Abstract
Domoic acid (DA), the causative agent for the human syndrome Amnesic Shellfish Poisoning (ASP), is a potent, naturally occurring neurotoxin produced by common marine algae. DA accumulates in seafood, and humans and wildlife alike can subsequently be exposed when consuming DA-contaminated shellfish or finfish. While strong regulatory limits protect people from the acute effects associated with ASP, DA is an increasingly significant public health concern, particularly for coastal dwelling populations, and there is a growing body of evidence suggesting that there are significant health consequences following repeated exposures to levels of the toxin below current safety guidelines. However, gaps in scientific knowledge make it difficult to precisely determine the risks of contemporary low-level exposure scenarios. The present review characterizes the toxicokinetics and neurotoxicology of DA, discussing results from clinical and preclinical studies after both adult and developmental DA exposure. The review also highlights crucial areas for future DA research and makes the case that DA safety limits need to be reassessed to best protect public health from deleterious effects of this widespread marine toxin.
Keywords: domoic acid, amnesic shellfish poisoning, neurotoxicity, development, chronic exposure, public health
1. Introduction
Domoic acid (DA), the excitotoxic glutamate receptor agonist known to cause an acute neurotoxic syndrome called Amnesic Shellfish Poisoning (ASP), is produced by marine algae in the genus Pseudo-nitzschia, found worldwide (Bates, 2000; Bates et al., 1989; Bates, Hubbard, Lundholm, Montresor, & Leaw, 2018; Bates & Trainer, 2006; Perl, Bedard, Kosatsky, Hockin, & Todd, 1990; Todd, 1993). When these toxigenic algae divide rapidly, high-density toxic “blooms” emerge in marine waters, where they can persist for months (McCabe et al., 2016; Trainer et al., 2012). Production of DA, however, is variable, and, while some environmental conditions seem to enhance production, it remains unclear as to why these algae produce the toxin (Brunson et al., 2018). When DA is present in the environment, filter feeding marine life, such as clams, oysters, mussels, crabs, and anchovies, can accumulate DA and pass the toxin to humans and wildlife (D’Agostino et al., 2017; Fire et al., 2010; Kvitek, Goldberg, Smith, Doucette, & Silver, 2008; Lefebvre, Bargu, Kieckhefer, & Silver, 2002; Lefebvre, Silver, Coale, & Tjeerdema, 2002).
While regulations developed in the late 1980s have prevented acute human DA poisonings (i.e. ASP), other exposure scenarios have been of increasing concern (Lefebvre & Robertson, 2010; Wekell, Jurst, & Lefebvre, 2004). With the intensification of algal bloom conditions due to climate change (McKibben et al., 2017; Trainer et al., 2020; Wells et al., 2020, 2015) and recent consumption surveys identifying that many shellfish harvesters may be regularly exposed to low levels of DA (Andjelkovic, Vandevijvere, Van Klaveren, Van Oyen, & Van Loco, 2012; Ferriss, Marcinek, Ayres, Borchert, & Lefebvre, 2017), there is an urgent need to comprehensively understand the health impacts associated with chronic, low-level exposure to this prevalent neurotoxin. The following review synthesizes the evidence from epidemiological and in vivo laboratory studies on DA toxicity, while identifying persistent data gaps that hinder our understanding of the present-day public health risk of DA.
2. A Human Domoic Acid Poisoning Event
DA is a small amino acid, structurally similar to the neurotoxin, kainic acid (KA), and the endogenous neurotransmitter, glutamate (Fig. 1) (Wright et al., 1989). DA was first identified in the 1950s in Japan, when it was characterized as an anti-parasitic treatment, administered in doses of 20 mg (Takemoto & Daigo, 1958). It was not until nearly thirty years later, in 1987, when the potent neurotoxicity of the compound was revealed. In early December of that year, a national health bulletin was posted on Prince Edward Island, Canada, warning of a new mussel-associated intoxication, after three people were hospitalized with symptoms of confusion, disorientation, and memory loss after consuming mussels contaminated with 310–1280 ppm DA. In total, over 150 people were sickened and four people died after very high levels of DA exposure (estimated up to 290 mg/patient) (Perl, Bedard, Kosatsky, Hockin, & Todd, 1990; Perl, Bedard, Kosatsky, Hockin, Todd, et al., 1990). DA was not detected in blood or cerebral spinal fluid; instead, cases were considered positive if respondents experienced symptoms within 48 h of consuming shellfish (Perl, Bedard, Kosatsky, Hockin, & Todd, 1990). Of those who met this case definition, most reported upset stomachs, vomiting and diarrhea that developed within 4–5 h of exposure. Nearly a fifth of the poisoning cases were admitted to the hospital with seizures and a host of other neurological symptoms, which ranged from uncontrollable emotionality to coma. The term “ASP” is now widely used to refer to the clinical symptoms associated with acute DA toxicity (Perl, Bedard, Kosatsky, Hockin, Todd, et al., 1990). Neuropsychological examinations of some of the most severely affected ASP patients revealed a unique pattern of functional losses consistent with anterograde amnesia (Todd, 1993), which is characterized by the lack of ability to form new memories (Tulving, 1983). In extreme DA poisoning cases, patients with amnesia had persistent and long-term memory deficits (Zatorre, 1990).
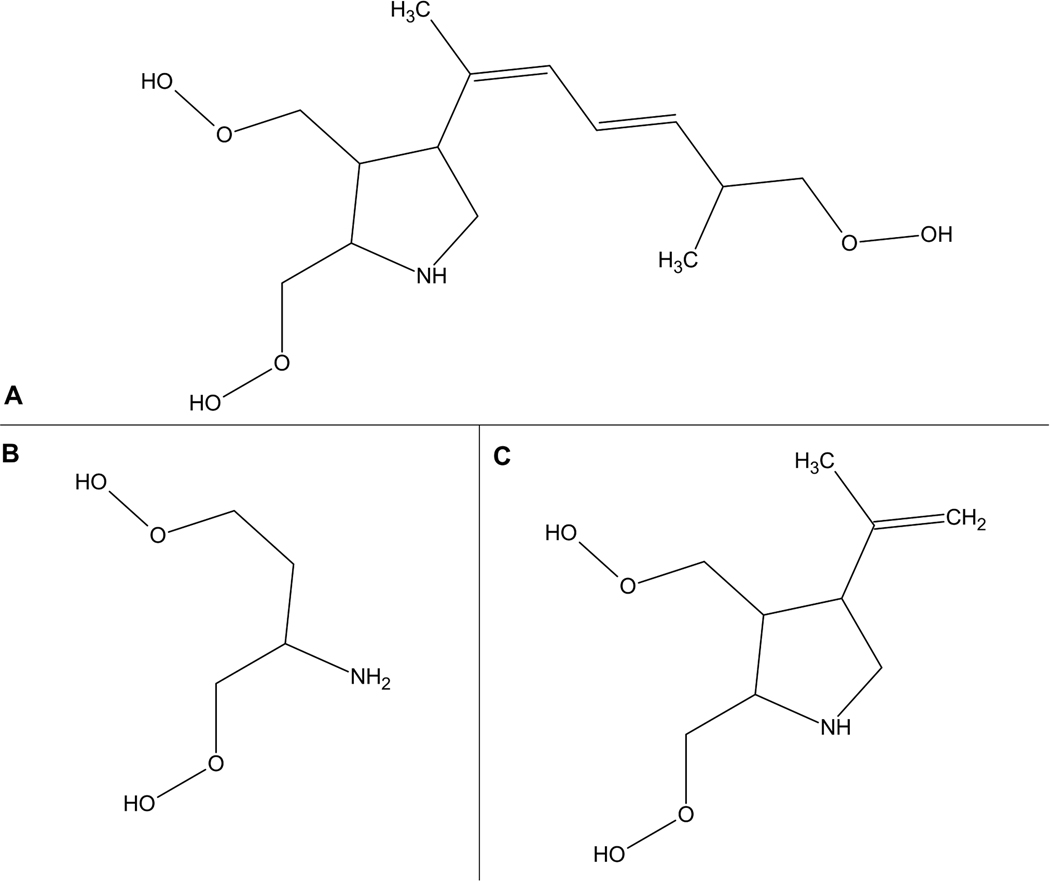
Figure 1:
Reprinted from Petroff, 2020. Chemical structures of domoic acid and analogues. A) domoic acid; B) glutamate; C) kainic acid
Several individuals sickened by DA underwent magnetic resonance imaging (MRI), positron emission tomography (PET) scans, and electroencephalography (EEG) assessments. MRI and other imaging results from patients indicated that those affected had acute neuronal death in the amygdala and parahippocampal gyrus, as well as moderate to severe disturbances in electrophysiology, as observed by spikes and seizure-like activity on EEG exams (Gjedde & Evans, 1990; Teitelbaum, Zatorre, Carpenter, Gendron, & Cashman, 1990). In addition to the three patients that died shortly after initial DA exposure, one patient survived the poisoning, but later developed temporal lobe epilepsy and died within a year (Cendes, Andermann, Carpenter, Zatorre, & Cashman, 1995). Histopathological follow-up in deceased patients revealed extensive neurotoxic injury in the amygdala and hippocampus, with neuronal death and astrocyte reactivity noted in the amygdala, hippocampus, olfactory cortex, and thalamus (Carpenter, 1990), reflecting the potent neurotoxic nature of DA.
Following the Prince Edward Island poisoning, public health officials implemented DA monitoring programs for seafood and instituted a 20 ppm DA action level for closing beaches to shellfish harvesting (see Section 8 for details). There have been no episodes of ASP since the 1987 poisoning episode.
3. Sea Lions as Sentinels for Health Impacts of Domoic Acid
While humans have been protected by this action level, multiple DA poisoning events have occurred in naturally exposed marine mammals over the past three decades. In May and June of 1998, California sea lions (CSLs) along the Pacific coast of California were observed exhibiting seizures, ataxia, abnormal scratching, and related neurological symptoms (Gulland, 2000; Scholin et al., 2000). Analysis of blood, urine, and feces from subsets of the estimated hundreds of impacted animals identified the presence of DA (Lefebvre et al., 1999; Scholin et al., 2000). This, in conjunction with a simultaneous Pseudo-nitzschia algal bloom, as well as the detection of both DA and the DA-producing algae in sea lion feces and the anchovy prey of CSLs, collectively led to the first documentation of DA poisoning in a marine mammal species (Lefebvre et al., 1999; Scholin et al., 2000). Since then, dozens to hundreds of CSLs off the coast of California are diagnosed with DA poisoning each year (Bargu, Goldstein, Roberts, Li, & Gulland, 2012; Bargu, Silver, Goldstein, Roberts, & Gulland, 2010; Greig, Gulland, & Kreuder, 2005).
In a 1998 poisoning event and subsequent follow-up, CSLs with acute DA toxicosis consistently exhibited excitotoxic cell death in the hippocampus (Gulland et al., 2002; Scholin et al., 2000; Silvagni, Lowenstine, Spraker, Lipscomb, & Gulland, 2005). Researchers have also identified an additional, long-lasting, DA-associated clinical syndrome in CSLs, characterized by reoccurring seizures following sublethal exposure (Goldstein et al., 2008; Ramsdell & Gulland, 2014). Persistent seizures are often accompanied with other lingering, adverse effects of DA. These include poor performance on spatial memory challenges, MRI changes in hippocampal structure and connectivity (Cook, Berns, Colegrove, Johnson, & Gulland, 2018; Cook et al., 2015), and aberrant behavior, including impaired spatial navigation, repetitive behaviors, and unusual aggression (Cook, Reichmuth, & Gulland, 2011; Cook et al., 2016; Goldstein et al., 2008). In instances of chronic seizures and related effects after DA exposure, researchers often observe unilateral hippocampal atrophy that is distinct from direct DA-associated atrophy (Buckmaster, Wen, Toyoda, Gulland, & Van Bonn, 2014; Goldstein et al., 2008).
CSLs have been an invaluable sentinel species in DA research, as marine mammal exposures are similar to the human oral exposure route, and the symptoms of acute CSL toxicosis syndrome are analogous to ASP (Goldstein et al., 2008). Given the expanding reports detailing the prolonged effects related to sub-lethal DA exposure described above, researchers should consider expanding investigations of CSLs to examine the effects of chronic, low-level DA exposures in both adult and developing marine mammals.
4. Toxicokinetic Properties of Domoic Acid
An important factor in cross-species comparisons of chemical exposures and effects is the toxicokinetics (TK) in humans and animal models. DA is a water soluble (logP = −0.23), small molecule compound (molecular weight: 331.33 g/mol) that is ionized with 3 negative charges and 1 positive charge at physiological pH of 7.4 (Walter, Leek, & Falk, 1992). Consistent with its hydrophilicity and ionization state at physiological pH, the plasma protein binding of DA is negligible (fraction unbound (fu) = 1), as measured in monkey and human plasma (Jing et al., 2018), and the transcellular permeability of DA is low, as shown in Caco-2 cells (Kimura, Kotaki, Hamaue, Haraguchi, & Endo, 2011). Based on these physicochemical properties, DA is not expected to distribute widely in the body and is mainly eliminated unchanged in the urine through glomerular filtration. Unfortunately, the TK of DA in humans is not known. During the 1987 Prince Edward Island DA poisoning, clinical specimens of blood and cerebral spinal fluid were collected from patients, but DA was not detected in any of these samples, likely due to the delayed sampling time (1–2 weeks after hospital admission) and inadequate sensitivity of the detection method (Todd, 1993). The TK of DA in laboratory animal models, however, have been described.
The TK of DA following intravenous (iv) dosing has been reported in multiple preclinical animal models (Table 1). As expected, based on the physicochemical properties, DA was rapidly eliminated in urine following an iv dose and has a short plasma half-life (1–2 h) in both monkeys and rats (Jing et al., 2018; Suzuki & Hierlihy, 1993; Truelove & Iverson, 1994). The volume of distribution (Vss) of DA was reported as less than the total body water content (60–70%) in both monkeys and rats over a wide range of doses, suggesting that DA is not widely distributed in the body and the distribution is independent of dose. Consistent with the low Vss, the brain-to-blood ratio of DA was low (0.04–0.06) in rats following a single iv and intraperitoneal (ip) dose. The same ratio following repeated dosing has not been reported.
Table 1:
Toxicokinetic Parameters of Domoic Acid
| Intravenous (iv) Toxicokinetics of DA | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Species | Route of Exposure | Dose (μg/kg) | Vss (mL/kg) | CL (mL/min/kg) | CLr (mL/min/kg) | MRT (h) | Half-life (h) | Citation |
|
| ||||||||
| Monkey | Single iv | 50 | 159 ± 29 | 1.3 ± 0.5 | -- | 2.48 ± 1.31 | 1.91 ± 0.98 | Truelove & Iverson, 1994 |
|
| ||||||||
| Monkey | Single iv | 5 | 131 ± 71 | 2.1 ± 1.2 | 0.60 ± 0.50 | -- | 1.2 ± 1.1 | Jing et al., 2018 |
|
| ||||||||
| Rat | Single iv | 500 | 272 ± 58 | 10.8 ± 1.2 | 12.2 ± 1.73 | -- | -- | Suzuki & Hierlihy, 1993 |
| 2000 | 244 ± 31 | 7.8 ± 1.6 | 8.8 ± 1.6 | -- | -- | |||
|
| ||||||||
| Rat | Single iv | 500 | 311 | 10.2 | -- | 0.51 | 0.38 | Truelove & Iverson, 1994 |
| 1000 | 229 ± 88 | 7.8 ± 2.7 | -- | 0.50 ± 0.09 | 0.35 ± 0.05 | |||
|
| ||||||||
| Rat (pregnant) | Single iv | 1000 | 494.1 | 6.5 | -- | 1.27 | 0.49 | Maucher Fuquay et al., 2012 |
| Rat (fetus) | Single iv | 1000 (maternal) | -- | -- | -- | 1.5 | 9.22 | |
|
| ||||||||
| Oral Toxicokinetics of DA | ||||||||
|
| ||||||||
| Species | Route of Exposure | Dose (μg/kg) | CL/F (mL/min/kg) | CLr (mL/min/kg) | Half-life (h) | Bioavailability (%) | fe (%) | Citation |
|
| ||||||||
| Monkey | Multiple oral | 500, 750 | -- | -- | -- | -- | 5.8 ± 1.7 | Truelove et al., 1997 |
|
| ||||||||
| Monkey | Single oral | 75 | 33 ± 12 | 1.6 ± 0.8 | 11.3 ± 2.4 | 6 ± 4 | 4 ± 2 | Jing et al., 2018 |
| 150 | 27 ± 15 | -- | 9.8 ± 5.9 | 7 ± 5 | -- | |||
|
| ||||||||
| Monkey | Single oral | 75 | -- | 5.2 (2.8 – 9.9) | -- | -- | -- | Shum et al., 2020 |
| 150 | -- | 6.4 (4.9 – 8.2) | -- | -- | -- | |||
| Multiple oral | 75 | 61 (49 – 76) | 6.2 (5.3 – 7.4) | -- | -- | 2.7 (2.1 – 3.4) | ||
| 150 | 39 (29–52) | 6.2 (4.7 – 8.2) | -- | -- | 4.2 (3.0 – 5.9) | |||
| Monkey (pregnant) | Multiple oral | 75 | 92 (58–146) | 11 (7.3 – 17) | -- | -- | 3.2 (2.5 – 4.1) | |
| 150 | 54 (38–76) | 9.4 (6.6 – 13) | -- | -- | 4.5 (3.2 – 6.3) | |||
|
| ||||||||
| Tissue Distribution | ||||||||
|
| ||||||||
| Species | Route of Exposure | Dose (μg/kg) | Brain:Blood Ratio | Citation | ||||
|
| ||||||||
| Rat | Single ip | 500, 1000, 2000 | 0.04 ± 0.01 | Hesp et al., 2007 | ||||
|
| ||||||||
| Rat (pregnant) | Single iv | 1000 | 0.06 | Maucher Fuquay et al., 2012 | ||||
| Rat (fetus) | Single iv | 1000 (maternal) | 0.15 | |||||
|
| ||||||||
| Maternal-Fetal Disposition | ||||||||
|
| ||||||||
| Species | Route of Exposure | Dose (μg/kg) | Fetal:Maternal Ratio | Amniotic Fluid:Blood Ratio | Citation | |||
|
| ||||||||
| Monkey | Multiple oral | 75 | 0.3 | 0.9 | Shum et al., 2020 | |||
| 150 | 0.3 | 3.1 | ||||||
|
| ||||||||
| Rat | Single iv | 1000 | 0.3 | 1.48 | Maucher Fuquay et al., 2012 | |||
Abbreviations: CL – total body clearance; CL/F – total body clearance after oral administration; CLr – renal clearance; fe – fraction excreted unchanged in urine; ip – intraperitoneal; iv – intravenous; MRT – mean residence time; Vss - volume of distribution at steady-state
Species differences have been observed in the pathways of elimination of DA. In monkeys, 30–70% of the iv dosed DA was excreted through the urine, suggesting extrarenal elimination (Jing et al., 2018; Truelove & Iverson, 1994). The remaining fraction of the dose was attributed to be eliminated through biliary excretion, as DA was detected in primate feces following an iv dose (Jing et al., 2018). The renal clearance of DA in monkeys was reported to be about 60% of the creatinine clearance (Jing et al., 2018), indicating tubular reabsorption of DA. In contrast, in rats, DA was predominantly (~100%) eliminated through urine following an iv dose (Suzuki & Hierlihy, 1993; Truelove & Iverson, 1994). Moreover, the renal clearance of DA has been reported to be similar to inulin clearance in rats, suggesting minimal tubular reabsorption of DA (Suzuki & Hierlihy, 1993).
Although the onset and duration of the toxicological effects have been shown to be significantly different following iv and oral dose (Tryphonas, Truelove, & Iverson, 1990; Tryphonas, Truelove, Todd, Nera, & Iverson, 1990), the oral TK of DA has not been reported until recently in cynomolgus monkeys (Jing et al., 2018; Shum et al., 2020). In this species, DA was absorbed slowly in the gut, limiting its oral bioavailability to less than 10% (Jing et al., 2018; Truelove et al., 1997). This observation is consistent with previous observation in rats that ~100% of orally dosed DA was recovered in feces (Iverson et al., 1989). This slow absorption significantly increased the apparent half-life of DA (10 h) in monkeys, indicating that DA follows flip-flop kinetics (when the rate of absorption is greater than the rate of elimination) after an oral dose (Jing et al., 2018; Shum et al., 2020). In cynomolgus monkeys, the slow absorption following an oral dose may also explain the slow onset and longer duration of toxicological effects following an oral dose compared to iv dose, which was supported by the predicted brain concentration-time profile using a physiologically-based pharmacokinetic (PBPK) model of DA. Furthermore, a more-than-dose-proportional increase in AUC has been observed in cynomolgus monkeys following oral doses of DA suggesting potential saturation kinetics in either the absorption processes, elimination processes, or both (Shum et al., 2020). This observation suggests that drug transporters may play an important role in the disposition of DA and may contribute to species differences in the TK of DA.
Another major concern of DA toxicity is its toxicological effect on the developing fetal brain, as DA has been shown to distribute to the fetus following maternal exposure in CSLs (Brodie et al., 2006; Lefebvre et al., 2018), monkeys (Shum et al., 2020), and rodents (Maucher Fuquay, Muha, Wang, & Ramsdell, 2012). Maternal-fetal disposition of DA has been reported in monkeys following repeated oral doses and in rats following a single iv dose. The TK of DA is not significantly altered during pregnancy suggesting that the DA exposure in pregnant animals is similar to that of the nonpregnant animals (Maucher Fuquay et al., 2012; Shum et al., 2020). The fetal/maternal AUC ratio was reported to be less than one (F/M ratio: 0.3) in both monkeys and rats, indicating that placental efflux transport is limiting fetal exposure. On the other hand, DA has been shown to accumulate in the amniotic fluid, acting as a distribution compartment for the fetus (DA recirculates to the fetus through fetal swallowing of amniotic fluid) (Lefebvre et al., 2018; Maucher Fuquay et al., 2012; Maucher & Ramsdell, 2007; Shum et al., 2020). The distribution kinetics between amniotic fluid and the fetus have been shown to increase the apparent fetal plasma half-life in monkeys and rats (Maucher Fuquay et al., 2012; Shum et al., 2020), which may increase the risk of fetal toxicity following repeated dosing to the mom.
Neonatal exposure to DA through breast milk has been estimated following iv exposure in lactating rats (Maucher & Ramsdell, 2005) and oral exposure in lactating CSLs (Rust, Gulland, Frame, & Lefebvre, 2014). Unequivocally, both studies demonstrated that DA was detected in breast milk, even when DA could no longer be detected in plasma and urine, indicating a long retention time of DA in breast milk. Despite this, DA concentrations in breast milk were quite low, thus minimizing the risk of neonatal exposure through breast milk. With a maternal exposure of 1 mg/kg DA iv, the neonatal rat was exposed to an estimated 60 ng DA/kg through breast milk, or 0.006% of the maternal dose (Maucher & Ramsdell, 2005). Although the relative infant dose is not known following an oral dose, it is likely lower than 0.006%, based on the low oral bioavailability of DA. Therefore, DA exposure through breast milk poses a minor risk to neonates.
These TK concepts, with recent improvements in the sensitivity of bioanalytical methods (Shum et al., 2018), may be useful for the development of a biomarker of exposure for DA. In humans, DA has been detected in urine from those who consumed razor clams containing low levels of DA up to 9 days before urine collection (Lefebvre et al., 2019). These results suggest that urine may be applicable to estimate recent exposure from consuming contaminated seafood. In the same study, a DA-specific antibody was also detected in the serum from subjects who regularly consume razor clams known to contain low levels of DA year-round, suggesting that the antibody may be a viable biomarker for chronic DA exposure. These new methods to estimate recent and chronic DA exposure will facilitate the understanding of the dose-response relationship of DA in humans.
Summary and Future Directions
DA is not widely distributed in the body and is mainly eliminated unchanged in the urine. Most relevant for humans, the TK of DA following oral dose in cynomolgus monkeys follow flip-flop kinetics, which are a result of slow intestinal absorption. Other kinetic data suggest that drug transporters may play an important role in the TK of DA and may contribute to species difference of DA disposition. The maternal-fetal kinetics suggests that the placenta acts as a partial barrier, thus limiting fetal exposure to DA, but DA can accumulate in amniotic fluid, which prolongs the exposure to the fetus. The neonatal exposure to DA through breast milk is expected to be minimal based on the low estimated relative infant dose.
Future mechanistic studies are warranted to further elucidate the role of drug transporters in the TK and maternal-fetal kinetics of DA. New advances have pioneered two potential biomarkers (e.g. urine DA levels, DA-specific antibody) to aid in understanding the human dose-response relationship, but additional data are necessary to confirm these results in broader human populations. Additionally, breast milk concentration in lactating women chronically exposed to DA should be measured to confirm that this route poses a low risk of neonatal exposure in humans. Building off these data, standardized biomarkers, could facilitate the diagnosis of health effects associated with chronic, low-level DA exposure.
5. Neurological Effects of Domoic Acid in Adults
Uncovering Adult Neurological Responses to Domoic Acid
Since the 1987 human DA poisoning, ample laboratory research has been conducted to identify the effects of DA in the adult nervous system. Early traditional toxicology experiments were aimed at disseminating information on the acute toxicity of this poison and revealed several key findings related to overt toxicity. Over the past 30 years, research has moved away from acute toxicity testing and towards assessing DA effects following chronic, low-level exposures, such as those observed in coastal populations. Results of studies reporting neurotoxic effects of DA in adult subjects are summarized below and in Table 2, including parts A, B, C, and D. Summaries of human epidemiological studies, as well as experimental studies using nonhuman primates, rodents, and fish laboratory models are presented in the following sections: overt neurotoxicity; functional effects on cognition, emotionality, motor responses, and neuroimaging; neuropathology; and neurochemical and molecular responses.
Table 2A:
Adult Clinical and Preclinical Neurotoxic Effects of Domoic Acid
| Route of Exposure | Dose | Duration of Exposure | Biomarker of Exposure | Subject Characteristics | Overt Neurotoxic Effects | Time Onset^ | Functional Neurotoxic Effects | Time Neurotoxic Effects Examined^ | Neuropathological Effects | Time Pathology Examined^ | Citation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Humans | |||||||||||
| dietary | up to 290 mg | 1x | Blood: none detected CSF: none detected | Prince Edward Island adults | Vomiting, diarrhea, headache, memory loss, seizures, coma, death | <38 h | ↓ memory performance | 4 mth-1 yr | Hippocampus, amygdala, septal area, olfactory area, frontal cortex: ND and astrocyte reaction | 7–98 d | Hynie & Todd, 1990; Perl, Bedard, Kosatsky, Hockin, & Todd, 1990 |
| dietary | >15 razor clams/ mth | 1+ yr | CoASTAL cohort | None | ↓ cognitive and memory performance | 1+ yr | Grattan et al., 2016 | ||||
| dietary | Not reported | Either 1 wk or 1 yr | CoASTAL cohort | None | Both 1 wk and 1 yr: ↑ problems in everyday memory | 10 d post-start of target week | Grattan et al., 2018 | ||||
| dietary | ~324 ng/kg/d | 1+ mth | CoASTAL cohort | None | ↓ memory performance on verbal recall tests | At least 6 mth after start of exposure | Stuchal et al., 2020 | ||||
| Monkeys | |||||||||||
| oral | 5.2–10 mg/kg | 1x | Females | Salivation, gagging, vomiting, diarrhea | 7–96 min | Hippocampus: ND | 4–44 d | Tryphonas, Truelove, Todd, et al., 1990 | |||
| oral | 0.5 mg/kg for 15 d, then 0.75 mg/kg for 15 d | Daily, for 30 d | Serum:10–60 ng/ml; Urine: 1–11% of dose | Females | None | Whole brain: no changes in temporal cortex, amygdala, hypothalamus, hippocampus, thalamus, cerebellum | 30 d | Truelove et al., 1997 | |||
| oral | 0.075 and 0.15 mg/kg | Daily, for 8–10 mth | Plasma: 0.93 ng/ml and 2.93 ng/ml | Females | Upper limb tremors | 1 mth+ | Burbacher et al., 2019 | ||||
| oral | 0.075 and 0.15 mg/kg | Daily, for 1–2 yr | Plasma: 0.93 ng/ml and 2.93 ng/ml | Females | Upper limb tremors | 1 mth+ | MRI: ↓ white matter integrity in fornix and internal capsule; MRS: ↑ lactate in thalamus | 1+ yr | Petroff et al., 2019 | ||
| oral | 0.075 and 0.15 mg/kg | Daily, for 1–2 yr | Plasma: 0.93 ng/ml and 2.93 ng/ml | Females | Upper limb tremors | 1 mth+ | EEG power differences; ↓ delta, ↑ alpha, theta, beta | 1+ yr | Petroff et al., 2020 | ||
| iv and ip | 0.025–0.5 mg/kg iv; 4 mg/kg ip | 1x | Salivation, gagging, vomiting, tremors, death | 3–4 min | Hippocampus and hypothalamus: ND and astrocyte reaction at >0.2 mg/kg | 3.5–5 h | Tryphonas, Truelove, & Iverson, 1990 | ||||
| iv | 0.25–4.0 mg/kg | 1x | Adult and Juvenile Males and Females | Gagging, vomiting, tremors, 4 deaths of adults >1 mg/kg | 8–75 min | Hippocampus: ND and astrocyte reaction; CA1: ↑ c-Fos at >0.5 mg/kg | 2–7 h (for morbidund), otherwise 1 wk | Scallet et al., 1993 | |||
| iv | 0.25–4.0 mg/kg | 1x | Adult and Juvenile Males and Females | Gagging, vomiting, tremors, 4 deaths of adults >1 mg/kg | 8–75 min | Hippocampus, entorhinal and piriform cortices, subiculum, lateral septum, and thalamus: ND at >0.5 mg/kg | 2–7 h (for morbidund), otherwise 1 wk | Schmued et al., 1995 | |||
| Rats | |||||||||||
| oral | 60–80 mg/kg | 1x | Head on floor, inactivity at 60 mg/kg; rolling, seizures, and death at 80 mg/kg | 5 min-5 h | Hippocampus and olfactory bulb: mild ND and astrocyte reaction at 80 mg/kg | 1–54 h | Tryphonas, Truelove, Todd, et al., 1990 | ||||
| oral | 0.1 and 5 mg/kg | Daily, for 64 d | Serum: Below the limit of detection | Males and Females | None | No changes in urine or blood chemistry | Urinalysis at 55 d, blood chemistry at 64 d | Whole brain: no changes in electron microscopic histopathology or GFAP | 64 d | Truelove et al., 1996 | |
| ip and oral | 1–9.5 mg/kg ip; 60–82 mg/kg oral | 1x | Urine: not detected; Feces: 98% of dose | Scratching, seizures, and death at >4 mg/kg ip; chewing at 70 mg/kg oral, death at >80 mg/kg oral | <4 h | Hippocampus and retina: ND at 4–7 mg/kg ip and 80 mg/kg oral | After death (within 4 h) | Iverson et al., 1989 | |||
| ip | 1–10 mg/kg | 1x | Males | Wet-dog shakes and seizures in all dose groups, death at >5 mg/kg | 2 h | Electrographic spikes and generalized epileptic status | 3–24 h, depending on morbidity | CA3: ND at all doses; Other hippocampal areas: damage at >5 mg/kg | 3–24 h, depending on morbidity | Fujita et al., 1996 | |
| ip | 2.25 mg/kg | 1x | Males | Hindlimb scratching, convulsions, and death | 45 min-3 h | CA1–4, pyramidal tracts, thalamus, amygdala, and olfactory bulb: ND, astrocyte reaction, and potential microglia response | 7 d | Appel et al., 1997 | |||
| ip | 2.5 mg/kg | 1x | Immunohistochemical DA detected in hippocampal neurons at 6–10 h post exposure | Females | Hyperactivity, scratching, tremors, head jerks | <6 h | No changes at 6–24 h; CA1/3: ND, astrocytosis, microglia reactivity at 5 d; Hippocampus and thalamus: ND, astrocyte reaction, microglia reactivity, and ↑ NOS at 54 d | 6 h, 10 h, 24 h, 5 d, and 54 d | Vieira et al., 2015 | ||
| ip | 2.2 and 4.4 mg/kg | 1x | Males | Hindlimb scratching, wet-dog shakes, salivation, and seizures at both doses | <150 min | Electrographic seizures and ↑ delta, theta, alpha, and beta power at both doses | Daily, over 7 d | Hippocampus, DG, and olfactory bulb: ↑ c-Fos+ neurons at both doses | 2 h | Binienda et al., 2011; Scallet et al., 2004 | |
| ip | 1 and 1.8 mg/kg | 1x | Males and Females | Signs of severe toxicity in 2/7 males | <3 h | ↓ activity, then ↑ locomotion, grooming, and stereotypic behaviors | Over 3 h | Hippocampus and olfactory bulb: no changes in GFAP+ astrocytes or pCREB | 3 d | Baron et al., 2013 | |
Overt Neurotoxicity
Similar to the human syndrome ASP, laboratory mammals exposed to acute, high doses of DA exhibit a common pattern of symptoms. Macaques administered >0.25 mg/kg iv (Scallet et al., 1993; Schmued, Scallet, & Slikker, 1995; Tryphonas, Truelove, & Iverson, 1990), 4 mg/kg ip (Tryphonas, Truelove, & Iverson, 1990), or oral doses of >5 mg/kg (Tryphonas, Truelove, Todd, et al., 1990), exhibited an explicit progression of toxicity, beginning with increased vocal expression (chirping), quickly moving to gastrointestinal distress (excessive salivation, gagging, vomiting), unusual motor activity (so called “wet-dog shakes”), postural positioning somewhat indicative of praying, and ending with tremors, seizures, and death.
In rodent models, this pattern of symptomology is repeated, with some slight discrepancies. Acute toxicity in both rats and mice is dose-dependent and has been well documented in the literature. The median ip toxic dose in mice is approximately 3–4 mg/kg and in rats is near 1 mg/kg (Fujita et al., 1996; Iverson et al., 1989; Sobotka et al., 1996; Tasker, Connell, & Strain, 1991). After DA administration, rodents demonstrate a short period of hypoactivity, which is quickly followed by a deeper sedative state. Advancing symptoms have been described as a sudden increase in activity, with signs of stereotypic behaviors, a loss of postural control and tremors and convulsions. Reported stereotypic behaviors include head-bobbing or weaving, circling, and hindlimb scratching near the ear. The appearance of the hindlimb scratching is so distinctive of this toxin, that it has been used as the primary assay for acute DA toxicity (Iverson & Truelove, 1994). Vomiting, one of the primary symptoms associated with DA in primates, is noticeably lacking in the progression, as rodents cannot vomit. Rats are more sensitive than mice to overt signs of toxicity, which may be due to differences in physiological parameters or pharmacological response (Iverson et al., 1989).
There is some evidence that DA effects vary depending on the sex and age of the subject, with male (Baron et al., 2013; Wetmore & Nance, 1991) and older (Hesp, Clarkson, Sawant, & Kerr, 2007) mice and rats responding more severely to the toxin. This apparent sensitivity in older males was also noted in the 1987 human poisoning (Perl, Bedard, Kosatsky, Hockin, & Todd, 1990). At the time, researchers postulated that sex-based differences in seafood consumption and age-related changes in kidney function may have contributed to variations in toxic responses.
Summary.
Following acute DA exposure, laboratory models exhibit progressive symptoms similar to those of ASP in humans, with effects that include activity level changes, gastrointestinal distress, stereotypic behaviors, seizures, and death. The potential for both sex-specific and age-related susceptibilities to DA exposure effects is notable and under active, ongoing investigation (Personal Communication, Dr. David Marcinek).
Functional Effects
Studies of adult humans, as well as nonhuman primates and rodents have also investigated the association between low-level DA exposure and more subtle neurological effects, such as changes in cognition, emotionality, or motor responses.
Effects on Cognition.
Cognitive effects have been the focus of both clinical and preclinical research, as memory loss was the hallmark symptom of acute DA poisoning in human episodes of ASP (Perl, Bedard, Kosatsky, Hockin, Todd, et al., 1990). The only human cohort study dedicated to understanding the health effects of DA is the Communities Advancing the Studies of Tribal Nations Across the Lifespan (CoASTAL) cohort. The CoASTAL cohort is comprised of volunteer Native American adults who live on the coast of Washington (WA) State and regularly consume shellfish that contain DA (Tracy, Boushey, Roberts, Morris, & Grattan, 2016). In this group, 97% of adults frequently consume fish or shellfish, and many of these adults eat more than one meal/month of razor clams (Tracy et al., 2016), a filter feeder known to have persistent DA concentrations up to a year after a toxic marine event ends (Wekell, Gauglitz, Barnett, Hatfield, & Eklund, 1994). Preliminary studies using the verbal cognitive CVLT-II Standard test in 513 adults suggested a subtle decrease in cognitive performance in those who consumed more than 15 clams/month (Grattan et al., 2016). A follow-up study of a subset of CoASTAL adults used additional surveys to assess everyday memory, a measure of the frequency of memory “failures” in day-to-day life (Grattan et al., 2018). Adults who consumed above the group median amount of razor clams in the past week, but not the past year, were nearly 4× more likely to report problems with everyday memory. While the median level of consumption was not reported, DA levels in clams were between 4–14 ppm. Most recently, results from a study in over 100 CoASTAL adults suggests that low-level DA exposure (~324 ng DA/kg/day over one month) was linked to decreased verbal memory recall, but not to measures of intelligence (Stuchal et al., 2020). The authors postulated that this memory deficit was an attenuated form of ASP in adults.
In rodents, learning and memory effects have been described after sub-lethal doses of DA. Rats given 0.04 mg/kg DA iv performed poorly on a radial maze, with a longer time to achieve success (Nakajima & Potvin, 1992), while mice administered a single of dose of 2 mg/kg ip had prolonged latencies and difficulties in finding the platform on the Morris Water Maze (MWM) test (Petrie, Pinsky, Standish, Bose, & Glavin, 1992). In a series of research studies designed to assess potential compounds that ameliorate the decrements of DA, mice given 2 mg/kg/day ip for 21–28 d also demonstrated decreased spatial memory, as noted by decreased object recognition as well as increased latencies both in the ability to find the platform on the MWM and in the step-through passive avoidance task (Lu et al., 2013; D. Wang, Zhao, Li, Shen, & Hu, 2018; Wu et al., 2013, 2012). A single dose of 1.32 mg/kg ip in rats did not, however, result in changes in passive or active avoidance tasks in other research (Sobotka et al., 1996). Working memory on a match-to-sample task was decreased in animals given single doses of 1 and 2 mg/kg ip (Clayton, Peng, Means, & Ramsdell, 1999). In the same study, memory effects were replicated with repeated exposure to doses of 1 mg/kg ip, but not doses of 2 mg/kg ip, delivered every other day for 7 d (total of 4 doses). This suggests that there may be a potential resistance to the effects of multiple, higher doses. In all of these rodent studies, however, animals displayed some signs of overt toxicity (e.g. changes in locomotion, stereotypic behaviors, hindlimb scratching) in addition to learning and memory deficits.
Only one laboratory study has been designed to assess learning and memory at doses below those that produce overt toxicities. Lefebvre and colleagues conducted a long-term study using low-level exposure (~0.75 mg/kg ip), where mice were exposed to DA once a week for up to 25 weeks (Lefebvre et al., 2017). After 25 weeks, animals had fewer successful trials on the radial water tread maze, but this deficit in learning and memory was reversed after a 9-week wash-out period. Authors additionally noted that recovered mice maintained their ability to navigate the maze throughout old age, suggesting that a chronic, low-level exposure in mice may produce subtle changes in memory that are recoverable after cessation of exposure.
In studies with adult humans and animal models, DA-related effects on cognition are evident. Importantly, deficits in learning and memory occurred in both humans and animals in absence of signs of overt toxicity.
Effects on Emotionality.
DA effects on emotionality have been studied in a small set of research projects using adult animal models, mostly using observations of behavior in an open field. Rats exposed to 1.8 mg/kg ip demonstrated more grooming behaviors and other stereotypic actions in an open field test, in absence of overt DA toxicity (Baron et al., 2013). Authors suggested that this was indicative of heighted emotionality or distress. Similar results of longer habituation times and increases in grooming behaviors in open field test were reported in another study with rats exposed to 1 mg/kg ip DA (Schwarz et al., 2014). In a separate rodent study designed to create a model for epilepsy, doses of 1 mg/kg ip given at least 2× (once per h, for up to 5 h) produced increases in defensive aggression in epileptic rats (Tiedeken & Ramsdell, 2013). While emotional effects of DA in adult models are inadequately documented, the findings presented here encourage additional investigation.
Effects on Motor Responses.
Laboratory studies using adult animal models exposed to DA have also investigated effects on the motor system and associated reflexes. A recent seminal study used macaque monkeys exposed to daily, oral doses of 0.075 and 0.15 mg/kg/day for up to 11 months to study the maternal reproductive and offspring neurodevelopmental effects of DA (Burbacher et al., 2019). Findings from this cohort documented an increased incidence in subtle upper limb tremors in adult females, when performing a reaching task. In rats given daily doses of 0.2–1.6 mg/kg ip for 30 days, motor coordination was also decreased after just 10 days of exposure (Xu et al., 2008). Another study documented an exaggerated auditory startle response in rats exposed to 1.32 mg/kg ip, which was paired with signs of overt locomotive toxicity (Sobotka et al., 1996). In these studies, however, few researchers have sought to clarify whether changes in motor measures are driven by damage directly to the motor neurons or other neurotoxic effects. This point may be more salient when considering that DA has been shown to directly damage the spinal cord in rodents (Xu et al., 2008). At present, there is evidence that lower levels of DA can cause motor effects, but these effects are subtle, and the origin of motor changes is unknown.
Effects from Neuroimaging Studies.
Seizures and electrophysiological changes are known to occur after DA exposure in humans and animals (Cendes et al., 1995; Perl, Bedard, Kosatsky, Hockin, & Todd, 1990; Tasker et al., 1991), but only a few studies have employed neuroimaging to investigate these changes. Adult monkeys in the aforementioned reproductive study, who were orally exposed to 0.075 and 0.15 mg/kg/day for 1–2 years, underwent structural MRI, magnetic resonance spectroscopy (MRS) and EEG assessments (Petroff et al., 2020, 2019). Structural MRI scans in a subset of these macaques suggested that DA-related tremors observed during a reaching task were connected to decreased white matter integrity in key white matter motor tracts and increased lactate in the thalamus (Petroff et al., 2019). DA-exposed animals, on average, also had decreased delta power and increased theta, alpha, and beta power on resting, sedated EEG exams (Petroff et al., 2020).
EEG imaging has been used to examine the effects of DA exposure in rats. Doses of 1–10 mg/kg ip (Binienda, Beaudoin, Thorn, & Ali, 2011; Fujita et al., 1996; Sawant, Tyndall, et al., 2010; Scallet, Kowalke, Rountree, Thorn, & Binienda, 2004) and intrahippocampal exposure to 10–300 pmol of DA (Dakshinamurti, Sharma, & Sundaram, 1991; Sawant, Mountfort, & Kerr, 2010) led to extensive activation in the hippocampus, increased electrographic spiking and seizures, and increased delta, theta, alpha, and beta power. Changes like these are indicative of subtle neuroelectric variations that have been linked to deficits in learning and memory and the diagnosis of neurodegenerative disorders (Harmony, 2013; Newson & Thiagarajan, 2019). Further analyses of these similarities may reveal more about the underlying functional and cellular effects of the DA-induced neuroimaging changes reported here.
Summary.
Due to its prominent role in ASP, memory has been the focus of the majority of DA research. Both symptomatic and asymptomatic DA doses are known to cause adverse learning and memory outcomes, which were reversible in asymptomatic rodents. Effects on other functional domains have not been studied well, but results from a few recent studies suggest that anxiety-related behaviors and motor function are impacted after low-level, asymptomatic exposure. Subtle electrophysiological, neurochemical, or structural changes in the brain may underlie these functional changes.
Neuropathological Effects
Effects on Neurons.
After acute, high-dose DA exposures, neuronal degeneration and gross lesions have been documented in several mammalian brain regions. DA most notably causes damage in the hippocampus, the memory center of the brain. In monkeys given single doses of DA >0.2 mg/kg iv (Tryphonas, Truelove, & Iverson, 1990) or >6 mg/kg oral (Tryphonas, Truelove, Todd, et al., 1990), large neuropathic lesions are evident in the hippocampus (including CA1, 3 and 4), hypothalamus, and medulla, but not other regions in the brain. Lower amounts of neuronal degeneration have also been documented in the hippocampus, subiculum, thalamus, and lateral septum, as well as the entorhinal and piriform cortices after doses >0.5 mg/kg iv (Schmued et al., 1995). In rats given >2 mg/kg ip (Appel, Rapoport, O’Callaghan, Bell, & Freed, 1997) or mice given > 4 mg/kg ip (Peng, Taylor, Finch, Switzer, & Ramsdell, 1994; J. C. Ryan, Cross, & Van Dolah, 2011; Strain & Tasker, 1991), similar persistent lesions and neuronal damage occur in the hippocampus, hypothalamus, thalamus, amygdala, olfactory and piriform cortices, and septal area. A comprehensive brain survey of DA damage in rodents largely confirmed these results, while also suggesting that individual regions in target areas, such as the dentate gyrus of the hippocampus, are largely unaffected by acute DA exposures at 4 mg/kg ip (Colman, Nowocin, Switzer, Trusk, & Ramsdell, 2005).
Results from studies that examine pathology at multiple time points after the initial exposure suggest that the complete picture of neuronal degeneration in the brain may only be visible sometime after sub-lethal doses (2–7 mg/kg ip and 0.75 mg/kg iv), with rats not expressing any neuronal damage until at least 2 days after the initial exposure (Ananth, Thameem Dheen, Gopalakrishnakone, & Kaur, 2001; Bruni, Bose, Pinsky, & Glavin, 1991; Vieira et al., 2015). Thus, histopathology conducted less than 24 h after sub-lethal, but symptomatic, DA exposure may not be the most useful way of assessing neuropathological changes. Histopathology after asymptomatic exposures in rodents has not revealed any gross neuronal effects (Lefebvre et al., 2017; Moyer et al., 2018).
Effects on Axons.
Limited evidence suggests that axonal damage is typically less extensive than damage to the neuronal body. In adult monkeys, axon terminal degeneration was reported after exposure to 1 and 1.25 mg/kg DA iv (Scallet et al., 1993; Schmued et al., 1995). Authors suggested that the injury may have been caused by the death of the cell body and not by damage directly to the axon. In rats, a single exposure of 2.25 mg/kg led to axonal damage in the hippocampus (Appel et al., 1997), whereas repeated exposure to 1 mg/kg ip was connected with axonal injury in both the olfactory bulb and thalamus (Tiedeken, Muha, & Ramsdell, 2013). Results from another study with mice given 4 mg/kg ip indicated axonal damage in the same regions, as well as in the septal area, but not the amygdala (Colman et al., 2005). However, other studies have not reported axonal damage after similar exposures in rodents (Clayton et al., 1999; Peng et al., 1994). Further, lower exposures (2 mg/kg ip) do not appear to impact axons or the associated myelination (Scallet, Schmued, & Johannessen, 2005).
Effects on Glia.
Important glial responses to DA have been documented in early studies using animal models. Most commonly, in acute, high-dose DA exposure, a marked astrocytic reaction, detected typically with glial fibrillary acidic protein (GFAP) immunohistochemistry, has been observed in symptomatic monkeys (>0.2–0.5 mg/kg iv) (Scallet et al., 1993; Tryphonas, Truelove, & Iverson, 1990) and rodents (rats: >1 mg/kg ip (Sobotka et al., 1996; Vieira et al., 2015); mice: >2 mg/kg ip (Lu et al., 2013)). In zebrafish, however, asymptomatic exposure to DA for up to 6 weeks did not alter whole brain GFAP expression (Hiolski et al., 2014), suggesting that either GFAP-positive cell responses are highly regional, species-dependent, or do not change after asymptomatic toxic exposures. Several studies in rodents have also documented a potential microglial reaction and suggest that microglial pathology may only be observable at least 2–7 days after initial DA exposure (Ananth, Gopalakrishnakone, & Kaur, 2003a, 2003b; Ananth et al., 2001; Appel et al., 1997; Vieira et al., 2015). This finding is contrary to early studies, which did not observe microglia differences, but examined histopathology immediately after overt behavioral signs of toxicity.
Summary.
High-exposure DA toxicity leads to neuronal degeneration and the formation of lesions, most recognizably in the hippocampus. Additional brain areas, such as the amygdala, thalamus, and olfactory areas, may be of concern in particular species and exposure scenarios. Axons and myelin do not appear to be impacted in either acute or sub-acute exposure scenarios, but limited findings from studies with glia suggest that there may be astrocyte responses after acute exposures and microglia responses after either acute or low-level exposures, but the timing of histopathological examination is an important factor in these findings.
Neurochemical and Molecular Responses
A combination of in vivo and in vitro studies has demonstrated that DA binds to α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and KA-type glutamate receptors (Berman & Murray, 1997; Hampson, Huang, Wells, Walter, & Wright, 1992; Hampson & Manalo, 1998; Qiu, Pak, & Currás-Collazo, 2006; Stewart, Zorumski, Price, & Olney, 1990; Watanabe et al., 2011), triggering a series of events typical of glutamate-derived excitotoxicity (Fig. 2) (Y. Wang & Qin, 2010). In acute, high-dose DA exposure scenarios (up to 1000 μM) with in vitro cell culture experiments, activated AMPA and KA receptors allow both an influx of Na+ into the cell and the release of glutamate into the synapse. N-methyl-D-aspartic acid (NMDA) receptors are then indirectly activated via the released glutamate, and Ca+2 ions subsequently flood into the cell. This potent activation causes the depolarization of the post-synaptic cell and leads to excessive production of reactive oxygen species (ROS) via the disruption of normal mitochondria function, ultimately activating necrotic cell death pathways.
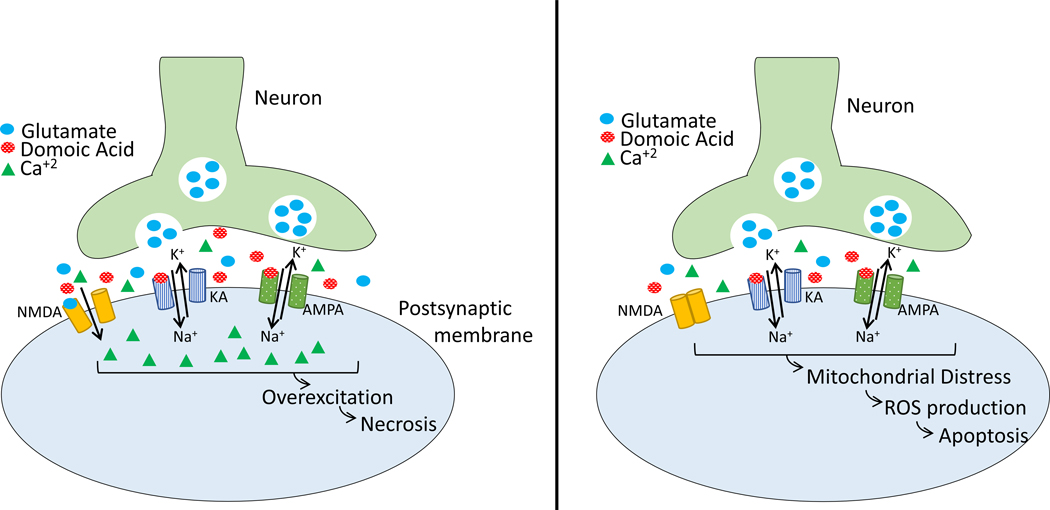
Figure 2:
Reprinted from Petroff, 2020. Proposed mechanism of action for domoic acid (DA). LEFT: Acute exposures to DA involve the activation of KA- and AMPA-type glutamate receptors, resulting in an influx of Na+ into the postsynaptic membrane, and the release of glutamate into the synapse. Glutamate activates NMDA receptors, allowing an influx of Ca+2 and leading to necrotic cell death. RIGHT: Lower-level exposures do not involve the NMDA receptors, and therefore, lead to mitochondrial distress, the production of ROS, and apoptosis. Abbreviations: AMPA – α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; Ca+2 – calcium; DA – domoic acid; K+ – potassium; KA – kainic acid; Na+ – sodium; NMDA – N-methyl-D-aspartic acid; ROS – reactive oxygen species
Consensus on the acute mechanism of toxicity is well established, but the mechanism of action after lower-level DA exposures is still under active investigation (Costa, Giordano, & Faustman, 2010; Lefebvre & Robertson, 2010; Pulido, 2008). Current in vivo rodent evidence suggests that sub-lethal and symptomatic or repeat exposures at 0.3–2 mg/kg ip or 0.75 mg/kg iv may not lead to necrotic cell death, but instead produce smaller increases in ROS (Tsunekawa et al., 2013) and related nitric oxide synthase (NOS) products (Ananth et al., 2003a, 2001; Lu et al., 2013; Vieira et al., 2015), which can disrupt normal mitochondrial function (Wu et al., 2013; Xu et al., 2008).
Other sub-cellular effects have increasingly become a focus of investigation, with some studies probing the effects of DA on specific target genes and related products, while additional studies have assessed changes in large-scale gene expression profiles (Hiolski et al., 2014; Lefebvre et al., 2009; J. C. Ryan, Morey, Ramsdell, & Van Dolah, 2005). Fos genes and related proteins, a key signal in cell proliferation and apoptotic cell death pathways, were upregulated in the hippocampi of monkeys, mice, and rats, and the brains of fish after both asymptomatic and symptomatic exposure (Lefebvre et al., 2009; Peng & Ramsdell, 1996; Peng et al., 1994; J. C. Ryan et al., 2005; Salierno et al., 2006; Scallet et al., 1993, 2004). To act as a regulatory protein, fos dimerizes with jun proteins, and Jun-family gene expressions have been similarly upregulated after DA exposures in rats and zebrafish (Lefebvre et al., 2009; J. C. Ryan et al., 2005; Scallet et al., 2005). Studies have also suggested in vivo alterations in important cell signaling and mitochondrial genes and gene products including those in the FOX family (Lefebvre et al., 2009; J. C. Ryan et al., 2005; Wu et al., 2013), MAP-2 (Vieira et al., 2015), MAPK (Lefebvre et al., 2009; J. C. Ryan et al., 2005; Tsunekawa et al., 2013), and Bax/Bcl-2 (Ananth et al., 2001; Hiolski et al., 2014). Gene expression differences in important neuronal health genes like APOE, APP, NRXN, GABARAP, and NPTX have also been described in whole-brain zebrafish studies (Hiolski et al., 2014; Lefebvre et al., 2009). Notably, gene expression differences are highly dependent on the dose (Lefebvre et al., 2009), exposure duration (Hiolski et al., 2014), and the time between the end of exposure and gene analysis in both mice and fish (J. C. Ryan et al., 2005). These divergent responses are particularly striking when comparing expression differences in symptomatic and asymptomatic animals.
Summary.
The mechanism of acute DA toxicity is well established, involving the activation of AMPA and KA-type glutamate receptors, subsequent activation of NMDA receptors, and necrosis processes. Mechanisms of action at lower levels of DA exposure are still under investigation. Future research on DA adult neurotoxicity should work to understand the potential cell death compensatory mechanisms or other means of cell protection that may lead to differences in response, which may include neurogenesis (Pérez-Gómez & Tasker, 2012, 2013), synaptic protein expression changes (Moyer et al., 2018), alterations in the balance of glutamatergic and GABA(γ-aminobutyric acid)ergic neuron functioning (Dakshinamurti et al., 1991; Hiolski et al., 2016; Moyer et al., 2018), and the upregulation of neuroprotection pathways (Giordano, Kavanagh, Faustman, White, & Costa, 2013).
Future Directions
Adult neurotoxicity has been thoroughly described after acute exposure scenarios, but there is only a small body of research on the effects of DA in absence of overt toxicity. Going forward, studies focused on the functional effects of DA should be a priority area of research, especially when considering the new evidence detailing human health consequences from chronic, low-level exposure to DA in the CoASTAL cohort study. Additional avenues of research in the potential sex- and age-variability of responses as well as in molecular and neuroprotective mechanistic pathways should also be pursued. Future studies should include quantitative biomarkers of DA exposure (e.g. blood, urine) to better translate results to public-health risk assessment and policy.
6. Neurodevelopmental Effects of Domoic Acid Exposure
Exploring the Consequences of Prenatal and Neonatal Domoic Acid Exposure
It is a well-established tenet of neurotoxicology that age is an important determinant of exposure-driven outcomes. Frequently, the embryo and fetus exhibit heightened sensitivity to the deleterious effects of chemical exposures. Early exposure to toxic agents has the potential to disrupt brain development in ways that may not be immediately expressed, and some effects may not be manifest until adolescence or adulthood (Kraft et al., 2016). The data from animal laboratory studies on DA collectively suggest that the fetus and neonate have an exaggerated vulnerability to the adverse effects of exposure, and early-life central nervous system injuries can be both progressive and persistent (Costa et al., 2010; Grant, Burbacher, Faustman, & Grattan, 2010). There is compelling evidence that DA is a developmental neurotoxin, causing behavioral and pathological effects, at levels of exposure that do not produce toxicity in adults (Doucette & Tasker, 2016). The adverse consequences of early-life DA exposure are not limited to one developmental system and effects have been found on multiple domains of behavior. To facilitate an understanding of how DA affects developing organisms, the data presented herein are organized as follows: overt neurological toxicities; functional effects on physical development and neurological domains of reflexes, sensory processing, cognition, emotionality, activity/motor function, and social behavior; and neuropathology. To date, no reports of DA-exposed children have been published, but there are studies modeling developmental exposure in macaque monkeys, rodents, and zebrafish. The experimental details of these studies are provided in Tables 3 and 4.
Table 3:
Prenatal Exposure and Neurodevelopmental Effects of Domoic Acid
| Route of Exposure | Dose | Duration of Exposure | Maternal Toxicities | Offspring Sex | Offspring Biomarker of Exposure | Period of Study | Overt Signs of Neurotoxicity | Affected Developmental Domains | Unaffected Developmental Domains | Neuropathological Effects | Citation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Monkeys | |||||||||||
| maternal, oral | 0.075 or 0.15 mg/kg/day | 1x/d in gestation (~5–6 mth) | Tremors | Males and Females | Plasma: 0.44 ng/ml and 1.26 ng/ml | Infancy | None | Physical | Burbacher et al., 2019 | ||
| maternal, oral | 0.075 or 0.15 mg/kg/day | 1x/d in gestation (~5–6 mth) | Tremors | Males and Females | Plasma: 0.44 ng/ml and 1.26 ng/ml | Infancy | None | Cognition | Early Reflexes | Grant et al., 2019 | |
| Rats | |||||||||||
| maternal, sc | 0.3, 0.6 or 1.2 mg/kg | 1x on GD13 | None | Males and Females | Weaning- adulthood | None | Activity, Cognition | Physical | E. D. Levin et al., 2005 | ||
| Mice | |||||||||||
| maternal, oral | 1 or 3 mg/kg/d | 1x on GD10–17 | None | Males and Females | Neonatal period- adulthood | None | Physical, Emotionality, Activity | Physical, Early Reflexes, Sensory/Motor Processing, Cognition | Shiotani et al., 2017 | ||
| maternal, ip | 1 mg/kg | 1x on GD11.5, 14.5 and 17.5 | Males | Adulthood | Emotionality, Cognition, Activity | Activity | Whole brain: no pathology changes; Cortex: ↓ MAG; ↑ MAP; CA3: ↑ MAP | Tanemura et al., 2009 | |||
| maternal, sc | 1.5 mg/kg | 1x on GD16 | Males and Females | Behavior: adolescence; MRI: adulthood | Social Behavior | Emotionality | MRI tracts of anterior cingulate cortex and infralimbic and orbital regions: ↑ connectivity; MRI tracts of dorsal retrosplenial and CA3: ↓ connectivity | Mills et al., 2016 | |||
| maternal, sc | 1.5 mg/kg | 1x on GD16 | Males and Females | Adolescence | Sensory/Motor Processing, Social Behavior, Emotionality | None | CA1/3, basolateral amygdala, infralimbic and prelimbic cortices: no changes in parvalbumin+ cells; Lateral amygdala and DG: ↑ in parvalbumin+ cells | Zuloaga et al., 2016 | |||
| maternal, iv | 0.6 or 1.2 mg/kg | 1x on GD13 | Hindlimb scratching, seizures, death | Males (0.06 mg/kg) and Females (1.2 mg/kg) | Adolescence and adulthood | Spontaneous Seizures | Hippocampus: no change in hilar neurons or mossy fiber sprouting | Demars et al., 2018 | |||
| maternal, iv | 0.6 mg/kg | 1x on GD13 | Slight hypoactivity, temporary immobilization | Males and Females | Neonatal period, weaning, and adolescence | None | Electrophysiology (EEG, ↓ seizure threshold) | Physical | Age-related, selective ND: PND1-No obvious cellular damage; PND14- In CA3 and DG: ↑ cell damage; PND30- Hippocampus and cerebral cortex:↓ GABA and ↑ glutamate | Dakshinamurti et al., 1993 | |
| Zebrafish | |||||||||||
| in ovo | 0.12 to 17 mg/kg (DA/egg weight) | Microinjection of fertilized eggs at the 128-to 512 cell stages | N/A | Hatching-5 d postfertilization | Seizures, rapid and continuous fin movement | Early Reflexes | Tiedeken et al., 2005 | ||||
| in ovo | 0.12 to 1.26 ng/mg (DA/egg weight) | Microinjection of fertilized eggs at 1K-cell and high-oblong cell stages | N/A | 7 d | Seizures, rapid and continuous fin movement | Physical, Early Reflexes, Activity, Electrophysiology (EEG, ↓ seizure threshold) | ↑ spinal deformities | Tiedeken & Ramsdell, 2007 | |||
| in ovo | 1, 10, 100, and 1000 ng/L | Cocultured for 72 h | N/A | 72 h postfertilization | Death | ↑ spinal deformities, yolk sac edema | Hong et al., 2015 | ||||
| iv | 0.09–0.18 ng/fish | 1x on a single day within 1–4 d postfertilization | 5–7 d postfertilization | Fin flapping and convulsions at some doses/times | Physical, Early Reflexes | Whole brain: widespread necrosis at highest dose; reduced myelin and altered structure at some timepoints; 10 differentially expressed genes at all timepoints | Panlilio, Aluru, & Hahn, 2020 | ||||
Abbreviations: GABA - γ-aminobutyric acid; GD – gestational day; ip – interparental; iv – intravenous; MRI – magnetic resonance imaging; PND – postnatal day; sc – subcutaneous
Table 4:
Postnatal Exposure and Neurodevelopmental Effects of Domoic Acid*
| Route of Exposure | Dose | Duration of Exposure | Period of Study | Sex | Overt Signs of Neurotoxicity | Affected Developmental Domains | Unaffected Developmental Domains | Neuropathological Effects | Citation |
|---|---|---|---|---|---|---|---|---|---|
| Rats | |||||||||
| ip | 0.05–0.4 mg/kg | 1x on PND2, 5, or 10 | Neonatal | Males and Females | Dose-dependent hyperactivity, scratching, seizures | Hippocampus: no morphological changes; Whole brain: ↑ c-fos mRNA | Xi et al., 1997 | ||
| sc | 0.1–0.50 mg/kg | 1x on PND7 | Neonatal | Males and Females | Paralysis, tremors, seizures, death | Electrophysiology (EEG) | Whole brain: no pathology changes; Spinal cord: ↑ lesions | J. Wang et al., 2000 | |
| sc | 25–100 μg/kg | 2x/d on PND1 and 2 | Neonatal period- adulthood | Males and Females | Death (highest dose) | Activity | Physical, Cognition | E. D. Levin et al., 2006 | |
| sc | 20 or 60 μg/kg | 1x/d on PND8–14 | Neonatal period- adulthood | Males | None | Emotionality | Physical, Activity, Social behavior, Emotionality | Limbic areas: ↑ binding of yohimbine to α2- adrenoceptors in at 20 μg/kg and ↓ binding at 60 μg/kg | Thomsen et al., 2016 |
| sc | 30 μg/kg | 1x/d on PND10–14 | Adolescence and adulthood | Males | None | Activity | Physical | Jandová et al., 2014 | |
| sc | 20 μg/kg | 1x/d on PND8–14 | Adulthood | Males | Electrophysiology (EEG) | D. A. Gill et al., 2009 | |||
| sc | 20 μg/kg | 1x/d on PND8–14 | Adulthood | Males | Electrophysiology (EEG), ↓ threshold to chemically induced seizures | DG and CA3: ↑ MFS | D. A. Gill, Bastlund, et al., 2010 | ||
| sc | 20 μg/kg | 1x/d on PND8–14 | Neonatal period- adulthood | Males and Females | None | Cognition | Physical, Early reflexes | Adams et al., 2009 | |
| sc | 20 μg/kg | 1x/d on PND8–14 | Neonatal period- adulthood | Males and Females | None | Physical, Early reflexes | Hippocampus: ↑ in MFS; ↑ density of trkB receptors; no cell loss | Bernard et al., 2007 | |
| sc | 20 μg/kg | 1x/d on PND8–14 | Neonatal period- adulthood | Males and Females | None | Physical, Activity, Drug-seeking behavior | Physical, Early reflexes | Burt et al., 2008a | |
| sc | 20 μg/kg | 1x/d on PND8–14 | Adolescence | Males and Females | None | Drug-seeking behavior | Activity | Burt et al., 2008b | |
| sc | 20 μg/kg | 1x/d on PND8–14 | Neonatal period-adulthood | Males and Females | None | Cognition, Emotionality, Activity, Behavioral seizures | Physical, Early reflexes, Cognition | Hippocampus and amygdala: Sex-specific alterations α2 adrenoceptors; no changes in corticotropin-releasing factor receptors I/II or D2 receptors | D. A. Gill et al., 2012 |
| sc | 20 μg/kg | 1x/d on PND8–14 | Adulthood | Males and Females | None | Ventral hippocampus: ↓ GAD; Dorsal/mid hippocampus: sex-specific ↓ parvalbumin+ cells; no changes in somatostatin | D. A. Gill, Ramsay, & Tasker, 2010 | ||
| sc | 20 μg/kg | 1x/d on PND8–14 | Neonatal period- adulthood | Males and Females | None | Cognition, Sensory/Motor Processing | Physical, Early reflexes | Marriott et al., 2012 | |
| sc | 20 μg/kg | 1x/d on PND8–14 | Adulthood | Males and Females | Cognition | Marriott et al., 2014 | |||
| sc | 20 μg/kg | 1x/d on PND8–14 | Adulthood | Males and Females | None | Cognition | Physical | Hippocampus and prefrontal cortex: no changes in D1, D2, TH, GAD65 or GAD67 proteins | Marriott et al., 2016 |
| sc | 20 μg/kg | 1x/d on PND8–14 | Neonatal period-adulthood | Males and Females | None | Behavioral seizures | Physical, Early reflexes | Hippocampus and hypothalamus: no change in glucocorticoid and mineralocorticoid receptors | Perry et al., 2009 |
| sc | 20 μg/kg | 1x/d on PND8–14 | Adulthood | Males and Females | None | Social behavior | Physical | C. L. Ryan et al., 2011 | |
| sc | 20 μg/kg | 1x/d on PND8–14 | Neonatal | Males and Females | None | Cognition | Tasker et al., 2005 | ||
| sc | 5 or 20 μg/kg | 1x/d on PND8–14 | Neonatal period-preadolescence | Males and Females | None | Physical, Cognition | Physical, Early reflexes, Emotionality, Activity | Doucette et al., 2003 | |
| sc | 5 or 20 μg/kg | 1x/d on PND8–14 | Adulthood | Males and Females | None | Behavioral seizures | Physical | Hippocampus: ↑ MFS, ↓ cell counts, ↑ brain derived neurotrophic factor (BDNF) | Doucette et al., 2004 |
| sc | 5 or 20 μg/kg | 1x/d on PND8–14 | Adulthood | Males and Females | None | Cognition, Emotionality | Doucette et al., 2007 | ||
No biomarkers of exposure were reported.
Abbreviations: D – dopamine; GABA - γ-aminobutyric acid; GAD – glutamic acid decarboxylase; MFS – mossy fiber sprouting; PND – postnatal day; TH – tyrosine hydroxylase
Overt Neurotoxicity
In preclinical animal models with adults, seizures are a hallmark sign of overt DA neurotoxicity. This neurological outcome has also been studied in animals and fish developmentally exposed to DA. No evidence of spontaneous seizures or epilepsy was observed in a rodent study of EEG recordings that used maternal iv doses of 0.6 mg/kg or 1.2 mg/kg DA on gestational day (GD) 13 (Demars, Clark, Wyeth, Abrams, & Buckmaster, 2018). In contrast, a separate study found that a single maternal dose of 0.6 mg/kg DA iv on GD 13 resulted in abnormal basal EEGs (Dakshinamurti, Sharma, Sundaram, & Watanabe, 1993). When challenged with a postnatal dose of DA, animals with a history of prenatal DA exposure exhibited a reduced threshold for seizures. A decreased threshold to chemically-induced seizures has also been observed in zebrafish embryonically exposed to DA (Tiedeken & Ramsdell, 2007). In a recent publication that examined developmental DA exposure over a range of doses in zebrafish, treated larvae displayed pectoral fin flipping and convulsions that were dose and time dependent (Panlilio, Aluru, & Hahn, 2020).
Neonatal exposure to ip doses ranging from 0.05 to 0.4 mg/kg DA on postnatal day (PND) 2, 5 or 10 resulted in hyperactivity, stereotypic scratching, paralysis and tonic/clinic seizures, suggesting a heighten sensitivity of young rat pups to the toxic, even lethal, effects of DA when compared to adult animals (Xi, Peng, & Ramsdell, 1997). An interesting phenomenon referred to as “behavioral seizures” has been replicated in a number of postnatal DA studies with rodent models (Doucette et al., 2004; D. A. Gill, Perry, McGuire, Pérez-Gómez, & Tasker, 2012; Perry, Ryan, & Tasker, 2009). Animals treated with subcutaneous (sc) doses of 5 or 20 μg/kg DA on PND 8–14 displayed low-grade seizure behavior that was not spontaneous, but rather, triggered by the presentation of challenging cognitive tasks. The authors suggest that in rats, neonatal DA exposure may increase susceptibility to stress, which is behaviorally manifested as repetitive squinting, mastication, and head bobbing.
Summary.
In mammals, prenatal DA exposure has been linked to abnormalities in electrophysiology and a reduced threshold for chemically induced seizures in some studies. Postnatal DA exposure can induce early spontaneous seizures, but seizure-like behaviors can also be triggered by challenging tests of learning and memory in adulthood. In zebrafish embryonically exposed to DA, repetitive fin-flipping and convulsions have been reported.
Ample studies in different animal models have been conducted to characterize overt neurotoxicity in offspring (seizures, hyperactivity, stereotypic scratching, squinting, mastication, head-bobbing, paralysis, death) following high-dose in utero or early postnatal DA exposure. Future studies focused on the high-dose acute toxic effects of DA should seek to characterize the mechanistic aspects of these responses.
Functional Effects
Effects on Physical Development.
DA effects on physical development have been studied in several animal models including nonhuman primates. Infant macaques exposed prenatally to maternal oral doses of 0.075 or 0.15 mg/kg/day DA throughout gestation showed no evidence of congenital anomalies or effects on birth size (birthweight, crown-rump length, head width, length and circumference) (Burbacher et al., 2019). DA-exposed offspring also exhibited normal weight gain during their first year of life (Dr. Thomas Burbacher, personal communication).
Studies of physical development in prenatally-exposed rodents using maternal iv or ip doses of 0.6 mg/kg or 0.3–1.2 mg/kg DA on GD 13 did not find significant adverse effects on key variables such as gestation length, litter size, birthweight, and neonatal growth (Dakshinamurti et al., 1993; E. D. Levin, Pizarro, Pang, Harrison, & Ramsdell, 2005). In the only study of prenatal exposure in rodents using maternal oral exposures (1 or 3 mg/kg/day on GD 10–17), early physical development in offspring was regularly assessed by evaluating the timing of hair emergence, incisor eruption, eye opening, descent of testes, and vaginal opening (Shiotani et al., 2017). There were no differences between exposed and control animals in achieving these physical milestones, but weight gain during the preweaning period was greater in DA-exposed pups. Studies of postnatal DA treatment generally do not report changes in physical development as well. Weight gain was unaffected in rat pups exposed to 25–100 μg/kg sc DA on PND 1–2 (E. D. Levin et al., 2006). A series of neonatal exposure studies conducted at the University of Prince Edward Island carefully investigated weight gain and day of eye opening after 20 μg/kg sc DA on PND 8–14 in the rat model. DA did not negatively impact these indices of physical development (Adams, Doucette, James, & Ryan, 2009; Bernard, MacDonald, Gill, Ryan, & Tasker, 2007; Doucette et al., 2004; D. A. Gill et al., 2012; Marriott, Ryan, & Doucette, 2012; Perry et al., 2009; C. L. Ryan et al., 2011; Tasker, Perry, Doucette, & Ryan, 2005), nor did it influence sexual maturation (Burt, Ryan, & Doucette, 2008a). While early postnatal DA exposure does not appear to adversely influence physical development, precocious attainment of eye opening has been documented in exposed pups. In two studies, doses of either 5 or 20 μg/kg sc on PND 8–14 did not affect weight gain, but treated pups, especially females, reached criterion on eye opening before their saline-treated counterparts (Burt et al., 2008a; Doucette, Bernard, Yuill, Tasker, & Ryan, 2003).
In zebrafish models, embryonic DA exposure resulted in defects of the heart and spinal cord (Hong, Zhang, Zuo, Zhu, & Gao, 2015; Tiedeken, Ramsdell, & Ramsdell, 2005). More recently, DA exposure at two days post-fertilization was associated with a high prevalence of uninflated swim bladders (a physical milestone that is essential to survival) when measured with imaging techniques at five days post-fertilization (Panlilio et al., 2020). High dose exposure at four days post-fertilization was related to an abnormal opaque appearance of the brain, suggesting frank neurotoxicity at this dose (0.18 ng DA).
In summary, most investigations conducted with mammals do not report an association between developmental DA exposure and congenital anomalies or deficits in physical growth. Reported effects on day of eye opening, an early physical landmark, are mixed, with reports of either no effect or an accelerated effect. New results with zebrafish indicate structural malformations in exposed larvae and highlight the potential importance of this model for future studies of this toxin.
Effects on Reflexes.
The assessment of reflexes in infancy provides a measure of nervous system maturity. In the nonhuman primate study described above, the development of early survival reflexes and responsivity to the environment during the first two weeks of life was unaffected by maternal oral exposure throughout gestation to 0.075 mg/kg or 0.15 mg/kg day DA (Grant et al., 2019). Similarly, righting, cliff avoidance, negative geotaxis, and auditory startle in rodent pups were not adversely impacted by maternal oral exposure to 1 or 3 mg/kg on GD 10–17 (Shiotani et al., 2017). Results of postnatal exposure studies also showcase the resilience of this developmental domain to early-life DA exposure. The auditory startle reflex was not diminished by postnatal DA exposure to doses of either 5 or 20 μg/kg sc DA on PND 8–14 in neonatal rodents (Burt et al., 2008a; Doucette et al., 2004; D. A. Gill et al., 2012; Marriott et al., 2012; Perry et al., 2009).
Two investigations using zebrafish found that developmental exposure to DA abolished the “touch response” reflex (Panlilio et al., 2020; Tiedeken et al., 2005). This survival reflex is elicited when zebrafish are touched, triggering movement to quickly change orientation and swim away. Abnormal startle responses have also been reported in DA-exposed larvae (Panlilio et al., 2020).
The collective results on reflex development in monkeys and rodents suggest that DA does not adversely impact the presence and strength of reflexive behaviors, but, in zebrafish, key survival reflexes are adversely affected and, in some cases, completed eliminated.
Effects on Sensory and Motor Processing.
Pre-pulse inhibition (PPI) refers to the phenomenon whereby a weakened pre-stimulus inhibits the subsequent reaction to a stronger reflex-eliciting stimulus. In animal work, PPI is frequently evaluated in the context of auditory startle testing and is used as a measure of both sensory-motor gating and early information processing. Prenatal exposure to maternal sc doses of 1.5 mg/kg DA on GD 16 decreased PPI in exposed male pups, suggesting sex-specific impairments in this outcome (Zuloaga et al., 2016), but this finding was not replicated in a study using maternal oral doses of 1 or 3 mg/kg on GD 10–17 (Shiotani et al., 2017). PPI has also been studied in rodents after postnatal DA exposure, primarily in the context of animal model development for schizophrenia. Using a 20 μg/kg sc dose on PND 8–14, investigators found an association between DA treatment and PPI deficits that was dependent on sex and time of day but the baseline startle response and habituation were not affected (Marriott et al., 2012).
A limited number of studies suggest an association between developmental DA exposure and the presence of sex-dependent shifts in the processing of sensory and motor information. Any effect of DA on PPI appears to be independent from the integrity of reflexive behaviors, like the startle response, that are used to measure this psychological construct.
Effects on Cognition.
Memory is considered to be a key outcome that is sensitive to the effects of DA exposure across species. In the only study of DA exposure and cognition in primates (as described in Section 5), visual recognition memory was assessed in prenatally exposed infant macaques (0.075 or 0.15 mg/kg/day maternal oral DA throughout gestation) using a test paradigm based on the Fagan Test of Infant Intelligence (Grant et al., 2019). Scores on this test were not affected by DA exposure when test problems were relatively easy to solve. However, when the problems became more difficult and required processing complex social stimuli (faces), high-dose DA exposed infants performed poorly and failed to provide empirical evidence of memory when compared to their control and low-dose counterparts.
Results from rodent studies of prenatal DA exposure have employed a range of testing paradigms, particularly mazes, to evaluate effects on cognition. Using the radial arm maze to measure spatial cognition, rodents prenatally exposed to maternal sc doses of 0.3, 0.6 or 1.2 mg/kg DA on GD 13 showed no deficits in learning, but normal sex-specific differences in performance were attenuated (E. D. Levin et al., 2005). A chemical challenge with scopolamine, conducted when behavioral testing was complete, indicated greater working memory deficits in the most highly exposed animals. In other rodent studies of prenatal exposure, a cued-fear conditioning test has been used to study the effects of DA on associative learning and memory. Significant decreases in freezing behavior were documented in animals after exposure to maternal ip doses of 1 mg/kg DA on GD 11.5, 14.5 and 17.5 (Tanemura et al., 2009), but these effects were not found in a separate study using maternal oral doses of 1 or 3 mg/kg on GD 10–17 (Shiotani et al., 2017).
The effects of DA on cognition have also been examined after neonatal exposure. A study of rat pups exposed to 25–100 μg/kg sc DA on PND 1–2 found no adverse effects of DA on learning in the radial arm maze (E. D. Levin et al., 2006). However, an investigation using three different types of mazes (elevated plus maze, H-water maze, MWM) found that animals treated with doses of 20 μg DA sc on PND 8–14 solved problems of limited difficulty as adeptly as controls, but significant differences in cognition were revealed when exposed animals were challenged with more complex test environments (D. A. Gill et al., 2012). All DA-treated animals displayed increased perseverative behavior on reversal problems, and, in males, the ability to relearn previously mastered material was impaired relative to controls. In a separate study that also used the MWM, marked learning deficits were identified in females with a history of neonatal exposure (5 or 20 μg/kg sc on PND 8–14) (Doucette, Ryan, & Tasker, 2007).
While these published reports provide evidence of learning impairments after early postnatal DA treatment, other studies using similar dosing paradigms have found accelerated performance on tests of cognition. Young rat pups exposed to 5 or 20 μg/kg sc DA on PND 8–14 demonstrated superior neonatal learning on an olfactory conditioning task (Doucette et al., 2003), while adolescent rats exposed to 20 μg/kg sc DA on PND 8–14 showed improved choice accuracy on the radial arm maze (Adams et al., 2009).
The nicotine-induced condition place preference paradigm is designed to study behavioral responses to appetitive rewards. In studies examining how postnatal treatment to 20 μg/kg sc DA on PND 8–14 affected drug seeking behavior on this task, exposed males did not develop a place preference for nicotine, but exposed females showed an increased sensitivity to the rewarding properties of nicotine in one investigation (Burt et al., 2008a; Burt, Ryan, & Doucette, 2008b).
Finally, suppression of latent inhibition behavior, a measure of attentional processing, has been documented in rodents after 20 μg/kg sc DA on PND 8–14 exposure (Marriott et al., 2012), and males appear to be more adversely impacted than females (Marriott, Tasker, Ryan, & Doucette, 2014).
The effects of DA exposure on cognition are bidirectional, as studies have found both negative and positive effects on performance. There is, however, sound evidence that DA exposure early in life can result in subtle but persistent changes in learning and memory. Treatment effects are often gender-specific, and some study results suggest that deficits are most likely to be revealed when challenging test problems are presented (e.g. complex test stimuli, reversal tasks). Prenatal DA treatment appears to result in more serious effects than postnatal exposure.
Effects on Emotionality.
While emotionality is difficult to quantify in animals, ultrasonic vocalizations in neonatal rats and mice can be used to measure early social communications. Ultrasonic vocalizations, also referred to as isolation calling responses, are emitted by pups when separated from their dam or littermates and are used as a proxy to quantify emotionality. While prenatal DA exposure to a maternal dose of 1.5 mg/kg sc on GD 16 did not affect ultrasonic vocalizations at multiple postnatal time points in one study (Mills et al., 2016), the same exposure paradigm resulted in a significant reduction of the number of calls in another (Zuloaga et al., 2016). Prenatal exposure to a maternal ip dose of 1 mg/kg on GD 11.5, 14.5 and 17.5 in rodents was associated with the presence of anxiety-like behaviors on the open field test and elevated plus maze (Tanemura et al., 2009). Changes in anxiety were also documented in a study using maternal oral doses of 1 or 3 mg/kg DA on GD 10–17, but in this case, prenatally exposed male rats displayed reduced anxiety, while treated females displayed increased anxiety on the elevated plus maze (Shiotani et al., 2017). Increased anxiety-related behaviors have also been observed in postnatal rodent studies using doses of 5 or 20 μg/kg sc DA on PND 8–14 with the elevated plus maze, and females appear to be more affected than males (Doucette et al., 2007; D. A. Gill et al., 2012). Finally, treatment with 20 or 60 μg/kg sc DA on PND 8–14 did not increase depression-like behavior on the forced swim assay, but animals appeared more anxious during the open field test (Thomsen et al., 2016).
The body of information on emotionality is limited to the rodent animal model. The primary messages from studies on developmental DA exposure and emotionality point to heightened anxiety and increased susceptibility to stress as sensitive outcome measures. The manifestation of these effects is strongly gender- and dose-dependent.
Effects on Activity.
Levels of activity are important indicators of developing neurological function and have been studied in DA research with rodent models. Significant changes in locomotor activity patterns were found on the Figure-8 maze and open field test in prenatal exposure studies using maternal sc or ip doses ranging from 0.3–1.2 mg/kg DA on GD 11.5–17.5 (E. D. Levin et al., 2005; Tanemura et al., 2009). Treatment-related effects on circadian activity levels and motor function (coordination and gait) were also identified in a study using maternal oral doses of 1 or 3 mg/kg DA on GD 10–17 on the open field, Rotarod and CatWalk assessments (Shiotani et al., 2017).
A postnatal exposure study, using sc doses from 25–100 μg/kg DA on PND 1–2, found a significant reduction in locomotor activity on the Figure-8 maze (E. D. Levin et al., 2006). Subcutaneous exposure occurring later in the neonatal period (PND 8–14) has been associated with increased activity in female rats on the elevated plus maze (D. A. Gill et al., 2012), as well as increased activity in females and decreased activity in males on the open field test (Burt et al., 2008a). In other investigations, however, activity levels in open field arenas were not altered after DA treatment with doses ranging from 5–60 μg/kg sc on PND 8–14 (Doucette et al., 2004; J. C. Ryan et al., 2011; Thomsen et al., 2016).
Findings from prenatal and neonatal exposure studies suggest that DA can influence normal activity levels in complex ways and locomotor activity can be increased (particularly in females), decreased, or unaffected.
Effects on Social Behavior.
Much of the research focused on social behavior and developmental DA exposure has been conducted in an effort to develop a new rodent model of human psychiatric conditions (e.g. schizophrenia) and neurodevelopmental disorders (e.g. Autism Spectrum Disorder). In rodent offspring exposed in utero to maternal doses of 1.5 mg/kg DA sc on GD 16, time spent in social interactions was significantly reduced compared to controls, and this treatment effect was primarily observed in males (Mills et al., 2016; Zuloaga et al., 2016). The results from studies of postnatal exposure using a DA dose of 20 μg/kg sc on PND 8–14 are mixed. In one investigation, exposed males spent more time engaged in social withdrawal behaviors and less time in social contact with conspecifics (C. L. Ryan et al., 2011) while another found no treatment effects on social interactions (Thomsen et al., 2016).
Data from developmental DA studies suggest that exposure is associated with changes in social interactions that include increased withdrawal and avoidance behaviors. Males appear to be more sensitive to this treatment-driven change than females.
Summary.
In mammalian models, developmental DA exposure does not result in congenital anomalies or adversely impact physical growth trajectories. Reflex development is likewise, unaffected. Researchers have, however, noted subtle but persistent changes in learning and memory, often observed as animals are presented with increasingly challenging tasks. DA exposure is also associated with deficits in social behavior that are characterized by increased withdrawal and avoidance behaviors. Finally, heightened emotionality and susceptibility to stress have been identified as sensitive outcome measures in animals with a history of early-life DA exposure.
Neuropathological Effects
Several studies using animal models have examined the brains of asymptomatic offspring exposed to DA during gestation using a variety of histological and neuroimaging methods. In a seminal mouse study involving prenatal DA exposure to a maternal dose of 0.6 mg/kg iv on GD 13, Dakshinamurti and colleagues (1993) found evidence of progressive hippocampal injury. No cellular damage was observed on PND 1, but damage to hippocampal CA3 and dentate gyrus regions was detected on PND 10, and decreased regional GABA and increased glutamate levels in the cerebral cortex and hippocampus were documented on PND 30. Treatment-related damage to the hippocampus may contribute to the memory deficits observed in exposed offspring. In a separate study, a single maternal dose of 1.5 mg/kg sc DA administered on GD 16 resulted in a significant increase in the number of parvalbumin-positive cells in the lateral amygdala (both sexes) and in the dentate gyrus (males only) (Zuloaga et al., 2016). These cellular effects, suggesting an increase in GABAergic neurons, were observed in offspring with deficits in social behavior and sensorimotor gating. MRI was used in a study of mice exposed to a maternal dose of 1.5 mg/kg DA sc on GD 16, and investigators found an atypical pattern of connectivity in the anterior cingulate cortex (Mills et al., 2016). Treated animals showed overconnectivity from anterior cingulate cortex to infralimbic and orbital regions and underconnectivity to dorsal retrosplenial cortex and CA3 region of hippocampus. It is possible that changes in anterior cingulate cortex connectivity, known to play an important role in emotional regulation, are related to the heightened emotionality that has been observed after developmental DA exposure. Routine histological examinations of exposed brains were normal in a separate study using a maternal dose of 1 mg/kg ip on GD 11.5, 14.5 and 17.5, but evidence of long-term abnormalities in myelination and the overgrowth of neuronal processes in the cerebral cortex and hippocampus were identified using immunohistochemical methods (Tanemura et al., 2009).
Early postnatal exposure has also been associated with neuropathology. Mossy fiber axon sprouting (MFS), a finding commonly associated with temporal lobe epilepsy, has been studied after neonatal DA exposure, and increased MFS was found in the hippocampus of animals exposed to doses of 5 or 20 μg/kg sc on PND 8–14 (Bernard et al., 2007; Doucette et al., 2004; D. A. Gill, Bastlund, et al., 2010). Despite the presence of increased MFS, the clinical presentation of behaviors that resemble temporal lobe epilepsy have not been documented in neonatally-DA exposed animals (Demars et al., 2018). Doses of 20 or 60 μg/kg sc DA on PND 8–14 in rat pups produced long-term changes in α2-adrenoceptor binding in limbic brain regions, but the effects were bidirectional and highly dose-dependent (Thomsen et al., 2016). The observed neurochemical effects were detected in the absence of functional alterations in behavior in the low dose animals. Sex-specific variations in protein expression have been described in a study of 20 μg/kg sc DA on PND 8–14 (D. A. Gill et al., 2012). In this investigation, DA-treated male rats showed increased expression of several important stress-related receptors, including the adrenergic receptor subtypes α2a and α2c, in hippocampal and non-hippocampal brain areas. Other rodent studies using a similar dosing regimen have found no treatment effects on the expression of important dopamine receptors or enzymes related to tyrosine and glutamate in the prefrontal cortex and hippocampus (Marriott, Tasker, Ryan, & Doucette, 2016) or on glucocorticoid and mineralocorticoid receptors in the hippocampus and hypothalamus (Perry et al., 2009).
Summary.
At high exposures, the effects of DA on the developing brain are similar to the neuropathological changes observed in adults, and include neuronal damage and cell loss, particularly in the hippocampus. Lower-level developmental exposures appear to have unique pathological findings with differences in axonal sprouting, connectivity, and more subtle effects in neural protein and receptor expression. Importantly, these effects are dependent on the timing of exposure and may differ based on exposure duing specific windows of developmental susceptibility.
Future Directions
Research findings from animal models have indicated a heightened sensitivity to the adverse effects of DA in the fetus and neonate when compared to adults. Data from the only nonhuman primate study of developmental exposure suggest that subtle changes in early memory are important, but studies of human infants will be required to determine the translational value of the results from animal models. Future investigations in humans and animal models should prioritize the systematic collection of DA biomarkers (e.g. blood, urine) during pregnancy and in exposed offspring to characterize the relationship between increasing body burden of DA and related neurodevelopmental effects.
7. Other Toxicities from Domoic Acid Exposure
While the preponderance of studies examining the effects of DA exposure have focused on the central nervous system, studies of DA impacts on many peripheral organs have also been conducted. Like the nervous system, other organs, including the heart, kidney, spleen, liver, lung, and both male and female reproductive organs, have some level of glutamate receptor expression, which may interact with DA (S. S. Gill, Barker, & Pulido, 2008; S. S. Gill, Mueller, McGuire, & Pulido, 2000; S. S. Gill & Pulido, 2001). Cardiac effects of DA were first noted in the original human poisoning in Canada (Todd, 1993), as well as in wild sea lion populations poisoned by DA (Zabka et al., 2009). Two other research groups used both in vivo and in vitro models to demonstrate that DA can accumulate in the heart of rats after exposure to a single dose (2 mg/kg ip), intrahippocampal infusion (100 pmol) (Vranyac-Tramoundanas, Harrison, Sawant, Kerr, & Sammut, 2011), and two doses of 2.5 mg/kg ip, spaced 30 days apart (Vieira et al., 2016). In both studies, exposed animals expressed myocardial injuries and damaged cardiac mitochondria, but visible damage was subtle. In vitro, DA leads to the uncoupling of rat cardiac mitochondria, but this does not produce ROS, suggesting that the function, but not structure, of cardiomyocytes may be predisposed to DA toxicity (Vranyac-Tramoundanas et al., 2008).
Kidney damage was also noted in the original DA poisoning event, but only one study, using mice exposed to 3 single doses of DA (0.1–2.5 mg/kg ip) over three days, has examined the renal effects of this toxin (Funk et al., 2014). Animals demonstrated signs of kidney damage, with increased urinary biomarkers of KIM-1 and NGAL and evidence of increased cell death in proximal tubules of the kidney. Authors suggested that these effects may be most important in human populations with pre-existing renal disease or compromised renal function, such as aged or diabetic populations, as even low-level DA exposure could exacerbate existing kidney damage. DA may also cause subtle immunomodulatory effects in vivo, but results are limited. Infusions of 0.15 μg of DA directly into the lateral septal area of the brain caused neurotoxic lesions and subsequent modulatory effects in the endocrine system of female mice, but not male mice (Wetmore & Nance, 1991). A single dose exposure study conducted with mice given 2.5 mg /kg ip DA reported altered monocyte activity, decreased neutrophil phagocytosis, and decreased T-cell proliferation (M. Levin, Leibrecht, Ryan, Van Dolah, & De Guise, 2008). Immunomodulatory effects have also been reported in sea lions with DA poisoning (M. Levin et al., 2010). Studies that assessed standard serum and urine chemistry, which include biomarkers for both kidney and immune function, found few changes, however, after daily, oral, sub-chronic dosing in both rats exposed to 0.1 and 5 mg DA/kg (Truelove, Mueller, Pulido, & Iverson, 1996) or monkeys given 0.5 mg/kg DA for 15 days and then 0.75 mg/kg for another 15 days (Truelove et al., 1997).
Summary and Future Directions
The limited number of studies on the peripheral organ toxicity of DA collectively suggest that there may be many other, frequently overlooked effects from exposure to this toxin. Future research into the cardiac, renal, and immunomodulatory effects of DA should aim to better characterize these effects, especially considering chronic exposure. Results from these studies will also help reveal the human sub-populations with pre-existing conditions who may be more vulnerable to the toxic effects of this compound.
8. Current Exposures and Public Health Safety
Estimates of Human Exposures
Surveys to estimate real-world DA exposure have been conducted in high-seafood consumption populations in the USA and Europe. One of the first surveys targeted at elucidating DA consumption reported DA levels in commonly caught fish species and mussels consumed by fishers in the state of California (Mazzillo et al., 2010). DA consumption was highly dependent on the type of seafood consumed; mussels collected for the survey had no DA detected, whereas anchovies had levels up to 28.3 mg/kg fish. Researchers reported that those fishers self-reporting whole anchovy consumption may be at highest risk of low-level DA exposure, at up to 1.43 mg DA/meal. Using standardized consumption rates of 50 g fish/meal and a bodyweight (bw) of 60 kg, this equates to 0.024 mg DA/kg bw. Another survey of just 16 fishers in Bulgaria assessed DA exposure via mussels, and found that, while DA was detected in all mussels, the highest exposure in this group was estimated to be 0.27 mg DA/meal, or 0.0024 mg/kg bw (Peteva, Georgieva, Stancheva, & Makedonski, 2017).
A more recent survey of recreational fishers in WA, focused only on the risk of DA exposure after razor clam consumption (Ferriss et al., 2017). This survey aimed to assess the patterns DA exposure throughout the year in different ages and sexes. Data from the survey revealed that the reported number of clams eaten per meal may be much higher than previously estimated, over 7 clams/meal in some age groups. This, in combination with high levels of DA in the shellfish, lead to higher-than-expected exposure levels, ranging from 0.05–0.1 mg DA/kg bw/day. Using models to predict what long-term exposures may look like, researchers further identified that predicted consumption of DA was highest in the springtime and in younger groups (10–20 years), although they note that some of the highest shellfish consumer groups were underrepresented in their survey.
In Belgium, data from a nationwide dietary survey and samples of mussels, oysters, and scallops to quantify average DA concentrations and data to estimate average exposures (Andjelkovic et al., 2012). DA was detected in 11% of seafood samples and ranged from 0.8–203.4 ppm in shellfish meat. When consumed at the nationally reported levels, these concentrations equated to up to 0.013 mg DA/kg bw/day.
One group at risk for higher exposures includes those of coastal Native American Nations in WA State. In this state, as well as other locations, Indigenous Peoples of coastal Nations share a historical, cultural, and economic connection to the ocean and marine foods, including those contaminated with DA (Crosman, Petrou, Rudd, & Tillotson, 2019). Many coastal Native Americans in WA regularly consume Pacific razor clams (Fialkowski et al., 2010) and are concerned about the health effects of consuming these clams (Roberts et al., 2016). Dietary surveys and measures from 6-month records of DA concentrations in WA clams have estimated that the average monthly DA consumption rates in Native American adults were approximately 0.000218 ng/kg bw/day or 0.00322 ng DA/kg bw/meal (Stuchal et al., 2020). These rates, while below the current regulatory limit, were still connected with adverse health outcomes, demonstrating the necessity of including groups at high risk of DA exposure in regulatory considerations.
While these reports demonstrate the low-level and persistent exposure to DA in many populations today, DA concentrations vary by seafood species (Andjelkovic et al., 2012; Mazzillo et al., 2010), location (Wekell, Trainer, Ayres, & Simons, 2002), and time of the year (Smith et al., 2018). Further, DA does not degrade with typical cooking and freezing methods (McCarron & Hess, 2006; Vidal, Correa, & Blanco, 2009). Going forward, exposure assessments should include considerations for these variable factors and look towards the use of a biomarker to confirm DA exposures.
Domoic Acid Regulation and Safety Recommendations
Estimates of DA exposure from the Prince Edward Island poisoning were used to establish limits for shellfish harvesting to protect public health (Hynie & Todd, 1990; Todd, 1993; Wekell et al., 2004). Shellfish harvesting is closed when monitoring programs indicate DA concentrations in shellfish of 20 mg/kg or greater. This action level was derived from estimates of DA concentrations in mussels from the Prince Edward Island poisoning (200 mg/kg mussel tissue) and applied with a 12-fold safety factor. This limit was suggested to be well below the approximate no-effect-level in mice, and, therefore, thought to be protective of acute human exposures (Iverson & Truelove, 1994). Continued research after the establishment of this regulation estimated that the limit in seafood is approximately equivalent to 0.075–0.1 mg/kg bw in adults (Alexander et al., 2009; Mariën, 1996; Toyofuku, 2006). This threshold is based solely on information from the single episode of high-dose, catastrophic exposure and does not address the health risks associated with lower dose or chronic exposure.
Since the establishment of the regulatory threshold, several research groups have calculated other consumption limits, by incorporating newly available toxicological data, seafood consumption rates and patterns, and additional protective safety and uncertainty factors (Table 4). The results of these assessments vary significantly from daily consumption limits consistent with the current estimate of 0.075 mg/kg bw (Mariën, 1996; Toyofuku, 2006) to limits approximately 2- to 4-times lower (0.018 to 0.034 mg/kg bw) (Alexander et al., 2009; Slikker, Scallet, & Gaylor, 1998). Most of these assessments, however, indicated that there were not enough data to develop safety limits for chronic consumers of DA (Alexander et al., 2009; Kumar, Kumar, & Nair, 2009; Toyofuku, 2006).
Recently, however, a seafood safety limit considering chronic exposure was developed with data from the CoASTAL cohort study (Stuchal et al., 2020). Using estimates of shellfish intake from questionnaires given to study participants and DA levels in shellfish from the study site, the authors estimated a daily consumption limit of 0.003 mg/kg bw/day would be needed to protect adult consumers from the effects observed in the study (decreased verbal memory recall), a threshold well below the present regulatory action level.
The wide range of results across these studies are the product of different variations and approaches to risk assessment. None of the studies, however, include quantitative data on sensitive groups, such as young, aged, or other biologically compromised populations. These characteristics demand more attention going forward, so that updated regulations can better protect the most vulnerable populations. Future regulatory guidelines should be established with a focus on chronic and low-level effects, particularly in vulnerable and highly exposed populations, to best protect the health of all shellfish consumers. In the promotion of environmental justice, the input and consideration of key stakeholders, including Native American and Indigenous populations as well as other high-risk groups, should be considered vital in the reassessment and establishment of future regulations (Burger & Gochfeld, 2011). Interim guidance limiting chronic seafood consumption, such as that released by the WA State Department of Health, can help promote public health until such regulatory thresholds are adopted (Washington State Department of Health, n.d.).
9. Summary and Conclusion
DA causes overt excitotoxicity in adult mammals, producing striking behavioral symptoms and pathology that primarily manifests in the hippocampus. Since the 1987 human poisoning and subsequent regulation of DA, there have been no documented incidents of acute human ASP, but continued research has deepened our understanding of the perilous nature of this toxin. The compelling body of research collectively detailed in this review illuminates the worrisome effects of DA, even at levels deemed as “safe” under current regulatory limits. The results of preclinical studies indicate that chronic exposure to levels of DA near the human regulatory limit do not cause overt neuroinjury but can cause subtle, neurotoxic effects that impact the function, structure, physiology, and cellular response of the brain. Recent epidemiological studies have also provided new evidence of harm from chronic, low-level DA exposure, highlighting the importance of studies focused on the health effects from repeated exposure to this toxin at levels below the current regulatory limit. Future research efforts should aim to further explore these themes, by designing studies aimed at understanding the underlying mechanisms of toxicity associated with low-level and chronic DA exposure. Potential mechanisms for tolerance should be explored as well. Special considerations for differences in responses based on sex and age should be another focus, to best understand the risk to certain populations.
Health effects have also been documented following DA exposure in vulnerable, developmental laboratory models. If given during development, DA generally does not appear to cause congenital or other physical defects, but perinatal exposure to this toxin has been linked with deficits in measures of learning and memory, as well as aberrant behavior related to social and emotional domains. These changes were observed even in some studies using very low-level DA exposure paradigms administered during early life. Effects such as these may be caused, in part, by irregular mossy fiber sprouting in the hippocampus and altered connectivity in the brain. Other vulnerable populations that have not been well studied may include both those with diminished kidney, cardiac, or immune function, as limited evidence suggests DA may also impact these systems.
Up to now, few studies have included a biomarker of DA exposure. Results from DA studies in adult female nonhuman primates and their offspring (Shum et al., 2018) as well as results from a study of adult humans who chronically consume shellfish (Lefebvre et al., 2012, 2019) have provided evidence indicating that urine or a DA-specific antibody may be useful biomarkers for DA exposure. Future studies in human populations and preclinical models should develop strategies such as these to provide critical data regarding the relationship between DA body burden and related health effects in both the nervous system and other critical off-target organs.
In conclusion, the current literature on the health effects of DA exposure provides strong evidence that the current regulatory limit does not adequately protect populations that are chronic consumers of shellfish, particularly those individuals who may be sensitive to DA effects, such as developing young or aged individuals, as well as those with other comorbidities. New interim guidance in WA suggests limiting the consumption of razor clams to 15 per month for everyone, but particularly for “women who are or might become pregnant, nursing mothers, children, the elderly, and people with compromised renal function” (Washington State Department of Health, n.d.). This is especially pertinent because the health of the highest-exposed groups may already be disproportionately impacted by other environmental contaminants. Current regulatory limits should be reexamined and reestablished, with cooperation from regulators and representation from high-risk communities to best protect the health of populations chronically exposed to this common marine contaminant.
Table 2B:
Adult Clinical and Preclinical Neurotoxic Effects of Domoic Acid, continued
| Route of Exposure | Dose | Duration of Exposure | Biomarker of Exposure | Subject Characteristics | Overt Neurotoxic Effects | Time Onset^ | Functional Neurotoxic Effects | Time Neurotoxic Effects Examined^ | Neuropathological Effects | Time Pathology Examined^ | Citation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rats, continued | |||||||||||
| ip | 1 mg/kg | 1x | Males | None | N/A | ↑ locomotion immediately; ↑ time to habituate in novel field at 8 and 15 d | Immediately, 7 d, and 15 d | Hippocampus: no signs of damage or apoptosis | 15 d | Schwarz et al., 2014 | |
| ip | 0.3–3 mg/kg | 1x | Brain: mean 7.2 ng/g | Males | CA1: ND and apoptosis; ↑ WDR35 and p38 MAPK phosphorylation at 1 mg/kg | 1 and 5 d | Tsunekawa et al., 2013 | ||||
| ip | 0.22–1.32 mg/kg | 1x | Males | ↓ weight at >0.65 mg/kg; hindlimb scratching, seizures, death at 1.32 mg/kg | <24 h | Motor depression and exaggerated auditory startle at 1.32 mg/kg, no changes in passive or active avoidance | 1–8 days | Hippocampus: ND and astrocytosis at 1.32 mg/kg | 8 d | Sobotka et al., 1996 | |
| ip | 0.2 mg/kg | 1x | Males | CA4: ND; CA1/4: ↑ c-Fos and c-Jun; CA1/3/4: no changes in myelination, DG: astrocyte reaction; ↓ BBB integrity | 3 d | Scallet et al., 2005 | |||||
| ip | 0.5–2 mg/kg | 1x and preconditioning with 0.125–0.25 mg/kg before | Serum: 24–6133 ng/ml; Brain: 1.2–88.8 ng/g, with aged animals significantly higher | Young adult and aged adult males | Jerks, hindlimb scratching, and wet-dog shakes at 1–2 mg/kg; seizures and death at 2 mg/kg in aged animals | <3 h | Hesp et al., 2007 | ||||
| ip | 1 and 5 mg/kg | At least 2x, 1 dose per h, for up to 5 h | Males | Seizures and chronic epilepsy | <3 h | Muha & Ramsdell, 2011 | |||||
| ip | 1 mg/kg | At least 2x, 1 dose per h, for up to 5 h | Males | Hindlimb scratching, seizures, and chronic epilepsy | <3 h | Hippocampus, amygdala, thalamus, olfactory, prefrontal, and piriform areas: damaged cell bodies; Thalamus: axonal damage | 7 d | Tiedeken et al., 2013 | |||
| ip | 1 mg/kg | At least 2x, 1 dose per h, for up to 3 h | Males | Seizures and chronic epilepsy | <3 h | Motor seizures and dominant/ defensive aggression | Regularly, throughout 12 wk | Hippocampus, amygdala, thalamus, piriform, and olfactory areas: ND | 12 wk | Tiedeken & Ramsdell, 2013 | |
| sc | 0.6–3 mg/kg | 1x | Males | Hypoactivity at all doses; hindlimb scratching, wet-dog shakes, seizures at >2 mg/kg | <30 min | Hippocampus, amygdala, thalamus, and cortex: neural and glial swelling | 3.5–8.5 h | Stewart et al., 1990 | |||
| iv | 0.75 mg/kg | 1x | Males | CA1/3: ND and microglia reactivity; hypertrophic microglia expressing MHC-II; ↑ Bax/BCL-2; no changes in caspase-3 | 1, 2, 5, 14, and 21 d | Ananth et al., 2001 | |||||
| iv | 0.75 mg/kg | 1x | Males | Hippocampus: ND and astrogliosis at 5 d; NOS induction and activated microglia at 5 d and 3 mth | 5 d and 3 mth | Ananth et al., 2003a | |||||
| iv | 0.75 mg/kg | 1x | Males | CA1/3: ND; ↑GFAP+ astrocytes and OX-42+ microglia; ↑ iNOS | 5 d | Ananth et al., 2003b | |||||
| iv and intra-ventricular | 0.5–1 mg/kg iv; 0.04–0.08 mg/kg intraventricular | 1x, and repeated until EEG observations | Hindlimb scratching, seizures, and death | 3–7 min | ↓ memory and learning in 0.04 mg/kg intraventricular | <5 min | No changes reported in iv treatments; CA1/3/4: ND in intraventricular treatments | 3 d | Nakajima & Potvin, 1992 | ||
| intra-hippocampal | 10–300 pmol | 1x | Males | Seizures at >100 pmol | 0–7 min | ECoG spikes at 10–50 pmol; bilateral persistent spiking with seizures at >100 pmol | 7–45 min | Hippocampus and cortex: ↓ GABA at 100 pmol | 150 min | Dakshinamurti et al., 1991 | |
| intra-hippocampal | 30–1000 pmol | 1x | Males | Seizures at >130 pmol | <2.5 h | Sawant, Tyndall, et al., 2010 | |||||
| intra-hippocampal | 112.5 pmol | 1x at 3 sites | Males | Seizures | ↓ learning and memory performance | 10 days | Hippocampus and subiculum: ND; Thalamus: astrocyte reaction | 10 d | Sutherland et al., 1990 | ||
| intra-hippocampal | 3–8000 pmol | 1x and preconditioning with 15 pmol | Males | Freezing, wet-dog shakes, jerking, hindlimb scratching and seizures | <2.5 h | Sawant et al., 2008 | |||||
| intra-hippocampal | 100 pmol | 1x and preconditioning with 15 pmol | Males | Seizures in all treatments, fewer with preconditioning | <2.5 h | ↑ ECoG power with no precondition, normal power with preconditioning | 2 h | Sawant, Mountfort, et al., 2010 | |||
| micro-iontophoretic | 0.1 mM | 1x | Males | Electrical hippocampal activation in CA1/3 | Immediately | Debonnel et al., 1989 | |||||
Table 2C:
Adult Clinical and Preclinical Neurotoxic Effects of Domoic Acid, continued
| Route of Exposure | Dose | Duration of Exposure | Biomarker of Exposure | Subject Characteristics | Overt Neurotoxic Effects | Time Onset^ | Functional Neurotoxic Effects | Time Neurotoxic Effects Examined^ | Neuropathological Effects | Time Pathology Examined^ | Citation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mice | |||||||||||
| Oral and ip | 8.8–104 mg/kg oral; 12–233 mg/kg ip | 1x | Urine: not detected; Feces: 102% of dose | Males and Females | Hindlimb scratching, seizures, and death at >23mg/kg ip; death at >47 mg/kg oral | <30 min | Hippocampus and hypothalamus: ND at 5 mg/kg ip and 35 mg/kg oral | ~1 h | Iverson et al., 1989 | ||
| ip | 55–630 mg of shellfish protein extract/kg | 1x | Males | Motor activity, stereotypic behaviors, convulsions, respiratory distress, deaths | <60 min | Bose et al., 1989 | |||||
| ip | 5–20 mg/kg | 1x | Females | Forelimb tremors at 5 mg/kg, hypoactivity at 20 mg/kg | <60 min | Munday et al., 2008 | |||||
| ip | 4 mg/kg | 1x | Females | Catatonic state, hindlimb scratching, convulsions, death | 1 min | Hippocampus: ND at 4 h, largest damaged area at 168 h | 4,24,48, 72, and 168 h | Strain & Tasker, 1991 | |||
| ip | 4 mg/kg | 1x | Males | Hypo-/hyperactivity, hindlimb scratching, seizures, death | <1 h | Hippocampus, olfactory, and septal areas: ND | 48 h | J. C. Ryan et al., 2011 | |||
| ip | 4 mg/kg | 1x | Females | Hippocampus, amygdala, olfactory areas, septal area: ND and axonal degeneration | 72 h | Colman et al., 2005 | |||||
| ip | 2, 3, and 7 mg/kg | 1x | Hypothalamus and area postrema: No changes at 2–3 mg/kg; ND at 7 mg/kg | 30 min, 60 min, 24 h, 48 h, and 3 d | Bruni et al., 1991 | ||||||
| ip | 2 mg/kg | 1x | Males and Females | Behavioral toxicity, not otherwise described | ↓ learning and memory performance | 1–14 d | Petrie et al., 1992 | ||||
| ip | 1–15 mg/kg | 1x | Females | Hypoactivity, stereotypic hindlimb scratching, tremors, and death at >4 mg/kg | 1 min | Tasker et al., 1991 | |||||
| ip | ~1.3–50 mg/kg | 1x | Females | Hypoactivity, hindlimb scratching, death | <1 h | Grimmelt et al., 1990 | |||||
| ip | 1–4 mg/kg | 1x | Females | CA1/2/3: ND; ↑ transient c-Fos at 4 mg/kg | 15–240 min | Peng et al., 1994 | |||||
| ip | 1 and 4 mg/kg | 1x | Males and Females | Hypoactivity, hindlimb scratching, head weaving, tremors, convulsion | <30 min | Whole brain: 800 differentially expressed genes analysis per time point | 30, 60, and 240 min | J. C. Ryan et al., 2005 | |||
| ip | 0.25–4.0 mg/kg | 1x | Serum: 50–1000 ng/ml for low and high dose, 60 min post-exposure | Females | Hypoactivity, hindlimb scratching at >1 mg/kg, tremors, convulsions, death, convulsions at >2 mg/kg | <1 h | Whole brain: ↑ c-Fos mRNA at >1 mg/kg | 60 min | Peng & Ramsdell, 1996 | ||
| ip | 1 and 2 mg/kg | 1x and 4x over 7 d | Males | Hypoactivity, hindlimb scratching at >1 mg/kg, tremors, convulsions, death, convulsions at >2 mg/kg with repeat and single doses | <1 h | ↓ working memory performance for both doses of single exposures and 1 mg/kg multiple exposure | 7 d | Whole brain: ND at both 2 mg/kg, single and multiple exposures | 25 d | Clayton et al., 1999 | |
| ip | 0.5 and 2 mg/kg | 1x and 4x with 48 h between doses | Serum: ~550–1230 ng/ml for low and high dose, 60 min post exposure for repeat and single doses | Females | Hindlimb scratching, tremors, convulsions, death at <2 mg/kg; no symptoms at 0.5 mg/kg | <1 h | Peng et al., 1997 | ||||
| ip | 2 mg/kg | Daily, for 21 d | Males | Seizures, but not death | ↓ learning and memory | 5 wk | Hippocampus: ND; ↑ GFAP+ astrocytes; ↑ IL-1b, TNF-α, Cox-2, and NOS | 6 wk | Lu et al., 2013 | ||
| ip | 2 mg/kg | Daily, for 28 d | Males | Seizures, but not death | ↓ learning and memory | 5 wk | Hippocampus: ND; ↑ NADPH/ROS; ↑ SAPK/JNK/ FoxO1/Fas | 6 wk | Wu et al., 2012 | ||
| ip | 2 mg/kg | Daily, for 28 d | Males | Seizures, but not death | ↓ learning and memory | 5 wk | Hippocampus: ND; ↑ ROS and mitochondrial dysfunction | 6 wk | Wu et al., 2013 | ||
| ip | 2 mg/kg | Daily, for 28 d | Males | Not reported | ↓ object recognition, learning and memory | Hippocampus: ↑ mitochondrial dysfunction; ↑ ROS; ↓ NRF-1 | 5 wk | D. Wang et al., 2018 | |||
Table 2D:
Adult Clinical and Preclinical Neurotoxic Effects of Domoic Acid, continued
| Route of Exposure | Dose | Duration of Exposure | Biomarker of Exposure | Subject Characteristics | Overt Neurotoxic Effects | Time Onset^ | Functional Neurotoxic Effects | Time Neurotoxic Effects Examined^ | Neuropathological Effects | Time Pathology Examined^ | Citation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mice, continued | |||||||||||
| ip | 2 mg/kg | Daily, for 30 d | Males | Seizures | ↓ learning and memory | Hippocampus: ND and ↓ PCG-1a, NRF-1, TFAM; ↓ mitochondrial and antioxidant activity | 30 d | Lu et al., 2012 | |||
| ip | 0.2–1.6 mg/kg | Daily, up to 30 d | Females | None | ↓ hind limb motor coordination | 10 d | Spinal cord: lesions with TUNEL staining, ↓ proteasome activity after 10 days, ↑ ROS production | 0, 7, 15, 20, 25 and 30 d | Xu et al., 2008 | ||
| ip | 0.75–0.82 mg/kg | 1x/wk, up to 25 wk | Females | None | ↓ memory on the radial water tread maze and increased locomotion in open field at 25 wk, recoverable after 9 wk without exposure | 1, 6, 25 wk and 9 wk | Hippocampus: No changes in cell count for both young and aged mice | 22 wk | Lefebvre et al., 2017 | ||
| ip | 0.75–0.82 mg/kg | 1x/wk, for 22 wk | Females | None | Hippocampus: No changes in neurons or astrocytes with NeuN and GFAP; no changes in VGluT2 or VGAT; CA1: ↑ VGluT1 | 22 wk | Moyer et al., 2018 | ||||
| intracisternal | 25 ng | 1x | Males | Scratching, running, seizures | <10 min | Wood et al., 1982 | |||||
| Fish | |||||||||||
| oral and ic | 0.8 mg, oral; 38–194 mg/kg, ip | 2.7 mg/kg in liver; 0.4 mg/kg in body | Anchovies | Spiral swimming, head shaking, death | 40 min | Lefebvre et al, 2001 | |||||
| ip | 5 mg/kg | 1x | DA detection by immunohistochemistry | Killifish | Hyperactivity, spiral swimming | <3 h | Optic areas: ↑ c-Fos | 3 h | Salierno et al., 2006 | ||
| ic | 0.47 or 1.2 mg/kg | 1x | Zebrafish | Erratic darting, circle and spiral swimming, death | <20 min | Whole brain: 223 and 106 differentially expressed in 0.47 and 1.2 mg/kg, respectively | 6 h | Lefebvre et al., 2009 | |||
| ic | 0.31 mg/kg for 6 wks followed by 0.18 mg/kg | 1x/wk for 6 wk, plus 1x/every other wk for another 6–30 wk | Zebrafish | None | Whole brain: no changes in pathology at 36 wk; 52–239 differentially expressed genes per timepoint; ↓ mitochondrial respiration capacity | 2–36 wk | Hiolski et al., 2014 | ||||
reported in time post-initial dose
Abbreviations: BBB – blood-brain barrier; DG – dentate gyrus; ECoG – electrocorticography; EEG – electroencephalogram; GABA - γ-aminobutyric acid; GFAP – glial fibrillary acidic protein; ic – intracoelomic; ip – interparental; iv – intravenous; ND – neuronal degeneration; NOS – nitric oxide synthase; ROS – reactive oxygen species; sc - subcutaneous
Table 5:
Domoic Acid Human Health Risk Assessments and Suggested Regulatory Limit
| Suggested DA limit in shellfish (ppm) | Estimated Seafood Consumption in 1 Meal (g) | Suggested Consumption Limit (mg DA/kg bw) | Citation |
|---|---|---|---|
|
| |||
| 19.4 | 270 | 0.075 | Mariën, 1996 |
| 16–24 | 250–380 | 0.1 | WHO - Toyofuku, 2006 |
| 12 | 200 | 0.03 | LOAEL - Slikker et al., 1998 |
| 10 | 200 | 0.03 | 1 in 10,000 risk - Slikker et al., 1998 |
| 6.4 | 200 | 0.018 | Benchmark Dose - Slikker et al., 1998 |
| 4.5 | 400 | 0.03 | EFSA - Alexander et al., 2009 |
| 2 | 135 | 0.003 | Benchmark Dose – Stuchal et al., 2020 |
Abbreviations: bw – body weight; EFSA – European Food Safety Authority; LOAEL – lowest-observed-adverse-effect-level; WHO – World Health Organization
Acknowledgements
The publication of this review was supported in part by the following grants from the National Institutes of Health: NIEHS T32ES007062 (PI: Stuart Batterman); NIEHS T32ES007032 (PI: Michael Rosenfeld); NIEHS R01 ES030319 (PI: David Marcinek); NIEHS R01 ES023043 (PI: Thomas Burbacher); P51 OD010425 (PI: Sean Sullivan); NICHD HD083091 (PI: Michael Guralnick), and by the National Science Foundation (NSF) R01 OCE-1839041 (PI: David Marcinek). Partial support was also provided by The Teratogen Information System (TERIS) at the University of Washington. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of these supporting bodies.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ASP
Amnesic Shellfish Poisoning
- BBB
blood-brain barrier
- bw
body weight
- CL
total body clearance
- CL/F
total body clearance after oral administration
- CLr
renal clearance
- CSL
California Sea Lions
- CoASTAL
Communities Advancing the Studies of Tribal Nations Across the Lifespan
- D
dopamine
- DA
domoic acid
- DG
dentate gyrus
- ECoG
electrocorticography
- EEG
electroencephalography
- EFSA
European Food Safety Authority
- fe
fraction excreted unchanged in urine
- GABA
γ-aminobutyric acid
- GAD
glutamic acid decarboxylase
- GD
gestational day
- GFAP
glial fibrillary acidic protein
- ic
intracoelomic
- ip
intraperitoneal
- iv
intravenous
- KA
kainic acid
- LOAEL
lowest-observed-adverse-effect-level
- MFS
mossy fiber sprouting
- MRI
magnetic resonance imaging
- MRT
mean residence time
- MWM
Morris water maze
- ND
neuronal degeneration
- NMDA
N-methyl-D-aspartic acid
- NOS
nitric oxide synthase
- PND
postnatal day
- PPI
pre-pulse inhibition
- ROS
reactive oxygen species
- sc
subcutaneous
- TH
tyrosine hydroxylase
- TK
toxicokinetic
- Vss
volume of distribution at steady-state
- WA
Washington
- WHO
World Health Organization
Footnotes
Note to Reader
The authors of the present manuscript have established a tissue repository of adult and juvenile nonhuman primates chronically exposed to low-levels of DA (Supplement 1), from the study described in Burbacher et al., 2019 (NIEHS R01 ES023043). The authors would like to invite researchers interested in utilizing these samples to contact Dr. Burbacher (the corresponding author) for future collaborative investigations on DA toxicity.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Adams AL, Doucette TA, James R, & Ryan CL (2009). Persistent changes in learning and memory in rats following neonatal treatment with domoic acid. Physiology & Behavior, 96(4–5), 505–512. 10.1016/j.physbeh.2008.11.019 [DOI] [PubMed] [Google Scholar]
- Alexander J, Benford D, Boobis A, Ceccatelli S, Cravedi J-P, Domenico A. Leeuwen Di, Van R. (2009). Marine biotoxins in shellfish – Domoic acid. The EFSA Journal, 1181(7), 1–61. 10.2903/j.efsa.2008.723 [DOI] [Google Scholar]
- Ananth C, Gopalakrishnakone P, & Kaur C. (2003a). Induction of inducible nitric oxide synthase expression in activated microglia following domoic acid (DA)-induced neurotoxicity in the rat hippocampus. Neuroscience Letters, 338(1), 49–52. 10.1016/S0304-3940(02)01351-4 [DOI] [PubMed] [Google Scholar]
- Ananth C, Gopalakrishnakone P, & Kaur C. (2003b). Protective role of melatonin in domoic acid-induced neuronal damage in the hippocampus of adult rats. Hippocampus, 13(3), 375–387. 10.1002/hipo.10090 [DOI] [PubMed] [Google Scholar]
- Ananth C, Thameem Dheen S, Gopalakrishnakone P, & Kaur C. (2001). Domoic acid-induced neuronal damage in the rat hippocampus: Changes in apoptosis related genes (Bcl-2, Bax, Caspase-3) and microglial response. Journal of Neuroscience Research, 66(2), 177–190. 10.1002/jnr.1210 [DOI] [PubMed] [Google Scholar]
- Andjelkovic M, Vandevijvere S, Van Klaveren J, Van Oyen H, & Van Loco J. (2012). Exposure to domoic acid through shellfish consumption in Belgium. Environment International, 49, 115–119. 10.1016/j.envint.2012.08.007 [DOI] [PubMed] [Google Scholar]
- Appel NM, Rapoport SI, O’Callaghan JP, Bell JM, & Freed LM (1997). Sequelae of parenteral domoic acid administration in rats: comparison of effects on different metabolic markers in brain. Brain Research, 754(1–2), 55–64. 10.1016/S0006-8993(97)00042-5 [DOI] [PubMed] [Google Scholar]
- Bargu S, Goldstein T, Roberts K, Li C, & Gulland F. (2012). Pseudo-nitzschia blooms, domoic acid, and related California sea lion strandings in Monterey Bay, California. Marine Mammal Science, 28(2), 237–253. 10.1111/j.1748-7692.2011.00480.x [DOI] [Google Scholar]
- Bargu S, Silver M, Goldstein T, Roberts K, & Gulland F. (2010). Complexity of domoic acid-related sea lion strandings in Monterey Bay, California: Foraging patterns, climate events, and toxic blooms. Marine Ecology Progress Series, 418, 213–222. 10.3354/meps08816 [DOI] [Google Scholar]
- Baron AW, Rushton SP, Rens N, Morris CM, Blain PG, & Judge SJ (2013). Sex differences in effects of low level domoic acid exposure. NeuroToxicology, 34(1), 1–8. 10.1016/j.neuro.2012.10.010 [DOI] [PubMed] [Google Scholar]
- Bates SS (2000). Domoic-acid-producing diatoms: Another genus added! Journal of Phycology, 36(6), 978–983. 10.1046/j.1529-8817.2000.03661.x [DOI] [Google Scholar]
- Bates SS, Bird CJ, de Freitas ASW, Foxall R, Gilgan M, Hanic LA, … Wright JLC (1989). Pennate Diatom Nitzschia pungens as the Primary Source of Domoic Acid, a Toxin in Shellfish from Eastern Prince Edward Island, Canada. Canadian Journal of Fisheries and Aquatic Sciences, 46(7), 1203–1215. 10.1139/f89-156 [DOI] [Google Scholar]
- Bates SS, Hubbard KA, Lundholm N, Montresor M, & Leaw CP (2018). Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae, 79, 3–43. 10.1016/j.hal.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Bates SS, & Trainer VL (2006). The Ecology of Harmful Diatoms. In Ecology of Harmful Algae (pp. 81–93). 10.1007/978-3-540-32210-8_7 [DOI] [Google Scholar]
- Berman FW, & Murray TF (1997). Domoic acid neurotoxicity in cultured cerebellar granule neurons is mediated predominantly by NMDA receptors that are activated as a consequence of excitatory amino acid release. Journal of Neurochemistry, 69(2), 693–703. 10.1046/j.1471-4159.1997.69020693.x [DOI] [PubMed] [Google Scholar]
- Bernard PB, MacDonald DS, Gill DA, Ryan CL, & Tasker RA (2007). Hippocampal mossy fiber sprouting and elevated trkB receptor expression following systemic administration of low dose domoic acid during neonatal development. Hippocampus, 17(11), 1121–1133. 10.1002/hipo.20342 [DOI] [PubMed] [Google Scholar]
- Binienda ZK, Beaudoin MA, Thorn BT, & Ali SF (2011). Analysis of electrical brain waves in neurotoxicology: γ-hydroxybutyrate. Current Neuropharmacology, 9(1), 236–239. 10.2174/157015911795017209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose RJ, Glavin GB, & Pinsky C. (1989). Neurotoxicity and lethality of toxic extracts from atlantic coast shellfish. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 13(3–4), 559–562. 10.1016/0278-5846(89)90146-2 [DOI] [PubMed] [Google Scholar]
- Brodie EC, Gulland FMD, Greig DJ, Hunter M, Jaakola J, Leger J. Van Dolah FM (2006). Domoic Acid Causes Reproductive Failure in California Sea Lions (Zalophus Californianus). Marine Mammal Science, 22(3), 700–707. 10.1111/j.1748-7692.2006.00045.x [DOI] [Google Scholar]
- Bruni JE, Bose R, Pinsky C, & Glavin G. (1991). Circumventricular organ origin of domoic acid-induced neuropathology and toxicology. Brain Research Bulletin, 26(3), 419–424. 10.1016/0361-9230(91)90016-D [DOI] [PubMed] [Google Scholar]
- Brunson JK, Mckinnie SMK, Chekan JR, Mccrow JP, Miles ZD, Bertrand EM, … Moore BS (2018). Biosynthesis of the neurotoxin domoic acid in a bloom-forming diatom. Science, 365(6409), 1356–1358. 10.1126/SCIENCE.AAU0382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Wen X, Toyoda I, Gulland FMD, & Van Bonn W. (2014). Hippocampal neuropathology of domoic acid-induced epilepsy in California sea lions (Zalophus californianus). Journal of Comparative Neurology, 522(7), 1691–1706. 10.1002/cne.23509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbacher T, Grant K, Petroff R, Crouthamel B, Stanley C, McKain N, … Isoherranen N. (2019). Effects of chronic, oral domoic acid exposure on maternal reproduction and infant birth characteristics in a preclinical primate model. Neurotoxicology and Teratology, 440354. 10.1101/440354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, & Gochfeld M. (2011). Conceptual environmental justice model for evaluating chemical pathways of exposure in low-income, minority, Native American, and other unique exposure populations. American Journal of Public Health, 101(SUPPL. 1). 10.2105/AJPH.2010.300077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt MA, Ryan CL, & Doucette TA (2008a). Altered responses to novelty and drug reinforcement in adult rats treated neonatally with domoic acid. Physiology & Behavior, 93(1–2), 327–336. 10.1016/j.physbeh.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Burt MA, Ryan CL, & Doucette TA (2008b). Low dose domoic acid in neonatal rats abolishes nicotine induced conditioned place preference during late adolescence. Amino Acids, 35(1), 247–249. 10.1007/s00726-007-0584-2 [DOI] [PubMed] [Google Scholar]
- Carpenter S. (1990). The Human Neuropathology of Encephalopathic Mussel Toxin Poisoning. Symposium Domoic Acid Toxicity, 73–34. [PubMed] [Google Scholar]
- Cendes F, Andermann F, Carpenter S, Zatorre RJ, & Cashman NR (1995). Temporal lobe epilepsy caused by domoic acid intoxication: Evidence for glutamate receptor–mediated excitotoxicity in humans. Annals of Neurology, 37(1), 123–126. 10.1002/ana.410370125 [DOI] [PubMed] [Google Scholar]
- Clayton EC, Peng Y-G, Means LW, & Ramsdell JS (1999). Working memory deficits induced by single but not repeated exposures to domoic acid. Toxicon, 37(7), 1025–1039. 10.1016/S0041-0101(98)00230-X [DOI] [PubMed] [Google Scholar]
- Colman JR, Nowocin KJ, Switzer RC, Trusk TC, & Ramsdell JS (2005). Mapping and reconstruction of domoic acid-induced neurodegeneration in the mouse brain. Neurotoxicology and Teratology, 27(5), 753–767. 10.1016/j.ntt.2005.06.009 [DOI] [PubMed] [Google Scholar]
- Cook PF, Berns GS, Colegrove K, Johnson S, & Gulland FMD (2018). Postmortem DTI reveals altered hippocampal connectivity in wild sea lions diagnosed with chronic toxicosis from algal exposure. Journal of Comparative Neurology, 526(2), 216–228. 10.1002/cne.24317 [DOI] [PubMed] [Google Scholar]
- Cook PF, Reichmuth C, & Gulland FMD (2011). Rapid behavioural diagnosis of domoic acid toxicosis in California sea lions. Biology Letters, 7(4), 536–538. 10.1098/rsbl.2011.0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PF, Reichmuth C, Rouse AA, Libby LA, Dennison SE, Carmichael OT, … Ranganath C. (2015). Algal toxin impairs sea lion memory and hippocampal connectivity, with implications for strandings. Science, 350(6267), 1545–1547. 10.1126/science.aac5675 [DOI] [PubMed] [Google Scholar]
- Cook PF, Reichmuth C, Rouse A, Dennison S, Van Bonn B, & Gulland FMD (2016). Natural exposure to domoic acid causes behavioral perseveration in Wild Sea lions: Neural underpinnings and diagnostic application. Neurotoxicology and Teratology, 57, 95–105. 10.1016/j.ntt.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G, & Faustman EM (2010). Domoic acid as a developmental neurotoxin. NeuroToxicology, 31(5), 409–423. 10.1016/j.neuro.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosman KM, Petrou EL, Rudd MB, & Tillotson MD (2019). Clam hunger and the changing ocean: characterizing social and ecological risks to the quinault razor clam fishery using participatory modeling. Ecology and Society, 24(2). 10.5751/ES-10928-240216 [DOI] [Google Scholar]
- D’Agostino VC, Degrati M, Sastre V, Santinelli N, Krock B, Krohn T, … Hoffmeyer MS (2017). Domoic acid in a marine pelagic food web: Exposure of southern right whales Eubalaena australis to domoic acid on the Península Valdés calving ground, Argentina. Harmful Algae, 68, 248–257. 10.1016/j.hal.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Dakshinamurti K, Sharma SK, & Sundaram M. (1991). Domoic acid induced seizure activity in rats. Neuroscience Letters, 127(2), 193–197. 10.1016/0304-3940(91)90792-R [DOI] [PubMed] [Google Scholar]
- Dakshinamurti K, Sharma SK, Sundaram M, & Watanabe T. (1993). Hippocampal changes in developing postnatal mice following intrauterine exposure to domoic acid. Journal of Neuroscience, 13(10), 4486–4495. 10.1523/JNEUROSCI.13-10-04486.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debonnel G, Beauchesne L, & de Montigny C. (1989). Domoic acid, the alleged mussel toxin, might produce its neurotoxic effect through kainate receptor activation: an electrophysiological study in the rat dorsal hippocampus. Canadian Journal of Physiology and Pharmacology, 67(1), 29–33. 10.1139/y89-005 [DOI] [PubMed] [Google Scholar]
- Demars F, Clark K, Wyeth MS, Abrams E, & Buckmaster PS (2018). A single subconvulsant dose of domoic acid at mid-gestation does not cause temporal lobe epilepsy in mice. NeuroToxicology, 66, 128–137. 10.1016/j.neuro.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette TA, Bernard PB, Husum H, Perry MA, Ryan CL, & Tasker RA (2004). Low doses of domoic acid during postnatal development produce permanent changes in rat behaviour and hippocampal morphology. Neurotoxicity Research, 6(7–8), 555–563. 10.1007/BF03033451 [DOI] [PubMed] [Google Scholar]
- Doucette TA, Bernard PB, Yuill PC, Tasker RA, & Ryan CL (2003). Low doses of non-NMDA glutamate receptor agonists alter neurobehavioural development in the rat. Neurotoxicology and Teratology, 25(4), 473–479. 10.1016/S0892-0362(03)00034-5 [DOI] [PubMed] [Google Scholar]
- Doucette TA, Ryan CL, & Tasker RA (2007). Gender-based changes in cognition and emotionality in a new rat model of epilepsy. Amino Acids, 32(3), 317–322. 10.1007/s00726-006-0418-7 [DOI] [PubMed] [Google Scholar]
- Doucette TA, & Tasker RA (2016). Perinatal domoic acid as a neuroteratogen. In Current Topics in Behavioral Neurosciences (Vol. 29, pp. 87–110). 10.1007/7854_2015_417 [DOI] [PubMed] [Google Scholar]
- Ferriss BE, Marcinek DJ, Ayres D, Borchert J, & Lefebvre KA (2017). Acute and chronic dietary exposure to domoic acid in recreational harvesters: A survey of shellfish consumption behavior. Environment International, 101, 70–79. 10.1016/j.envint.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkowski MK, McCrory MA, Roberts SM, Tracy JK, Grattan LM, & Boushey CJ (2010). Evaluation of Dietary Assessment Tools Used to Assess the Diet of Adults Participating in the Communities Advancing the Studies of Tribal Nations Across the Lifespan Cohort. Journal of the American Dietetic Association, 110(1), 65–73. 10.1016/j.jada.2009.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire SE, Wang Z, Berman M, Langlois G, Morton SL, Sekula-Wood E, & Benitez-Nelson CR (2010). Trophic transfer of the harmful algal toxin domoic acid as a cause of death in a minke whale (Balaenoptera acutorostrata) stranding in southern California. Aquatic Mammals, 36(4), 342–350. 10.1578/AM.36.4.2010.342 [DOI] [Google Scholar]
- Fujita T, Tanaka T, Yonemasu Y, Cendes F, Cashman NR, & Andermann F. (1996). Electroclinical and pathological studies after parenteral administration of domoic acid in freely moving nonanesthetized rats: An animal model of excitotoxicity. Journal of Epilepsy, 9(2), 87–93. 10.1016/0896-6974(95)00075-5 [DOI] [Google Scholar]
- Funk JA, Janech MG, Dillon JC, Bissler JJ, Siroky BJ, & Bell PD (2014). Characterization of Renal Toxicity in Mice Administered the Marine Biotoxin Domoic Acid. Journal of the American Society of Nephrology, 1–11. 10.1681/ASN.2013080836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DA, Bastlund JF, Anderson NJ, & Tasker RA (2009). Reductions in paradoxical sleep time in adult rats treated neonatally with low dose domoic acid. Behavioural Brain Research, 205(2), 564–567. 10.1016/j.bbr.2009.07.018 [DOI] [PubMed] [Google Scholar]
- Gill DA, Bastlund JF, Watson WP, Ryan CL, Reynolds DS, & Tasker RA (2010). Neonatal exposure to low-dose domoic acid lowers seizure threshold in adult rats. Neuroscience, 169(4), 1789–1799. 10.1016/j.neuroscience.2010.06.045 [DOI] [PubMed] [Google Scholar]
- Gill DA, Perry MA, McGuire EP, Pérez-Gómez A, & Tasker RA (2012). Low-dose neonatal domoic acid causes persistent changes in behavioural and molecular indicators of stress response in rats. Behavioural Brain Research, 230(2), 409–417. 10.1016/j.bbr.2012.02.036 [DOI] [PubMed] [Google Scholar]
- Gill DA, Ramsay SL, & Tasker RA (2010). Selective reductions in subpopulations of GABAergic neurons in a developmental rat model of epilepsy. Brain Research, 1331, 114–123. 10.1016/j.brainres.2010.03.054 [DOI] [PubMed] [Google Scholar]
- Gill SS, Barker M, & Pulido O. (2008). Neuroexcitatory targets in the female reproductive system of the nonhuman primate (Macaca fascicularis). Toxicologic Pathology, 36(3), 478–484. 10.1177/0192623308315663 [DOI] [PubMed] [Google Scholar]
- Gill SS, Mueller R, McGuire PF, & Pulido O. (2000). Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicologic Pathology, 28(2), 277–284. 10.1177/019262330002800207 [DOI] [PubMed] [Google Scholar]
- Gill SS, & Pulido O. (2001). Glutamate Receptors in Peripheral Tissues: Current Knowledge, Future Research, and Implications for Toxicology. Toxicologic Pathology, 29(2), 208–223. 10.1080/019262301317052486 [DOI] [PubMed] [Google Scholar]
- Giordano G, Kavanagh TJ, Faustman EM, White CC, & Costa LG (2013). Low-level domoic acid protects mouse cerebellar granule neurons from acute neurotoxicity: Role of glutathione. Toxicological Sciences, 132(2), 399–408. 10.1093/toxsci/kft002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjedde A, & Evans AC (1990). PET Studies of Domoic Acid Poisoning in Humans: Excitotoxic Destruction of Brain Glutamatergic Pathways, Revealed in Measurements of Glucose Metabolism by Positron Emission Tomography. Symposium Domoic Acid Toxicity, 105–109. 10.2174/1568026616666160405 [DOI] [PubMed] [Google Scholar]
- Goldstein T, Mazet JAK, Zabka TS, Langlois G, Colegrove KM, Silver M, … Gulland FMD (2008). Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): an increasing risk to marine mammal health. Proceedings of The Royal Society, 275(1632), 267–276. 10.1098/rspb.2007.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KS, Burbacher TM, Faustman EM, & Grattan LM (2010). Domoic acid: Neurobehavioral consequences of exposure to a prevalent marine biotoxin. Neurotoxicology and Teratology, 32(2), 132–141. 10.1016/j.ntt.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KS, Crouthamel B, Kenney C, McKain N, Petroff R, Shum S, … Burbacher TM (2019). Preclinical modeling of exposure to a global marine bio-contaminant: Effects of in utero Domoic acid exposure on neonatal behavior and infant memory. Neurotoxicology and Teratology, 73, 1–8. 10.1016/j.ntt.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan LM, Boushey CJ, Liang Y, Lefebvre KA, Castellon LJ, Roberts KA, … Morris JGJ (2018). Repeated dietary exposure to low levels of domoic acid and problems with everyday memory: Research to public health outreach. Toxins, 10(3), 103. 10.3390/toxins10030103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan LM, Boushey CJ, Tracy K, Trainer VL, Roberts SM, Schluterman N, & Morris JGJ (2016). The association between razor clam consumption and memory in the CoASTAL cohort. Harmful Algae, 57(B), 20–25. 10.1016/j.hal.2016.03.011 [DOI] [PubMed] [Google Scholar]
- Greig DJ, Gulland FMD, & Kreuder C. (2005). A Decade of Live California Sea Lion (Zalophus californianus) Strandings Along the Central California Coast: Causes and Trends, 1991–2000. Aquatic Mammals, 31(1), 11–22. 10.1578/am.31.1.2005.11 [DOI] [Google Scholar]
- Grimmelt B, Nijjar MS, Brown J, Macnair N, Wagner S, Johnson GR, & Amend JF (1990). Relationship between domoic acid levels in the blue mussel (Mytilus edulis) and toxicity in mice. Toxicon, 28(5), 501–508. 10.1016/0041-0101(90)90294-H [DOI] [PubMed] [Google Scholar]
- Gulland FMD (2000). Domoic Acid Toxicity in California Sea Lions (Zalophus californianus) Stranded Along the Central California Coast, May-October 1998 | NOAA Fisheries. Report to the National Marine Fisheries Service Working Group on Unusual Marine Mammal Mortality Events. NOAA Technical Memorandum NMFS-OPR-17. Retrieved from https://www.fisheries.noaa.gov/resource/document/domoic-acid-toxicity-california-sea-lions-zalophus-californianus-stranded-along [Google Scholar]
- Gulland FMD, Haulena M, Fauquier D, Langlois G, Lander ME, Zabka TS, & Duerr R. (2002). Domoic acid toxicity in Californian sea lions (Zalophus californianus): clinical signs. Veterinary Record, 150, 475–480. 10.1136/vr.150.15.475 [DOI] [PubMed] [Google Scholar]
- Hampson DR, Huang X, Wells JW, Walter JA, & Wright JLC (1992). Interaction of domoic acid and several derivatives with kainic acid and AMPA binding sites in rat brain. European Journal of Pharmacology, 218(1), 1–8. 10.1016/0014-2999(92)90140-Y [DOI] [PubMed] [Google Scholar]
- Hampson DR, & Manalo JL (1998). The activation of glutamate receptors by kainic acid and domoic acid. Natural Toxins, Vol. 6, pp. 153–158. [DOI] [PubMed] [Google Scholar]
- Harmony T. (2013). The functional significance of delta oscillations in cognitive processing. Frontiers in Integrative Neuroscience, Vol. 7. 10.3389/fnint.2013.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesp BR, Clarkson AN, Sawant PM, & Kerr DS (2007). Domoic acid preconditioning and seizure induction in young and aged rats. Epilepsy Research, 76(2–3), 103–112. 10.1016/j.eplepsyres.2007.07.003 [DOI] [PubMed] [Google Scholar]
- Hiolski EM, Ito S, Beggs JM, Lefebvre KA, Litke AM, & Smith DR (2016). Domoic acid disrupts the activity and connectivity of neuronal networks in organotypic brain slice cultures. NeuroToxicology, 56, 215–224. 10.1016/j.neuro.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiolski EM, Kendrick PS, Frame ER, Myers MS, Bammler TK, Beyer RP, … Lefebvre KA (2014). Chronic low-level domoic acid exposure alters gene transcription and impairs mitochondrial function in the CNS. Aquatic Toxicology, 155, 151–159. 10.1016/j.aquatox.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Zhang Y, Zuo Z, Zhu R, & Gao Y. (2015). Influences of Domoic Acid Exposure on Cardiac Development and the Expression of Cardiovascular Relative Genes in Zebrafish ( Daniorerio ) Embryos. Journal of Biochemical and Molecular Toxicology, 29(6), 254–260. 10.1002/jbt.21692 [DOI] [PubMed] [Google Scholar]
- Hynie I, & Todd ECD (Eds.). (1990). Proceedings of Symposium on Domoic Acid Toxicity. Canada: Disease Weekly Reports. [Google Scholar]
- Iverson F, & Truelove J. (1994). Toxicology and seafood toxins: Domoic acid. Natural Toxins, 2(5), 334–339. 10.1002/nt.2620020514 [DOI] [PubMed] [Google Scholar]
- Iverson F, Truelove J, Nera E, Tryphonas L, Campbell J, & Lok E. (1989). Domoic acid poisoning and mussel-associated intoxication: Preliminary investigations into the response of mice and rats to toxic mussel extract. Food and Chemical Toxicology, 27(6), 377–384. 10.1016/0278-6915(89)90143-9 [DOI] [PubMed] [Google Scholar]
- Jandová K, Kozler P, Langmeier M, Marešová D, Pokornỳ J, & Riljak V. (2014). Influence of low-dose neonatal domoic acid on the spontaneous behavior of rats in early adulthood. Physiological Research, 63, S521–S528. 10.33549/physiolres.932936 [DOI] [PubMed] [Google Scholar]
- Jing J, Petroff R, Shum S, Crouthamel B, Topletz AR, Grant KS, … Isoherranen N. (2018). Toxicokinetics and physiologically based pharmacokinetic modeling of the shellfish toxin domoic acid in nonhuman primates. Drug Metabolism and Disposition, 46(2), 155–165. 10.1124/dmd.117.078485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura O, Kotaki Y, Hamaue N, Haraguchi K, & Endo T. (2011). Transcellular transport of domoic acid across intestinal Caco-2 cell monolayers. Food and Chemical Toxicology, 49(9), 2167–2171. 10.1016/j.fct.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Kraft AD, Aschner M, Cory-Slechta DA, Bilbo SD, Caudle WM, & Makris SL (2016). Unmasking silent neurotoxicity following developmental exposure to environmental toxicants. Neurotoxicology and Teratology, 55, 38–44. 10.1016/j.ntt.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Kumar KP, Kumar SP, & Nair GA (2009). Risk Assessment of the amnesiac shellfish poison, domoic acid, on animals and humans. Journal of Environmental Biology, 30(May), 319–325. 10.1016/j.automatica.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Kvitek RG, Goldberg JD, Smith GJ, Doucette GJ, & Silver MW (2008). Domoic acid contamination within eight representative species from the benthic food web of Monterey Bay, California, USA. Marine Ecology Progress Series, 367, 35–47. 10.3354/meps07569 [DOI] [Google Scholar]
- Lefebvre KA, Bargu S, Kieckhefer T, & Silver MW (2002). From sanddabs to blue whales: the pervasiveness of domoic acid. Toxicon, 40(7), 971–977. 10.1016/S0041-0101(02)00093-4 [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Dovel SL, & Silver MW (2001). Tissue distribution and neurotoxic effects of domoic acid in a prominent vector species, the northern anchovy Engraulis mordax. Marine Biology, 138(4), 693–700. 10.1007/s002270000509 [DOI] [Google Scholar]
- Lefebvre KA, Frame ER, Gulland FMD, Hansen JD, Kendrick PS, Beyer RP, … Marcinek DJ (2012). A novel antibody-based biomarker for chronic algal toxin exposure and sub-acute neurotoxicity. PLoS ONE, 7(5), 1–7. 10.1371/journal.pone.0036213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre KA, Hendrix A, Halaska B, Duignan P, Shum S, Isoherranen N, … Gulland FMD (2018). Domoic acid in California sea lion fetal fluids indicates continuous exposure to a neuroteratogen poses risks to mammals. Harmful Algae, 79, 53–57. 10.1016/j.hal.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre KA, Kendrick PS, Ladiges W, Hiolski EM, Ferriss BE, Smith DR, & Marcinek DJ (2017). Chronic low-level exposure to the common seafood toxin domoic acid causes cognitive deficits in mice. Harmful Algae, 64, 20–29. 10.1016/j.hal.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre KA, Powell CL, Busman M, Doucette GJ, Moeller PD, Silver JB, … Tjee (1999). Detection of domoic acid in northern anchovies and California sea lions associated with an unusual mortality event. Natural Toxins, 7(3), 85–92. [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, & Robertson A. (2010). Domoic acid and human exposure risks: A review. Toxicon, 56(2), 218–230. 10.1016/j.toxicon.2009.05.034 [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Silver M, Coale SL, & Tjeerdema RS (2002). Domoic acid in planktivorous fish in relation to toxic Pseudo-nitzschia cell densities. Marine Biology, 140(3), 625–631. 10.1007/s00227-001-0713-5 [DOI] [Google Scholar]
- Lefebvre KA, Tilton SC, Bammler TK, Beyer RP, Srinouanprachan S, Stapleton PL, … Gallagher EP (2009). Gene expression profiles in zebrafish brain after acute exposure to domoic acid at symptomatic and asymptomatic doses. Toxicological Sciences, 107(1), 65–77. 10.1093/toxsci/kfn207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre KA, Yakes BJ, Frame E, Kendrick P, Shum S, Isoherranen N, … Grattan L. (2019). Discovery of a Potential Human Serum Biomarker for Chronic Seafood Toxin Exposure Using an SPR Biosensor. Toxins, 11(5), 293. 10.3390/toxins11050293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Pang WG, Harrison J, Williams P, Petro A, & Ramsdell JS (2006). Persistent neurobehavioral effects of early postnatal domoic acid exposure in rats. Neurotoxicology and Teratology, 28(6), 673–680. 10.1016/j.ntt.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Levin ED, Pizarro K, Pang WG, Harrison J, & Ramsdell JS (2005). Persisting behavioral consequences of prenatal domoic acid exposure in rats. Neurotoxicology and Teratology, 27(5), 719–725. 10.1016/j.ntt.2005.06.017 [DOI] [PubMed] [Google Scholar]
- Levin M, Joshi D, Draghi A, Gulland FM, Jessup D, & De Guise S. (2010). Immunomodulatory effects upon in vitro exposure of California Sea lion and Southern sea otter peripheral blood leukocytes to domoic acid. Journal of Wildlife Diseases, 46(2), 541–550. 10.7589/0090-3558-46.2.541 [DOI] [PubMed] [Google Scholar]
- Levin M, Leibrecht H, Ryan JC, Van Dolah FM, & De Guise S. (2008). Immunomodulatory effects of domoic acid differ between in vivo and in vitro exposure in mice. Marine Drugs, 6(4), 636–659. 10.3390/md6040636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wu D. -m., Zheng Y. -l., Hu B, Cheng W, Zhang Z. -f., & Li M. -q. (2013). Troxerutin Counteracts Domoic Acid-Induced Memory Deficits in Mice by Inhibiting CCAAT/Enhancer Binding Protein -Mediated Inflammatory Response and Oxidative Stress. The Journal of Immunology, 190(7), 3466–3479. 10.4049/jimmunol.1202862 [DOI] [PubMed] [Google Scholar]
- Lu J, Wu D, Zheng Y, Hu B, Cheng W, & Zhang Z. (2012). Purple sweet potato color attenuates domoic acid-induced cognitive deficits by promoting estrogen receptor-α-mediated mitochondrial biogenesis signaling in mice. Free Radical Biology and Medicine, 52(3), 646–659. 10.1016/j.freeradbiomed.2011.11.016 [DOI] [PubMed] [Google Scholar]
- Mariën K. (1996). Establishing tolerable dungeness crab (Cancer magister) and razor clam (Siliqua patula) domoic acid contaminant levels. Environmental Health Perspectives, 104(11), 1230–1236. 10.1289/ehp.961041230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott AL, Ryan CL, & Doucette TA (2012). Neonatal domoic acid treatment produces alterations to prepulse inhibition and latent inhibition in adult rats. Pharmacology Biochemistry and Behavior, 103(2), 338–344. 10.1016/j.pbb.2012.08.022 [DOI] [PubMed] [Google Scholar]
- Marriott AL, Tasker RA, Ryan CL, & Doucette TA (2014). Neonatal domoic acid abolishes latent inhibition in male but not female rats and has differential interactions with social isolation. Neuroscience Letters, 578, 22–26. 10.1016/j.neulet.2014.06.025 [DOI] [PubMed] [Google Scholar]
- Marriott AL, Tasker RA, Ryan CL, & Doucette TA (2016). Alterations to prepulse inhibition magnitude and latency in adult rats following neonatal treatment with domoic acid and social isolation rearing. Behavioural Brain Research, 298, 310–317. 10.1016/j.bbr.2015.11.009 [DOI] [PubMed] [Google Scholar]
- Maucher Fuquay J, Muha N, Wang Z, & Ramsdell JS (2012). Toxicokinetics of domoic acid in the fetal rat. Toxicology, 294(1), 36–41. 10.1016/j.tox.2012.01.012 [DOI] [PubMed] [Google Scholar]
- Maucher JM, & Ramsdell JS (2005). Domoic acid transfer to milk: Evaluation of a potential route of neonatal exposure. Environmental Health Perspectives, 113(4), 461–464. 10.1289/ehp.7649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maucher JM, & Ramsdell JS (2007). Maternal-Fetal Transfer of Domoic Acid in Rats at Two Gestational Time Points. Environmental Health Perspectives, 115(12), 1743–1746. 10.1289/ehp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzillo FFM, Pomeroy C, Kuo J, Ramondi PT, Prado R, & Silver MW (2010). Angler exposure to domoic acid via consumption of contaminated fishes. Aquatic Biology, 9(1), 1–12. 10.3354/ab00238 [DOI] [Google Scholar]
- McCabe RM, Hickey BM, Kudela RM, Lefebvre KA, Adams NG, Bill BD, … Trainer VL (2016). An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophysical Research Letters, 43(19), 10,366–10,376. 10.1002/2016GL070023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron P, & Hess P. (2006). Tissue distribution and effects of heat treatments on the content of domoic acid in blue mussels, Mytilus edulis. Toxicon, 47(4), 473–479. 10.1016/j.toxicon.2006.01.004 [DOI] [PubMed] [Google Scholar]
- McKibben SM, Peterson W, Wood AM, Trainer VL, Hunter M, & White AE (2017). Climatic regulation of the neurotoxin domoic acid. Proceedings of the National Academy of Sciences, 114(2), 239–244. 10.1073/pnas.1606798114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills BD, Pearce HL, Khan O, Jarrett BR, Fair DA, & Lahvis GP (2016). Prenatal domoic acid exposure disrupts mouse pro-social behavior and functional connectivity MRI. Behavioural Brain Research, 308, 14–23. 10.1016/j.bbr.2016.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer CE, Hiolski EM, Marcinek DJ, Lefebvre KA, Smith DR, & Zuo Y. (2018). Repeated low level domoic acid exposure increases CA1 VGluT1 levels, but not bouton density, VGluT2 or VGAT levels in the hippocampus of adult mice. Harmful Algae, 79, 74–86. 10.1016/j.hal.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muha N, & Ramsdell JS (2011). Domoic acid induced seizures progress to a chronic state of epilepsy in rats. Toxicon, 57(1), 168–171. 10.1016/j.toxicon.2010.07.018 [DOI] [PubMed] [Google Scholar]
- Munday R, Holland PT, McNabb P, Selwood AI, & Rhodes LL (2008). Comparative toxicity to mice of domoic acid and isodomoic acids A, B and C. Toxicon, 52(8), 954–956. 10.1016/j.toxicon.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Nakajima S, & Potvin JL (1992). Neural and behavioural effects of domoic acid, an amnesic shellfish toxin, in the rat. Canadian Journal of Psychology, 46(4), 569–581. 10.1037/h0084334 [DOI] [PubMed] [Google Scholar]
- Newson JJ, & Thiagarajan TC (2019). EEG Frequency Bands in Psychiatric Disorders: A Review of Resting State Studies. Frontiers in Human Neuroscience, 12. 10.3389/fnhum.2018.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio JM, Aluru N, & Hahn ME (2020). Developmental Neurotoxicity of the Harmful Algal Bloom Toxin Domoic Acid: Cellular and Molecular Mechanisms Underlying Altered Behavior in the Zebrafish Model. Environmental Health Perspectives, 128(11), 117002. 10.1289/EHP6652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YG, Clayton EC, Means LW, & Ramsdell JS (1997). Repeated independent exposures to domoic acid do not enhance symptomatic toxicity in outbred or seizure-sensitive inbred mice. Fundamental and Applied Toxicology, 40(1), 63–67. 10.1093/toxsci/40.1.63 [DOI] [PubMed] [Google Scholar]
- Peng YG, & Ramsdell JS (1996). Brain Fos induction is a sensitive biomarker for the lowest observed neuroexcitatory effects of domoic acid. Fundamental and Applied Toxicology, 31(2), 162–168. 10.1006/faat.1996.0087 [DOI] [PubMed] [Google Scholar]
- Peng YG, Taylor TB, Finch RE, Switzer RC, & Ramsdell JS (1994). Neuroexcitatory and neurotoxic actions of the amnesic shellfish poison, domoic acid. NeuroReport, 5(8), 981–985. 10.1097/00001756-199404000-00032 [DOI] [PubMed] [Google Scholar]
- Pérez-Gómez A, & Tasker RA (2012). Enhanced neurogenesis in organotypic cultures of rat hippocampus after transient subfield-selective excitotoxic insult induced by domoic acid. Neuroscience, 208, 97–108. 10.1016/j.neuroscience.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Pérez-Gómez A, & Tasker RA (2013). Transient domoic acid excitotoxicity increases BDNF expression and activates both MEK- and PKA-dependent neurogenesis in organotypic hippocampal slices. BMC Neuroscience, 14(1), 72. 10.1186/1471-2202-14-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl TM, Bedard L, Kosatsky T, Hockin JC, & Todd ECD (1990). An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. New England Journal of Medicine, 322(25), 1775–1780. 10.1056/NEJM199006213222504 [DOI] [PubMed] [Google Scholar]
- Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd EC, McNutt LA, & Remis RS (1990). Amnesic shellfish poisoning: a new clinical syndrome due to domoic acid. Canada Diseases Weekly Report, 16 Suppl 1, 7–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2101742 [PubMed] [Google Scholar]
- Perry MA, Ryan CL, & Tasker RA (2009). Effects of low dose neonatal domoic acid administration on behavioural and physiological response to mild stress in adult rats. Physiology & Behavior, 98(1–2), 53–59. 10.1016/j.physbeh.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Peteva ZV, Georgieva S, Stancheva M, & Makedonski L. (2017). Recreational angler exposure to domoic acid via consumption of contaminated shellfish from the Black Sea, Bulgaria: a preliminary study. Archives of the Balkan Medical Union, 52(3), 291–297. Retrieved from http://umbalk.org/wp-content/uploads/2017/09/08.Recreational_angler_exposer_to_domoic.pdf [Google Scholar]
- Petrie BF, Pinsky C, Standish NM, Bose R, & Glavin G. (1992). Parenteral domoic acid impairs spatial learning in mice. Pharmacology Biochemistry and Behavior, 41(1), 211–214. 10.1016/0091-3057(92)90084-S [DOI] [PubMed] [Google Scholar]
- Petroff R. (2020). Neurological Effects of Low-Level, Chronic Domoic Acid Exposure in a Nonhuman Primate Model. University of Washington. [Google Scholar]
- Petroff R, Murias M, Grant KS, Crouthamel B, McKain N, Shum S, … Burbacher TM (2020). Power spectrum analysis of EEG in a translational nonhuman primate model after chronic exposure to low levels of the common marine neurotoxin, domoic acid. NeuroToxicology, 80, 124–129. 10.1016/j.neuro.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff R, Richards T, Crouthamel B, Mckain N, Stanley C, Grant KS, … Burbacher TM (2019). Chronic, low-level oral exposure to marine toxin, domoic acid, alters whole brain morphometry in nonhuman primates. NeuroToxicology, 72, 114–124. 10.1016/j.neuro.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido O. (2008). Domoic acid toxicologic pathology: A review. Marine Drugs, 6(2), 180–219. 10.3390/md20080010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Pak CW, & Currás-Collazo MC (2006). Sequential involvement of distinct glutamate receptors in domoic acid-induced neurotoxicity in rat mixed cortical cultures: Effect of multiple dose/duration paradigms, chronological age, and repeated exposure. Toxicological Sciences, 89(1), 243–256. 10.1093/toxsci/kfj008 [DOI] [PubMed] [Google Scholar]
- Ramsdell JS, & Gulland FMD (2014). Domoic acid epileptic disease. Marine Drugs, 12(3), 1185–1207. 10.3390/md12031185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SM, Grattan LM, Toben AC, Ausherman C, Trainer VL, Tracy K, & Morris JGJ (2016). Perception of risk for domoic acid related health problems: A cross-cultural study. Harmful Algae, 57(B), 39–44. 10.1016/j.hal.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust L, Gulland F, Frame E, & Lefebvre K. (2014). Domoic acid in milk of free living California marine mammals indicates lactational exposure occurs. Marine Mammal Science, 30(3), 1272–1278. 10.1111/mms.12117 [DOI] [Google Scholar]
- Ryan CL, Robbins MA, Smith MT, Gallant IC, Adams-Marriott AL, & Doucette TA (2011). Altered social interaction in adult rats following neonatal treatment with domoic acid. Physiology & Behavior, 102(3–4), 291–295. 10.1016/j.physbeh.2010.11.020 [DOI] [PubMed] [Google Scholar]
- Ryan JC, Cross CA, & Van Dolah FM (2011). Effects of COX inhibitors on neurodegeneration and survival in mice exposed to the marine neurotoxin domoic acid. Neuroscience Letters, 487(1), 83–87. 10.1016/j.neulet.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Ryan JC, Morey JS, Ramsdell JS, & Van Dolah FM (2005). Acute phase gene expression in mice exposed to the marine neurotoxin domoic acid. Neuroscience, 136(4), 1121–1132. 10.1016/J.NEUROSCIENCE.2005.08.047 [DOI] [PubMed] [Google Scholar]
- Salierno JD, Snyder NS, Murphy AZ, Poli M, Hall S, Baden D, & Kane AS (2006). Harmful algal bloom toxins alter c-Fos protein expression in the brain of killifish, Fundulus heteroclitus. Aquatic Toxicology, 78(4), 350–357. 10.1016/j.aquatox.2006.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant PM, Holland PT, Mountfort DO, & Kerr DS (2008). In vivo seizure induction and pharmacological preconditioning by domoic acid and isodomoic acids A, B and C. Neuropharmacology, 55(8), 1412–1418. 10.1016/j.neuropharm.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Sawant PM, Mountfort DO, & Kerr DS (2010). Spectral analysis of electrocorticographic activity during pharmacological preconditioning and seizure induction by intrahippocampal domoic acid. Hippocampus, 20(8), 994–1002. 10.1002/hipo.20698 [DOI] [PubMed] [Google Scholar]
- Sawant PM, Tyndall JDA, Holland PT, Peake BM, Mountfort DO, & Kerr DS (2010). In vivo seizure induction and affinity studies of domoic acid and isodomoic acids-D, -E and -F. Neuropharmacology, 59(3), 129–138. 10.1016/j.neuropharm.2010.03.019 [DOI] [PubMed] [Google Scholar]
- Scallet AC, Binienda ZK, Caputo FA, Hall S, Paule MG, Rountree RL, … Slikker W. (1993). Domoic acid-treated cynomolgus monkeys (M. fascicularis): Effects of Dose on Hippocampal Neuronal and Terminal Degeneration. Brain Research, 627(2), 307–313. 10.1016/0006-8993(93)90335-K [DOI] [PubMed] [Google Scholar]
- Scallet AC, Kowalke PK, Rountree RL, Thorn BT, & Binienda ZK (2004). Electroencephalographic, behavioral, and c-fos responses to acute domoic acid exposure. Neurotoxicology and Teratology, 26(2), 331–342. 10.1016/j.ntt.2003.10.004 [DOI] [PubMed] [Google Scholar]
- Scallet AC, Schmued LC, & Johannessen JN (2005). Neurohistochemical biomarkers of the marine neurotoxicant, domoic acid. Neurotoxicology and Teratology, 27(5), 745–752. 10.1016/j.ntt.2005.06.018 [DOI] [PubMed] [Google Scholar]
- Schmued LC, Scallet AC, & Slikker W. (1995). Domoic acid-induced neuronal degeneration in the primate forebrain revealed by degeneration specific histochemistry. Brain Research, 695(1), 64–70. 10.1016/0006-8993(95)00799-V [DOI] [PubMed] [Google Scholar]
- Scholin CA, Gulland F, Doucette GJ, Benson S, Busman M, Chavez FP, … Van Dolah FM (2000). Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature, 403(6765), 80–84. 10.1038/47481 [DOI] [PubMed] [Google Scholar]
- Schwarz M, Jandová K, Struk I, Marešová D, Pokorný J, & Riljak V. (2014). Low dose domoic acid influences spontaneous behavior in adult rats. Physiological Research, 63(3), 369–376. https://doi.org/932636 [pii] [DOI] [PubMed] [Google Scholar]
- Shiotani M, Cole TB, Hong S, Park JJY, Griffith WC, Burbacher TM, … Faustman EM (2017). Neurobehavioral assessment of mice following repeated oral exposures to domoic acid during prenatal development. Neurotoxicology and Teratology, 64(June), 8–19. 10.1016/J.NTT.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Shum S, Jing J, Petroff R, Crouthamel B, Grant KS, Burbacher TM, & Isoherranen N. (2020). Maternal-fetal disposition of domoic acid following repeated oral dosing during pregnancy in nonhuman primate. Toxicology and Applied Pharmacology, 398(March), 115027. 10.1016/j.taap.2020.115027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum S, Kirkwood JS, Jing J, Petroff R, Crouthamel B, Grant KS, … Isoherranen N. (2018). Validated HPLC-MS/MS Method To Quantify Low Levels of Domoic Acid in Plasma and Urine after Subacute Exposure. ACS Omega, 3(9), 12079–12088. 10.1021/acsomega.8b02115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvagni PA, Lowenstine LJ, Spraker T, Lipscomb TP, & Gulland FMD (2005). Pathology of domoic acid toxicity in California sea lions (Zalophus californianus). Veterinary Pathology, 42(2), 184–191. 10.1354/vp.42-2-184 [DOI] [PubMed] [Google Scholar]
- Slikker W, Scallet AC, & Gaylor DW (1998). Biologically-based dose-response model for neurotoxicity risk assessment. Toxicology Letters, 102–103, 429–433. 10.1016/S0378-4274(98)00335-X [DOI] [PubMed] [Google Scholar]
- Smith J, Connell P, Evans RH, Gellene AG, Howard MDA, Jones BH, … Caron DA (2018). A decade and a half of Pseudo-nitzschia spp. and domoic acid along the coast of southern California. Harmful Algae, 79, 87–104. 10.1016/j.hal.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Sobotka TJ, Brown R, Quander DY, Jackson R, Smith M, Long SA, … Scallet AC (1996). Domoic acid: Neurobehavioral and neurohistological effects of low-dose exposure in adult rats. Neurotoxicology and Teratology, 18(6), 659–670. 10.1016/S0892-0362(96)00120-1 [DOI] [PubMed] [Google Scholar]
- Stewart GR, Zorumski CF, Price MT, & Olney JW (1990). Domoic acid: A dementia-inducing excitotoxic food poison with kainic acid receptor specificity. Experimental Neurology, 110(1), 127–138. 10.1016/0014-4886(90)90057-Y [DOI] [PubMed] [Google Scholar]
- Strain SM, & Tasker RA (1991). Hippocampal damage produced by systemic injections of domoic acid in mice. Neuroscience, 44(2), 343–352. 10.1016/0306-4522(91)90059-W [DOI] [PubMed] [Google Scholar]
- Stuchal LD, Grattan LM, Portier KM, Kilmon KA, Manahan LM, Roberts SM, & Morris JG (2020). Dose-response assessment for impaired memory from chronic exposure to domoic acid among native American consumers of razor clams. Regulatory Toxicology and Pharmacology, 117, 104759. 10.1016/j.yrtph.2020.104759 [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Hoesing JM, & Whishaw IQ (1990). Domoic acid, an environmental toxin, produces hippocampal damage and severe memory impairment. Neuroscience Letters, 120(2), 221–223. 10.1016/0304-3940(90)90043-9 [DOI] [PubMed] [Google Scholar]
- Suzuki CAM, & Hierlihy SL (1993). Renal clearance of domoic acid in the rat. Food and Chemical Toxicology, 31(10), 701–706. 10.1016/0278-6915(93)90140-T [DOI] [PubMed] [Google Scholar]
- Takemoto T, & Daigo K. (1958). Constituents of Chondria armata. Chemical and Pharmaceutical Bulletin, 6(5), 578–580. 10.1248/cpb.6.578b [DOI] [Google Scholar]
- Tanemura K, Igarashi K, Matsugami T-R, Aisaki K, Kitajima S, & Kanno J. (2009). Intrauterine environment-genome interaction and Children’s development (2): Brain structure impairment and behavioral disturbance induced in male mice offspring by a single intraperitoneal administration of domoic acid (DA) to their dams. The Journal of Toxicological Sciences, 34(Special), SP279–SP286. 10.2131/jts.34.SP279 [DOI] [PubMed] [Google Scholar]
- Tasker RA, Connell BJ, & Strain SM (1991). Pharmacology of systemically administered domoic acid in mice. Canadian Journal of Physiology and Pharmacology, 69(3), 378–382. 10.1139/y91-057 [DOI] [PubMed] [Google Scholar]
- Tasker RA, Perry MA, Doucette TA, & Ryan CL (2005). NMDA receptor involvement in the effects of low dose domoic acid in neonatal rats. Amino Acids, 28(2), 193–196. 10.1007/s00726-005-0167-z [DOI] [PubMed] [Google Scholar]
- Teitelbaum J, Zatorre RJ, Carpenter S, Gendron D, & Cashman NR (1990). Neurological Sequelae of Domoic Acid Intoxication. Symposium Domoic Acid Toxicity, 16(1E), 9–12. 10.2174/13816128236661701241 [DOI] [PubMed] [Google Scholar]
- Thomsen MB, Lillethorup TP, Jakobsen S, Nielsen EH, Simonsen M, Wegener G, … Tasker RA (2016). Neonatal domoic acid alters in vivo binding of [11C]yohimbine to α2-adrenoceptors in adult rat brain. Psychopharmacology, 233(21–22), 3779–3785. 10.1007/s00213-016-4416-5 [DOI] [PubMed] [Google Scholar]
- Tiedeken JA, Muha N, & Ramsdell JS (2013). A cupric silver histochemical analysis of domoic acid damage to olfactory pathways following status epilepticus in a rat model for chronic recurrent spontaneous seizures and aggressive behavior. Toxicologic Pathology, 41(3), 454–469. 10.1177/0192623312453521 [DOI] [PubMed] [Google Scholar]
- Tiedeken JA, & Ramsdell JS (2007). Embryonic exposure to domoic acid increases the susceptibility of zebrafish larvae to the chemical convulsant pentylenetetrazole. Environmental Health Perspectives, 115(11), 1547–1552. 10.1289/ehp.10344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedeken JA, & Ramsdell JS (2013). Persistent neurological damage associated with spontaneous recurrent seizures and atypical aggressive behavior of domoic acid epileptic disease. Toxicological Sciences, 133(1), 133–143. 10.1093/toxsci/kft037 [DOI] [PubMed] [Google Scholar]
- Tiedeken JA, Ramsdell JS, & Ramsdell AF (2005). Developmental toxicity of domoic acid in zebrafish (Danio rerio). Neurotoxicology and Teratology, 27(5), 711–717. 10.1016/j.ntt.2005.06.013 [DOI] [PubMed] [Google Scholar]
- Todd ECD (1993). Domoic Acid and Amnesic Shellfish Poisoning - A Review. Journal of Food Protection, 56(1), 69–83. 10.4315/0362-028x-56.1.69 [DOI] [PubMed] [Google Scholar]
- Toyofuku H. (2006). Joint FAO/WHO/IOC activities to provide scientific advice on marine biotoxins (research report). Marine Pollution Bulletin, 52(12), 1735–1745. 10.1016/j.marpolbul.2006.07.007 [DOI] [PubMed] [Google Scholar]
- Tracy K, Boushey CJ, Roberts SM, Morris JG, & Grattan LM (2016). Communities advancing the studies of Tribal nations across their lifespan: Design, methods, and baseline of the CoASTAL cohort. Harmful Algae, 57(B), 9–19. 10.1016/j.hal.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainer VL, Bates SS, Lundholm N, Thessen AE, Cochlan WP, Adams NG, & Trick CG (2012). Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae, 14, 271–300. 10.1016/j.hal.2011.10.025 [DOI] [Google Scholar]
- Trainer VL, Moore SK, Hallegraeff G, Kudela RM, Clement A, Mardones JI, & Cochlan WP (2020). Pelagic harmful algal blooms and climate change: Lessons from nature’s experiments with extremes. Harmful Algae, 91, 101591. 10.1016/j.hal.2019.03.009 [DOI] [PubMed] [Google Scholar]
- Truelove J, & Iverson F. (1994). Serum Domoic Acid Clearance and Clinical Observations in the Cynomolgus Monkey and Sprague-Dawley Rat following a Single IV Dose. Bulletin of Environmetnal Contamination and Toxicology, 52(4), 479–486. [DOI] [PubMed] [Google Scholar]
- Truelove J, Mueller R, Pulido O, & Iverson F. (1996). Subchronic toxicity study of domoic acid in the rat. Food and Chemical Toxicology, 34(6), 525–529. 10.1016/0278-6915(96)81814-X [DOI] [PubMed] [Google Scholar]
- Truelove J, Mueller R, Pulido O, Martin L, Fernie S, & Iverson F. (1997). 30-day oral toxicity study of domoic acid in cynomolgus monkeys: Lack of overt toxicity at doses approaching the acute toxic dose. Natural Toxins, 5(3), 111–114. 10.1002/nt.5 [DOI] [PubMed] [Google Scholar]
- Tryphonas L, Truelove J, & Iverson F. (1990). Acute neurotoxicity of domoic acid in cynomolgus monkeys (M. fascicularis). Toxicologic Pathology, 18(2), 297–303. 10.1177/019262339001800101 [DOI] [PubMed] [Google Scholar]
- Tryphonas L, Truelove J, Todd ECD, Nera E, & Iverson F. (1990). Experimental oral toxicity of domoic acid in cynomolgus monkeys (Macaca fascicularis) and rats. Food and Chemical Toxicology, 28(10), 707–715. 10.1016/0278-6915(90)90147-F [DOI] [PubMed] [Google Scholar]
- Tsunekawa K, Kondo F, Okada T, Feng GG, Huang L, Ishikawa N, & Okada S. (2013). Enhanced expression of WD repeat-containing protein 35 (WDR35) stimulated by domoic acid in rat hippocampus: Involvement of reactive oxygen species generation and p38 mitogen-activated protein kinase activation. BMC Neuroscience, 14. 10.1186/1471-2202-14-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. (1983). Elements of Episodic Memory. Canadian Psychology, 26(3), 351. [Google Scholar]
- Vidal A, Correa J, & Blanco J. (2009). Effect of some habitual cooking processes on the domoic acid concentration in the cockle (Cerastoderma edule) and Manila clam (Ruditapes philippinarum). Food Additives & Contaminants: Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment., 26(7), 1089–1095. 10.1080/02652030902855422 [DOI] [PubMed] [Google Scholar]
- Vieira AC, Alemañ N, Cifuentes JM, Bermúdez R, Peña ML, & Botana LM (2015). Brain pathology in adult rats treated with domoic acid. Veterinary Pathology, 52(6), 1077–1086. 10.1177/0300985815584074 [DOI] [PubMed] [Google Scholar]
- Vieira AC, Cifuentes JMJM, Bermúdez R, Ferreiro SF, Castro ARR, & Botana LM (2016). Heart Alterations after Domoic Acid Administration in Rats. Toxins, 8(3), 68. 10.3390/toxins8030068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranyac-Tramoundanas A, Harrison JC, Clarkson AN, Kapoor M, Winburn IC, Kerr DS, & Sammut IA (2008). Domoic acid impairment of cardiac energetics. Toxicological Sciences, 105(2), 395–407. 10.1093/toxsci/kfn132 [DOI] [PubMed] [Google Scholar]
- Vranyac-Tramoundanas A, Harrison JC, Sawant PM, Kerr DS, & Sammut IA (2011). Ischemic cardiomyopathy following seizure induction by domoic acid. American Journal of Pathology, 179(1), 141–154. 10.1016/j.ajpath.2011.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter JA, Leek DM, & Falk M. (1992). NMR study of the protonation of domoic acid. Canadian Journal of Chemistry, 70(4), 1156–1161. 10.1139/v92-151 [DOI] [Google Scholar]
- Wang D, Zhao J, Li S, Shen G, & Hu S. (2018). Quercetin attenuates domoic acid-induced cognitive deficits in mice. Nutritional Neuroscience, 21(2), 123–131. 10.1080/1028415X.2016.1231438 [DOI] [PubMed] [Google Scholar]
- Wang GHJ, Schmued LC, Andrews AM, Scallet AC, Slikker WH, & Binienda ZH (2000). Systemic Administration of Domoic Acid-Induced Spinal Cord Lesions in Neonatal Rats. Journal of Spinal Cord Medicine, 23(1), 31–39. 10.1080/10790268.2000.11753506 [DOI] [PubMed] [Google Scholar]
- Wang Y, & Qin ZH (2010). Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis, 15(11), 1382–1402. 10.1007/s10495-010-0481-0 [DOI] [PubMed] [Google Scholar]
- Washington State Department of Health. (n.d.). Domoic Acid in Razor Clams. Retrieved May 10, 2020, from https://www.doh.wa.gov/CommunityandEnvironment/Shellfish/RecreationalShellfish/Illnesses/Biotoxins/DomoicAcidinRazorClams
- Watanabe KH, Andersen ME, Basu N, Carvan MJ, Crofton KM, King KA, … Schultz IR (2011). Defining and modeling known adverse outcome pathways: Domoic acid and neuronal signaling as a case study. Environmental Toxicology and Chemistry, 30(1), 9–21. 10.1002/etc.373 [DOI] [PubMed] [Google Scholar]
- Wekell JC, Gauglitz EJ, Barnett HJ, Hatfield CL, & Eklund M. (1994). The occurrence of domoic acid in razor clams (Siliqua patula), Dungeness crab (Cancer magister), and achovies (Engraulis mordax). Journal of Shellfish Research, 13(2), 587–593. 10.2983/035.029.0302 [DOI] [Google Scholar]
- Wekell JC, Jurst J, & Lefebvre KA (2004). The origin of the regulatory limits for PSP and ASP toxins in shellfish. Journal of Shellfish Research, 23(3), 927–930. [Google Scholar]
- Wekell JC, Trainer VL, Ayres D, & Simons D. (2002). A study of spatial variability of domoic acid in razor clams: Recommendations for resource management on the Washington coast. Harmful Algae, 1(1), 35–43. 10.1016/S1568-9883(02)00004-5 [DOI] [Google Scholar]
- Wells ML, Karlson B, Wulff A, Kudela R, Trick C, Asnaghi V, … Trainer VL (2020). Future HAB science: Directions and challenges in a changing climate. Harmful Algae, 91, 101632. 10.1016/j.hal.2019.101632 [DOI] [PubMed] [Google Scholar]
- Wells ML, Trainer VL, Smayda TJ, Karlson BSO, Trick CG, Kudela RM, … Cochlan WP (2015). Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae, 49, 68–93. 10.1016/j.hal.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore L, & Nance DM (1991). Differential and sex-specific effects of kainic acid and domoic acid lesions in the lateral septal area of rats on immune function and body weight regulation. Experimental Neurology, 113(2), 226–236. 10.1016/0014-4886(91)90179-G [DOI] [PubMed] [Google Scholar]
- Wood PL, Richard JW, Pilapil C, & Nair NPV (1982). Antagonists of excitatory amino acids and cyclic guanosine monophosphate in cerebellum. Neuropharmacology, 21(12), 1235–1238. 10.1016/0028-3908(82)90126-5 [DOI] [PubMed] [Google Scholar]
- Wright JLC, Boyd RK, de Freitas ASW, Falk M, Foxall RA, Jamieson WD, … Walter JA (1989). Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from eastern Prince Edward Island. Canadian Journal of Chemistry, 67, 481–490. 10.1139/v89-075 [DOI] [Google Scholar]
- Wu D, Lu J, Zhang Y, Zheng Y, Hu B, Cheng W, … Li M. (2013). Ursolic acid improves domoic acid-induced cognitive deficits in mice. Toxicology and Applied Pharmacology, 271(2), 127–136. 10.1016/j.taap.2013.04.038 [DOI] [PubMed] [Google Scholar]
- Wu D, Lu J, Zheng YL, Zhang YQ, Hu B, Cheng W, … Li MQ (2012). Small interfering RNA-mediated knockdown of protein kinase C zeta attenuates domoic acid-induced cognitive deficits in mice. Toxicological Sciences, 128(1), 209–222. 10.1093/toxsci/kfs124 [DOI] [PubMed] [Google Scholar]
- Xi D, Peng Y-G, & Ramsdell JS (1997). Domoic acid is a potent neurotoxin to neonatal rats. Natural Toxins, 5(2), 74–79. [DOI] [PubMed] [Google Scholar]
- Xu R, Tao Y, Wu C, Yi J, Yang Y, Yang R, & Hong D. (2008). Domoic acid induced spinal cord lesions in adult mice: Evidence for the possible molecular pathways of excitatory amino acids in spinal cord lesions. NeuroToxicology, 29(4), 700–707. 10.1016/j.neuro.2008.04.011 [DOI] [PubMed] [Google Scholar]
- Zabka TS, Goldstein T, Cross C, Mueller RW, Kreuder-Johnson C, Gill S, & Gulland FMD (2009). Characterization of a degenerative cardiomyopathy associated with domoic acid toxicity in California sea lions (Zalophus californianus). Veterinary Pathology, 46(1), 105–119. 10.1354/vp.46-1-105 [DOI] [PubMed] [Google Scholar]
- Zatorre R. (1990). Memory Loss Following Domoic Acid Intoxication from Ingestion of Toxic Mussels. Symposium Domoic Acid Toxicity, 101–103. [PubMed] [Google Scholar]
- Zuloaga DG, Lahvis GP, Mills B, Pearce HL, Turner J, & Raber J. (2016). Fetal domoic acid exposure affects lateral amygdala neurons, diminishes social investigation and alters sensory-motor gating. NeuroToxicology, 53, 132–140. 10.1016/j.neuro.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]