Abstract
Background
Campylobacteriosis is currently the most frequently reported zoonosis. Dogs, especially puppies or those with diarrhea, are considered a possible source of human infection. Probiotic bacteria, such as Lactobacillus species, seem to be a valuable tool in controlling of intestinal pathogenic microorganisms in dogs. The main purpose of this study was to assess the anti-Campylobacter activity and some probiotic properties, like ability to produce H2O2, bile salt and low pH tolerance of Lactobacillus strains isolated from gastrointestinal tract of healthy dogs.
Results
A total of 39 rectal swabs derived from healthy dogs and 19 from dogs with diarrhea were examined to detect Lactobacillus and Campylobacter bacteria respectively. In total, 30 strains of Lactobacillus genus and four strains of Campylobacter genus were isolated and identified. Of the 30 strains of Lactobacillus, 22 showed an inhibitory effect towards Campylobacter. Four strains with the strongest antagonism towards Campylobacter bacteria (L. salivarius 25 K/L/1, L. rhamnosus 42 K/L/2, L. sakei 50 K/L/1 and L. agilis 55 K/L/1) were selected to assess their potential probiotic traits. Three out of four analyzed strains produced extracellular H2O2. All displayed very good or moderate survival at pH 3.0 and 2.0 and showed high tolerance to 0.5% and 1% bile salts.
Conclusions
Among selected Lactobacillus strains, all may have a potential probiotic application in reducing Campylobacter spp. in dogs and thus prevent transmission of infection to humans, although the best candidate for probiotic seems to be L. sakei 50 K/L/1. Further in vitro and in vivo studies are needed.
Keywords: Campylobacter, Dog, Antibacterial activity, Lactobacillus, Probiotics
Background
Campylobacter is a Gram-negative, microaerophilic bacteria causing one of the most common bacterial gastroenteritis. This organism is frequently found in the alimentary tract of numerous host species, including companion animals. For humans, infections with Campylobacter species, most commonly C. jejuni and C. coli, are the main cause of food-borne diarrhea [1]. In 2019, in European Union, there were noted 220,682 cases of campylobacteriosis, while the numbers of Salmonella cases were 87,923 [2]. The most common sources of infections are consumption of raw and undercooked poultry, unpasteurized milk, contaminated water, and direct animal contact [3]. It is estimated (data from 2017) that, around 6% cases of human campylobacteriosis are caused by contact with pets [4]. In recent years, it has been demonstrated that dogs, especially those less than 6 months of age, should be regarded as a potential source of Campylobacter infections [5, 6]. Predominant Campylobacter species detected in feces of dogs are C. upsaliensis, C. jejuni, C. helveticus and C. coli [5, 7, 8].
Dogs and cats are generally considered as asymptomatic carriers of Campylobacter. However, this pathogen can cause severe, gastrointestinal disease, particularly in young or immune suppressed animals [9]. Unfortunately, the extended treatment of bacterial-associated diarrhea with broad-spectrum antibiotics can result in increased antimicrobial resistance [10]. Therefore, there is a need for alternative therapies, such as probiotics.
Bacteria of the genus Lactobacillus are recognized candidates for probiotics. They are non-pathogenic organisms that may eliminate unfavorable microflora by several mechanisms such as the production of antimicrobial substances (lactic acid, bacteriocins, hydrogen peroxide), inhibition of bacterial adhesion to the mucosa, competition for nutrients, and stimulation of immunity [11]. There are several studies investigating the antimicrobial activity and metabolic potentials of Lactobacillus species isolated from the intestinal tract of dogs [12–14]. Currently, most of the probiotic microorganisms widely used in application studies on dogs are mainly of human origin [15, 16]. Potential probiotic strains for dogs are preferred to be of canine intestinal origin, since such strains exhibit host specificity [17]. Therefore, the aim of this study was to isolate, identify and evaluate the anti-Campylobacter activity of Lactobacillus spp. strains derived from the intestinal tract of healthy dogs, as well as to assess some probiotic traits of selected strains, like ability to produce H2O2, and bile salt, and low pH tolerance. To our knowledge there are no published studies so far, which assess anti-Campylobacter activity of canine-originated Lactobacillus bacteria.
Results
Identification of Lactobacillus and Campylobacter strains
From 39 rectal swabs of healthy dogs, a total of 30 strains of the genus Lactobacillus were isolated, which was confirmed by genus-specific Polymerase Chain Reaction (PCR) analysis. Using API 50CH test and species-specific PCR assays, 28 isolates were classified into six species, i.e., L. brevis (n = 4), L. casei (n = 4), L. crispatus (n = 2), L. delbrueckii (n = 3), L. rhamnosus (n = 8) and L. salivarius (n = 7). In the case of two Lactobacillus isolates, species identification using previously mentioned methods was ambiguous, so these strains were classified by MALDI-TOF MS. One of them was classified as L. sakei and the other as L. agilis with a Biotyper log (score) values greater than 2.0 (high-confidence identification).
Campylobacter strains were isolated from 4 (21%) out of 19 dogs with diarrhea (one strain from each sample). By API Campy system and species-specific PCR technique three strains were identified as C. jejuni and one as C. upsaliensis.
Agar slab method
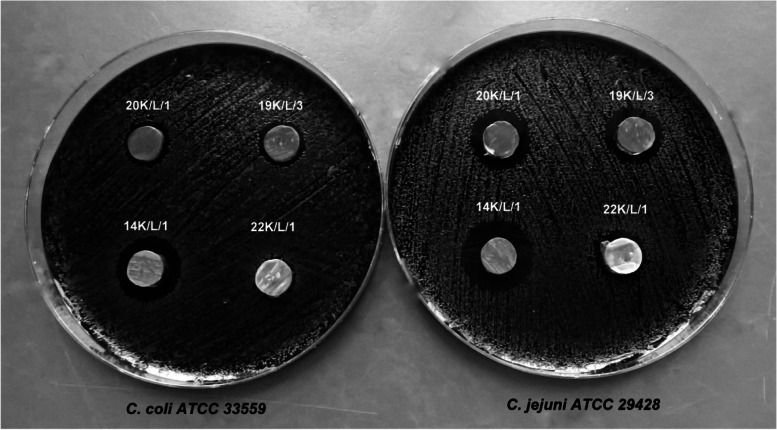
Results obtained by the agar slab method are presented in Table 1 as the mean diameter of the inhibition zone ± SD (standard deviation). Among 30 strains of Lactobacillus, 22 showed an inhibitory effect towards two reference Campylobacter strains (C. jejuni ATCC 29,428 and C. coli ATCC 33,559). The diameter of the growth inhibition zones induced by all Lactobacillus strains ranged from 9.0 ± 0.0 mm to 21.25 ± 1.1 mm, where 9 mm was the diameter of the slab (Fig. 1). The activity of the tested Lactobacillus strains against indicator bacteria was mainly correlated with the species. All analyzed isolates of L. delbrueckii (n = 3) exhibited no antagonistic properties (9.0 ± 0.0 mm) towards C. jejuni and C. coli, but strains of L. rhamnosus (n = 8) or L. brevis (n = 4) were all active against these pathogens. For strains belonging to the species L. salivarius, there was observed a large heterogeneity of the size of inhibitory zones. In this group, there was four out of seven strains that exhibited no antagonistic properties towards indicator strains and one isolate with the strongest inhibition of Campylobacter growth (mean inhibition zone 20.25 ± 1.4 mm).
Table 1.
Anti-Campylobacter activity of Lactobacillus isolates
Results of agar slab method are presented as the mean diameter of the inhibition zone ± SD. Means with different letters within the same column indicate significant difference at P < 0.05
Grey highlights indicate strains that have been selected for further research
Fig. 1.
Antagonistic activity of some Lactobacillus isolates against reference strains of Campylobacter in the agar slab method
The average diameter of inhibition zones for C. jejuni was 13.6 ± 3.8 mm, and for C. coli 12.6 ± 3.4 mm. There were no statistically significant differences (P < 0.05) in susceptibility of these two species of Campylobacter to the antagonistic substances produced by Lactobacillus strains.
Serial dilution method
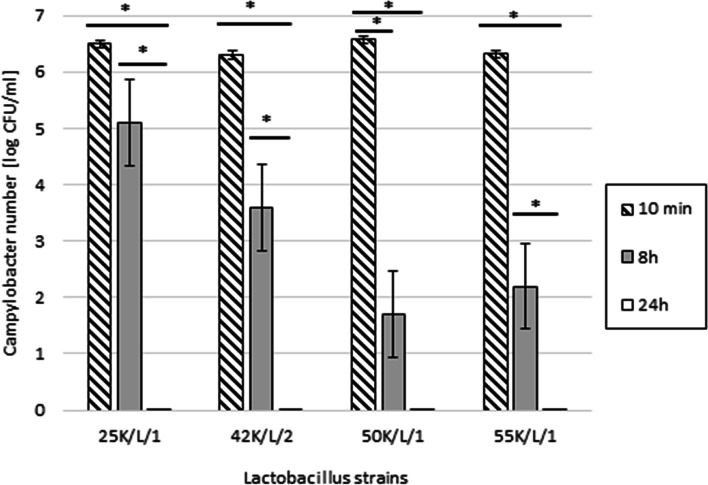
Among four selected Lactobacillus strains: 25 K/L/1 (L. salivarius), 42 K/L/2 (L. rhamnosus), 50 K/L/1 (L. sakei) and 55 K/L/1 (L. agilis), that had induced the biggest growth inhibition zones in the agar slab method, all confirmed strong inhibitory properties towards Campylobacter strains isolated from dogs. During the experiment, the Campylobacter number changed significantly (Fig. 2). After 8 h since the start of the procedure, the Campylobacter counts decreased by 1–5 logarithms (depending on the Lactobacillus strains). The most active strain turned out to be L. sakei 50 K/L/1. Only its activity contributed to a statistically significant reduction in the number of Campylobacter cells during the first 8 h of the experiment (P < 0.05). The remaining strains of Lactobacillus also showed high antimicrobial activity. Within 24 h all resulted in a complete inhibition of the growth of Campylobacter bacteria.
Fig. 2.
Relation of Campylobacter number [log CFU/ml] with the antibacterial activity of Lactobacillus strains. Error bars represent standard errors. Asterisks indicate the statistical differences: * P < 0.05
Well diffusion method
The pH values of native acidified supernatants obtained from 24 h cultures of Lactobacillus isolates ranged from 3.8 to 4.5. The average diameter of the growth inhibition zones induced by native acidified supernatants of tested 4 Lactobacillus strains was 16.26 ± 0.79 mm, where the well diameter was 9 mm. The average diameter of the growth inhibition zones induced by these strains in agar slab method was 19.9 ÷ 1.05. This difference is statistically significant (P < 0.05). Supernatants of Lactobacillus with neutralized acids (pH 6.8—7.0) did not exhibit any antagonistic activity towards Campylobacter bacteria.
Production of H2O2
Three out of four mentioned above strains of Lactobacillus produced extracellular H2O2. The highest rate of production (10 mg/L after 10 min from the start of the experiment and 30 mg/L 24 h later) was observed in 50 K/L/1 strain. Strains 25 K/L/1 and 55 K/L/1 after 24 h of incubation showed lower H2O2 production, 1 mg/L and 3 mg/L respectively. Detailed results are shown in Table 2.
Table 2.
Production of H2O2 by selected Lactobacillus strains
| Strain | Species | Ability to produce H2O2 (mg/L) | ||
|---|---|---|---|---|
| 10 min | 4 h | 24 h | ||
| 25 K/L/1 | L. salivarius | 1 | 1 | 1 |
| 42 K/L/2 | L. rhamnosus | 0 | 0 | 0 |
| 50 K/L/1 | L. sakei | 10 | 10 | 30 |
| 55 K/L/1 | L. agilis | 1 | 1 | 3 |
SD data were negligible
Tolerance to low pH
All selected Lactobacillus strains were tolerant to acidic environment. The highest tolerance to low pH showed two strains: 50 K/L/1 (89.9% of viability at pH 3.0 and 69.1% at pH 2.0) and 42 K/L/2 (80.1% of viability at pH 3.0 and 79.5% at pH 2.0). Strains 25 K/L/1 and 55 K/L/1 had a good survival rate at pH 3.0 (percentage of the viability was 85.4 and 83.4% respectively) and a poorer survival rate at pH 2.0 (percentage of the viability was 50.1 and 28.6% respectively). Detailed results are shown in Table 3.
Table 3.
The ability to survive of tested Lactobacillus strains in the different pH values
| Strain | Ability to survive in low pH values | ||||
|---|---|---|---|---|---|
| pH 6.5 (control) | pH 3.0 | pH 2.0 | |||
| log (CFU/ml)a | log (CFU/ml)a | viability [%] | log (CFU/ml)a | viability [%] | |
| 25 K/L/1 | 8.71 ± 0.03b | 7.44 ± 0.57a | 85.4 | 4.44 ± 0.33c | 50.1 |
| 42 K/L/2 | 9.02 ± 0.03a | 7.23 ± 0.16a | 80.1 | 7.17 ± 0.18a | 79.5 |
| 50 K/L/1 | 8.29 ± 0.06c | 7.45 ± 0.16a | 89.9 | 5.73 ± 0.17b | 69.1 |
| 55 K/L/1 | 8.62 ± 0.05b | 7.19 ± 0.16a | 83.4 | 2.47 ± 0.21d | 28.6 |
aValues are mean ± standard deviations, N = 2
Means with different letters within the same column indicate significant difference at P < 0.05
Bile salt tolerance
The tested isolates of lactic acid bacteria were exposed to bile salts in concentrations of 0.5% and 1%. All four Lactobacillus strains employed in the study were tolerant to both bile salts concentrations (percentage of the viability ranged from 78.2 to 88.8%). The highest resistance to bile was demonstrated by strain 50 K/L/1 (88.8% of viability at 0.5% bile salts concentration and 86.4% of viability at 1% bile salts concentration). Survival of all strains is presented in Table 4.
Table 4.
The ability to survive of tested Lactoabcillus strains in the different concentration of bile salts
| Strain | Ability to survive in the presence of bile salts | ||||
|---|---|---|---|---|---|
| MRS (control) | MRS + 0.5% bile | MRS + 1% bile | |||
| log CFU/mla | log (CFU/ml)a | viability [%] | log (CFU/ml)a | viability [%] | |
| 25 K/L/1 | 8.49 ± 0.41abc | 6.94 ± 0.76a | 81.7 | 6.64 ± 0.57a | 78.2 |
| 42 K/L/2 | 8.74 ± 0.08ab | 7.20 ± 0.12a | 82.4 | 7.06 ± 0.16a | 80.8 |
| 50 K/L/1 | 8.15 ± 0.15c | 7.24 ± 0.17a | 88.8 | 7.04 ± 0.15a | 86.4 |
| 55 K/L/1 | 8.81 ± 0.12a | 7.31 ± 0.23a | 82.9 | 7.28 ± 0.28a | 82.6 |
aValues are mean ± standard deviations, N = 2
Means with different letters within the same column indicate significant difference at P < 0.05
Discussion
In recent years, there has been an increased interest in using probiotics in small animal veterinary medicine. In the literature only a few studies can be found, where probiotic strains have been isolated from dogs [17–19]. In this study, 30 strains of Lactobacillus spp. were isolated from rectal swabs of healthy dogs and assessed for their antagonistic activity against Campylobacter spp. strains.
The results of agar slab method showed that most of the tested Lactobacillus strains were able to inhibit the growth of C. jejuni and C. coli. To confirm the capacity to produce some antimicrobial substances by four selected strains, we analyzed the activity of cell-free broth using well diffusion and serial dilution methods and we assessed the ability to synthesize H2O2. The results of the serial dilution method indicated that all four Lactobacillus strains show strong inhibitory effect towards Campylobacter bacteria of canine origin: C. upsaliensis and C. jejuni, but the strongest was exhibited by L. sakei 50 K/L/1. The results of the well diffusion method indicated that the reduced pH of the supernatant (due to lactic acid) play a key role in inhibiting the growth of Campylobacter bacteria. Neal-McKinney et al. showed that production of antimicrobial substances, like lactic acid, by Lactobacillus strains is responsible for disrupting the membrane of Campylobacter and reducing the growth of these pathogens [20]. The pH-dependent, anti-Campylobacter activity of cell-free supernatants of Lactobacillus strains has also been demonstrated by Bratz et al. [21].
L. sakei 50 K/L/1 turned out to be not only the most active isolate, but also the only one, at which a relationship between antimicrobial effect and high production of hydrogen peroxide was observed. Hydrogen peroxide is a very potent, biologically active substance that react with lipids, proteins and nucleic acids causing oxidative cell damage [22]. Antimicrobial activity of H2O2-producing Lactobacillus has been already proven [23–25]. The quantity of H2O2 produced by different Lactobacillus species varies, depending on the strain and for some of them production is not observed [26]. Some results indicate that there is no correlation between antimicrobial activity and H2O2 production of strains [27, 28]. It has also been confirmed in this study. Strains other than L. sakei 50 K/L/1, exhibited strong inhibition of Campylobacter growth, but their production of H2O2 was generally weak. The lack of relationship between amounts of hydrogen peroxide production and the antimicrobial activity of Lactobacillus strains isolated from fecal microbiota of dogs has also been described previously [29]. It is suggested that synthesis of hydrogen peroxide is a rare feature of intestinal Lactobacillus, as it is mainly related to vaginal isolates [30].
To check the capacity of Lactobacillus strains to act as probiotics, it is essential to determine their ability to survive in the low pH and in the high concentration of bile salt that are present in the intestinal tract. Thus, we decided to check whether the chosen strains display these properties. The pH levels of gastric juice may vary from 2.5 to 3.0 depending on the kind and feeding time, the growing stage, as well as the kind of animal [31]. Therefore, the survival of Lactobacillus strains was tested in deMan-Rogosa-Sharpe (MRS) medium with pH adjusted to 2.0, 3.0 and 6.5 (optimal conditions). Bacterial growth under low pH was monitored for 3 h, as it simulates bacterial residency in the stomach [32]. The results on acid tolerance showed that all tested Lactobacillus strains were tolerant to acidic environment. All strains had a good survival rate at pH 3.0 (< 20% of inhibition), but a moderate survival rate at pH 2.0. The highest tolerance to pH 2.0 showed two strains 50 K/L/1 and 42 K/L/2. After 3 h of incubation at pH value 2.0, they exhibited respectively 69,1% and 79,5% of viability. The results described in other studies confirm that after 3 h of exposure to a pH ≤ 2, the viability count of lactic acid bacteria is significantly reduced [33–35]. According to Liong and Shah reports, the survival of Lactobacillus strains at pH 3.0 for 2–3 h is acceptable as one of the requirements for the bacteria to be considered as probiotics [36]. Although most bacteria survive poorly at low pH values, it is suggested, that bacteria of intestinal origin tend to be more resistant to gastric pH, what is in accordance with our results [37]. The results obtained in the current study suggest also, that the tolerance of low pH values, is primarily strain-dependent feature.
Resistance to bile is also very important feature for probiotic strains, which affects their survival and the ability to reach the large intestine. The mechanism of toxic depletion of bile salts onto bacterial cells is not fully understood. However, it is known that these are amphipathic molecules which act as detergents that damage cell walls demonstrating a strong antimicrobial activity [38]. There is little information regarding bile concentration in the canine intestine. In the literature, for similar studies, the bile concentration of 0.3% is most often used as the corresponding amount of this component in the human small intestine [31, 39]. However, the variability of this parameter is emphasized in relation to both humans and animals. Strompfova et al. checked the survival rate of L. fermentum AD1 of canine origin in the presence of 1% bile [40]. In turn, Coman et al. assessed the tolerance of probiotic strains isolated from dog faecal samples to bile salts at concentration 0.1%, 0.3% and 0.5% [12]. Based on the above literature data, Lactobacillus strains in the current study were treated with bile salts at a concentration 0.5% and 1%. All tested Lactobacillus strains were very tolerant to both concentrations of bile, but the best survival rate showed strain 50 K/L/1.
Conclusions
This study showed that all four selected strains of Lactobacillus may have potential application in reducing the level of canine intestine colonization by Campylobacter spp. and thus prevent infections in both dogs and humans. All strains possess characteristics of a probiotic candidates, but the most promising seems to be L. sakei 50 K/L/1. This strain turned out to be the most active against Campylobacter bacteria and possessed the highest tolerance for bile salts and acidic pH. Moreover, it produces high concentrations of hydrogen peroxide, which is a desirable feature of probiotic bacteria and unique in strains that usually live in the intestine. Further experiments are needed to investigate antibiotic susceptibility, biofilm formation, the adherence properties using epithelium cell lines and animal models.
Methods
Isolation and growth conditions of Lactobacillus strains
Rectal swabs taken from 39 of healthy dogs were examined to isolate Lactobacillus strains. In this study, all samples were obtained during routine investigations by practicing veterinarians from veterinary clinics located in two cities of Poland: Krakow and Tarnow. Specimens were transported to the University Centre of Veterinary Medicine, Jagiellonian University – Agricultural University in Krakow at refrigerator temperature (2–8 °C), then plated on MRS agar (Oxoid, UK) and cultured at 37 °C for 48 h in anaerobic conditions (Genbox anaer, bioMerieux, France). The cultivated colonies were identified for genus and species using Gram staining, commercially available API 50CH (bioMerieux, France) system and PCR assays (primers and protocols listed in Table 5). In the case of ambiguous species identification results, MALDI-TOF mass spectrometry (MS) was additionally used.
Table 5.
Primer sequences used for this study
| Microorganism | Primer name | Sequence (5' to 3') | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| Campylobacter spp. |
cadF cadR |
TTG AAG GTA ATT TAG ATA TG CTA ATA CCT AAA GTT GAA AC |
400 | [41] |
| Campylobacter jejuni |
CJF CJR |
ACT TCT TTA TTG CTT GCT GC GCC ACA ACA AGT AAA GAA GC |
323 | [42] |
| Campylobacter upsaliensis |
CUF CUR |
AAT TGA AAC TCT TGC TAT CC TCA TAC ATT TTA CCC GAG CT |
204 | [42] |
| Lactobacillus spp. | LbLMA-1 | CTC AAA ACT AAA CAA AGT TTC | 250 | [43] |
| R-161 | CTT GTA CAC ACC GCC CGT CA | |||
| Lactobacillus brevis |
LbrevF LbrevR |
CTT GCA CTG ATT TTA ACA GGG CGG TGT GTA CAA GGC |
1340 | [44] |
| Lactobacillus casei—group |
IDL11F IDL03R |
TGG TCG GCA GAG TAA CTG TTG TCG CCA CCT TCC TCC GGT TTG TCA |
727 | [45] |
| Lactobacillus crispatus |
Cri 16SI Cri 16SII |
GTA ATG ACG TTA GGA AAG CG ACT ACC AGG GTA TCT AAT CC |
734 | [46] |
| Lactobacillus delbrueckii |
IDL31F IDL03R |
CCA CCT TCC TCC GGT TTG TCA CTG TGC TAC ACC TAG AGA TAG GTG G |
184 | [45] |
| Lactobacillus rhamnosus | PrI | CAG ACT GAA AGT CTG ACG G | 190 | [46] |
| RhaII | GCG ATG CGA ATT TCT ATT ATT | |||
| Lactobacillus salivarius |
Lsal-1 Lsal-2 |
AAT CGC TAA ACT CAT AAC CT CAC TCT CTT TGG CTA ATC TT |
411 | [47] |
Isolation and growth conditions of Campylobacter strains
Rectal swabs isolated from 19 dogs with diarrhea were examined for Campylobacter species. The samples were plated on Campylobacter blood-free selective agar CCDA (Oxoid, UK) and cultured for 48 h at 42 ± 1 °C under microaerophilic conditions (Genbox microaer, bioMerieux, France). Species identification of the grown colonies was performed using a commercial, standardized system for the identification of Campylobacter—API Campy (bioMerieux, France) and PCR technique, performed according to protocols described previously (Table 5).
Anti-Campylobacter activity of Lactobacillus – agar slab method
In total, 30 strains of Lactobacillus were collected. All isolates were suspended in 0.9% NaCl so that the optical density (OD) of the suspension at 600 nm was 0.5. The suspensions were seeded onto MRS agar and incubated for 24 h at 37 °C in anaerobic conditions. Then, agar slabs were cut (9 mm in diameter) and placed on Campylobacter blood-free selective agar CCDA inoculated with 0.5 ml of the Campylobacter indicator strain suspended in 0.9% NaCl (OD600 = 0.5). As indicator strains, two reference Campylobacter strains were used: C. jejuni ATCC 29,428 and C. coli ATCC 33,559. The plates were incubated at 42 ± 1 °C for 48 h in microaerophilic conditions. After incubation, the diameter of the zone of growth inhibition was measured. The experiment was performed in duplicate.
Anti-Campylobacter activity of Lactobacillus– serial tenfold dilution method
The current and the subsequent experiments were carried out on four selected Lactobacillus strains that had induced significant growth inhibition zones in the agar slab method: 25 K/L/1 (L. salivarius), 42 K/L/2 (L. rhamnosus), 50 K/L/1 (L. sakei) and 55 K/L/1 (L. agilis). Four strains of Campylobacter genus that had been isolated in this study from faeces of dogs with diarrhea: 18 K/C/1 (C. jejuni), 25 K/C/1 (C. jejuni), 27 K/C/1 (C. jejuni) and 28 K/C/1 (C. upsaliensis) were used as target strains to test the inhibitory activity of selected Lactobacillus.
Tested Lactobacillus strains were cultured in 10 ml of TSB (Tryptic Soy Broth, Biocorp, Poland) for 24 h at 37 °C under anaerobic condition to get a final concentration of 107 CFU/ml (colony-forming units per milliliter). Then, the suspensions were centrifuged (12,000 × g, 15 min, 20 °C) and the supernatants were sterile-filtered using a 0.22 μm Millipore filter (VWR, Germany). Strains of Campylobacter were also cultured in 10 ml of TSB for 24 h, at 42 ± 1 °C under microaerophilic condition. The final concentration of suspensions was 107 CFU/ml as well. The supernatants of Lactobacillus strains and the suspensions of Campylobacter cultures were mixed in the ratio of 9:1. The mixtures of Lactobacillus supernatants and Campylobacter cultures were spread over Columbia Agar with 5% sheep blood (Biocorp, Poland) in the following dilutions: 0, -2 and –4, respectively within 10 min., 8 h and 24 h after the moment they had been mixed. The plates were incubated for 24 h under microaerophilic conditions at 42 ± 1 °C. Campylobacter colonies were enumerated and expressed as CFU/ml. In this experiment 16 samples wereanalyzed in duplicate.
Anti-Campylobacter activity of Lactobacillus – well diffusion method
This experiment was performed to determine the mechanism of the antimicrobial activity of selected Lactobacillus strains. Isolates were grown in 10 ml of MRS broth for 24 h at 37 °C in anaerobic conditions to get the final concentration of 107 CFU/ml. The suspensions were centrifuged (12,000 × g, 15 min, 20 °C) and the supernatants were sterile-filtered using a 0.22 μm Millipore filter. Then, each sample of supernatant was divided into 2 equal volumes. In half of the samples the pH was adjusted to 6.8—7.0 using 1 M NaOH (to eliminate the influence of organic acids and low pH).
Strains of Campylobacter were cultured in 10 ml of TSB for 24 h at 42 °C under microaerophilic conditions and inoculated on Columbia Agar with 5% sheep blood. Then, agar slabs were cut (9 mm in diameter) and filled with 200 ul of the supernatants with neutralized acids, as well as native acidified supernatants (control). After 48 h of incubation at 42 ± 1 °C under microaerophilic conditions, the plates were checked for inhibition zones. The experiment involving 16 samples was run in duplicate.
Production of H2O2
The ability of selected Lactobacillus strains to produce H2O2 was determined by semiquantitative Peroxide Test Strip method (Merck, Germany). For this assay, Lactobacillus strains were cultured in 10 ml of MRS broth for 24 h at 37 °C in aerobic conditions. The mean density of bacteria at the beginning of an experiment was estimated approximately as 1 × 106 CFU/ml. H2O2 measurement was performed in three-time intervals: 10 min., 4 h, and 24 h after the start of culture. The Peroxide Test Strip indicates the presence of H2O2 by a color change on an indicator strip. The results were compared to a provided color scale (detection scale between 0 and 100 mg/L). The experiment involving 16 samples was run in duplicate.
Tolerance for acidic pH
The group of selected Lactobacillus strains were grown overnight in MRS broth at 37 °C in anaerobic conditions and then centrifuged (12,000 × g, 5 min, 20 °C). Pellets were washed with PBS and resuspended in 1 ml of PBS (the final suspensions had a value of 1 × 108 CFU/ml). 100 ul of the suspensions were added to 900 ul of MRS broth with pH adjusted to 2.0 and 3.0 with 1 M HCl. Moreover 100 ul of the suspensions were added to 900 ul of MRS broth with pH 6.5 (positive control). The bacteria were incubated at 37 °C in anaerobic conditions. Samples were taken after 3 h and the viable number of bacteria were determined by standard serial tenfold dilution method on MRS agar. The survival of bacteria was expressed as a percentage calculated from the logarithms of the number of CFU after 3 h of incubation in environment with pH 2.0 or 3.0 compared to the logarithms of the number of CFU after the same time intervals in an optimal pH environment (6.5). The experiment involving 16 samples was run in duplicate.
Bile tolerance test
The bile tolerance test of Lactobacillus isolates was performed by addition of bile salts (Merck, Germany) to MRS medium, to the final concentration of bile salts 0.5% and 1% and then the mixture was inoculated with bacteria for 1 × 108 CFU/ml. For the control samples, distilled water was added to MRS medium instead of bile salts. Lactobacillus bacteria were incubated in this medium at 37 °C in anaerobic conditions. The samples were collected at time 3 h and to estimate the bacteria survival a standard serial tenfold dilution method on MRS agar was performed. The survival of bacteria was expressed as a percentage calculated from the logarithms of the number of CFU after 3 h of incubation with the addition of bile salts (0.5% or 1%) relative to the logarithms of the number of CFU after the same time intervals without adding the bile salts to the medium. The experiment involving 16 samples was run in duplicate.
Statistical analysis
All experiments were carried out in duplicate, and the results were expressed as a mean ± standard deviation. To analyze the obtained results, the following statistical tests were used: the one-way analysis of variance (ANOVA) to compare the mean diameters of the inhibition zones for indicator Campylobacter strains that were determined to be sensitive to various Lactobacillus species and the paired t-test for comparing differences between the counts of Campylobacter in serial dilution method after following hours of the experiment. P values lower than 0.05 were considered significant. All calculations were performed using JMP 7.0.2 (SAS, United States) software package.
Acknowledgements
The authors thank DVM Jacek Jakubek and his team from Veterinary Clinic Gumniska 11, Tarnów, Poland as well as the staff of the Veterinary Clinic “Vetika” Lipska 49, Kraków, Poland for their commitment and help in samples collecting.
Abbreviations
- C.F.U
Colony Forming Unit
- MRS
De Man Rogosa Sharpe
- PBS
Phosphate Buffered Saline
- PCR
Polymerase Chain Reactin
- SD
Standard Deviation
- TSB
Tryptic Soy Broth
Authors' contributions
ATP: designed the study and wrote the manuscript, MM and SR: conducted the experiment and analyzed the data MS: supervised the project and revised the manuscript, ZA: participated in project implementation and contributed with data analysis. All authors have reviewed and approved the manuscript.
Funding
This study was supported by University Center of Veterinary Medicine Ju-AU (grant no. K/ZDS/007692). The funding agency did not participate in study design, data collection, analysis and interpretation or writing of the manuscript.
Availability of data and materials
The data and materials are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethics approval is deemed unnecessary by the 2nd local Institutional Animal Care and Use Committee in Krakow.. All experiments were carried out in accordance with relevant guidelines and regulations, and the study was carried out in compliance with the ARRIVE guidelines. Rectal swabs were obtained by veterinarians as part of routine clinical veterinary procedures, and with the informed consent of the animal’s owner. The collection of rectal swabs does not require anesthesia.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. 2015. Available online at: https://apps.who.int/iris/handle/10665/199350. Accessed 09 Nov 2021.
- 2.EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) The European Union One Health 2019 Zoonoses Report. EFSA J. 2021;19:6406. doi: 10.2903/j.efsa.2021.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heredia N, Garcia S. Animals as sources of food-borne pathogens: A review. Anim Nutr. 2018;4(3):250–255. doi: 10.1016/j.aninu.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iannino F, Di Donato G, Ruggieri E, Salucci S, De Massis F, Di Giannatale E. Campylobacter infections, a significant issue of veterinary urban hygiene: dog-related risk factors. Vet Ital. 2017;53(2):111–120. doi: 10.12834/VetIt.904.4615.2. [DOI] [PubMed] [Google Scholar]
- 5.Kolackova I, Duskova M, Vojkovska H, Bardon J, Pudova V, Karpiskova R. Dogs as a possible source of human Campylobacter infections. Klin Mikrobiol Infekc Lek. 2015;21(2):36–40. [PubMed] [Google Scholar]
- 6.Moffatt C, Appuhamy R, Andrew W, Wynn S, Roberts J, Kennedy K. An assessment of risk posed by a Campylobacter-positive puppy living in an Australian residential aged-care facility. Western Pac Surveill Response J. 2014;5(3):1–6. doi: 10.5365/WPSAR.2014.5.2.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acke E. Campylobacteriosis in dogs and cats: a review. N Z Vet J. 2018;66(5):221–228. doi: 10.1080/00480169.2018.1475268. [DOI] [PubMed] [Google Scholar]
- 8.Chaban B, Ngeleka M, Hill JE. Detection and quantification of 14 Campylobacter species in pet dogs reveals an increase in species richness in feces of diarrheic animals. BMC Microbiol. 2010;10:73. doi: 10.1186/1471-2180-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taema MM, Bull JC, Macgregor SK, Flach EJ, Boardman WS, Routh AD. Retrospective Study of Campylobacter Infection in a Zoological Collection. Appl Environ Microbiol. 2008;74(5):1332–1338. doi: 10.1128/AEM.02060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEwen SA, Fedorka-Cray PJ. Antimicrobial use and resistance in animals. Clin Infect Dis. 2002;34(3):S93–S106. doi: 10.1086/340246. [DOI] [PubMed] [Google Scholar]
- 11.Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of Action of Probiotics Adv Nutr. 2019;10(1):S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coman MM, Verdenelli MC, Cecchini C, Bela B, Gramenzi A, Orpianesi C, et al. Probiotic characterization of Lactobacillus isolates from canine faeces. J Appl Microbiol. 2019;126(4):1245–1256. doi: 10.1111/jam.14197. [DOI] [PubMed] [Google Scholar]
- 13.Lin CF, Lin MY, Lin CN, Chiou MT, Chen JW, Yang KC, et al. Potential probiotic of Lactobacillus strains isolated from the intestinal tract of pigs and feces of dogs with antibacterial activity against multidrug-resistant pathogenic bacteria. Arch Microbiol. 2020;202(7):1849–1860. doi: 10.1007/s00203-020-01908-w. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez-Trigos E, Toquet M, Barba M, Gómez-Martín A, Quereda JJ, Bataller E. Search of antimicrobial lactic acid bacteria from Salmonella-negative dogs. BMC Vet Res. 2022;18(1):12. doi: 10.1186/s12917-021-03070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weese JS, Anderson MEC. Preliminary evaluation of Lactobacillus rhamnosus strain GG, a potential probiotic in dogs. Can Vet J. 2002;43(10):771–774. [PMC free article] [PubMed] [Google Scholar]
- 16.Ziese AL, Suchodolski JS, Hartmann K, Busch K, Anderson A, Sarwar F, et al. Effect of probiotic treatment on the clinical course, intestinal microbiome and toxigenic Clostridium perfringens in dogs with acute hemorrhagic diarrhea. PLoS ONE. 2018;13(9):e0204691. doi: 10.1371/journal.pone.0204691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCoy S, Gilliland SE. Isolation and characterization of Lactobacillus species having potential for use as probiotic cultures for dogs. J Food Sci. 2007;72(3):94–97. doi: 10.1111/j.1750-3841.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 18.Martin R, Olivares M, Perez M, Xaus J, Torre C, Fernandez L, et al. Identification and evaluation of the probiotic potential of lactobacilli isolated from canine milk. Vet J. 2010;185(2):193–198. doi: 10.1016/j.tvjl.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 19.O'Mahony D, Murphy KB, MacSharry J, Boileau T, Sunvold G, Reinhart G, et al. Portrait of a canine probiotic Bifidobacterium - from gut to gut. Vet Microbiol. 2009;139(1–2):106–112. doi: 10.1016/j.vetmic.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Neal-McKinney JM, Lu X, Duong T, Larson CL, Call DR, Shah DH, et al. Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS ONE. 2012;7(9):e43928. doi: 10.1371/journal.pone.0043928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bratz K, Golz G, Janczyk P, Nockler K, Alter T. Analysis in vitro and in vivo effects of probiotics against Campylobacter spp. Berl Munch Tierarztl Wochenschr. 2015;128(3–4):155–162. [PubMed] [Google Scholar]
- 22.Imlay JA. How oxygen damages microbes: oxygen tolerance and obligate anaerobiosis. Adv Microb Physiol. 2002;46:111–153. doi: 10.1016/s0065-2911(02)46003-1. [DOI] [PubMed] [Google Scholar]
- 23.Strus M, Brzychczy-Włoch M, Gosiewski T, Kochan P, Heczko PB. The in vitro effect of hydrogen peroxide on vaginal microbial communities. FEMS Immunol Med Microbiol. 2006;48(1):56–63. doi: 10.1111/j.1574-695X.2006.00120.x. [DOI] [PubMed] [Google Scholar]
- 24.Pridmore RD, Pittet AC, Praplan F, Cavadini C. Hydrogen peroxide production by Lactobacillus johnsonii NCC 533 and its role in anti-Salmonella activity. FEMS Microbiol Lett. 2008;283(2):210–215. doi: 10.1111/j.1574-6968.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- 25.Ito A, Sato Y, Kudo S, Sato S, Nakajima H, Toba T. The screening of hydrogen peroxide-producing lactic acid bacteria and their application to inactivating psychrotrophic food-borne pathogens. Curr Microbiol. 2003;47(3):231–236. doi: 10.1007/s00284-002-3993-1. [DOI] [PubMed] [Google Scholar]
- 26.Sabir F, Beyatli Y, Cokmus C, Onal-Darilmaz D. Assessment of potential probiotic properties of Lactobacillus spp., Lactococcus spp., and Pediococcus spp. Strains isolated from kefir. J Food Sci. 2010;75:M568–M573. doi: 10.1111/j.1750-3841.2010.01855.x. [DOI] [PubMed] [Google Scholar]
- 27.Annuk H, Shchepetova T, Kullisaar T, Songisepp E, Zilmer M, Mikelsaar M. Characterization of intestinal lactobacilli as putative probiotic candidates. J Appl Microbiol. 2003;94:403–412. doi: 10.1046/j.1365-2672.2003.01847.x. [DOI] [PubMed] [Google Scholar]
- 28.Aslim B, Kilic E. Some probiotic properties of vaginal lactobacilli isolated from healthy women. Jpn J Infect Dis. 2006;59(4):249–253. [PubMed] [Google Scholar]
- 29.Silva BC, Jung LRC, Sandes SHC, Alvim LB, Bomfim MRQ, Nicoli JR, et al. In vitro assessment of functional properties of lactic acid bacteria isolated from faecal microbiota of healthy dogs for potential use as probiotics. Benef Microbes. 2013;4(3):267–275. doi: 10.3920/BM2012.0048. [DOI] [PubMed] [Google Scholar]
- 30.Martin R, Suarez JE. Biosynthesis and degradation of H2O2 by vaginal lactobacilli. Appl Environ Microbiol. 2010;76(2):400–405. doi: 10.1128/AEM.01631-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu B, Tsen HY. Lactobacillus cells in the rabbit digestive tract and the factors affecting their distribution. J Appl Bacteriol. 1993;75(3):269–275. doi: 10.1111/j.1365-2672.1993.tb02776.x. [DOI] [PubMed] [Google Scholar]
- 32.Sahadeva RPK, Leong SF, Chua KH, Tan CH, Chan H, Tong EV, et al. Survival of commercial probiotic strains to pH and bile. Int Food Res J. 2011;18(4):1515–1522. [Google Scholar]
- 33.Mulaw G, Tessema TS, Muleta D, Tesfaye A. In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented Ethiopian food products. Int J Microbiol. 2019;2019:7179514. doi: 10.1155/2019/7179514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng Y, Qiao L, Liu R, Yao H, Gao C. Potential probiotic properties of lactic acid bacteria isolated from the intestinal mucosa of healthy piglets. Ann Microbiol. 2017;67:239–253. [Google Scholar]
- 35.Hassanzadazar H, Ehsani A, Mardani K, Hesari J. Investigation of antibacterial, acid and bile tolerance properties of lactobacilli isolated from Koozeh cheese. Vet Res Forum. 2012;3(3):181–185. [PMC free article] [PubMed] [Google Scholar]
- 36.Liong MT, Shah NP. Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. J Dairy Sci. 2005;88(1):55–66. doi: 10.3168/jds.S0022-0302(05)72662-X. [DOI] [PubMed] [Google Scholar]
- 37.Morelli L. In vitro selection of probiotic lactobacilli: a critical appraisal. Curr Issues Intest Microbiol. 2000;1(2):59–67. [PubMed] [Google Scholar]
- 38.Lebeer S, Vanderleyden J, De Keersmaecker SC. Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev. 2008;72(4):728–764. doi: 10.1128/MMBR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shehata MG, El Sohaimy SA, El-Sahn MA, Youssef MM. Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Ann Agric Sci. 2016;61(1):65–75. [Google Scholar]
- 40.Strompfova V, Marcinakova M, Simonova M, Bogovic-Matijasic B, Laukova A. Application of potential probiotic Lactobacillus fermentum AD1 strain in healthy dogs. Anaerobe. 2006;12(2):75–79. doi: 10.1016/j.anaerobe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Amri A, Senok AC, Ismaeel AY, Al-Mahmeed AE, Botta GA. Multiplex PCR for direct identification of Campylobacter spp in human and chicken stools. J Med Microbiol. 2007;56(Pt 10):1350–1355. doi: 10.1099/jmm.0.47220-0. [DOI] [PubMed] [Google Scholar]
- 42.Wang G, Clark CG, Taylor TM, Pucknell C, Barton C, Price L, et al. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni C coli C lari C upsaliensis and C fetus subsp fetus. J Clin Microbiol. 2002;40(12):4744–4747. doi: 10.1128/JCM.40.12.4744-4747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubernet S, Desmasures N, Gueguen M. A PCR-based method for identification of lactobacilli at the genus level. FEMS Microbiol Lett. 2002;214(2):271–275. doi: 10.1111/j.1574-6968.2002.tb11358.x. [DOI] [PubMed] [Google Scholar]
- 44.Guarneri T, Rossetti L, Giraffa G. Rapid identification of Lactobacillus brevis using the polymerase chain reaction. Lett Appl Microbiol. 2001;33(5):377–381. doi: 10.1046/j.1472-765x.2001.01014.x. [DOI] [PubMed] [Google Scholar]
- 45.Kwon H, Yang EH, Yeon SW, Kang BH, Kim TY. Rapid identification of probiotic Lactobacillus species by multiplex PCR using species-specific primers based on the region extending from 16S rRNA through 23S rRNA. FEMS Microbiol Lett. 2004;239(2):267–275. doi: 10.1016/j.femsle.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 46.Walter J, Tannock GW, Tilsala-Timisjarvi A, Rodtong S, Loach DM, Munro K, et al. Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl Environ Microbiol. 2000;66(1):297–303. doi: 10.1128/aem.66.1.297-303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song Y, Kato N, Liu C, Matsumiya Y, Kato H, Watanabe K. Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group- and species-specific primers derived from the 16S–23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol Lett. 2000;187(2):167–173. doi: 10.1111/j.1574-6968.2000.tb09155.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials are available from the corresponding author on reasonable request.