its influence on self efficacy and adherence. |
| Outcomes | Health related Quality of Life at 2 weeks and 4 weeks during the intervention, immediately post‐intervention and at 6 months and 12 months, secondary outcomes include self care and self efficacy at these time points. |
| Starting date | Dec 2005 |
| Contact information | Contacts: Anthony F Jerant, MD, Contact: Monique Moore Hill, BA |
| Notes | Self efficacy is the beliefs patients have about their own abilities to successfully execute the actions required to achieve valued health outcomes. |
Mair 2001.
| Trial name or title | Acute Chest Triage Rapid Intervention Guided by Home Care or Telecare (ACTRIGHT) |
| Methods | A team of specialist nurses intercepts patients warranting admission with COPD exacerbation. They perform clinical assessment of the patient's suitability to be cared for at home. They are then randomised to either telecare or face‐to‐face nurse home visits. |
| Participants | All COPD patients presenting to the A and E department of Aintree University Hospital or Royal Liverpool Hospital were assessed for participation. |
| Interventions | Patients receive a medication package which includes antibiotics steroids and nebulised bronchodilators and social service report if required until the patient is stable. |
| Outcomes | Clinical outcomes and costs |
| Starting date | Ongoing |
| Contact information | University of Liverpool |
| Notes | Limited information available online |
Victor 2008.
| Trial name or title | A pilot study on the effect of telehealth on COPD patients in the Community |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | The number of emergency admissions owing to COPD in the intervention group as compared with the control group |
| Starting date | |
| Contact information | Doncaster Central, East and West Primary Care Trusts and Bassetlaw Primary Care Trust |
| Notes | No further information found |
Whiteford 2002.
| Trial name or title | Evaluation of the effect of a home‐based cognitive‐behavioural pulmonary rehabilitation programme on physiological and psychosocial outcomes in COPD patients. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | Aerobic and functional capacity , activity levels health status, quality of life, dyspnoea rating, lung function, self‐efficacy, stage and process of behavioural change. |
| Starting date | |
| Contact information | North Glasgow University Hospitals NHS Trust |
| Notes | No further information |
Contributions of authors
SM wrote the protocol with guidance and revisions from AS, JL and CP. SM and JL selected studies for inclusion. SM and UN undertook the data extraction.
Sources of support
Internal sources
No sources of support supplied
External sources
NHS Connecting for Health Evaluation Programme (NHS CFHEP 001), Not specified.
Edinburgh MRC Trials Methodology Hub (G0800803), Not specified.
NHS Education for Scotland, Clinical Fellowship, Not specified.
Declarations of interest
All of the authors are working on other projects in telehealth and e‐health funded by the NHS Connecting for Health Evaluation Programme. In addition, JL has worked on a Medicaid Funded project. SM was funded by a clinical fellowship from NHS Education for Scotland.
Edited (no change to conclusions)
References
References to studies included in this review
Bourbeau 2003 {published data only}
- Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease. Archives of Internal Medicine 2003;163:585‐91. [DOI] [PubMed] [Google Scholar]
Casas 2006 {published data only}
- Casas A, Troosters T, Garcia‐Aymerich J, Roca J, Hernandez C, Alonso A, et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. European Respiratory Journal 2006;28:123‐30. [DOI] [PubMed] [Google Scholar]
Chandler 1990 {published data only}
- Chandler MHH, Clifton GD, Louis BA, Coons SJ, Foster TS, Philips BA. Home monitoring of theophylline levels: a novel therapeutic approach. Pharmacotherapy 1990;10(4):294‐300. [PubMed] [Google Scholar]
de Toledo 2006 {published data only}
- Toledo P, Jimenez S, Pozo F, Roca J, Alonso A, Hernandez C. Telemedicine experience for chronic care in COPD. IEEE Transactions of Information Technology in Biomedicine July 2006;10(3):567‐73. [DOI] [PubMed] [Google Scholar]
Finkelstein 2004 {published data only}
- Finkelstein SM, Speedie SM, Demiris G, Veen M, Lundgren JM, Potthoff S. Telehomecare: quality, perception, satisfaction. Telemedicine Journal and e‐Health 2004;10(2):122‐8. [DOI] [PubMed] [Google Scholar]
Finkelstein 2006 {published data only}
- Finkelstein SM, Speedie SM, Potthoff S. Home telehealth improves clinical outcomes at lower cost for home healthcare. Telemedicine and eHealth 2006;12(2):128‐36. [DOI] [PubMed] [Google Scholar]
Garcia‐Aymerich 2007 {published data only}
- Garcia‐Aymerich J, Hernandez C, Alonso A, Casas Al, Rodriguez‐Roisin R, Anto JM, et al. Effects of an integrated care intervention on risk factors of COPD readmission. Respiratory Medicine 2007;101:1462‐9. [DOI] [PubMed] [Google Scholar]
Johnston 2000 {published data only}
- Johnston B, Wheeler L, Deuser J, Sousa KH. Outcomes of the Kaiser Permanente tele‐home health research project. Archives of Family Medicine 2000;9:40‐5. [DOI] [PubMed] [Google Scholar]
Nguyen 2008 {published data only}
- Nguyen HQ, Doneshy‐Cuenco D, Wolpin S, Reinke LF, Benditt JO, Paul SM, et al. Randomized controlled trial of an internet‐based versus face to face dyspnoea self‐management program for patients with chronic obstructive pulmonary disease: pilot study. Journal of Medical Internet Research 2008;10(2):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vitacca 2009 {published data only}
- Vitacca M, Bianchi L, Guerra A, Fracchia C, Spanevello A, Balbi B, et al. Tele‐assistance in chronic respiratory failure patients: a randomised clinical trial. European Respiratory Journal 2009;33:411‐8. [DOI] [PubMed] [Google Scholar]
Whitten 2007 {published data only}
- Whitten P, Mickus M. Home telecare for COPD/CHF patients outcomes and perceptions. Journal of Telemedicine and Telecare 2007;13:69‐73. [DOI] [PubMed] [Google Scholar]
Wong 2005 {published data only}
- Wong KW, Wong FKY, Chan MF. Effects of nurse‐initiated telephone follow‐up on self‐efficacy among patients with chronic obstructive pulmonary disease. Journal of Advanced Nursing 2005;49(2):210‐22. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aiken 2006 {published data only}
- Aiken LS, Butner J, Lockhart CA, Volk‐Craft BE, Hamilton G, Williams FG. Outcome evaluation of a randomised trial of the PhoenixCare Intervention: program of case management and coordinated care for the seriously chronically Ill. Journal of Palliative Medicine 2006;9(1):111‐26. [DOI] [PubMed] [Google Scholar]
Balas 1997 {published data only}
- Balas EA, Jaffrey F, Kuperman GJ, Boren SA, Brown GD, Pinicirolif F, et al. Electronic communication with patients : evaluation of distance medicine technology. JAMA 1997;278(2):152‐9. [PubMed] [Google Scholar]
Brooks 2002 {published data only}
- Brooks D. The effect of post‐rehabilitation programmes among individuals with chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(1):20‐9. [DOI] [PubMed] [Google Scholar]
Carrieri 2005 {published data only}
- Carrieri‐Kohlman V, Nguyen HQ, Donesky‐Cuenco D, Demier‐Deviren S, Neuhaus J Stulbarg MS. Impact of brief or extended exercise training on the benefit of a dyspnoea self‐management program in COPD. Journal of Cardiopulmonary Rehabilitation 2005;25(5):275‐84. [DOI] [PubMed] [Google Scholar]
Demiris 2003 {published data only}
- Demiris G, Speedie S, Finkelstein S, Harris I. Communication patterns and technical quality of virtual visits in home care. Journal of telemedicine and telecare 2003;9(4):210‐5. [DOI] [PubMed] [Google Scholar]
Donesky‐Cuenco 2003 {published data only}
- Donesky‐Cuenco D, Janson S, Neuhaus J, Neilands TB, Carrieri‐Kohlman V. Adherence to a home‐walking prescription in patients with chronic obstructive pulmonary disease [From dissertation: Adherence to exercise in patients with chronic obstructive pulmonary disease [Dissertation]]. Cardiovascular Prevention & Rehabilitation 2003;36(5):348‐63. [DOI] [PubMed] [Google Scholar]
Egan 2002 {published data only}
- Egan E, Clavarino A, Burridge L, Tueuwen M, White E. A randomised control trial of nursing‐based case management for patients with chronic obstructive pulmonary disease. Lippincotts Case Management 2002;7:170‐9. [DOI] [PubMed] [Google Scholar]
Farrero 2001 {published data only}
- Farrero E, Escarrabill J, Prats E, Maderal M, Manresa F. Impact of a hospital‐based home‐care program on the management of COPD patients receiving long‐term oxygen therapy. Chest 2001;119(2):364‐9. [DOI] [PubMed] [Google Scholar]
Gadoury 2005 {published data only}
- Gadoury MA. A disease specific self‐management intervention reduces hospital utilization in patients with COPD: the effect remains at two years [MSc Thesis]. McGill University 2004.
- Gadoury MA, Schwartzman K, Rouleau M, Maltais F, Julien M, Beaupré A, et al. Self‐management reduces both short‐ and long‐term hospitalisation in COPD. European Respiratory Journal 2005;26(5):853‐7. [DOI] [PubMed] [Google Scholar]
Griffiths 2005 {published data only}
- Griffiths C, Motlib J, Azad A, Ramsay J, Eldridge S, Feder G, et al. Randomised controlled trial of a lay‐led self management programme for Bangladeshi patients with chronic disease. British Journal of General Practice 2005;55(520):831‐7. [PMC free article] [PubMed] [Google Scholar]
Hernandez 2003 {published data only}
- Hernandez C, Casas A, Escarrabill J, Alonso J, Puig‐Junoy J, Farrero E, et al. Home hospitalisation of exacerbated chronic obstructive pulmonary disease patients. European Respiratory Journal 2003;21:58‐67. [DOI] [PubMed] [Google Scholar]
Hibbert 2003 {published data only}
- Hibbert D, Mair F, Angus R, May C, Boland A, Haycox A, et al. Lessons from the implementation of a home telecare service. Journal of Telemedicine and Telecare 2003;9(Suppl 1):55‐6. [DOI] [PubMed] [Google Scholar]
Hopp 2006 {published data only}
- Hopp F, Woodbridge P, Subramanian U, Copeland L, Smith D, Lowery J. Outcomes associated with a home care telehealth intervention. Telemedicine and eHealth 2006;12(3):297‐307. [DOI] [PubMed] [Google Scholar]
Jerant 2008 {published data only}
- Jerant A, Moore M, Lorig K, Franks P. Perceived control moderated the self‐efficacy‐enhancing effects of a chronic illness self‐management intervention. Chronic Illness 2008;4(3):173‐82. [DOI] [PubMed] [Google Scholar]
Jimison 2008 {published data only}
- Jimison H, Gorman P, Woods S, Nygren P, Walker M, Norris S, et al. Barriers and drivers of health information technology use for the elderly, chronically ill, and underserved. Evidence Report ‐ Technology Assessment 2008;175:1‐1422. [PMC free article] [PubMed] [Google Scholar]
Mair 1999 {published data only}
- Mair FS, Wilkinson M, Bonnar SA, Wootton, R, Angus RM. The role of telecare in the management of exacerbations of chronic obstructive pulmonary disease in the home. Journal of Telemedicine and Telecare 1999;5(Suppl 1):66‐7. [DOI] [PubMed] [Google Scholar]
Mair 2000a {published data only}
- Mair FS, Haycox A, May C, Williams T. A review of telemedicine cost‐effectiveness studies. Journal of Telemedicine and Telecare 2000;6(Suppl 1):S38‐40. [DOI] [PubMed] [Google Scholar]
Mair 2002 {published data only}
- Mair F, Boland A, Angus R, Haycox A, Hibbert D, Bonner S, et al. A randomized controlled trial of home telecare. Journal of Telemedicine and Telecare 2002;8(Suppl 2):S58‐60. [DOI] [PubMed] [Google Scholar]
Mair 2005 {published data only}
- Mair F, Goldstein P, May C, Angus R, Shiels C, Hibbert D, et al. Patient and provider perspectives on home telecare: preliminary results from a randomized controlled trial. Journal of Telemedicine and Telecare 2005;11(Suppl 1):S1:95‐7. [DOI] [PubMed] [Google Scholar]
Maltais 2005 {published data only}
- Maltais F, Bourbeau J, Lacasse Y, Shapiro S, Perrault H, Penrod J, et al. A Canadian multicentre randomized clinical trial of home‐based pulmonary rehabilitation in chronic obstructive pulmonary disease: Rationale and methods. Canadian Respiratory Journal 2005;12(4):193‐8. [DOI] [PubMed] [Google Scholar]
Martens 2007 {published data only}
- Martens JD, Weijden T, Severens JL, Clercq PA, Bruijn DP, Kester AD, et al. The effect of computer reminders on GP's prescribing behaviour: a cluster‐randomised trial. International Journal of Medical Informatics 2007;76(Suppl 3):S403‐16. [DOI] [PubMed] [Google Scholar]
Moore 2007 {published data only}
- Moore J, Fiddler H, Grant A, Johnson L, Jolley C, Seymour J, et al. A randomised controlled pilot study to assess the impact of a home exercise video on exercise tolerance and dyspnoea in COPD [Poster abstract]. European Respiratory Society Congress. 2007:[E3084].
Moxam 2007 {published data only}
- Moxham J. The effects of a home exercise video programme for patients with chronic obstructive pulmonary disease: pilot study. www.clinicaltrials.gov 2007. [ClinicalTrials.gov Identifier: NCT00542932]
Oh 2003 {published data only}
- Oh EG. The effects of home‐based pulmonary rehabilitation in patients with chronic lung disease. International Journal of Nursing Studies 2003;40(8):873‐9. [DOI] [PubMed] [Google Scholar]
Pare 2006 {published data only}
- Pare G, Sicotte C, St‐Jules D, Gauthier R. Cost‐minimization analysis of a telehomecare program for patients with chronic obstructive pulmonary disease. Telemedicine and eHealth 2006;12(2):114‐21. [DOI] [PubMed] [Google Scholar]
Petty 2006 {published data only}
- Petty TL, Dempsey EC, Collins T, Pluss W, Lipkus I, Cutter GR, et al. Impact of customized videotape education on quality of life in patients with chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation 2006;26(2):112‐7. [DOI] [PubMed] [Google Scholar]
Poels 2008 {published data only}
- Poels PJ, Schermer TR, Schellekens DP, Akkermans RP, Vries Robbé PF, Kaplan A, et al. Impact of a spirometry expert system on general practitioners' decision making. European Respiratory Journal 2008;31(1):84‐92. [DOI] [PubMed] [Google Scholar]
Rebuck 1996 {published data only}
- Rebuck DA, Hanania NA, D'Urzo AD, Chapman KR. The accuracy of a handheld portable spirometer. Chest 1996;109(1):152‐7. [DOI] [PubMed] [Google Scholar]
Ries 1995 {published data only}
- Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Annals of Internal Medicine 1995;122(11):823‐32. [DOI] [PubMed] [Google Scholar]
Ries 2003 {published data only}
- Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2003;167(6):880‐8. [DOI] [PubMed] [Google Scholar]
Sridhar 2008 {published data only}
- Sridhar M, Taylor R, Dawson S, Roberts NJ, Partridge MR. A nurse led intermediate care package in patients who have been hospitalised with an acute exacerbation of chronic obstructive pulmonary disease. Thorax 2008;63(3):194‐200. [DOI] [PubMed] [Google Scholar]
Tierney 2005 {published data only}
- Tierney WM, Overhage JM, Murray MD, Harris LE, Zhou XH, Eckert GJ, et al. Can computer‐generated evidence‐based care suggestions enhance evidence‐based management of asthma and chronic obstructive pulmonary disease? A randomized, controlled trial. Health Services Research 2005;40(2):477‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trappenburg 2008 {published data only}
- Trappenburg JC, Niesink A, Weert‐van Oene GH, Zeijden H, Snippenburg R, Peters A, et al. Effects of telemonitoring in patients with chronic obstructive pulmonary disease. Telemedicine and eHealth 2008;14(2):138‐46. [DOI] [PubMed] [Google Scholar]
Wasson 1992 {published data only}
- Wasson J, Gaudette C, Whaley F, Sauvigne A, Baribeau P, Welch HG. Telephone care as a substitute for routine clinic follow‐up. Journal of the American Medical Association 1992;267:1788‐3. [PubMed] [Google Scholar]
Welch 2000 {published data only}
- Welch HG, Johnson DJ, Edson R. Telephone care as an adjunct to routine medical follow‐up: a negative randomized trial. Effective Clinical Practice 2000;3:123‐30. [PubMed] [Google Scholar]
Whitten 2002 {published data only}
- Whitten PS, Mair FS, Haycox A, May CR, Williams TL, Hellmich S. Systematic review of cost effectiveness studies of telemedicine interventions. BMJ 2002;324(7351):1434‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN 12608000112369 {published data only}
- Walters J, Wood‐Baker R. Pathways to lung health:a comprehensive self‐management programme for chronic obstructive pulmonary disease in the community. Australian New Zealand Clinical Trials Registry (www. anzctr.org.au).
ACTRN 12609000428268 {published data only}
- Smith J. Telehealth Research Across the Community (TRAC:) An evaluation of telehealth home monitoring of home care clients with chronic obstructive pulmonary disease compared to usual care. www.anzctr.org.au.
Alonso 2004 {published data only}
- Alonso A. A new model for home care for COPD. Studies in Health Technology and Informatics 2004;103:368‐73. [PubMed] [Google Scholar]
Battaglia 2007 {published data only}
- Battaglia E, Madonini E, Amaducci S. The AIRTEM project: home telemonitoring of patients affected by COPD and chronic respiratory failure [Abstract]. European Respiratory Journal 2007;30(Suppl 51):779s. [Google Scholar]
Battaglia 2008 {published data only}
- Battaglia E, Luliano A, Amaducci S. Home telemonitoring program of patients affected by COPD and chronic respiratory failure. American Thoracic Society International Conference. 2008:Poster F3.
ISRCTN41424840 {published data only}
- Warm D, Lewis K. A new model for continuous care of chronic patients ‐ eCare and eLearning for patients with COPD. www.controlled‐trials.com 2008.
ISRCTN96634935 {published data only}
- Pinnock H. COPD: The impact of a telemetric COPD monitoring service TELESCOT. www.controlled‐trials.com 2009.
Kalter‐Leibovici 2009 {published data only}
- Kalter‐Leibovici O. Comprehensive disease management program in COPD patients in the community (COPD‐CDM). www.clinicaltrials.gov 2009. [NCT00982384]
Koff 2006 {published data only}
- Koff PB, Stevens CC, Cashman J, Greene KE, Jones RH, Vandivier RW, et al. Telemonitoring/ehealth management improves quality of life and healthcare expenditures in COPD [Abstract]. Proceedings of the American Thoracic Society. 2006:A123 [Poster J87].
NCT 00918905 {published data only}
- Madsen H. Nurse tele‐consultations with discharged COPD patients reduce the numbers of readmissions. ClinicalTrials.gov 2009. [ClinicalTrials.gov Identifier: NCT00918905]
Prior 2009 {published data only}
- Prior H. Randomized controlled trial of home telemonitoring for elderly people (DREAMING). www.clinicaltrials.gov 2009. [ClinicalTrials.gov Identifier: NCT00893685]
Troosters 2003 {published data only}
- Troosters T, Celis G, Deprez S, Spruit MA, Gosselink R, Decramer M. Telephone supported discharge reduces readmission after acute exacerbations in COPD. American Thoracic Society 99th International Conference. 2003:Poster C72.
Vandivier 2010 {published data only}
- Vandivier W. Advanced ehealth for chronic obstructive pulmonary disease in Colorado. www.clinicaltrials.gov 2010: NCT01044927. [ClinicalTrials.gov Identifier: NCT01044927]
References to ongoing studies
Brown 2009 {published data only}
- Brown RS, Magee CA. Evaluation of programs of coordinated care and disease management (COCA). www.clinicaltrials.gov 2009. [ClinicalTrials.gov Identifier: NCT00627029]
Carrieri‐Kohlman 2005 {published data only}
- Carrieri‐Kohlman V, Nguyen HQ. Comparing the effects of an internet‐based to an established dyspnea self‐management program on dyspnea, exercise behavior, and pulmonary exacerbations in patients with COPD. www.clinicaltrials.gov 2005.
Finkelstein 2009 {published data only}
- Finkelstein J. Effectiveness of home automated telemanagement in chronic obstructive pulmonary disorder. www.clinicaltrials.gov 2009. [ClinicalTrials.gov Identifier: NCT00752531]
ISRCTN 18443546 {published data only}
- Lewis K. Home telemonitoring for patients with COPD. www.controlled‐trials.com.
Jerant 2005 {published and unpublished data}
- Jerant A, Hill M. Homing in on Health: study of a home delivered chronic disease self management programme. www.clinicaltrials.gov.
Mair 2001 {published data only}
- Mair F. Acute chest triage rapid intervention guided by home care or telecare (ACTRIGHT). National Research Register (UK) 2002. [NRR Identifier: M0009108189]
Victor 2008 {published data only}
- Victor J. A pilot study on the effects of telehealth on copd patients in the community (TELECCOM). National Research Register (UK) 2007. [NRR Identifier: N0601191999]
Whiteford 2002 {published data only}
- Whiteford S. Evaluation of the effect of a home‐based cognitive‐behavioural pulmonary rehabilitation programme on physiological and psychosocial outcomes in COPD patients. National Research Register (UK) 2003. [NRR Identifier: N0394118760]
Additional references
Bourbeau 2006
- Bourbeau J, Collet JP, Schwartzman K, Ducruet T, Nault D, Bradley C. Economic benefits of self‐management eduction in COPD. Chest 2006;130:1704‐11. [DOI] [PubMed] [Google Scholar]
Busey 2008
- Busey C, Michael P. Telehealth opportunities and pitfalls. Journal of the American Dietetic Association 2008;108(8):1296‐301. [DOI] [PubMed] [Google Scholar]
Currell 2008
- Currell R, Urquhar C, Wainwright P, Lewis R. Telemedicine versus face to face patient care:effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002098] [DOI] [PubMed] [Google Scholar]
Darzi 2008
- Lord Darzi of Denham. High quality care for all: NHS Next Stage Review final report (Command paper CM 7432). Department of Health 2008.
Finkelstein 2000
- Finkelstein J, Friedman RH. Potential role of telecommunication technologies in the management of chronic health conditions. Disease Management and Health Outcomes 2000;2:57‐63. [Google Scholar]
GOLD 2007
- Global Initiative for Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of COPD. www.goldcopd.com 2007.
Hajiro 2002
- Hajiro T, Nishimura K. Minimal clinically significant difference in health status: the thorny path of health status measures. European Respiratory Journal 2002;19:390‐1. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. [Website: http://www.cochrane.org/resources/handbook/hbook.htm] [Google Scholar]
HRSA
- USA Health Resources and Services Administration. http://www.hrsa.gov/telehealth/ (accessed 26th Sept 2008).
Lorentz 2008
- Lorentz M. Telenursing and home healthcare. Home Healthcare Nurse 2008;4:26. [DOI] [PubMed] [Google Scholar]
Mahen 2006
- Mahen MM, Allen A. E‐Health & Telehealth Glossary. http://telehealth.net/glossary.html (accessed 26th Sept 2008) 2006.
Mair 2000b
- Mair F, Whitten P. Systematic review of studies of patient satisfaction with telemedicine. BMJ 2000;320:1517‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mannino 2007
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765‐73. [DOI] [PubMed] [Google Scholar]
Miller 2007
- Miller E. Solving the disjuncture between research and practice; Telehealth trends in the 21st Century. Health Policy 2007;82:133‐41. [DOI] [PubMed] [Google Scholar]
Murray 2006
- Murray S, Pinnock H, Sheikh A. Palliative care for COPD: we need to meet the challenge. Primary Care Respiratory Journal 2006;15(6):362‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polisena 2010
- Polisena J, Tran K, Cimon K, Hutton B, McGill S, Palmer K, et al. Home telehealth for chronic obstructive pulmonary disease: a systematic review and meta‐analysis. Journal of Telemedicine and Telecare 2010;16:120‐7. [DOI] [PubMed] [Google Scholar]
Pride 2002
- Pride NB, Soriano JB. Chronic obstructive pulmonary disease in the United Kingdom: trends in mortality, morbidity and smoking. Current Opinion in Pulmonary Medicine 2002;8:95‐101. [DOI] [PubMed] [Google Scholar]
Puhan 2006
- Puhan M, Soesilo I, Guyatt G, Schunemann HJ. Combining scores from different patient reported outcome measures in meta‐analyses: when is it justified?. Health and Quality of Life Outcomes 2006;4:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
The Lancet 2007
- The Editors. Beyond the lungs ‐ a new view of COPD. The Lancet 2007;370:713. [DOI] [PubMed] [Google Scholar]


