Abstract
Childhood obesity has been linked to early puberty in girls but the mechanism(s) by which overnutrition triggers pubertal onset remain unclear. In a recent issue of Cell Metabolism, Heras et al., 2020 implicate a non-canonical central ceramide to ovarian sympathetic innervation pathway as a novel mediator of obesity-induced pubertal acceleration in female rats.
Puberty marks an important physical and psychosocial milestone when children develop secondary sexual characteristics and attain reproductive capability. Pubertal timing is signaled by activation of pulsatile hypothalamic gonadotropin-releasing hormone (GnRH) secretion, which in turn drives pituitary gonadotropin secretion and downstream gonadal maturation (Navarro, 2020) (Figure 1). Intricate neuroendocrine pathways govern the co-ordinated GnRH neurosecretory activity by integrating nutritional, metabolic, hormonal, and environmental cues. Among these factors, the modulatory activity of nutrition and adiposity on pubertal timing has been long known. Nearly five decades ago, Frisch and Revelle framed the “critical weight hypothesis” as a key determinant of pubertal maturation in girls (Frisch and Revelle, 1970). The recent worldwide rise in childhood obesity has sparked a renewed interest into the effects of adiposity on pubertal development. Several cross-sectional and longitudinal studies have linked childhood obesity with earlier pubertal onset, especially in girls (Huang et al., 2020). Recent genome-wide association studies (GWAS) in humans have identified multiple BMI-increasing alleles that also associate with earlier age of menarche, confirming genetic co-regulation (Perry et al., 2014). Further, Mendelian randomization studies imply a strong causal effect of higher childhood BMI on the risk of early menarche (Mumby et al., 2011). Despite these strong epidemiologic and genetic links, the precise mechanism(s) underlying obesity-related early pubertal onset have remained elusive. Although brain circuits involving the hypothalamic kisspeptin neurons had been postulated to be a key nodal nexus between metabolism and puberty (Navarro, 2020), several preclinical models of postnatal overnutrition (ON) failed to detect any consistent association between adiposity and hypothalamic kisspeptin (encoded by Kiss1 gene) expression (Vazquez et al., 2019). Thus, alternative pathway mediators of obesity-induced precocious puberty (PP) need to be explored.
Figure 1. Regulatory Pathways Governing Metabolic Control of Puberty Discerned from Rodent, Nonhuman Primate, and Human Studies.
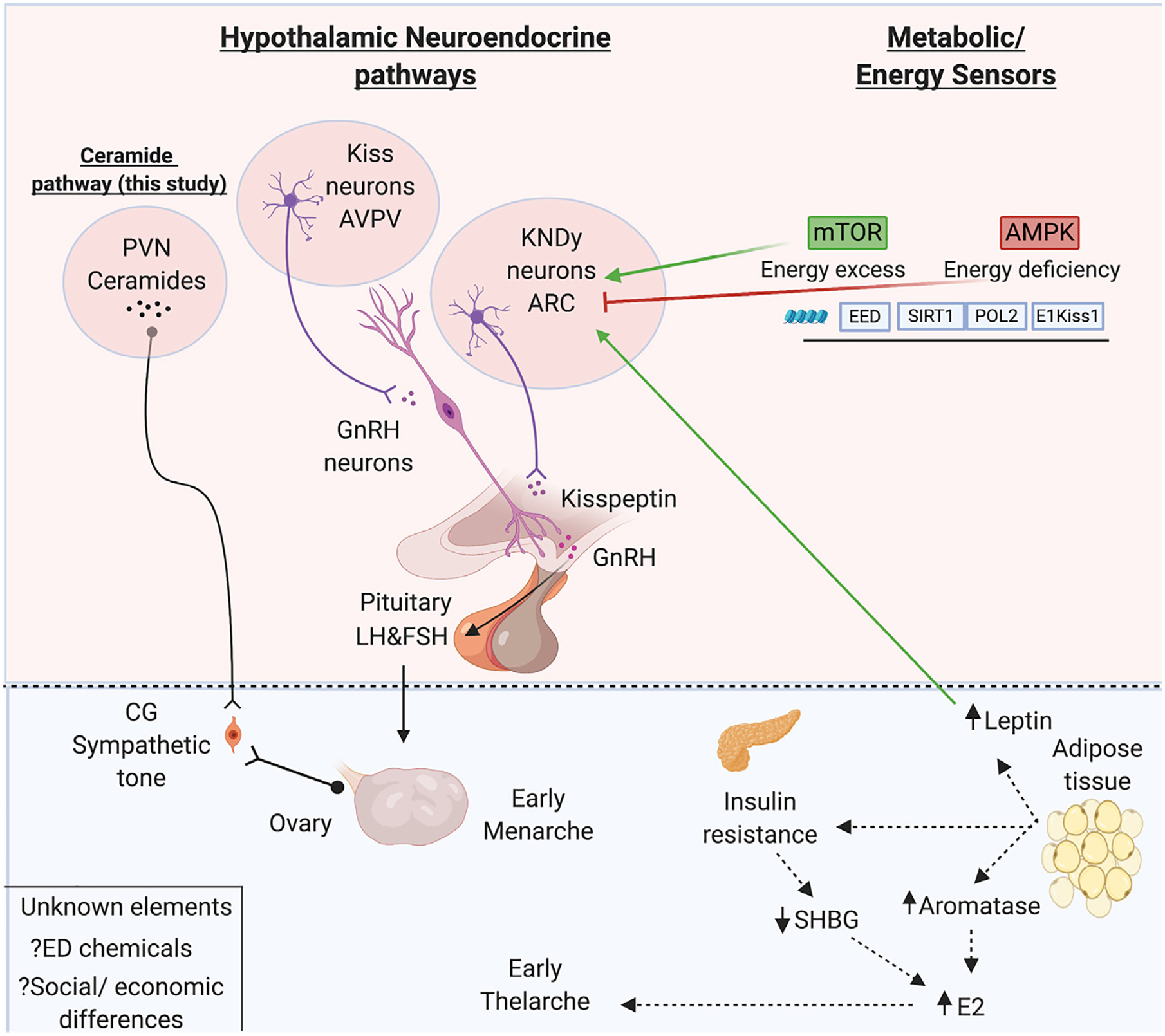
The hypothalamo-pituitary-gonadal axis: pulsatile hypothalamic secretion of GnRH into the hypophyseal-portal circulation drives pituitary gonadotropin (LH and FSH) secretion. Circulating LH and FSH in turn regulate ovarian sex steroid production and trigger ovulation. Upstream of GnRH, the kisspeptin-neurokinin B-dynorphin (KNDy) neurons from the ARC nucleus project to the GnRH terminals to coordinately regulate the pulsatile release of GnRH while kisspeptin neurons from the AVPV project to GnRH somas and orchestrate the pre-ovulatory LH surge in females (Navarro, 2020). The metabolic/energy sensors including mTOR, AMPK, SIRT, and leptin act either directly or indirectly on the ARC KNDy neurons, regulating GnRH neurosecretory activity based on the energy/nutrition status (Vazquez et al., 2019). Heras et al. implicate a novel central/ceramide pathway from the PVN that causes PP via ovarian sympathetic activation. In the periphery, excess adiposity increases production of aromatase from adipose tissue, induces insulin resistance, and lowers SHBG levels, leading to increased bioavailability of E2 to initiate premature thelarche (breast development) (Huang et al., 2020). Other unknown factors that may affect the pubertal timing via any of the pathways described above include ED chemicals and social-economic status. AMPK, AMP-activated protein kinase; ARC, arcuate; AVPV, anteroventral periventricular; CG, celiac ganglion; EED, embryonic ectoderm development; E2, estradiol; ED, endocrine disruptor; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; HPG, hypothalamic pituitary gonadal; KNDy, Kiss1, neurokinin B, and dynorphin; LH, luteinizing hormone; mTOR, mammalian target of rapamycin; POL2, DNA polymerase epsilon catalytic subunit A; PVN, periventricular nucleus; SIRT, sirtuin; SHBG, sex hormone binding globulin. Created with https://biorender.com/.
Taking a cue from the impact of ceramides, a family of lipid-signaling molecules, on hypothalamic feeding circuits (Gao et al., 2011), Heras et al. perform a series of elegantly designed studies that unveil a central ceramide signaling pathway as a novel mediator of obesity-induced PP in female rats. This non-canonical pathway involves the hypothalamic paraventricular nucleus (PVN) and sympathetic ovarian innervation. First, the authors demonstrate that female rats subject to ON exhibit advanced pubertal onset and concomitantly show an increase in total hypothalamic ceramide content. To implicate central ceramide signaling in pubertal disruption, they then perform in-tracerebroventricular (i.c.v.) pharmacologic manipulations in lean female rats and show that increasing ceramide synthesis (using CER:C6, a ceramide precursor) accelerated puberty, while persistently blocking ceramide production (using myriocin [MYR]) delayed puberty.
In search of mechanisms by which ceramides impact puberty, the authors examine the interplay between ceramide signaling and kisspeptin using multiple rodent models and ex vivo and in vivo experimental approaches. Intriguingly, while central ceramide signaling was permissive for stimulatory effects of kisspeptin, it did not alter the hypothalamic Kiss1 expression at its two major hypothalamic sites, namely the arcuate (ARC) and the anteroventral periventricular (AVPV) nuclei (Figure 1), nor did it influence kisspeptin-induced GnRH/luteinizing hormone secretion, implying that ceramide signaling utilizes an alternative non-canonical effector pathway for mediating pubertal maturation.
To define this alternate pathway, the authors turn their attention to the PVN, a hypothalamic region implicated in conveying sympathetic neural information to the ovaries to regulate their maturation (Gerendai et al., 1998). First, they show increased expression of serine palmitoyltransferase long-chain base subunit 1 (SPTLC1), a component that is crucial for de novo ceramide synthesis, increased SPTLC1 immunoreactivity (ir), and increased ceramide-ir within the PVN of obese ON female rats displaying PP. Surprisingly, kisspeptin-ir fibers in the PVN were significantly reduced, which the authors suppose reflects increased secretory dynamics of the peptide rather than decreased release per se. Next, they evaluate the role of ceramides within the PVN and resultant ovarian sympathetic output in female ON rats with PP at postnatal day 25. By examining two sympathetic innervation markers, norepinephrine (NE) and its metabolite, 3-metoxy-4-hydroxyphnylglycol (MHPG), at the celiac ganglion and ovary, they show these animals exhibit increased NE and MHPG on day 25 but decreased NE and MHPG on postnatal day 29, confirming early sympathetic activation and early ovarian maturation, respectively. Further, chronic blockade of ceramide synthesis with MYR (ON +MYR) normalized not only the timing of puberty, but also the ovarian sympathetic tone on postnatal day 29. Finally, the authors performed virogenetic silencing of SPTLC1 in the PVN to reduce de novo ceramide synthesis and successfully prevented PP without any change in body weight or food intake in these animals. These SPTLC1-targeted animals also showed normalization of their ovarian sympathetic tone.
The metabolic control of puberty has been postulated to be orchestrated through both central and peripheral mechanisms (Figure 1). Centrally, a dynamic interplay between energy/metabolic sensors (leptin, mTOR, AMPK, sirtuins) and hypothalamic pathways (primarily kisspeptin) has been shown to modulate GnRH neurosecretory activity either directly or indirectly (Vazquez et al., 2019). Given the raging childhood epidemic that poses a significant health challenge globally, the current study by Heras et al. provides compelling data implicating a novel brain circuitry involving an alternative PVN-ovarian sympathetic innervation pathway and greatly expanding our current understanding of the metabolic control of pubertal timing, specifically in females. However, these findings also raise several important questions: (1) What is the role of the PVN-ovarian sympathetic innervation pathway (i.e., putatively gonadotropin independent) in normal pubertal activation in humans? (2) Why does ceramide signaling differ between sexes in obesity-induced modulation of pubertal timing and could this explain the heightened susceptibility of girls to obesity-induced early puberty compared to boys? (3) How does ceramide signaling interplay with the other known metabolic sensors (e.g., leptin)? (4) Finally, what is role of ceramide signaling in other reproductive disorders? In this regard, it is tempting to implicate ceramide signaling in polycystic ovarian syndrome (PCOS), a condition previously associated with enhanced ovarian sympathetic outflow (Stener-Victorin et al., 2020). Moreover, increased ceramide subclasses have also been identified as novel lipidomic biomarkers in PCOS (Jiang et al., 2020). Answering these questions holds promise in discerning the role of ceramide signaling in both sexes and across a broader realm of reproductive physiology and pathophysiology.
ACKNOWLEDGMENTS
R.B. is funded by grants from the Eunice Kennedy National Institute of Child Health and Development (R01HD096324, P50HD028138, R15HD096411, and R01HD097331).
REFERENCES
- Frisch RE, and Revelle R (1970). Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science 169, 397–399. [DOI] [PubMed] [Google Scholar]
- Gao S, Zhu G, Gao X, Wu D, Carrasco P, Casals N, Hegardt FG, Moran TH, and Lopaschuk GD (2011). Important roles of brain-specific carnitine palmitoyltransferase and ceramide metabolism in leptin hypothalamic control of feeding. Proc. Natl. Acad. Sci. USA 108, 9691–9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerendai I, Tóth IE, Boldogkoi Z, Medveczky I, and Halász B (1998). Neuronal labeling in the rat brain and spinal cord from the ovary using viral transneuronal tracing technique. Neuroendocrinology 68, 244–256. [DOI] [PubMed] [Google Scholar]
- Heras V, Castellano JM, Fernandois D, Velasco I, Rodríguez-Vazquez E, Roa J, Vazquez MJ, Ruiz-Pino F, Rubio M, Pineda R, et al. (2020). Central ceramide signaling mediates obesity-induced precocious puberty. Cell Metab. 32, 951–966. [DOI] [PubMed] [Google Scholar]
- Huang A, Reinehr T, and Roth CL (2020). Connections between obesity and puberty: invited by Manuel Tena-Sempere, Cordoba. Curr Opin Endocr Metab Res 14, 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Qi J, Xue X, Huang R, Zheng J, Liu W, Yin H, and Li S (2020). Ceramide subclasses identified as novel lipid biomarker elevated in women with polycystic ovary syndrome: a pilot study employing shotgun lipidomics. Gynecol. Endocrinol 36, 508–512. [DOI] [PubMed] [Google Scholar]
- Mumby HS, Elks CE, Li S, Sharp SJ, Khaw KT, Luben RN, Wareham NJ, Loos RJ, and Ong KK (2011). Mendelian randomisation study of childhood BMI and early menarche. J. Obes 2011, 180729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM (2020). Metabolic regulation of kisspeptin - the link between energy balance and reproduction. Nat. Rev. Endocrinol 16, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JR, Day F, Elks CE, Sulem P, Thompson DJ, Ferreira T, He C, Chasman DI, Esko T, Thorleifsson G, et al. ; Australian Ovarian Cancer Study; GENICA Network; kConFab; LifeLines Cohort Study; InterAct Consortium; Early Growth Genetics (EGG) Consortium (2014). Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature 514, 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stener-Victorin E, Padmanabhan V, Walters KA, Campbell RE, Benrick A, Giacobini P, Dumesic DA, and Abbott DH (2020). Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr. Rev 41, 538–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez MJ, Velasco I, and Tena-Sempere M (2019). Novel mechanisms for the metabolic control of puberty: implications for pubertal alterations in early-onset obesity and malnutrition. J. Endocrinol 242, R51–R65. [DOI] [PubMed] [Google Scholar]


