Abstract
Background
Inflammation plays a crucial role in coronary atherosclerosis progression, and growing evidence has demonstrated that the fibrinogen-to-albumin ratio (FAR), as a novel inflammation biomarker, is associated with the severity of coronary artery disease (CAD). However, the long-term risk of cardiovascular events remains indistinct in patients with different level of FAR and different glycemic metabolism status. This study was to assess 5-year clinical outcomes of diabetic and non-diabetic patients who underwent percutaneous coronary intervention (PCI) with different level of FAR.
Methods
We consecutively enrolled 10,724 patients with CAD hospitalized for PCI and followed up for the major adverse cardiac and cerebrovascular events (MACCE) covering all-cause mortality, cardiac mortality, non-fatal myocardial infarction, non-fatal ischemic stroke, and unplanned coronary revascularization. FAR was computed using the following formula: Fibrinogen (g/L)/Albumin (g/L). According to the optimal cut-off value of FAR for MACCE prediction, patients were divided into higher level of FAR (FAR-H) and lower level of FAR (FAR-L) subgroups, and were further categorized into four groups as FAR-H with DM and non-DM, and FAR-L with DM and non-DM.
Results
5298 patients (58.36 ± 10.36 years, 77.7% male) were ultimately enrolled in the present study. A total of 1099 (20.7%) MACCEs were documented during the 5-year follow-up. The optimal cut-off value of FAR was 0.0783 by the surv_cutpoint function. Compared to ones with FAR-H and DM, patients with FAR-L and non-DM, FAR-H and non-DM, FAR-L and DM had decreased risk of MACCEs [adjusted hazard ratio (HR): 0.75, 95% confidence interval (CI) 0.64–0.89, P = 0.001; HR: 0.78, 95% CI 0.66–0.93, P = 0.006; HR: 0.81, 95% CI 0.68–0.97, P = 0.019; respectively]. Notably, non-diabetic patients with lower level of FAR also had lower all-cause mortality and cardiac mortality risk than those in the FAR-H/DM group (HR: 0.41, 95% CI 0.27–0.63, P < 0.001; HR: 0.30, 95% CI 0.17–0.53, P < 0.001; respectively). Multivariate Cox proportional hazards regression analysis also indicated the highest risk of MACCEs in patients with FAR-H and DM than others (P for trend = 0.005). In addition, post-hoc analysis revealed consistent effects on 5-year MACCE across various subgroups.
Conclusion
In this real-world cohort study, higher level of FAR combined with DM was associated with worse 5-year outcomes among patients with CAD undergoing PCI. The level of FAR may help to identify high-risk individuals in this specific population, where more precise risk assessment should be performed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01477-w.
Keywords: Fibrinogen‑to‑albumin ratio, Type 2 diabetes mellitus, Prognosis, Percutaneous coronary intervention
Introduction
Inflammation plays a crucial role in coronary atherosclerosis progression [1]. Increasing evidence has indicated that higher levels of inflammatory biomarkers are associated with increased adverse cardiovascular events in patients with coronary artery disease (CAD) [2–5]. Previous studies suggested that fibrinogen (FIB), a biomarker of inflammation as well as a core component in the coagulation pathway, was an independent risk factor and might predict cardiovascular events in patients with CAD [3, 6]. Besides, our prior findings also indicated that higher level of FIB was strongly related to increased risk of long-term all-cause and cardiac mortality among CAD patients undergoing percutaneous coronary intervention (PCI), especially in those with diabetes mellitus (DM) [7]. As the most abundant plasma protein, albumin is a negative acute‑phase reactant produced in the liver, whose serum concentration is associated with inflammatory and hemostatic processes [8, 9]. Moreover, serum albumin level was inversely associated with cardiovascular mortality and hypoalbuminemia might predict the no-flow phenomenon in patients with acute myocardial infarction (AMI) after PCI [10, 11]. Therefore, both fibrinogen and albumin are important equivalent of hemorheological and inflammatory alterations. Recently, several publications confirmed that fibrinogen-to-albumin ratio (FAR), which comprised these two indicators above, was a well-established prognostic factor in esophageal, liver and breast cancers [12–14], and had a closely association with the severity of coronary lesions as well as short-term prognosis in patients with CAD [15–17].
Inflammation is regarded as the common antecedent of atherosclerosis and diabetes [2, 18]. Type 2 diabetes mellitus, a well-established risk factor of CAD, has been previously demonstrated to be closely associated with greater atherosclerotic plaque burden and increased risk of adverse cardiovascular events [19–21]. However, to date, insufficient literature has investigated the relationship between the level of FAR, glucose metabolism and long-term prognosis in the CAD population after PCI. In the light of the above, the present study was conducted to evaluate the relationship between FAR and glycemic metabolism indices, and further determine the joint effect of FAR and DM on long-term major adverse cardiac and cerebrovascular events (MACCE) in CAD patients undergoing PCI.
Methods
Study design
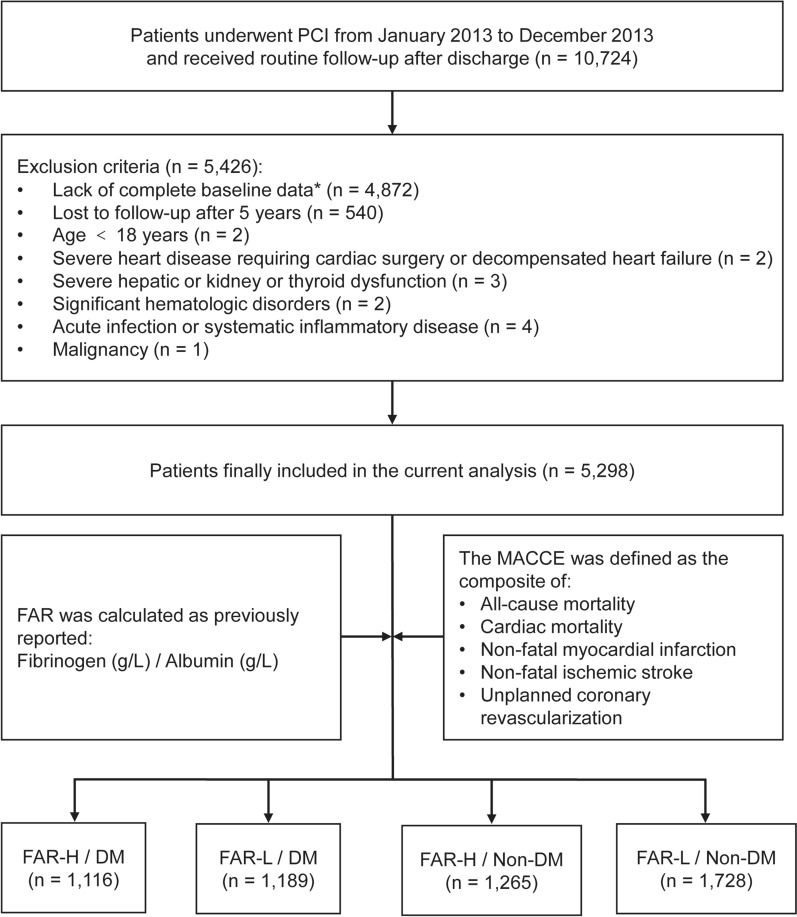
From January 2013 to December 2013, a total of 10,724 consecutive CAD patients were hospitalized for PCI in Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Beijing, China. Patients with missing baseline and follow-up data, age < 18 years and/or other exclusion criteria were excluded (detailed recruitment process shown in Fig. 1). A comparison of the baseline characteristics and crude outcomes between the non-participants and participants was presented in Additional file 1: Table S1. Overall, 5298 patients were ultimately included in the present analysis and assigned to the FAR-H/DM (n = 1116), FAR-L/DM (n = 1189), FAR-H/Non-DM (n = 1265) and FAR-L/Non-DM (n = 1728) groups, due to the optimal cut-off value of FAR and different glycemic metabolism status.
Fig. 1.
Study flowchart. PCI percutaneous coronary intervention, MACCE major adverse cardiac and cerebrovascular events, FAR fibrinogen-to-albumin ratio, DM diabetes mellitus * 4607 patients with missing fibrinogen values and 265 patients with missing fasting blood glucose or HbA1c levels were excluded
Enrolled patients were routinely followed up through telephone interview or examination of medical records at five time points (1-month, 6-month, 1-year, 2-year, and 5-year after discharge) by well-trained research coordinators, who were blinded to the objectives of the current study. The primary endpoint was defined as a composite of the MACCE covering all-cause mortality, cardiac mortality, non-fatal myocardial infarction, non-fatal ischemic stroke and unplanned coronary revascularization [22]. The secondary outcome was each component of MACCE. All events were adjudicated centrally by 2 independent and experienced cardiologists, who were unaware of the study protocol. Conflicts were resolved by turning to a third experienced cardiologist.
This study complied with the Declaration of Helsinki and was endorsed by the Ethics Committee of The Fuwai Hospital, National Center for Cardiovascular Diseases, Beijing, China. The informed written consent was obtained from all patients before the intervention.
Treatment and procedure
PCI was performed by interventional cardiologists who were blind to the study protocol, in line with current practice guidelines in China. The choice of equipment and detailed strategies during coronary intervention was at the discretion of the operating physicians. Before the procedure, elective PCI patients received oral administration of aspirin (300 mg) and ticagrelor (loading dose 180 mg) or clopidogrel (loading dose 300 mg) at least 24 h, unless on long-term P2Y12 inhibitor treatment. Patients presenting as acute coronary syndrome (ACS) scheduled for PCI received the same dose of aspirin and ticagrelor (loading dose 180 mg) or clopidogrel (loading dose 300–600 mg) as soon as possible. Unfractionated heparin (100 U/kg) was administered before PCI, however, the use of glycoprotein IIb/IIIa inhibitors was at the cardiologist’s judgment during the procedure. After the catheterization, the dual antiplatelet therapy including aspirin (100 mg daily) and ticagrelor (90 mg, twice daily) or clopidogrel (75 mg, daily) were recommended for at least 12 months.
Definitions
Diabetes mellitus was diagnosed by fasting blood glucose (FBG) ≥ 7.0 mmol/L (126 mg/dL), or hemoglobin A1c (HbA1c) levels ≥ 6.5%, or 2-h blood glucose of oral glucose tolerance test ≥ 11.1 mmol/L (200 mg/dL), or previous definite diagnosis of DM with hypoglycemic drugs treatment [23]. FAR was defined as the ratio of the preoperative plasma fibrinogen concentration (g/L) to the preoperative plasma albumin level (g/L). Hypertension (HT) was defined as newly confirmation more than twice on different days by systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg during the baseline hospitalization, or known HT with antihypertensive therapy [24]. Fasting total cholesterol (TC) ≥ 5.2 mmol/L, low-density lipoprotein cholesterol (LDL-C) ≥ 3.4 mmol/L, high-density lipoprotein cholesterol (HDL-C) < 1.0 mmol/L, triglyceride (TG) ≥ 1.7 mmol/L, and/or long-term treatment with lipid-lowering drugs were considered criteria for dyslipidemia [25]. Body mass index (BMI) was calculated as weight (kg)/[height (m)]2. Previous medical history of MI, PCI, stroke, and peripheral artery disease (PAD), smoking history, family history of CAD, and medications at admission were collected from self-reported information and then confirmed by relevant medical records.
Coronary procedural information was interpreted and recorded by two independent experienced operating physicians and disagreement was resolved by consensus. Based on the coronary angiography, left main disease was defined as stenosis of ≥ 50% in left main coronary artery and three-vessel disease was diagnosed by stenosis of ≥ 50% in all three main coronary arteries (left anterior coronary artery, left circumflex artery and right coronary artery). Chronic total occlusion (CTO) was defined as complete obstruction of a native coronary artery for more than 3 months with thrombolysis in myocardial infarction (TIMI) flow grade 0 [26]. The synergy between PCI with taxus and cardiac surgery (SYNTAX) score was calculated using an online calculator (http://www.syntaxscore.com/) to evaluate the coronary lesion complexity by a dedicated research group blinded to the clinical data. Complete revascularization was considered successful if residual stenosis < 30% with TIMI flow grade 3 at the end of the PCI procedure was obtained according to visual estimation of the angiograms [26].
Laboratory tests measurements
Fasting blood samples were drawn from each patient within 24 h after admission and all of them were stored in −80 °C refrigerators until test. Stago autoanalyzer with the STA fibrinogen kit (Diagnostica Stago, Taverny, France) was used to measure the concentrations of plasma fibrinogen. Serum albumin was measured using an automated chemistry analyzer (AU5400, Olymus, Japan) by the bromocresol green dye method. Tosoh Automated Glycohemoglobin Analyzer (HLC-723G8, Tokyo, Japan) was used to measure the HbA1c levels. The concentrations of fast blood glucose were measured by the enzymatic hexokinase method. Other laboratory indices, including lipid profiles (TG, TC, HDL-C and LDL-C), estimated glomerular filtration rate (eGFR), creatinine, high-sensitivity C-reactive protein (hs-CRP) were examined with standard biochemical techniques at the core laboratory in Fuwai Hospital. According to modified Simpson’s rule, left ventricular ejection fraction (LVEF) was measured from two-dimensional echocardiography.
Statistical analyses
Continuous variates were described as mean with standard deviation and nominal variates were summarized as frequency with percentage. Comparison of continuous and categorical variates among different groups was analyzed by Student’s t-test or Analysis of variance and Chi-square test or Fisher’s exact test, as appropriate. The surv_cutpoint function was used to determine the optimal cut-off value for FAR. Pearson correlation and linear regression analysis were constructed to evaluate the correlation between FAR and glycemic metabolism indices (FBG and HbA1c). The risks of MACCE in different subgroups were presented by Kaplan–Meier survival curves and compared by log-rank test. The associations among FAR, DM, and 5-year clinical outcomes were analyzed by unadjusted and adjusted Cox regression analyses. Hazard ratios (HRs) and 95% confidence interval (CI) were reported. In the multivariate Cox analysis, age, sex, BMI, hypertension, previous MI, previous PCI, previous stroke, eGFR, LVEF, Left main (LM)/three-vessel disease, and SYNTAX score were adjusted because of their statistical significance (P < 0.05) in univariate analysis or clinical importance (Additional file 1: Table S2). Furthermore, exploratory analyses were performed to assess the effect of FAR and glycemic metabolism status in different subgroups on the primary endpoint in specific subsets and shown as the forest plot. In addition, the continuous relationship between FAR and the risk of MACCE was illustrated by restricted cubic spline and examined by the likelihood ratio test. Statistical analyses were performed using SPSS software (version 26.0; IBM Corp., Armonk, N.Y., United States), RStudio software (version 2021.09.0; http://www.rstudio.org/), and GraphPad Prism software (version 8.0.1; GraphPad Software, Inc., La Jolla, CA, United States). A two-tailed P value < 0.05 was considered statistical significance.
Results
Overall, 5298 patients (58.36 ± 10.36 years, 77.7% male) who met the enrollment criteria and completed the 5-year follow-up were ultimately enrolled in the present study. During the 5-year follow-up (interquartile range: 5.0–5.1 years), 206 (3.89%) all-cause mortality, 310 (5.85%) non-fatal MI, 184 (3.47%) non-fatal ischemic stroke, 671 (12.67%) unplanned coronary revascularization and 1099 (20.7%) MACCEs were recorded.
Baseline characteristics
The baseline characteristics of enrolled patients and groups stratified by the occurrence of MACCEs were summarized in Table 1. Patients with each component of the primary endpoint presented older and higher level of FAR, FIB, FBG, HbA1c, hs-CRP, creatinine, eGFR and LVEF. In addition, they also had higher prevalence of DM, HT, previous MI, previous PCI, previous stroke, nitrate at admission and insulin before hospitalization. As for the angiographic findings, patients in the MACCEs subset were more likely to have LM/three-vessel disease, and target lesions in RCA and LCX. Moreover, the SYNTAX score was significantly higher in participants with adverse prognosis.
Table 1.
Baseline demographics and angiographic characteristics of entire population stratified by the primary endpoint
| Variable | Total population (n = 5298) | Non-MACCEs (n = 4199) | MACCEs (n = 1099) | P value |
|---|---|---|---|---|
| FAR | 0.081 ± 0.022 | 0.080 ± 0.022 | 0.082 ± 0.024 | 0.010 |
| Four subgroups, n (%) | < 0.001 | |||
| FAR-H/DM | 1116 (21.1) | 829 (19.7) | 287 (26.1) | |
| FAR-L/DM | 1189 (22.4) | 941 (22.4) | 248 (22.6) | |
| FAR-H/Non-DM | 1265 (23.9) | 1018 (24.2) | 247 (22.5) | |
| FAR-L/Non-DM | 1728 (32.6) | 1411 (33.6) | 317 (28.8) | |
| Baseline characteristics | ||||
| Age, years | 58.36 ± 10.36 | 58.12 ± 10.26 | 59.30 ± 10.68 | 0.001 |
| Male, n (%) | 4,119 (77.7) | 3,246 (77.3) | 873 (79.4) | 0.130 |
| BMI, kg/m2 | 25.89 ± 3.15 | 25.92 ± 3.15 | 25.81 ± 3.15 | 0.338 |
| DM, n (%) | 2305 (43.5) | 1770 (42.2) | 535 (48.7) | < 0.001 |
| Hypertension, n (%) | 3386 (63.9) | 2629 (62.6) | 757 (68.9) | < 0.001 |
| Dyslipidemia, n (%) | 3657 (69.0) | 2889 (68.8) | 768 (69.9) | 0.491 |
| Smoking history, n (%) | 3107 (58.6) | 2435 (58.0) | 672 (61.1) | 0.059 |
| Family history of CAD, n (%) | 1222 (23.1) | 948 (22.6) | 274 (24.9) | 0.101 |
| Previous MI, n (%) | 1036 (19.6) | 791 (18.8) | 245 (22.3) | 0.010 |
| Previous PCI, n (%) | 1229 (23.2) | 914 (21.8) | 315 (28.7) | < 0.001 |
| Previous stroke, n (%) | 556 (10.5) | 419 (10.0) | 137 (12.5) | 0.017 |
| Previous PAD, n (%) | 150 (2.8) | 120 (2.9) | 30 (2.7) | 0.820 |
| Clinical presentation, n (%) | 0.435 | |||
| CCS | 2258 (42.6) | 1801 (42.9) | 457 (41.6) | |
| ACS | 3040 (57.4) | 2398 (67.1) | 642 (58.4) | |
| Laboratory tests | ||||
| FIB, g/L | 3.38 ± 0.83 | 3.37 ± 0.82 | 3.43 ± 0.85 | 0.047 |
| Albumin, g/L | 42.40 ± 3.83 | 42.45 ± 3.84 | 42.22 ± 3.79 | 0.079 |
| FBG, mmol/L | 6.02 ± 1.97 | 5.99 ± 1.93 | 6.16 ± 2.11 | 0.012 |
| HbA1c, % | 6.59 ± 1.21 | 6.56 ± 1.21 | 6.72 ± 1.21 | < 0.001 |
| TG, mmol/L | 1.80 ± 1.09 | 1.80 ± 1.10 | 1.80 ± 1.02 | 0.895 |
| TC, mmol/L | 4.17 ± 1.09 | 4.17 ± 1.08 | 4.16 ± 1.90 | 0.883 |
| HDL-C, mmol/L | 1.02 ± 0.27 | 1.02 ± 0.28 | 1.01 ± 0.27 | 0.158 |
| LDL-C, mmol/L | 2.48 ± 0.91 | 2.48 ± 0.91 | 2.48 ± 0.91 | 0.971 |
| hs-CRP, mg/L | 3.07 ± 3.68 | 2.99 ± 3.63 | 3.36 ± 3.87 | 0.006 |
| Creatinine, μmol/L | 75.33 ± 15.95 | 74.98 ± 15.50 | 76.68 ± 17.52 | 0.004 |
| eGFR, mL/min/1.73 m2 | 91.63 ± 15.10 | 92.01 ± 14.72 | 90.18 ± 16.41 | 0.001 |
| LVEF, % | 63.23 ± 7.04 | 63.38 ± 6.92 | 62.67 ± 7.47 | 0.005 |
| Medications at admission | ||||
| Aspirin, n (%) | 5245 (99.0) | 4157 (99.0) | 1088 (99.0) | 0.998 |
| Clopidogrel, n (%) | 5286 (99.8) | 4190 (99.8) | 1096 (99.7) | 0.716 |
| β-blocker, n (%) | 4829 (91.1) | 3822 (91.0) | 1007 (91.6) | 0.528 |
| CCB, n (%) | 2485 (46.9) | 1941 (46.2) | 544 (49.5) | 0.053 |
| Statins, n (%) | 5112 (96.5) | 4054 (96.5) | 1058 (96.3) | 0.656 |
| Nitrate, n (%) | 5162 (97.4) | 4082 (97.2) | 1080 (98.3) | 0.048 |
| Insulin, n (%) | 613 (11.6) | 456 (10.9) | 157 (14.3) | 0.002 |
| Coronary procedural information | ||||
| LM/three-vessel disease, n (%) | 2382 (45.0) | 1806 (43.0) | 576 (52.4) | < 0.001 |
| Chronic total occlusion, n (%) | 377 (7.1) | 291 (6.9) | 86 (7.8) | 0.304 |
| Target vessel territory, n (%) | < 0.001 | |||
| LAD | 2512 (47.4) | 2073 (49.4) | 439 (39.9) | |
| LCX | 955 (18.0) | 739 (17.6) | 216 (19.7) | |
| RCA | 1757 (33.2) | 1334 (31.8) | 423 (38.5) | |
| Number of stents | 1.77 ± 0.89 | 1.76 ± 0.90 | 1.79 ± 0.86 | 0.303 |
| SYNTAX score | 11.79 ± 9.06 | 11.61 ± 7.90 | 12.47 ± 8.62 | 0.003 |
| Complete revascularization, n (%) | 5268 (99.4) | 4179 (99.5) | 1089 (99.1) | 0.088 |
| DES implantation, n (%) | 5226 (98.6) | 4148 (98.8) | 1078 (98.1) | 0.076 |
MACCE major adverse cardiac and cerebrovascular events, FAR fibrinogen-to-albumin ratio, BMI body mass index, DM diabetes mellitus, CAD coronary artery disease, MI myocardial infarction, PCI percutaneous coronary intervention, PAD peripheral artery disease, CCS chronic coronary syndrome, ACS acute coronary syndrome, FIB fibrinogen, FBG fasting blood glucose, HbA1c glycosylated hemoglobin A1c, TG triglyceride, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, hs-CRP high-sensitivity C-reactive protein, eGFR estimated glomerular filtration rate, LVEF left ventricular ejection fraction, CCB calcium channel blocker, LM left main artery, LAD left anterior descending artery, LCX left circumflex artery, RCA right coronary artery, SYNTAX synergy between PCI with taxus and cardiac surgery, DES drug-eluting stent
Comparison of clinical data among four groups
Based on the surv_cutpoint function of the R package survminer in the R programming language, the optimal cut-off point of FAR is 0.0783. Thus, baseline characteristics of four subgroups according to the level of FAR and glycemic metabolism status were shown in Table 2. Compared with patients in the FAR-H/DM group, those in other three subgroups tended to be younger and male, with a lower proportion of comorbidities, such as HT, dyslipidemia, and previous stroke. Laboratory indices including FIB, FBG, HbA1c, TC, LDL-C, hs-CRP, creatine, eGFR and LVEF were significantly higher in patients with higher level of FAR combined with DM, while the level of HDL-C was relatively lower. Meanwhile, patients in other three subsets were less likely to have LM/three-vessel disease, target lesions in RCA and LCX, and higher SYNTAX score, when compared with those in the FAR-H/DM group.
Table 2.
Baseline demographics and angiographic characteristics stratified by low or high FAR and different glycemic metabolism status
| Variable | FAR-H/DM (n = 1116) | FAR-L/DM (n = 1189) | FAR-H/Non-DM (n = 1265) | FAR-L/Non-DM (n = 1728) | P value |
|---|---|---|---|---|---|
| FAR | 0.099 ± 0.021 | 0.067 ± 0.009 | 0.098 ± 0.021 | 0.066 ± 0.008 | < 0.001 |
| Baseline characteristics | |||||
| Age, years | 60.86 ± 10.03 | 58.20 ± 9.70 | 59.54 ± 10.75 | 56.01 ± 10.21 | < 0.001 |
| Male, n (%) | 779 (69.8) | 957 (80.5) | 937 (74.1) | 1,146 (83.7) | < 0.001 |
| BMI, kg/m2 | 26.20 ± 3.22 | 26.37 ± 3.01 | 25.54 ± 3.09 | 25.63 ± 3.18 | < 0.001 |
| DM, n (%) | 1116 (48.4) | 1189 (51.6) | – | – | < 0.001 |
| Hypertension, n (%) | 784 (70.3) | 807 (67.9) | 788 (62.3) | 1007 (58.3) | < 0.001 |
| Dyslipidemia, n (%) | 826 (74.0) | 868 (73.0) | 818 (64.7) | 1145 (66.3) | < 0.001 |
| Smoking history, n (%) | 624 (55.9) | 697 (58.6) | 732 (57.9) | 1054 (61.0) | 0.053 |
| Family history of CAD, n (%) | 230 (20.6) | 276 (23.2) | 282 (22.3) | 434 (25.1) | 0.040 |
| Previous MI, n (%) | 236 (21.1) | 258 (21.7) | 198 (15.7) | 344 (19.9) | 0.001 |
| Previous PCI, n (%) | 274 (24.6) | 341 (28.7) | 220 (17.4) | 394 (22.8) | < 0.001 |
| Previous stroke, n (%) | 162 (14.5) | 133 (11.2) | 120 (9.5) | 141 (8.2) | < 0.001 |
| Previous PAD, n (%) | 41 (3.7) | 43 (3.6) | 24 (1.9) | 42 (2.4) | 0.014 |
| Clinical presentation, n (%) | < 0.001 | ||||
| CCS | 443 (39.7) | 583 (49.0) | 438 (34.6) | 794 (45.9) | |
| ACS | 673 (60.3) | 606 (51.0) | 827 (65.4) | 934 (54.1) | |
| Laboratory tests | |||||
| FIB, g/L | 4.05 ± 0.77 | 2.91 ± 0.42 | 3.99 ± 0.78 | 2.84 ± 0.39 | < 0.001 |
| Albumin, g/L | 41.22 ± 3.75 | 43.52 ± 3.57 | 41.12 ± 3.67 | 43.34 ± 3.66 | < 0.001 |
| FBG, mmol/L | 7.28 ± 2.43 | 7.22 ± 2.40 | 5.07 ± 0.56 | 5.08 ± 0.58 | < 0.001 |
| HbA1c, % | 7.67 ± 1.40 | 7.36 ± 1.21 | 5.93 ± 0.31 | 5.86 ± 0.35 | < 0.001 |
| TG, mmol/L | 1.85 ± 1.13 | 1.97 ± 1.40 | 1.68 ± 0.85 | 1.72 ± 0.93 | < 0.001 |
| TC, mmol/L | 4.22 ± 1.13 | 4.14 ± 1.09 | 4.19 ± 1.03 | 4.14 ± 1.09 | 0.173 |
| HDL-C, mmol/L | 0.98 ± 0.26 | 1.02 ± 0.26 | 1.01 ± 0.27 | 1.06 ± 0.29 | < 0.001 |
| LDL-C, mmol/L | 2.54 ± 0.94 | 2.41 ± 0.89 | 2.52 ± 0.88 | 2.45 ± 0.93 | 0.002 |
| hs-CRP, mg/L | 5.08 ± 4.48 | 1.71 ± 2.03 | 4.82 ± 4.38 | 1.42 ± 1.73 | < 0.001 |
| Creatinine, μmol/L | 76.44 ± 19.39 | 74.83 ± 15.27 | 75.71 ± 16.55 | 74.70 ± 13.24 | 0.019 |
| eGFR, mL/min/1.73 m2 | 88.16 ± 17.12 | 92.39 ± 14.89 | 90.03 ± 15.15 | 94.52 ± 13.09 | < 0.001 |
| LVEF, % | 62.12 ± 7.45 | 63.22 ± 6.85 | 62.90 ± 7.50 | 64.19 ± 6.40 | < 0.001 |
| Medications at admission, n (%) | |||||
| Aspirin, n (%) | 1104 (98.9) | 1177 (99.0) | 1247 (98.6) | 1717 (99.4) | 0.199 |
| Clopidogrel, n (%) | 1114 (99.8) | 1185 (99.7) | 1264 (99.9) | 1723 (99.7) | 0.517 |
| β-blocker, n (%) | 1108 (91.2) | 1115 (93.8) | 1146 (90.6) | 1550 (89.7) | 0.002 |
| CCB, n (%) | 566 (50.7) | 583 (49.0) | 704 (55.7) | 960 (55.6) | < 0.001 |
| Statins, n (%) | 1069 (95.8) | 1140 (95.9) | 1226 (96.9) | 1677 (97.0) | 0.157 |
| Nitrate, n (%) | 1088 (97.5) | 1153 (97.0) | 1232 (97.4) | 1689 (97.7) | 0.638 |
| Insulin, n (%) | 319 (28.6) | 294 (24.7) | – | – | < 0.001 |
| Coronary procedural information | |||||
| LM/three-vessel disease, n (%) | 604 (54.1) | 568 (47.8) | 525 (41.5) | 685 (39.6) | < 0.001 |
| Chronic total occlusion, n (%) | 66 (5.9) | 86 (7.2) | 98 (7.7) | 127 (7.3) | 0.338 |
| Target vessel territory, n (%) | 0.001 | ||||
| LAD | 471 (42.2) | 540 (45.4) | 623 (49.2) | 878 (50.8) | |
| LCX | 232 (20.8) | 211 (17.7) | 207 (16.4) | 305 (17.7) | |
| RCA | 396 (35.5) | 426 (35.8) | 415 (32.8) | 520 (30.1) | |
| Number of stents | 1.79 ± 0.86 | 1.77 ± 0.86 | 1.76 ± 0.94 | 1.75 ± 0.89 | 0.677 |
| SYNTAX score | 12.76 ± 8.47 | 11.77 ± 8.27 | 12.14 ± 8.22 | 10.92 ± 7.41 | < 0.001 |
| Complete revascularization, n (%) | 1106 (99.1) | 1185 (99.7) | 1256 (99.3) | 1721 (99.6) | 0.209 |
| DES implantation, n (%) | 1099 (98.5) | 1175 (98.8) | 1253 (99.1) | 1699 (98.3) | 0.331 |
MACCE major adverse cardiac and cerebrovascular events, FAR fibrinogen-to-albumin ratio, BMI body mass index, DM diabetes mellitus, CAD coronary artery disease, MI myocardial infarction, PCI percutaneous coronary intervention, PAD peripheral artery disease, CCS chronic coronary syndrome, ACS acute coronary syndrome, FIB fibrinogen, FBG fasting blood glucose, HbA1c glycosylated hemoglobin A1c, TG triglyceride, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, hs-CRP high-sensitivity C-reactive protein, eGFR estimated glomerular filtration rate, LVEF left ventricular ejection fraction, CCB calcium channel blocker, LM left main artery, LAD left anterior descending artery, LCX left circumflex artery, RCA right coronary artery, SYNTAX synergy between PCI with taxus and cardiac surgery, DES drug-eluting stent
Relationship between FAR and FBG or HbA1c
Linear regression analysis was conducted to evaluate the correlation between FAR and glycemic metabolism indices (Additional file 1: Table S3). The results showed that both admission FBG (R2 = 0.003, Standard β = 0.061, P < 0.001) and HbA1c (R2 = 0.019, Standard β = 0.139, P < 0.001) were positively associated with FAR in the whole cohort. Furthermore, both in the DM subgroup and in the non-DM subgroup, there was also a positive relationship between FAR and HbA1c (R2 = 0.025, Standard β = 0.101, P < 0.001; R2 = 0.010, Standard β = 0.003, P < 0.001, respectively).
Predictive value of FAR combined with glycemic metabolism status on MACCE and each component
The incidence of the primary endpoint in FAR-H with DM or non-DM and FAR-L with DM or non-DM group was 25.7% (287/1116), 20.9% (248/1189), 19.5% (247/1265) and 18.3% (317/1728), respectively. As shown in Fig. 2, the Kaplan–Meier analysis curves revealed the highest risk of MACCEs in patients with FAR-H and DM compared with other groups (log-rank test P < 0.001). Furthermore, we assessed the prognostic utility in enrolled patients presented different level of FAR with or without DM by Cox regression analysis (Table 3). In the unadjusted model, patients with FAR-L and DM, FAR-H and non-DM, and FAR-L and non-DM showed decreased risk of MACCEs (HR: 0.80, 95% CI 0.67–0.95, P = 0.009; HR: 0.74; 95% CI 0.63–0.88, P = 0.001; HR: 0.69, 95% CI 0.59–0.81, P < 0.001; respectively) compared to those in FAR-H and DM group (as reference). The results remained statistical significance in multivariate analysis after adjusted for age, sex, BMI, hypertension, previous MI, previous PCI, previous stroke, eGFR, LVEF, LM/three-vessel disease, and SYNTAX score (Table 3; adjusted HR: 0.81, 95% CI 0.68–0.97, P = 0.019; adjusted HR: 0.78, 95% CI 0.66–0.93, P = 0.006; adjusted HR: 0.75, 95% CI 0.64–0.89, P = 0.001; respectively). Particularly, there was a significant risk of the all-cause mortality and the cardiac mortality in patients with FAR-H/DM and FAR-L/Non-DM (Table 3; adjusted HR: 0.75, 95% CI 0.64–0.89, P < 0.001; adjusted HR: 0.30, 95% CI 0.17–0.53, P < 0.001; respectively). Multivariate Cox proportional hazards regression analysis also indicated the highest risk of MACCEs in patients with FAR-H and DM than others (Fig. 3; P for trend = 0.005). Moreover, restricted cubic spline analysis elucidated that there was a linear association between FAR and the risk of MACCE, regardless of the unadjusted and adjusted model (Additional file 1: Fig. S1; all P for non-linear association > 0.05).
Fig. 2.
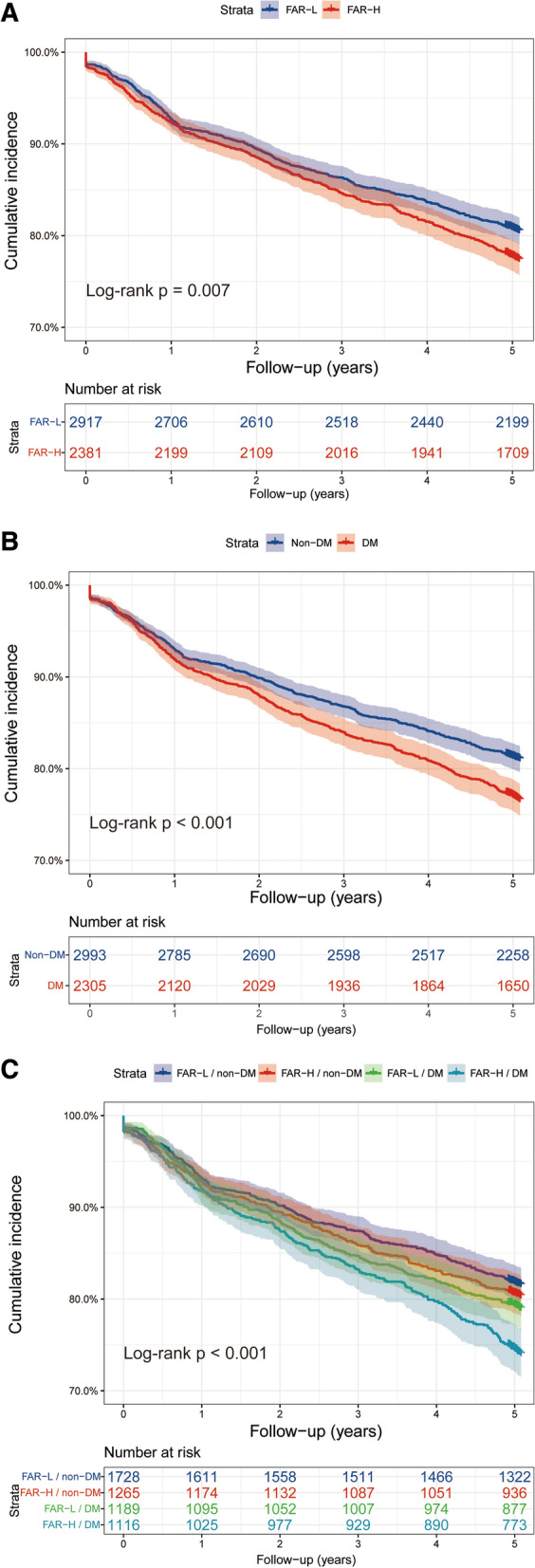
Kaplan–Meier analysis for MACCE according to different FAR levels (A), glycemic metabolism status (B), and status of both FAR levels and glycemic metabolism (C)
Table 3.
Predictive value of the FAR level and different glycemic metabolism status for primary endpoint and each component in univariate and multivariate analysis
| Variables | Events/subjects | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| MACCE | 1099/5298 | ||||
| FAR-H/DM | 287/1116 | Reference | – | Reference | – |
| FAR-L/DM | 248/1189 | 0.80 (0.67–0.95) | 0.009 | 0.81 (0.68–0.97) | 0.019 |
| FAR-H/Non-DM | 247/1265 | 0.74 (0.63–0.88) | 0.001 | 0.78 (0.66–0.93) | 0.006 |
| FAR-L/Non-DM | 317/1728 | 0.69 (0.59–0.81) | < 0.001 | 0.75 (0.64–0.89) | 0.001 |
| All-cause mortality | 206/5298 | ||||
| FAR-H/DM | 71/1116 | Reference | – | Reference | – |
| FAR-L/DM | 44/1189 | 0.57 (0.39–0.84) | 0.004 | 0.68 (0.47–1.00) | 0.053 |
| FAR-H/Non-DM | 56/1265 | 0.69 (0.49–0.98) | 0.040 | 0.72 (0.50–1.03) | 0.076 |
| FAR-L/Non-DM | 35/1728 | 0.31 (0.21–0.47) | < 0.001 | 0.41 (0.27–0.63) | < 0.001 |
| Cardiac mortality | 125/5298 | ||||
| FAR-H/DM | 49/1116 | Reference | – | Reference | – |
| FAR-L/DM | 27/1189 | 0.51 (0.32–0.82) | 0.005 | 0.62 (0.39–1.00) | 0.052 |
| FAR-H/Non-DM | 35/1265 | 0.63 (0.41–0.97) | 0.035 | 0.67 (0.43–1.05) | 0.077 |
| FAR-L/Non-DM | 17/1728 | 0.22 (0.13–0.38) | < 0.001 | 0.30 (0.17–0.53) | < 0.001 |
| Non-fatal MI | 310/5298 | ||||
| FAR-H/DM | 77/1116 | Reference | – | Reference | – |
| FAR-L/DM | 67/1189 | 0.82 (0.61–1.10) | 0.187 | 0.81 (0.58–1.13) | 0.221 |
| FAR-H/Non-DM | 66/1265 | 0.75 (0.54–1.04) | 0.084 | 0.80 (0.57–1.12) | 0.185 |
| FAR-L/Non-DM | 100/1728 | 0.80 (0.58–1.11) | 0.188 | 0.87 (0.64–1.19) | 0.376 |
| Non-fatal ischemic stroke | 184/5298 | ||||
| FAR-H/DM | 48/1116 | Reference | – | Reference | – |
| FAR-L/DM | 44/1189 | 0.85 (0.57–1.28) | 0.447 | 0.92 (0.60–1.40) | 0.702 |
| FAR-H/Non-DM | 47/1265 | 0.86 (0.57–1.28) | 0.455 | 0.89 (0.59–1.35) | 0.593 |
| FAR-L/Non-DM | 45/1728 | 0.59 (0.39–0.89) | 0.011 | 0.70 (0.46–1.08) | 0.105 |
| Unplanned coronary revascularization | 671/5298 | ||||
| FAR-H/DM | 160/1116 | Reference | – | Reference | – |
| FAR-L/DM | 155/1189 | 0.90 (0.72–1.12) | 0.333 | 0.85 (0.68–1.07) | 0.165 |
| FAR-H/Non-DM | 142/1265 | 0.77 (0.62–0.97) | 0.024 | 0.79 (0.63–1.00) | 0.050 |
| FAR-L/Non-DM | 214/1728 | 0.84 (0.69–1.04) | 0.103 | 0.82 (0.66–1.02) | 0.070 |
Model adjusted for age, sex, BMI, hypertension, previous MI, previous PCI, previous stroke, eGFR, LVEF, LM/three-vessel disease, and SYNTAX score
MACCE major adverse cardiac and cerebrovascular events, FAR fibrinogen-to-albumin ratio, CI confidence interval, DM diabetes mellitus, MI myocardial infarction
Fig. 3.
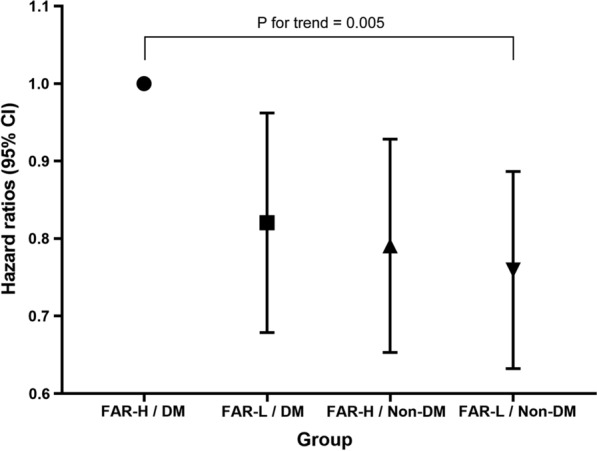
Hazard ratios (95% CIs) for MACCE according to four groups after adjusting for age, sex, BMI, hypertension, previous MI, previous PCI, previous stroke, eGFR, LVEF, LM/three-vessel disease, and SYNTAX score
Subgroup analysis
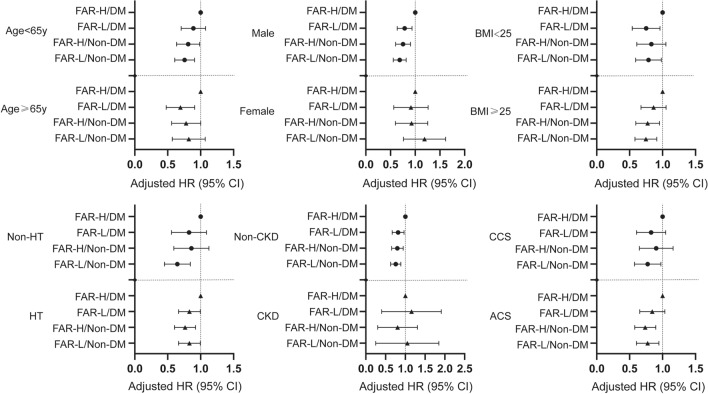
Further evaluation of post-hoc subgroup analysis presented comparable interactions following MACCEs between four subsets and those covariates (age, sex, BMI, hypertension, chronic kidney disease, and clinical presentation) (Fig. 4 & Additional file 1: Table S5; all P for interaction > 0.05). Interestingly, patients with FAR-L/DM, FAR-H/non-DM and FAR-L/non-DM showed significantly consistent characteristics in certain subsets (male and non-CKD), when compared to ones in the FAR-H/DM group.
Fig. 4.
Forest plot of MACCE according to different subgroups. Adjusted model included age, sex, BMI, hypertension, previous MI, previous PCI, previous stroke, eGFR, LVEF, LM/three-vessel disease, and SYNTAX score
Discussion
In this real-world, perspective, observational study on a large-scale cohort from China with 5-year follow-up, we examined the prognostic association of type 2 diabetes mellitus with adverse outcomes in patients with different level of the fibrinogen-to-albumin ratio. Our data demonstrated that diabetic patients with higher level of FAR were associated with significantly increased risks of long-term MACCEs compared to ones in other three groups. Furthermore, non-diabetic patients with lower level of FAR also had lower all-cause mortality and cardiac mortality risk than those in the FAR-H/DM group. Multivariate Cox analysis also revealed the highest risk of MACCEs in patients with FAR-H and DM than others. Additionally, compared with patients in the FAR-H/DM group, patients in other three groups showed significantly consistency in certain subsets (male and non-CKD), which may conduct clinical trials for certain therapies. Our findings suggested that more precise risk assessment should be performed in diabetic patients with higher plane of FAR.
FIB, a serum glycoprotein synthesized by the liver with a dimeric molecular structure, plays a crucial role in the inflammatory and coagulation cascade, which contribute to the pathogenesis of coronary atherosclerosis [2, 3]. Many literatures have reported the association between FIB and inflammation involved in progression of CAD previously [6, 27]. For example, a recent prospective study from our team indicated FIB was strongly associated with long-term cardiac and all-cause mortality among CAD patients undergoing PCI, especially when complicated with DM and Pre-DM [7]. Albumin, which is the most abundant protein in human extracellular fluid, plays significant physiological functions as a negative inflammation biomarker, an inhibitor of platelet activation and aggregation, and a mediator of platelet-induced CAD [9, 17]. Many observational studies and meta-analysis have reported a negative correlation between serum albumin levels and cardiovascular outcomes [11, 28, 29]. Considering both plasma FIB and albumin are useful inflammatory biomarkers and strongly associated with cardiovascular events, additional studies are warranted to further evaluate whether their reciprocal relationship, like FAR, could be helpful to identify high-risk individuals in CAD population undergoing PCI, such as diabetic patients.
The fibrinogen-to-albumin ratio, comprising the two easily detective biomarkers mentioned above, is a well-known indicator for assessing the prognosis of patients with some cancers [12–14]. As a promising serum biomarker, FAR has been proved to have better sensitivity and specificity in predicting MACE than FIB and albumin alone [30, 31]. To date, several publications have been conducted on the prognostic value of FAR in different clinical settings [15, 16, 30]. For example, Li et.al found that the value of FAR was associated independently with the severity of CAD and long-term prognosis, which might contribute to improve risk stratification in non-ST-segment elevation myocardial infarction (NSTEMI) patients initially implanted with drug-eluting stents [16]. Recently, Karahan and co-workers reported that FAR was significantly associated with SYNTAX score in predicting the severity of CAD in patients with STEMI [30]. As described above, previous studies have demonstrated that the value of FAR at admission was associated with the severity of CAD and major cardiovascular adverse outcomes in CAD patients after PCI [15, 16, 30]. What’s more, considerable evidence has demonstrated that chronic inflammation is a recognized pathological mechanism of both DM and CAD [2, 18]. It has been previously reported that DM independently increased the risk of adverse events in CAD patients [21]. Our study showed that FAR was positively associated with glycemic metabolism indices (HbA1c and FBG) in our PCI cohort. It is important to investigate the joint effect of FAR and DM on long-term MACCE in CAD patients undergoing PCI. However, there is insufficient literature on the relationship between FAR, different glycemic metabolism and cardiovascular events.
To the best of our knowledge, our study demonstrated that higher level of FAR combined with impaired glycemic metabolism was strongly related to increased risks of long-term MACCEs, for the first time. Furthermore, non-diabetic patients with lower level of FAR also had lower all-cause mortality and cardiac mortality risk than those in the FAR-H/DM group. Multivariate Cox analysis also indicated the highest risk of MACCEs in patients with FAR-H and DM than others (P for trend = 0.005). Interestingly, we observed there were no significant differences among groups in the risk of other components of MACCE, which deserves further exploration in future studies. Given the clinical burden that both diabetes and inflammation exert on cardiovascular risk complications, the joint evaluations of diabetes and FAR might be of clinical significance for management of high-risk individuals in the FAR-H/DM group. However, till now, no treatment can specifically control the level of FAR on a long-term basis [32]. Further studies are warranted to evaluate whether CAD patients undergoing PCI, especially combined with DM, could benefit from medication targeting FAR in the future.
In this study, subgroup analysis was conducted to further explore some common factors involved and to identify in which subsets patients with FAR-H/DM is more meaningful. We found that patients with FAR-L/DM, FAR-H/non-DM and FAR-L/non-DM showed significantly consistency in certain subsets (male and non-CKD). Moreover, it may allow researchers to conduct clinical trials for certain therapies (e.g. colchicine, IL-6 inhibition, or low dose rivaroxaban 2.5 mg) for these subgroups to better guide subsequent treatment towards FAR-H/DM individuals. For instance, the CADENCE trial (NCT04181996) was conducted to determine if the drug colchicine had an effect on plaque inflammation in patients at high risk for events (patients with DM or Pre-DM and recent MI, stroke or transient ischemic attacks). The results focusing on patients with recent MI in this RCT have confirmed the safety and efficacy of colchicine [33]. Combined with the findings of our research, we are looking forward to the favorable results from the diabetic subgroup in the CADENCE trial. The ZEUS trial (NCT05021835) was designed to address whether IL-6 inhibition would reduce cardiovascular events in patients with both CKD and residual inflammatory risk [34]. The ongoing TRACK trial (NCT03969953) will hopefully answer whether low dose rivaroxaban therapy could bring favorable benefit in patients with both CAD and CKD. However, according to subgroup analysis in this study, it seems that in patients without CKD, controlling inflammation and treating diabetes also remain significant, which deserves our attention. Nevertheless, any recommendations made here are tentative, due to the limitations about the observational research.
Another issue to be discussed is the potential mechanisms underlying the association of FAR and DM with unfavorable prognosis. Firstly, previous studies confirmed that FIB could upregulate the expression of proinflammatory cytokines, like interleukin-1 and tumor necrosis factor-α, induce vascular inflammation and endothelial dysfunction, facilitate monocyte or macrophage adhesion, stimulate the proliferation and migration of vascular smooth muscle cells and eventually lead to the formation and vulnerability of atherosclerotic plaque [7, 35]. In addition, evidence from previous literatures suggested that higher concentrations of plasma FIB might contribute to the increase of blood viscosity and peripheral resistance, thereby increasing the risk of thrombosis and ischemic events during follow-up [28, 36]. Secondly, some basic researches proved that physiological concentration of serum albumin could inhibit the expression of vascular cell adhesion molecule-1, increase the elimination of oxygen-free radicals and finally reduce the inflammatory response, suggesting albumin was a protective anti-inflammatory property [17, 37]. Thirdly, inflammation is the shared antecedent of diabetes and atherosclerosis [2, 18]. In line with the previous studies, FAR was positively associated with glycemic metabolism indices and the potential association between DM and FAR had been discussed above [38, 39]. Therefore, further studies are required to elucidate the potential mechanisms. Meanwhile, the importance of routine screening for both novel inflammation biomarkers and impaired glycemic metabolism indices could not be neglected.
This study has several limitations. Firstly, the level of FAR was only calculated at baseline. Dynamic changes in this novel biomarker during follow-up are missing. Secondly, as the nature of the observational studies, potential confounders could not be adequately adjusted. Further randomized clinical trials are necessary to confirm our findings. Thirdly, this study was conducted in Chinese patients with CAD undergoing PCI and whether the findings could be generalized to other populations remains unknown. Fourthly, there was a degree of selection bias because of the strict exclusion criteria that excluded most of the individuals mainly due to FIB data unavailable.
Conclusion
In this real-world cohort study, higher level of FAR combined with DM was associated with worse 5-year outcomes among patients with CAD undergoing PCI. The level of FAR may help to identify high-risk individuals in this specific population, where more precise risk assessment should be performed.
Supplementary Information
Additional file 1: Table S1. Comparison of baseline characteristics and crude outcomes of participants and non-participants due to exclusion criteria. Table S2. Univariate Cox proportional hazard analysis for primary endpoint. Table S3. Correlation analysis between glycemic metabolism and FAR in patients with DM, without DM and whole. Table S4. Subgroup analysis for the primary endpoint as the unadjusted model. Table S5. Subgroup analysis for the primary endpoint as the adjusted model. Fig. S1. Restricted cubic splines of FAR levels in relation to crude HR(A) and adjusted HR(B) for the risk of MACCE. Model adjusted for age, sex, BMI, hypertension, previous MI, previous PCI, previous stroke, eGFR, LVEF, LM/three-vessel disease, and SYNTAX score. Red line with 95% confidence interval shaded in light red. HR hazard ratio, CI confidence interval, FAR fibrinogen to albumin ratio, MACCE major adverse cardiac and cerebrovascular events.
Acknowledgements
We give special thanks to the staff in the Department of Cardiology and Catheterization Laboratory, Fuwai Hospital for their research contributions.
Authors' contributions
PW and DY contributed to the study design and interpretation of the results. CZ, PZ, SJ, YS, XT, JX, TL, GZ contributed to the collection, analysis, or interpretation of data. PW and DY prepared the manuscript. JY, RG, BX, YY and XZ critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by grants from the Ministry of Science and Technology of the People’s Republic of China (2016YFC1301300 and 2016YFC1301301), National Natural Science Foundation of China (No. 81770365 and Grant No. 81900323), National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Sciences (Grant No. NCRC2020013), and the CAMS Innovation Fund for Medical Sciences (CIFMS): 2020-I2M-C&T-B-049.
Availability of data and materials
Due to ethical restrictions related to the consent given by subjects at the time of study commencement, our datasets are available from the corresponding author upon reasonable request after permission of the Institutional Review Board of State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the Fuwai hospital’s Research Ethics Committee. The Institutional Review Board approved the study protocol and all of the participants provided written informed consent.
Consent for publication
The manuscript was approved by all authors for publication.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peizhi Wang and Deshan Yuan contributed equally to this work
References
- 1.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 2.Sharif S, Van der Graaf Y, Cramer MJ, Kapelle LJ, de Borst GJ, Visseren FLJ, Westerink J. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc Diabetol. 2021;20(1):220. doi: 10.1186/s12933-021-01409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowe GD. Fibrinogen and cardiovascular disease: historical introduction. Eur Heart J. 1995;16(Suppl A):2–5. doi: 10.1093/eurheartj/16.suppl_A.2. [DOI] [PubMed] [Google Scholar]
- 4.Omland T, White HD. State of the art: blood biomarkers for risk stratification in patients with stable ischemic heart disease. Clin Chem. 2017;63(1):165–176. doi: 10.1373/clinchem.2016.255190. [DOI] [PubMed] [Google Scholar]
- 5.Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367(14):1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294(14):1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 7.Yuan D, Jiang P, Zhu P, Jia S, Zhang C, Liu Y, Liu R, Xu J, Tang X, Zhao X, et al. Prognostic value of fibrinogen in patients with coronary artery disease and prediabetes or diabetes following percutaneous coronary intervention: 5-year findings from a large cohort study. Cardiovasc Diabetol. 2021;20(1):143. doi: 10.1186/s12933-021-01335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oettl K, Stauber RE. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br J Pharmacol. 2007;151(5):580–590. doi: 10.1038/sj.bjp.0707251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 10.Zheng YY, Wu TT, Chen Y, Hou XG, Yang Y, Ma X, Ma YT, Zhang JY, Xie X. Gamma-glutamyl transferase to albumin ratio as a novel predictor of bleeding events and mortality in patients after percutaneous coronary intervention: a retrospective cohort study. Catheter Cardiovasc Interv. 2020;95(Suppl 1):572–578. doi: 10.1002/ccd.28696. [DOI] [PubMed] [Google Scholar]
- 11.Kurtul A, Ocek AH, Murat SN, Yarlioglues M, Demircelik MB, Duran M, Ergun G, Cay S. Serum albumin levels on admission are associated with angiographic no-reflow after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Angiology. 2015;66(3):278–285. doi: 10.1177/0003319714526035. [DOI] [PubMed] [Google Scholar]
- 12.Tan Z, Zhang M, Han Q, Wen J, Luo K, Lin P, Zhang L, Yang H, Fu J. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the Fibrinogen/Albumin Ratio. J Cancer. 2017;8(6):1025–1029. doi: 10.7150/jca.16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang L, Mo Z, Hu Z, Zhang L, Qin S, Qin X, Li S. Diagnostic value of fibrinogen to prealbumin ratio and gamma-glutamyl transpeptidase to platelet ratio in the progression of AFP-negative hepatocellular carcinoma. Cancer Cell Int. 2020;20:77. doi: 10.1186/s12935-020-1161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y, Wu C, Yan H, Chen S. Prognostic value of combined preoperative fibrinogen-albumin ratio and platelet-lymphocyte ratio score in patients with breast cancer: a prognostic nomogram study. Clin Chim Acta. 2020;506:110–121. doi: 10.1016/j.cca.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Duan Z, Luo C, Fu B, Han D. Association between fibrinogen-to-albumin ratio and the presence and severity of coronary artery disease in patients with acute coronary syndrome. BMC Cardiovasc Disord. 2021;21(1):588. doi: 10.1186/s12872-021-02400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M, Tang C, Luo E, Qin Y, Wang D, Yan G. Relation of fibrinogen-to-albumin ratio to severity of coronary artery disease and long-term prognosis in patients with non-ST elevation acute coronary syndrome. Biomed Res Int. 2020;2020:1860268. doi: 10.1155/2020/1860268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deveci B, Gazi E. Relation between globulin, fibrinogen, and albumin with the presence and severity of coronary artery disease. Angiology. 2021;72(2):174–180. doi: 10.1177/0003319720959985. [DOI] [PubMed] [Google Scholar]
- 18.Winzap P, Davies A, Klingenberg R, Obeid S, Roffi M, Mach F, Räber L, Windecker S, Templin C, Nietlispach F, et al. Diabetes and baseline glucose are associated with inflammation, left ventricular function and short- and long-term outcome in acute coronary syndromes: role of the novel biomarker Cyr 61. Cardiovasc Diabetol. 2019;18(1):142. doi: 10.1186/s12933-019-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosenzon O, Alguwaihes A, Leon JLA, Bayram F, Darmon P, Davis TME, Dieuzeide G, Eriksen KT, Hong T, Kaltoft MS, et al. CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol. 2021;20(1):154. doi: 10.1186/s12933-021-01344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjörnsdottir S, Wedel H, Clements M, Dahlqvist S, Lind M. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 21.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 22.Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards) Circulation. 2015;132(4):302–361. doi: 10.1161/CIR.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 23.Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, Green J, Huang E, Isaacs D, Kahan S, et al. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Supplement_1):17–38. doi: 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 24.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 25.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 26.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 27.Yano K, Grove JS, Chen R, Rodriguez BL, Curb JD, Tracy RP. Plasma fibrinogen as a predictor of total and cause-specific mortality in elderly Japanese-American men. Arterioscler Thromb Vasc Biol. 2001;21(6):1065–1070. doi: 10.1161/01.ATV.21.6.1065. [DOI] [PubMed] [Google Scholar]
- 28.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279(18):1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 29.González-Pacheco H, Amezcua-Guerra LM, Sandoval J, Martínez-Sánchez C, Ortiz-León XA, Peña-Cabral MA, Bojalil R. Prognostic implications of serum albumin levels in patients with acute coronary syndromes. Am J Cardiol. 2017;119(7):951–958. doi: 10.1016/j.amjcard.2016.11.054. [DOI] [PubMed] [Google Scholar]
- 30.Karahan O, Acet H, Ertaş F, Tezcan O, Çalişkan A, Demir M, Kaya AF, Demirtaş S, Çevik MU, Yavuz C. The relationship between fibrinogen to albumin ratio and severity of coronary artery disease in patients with STEMI. Am J Emerg Med. 2016;34(6):1037–1042. doi: 10.1016/j.ajem.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Xiao L, Jia Y, Wang X, Huang H. The impact of preoperative fibrinogen-albumin ratio on mortality in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Clin Chim Acta. 2019;493:8–13. doi: 10.1016/j.cca.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Sahebkar A, Serban MC, Mikhailidis DP, Toth PP, Muntner P, Ursoniu S, Mosterou S, Glasser S, Martin SS, Jones SR, et al. Head-to-head comparison of statins versus fibrates in reducing plasma fibrinogen concentrations: a systematic review and meta-analysis. Pharmacol Res. 2016;103:236–252. doi: 10.1016/j.phrs.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Rane M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ Res. 2021;128(11):1728–1746. doi: 10.1161/CIRCRESAHA.121.319077. [DOI] [PubMed] [Google Scholar]
- 35.Jensen T, Kierulf P, Sandset PM, Klingenberg O, Joø GB, Godal HC, Skjønsberg OH. Fibrinogen and fibrin induce synthesis of proinflammatory cytokines from isolated peripheral blood mononuclear cells. Thromb Haemost. 2007;97(5):822–829. doi: 10.1160/TH07-01-0039. [DOI] [PubMed] [Google Scholar]
- 36.Lowe GD, Fowkes FG, Dawes J, Donnan PT, Lennie SE, Housley E. Blood viscosity, fibrinogen, and activation of coagulation and leukocytes in peripheral arterial disease and the normal population in the Edinburgh Artery Study. Circulation. 1993;87(6):1915–1920. doi: 10.1161/01.CIR.87.6.1915. [DOI] [PubMed] [Google Scholar]
- 37.Prajapati KD, Sharma SS, Roy N. Current perspectives on potential role of albumin in neuroprotection. Rev Neurosci. 2011;22(3):355–363. doi: 10.1515/rns.2011.028. [DOI] [PubMed] [Google Scholar]
- 38.Odegaard AO, Jacobs DR, Jr, Sanchez OA, Goff DC, Jr, Reiner AP, Gross MD. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc Diabetol. 2016;15:51. doi: 10.1186/s12933-016-0369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunutsor SK, Khan H, Laukkanen JA. Serum albumin concentration and incident type 2 diabetes risk: new findings from a population-based cohort study. Diabetologia. 2015;58(5):961–967. doi: 10.1007/s00125-015-3520-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Comparison of baseline characteristics and crude outcomes of participants and non-participants due to exclusion criteria. Table S2. Univariate Cox proportional hazard analysis for primary endpoint. Table S3. Correlation analysis between glycemic metabolism and FAR in patients with DM, without DM and whole. Table S4. Subgroup analysis for the primary endpoint as the unadjusted model. Table S5. Subgroup analysis for the primary endpoint as the adjusted model. Fig. S1. Restricted cubic splines of FAR levels in relation to crude HR(A) and adjusted HR(B) for the risk of MACCE. Model adjusted for age, sex, BMI, hypertension, previous MI, previous PCI, previous stroke, eGFR, LVEF, LM/three-vessel disease, and SYNTAX score. Red line with 95% confidence interval shaded in light red. HR hazard ratio, CI confidence interval, FAR fibrinogen to albumin ratio, MACCE major adverse cardiac and cerebrovascular events.
Data Availability Statement
Due to ethical restrictions related to the consent given by subjects at the time of study commencement, our datasets are available from the corresponding author upon reasonable request after permission of the Institutional Review Board of State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases.