Abstract
Background
There is a lack of data comparing azithromycin to alternative antibiotic choices in managing COPD exacerbations, making appropriate antibiotic selection controversial.
Objective
To compare treatment failure in hospitalized patients with acute exacerbations of chronic obstructive pulmonary disease (AECOPD) receiving azithromycin or beta-lactams.
Design
Retrospective, multicenter cohort study using logistic regression for multivariable analysis. Patients were included if they were at least 18 years old, admitted with AECOPD, and received at least two consecutive days of either a beta-lactam or azithromycin. Patients were excluded if they received concomitant azithromycin and beta-lactam antibiotics during the first 2 days, had a history of other severe underlying pulmonary diseases, pregnancy, COVID-19, alpha-1 antitrypsin deficiency, or received a corticosteroid for a diagnosis other than COPD.
Participants
Five hundred ninety-five patients were included, of which 428 (72%) received azithromycin and 167 patients (28%) received a beta-lactam.
Main Measures
The primary endpoint was treatment failure rate in patients receiving azithromycin versus beta-lactams, which was a composite endpoint defined as in-hospital mortality, admission to intensive care, initiation of invasive mechanical ventilation, initiation of a new antibiotic, steroid therapy escalation, or readmission due to AECOPD within 30 days.
Key Results
The composite primary outcome occurred in 84 patients (19.6%) in the azithromycin group and 54 (32.3%) in the beta-lactam group (p<0.01). The difference in the composite outcome was a result of higher rates of new antibiotics during admission (12.6% vs 4.2%; p<0.01) and higher readmission within 30 days (19.3% vs 12.4%; p=0.032). After controlling for potential confounders, beta-lactams continued to demonstrate a higher risk for treatment failure (OR, 2.30; 95% CI, 1.46–3.63). There was no difference in adverse effects between the groups.
Conclusion
Azithromycin was associated with less treatment failure in AECOPD which was driven by lower readmission rates and prescription of new antimicrobials.
KEY WORDS: Macrolides, Beta-lactams, Azithromycin, COPD (pulmonary disease, chronic obstructive)
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of death globally, with rising mortality and economic cost.1–3Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are associated with significant morbidity and mortality, as well as a frequent cause of inpatient hospitalizations and healthcare expenditure. Pharmacotherapeutic management of AECOPD generally consists of systemic corticosteroids, bronchodilators, anticholinergics, and antibiotics.4 The etiology of AECOPD is thought to be bacterial, viral, and/or environmental in nature. 2 Appropriate antibiotic therapy is critical, as bacterial infections cause approximately 50% of AECOPD.5Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and atypical pathogens are the most common bacterial organisms isolated in AECOPD.6,7
The 2021 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend treatment with antibiotics when patients present with three cardinal symptoms, also known as the Winnipeg criteria8 (sputum purulence, sputum production, and dyspnea) or if there is increased sputum purulence and one other cardinal symptom, or the patient requires mechanical ventilation.2 The choice of antibiotic is more complex. Clinical trials comparing antimicrobial therapies for AECOPD are limited; hence, current guidelines recommend a wide range of empirical treatment options such as penicillins, macrolides, cephalosporins, and tetracyclines.2,5 However, broad spectrum antibiotics spectrum antibiotics for AECOPD are not associated with improved clinical outcomes and may increase the risk for adverse effects. 9
Antibiotic selection is vital in the setting of growing antimicrobial resistance and should target common organisms in AECOPD. Azithromycin demonstrates activity against common respiratory tract pathogens including atypical organisms.10,11 Unfortunately, increasing azithromycin resistance in Streptococcus pneumoniae has been associated with higher rates of treatment failure in lower respiratory tract infections.12 Beta-lactams, in contrast, lack activity against atypical bacteria, but display lower rates of resistance for Streptococcus pneumoniae. One additional factor that may improve treatment success in AECOPD is the anti-inflammatory effect of azithromycin, which is not observed with beta-lactam therapy.10 However, the ideal strategy of targeting anti-inflammatory effects or resistance patterns is uncertain as the pathophysiology of AECOPD is not always bacterial in nature.
Recently our healthcare system order sets for AECOPD were changed in December of 2019: azithromycin was removed as an option for inpatient treatment, and the new order set only includes beta-lactams options, hence, conflicting with guideline recommendations. Currently, there is a lack of data comparing azithromycin to alternative antibiotic choices in managing COPD exacerbations, making appropriate antibiotic selection controversial. The purpose of this study is to compare the effectiveness of azithromycin versus beta-lactams in the treatment of hospitalized patients with AECOPD.
METHODS
This was a multicenter, retrospective, observational study conducted across six hospitals in Southeast Michigan between January 1st, 2015, and October 15th, 2020. A list of patients with an ICD-10 inpatient diagnosis of COPD exacerbation (J44.0, J44.1, J44.9) who received azithromycin or beta-lactams (ceftriaxone, cefuroxime, cefepime, cephalexin, cefazolin, amoxicillin-clavulanic acid, ampicillin-sulbactam, piperacillin-tazobactam) were identified from an institutional database. Patients were included if they were at least 18 years old, admitted with AECOPD, and received at least two consecutive days of either a beta-lactam or azithromycin. Only the first patient admission during the study period was included. Patients were excluded if they received concomitant azithromycin and beta-lactam antibiotics during the first 2 days, had a history of other severe underlying pulmonary diseases (cystic fibrosis, active tuberculosis, lung cancer), pregnancy, COVID-19, directly admitted to the intensive care unit (ICU), alpha-1 antitrypsin deficiency, or received a corticosteroid for a diagnosis other than COPD. Patients who were initially diagnosed with pneumonia were excluded; however, patients who were diagnosed with pneumonia during their hospitalization were included. IRB approval was obtained from Ascension St. John Hospital IRB prior to the start of the study.
The primary outcome was proportion of patients who experienced treatment failure. Treatment failure was a composite endpoint defined as in-hospital mortality, admission to the ICU, initiation of invasive mechanical ventilation (after one dose of initial antibiotic), initiation of a new antibiotic, steroid therapy escalation (after 2 days of initial antibiotic), or readmission due to AECOPD within 30 days of discharge.13,14 Secondary endpoints included each individual component of the composite of treatment failure and hospital length of stay. Safety outcomes included antibiotic-associated diarrhea (AAD) and clostridium-difficile-associated diarrhea (CDAD) were collected. Antibiotic-associated diarrhea was defined as three or more loose stools per day for two or more consecutive days. CDAD was defined as unexplained and new-onset of at least three unformed stools in 24 h and either a stool test positive for C. difficile toxins or detection of toxigenic C. difficile.15,16
Data was extracted from the electronic medical record. Demographic data included age, sex, race, and general data collection included weight, height, smoking history, and body mass index. Disease state and clinical data included concomitant bacterial infection, Charlson Weighted Index of Comorbidity, usage of supplemental oxygen (including invasive and non-invasive support), home oxygen, and admission serum creatinine. Risk factors for multidrug-resistant organisms were collected, including chronic steroid use, failure of outpatient antibiotic therapy specific to AECOPD, any antibiotics prior to admission, and hospitalization in the previous 90 days. Medication-related data included usage of short- and long-acting bronchodilators, intranasal corticosteroids, theophylline, roflumilast, and corticosteroids. Data on corticosteroid therapy was collected, including steroid therapy escalation (yes/no), duration of therapy, and cumulative dose (prednisone equivalents).
Sample Size
Based on previously reported treatment failure rates of 17% for azithromycin and 9.4% for cephalosporins 10,18 utilizing a 2:1 ratio, 428 azithromycin cases and 214 beta-lactam cases were needed for a total sample size of 642 patients (alpha=0.05, beta=0.2).
Statistical Analysis
Descriptive statistics were utilized to characterize the study groups. Continuous variables were presented as means with standard deviation, non-normally distributed variables were presented as medians with interquartile range, and categorical variables were expressed as proportions. Differences between the azithromycin and beta-lactam groups were assessed using the Student’s t-test for continuous variables and chi-square tests or Fisher exact tests for categorical variables. Logistic regression was performed to evaluate the effect of azithromycin or beta-lactams as the primary predictor of interest on treatment failure. Variables were included in the model if there was a difference in baseline characteristics (p < 0.05), with the exception of variables displaying multicollinearity. Additionally, a sensitivity analysis was performed utilizing propensity matching score and examining the timer period before and after order set changes. For the propensity analysis, matching was performed in a 1:1 ratio within 0.2 of the logit propensity score standard deviation. Logistic regression was used to estimate propensity score and included variables which were expected to be confounders as independent variables with receipt of treatment as the dependent variable. Propensity score was assessed after matching by evaluating balancing of groups and distribution of propensity scores in quintiles, across the area of common support, and across the entire distribution. All data were analyzed using SPSS v. 27.0 and a p-value of 0.05 or less was considered to indicate statistical significance.
RESULTS
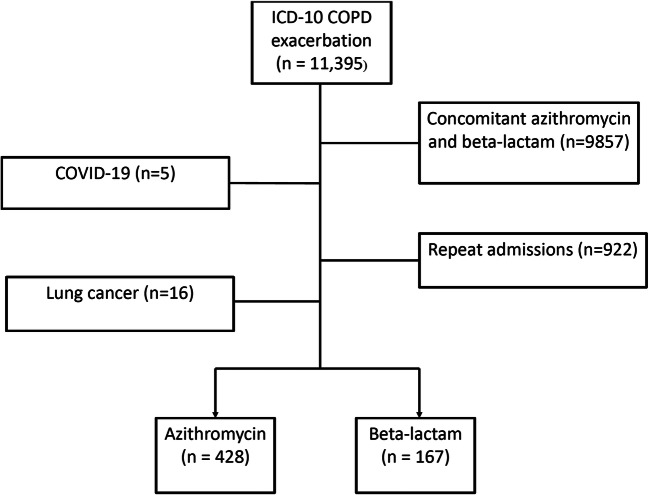
Overall, 595 met inclusion criteria. Of those, 428 (72%) were treated with azithromycin and 167 patients (28%) were treated with a beta-lactam (Figure 1). The beta-lactams most commonly prescribed were ceftriaxone (70.7%) followed by cefuroxime (21.0%). The study population was predominantly female (60.6%) with an average age of 66 and at least half of the patients were active smokers. Differences in baseline characteristics between the two groups were observed (Table 1). Patients in the azithromycin group were younger and more likely to be active smokers, and require increased oxygen compared to their baseline requirements. Additionally, patients treated with azithromycin were more likely to receive long-acting bronchodilators both during admission and upon discharge (Table 2). Nearly all patients received a short acting bronchodilator (99.8% in the azithromycin group vs 99.4% in the beta-lactam group) and systemic corticosteroids (98.6% in the azithromycin group vs 98.2% in the beta-lactam group) during admission. However, less than 50% of patients in both groups received long-acting bronchodilators when discharged from the hospital. Propensity matching was successful for 160 matched pairs of azithromycin and beta lactams. Groups were well matched post propensity matching (standardized mean differences <0.1)
Figure 1.
Flowchart of study participants.
Table 1.
Baseline Characteristics
| Variables | Azithromycin (n = 428) | Beta-lactam (n = 167) | p-value |
|---|---|---|---|
| Age, years (mean ± SD) | 65 ± 11.0 | 69 ± 11.9 | < 0.001 |
| Body mass index (kg/m2), (mean ± SD) | 30 ± 10.2 | 31 ± 11.8 | 0.31 |
| Male, n (%) | 177 (41.4) | 57 (34.1) | 0.11 |
| Smoking History, n (%) | 0.03 | ||
| Active | 233 (54.4) | 75 (44.9) | |
| Non-active | 190 (44.4) | 86 (51.5) | |
| Unknown | 5 (1.2) | 6 (3.6) | |
| Comorbidities, n (%) | |||
| Myocardial infarction | 67 (15.7) | 31 (18.6) | 0.39 |
| Congestive heart failure | 140 (32.7) | 53 (31.7) | 0.82 |
| Cerebrovascular accident | 59 (13.8) | 23 (13.8) | 0.99 |
| Dementia | 13 (3) | 9 (5.4) | 0.17 |
| Ulcer | 104 (24.3) | 38 (22.8) | 0.69 |
| Acquired immune deficiency syndrome | 1 (0.2) | 0 (0.0) | 0.53 |
| Diabetes | 131 (30.6) | 60 (35.9) | 0.21 |
| Mild liver disease | 12 (2.8) | 3 (1.8) | 0.48 |
| Moderate or severe liver disease | 8 (1.9) | 5 (3) | 0.39 |
| Moderate or severe renal disease | 90 (21) | 53 (31.7) | 0.08 |
| Non-metastatic tumor | 46 (10.7) | 10 (6) | 0.07 |
| Leukemia or lymphoma | 3 (0.7) | 1 (0.6) | 0.89 |
| Metastatic solid tumor | 4 (0.9) | 1 (0.6) | 0.68 |
| Connective tissue | 15 (3.5) | 4 (2.4) | 0.49 |
| Peripheral vascular disease | 26 (6.1) | 11 (6.6) | 0.82 |
| Charlson Weighted Index of Comorbidity (CWIC) score (mean ± SD) | 2.4 ± 1.7 | 2.4 ± 1.6 | 0.89 |
| Prior to admission therapies | |||
| Antibiotics, n (%) | 34 (7.9) | 27 (16.2) | 0.004 |
| Home oxygen therapy, n (%) | 135 (31.6) | 52 (31.1) | 0.63 |
| Systemic corticosteroids, n (%) | 44 (10.3) | 24 (14.4) | 0.16 |
| Chronic steroids use, n (%) | 16 (3.7) | 3 (1.8) | 0.23 |
| Failed outpatient antibiotic therapy, n (%) | 14 (3.3) | 20 (12) | < 0.001 |
| Hospitalization in past 90 days, n (%) | 150 (35) | 69 (41.3) | 0.15 |
Table 2.
Therapies Received During Admission
| Variables | Azithromycin (n = 428) | Beta-lactam (n = 167) |
p-value CI |
|---|---|---|---|
| Respiratory medications during admission | |||
| Short acting beta-2 agonist (SABA), n (%) | 427 (99.8) | 166 (99.4) | 0.49 |
| Short acting muscarinic antagonist (SAMA), n (%) | 422 (98.6) | 167 (100) | 0.12 |
| Long acting beta-2 agonist (LABA), n (%) | 98 (22.9) | 22 (13.2) | 0.008 |
| Long acting muscarinic antagonist (LAMA), n (%) | 68 (15.9) | 7 (4.2) | < 0.001 |
| Inhaled corticosteroid (ICS), n (%) | 141 (32.9) | 63 (37.7) | 0.27 |
| Theophylline | 4 (0.9) | 1 (0.6) | 0.69 |
| Roflumilast | 0 (0.0) | 1 (0.6) | 0.11 |
|
Corticosteroids, n (%) IV therapy PO therapy |
422 (98.6) 403 95.5) 322 (76.3) |
164 (98.2) 159 (97) 91 (55.5) |
0.95 0.56 < 0.001 |
| Duration of therapy (days), (mean ± SD) | 4.2 ± 2.9 | 5.2 ± 2.7 | < 0.001 |
| Cumulative equivalent dose of prednisone/day (mg), (mean ± SD) | 127 ± 50.6 | 132 ± 61.3 | 0.33 |
| Escalation of O2 requirement | 252 (58.9) | 52 (31.1) | < 0.001 |
| Respiratory medications upon discharge | |||
| Long-acting beta-agonist, n (%) | 255 (59.6) | 59 (35.3) | < 0.001 |
| Long-acting muscarinic antagonist, n (%) | 176 (41.1) | 32 (19.2) | < 0.001 |
| Bacterial infections during hospital stay, n (%) | 8 (1.9) | 10 (6) | 0.01 |
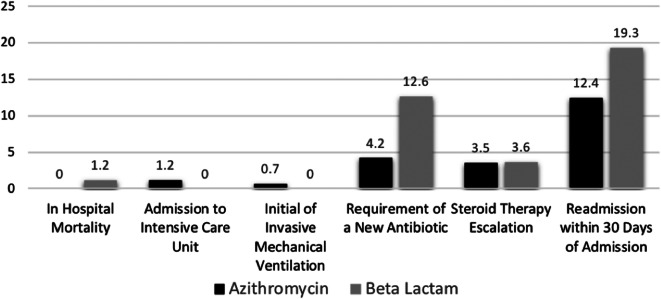
The composite primary outcome of treatment failure occurred in 84 of 428 patients (19.6%) in the azithromycin group and 54 of 167 (32.3%) in the beta-lactam group (p= 0.001). After controlling for smoking history, age, failure of outpatient therapy, and receipt of long-acting bronchodilators and anticholinergics upon discharge, beta-lactam use was associated with a higher rate of treatment failure (OR, 2.30; 95% CI, 1.46–3.63) (Table 3). In both time periods, beta lactams were associated with increased risk of treatment failure; before (OR 2.28, 95% CI 1.31 to 3.97) and after (OR 3.23, 95% CI 1.27 to 8.25) order set implementation. Furthermore, in the propensity-matched analysis beta lactams remained a predictor of higher rates of treatment failure (OR, 2.0; 95% CI 1.2 to 3.3). The difference in the composite outcome of treatment failure between beta-lactams and azithromycin was driven by increased likelihood for patients receiving beta-lactams to require a new antibiotic during admission (12.6% vs 4.2%; p < 0.001) and higher readmission within 30 days of discharge due to AECOPD (19.3% vs 12.4%; p = 0.03) (Figure 2). Similar results were observed in the propensity analysis with new antibiotics during admission (12.5% vs 5.6%, p<0.01) and readmission within 30 days (19.5% vs. 11.9%, p=0.07) driving the composite outcome. Length of stay was significantly shorter in the azithromycin group compared to the beta-lactam group (3.9 vs 5, p < 0.001) and this observation persisted in the propensity-matched analysis (4.1 vs. 5.2 days, p<0.01). There were no differences in the rates of AAD (1.4% in the azithromycin group vs 0.0% in the beta-lactam group, p = 0.6) nor CDAD among both groups (0.5% in the azithromycin group vs 0.0% in the beta-lactam group, p = 1.00).
Table 3.
Logistic Regression of Beta-lactam Versus Azithromycin and Treatment Failure
| Treatment failure aOR (95% CI) |
Significance | |
|---|---|---|
| Beta-lactam therapy | 2.30 (1.458–3.629) | < 0.01 |
| Variables in model | ||
| Smoking history | 1.01 (0.667–1.535) | 0.96 |
| Age | 1.02 (0.997–1.035) | 0.09 |
| Long-acting beta-agonist at discharge | 1.09 (0.705–1.669) | 0.71 |
| Long-acting muscarinic antagonist at discharge | 1.31 (0.843–2.047) | 0.23 |
| Failed outpatient therapy | 1.33 (0.608–2.902) | 0.48 |
| Escalation of O2 | 1.86 (1.22–2.83) | 0.04 |
Figure 2.
Individual components of composite outcome.
DISCUSSION
We observed increased rates of treatment failure with beta-lactams compared to azithromycin. This occurred despite increasing Streptococcus pneumoniae resistance across the USA, suggesting that the anti-inflammatory properties of azithromycin may play an important role in management of AECOPD. Additionally, this is the largest study to date comparing the effectiveness of azithromycin to beta-lactams. Treatment failure differences were driven by increases in readmission and higher rates of new antimicrobial prescription with beta-lactams.
Our results contrast with previous research outside of the ICU, as patients treated with azithromycin and beta-lactams had similar outcomes. However, previous studies were limited in size and potentially underpowered. One open-label, randomized study published in 1993 compared the efficacy and safety of azithromycin to cefaclor.10 The authors concluded that azithromycin was as effective as cefaclor in the treatment of patients with acute exacerbations of COPD. However, rates were numerically higher for cure or improvement with azithromycin versus cefaclor (100% vs 92%). Rates of treatment success may have been influenced by differences in resistance patterns and/or clinical outcome definitions, which were not objectively defined. Another small study of 102 patients conducted in 2007 compared azithromycin and amoxicillin and found similar rates of improvement or cure (85% vs. 78%, respectively) with no differences in the incidence of adverse events.17 This study had limitations similar to the previous study, as clinical outcome definitions were not explicitly defined. Finally, one trial of 106 patients compared four antibiotics (azithromycin, ampicillin-sulbactam, ciprofloxacin, and cefaclor) and found no difference in clinical improvement. Azithromycin rates of clinical improvement were 93.3% versus 83.3% with ampicillin-sulbactam and 82.3% with cefaclor.18 Clinical improvement was defined utilizing the degree of coughing, sputum production, and dyspnea. The subjectivity of the outcome may have influenced the study findings. Interestingly, in ICU patients azithromycin was associated with a reduction in 30-day readmissions compared to other antibiotics, which is similar to our findings.19
The main implication of our findings is that azithromycin should remain a viable treatment option for AECOPD in the inpatient setting. Macrolides including azithromycin not only have anti-bacterial activities but also anti-inflammatory activities which can be advantageous in AECOPD.20 There are several randomized controlled trials that evaluated the effect of long-term treatment with azithromycin in decreasing the frequency of AECOPD. For instance, maintenance therapy with azithromycin has shown to decrease the frequency of exacerbations in high-risk patients. Although long-term use of azithromycin can be beneficial in reducing AECOPD, it is important to note the adverse effects associated with azithromycin use including bacterial resistance and QTc prolongation.21,22
These results also highlight the lack of understanding of the interplay between the inflammatory and infectious pathophysiology of AECOPD. Current management of AECOPD is primarily based on sputum purulence, production, and shortness of breath which may be present to varying degrees irrespective of the underlying cause of AECOPD. More research is necessary to better differentiate between patients with an infectious versus noninfectious cause of AECOPD, which may help optimize treatment selection.
Our study has several limitations. First, the study was observational and thus may be susceptible to information bias. However, we hypothesize this would be non-differential and would have made the results more likely to show no difference. Second, our results may only be applicable to patients receiving ceftriaxone or cefuroxime, as these were the most commonly used beta-lactams. Third, we were unable to collect information about the number of exacerbations, although we did collect information on hospitalizations in the past 90 days. Fourth, there was a higher rate of patients receiving long-acting inhalers at discharge in the azithromycin group. It is important to note that using long-acting inhalers at discharge may have had a major role in the reduction of readmission rates in patients receiving azithromycin which in turn may have had an impactful effect in lowering treatment failure rate since readmission within 30 days of discharge was one of the driven factors in the rate of treatment failure. However, similar findings were observed in the propensity analysis. Fifth, many patients did not receive long-acting bronchodilators at discharge and steroid doses utilized were higher than 40 mg daily of prednisone equivalent, which conflicts with evidence-based guidelines. However, this is reflective of the practice patterns in our community, although may impact external validity. Fifth, this was not a randomized study, so the possibility of unmeasured confounding factors and selection bias exists, although we attempted to collect all theoretically plausible confounders. Lastly, many patients could not be included as they received concomitant azithromycin and beta-lactams as empiric therapy.
CONCLUSION
Our study suggests that azithromycin is associated with less treatment failure rate compared to beta-lactams in AECOPD. Azithromycin use was associated with lower readmission rates within 30 days due to AECOPD and lower rates of subsequent antibiotics. These findings suggest azithromycin remains a viable option for the management of AECOPD. However, azithromycin and beta-lactams were equally tolerable. Randomized clinical trials should be performed to confirm these findings.
Acknowledgements
The authors would like to thank Dr. Szpunar Susan, PhD, and Othuke Abada, MS, for providing the data analysis. The authors would also like to thank Bianca Aprilliano for her assistance in data collection
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- AECOP
Acute exacerbations of chronic obstructive pulmonary disease
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- AAD
Antibiotic associated diarrhea
- CDAD
Clostridium-difficile associated diarrhea
Declarations
Conflict of interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nour Baalbaki, Email: nour.baalbaki@ascension.org.
Christopher Giuliano, Email: Ek2397@wayne.edu.
Carrie L. Hartner, Email: carrie.hartner@ascension.org.
Pramodini Kale-Pradhan, Email: pramodine.kale@ascension.org.
Leonard Johnson, Email: leonard.johnson@ascension.org.
References
- 1.Rosenberg SR, Kalhan R, Mannino DM. Epidemiology of chronic obstructive pulmonary disease: prevalence, morbidity, mortality, and risk factors. Semin Respir Crit Care Med. 2015;36:457–469. doi: 10.1055/s-0035-1555607. [DOI] [PubMed] [Google Scholar]
- 2.GOLD. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2021 Report. Available at https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf. Accessed June 1, 2021.
- 3.Naderi N, Assayag D, Mostafavi-Pour-Manshadi SM, et al. Long-term azithromycin therapy to reduce acute exacerbations in patients with severe chronic obstructive pulmonary disease. Respir Med. 2018;138:129–136. doi: 10.1016/j.rmed.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 4.Zhang HL, Tan M, Qiu AM, Tao Z, Wang CH. Antibiotics for treatment of acute exacerbation of chronic obstructive pulmonary disease: a network meta-analysis. BMC Pulm Med. 2017;17:196. doi: 10.1186/s12890-017-0541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 6.Feldman C, Richards G. Appropriate antibiotic management of bacterial lower respiratory tract infections. F1000Res. 2018;7:F1000 Faculty Rev-1121. Published 2018 Jul 23. 10.12688/f1000research.14226.1 [DOI] [PMC free article] [PubMed]
- 7.Yoon HI, Lee CH, Kim DK, et al. Efficacy of levofloxacin versus cefuroxime in treating acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:329–334. doi: 10.2147/COPD.S41749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacIntyre N, Huang YC. Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):530–535. doi: 10.1513/pats.200707-088ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyner KR, Walkerly A, Seidel K, et al. Comparison of narrow-versus broad-spectrum antibiotics in elderly patients with acute exacerbations of chronic obstructive pulmonary disease. J Pharm Pract. 2022;35(1):26–31. doi: 10.1177/0897190020938190. [DOI] [PubMed] [Google Scholar]
- 10.Dark D. Azithromycin versus cefaclor in the treatment of acute exacerbations of chronic obstructive pulmonary disease. Current therapeutic research. 1993;53:203–211. doi: 10.1016/S0011-393X(05)80247-4. [DOI] [Google Scholar]
- 11.Dark D. Multicenter evaluation of azithromycin and cefaclor in acute lower respiratory tract infections. Am J Med. 1991;91:31S–35S. doi: 10.1016/0002-9343(91)90399-I. [DOI] [PubMed] [Google Scholar]
- 12.Klugman KP. Bacteriological evidence of antibiotic failure in pneumococcal lower respiratory tract infections. Eur Respir J. 2002;36:3s-8s. [DOI] [PubMed]
- 13.Vermeersch K, Gabrovska M, Deslypere G, et al. The Belgian trial with azithromycin for acute COPD exacerbations requiring hospitalization: an investigator-initiated study protocol for a multicenter, randomized, double-blind, placebo-controlled trial. Int J Chron Obstruct Pulmon Dis. 2016;11:687–696. doi: 10.2147/COPD.S95501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Comparative effectiveness of macrolides and quinolones for patients hospitalized with acute exacerbations of chronic obstructive pulmonary disease (AECOPD) J Hosp Med. 2010;5:261–267. doi: 10.1002/jhm.628. [DOI] [PubMed] [Google Scholar]
- 15.Wiström J, Norrby SR, Myhre EB, et al. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: a prospective study. J Antimicrob Chemother. 2001;47:43–50. doi: 10.1093/jac/47.1.43. [DOI] [PubMed] [Google Scholar]
- 16.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66:e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andre-Alves MR, Jardim JR, Frare e Silva R, Fiss E, Freire DN, Teixeira PJ. Comparison between azithromycin and amoxicillin in the treatment of infectious exacerbation of chronic obstructive pulmonary disease. J Bras Pneumol. 2007;33:43-50. [DOI] [PubMed]
- 18.Umut S, Tutluoglu B, Aydin Tosun G, et al. Determination of the etiological organism during acute exacerbations of COPD and efficacy of azithromycin, ampicillin-sulbactam, ciprofloxacin and cefaclor. Turkish Thoracic Society COPD Working Group. J Chemother. 1999;11(3):211–214. doi: 10.1179/joc.1999.11.3.211. [DOI] [PubMed] [Google Scholar]
- 19.Kiser TH, Reynolds PM, Moss M, et al. Impact of macrolide antibiotics on hospital readmissions and other clinically important outcomes in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease: a propensity score-matched cohort study. Pharmacotherapy. 2019;39(3):242–252. doi: 10.1002/phar.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwiatkowska B, Maślińska M. Macrolide therapy in chronic inflammatory diseases. Mediators Inflamm. 2012;2012:636157. doi: 10.1155/2012/636157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD [published correction appears in N Engl J Med. 2012 Apr 5;366(14):1356] N Engl J Med. 2011;365(8):689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2(5):361–368. doi: 10.1016/S2213-2600(14)70019-0. [DOI] [PubMed] [Google Scholar]