Abstract
Background
A significant number of patients who suffer with anxiety and related disorders (that is post‐traumatic stress disorder (PTSD), social anxiety disorder (SAnD), panic disorder with or without agoraphobia (PD), specific phobia (SPh) and obsessive compulsive disorder (OCD)) fail to respond optimally to first‐line treatment with medication or cognitive and behavioural therapies. The addition of d‐cycloserine (DCS) to cognitive and behavioural therapies may improve treatment response by impacting the glutamatergic system. This systematic review aimed to investigate the effects of adding DCS to cognitive and behavioural therapies by synthesising data from relevant randomised controlled trials and following the guidelines recommended by Cochrane.
Objectives
To assess the effect of DCS augmentation of cognitive and behavioural therapies compared to placebo augmentation of cognitive and behavioural therapies in the treatment of anxiety and related disorders. Additionally, to assess the efficacy and tolerability of DCS across different anxiety and related disorders.
Search methods
This review fully incorporates studies identified from a search of the Cochrane Depression, Anxiety and Neurosis Controlled Trials Register (CCDANCTR) to 12 March 2015. This register includes relevant randomised controlled trials (RCTs) from: the Cochrane Library (all years), EMBASE (1974 to date), MEDLINE (1950 to date), PsycINFO (1967 to date), the World Health Organization’s trials portal (ICTRP) and ClinicalTrials.gov . Reference lists from previous meta‐analyses and reports of RCTs were also checked. No restrictions were placed on language, setting, date or publication status.
Selection criteria
All RCTs of DCS augmentation of cognitive and behavioural therapies versus placebo augmentation of cognitive and behavioural therapies for anxiety and related disorders were included.
Data collection and analysis
Two authors (RO and TA) independently assessed RCTs for eligibility and inclusion, extracted outcomes and risk of bias data and entered these into a customised extraction form. Investigators were contacted to obtain missing data. In addition, data entry and analysis were performed by two review authors (KSW and HB).
Main results
Twenty‐one published RCTs, with 788 participants in outpatient settings, were included in the review. Sixteen studies had an age range of 18 to 75 years, while four investigated paediatric populations aged 8 to 17 years and one included children, adolescents and adults. The 21 RCTs investigated OCD (number of RCTs (N) = 6), PTSD (N = 5), SAnD (N = 5), SPh (N = 3) and PD (N = 2). Most information from the studies was rated as having either low risk or unclear risk of bias.
There was no evidence of a difference between DCS augmentation of cognitive and behavioural therapies and placebo augmentation of cognitive and behavioural therapies for the treatment of anxiety and related disorders in adults at the endpoint (treatment responders, N = 9, risk ratio (RR) 1.10; 95% confidence interval (CI) 0.89 to 1.34; number of participants (n) = 449; low quality evidence) and between 1 and 12 months follow‐up (N = 7, RR 1.08; 95% CI 0.90 to 1.31; n = 383). DCS augmentation of cognitive and behavioural therapies was not superior to placebo augmentation of cognitive and behavioural therapies for children and adolescents, both at the endpoint (N = 4, RR 1.01; 95% CI 0.78 to 1.31; n = 121; low quality evidence) and between 3 and 12 months follow‐up (N = 3, RR 0.86; 95% CI 0.67 to 1.09; n = 91).
There was no evidence of a difference in treatment acceptability for DCS augmentation of cognitive and behavioural therapies compared with placebo augmentation of cognitive and behavioural therapies in adults (N = 16, RR 0.88; 95% CI 0.61 to 1.25; n = 740), nor in children and adolescents (N = 4, RR 0.90; 95% CI 0.17 to 4.69; n = 131). These conclusions were based on moderate quality evidence for adults, and very low quality evidence for children and adolescents. Although the observed difference was small, it is noteworthy that there was a high efficacy of exposure‐based therapies alone in the included trials. Due to the limited number of studies, subgroup analysis of moderating factors for clinical and methodological effect could not take place.
Authors' conclusions
This review found no evidence of a difference between DCS augmentation of cognitive and behavioural therapies and placebo augmentation of cognitive and behavioural therapies for treating anxiety and related disorders in children, adolescents and adults. These findings are based on low quality evidence from heterogenous studies with small sample sizes and incomplete data for clinical response, which precludes us from drawing conclusions on the use of DCS augmentation of cognitive and behavioural therapies at this stage. Given there is some promising preliminary data from individual studies, further research is necessary to assess DCS compared with placebo augmentation of cognitive and behavioural therapies, and determine mechanisms of action as well as magnitude of effect in anxiety and related disorders.
Keywords: Adolescent, Adult, Aged, Child, Humans, Middle Aged, Anxiety Disorders, Anxiety Disorders/therapy, Cognitive Behavioral Therapy, Cognitive Behavioral Therapy/methods, Combined Modality Therapy, Combined Modality Therapy/methods, Cycloserine, Cycloserine/therapeutic use, Randomized Controlled Trials as Topic
Plain language summary
Addition of d‐cycloserine to cognitive and behavioural therapies for the treatment of anxiety and related disorders
Why is this review important?
Many people suffer from anxiety and related disorders (post‐traumatic stress disorder, social anxiety disorder, panic disorder with or without agoraphobia, specific phobia and obsessive compulsive disorder). These disorders are disabling and can affect a person’s ability to function well at work and in social situations. Current treatment options include talking therapies such as cognitive and behavioural therapies. Many patients, however, do not respond as well as hoped to these treatments. Using cognitive and behavioural therapies in combination with certain medicines, for example d‐cycloserine (DCS), is one option that may improve treatment response. In this review we examined the evidence for DCS combined with cognitive behavioural therapies as a treatment for anxiety and related disorders in children, adolescents and adults.
Who may be interested in this review?
‐ People with anxiety and related disorders.
‐ Families and friends of people who suffer from anxiety and related disorders.
‐ General practitioners, psychiatrists, psychologists and pharmacists.
‐ Professionals working in adult as well as child and adolescent mental health services.
What does this review aim to answer?
‐ Is treatment with DCS in combination with cognitive and behavioural therapies more effective than treatment with placebo (dummy pill) and cognitive and behavioural therapies for anxiety and related disorders?
‐ Is treatment with a combination of DCS and cognitive and behavioural therapies more effective in some anxiety and related disorders compared to others?
‐ How acceptable is DCS to patients and do people withdraw from treatment?
Which studies were included in the review?
We searched medical databases to find reports of clinical trials (specifically randomised controlled trials) published up to 12 March 2015 that investigated the treatment of anxiety and related disorders using DCS combined with cognitive and behavioural therapies. To be included in the review, trials had to compare the combined treatment of DCS and a cognitive and behavioural therapy with combined treatment of a placebo and a cognitive and behavioural therapy for anxiety and related disorders. We included studies with participants of all ages.
We included 21 studies in the review, with a total of 788 participants.
What does the evidence from the review tell us?
There was no evidence of a difference between combined treatment with DCS and cognitive and behavioural therapies, and combined treatment with placebo and cognitive and behavioural therapies for anxiety and related disorders in children, adolescents or adults. This conclusion was based on low quality evidence mainly due to small sample sizes and inconsistency across studies.
There was no evidence of a difference in the number of children, adolescents and adults who withdrew from treatment with DCS in addition to cognitive behavioural therapies, and those who withdrew from treatment with placebo in addition to psychological therapies.
What should happen next?
More trials are needed to enable a clearer understanding of the effect of treatment with DCS in combination with cognitive and behavioural therapies.
Summary of findings
Summary of findings for the main comparison. DCS and cognitive and behavioural therapies compared to placebo and cognitive and behavioural therapies for anxiety disorders in adults.
| Augmentation of cognitive and behavioural therapies with DCS compared to placebo for anxiety disorders in adults at end of treatment | ||||||
|
Patient or population: Adults with anxiety disorders
Settings: Outpatient settings in Australia, Germany, the Netherlands and the USA
Intervention: Augmentation of cognitive and behavioural therapies with DCS Comparison: Cognitive and behavioural therapies and placebo pill | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo and cognitive and behavioural therapies | DCS and cognitive and behavioural therapies | |||||
|
Treatment efficacy: treatment responders As assessed per study |
59 per 100 | 65 per 100 (53 to 79) | RR 1.1 (0.89 to 1.34) | 449 (9 studies) | ⊕⊕⊝⊝ low1,2 | Subgroups included: OCD (1 study), PD (1), PTSD (3), SAnD (1), and SPh (3). For PD a single study showed an improvement with DCS compared to placebo, RR 2.25 (1.04 to 4.86) |
| Treatment acceptability: withdrawals from treatment | 23 per 100 | 20 per 100 (14 to 28) | RR 0.88 (0.61 to 1.25) | 740 (16 studies) | ⊕⊕⊕⊝ moderate3 | Subgroups included: OCD (3), PD (1), PTSD (4), SAnD (5), and SPh (3) |
|
In remission As assessed per study |
30 per 100 | 35 per 100 (24 to 52) | RR 1.16 (0.79 to 1.71) | 292 (5 studies) | ⊕⊕⊕⊝ moderate4 | Subgroups included: OCD (1), PTSD (2), SAnD (1), and SPh (1) |
| Condition‐specific anxiety symptoms As assessed by LSAS5 (scale from: 0 to 144, better indicated by a lower score. 55 to 65 points = moderate social anxiety disorder (Liebowitz 1987)) | The mean condition‐specific anxiety symptoms in the control groups was 57.79 points | The mean condition‐specific anxiety symptoms in the intervention groups was 6.55 points lower (11.88 to 1.43 lower), which may represent a clinically important improvement since the mean in the control group was 57.79 points (moderate social anxiety on the LSAS scale) and the mean in the intervention group was 6.55 points lower (below the cut‐off for moderate social anxiety on the LSAS scale). |
MD** ‐6.55 (‐11.88 to ‐1.43) |
735 (17 studies) | ⊕⊕⊕⊝ moderate6 | Subgroups included: OCD (3), PD (2), PTSD (4), SAnD (5), and SPh (3). Little or no difference was found with DCS compared to placebo for OCD, SMD ‐0.14 (‐0.61 to 0.33); PTSD, SMD ‐0.06 (‐0.52 to 0.39); SAnD, SMD ‐0.39 (‐0.99 to 0.21); and SP, SMD ‐0.51 (‐1.14 to 0.13) |
| Co‐morbid symptoms of depression As assessed by BDI‐II7 (scale from: 0 to 63, better indicated by a lower score) | The mean co‐morbid symptoms of depression in the control groups was 10.73 points | The mean co‐morbid symptoms of depression in the intervention groups was 2.25 points lower (7.2 lower to 2.79 higher) |
MD** ‐2.25 (‐7.22 to 2.79) |
178 (5 studies) | ⊕⊕⊕⊝ moderate8 | Subgroups included: OCD (2), PD (1), and PTSD (2). For OCD two studies found an improvement with DCS compared to placebo, SMD ‐1.64 (‐1.23 to ‐0.04) |
| Co‐morbid anxiety symptoms As assessed by BAI9 (scale from: 0 to 63, better indicated by a lower score. 8 to 15 points = mild anxiety, 16 to 25 points = moderate anxiety (Beck 1993)) | The mean co‐morbid anxiety symptoms in the control groups was 19.5 points | The mean co‐morbid anxiety symptoms in the intervention groups was 8.82 points lower (13.85 to 3.64 lower), which may represent a clinically important improvement since the mean in the control group was 19.5 points (moderate anxiety on the BAI scale) and the mean in the intervention group was 8.82 points lower (mild anxiety on the BAI scale). |
MD** ‐8.82 (‐13.85 to ‐3.64) |
122 (3 studies) | ⊕⊕⊝⊝ low10,11 | Subgroups included: PD (1), PTSD (1) and (SAnD (1). Little or no difference was found with DCS compared to placebo for PD, MD ‐1.52 (‐1.16 to 0.12), and for SAnD, MD ‐0.70 (‐1.73 to 0.32) |
| Quality of life Assessed by LIS (scale from: 0 to 48, better indicated by a lower score) | The mean quality of life in the control group was 30.75 points | The mean quality of life in the intervention group was 5.32 points lower (9.87 to 0.77 lower) |
MD ‐5.32 (‐9.87 to ‐0.77) |
56 (1 study) | ⊕⊝⊝⊝ very low12,13 | Subgroups included: SAnD (1) |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** Back‐estimated from the SMD, see footnotes for further details. BAI: Beck Anxiety Inventory; BDI‐II: Beck Depression Inventory‐II; CI: Confidence interval; DCS: d‐cycloserine; LSAS: Liebowitz Social Anxiety Scale; LIS: Life Interference Scale; MD: mean difference; OCD: Obsessive compulsive disorder; PD: Panic disorder; PTSD: Post‐traumatic stress disorder; RR: Risk ratio; SAnD: social anxiety disorder; SMD: standardised mean difference; SPh: Specific phobia | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded 1 step due to risk of bias: five out of the nine included studies did not report on method of allocation concealment sufficiently. 2 Downgraded 1 step due to inconsistency: there was substantial heterogeneity between subgroups (I2 = 57%). 3 Downgraded 1 step for indirectness: withdrawals from treatment is not a direct measure of treatment acceptability to the participants. There could be other reasons for dropping out.
4 Downgraded 1 step for risk of bias: In remission is at risk of selective reporting bias as only five out of 16 studies reported on this important outcome.
5Five of the 16 studies used the LSAS. Scores were back‐estimated to the LSAS from the SMD ‐0.32 (‐0.58 to ‐0.07) using the control group SD 20.4802 from the representative study Hofmann 2013. 6 Downgraded 1 step for inconsistency: there was substantial heterogeneity between subgroups (I2 = 60%).
7 Three of the five studies used the BDI‐II. Scores were back‐estimated to the BDI‐II from the SMD ‐0.25 (‐0.80 to 0.31) using the control group SD 9.0 from the representative study Storch 2007. 8 Downgraded 1 step for inconsistency: there was substantial heterogeneity between subgroups (I2 = 68%). 9 One of the two studies used the BAI. Scores were back‐estimated to the BAI from the SMD ‐0.63 (‐0.99 to ‐0.26) using the control group SD 13.9921 from the representative study Siegmund 2011.
10 Downgraded 1 step for risk of bias: Two out of the three included studies had a high drop‐out rate and one study was of high risk of bias for allocation concealment and blinding.
11 Downgraded 1 step for imprecision: the total sample size is lower than the calculated optimal information size. 12 Downgraded 2 steps for imprecision: the total sample size is lower than the calculated optimal information size, and one study reported on this outcome. 13 Downgraded 1 step for indirectness: measuring the impact of an individual’s social fears on various components of their life is not a direct measure of quality of life, which includes many more factors.
Summary of findings 2. DCS and cognitive and behavioural therapies compared to placebo and cognitive and behavioural therapies for anxiety disorders in children and adolescents.
| Augmentation of cognitive and behavioural therapies with DCS compared to placebo for anxiety disorders in children and adolescents at end of treatment | ||||||
|
Patient or population: Children and adolescents with anxiety disorders
Settings: Outpatient settings in Australia, the UK and the USA
Intervention: Augmentation of cognitive and behavioural therapies with DCS Comparison: Cognitive and behavioural therapies and placebo pill | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo and cognitive and behavioural therapies | DCS and cognitive and behavioural therapies | |||||
|
Treatment efficacy: treatment responders As assessed per study |
77 per 100 | 78 per 100 (60 to 100) | RR 1.01 (0.78 to 1.31) | 121 participants (4 studies) | ⊕⊕⊝⊝ low1 | Subgroups included OCD (3 studies) and PTSD (1) |
| Treatment acceptability: withdrawals from treatment | 9 per 100 | 8 per 100 (2 to 43) | RR 0.90 (0.17 to 4.69) | 131 participants (4 studies) | ⊕⊝⊝⊝ very low1,2 | Subgroups included OCD (3) and PTSD (1) |
|
In remission As assessed per study |
45 per 100 | 54 per 100 (30 to 98) | RR 1.19 (0.66 to 2.16) | 44 participants (2 studies) | ⊕⊕⊝⊝ low1 | Subgroups included OCD (2) |
| Condition‐specific anxiety symptoms As assessed by Y‐BOCS3 (scale from 0 to 40, better indicated by a lower score) | The mean condition‐specific anxiety symptoms ranged across control groups from 11‐13.8 points | The mean condition‐specific anxiety symptoms in the intervention groups was 0.46 higher (3.63 lower to 4.55 higher) | MD** 0.46 (‐3.63 to 4.55) | 131 participants (4 studies) | ⊕⊝⊝⊝ very low1,4 | Subgroups included: OCD (3) and PTSD (1). For OCD one study found an improvement with placebo compared to DCS, SMD 0.70 (0.17 to 1.24) |
| Co‐morbid symptoms of depression As assessed by CDI5 (scale from 0 to 54, better indicated by a lower score) | The mean co‐morbid symptoms of depression ranged across control groups from 2.2‐16.2 points | The mean co‐morbid symptoms of depression in the intervention groups was 0.62 higher (4.06 lower to 5.38 higher) | MD** 0.62 (‐4.06 to 5.38) | 114 participants (3 studies) | ⊕⊝⊝⊝ very low1,6 | Subgroups included OCD (2) and PTSD (1). For PTSD one study found an improvement with placebo compared to DCS, SMD 0.60 (0.06 to 1.13) |
| Co‐morbid anxiety symptoms As assessed by each study | See comment | 104 participants (3 studies) | ⊕⊝⊝⊝ very low1,7,8 | Heterogeneity was considerable (I2 = 77%), consequently no pooled estimate was calculated. Subgroups included OCD, SMD ‐0.35 (‐0.93 to 0.23, 2 studies, 47 participants) and PTSD, SMD 0.80 (0.26 to 1.34, 1 study, 57 participants) | ||
| Quality of life | No study was found that reported on this outcome. | |||||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **Back‐estimated from the SMD, see footnotes for further details. CDI: Child Depression Inventory; CI: Confidence interval; DCS: d‐cycloserine; MASC: Multidimensional Anxiety Scale for Children; OCD: Obsessive compulsive disorder; PD: Panic disorder; PTSD: Post‐traumatic stress disorder; RR: Risk ratio; SAnD: social anxiety disorder; SMD: standardised mean difference; Y‐BOCS: Yale‐Brown Obsessive Compulsive Scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded 2 steps for imprecision: the total sample size is lower than the calculated optimal information size, and the confidence intervals are wide including both appreciable benefit and no effect. 2 Downgraded 1 for indirectness: withdrawals from treatment is not a direct measure of treatment acceptability. 3 Three of the four studies used the Y‐BOCS. Scores were back‐estimated to the Y‐BOCS from the SMD 0.07 (‐0.55 to 0.69) using the control group SD 6.6 from the representative study Storch 2010. 4 Downgraded 1 step for inconsistency: there was substantial heterogeneity between subgroups (I2 = 66%). 5 Two of the three studies used the CDI. Scores were back‐estimated to the CDI from the SMD 0.08 (‐0.52 to 0.69) using the control group SD 7.8 from the representative study Scheeringa 2014. 6 Downgraded 1 step for inconsistency: there was substantial heterogeneity between subgroups (I2 = 60%). 7 Downgraded 1 step for risk of bias: two of the three included studies did not report details on allocation concealment. 8 Downgraded 1 step for inconsistency: there was considerable heterogeneity between subgroups (I2 = 77%), consequently the subgroups were not pooled.
Background
Description of the condition
Anxiety and related disorders including generalized anxiety disorder (GAD), obsessive compulsive disorder (OCD), panic disorder (PD), post‐traumatic stress disorder (PTSD), specific phobia (SPh), and social anxiety disorder (SAnD) are the most prevalent class of psychiatric disorders, affecting up to 29% of United States of America (US) citizens at some point in their lives (Kessler 2005) and 13.6% of Europeans (Alonso 2004). The anxiety disorders are associated with significant co‐morbidity (Kessler 1994; Kessler 2005), disability, and impaired quality of life (Mendlowicz 2000) and also contribute significantly to the global burden of disease and disability adjusted life years (Murray 2013).
Anxiety disorders are identified after careful history taking and physical history and examination to exclude general medical conditions as the cause of symptoms. They are diagnosed according to criteria specified in the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5), or the International Classification of Diseases and Related Health Problems, 10th revision (ICD‐10) (APA 2013; WHO 1993).
From a biological perspective, anxiety disorders have been associated with disrupted modulation of various central neurotransmitter systems, including the gamma‐aminobutyric acid (GABA), noradrenergic and serotonergic systems (Ressler 2000). From a psychological perspective, learning and cognitive theories largely predominate in the explanation and treatment of anxiety disorders. According to learning theory, individuals develop associations between threatening stimuli (conditioned stimulus) and adverse outcomes (unconditioned stimulus). Anxiety disorders develop when individuals develop irrational associations or associations that lead to excessive symptoms (Yates 2012).
Description of the intervention
Fear conditioning occurs as a conditioned response (CR) and is created when a conditioned stimulus (CS) is paired with an aversive unconditioned stimulus (US). These CRs can be decreased, or extinguished, by the repeated presentation of the CS in the absence of the US. Pavlovian fear conditioning and extinction are relevant to the neurobiology of anxiety and related disorders (Rothbaum 2003). Further, extinction learning refers to the gradual, within‐session decrements of conditioned fear responses (Quirk 2000). This forms the basis for exposure and response prevention (ERP) therapies. The cognitive behaviour therapy (CBT) model suggests that dysfunctional thoughts are causally related to emotional distress, and that correcting these dysfunctional thoughts results in improvement of the distress and maladaptive behaviours. CBT is a short‐term individual or group treatment. The treatment introduces cognitive restructuring techniques and the exposure rationale. Specifically, patients practise identifying maladaptive cognitions (automatic thoughts), observing the association between anxious mood and automatic thoughts, examining the errors of logic, and formulating rational alternatives to their automatic thoughts. Patients also learn to identify avoidance strategies and to eliminate them while exposing themselves to anxiety‐provoking situations. Patients then confront increasingly difficult feared situations while applying cognitive restructuring techniques and eliminating any forms of avoidance strategies. Behavioural experiments are utilized to confront specific reactions to exposure experiences (Hofmann 2011).
CBT has been shown to be successful in reducing the severity of anxiety symptoms in PTSD, OCD, SAnD, PD and SPh (NICE 2011). A number of specific pharmacologic agents, such as the selective serotonin reuptake inhibitors (SSRIs) and the noradrenergic and serotonin selective reuptake inhibitors (SNRIs), have also proven to have efficacy as first‐line treatments for anxiety disorders and are recommended by expert consensus for this purpose (Baldwin 2011; Bandelow 2008; CPA 2006).
Despite the availability of efficacious pharmacotherapy and cognitive and behavioural therapies for anxiety disorders, a significant number of patients with these conditions fail to respond optimally to first‐line interventions. Treatment failure rates are estimated at 40% to 60% in OCD (Pallanti 2002) and 20% to 40% in PD (Bandelow 2004). Poor patient adherence may limit the efficacy of treatment, while treatment with certain agents (that is benzodiazepines) carries the risk of dependency (Shader 1993).
Multimodal treatment regimes combining cognitive and behavioural therapies with pharmacotherapy represent one potential approach to maximise treatment response. However, the results of clinical trials employing this approach have been mixed (Black 2006; Furukawa 2006; Mitte 2005). Where there is evidence in some trials of an increased response to combination treatment, this may be jeopardised by higher relapse rates once treatment has been discontinued (Barlow 2000; Marks 1993). In general, it has been found that combination treatments are not more efficacious than monotherapies (Hofmann 2009).
The finding that the N‐methyl‐D‐aspartate (NMDA) receptor complex is critical for associative learning suggests that a pharmacological agent might usefully be employed to potentiate the learning effects of cognitive and behavioural therapies. Animal studies have demonstrated that NMDA antagonists prevent both acquisition and extinction of conditioned responses (Lee 1998; Lee 2006; Szapiro 2003). Conversely, administration of d‐cycloserine (DCS), a partial agonist that binds to the glycine site at the NMDA receptor, has been associated with enhanced fear extinction in multiple animal studies employing diverse paradigms (Ledgerwood 2003; Ledgerwood 2005; Walker 2002). DCS may therefore possess clinical potential in augmenting responses to cognitive and behavioural therapies for anxiety disorders. Findings from Jones 2002 and Tiihonen 2006 would not necessarily be relevant to this hypothesis, since both tested DCS only as monotherapy for treating psychiatric disorders.
Research on cognitive enhancers for the CBT of anxiety and related disorders is ongoing and includes DCS, methylene blue, catecholamines (dopamine and noradrenaline), yohimbine (selective competitive alpha2‐adrenergic receptor antagonist), modafinil, endocannabinoids, cortisol, and nutrients and botanicals (omega‐3 fatty acids, caffeine and nicotine) as potential cognitive enhancers. Of these substances, DCS has so far been the best studied (Hofmann 2009; Sulkowski 2014).
How the intervention might work
While the exact mechanism of action of DCS is unknown, there is some evidence that it achieves its effects during the consolidation stage of the formation of new memories (Richardson 2004) through the downstream modulation of protein synthesis in the amygdala, a region of the brain implicated in the processing of fearful stimuli (Yang 2005). Further, there is evidence that the chronic application of partial agonists desensitises the glycine site of the NMDA receptor (Boje 1993). This is consistent with clinical findings of the reduced efficacy of DCS in treating anxiety disorders when given over extended periods of time (Kushner 2007). However, to date, we do not know what the optimal dosing schedule is for DCS administration in conjunction with exposure therapy. In contrast, the administration of anti‐depressants typically only results in detectable improvements in symptoms after weeks of sustained treatment.
Why it is important to do this review
A meta‐analysis of the efficacy of augmenting fear extinction and exposure therapy with DCS (Norberg 2008) included data from a number of published randomised controlled trials (RCTs) that addressed the efficacy of cognitive and behavioural therapies (Guastella 2008; Hofmann 2006; Kushner 2007; Ressler 2004; Storch 2007). This meta‐analysis observed a moderate treatment effect (Cohen's d = 0.6) for patients diagnosed with a range of anxiety and related disorders (PD, OCD, SAnD, SPh) who received exposure‐based therapy augmented with DCS. A robust association was detected between the magnitude of the effect and the timing of medication administration, with medication administered closer to the start of the exposure therapy being more efficacious. Similar results were found with Rodrigues 2014 where DCS appeared to be efficacious when administered a limited number of times closer to the exposure therapy and at low doses. These findings are in contrast to a meta‐analysis by Bontempo 2012 in which no evidence of an effect of dose timing, number or dosage of D‐cycloserine was seen on reported efficacy in the ranges assessed.
The synthesis of data from RCTs of DCS augmentation of cognitive and behavioural therapies for the treatment of anxiety and related disorders would therefore allow one to obtain a more reliable estimate of the magnitude of the treatment effect, as well as allow investigation of clinical and methodological mediators of this strategy. A meta‐analysis would also help determine the extent to which the efficacy of DCS is consistent across anxiety disorders. Moreover, following the guidelines recommended by The Cochrane Collaboration to minimise systematic sources of bias provides some assurance of the accuracy of the effect size estimates obtained in this review.
The outcome of the review would be beneficial for clinicians deciding on a treatment approach, particularly in patients who have a limited response to combination treatments with cognitive and behavioural therapies and pharmacological agents. Patients in turn would also be informed about treatment options and the potential for both positive and negative outcomes. Families and friends of patients with anxiety disorders will have a basis of knowledge on treatment options available.
Objectives
To assess the effect of DCS augmentation of cognitive and behavioural therapies compared to placebo augmentation of cognitive and behavioural therapies in the treatment of anxiety and related disorders. Additionally, to assess the efficacy and tolerability of DCS across different anxiety and related disorders.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), cluster randomised controlled trials, cross‐over trials and studies with multiple treatment groups were considered for inclusion. Both published and unpublished trials were considered. No restrictions were placed on language, setting, date or publication status.
Types of participants
Participant characteristics
All patients diagnosed with an anxiety disorder according to DSM‐III (APA 1980), DSM‐IV (APA 1994), DSM‐IV‐TR (APA 2000) and DSM‐V (APA 2013) criteria, irrespective of age, gender or ethnicity were included.
Diagnosis
The following anxiety diagnoses were included.
Generalized anxiety disorder (GAD).
Obsessive compulsive disorders (OCD).
Panic disorder ± agoraphobia (PD and PD&A).
Post‐traumatic stress disorder (PTSD).
Social anxiety disorder (SAnD).
Specific phobias (SPh).
Co‐morbidities
Patients diagnosed with a comorbid DSM Axis I anxiety disorder were included on the condition that the primary anxiety disorder was the most significant source of distress. Participants who were receiving pharmacotherapy were also included if trial investigators identified them as having achieved a stable dose of medication.
Setting
No restrictions were placed on setting.
Types of interventions
Experimental interventions
Cognitive and behavioural therapies* augmented with DCS
Comparator interventions
Cognitive and behavioural therapies* augmented with placebo pill
There were no restrictions placed on dose, duration or co‐interventions. These details were reported in Characteristics of included studies. We aimed to investigate the possible impact on the results of DCS dose and co‐medication in Subgroup analysis and investigation of heterogeneity. In addition, we could not address the additional two comparators (that is d‐cycloserine and psychotherapy versus wait‐list and psychotherapy, and d‐cycloserine and psychotherapy versus psychotherapy only) as specified previously in the protocol (for more details see Differences between protocol and review).
*As per protocol, we limited the interventions included in this review to those psychological therapies containing a form of exposure‐based learning. These therapies are behavioural or cognitive behavioural, or both, in approach and include the following:
Exposure and response prevention therapy (ERP)
ERP is a type of cognitive behavioural therapy (CBT). It is focused on facilitating fear extinction through systematic, prolonged exposure to anxiety‐provoking stimuli while simultaneously preventing fear‐reducing physical and mental actions in the patient. ERP has been found to be very effective for the treatment of OCD; patients who completed 10 to 20 sessions reported a symptom reduction of 85% (Jenike 2004). This form of therapy can be performed in a manualised method of CBT (Scheeringa 2014).
Exposure therapy (ET) (individual or group)
ET is a type of CBT that is used in the treatment of anxiety disorders, including PTSD and SAnD. Patients undergoing ET are repeatedly exposed to their traumatic or anxiety‐producing stimuli via imaginal exposure. They may also be exposed to real time anxiety‐provoking stimuli (in vivo exposure). Fear is extinguished through the effective emotional processing of the traumatic memory and with the incorporation of corrective information (Foa 1986).
Prolonged exposure therapy (PE)
PE is a type of CBT that is used in the treatment of PTSD. It is a manualised treatment focused on extinguishing fears through efficacious emotional processing of the traumatic memory. It is based on Emotional Processing Theory, which states that PTSD stems from cognitive and behavioural avoidance of trauma‐related thoughts, activities and situations. With PE treatment, clients re‐process and reorganize their experience of trauma via imaginal and in vivo exposure (Foa 2007).
Virtual reality exposure therapy (VRE)
VRE is a form of CBT that is used in the treatment of anxiety disorders. VRE uses a multisensory virtual reality environment as a form of exposure. Whereas patients undergoing exposure via PE or ET are generally imagining their traumatic experience with their eyes closed, patients participating in VRE are exposed to visual and auditory stimuli related to their traumatic past (Difede 2014(a)).
Types of outcome measures
Primary outcomes
1. Treatment efficacy: treatment responders as defined by each study, for instance using the Clinical Global Impressions scale ‐ Improvement item (CGI‐I), a widely used global outcome measure (Guy 1976).
2. Treatment acceptability: withdrawals from treatment, indicating the number of participants who dropped out of cognitive and behavioural therapies for any reason. This served as a surrogate measure of treatment acceptability in the absence of other more direct indicators of acceptability.
All primary outcome measures were binary in nature.
Secondary outcomes
3. Remission, as defined by each study.
4. Anxiety symptoms specific to the condition: determined from a variety of outcome measures tailored to each anxiety disorder, such as the Liebowitz Social Anxiety Scale (LSAS) (Liebowitz 1987), the Yale‐Brown Obsessive Compulsive Scale (Y‐BOCS) (Goodman 1989) and the Clinician Administered PTSD Scale (CAPS) (Blake 1990).
5. Co‐morbid symptoms of depression: these were assessed using scales such as the Beck Depression Inventory (BDI) (Beck 1961) and the Child Depression Inventory (CDI) (Kovacs 1992).
6. Co‐morbid symptoms of anxiety: assessed using clinician‐rated measures of anxiety such as the Beck Anxiety Inventory (BAI) (Beck 1993), the State‐Trait Anxiety Inventory (STAI) (Spielberger 1970), the Multidimensional Anxiety Scale for Children (MASC) (March 1997) and the Screen for Child Anxiety Related Disorders (SCARED) (Birmaher 2003).
7. Quality of life: the efficacy of treatment was determined using measures of quality of life as well as measures of functional disability, such as the Life Interference Scale (LIS), which provides a measure of the impact of an individual’s social fears on various components of their life (Rapee 2007).
8. Adverse events leading to discontinuation or hospitalisation.
9. The most common adverse events (defined as those occurring in at least 20% of the participants), as well as significant differences in the rate of occurrence of drug‐related adverse events between intervention and control groups.
Secondary outcomes 6 and 7 were continuous outcome measures whereas 8 and 9 were reported as binary outcomes.
Main outcomes in 'summary of findings’ tables
We used the GRADE approach to summarise and interpret findings (Schünemann 2008) and the GRADE profiler to import data from RevMan to create summary of findings tables. These tables provide outcome‐specific information concerning within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, risk of publication bias and the sum of available data on all outcomes rated as important to patient care and decision making. The GRADE approach specifies four levels of quality. The highest quality rating is for randomised trial evidence. We included the following outcomes in the summary of findings table.
Treatment efficacy: treatment responders.
Treatment acceptability: withdrawals from treatment.
Remission.
Condition‐specific anxiety symptoms.
Co‐morbid symptoms of depression.
Co‐morbid anxiety symptoms.
Quality of life.
Timing of outcome assessment
Outcome measures were collected at end of treatment and end of follow‐up, and the duration of follow‐up was noted. We report the end‐of‐treatment outcomes in the summary of findings tables.
Hierarchy of outcome measures
Where there were several possible measures for one outcome, we selected the measures or scales in the order laid out in the Primary outcomes and Secondary outcomes, and any other validated scales after those. We chose clinician‐rated scales over self‐reported scales.
Search methods for identification of studies
CCDAN Specialised Register (CCDANCTR)
The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) maintain two clinical trials registers at their editorial base in Bristol, UK, a references register and a studies based register. The CCDANCTR‐References Register contains over 37,500 reports of randomised controlled trials in depression, anxiety and neurosis. Approximately 60% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual.
Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (January 1950 to date), EMBASE (January 1974 to date) and PsycINFO (January 1967 to date); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review specific searches of additional databases. Reports of trials are also sourced from international trials registers care of the World Health Organisation’s trials portal (ICTRP), ClinicalTrials.gov, drug companies, the hand‐searching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCDAN's generic search strategies can be found on the Group's website.
Electronic searches
The CCDANCTR (Studies and References Register) was initially searched (2014‐03‐25) using the following search terms: (*cycloserine or Seromycin).
No restriction on date, language or publication status was applied to the search.
The CCDANCTR already contained relevant records from ClinicalTrials.gov and the WHO trials portal (ICTRP), so it was not necessary to re‐search these registries.
An update search was conducted (2015‐03‐12) using a more precise search strategy. CCDANCTR: ((antibiotic or *cycloserine or DCS or seromycin) NEAR (“add on“ or add‐on or adjunct* or augment* or combin* or enhanc*)):ti,ab
Searching other resources
Reference lists
The bibliographies of all identified trials were scanned for additional studies.
Correspondence
Attempts were made to obtain published and unpublished trials, as identified by the frequency with which they were cited in the bibliographies of RCTs and open‐label studies.
Authors or pharmaceutical companies were contacted if further information was needed. These were identified through the source of funding cited in published RCTs, as well as through author affiliations.
Data collection and analysis
Selection of studies
RCTs identified from the search were independently assessed for inclusion by two authors (RO and TA), based on information included in the abstract or method section of the trial report. These two authors were also independently involved in full text screening. The authors independently collated the data listed under Data extraction and management from RCTs that they both regarded as satisfying the inclusion criteria specified in the Criteria for considering studies for this review. Studies for which additional information was required in order to determine their suitability for inclusion in the review were listed in Characteristics of studies awaiting classification, pending the availability of this information. Any disagreements in the independent trial assessment and data collation procedures were resolved by discussion with a third review author (DS).
Data extraction and management
Spreadsheet forms were designed for the purpose of recording descriptive information, summary statistics of the outcome measures, the risk of bias ratings, and associated commentary. Data were collected independently by two review authors (RO and TA). Any disagreements were resolved in discussion with a third review author (DS). Where data were presented in graphs, we used digitizing software (http://www.getdata‐graph‐digitizer.com/) to acquire data points, and reported where this had been done in the analyses footnotes and Characteristics of included studies. Where information was missing, the review authors contacted the study investigators in an attempt to obtain the information.
The following study characteristics were collated from each trial.
Description of the trials: study design, duration, follow‐up and country.
Characteristics of the participants: sample size, recruitment method, diagnostic classification criteria, rating scale inclusion criteria, included disorders, co‐morbidities (especially major depressive disorder), gender, mean age, ethnicity and pharmacotherapy during the study.
Characteristics of the interventions: description of the intervention and comparison conditions including number of participants randomised to each condition, dose and timing of medication, number of sessions and description of therapists.
Outcome measures: we listed outcome measures (primary and secondary), summary continuous (means and standard deviations) and dichotomous (number of responders) data, and whether data reflected the intention to treat, with methods of estimating the outcome for participants who dropped out of the study (such as last observation carried forward (LOCF) or mixed effects (ME) model, or completer/observed cases (OC)) sample.
Notes: funding from industry, whether medication was supplied by industry, whether any author worked for industry and study ID were also recorded for each study.
Main comparisons
D‐cycloserine (DCS) augmentation of cognitive and behavioural therapies versus placebo augmentation of cognitive and behavioural therapies for adults and children.
We made a post hoc decision to analyse studies conducted with adult participants separately from those conducted with children and adolescents. This decision was made as the disorders profile and treatment response is different per group (see Differences between protocol and review).
Outcomes were also stratified by type of anxiety disorder (GAD, OCD, PD and PD&A, PTSD, SAnD, or SPh). In addition, we could not address the additional two comparators (that is DCS and psychotherapy versus wait‐list and psychotherapy, and DCS and psychotherapy versus psychotherapy only) as specified previously in the protocol (for more details see Differences between protocol and review).
Assessment of risk of bias in included studies
The quality of the trials was assessed independently by two review authors (RO and TA) using the Cochrane Collaboration's risk of bias tool (Higgins 2008a). Any disagreements were discussed with a third and fourth review author (KSW and HB). Where necessary, the authors of the studies were contacted for further information, see Risk of bias in included studies. The Cochrane risk of bias instrument consists of items assessing six potential domains of systematic bias, including the following.
Random sequence generation: referring to a random number table or using a computer random number generator?
Allocation concealment: was the medication allocation sequentially numbered, sealed or placed in opaque envelopes?
Blinding of a) participants, personnel, and b) outcome assessors for each main outcome or class of outcomes: was knowledge of the allocated treatment or assessment adequately prevented during the study?
Incomplete outcome data for each main outcome or class of outcomes: were missing or excluded outcome data adequately addressed?
Selective outcome reporting: were the reports of the study free of suggestion of selective outcome reporting?
Other sources of bias: was the study apparently free of other problems that could put it at a 'high' risk of bias.
Studies were rated as: 'low', 'high' or 'unclear' risk of bias for these domains.
Measures of treatment effect
Categorical data
For dichotomous data, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). Although odds ratios possess mathematical characteristics that are advantageous with respect to modelling treatment effects, especially in small samples (Greenland 1987), they are frequently confused with RRs leading to inflated estimates of treatment effects (Deeks 2008).
Continuous data
Mean differences (MD) were calculated for continuous summary data derived from the same scale, such as the CAPS. When a range of scales were employed for each outcome, such as in the assessment of symptoms on the LSAS and CAPS, as well as in the assessment of co‐morbid depression on the Montgomery‐Asberg Depression Rating Scale (MADRS) and Hamilton Rating Scale for Depression (HAM‐D), the standardised mean difference (SMD) was determined for each outcome. This method of analysis standardises the differences between the means of the treatment and control groups in terms of the variability observed in the trial.
To facilitate interpretation of SMDs, for meta‐analyses where results were statistically significant we estimated whether the magnitude of effect was of minimal clinical importance. The SMDs were converted to MDs using a representative study for the most frequently reported scale within that outcome (section 12.6.4 in Higgins 2011). The pooled effect was thus re‐expressed in the original units of that particular scale and the clinical relevance and impact of the intervention effect were interpreted. Please note this was a post hoc change to the protocol methods (see Differences between protocol and review).
Unit of analysis issues
Studies with multiple treatment groups
The potential bias introduced through comparing the summary statistics for multiple groups against the same placebo control in dose comparison studies was avoided by pooling the means and standard deviations across all of the treatment arms as a function of the number of participants in each arm.
Cross‐over trials
Cross‐over trials were only included in the calculation of summary statistics when it was: (a) possible to extract treatment and placebo or comparator data from the first treatment period, or (b) when the inclusion of these data from both treatment periods was justified through a wash‐out period of sufficient duration to minimise the risk of carry‐over effects (Higgins 2011). No cross‐over trials were found in the study search.
Cluster randomised trials
Cluster randomised trials were included as long as the clustering effect was properly adjusted for in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008b). No cluster randomised trials were included in this review. To prevent unit of analysis errors in future updates of this review, we plan to divide the sample size of each comparison group in trials that do not adjust for clustering by the design effect metric (Higgins 2011). For these analyses the intraclass correlation coefficient (ICC) that is incorporated within the design effect will be set equivalent to the median ICC from published cluster randomised pharmacotherapy RCTs for anxiety and related disorders.
Dealing with missing data
We extracted data to allow an intention‐to‐treat analysis in which all randomised participants were analysed in the groups to which they were originally assigned. For continuous outcomes, we calculated missing standard deviations from other available data such as confidence intervals, standard errors, P, T or F values, as detailed in the Cochrane Handbook for Systemic reviews of Interventions, section 7.7.3 (Higgins 2011). If such statistics were unavailable, we imputed SDs using the average SD of the other included studies (section 16.1.3.1 in Higgins 2011). In trial reports in which multiple forms of data imputation were conducted, we gave preference to the inclusion of summary statistics for continuous outcome measures derived from mixed‐effects models (ME), followed by last observation carried forward (LOCF) and observed cases (OC) summary statistics (in that order). If data on studies, outcomes, summary data, participants or study‐level characteristics were missing, we contacted the original investigators.
Assessment of heterogeneity
Heterogeneity was assessed by means of the Chi² test of heterogeneity to assess whether observed differences in results were compatible with chance alone. If the Chi² test had a P value of less than 0.10, this was interpreted as evidence of heterogeneity, given the low power of the Chi² statistic when the number of trials is small (Deeks 2008).
In addition, the I² heterogeneity statistic was used to quantify the inconsistency of the trial results within each analysis (Higgins 2003). The I² statistic was interpreted as follows:
0% to 40%, might not be important;
30% to 60%, may represent moderate heterogeneity;
50% to 90%, may represent substantial heterogeneity;
75% to 100%, considerable heterogeneity (Higgins 2011).
We used the Tau², the estimated standard deviation of underlying effects across studies in random‐effects model meta‐analyses (section 9.5.4 in Higgins 2011) to estimate between‐study variance. As a rough indication, we interpreted 4*Tau as the width of the prediction interval that contains 95% of the true effects of future studies, assuming the sample size was large enough (Higgins 2009).
Assessment of reporting biases
Funnel plots provide a graphical illustration of the effect estimates of an intervention from individual studies against some measure of the precision of that estimate. Tests for funnel plot asymmetry should be used only when there are at least 10 studies included in the meta‐analysis (see section 10.4.3.1 in Higgins 2011). We visually inspected publication bias from the funnel plot for treatment acceptability and condition‐specific anxiety disorders for adults at end of treatment, the only two outcomes with at least 10 studies, with consideration of confounding selection bias, poor methodological quality, true heterogeneity, artefact and chance.
Data synthesis
Categorical and continuous treatment effects were obtained from a random‐effects model. Random‐effects analytic models include both within‐study sampling error and between‐study variation in determining the precision of the confidence interval around the overall effect size. A random‐effects meta‐analysis model involves an assumption that the effects being estimated in the different studies are not identical, but follow some distribution. The outcomes were expressed in terms of an average effect size as well as by means of 95% confidence intervals. Outcomes were stratified by type of anxiety disorder.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to assess the degree to which methodological differences between trials might have systematically influenced differences observed in the primary treatment outcomes (Thompson 1994). Current guidelines recommend at least 10 studies per characteristic used for stratifying subgroups (Deeks 2011). Accordingly, we did not conduct subgroup analyses to determine differences in dosage, isolated versus chronic treatment with DCS, and timing of drug administration (see Differences between protocol and review).
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcomes to determine:
1) Whether studies that imputed data influenced the results. Studies in which missing data were imputed would be excluded from this analysis;
2) Whether study quality influenced the findings. Studies with high risk of bias for allocation concealment and studies with at least two high risk of bias judgements would be excluded in this analysis.
As four studies at most were included for any single outcome, we did not find any meaningful sensitivity analyses that could be undertaken with so few studies per outcome.
Summary of findings tables
Summary of findings tables were compiled to summarise the best evidence for all relevant outcomes (that is experimental versus comparator interventions). These consisted of the following six elements, using a fixed format (Higgins 2011).
A list of all important outcomes, both desirable and undesirable.
A measure of the typical burden of these outcomes (e.g. illustrative risk, or illustrative mean, on control intervention).
Absolute and relative magnitude of effect (if both are appropriate).
Numbers of participants and studies addressing these outcomes.
A grade of the overall quality of the body of evidence for each outcome.
Space for comments.
Evidence for downgrading studies was based on five factors. If we found a reason for downgrading the evidence, we classified the factor as ’serious’ (downgrading the quality rating by one level) or ’very serious’ (downgrading the quality grade by two levels).
Limitations in the design and implementation of the trial.
Indirectness of evidence.
Unexplained heterogeneity or inconsistency of results.
Imprecision of results.
High probability of publication bias.
The quality of evidence was classified for each outcome according to the following categories.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate.
Results
Description of studies
Results of the search
The search of the CCDAN Specialised Register (see Search methods for identification of studies) yielded 116 references (all years to 12 March 2015). After removing one duplicate record, 27 records were excluded at abstract level screening, 88 full text documents were assessed for eligibility and a further 14 records excluded at this stage. Twenty‐one studies (48 records including secondary reports of same trial) and 788 participants were included. Four studies are awaiting classification (7 records) and 18 studies are ongoing. See Figure 1 for a flow chart of the screening process.
1.
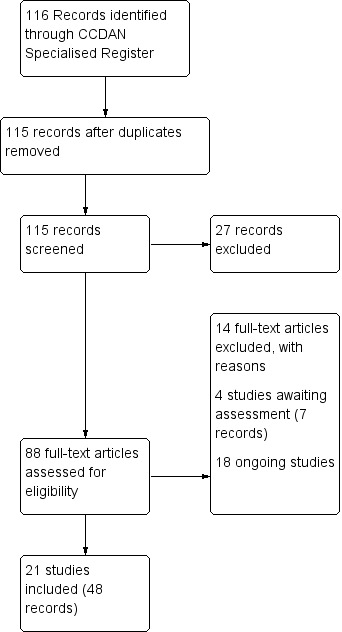
Study flow diagram.
Included studies
See Characteristics of included studies for full details of the included studies, and Table 3 for an overview of the studies' main characteristics.
1. Studies overview.
|
Study ID Country |
Who was treated? | How long was the treatment? | How long was the follow up? | What disorders? | Anti‐depressant co‐medication? | What was the treatment? | Number of sessions | When DCS was given? | Which dose of DCS? | Was the therapist trained? |
|
Cameron 2005 USA |
Children adolescents and adults | 12 weeks | 3 months after treatment completed | SAnD | No | CBT + DCS versus CBT + placebo | 12 (weekly) | 1 to 2 hours prior each CBT session | 50 mg | NR |
|
de Kleine 2012 Netherlands |
Adults | 10 weeks | 3 months after treatment completed | PTSD | Yes, 17/67 on anti‐depressants | PE + DCS versus PE + placebo | 10 (weekly) | 1 hour prior each session | 50 mg | Psychologists trained in PE |
|
Difede 2014 USA |
Adults | 12 weeks | 6 months after treatment completed | PTSD | Yes, numbers not reported | VRE + DCS versus VRE + placebo | 12 (weekly) | 1.5 hours prior sessions 2 to 11 | 100 mg | NR |
|
Farrell 2013 Australia |
Children and adolescents | 9 weeks | 3 months after treatment completed | OCD (difficult to treat) | Yes, 13/17 on medication | ERP + DCS versus ERP + placebo | 9 (weekly) | 1 hour prior sessions 5 to 9 | 25 to 50 mg* | Postgraduate level clinicians with previous experience in CBT for OCD |
|
Guastella 2008 Australia |
Adults | 5 weeks | 1 month after treatment completed | SAnD | Yes, 5/56 on anti‐depressants | ET + DCS versus ET + placebo | 5 (weekly) | 1 hour prior session 2 to 5 | 50 mg | Clinical psychologists |
|
Hofmann 2006 USA |
Adults | 5 weeks | 1 month after treatment completed | SAnD (public speaking anxiety) | Yes, 9/27 on anti‐depressants | GET + DCS versus GET + placebo | 5 (weekly) | 1 hour prior session 2 to 5 | 50 mg | Therapists trained and supervised |
|
Hofmann 2013 USA |
Adults | 12 weeks | 6 months after treatment completed | Generalized SAnD | No | CBT + DCS versus CBT + placebo | 12 (weekly) | 1 hour prior sessions 3 to 7 | 50 mg | Therapists trained and supervised |
|
Kushner 2007 USA |
Adults | 5 weeks | 3 months after treatment completed | OCD | Yes, numbers not reported | CBT + DCS versus CBT + placebo | 10 (twice week) | 2 hours prior each session | 125 mg | Trained and supervised psychologists |
|
Litz 2012 USA |
Adults | 6 weeks | 6 months after treatment completed | PTSD | Yes, numbers not reported | BET + DCS versus BET + placebo | 6 (weekly) | 0.5 hour prior sessions 2 to 5 | 50 mg | Doctoral‐level clinicians with previous experience and training in CBT |
|
Mataix‐Cols 2014 UK |
Adolescents | 17 weeks | 12 months after treatment completed | OCD | Yes, 6/27 on anti‐depressants | ERP + DCS versus ERP + placebo | 14 (weekly?) | Immediately after sessions 3 to 12 | 50 mg | Experienced therapists |
|
Nave 2012 USA |
Adults | 1 week | 1 week after treatment completed | Snake phobia | Yes, 5/20 on medication | GET + DCS versus GET + placebo | 1 | 1 hour prior single session | 50 mg | NR |
|
Otto 2010 USA |
Adults | 5 weeks | 1 month after treatment completed | Panic disorder with or without agoraphobia | Yes, 19/31 on anti‐depressants | CBT + DCS versus CBT + placebo | 5 (weekly) | 1 hour prior sessions 3 to 5 | 50 mg | Doctoral‐ and graduate‐level clinicians trained and supervised |
|
Ressler 2004 USA |
Adults | 2 weeks | 3 months after treatment completed | Acrophobia | Not reported | VRE + DCS versus VRE + placebo | 2 (weekly) | Acutely prior each session | 50 or 500 mg | NR |
|
Rothbaum 2014 USA |
Adults | 6 weeks | 12 months after treatment completed | PTSD | Yes, 56% on psychotropic medication | VRE + DCS versus VRE + alprazolam versus VRE + placebo | 6 (weekly) | 0.5 hour prior sessions 2 to 5 | 50 mg (DCS) 0.25 mg (alprazolam) |
Masters‐level clinicians |
|
Scheeringa 2014 USA |
Children and adolescents | 12 weeks | 3 months after treatment completed | PTSD | Yes, numbers not reported | CBT + DCS versus CBT + placebo | 12 (weekly?) | 1 hour prior sessions 5 to 11 | 50 mg | Masters level therapists trained in CBT and supervised |
|
Sheerin 2014 USA |
Adults | 10 weeks | 6 months after treatment completed | SAnD | No | CBT + DCS versus CBT + placebo | 10 (weekly) | Immediately after each session | 250 mg | Doctoral graduate student therapists with a minimum of one year of supervised clinical experience |
|
Siegmund 2011 Germany |
Adults | 1 month | 5 months after treatment completed | Panic disorder with agoraphobia | Yes, 12/44 on anti‐depressants | CBT + DCS versus CBT + placebo | 11 (twice week) | 1 hour prior each session | 50 mg | Certified psychologist |
|
Storch 2007 USA |
Adults | 12 weeks | 2 months after treatment completed | OCD | Yes, 50% on anti‐depressants | ERP + DCS versus ERP + placebo | 12 (weekly) | 4 hours prior sessions 3 to 12 | 250 mg | Doctoral fellows or trainees under supervision |
|
Storch 2010 USA |
Children and adolescents | 8 weeks | Post treatment | OCD | Yes, 36% on anti‐depressants | CBT + DCS versus CBT + placebo | 4 (twice week) 6 (weekly) |
1 hour prior sessions 4 to 10 | 25 to 50 mg* | Experienced therapists |
|
Tart 2013 USA |
Adults | 2 weeks | 1 month after treatment completed | Acrophobia | No | VRE + DCS versus VRE + placebo | 2 (weekly) | Immediately after each session | 50 mg | Advanced doctoral‐student level therapists trained and supervised |
|
Wilhelm 2008 USA |
Adults | 5 weeks | 1 month after treatment completed | OCD | Yes, 14/33 on anti‐depressants | CBT + DCS versus CBT + placebo | 10 (twice week) | 1 hour prior each session | 100 mg | Advanced trainees, under the supervision of licensed psychologists |
CBT= Cognitive behaviour therapy; DCS = d‐cycloserine; ERP = Exposure and response prevention; BET = Behaviour exposure therapy; GET = Group exposure therapy; OCD = Obsessive compulsive disorder; PE = Prolonged exposure therapy; PTSD = Post‐traumatic stress disorder; SAnD = Social anxiety disorder; VRE = Virtual reality exposure
* 25 mg for children ≤ 45 kg, and 50 mg for children > 45 kg
Design
The review included 21 RCTs of DCS augmentation of cognitive and behavioural therapies versus placebo augmentation of cognitive and behavioural therapies for the treatment of anxiety and related disorders in children, adolescents and adults. Treatment was provided over 1 to 17 weeks, and most studies had 1 to 3 months follow‐up. Two studies reported a follow‐up of one year (Mataix‐Cols 2014; Rothbaum 2014). No cross‐over trials were found in the study search. Each study was published in English.
Sample size
The sample size for studies ranged from 16 (Sheerin 2014) to 169 (Hofmann 2013).
Setting
The majority of the studies were conducted in the United States of America (USA), two in Australia (Farrell 2013; Guastella 2008), and one each in Germany (Siegmund 2011), the Netherlands (de Kleine 2012) and the United Kingdom (UK) (Mataix‐Cols 2014). All participants were outpatients.
Participants
Age
Most studies included adults above the age of 18 years. Three studies included children and adolescents (Farrell 2013; Scheeringa 2014; Storch 2010) and one study included adolescents only (Mataix‐Cols 2014). One study included children, adolescents and adults, with 36% below the age of 18 years and the remaining participants above 18 years (Cameron 2005); this study was analysed within the adult category. One study did not report the age of participants (Kushner 2007) and was also placed in this category.
Gender
The proportion of male participants in the studies ranged from 37% (Sheerin 2014) to 100% (Litz 2012).
Diagnosis
Obsessive compulsive disorder (OCD)
Six studies included participants with a primary diagnosis of OCD (Farrell 2013; Kushner 2007; Mataix‐Cols 2014; Storch 2007; Storch 2010; Wilhelm 2008). A diagnosis was made according to the DSM‐IV criteria for OCD in four studies (Kushner 2007; Mataix‐Cols 2014; Storch 2010; Wilhelm 2008), whereas one study used the revised version (Storch 2007). The additional study by Farrell 2013 did not report which diagnostic measure was used to diagnose participants.
Panic disorder ± agoraphobia (PD and PD&A)
Two studies included participants with a primary diagnosis of PD and PD&A: panic disorder with or without agoraphobia (Otto 2010) and PD&A (Siegmund 2011). Both studies used the DSM‐IV to diagnose participants.
Post‐traumatic stress disorder (PTSD)
Five studies included participants with PTSD (de Kleine 2012; Difede 2014; Litz 2012; Rothbaum 2014; Scheeringa 2014). The participants were diagnosed using the DSM‐IV in four studies. Scheeringa 2014 did not report the diagnostic measure used.
Social anxiety disorder (SAnD)
Five studies included participants with SAnD and all studies followed the DSM‐IV criteria (Cameron 2005; Guastella 2008; Hofmann 2006; Hofmann 2013; Sheerin 2014).
Specific phobias (SPh)
Two studies included participants with acrophobia (Ressler 2004; Tart 2013) and one with snake phobia (Nave 2012). Ressler 2004 used the DSM‐III‐R, Nave 2012 the DSM‐IV, and Tart 2013 the DSM‐IV‐TR to diagnose the disorder.
Interventions
All of the studies used some form of exposure‐based learning, although this was not explicitly stated in Cameron 2005. Just over a third of the studies used ET, one study used PE (de Kleine 2012), one study ERP (Storch 2007) and another used ERP with CBT (Farrell 2013). VRE was used alone in three studies (Ressler 2004; Rothbaum 2014; Tart 2013) and with CBT in one study (Difede 2014). Five studies used exposure‐based CBT (Hofmann 2013; Mataix‐Cols 2014; Otto 2010; Sheerin 2014; Storch 2010), and one used manualised trauma‐focused CBT (Scheeringa 2014). The number of exposure sessions ranged from 12 (Cameron 2005; Difede 2014; Hofmann 2013; Storch 2007) to one session (Nave 2012). Kushner 2007 provided exposure therapy until all Subjective Units of Distress (SUDS) ratings were reduced by 50%, or when 10 sessions were completed, whichever came sooner. In most studies DCS was given before the therapy session, ranging from 30 minutes (Litz 2012; Rothbaum 2014) to 4 hours (Storch 2007). Three studies gave DCS immediately after the session (Mataix‐Cols 2014; Sheerin 2014; Tart 2013). The dose of DCS given to adults ranged from 50 mg, which was used in the majority of studies, to 500 mg (Ressler 2004). For children and adolescents, the dose was 50 mg in two studies (Mataix‐Cols 2014; Scheeringa 2014) and in the other two studies it was 25 mg or 50 mg depending on the weight of the participants (Farrell 2013; Storch 2010). The number of DCS augmentation of cognitive and behavioural therapy sessions also ranged from 1 (Nave 2012) to 12 (Cameron 2005). Six of the 21 studies administered DCS augmentation of cognitive and behavioural therapy over 10 sessions (de Kleine 2012; Difede 2014; Kushner 2007; Mataix‐Cols 2014; Sheerin 2014; Wilhelm 2008).
Outcomes
Primary outcomes
Twelve studies provided data on response to treatment at end of treatment, and nine at end of follow‐up. Nave 2012, Ressler 2004, Storch 2007, Storch 2010 and Tart 2013 defined response to treatment as ‘very much improved’ or ‘much improved’ on the CGI‐I scale. Other studies defined response to treatment as a pre‐specified reduction on the scale measuring anxiety for each condition: Farrell 2013 > 25% and Mataix‐Cols 2014 > 35% reduction on the Children's Y‐BOCS (CY‐BOCS) (Scahill 1997), Scheeringa 2014 > 50% reduction in joint (parent and child ratings) Child PTSD Symptom Scale (CPSS) (Foa 2001) scores, de Kleine 2012 and Litz 2012 as a > 10 point reduction on the CAPS, and Hofmann 2013 as a score of 1 or 2 on the Social Phobic Disorders Severity and Change Form (SPDSCF) ‐ improvement score (Liebowitz 1992). Rothbaum 2014 reported how many participants met the PTSD criteria after treatment; we included these data as non‐response to treatment.
All studies provided data on withdrawals from treatment, except Otto 2010, which did not report how many participants withdrew from each treatment group.
Secondary outcomes
Seven studies provided data on remission at end of treatment and at end of follow‐up. Storch 2007 defined remission as a severity rating on the Anxiety Disorder Interview Schedule–Fourth Edition (ADIS‐IV) (Brown 1989) of ≤ 3 and on the CY–BOCS as ≤ 10, de Kleine 2012 on the CAPS as < 20, Difede 2014 as ≤ 20 with minimal or no impairment in social, occupational and other important areas of functioning (CAPS items F21 and F22 ≤ 1), Hofmann 2013 as an improvement score of 1 or 2 on the SPDSCF and a score of < 30 on the LSAS, Tart 2013 as 'normal' or 'minimally ill' on the Clinical Global Impressions scale ‐ Severity item (CGI‐S) (score ≤ 2) (Guy 1976), Farrell 2013 as > 50% reduction on the CY‐BOCS combined with a CY‐BOCS score of < 14, and Mataix‐Cols 2014 as ≤ 10 on the CY‐BOCS.
All studies measured anxiety symptoms using various condition‐specific scales at end of treatment. Only four studies reported follow‐up data for this outcome (Cameron 2005; Kushner 2007; Nave 2012; Storch 2010). OCD was measured using the Y‐BOCS (Farrell 2013; Kushner 2007; Mataix‐Cols 2014; Storch 2007; Storch 2010; Wilhelm 2008), PD with the Panic and Agoraphobia Scale (PAS) (Siegmund 2011) with the Panic Disorder Severity Scale (PDSS) (Otto 2010), PTSD with the CAPS (de Kleine 2012; Difede 2014; Litz 2012; Rothbaum 2014) and CPSS (Scheeringa 2014), SAnD with the LSAS (Cameron 2005; Guastella 2008; Hofmann 2006; Hofmann 2013; Sheerin 2014), and SPh with the Acrophobia Anxiety Questionnaire (AAQ) (Ressler 2004; Tart 2013) and Snake Questionnaire (Klorman 1974).
Eight studies measured co‐morbid symptoms of depression at end of treatment. Only one of the eight studies reported follow‐up data (Storch 2010) for this outcome. All studies with adult participants used the BDI (de Kleine 2012; Siegmund 2011) or BDI‐II scale (Litz 2012; Storch 2007; Wilhelm 2008). The studies on children and adolescents used the Beck Depression Inventory for Youth (BDI‐Y) (Beck 2001) (Mataix‐Cols 2014) or the CDI (Scheeringa 2014; Storch 2010).
Six studies measured co‐morbid anxiety symptoms at end of treatment, although data were only available at follow‐up for two of these studies (Farrell 2013; Storch 2010). Studies with adults used the BAI (Siegmund 2011) or STAI (de Kleine 2012; Sheerin 2014), whereas studies with children and adolescents used the MASC (Farrell 2013; Storch 2010) or SCARED (Scheeringa 2014).
Only Guastella 2008 measured quality of life, both at end of treatment and at follow‐up, using the LIS.
We analysed data for adverse events for all but three studies: de Kleine 2012 merely reported that there was no difference between groups, and Nave 2012 and Rothbaum 2014 did not report on adverse events. In addition, Hofmann 2013 reported on those hospitalised due to adverse events, and Siegmund 2011 on those that discontinued due to adverse events.
Excluded studies
Fourteen studies were excluded from the review. Eight studies were excluded because they did not include cognitive and behavioural therapies (Behar 2010; Evins 2012; Gutner 2012; Heresco‐Levy 2002; Inslicht 2013; Levinson 2013; Rajabi 2013; Rodebaugh 2013), whereas five studies did not meet the inclusion criteria for an anxiety disorder: subclinical fear of public speaking in Galovic 2010, subclinical spider fear in Guastella 2007(a) and Guastella 2007(b), children with food refusal in Sharp 2013, and anorexia in Steinglass 2007. One study was a prevention study (NCT00257361 2005) (also see Characteristics of excluded studies).
Studies awaiting classification
Four studies are awaiting classification as additional information is required in order to determine their suitability for inclusion. A record of Guay 2007 was traced through ClinicalTrials.gov. It aimed to compare CBT plus DCS to CBT plus placebo in the treatment of PTSD in adults. The main hypothesis of this study is that the efficacy of CBT for PTSD will be increased when combined with DCS compared to a placebo. Anxiety severity was measured with SCID and CAPS. Additional information necessary to classify the study was not available. Strohle 2011 is a completed randomised, double‐blind, parallel assignment study involving participants aged 18 to 75 years with a diagnosis of agoraphobia. Participants received 12 sessions of CBT with 50 mg DCS or placebo pill administered three times directly after exposure. The severity of anxiety symptoms was measured with the PAS and BAI. Additional information necessary to classify the study was not available. A collaborative project in the Netherlands assessed DCS enhancement in exposure therapy for patients with PD&A (Cath 2010a) (conference abstract) or OCD (Cath 2010b.) (see Characteristics of studies awaiting classification).
Ongoing studies
Eighteen ongoing randomised double‐blinded trials, investigating augmentation of cognitive and behavioural therapies with DCS or placebo, in various phases were identified. Seven studies include participants diagnosed with OCD, three with PTSD, four with SPh, four with PD, and one with SAnD. Studies on OCD used either the Y‐BOCS or CY‐BOCS as a measure of severity. Dosages of DCS ranged from 25 mg to 125 mg one hour prior to exposure sessions (Arman 2013; Bergman 2012; Cath 2010a; Farrell 2014; de Leeuw 2008; Ruck 2012; Storch 2011). Of the three studies on PTSD only, Difede 2009 stated that the CAPS scale would be used. All studies stated single doses prior to the exposure session (Difede 2009; Difede 2011a). Pollack 2014 differed from other studies as it aimed to assess the optimal dose timing of DCS to augment treatment for SAnD in adults. Participants would receive five weeks of CBT for social anxiety and two pills (one placebo before and one DCS or placebo after the session), or five weeks of CBT and two pills (one DCS before and one placebo after), or five weeks of CBT and two pills (one placebo before and one placebo after) or five weeks of CBT for SAnD and two pills (one DCS before and one DCS after). Little information was provided on the remaining studies (Guastella 2006; Otto 2008; Reinecke 2012; Sirbu 2009; Smits 2013). Most ongoing studies include adults only. One study included adolescents (Arman 2013), five studies children and adolescents (Bergman 2012; Farrell 2012; Farrell 2014; Geller 2011; Storch 2011), and two studies include children (Rapee 2010; Rapee 2011). See Characteristics of ongoing studies for more details.
Risk of bias in included studies
Risk of bias was assessed using the Cochrane Collaboration's 'risk of bias' tool for allocation concealment, blinding, incomplete outcome data, selective reporting and other potential sources of bias. Most information was from studies rated at 'low' or 'unclear' risk of bias (see Characteristics of included studies; Figure 2 and Figure 3).
2.
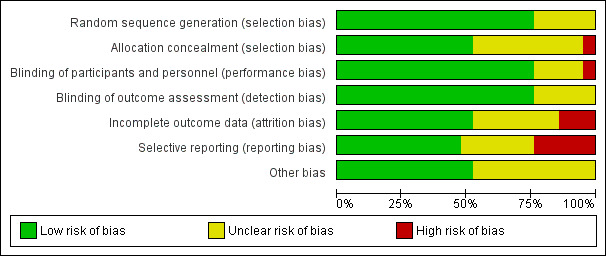
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
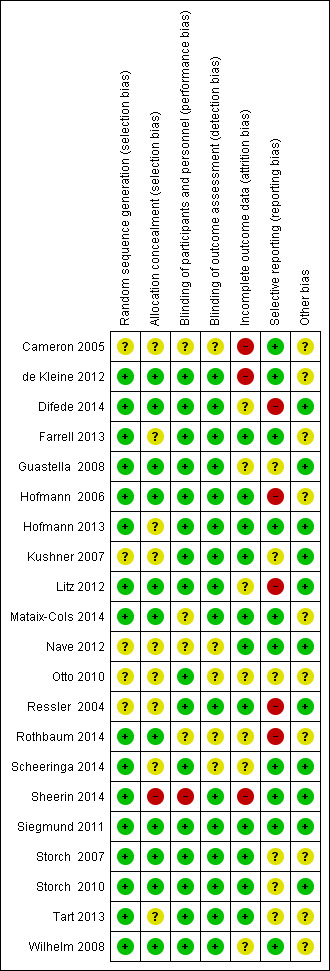
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation
We classified 16 of the 21 studies as 'low' risk of bias. Of the 16 studies, 10 employed computer generated random block sequences (de Kleine 2012; Farrell 2013; Hofmann 2013; Litz 2012; Mataix‐Cols 2014; Scheeringa 2014; Sheerin 2014; Siegmund 2011; Storch 2010; Tart 2013). Difede 2014, Hofmann 2006, Storch 2007, Wilhelm 2008, Guastella 2008 and Rothbaum 2014 were regarded as low risk as other forms of bias were minimised. The remaining studies were rated 'unclear' as the authors did not report on the method of randomisation.
Allocation concealment
Eleven of the studies provided sufficient information to be considered at 'low' risk for selection bias. Active and placebo medications were dispensed by a pharmacist in numbered containers according to a randomly generated list in eight studies (de Kleine 2012; Difede 2014; Guastella 2008; Hofmann 2006; Litz 2012; Rothbaum 2014; Storch 2010; Wilhelm 2008). One study (Sheerin 2014) was rated 'high' risk of bias because the psychiatrist, who met with patients, was not blinded to allocation. The additional studies were rated 'unclear' because there was insufficient information to determine how allocation was concealed.
Blinding
Blinding of participants and personnel
Sixteen of the studies reported that the participants and personnel were blinded, and used an adequate method; these were therefore rated as being at 'low' risk of bias. Medication was administered in a double‐blind fashion and the research pharmacist oversaw the randomisation (de Kleine 2012; Difede 2014; Farrell 2013; Guastella 2008; Hofmann 2006; Hofmann 2013; Kushner 2007; Litz 2012; Otto 2010; Ressler 2004; Scheeringa 2014; Siegmund 2011; Storch 2007; Storch 2010; Tart 2013; Wilhelm 2008). One study (Sheerin 2014) was rated 'high' risk of bias because the psychiatrist, who met with patients, had access to the list with participants' treatment conditions. The remaining four studies were given a rating of 'unclear' for risk of bias: Cameron 2005 and Rothbaum 2014 reported that they were double‐blind but did not provide further details; Mataix‐Cols 2014 and Nave 2012 reported that identical pills were used but other details to assure blinding were not reported.
Blinding of outcome assessors
Five studies were rated as at 'unclear' risk of bias. These studies were described as double‐blind but no information was provided on how the outcome assessors were blinded (Cameron 2005; Nave 2012; Otto 2010; Rothbaum 2014; Scheeringa 2014). The remaining 16 studies were rated 'low' risk of bias as outcome assessors were blinded in these studies. It was stated in these studies that the psychologist or therapist who was blind to the medication condition conducted the assessments.
Incomplete outcome data
Eleven studies were classified as 'low' risk of bias. Each of these studies had no missing data and missing outcome data were balanced in numbers across the intervention groups. Three studies were rated as 'high' risk of bias (Cameron 2005; de Kleine 2012; Sheerin 2014). In Cameron 2005 five participants were not included in the analysis with no reason given, in de Kleine 2012 there was a high dropout rate with approximately one in three patients ending treatment prematurely, and in Sheerin 2014 only 7 out of 16 participants that started treatment completed, which may have introduced bias in the results. We classified the remaining studies as 'unclear'.
Selective reporting
Ten studies were rated as 'low' risk of bias. The protocols for each of these 10 studies were available and all pre‐specified outcomes were reported. Seven studies were rated as 'unclear' risk of bias. In five of these this was because the study protocol was not available, in Otto 2010 the protocol was available but did not list the specific scales that would be used to measure the outcomes, and Storch 2010 had a protocol available but reported additional outcomes in the study report. Four studies were rated 'high' for reporting bias. In three of these studies outcomes were missing that had been pre‐specified in the protocol (Hofmann 2006; Litz 2012; Ressler 2004; Rothbaum 2014); whereas in Difede 2014 two outcome measures were not reported sufficiently with means and standard deviations (SDs).
Other potential sources of bias
Ten studies were noted to have 'unclear' risk for other sources of bias. In six of those studies a large percentage of patients were receiving concomitant medications, which might have confounded the results. In de Kleine 2012, when patients completed the homework assignments they were not taking DCS, which may have diluted DCS‐related effects and influenced the treatment outcomes. Cameron 2005 and Mataix‐Cols 2014 were brief reports and insufficient information was provided to assess other biases. The remaining eleven studies were rated 'low' risk bias because no sources of other bias were identified.
Effects of interventions
Comparison 1: DCS augmentation of cognitive and behavioural therapies (CBT) versus placebo augmentation of CBT for adults
See: Table 1.
Primary outcomes
1.1 Treatment efficacy: treatment responders at end of treatment
There was no evidence of a difference between DCS augmentation of CBT compared to placebo augmentation of CBT (N = 9, risk ratio (RR) 1.10; 95% confidence interval (CI) 0.89 to 1.34; n = 449; see Analysis 1.1) for the treatment of anxiety and related disorders in adults. The evidence was low in quality. In addition, no evidence of a difference was found within the subgroups that reported on this outcome (OCD, PTSD, SAnD, SPh), except for PD where DCS augmentation of CBT was found to be more efficacious than placebo augmentation of CBT (N = 1, RR 2.25; 95% CI 1.04 to 4.86; n = 31). There were moderate levels of heterogeneity in the overall results (I² = 45%, Tau² = 0.03).
1.1. Analysis.
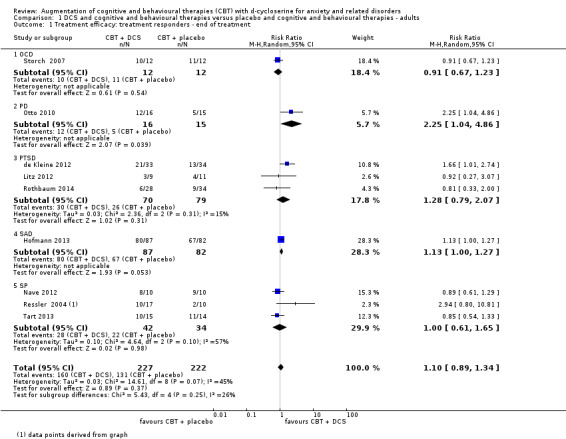
Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 1 Treatment efficacy: treatment responders ‐ end of treatment.
1.2 Treatment efficacy: treatment responders at 1 to 12 months follow‐up
No evidence of a difference between DCS augmentation of CBT compared to placebo augmentation of cognitive and behavioural therapies was found at follow‐up (N = 7, RR 1.08; 95% CI 0.90 to 1.31; n = 383; see Analysis 1.2). In addition, no evidence of a difference was found within the subgroups that reported on this outcome (OCD, PD, PTSD, SAnD, SPh). Substantial heterogeneity was observed within some of the subgroups.
1.2. Analysis.
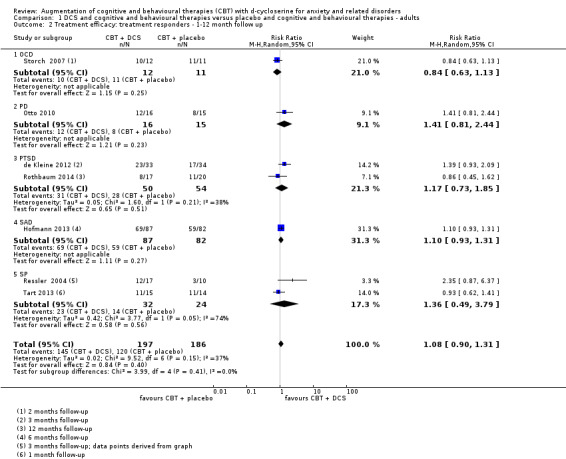
Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 2 Treatment efficacy: treatment responders ‐ 1‐12 month follow up.
1.3 Treatment acceptability: withdrawals from treatment at end of treatment
There was no evidence of a difference in treatment acceptability for DCS augmentation of CBT with placebo augmentation of CBT overall (N = 16, RR 0.88; 95% CI 0.61 to 1.25; n = 740; see Analysis 1.3). This conclusion was based on moderate quality evidence. In addition, no evidence of a difference between the experimental and control interventions was found within the subgroups that reported on this outcome (OCD, PD, PTSD, SAnD, SPh). Substantial heterogeneity was observed within some of the subgroups.
1.3. Analysis.
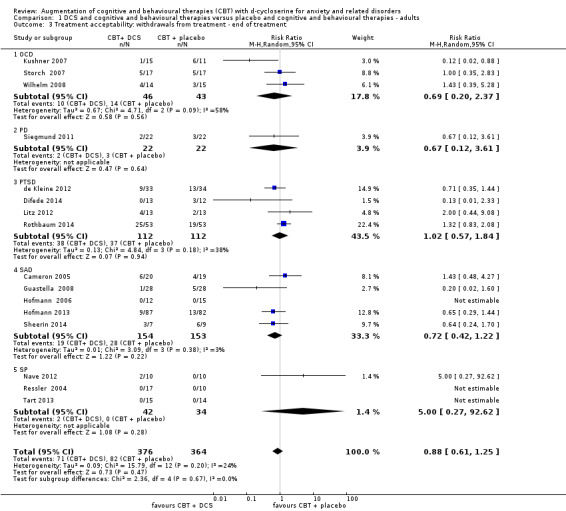
Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 3 Treatment acceptability: withdrawals from treatment ‐ end of treatment.
1.4 Treatment acceptability: withdrawals from treatment at 1 to 12 months follow‐up
This outcome was not applicable in the comparison of DCS augmentation of CBT versus placebo augmentation of CBT for adults at follow‐up.
Secondary outcomes
1.5 In remission at end of treatment
There was no evidence of a difference in rate of remission between DCS augmentation of CBT and placebo augmentation of CBT (N = 5, RR 1.16, 95% CI 0.79 to 1.71; n = 292; see Analysis 1.4). This conclusion was based on moderate quality evidence. In addition, no evidence of a difference was found within the subgroups that reported on this outcome (OCD, PTSD, SAnD, SPh). Substantial heterogeneity was observed within some of the subgroups.
1.4. Analysis.
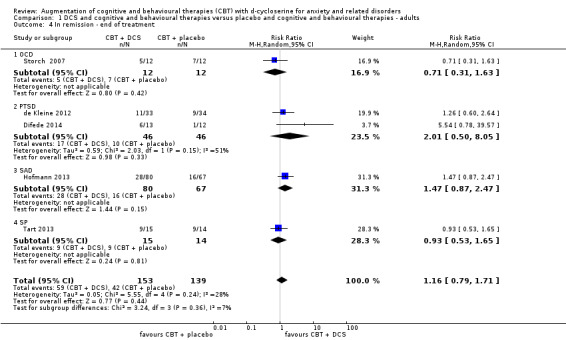
Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 4 In remission ‐ end of treatment.
1.6 In remission at one to six months follow‐up
No evidence of a difference in rate of remission for DCS augmentation of CBT compared with placebo augmentation of CBT was found (N = 5, RR 1.29; 95% CI 0.79 to 2.10; n = 272; see Analysis 1.5). In addition, no evidence of a difference was found for the subgroups that reported on this outcome (OCD, PTSD, SAnD, SPh), except for PTSD where DCS augmentation of CBT was more efficacious than placebo augmentation of CBT (N = 2, RR 2.58; 95% CI 1.34 to 4.99; n = 92). There was substantial heterogeneity in the overall results (I² = 53%, Tau² = 0.16).
1.5. Analysis.
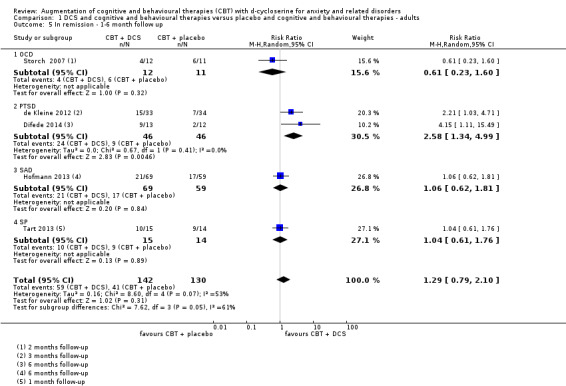
Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 5 In remission ‐ 1‐6 month follow up.
1.7 Condition‐specific anxiety symptoms at end of treatment
DCS augmentation of cognitive and behavioural therapies was found to be more efficacious than placebo augmentation of CBT in reducing condition‐specific anxiety symptoms (N = 17, standardised mean difference (SMD) ‐0.32; 95% CI ‐0.58 to ‐0.07; n = 735; see Analysis 1.6). The evidence was moderate in quality. To investigate whether the magnitude of effect was of clinical importance, the SMD was converted to mean difference (MD) using a representative study (Hofmann 2013) for the LSAS scale. Compared to the mean 57.79 points for the control group, the converted MD (6.55 points lower) exceeded the minimal clinically important difference on the LSAS scale (55 to 65 points = moderate social phobia, lower scores indicate a better outcome). The 95% CI lower value (11.88 points lower) also showed a clinically important difference, whereas the upper value (1.43 points lower) did not. No evidence of a difference was found for the subgroups that reported on this outcome (OCD, PTSD, SAnD, SPh), except for PD where DCS augmentation of CBT was found to be more efficacious than placebo augmentation of CBT (N = 2, SMD ‐0.84; 95% CI ‐1.33 to ‐0.34; n = 70). There was substantial heterogeneity in the overall results (I² = 60%, Tau² = 0.15).
1.6. Analysis.
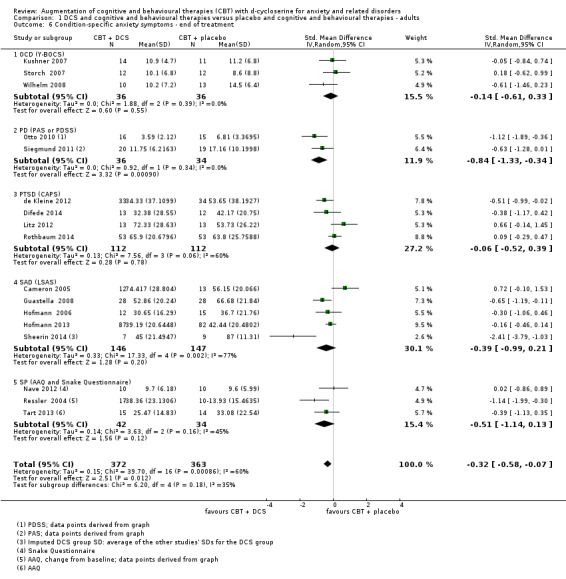
Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 6 Condition‐specific anxiety symptoms ‐ end of treatment.
1.8 Condition‐specific anxiety symptoms at 1 to 12 months follow‐up
DCS augmentation of cognitive and behavioural therapies was found to be more efficacious than placebo augmentation of CBT in reducing condition‐specific anxiety symptoms (N = 13, SMD ‐0.27; 95% CI ‐0.47 to ‐0.06; n = 641; see Analysis 1.7). To investigate whether the magnitude of effect was of clinical importance, the SMD was converted to MD using a representative study (Hofmann 2013) for the LSAS scale. Compared to the mean 46.84 points for the control group, the converted MD (6.43 points lower; 95% CI 11.21 to 1.43) did not exceed the minimal clinically important difference on the LSAS scale (55 to 65 points = moderate social phobia, lower scores indicate a better outcome). No evidence of a difference was found within the subgroups that reported on this outcome (OCD, PD, PTSD, SAnD), except for SPh where DCS augmentation of CBT was found to be more efficacious than placebo augmentation of CBT (N = 2, SMD ‐0.66; 95% CI ‐1.21 to ‐0.11; n = 56).
1.7. Analysis.
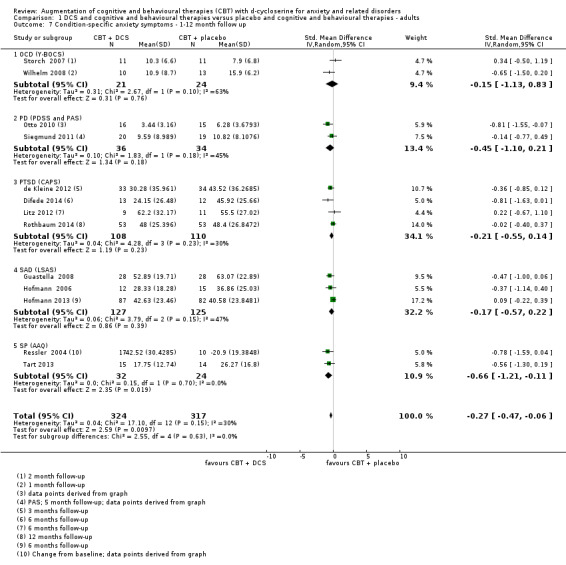
Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 7 Condition‐specific anxiety symptoms ‐ 1‐12 month follow up.
1.9 Co‐morbid symptoms of depression at end of treatment
There was no evidence of a difference in co‐morbid symptoms of depression between DCS augmentation of CBT and placebo augmentation of CBT (N = 5, SMD ‐0.25, 95% CI ‐0.80 to 0.31; n = 178; see Analysis 1.8). This conclusion was based on moderate quality evidence. In addition, no evidence of a difference was found within the subgroups that reported on this outcome (PD, PTSD), except for OCD for which DCS augmentation of CBT was more efficacious than placebo augmentation of CBT (N = 2, SMD ‐0.64, 95% CI ‐1.23 to ‐0.04; n = 47). There was substantial heterogeneity in the overall results (I² = 68%, Tau² = 0.26).
1.8. Analysis.
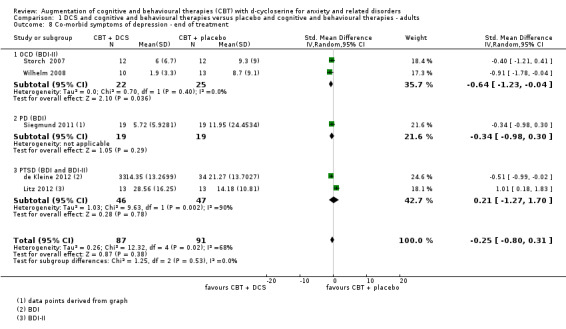
Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 8 Co‐morbid symptoms of depression ‐ end of treatment.
1.10 Co‐morbid symptoms of depression at one to six months follow‐up
There was no evidence of a difference in co‐morbid symptoms of depression with DCS augmentation of CBT compared to placebo augmentation of CBT (N = 5, SMD ‐0.12; 95% CI ‐0.47 to 0.24; n = 171; see Analysis 1.9). In addition, no evidence of a difference was found within the subgroups that reported on this outcome (OCD, PD, PTSD). Moreover, there was substantial heterogeneity within some of the subgroups.
1.9. Analysis.
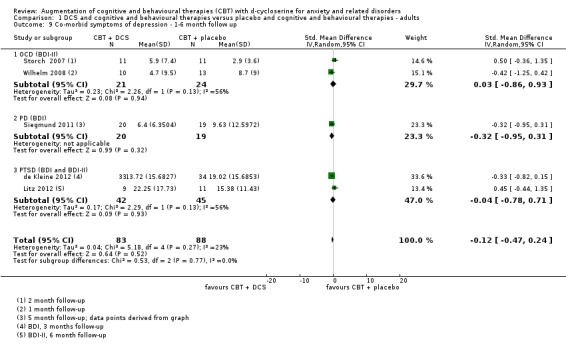
Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 9 Co‐morbid symptoms of depression ‐ 1‐6 month follow up.
1.11 Co‐morbid anxiety symptoms: end of treatment
There was evidence of a difference showing that DCS augmentation of CBT was more efficacious in reducing co‐morbid anxiety symptoms than placebo augmentation of CBT (N = 3, SMD ‐0.63; 95% CI ‐0.99 to ‐0.26; n = 122; see Analysis 1.10). This conclusion was based on low quality evidence. To investigate whether the magnitude of effect was of clinical importance, the SMD was converted to MD using a representative study (Siegmund 2011) for the BAI scale. Compared to the mean 19.5 points for the control group, the converted MD (8.82 points lower; 95% CI 13.85 to 3.64 points) may represent a clinically important improvement for the intervention group in co‐morbid anxiety, from moderate anxiety to mild anxiety on the BAI scale (8 to 15 points = mild anxiety, 16 to 25 points = moderate anxiety). One of the three subgroups that reported on this outcome (PTSD) also found that DCS augmentation of CBT was more efficacious than placebo augmentation of CBT (N = 1, SMD ‐0.67; 95% CI ‐1.16 to ‐0.18; n = 67). The other subgroups, however, found no evidence of a difference for DCS over placebo (PD: N = 1, SMD ‐0.52; 95% CI ‐1.16 to 0.12; n = 39; SAnD: N = 1, SMD ‐0.70; 95% CI ‐1.73 to 0.32; n = 16; see Analysis 1.10).
1.10. Analysis.
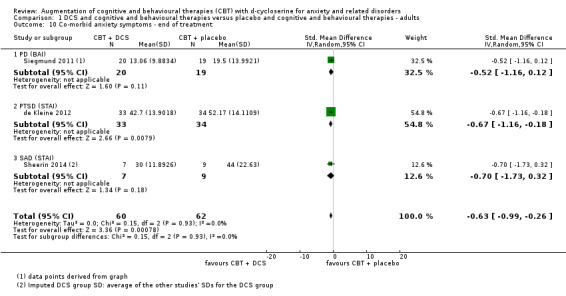
Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 10 Co‐morbid anxiety symptoms ‐ end of treatment.
1.12 Co‐morbid anxiety symptoms at three to five months follow‐up
No evidence of a difference in the reduction of co‐morbid anxiety symptoms with DCS augmentation of CBT compared with placebo augmentation of CBT was found (N = 2 ,SMD ‐0.29; 95% CI ‐0.68 to 0.09; n = 106; see Analysis 1.11). In addition, no evidence of a difference was found within the subgroups that reported on this outcome (PD, PTSD).
1.11. Analysis.
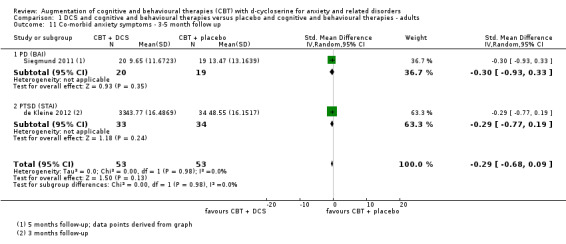
Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 11 Co‐morbid anxiety symptoms ‐ 3‐5 month follow up.
1.13 Quality of life at end of treatment
There was very low quality evidence that DCS augmentation of CBT was more efficacious in improving quality of life than placebo augmentation of CBT (N = 1, MD ‐5.32; 95% CI ‐9.87 to ‐0.77; n = 56; see Analysis 1.12).
1.12. Analysis.

Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 12 Quality of life ‐ end of treatment.
1.14 Quality of life at one month follow‐up
DCS augmentation of CBT was found to be more efficacious in increasing quality of life than placebo augmentation of CBT (N = 1, MD ‐5.71; 95% CI ‐11.12 to ‐0.30; n = 56; see Analysis 1.13).
1.13. Analysis.
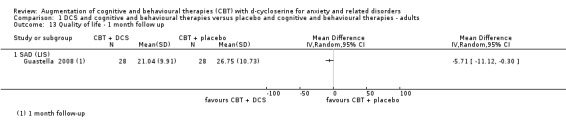
Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 13 Quality of life ‐ 1 month follow up.
1.15 Adverse events leading to discontinuation or hospitalisation at end of treatment
No evidence of a difference was noted between DCS augmentation of CBT and placebo augmentation of CBT (N = 2, RR 0.96; 95% CI 0.10 to 9.00; n = 213; see Analysis 1.14). In addition, no evidence of a difference was found within the subgroups that reported on this outcome (PD, SAnD).
1.14. Analysis.
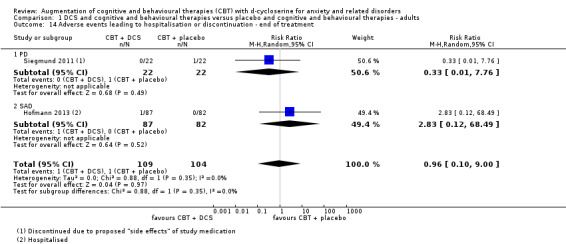
Comparison 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, Outcome 14 Adverse events leading to hospitalisation or discontinuation ‐ end of treatment.
1.16 Adverse events leading to discontinuation or hospitalisation at follow‐up
This outcome measure was not applicable during the follow‐up period.
1.17 Commonly occurring or treatment‐related adverse events at end of treatment
No studies were found that specifically reported on commonly occurring or treatment‐related adverse events.
None of the five studies that reported on 'any' or 'all' adverse events found any evidence of a difference between DCS augmentation of CBT and placebo augmentation of cognitive and behavioural therapies in anxiety and related disorders. A further eight studies reported no events. See Table 4 for detailed results on adverse events.
2. Adverse events results.
| Sub‐group | Study | Type of adverse event | DCS | Placebo | RR (95% CI) | ||
| Events | Total | Events | Total | ||||
| Adults OCD | Kushner 2007 | “Any” or “all” adverse events | 4 | 15 | 3 | 17 | 1.51 (0.40 to 5.69) |
| Storch 2007 | “Any” or “all” adverse events | 3 | 12 | 3 | 12 | 1.00 (0.25 to 4.00) | |
| Wilhelm 2008 | “Any” or “all” adverse events | No events were reported | |||||
| Adults PD | Otto 2010 | “Any” or “all” adverse events | No events were reported | ||||
| Siegmund 2011 | "Harms" or "unintended effects" | No events were reported | |||||
| Adults PTSD | Difede 2014 | “Any” or “all” adverse events | No events were reported | ||||
| Litz 2012 | Serious adverse events | No events were reported | |||||
| Adults SAnD | Cameron 2005 | Any adverse events excluding serious adverse events | 10 | 20 | 12 | 19 | 0.79 (0.45 to 1.38) |
| Guastella 2008 | “Any” or “all” adverse events | No events were reported | |||||
| Hofmann 2006 | Vivid nightmares in 1 patient and euphoric mood and increased energy in another | 2 | 12 | 0 | 15 | 6.15 (0.32 to 117.21) | |
| Sheerin 2014 | “Any” or “all” adverse events, mild (dizziness, fatigue) | 0 | 7 | 2 | 9 | 0.25 (0.01 to 4.50) | |
| Adults SPh | Ressler 2004 | “Any” or “all” adverse events, not systematically obtained | No events were reported | ||||
| Tart 2013 | “Any” or “all” adverse events | No events were reported | |||||
| Children and adolescents OCD | Farrell 2013 | Treatment related adverse events | No events were reported | ||||
| Mataix‐Cols 2014 | Drug related adverse events | No events were reported | |||||
| Storch 2010 | “Any” or “all” adverse events | No events were reported | |||||
| Children and adolescents PTSD | Scheeringa 2014 | “Any” or “all” adverse events | 7 | 29 | 7 | 28 | 0.97 (0.39 to 2.40) |
1.18 Commonly occurring or treatment‐related adverse events at follow‐up
This outcome measure was not applicable during the follow‐up period.
Comparison 2: DCS augmentation of cognitive and behavioural therapies (CBT) versus placebo augmentation of CBT for children and adolescents
See: Table 2.
Primary outcomes
2.1 Treatment efficacy: treatment responders at end of treatment
There was no evidence of a difference between DCS augmentation of CBT and placebo augmentation of CBT for the treatment of anxiety and related disorders in children and adolescents (N = 4, RR 1.01; 95% CI 0.78 to 1.31; n = 121; see Analysis 2.1). The evidence was low in quality. In addition, no evidence of a difference was found within the subgroups that reported on this outcome (OCD, PTSD).
2.1. Analysis.
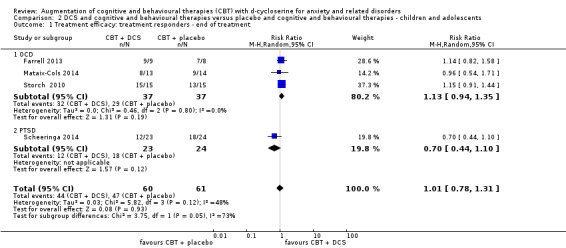
Comparison 2 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ children and adolescents, Outcome 1 Treatment efficacy: treatment responders ‐ end of treatment.
2.2 Treatment efficacy: treatment responders at 3 to 12 months follow‐up
No evidence of a difference between DCS augmentation of CBT and placebo augmentation of cognitive and behavioural therapies was found (N = 3, RR 0.86; 95% CI 0.76 to 1.09; n = 91; see Analysis 2.2). In addition, no evidence of a difference was found within the subgroups that reported on this outcome (OCD, PTSD).
2.2. Analysis.
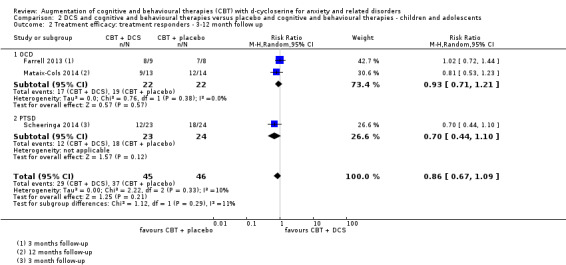
Comparison 2 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ children and adolescents, Outcome 2 Treatment efficacy: treatment responders ‐ 3‐12 month follow up.
2.3 Treatment acceptability: withdrawals from treatment at end of treatment
There was no evidence of a difference in treatment acceptability for DCS augmentation of CBT compared with placebo augmentation of CBT (N = 4, RR 0.90; 95% CI 0.17 to 4.69; n = 131; see Analysis 2.3). The evidence was very low in quality. In addition, no evidence of a difference was found within the subgroups that reported on this outcome (OCD, PTSD).
2.3. Analysis.
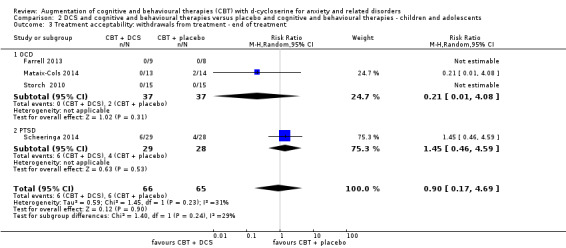
Comparison 2 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ children and adolescents, Outcome 3 Treatment acceptability: withdrawals from treatment ‐ end of treatment.
2.4 Treatment acceptability: withdrawals from treatment at follow‐up
This outcome was not applicable for DCS augmentation of CBT versus placebo augmentation of CBT for children and adolescents at follow‐up.
Secondary outcomes
2.5 In remission at end of treatment
There was no evidence of a difference in rate of remission between DCS augmentation of CBT and placebo augmentation of CBT for the 2 studies including participants with OCD (RR 1.19; 95% CI 0.66 to 2.16; n = 44; see Analysis 2.4). This conclusion was based on low quality evidence.
2.4. Analysis.
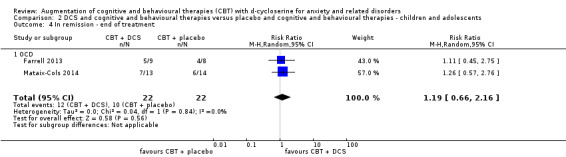
Comparison 2 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ children and adolescents, Outcome 4 In remission ‐ end of treatment.
2.6 In remission at 3 to 12 months follow‐up
There was no evidence of a difference between DCS augmentation of CBT and placebo augmentation of CBT for participants with OCD in the studies that reported on rate of remission (RR 1.05; 95% CI 0.69 to 1.61; n = 44; see Analysis 2.5).
2.5. Analysis.
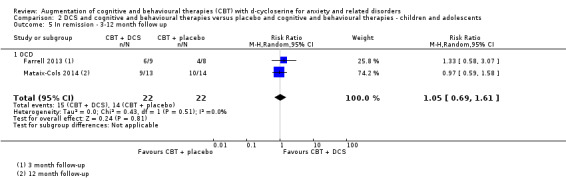
Comparison 2 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ children and adolescents, Outcome 5 In remission ‐ 3‐12 month follow up.
2.7 Condition‐specific anxiety symptoms at end of treatment
There was no evidence of a difference between DCS augmentation of CBT compared with placebo augmentation of CBT for condition‐specific anxiety symptoms (N = 4, SMD 0.07; 95% CI ‐0.55 to 0.69; n = 131; see Analysis 2.6). The evidence was very low in quality. There was, however, evidence of a small difference for children and adolescents with PTSD in favour of placebo (N = 1, SMD 0.70; 95% CI 0.17 to 1.24; n = 57). There was substantial heterogeneity in the overall results (I² = 66%, Tau²= 0.26).
2.6. Analysis.
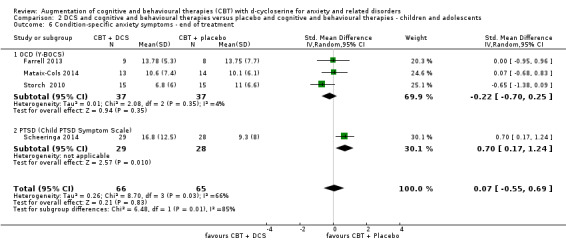
Comparison 2 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ children and adolescents, Outcome 6 Condition‐specific anxiety symptoms ‐ end of treatment.
2.8 Condition‐specific anxiety symptoms at 3 to 12 months follow‐up
No evidence of a difference in condition‐specific anxiety symptoms for DCS augmentation of CBT compared with placebo augmentation of CBT was found (N = 3, SMD 0.23; 95% CI ‐0.32 to 0.78; n = 91; see Analysis 2.7). There was, however, evidence of a small difference for children and adolescents with PTSD at follow‐up in favour of placebo augmentation of CBT (N = 1, SMD 0.62; 95% CI 0.03 to 1.21; n = 57).
2.7. Analysis.
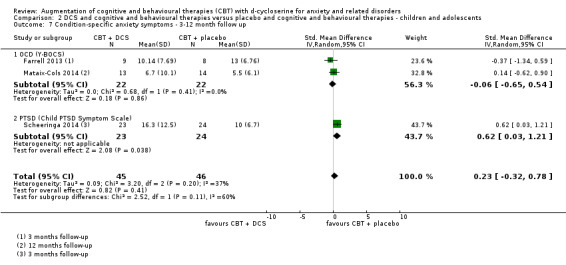
Comparison 2 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ children and adolescents, Outcome 7 Condition‐specific anxiety symptoms ‐ 3‐12 month follow up.
2.9 Co‐morbid symptoms of depression at end of treatment
There was no difference between DCS augmentation of CBT compared with placebo augmentation of CBT for co‐morbid symptoms of depression (N = 3, SMD 0.08; 95% CI ‐0.52 to 0.69; n = 114; see Analysis 2.8). Evidence of a small difference for children and adolescents with PTSD was found in favour of placebo augmentation of CBT (N = 1, SMD 0.60; 95% CI 0.06 to 1.13; n = 57). There was substantial heterogeneity in the overall results (I² = 60%, Tau² = 0.17).
2.8. Analysis.
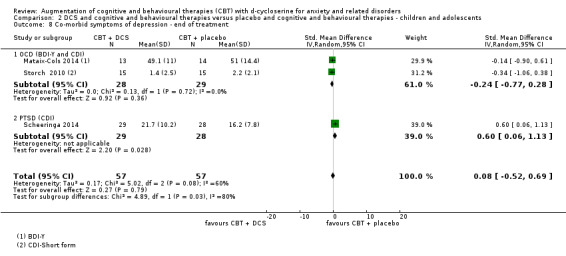
Comparison 2 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ children and adolescents, Outcome 8 Co‐morbid symptoms of depression ‐ end of treatment.
2.10 Co‐morbid symptoms of depression at 3 to 12 months follow‐up
No evidence of a difference in the reduction of co‐morbid symptoms of depression in DCS augmentation of CBT compared with placebo augmentation of CBT was found (N = 2, SMD 0.09; 95% CI ‐0.56 to 0.74; n = 84; see Analysis 2.9). In addition, no evidence of a difference was found within the subgroups that reported on this outcome (OCD, PTSD). There was also substantial heterogeneity in the overall results (I² = 51%, Tau² = 0.11).
2.9. Analysis.
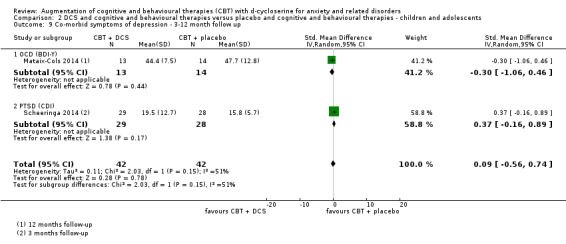
Comparison 2 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ children and adolescents, Outcome 9 Co‐morbid symptoms of depression ‐ 3‐12 month follow up.
2.11 Co‐morbid anxiety symptoms at end of treatment
Results were not pooled between subgroups as heterogeneity was considerable (I² = 77%, Tau² = 0.45). The following was found (see Analysis 2.10).
2.10. Analysis.
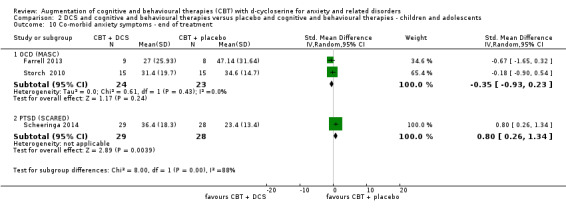
Comparison 2 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ children and adolescents, Outcome 10 Co‐morbid anxiety symptoms ‐ end of treatment.
2.11.1 OCD
In this subgroup we identified two relevant trials that used MASC to measure co‐morbid anxiety symptoms and found no evidence of a difference between DCS augmentation of CBT and placebo augmentation of CBT (SMD ‐0.35; 95% CI ‐0.93 to 0.23; n = 47).
2.11.2 PTSD
In this subgroup we identified one relevant trial that found placebo augmentation of CBT to be more efficacious than DCS augmentation of CBT in reducing co‐morbid anxiety symptoms, using the SCARED measurement (SMD 0.80; 95% CI 0.26 to 1.34; n = 57).
2.12 Co‐morbid anxiety symptoms at three months follow‐up
Placebo augmentation of CBT was found to be more efficacious than DCS augmentation of CBT for the single study including participants with PTSD that reported on co‐morbid anxiety symptoms (MD 10.1; 95% CI 1.88 to 18.32; n = 57; see Analysis 2.11).
2.11. Analysis.
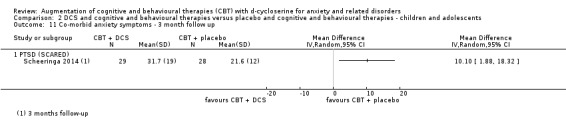
Comparison 2 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ children and adolescents, Outcome 11 Co‐morbid anxiety symptoms ‐ 3 month follow up.
2.13 Quality of life at end of treatment
No studies were found that reported on quality of life.
2.14 Quality of life at follow‐up
No studies were found that reported on quality of life.
2.15 Adverse events leading to discontinuation or hospitalisation at end of treatment
No studies were found that reported on adverse events leading to hospitalisation or discontinuation.
2.16 Adverse events leading to discontinuation or hospitalisation at follow‐up
This outcome was not applicable to the follow‐up period.
2.17 Commonly occurring or treatment related adverse events at end of treatment
Two studies that reported on treatment or drug‐related adverse events reported no events (Farrell 2013; Mataix‐Cols 2014), see Table 4.
The one study that reported on 'any' or 'all' adverse events did not find any evidence of a difference for DCS augmentation of CBT compared to placebo augmentation of CBT, whereas the other study reported no events.
2.18 Commonly occurring or treatment related adverse events at follow‐up
This outcome was not applicable to the follow‐up period.
3. Heterogeneity
Assessment of the primary outcome measure of treatment efficacy (treatment response) indicated Tau² = 0.03; Chi² = 14.61, df = 8 (P = 0.07); I² = 45%. This suggested that the result may present moderate heterogeneity.
4. Subgroup analysis
Subgroup analyses were planned to assess the degree to which methodological differences between trials might have systematically influenced differences observed in primary treatment outcomes. Analyses of medication dosages, isolated versus chronic treatment with DCS, timing of drug administration, and the effect of the inclusion of patients on a stable dose of anti‐depressants were intended, however there were insufficient studies to conduct the subgroup analyses (fewer than 10).
5. Sensitivity analysis
There were insufficient studies (fewer than ten) to conduct planned sensitivity analyses. For Sheerin 2014, SDs for the DCS group were not reported for two outcomes (condition‐specific anxiety symptoms and co‐morbid anxiety symptoms). As stated in Dealing with missing data, we imputed the DCS group SDs based on an average of the other studies' SDs. However, as the placebo group SD was available for this study and differed from the average SDs, we carried out sensitivity analyses to investigate whether using the placebo group SDs would impact on the overall results for these outcomes. We found that they did not significantly change the total effect or 95% CI (data not reported).
6. Publication bias
There was no evidence of possible funnel plot asymmetry for the outcomes that included at least 10 studies: treatment acceptability for adults at end of treatment, and condition‐specific anxiety disorders for adults at the end of treatment and at follow‐up. The graphs appeared to be symmetrical and disorders were normally distributed above and below the mean (see Figure 4; Figure 5; Figure 6).
4.
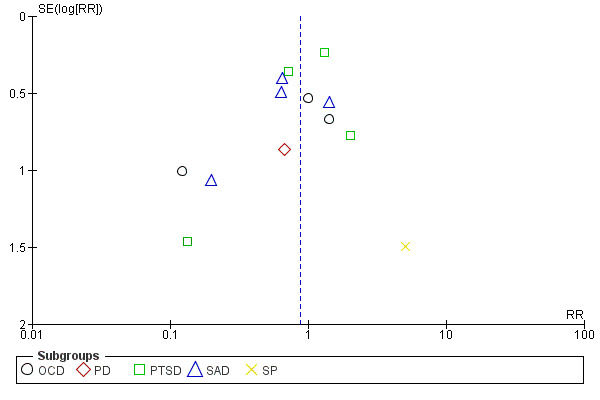
Funnel plot of comparison: 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults at end of treatment, outcome: 1.3 Treatment acceptability: withdrawals from treatment ‐ end of treatment.
5.
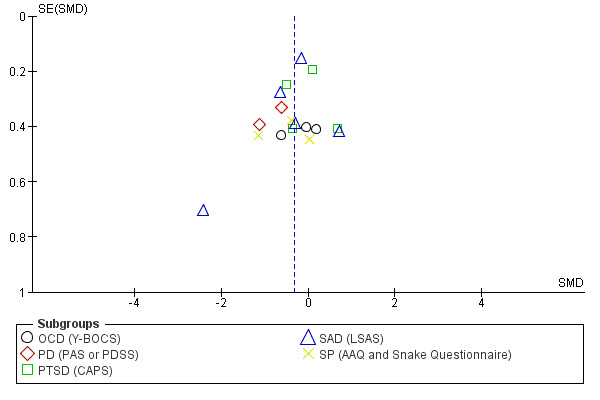
Funnel plot of comparison: 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, outcome: 1.6 Condition‐specific anxiety symptoms ‐ end of treatment.
6.
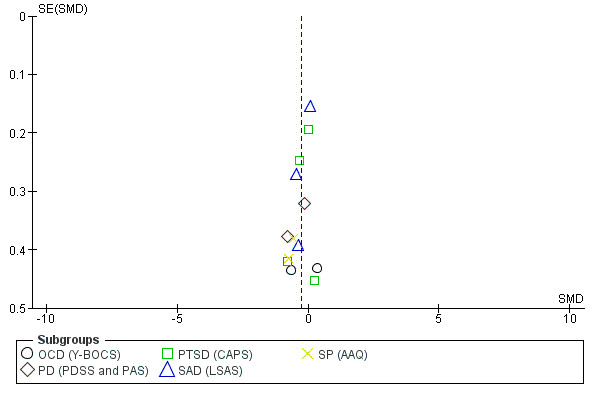
Funnel plot of comparison: 1 DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults, outcome: 1.7 Condition‐specific anxiety symptoms ‐ 1 to 12 month follow‐up.
Discussion
Summary of main results
This review aimed to assess the efficacy and acceptability of DCS augmented cognitive and behavioural therapies compared to placebo augmented cognitive and behavioural therapies for anxiety and related disorders in children, adolescents and adults. Exposure‐based forms of cognitive and behavioural therapies were included in the review, namely ERP, ET (group or individual), PE and VRE. Overall, 21 studies were included in the review (788 participants). The 21 RCTs investigated obsessive compulsive disorder (OCD, N = 6), post‐traumatic stress disorder (PTSD, N = 5), social anxiety disorder (SAnD, N = 5), specific phobia (SPh, N = 3) and panic disorder (PD, N = 2).
Although DCS augmentation of cognitive and behavioural therapies was efficacious in individual studies, the combined effect size indicated that there was no difference in terms of efficacy, response or remission. These findings were seen in the adult group at the end of treatment and of follow‐up. Further, DCS augmentation of cognitive and behavioural therapies for children and adolescents, both at post‐treatment and follow‐up, did not appear to be superior to placebo augmentation of cognitive and behavioural therapies. No evidence of a difference was found within the subgroups that reported on treatment efficacy (OCD, PTSD, SAnD, SPh), except for PD where one small adult study found DCS augmented cognitive and behavioural therapies to be more efficacious than placebo augmented cognitive and behavioural therapies. Moderate heterogeneity was detected amongst studies, particularly for the primary outcome assessment of treatment efficacy in the adult comparator group.
There was no evidence of difference in treatment acceptability for DCS augmentation of cognitive and behavioural therapies compared with placebo augmentation of cognitive and behavioural therapies overall. No studies were found that specifically reported on commonly occurring or treatment ‐related adverse events.
Findings on secondary outcome measures were largely consistent with those for the primary outcome measures. A reduction in condition‐specific anxiety symptoms was found in the DCS arm compared to the placebo arm for adults, both at the end of treatment and at follow‐up. There is a noted statistically significant improvement with DCS augmentation of CBT over placebo augmentation of CBT (moderate quality evidence). This could be interpreted as a clinically important effect, due to the overall SMD that was back transformed to one scale, the LSAS scale. The LSAS scale was chosen as it was included in most studies. Further, Hofmann 2013 was chosen as the representative study as it was relatively large with low risk of bias. This method is based on many assumptions and the clinical importance should be interpreted with caution. Overall the upper CI value showed no clinically important difference for this outcome. There was no evidence of a difference in condition‐specific anxiety symptom for children and adolescents. The reduction of co‐morbid symptoms of depression was not found in the DCS arm compared to the placebo arm. Subgroup analysis in the adult comparator group showed no evidence of a difference except for the OCD group where two small studies showed DCS augmentation of cognitive and behavioural therapies to be more efficacious than placebo augmentation of cognitive and behavioural therapies. In the child and adolescent comparison group, DCS augmentation of cognitive and behavioural therapies was successful in reducing co‐morbid depression. Evidence of a difference was also found for children, adolescents and adults with PTSD. No evidence of a difference was found at follow‐up across all age groups, however. Furthermore, there was no evidence of a difference between DCS augmentation of cognitive and behavioural therapies compared with placebo augmentation of cognitive and behavioural therapies in the reduction of disability for adult participants.
Overall completeness and applicability of evidence
Although every effort was made to identify relevant trials, the number of studies included in this review is small. There are a number of factors limiting the strength of the conclusions that can be supported by this review. Missing outcome data for numerous studies prevented the addition of their data into the analysis of treatment efficacy, despite requests to trial investigators for this information. Of the 21 included studies in the review, only nine studies in the adult comparator groups provided responder rates, and four studies in the child and adolescent group. Further, in the child and adolescent group only studies on OCD and PTSD were found, which limits the completeness and applicability of the findings. Limited conclusions can be drawn around acceptability of treatment, as treatment withdrawals were used as an indirect measure. Quality of life outcomes could not be determined as only one study reported on this outcome. Two trials did not report on the reasons why participants were excluded from the analysis and one noted a large dropout rate with a premature end to treatment.
The sample size of each trial (mean 40 participants) and the review (788 participants) were small. This raises concerns around the interpretation of findings given that (1) larger studies tend to give more precise estimates of effects (and hence have narrower CIs) than smaller studies; (2) the statistical significance of an effect of a particular magnitude will be greater (the P value will be smaller) in a larger study than in a smaller study; and (3) the Chi² test will be low in power in the (common) situation of a meta‐analysis where studies have small sample sizes or are few in number (Hofmann 2011).
Insufficient data are currently available to carry out subgroup analysis, as a minimum of 10 studies per outcome is required. This limits the overall completeness of the evidence presented. Numerous disorders (OCD, PTSD, SAnD, SPh, PD) were included in the review and compared. Marked heterogeneity exists between these disorders in clinical presentation and response to treatment.
The amount of DCS given amongst the disorders varied significantly. The timing of DCS was another significant methodological factor as DCS was given from between four hours before the exposure session to half an hour before. Studies also employed post‐exposure treatment administration of DCS. When comparing reduction in symptoms, clinician assessed scales were not uniformly used, namely the AAVQ, which is a self‐report scale, could have possibly skew the results.
A lack of generalizability of the results is noted. Most participants were in an outpatient rather than primary care setting. Of the selected study population, only three studies included children and adolescents, and one adolescents only. These studies were also limited to OCD and PTSD. It may be possible that the population selected, given the stringent exclusion criteria employed in the studies, may not have been representative of the 'real world' sample. All included studies in the review excluded participants who were pregnant or lactating, had a history of substance abuse, and a history of a serious general medical condition, and thus participants may not be representative of the general population. Often 'real' patients with anxiety disorders have several co‐morbidities, and this may not be accurately reflected in the severity of the condition evident in the participants in these trials. Only 55% of studies reported on co‐morbidities (de Kleine 2012; Farrell 2013; Guastella 2008; Hofmann 2013; Litz 2012; Mataix‐Cols 2014; Nave 2012; Rothbaum 2014; Storch 2007; Storch 2010; Wilhelm 2008). These rates varied from a total of 70.1% of the sample having an additional diagnosis (de Kleine 2012), 65% having a second diagnosis and 53% having a third diagnosis in Farrell 2013, and only one participant in Nave 2012 with an additional diagnosis. Further, all the trials were conducted in first world settings, limiting the applicability to developing and resource limited countries.
Quality of the evidence
The quality of the evidence was assessed across seven outcomes at the end of treatment, namely treatment efficacy, treatment acceptability, remission, condition‐specific anxiety symptoms, co‐morbid depression, co‐morbid anxiety and quality of life. We constructed two separate summary of findings tables, one for DCS and cognitive and behavioural therapies compared to placebo and cognitive and behavioural therapies for anxiety disorders in adults (see Table 1) and the other in children and adolescents (see Table 2). In the adult comparison the main outcome of treatment efficacy was marked as low quality mainly due to the missing data reported on allocation concealment in five studies. This indicates that future research is likely to have an important impact on our confidence in the estimate of effect and is likely to change this effect. The quality of evidence for the main outcome comparison of treatment efficacy in the children and adolescent group was also regarded as low. The quality of information was downgraded as the total sample size was lower than the calculated optimal information size; and the confidence intervals are wide, including both appreciable benefit and no effect.
Study limitations (risk of bias)
Treatment efficacy in adults was marked as having a high risk of bias due to insufficient reporting on the method of allocation concealment. This risk of bias was also seen in comparisons of co‐morbid anxiety symptoms in children and adolescents. Outcome assessment of remission in adults was downgraded to a moderate quality due to concerns around selective reporting.
Consistency of effect
Studies showed marked heterogeneity over a range of outcomes in both adult and children and adolescent comparator groups. This may be due to the variability in the types of anxiety disorders compared, the participants, and the severity of illness within each group. Methodological diversity may also account for this result as different dosages, timing and frequency of DCS were administered. Further, the number and duration of exposure‐based therapy sessions varied amongst studies. Treatment acceptability in adults was downgraded to very low quality. In the child and adolescent comparator groups assessment of co‐morbid depression and co‐morbid anxiety was downgraded to very low quality of evidence suggesting uncertainty about the overall estimate.
Indirectness
The studies included in our review were conducted in outpatient settings and some populations were regarded as difficult to treat, so results may not be generalizable to the primary care setting. In addition, withdrawal from treatment was used as an indirect measure of treatment acceptability to patients. The quality of evidence was thus downgraded by one level as there could have been other reasons why participants dropped out. Quality of life outcomes were downgraded by one step for indirectness. Measuring the impact of an individual's fears on various components of their life can not be considered the sole measure of quality of life.
Imprecision
The results on co‐morbid anxiety symptoms and quality of life were downgraded by two levels for imprecision. The total sample size was lower than the calculated optimal information size and two or fewer studies reported this outcome for adults. Treatment efficacy or the measurement of treatment responders in children and adolescents was downgraded two levels as the total sample size was also lower than the optimal informational size and the CIs were wide, including both appreciable benefit and no treatment effect.
Publication bias
A funnel plot was used to assess condition‐specific anxiety symptoms at treatment and follow‐up. This was the only outcome with more than 10 studies. The funnel plots indicated no evidence asymmetry, therefore this outcome was not downgraded (see Figure 4; Figure 5).
Potential biases in the review process
Some biases and limitations can be noted:
1. We tried to perform a thorough article search, however it is possible that we missed some relevant studies, including unpublished trials. We did not consider searching for publicly accessible reports of drug trials in the FDA and EMA databases, and this may have limited the study findings.
2. The post hoc addition of analysing adults and children separately may have introduced bias.
3. Only 9 of 16 studies in the adult comparator group reported on responder rates and this could have introduced bias in the results.
4. Two studies were rated as high risk of bias, in Cameron 2005 five participants were not included in the analysis with no reason given, and in de Kleine 2012, there was a high dropout rate with approximately one in three patients ending treatment prematurely.
5. Six included studies had a large percentage of patients receiving concomitant medications, which might have confounded the results. In de Kleine 2012, when patients completed the homework assignments they were not taking DCS, which may have diluted the DCS‐related effects and influenced treatment outcomes. There was also insufficient information provided on other biases.
6. The extraction of data points from graphs using digitizing software may have introduced bias as any adjustment of the analysis in the graph might have made it difficult to match the effect size exactly. To ensure that our estimations were not too far off, we compared the P values from our estimations with those reported in the papers (when P values were reported). This applied to continuous outcomes for Otto 2010, Ressler 2004 and Siegmund 2011. Standard deviations, standard errors or CIs for the treatment effects were not reported for these studies.
Agreements and disagreements with other studies or reviews
Our findings are similar to research conducted on the effects of DCS by Bontempo 2012. Bontempo 2012 found no significant differences in methodological features, however the authors found that DCS may still have significant effects in improving outcomes in patients using CBT for anxiety and related disorders. Our current findings contrast with this conclusion, possibly due to the inclusion of more studies without significant clinical effects of DCS compared with placebo.
A meta‐analysis by Rodrigues 2014 suggests that DCS enhances the effects of exposure therapy in anxiety and related disorders. The observed effect size was, however, small to moderate (Cohen’s d = ‐0.34) and showed low heterogeneity. Our results may have differed as, despite Rodrigues 2014 being published in 2014, the search results only included studies up to 2012, and seven new studies were not included. Of these new studies, five indicated that there was no clinically significant difference between DCS augmentation of cognitive and behavioural therapies compared to placebo augmentation of cognitive and behavioural therapies for treatment response in anxiety and related disorders (Cameron 2005; Hofmann 2013; Mataix‐Cols 2014; Rothbaum 2014; Scheeringa 2014). In addition, Hofmann 2013, which indicated similar response rates between the placebo and DCS arms, is the largest trial to date, with 144 participants, and was not included in the analysis. The remaining two new studies (Difede 2014; Farrell 2013) that did show a response in favour of DCS were smaller trials of 25 and 30 participants respectively. Farrell 2013 also stated that both groups showed significant improvement of anxiety symptoms, with 94% of the total sample regarded as responders.
These nuances include claims made about DCS around effects on the speed of treatment gains, effects of dosing and dose timing, and effects on fear memory reconsolidation (Hofmann 2015). Of these claims, this review aimed to primarily assess the response for DCS augmentation of CBT compared with placebo augmentation of CBT for anxiety and related disorders. Nevertheless, other reviews and updates have suggested a trend toward acceleration in symptom reduction, particularly in more severe symptoms (Hofmann 2015; Siegmund 2011). Hofmann 2015 suggests three important issues that may influence the therapeutic action of DCS, namely that DCS primarily acts by accelerating CBT; the dosage and timing of administration, and under conditions of poor exposure therapy; and that DCS can worsen symptoms by enhancing fear memory reconsolidation. Declining benefit of DCS across weeks of treatment, as seen in Kushner 2007, Siegmund 2011 and Wilhelm 2008, suggests an accelerated response rather than an increased magnitude of effect. This was further replicated in Hofmann 2013, where both DCS and placebo augmented CBT groups were associated with similar response and remission rates at the end of treatment and follow‐up, but DCS was associated with a 24% to 33% faster rate of improvement over 12 weeks relative to placebo. This has important implications as patients who may experience early treatment gains are less likely to discontinue treatment. Some studies also show that increased doses, from 500 mg to 1 g, have a weaker effect; and sometimes NMDA antagonistic effects are seen at different concentrations (Davis 2006). The authors conclude that there is currently not enough information to provide clear guidance on the most efficacious dose, number of exposure therapy sessions, and the timing of administration of DCS. Further considerations provided by Lee 2006 and Hofmann 2014 are that, in addition to strengthening extinction memory, DCS can be employed to strengthen reconsolidation of fear memory. It has been proposed that DCS may worsen symptoms if the within‐session decrease of fear is insufficient, and may result in fear consolidation. This suggests that DCS augmentation should only be administered if the exposure sessions were regarded as successful. Further, this current review aimed to assess the efficacy of DCS augmented cognitive and behavioural therapies compared to placebo and cognitive and behavioural therapies at the end of treatment, rather than per session reduction of anxiety symptoms. We, therefore, cannot support these conclusions but suggest future work in the direction of assessing within session reduction of anxiety symptoms and thus speed of treatment gains.
Authors' conclusions
Implications for practice.
There is currently no evidence of a difference between DCS augmentation of cognitive and behavioural therapies and placebo augmentation of cognitive and behavioural therapies in children, adolescents and adults. These findings were based on low quality evidence due to incomplete data, with many studies not presenting response, and small sample sizes. It is noteworthy that whilst there is no evidence of an effect of DCS augmentation of cognitive and behavioural therapies in the treatment of anxiety and related disorders, there is also no evidence against its use. On balance we believe there is not sufficient evidence to draw conclusions on its use at this stage.
Implications for research.
The evidence from the studies of DCS augmentation of cognitive and behavioural therapies for the treatment of anxiety and related disorders included in this review is limited by the small sample sizes used. Larger trials and additional meta‐analysis would allow for a more precise estimate of the treatment effects of DCS, as well as a more comprehensive look at sources of heterogeneity between study results. Methodological differences in therapy techniques in trials and particular types of anxiety disorders may be important causes of heterogeneity between trials, and deserve further research.
More experimental work should be undertaken to disentangle extinction learning, fear memory reconsolidation and DCS enhancement. Increasing evidence shows that DCS enhances cognitive processes during extinction learning, as well as in fear memory reconsolidation. This evidence suggests that administration of DCS based on the level of fear reduction at the end of an exposure session may be efficacious. However, it is not yet known whether this is a desirable augmentation strategy in humans.
Several aspects of DCS augmentation need further investigation in order to establish optimal augmentation strategies, such as dosage, timing of administration (for example before or after therapy sessions), number of administrations and individual differences in response. Studies should also include quality of life as an outcome measure. Only one included study reported quality of life despite its clinical value in understanding treatment strategies for these disorders.
DCS acts as a cognitive enhancer but may not be the only agent that has potential benefit for augmenting cognitive and behavioural therapies processes. Other agents have been identified, such as yohimbine (a selective, competitive alpha2‐adrenergic receptor antagonist), thus future research may be employed to determine mechanisms of action as well as magnitude of effect.
Acknowledgements
We would like to thank Rachel Churchill for her ongoing support and assistance throughout the review. In addition, we would like to acknowledge Dr Eric Storch for responding to our request for missing data for inclusion in the review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Depression, Anxiety and Neurosis Group. The views and opinions expressed therein are those of the authors and do not reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
CRG Funding Acknowledgement
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Depression, Anxiety and Neurosis Group.
Disclaimer
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: treatment responders ‐ end of treatment | 9 | 449 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.89, 1.34] |
| 1.1 OCD | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.67, 1.23] |
| 1.2 PD | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [1.04, 4.86] |
| 1.3 PTSD | 3 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.79, 2.07] |
| 1.4 SAD | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.00, 1.27] |
| 1.5 SP | 3 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.61, 1.65] |
| 2 Treatment efficacy: treatment responders ‐ 1‐12 month follow up | 7 | 383 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.90, 1.31] |
| 2.1 OCD | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.63, 1.13] |
| 2.2 PD | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.81, 2.44] |
| 2.3 PTSD | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.73, 1.85] |
| 2.4 SAD | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.93, 1.31] |
| 2.5 SP | 2 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.49, 3.79] |
| 3 Treatment acceptability: withdrawals from treatment ‐ end of treatment | 16 | 740 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.61, 1.25] |
| 3.1 OCD | 3 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.20, 2.37] |
| 3.2 PD | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.61] |
| 3.3 PTSD | 4 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.57, 1.84] |
| 3.4 SAD | 5 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.42, 1.22] |
| 3.5 SP | 3 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.27, 92.62] |
| 4 In remission ‐ end of treatment | 5 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.79, 1.71] |
| 4.1 OCD | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.31, 1.63] |
| 4.2 PTSD | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.50, 8.05] |
| 4.3 SAD | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.87, 2.47] |
| 4.4 SP | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.53, 1.65] |
| 5 In remission ‐ 1‐6 month follow up | 5 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.79, 2.10] |
| 5.1 OCD | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.23, 1.60] |
| 5.2 PTSD | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 2.58 [1.34, 4.99] |
| 5.3 SAD | 1 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.62, 1.81] |
| 5.4 SP | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.61, 1.76] |
| 6 Condition‐specific anxiety symptoms ‐ end of treatment | 17 | 735 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.58, ‐0.07] |
| 6.1 OCD (Y‐BOCS) | 3 | 72 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.61, 0.33] |
| 6.2 PD (PAS or PDSS) | 2 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.33, ‐0.34] |
| 6.3 PTSD (CAPS) | 4 | 224 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.52, 0.39] |
| 6.4 SAD (LSAS) | 5 | 293 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.99, 0.21] |
| 6.5 SP (AAQ and Snake Questionnaire) | 3 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.14, 0.13] |
| 7 Condition‐specific anxiety symptoms ‐ 1‐12 month follow up | 13 | 641 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.47, ‐0.06] |
| 7.1 OCD (Y‐BOCS) | 2 | 45 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐1.13, 0.83] |
| 7.2 PD (PDSS and PAS) | 2 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.10, 0.21] |
| 7.3 PTSD (CAPS) | 4 | 218 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.55, 0.14] |
| 7.4 SAD (LSAS) | 3 | 252 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.57, 0.22] |
| 7.5 SP (AAQ) | 2 | 56 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.21, ‐0.11] |
| 8 Co‐morbid symptoms of depression ‐ end of treatment | 5 | 178 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.80, 0.31] |
| 8.1 OCD (BDI‐II) | 2 | 47 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.23, ‐0.04] |
| 8.2 PD (BDI) | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.98, 0.30] |
| 8.3 PTSD (BDI and BDI‐II) | 2 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐1.27, 1.70] |
| 9 Co‐morbid symptoms of depression ‐ 1‐6 month follow up | 5 | 171 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.47, 0.24] |
| 9.1 OCD (BDI‐II) | 2 | 45 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.86, 0.93] |
| 9.2 PD (BDI) | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.95, 0.31] |
| 9.3 PTSD (BDI and BDI‐II) | 2 | 87 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.78, 0.71] |
| 10 Co‐morbid anxiety symptoms ‐ end of treatment | 3 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.99, ‐0.26] |
| 10.1 PD (BAI) | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.16, 0.12] |
| 10.2 PTSD (STAI) | 1 | 67 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.16, ‐0.18] |
| 10.3 SAD (STAI) | 1 | 16 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.73, 0.32] |
| 11 Co‐morbid anxiety symptoms ‐ 3‐5 month follow up | 2 | 106 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.68, 0.09] |
| 11.1 PD (BAI) | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.93, 0.33] |
| 11.2 PTSD (STAI) | 1 | 67 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.77, 0.19] |
| 12 Quality of life ‐ end of treatment | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1 SAD (LIS) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Quality of life ‐ 1 month follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.1 SAD (LIS) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Adverse events leading to hospitalisation or discontinuation ‐ end of treatment | 2 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.10, 9.00] |
| 14.1 PD | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.76] |
| 14.2 SAD | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.12, 68.49] |
Comparison 2. DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies ‐ children and adolescents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment efficacy: treatment responders ‐ end of treatment | 4 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.31] |
| 1.1 OCD | 3 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.94, 1.35] |
| 1.2 PTSD | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.44, 1.10] |
| 2 Treatment efficacy: treatment responders ‐ 3‐12 month follow up | 3 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.67, 1.09] |
| 2.1 OCD | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.21] |
| 2.2 PTSD | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.44, 1.10] |
| 3 Treatment acceptability: withdrawals from treatment ‐ end of treatment | 4 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.17, 4.69] |
| 3.1 OCD | 3 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.08] |
| 3.2 PTSD | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.46, 4.59] |
| 4 In remission ‐ end of treatment | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.66, 2.16] |
| 4.1 OCD | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.66, 2.16] |
| 5 In remission ‐ 3‐12 month follow up | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.69, 1.61] |
| 5.1 OCD | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.69, 1.61] |
| 6 Condition‐specific anxiety symptoms ‐ end of treatment | 4 | 131 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.55, 0.69] |
| 6.1 OCD (Y‐BOCS) | 3 | 74 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.70, 0.25] |
| 6.2 PTSD (Child PTSD Symptom Scale) | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.70 [0.17, 1.24] |
| 7 Condition‐specific anxiety symptoms ‐ 3‐12 month follow up | 3 | 91 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.32, 0.78] |
| 7.1 OCD (Y‐BOCS) | 2 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.65, 0.54] |
| 7.2 PTSD (Child PTSD Symptom Scale) | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [0.03, 1.21] |
| 8 Co‐morbid symptoms of depression ‐ end of treatment | 3 | 114 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.52, 0.69] |
| 8.1 OCD (BDI‐Y and CDI) | 2 | 57 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.77, 0.28] |
| 8.2 PTSD (CDI) | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [0.06, 1.13] |
| 9 Co‐morbid symptoms of depression ‐ 3‐12 month follow up | 2 | 84 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.56, 0.74] |
| 9.1 OCD (BDI‐Y) | 1 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.06, 0.46] |
| 9.2 PTSD (CDI) | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.16, 0.89] |
| 10 Co‐morbid anxiety symptoms ‐ end of treatment | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 OCD (MASC) | 2 | 47 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.93, 0.23] |
| 10.2 PTSD (SCARED) | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.80 [0.26, 1.34] |
| 11 Co‐morbid anxiety symptoms ‐ 3 month follow up | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 PTSD (SCARED) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cameron 2005.
| Methods | Design: Randomised double‐blind placebo controlled trial Study duration: 12 weeks Follow‐up: 3 months Country: USA |
|
| Participants | Sample size: 39 children, adolescents and adults were randomised Recruitment: Not reported Inclusion criteria ‐ diagnostic classification criteria: DSM‐IV symptoms of social phobia, generalized or specific type Inclusion criteria ‐ rating scales: Not reported Included disorders: Social anxiety disorder Co‐morbidities: co‐morbid major depressive disorder was an exclusion criteria Gender: 51% male Mean age: Not reported; 36% were below 18 years old, the remaining participants were 18 to 65 years old Ethnicity: 5% Asian, 26% Black, 62% White, 5% not reported, 3% multiracial Pharmacotherapy during the study: Naturalistic prescribing not allowed |
|
| Interventions |
Therapists: Not reported |
|
| Outcomes | Withdrawals; anxiety symptoms: LSAS; adverse events | |
| Notes | Funding from industry: No Medication supplied by industry: Not reported Any author work for industry: Not reported Study ID: NCT00128401 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised" but does not report method |
| Allocation concealment (selection bias) | Unclear risk | Details not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)". Further details not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)". Further details not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study stated that 14 participants in the DCS group (70%) and 15 participants from the placebo group (79%) completed the study, but only 12 participants from each group (60%) were analysed for the CGI‐S and LSAS with no reasons given for excluding data |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in protocol reported on with data provided |
| Other bias | Unclear risk | Insufficient information to assess other bias (record from www.ClinicalTrials.gov) |
de Kleine 2012.
| Methods | Design: Randomized, double‐blind, placebo controlled study Study duration: 10 weeks Follow‐up: 3 months Country: Netherlands |
|
| Participants | Sample size: 67 adult patients eligible and agreed to participate Recruitment: recruited from referrals to two Dutch outpatient clinics Inclusion criteria ‐ diagnostic classification criteria: DSM‐IV primary diagnosis of PTSD Inclusion criteria ‐ rating scales: Mini‐International Neuropsychiatric Interview (MINI) Included disorders: PTSD and any co‐morbid diagnoses. The traumatic events underlying PTSD were mixed and comprised sexual assault including childhood sexual abuse (n = 35), violent nonsexual assault (n = 20), a road traffic or other accident (n = 3), war‐zone experiences (n = 2), and miscellaneous (n = 7) Co‐morbidities: 70.1% (n = 47) had additional diagnosis (mean 2.0) ‐mMajor depressive disorder (53.7%) and anxiety disorders (41.8%) Gender: 80.6% female Mean age: 38.3 years (SD 11.4 years) Ethnicity: Not reported Pharmacotherapy during the study: Naturalistic prescribing allowed; 11 participants were taking a benzodiazepine, 8 participants were taking an anti‐depressant, 9 participants were taking a benzodiazepine and an antidepressant |
|
| Interventions |
Therapists: Psychologists trained in prolonged exposure therapy (PE) administered the PE |
|
| Outcomes | Responders; withdrawals; remission; anxiety: CAPS; co‐morbid depression: BDI; co‐morbid anxiety: STAI | |
| Notes | Funding from Industry: Unclear Medication funded by industry: No, DCS purchased from Duchefa Farma Any authors work for industry: Unclear. This work was supported by Stichting Achmea Slachtoffer en Samenleving (to GH and AvM) and Vereniging tot Christelijke Verzorging van Geestes‐ en Zenuwzieken (to GH and AvM) Study ID: NTR1184 (Nederlands trial register: http://www.trialregister.nl/) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using standard software, an independent statistician generated a randomisation list using random blocks with a maximum of 1 number each |
| Allocation concealment (selection bias) | Low risk | The active and placebo capsules were dispensed by the pharmacist in numbered containers in accordance with the randomisation list.The compounding chemist purchased DCS from Duchefa Farma (Haarlem, the Netherlands) to make the 50 mg DCS capsules along with the identical‐looking placebo |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Everyone involved in the study (i.e. researchers, participants, therapists, and assessors) were blind to the treatment condition until all follow‐up assessments were completed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Everyone involved in the study (i.e. researchers, participants, therapists, and assessors) were blind to the treatment condition until all follow‐up assessments were completed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Eight dropped out before the first exposure session, leaving 67 participants receiving the allocated intervention. The treatment protocol was completed by 45 participants, whereas 40 completers and 5 dropouts completed the 3‐month follow‐up assessment. High dropout rate: in both groups, approximately one in three patients ended treatment prematurely. This may have introduced a bias in the results. Eight participants never received any exposure treatment and were not included in the analysis, thus not a true intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes pre‐specified in protocol were reported |
| Other bias | Unclear risk | When patients completed the homework assignments they were not taking DCS, which may have diluted DCS‐related effects |
Difede 2014.
| Methods | Design: Randomised, placebo controlled, double‐blind trial Study duration: 12 weeks Follow‐up: 6 months Country: USA |
|
| Participants | Sample size: Estimated 40 adult participants who developed posttraumatic stress disorder (PTSD) following either the events of September 11, 2001, or military service in the war in Iraq Sample size: 25 participants were randomised Recruitment: Participants were recruited between 2005 and 2011 by publicizing the study in medical centres and the general community Inclusion criteria ‐ diagnostic classification criteria: PTSD, according to DSM‐IV criteria, following exposure to the World Trade Center (WTC) attacks Inclusion criteria ‐ rating scales: Clinician Administered PTSD Scale (CAPS) Included disorders: PTSD Co‐morbidities: Not reported Gender: 76% male Mean age: 45.84 years (SD = 10.50, range 25 to 70 years) Ethnicity: 84% White Pharmacotherapy during the study: Naturalistic prescribing allowed. Participants had to be on a stable dose for at least 2 months before enrolment and maintain their medication regimen throughout the study. Prescriptions included: Paxil, Xanax, Klonopin, Lexapro, Prozac, Wellbutrin, Effexor, Ativan, Nortriptyline, Celexa, Cymbalta, and Zoloft. Numbers of participants taking each medication not reported |
|
| Interventions |
Therapists: Not reported |
|
| Outcomes | Withdrawals; remission; anxiety: CAPS; adverse events | |
| Notes | Funding from industry: "Partial funding support was provided by DeWitt‐Wallace Fund of the New York Community Trust, which had no role in study design, data collection, analysis and interpretation, or writing of this paper" Medication supplied by industry: Not reported Any author work for industry: Dr Altemus has consulted for Ironwood Pharmaceuticals and Corcept Therapeutics, and has received research support from the Fisher Family Foundation Study ID: NCT00632632 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "randomised" but does not report method of sequence generation. However, since allocation concealment was adequate we assume that randomisation method also was adequate |
| Allocation concealment (selection bias) | Low risk | Central allocation: "the research pharmacy oversaw randomisation" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "100 mg DCS capsules and matching placebo capsules containing lactose were prepared. Medication was administered double‐blind; the research pharmacy oversaw randomisation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A psychologist who was blind to the medication condition conducted assessments" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A total of 22/25 (88%) completed intervention and follow‐up process. Although the number of dropouts was small, all dropouts were in the intervention group and reasons for dropping out were not provided. "Three participants dropped out (after sessions 3, 6, and 7); all were in the VRE‐placebo group". "All analyses adhered to intent‐to‐treat principle, with the last available observation used as the outcome data (ITT/LOCF)." |
| Selective reporting (reporting bias) | High risk | Two outcome measures (PCL and BDI‐II) were not reported sufficiently with means and SDs to be included in a meta‐analysis |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Farrell 2013.
| Methods | Design: Randomised double‐blind placebo controlled pilot trial Study duration: 9 weeks Follow‐up: 3 months Country: Australia |
|
| Participants | Sample size: 17 children and adolescents were enrolled Recruitment: Children and adolescents with a primary diagnosis of OCD were enrolled at Griffith University between May 2009 and September 2010 Inclusion criteria ‐ diagnostic classification criteria: Primary diagnosis of OCD, child meeting criteria for "difficult‐to‐treat OCD", specific diagnostic criteria not reported Inclusion criteria ‐ rating scales: CY‐BOCS score of ≥ 19 Included disorders: OCD Co‐morbidities: 65% of the sample was presented with a secondary co‐morbid diagnosis and 53% presented with a tertiary diagnosis Diagnoses included: Specific phobia (3), GAD (8), MDD (2), SAnD (1), Social phobia (3), PTSD (1), ADD/ADHD (4) Gender: 41% male Mean age: mean age = 13.11 years (SD 3.33, range 8 to 18) Ethnicity: 94% Caucasian, 6% Asian Pharmacotherapy during the study: Naturalistic prescribing allowed. At study entry, 13 youth (76%) were on SRI medication and were stable on medication for at least 4 weeks, mean 51 weeks (range 4 to 240) and remained stable throughout the trial |
|
| Interventions |
Therapists: Therapists were all postgraduate‐level clinicians with previous experience in CBT for OCD. All clinicians received formal weekly supervision, wherein clinicians reported client progress, adherence to the treatment protocol, and provided and had an opportunity to ask questions and problem solve treatment difficulties or process issues |
|
| Outcomes | Responders; withdrawals; remission; anxiety: CY‐BOCS; co‐morbid anxiety: MASC; adverse events | |
| Notes | Funding from industry: No, this trial was supported by an Australian Rotary Health Research Fund grant Medication supplied by industry: Not reported Any author work for industry: Not reported Study ID: ACTRN12609000370202 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Children were randomised using a computer‐generated list of randomly permuted blocks of pairs, with an allocation of 1:1 to either ERP + DCS or ERP + PBO" |
| Allocation concealment (selection bias) | Unclear risk | Details not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All other investigators were blind, as were assessors, therapists, and all participants." "Pills were compounded to be identical in size and colour, and were dispensed by the study pharmacist corresponding to randomisation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Therapists and assessors were blinded. Additionally, some outcome assessments were self‐reports |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All children enrolled in the trial completed treatment" |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the protocol were reported |
| Other bias | Unclear risk | Greater than 75% of patients (13/17 patients) were receiving concomitant medications, which might have confounded the results |
Guastella 2008.
| Methods | Design: Randomised, double‐blind, placebo controlled study Study duration: 5 weeks Follow‐up: 1 month Country: Australia |
|
| Participants | Sample size: 56 adult patients eligible and agreed to participate Recruitment: recruited from the community Inclusion criteria ‐ diagnostic classification criteria: DSM‐IV primary diagnosis of SAnD Inclusion criteria ‐ rating scales: Anxiety Disorder Interview Schedule for Adults (ADIS‐IV) and self‐reported fear of public speaking Included disorders: SAnD and co‐morbid diagnoses Co‐morbidities: 30.34% of the participants (n = 17) had secondary diagnosis, 8 = additional anxiety disorder, 8 = additional secondary mood disorder, 1 = additional anxiety and mood disorder Gender: 57% male Mean age: 35.48 years (SD 11.35 years, range 18 to 60 years) Ethnicity: 76.8% Caucasian Pharmacotherapy during the study: Naturalistic prescribing allowed; 5 participants were taking an antidepressant, 2 participants were taking an immune suppressant, 2 participants were taking an appetite suppressant, 2 participants were taking blood pressure medication, and 1 participant was taking an herbal preparation |
|
| Interventions |
Therapists: Sessions were administered by therapists who were registered clinical psychologists or provisionally registered clinical psychologists. All therapists were supervised and trained by a senior clinical psychologist |
|
| Outcomes | Withdrawals; anxiety: LSAS; quality of life: LIS; adverse events | |
| Notes | Funding from Industry: No Medication supplied by industry: No, DCS purchased from Eli‐Lilly Any of the authors work for industry: No Study ID: Australian Clinical Trials Registry: 012606000352505 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random component in sequence generation performed by chemist, the method was not stated. However, since allocation concealment was adequate we assume that the randomisation method also was adequate |
| Allocation concealment (selection bias) | Low risk | A random allocation sequence was generated by numbering containers with the medication. This randomisation sequence was developed by the compounding chemist before the trial and concealed from all individuals involved in patient care, evaluation, or supervision until follow‐up assessments were completed. The compounding chemist purchased DCS to make 50 mg DCS capsules, along with identical placebo |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and assessor were blinded until follow‐up assessments were completed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and assessor were blinded until follow‐up assessments were completed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | After drug assignment, 6 participants (n = 1 DCS; n = 5 placebo) failed to attend at least three group exposure sessions between session 2 and 5 and dropped out of treatment. The x 2 analysis showed the difference between the two groups in dropout rates after drug assignment approached significance (P = 0.08). No dropouts occurred over the 1‐month follow‐up assessment period. LOCF used, with ITT principle |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available (the trial was registered retrospectively) thus unsure if all of study's pre‐specified outcomes of interest reported in pre‐specified way |
| Other bias | Low risk | No sources of other bias were identified |
Hofmann 2006.
| Methods | Design: Randomised, double‐blind, placebo controlled augmentation trial Study duration: 5 weeks Follow‐up: 1 month Country: USA |
|
| Participants | Sample size: 27 adult patients eligible and agreed to participate Recruitment: self‐referred from the community to one of three research clinics Inclusion criteria ‐ diagnostic classification criteria: DSM‐IV primary diagnosis of SAnD with significant public speaking anxiety Inclusion criteria ‐ rating scales: Anxiety Disorder Interview Schedule for Adults (ADIS‐IV) and self‐reported fear of public speaking Included disorders: SAnD and co‐morbid diagnoses Co‐morbidities: 11 individuals had at least 1 additional DSM‐IV Axis I diagnosis; 9 had an additional anxiety disorder and 4 had an additional mood disorder Gender: 70% male Mean age: 33.70 years (SD 10.02 years) Ethnicity: 59.3% White, 14.8% Asian, 11.1% Hispanic, 11.1% African American Pharmacotherapy during the study: Naturalistic prescribing allowed; 1 participant was taking a benzodiazepine, 9 participants were taking an antidepressant, 1 participant was taking a beta‐blocker, and 3 participants were taking stimulants |
|
| Interventions |
Therapists: Sessions were administered by therapists who were supervised and trained by 2 of the article authors |
|
| Outcomes | Withdrawals; anxiety: LSAS; adverse events | |
| Notes | Funding from Industry: Unclear Medication funded by industry: Unclear Any authors work for industry: Unclear Study ID: NCT00515879 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were randomised to either adjunctive DCS or pill placebo administered as a 50‐mg pill on each of 4 occasions.' Study was randomised but method of sequence generation not stated. However, since allocation concealment was adequate we assume that randomisation method was adequate |
| Allocation concealment (selection bias) | Low risk | 'The random allocation sequence was generated by numbering containers with the medication. The sequence was generated prior to allocating participants and was concealed until the end of the study.' Matching d‐cycloserine or placebo was given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'All of the individuals involved inpatient care, evaluation, or study supervision were blind to group assignment until the end of the study.' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'All of the individuals involved inpatient care, evaluation, or study supervision were blind to group assignment until the end of the study.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 32 eligible participants, 5 had to be excluded from analysis for the following reasons: 4 patients withdrew after signing the consent form or after the initial treatment session, and 1 patient was excluded owing to a protocol violation. Twenty‐seven patients (12 who received exposure therapy plus DCS and 15 who received exposure therapy plus placebo) completed the 5‐session treatment. Twenty‐three patients (10 who received exposure therapy plus DCS and 13 who received exposure therapy plus placebo) completed the 1‐month follow‐up assessment. |
| Selective reporting (reporting bias) | High risk | No information was provided on the following outcomes as per protocol ‐ Social Phobic Disorders Severity and Change Form, Quality of Life Enjoyment and Satisfaction Questionnaire, Range of Impaired Functioning Tool |
| Other bias | Unclear risk | A large percentage of patients (11 patients, 40.7%) were receiving concomitant medications, which might have confounded the results |
Hofmann 2013.
| Methods | Design: Randomised double‐blind placebo controlled trial Study duration: 12 weeks Follow‐up: 6 months Country: USA |
|
| Participants | Sample size: 169 participants were randomised Recruitment: Participants were recruited between September 2007 and June 2011 through referrals to the three study sites (Boston University, Massachusetts General Hospital, and Southern Methodist University) from other area clinical facilities and programs, and through advertisements Inclusion criteria ‐ diagnostic classification criteria: DSM‐IV diagnosis of generalized social anxiety disorder Inclusion criteria ‐ rating scales: score > 60 on the Liebowitz Social Anxiety Scale Included disorders: SAnD Co‐morbidities: entry of patients with other mood or anxiety disorders was permitted if the social anxiety disorder was judged to be the predominant disorder Gender: DCS group 64.4% male; placebo group 48.8% male Mean age: DCS group 34.6 years; placebo group 30.5 years Ethnicity: DCS group 70.1% White, 9.2% African‐American, 15% Asian, 5.7% other, 10.3 Hispanic or Latino; placebo group 74.4% White, 9.8% African‐American, 8.5% Asian, 7.3% other, 11.0% Hispanic or Latino Pharmacotherapy during the study: Naturalistic prescribing not allowed |
|
| Interventions |
Therapists: All therapists were trained and supervised by senior clinicians and participated in weekly cross‐site supervision |
|
| Outcomes | Response; withdrawals; remission; anxiety: LSAS; adverse events | |
| Notes | Funding from industry: No, supported by NIMH grant Medication supplied by industry: Not reported Any author work for industry: Dr Hofmann has received royalties from multiple publishers, including Routledge, the publisher of the CBT manual used in the study. Dr Otto has also received royalties from Routledge. Dr Pollack has served as a consultant for Bristol‐Myers Squib, Euthymics, Eli Lilly, Forest Laboratories, GlaxoSmithKline, Medavante, and Targia Pharmaceuticals and has equity in Medavante, Mendsante, Mindsite and Targia Pharmaceuticals Study ID: NCT00633984 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Assignment to treatment condition was determined by a computer‐generated allocation schedule with stratification by baseline severity of social anxiety disorder." |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All capsules were identical in appearance to maintain the blind." " patients were asked to indicate whether they believed the pill contained d‐cycloserine or placebo or whether they were unable to guess. Approximately one‐third to one‐half of all patients (30.9%–46.2%, depending on group and session) in both conditions reported that they were unable to guess their treatment condition (all chi‐square tests, n.s.). Among patients who guessed either of the two drug conditions, those who received d‐cycloserine did not differ significantly from those who received placebo in their guess that they received d‐cycloserine, in any of the sessions". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The blinded assessments were conducted by a master’s‐level or doctoral‐level clinician trained in these assessments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Attrition rates during the 12‐week treatment phase were low and did not differ significantly between groups (10.3% for the DCS group and 15.9% for the placebo group)." "Attrition was low during the follow‐up phase (11.5% and 11.)% for the DCS and placebo groups, respectively)." |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the protocol were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kushner 2007.
| Methods | Design: Randomised, double‐blind, placebo controlled trial Study duration: 5 weeks Follow‐up: 3 months Country: USA |
|
| Participants | Sample size: 32 adult participants eligible and agreed to participate Recruitment: 150 individuals responded to newspaper ads about study; 63 failed to meet inclusion/ exclusion criteria and 55 chose not to participate or could not be contacted Inclusion criteria ‐ diagnostic classification criteria: primary diagnosis of OCD, DSM‐IV Inclusion criteria ‐ rating scales: Structured Clinical Interview (SCID‐IV) and Yale‐Brown Obsessive Compulsive Scale (Y‐BOCS) Included disorders: OCD and co‐morbid diagnoses, excluding substance abuse disorders, major depressive disorder, or primary hoarding or ordering ritual behaviours. Co‐morbidities: Not reported Gender: Not reported Mean age: Not reported, adults Ethnicity: Not reported Pharmacotherapy during the study: Naturalistic prescribing allowed if participant had been stable for at least 2 months prior to beginning of study |
|
| Interventions |
Therapists: Sessions were administered by psychologists who were supervised and trained by 2 of the article authors |
|
| Outcomes | Withdrawals; anxiety: Y‐BOCS; adverse events | |
| Notes | Funding from Industry: Unclear‐ This work was supported, in part, by a grant to the first author from the Obsessive‐Compulsive Foundation (#450709) Medication supplied by industry: Unclear Any authors work for industry: Unclear Study ID: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'participants allocated (to intervention) in a random double‐blind fashion.' Likely that adequate sequence generation performed, but method not stated |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded. "We dispensed to each subject 10 doses of 125 mg DCS or 10 identical‐looking placebo doses in a random double‐blind fashion" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | '7 dropouts. We also found that those given DCS were about one sixth as likely to drop out of the EX/RP therapy as those given placebo 6% (1) vs. 35% (6). Baseline scores of both completers and non‐completers provided'. Outcome data for completers and non‐completers provided |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available thus unsure if all of study's outcomes of interest reported in pre‐specified way |
| Other bias | Low risk | No sources of other bias were identified |
Litz 2012.
| Methods | Design: Randomised, double‐blind, placebo controlled trial Study duration: 6 weeks Follow‐up: 6 months Country: USA |
|
| Participants | Sample size: 26 adult participants eligible and agreed to participate Recruitment: unclear Inclusion criteria ‐ diagnostic classification criteria: primary diagnosis of PTSD, DSM‐IV Inclusion criteria ‐ rating scales: Structured Clinical Interview (SCID‐IV) Included disorders: PTSD and co‐morbid diagnoses Co‐morbidities: MDD (n = 7), alcohol use (n = 5), SAnD (n = 2) Gender: 100% male Mean age: 32.19 years (SD 9.31 years) Ethnicity: 76.9% White, 15.4% Black, 3.% Hispanic, 11.18% Pacific Islander, 3.8% Haitian Pharmacotherapy during the study: Naturalistic prescribing allowed |
|
| Interventions |
Therapists: Therapists were doctoral‐level clinicians with previous experience and training in CBT for anxiety disorders |
|
| Outcomes | Response; withdrawals; anxiety: CAPS; co‐morbid depression: BDI‐II; adverse events | |
| Notes | Funding from Industry: No, this randomised controlled trial was funded by the VA as part of a joint VA/NIMH solicitation for R‐34 type PTSD trials Medication supplied by industry: No Any authors work for industry: Yes Study ID: NCT00371176 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation list. Randomisation was blocked and stratified based on PTSD scores (CAPS scores < 75 or > 75) |
| Allocation concealment (selection bias) | Low risk | The randomisation allocation sequence was implemented by a pharmacist (not part of the research team) who assigned participants to conditions according to a computer generated randomisation list |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All research team members, therapists, assessors, and participants were blind to condition |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All research team members, therapists, assessors, and participants were blind to condition |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Six participants were lost to follow‐up. Insufficient reporting of attrition to permit judgement |
| Selective reporting (reporting bias) | High risk | Quality of Life Enjoyment and Satisfaction Questionnaire, an outcome relevant for this review, was pre‐stated in the protocol but the results not reported |
| Other bias | Low risk | No sources of other bias were identified |
Mataix‐Cols 2014.
| Methods | Design: double‐blind randomised placebo controlled trial Study duration: 17 weeks Follow‐up: 1 year Country: UK |
|
| Participants | Sample size: 27 adolescent participants were randomised Recruitment: youth with a principle diagnosis of OCD were recruited from the OCD Clinic for Young People at the Maudsley Hospital, London Inclusion criteria ‐ diagnostic classification criteria: DSM‐IV principle diagnosis of OCD Inclusion criteria ‐ rating scales: Children's Yale‐Brown Obsessive Compulsive Scale (Y‐BOCS) score > 16 Included disorders: OCD Co‐morbidities: for DCS group co‐morbidities included: social anxiety disorder (3), specific phobia (5), GAD (2), body dysmorphic disorder (1), MDD (1). For placebo group, co‐morbidities included: SAnD (3), specific phobia (2), GAD (1), dysthymia(2), tic disorder (3), ADHD (1) Gender: DCS group 38% male; placebo group 64% male Mean age: DCS group ‐ mean age 14.7, SD 2.1. Placebo group mean age 15.2, SD 2.0 Ethnicity: Not reported Pharmacotherapy during the study: Naturalistic prescribing allowed; participants must have been stable on medication for at least 12 weeks. At baseline 4/13 subjects in the DCS group and 1/14 subjects in the placebo group were treated with SSRI and 1/14 subjects in the placebo group was treated with risperidone. In the DCS group, for 1 participant, SSRI dose was increased at 3‐month follow‐up; another started fluoxetine at 6‐month follow‐up; one participant discontinued medication at the end of treatment; another's SSRI dose was reduced at 6‐month follow‐up and stopped completely at 12‐month follow‐up. For 1 participant (placebo), SSRI dose was reduced at 5‐month follow‐up and stopped completely at 8‐month follow‐up |
|
| Interventions |
Therapists: "treatment was delivered by experienced therapists" |
|
| Outcomes | Response; withdrawals; remission; anxiety: CY‐BOCS; co‐morbid depression: BDI‐Y; adverse events | |
| Notes | Funding from industry: No, this work was funded by the Department of Health Medication supplied by industry: Not reported Any author work for industry: Unclear Study IDs: ISRCTN70977225; EUCTR2008‐006947‐38‐GB |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly allocated via an external computer allocation system to receive either 50 mg DCS or placebo in a double‐blind design." |
| Allocation concealment (selection bias) | Low risk | "Participants were randomly allocated via an external computer allocation system to receive either 50 mg DCS or placebo in a double‐blind design." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | DCS and placebo pills were "identical". Additional procedures for assurance of blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A masked rater administered the CY‐BOCS at the beginning of each session, providing session‐by‐session data. Double‐blind follow‐up assessments were completed at 3, 6, and 12 months post‐treatment. Unmasking took place after the last patient had completed the 12‐month follow‐up." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant dropped out from the DCS group and two from the placebo group. ITT analyses of all participants randomised were presented |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the protocol were reported |
| Other bias | Unclear risk | Insufficient information to assess other bias (very brief report) |
Nave 2012.
| Methods | Design: double‐blind, randomised pilot study Study duration: 1 week (1 session, plus assessment 1 week before) Follow‐up: 1 week Country: USA |
|
| Participants | Sample size: 20 adult participants were randomised Recruitment: Not reported Inclusion criteria ‐ diagnostic classification criteria: DSM‐IV Specific Phobia Inclusion criteria ‐ rating scales: Mini‐International Neuropsychiatric Interview (MINI), Snake Questionnaire Included disorders: specific phobia: snake phobia Co‐morbidities: 1 participant in DCS group had co‐morbid depressive disorder Gender: 40% male (both groups) Mean age: placebo group mean age 39.00 years (SD 13.91); DCS group mean age 34.60 years (SD 12.69) Ethnicity: 80% of placebo group was White; 60% of DCS group was White Pharmacotherapy during the study: Naturalistic prescribing allowed. 3 participants in placebo group were taking medication; 2 participants in DCS group were taking medication (types of medication not reported) |
|
| Interventions |
Therapists: Not reported |
|
| Outcomes | Response; withdrawals; anxiety: Snake Questionnaire | |
| Notes | Funding from industry: No, study was funded by departmental funds at Hartford Hospital Medication supplied by industry: Not reported Any author work for industry: No Study ID: NCT01450306 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but does not report method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subjects received DCS or "an identically packaged placebo capsule". Other details to assure blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details regarding blinding of assessors not provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised subjects participated in study and clinical follow‐up assessment |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in protocol were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Otto 2010.
| Methods | Design: Randomised, double‐blind, placebo controlled augmentation trial Study duration: 5 weeks Follow‐up: 1 month Country: USA |
|
| Participants | Sample size: 31 participants eligible and agreed to participate Recruitment: participants recruited from 3 outpatient treatment centres Inclusion criteria ‐ diagnostic classification criteria: primary diagnosis of panic disorder with or without agoraphobia, DSM‐IV Inclusion criteria ‐ rating scales: Structured Clinical Interview (SCID‐IV) and Clinical Global Impression‐Severity Scale (CGI‐S) Included disorders: Panic Disorder and co‐morbid diagnoses, excluding history of bipolar disorder, psychosis or delusional disorders, or substance abuse or dependence (other than nicotine) in the last 3 months, current PTSD, current MDD with severity more than mild to moderate, and severe agoraphobia Co‐morbidities: Not reported Gender: 50% male Mean age: 35.0 years (SD 11.0 years) Ethnicity: 100.0% White, 7% Hispanic ethnicity Pharmacotherapy during the study: Naturalistic prescribing allowed; 12 participants were taking an antidepressant and benzodiazepine, 7 participants were taking an antidepressant alone, 3 participants were taking a benzodiazepine, and 1 participant was taking gabapentin and atomoxetine |
|
| Interventions |
Therapists: Therapists were doctoral and graduate‐level providers trained and supervised by the paper’s first and second authors. *The study did not report number randomised, allocated or analysed per group. We requested clarification from the study investigators, but no further information was available at the time this review was prepared. We assumed 1:1 randomisation and divided the participants accordingly in the analyses |
|
| Outcomes | Response; anxiety: PDSS (data points derived from graph); adverse events | |
| Notes | Funding from Industry: Unclear ‐ authors declared funding from various organisations, however not stated if this study funded from those proceeds Medication supplied by industry: Unclear Any authors work for industry: Yes Study ID: NCT00131339 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised, but no details provided on how random sequence generated |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'Blinding occurred for both participant and personnel.' 'Doses of study drug (50 mg of DCS or matching placebo) were administered by study personnel in a double‐blind fashion' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of outcome assessor blinding were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rates: Five patients discontinued participation (two before randomisation at week 3 of the protocol, three after randomisation). Reasons for discontinuation and which group they belonged to were not reported. Only those patients who completed the 1‐month follow‐up assessment were included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | The protocol states that the primary outcome is "significant reduction in panic symptoms after completion of treatment", the paper reports of the PDSS and CGI‐S scales |
| Other bias | Unclear risk | Greater than 60% of patients (19/31 patients) were receiving concomitant anti‐depressants, which might have confounded the results |
Ressler 2004.
| Methods | Design: Randomised, double‐blind, placebo controlled trial Study duration: 2 weeks Follow‐up: 3 months Country: USA |
|
| Participants | Sample size: 28 adult participants eligible and agreed to participate Recruitment: participants recruited from the general community to a research clinic Inclusion criteria ‐ diagnostic classification criteria: primary diagnosis of acrophobia, DSM‐III‐R Inclusion criteria ‐ rating scales: Structured Clinical Interview (SCID‐III‐R) Included disorders: Acrophobia Co‐morbidities: Not reported Gender: 59.3% female Mean age: DCS mean age 46.4 years (SD 2.8); placebo mean age 44.8 years (SD 2.3) Ethnicity: Not reported Pharmacotherapy during the study: Not reported |
|
| Interventions |
Therapists: Information about therapists administering treatment not provided |
|
| Outcomes | Response (data points derived from graph); withdrawals; anxiety: AAQ (data points derived from graph); adverse events | |
| Notes | Funding from Industry: No ‐ Supported by grant IBN‐987675 from the Science and Technology Center Program, Center for Behavioral Neuroscience, National Science Foundation, Arlington, Va Medication supplied by industry: Unclear ‐ d‐cycloserine (Seromycin, 250 mg; Eli Lilly and Co, Indianapolis, Ind) was reformulated into 50 mg or 500 mg with identical placebo capsules Any authors work for industry: Yes Study ID: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | '27 were randomly assigned, via a predetermined and blinded order of treatment assignment'. The process of sequence generation was however not described |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'Treatment condition was double‐blinded, such that the subjects, therapists, and assessors were not aware of the assigned study medication condition.' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Treatment condition was double‐blinded, such that the subjects, therapists, and assessors were not aware of the assigned study medication condition.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates: 'Twenty‐one of the 27 completing participants returned for follow‐up assessment (8 placebo (80% of enrolled), 13 DCS (77% of enrolled)),' however all enrolled participants completed intervention.' Analysis of the pretreatment data and the 1‐week post‐treatment assessments showed that there were no significant pretreatment or post‐treatment differences on anxiety or fear measures between those who returned for follow‐up and the six who did not.' |
| Selective reporting (reporting bias) | High risk | Results for BDI (co‐morbid depression) and STAI (co‐morbid anxiety) scales were not reported |
| Other bias | Low risk | Small sample size ‐ not adequately powered to demonstrate significant differences between the DCS doses used. Psychological measures are by definition subjective, and the physiological measure of skin conductance fluctuation may also be affected by external stimuli and the subjects’ movements, however every attempt to control for these issues and to demonstrate that the physiological and subjective measures of fear were correlated (stated in methods) |
Rothbaum 2014.
| Methods | Design: Randomised, double blind, placebo controlled trial Study duration: 6 weeks Follow‐up: 12 months Country: USA |
|
| Participants | Sample size: 106 adult participants were randomised Recruitment: Not reported Inclusion criteria ‐ diagnostic classification criteria: DSM‐IV criteria for PTSD due to Iraq military trauma Inclusion criteria ‐ rating scales: CAPS to assess PTSD diagnosis status and MINI to assess co‐morbidities Included disorders: PTSD Co‐morbidities: 22.6% of participants in the DCS group and 35.8% of placebo group had co‐morbid mood disorder Gender: 99 males, 7 females Mean age: DCS 34.9, placebo 34.3 years Ethnicity: DCS: 50.9% Black, 41.5% White, 5.7% Hispanic, 1.9% other; Placebo: 52.8% Black, 37.7% White, 3.8% Hispanic, 1.9% Asian, 3.8% other Pharmacotherapy during the study: About half of participants (n = 14, 56%) were on a stable dose of psychotropic medications (n = 9 in the VRE‐DCS group, n = 5 in the VRE‐placebo group) |
|
| Interventions |
Therapists: Doctoral‐level clinicians The study also included an active comparison group where participants received VRE therapy and alprazolam. This group was not included in the review |
|
| Outcomes | Response; withdrawals; anxiety: CAPS | |
| Notes | Funding from Industry: No, supported by NIMH grant R01 MH‐70880 to Dr Rothbaum Medication supplied by industry: Not reported Any authors work for industry: Drs Ressler and Davisare founding members of Extinction Pharmaceuticals/Therapade Technologies, which seek to develop d‐cycloserine and other compounds for use to augment the efficacy of cognitive and behavioural therapies; they have received no equity or income from this relationship within the last 3 years Study ID: NCT00356278 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subjects were randomly assigned to treatment and comparison, but the procedure was not specified. However, since allocation concealment was adequate we assume that the randomisation method also was adequate |
| Allocation concealment (selection bias) | Low risk | Central allocation. "The compounding pharmacy randomly assigned patients to the medications in blocks of 30" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The study staff were blind to medication condition", further, the study was described as 'double‐blind', though no specific information was provided on blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study was described as 'double‐blind', though no information was provided on blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was insufficient information to determine dropout rates for the two groups separately. Reasons for withdrawals were not provided. Quote: "Dropouts did not significantly differ from completers on baseline demographic characteristics or symptom variables. Weaknesses of the current study include the high dropout rate. It should be noted that 31 participants dropped out before the first treatment session" |
| Selective reporting (reporting bias) | High risk | No information was provided on the following scales as per protocol ‐ Quality of Life Inventory, State‐Trait Anxiety Inventory, Beck Depression Inventory |
| Other bias | Unclear risk | A total of 56% of patients were receiving concomitant psychotropic medications, which might have confounded the results |
Scheeringa 2014.
| Methods | Design: Randomised, placebo controlled, double‐blind study Study duration: 12 weeks (4 weeks CBT only) Follow‐up: 3 months Country: USA |
|
| Participants | Sample size: 57 children and adolescents were randomised Recruitment: Investigators attempted to contact a total of 644 potential participants: 30% were referred by other professionals, 14% referred themselves from radio and television advertisements, and 56% were contacted from the local level I trauma centre registry Inclusion criteria ‐ diagnostic classification criteria: 5 or more PTSD symptoms plus functional impairment, specific diagnostic criteria not reported Inclusion criteria ‐ rating scales: National Institute of Mental Health Diagnostic Interview Schedule for Children‐IN (DISC‐IV) Included disorders: PTSD Co‐morbidities: Not reported Gender: DCS group 66% female; placebo group 46% female Mean age: DCS group mean age 12.4 (SD 3.3); placebo group mean age 12.6 (SD 3.4) Ethnicity: DCS group 41% Black, 41% White, 14% mixed, 3% other; placebo group 43% Black, 39% White, 14% mixed, 4% other Pharmacotherapy during the study: Naturalistic prescribing allowed. Subjects must have been stable on medication for at least 4 weeks prior to treatment |
|
| Interventions |
Therapists: Therapy was delivered by 2 masters‐level therapists trained in CBT and supervised by the authors |
|
| Outcomes | Response; withdrawals; anxiety: CPSS; co‐morbid depression: CDI; co‐morbid anxiety: SCARED | |
| Notes | Funding from industry: No. Financial support for this study was provided by National Institute of Mental Health grant 5RC1MH088969‐02 and a 2009 National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award (principal investigator: M.S.S.) Medication supplied by industry: Not reported Any author work for industry: Unclear, "No competing financial interests exist." Study ID: NCT01157416 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "For each age group, we created a list of randomised numbers using the Microsoft Excel 2007 random number generator. Block randomisation in sets of four was used. Within the first set of four numbers, two were randomly assigned to CBT and DCS and two to CBT and placebo." |
| Allocation concealment (selection bias) | Unclear risk | Details on allocation concealment were not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All research personnel were blinded except the pharmacist, who had no contact with subjects." "The study was triple‐blind as the Board, the participants, and the investigators were blind to allocation status." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "All research personnel were blinded except the pharmacist, who had no contact with subjects." It is not specifically stated that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A total of 72% of DCS group completed treatment and follow‐up; 64% of placebo group completed treatment and follow‐up. Reasons were not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the protocol were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sheerin 2014.
| Methods | Design: Randomised double‐blind placebo controlled study Study duration: 10 weeks Follow‐up: 6 months Country: USA |
|
| Participants | Sample size: 16 adult participants were found eligible and agreed to participate. Recruitment: flyers were posted around a university campus with relevant information about the nature of the study, what types of participants were being recruited, who was conducting the study, who to contact for more information, and information needed to schedule an initial assessment. Inclusion criteria ‐ diagnostic classification criteria: participants who met criteria for a current DSM‐IV diagnosis of social anxiety disorder. Inclusion criteria ‐ rating scales: Anxiety Disorders Interview Schedule (ADIS), supported by results of the Liebowitz Social Anxiety Scale (LSAS), with a minimum score (minimum 55) required for designation of moderate social anxiety. Included disorders: social anxiety disorder. Co‐morbidities: exclusion criteria included current major depressive episode, obsessive‐compulsive disorder, panic disorder, and post‐traumatic stress disorder, as well as diagnosis of bipolar disorder or psychosis, assessed by the ADIS‐IV. Additionally, anyone with a drug allergy that included medications in the class with DCS was excluded for health reasons, as well as anyone who was pregnant (determined by pregnancy test given at the initial screening), breast feeding, as was anyone who had medical conditions contraindicated for the experimental drug (e.g., a heart condition or epilepsy). Also, individuals who reported currently drinking alcohol on a daily basis were excluded. Gender: 63% female Mean age: 19.81 (SD 1.91), range not reported. Ethnicity: 63% non‐Hispanic white. Pharmacotherapy during the study: Potential participants were excluded if they were currently taking anti‐anxiety or anti‐depressant medications, currently using illicit substances, reported currently drinking alcohol on a regular basis or were on a medication that could potentially interact with DCS. |
|
| Interventions |
Therapists: sessions were conducted by doctoral graduate student therapists with a minimum of one year of supervised clinical experience. |
|
| Outcomes | Withdrawals: anxiety: LSAS (imputed SDs for DCS group); co‐morbid anxiety: STAI (imputed SDs for DSC group); adverse events. | |
| Notes | Funding from industry: unclear. Medication supplied by industry: no, DCS and placebo purchased from a local pharmacy. Any author work for industry: unclear (PhD dissertation). Study ID: not stated. A dissertation submitted to the Graduate College in partial fulfilment of the requirements for the degree of Doctor of Philosophy ‐ West Michigan University. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number generator was used to randomly assign participants to one of two conditions. |
| Allocation concealment (selection bias) | High risk | "All participants, research assistants, and therapists were blind to the condition; however, the psychiatrist and pharmacy staff retained a list of all participants and those who were receiving DCS and placebo." Risk of bias is considered high, since the psychiatrist, whom all potential participants met with individually for a medical screening, was not blind to the allocation. Further, it is not specified in what way the blinding was carried out. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "All participants, research assistants, and therapists were blind to the condition; however, the psychiatrist and pharmacy staff retained a list of all participants and those who were receiving DCS and placebo." Risk of bias is considered high, since the psychiatrist, whom all potential participants met with individually for a medical screening, was not blind to the allocation. Further, it is not specified in what way the blinding was carried out. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All participants, research assistants, and therapists were blind to the condition." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Of the 24 randomized participants, 16 entered treatment, with 7 in the DCS group and 9 in the placebo group." "Except where noted, all analyses were conducted using the intent‐to‐treat sample (N = 16)." "Of participants who entered treatment, 7 completed and 9 dropped out prior to session completion." Although the data for all intention‐to‐treat patients is reported and the reasons for attrition were reported, the high dropout rate (8 out of 24 before treatment and 9 out of 16 prior to treatment completion) could well have introduced a bias in the results, especially given the small sample size. Furthermore, only two participants completed the pre‐planned 6 months follow‐up assessment. |
| Selective reporting (reporting bias) | Low risk | All outcomes were completed and presented, as proposed. |
| Other bias | Low risk | No sources of other bias were identified. |
Siegmund 2011.
| Methods | Design: Randomised, double‐blind, placebo controlled study Study duration: 1 month Follow‐up: 5 months Country: Germany |
|
| Participants | Sample size: 44 adult participants eligible and agreed to participate Recruitment: Not reported Inclusion criteria ‐ diagnostic classification criteria: primary diagnosis of panic disorder with agoraphobia, DSM‐IV Inclusion criteria ‐ rating scales: Clinical Global Improvement Scale (CGI‐I) Included disorders: panic disorder with agoraphobia Co‐morbidities: Not reported, though "severe other mental disorders" excluded Gender: DCS group 40% male; comparison group 68% male Mean age: DCS group 37.85; comparison group 37.32 Ethnicity: Not reported Pharmacotherapy during the study: Naturalistic prescribing allowed; 9 participants were taking an antidepressant, 1 participant was taking 2 antidepressants, 2 patients were taking benzodiazepines, 2 participants were taking an anti‐depressant and a benzodiazepine, and 1 participant was taking pregabalin |
|
| Interventions |
Therapists: Therapy conducted by a certified psychologist accompanied by a co‐therapist |
|
| Outcomes | Withdrawals; anxiety: PAS (data points derived from graph); co‐morbid depression: BDI (data points derived from graph); co‐morbid anxiety: BAI (data points derived from graph); adverse events | |
| Notes | Funding from Industry: No. This work was funded by a research grant of the German Federal Ministry of Education and Research to Andreas Ströhle (01GV0612). Medication supplied by industry: Unclear ‐ 50 mg of d‐cycloserine (Seromycin, Eli Lilly, USA) Any authors work for industry: Yes Study ID: ISRCTN44960833 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomisation was performed by pharmacy which prepared the study medication using the method of randomly permuted blocks of pairs.' |
| Allocation concealment (selection bias) | Low risk | 'The study medication was handed out by the study staff in consecutive numbers, according to the individual time arrangements for exposure therapy (next exposure received next container). The randomisation sequence was kept in the pharmacy inaccessible to study staff until the last follow‐up data had been assessed and monitored.' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'All study staff (including those who did recruitment, assessments and therapy) and all participants were blind to the random allocation sequence.' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'All study staff (including those who did recruitment, assessments and therapy) and all participants were blind to the random allocation sequence.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced in numbers across intervention groups, with similar reasons for missing data across groups, n = 2 in DCS group declined to participate in intervention, in placebo group n = 3 did not participate, n = 2 due to family problems and n = 1 worried about possible side effects. At 5 months post‐treatment, n = 4 lost for DCS group, n = 2 for further cognitive and behavioural therapies, n = 1 further pharmacotherapy, n = 1 for further pharmacotherapy and cognitive and behavioural therapies. In placebo group,n = 4 lost at five months, n = 3 for further pharmacotherapy and n = 1 for further pharmacotherapy and cognitive and behavioural therapies |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the protocol were reported on with data provided |
| Other bias | Low risk | No sources of other bias were identified |
Storch 2007.
| Methods | Design: Randomised, double‐blind, placebo controlled trial Study duration: 12 weeks Follow‐up: 2 months Country: USA |
|
| Participants | Sample size: 24 participants eligible and agreed to participate Recruitment: Participants were recruited from patients who presented at the University of Florida OCD Program for treatment Inclusion criteria ‐ diagnostic classification criteria: primary diagnosis of OCD, DSM‐IV‐TR Inclusion criteria ‐ rating scales: Yale‐Brown Obsessive Compulsive Scale (YBOC‐S) Included disorders: OCD Co‐morbidities: GAD 50%, MDD 25%, dysthymia 16%, social phobia 25%, panic disorder 21%, tricotillomania 4% Gender: 50% male Mean age: 29.9 years (SD 9.9 years) Ethnicity: 92% White, 4% African American, 4% Asian Pharmacotherapy during the study: 50% of participants were taking SSRIs |
|
| Interventions |
Therapists: Therapy was conducted by the first author or doctoral fellows or trainees under his supervision |
|
| Outcomes | Response; withdrawals; remission; anxiety: Y‐BOCS; co‐morbid depression: BDI‐II; adverse events | |
| Notes | Funding from Industry: This work was supported by a grant to the first author from the Obsessive–Compulsive Foundation Medication supplied by industry: Unclear, Seromycin, Eli Lilly, USA Authors work for industry: Unsure Study ID: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Participants were randomised to receive DCS or placebo.' Sequence generation not stated. Marked as low risk, as other included publications by author used computer generated random number generation (Storch 2010) therefore likely to be similar |
| Allocation concealment (selection bias) | Low risk | 'The rater, the treatment teams, the patients and their families were unaware of and unable to determine, the study drug assignment by appearance or otherwise' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'The rater, the treatment teams, the patients and their families were unaware of and unable to determine, the study drug assignment by appearance or otherwise' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'The rater, the treatment teams, the patients and their families were unaware of and unable to determine, the study drug assignment by appearance or otherwise' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced in numbers across intervention groups with similar reasons for missing data across groups.' Thirty‐four participants randomised with n = 17 across each arm; n = 12 received allocation in each arm as n = 5 for each arm withdrew. Exactly the same number and reasons sited across both groups; n = 3 for each group refusing to sign consent form, n = 1 for each group withdrawing before the second session, n = 1 for each arm withdrawing as unwilling to receive cognitive and behavioural therapies; n = 1 for each arm lost to follow‐up as unable to be contacted |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available thus unsure if all of study's pre‐specified outcomes of interest reported in pre‐specified way |
| Other bias | Unclear risk | A total of 50% of patients were receiving concomitant anti‐depressants, which might have confounded the results |
Storch 2010.
| Methods | Design: Randomised, double‐blind, placebo controlled augmentation trial Study duration: 8 weeks Follow‐up: post‐treatment Country: USA |
|
| Participants | Sample size: 30 children and adolescent participants eligible and agreed to participate Recruitment: Not reported Inclusion criteria ‐ diagnostic classification criteria: primary diagnosis of OCD, DSM‐IV Inclusion criteria ‐ rating scales: Anxiety Disorder Interview Schedule (ADIS) and Children’s Yale–Brown Obsessive Compulsive Scale CY‐BOCS Included disorders: OCD Co‐morbidities: ADHD 47%, GAD 17%, ODD 13%, Tourette Syndrome 10%, MDD 10%, social phobia 7%, enuresis 7%, specific phobia 3% Gender: 63% male Mean age: 12.2 (SD 2.8) Ethnicity: 97% Caucasian, 3% Hispanic Pharmacotherapy during the study: SSRI 30%, atomoxetine 7%, alpha2‐adrenergic agonist 7%, tricyclic 3%, SNRI 3%, stimulant 3% |
|
| Interventions |
Therapists: Therapy was provided by experienced therapists supervised by the first or ninth author |
|
| Outcomes | Response to treatment, defined as "very much improved" or "much improved" on CGI‐I (information received from study investigator E Storch in an e‐mail to RO on 15/09/2014); withdrawals; anxiety: CY‐BOCS; co‐morbid depression: CDI; co‐morbid depression: MASC; adverse events | |
| Notes | Funding from Industry: No. This work was supported by grants to the first author from the National Institutes of Health (Grant Nos. MH076775 and L40 MH081950‐02) and National Alliance for Research on Schizophrenia and Affective Disorders (Robidoux Foundation Young Investigator Award) Medication supplied by industry: No Authors work for Industry: Yes Study ID: NCT00864123 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomized by a computer‐generated program maintained in the site research pharmacy, in a double‐blinded fashion to CBT + DCS or CBT + Placebo.' |
| Allocation concealment (selection bias) | Low risk | 'Identical placebo and DCS given in double‐blinded fashion.' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was described as double‐blind. Further information from the study investigators (e‐mail from E Storch to RO on 15/09/2014) states "DCS/PBO were matched in every fashion in a double blinded manner" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Assessments were conducted by trained blinded raters at pretreatment, after Session 6, and within 1 week post‐treatment.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants allocated to each treatment arm completed the intervention and were analysed in study findings |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the protocol reported on with data provided. In addition, the Multidimensional Anxiety Scale for Children and the Children's Depression Inventory are reported |
| Other bias | Low risk | No sources of other bias were identified |
Tart 2013.
| Methods | Design: Randomised, double‐blind, placebo controlled augmentation trial Study duration: 2 weeks Follow‐up: 1 month Country: USA |
|
| Participants | Sample size: 29 adult participants eligible and agreed to participate Recruitment: Participants recruited via study advertisements Inclusion criteria ‐ diagnostic classification criteria: primary diagnosis of acrophobia, DSM‐IV‐TR Inclusion criteria ‐ rating scales: Subjective Units of Distress Scale (SUDS) Included disorders: Acrophobia Co‐morbidities: Individuals with co‐morbidities excluded from study Gender: Not reported. Mean age: DCS group 29.33 (SD 14.67); comparison group 37.71 (SD 16.81) Ethnicity: Not reported Pharmacotherapy during the study: Naturalistic prescribing not permitted |
|
| Interventions |
Therapists: Therapists were advanced doctoral student‐level therapists trained and supervised by the senior author |
|
| Outcomes | Response; withdrawals; remission; anxiety: AAQ; adverse events | |
| Notes | Funding from industry: No. This work was supported by Diversity Supplement to R01MH075889 from the National Institute of Mental Health (NIMH) Medication supplied by industry: No Authors work for Industry: Yes Study ID: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Done by research staff not involved in trial using minimisation procedures and stratifying on gender, therapist, time of day of treatment sessions.' |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and personal were blinded. 'All capsules were identical in appearance to maintain blinding.' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Outcome assessors blind to group assignment.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data in groups; 'n = 15 assigned to DCs and cognitive and behavioural therapies arm and n = 14 to placebo and cognitive and behavioural therapies arm, with n = 3 withdrawing from placebo arm by post‐treatment, n = 3 withdrawing from DCS arm at follow‐up and n = 1 withdrawing from follow‐up in placebo arm |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available thus unsure if all of study's pre‐specified outcomes of interest reported in pre‐specified way |
| Other bias | Unclear risk | CGI ratings were completed by a clinician who was blind to group assignment but not an independent evaluator. May have introduced a bias resulting in larger overall clinician‐rated improvement rates |
Wilhelm 2008.
| Methods | Design: Randomised, double‐blind, placebo controlled trial Study duration: 5 weeks Follow‐up: 1 month Country: USA |
|
| Participants | Sample size: 33 adult participants eligible and agreed to participate, 29 initiated treatment Recruitment: participants were recruited through flyers posted in the community and in area outpatient clinics Inclusion criteria ‐ diagnostic classification criteria: primary diagnosis of OCD, DSM‐IV Inclusion criteria ‐ rating scales: Not reported Included disorders: OCD and co‐morbid diagnoses Co‐morbidities: MDD (N = 3), social phobia (N = 3), specific phobia (N = 3), dysthymia (N = 2), GAD (N = 2), anxiety disorder not otherwise specified (N = 1), depressive disorder not otherwise specified (N = 1), and panic disorder with agoraphobia (N = 1) Gender: not reported Mean age: mean age of DCS group 40.0 years (SD 13.4); mean age of placebo group 38.2 years (SD 13.0) Ethnicity: Not reported Pharmacotherapy during the study: Naturalistic prescribing allowed; 14 participants were taking an anti‐depressant, 5 participants were taking a benzodiazepine, and 1 participant was taking an antipsychotic |
|
| Interventions |
Therapists: Advanced trainees, under the supervision of licensed psychologists, administered the ERP |
|
| Outcomes | Withdrawals; anxiety: Y‐BOCS; co‐morbid depression: BDI‐II, adverse events | |
| Notes | Funding from Industry: No. Supported by internal funding provided by Massachusetts General Hospital Medication supplied by industry: No Authors work for industry: Unclear Study ID: NCT00126282 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study was randomised but not stated how random sequence generation was done. However, since allocation concealment was adequate we assume that randomisation method also was adequate |
| Allocation concealment (selection bias) | Low risk | 'The research pharmacies at Massachusetts General Hospital and the Institute of Living prepared and dispensed the study medication (100 mg d‐cycloserine or placebo) and maintained the coded random assignment schedule for the double‐blind design.' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Assessors blinded to treatment condition.' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Thirty‐three patients signed consent form of which three did not meet inclusion criteria, one refused the treatment because he reported that he was “not ready for change”, and six discontinued treatment before the mid‐treatment evaluation. Twenty‐two patients completed the treatment and were included in the statistical analysis. One patient dropped out after the mid‐treatment evaluation; his data were carried forward. Reasons for dropping out before mid‐treatment were not provided, and all randomised patients not included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in protocol were reported |
| Other bias | Unclear risk | More than 40% of patients (14/33 patients) were receiving concomitant anti‐depressants, which might have confounded the results |
(ADIS‐IV) Anxiety Disorder Interview Schedule for Adults, (SPAI) Social Phobia and anxiety Inventory, (LSAS) Liebowitz Social Anxiety Scale, (BFNES) Brief Fear of Negative Evaluation Scale, (LIS) Life Interference Scale, (GAF) Global Assessment of Functioning Scale, (SCID) Structured Clinical Interview for DSM‐IV, (YBOC‐S) Yale‐Brown Obsessive Compulsive Scale, (SUDS) Subjective Units of Distress Scale, (CGI‐S) Clinican's Global impressions of Severity Scale, (PDSS) Panic Disorder Severity Scale, (CAPS) Clinician Administered Post Traumatic Stress Disorder Scale, (PCL) PTSD Checklist, (BDI‐II) Beck's Depression Inventory, (PSS‐SR) Posttraumatic Stress Symptom Scale—Self Report, (STAI) State and Trait Anxiety Inventory, (SL90) Symptom Checklist 90, (AAVQ) Acrophobia Questionnaire with Avoidance, (AAQ) Anxiety sub‐scales, (ATHI) Attitudes Toward Heights Inventory, (BAT) Behavioural Avoidance Test, (PAS) Panic and agoraphobia scale, (MI) Mobility Index, (BAI) Beck's Anxiety Inventory, (HAM‐D) Hamilton Depression Scale, (CGI), Clinical Global improvement scale, (OCI‐R) Obsessive‐Compulsive Inventory Revised, (ADIS‐IV‐P) Anxiety Disorders Interview Schedule for DSM‐IV: Parent Version, (CY‐BOCS) Children’s Yale–Brown Obsessive Compulsive Scale, (MASC) Multidimensional Anxiety Scale for Children
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Behar 2010 | Cognitive and behavioural therapies were not used in the study, instead attentional therapy was augmented with d‐cycloserine in trait anxiety, which did not meet Diagnostic and Statistical Manual Criteria for Anxiety Disorders |
| Evins 2012 | No cognitive and behavioural therapies |
| Galovic 2010 | Participants had fear of public speaking which did not meet inclusion criteria |
| Guastella 2007(a) | Spider fear was tested with subclinical anxiety and participants did not meet criteria for specific phobia |
| Guastella 2007(b) | Subclinical spider fear ‐ does not meet criteria for specific phobia |
| Gutner 2012 | No cognitive and behavioural therapies |
| Heresco‐Levy 2002 | No cognitive and behavioural therapies comparison |
| Inslicht 2013 | No cognitive and behavioural therapies |
| Levinson 2013 | No cognitive and behavioural therapies |
| NCT00257361 2005 | This study was withdrawn prior to participant enrolment |
| Rajabi 2013 | Most participants did not receive cognitive and behavioural therapies in addition to d‐cycloserine or placebo pill. |
| Rodebaugh 2013 | No cognitive and behavioural therapies |
| Sharp 2013 | Participants were children with food refusal, which did not meet inclusion criteria |
| Steinglass 2007 | Participants were patients with anorexia, which did not meet inclusion criteria |
Characteristics of studies awaiting assessment [ordered by study ID]
Cath 2010a.
| Methods | Randomised, double blind placebo controlled trial |
| Participants | 60 participants meeting DSM‐IV‐TR criteria for PD with or without agoraphobia |
| Interventions | All participants receive 12 ERP standard sessions, and in the first 6 sessions they receive study medication either directly pre or post session: Group 1, n = 20 receive DCS pre and placebo post session; Group 2, n=20 receive placebo pre and DCS post‐session; and Group 3, n = 20 receive placebo both pre‐ and post‐sessions |
| Outcomes | Panic disorder and agoraphobia: the Mobility Inventory |
| Notes | Study ID: Euctr‐021198‐35‐Nl, NTR2050 (Nederlands trial register: http://www.trialregister.nl/) |
Cath 2010b.
| Methods | Randomised, double‐blind placebo controlled trial |
| Participants | 60 participants meeting DSM‐IV‐TR criteria for OCD |
| Interventions | All participants receive 12 ERP standard sessions, and in the first 6 sessions they receive study medication either directly pre‐ or post‐session: Group 1, n = 20 receive DCS pre‐ and placebo post‐session; Group 2, n = 20 receive placebo pre‐ and DCS post‐session; and Group 3, n = 20 receive placebo both pre‐ and post‐sessions |
| Outcomes | Y‐BOCS |
| Notes | Study ID: NTR2050 (Nederlands trial register: http://www.trialregister.nl/) |
Guay 2007.
| Methods | Randomised, double‐blinded, placebo controlled trial |
| Participants | Adults with a confirmed diagnosis of PTSD |
| Interventions | Compare CBT and d‐cycloserine to CBT or placebo in the treatment of PTSD. The main hypothesis of the current study is that the efficacy of CBT for PTSD will be increased when combined with d‐cycloserine compared to a placebo |
| Outcomes | CAPS, SCID |
| Notes | The recruitment status of this study is unknown as the study information has not been updated since 2007 Study ID: NCT00452231 |
Strohle 2011.
| Methods | Randomised, double‐blind parallel assignment study |
| Participants | Subjects aged 18 to 75 years with diagnosis of agoraphobia |
| Interventions | 12 sessions of CBT with 50 mg DCS or placebo pill administered 3x, directly after exposure |
| Outcomes | Panic and Agoraphobia Rating Scale (PAS), Beck Anxiety Inventory (BAI), Clinical Global Index (CGI), Agoraphobic Cognitions, Body Sensations Questionnaire and Mobility Inventory (AKV), Anxiety Sensitivity Index (ASI), Beck Depression Inventory II (BDI II), Brief Symptom Inventory (BSI) |
| Notes | Study ID: NCT01928823 |
Characteristics of ongoing studies [ordered by study ID]
Arman 2013.
| Trial name or title | The efficacy of D‐Cycloserine and cognitive‐behavioral therapy on symptoms improvement in the adolescents with one type of anxiety disorders: A double‐blind randomised controlled trial |
| Methods | Double‐blind, randomised controlled trial |
| Participants | Adolescents aged 12 to 20 years with anxiety disorders. Target sample size = 36 |
| Interventions | 50 mg DCS or placebo daily for a month in addition to cognitive and behavioural therapies |
| Outcomes | SCARED score, CATS questionnaire |
| Starting date | September 2011 |
| Contact information | soroorarman@yahoo.com |
| Notes | Study ID: IRCT2012071610297N1 |
Bergman 2012.
| Trial name or title | The Use of D‐Cycloserine to Augment Intensive Cognitive Behavioral Therapy for Pediatric Obsessive Compulsive Disorder |
| Methods | Randomised double‐blind parallel assignment study |
| Participants | 26 subjects aged 7 to 17 with a primary diagnosis of DSM IV criteria for OCD |
| Interventions | 50 mg of DCS or placebo 1 hour prior to each treatment session, 4 days per week for 2 weeks |
| Outcomes | Children's Yale‐Brown Obsessive‐Compulsive Scale (CY‐BOCS) |
| Starting date | July 2012 |
| Contact information | lbergman@mednet.ucla.edu |
| Notes | Study ID: NCT01687140 |
de Leeuw 2008.
| Trial name or title | The effect of the addition of D‐cycloserine to exposure sessions in the treatment of patients with obsessive‐compulsive disorder. A randomised, placebo‐controlled trial |
| Methods | Randomised placebo controlled, double‐blind, parallel trial |
| Participants | Subjects aged 18 years and up with a primary diagnosis of OCD as established with Structural Clinical Interview for axis I DSM‐IV Disorders (SCID‐I) |
| Interventions | DCS capsule in oral form |
| Outcomes | Y‐BOCS |
| Starting date | February 2008 |
| Contact information | aleeuw@meerkanten.nl |
| Notes | Study ID: NTR1189; EUCTR2007‐000367‐18‐NL |
Difede 2009.
| Trial name or title | Imaginal exposure and D‐cycloserine for Post Traumatic Stress Disorder |
| Methods | Randomised placebo controlled double‐blind study |
| Participants | 124 estimated participants with PTSD |
| Interventions | Assignment to one of two treatment groups: imaginal exposure (IE) plus DCS (100mg) or IE plus placebo (sugar pill) as well as 12 to 14 weekly individual (one‐on‐one) sessions of imaginal exposure, graduated in vivo exposure. In addition, all participants will be genotyped once for the BDNF SNP (Val66Met) using a non‐invasive saliva sample |
| Outcomes | CAPS, BDI‐II |
| Starting date | April 2009 |
| Contact information | jdifede@med.cornell.edu |
| Notes | Study ID: NCT00875342 |
Difede 2011a.
| Trial name or title | Enhancing Exposure Therapy for Post Traumatic Stress Disorder (PTSD): Virtual Reality and Imaginal Exposure With a Cognitive Enhancer |
| Methods | Randomised, double‐blind, parallel efficacy study |
| Participants | War veterans with PTSD |
| Interventions | DCS (d‐cycloserine ) + prolonged imaginal exposure
DCS (d‐cycloserine ) + virtual reality exposure Placebo + prolonged imaginal exposure Placebo (sugar pill) + virtual reality exposure Genetic polymorphism (BDNF Val66Met) obtained from a saliva sample will be examined |
| Outcomes | CAPS |
| Starting date | May 2011 |
| Contact information | jdifede@med.cornell.edu |
| Notes | Phase 3 Study ID: NCT01352637 |
Farrell 2012.
| Trial name or title | Novel treatment of phobias in children and teenagers |
| Methods | Randomised, controlled parallel trial |
| Participants | Individuals aged 7 to 17 years with a DSM‐IV‐TR diagnosis of any anxiety disorders and specific phobia |
| Interventions | DCS and exposure therapy versus placebo pill and exposure therapy. Participants receive 1 session of exposure therapy with 35 or 70 mg of DCS (dose dependant on child's weight) at the beginning of the session |
| Outcomes | Anxiety Disorders Interview Schedule (ADIS), Childrens Global Assessment Scale (CGAS), Fear Survey Schedule for Children Revised, Spence Children's Anxiety Scale |
| Starting date | March 2012 |
| Contact information | l.farrell@griffith.edu.au |
| Notes | Study ID: ACTRN12612000420842 |
Farrell 2014.
| Trial name or title | Intensive treatment of Pediatric Obsessive Compulsive Disorder (OCD): Improving access and outcomes |
| Methods | Randomised controlled trial, double‐blind |
| Participants | Individuals aged 7 to 17 years with a primary diagnosis of OCD (CYBOCS score of ≥ 16). Target sample size = 60 |
| Interventions | DCS pill and intensive exposure therapy or placebo and intensive exposure therapy |
| Outcomes | Children's Yale‐Brown Obsessive‐Compulsive Scale (CY‐BOCS) |
| Starting date | January 2014 |
| Contact information | l.farrell@griffith.edu.au |
| Notes | Study ID: ACTRN12614000140651 |
Geller 2011.
| Trial name or title | 2/2 D‐Cycloserine Augmentation of CBT for Pediatric OCD |
| Methods | Randomised, double‐blind placebo controlled trial |
| Participants | 75 youth aged between 7 and 17 years with clinical diagnosis of OCD as primary or co‐primary diagnosis with score on CY‐BOCS of 16 or greater and Full Scale IQ greater than or equal to 85 |
| Interventions | 25 mg dose of DCS for children weighing between 22.5 kg and 45 kg (dose = approximately 0.7 mg/kg), 50 mg dose for children weighing greater than 46 kg (dose = approximately 0.7 mg/kg). Dose given 7 times, every 7 days, except for the third dose, which will be given three days after the second dose. All doses given 1 hour prior to therapy session. The placebo comparator will be a sugar pill |
| Outcomes | CY‐BOCS, CGI |
| Starting date | July 2011 |
| Contact information | amstark@partners.org |
| Notes | Study ID: NCT01404208 |
Guastella 2006.
| Trial name or title | The role of D‐Cycloserine in combination with exposure therapy in the treatment of Panic Disorder to improve the severity of Panic Symptoms |
| Methods | Randomised parallel controlled trial |
| Participants | Subjects aged 18 years and older with a primary diagnosis of panic disorder |
| Interventions | DCS and exposure therapy. Participants receive 3 weekly sessions of group‐based exposure therapy. One group of participants receives 50 mg of DCS before each session |
| Outcomes | Severity of panic symptoms: global functioning such as their general health, depression and stress levels and current diagnosis |
| Starting date | June 2006 |
| Contact information | a.guastella@unsw.edu.au |
| Notes | Study ID: ACTRN12606000351516 |
Otto 2008.
| Trial name or title | Exposure, D‐Cycloserine Enhancement, and Genetic Modulators in Panic Disorder |
| Methods | Double‐blind, randomised placebo controlled trial |
| Participants | 192 patients who meet criteria for panic disorder, will be followed for over 5 years |
| Interventions | Patient with panic disorder will randomly receive DCS or placebo 1 hour prior to sessions 3 to 5 of a 5‐session CBT protocol that includes 2 additional booster sessions over the course of follow‐up |
| Outcomes | MADRS, CGI |
| Starting date | April 2008 |
| Contact information | Michael Otto, National Institute on Drug Abuse |
| Notes | Phase 2 Study ID: NCT00790868 |
Pollack 2014.
| Trial name or title | Dose timing of D‐cycloserine to augment CBT for social anxiety disorder |
| Methods | Randomised double‐blind parallel study |
| Participants | Outpatients aged 18 to 70 years with a primary diagnosis of social anxiety disorder meeting DSM‐V criteria |
| Interventions | Five weeks of CBT for social anxiety and 2 pills (1 placebo before and one DCS or placebo after the session) or 5 weeks of CBT for social anxiety disorder and 2 pills (1 DCS before and 1 placebo after) or 5 weeks of CBT for social anxiety disorder and 2 pills (1 placebo before and 1 placebo after) or 5 weeks of CBT for social anxiety disorder and 2 pills (1 DCS before and 1 DCS after) |
| Outcomes | Liebowitz Social Anxiety Scale (LSAS) and the Social Phobic Disorders Severity and Change Form (SPD‐SC Form) |
| Starting date | April 2014 |
| Contact information | scarlett.baird@utexas.edu |
| Notes | Study ID: NCT02066792 |
Rapee 2010.
| Trial name or title | Examining the efficacy of D‐cycloserine for augmenting exposure therapy in children with spider phobia and dog phobia: A randomised controlled trial |
| Methods | Randomised controlled parallel trial |
| Participants | Children aged 8 to 14 with DSM‐IV diagnosis of spider or dog phobia |
| Interventions | 50 mg DCS administered 1 hour before single exposure therapy session |
| Outcomes | Behavioural Avoidance Test: BAT, Children's State‐Trait Anxiety Inventory, Spider Phobia Questionnaire or Dog Phobia Questionnaire, Spider or Dogs Beliefs Questionnaire "Harm Subscale", Spence Child Anxiety Scale |
| Starting date | August 2010 |
| Contact information | rrapee@psy.mq.edu.au |
| Notes | Study ID: ACTRN12610000490077 |
Rapee 2011.
| Trial name or title | d‐cycloserine versus placebo combined with in‐vivo exposure in the reduction of specific fears among children with broad‐based anxiety disorders |
| Methods | Randomised controlled parallel trial |
| Participants | Subjects aged 7‐14 years with DSM diagnosis of anxiety disorder |
| Interventions | 50 mg DCS or placebo taken on 5 weekly occasions 1 hour before session |
| Outcomes | Behavioural avoidance test, Spence Children's Anxiety Scale |
| Starting date | September 2011 |
| Contact information | Ron.Rapee@mq.edu.au |
| Notes | Study ID: ACTRN12611000660987 |
Reinecke 2012.
| Trial name or title | The Effect of a Single‐dose of D‐cycloserine on the Basic Effects of Cognitive‐behaviour Therapy for Panic Disorder ‐ a Randomized Placebo‐controlled Trial |
| Methods | Randomised, double‐blind, parallel assignment study |
| Participants | Subjects aged 18 to 65 years with clinical diagnosis of panic disorder and at least moderate agoraphobic evidence |
| Interventions | 250 mg of DCS or placebo pill with CBT |
| Outcomes | Self‐reported and clinician‐rated anxiety and depression measures |
| Starting date | October 2012 |
| Contact information | andrea.reinecke@psych.ox.ac.uk |
| Notes | Study ID: NCT01680107 |
Ruck 2012.
| Trial name or title | D‐Cycloserine as an Adjunct to Internet‐CBT for OCD |
| Methods | Randomised, placebo controlled, double‐blind study |
| Participants | Fulfilling diagnostic criteria of OCD not associated with hoarding. Number of subjects: 128 |
| Interventions | The purpose of this study is to examine if d‐cycloserine is an efficacious adjunct to Internet‐based cognitive behaviour therapy for patients with obsessive compulsive disorder |
| Outcomes | YBOC‐S, OCI‐R,CGI, GAF |
| Starting date | August 2012 |
| Contact information | |
| Notes | Study ID: NCT01649895 |
Sirbu 2009.
| Trial name or title | D‐Cycloserine‐Enhancer of One‐Session Treatment for Phobia of Heights |
| Methods | Randomised placebo controlled double‐blind study |
| Participants | 80 participants with acrophobia |
| Interventions | One of the 4 treatment conditions: one‐session VRET (3 hours) and 50 mg d‐cycloserine (VR‐OST + DCS) (N1 = 20); one‐session VRET (3 hours) and placebo (VR‐OST + Pl (N2 = 20); one‐session IVET (3 hours) and placebo (IV + Pl) (N3 = 20); or one‐session IVET (3 hours) and 50 mg d‐cycloserine (IV + DCS) (N4 = 20). For 20 participants (5 from each group) the treatment will be delayed for 3 weeks and an additional assessment will be conducted as they will comprise a wait list (WL) control group |
| Outcomes | ADIS‐IV, Subjective Units of Distress during a Behavioral Avoidance Test (BAT) |
| Starting date | July 2009 |
| Contact information | cristian.sirbu@camc.org |
| Notes | Study ID: NCT01037101 |
Smits 2013.
| Trial name or title | Enhancing Panic and Smoking Reduction Treatment With D‐Cycloserine |
| Methods | Randomised, parallel assignment, double‐blind study |
| Participants | 18 to 65 years old, daily smoker for at least 1 year, currently smoking average of at least 10 cigarettes per day, evidence of panic attack within past year |
| Interventions | 7 weeks of panic and smoking reduction treatment (PSRT) and one pill of DCS or placebo 1 hour prior to sessions 3, 4, 5 and nicotine replacement therapy as part of PSRT |
| Outcomes | Not reported |
| Starting date | October 2013 |
| Contact information | scarlett.baird@utexas.edu |
| Notes | Study ID: NCT01944423 |
Storch 2011.
| Trial name or title | 1/2 D‐cycloserine Augmentation of CBT for Pediatric OCD |
| Methods | Randomised double‐blind placebo controlled trial |
| Participants | 150 youths aged 7 to 17 years with obsessive compulsive disorder and a CY‐BOCS score ≥ 16 and Full Scale IQ ≥ 85 as assessed will be included |
| Interventions | To examine the relative benefit of 10 cognitive and behavioural therapies sessions of which sessions 4 to 10 will be augmented with weight‐adjusted doses of DCS (25, 50 mg) compared to CBT augmented with placebo in paediatric OCD |
| Outcomes | CY‐BOCS, CGI |
| Starting date | June 2011 |
| Contact information | estorch@health.usf.edu |
| Notes | Study ID: NCT01411774 |
(YBOC‐S) Yale Brown Obsessive Compulsive Scale, (OCI‐R) Obsessive Compulsive Scale ‐ Revised, (MADRS‐S) Montgomery Asberg Depression Rating Scale Self‐rating, (CGI) Clinical Global Impression Scale, (GAF) Global assessment of Functioning Scale, CAPS (Clinician Administered PTSD Scale), (AAVQ) Acrophobia Questionnaire With Avoidance, (ATHQ) Attitudes Towards Heights Questionnaire, (BAT) Behavioral Avoidance Test, (ADIS‐ IV) Anxiety Disorders Interview Schedule‐IV, (BDI‐II) Beck's Depression Inventory, (MADRS) Montgomery‐ Asberger Depression rating scale, (STAXI‐2) State Trait Anger Expression Inventory, (PSS‐SR) Post‐Traumatic Stress Symptom Self‐Report Scale, (QLI) Quality of Life Inventory, (STAI) State‐Trait Anxiety Inventory
Differences between protocol and review
The title of the review was changed from 'Augmentation of psychotherapy with d‐cycloserine for anxiety disorders' to include 'anxiety and related disorders'. This terminology is in keeping with the new DSM‐5 chapter on anxiety and related disorders.
The title of the review was changed from 'Augmentation of psychotherapy with d‐cycloserine for anxiety disorders' to 'Augmentation of cognitive and behavioural therapies (CBT) with d‐cycloserine for anxiety and related disorders', to better reflect that (as stipulated in the protocol for this review) the interventions included in this review are limited to exposure‐based treatments, which are behavioural and cognitive‐behavioural in approach.
For clarity, a list of exposure‐based learning interventions used to treat anxiety disorders was added to the methods.
The reduction of symptom severity was stated in the protocol as a primary outcome measure. The Cochrane Handbook for Systematic Reviews of Interventions recommends including one primary outcome for benefit and one for harm, so this outcome measure was changed to a secondary outcome in the review but was still determined by the prespecified assessment of a variety of validated continuous outcome measures. These outcomes were still assessed at trial endpoint and follow‐up. Treatment acceptability as assessed by withdrawals from treatment was described in the protocol as an intended secondary measure. The authors however felt that it should be regarded as a primary outcome in the review (in keeping with the aforementioned recommendation in the Cochrane Handbook). Remission was added as a secondary outcome by the authors. This measure was used to assess the number of participants that achieved a diminution of symptoms at treatment endpoint and to assess whether this effect was still seen at follow‐up.
Planned treatment comparisons included DCS and cognitive and behavioural therapies versus placebo and cognitive and behavioural therapies; DCS and cognitive and behavioural therapies versus wait‐list and cognitive and behavioural therapies, and DCS and cognitive and behavioural therapies versus cognitive and behavioural therapies only. Only the first comparison was used in the review as all the included studies compared DCS and cognitive and behavioural therapies with placebo and cognitive and behavioural therapies.
Unit of analysis issues were not encountered in the review as none of the included studies contained summary statistics for multiple groups against the same placebo control. Standard deviations and means therefore did not need to be pooled to avoid potential bias.
In the protocol we planned to exclude cross‐over trials due to concerns regarding the likelihood that results reported by studies employing this design will be biased (Higgins 2008b). However, for the full review we deemed cross‐over trials eligible for inclusion in the calculation of summary statistics when it was: (a) possible to extract treatment and placebo or comparator data from the first treatment period, or (b) when the inclusion of these data from both treatment periods was justified through a wash‐out period of sufficient duration as to minimise the risk of carry‐over effects (Higgins 2011). No cross‐over trials were found in the study search.
Following recommendation by a peer reviewer, we decided to convert SMDs to MDs in the 'Effects of interventions; section for outcomes that are statistically significant, in order to facilitate interpretation.
We made a post hoc decision to analyse the included studies for children and adults separately, given that the disorder profiles and treatment responses are different per group.
We added a line to clarify how we imputed SDs ('If such statistics were unavailable, we imputed SDs using the average SD of the other included studies (section 16.1.3.1 in Higgins 2011)').
The possibility that a more rapid onset response to therapy following augmentation with DCS may increase the rates of treatment compliance could not be assessed. There was incomplete data regarding the number of treatment sessions attended by responders and non‐responders.
Subgroup analyses were planned to assess the degree to which methodological differences between trials might have systematically influenced differences observed in the primary treatment outcomes. Analyses of medication dosages, isolated versus chronic treatment with DCS, timing of drug administration, and the effect of the inclusion of patients on a stable dose of anti‐depressants were intended. However, there were insufficient studies to conduct the subgroup analyses (fewer than 10) to assess for treatment effect on the timing of dosing as well as the assessment of clinical sources of heterogeneity.
A sensitivity analysis was planned in the protocol, however this was not undertaken as all RCTs included all participants randomised to the treatment group in the responder analysis (less than 10 studies).
Contributions of authors
Dan Stein co‐ordinated work on the protocol, and provided feedback on draft versions of the protocol. Jonathan Ipser wrote substantial parts of the protocol and provided methodological support for the review. Karla Soares‐Weises and Hanna Bergman were responsible for entering data into RevMan, writing the results and summary of findings tables. Taryn Amos and Rasmita Ori assisted with data extraction, data analysis, the discussion and the abstract.
Sources of support
Internal sources
University of Cape Town, Cape Town, South Africa.
External sources
MRC Research Unit on Anxiety and Stress Disorders, Cape Town, South Africa.
Declarations of interest
Dan Stein has received research grants and consultancy honoraria from AstraZeneca, Eli‐Lilly, GlaxoSmithKline, Lundbeck, Orion, Pfizer, Pharmacia, Roche, Servier, Solvay, Sumitomo, and Wyeth. He has participated in a number of ongoing trials, and has presented data from some of these trials on behalf of the sponsoring companies. Dan Stein is supported by the MRC.
Jonathan Ipser has no known conflicts of interest.
Taryn Amos has no known conflicts of interest outside of her employment by the MRC Unit on Cross‐University Brain & Behaviour.
Hanna Bergman and Karla Soares‐Weiser currently work for Enhance Reviews Ltd, a company that carries out systematic reviews mostly for the public sector; it currently does not provide services for the pharmaceutical industry.
Rasmita Ori has no known conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Cameron 2005 {published data only}
- Cameron JA. Use of an Antibiotic as an Enhancer for the Treatment of Social Phobia NCT00128401. ClinicalTrials.gov [www.clinicaltrials.gov] 2005 (accessed on 26 June 2014).
de Kleine 2012 {published data only}
- Kleine RA, Hendriks G‐J, Kusters WJC, Broekman TG, Minnen A. A randomized placebo‐controlled trial of d‐cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biological Psychiatry 2012;71(11):962‐8. [DOI] [PubMed] [Google Scholar]
- Kleine RA, Hendriks GJ, Smits JA, Broekman TG, Minnen A. Prescriptive variables for d‐cycloserine augmentation of exposure therapy for posttraumatic stress disorder. Journal of Psychiatric Research 2014;48(1):40‐6. [DOI] [PubMed] [Google Scholar]
- Minnen A. Augmentation of (imaginal) exposure therapy with D‐cycloserine for patients with Posttraumatic Stress Disorder (PTSD); A Randomized Placebo controlled Study. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/Trial.aspx?TrialID=NTR1184] 2007.
Difede 2014 {published data only}
- Difede J. Combined Exposure Therapy and D‐Cycloserine vs. Placebo for Posttraumatic Stress Disorder. ClinicalTrials.gov [http://clinicaltrials.gov/show/NCT00632632] 2005 (accessed on 26 June 2014).
- Difede J, Cukor J, Wyka K, Olden M, Hoffman H, Lee FS, Altemus M. D‐cycloserine augmentation of exposure therapy for post‐traumatic stress disorder: a pilot randomized clinical trial. Neuropsychopharmacology 2014;39(5):1052‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farrell 2013 {published data only}
- Farrell L. A randomised controlled trial to evaluate the effects of D‐Cycloserine in combination with Exposure Therapy, versus pill placebo and Exposure Therapy, in the treatment of refractory pediatric Obsessive‐Compulsive Disorder (OCD) to improve the severity of obsessive‐compulsive (OC) symptoms. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/Trial.aspx?TrialID=ACTRN12609000370202] 2009 (accessed on 26 June 2014).
- Farrell LJ, Waters AM, Boschen MJ, Hattingh L, McConnell H, Milliner EL, et al. Difficult‐to‐treat pediatric obsessive‐compulsive disorder: feasibility and preliminary results of a randomized pilot trial of D‐cycloserine‐augmented behavior therapy. Journal of Depression and Anxiety 2013;30(8):723‐31. [DOI] [PubMed] [Google Scholar]
Guastella 2008 {published data only}
- Guastella A. A randomised controlled trial to evaluate the effect of D‐Cycloserine in combination with exposure therapy in the treatment of social phobia to improve the severity of social phobia symptoms. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/Trial.aspx?TrialID=ACTRN12606000352505] 2006 (accessed on 26 June 2014).
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of D‐cycloserine enhancement of exposure therapy for social anxiety disorder. Biological Psychiatry 2008;63(6):544‐9. [DOI] [PubMed] [Google Scholar]
Hofmann 2006 {published data only}
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, et al. Augmentation of exposure therapy with D‐cycloserine for social anxiety disorder. Archives of General Psychiatry 2006;63(3):298‐304. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Pollack MH, Smits JA. D‐cycloserine Enhancement of Exposure in Social Phobia. ClinicalTrials.gov [http://clinicaltrials.gov/show/NCT00515879] 2007 (accessed on 26 June 2014).
Hofmann 2013 {published data only}
- Hofmann SG, Smits JA, Rosenfield D, Simon N, Otto MW, Meuret AE, et al. D‐cycloserine as an augmentation strategy with cognitive‐behavioral therapy for social anxiety disorder. American Journal of Psychiatry 2013;170(7):751‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00633984. D‐Cycloserine Enhancement of Exposure in Social Phobia. ClinicalTrials.gov [http://clinicaltrials.gov/show/NCT00633984] 2008 (accessed on 26 June 2014).
- Pollack MH, Smits J, Simon NM, Meuret A, Marques L, Otto MW, et al. D‐Cycloserine (DCS) augmentation of CBT for social anxiety disorder: Results from an RCT abstract. Biological Psychiatry. Abstracts of the 67th annual scientific convention and meeting of the Society of Biological Psychiatry May 3‐5, 2012. Philadelphia, PA United States, 2012; Vol. 71:555.
- Smits J. Session success moderates the efficacy of cognitive enhancers for improving exposure therapy outcomes [conference abstract]. Biological Psychiatry 2014;75(9 Suppl 1):30S. [Google Scholar]
- Smits JA, Hofmann SG, Rosenfield D, DeBoer LB, Costa PT, Simon NM, et al. D‐cycloserine augmentation of cognitive behavioral group therapy of social anxiety disorder: prognostic and prescriptive variables. Journal of Consulting Psychology 2013;81(6):1100‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, Otto MW, Marques L, Davis ML, Meuret AE, et al. D‐cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. Journal of Psychiatric Research 2013;47(10):1455‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalta AK, Dowd S, Rosenfield D, Smits JA, Otto MW, Simon NM, et al. Sleep quality predicts treatment outcome in CBT for social anxiety disorder. Journal of Depression and Anxiety 2013;30(11):1114‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kushner 2007 {published data only}
- Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, et al. D‐cycloserine augmentated exposure therapy for obsessive‐compulsive disorder. Biological Psychiatry 2007;62(9):835‐8. [DOI] [PubMed] [Google Scholar]
Litz 2012 {published data only}
- Litz BT. A Placebo‐Controlled Trial of D‐Cycloserine and Exposure Therapy for Combat‐PTSD. ClinicalTrials.gov [http://clinicaltrials.gov/show/NCT00371176] 2006 (accessed 26 June 2014).
- Litz BT, Salters‐Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, Hofmann SG. A randomized placebo‐controlled trial of D‐cycloserine and exposure therapy for posttraumatic stress disorder. Journal of Psychiatric Reseach 2012;46:1184‐90. [DOI] [PubMed] [Google Scholar]
Mataix‐Cols 2014 {published data only}
- Euctr‐006947‐38‐Gb. A randomised double‐blind placebo‐controlled pilot study of D‐cycloserine‐augmented exposure therapy in adolescents with obsessive‐compulsive disorder. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/Trial.aspx?TrialID=EUCTR2008‐006947‐38‐GB] 2009 (accessed on 26 June 2014).
- Mataix‐Cols D, Turner C, Monzani B, Isomura K, Murphy C, Krebs G, Heyman I. Cognitive‐behavioural therapy with post‐session D‐cycloserine augmentation for paediatric obsessive‐compulsive disorder: Pilot randomised controlled trial. British Journal of Psychiatry 2014;204(1):77‐8. [DOI] [PubMed] [Google Scholar]
Nave 2012 {published data only}
- NCT01450306. Exposure, D‐cycloserine Enhancement, and fMRI in Snake Phobics. ClinicalTrials.gov [http://clinicaltrials.gov/show/NCT01450306] 2010 (accessed on 26 June 2014).
- Nave AM, Tolin DF, Stevens MC. Exposure therapy, D‐cycloserine, and functional magnetic resonance imaging in patients with snake phobia: a randomized pilot study. Journal of Clinical Psychiatry 2012;73(9):1179‐86. [DOI] [PubMed] [Google Scholar]
Otto 2010 {published data only}
- Otto MW. Placebo‐Controlled Evaluation of the Efficacy of D‐Cycloserine for Enhancing the Effects of CBT for Panic Disorder. ClinicalTrials.gov [http://clinicaltrials.gov/show/NCT00131339] 2004 (accessed on 26 June 2014).
- Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, et al. Efficacy of d‐cycloserine for enhancing response to Cognitive‐Behaviour Therapy for Panic Disorder. Biological Psychiatry 2010;67(4):365‐70. [DOI] [PubMed] [Google Scholar]
Ressler 2004 {published data only}
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D‐cycloserine in phobic individuals to facilitate extinction of fear. Archives of General Psychiatry 2004;61(11):1136‐44. [DOI] [PubMed] [Google Scholar]
Rothbaum 2014 {published data only}
- Granoff AL. The impact of benzodiazepine management in the randomized, double‐blind evaluation of D‐cycloserine or alprazolam combined with virtual reality exposure therapy [letter]. American Journal of Psychiatry 2014;171(11):1222. [Other comments/author response in: 'letters to the editor', http://ajp.psychiatryonline.org/toc/ajp/171/11] [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm S, Gerardi M, Breazeale K, Davis M, Duncan E, et al. Psychophysiological and cortisol reactivity predicts PTSD treatment outcome in virtual reality exposure therapy with D‐cycloserine. Neuropsychopharmacology [abstracts of the 53rd Annual Meeting of the American College of Neuropsychopharmacology, ACNP; 2014 Dec 7‐11; Phoenix, AZ United States] 2014:S369‐70. [Google Scholar]
- Rothbaum BO. D‐cycloserine and Virtual Reality Exposure to Treat Iraq War Veterans With PTSD. http://clinicaltrials.gov/show/NCT00356278 2006.
- Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, et al. A randomized, double‐blind evaluation of D‐cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. American Journal of Psychiatry 2014;171(6):640‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheeringa 2014 {published data only}
- Scheeringa MS. Effect of D‐cycloserine on Treatment of PTSD in Youth. ClinicalTrials.gov [http://www.clinicaltrials.gov/ct2/results?term=NCT01157416&Search=Search] 2010 (accessed on 26 June 2014).
- Scheeringa MS, Weems CF. Randomized placebo‐controlled D‐cycloserine with cognitive behavior therapy for pediatric posttraumatic stress. Journal of Child and Adolescent Psychopharmacology 2014;24(2):69‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheerin 2014 {published data only}
- Sheerin CM. Slope of change through D‐cycloserine facilitation of exposure therapy in a social anxiety population. Dissertation Abstracts International: Section B: the Sciences and Engineering Dissertation Abstracts International 2014 75( 6‐B(E)) 2014. [Google Scholar]
Siegmund 2011 {published data only}
- Arolt V, Zwanzger P, Ströhle A, Hamm A, Gerlach A, Kircher T, Deckert J. The research network PANIC‐NET: improving the treatment of panic disorder ‐ from a better understanding of fear circuit mechanisms to more effective psychological treatment and routine care. Psychotherapie, Psychosomatik, Medizinische Psychologie 2009;59(3):124‐31. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Golfels F, Finck C, Halisch A, Rath D, Plag J, Strohle A. D‐Cycloserine does not improve but might slightly speed up the outcome of in‐vivo exposure therapy in patients with severe agoraphobia and panic disorder in a randomized double blind clinical trial. Journal of Psychiatric Research 2011;45(8):1042‐7. [DOI] [PubMed] [Google Scholar]
- Strohle A. D‐cycloserine‐supported exposure in patients with panic disorder. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/Trial.aspx?TrialID=ISRCTN44960833] 2007 (accessed on 26 June 2014).
Storch 2007 {published data only}
- Storch EA, Merlo LJ, Bengtson M, Murphy TK, Lewis MH, Yang MC, et al. D‐cycloserine does not enhance exposure‐response prevention therapy in obsessive‐compulsive disorder. International Clinical Psychopharmacology 2007;22(4):230‐7. [DOI] [PubMed] [Google Scholar]
Storch 2010 {published and unpublished data}
- Park JM, Small BJ, Geller DA, Murphy TK, Lewin AB, Storch EA. Does D‐cycloserine augmentation of CBT improve therapeutic homework compliance for pediatric obsessive‐compulsive disorder?. Journal of Child and Family Studies 2014;23(5):863‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch E. D‐Cycloserine Augmentation of Therapy for Pediatric Obsessive‐Compulsive Disorder. ClinicaTrials.gov [http://clinicaltrials.gov/show/NCT00864123] 2008 (accessed 26 June 2014).
- Storch EA, Murphy TK, Goodman WK, Geffken GR, Lewin AB, Henin A, et al. A preliminary study of d‐cycloserine augmentation of cognitive‐behavioural therapy in pediatric obsessive‐compulsive disorder. Biological Psychiatry 2010;68(11):1073‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tart 2013 {published data only}
- Smits JA, Rosenfield D, Otto MW, Powers MB, Hofmann SG, Telch MJ, et al. D‐cycloserine enhancement of fear extinction is specific to successful exposure sessions: Evidence from the treatment of height phobia. Biological Psychiatry 2013;73(11):1054‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tart CA, Handelsman PR, DeBoer LB, Rosenfield D, Pollack MH, Hofmann SG, et al. Augmentation of exposure therapy with post‐session administration of d‐cycloserine. Journal of Psychiatric Research 2013;47(2):168‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilhelm 2008 {published data only}
- Chasson GS, Buhlmann U, Tolin DF, Rao SR, Reese HE, Rowley T, et al. Need for speed: Evaluating the slopes of OCD recovery in behaviour therapy enhanced with D‐cycloserine. Behavioural Research and Therapy 2010;48(7):675‐9. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, et al. Augmentation of behaviour therapy with d‐cycloserine for obsessive‐compulsive disorder. American Journal of Psychiatry 2008;165(3):335‐41. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Tolin D. A Randomized, Double‐blind, Placebo‐controlled Medication Trial With D‐Cycloserine for Individuals With Obsessive‐compulsive Disorder Currently Receiving Behavior Therapy. ClinicalTrials.gov [http://clinicaltrials.gov/show/NCT00126282] 2003 (accessed on 26 June 2014).
References to studies excluded from this review
Behar 2010 {published data only}
- Behar E, McHugh RK, Peckham A, Otto MW. D‐cycloserine for the augmentation of an attentional training intervention for trait anxiety. Journal of Anxiety Disorders 2010;24(4):440‐5. [DOI] [PubMed] [Google Scholar]
Evins 2012 {published data only}
- Evins AE. Cognitive remediation with D‐cycloserine added to cue exposure therapy. 51st Annual Meeting of the American College of Neuropsychopharmacology, Acnp; 2012 Feb 2‐6; Hollywood, FL United States. 2012.
Galovic 2010 {published data only}
- Galovic N, Rapee R. The effect of D‐Cycloserine on imaginal and in‐vivo exposure therapy for public speaking anxiety. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/Trial.aspx?TrialID=ACTRN12610000587000] 2010.
Guastella 2007(a) {published data only}
- Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of d‐cycloserine on exposure therapy for spider fear. Journal of Psychiatric Research 2007;41(6):466‐71. [DOI] [PubMed] [Google Scholar]
Guastella 2007(b) {published data only}
- Guastella A, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomised control trial of the effect of D‐cycloserine on extinction and fear conditioning in humans. Behaviour Research and Therapy 2007;45(4):663‐72. [DOI] [PubMed] [Google Scholar]
Gutner 2012 {published data only}
- Gutner CA, Weinberger J, Hofmann SG. The effect of D‐cycloserine on subliminal cue exposure in spider fearful individuals. Cognitive Behaviour Therapy 2012;41(4):335‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heresco‐Levy 2002 {published data only}
- Heresco‐Levy U, Kremer I, Javitt DC, Goichman R, Reshef A, Blanaru M, Cohen T. Pilot‐controlled trial of D‐cycloserine for the treatment of post‐traumatic stress disorder. International Journal of Neuropsychopharmacology 2002;5(4):301‐7. [DOI] [PubMed] [Google Scholar]
Inslicht 2013 {published data only}
- Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, et al. Sex differences in fear conditioning in posttraumatic stress disorder. Journal of Psychiatric Research 2013;47(1):64‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levinson 2013 {published data only}
- Levinson CA. A Double Blind Clinical Trial of DCS for Food Anxiety for Patients With Anorexia and Bulimia Nervosa. http://clinicaltrials.gov/show/NCT01996644 2013.
NCT00257361 2005 {unpublished data only}
- NCT00257361. D‐Cycloserine Augmentation of Exposure and Response Prevention Treatment for Obsessive‐Compulsive Disorder. ClinicalTrials.gov [http://clinicaltrials.gov/show/NCT00257361] 2005.
Rajabi 2013 {published data only}
- Attari A, Rajabi F, Maracy MR. D‐cycloserine for treatment of numbing and avoidance in chronic post traumatic stress disorder: A randomized, double blind, clinical trial. Journal of Research in Medical Sciences 2014;19(7):592‐8. [PMC free article] [PubMed] [Google Scholar]
- Rajabi F. Efficacy of D‐Cycloserine for Treatment of Numbing and Avoidance in patients with Chronic PTSD ‐ D‐Cycloserine for Numbing and Avoidance in Chronic PTSD. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/] 2013.
Rodebaugh 2013 {published data only}
- Rodebaugh TL, Levinson CA, Lenze EJ. A high‐throughput clinical assay for testing drug facilitation of exposure therapy. Depression and Anxiety 2013; Vol. 30:631‐7. [DOI] [PMC free article] [PubMed]
Sharp 2013 {published data only}
- Sharp WG. Use of D‐cycloserine to Facilitate Extinction of Food Aversion in Pediatric Populations. http://clinicaltrials.gov/show/NCT01923896 2013.
Steinglass 2007 {published data only}
- Steinglass J, Sysko R, Schebendach J, Broft A, Strober M, Walsh BT. The application of exposure therapy and D‐cycloserine to the treatment of anorexia nervosa: a preliminary trial. Journal of Psychiatric Research 2007;13(4):238‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cath 2010a {published data only}
- Cath DC. D‐cycloserine enhancement of exposure and response prevention therapy in OCD and panic disorder with agoraphobia: a randomised controlled trial. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2050 2009. [Google Scholar]
- Euctr‐021198‐35‐Nl. D‐cycloserine (DCS) enhancement of exposure therapy in panic disorder with agoraphobia: a randomized controlled trial ‐ D‐cycloserine enhancement of exposure therapy. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/Trial.aspx?TrialID=EUCTR2010‐021198‐35‐NL] 2010 (accessed on 26 June 2014).
- Klein Hofmeijer M, Duits P, Rijkeboer MM, Hoogendoorn A, Megen H, Balkom AJLM, et al. D‐Cycloserine enhancement in exposure therapy for patients with a panic disorder with agoraphobia; a randomised controlled trial. European Neuropsychopharmacology 2014 S617 [abstracts of the 27th European College of Neuropsychopharmacology, ECNP Congress; 2014 Oct 18‐21; Berlin Germany] 2014. [Google Scholar]
Cath 2010b {published data only}
- Cath DC. D‐cycloserine enhancement of exposure and response prevention therapy in OCD and panic disorder with agoraphobia: a randomised controlled trial. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2050 2009.
Guay 2007 {published data only}
- Guay S. RCT of CBT combined with d‐cycloserine for treating PTSD or comparative study of the efficacy of a cognitive‐behavioral therapy for post‐traumatic stress disorder with or without d‐cycloserine NCT00452231. Clinicaltrials.gov [www.clinicaltrials.gov] 2007.
Strohle 2011 {published data only}
- Strohle A. Augmentation of Exposure Therapy With D‐Cycloserine in Patients With Agoraphobia With or Without Panic Disorder. http://clinicaltrials.gov/show/NCT01928823 2011.
References to ongoing studies
Arman 2013 {published data only}
- Arman S, Soheilimehr A. The efficacy of augment of D‐cycloserine and cognitive‐behavioral therapy on symptoms improvement in the adolescents with one type of anxiety disorders: A double‐blind randomized controlled trial. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/Trial.aspx?TrialID=IRCT2012071610297N1] 2013. [DOI] [PMC free article] [PubMed]
Bergman 2012 {published data only}
- Bergman L. The Use of D‐Cycloserine to Augment Intensive Cognitive Behavioral Therapy for Pediatric Obsessive Compulsive Disorder. http://clinicaltrials.gov/show/NCT01687140 2012.
de Leeuw 2008 {published data only}
- Meerkanten GGZ. The effect of the addition of D‐cycloserine to exposure sessions in the treatment of patients with obsessive‐compulsive disorder. A randomized, placebo‐controlled trial. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/Trial.aspx?TrialID=EUCTR2007‐000367‐18‐NL] 2007.
- Leeuw AS. The effect of the addition of D‐cycloserine to exposure sessions in the treatment of patients with obsessive‐compulsive disorder. A randomized, placebo‐controlled trial [Onderzoek naar het effect van de toevoeging van D‐cycloserine aan exposure sessies bij de behandeling van patiënten met een obsessieve‐compulsieve stoornis]. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1189 2008.
Difede 2009 {published data only}
- Difede J. D‐cycloserine Enhanced Imaginal ExposureTherapy for Posttraumatic Stress Disorder (PTSD). http://clinicaltrials.gov/show/NCT00875342 2009.
Difede 2011a {published data only}
- Difede J. Enhancing Exposure Therapy for Post Traumatic Stress Disorder (PTSD): Virtual Reality and Imaginal Exposure With a Cognitive Enhancer. http://clinicaltrials.gov/show/NCT01352637 2011. [DOI] [PMC free article] [PubMed]
Farrell 2012 {published data only}
- Farrell L. A randomised controlled trial to evaluate the effects of D‐cycloserine in combination with exposure therapy, versus pill placebo and exposure therapy, in the treatment of pediatric Specific Phobia to improve symptoms of anxiety, fear and phobic avoidance. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/Trial.aspx?TrialID=ACTRN12612000420842] 2012.
Farrell 2014 {published data only}
- Farrell I. A randomised controlled trial to evaluate the effects of D‐cycloserine in combination with intensive exposure therapy, versus pill placebo in combination with intensive exposure therapy in the treatment of pediatric Obsessive Compulsive Disorder to improve the severity of obsessive compulsive symptoms. http://www.anzctr.org.au/ACTRN12614000140651.aspx 2014.
Geller 2011 {published data only}
- Geller DA. 2/2 D‐Cycloserine Augmentation of CBT for Pediatric OCD. http://clinicaltrials.gov/show/NCT01404208 2011.
Guastella 2006 {published data only}
- Guastella A. A randomised controlled trial to evaluate the effects of D‐Cycloserine in combination with Exposure Therapy in the treatment of panic disorder to improve the severity of Panic Symptoms. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/Trial.aspx?TrialID=ACTRN12606000351516] 2006.
Otto 2008 {published data only}
- Otto MW, Tolin DF, Pollack MH. Exposure, D‐Cycloserine Enhancement, and Genetic Modulators in Panic Disorder. http://clinicaltrials.gov/show/NCT00790868 2008.
Pollack 2014 {published data only}
- Pollack M, Hofmann S, Smits JA. Dose Timing of D‐Cycloserine to Augment CBT for Social Anxiety Disorder. http://clinicaltrials.gov/show/NCT02066792 2014.
Rapee 2010 {published data only}
- Rapee R, Byrne S. Among Spider and Dog Phobic Children is D‐Cycloserine Augmented Exposure Therapy more Effective than Exposure Therapy alone as Measured by Performance on Behavioural Approach Tests. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/Trial.aspx?TrialID=ACTRN12610000490077] 2010.
Rapee 2011 {published data only}
- Rapee R. D‐cycloserine vs placebo combined with in‐vivo exposure in the reduction of specific fears among children with broad‐based anxiety disorders. WHO International Clinical Trials Registry Platform [http://apps.who.int/trialsearch/Trial.aspx?TrialID=ACTRN12611000660987] 2011.
Reinecke 2012 {published data only}
- Reinecke A, Harmer C. The Effect of a Single‐dose of D‐cycloserine on the Basic Effects of Cognitive‐behaviour Therapy for Panic Disorder ‐ a Randomized Placebo‐controlled Trial. http://clinicaltrials.gov/show/NCT01680107 2012.
Ruck 2012 {published data only}
- Ruck C. Internet‐based Cognitive Behavior Therapy in Combination With D‐Cycloserine for Obsessive Compulsive Disorder: A Double Blinded Randomized Controlled Trial. http://clinicaltrials.gov/show/NCT01649895 2012.
Sirbu 2009 {published data only}
- Sirbu C, Kerr PL. D‐Cycloserine‐Enhancer of One‐Session Treatment for Phobia of Heights. http://clinicaltrials.gov/show/NCT01037101 2009.
Smits 2013 {published data only}
- Smits JA. Enhancing Panic and Smoking Reduction Treatment With D‐Cycloserine. http://clinicaltrials.gov/show/NCT01944423 2013.
Storch 2011 {published data only}
- Storch EA. 1/2 D‐cycloserine Augmentation of CBT for Pediatric OCD. http://clinicaltrials.gov/show/NCT01411774 2011.
Additional references
Alonso 2004
- Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, et al. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatrica Scandinavica 2004;420:21‐7. [DOI] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐III). 3rd Edition. Washington, DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM‐IV). 4th Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM‐IV‐TR). 4th Edition. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
Baldwin 2011
- Baldwin DS, Waldman S, Allgulander C. Evidence‐based pharmacological treatment of generalized anxiety disorder. International Journal of Neuropsychopharmacology 2011;14(5):697‐710. [DOI] [PubMed] [Google Scholar]
Bandelow 2004
- Bandelow B, Ruther E. Treatment‐resistant panic disorder. CNS Spectrums 2004;9(10):725‐39. [DOI] [PubMed] [Google Scholar]
Bandelow 2008
- Bandelow B, Zohar J, Hollander E, Kasper S, Möller H. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Pharmacological Treatment of Anxiety, Obsessive‐Compulsive and Post‐Traumatic Stress Disorders. Informa Healthcare 2008;9(4):248‐312. [DOI] [PubMed] [Google Scholar]
Barlow 2000
- Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive‐behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA 2000;283(19):2529‐36. [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
Beck 1993
- Beck AT, Steer RA. Beck Anxiety Inventory. San Antonio, TX: The Psychological Corporation, 1993. [Google Scholar]
Beck 2001
- Beck JS, Beck AT, Jolly JB. Beck Youth Inventories. San Antonio, TX: Psychological Corporation, 2001. [Google Scholar]
Birmaher 2003
- Birmaher B, Khetarpal S, Cully M, Brent DA, McKenzie S. Screen for Child Anxiety Related Disorders (SCARED)–Parent form and child form (8 years and older). Innovations in Clinical Practice: Focuson Children & Adolescents, edited by L. VandeCreek and T.L. Jackson. Sarasota, FL: Professional Resource Press/Professional Resource Exchange, 99–104, 2003. [Google Scholar]
Black 2006
- Black DW. Efficacy of combined pharmacotherapy and psychotherapy versus monotherapy in the treatment of anxiety disorders. CNS Spectrums 2006;11(10 Suppl 12):29‐33. [DOI] [PubMed] [Google Scholar]
Blake 1990
- Blake DD, Weathers FW, Nagy LM, et al. A clinician rating scale for assessing current and lifetime PTSD: The CAPS‐1. Behavior Therapy 1990;21:187‐8. [Google Scholar]
Boje 1993
- Boje KM, Wong G, Skolnick P. Desensitization of the NMDA receptor complex by glycinergic ligands in cerebellar granule cell cultures. Brain Research 1993;603(2):207‐14. [DOI] [PubMed] [Google Scholar]
Bontempo 2012
- Bontempo A, Panza KE, Bloch MH. D‐cycloserine augmentation of behaviour therapy for treatment of anxiety disorders: Meta‐analysis. Journal of Clinical Psychiatry 2012;73(4):533‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 1989
- Brown TA, Nardo PA, Barlow DH. Anxiety Disorders Interview Schedule for DSM‐IV. Albany, New York: University of Albany, 1989. [Google Scholar]
CPA 2006
- Canadian Psychiatric Association. Clinical practice guidelines. Management of anxiety disorders. Canadian Journal of Psychiatry 2006;51(8 Suppl 2):9S‐91S. [PubMed] [Google Scholar]
Davis 2006
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of d‐cycloserine on extinction: translation from preclinical to clinical work. Biological Psychiatry 2006;60:369‐75. [DOI] [PubMed] [Google Scholar]
Deeks 2008
- Deeks J, Higgins JPT, Altman D. editors. Analysing and presenting results. In: Higgins JPT, Green S editor(s). Cochrane Reviewers’ Handbook 5.0.0 [updated February] Section 9;. Chichester, UK: John Wiley & Sons, Ltd., 2008. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Difede 2014(a)
- Difede J, Cukor J, Wyka K, Olden M, Hoffman H, Lee FS, Altemus M. D‐cycloserine augmentation of exposure therapy for post‐traumatic stress disorder: a pilot randomized clinical trial. Neuropsychopharmacology 2014;39(5):1052‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foa 1986
- Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychological Bulletin 1986;99(1):20‐35. [PubMed] [Google Scholar]
Foa 2001
- Foa EB, Johnson KM, Feeny NC, Treadwell KRH. The child PTSD symptom scale (CPSS): A preliminary examination of its psychometric properties. Journal of Clinical Child Psychology 2001;30:376‐84. [DOI] [PubMed] [Google Scholar]
Foa 2007
- Foa EB, Hembree E, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide. Oxford University Press, 2007. [Google Scholar]
Furukawa 2006
- Furukawa TA, Watanabe N, Churchill R. Psychotherapy plus antidepressant for panic disorder with or without agoraphobia: systematic review. British Journal of Psychiatry 2006;188:305‐12. [DOI] [PubMed] [Google Scholar]
Goodman 1989
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale‐Brown Obsessive Compulsive Scale, I: development, use, and reliability. Archives of General Psychiatry 1989;46:1006‐11. [DOI] [PubMed] [Google Scholar]
Greenland 1987
- Greenland S. Interpretation and choice of effect measures in epidemiologic analyses. American Journal of Epidemiology 1987;125(5):761‐8. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. ECDEU assessment manual for psychopharmacology. Washington, DC: U.S. National Institute of Health, 1976. [Google Scholar]
Higgins 2003
- Higgins JTP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(6):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008a
- Higgins JPT, Altman DG. Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Reviewers’ Handbook 5.0.0 [updated February] Section 8;. Chichester, UK: John Wiley & Sons, Ltd., 2008. [Google Scholar]
Higgins 2008b
- Higgins JPT, Deeks JJ, Altman DG. Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Reviewers’ Handbook 5.0.0 [updated February] Section 16. Chichester, UK: John Wiley & Sons, Ltd, 2008. [Google Scholar]
Higgins 2009
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009 Jan;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
Hofmann 2009
- Hofmann SG, Sawyer AT, Korte KJ, Smits JAJ. Is it beneficial to add pharmacotherapy to cognitive‐behavioral therapy when treating anxiety disorders? A meta‐analytic review. International Journal of Cognitive Therapy 2009;2:160‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmann 2011
- Hofmann SG, Smits JA, Asnaani A, Gutner CA, Otto MW. Cognitive Enhancers for Anxiety Disorders. Pharmacology Biochemistry and Behavior 2011;99(2):275‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmann 2014
- Hofmann SG. d‐Cycloserine for treatment of anxiety disorders ‐ making good exposures better and bad exposures worse. Depression and Anxiety 2014 March;31(3):175‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmann 2015
- Hofmann SG, Otto MW, Pollack MH, Smits JA. d‐Cycloserine Augmentation of Cognitive Behaviour Therapy for Anxiety disorders: an update. Current Psychiatry Reports 2015;17:532. [DOI] [PubMed] [Google Scholar]
Jenike 2004
- Jenike MA. Clinical practice. Obsessive‐compulsive disorder. New England Journal of Medicine 2004;350(3):259‐65. [DOI] [PubMed] [Google Scholar]
Jones 2002
- Jones R, Laake K, Oeksengaard AR. D‐cycloserine for Alzheimer's disease. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD003153; CD003153] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 1994
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12‐month prevalence of DSM‐III‐R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry 1994;51(1):8‐19. [DOI] [PubMed] [Google Scholar]
Kessler 2005
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry 2005;62(6):593‐602. [DOI] [PubMed] [Google Scholar]
Klorman 1974
- Klorman R, Weerts TC, Hastings JE, Melamed BG, Lang PG. Psychometric description of some specific fear questionnaires. Behavior Therapy 1974;5(3):401‐9. [Google Scholar]
Kovacs 1992
- Kovacs M. Children’s Depression Inventory Manual. New York: Multi‐Health Systems, 1992. [Google Scholar]
Ledgerwood 2003
- Ledgerwood L, Richardson R, Cranney J. Effects of D‐cycloserine on extinction of conditioned freezing. Behavioral Neuroscience 2003;117(2):341‐9. [DOI] [PubMed] [Google Scholar]
Ledgerwood 2005
- Ledgerwood L, Richardson R, Cranney J. D‐cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biological Psychiatry 2005;57(8):841‐7. [DOI] [PubMed] [Google Scholar]
Lee 1998
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear‐conditioned rats. The Journal of Neuroscience 1998;18(20):8444‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2006
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. The Journal of Neuroscience 2006;26(39):10051‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liebowitz 1987
- Liebowitz MR. Social phobia. Modern Problems in Pharmacopsychiatry 1987;22:141‐73. [DOI] [PubMed] [Google Scholar]
Liebowitz 1992
- Liebowitz MR, Schneier FR, Campeas R, Hollander E, Hatterer J, Fyer A, et al. Phenelzinevs atenolol in social phobia: a placebo‐controlled comparison. Archives of General Psychiatry 1992;49:290‐300. [DOI] [PubMed] [Google Scholar]
March 1997
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children: Factor structure, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36:554‐65. [DOI] [PubMed] [Google Scholar]
Marks 1993
- Marks IM, Swinson RP, Başoğlu M, Kuch K, Noshirvani H, O'Sullivan G, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. British Journal of Psychiatry 1993;162:776‐87. [DOI] [PubMed] [Google Scholar]
Mendlowicz 2000
- Mendlowicz MV, Stein MB. Quality of life in individuals with anxiety disorders. American Journal of Psychiatry 2000;157(5):669‐82. [DOI] [PubMed] [Google Scholar]
Mitte 2005
- Mitte K. A meta‐analysis of the efficacy of psycho‐ and pharmacotherapy in panic disorder with and without agoraphobia. Journal of Affective Disorders 2005 Sept;88(1):27‐45. [DOI] [PubMed] [Google Scholar]
Murray 2013
- Murray CJL, Lopez AD. Measuring the Global burden of disease. New England Journal of Medicine 2013;369:448‐57. [DOI] [PubMed] [Google Scholar]
NICE 2011
- NICE. Generalised Anxiety Disorder and Panic Disorder (With Or Without Agoraphobia) in Adults: Management in Primary, Secondary and Community Care. NICE clinical guideline 113. Available at www.nice.org.uk/CG113[NICE guideline] 2011;113. [Google Scholar]
Norberg 2008
- Norberg MM, Krystal JH, Tolin DF. A meta‐analysis of D‐cycloserine and the facilitation of fear extinction and exposure therapy. Biological Psychiatry 2008;63(12):1118‐26. [DOI] [PubMed] [Google Scholar]
Pallanti 2002
- Pallanti S, Hollander E, Bienstock C, Koran L, Leckman J, Marazziti D, et al. Treatment non‐response in OCD: methodological and operational definitions. International Journal of Neuropsychopharmacology 2002;5:181‐91. [DOI] [PubMed] [Google Scholar]
Quirk 2000
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. The Journal of Neuroscience Aug 15;20(16):6225‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rapee 2007
- Rapee RM, Abbott MJ, Baillie AJ, Gaston JE. Treatment of social phobia through pure self help and therapist‐augmented self help. British Journal of Psychiatry 2007;191:246‐52. [DOI] [PubMed] [Google Scholar]
Ressler 2000
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depression and anxiety November 2000;12 Suppl 1:2‐19. [DOI] [PubMed] [Google Scholar]
Richardson 2004
- Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by D‐cycloserine: theoretical and clinical implications. Learning and Memory 2004;11(5):510‐6. [DOI] [PubMed] [Google Scholar]
Rodrigues 2014
- Rodrigues H, Figueira I, Lopes A, Gonçalves R, Mendlowicz MV, Coutinho ESF, Ventura P. Does D‐cycloserine enhance exposure therapy for anxiety disorders in humans? A meta‐analysis. PLOS ONE July 2014. [DOI] [PMC free article] [PubMed]
Rothbaum 2003
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post‐trauma reactions. Annals of the New York Academy of Sciences Dec 2003;1008:112‐21. [DOI] [PubMed] [Google Scholar]
Scahill 1997
- Scahill L, Riddle MA, McSwiggan‐Hardin MT, et al. The Children’s Yale‐Brown Obsessive‐Compulsive Scale: preliminary report of reliability and validity. JAACAP 1997;36:844‐53. [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Interpreting results and drawing conclusions. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83. [Google Scholar]
Shader 1993
- Shader RI, Greenblatt DJ. Use of benzodiazepines in anxiety disorders. New England Journal of Medicine 1993;328:1398‐405. [DOI] [PubMed] [Google Scholar]
Spielberger 1970
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, 1970. [Google Scholar]
Sulkowski 2014
- Sulkowski ML, Geller DA, Lewin AB, Murphy TK, Mittleman A, Brown A, Storch EA. The future of D‐cycloserine and other cognitive modifiers in obsessive ‐ compulsive and related disorders. Current Psychiatry Reviews 2014;10(4):317‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Szapiro 2003
- Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus 2003;13(1):53‐8. [DOI] [PubMed] [Google Scholar]
Thompson 1994
- Thompson SG. Why sources of heterogeneity in meta analysis should be investigated. BMJ 1994;309(6965):1351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tiihonen 2006
- Tiihonen J, Wahlbeck K. Glutamatergic drugs for schizophrenia. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003730.pub2; CD003730] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2002
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra‐amygdala infusions of D‐cycloserine as assessed with fear‐potentiated startle. Journal of Neuroscience 2002;22:2343‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1993
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. World Health Organization 1993.
Yang 2005
- Yang YL, Lu KT. Facilitation of conditioned fear extinction by d‐cycloserine is mediated by mitogen‐activated protein kinase and phosphatidylinositol 3‐kinase cascades and requires de novo protein synthesis in basolateral nucleus of amygdala. Neuroscience 2005;134(1):247‐60. [DOI] [PubMed] [Google Scholar]
Yates 2012
- Yates RW, David Bienenfeld D, Bessman E, Brenner B, Daniels C, Erickson M, Friedman S. Anxiety Disorders. Medscape Emedicine January 2012.


