Abstract
Background
Increased epigenetic age acceleration (EAA) in survivors of childhood cancer is associated with specific treatment exposures, unfavorable health behaviors, and presence of certain chronic health conditions. To better understand inter-individual variability, we investigated the genetic basis underlying EAA.
Methods
Genome-wide association studies of EAA based on multiple epigenetic clocks (Hannum, Horvath, PhenoAge, and GrimAge) were performed. MethylationEPIC BeadChip array and whole-genome sequencing data were generated with blood-derived DNA from participants in the St. Jude Lifetime Cohort Study (discovery: 2138 pre-existing and 502 newly generated data, all survivors; exploratory: 282 community controls). Linear regression models were fit for each epigenetic age against the allelic dose of each genetic variant, adjusting for age at sampling, sex, and cancer treatment exposures. Fixed-effects meta-analysis was used to combine summary statistics from two discovery data sets. LD (Linkage disequilibrium) score regression was used to estimate single-nucleotide polymorphism (SNP)-based heritability.
Results
For EAA-Horvath, a genome-wide significant association was mapped to the SELP gene with the strongest SNP rs732314 (meta-GWAS: β=0.57, P=3.30×10-11). Moreover, the stratified analysis of the association between rs732314 and EAA-Horvath showed a substantial heterogeneity between children and adults (meta-GWAS: β=0.97 vs. 0.51, I2=73.1%) as well as between survivors with and without chest/abdominal/pelvic-RT exposure (β=0.64 vs. 0.31, I2=66.3%). For EAA-Hannum, an association was mapped to the HLA locus with the strongest SNP rs28366133 (meta-GWAS: β=0.78, P=3.78×10-11). There was no genome-wide significant hit for EAA-PhenoAge or EAA-GrimAge. Interestingly, among community controls, rs732314 was associated with EAA-Horvath (β=1.09, P=5.43×10-5), whereas rs28366133 was not associated with EAA-Hannum (β=0.21, P=0.49). The estimated heritability was 0.33 (SE=0.20) for EAA-Horvath and 0.17 (SE=0.23) for EAA-Hannum, but close to zero for EAA-PhenoAge and EAA-GrimAge.
Conclusions
We identified novel genetic variants in the SELP gene and HLA region associated with EAA-Horvath and EAA-Hannum, respectively, among survivors of childhood cancer. The new genetic variants in combination with other replicated known variants can facilitate the identification of survivors at higher risk in developing accelerated aging and potentially inform drug targets for future intervention strategies among vulnerable survivors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13073-022-01038-6.
Keywords: Genome-wide association study, Epigenetic age acceleration, Childhood cancer, Survivorship, SELP, HLA
Background
A 5-year survival for children diagnosed with cancer has increased to ~85% due to remarkable progress in treatment [1]. Thus, the population of childhood cancer survivors has grown rapidly and is estimated to exceed 500,000 in the USA [2]. Survivors of childhood cancer experience accelerated aging [3, 4], which may not only correlate with increased risk of chronic health conditions (CHCs), but likely reflect the influences of genetics, treatment/environmental exposures, and behavioral factors that, in combination, impact physiological health [5, 6]. Understanding biological pathways and determinants underpinning the aging process among childhood cancer survivors will facilitate the identification of individuals at the greatest risk and inform molecular candidates and biological processes for those who may benefit from targeted therapies soon after curative treatment or later among long-term survivors.
Several molecular biomarkers of aging are available [5–10], including DNA methylation (DNAm)-based epigenetic age. Epigenetic age acceleration (EAA) (i.e., the difference between epigenetic and chronological ages) demonstrates excellent predictive accuracy for physiological aging and age-related disease risks in the general population [11]. We recently reported that EAA based on PhenoAge (i.e., Levine’s clock) [12] is statistically significantly higher in survivors of childhood cancer than community controls and is associated with specific treatment exposures, unfavorable health behaviors, and presence of different CHCs such as hypertension, myocardial infarction, obesity, obstructive pulmonary deficit, and peripheral sensory neuropathy [6]. However, the genetic basis underpinning EAA has not yet been investigated among the childhood cancer survivors.
A meta-analysis of genome-wide association studies (GWASs) on 9907 individuals demonstrated that the EAA derived from various epigenetic clocks is a trait with a moderate heritability (h2=0.19) in the general population [13] and identified five loci associated with IEAA-Horvath (Intrinsic Epigenetic Age Acceleration) and three associated with EEAA-Hannum (Extrinsic Epigenetic Age Acceleration) [13]. Gilson et al. performed single-nucleotide polymorphism (SNP)-based and gene-based GWAS of both IEAA-Horvath and EEAA-Hannum on 13,493 individuals of European ancestry and identified 10 independent SNPs and 21 genes associated with IEAA-Horvath including the notable PIK3CB related to human longevity, and one SNP and 12 genes associated with EEAA-Hannum including CISD2 related to lifespan control [14]. Another large-scale GWAS study comprised of more than 40,000 individuals identified a bulk of 137 loci associated with DNAm biomarkers of aging [15], which enhanced knowledge about the genetic architecture underlying EAA. In addition to germline variants, clonal hematopoiesis of indeterminant potentials (CHIP), which were somatically acquired genetic factors and predictive of the development of leukemia (particularly, acute myeloid leukemia in the elderly) [16], were associated with EAA derived from multiple epigenetic clocks [17].
Thus, to advance the understanding about the genetic factors underlying EAA among childhood cancer survivors, we performed GWAS with Infinium MethylationEPIC BeadChip array and whole-genome sequencing (WGS) data generated with blood-derived DNA from participants of the St. Jude Lifetime Cohort Study (SJLIFE) [18, 19]. Specifically, we aimed to search for novel genetic loci, evaluate previous findings reported in the general population, and characterize genetic contributions to EAA in childhood cancer survivors.
Methods
Study population
SJLIFE participants (n=2922) with pre-exiting whole-genome sequencing data [20, 21] were included in the current study. The first discovery data set, denoted as SJLIFE1 Survivors, included 2138 survivors previously scanned with EPIC array [6]. The second discovery data set, denoted as SJLIFE2 survivors, included 502 survivors newly scanned with EPIC array in an expansion study focused primarily on childhood and adolescent survivors. The third exploratory data set, denoted Community Controls, included 282 controls with no history of childhood cancer, who were enrolled in SJLIFE study and frequency matched to the survivors by age, sex, and race. This set of controls was also previously scanned with an EPIC array [6].
Demographic, diagnostic, and treatment data
Demographic characteristics (sex and race/ethnicity) and clinical information (primary diagnosis, age at diagnosis, and treatment exposures) were abstracted using a structured protocol [19]. Region-specific radiotherapy (RT) dosimetry, including brain-RT, chest-RT, and abdomen/pelvic-RT, were estimated using radiation oncology treatment records [19]. Cumulative doses of individual chemotherapeutic agents, including alkylating agents, anthracyclines, epipodophyllotoxins, glucocorticoids, platinum, and vincristine, were abstracted from medical records.
DNA methylation measurement
Genome-wide methylation data were generated using Infinium MethylationEPIC BeadChip array on whole blood-derived DNA. Laboratory work followed the standard procedure as described previously [6, 21], including DNA extraction, bisulfite treatment, array hybridization, and scanning. The raw intensity was exported from Illumina Genome Studio and analyzed in R (version 3.6.3) using the minfi package [22]. Detailed quality controls (QCs) and data normalization were described previously [23]. After QCs, the data set comprised beta-values for 689,419 CpGs.
Epigenetic age and epigenetic age acceleration
Epigenetic age estimates based on different clocks, including those of Horvath [24], Hannum [25], PhenoAge [12], and GrimAge [26], were obtained from the online New Methylation Age Calculator (https://dnamage.genetics.ucla.edu/new) [24]. Normalized DNA methylation beta-values and the sample annotation files were submitted to the calculator, using the “Advanced Analysis” option. Blood cell abundance measures were also estimated by the calculator, based on DNA methylation levels, as described previously [27, 28]. EAAs were estimated as residuals from a linear regression model of the estimated epigenetic age against the chronological age (i.e., age at DNA sampling). Additionally, another variation of EAA was estimated based on the Horvath clock with adjustments for leukocyte subtype proportions, commonly referred to as IEAA, which captures cellular intrinsic DNAm changes [29, 30]. In contrast, EEAA, which tracks age-related changes in blood cell composition and cellular intrinsic DNAm level [29, 30], was calculated as residuals from regressing BioAge4HAStatic (Hannum clock with up-weighting of blood cell counts) against the chronological age.
Genotyping based on whole-genome sequencing (WGS)
WGS data (n=2922) for this study were obtained from a previous large effort to sequence blood-derived DNA from 4402 SJLIFE survivors as previously described [20, 21], including the first set of 3006 survivors sequenced by using HiSeq X Ten System with 36.8-fold average genome-wide coverage per sample, and the second set of 1396 survivors sequenced on Illumina NovaSeq with similar (38.7-fold) average genome-wide coverage. Sequencing reads were aligned to the GRCh38 human reference genome assembly with BWA (v0.7.12-r1039) [31] using default settings, and variant calls were processed with the GATK v3.4 pipeline by following its recommended best practices including VQSR (variant quality score recalibration) filtering [32]. The entire collection of WGS data for 4402 survivors, including raw sequence reads, aligned BAM files, and joint genotype calls (gVCFs), is accessible through St. Jude Cloud (https://stjude.cloud). Additional quality control was performed when genotypes for the subset of survivors (n=2640 also with DNA EPIC array data available) were extracted with VCFtools v0.1.15 [33], including the following criteria for keeping the variants or genotypes: (1) minimum genotype quality score of 20, (2) minimum depth of 5, (3) minimum mean depth of 10, (4) Hardy Weinberg Equilibrium (HWE) P > 1×10-6, (5) maximum of missing rate is 10% across all samples, and (6) minor allele frequency (MAF) > 0.01. Genotypes for a set of community controls (n=282) was extracted from the existing WGS data and processed in the same manner. A total of 8.3 million autosomal single-nucleotide variants (SNVs) and small insertions and deletions (indels) were advanced for further association analysis.
Statistical and bioinformatic analyses
Population characteristics were compared between survivors in the SJLIFE1 and SJLIFE2 data sets, and between survivors and controls by chi-square test for categorical variables and t test for continuous variables. Pearson’s correlation coefficient (r) was used to measure the linear correlation between the estimated epigenetic age and the chronological age, and between a pair of the estimated epigenetic ages or EAAs based on different epigenetic clocks among survivors. For EAA GWAS, linear regression models were fit for each estimated epigenetic age against the additive dose of each genetic variant, adjusting for age at DNA sampling, sex, and cancer treatment exposures. EAA GWAS analysis was carried out for each of three data sets with both WGS and EPIC array data, respectively. Fixed-effects meta-analysis was used to combine the summary statistics of two discovery data sets. A fixed meta-GWAS P value threshold of 5×10-8 was set as the level of genome-wide significance. PLINK (1.90b) was used for the genetic association analysis [34]. Genetic heterogeneity between data sets was assessed by using I2 and P value (Phet) calculated from the Cochran’s Q statistic. Differentially methylated regions (DMR) between survivors and community controls were analyzed with DMRcate R package [35] using R 4.0.2 [30]. Other statistical analyses were performed with R 3.6.1 [36], and a two-sided P value <0.05 was considered as statistically significant.
Manhattan plots were generated using CMplot [37]. Regional SNP association results were visualized with the LocusZoom [38]. All linkage disequilibrium (LD) estimates were calculated using individuals of European ancestry from the 1000 genomes reference panel using LDlink [39]. LD score regression [40] was used to estimate SNP-based heritability (h2 and its standard error [SE]) and to calculate genetic correlations between EAA and other traits (n=855) through LD Hub [41].
Results
Characteristics of the study population
Demographics, primary diagnosis, and treatment information for the SJLIFE1 data set were described previously [6] and were included in Table 1, along with information for the SJLIFE2 data set with newly generated DNAm data. Among those in the SJLIFE2 data set, 53.2% were male and 97.8% were non-Hispanic. Primary diagnoses were leukemia (35.1%), lymphoma (7.2%), sarcoma (7.8%), central nervous system (CNS) tumors (16.5%), and other solid tumors (33.4%). Regarding treatments, 29.3% were exposed to brain-RT, 16.3% chest-RT, 16.1% abdominal/pelvic-RT, 51.2% alkylating agents, 51.2% anthracyclines, 33.1% epipodophyllotoxins, 39.6% glucocorticoids, 25.1% platinum, and 68.3% vincristine. The median ages at diagnosis and at DNA sampling were 3.1 (range= 0.0–19.9) and 16.5 (range= 7.3–66.6) years, respectively. Among the 282 noncancer controls, 48.6% were male and 2.1% Hispanic. The median age at DNA sampling was 35.0 (range = 18.7 to 70.2) years [6].
Table 1.
Characteristics of participants included in the study
| Characteristics | SJLIFE1 survivors No. (%) |
SJLIFE2 survivors No. (%) |
Non-cancer controls No. (%) |
SJLIFE1 vs. SJLIFE2 P |
Survivors vs. controls P |
|---|---|---|---|---|---|
| Total | 2138a | 502 | 282 | ||
| Sex | |||||
| Male | 1132 (53.0) | 267 (53.2) | 137 (48.6) | 0.17 | 0.19 |
| Female | 1006 (47.0) | 235 (46.8) | 145 (51.4) | ||
| Ethnicity | |||||
| Hispanic | 24 (1.1) | 11 (2.2) | 6 (2.1) | 0.18 | 0.15 |
| Non-Hispanic | 2114 (98.9) | 491 (97.8) | 276 (97.9) | ||
| Diagnosis | |||||
| Leukemia | 731 (34.2) | 176 (35.1) | |||
| Acute lymphoblastic leukemia | 671 (31.4) | 159 (31.7) | – | ||
| Acute myeloid leukemia | 58 (2.7) | 15 (3.0) | – | ||
| Other leukemia | 2 (0.1) | 2 (0.4) | – | ||
| Lymphoma | 460 (21.5) | 36 (7.2) | |||
| Hodgkin lymphoma | 296 (13.8) | 11 (2.2) | – | ||
| Non-Hodgkin lymphoma | 164 (7.7) | 25 (5.0) | – | ||
| Sarcoma | 283 (13.2) | 39 (7.8) | – | ||
| Ewing sarcoma | 76 (3.6) | 7 (1.4) | – | ||
| Osteosarcoma | 76 (3.6) | 5 (1.0) | |||
| Rhabdomyosarcoma | 73 (3.4) | 15 (3.0) | – | ||
| Nonrhabdomyosarcoma | 58 (2.7) | 12 (2.4) | – | ||
| CNS tumors | 245 (11.5) | 83 (16.5) | |||
| Astrocytoma or glioma | 113 (5.3) | 41 (8.2) | – | ||
| Medulloblastoma or PNET | 59 (2.8) | 15 (3.0) | – | ||
| Ependymoma | 27 (1.3) | 8 (1.6) | – | ||
| Other CNS tumors | 46 (2.2) | 19 (3.8) | – | ||
| Embryonal | 285 (13.3) | 82 (16.3) | |||
| Wilms tumor | 140 (6.5) | 32 (6.4) | – | ||
| Neuroblastoma | 109 (5.1) | 39 (7.8) | – | ||
| Germ cell tumor | 36 (1.9) | 11 (2.2) | – | ||
| Other | 134 (6.3) | 86 (17.1) | |||
| Retinoblastoma | 50 (2.3) | 67 (13.3) | – | ||
| Hepatoblastoma | 15 (0.7) | 2 (0.4) | – | ||
| Melanoma | 13 (0.6) | 4 (0.8) | – | ||
| Carcinomas | 24 (1.1) | 3 (0.6) | – | ||
| Others | 32 (1.5) | 10 (2.0) | – | ||
| Radiation | |||||
| Brain RT | 666 (31.2) | 147 (29.3) | – | ||
| Chest RT | 608 (28.4) | 82 (16.3) | – | ||
| Abdomen/pelvis RT | 479 (22.4) | 81 (16.1) | – | ||
| Chemotherapy | |||||
| Alkylating agent | 1269 (59.4) | 257 (51.2) | – | ||
| Anthracyclines | 1265 (59.2) | 257 (51.2) | – | ||
| Epipodophyllotoxins | 762 (35.6) | 166 (33.1) | – | ||
| Glucocorticoids | 1010 (47.2) | 199 (39.6) | – | ||
| Platinum | 271 (12.7) | 126 (25.1) | – | ||
| Vincristine | 1489 (69.6) | 343 (68.3) | – | ||
| Median age at diagnosis, years (range) | 7.3 (0.0, 23.6) | 3.1 (0.0, 19.9) | NA | ||
| Median age at DNA sampling, years (range) | 31.8 (6.0, 66.4) | 16.5 (7.3, 66.6) | 35.0 (18.7, 70.2) | <0.001 | <0.001 |
Abbreviations: SJLIFE1 survivors The first discovery data set of 2138 survivors included in our previously published study (ref. [6]), SJLIFE2 survivors The second discovery data set of 502 children and adolescent survivors, CNS Central nervous system, PNET Primitive neuroectodermal tumor, RT Radiotherapy
aStarting from 2139 survivors included in our previous publication (ref. [6]), a retinoblastoma survivor failed the minfi QC with low total intensity and hence was excluded from this current analysis
Epigenetic age and epigenetic age acceleration
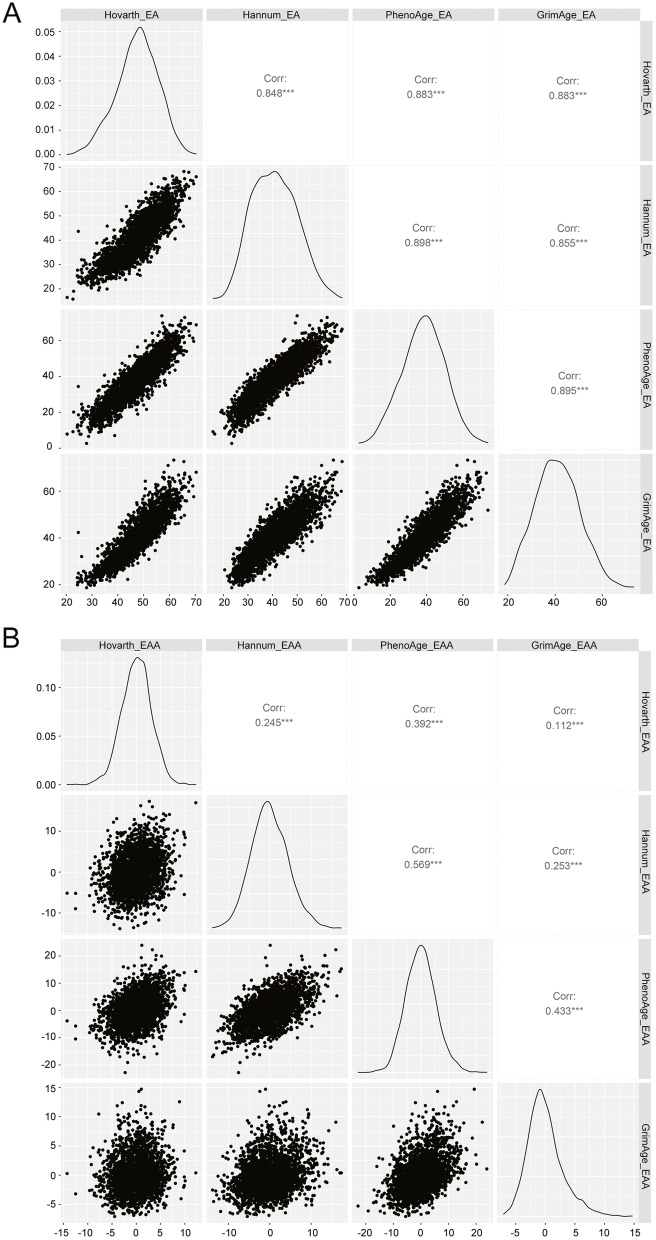
All four epigenetic age estimates had high Pearson’s correlation with chronological age (r=0.87–0.95) (Additional file 1: Fig. S1). Pair-wise comparisons among the four epigenetic age estimates also showed a high correlation (r=0.85–0.90) (Fig. 1A). However, the pair-wise comparison among the four EAA showed a range of correlations (r=0.11–0.57); the pair of EAA-Hannum and EAA-PhenoAge had a moderately strong correlation (r=0.57), whereas EAA-Horvath and EAA-GrimAge had weak correlation (r=0.11) (Fig. 1B).
Fig. 1.
Pairwise correlations of four epigenetic age (A) and epigenetic age acceleration (B). Abbreviations: epigenetic age (EA) and epigenetic age acceleration (EAA). Pair-wise Pearson correlation coefficients were shown with P< 0.001 denoted as ***
Genome-wide association analysis of EAA-Horvath
The overall association results (-log10 of P values) for the EAA based on the Horvath clock [24] in the meta-GWAS of two discovery data sets (SJLIFE1 and SJLIFE2) with a total of 2640 survivors were illustrated in Fig. 2A, with a genomic inflation factor of 1.01, suggesting little systematic inflation (Additional file 1: Fig. S2A). The strongest association was observed for rs732314 (combined: β=0.57, P=3.30×10-11, I2=54.36%; SJLIFE1: β=0.50, P=1.55×10-7; SJLIFE2: β=0.82, P=2.09×10-5), which is mapped to the first intron of SELP gene on chromosome 1, and this SNP also showed statistically significant association among the community controls (Table 2). There were 53 other variants reaching genome-wide significance (P<5×10-8) (Additional file 1: Table S1), and they are all mapped to the same genomic region with high LD with the index variant rs732314 (R2 > 0.62) based on CEU (Utah residence from North and West Europe) population in 1000 Genomes project (Fig. 3A).
Fig. 2.
Manhattan plots of genome-wide association study (GWAS) for epigenetic age acceleration (EAA)-Horvath (A) and EAA-Hannum (B). aEach dot represents the test result for one SNP. bX-axis is the genomic location along each chromosome, and Y-axis is -log10 of P value. cThe red horizontal line corresponds to the P value of 5×10-8
Table 2.
Top SNP significantly associated with EAA-Horvath and EAA-Hannum among survivors (SJLIFE1, SJLIFE2) and controls
| GWAS | SNP | Chr | Pos_hg38 | Effect allele | Other allele | Population | Effect size | (SE) | P | Phet | I2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EAA-Horvath | rs732314 | 1 | 169630016 | C | T | SJLIFE1 survivors | 0.50 | 0.10 | 1.55E−07 | ||
| SJLIFE2 survivors | 0.82 | 0.19 | 2.09E−05 | ||||||||
| Combined survivors | 0.57 | 0.09 | 3.30E−11 | 0.14 | 54.36 | ||||||
| Community controls | 1.09 | 0.27 | 5.43E−05 | ||||||||
| EAA-Hannum | rs28366133 | 6 | 31396299 | C | T | SJLIFE1 survivors | 0.76 | 0.14 | 5.30E−08 | ||
| SJLIFE2 survivors | 0.84 | 0.23 | 2.05E−04 | ||||||||
| Combined survivors | 0.78 | 0.12 | 3.78E−11 | 0.75 | 0.00 | ||||||
| Community controls | 0.21 | 0.30 | 4.88E−01 |
Abbreviations: GWAS Genome-wide association study, EAA Epigenetic age acceleration, Chr Chromosome, SE Standard error, SJLIFE1 survivors The first discovery data set of 2138 survivors included in our previously published study (ref. [6]), SJLIFE2 survivors The second discovery data set of 502 children and adolescent survivors, Combined survivors A combined set (meta-GWAS analysis) of the two discovery data sets (SJLIFE1 and SJLIFE2), Community controls A set of 282 community controls
Fig. 3.
Regional plots for genetic associations between SELP locus and epigenetic age acceleration (EAA)-Horvath (A), HLA locus, and EAA-Hannum (B). aEach dot represents the test result for one SNP. bX-axis is the genomic location along each chromosome, and Y-axis is -log10 of P value. cThe red horizontal line corresponds to the P value of 5×10-8. dSNP depicted in diamond is the index SNP for the region, and all other SNPs were depicted as circles and their correlations (i.e., pair-wise linkage disequilibrium) with the index SNP was warm/cool color-coded
In addition, we carried out an analysis with another variation of EAA based on the Horvath clock, i.e., IEAA [29]. The top findings were similar, but with one additional SNP rs3917679 reaching the genome-wide significance level (Additional file 1: Table S2). This SNP, which also mapped to the first intron of SELP, had high LD with rs732314 (R2=0.98) and perfect LD (R2=1) with rs3917647, suggesting that they share the same haplotype and presumably tag the same causative variant.
Genome-wide association analysis of EAA-Hannum
A similar analysis was performed for the EAA based on the Hannum clock [25]. The overall association results (-log10 of P values) across the genome in the meta-GWAS were provided in Fig. 2B. The genomic inflation factor was 1.02, suggesting little systematic inflation (Additional file 1: Fig. S2B). The most strongly associated SNP was rs28366133 (combined: β=0.78, P=3.78×10-11, I2=0; SJLIFE1: β=0.76, P=5.30×10-8; SJLIFE2: β=0.84, P=2.05×10-4), but this SNP did not show a statistically significant association among the community controls (Table 2). There were 10 other variants reaching genome-wide significance (Additional file 1: Table S1), and they were all mapped to the complex HLA region with a number of genes nearby and moderate to high LD with the index variant rs28366133 (R2 > 0.29) (Fig. 3B). To explore reasons for the heterogeneity, we analyzed and observed significant DMRs in the HLA locus between survivors and community controls (Additional file 1: Table S3). In further analysis conditioning on rs28366133, the strength of association with these ten variants was attenuated with the lowest P value >1.0×10-3, suggesting that a secondary signal in this locus is less likely.
In addition, we analyzed another variation of EAA based on the Hannum clock, i.e., EEAA. Only rs28366133 was associated with EEAA-Hannum at the genome-wide significant level (Additional file 1: Table S2).
There was no genome-wide significant association in our analysis for EAA based on PhenoAge and GrimAge.
Stratified analysis by chronologically defined age and sex
The fixed effects meta-analysis that showed substantial heterogeneity for the top EAA-Horvath associated SNP (I2: 54.36% for rs732314, Table 2) prompted us to conduct stratified analysis, given the difference in distributions of chronological age between the SJLIFE1 and SJLIFE2 data sets. Among children (<18 years old), the association between rs732314 and EAA-Horvath was stronger with a much larger effect size (combined: β=0.97, P=1.52×10-5, I2=0.0%; SJLIFE1: β=0.96, P=0.062; SJLIFE2: β=0.97, P=1.27×10-4) compared to adults (combined: β=0.51, P=1.39×10-8, I2=0.0%; SJLIFE1: β=0.51, P=1.14×10-7; SJLIFE2: β=0.51, P=0.05) (Table 3). It is notable that the effect size is highly consistent between the SJLIFE1 and SJLIFE2 data sets among children (0.96 vs. 0.97) or adults (0.51 vs. 0.51). Overall, a comparison between the children and adults showed substantial heterogeneity (combined: β=0.97 vs. 0.51, I2=73.1%, Phet=0.05).
Table 3.
Age-related, gender-, and treatment-stratified effect sizes of significant SNPs associated with EAA among different data sets
| GWAS | SNP | Population | − | Effect size | (SE) | P | Phet | I2 (%) |
|---|---|---|---|---|---|---|---|---|
| EAA-Horvath | rs732314 | survivors (SJLIFE1) <18 Y | 70 | 0.96 | 0.50 | 6.16E−02 | ||
| survivors (SJLIFE2) <18 Y | 314 | 0.97 | 0.25 | 1.27E−04 | ||||
| survivors (combined) <18 Y | 384 | 0.97 | 0.22 | 1.52E−05 | 0.98 | 0.00 | ||
| survivors (SJLIFE1) ≥18 Y | 2066 | 0.51 | 0.10 | 1.14E−07 | ||||
| survivors (SJLIFE2) ≥18 Y | 188 | 0.51 | 0.26 | 5.04E−02 | ||||
| survivors (combined) ≥18 Y | 2254 | 0.51 | 0.09 | 1.39E−08 | 1.00 | 0.00 | ||
| survivors (SJLIFE1) male | 1132 | 0.46 | 0.13 | 3.92E−04 | ||||
| survivors (SJLIFE2) male | 267 | 0.79 | 0.25 | 1.71E−03 | ||||
| survivors (combined) male | 1399 | 0.53 | 0.11 | 3.94E−06 | 0.24 | 28.57 | ||
| survivors (SJLIFE1) female | 1004 | 0.54 | 0.14 | 2.10E−04 | ||||
| survivors (SJLIFE2) female | 235 | 0.84 | 0.30 | 5.04E−03 | ||||
| survivors (combined) female | 1239 | 0.60 | 0.13 | 4.58E−06 | 0.35 | 0.00 | ||
| survivors (SJLIFE1) trunk-RT_Yes | 649 | 0.37 | 0.17 | 3.47E−02 | ||||
| survivors (SJLIFE2) trunk-RT_Yes | 91 | 0.03 | 0.39 | 9.39E−01 | ||||
| survivors (combined) trunk-RT_Yes | 740 | 0.31 | 0.16 | 4.99E−02 | 0.42 | 0.00 | ||
| survivors (SJLIFE1) trunk-RT_No | 1487 | 0.56 | 0.12 | 1.30E−06 | ||||
| survivors (SJLIFE2) trunk-RT_No | 411 | 0.91 | 0.22 | 3.46E−05 | ||||
| survivors (combined) trunk-RT_No | 1898 | 0.64 | 0.10 | 3.88E−10 | 0.16 | 49.83 | ||
| EAA-Hannum | rs28366133 | survivors (SJLIFE1) <18 Y | 70 | 0.17 | 0.73 | 8.20E−01 | ||
| survivors (SJLIFE2) <18 Y | 314 | 1.13 | 0.27 | 5.17E−05 | ||||
| survivors (combined) <18 Y | 384 | 1.01 | 0.26 | 8.80E−05 | 0.21 | 35.12 | ||
| survivors (SJLIFE1) ≥18 Y | 2066 | 0.80 | 0.14 | 2.54E−08 | ||||
| survivors (SJLIFE2) ≥18 Y | 188 | 0.28 | 0.40 | 4.78E−01 | ||||
| survivors (combined) ≥18 Y | 2254 | 0.74 | 0.13 | 3.69E−08 | 0.23 | 32.01 | ||
| survivors (SJLIFE1) male | 1131 | 0.74 | 0.19 | 1.40E−04 | ||||
| survivors (SJLIFE2) male | 267 | 0.91 | 0.32 | 4.43E−03 | ||||
| survivors (combined) male | 1398 | 0.79 | 0.17 | 1.97E−06 | 0.64 | 0.00 | ||
| survivors (SJLIFE1) female | 1005 | 0.77 | 0.20 | 1.39E−04 | ||||
| survivors (SJLIFE2) female | 235 | 0.71 | 0.33 | 3.47E−02 | ||||
| survivors (combined) female | 1240 | 0.76 | 0.17 | 1.23E−05 | 0.87 | 0.00 | ||
| survivors (SJLIFE1) chest-RT_Yes | 608 | 0.72 | 0.27 | 6.89E−03 | ||||
| survivors (SJLIFE2) chest-RT_Yes | 83 | 0.84 | 0.71 | 2.41E−01 | ||||
| survivors (combined) chest-RT_Yes | 691 | 0.74 | 0.25 | 3.14E−03 | 0.88 | 0.00 | ||
| survivors (SJLIFE1) chest-RT_No | 1528 | 0.77 | 0.16 | 2.48E−06 | ||||
| survivors (SJLIFE2) chest-RT_No | 419 | 0.88 | 0.24 | 3.02E−04 | ||||
| survivors (combined) chest-RT_No | 1947 | 0.80 | 0.14 | 2.56E−09 | 0.70 | 0.00 |
Abbreviations: GWAS Genome-wide association study, EAA Epigenetic age acceleration, Chr Chromosome, SE Standard error, N Number of samples, survivor (SJLIFE1) The first discovery data set of 2138 survivors included in our previously published study (ref. [6]), survivor (SJLIFE2) The second discovery data set of 502 children and adolescent survivors, survivors (combined) A combined set (meta-GWAS analysis) of the two discovery data sets (SJLIFE1 and SJLIFE2), trunk-RT Chest/abdominal/pelvic radiation therapy, chest-RT Chest radiation therapy
The association between rs28366133 and EAA-Hannum in children (combined: β=1.01, P=8.80×10-5, I2=35.12%; SJLIFE1: β=0.17, P=0.82; SJLIFE2: β=1.13, P=5.17×10-5) did not vary much from that in adults (combined: β=0.74, P=3.69×10-8, I2=32.01%; SJLIFE1: β=0.80, P=2.54×10-8; SJLIFE2: β=0.28, P=0.48) (Table 3). There was only moderate heterogeneity between SJLIFE1 and SJLIFE2 data sets among children (I2=35.12) or adults (I2=32.01).
Sex was significantly associated with EAA-Horvath or EAA-Hannum in the multivariable regression models (Additional file 1: Table S4). The association between rs732314 and EAA-Horvath was slightly stronger in females (β=0.60 vs. 0.53) whereas the association between rs28366133 and EAA-Hannum was slightly stronger in males (β=0.79 vs. 0.76) (Table 3).
Stratified analysis by treatment exposures
In the multivariable regression models, chest-RT, abdominal/pelvic-RT, and alkylators were significantly associated with EAA-Horvath, and chest-RT was significantly associated with EAA-Hannum (Additional file 1: Table S4). The association between rs732314 and EAA-Horvath was much stronger in survivors without exposure to chest/abdominal/pelvic-RT (combined: β=0.64 vs 0.31, I2=66.3%, Phet=0.08) whereas the association between rs28366133 and EAA-Hannum was slightly stronger in those without exposure to chest-RT (combined: β=0.80 vs. 0.74) (Table 3).
SNP-based heritability and genetic correlation with other traits
The estimated h2 using LD score regression was 0.33 (SE=0.20) and 0.17 (SE=0.23) for EAA-Horvath and EAA-Hannum, respectively. The point estimates were negative numbers and nearly zero for EAA-PhenoAge and EAA-GrimAge (Additional file 1: Table S5). Notably, there were significant positive genetic correlations between EAA-Horvath and heart rate, pulse rate, glycoprotein, falls in the last year, and a negative genetic correlation with creatinine (Additional file 1: Table S6).
Previously established loci in survivors of childhood cancer
Among the 39 SNPs associated with IEAA in the general population, 20 were replicated (P<0.05) based on our meta-analysis of the SJLIFE1 and SJLIFE2 data sets (Additional file 1: Table S7). Similarly, only a limited number of the SNPs associated in the general population with each EAA were replicated: none of the three SNPs for EEAA-Hannum, two of the nine SNPs for EAA-Hannum, one of the four SNPs for EAA-GrimAge, and two of the 12 SNPs for EAA-PhenoAge (Additional file 1: Table S7). Notably, the previous most intriguing finding of rs2736099 (TERT) had a P value of 0.55 in our data, suggesting substantial genetic heterogeneity. We observed multiple significant DMRs between survivors and controls in the TERT gene including its promoter region (Additional file 1: Table S8). A polygenic risk score (PRS) for IEAA was derived from the weighted sum of the number of risk alleles (13 independent SNPs including our novel SNP rs732314) carried by each survivor. IEAA increased across the PRS quintiles with positive correlation (r=0.23, P< 2.2×10-16) (Additional file 1: Fig. S3), suggesting that 5.3% of the variance of IEAA can be explained by PRS.
Discussion
It is well-established that adult survivors of childhood cancer are at risk for developing a high cumulative burden of age- and therapy-related CHCs and premature mortality, a phenomenon that might be indicative of accelerated aging [3, 4, 6, 42–48]. By leveraging the existing genetic (WGS) and epigenetic (DNAm) data in the SJLIFE cohort, an informative population of childhood cancer survivors, we performed GWAS analyses on EAA and identified two novel genome-wide significant associations. The findings identify genetic variations contributing to EAA, which may explain inter-individual variability beyond exposures of cancer treatment toxicity [6]. These novel genetic variations in combination with other replicated known hits can facilitate the identification of survivors at higher risk in accelerated aging and potentially inform drug targets for future intervention strategies in vulnerable survivors.
rs732314 was previously reported as a susceptibility locus for low high-density lipoprotein cholesterol and coronary heart disease [49]. rs732314 was also identified as a strong mQTL (methylation quantitative trait loci) with its C allele associated with a lower methylation level of cg01459453 across the human life course [50], potentially upregulating expression of the SELP gene (encoding P-selectin). Notably, cg01459453 was inversely correlated with chronological age and included as an aging predictive CpG in both Levine’s and Horvath’s epigenetic clocks [12, 24]. Furthermore, SELP, associated with IEAA-Horvath using gene-based association analysis [14], is the top-ranking aging-related gene consistently showing upregulated expression in hematopoietic stem cells across multiple studies [51]. SELPLG gene that encodes P-selectin glycoprotein ligand 1 had the highest level of expression in blood across all tissues analyzed in GTEx [52] and is upregulated in Alzheimer’s disease [53, 54]. We previously investigated persistent variations of DNAm-associated specific treatment exposures where cg00159243 was one of the borderline epigenome-wide significant CpGs and was inversely associated with RT exposure (raw P value: 8.50×10-11, genomic control adjusted P value: 2.28×10-7 for chest-RT, and similarly for abdominal/pelvic-RT). Specifically, survivors previously treated with chest-RT or abdominal/pelvic-RT had a low methylation level of cg00159243 and presumably had a higher expression level of the SELPLG gene. Moreover, cg00159243 was associated with low-grade chronic inflammation [55] and was an expression quantitative trait methylation ([eQTM], negative correlation) for SELPLG [56, 57]. Based on our new data and existing evidence from literature, we propose a potential molecular mechanism underlying the association between rs732314, chest/abdominal/pelvic-RT, and EAA-Horvath (Additional file 1: Fig. S4). The stratified analysis of the association between rs732314 and EAA-Horvath by age or chest/abdominal/pelvic-RT showed substantial heterogeneity between children and adults as well as between survivors with and without RT exposure. We postulate that higher expression of SELP (receptor) in adults or SELPLG (ligand) in irradiated survivors substantially weakens the association between rs732314 and EAA-Horvath.
The association between rs28366133 and EAA-Hannum association is novel and seems to be specific to the survivor population. Based on the GTEx database, rs28366133 is an expression quantitative trait locus (eQTL) for XXbac-BPG181B23.7 (a novel transcript) and MICA among other genes. It is also a splicing quantitative trait locus (sQTL) for multiple genes including MICA, HLA-B, HLA-C, and HLA-S across different tissues including the blood. rs3093956 is a known hit for EAA-Hannum [15]; however, it has low LD (r2=0.075) with rs28366133. Multiple striking DMRs were observed between survivors and controls in the HLA region, which might be due to the fact that genotoxic cancer treatments modified the epigenome among other physiological alterations in survivors and hence altered functional genomic links (e.g., eQTL, mQTL, and eQTM) [23, 58], which may lead to either disruption or introduction of genetic associations with EAA. For the same reason, a substantial proportion (e.g., 20 out of 39 IEAA-associated SNPs) of previously reported genetic associations, including the most notable rs2736099 (TERT), were not replicated in our study. One caveat in interpreting DMRs in the HLA region is that the inferred DMRs might be partly contributed by subtle differences in genetic variations between survivors and controls in this highly polymorphic genomic region.
Although our meta-GWAS study identified genetic variants associated with EAA at a genome-wide significance level, further validation is warranted by using our expanded cohort or other survivorship cohorts in the future.
Conclusions
In summary, we identified novel genetic variants in SELP gene and HLA region associated with EAA-Horvath and EAA-Hannum, respectively, among survivors of childhood cancer. This research represents the first EAA GWAS conducted in childhood cancer survivors, a unique clinical population who demonstrate accelerated aging. Future studies including larger and more racially and ethnically diverse populations, integrating multi-omics data such as RNA-seq and metabolomics profiling, are warranted for new discovery, association refinement, and elucidation of functional mechanisms.
Supplementary Information
Additional file 1: Fig. S1. The scatter plots between chronological age and epigenetic age based on four epigenetic clocks. Fig. S2. QQ plots of EAA GWAS based on four epigenetic clocks. Fig. S3. Distribution of intrinsic epigenetic age acceleration (IEAA) across five quintiles of polygenic risk score (PRS) for IEAA. Fig. S4. Molecular mechanism for associations between rs732314, chest/abdominal/pelvic-RT and EAA-Horvath. Table S1. SNPs (except the top one) significantly associated with EAA-Horvath and EAA-Hannum among survivors (SJLIFE1, SJLIFE2) and controls. Table S2. Single nucleotide polymorphisms (SNPs) with significant associations with IEAA-Horvath and EEAA-Hannum among survivors (SJLIFE1, SJLIFE2) and controls. Table S3. Differentially methylated regions (DMR) between the SJLIFE1 data set of survivors and controls overlapping with rs28366133 (+/- 500 kb) in HLA region. Table S4. Multivariable linear regression models for the two top SNPs. Table S5. Estimated SNP heritability of EAA based on each of four clock methods using single-trait LD Score Regression of the GWAS of the SJLIFE1 data set. Table S6. Estimated genetic correlation between EAA-Horvath and other traits using LD Score Regression of the GWAS of the SJLIFE1 data set (P<0.05). Table S7. Evaluation of the known loci in the current study. Table S8. DMR between the survivors (SJLIFE1 data set) and controls overlapping with TERT gene region.
Acknowledgements
The authors thank all individuals who participated in this study.
Abbreviations
- CHCs
Chronic health conditions
- DMR
Differentially methylated regions
- DNAm
DNA methylation
- EAA
Epigenetic age acceleration
- eQTL
Expression quantitative trait locus
- eQTM
Expression quantitative trait methylation
- EEAA
Extrinsic epigenetic age acceleration
- GWAS
Genome-wide association study
- IEAA
Intrinsic epigenetic age acceleration
- LD
Linkage disequilibrium
- mQTL
Methylation quantitative trait loci
- QC
Quality control
- RT
Radiotherapy
- SNP
Single-nucleotide polymorphism
- SJLIFE
St. Jude Lifetime Cohort Study
- TOPMed
Trans-omics for precision medicine
- WGS
Whole-genome sequencing
Authors’ contributions
ZW designed the study. LLR and MMH and KKN assisted in or provided support for data collection and recruitment of study participants. JE, HM, GN, EW, and JZ assisted in or supervised sample processing and performed DNA extraction and library preparation for whole-genome sequencing and the Infinium MethylationEPIC Array. QD, NS, NQ, and ZW performed the methylation analysis and statistical analysis. QD, NS, and ZW wrote the first draft of the manuscript. All authors contributed to data interpretation and writing of the manuscript draft. The authors read and approved the final manuscript.
Funding
This research was supported by funding from the American Lebanese Syrian Associated Charities to St. Jude Children’s Research Hospital, V Foundation (Grant # DT2020-014), and by grants (CA021765, CA195547) from the National Institutes of Health to St. Jude Children’s Research Hospital. The funders of the study had no role in the design and conduct of the study and were not involved in the collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Availability of data and materials
The DNA methylation data, demographics, and cancer treatment information generated and analyzed in this study are accessible at the NCBI GEO website under the accession number GSE197678 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE197678) [59]. Parts of these data have been previously published in Song et al. [23] and are accessible at the NCBI GEO website under the accession number GSE169156 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE169156). Additional clinical data about the study participants in the St. Jude Lifetime Cohort can be accessed via the survivorship portal (http://survivorship.stjude.cloud/). WGS data was previously published in Qin et al. [20] and Wang et al. [21] and is accessible through the St. Jude Cloud (https://stjude.cloud) under the accession number SJC-DS-1002. To protect patients’ privacy and confidentiality (in compliance with the IRB approval and the data sharing plan), WGS data download requires an application providing a purpose and project (medical or scientific research use only), completing a data access agreement, and a subsequent approval by the data access committee. Detailed instructions can be provided by contacting support@stjude.cloud.
Declarations
Ethics approval and consent to participate
The SJLIFE study protocol was approved by the Institutional Review Board (IRB) at St. Jude Children’s Research Hospital with a reference number (010882). All SJLIFE study participants provided written informed consent. This study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qian Dong and Nan Song contributed equally as co-first authors.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ness KK, Krull KR, Jones KE, Mulrooney DA, Armstrong GT, Green DM, Chemaitilly W, Smith WA, Wilson CL, Sklar CA, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31:4496–4503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ness KK, Wogksch MD. Frailty and aging in cancer survivors. Transl Res. 2020;221:65–82. doi: 10.1016/j.trsl.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song N, Li Z, Qin N, Howell CR, Wilson CL, Easton J, Mulder HL, Edmonson MN, Rusch MC, Zhang J, et al. Shortened leukocyte telomere length associates with an increased prevalence of chronic health conditions among survivors of childhood cancer: a report from the St. Jude Lifetime Cohort. Clin Cancer Res. 2020;26:2362–2371. doi: 10.1158/1078-0432.CCR-19-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin N, Li Z, Song N, Wilson CL, Easton J, Mulder H, Plyler E, Neale G, Walker E, Zhou X, et al. Epigenetic age acceleration and chronic health conditions among adult survivors of childhood cancer. J Natl Cancer Inst. 2021;113:597–605. doi: 10.1093/jnci/djaa147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh KM. Telomere attrition in childhood cancer survivors. Clin Cancer Res. 2020;26:2281–2283. doi: 10.1158/1078-0432.CCR-20-0380. [DOI] [PubMed] [Google Scholar]
- 8.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou-El-Ardat K, Schmid T, Brüne B, Wagner S, Serve H, et al. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol. 2019;4:25–33. doi: 10.1001/jamacardio.2018.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19:371–384. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- 12.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10:573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu AT, Xue L, Salfati EL, Chen BH, Ferrucci L, Levy D, Joehanes R, Murabito JM, Kiel DP, Tsai PC, et al. GWAS of epigenetic aging rates in blood reveals a critical role for TERT. Nat Commun. 2018;9:387. doi: 10.1038/s41467-017-02697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson J, Russ TC, Clarke TK, Howard DM, Hillary RF, Evans KL, Walker RM, Bermingham ML, Morris SW, Campbell A, et al. A meta-analysis of genome-wide association studies of epigenetic age acceleration. PLoS Genet. 2019;15:e1008104. doi: 10.1371/journal.pgen.1008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCartney DL, Min JL, Richmond RC, Lu AT, Sobczyk MK, Davies G, Broer L, Guo X, Jeong A, Jung J, et al. Genome-wide association studies identify 137 genetic loci for DNA methylation biomarkers of aging. Genome Biol. 2021;22:194. doi: 10.1186/s13059-021-02398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abelson S, Collord G, Ng SWK, Weissbrod O, Mendelson Cohen N, Niemeyer E, Barda N, Zuzarte PC, Heisler L, Sundaravadanam Y, et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature. 2018;559:400–404. doi: 10.1038/s41586-018-0317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nachun D, Lu AT, Bick AG, Natarajan P, Weinstock J, Szeto MD, Kathiresan S, Abecasis G, Taylor KD, Guo X, et al. Clonal hematopoiesis associated with epigenetic aging and clinical outcomes. Aging Cell. 2021;20:e13366. doi: 10.1111/acel.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudson MM, Ehrhardt MJ, Bhakta N, Baassiri M, Eissa H, Chemaitilly W, Green DM, Mulrooney DA, Armstrong GT, Brinkman TM, et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26:666–674. doi: 10.1158/1055-9965.EPI-16-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howell CR, Bjornard KL, Ness KK, Alberts N, Armstrong GT, Bhakta N, Brinkman T, Caron E, Chemaitilly W, Green DM, et al. Cohort profile: the St. Jude Lifetime Cohort Study (SJLIFE) for paediatric cancer survivors. Int J Epidemiol. 2021;50:39–49. doi: 10.1093/ije/dyaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin N, Wang Z, Liu Q, Song N, Wilson CL, Ehrhardt MJ, Shelton K, Easton J, Mulder H, Kennetz D, et al. Pathogenic germline mutations in DNA repair genes in combination with cancer treatment exposures and risk of subsequent neoplasms among long-term survivors of childhood cancer. J Clin Oncol. 2020;38:2728–2740. doi: 10.1200/JCO.19.02760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Wilson CL, Easton J, Thrasher A, Mulder H, Liu Q, Hedges DJ, Wang S, Rusch MC, Edmonson MN, et al. Genetic risk for subsequent neoplasms among long-term survivors of childhood cancer. J Clin Oncol. 2018;36:2078–2087. doi: 10.1200/JCO.2018.77.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, Irizarry RA. Minfi: a flexible and comprehensive bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30:1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song N, Hsu CW, Pan H, Zheng Y, Hou L, Sim JA, Li Z, Mulder H, Easton J, Walker E, et al. Persistent variations of blood DNA methylation associated with treatment exposures and risk for cardiometabolic outcomes in long-term survivors of childhood cancer in the St. Jude Lifetime Cohort. Genome Med. 2021;13:53. doi: 10.1186/s13073-021-00875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, Hou L, Baccarelli AA, Li Y, Stewart JD, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 2019;11:303–327. doi: 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, Wiencke JK, Kelsey KT. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horvath S, Levine AJ. HIV-1 infection accelerates age according to the epigenetic clock. J Infect Dis. 2015;212:1563–1573. doi: 10.1093/infdis/jiv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horvath S, Gurven M, Levine ME, Trumble BC, Kaplan H, Allayee H, Ritz BR, Chen B, Lu AT, Rickabaugh TM, et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17:171. doi: 10.1186/s13059-016-1030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen BH, Marioni RE, Colicino E, Peters MJ, Ward-Caviness CK, Tsai PC, Roetker NS, Just AC, Demerath EW, Guan W, et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY) 2016;8:1844–1865. doi: 10.18632/aging.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters TJ, Buckley MJ, Statham AL, Pidsley R, Samaras K, Lord RV, Clark SJ, Molloy PL. De novo identification of differentially methylated regions in the human genome. Epigenetics Chromatin. 2015;8:6. doi: 10.1186/1756-8935-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 37.Yin L, Zhang H, Tang Z, Xu J, Yin D, Zhang Z, et al. rMVP: a memory-efficient, visualization-enhanced, and parallel-accelerated tool for genome-wide association study. Genomics Proteomics Bioinformatics. 2021. 10.1016/j.gpb.2020.10.007. [DOI] [PMC free article] [PubMed]
- 38.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–3557. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, Duncan L, Perry JR, Patterson N, Robinson EB, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng J, Erzurumluoglu AM, Elsworth BL, Kemp JP, Howe L, Haycock PC, Hemani G, Tansey K, Laurin C, Pourcain BS, et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics. 2017;33:272–279. doi: 10.1093/bioinformatics/btw613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smitherman AB, Wood WA, Mitin N, Ayer Miller VL, Deal AM, Davis IJ, Blatt J, Gold SH, Muss HB. Accelerated aging among childhood, adolescent, and young adult cancer survivors is evidenced by increased expression of p16(INK4a) and frailty. Cancer. 2020;126:4975–4983. doi: 10.1002/cncr.33112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guida JL, Agurs-Collins T, Ahles TA, Campisi J, Dale W, Demark-Wahnefried W, Dietrich J, Fuldner R, Gallicchio L, Green PA, et al. Strategies to prevent or remediate cancer and treatment-related aging. J Natl Cancer Inst. 2021;113:112–122. doi: 10.1093/jnci/djaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henderson TO, Ness KK, Cohen HJ. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am Soc Clin Oncol Educ Book. 2014;34(1):e423–e430. doi: 10.14694/EdBook_AM.2014.34.e423. [DOI] [PubMed] [Google Scholar]
- 45.Daniel S, Nylander V, Ingerslev LR, Zhong L, Fabre O, Clifford B, Johnston K, Cohn RJ, Barres R, Simar D. T cell epigenetic remodeling and accelerated epigenetic aging are linked to long-term immune alterations in childhood cancer survivors. Clin Epigenetics. 2018;10:138. doi: 10.1186/s13148-018-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sulicka-Grodzicka J, Surdacki A, Seweryn M, Mikołajczyk T, Rewiuk K, Guzik T, Grodzicki T. Low-grade chronic inflammation and immune alterations in childhood and adolescent cancer survivors: a contribution to accelerated aging? Cancer Med. 2021;10:1772–1782. doi: 10.1002/cam4.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ariffin H, Azanan MS, Abd Ghafar SS, Oh L, Lau KH, Thirunavakarasu T, Sedan A, Ibrahim K, Chan A, Chin TF, et al. Young adult survivors of childhood acute lymphoblastic leukemia show evidence of chronic inflammation and cellular aging. Cancer. 2017;123:4207–4214. doi: 10.1002/cncr.30857. [DOI] [PubMed] [Google Scholar]
- 48.Pluijm SMF. Accelerated aging as a paradigm to understand the late effects of cancer therapies. Front Horm Res. 2021;54:16–24. doi: 10.1159/000518816. [DOI] [PubMed] [Google Scholar]
- 49.Peloso GM, Demissie S, Collins D, Mirel DB, Gabriel SB, Cupples LA, Robins SJ, Schaefer EJ, Brousseau ME. Common genetic variation in multiple metabolic pathways influences susceptibility to low HDL-cholesterol and coronary heart disease. J Lipid Res. 2010;51:3524–3532. doi: 10.1194/jlr.P008268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaunt TR, Shihab HA, Hemani G, Min JL, Woodward G, Lyttleton O, Zheng J, Duggirala A, McArdle WL, Ho K, et al. Systematic identification of genetic influences on methylation across the human life course. Genome Biol. 2016;17:61. doi: 10.1186/s13059-016-0926-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Svendsen AF, Yang D, Kim KM, Lazare SS, Skinder N, Zwart E, et al. A comprehensive transcriptome signature of murine hematopoietic stem cell aging. Blood. 2021;138:439-51. [DOI] [PubMed]
- 52.Melé M, Ferreira PG, Reverter F, DeLuca DS, Monlong J, Sammeth M, Young TR, Goldmann JM, Pervouchine DD, Sullivan TJ, et al. Human genomics. The human transcriptome across tissues and individuals. Science. 2015;348:660–665. doi: 10.1126/science.aaa0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haure-Mirande JV, Wang M, Audrain M, Fanutza T, Kim SH, Heja S, Readhead B, Dudley JT, Blitzer RD, Schadt EE, et al. Integrative approach to sporadic Alzheimer’s disease: deficiency of TYROBP in cerebral Abeta amyloidosis mouse normalizes clinical phenotype and complement subnetwork molecular pathology without reducing Abeta burden. Mol Psychiatry. 2019;24:431–446. doi: 10.1038/s41380-018-0255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giovagnoni C, Ali M, Eijssen LMT, Maes R, Choe K, Mulder M, Kleinjans J, Del Sol A, Glaab E, Mastroeni D, et al. Altered sphingolipid function in Alzheimer’s disease; a gene regulatory network approach. Neurobiol Aging. 2021;102:178–187. doi: 10.1016/j.neurobiolaging.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Ligthart S, Marzi C, Aslibekyan S, Mendelson MM, Conneely KN, Tanaka T, Colicino E, Waite LL, Joehanes R, Guan W, et al. DNA methylation signatures of chronic low-grade inflammation are associated with complex diseases. Genome Biol. 2016;17:255. doi: 10.1186/s13059-016-1119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonder MJ, Luijk R, Zhernakova DV, Moed M, Deelen P, Vermaat M, van Iterson M, van Dijk F, van Galen M, Bot J, et al. Disease variants alter transcription factor levels and methylation of their binding sites. Nat Genet. 2017;49:131–138. doi: 10.1038/ng.3721. [DOI] [PubMed] [Google Scholar]
- 57.Zhernakova DV, Deelen P, Vermaat M, van Iterson M, van Galen M, Arindrarto W, Hof P, Mei H, van Dijk F, Westra H-J, et al. Identification of context-dependent expression quantitative trait loci in whole blood. Nat Genet. 2017;49:139–145. doi: 10.1038/ng.3737. [DOI] [PubMed] [Google Scholar]
- 58.Im C, Qin N, Wang Z, Qiu W, Howell CR, Sapkota Y, Moon W, Chemaitilly W, Gibson TM, Mulrooney DA, et al. Generalizability of “GWAS Hits” in clinical populations: lessons from childhood cancer survivors. Am J Hum Genet. 2020;107:636–653. doi: 10.1016/j.ajhg.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong Q, Song N, Qin N, Chen C, Li Z, Sun X, Easton J, Mulder H, Plyler E, Neale G, et al Genome-wide association studies identify novel genetic loci for epigenetic age acceleration among survivors of childhood cancer. GSE197678, NCBI Gene Expression Omnibus. 2022. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE197678. Accesesed 9 Mar 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. The scatter plots between chronological age and epigenetic age based on four epigenetic clocks. Fig. S2. QQ plots of EAA GWAS based on four epigenetic clocks. Fig. S3. Distribution of intrinsic epigenetic age acceleration (IEAA) across five quintiles of polygenic risk score (PRS) for IEAA. Fig. S4. Molecular mechanism for associations between rs732314, chest/abdominal/pelvic-RT and EAA-Horvath. Table S1. SNPs (except the top one) significantly associated with EAA-Horvath and EAA-Hannum among survivors (SJLIFE1, SJLIFE2) and controls. Table S2. Single nucleotide polymorphisms (SNPs) with significant associations with IEAA-Horvath and EEAA-Hannum among survivors (SJLIFE1, SJLIFE2) and controls. Table S3. Differentially methylated regions (DMR) between the SJLIFE1 data set of survivors and controls overlapping with rs28366133 (+/- 500 kb) in HLA region. Table S4. Multivariable linear regression models for the two top SNPs. Table S5. Estimated SNP heritability of EAA based on each of four clock methods using single-trait LD Score Regression of the GWAS of the SJLIFE1 data set. Table S6. Estimated genetic correlation between EAA-Horvath and other traits using LD Score Regression of the GWAS of the SJLIFE1 data set (P<0.05). Table S7. Evaluation of the known loci in the current study. Table S8. DMR between the survivors (SJLIFE1 data set) and controls overlapping with TERT gene region.
Data Availability Statement
The DNA methylation data, demographics, and cancer treatment information generated and analyzed in this study are accessible at the NCBI GEO website under the accession number GSE197678 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE197678) [59]. Parts of these data have been previously published in Song et al. [23] and are accessible at the NCBI GEO website under the accession number GSE169156 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE169156). Additional clinical data about the study participants in the St. Jude Lifetime Cohort can be accessed via the survivorship portal (http://survivorship.stjude.cloud/). WGS data was previously published in Qin et al. [20] and Wang et al. [21] and is accessible through the St. Jude Cloud (https://stjude.cloud) under the accession number SJC-DS-1002. To protect patients’ privacy and confidentiality (in compliance with the IRB approval and the data sharing plan), WGS data download requires an application providing a purpose and project (medical or scientific research use only), completing a data access agreement, and a subsequent approval by the data access committee. Detailed instructions can be provided by contacting support@stjude.cloud.