Abstract
Background
Excessive blood loss and increased blood transfusion requirements may have significant impact on the short‐term and long‐term outcomes after liver transplantation.
Objectives
To compare the potential benefits and harms of different methods of decreasing blood loss and blood transfusion requirements during liver transplantation.
Search methods
We searched The Cochrane Central Register of Controlled Trials in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, and metaRegister of Controlled Trials until September 2011.
Selection criteria
We included all randomised clinical trials that were performed to compare various methods of decreasing blood loss and blood transfusion requirements during liver transplantation.
Data collection and analysis
Two authors independently identified the trials and extracted the data. We analysed the data with both the fixed‐effect and the random‐effects model using RevMan Analysis. For each outcome we calculated the risk ratio (RR), mean difference (MD), or standardised mean difference (SMD) with 95% confidence intervals (CI) based on available data analysis. We also conducted network meta‐analysis.
Main results
We included 33 trials involving 1913 patients. The sample size in the trials varied from 8 to 209 participants. The interventions included pharmacological interventions (aprotinin, tranexamic acid, epsilon amino caproic acid, antithrombin 3, recombinant factor (rFvIIa), oestrogen, prostaglandin, epinephrine), blood substitutes (blood components rather than whole blood, hydroxy‐ethyl starch, thromboelastography), and cardiovascular interventions (low central venous pressure). All the trials were of high risk of bias. Primary outcomes were reported in at least two trials for the following comparisons: aprotinin versus control, tranexamic acid versus control, recombinant factor VIIa (rFVIIa) versus control, and tranexamic acid versus aprotinin. There were no significant differences in the 60‐day mortality (3 trials; 6/161 (3.7%) in the aprotinin group versus 8/119 (6.7%) in the control group; RR 0.52; 95% CI 0.18 to 1.45), primary graft non‐function (2 trials; 0/128 (0.0%) in the aprotinin group versus 4/89 (4.5%) in the control group; RR 0.15; 95% CI 0.02 to 1.25), retransplantation (3 trials; 2/256 (0.8%) in the aprotinin group versus 12/178 (6.7%) in the control group; RR 0.21; 95% CI 0.02 to 1.79), or thromboembolic episodes (3 trials; 4/161 (2.5%) in the aprotinin group versus 5/119 (4.2%) in the control group; RR 0.59; 95% CI 0.19 to 1.84) between the aprotinin and control groups. There were no significant differences in the 60‐day mortality (3 trials; 4/83 (4.8%) in the tranexamic acid group versus 5/56 (8.9%) in the control group; RR 0.55; 95% CI 0.17 to 1.76), retransplantation (2 trials; 3/41 (7.3%) in the tranexamic acid group versus 3/36 (8.3%) in the control group; RR 0.79; 95% CI 0.18 to 3.48), or thromboembolic episodes (5 trials; 5/103 (4.9%) in the tranexamic acid group versus 1/76 (1.3%) in the control group; RR 2.20; 95% CI 0.38 to 12.64) between the tranexamic acid and control groups. There were no significant differences in the 60‐day mortality (3 trials; 8/195 (4.1%) in the recombinant factor VIIa (rFVIIa) group versus 2/91 (2.2%) in the control group; RR 1.51; 95% CI 0.33 to 6.95), thromboembolic episodes (2 trials; 24/185 (13.0%) in the rFVIIa group versus 8/81 (9.9%) in the control group; RR 1.38; 95% CI 0.65 to 2.91), or serious adverse events (2 trials; 90/185 (48.6%) in the rFVIIa group versus 30/81 (37.0%) in the control group; RR 1.30; 95% CI 0.94 to 1.78) between the rFVIIa and control groups. There were no significant differences in the 60‐day mortality (2 trials; 6/91 (6.6%) in the tranexamic acid group versus 1/87 (1.1%) in the aprotinin group; RR 4.12; 95% CI 0.71 to 23.76) or thromboembolic episodes (2 trials; 4/91 (4.4%) in the tranexamic acid group versus 2/87 (2.3%) in the aprotinin group; RR 1.97; 95% CI 0.37 to 10.37) between the tranexamic acid and aprotinin groups. The remaining outcomes in the above comparisons and the remaining comparisons included only only trial under the primary outcome or the outcome was not reported at all in the trials. There were no significant differences in the mortality, primary graft non‐function, graft failure, retransplantation, thromboembolic episodes, or serious adverse events in any of these comparisons. However, the confidence intervals were wide, and it is not possible to reach any conclusion on the safety of the interventions. None of the trials reported the quality of life in patients.
Secondary outcomes were reported in at least two trials for the following comparisons ‐ aprotinin versus control, tranexamic acid versus control, rFVIIa versus control, thromboelastography versus control, and tranexamic acid versus aprotinin. There was significantly lower allogeneic blood transfusion requirements in the aprotinin group than the control group (8 trials; 185 patients in aprotinin group and 190 patients in control group; SMD ‐0.61; 95% CI ‐0.82 to ‐0.40). There were no significant differences in the allogeneic blood transfusion requirements between the tranexamic acid and control groups (4 trials; 93 patients in tranexamic acid group and 66 patients in control group; SMD ‐0.27; 95% CI ‐0.59 to 0.06); rFVIIa and control groups (2 trials; 141 patients in rFVIIa group and 80 patients in control group; SMD ‐0.05; 95% CI ‐0.32 to 0.23); thromboelastography and control groups (2 trials; 31 patients in thromboelastography group and 31 patients in control group; SMD ‐0.73; 95% CI ‐1.69 to 0.24); or between the tranexamic acid and aprotinin groups (3 trials; 101 patients in tranexamic acid group and 97 patients in aprotinin group; SMD ‐0.09; 95% CI ‐0.36 to 0.19). The remaining outcomes in the above comparisons and the remaining comparisons included only only trial under the primary outcome or the outcome was not reported at all in the trials. There were no significant differences in the blood loss, transfusion requirements, hospital stay, or intensive care unit stay in most of the comparisons.
Authors' conclusions
Aprotinin, recombinant factor VIIa, and thromboelastography groups may potentially reduce blood loss and transfusion requirements. However, risks of systematic errors (bias) and risks of random errors (play of chance) hamper the confidence in this conclusion. We need further well‐designed randomised trials with low risk of systematic error and low risk of random errors before these interventions can be supported or refuted.
Keywords: Humans; Liver Transplantation; Liver Transplantation/mortality; Antifibrinolytic Agents; Antifibrinolytic Agents/therapeutic use; Aprotinin; Aprotinin/therapeutic use; Blood Loss, Surgical; Blood Loss, Surgical/prevention & control; Blood Transfusion; Blood Transfusion/statistics & numerical data; Factor VIIa; Factor VIIa/therapeutic use; Hemostatics; Hemostatics/therapeutic use; Randomized Controlled Trials as Topic; Recombinant Proteins; Recombinant Proteins/therapeutic use; Thrombelastography; Tranexamic Acid; Tranexamic Acid/therapeutic use
Plain language summary
Methods to decrease blood loss and transfusion requirements for liver transplantation
The liver is the powerhouse of the body. It acts as a store of energy and a centre of metabolic activity. Liver transplantation is the main treatment for severe liver disease resulting in destruction of the liver (which can happen suddenly or over a period of time) due to various causes including alcoholism, viral infections, and autoimmune diseases. Liver transplantation is a major surgical procedure and is associated with significant loss of blood. Various methods have been used to decrease blood loss and transfusion requirements in patients undergoing liver transplantation, with a view to improve the results of liver transplantation. We performed a detailed review of the medical literature (available until September 2011) to determine the benefits and harms of different methods of decreasing blood loss and transfusion requirements in patients undergoing liver transplantation. We sought evidence from randomised clinical trials only, as when conducted properly such studies provide the best evidence. Two authors independently identified the trials and obtained the information from the trials.
We included 33 trials involving 1913 patients. The number of patients included in the trials varied from 8 to 209. The comparisons included various drugs that affect the blood clotting (congealing) such as aprotinin, tranexamic acid; blood substitutes (blood components rather than whole blood); use of thromboelastography (a bedside measure of blood clot formation); and lowering the pressure in the veins with an aim to decrease the blood loss from veins. We found no significant difference in the risk of death or graft loss, or in the major complication rates between the compared groups in any of the comparisons. Quality of life was not reported in any of the trials. There does not appear to be any consistency in the results between blood loss and blood transfusion requirements. Aprotinin, tranexamic acid, recombinant factor VIIa, low central venous pressure, and thromboelastography may lower blood loss and transfusion requirements. However, these findings are based on few trials with a high risk of bias (systematic overestimation of benefits) and high risk of play of chance (random error due to small number of patients). There were no differences in the hospital stay or intensive care unit stay in any of the comparisons. Nor was there any significant difference in the intensive therapy unit stay, or hospital stay between the compared groups. Again, most of the trials were of high risk of systematic errors (a potential to arrive at wrong conclusions because of the way the trial was conducted) and random errors (a potential to arrive at wrong conclusions because of play of chance).
Aprotinin is a drug which has been withdrawn from market since there was a suspicion that it increased death after major heart operations. The results from this review do not reveal any increased mortality with aprotinin in the liver transplantation setting although one has to interpret this information with caution because of the few patients included in the trial. We are unable to advocate or refute any method of decreasing blood loss and transfusion requirements in patients undergoing liver transplantation. Further well designed trials with low risk of systematic error and low risk of random errors are necessary.
Summary of findings
Summary of findings for the main comparison. Intervention versus control for liver transplantation (mortality, primary graft non‐function, and retransplantation).
| Intervention versus control for liver transplantation | ||||||
| Patient or population: Patients with liver transplantation. Settings: Transplantation centre. Intervention: Intervention versus control. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intervention versus control | |||||
| 60‐day mortality ‐ Aprotinin versus control | Study population | RR 0.52 (0.18 to 1.45) | 280 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 67 per 1000 | 35 per 1000 (12 to 97) | |||||
| Moderate | ||||||
| 73 per 1000 | 38 per 1000 (13 to 106) | |||||
| 60‐day mortality ‐ Tranexamic acid versus control | Study population | RR 0.55 (0.17 to 1.76) | 139 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 89 per 1000 | 49 per 1000 (15 to 157) | |||||
| Moderate | ||||||
| 100 per 1000 | 55 per 1000 (17 to 176) | |||||
| 60‐day mortality ‐ Recombinant factor VIIa (rFVIIa) versus control | Study population | RR 1.51 (0.33 to 6.95) | 286 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 22 per 1000 | 33 per 1000 (7 to 153) | |||||
| Moderate | ||||||
| 16 per 1000 | 24 per 1000 (5 to 111) | |||||
| 60‐day mortality ‐ Tranexamic acid versus aprotinin | Study population | RR 4.12 (0.71 to 23.76) | 178 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 11 per 1000 | 47 per 1000 (8 to 273) | |||||
| Moderate | ||||||
| 8 per 1000 | 33 per 1000 (6 to 190) | |||||
| Primary graft non‐function ‐ Aprotinin versus control | Study population | RR 0.15 (0.02 to 1.25) | 217 (2 studies) | ⊕⊝⊝⊝ very low1,3,4 | ||
| 45 per 1000 | 7 per 1000 (1 to 56) | |||||
| Moderate | ||||||
| 45 per 1000 | 7 per 1000 (1 to 56) | |||||
| Retransplantation ‐ Aprotinin versus control | Study population | RR 0.21 (0.02 to 1.79) | 217 (2 studies) | ⊕⊝⊝⊝ very low1,3,4 | ||
| 67 per 1000 | 14 per 1000 (1 to 121) | |||||
| Moderate | ||||||
| 66 per 1000 | 14 per 1000 (1 to 118) | |||||
| Retransplantation ‐ Tranexamic acid versus control | Study population | RR 0.79 (0.18 to 3.48) | 77 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 83 per 1000 | 66 per 1000 (15 to 290) | |||||
| Moderate | ||||||
| 75 per 1000 | 59 per 1000 (14 to 261) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All trials were at high risk of bias, 2 The confidence intervals overlap 0.75 and 1.25. 3 Funnel plots could not be performed for any of the outcomes. 4 Although the confidence intervals do not overlap 0.75 and 1.25, the confidence intervals were wide.
Summary of findings 2. Intervention versus control for liver transplantation (thromboembolic episodes and other serious adverse events).
| Intervention versus control for liver transplantation | ||||||
| Patient or population: Patients with liver transplantation. Settings: Transplantation centre. Intervention: Intervention versus control. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intervention versus control | |||||
| Thromboembolic episodes ‐ Aprotinin versus control | Study population | RR 0.6 (0.18 to 1.96) | 280 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 42 per 1000 | 25 per 1000 (8 to 82) | |||||
| Moderate | ||||||
| 63 per 1000 | 38 per 1000 (11 to 123) | |||||
| Thromboembolic episodes ‐ Tranexamic acid versus control | Study population | RR 2.2 (0.38 to 12.64) | 179 (5 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 13 per 1000 | 29 per 1000 (5 to 166) | |||||
| Thromboembolic episodes ‐ Recombinant factor VIIa (rFVIIa) versus control | Study population | RR 1.38 (0.65 to 2.91) | 266 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 99 per 1000 | 136 per 1000 (64 to 287) | |||||
| Moderate | ||||||
| 101 per 1000 | 139 per 1000 (66 to 294) | |||||
| Serious adverse events ‐ Recombinant factor VIIa (rFVIIa) versus control | Study population | RR 1.3 (0.94 to 1.78) | 266 (2 studies) | ⊕⊝⊝⊝ very low1,3,4 | ||
| 370 per 1000 | 481 per 1000 (348 to 659) | |||||
| Moderate | ||||||
| 406 per 1000 | 528 per 1000 (382 to 723) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All trials were at high risk of bias, 2 The confidence intervals overlap 0.75 and 1.25. 3 Funnel plots could not be performed for any of the outcomes. 4 Although the confidence intervals do not overlap 0.75 and 1.25, the confidence intervals were wide.
Summary of findings 3. Intervention versus control for liver transplantation (blood loss).
| Intervention versus control for liver transplantation | ||||||
| Patient or population: Patients with liver transplantation. Settings: Transplantation centre. Intervention: Intervention versus control. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intervention versus control | |||||
| Blood loss ‐ Aprotinin versus control (litre) | The mean blood loss ‐ aprotinin versus control in the intervention groups was 1.36 lower (3.39 lower to 0.66 higher) | 195 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | |||
| Blood loss ‐ Tranexamic acid versus control (litre) | The mean blood loss ‐ tranexamic acid versus control in the intervention groups was 4.98 lower (10.18 lower to 0.23 higher) | 65 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | |||
| Blood loss ‐ Thromboelastography versus control (litre) | The mean blood loss ‐ thromboelastography versus control in the intervention groups was 1.13 lower (1.85 to 0.41 lower) | 62 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | |||
| Blood loss ‐ Tranexamic acid versus aprotinin | The mean blood loss ‐ tranexamic acid versus aprotinin in the intervention groups was 1.01 lower (2.31 lower to 0.29 higher) | 71 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All trials were at high risk of bias, 2 Sample size was less than 400 patients in both groups put together, 3 Funnel plots could not be performed for any of the outcomes.
Summary of findings 4. Intervention versus control for liver transplantation (red cell or whole blood allogeneic transfusion).
| Intervention versus control for liver transplantation | ||||||
| Patient or population: Patients with liver transplantation. Settings: Transplantation centre. Intervention: Intervention versus control. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intervention versus control | |||||
| Red cell or whole blood transfusion ‐ Aprotinin versus control | The mean red cell or whole blood transfusion ‐ aprotinin versus control in the intervention groups was 0.61 standard deviations lower (0.82 to 0.40 lower) | 375 (8 studies) | ⊕⊝⊝⊝ very low1,2,3 | SMD ‐0.61 (‐0.82 to ‐0.40) | ||
| Red cell or whole blood transfusion ‐ Tranexamic acid versus control | The mean red cell or whole blood transfusion ‐ tranexamic acid versus control in the intervention groups was 0.27 standard deviations lower (0.59 lower to 0.06 higher) | 159 (4 studies) | ⊕⊝⊝⊝ very low1,3,4 | SMD ‐0.27 (‐0.59 to 0.06) | ||
| Red cell or whole blood transfusion ‐ Recombinant factor VIIa (rFVIIa) versus control | The mean red cell or whole blood transfusion ‐ recombinant factor VIIa (rFVIIa) versus control in the intervention groups was 0.05 standard deviations higher (0.32 lower to 0.23 higher) | 221 (2 studies) | ⊕⊝⊝⊝ very low1,3,4 | SMD 0.05 (‐0.32 to 0.23) | ||
| Red cell or whole blood transfusion ‐ Thromboelastography versus control | The mean red cell or whole blood transfusion ‐ thromboelastography versus control in the intervention groups was 0.73 standard deviations lower (1.69 lower to 0.24 higher) | 62 (2 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | SMD ‐0.73 (‐1.69 to 0.24) | ||
| Red cell or whole blood transfusion ‐ Tranexamic acid versus aprotinin | The mean red cell or whole blood transfusion ‐ tranexamic acid versus aprotinin in the intervention groups was 0.09 standard deviations lower (0.36 lower to 0.19 higher) | 198 (3 studies) | ⊕⊝⊝⊝ very low1,3,4 | SMD ‐0.09 (‐0.36 to 0.19) | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All trials were at high risk of bias. 2 High heterogeneity. 3 Funnel plots could not be performed for any of the outcomes. 4 Sample size was less than 400 patients in both groups put together.
Background
Description of the condition
Liver transplantation is the replacement of a diseased recipient liver with a donor liver. Annually around 5700 liver transplantations are performed in Europe (ELTR 2011) and around 6000 in the United States of America (OPTN/SRTR 2009). Liver transplantation is performed mainly for end‐stage liver failure arising as a result of chronic liver disease (for example, alcoholic cirrhosis or viral disease), for acute liver failure (for example viral or due to drug overdose), or for tumour (Lim 2006). The model for end‐stage liver disease score (MELD score) has been suggested as one of the methods of determining the severity of end‐stage liver failure (Kamath 2001) and is being used as a tool for allocation of donor livers in some countries (Shiffman 2006). Worldwide, there is a demand for liver transplants in surplus of supply. Liver grafts are mainly harvested from cadavers (Koneru 2005; Cescon 2006), but due to shortage of cadaveric organs, there is an increasing interest in live donors who donate part of their liver to the recipients (Jeon 2010). Currently, around 4% of liver grafts are retrieved from live donors (OPTN/SRTR 2009). Recently, split liver transplantation (using one cadaveric donor liver for two recipients, ie, an adult and a paediatric recipient) has been suggested as a way to decrease the organ shortage for liver transplant (Corno 2006).
During liver transplantation, the diseased liver has to be removed. Because of portal hypertension and resulting variceal blood vessels as a consequence of end‐stage liver failure, blood loss during removal of the diseased liver can be high. In addition, because of clotting disorders related to liver failure, the blood loss during liver transplantation can be high. Peri‐operative blood loss is one of the causes of mortality due to liver transplantation (Bismuth 1987). In observational studies, differences in blood transfusion requirements are associated with increased short‐term morbidity such as infective episodes (Nardo 2005), re‐operations (Hendriks 2005), and differences in the long‐ term patient survival (Ramos 2003; Massicotte 2005; Boyd 2007; Boin 2008) in patients undergoing liver transplantation.
Description of the intervention
Various methods have been attempted with an aim to decrease the blood loss and allogeneic blood transfusion requirements during liver transplantation. These include interventions such as lowering central venous pressure (CVP) (Massicotte 2006); acute normovolemic haemodilution (Jabbour 2005); intra‐operative blood salvage (Sankarankutty 2006); thromboelastography (Wang 2010), and the use of pharmacological agents such as antifibrinolytics (aprotinin, tranexamic acid, epsilon amino caproic acid (EACA)) (Dalmau 2000; Dalmau 2004); prostaglandins (Himmelreich 1993); conjugated oestrogens (Frenette 1998); desmopressin (Pivalizza 2003); antithrombin III (Palareti 1991); or recombinant factor VIIa (Lodge 2005).
How the intervention might work
Different interventions work in different ways. Lowering central venous pressure aims to decrease the venous blood loss. Acute normovolemic haemodilution aims to decrease the blood loss by diluting the blood. Dilution of blood reduces blood transfusion requirements by decreasing loss of blood constituents, this means that even if the same volume of blood is lost, the proportion of this blood that requires replacing by transfusion is less. Intra‐operative blood salvage aims to recover the blood lost by the patient, process it, and transfuse the patient the same blood thereby decreasing the need for allogeneic blood (donated blood). Thromboelastography provides rapid information about global clotting and is performed in the operation theatre (point‐of‐care approach). It provides rapid results as opposed to laboratory results, which may not be available immediately (Wang 2010). Various pharmacological agents are aimed at decreasing the blood loss and transfusion requirements by altering the coagulation cascade.
Why it is important to do this review
Previous systematic reviews mainly assessed the role of antifibrinolytics and concluded that antifibrinolytics (mainly aprotinin) were safe and decreased blood loss but these studies have failed to demonstrate a reduction in the overall mortality or morbidity (Molenaar 2007; Liu 2008). There has been no Cochrane review assessing the different methods, specified above, to decrease blood loss for liver transplantation.
Objectives
To compare the benefits and harms of different intervention methods aimed at decreasing blood loss and/or allogeneic blood transfusion requirements during liver transplantation.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials irrespective of blinding, language, publication status, or sample size. We planned to exclude quasi‐randomised trials (eg, allocation by date of birth, day of the week, etc) with regards to the benefits of the intervention but include such studies with regards to harms.
Types of participants
Patients undergoing orthotopic liver transplantation irrespective of age, living donor or cadaveric donor, and reason for liver transplantation.
Types of interventions
The following comparisons were included.
Any intervention aimed at decreasing blood loss and/or allogeneic blood transfusion in recipient operation during liver transplantation versus no intervention or placebo.
Any intervention aimed at decreasing blood loss and/or allogeneic blood transfusion in recipient operation during liver transplantation versus the same intervention at a different dose or another intervention.
Interventions in living donor retrieval will not be included as they were assessed in other two Cochrane reviews (Gurusamy 2009a; Gurusamy 2009b).
Types of outcome measures
Primary outcomes
-
Mortality.
60‐day mortality.
At maximal follow‐up.
-
Graft failure/retransplantation.
Primary graft non‐function/failure.
Graft failure at maximal follow‐up.
Retransplantation for any cause.
Adverse events defined as any untoward medical occurrence not necessarily having a causal relationship with the treatment, but resulting in a dose reduction or discontinuation of treatment (ICH‐GCP 1997). Serious adverse events are defined as any event that would increase mortality; is life‐threatening; requires inpatient hospitalisation; results in a persistent or significant disability; or any important medical event, which might have jeopardised the patient or requires intervention to prevent it. Any surgery related morbidity such as re‐operation for any cause, sepsis, bile leak, renal failure, wound complications, chest infection are likely to result in prolongation of inpatient hospitalisation and were classified as serious adverse events. We obtained information for thromboembolic events such as hepatic artery thrombosis and portal vein thrombosis separately, since many of the interventions affect the coagulation cascade, which may result in increased thromboembolic episodes.
Quality of life.
Secondary outcomes
-
Blood loss.
Blood loss (however measured by authors).
Allogeneic transfusion requirements (blood, platelets, fresh frozen plasma).
-
Hospital stay.
Total hospital stay.
Intensive therapy unit (ITU) stay.
Search methods for identification of studies
Electronic searches
We searched The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded (Royle 2003). We also searched the metaRegister of Controlled Trials (mRCT) (http://www.controlled‐trials.com/mrct/). The meta‐register includes ISRCTN register and NIH ClinicalTrials.gov register among other registers. We have given the search strategies and the time span of the searches in Appendix 1.
Searching other resources
We searched the references of the identified trials to identify further relevant trials.
Data collection and analysis
We performed the systematic review following the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2011).
Selection of studies
Two authors (KG and TP) identified the trials for inclusion independently of each other. We have listed the excluded studies with the reasons for the exclusion. Any differences were resolved through discussion and arbitration by BRD.
Data extraction and management
Both authors independently extracted the following data.
Year and language of publication.
Country.
Year of conduct of the trial.
Inclusion and exclusion criteria.
Sample size.
Donor characteristics such as age, sex, living or cadaveric donor.
Participant characteristics such as age, sex, MELD score, reason for transplant.
Piggy‐back or conventional technique: During liver transplantation, the recipient inferior vena cava (IVC) is cross‐clamped in the conventional method of liver transplantation. This results in a decrease in venous return from the lower half of the body (Calne 1979). This can result in fall in blood pressure (Calne 1979). To overcome this decreased venous return, methods such as piggy‐back technique, use of veno‐venous bypass have been attempted. Till date, there is no evidence that these methods have any benefit in the reduction of mortality, morbidity, or blood transfusion requirements (Gurusamy 2011a; Gurusamy 2011b).
Use of veno‐venous by‐pass (please see above).
Risk of bias (described below).
Any unclear or missing information was sought by contacting the authors of the individual trials. If there was any doubt whether the trials share the same patients ‐ completely or partially (by identifying common authors and centres), we contacted the authors of the trials to clarify whether the trial report has been duplicated. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2011). According to empirical evidence (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008), the risk of bias of the trials will be assessed based on the following bias risk domains.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are adequate if performed by an independent adjudicator.
Uncertain risk of bias: the trial is described as randomised, but the method of sequence generation was not specified.
High risk of bias: the sequence generation method is not, or may not be, random. Quasi‐randomised studies, those using dates, names, or admittance numbers in order to allocate patients are inadequate and will be excluded for the assessment of benefits but not for harms.
Allocation concealment
Low risk of bias: allocation was controlled by a central and independent randomisation unit, sequentially numbered, opaque and sealed envelopes or similar, so that intervention allocations could not have been foreseen in advance of, or during, enrolment.
Uncertain risk of bias: the trial was described as randomised, but the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: if the allocation sequence was known to the investigators who assigned participants, or if the study was quasi‐randomised. We planned to exclude quasi‐randomised studies for the assessment of benefits but not for harms.
Blinding of participants and personnel
Low risk of bias: the trial was described as blinded and the method of blinding of participants and personnel was described so that knowledge of allocation was adequately prevented during the trial.
Uncertain risk of bias: the trial was described as blinded, but the method of blinding was not described so that knowledge of allocation was possible during the trial.
High risk of bias, the trial was not blinded so that the allocation was known during the trial.
Blinding of outcome assessors
Low risk of bias: the trial was described as blinded and the method of blinding of outcome assessors was described so that knowledge of allocation was adequately prevented during the trial.
Uncertain risk of bias: the trial was described as blinded, but the method of blinding was not described so that knowledge of allocation was possible during the trial.
High risk of bias, the trial was not blinded so that the allocation was known during the trial.
Incomplete outcome data
Low risk of bias: (the underlying reasons for missingness are unlikely to make treatment effects departure from plausible values, or proper methods have been employed to handle missing data).
Uncertain risk of bias: (there is insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data is likely to induce bias on the estimate of effect).
High risk of bias: (the crude estimate of effects (eg, complete case estimate) will clearly be biased due to the underlying reasons for missingness, and the methods used to handle missing data are unsatisfactory).
Selective outcome reporting
Low risk of bias: pre‐defined, or clinically relevant and reasonably expected outcomes are reported on.
Uncertain risk of bias: not all pre‐defined or clinically relevant and reasonably expected outcomes are reported on, or are not reported fully, or it is unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded.
Vested interest bias
Low risk of bias: the trial was conducted by parties that might have conflicting interest (eg, drug manufacturer).
Uncertain risk of bias: the source of funding was not clear.
High risk of bias: the trial was funded by a drug manufacturer.
We considered trials which are classified as low risk of bias in all the above domains as low bias‐risk trials.
Measures of treatment effect
For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence interval (CI). Risk ratio calculations do not include trials in which no events occurred in either group, whereas risk difference calculations do. We planned to report the risk difference if the results using this association measure were different from risk ratio. For continuous variables, we calculated the mean difference (MD) with 95% CI for outcomes such as hospital stay and standardised mean difference (SMD) with 95% CI for blood transfusion requirements (which can be reported as units transfused or as ml transfused) and quality of life (where different scales might be used). For time‐to‐event outcomes such as survival at maximal follow‐up, we planned to calculate the hazard ratio (HR) with 95% CI.
Unit of analysis issues
The unit of analysis were the aggregate data on patients who underwent liver transplantation according to randomised group.
Dealing with missing data
We performed an intention‐to‐treat analysis (Newell 1992) whenever possible. We imputed data for binary outcomes using various scenarios such as best‐case, worst‐case, best‐worst and worse‐best scenarios (Gurusamy 2009c; Gluud 2011).
For continuous outcomes, we used available‐case analysis. We impute the standard deviation from P values according to the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011) and use the median for the meta‐analysis when the mean is not available. If it was not possible to calculate the standard deviation from the P value or the confidence intervals, we imputed the standard deviation as the highest standard deviation in the other trials included under that outcome fully recognising that this form of imputation will decrease the weight of the study for calculation of mean differences and bias the effect estimate to no effect in case of standardised mean difference (Higgins 2011).
For time‐to‐event outcomes, if the hazard ratio and 95% confidence intervals were not reported, we planned to obtain the logarithm of hazard ratios (ln(HR)) and the standard error (SE) of ln(HR) according to the methods described by Parmar 1998 using the Excel sheet provided by Tierney 2007.
Assessment of heterogeneity
We explored heterogeneity by chi‐squared test with significance set at P value 0.10, and measure the quantity of heterogeneity by I2 (Higgins 2002).
Assessment of reporting biases
We planned to use visual asymmetry on a funnel plot to explore reporting bias in the presence of at least 10 trials (Egger 1997; Macaskill 2001). We planned to perform linear regression approach described by Egger 1997 to determine the funnel plot asymmetry.
Data synthesis
We performed the meta‐analysis using two different approaches. The first approach was the meta‐analyses using the software package Review Manager 5 (RevMan 2011) and following the recommendations of The Cochrane Collaboration (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2011). We performed the meta‐analysis when there were at least two trials that could be included in the comparison. We used both a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987) meta‐analyses. In case of discrepancy between the two models we have reported both results; otherwise we planned to report the results of the fixed‐effect model. We planned to use the generic inverse method to combine the hazard ratios for time‐to‐event outcomes. If there was only one trial that could be included for the comparison for a binary outcome, we used the Fisher's exact test and present the P value rather than the risk ratio or risk difference. We used the statistical software StatsDirect 2.7 to perform this.
The second approach was network meta‐analysis which takes the indirect comparisons into account. We performed the network meta‐analysis using SAS 9.2 statistical software. A sample code for a binary outcome and a continuous outcome is shown in Appendix 2. The starting value for the parameters was obtained by running a logistic regression and simple linear regression, which do not take the association of data in different arms at the study level into account.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses:
Trials with low risk of bias compared to trials with high risk of bias.
Different methods to decrease blood loss and blood transfusion requirements.
Trials using piggy‐back technique of liver transplantation versus those using the conventional method of liver transplantation.
Trials using veno‐venous bypass versus those that do not use veno‐venous bypass.
We planned to use the 'test for subgroup differences' to identify the differences between subgroups.
Trial sequential analysis
We used trial sequential analysis (Wetterslev 2008; Wetterslev 2009) using an alpha error of 0.05, beta error of 0.20, control event proportion considered to be 10% based on an approximation of Eurpeon Liver Transplant Registry data (ELTR 2011), and a relative risk reduction of 20% for mortality. The theoretical background and the procedures for trial sequential analysis has been described in detail (Thorlund 2011). We used the trial sequential analysis (TSA) program (TSA 2011).
Sensitivity analysis
We performed a sensitivity analysis by imputing data for binary outcomes using various scenarios such as good outcome analysis, bad outcome analysis, best‐case scenario, and worst‐case scenario (Gurusamy 2009c; Gluud 2011). For continuous outcomes, we performed a sensitivity analysis by excluding the trials in which the mean and the standard deviation were imputed. We also performed a sensitivity analysis by imputing the standard deviation as the average standard deviation from the trials that reported the outcome in order to assess the impact of the imputation of standard deviation.
Presentation of results
We have presented the results as data tables, forest plots, and as 'Summary of findings' tables in addition to narrative text. We have presented the summary of findings for mortality, primary graft non‐function (Table 1), thromboembolic episodes and other serious adverse events (Table 2), blood loss (Table 3), and red cell or whole blood transfusion (Table 4).
Results
Description of studies
Results of the search
We identified a total of 3511 bibliographic references through the electronic searches in The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (n = 472), MEDLINE (n = 910), EMBASE (n = 1281), and Science Citation Index Expanded (n = 848). No additional trials were identified through reference search or meta‐register of current controlled trials. We did not identify any reference from reference searching. We excluded 1143 duplicates and 2291 clearly irrelevant references through reading abstracts. Seventy‐seven references were retrieved for further assessment. We excluded 23 references for the reasons stated in 'Characteristics of excluded studies'. Fifty‐four references of 33 completed randomised clinical trials were included in the review.The reference flow is shown in Figure 1.
1.
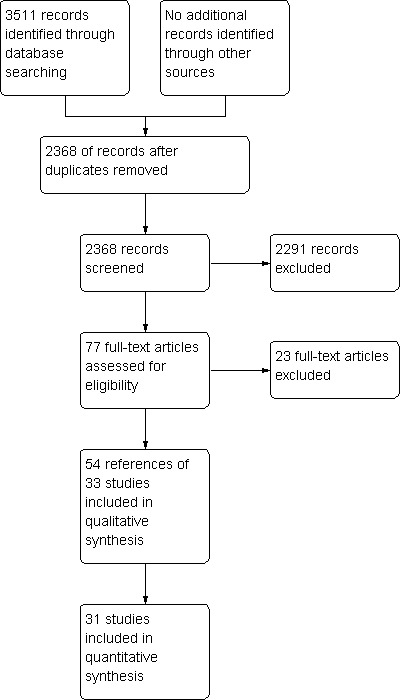
Study flow diagram.
Included studies
The characteristics of the patients included in the trials is shown in 'Characteristics of included studies'. All the included trials included patients undergoing liver transplantation. The average age ranged between 43 years and 58 years. The proportion of females ranged between 16% to 55%.
The interventions included pharmacological interventions (aprotinin, tranexamic acid, epsilon amino caproic acid (EACA), antithrombin 3, recombinant factor (rFvIIa), oestrogen, prostaglandin, or epinephrine), blood substitutes (blood components rather than whole blood, hydroxy‐ethyl starch), thromboelastography, or cardiovascular interventions (low central venous pressure).
There were 24 two‐armed trials. For the negative or inactive controls, 12 trials used placebo (Groh 1993; Ickx 1993; Yassen 1993; Milroy 1995; Boylan 1996; Marcel 1996; Garcia‐Huete 1997; Kaspar 1997; Merle 1997; Frenette 1998; Findlay 2001; Pugliese 2007) and nine trials used no intervention (Cottam 1991; Baudo 1992; Himmelreich 1993; Williamson 1999; Hei 2005; Ponnudurai 2005; Wang 2010; Feng 2010; Rummo 2010). Three trials compared one intervention with another (Laine 2003; Dalmau 2004; Ahn 2008). Three trials compared different doses of the same drug (Himmelreich 1992; Soilleux 1995; Lassale 1996). There were six trials with three or more intervention arms. Of these, three trials used different doses of the same drug and compared them with placebo (Porte 2000; Lodge 2005; Planinsic 2005). Three trials used different drugs and compared them with placebo (Ickx 1995; Dalmau 2000; Ickx 2006).
The drug doses used in the studies were highly variable. We calculated the total mean drug dose based on a mean duration of six hours for liver transplantation, using 70 kg patient for those trials in which the drug dose was based on the weight of the patient. The trials were considered to have different levels of doses based on information below. These were arbitrary decisions made after the data extraction was performed but before the data was analysed.
Aprotinin
High dose: > 2.5 million KIU
Medium dose: 1 to 2.5 million KIU
Low dose: < 1 million KIU
Tranexamic acid and recombinant factor VIIa (rFVIIa)
High dose: > =100 mg/Kg
Low dose: < 100 mg/Kg
The doses in the individual trial are shown in Table 5.
1. Doses used in the trials.
| Bolus | Continuous/hour | Additional boluses | Start time | End time | Approximate total including bolus | |
| Aprotinin (million KIU units) | ||||||
| Aprotinin versus control* | ||||||
| Cottam 1991 | 2 | 0.5 | 0.05 per unit transfused | induction | not stated | 5 |
| Findlay 2001 | 1 | 0.25 | None | not stated | end of surgery | 2.5 |
| Dalmau 2004 (control: tranexamic acid) | 2 | 0.5 | None | Beginning of surgery | 2 hours after reperfusion | 5 |
| Garcia‐Huete 1997 | 2 | 0.5 | None | induction | end of surgery | 5 |
| Groh 1993 | 2 | 0.5 | None | induction | end of surgery | 5 |
| Hei 2005 | None | 0.4 | None | induction | not stated | 2.4 |
| Ickx 1993 | None | 0.2 | None | Beginning of surgery | not stated | 1.2 |
| Ickx 1995 | 2 | 0.5 | None | anhepatic phase | not stated | 5 |
| Ickx 2006 (control: tranexamic acid) | 2 | 0.5 | None | anhepatic phase | 2 hours after reperfusion | 3.5 |
| Marcel 1996 | None | 0.2 | None | induction | not stated | 1.2 |
| Merle 1997 | 2 | 0.5 | None | Beginning of surgery | end of surgery | 5 |
| Milroy 1995 | 2 | 0.5 | None | induction | transfer to ITU | 5 |
| Porte 2000 (high dose A) | 2 | 1 | 1 million KIU half an hour before reperfusion | induction | 2 hours after reperfusion | 8 |
| Porte 2000 (high dose B) | 2 | 0.5 | None | induction | 2 hours after reperfusion | 5.5 |
| Different doses or methods of administration of aprotinin | ||||||
| Himmelreich 1992 (bolus method) | 0.5 | None | 0.5 during anhepatic phase and then at reperfusion | induction | reperfusion | 1 |
| Himmelreich 1992 (continuous method) | None | 0.2, increased to 0.4 during anhepatic phase and decreased to 0.1 from reperfusion | None | induction | end of surgery | 1.2 |
| Lassale 1996 (high dose) | 2 | 0.5 | None | induction | end of surgery | 5 |
| Lassale 1996 (low dose) | None | 0.1 | None | induction | end of surgery | 0.6 |
| Soilleux 1995 (high dose) | 2 | 0.5 | None | induction | transfer to ITU | 5 |
| Soilleux 1995 (medium dose) | 0.5 | 0.15 | None | induction | transfer to ITU | 1.4 |
|
Tranexamic acid versus control* Units: mg/kg/hr | ||||||
| Boylan 1996 | None | 40 | None | induction | reperfusion | 240 |
| Dalmau 2000 | None | 10 | None | induction | reperfusion | 60 |
| Dalmau 2004 (control: aprotinin) | None | 10 | None | Beginning of surgery | 2 hours after reperfusion | 60 |
| Ickx 1995 | 80 | 40 | None | anhepatic phase | not stated | 320 |
| Ickx 2006 (control: aprotinin) | 40 | 40 | None | anhepatic phase | 2 hours after reperfusion | 280 |
| Kaspar 1997 | None | 2 | None | Beginning of surgery | end of surgery | 12 |
| Yassen 1993 | 10 | 3 | None | anhepatic phase | transfer to ITU | 28 |
|
Epsilon amino caproic acid (EACA) versus control* Units: mg/kg/hour | ||||||
| Dalmau 2000 | None | 16 | None | induction | reperfusion | 96 |
|
Antithrombin versus control* Units: units per hour | ||||||
| Baudo 1992 | (100‐plasma activity level) per kg body weight | 1000 | None | induction | end of surgery | 6000 |
|
Recombinant Factor VIIa versus control* (mcg/kg) | ||||||
| Lodge 2005 (high dose A) | 60 | None | 60 mcg/kg every 2 hours to approximately 30 minutes before reperfusion and a final dose on wound closure | within 10 minutes of skin incision | None | 180 |
| Lodge 2005 (high dose B) | 120 | None | 120 mcg/kg every 2 hours to approximately 30 minutes before reperfusion and a final dose on wound closure | within 10 minutes of skin incision | None | 360 |
| Planinsic 2005 (low dose A) | 20 | None | None | within 10 minutes of skin incision (single bolus) | None | 20 |
| Planinsic 2005 (low dose B) | 40 | None | None | within 10 minutes of skin incision (single bolus) | None | 40 |
| Planinsic 2005 (low dose C) | 80 | None | None | within 10 minutes of skin incision (single bolus) | None | 80 |
| Pugliese 2007 | 40 | Just before induction | None | Just before induction | None | 40 |
|
Oestrogen versus control* Units: mg | ||||||
| Frenette 1998 | 100 | None | 100 mg 30 min after reperfusion | Beginning of surgery | None | 200 |
|
Prostaglandin E versus control* Units: microgram (mcg) | ||||||
| Himmelreich 1993 | None | 10 mcg increased to 40 mcg | None | Beginning of surgery | three post‐operative days | 2800 |
|
Norpeinephrine versus control* Units: mcg/min | ||||||
| Ponnudurai 2005 | None | 0.5 mcg/min increased by 1 mcg increments up to a maximum of 6 mcg/min to maintain a systolic blood pressure and pulmonary capillary wedge pressure more than 80% of baseline values. | ||||
* Control was placebo or no intervention unless stated. ITU = intensive therapy unit.
Risk of bias in included studies
The risk of bias is summarised in Figure 2 and Figure 3.
2.
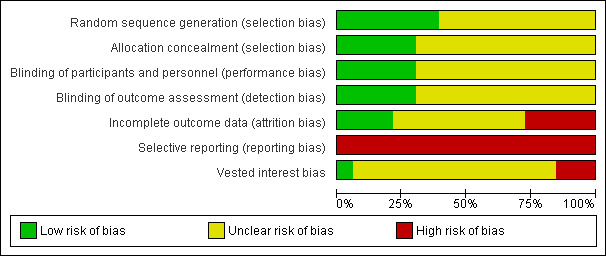
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
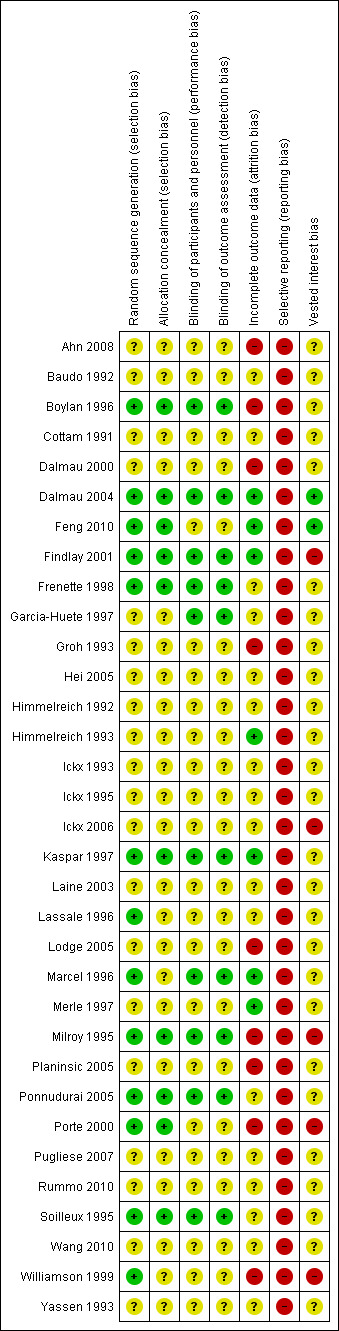
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Aprotinin versus control
Eight trials were included under this comparison (Groh 1993; Ickx 1993; Marcel 1996; Garcia‐Huete 1997; Merle 1997; Porte 2000; Findlay 2001; Hei 2005). All the trials were of high risk of bias.
Tranexamic acid versus control
Five trials were included under this comparison (Yassen 1993; Boylan 1996; Kaspar 1997; Dalmau 2000; Pugliese 2007). All the trials were of high risk of bias.
Epsilon amino caproic acid versus control
Only one trial was included under this comparison (Dalmau 2000). This trial was of high risk of bias.
Antithrombin III versus control
Only one trial was included under this comparison (Baudo 1992). This trial was of high risk of bias.
Recombinant factor VIIa versus control
Three trials were included under this comparison (Lodge 2005; Planinsic 2005; Pugliese 2007). All the trials were of high risk of bias.
Oestrogen versus control
Only one trial was included under this comparison (Frenette 1998). This trial was of high risk of bias.
Prostaglandin versus control
Only one trial was included under this comparison (Himmelreich 1993). This trial was of high risk of bias.
Norepinephrine versus control
Only one trial was included under this comparison (Ponnudurai 2005). This trial was of high risk of bias.
Thromboelastography versus control
Two trials were included under this comparison (Rummo 2010; Wang 2010). Both the trials were of high risk of bias.
Low central venous pressure (CVP) versus control
Only one trial was included under this comparison (Feng 2010). This trial was of high risk of bias.
Aprotinin: bolus versus continuous infusion
Only one trial was included under this comparison (Himmelreich 1992). This trial was of high risk of bias.
Aprotinin: high dose versus medium dose
Only one trial was included under this comparison (Soilleux 1995). This trial was of high risk of bias.
Aprotinin: high dose versus low dose
Only one trial was included under this comparison (Lassale 1996). This trial was of high risk of bias.
Whole blood versus blood components
Only one trial was included under this comparison (Laine 2003). This trial was of high risk of bias.
Solvent detergent plasma versus standard fresh frozen plasma
Only one trial was included under this comparison (Williamson 1999). This trial was of high risk of bias.
Tranexamic acid versus epsilon amino caproic acid
Only one trial was included under this comparison (Dalmau 2000). This trial was of high risk of bias.
Tranexamic acid versus aprotinin
Three trials were included under this comparison (Ickx 1995; Dalmau 2004; Ickx 2006). All the trials were of high risk of bias.The trials were included for the outcomes that they reported.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Interventions versus inactive controls
Aprotinin versus control
Eight trials were included under this comparison (Groh 1993; Ickx 1993; Marcel 1996; Garcia‐Huete 1997; Merle 1997; Porte 2000; Findlay 2001; Hei 2005). All the trials were of high risk of bias.The trials were included for the outcomes that they reported.
Primary outcomes
Mortality
There was no significant difference in the 60‐day mortality between the aprotinin groups versus the control (RR 0.52; 95% CI 0.18 to 1.45) (Analysis 1.1).
1.1. Analysis.
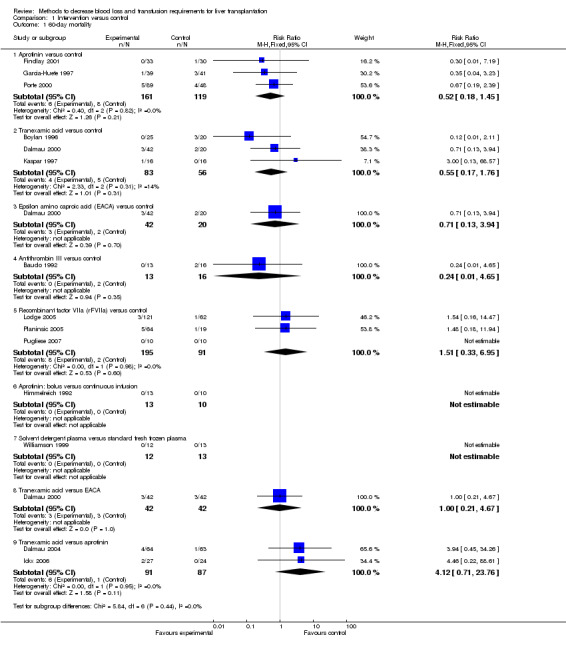
Comparison 1 Intervention versus control, Outcome 1 60‐day mortality.
Graft failure and retransplantation There was no significant difference in the primary graft non‐function (RR 0.15; 95% CI 0.02 to 1.25) (Analysis 1.3). Long‐term graft failure was not reported in any of the trials. The proportion of patients undergoing retransplantation was significantly lower in patients receiving aprotinin compared to placebo by the fixed‐effect model (RR 0.18; 95% CI 0.03 to 0.90) (Analysis 1.4) but not by random‐effects model (RR 0.21; 95% CI 0.02 to 1.79). There was no significant statistical heterogeneity (I2 = 26%; P = 0.25).
1.3. Analysis.
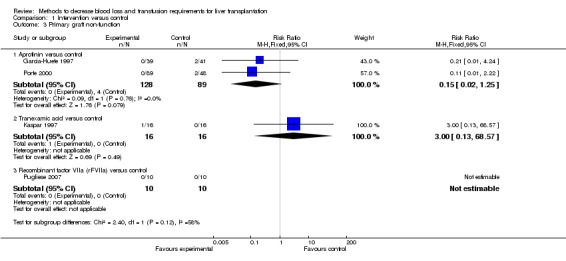
Comparison 1 Intervention versus control, Outcome 3 Primary graft non‐function.
1.4. Analysis.
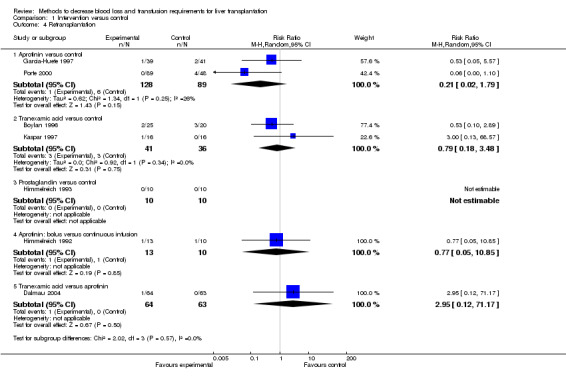
Comparison 1 Intervention versus control, Outcome 4 Retransplantation.
Serious adverse events There was no significant difference in the proportion of patients who developed thromboembolic episodes (RR 0.59; 95% CI 0.19 to 1.84) (Analysis 1.5) or other serious adverse events between the groups (Fisher's exact test: P > 0.99).
1.5. Analysis.
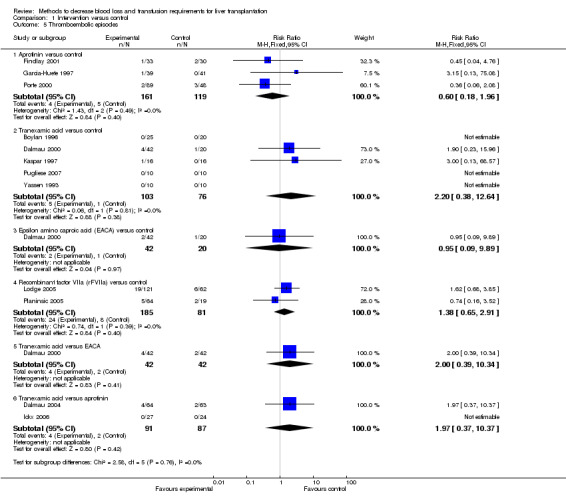
Comparison 1 Intervention versus control, Outcome 5 Thromboembolic episodes.
Quality of life This outcome was not reported in any of the trials.
Secondary outcomes
Blood loss and allogenic transfusion requirements There was no significant difference in the blood loss between the two groups (MD ‐1.36 litre; 95% CI ‐3.39 to 0.66) (Analysis 1.7). There was no significant difference in the proportion of patients requiring allogeneic red cell blood transfusion (Fisher's exact test: P = 0.18). There was significantly lower allogeneic blood transfusion requirements, amount of platelets, fresh frozen plasma, and cryoprecipitate transfused in the aprotinin group than the control group (SMD ‐0.59; 95% CI ‐0.80 to ‐0.39 (Analysis 1.9); SMD ‐0.44; 95% CI ‐0.73 to ‐0.14 (Analysis 1.10); SMD ‐0.33; 95% CI ‐0.53 to ‐0.13 (Analysis 1.11); SMD ‐0.49; 95% CI ‐0.82 to ‐0.16 (Analysis 1.12) respectively).
1.7. Analysis.
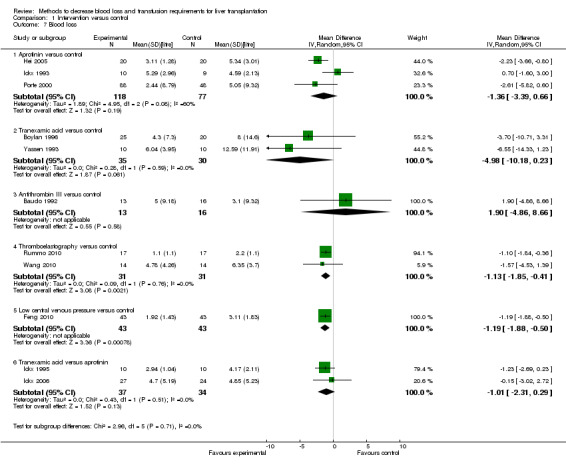
Comparison 1 Intervention versus control, Outcome 7 Blood loss.
1.9. Analysis.
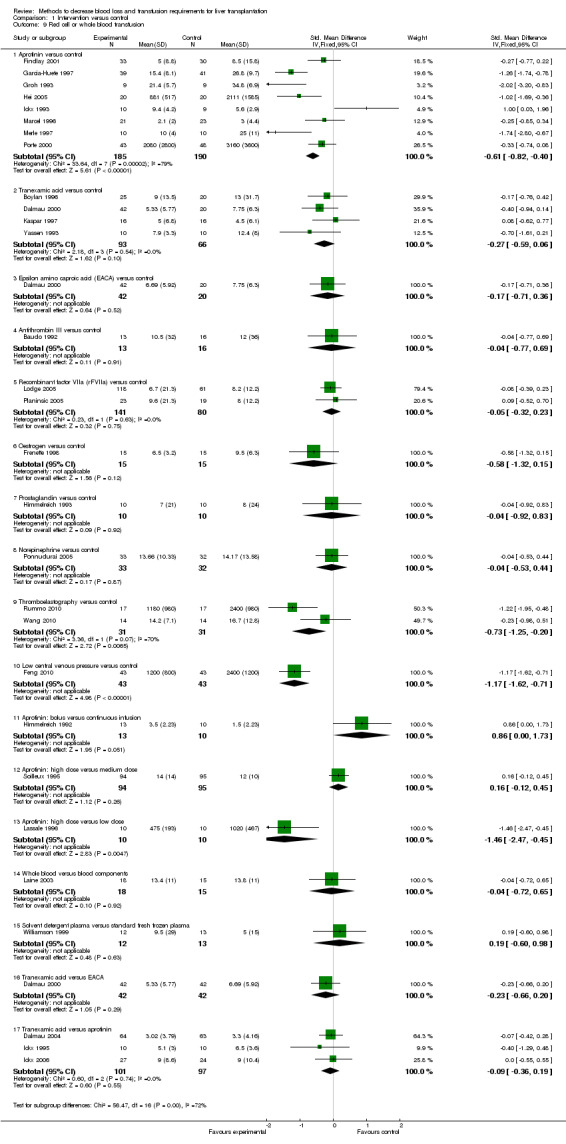
Comparison 1 Intervention versus control, Outcome 9 Red cell or whole blood transfusion.
1.10. Analysis.
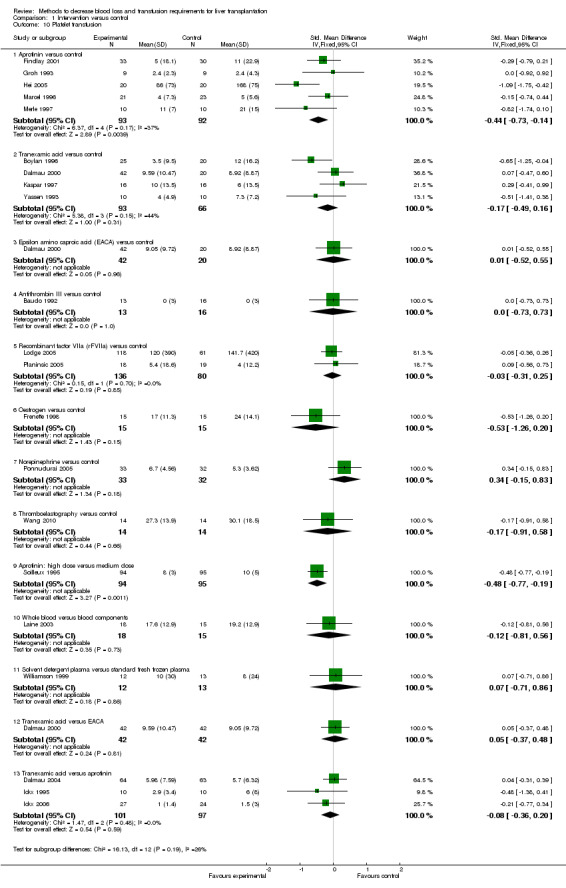
Comparison 1 Intervention versus control, Outcome 10 Platelet transfusion.
1.11. Analysis.
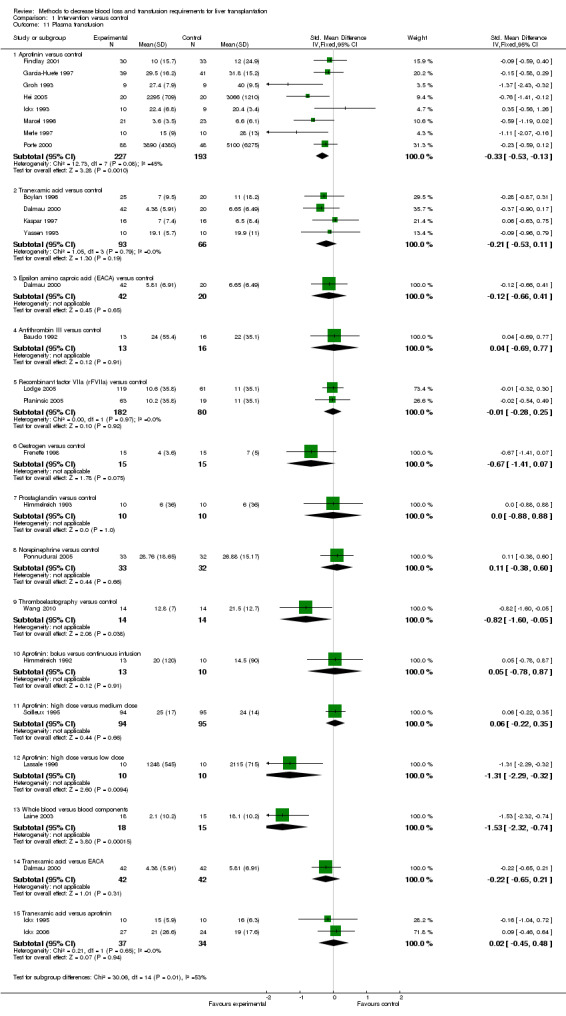
Comparison 1 Intervention versus control, Outcome 11 Plasma transfusion.
1.12. Analysis.
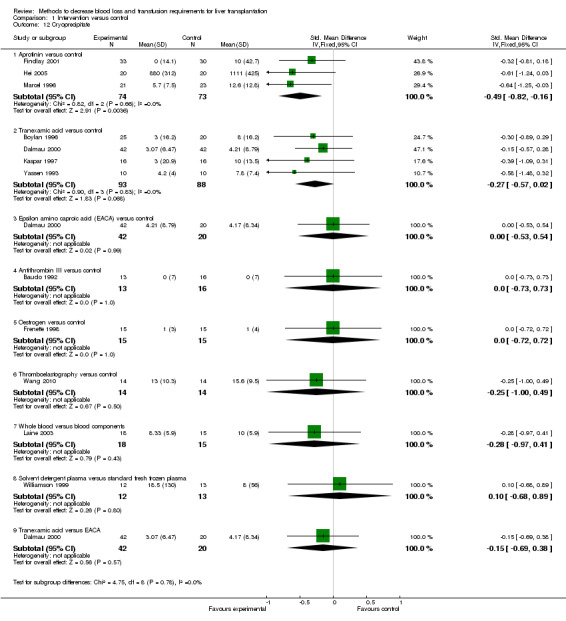
Comparison 1 Intervention versus control, Outcome 12 Cryoprecipitate.
Hospital stay There was no significant difference in the mean total hospital stay or ITU stay between the two groups (MD 0.00 days; 95% CI ‐4.94 to 4.94 (Analysis 1.13); MD ‐0.21 days; 95% CI ‐0.77 to 0.36 (Analysis 1.14) respectively).
1.13. Analysis.
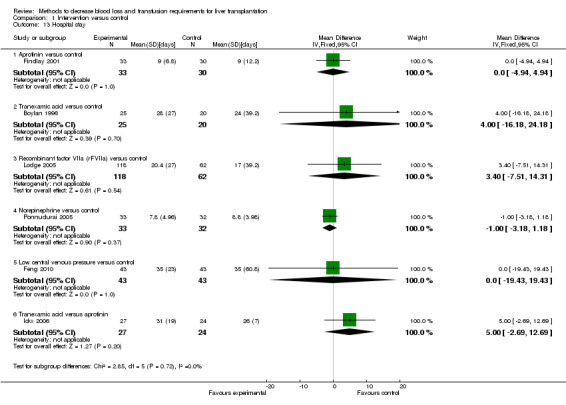
Comparison 1 Intervention versus control, Outcome 13 Hospital stay.
1.14. Analysis.
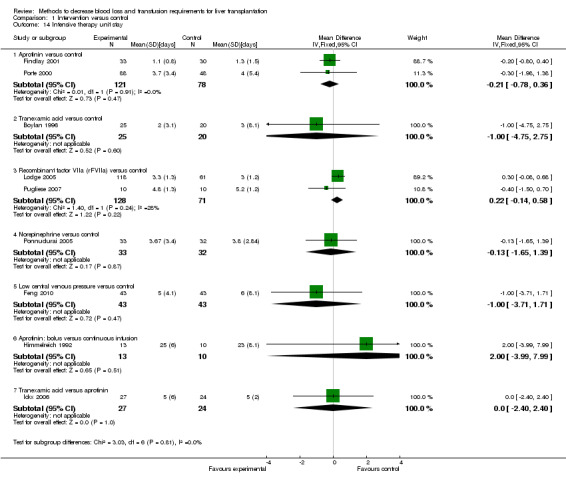
Comparison 1 Intervention versus control, Outcome 14 Intensive therapy unit stay.
Tranexamic acid versus control
Five trials were included under this comparison (Yassen 1993; Boylan 1996; Kaspar 1997; Dalmau 2000; Pugliese 2007). All the trials were of high risk of bias.The trials were included for the outcomes that they reported.
Primary outcomes
Mortality There was no significant difference in the 60‐day mortality between the tranexamic acid versus control groups (RR 0.55; 95% CI 0.17 to 1.76) (Analysis 1.1).
Graft failure and retransplantation There was no significant difference in the primary graft non‐function (Fisher's exact test: P > 0.99) (Kaspar 1997). Long‐term graft failure was not reported in any of the trials. There was no significant difference in the proportion of patients undergoing retransplantation (RR 0.79; 95% CI 0.18 to 3.48) (Analysis 1.4).
Serious adverse events There was no significant difference in the proportion of patients who developed thromboembolic episodes (RR 2.20; 95% CI 0.38 to 12.64) (Analysis 1.5). None of the trials reported other serious adverse events.
Quality of life This outcome was not reported in any of the trials.
Secondary outcomes
Blood loss and allogenic transfusion requirements
There was no significant difference in the blood loss between the two groups (MD ‐4.98 litre; 95% CI ‐10.18 to 0.23) (Analysis 1.7). There was no significant difference in the proportion of patients requiring allogeneic red cell blood transfusion (Fisher's exact test: P = 0.11) (Dalmau 2000). There was no significant difference in the allogeneic blood transfusion requirements, amount of platelets, fresh frozen plasma, or cryoprecipitate transfused (SMD ‐0.27; 95% CI ‐0.59 to 0.06 (Analysis 1.9); SMD ‐0.17; 95% CI ‐0.49 to 0.16 (Analysis 1.10); SMD ‐0.21; 95% CI ‐0.53 to 0.11 (Analysis 1.11); SMD ‐0.27; 95% CI ‐0.57 to 0.02 (Analysis 1.12) respectively) between the two groups.
Hospital stay
There was no significant difference in the mean total hospital stay or ITU stay between the two groups (MD 4.00 days; 95% CI ‐16.18 to 24.18 (Analysis 1.13); MD ‐1.00 days; 95% CI ‐4.75 to 2.75 (Analysis 1.14) respectively).
Epsilon amino caproic acid versus control
Only one trial was included under this comparison (Dalmau 2000). This trial was of high risk of bias.
Primary outcomes
Mortality
There was no significant difference in the 60‐day mortality between the groups (Fisher's exact test: P = 0.65).
Graft failure and retransplantation
None of these outcomes were reported in this trial.
Serious adverse events
There was no significant difference in the proportion of patients who developed thromboembolic episodes (Fisher's exact test: P > 0.99). Other serious adverse events were not reported in this trial.
Quality of life
This outcome was not reported in any of the trials.
Secondary outcomes
Blood loss and allogenic transfusion requirements
The amount of blood loss was not reported in this trial. There was no significant difference in the proportion of patients requiring allogeneic red cell blood transfusion (Fisher's exact test: P = 0.41). There was no significant difference in the allogeneic blood transfusion requirements, amount of platelets, fresh frozen plasma, or cryoprecipitate transfused (SMD ‐0.17; 95% CI ‐0.71 to 0.36 (Analysis 1.9); SMD 0.01; 95% CI ‐0.52 to 0.55 (Analysis 1.10); SMD ‐0.12; 95% CI ‐0.66 to 0.41 (Analysis 1.11); SMD 0.00; 95% CI ‐0.53 to 0.54 (Analysis 1.12) respectively) between the two groups.
Hospital stay
The mean total hospital stay or ITU stay were not reported by this trial.
Antithrombin III versus control
Only one trial was included under this comparison (Baudo 1992). This trial was of high risk of bias.
Primary outcomes
Mortality
There was no significant difference in the 60‐day mortality or mortality at maximal follow‐up between the antithrombin III versus the control groups (Fisher's exact test: P = 0.49 and P = 0.18 respectively).
Graft failure and retransplantation
None of these outcomes were reported in this trial.
Serious adverse events
The proportion of patients who developed thromboembolic episodes or other serious adverse events were not reported in this trial.
Quality of life
This outcome was not reported in this trial.
Secondary outcomes
Blood loss and allogenic transfusion requirements
There was no significant difference in the amount of blood loss between the two groups (MD 1.90 litre; 95% CI ‐4.86 to 8.66) (Analysis 1.7). The proportion of patients requiring allogeneic red cell blood transfusion was not reported in this trial. There was no significant difference in the allogeneic blood transfusion requirements, amount of platelets, fresh frozen plasma, or cryoprecipitate transfused (SMD ‐0.04; 95% CI ‐0.77 to 0.69 (Analysis 1.9); SMD 0.00; 95% CI ‐0.73 to 0.73 (Analysis 1.10); SMD 0.04; 95% CI ‐0.69 to 0.77 (Analysis 1.11); SMD 0.00; 95% CI ‐0.73 to 0.73 (Analysis 1.12) respectively) between the two groups.
Hospital stay
The mean total hospital stay or ITU stay were not reported in this trial.
Recombinant factor VIIa versus control
Three trials were included under this comparison (Lodge 2005; Planinsic 2005; Pugliese 2007). All the trials were of high risk of bias.
Primary outcomes
Mortality
There was no significant difference in the 60‐day mortality between the recombinant factor VIIa versus the control groups (RR 1.51; 95% CI 0.33 to 6.95).
Graft failure and retransplantation
There were no primary graft non‐function in the only trial that reported the outcome (Pugliese 2007). There was no significant difference in the graft failure (Fisher's exact test: P > 0.99). The proportion of patients who underwent retransplantation was not reported in any of the two trials.
Serious adverse events
There was no significant difference in the proportion of patients who developed thromboembolic episodes (RR 1.38; 95% CI 0.65 to 2.91) (Analysis 1.5) or other serious adverse events between the groups (RR 1.30; 95% CI 0.94 to 1.78) (Analysis 1.6).
1.6. Analysis.
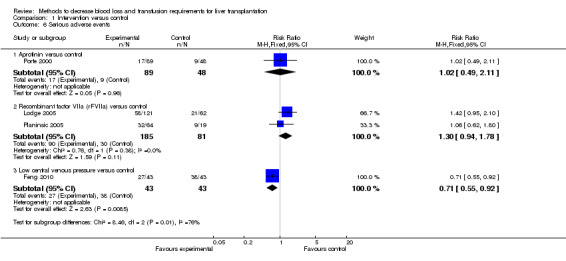
Comparison 1 Intervention versus control, Outcome 6 Serious adverse events.
Quality of life
This outcome was not reported in any of the trials.
Secondary outcomes
Blood loss and allogenic transfusion requirements
The amount of blood loss was not reported in the two trials. The proportion of patients requiring allogeneic red cell blood transfusion was lower in the rVIIIa group (Fisher's exact test: P = 0.02). There was no significant difference in the allogeneic blood transfusion requirements, amount of platelets, or fresh frozen plasma transfused (SMD ‐0.05; 95% CI ‐0.32 to 0.23 (Analysis 1.9); SMD ‐0.03; 95% CI ‐0.31 to 0.25 (Analysis 1.10); SMD ‐0.01; 95% CI ‐0.28 to 0.25 (Analysis 1.11) respectively) between the two groups.
Hospital stay
There was no significant difference in the mean total hospital stay or ITU stay between the two groups (MD 3.40 days; 95% CI ‐7.51 to 14.31(Analysis 1.13); MD 0.22 days; 95% CI ‐0.14 to 0.58 (Analysis 1.14) respectively).
Oestrogen versus control
Only one trial was included under this comparison (Frenette 1998). This trial was of high risk of bias.
Primary outcomes
Mortality
This outcome was not reported in this trial.
Graft failure and retransplantation
None of these outcomes were reported in this trial.
Serious adverse events
The proportion of patients who developed thromboembolic episodes or other serious adverse events were not reported in this trial.
Quality of life
This outcome was not reported in this trial.
Secondary outcomes
Blood loss and allogenic transfusion requirements
The amount of blood loss and the proportion of patients requiring allogeneic red cell blood transfusion were not reported in this trial. There was no significant difference in the allogeneic blood transfusion requirements, amount of platelets, fresh frozen plasma, or cryoprecipitate transfused (SMD ‐0.58; 95% CI ‐1.32 to 0.15 (Analysis 1.9); SMD ‐0.53; 95% CI ‐1.26 to 0.20 (Analysis 1.10); SMD ‐0.67; 95% CI ‐1.41 to 0.07 (Analysis 1.11); SMD 0.00; 95% CI ‐0.72 to 0.72 (Analysis 1.12) respectively) between the two groups.
Hospital stay
The mean total hospital stay or ITU stay were not reported by this trial.
Prostaglandin versus control
Only one trial was included under this comparison (Himmelreich 1993). This trial was of high risk of bias.
Primary outcomes
Mortality
This outcome was not reported in this trial.
Graft failure and retransplantation
Primary graft function and long‐term graft failure were not reported in this trial. There were no retransplantations in either group in this trial.
Serious adverse events
The proportion of patients who developed thromboembolic episodes or other serious adverse events were not reported in this trial.
Quality of life
This outcome was not reported in this trial.
Secondary outcomes
Blood loss and allogenic transfusion requirements
The amount of blood loss and the proportion of patients requiring allogeneic red cell blood transfusion were not reported in this trial. There was no significant difference in the allogeneic blood transfusion requirements or amount of cryoprecipitate transfused (SMD ‐0.04; 95% CI ‐0.92 to 0.83 (Analysis 1.9); SMD 0.00; 95% CI ‐0.88 to 0.88 (Analysis 1.11) respectively) between the two groups.
Hospital stay
The mean total hospital stay or ITU stay were not reported by this trial.
Norepinephrine versus control
Only one trial was included under this comparison (Ponnudurai 2005). This trial was of high risk of bias.
Primary outcomes
Mortality
This outcome was not reported in this trial.
Graft failure and retransplantation
None of these outcomes were reported in this trial.
Serious adverse events
The proportion of patients who developed thromboembolic episodes or other serious adverse events were not reported in this trial.
Quality of life
This outcome was not reported in this trial.
Secondary outcomes
Blood loss and allogenic transfusion requirements
The amount of blood loss and the proportion of patients requiring allogeneic red cell blood transfusion were not reported in this trial. There was no significant difference in the allogeneic blood transfusion requirements, amount of platelets, or fresh frozen plasma transfused between the norepinephrine versus the control group (SMD ‐0.04; 95% CI ‐0.53 to 0.44 (Analysis 1.9); SMD 0.34; 95% CI ‐0.15 to 0.83 (Analysis 1.10); SMD 0.11; 95% CI ‐0.38 to 0.60 (Analysis 1.11) respectively) between the two groups.
Hospital stay
There was no significant difference in the mean total hospital stay or ITU stay between the two groups (MD ‐1.00 days; 95% CI ‐3.18 to 1.18 (Analysis 1.13); MD ‐0.13 days; 95% CI ‐1.65 to 1.39 (Analysis 1.14) respectively).
Thromboelastography versus control
Two trials were included under this comparison (Rummo 2010; Wang 2010). Both the trials were of high risk of bias.
Primary outcomes
Mortality
There was no significant difference in the mortality at maximal follow‐up between the thromboelastography versus the control groups (Fisher's exact test: P > 0.99) (Wang 2010).
Graft failure and retransplantation
None of these outcomes were reported in either trial.
Serious adverse events
The proportion of patients who developed thromboembolic episodes or other serious adverse events were not reported in either trial.
Quality of life
This outcome was not reported in either trial.
Secondary outcomes
Blood loss and allogenic transfusion requirements There was no significant difference in the amount of blood loss between the two groups (SMD ‐0.73; 95% CI ‐1.69 to 0.24) (Analysis 1.7). The proportion of patients requiring allogeneic red cell blood transfusion was not reported in either trial. The allogeneic blood transfusion requirements were significantly lower in the thromboelastography group than controls by the fixed effect model (SMD ‐0.73; 95% CI ‐1.25 to ‐0.20) but not by the random‐effects model (SMD ‐0.73; 95% CI ‐1.69 to 0.24) (Analysis 1.9). There was significant statistical heterogeneity (I2 = 70%; P = 0.07). There was no significant difference in the amount of platelet transfusion, or cryoprecipitate (SMD ‐0.17; 95% CI ‐0.91 to 0.58 (Analysis 1.10) ; SMD ‐0.25; 95% CI ‐1.00 to 0.49 (Analysis 1.12) respectively) between the two groups.
The amount of fresh frozen plasma transfused was significantly lower in the thromboelastography group than control (SMD ‐0.82; 95% CI ‐1.60 to ‐0.05) (Analysis 1.11).
Hospital stay
The mean total hospital stay or ITU stay were not reported in this trial.
Low central venous pressure (CVP) versus control
Only one trial was included under this comparison (Feng 2010). This trial was of high risk of bias.
Primary outcomes
Mortality
This outcome was not reported in this trial.
Graft failure and retransplantation
None of these outcomes were reported in this trial.
Serious adverse events The proportion of patients who developed thromboembolic episodes was not reported in this trial. The proportion of patients with other serious adverse events was significantly lower in the low CVP group than the control group (Fisher's exact test: P = 0.01)
Quality of life
This outcome was not reported in this trial.
Secondary outcomes
Blood loss and allogenic transfusion requirements
The amount of blood loss was significantly lower in the low CVP group than control (MD ‐1.19 litre; 95% CI ‐1.88 to ‐0.50) (Analysis 1.7). The proportion of patients requiring allogeneic red cell blood transfusion was not reported in this trial. The allogeneic blood transfusion requirements were significantly lower in the low CVP group than the control group (SMD ‐1.17; 95% CI ‐1.62 to ‐0.71) (Analysis 1.9).
Hospital stay
There was no significant difference in the mean total hospital stay or ITU stay between the two groups (MD 0.00 days; 95% CI ‐19.43 to 19.43 (Analysis 1.13); (MD ‐1.00 days; 95% CI ‐3.71 to 1.71 (Analysis 1.14)).
One intervention versus another intervention
Aprotinin: bolus versus continuous infusion
Only one trial was included under this comparison (Himmelreich 1992). This trial was of high risk of bias.
Primary outcomes
Mortality
There was no mortality in either group.
Graft failure and retransplantation
Primary graft non‐function and long‐term graft failure were not reported in this trial. There was no significant difference in the proportion of patients requiring retransplantation between the two groups (Fisher's exact test: P > 0.99).
Serious adverse events The proportion of patients who developed thromboembolic episodes or other serious adverse events were not reported in this trial.
Quality of life
This outcome was not reported in this trial.
Secondary outcomes
Blood loss and allogenic transfusion requirements The amount of blood loss and the proportion of patients requiring allogeneic red cell blood transfusion were not reported in this trial. There was no significant difference in the allogeneic blood transfusion requirements or the amount of fresh frozen plasma transfused (SMD 0.86; 95% CI ‐0.00 to 1.73 (Analysis 1.9); SMD 0.05; 95% CI ‐0.78 to 0.87 (Analysis 1.11) respectively) between the two groups.
Hospital stay
The mean total hospital stay was not reported in this trial. The ITU stay was significantly lower in the bolus group than the continuous infusion group (MD 2.00 days; 95% CI ‐3.99 to 7.99 (Analysis 1.14) respectively).
Aprotinin: high dose versus medium dose
Only one trial was included under this comparison (Soilleux 1995). This trial was of high risk of bias.
Primary outcomes
Mortality This outcome was not reported in this trial.
Graft failure and retransplantation None of these outcomes were reported in this trial.
Serious adverse events The proportion of patients who developed thromboembolic episodes or other serious adverse events were not reported in this trial.
Quality of life This outcome was not reported in this trial.
Secondary outcomes
Blood loss and allogenic transfusion requirements The amount of blood loss and the proportion of patients requiring allogeneic red cell blood transfusion were not reported in this trial. There was no significant difference in the allogeneic blood transfusion requirements, amount of platelets, or fresh frozen plasma transfused (SMD 0.16; 95% CI ‐0.12 to 0.45 (Analysis 1.9); SMD ‐0.48; 95% CI ‐0.77 to ‐0.19 (Analysis 1.10); SMD 0.06; 95% CI ‐0.22 to 0.35 (Analysis 1.11) respectively) between the two groups.
Hospital stay The mean total hospital stay or ITU stay were not reported in this trial.
Aprotinin: high dose versus low dose
Only one trial was included under this comparison (Lassale 1996). This trial was of high risk of bias.
Primary outcomes
Mortality This outcome was not reported in this trial.
Graft failure and retransplantation None of these outcomes were reported in this trial.
Serious adverse events The proportion of patients who developed thromboembolic episodes or other serious adverse events were not reported in this trial.
Quality of life This outcome was not reported in this trial.
Secondary outcomes
Blood loss and allogenic transfusion requirements The amount of blood loss and the proportion of patients requiring allogeneic red cell blood transfusion were not reported in this trial. There was no significant difference in the allogeneic blood transfusion requirements or amount of fresh frozen plasma transfused (SMD ‐1.46; 95% CI ‐2.47 to ‐0.45 (Analysis 1.9); SMD ‐1.31; 95% CI ‐2.29 to ‐0.32 (Analysis 1.11) respectively) between the two groups.
Hospital stay The mean total hospital stay or ITU stay were not reported in this trial.
Whole blood versus blood components
Only one trial was included under this comparison (Laine 2003). This trial was of high risk of bias.
Primary outcomes
Mortality This outcome was not reported in this trial.
Graft failure and retransplantation None of these outcomes were reported in this trial.
Serious adverse events The proportion of patients who developed thromboembolic episodes or other serious adverse events were not reported in this trial.
Quality of life This outcome was not reported in this trial.
Secondary outcomes
Blood loss and allogenic transfusion requirements The amount of blood loss and the proportion of patients requiring allogeneic red cell blood transfusion were not reported in this trial. There was no significant difference in the allogeneic blood transfusion requirements or the amount of platelets, fresh frozen plasma, or cryoprecipitate transfused (SMD ‐0.04; 95% CI ‐0.72 to 0.65 (Analysis 1.9); SMD ‐0.12; 95% CI ‐0.81 to 0.56 (Analysis 1.10); SMD ‐1.53; 95% CI ‐2.32 to ‐0.74 (Analysis 1.11); SMD ‐0.28; 95% CI ‐0.97 to 0.41 (Analysis 1.12) respectively) between the two groups.
Hospital stay The mean total hospital stay or ITU stay were not reported in this trial.
Solvent detergent plasma versus standard fresh frozen plasma
Only one trial was included under this comparison (Williamson 1999). This trial was of high risk of bias.
Primary outcomes
Mortality There was no 60‐day mortality in either group in this trial.
Graft failure and retransplantation None of these outcomes were reported in this trial.
Serious adverse events The proportion of patients who developed thromboembolic episodes or other serious adverse events were not reported in this trial.
Quality of life This outcome was not reported in this trial.
Secondary outcomes
Blood loss and allogenic transfusion requirements: The amount of blood loss and the proportion of patients requiring allogeneic red cell blood transfusion were not reported in this trial. There was no significant difference in the allogeneic blood transfusion requirements or the amount of platelets, fresh frozen plasma, or cryoprecipitate transfused (SMD 0.19; 95% CI ‐0.60 to 0.98 (Analysis 1.9); SMD 0.07; 95% CI ‐0.71 to 0.86 (Analysis 1.10); SMD 0.10; 95% CI ‐0.68 to 0.89 (Analysis 1.12) respectively) between the two groups.
Hospital stay: The mean total hospital stay or ITU stay were not reported in this trial.
Tranexamic acid versus epsilon amino caproic acid
Only one trial was included under this comparison (Dalmau 2000). This trial was of high risk of bias.
Primary outcomes
Mortality There was no significant difference in the 60‐day mortality between the groups (Fisher's exact test: P > 0.99).
Graft failure and retransplantation None of these outcomes were reported in this trial.
Serious adverse events There was no significant difference in the proportion of patients who developed thromboembolic episodes (Fisher's exact test: P =0.68). Other serious adverse events were not reported in this trial.
Quality of life
This outcome was not reported in any of the trials.
Secondary outcomes
Blood loss and allogenic transfusion requirements The amount of blood loss was not reported in this trial. There was no significant difference in the proportion of patients requiring allogeneic red cell blood transfusion (Fisher's exact test: P = 0.12). There was no significant difference in the allogeneic blood transfusion requirements, amount of platelet transfusion, fresh frozen plasma, or cryoprecipitate (SMD ‐0.23; 95% CI ‐0.66 to 0.20 (Analysis 1.9); SMD 0.05; 95% CI ‐0.37 to 0.48 (Analysis 1.10); SMD ‐0.22; 95% CI ‐0.65 to 0.21 (Analysis 1.11); SMD ‐0.15; 95% CI ‐0.69 to 0.38) (Analysis 1.12) respectively) between the two groups.
Hospital stay The mean total hospital stay or ITU stay were not reported by this trial.
Tranexamic acid versus aprotinin
Three trials were included under this comparison (Ickx 1995; Dalmau 2004; Ickx 2006). All the trials were of high risk of bias.The trials were included for the outcomes that they reported.
Primary outcomes
Mortality There was no significant difference in the 60‐day mortality between the groups (RR 4.12; 95% CI 0.71 to 23.76) (Analysis 1.1).
Graft failure and retransplantation Primary graft non‐function and graft failure were not reported in any of the trials. There was no significant difference in the proportion of patients requiring retransplantation between the two groups (Fisher's exact test: P >0.99) (Dalmau 2004).
Serious adverse events There was no significant difference in the proportion of patients who developed thromboembolic episodes (Fisher's exact test: P =0.68) (Dalmau 2004). Other serious adverse events were not reported in this trial.
Quality of life
This outcome was not reported in any of the trials.
Secondary outcomes
Blood loss and allogenic transfusion requirements There was no significant difference in the amount of blood loss between the two groups (MD ‐1.01 litre; 95% CI ‐2.31 to 0.29). There was no significant difference in the proportion of patients requiring allogeneic red cell blood transfusion (Fisher's exact test: P = 0.72) (Dalmau 2004). There was no significant difference in the allogeneic blood transfusion requirements, amount of platelets, or fresh frozen plasma transfused (SMD ‐0.09; 95% CI ‐0.36 to 0.19 (Analysis 1.9); (SMD ‐0.08; 95% CI ‐0.36 to 0.20) (Analysis 1.10); (SMD 0.02; 95% CI ‐0.45 to 0.48) (Analysis 1.11) respectively) between the two groups.
Hospital stay There was no significant difference in the mean total hospital stay or ITU stay between the two groups (MD 5.00 days; 95% CI ‐2.69 to 12.69 (Analysis 1.13); MD 0.00 days; 95% CI ‐2.40 to 2.40) (Analysis 1.14).
Network meta‐analysis
The results of the network meta‐analysis are summarised in Table 6.
2. Network analysis results.
| P value | Effect estimate Point estimate and 95% confidence intervals (CI) | |
| 60‐day mortality | ||
| Solvent detergent FFP | 0.5483 | RR 0; 95% CI 0 to 257517954.02 |
| Aprotinin | 0.0489 | RR 0.09; 95% CI 0.01 to 0.99 |
| Tranexamic acid | 0.0237 | RR 0.44; 95% CI 0.22 to 0.88 |
| EACA | 0.387 | RR 0.45; 95% CI 0.06 to 3.16 |
| Antithrombin III | 0.546 | RR 0; 95% CI 0 to 225447979.34 |
| rFVIIa | 0.4494 | RR 0.31; 95% CI 0.01 to 8.19 |
| Mortality at maximal follow‐up | ||
| Not performed | ||
| Primary graft non‐function | ||
| Convergence not obtained | ||
| Graft failure | ||
| Not performed | ||
| Retransplantation | ||
| Convergence not obtained | ||
| Thromboembolic episodes | ||
| rFVIIa | 0.0842 | RR 2.23; 95% CI 0.88 to 5.69 |
| control | 0.2142 | RR 0.5; 95% CI 0.16 to 1.6 |
| Aprotinin | 0.9371 | RR 1.04; 95% CI 0.36 to 2.99 |
| Tranexamic acid | 0.8742 | RR 0.88; 95% CI 0.14 to 5.46 |
| Serious adverse events | ||
| Low CVP | 0.1552 | RR 0.29; 95% CI 0.03 to 3.13 |
| Aprotinin | 0.8512 | RR 0.91; 95% CI 0.13 to 6.24 |
| rFVIIa | 0.2517 | RR 1.54; 95% CI 0.48 to 4.88 |
| Blood loss | ||
| Aprotinin | 0.4028 | MD 0.3 litre; 95% CI ‐ 0.47 to 1.07 |
| Tranexamic acid | 0.6075 | MD 0.20 litre; 95% CI ‐0.63 to 1.03 |
| Antithrombin III | 0.9966 | MD 0.03 litre; 95% CI ‐14.17 to 14.22 |
| Thromboelastometry | 0.0003 | MD ‐1.83 litre; 95% CI ‐2.55 to ‐1.12 |
| Low CVP | 0.004 | MD ‐1.12 litre; 95% CI ‐1.19 to ‐0.49 |
| Proportion of patients requiring red cell or whole blood transfusion | ||
| rFVIIa | 0.2765 | RR 0.18; 95% CI 0 to 25.84 |
| Aprotinin | 0.1301 | RR 0.37; 95% CI 0.07 to 2.04 |
| Tranexamic acid | 0.0937 | RR 0.27; 95% CI 0.04 to 1.72 |
| EACA | 0.5714 | RR 0.67; 95% CI 0.05 to 8.95 |
| Amount of blood transfused | ||
| Convergence not obtained | ||
| Platelet transfusion | ||
| Aprotinin | 0.0633 | SMD ‐0.25; 95% CI ‐0.51 to 0.02 |
| Tranexamic acid | 0.0129 | SMD ‐0.33; 95% CI ‐0.58 to ‐0.08 |
| EACA | 0.0231 | SMD ‐0.5; 95% CI ‐0.92 to ‐0.08 |
| rFVIIa | 0.7865 | SMD ‐0.04; 95% CI ‐0.32 to 0.25 |
| Oestrogen | 0.438 | SMD 0.28; 95% CI ‐0.46 to 1.02 |
| Thromboelastometry | 0.1046 | SMD 0.62; 95% CI ‐0.14 to 1.38 |
| Norepinephrine | 0.1552 | SMD 0.36; 95% CI ‐0.15 to 0.87 |
| Solvent detergent FFP | 0.3926 | SMD 0.34; 95% CI ‐0.48 to 1.17 |
| Fresh frozen plasma | ||
| Aprotinin | 0.4663 | SMD ‐0.12; 95% CI ‐0.46 to 0.22 |
| Tranexamic acid | 0.7514 | SMD ‐0.06; 95% CI ‐0.47 to 0.35 |
| EACA | 0.9742 | SMD 0.01; 95% CI ‐0.56 to 0.58 |
| Antithrombin III | 0.9217 | SMD ‐0.05; 95% CI ‐1.17 to 1.07 |
| rFVIIa | 0.9748 | SMD 0.01; 95% CI ‐0.64 to 0.66 |
| Oestrogen | 0.5834 | SMD ‐0.3; 95% CI ‐1.4 to 0.81 |
| Prostaglandin | 0.8881 | SMD ‐0.08; 95% CI ‐1.31 to 1.14 |
| Thromboelastometry | 0.7947 | SMD 0.14; 95% CI ‐0.98 to 1.27 |
| Norepinephrine | 0.628 | SMD ‐0.23; 95% CI ‐1.18 to 0.73 |
| Cryoprecipate | ||
| Aprotinin | 0.2675 | SMD ‐0.32; 95% CI ‐0.94 to 0.3 |
| Tranexamic acid | 0.2572 | SMD ‐0.35; 95% CI ‐1.01 to 0.31 |
| EACA | 0.5333 | SMD ‐0.29; 95% CI ‐1.32 to 0.74 |
| Oestrogen | 0.3963 | SMD 0.36; 95% CI ‐0.56 to 1.27 |
| Thromboelastometry | 0.8342 | SMD 0.1; 95% CI ‐0.92 to 1.11 |
| Solvent detergent FFP | 0.4925 | SMD 0.31; 95% CI ‐0.68 to 1.29 |
| Hospital stay | ||
| Aprotinin | 0.8137 | MD 0.59 days; 95% CI ‐5.89 to 7.06 |
| Tranexamic acid | 0.1876 | MD 6.45 days; 95% CI ‐4.83 to 17.74 |
| rFVIIa | 0.5458 | MD 3.04 days; 95% CI ‐9.78 to 15.87 |
| Low CVP | 0.1629 | MD 11.91 days; 95% CI ‐7.46 to 31.28 |
| Norepinephrine | 0.3781 | MD ‐1.1 days; 95% CI ‐4.19 to 1.99 |
| Intensive therapy unit stay | ||
| Aprotinin | 0.723 | MD ‐0.11 days; 95% CI ‐0.8 to 0.59 |
| Tranexamic acid | 0.9696 | MD 0.04 days; 95% CI ‐2.35 to 2.43 |
| rFVIIa | 0.6915 | MD ‐0.21 days; 95% CI ‐1.45 to 1.03 |
| Low CVP | 0.6431 | MD 0.6 days; 95% CI ‐2.39 to 3.59 |
| Norepinephrine | 0.8102 | MD 0.19 days; 95% CI ‐1.67 to 2.06 |
CVP = central venous pressure. EACA = epsilon amino caproic acid. FFP = fresh frozen plasma. rFVIIa = recombinant factor VIIa.
Primary outcomes
Mortality The 60‐day mortality was significantly lower in the aprotinin (RR 0.09; 95% CI 0.01 to 0.99) and tranexamic acid (RR 0.44; 95% CI 0.22 to 0.88) groups compared with no intervention or placebo. We did not perform the network meta‐analysis for mortality at maximal follow‐up because of the few trials included under this outcome.
Graft failure and retransplantation Convergence was not obtained for primary graft non‐function and retransplantation. We did not perform the network meta‐analysis for graft failure since only one trial reported this outcome.
Serious adverse events The network meta‐analysis did not show any significant differences in thromboembolic episodes or in serious adverse events in the different interventions.
Quality of life None of the trials reported the quality of life in patients.
Secondary outcomes
Blood loss and allogenic transfusion requirements The blood loss was significantly lower in the thromboelastography group (MD ‐1.83 litre; 95% CI ‐2.55 to ‐1.12) and low CVP group (MD ‐1.12 litre; 95% CI ‐1.19 to ‐0.49). The network meta‐analysis did not show any intervention to be effective in decreasing the proportion of patients requiring allogeneic red cell or whole blood transfusion. Convergence was not obtained for the allogeneic red cell or whole blood transfusion. The platelet transfusion was significantly lower in the tranexamic acid group (SMD ‐0.33; 95% CI ‐0.58 to ‐0.08). The network meta‐analysis did not show any intervention to be effective in decreasing the amount of fresh frozen plasma transfused, or cryoprecipitate transfused.
Hospital stay The network meta‐analysis did not show any significant differences in the mean total hospital stay or ITU stay in the different interventions.
Variations in statistical analysis
Calculating the risk difference did not alter the interpretation of effectiveness of the interventions in any way for the binary outcomes.
Subgroup analysis
All trials were of high risk of bias. There were few trials included under each outcome and the details necessary for subgroup analysis was not available for many trials. So, subgroup analysis was not performed.
Sensitivity analysis
There was no alteration in the interpretation of effectiveness of the interventions by performing the various sensitivity analyses.
Assessment of reporting bias
Funnel plot was not performed because of the few trials that were included under each comparison for primary outcomes.
Trial sequential analysis
Trial sequential analysis was performed when there were at least two trials under the comparison. The comparisons for which trial sequential analysis was performed were aprotinin versus control, tranexamic acid versus control, recombinant factor VIIa versus control, and tranexamic acid versus aprotinin. The required information size was calculated using an alpha error of 0.05, power of 80%, control event proportion considered to be 10% based on an approximation of Eurpeon Liver Transplant Registry data (ELTR 2011), and a relative risk reduction of 20% for mortality and was adjusted for heterogeneity based on model variance using TSA 2011. The current information size, required information size, and the information fraction for the comparisons were 280/15,302 (1.83%) for aprotinin versus control, 139/18706 (0.74%) tranexamic acid versus control, 286/15.302 (1.87%) recombinant factor VIIa versus control, and 178/15,302 (1.16%) tranexamic acid versus aprotinin. This information was too little to represent the available information and draw a boundary for any of the comparisons (Figure 4; Figure 5; Figure 6; Figure 7).
4.
Trial sequential analysis (60‐day mortality) ‐ aprotinin versus control.
The blue line indicates the cumulative Z‐curve.
Current information size: 280 patients; required information size: 15,302. information fraction: 1.83%.
5.
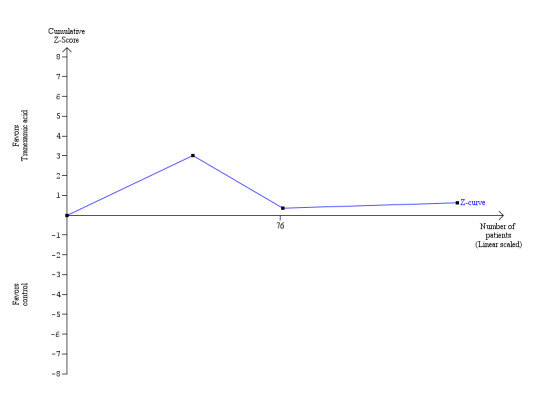
Trial sequential analysis (60‐day mortality) ‐ tranexamic acid versus control.
The blue line indicates the cumulative Z‐curve.
Current information size: 139 patients; required information size: 18,706. information fraction: 0.74%.
6.
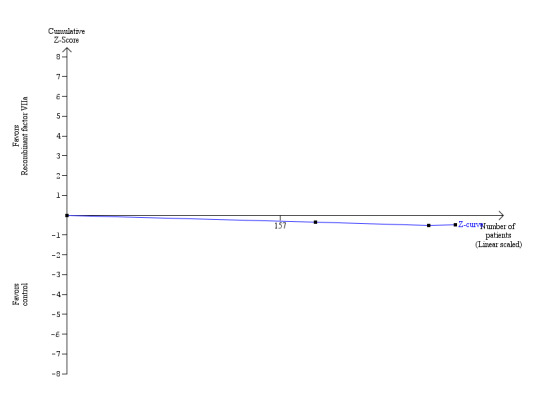
Trial sequential analysis (60‐day mortality) ‐ recombinant factor VIIa versus control.
The blue line indicates the cumulative Z‐curve.
Current information size: 286 patients; required information size: 15,302. information fraction: 1.87%.
7.
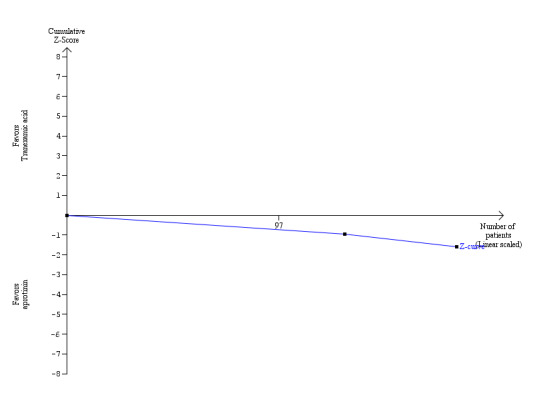
Trial sequential analysis (60‐day mortality) ‐ tranexamic acid versus aprotinin.
The blue line indicates the cumulative Z‐curve.
Current information size: 178 patients; required information size: 15,302. information fraction: 1.16%.
Discussion
Summary of main results
This systematic review was set out to evaluate all the interventions aimed at decreasing blood loss and transfusion requirements for liver transplantation. The direct meta‐analysis did not identify any significant differences in the mortality in any comparisons. The network meta‐analysis revealed a lower mortality in aprotinin and tranexamic acid groups. Thus, the results of direct analysis and network meta‐analysis are potentially different. Such differences may be due to heterogeneity or bias (Salanti 2008). As discussed previously in this review, the trials were all at high risk of bias. In addition, the trials spanned over several years during which the surgical experience and the results of liver transplantation have improved (ELTR 2011), resulting in heterogeneity in the trials. Thus, these results are not as reliable as results from direct analysis, but warrant further investigation (Mills 2011).
Mortality at maximal follow‐up was not reported in most trials. This was quite understandable considering that excessive blood loss affects mainly the peri‐operative outcomes. However, recent evidence from observational studies suggest that increased peri‐operative blood transfusion decreases long‐term survival after liver transplantation (Ramos 2003; Massicotte 2005; Boyd 2007; Boin 2008). So, new trials assessing interventions aimed at decreasing the blood transfusion requirements after liver transplantation should also consider evaluating the long‐term survival after liver transplantation.
There was no significant difference in the primary graft non‐function, graft failure, or retransplantation in any of the comparisons. There were no significant differences in the thromboembolic episodes or in serious adverse events in any of the comparisons. However, the confidence intervals were wide, and it is not possible to reach any conclusion on the safety of the interventions.
None of the trials reported the quality of life in patients. This is one of the main aspects of healthcare decisions using economic modelling in state‐funded healthcare systems. Future trials should include quality of life as an important outcome.
Overall completeness and applicability of evidence
None of the trials evaluated cell salvage method of decreasing allogeneic transfusion. Cell salvage may be an effective method of decreasing blood loss and transfusion requirements (Murphy 2004), it can be used in combination with anti‐fibrinolytics (Diprose 2005), and should be evaluated in future trials in liver transplantation.
Most of the trials were performed more than a decade ago. The results of liver transplantation has steadily improved during the last 10 to 15 years (ELTR 2011), although there is no evidence from this review that the blood transfusion requirements have decreased over time. Aprotinin has been withdrawn from market because of concerns about increased risk of death in high risk cardiac procedures (MHRA 2007; Fergusson 2008; Ray 2008). The significantly higher deaths in the aprotinin group compared with tranexamic acid or aminocaproic acid in this trial was because of cardiac reasons with no significant difference in the deaths due to other causes. Thus, the increased risk of death might be specific to the population. We noted no such increase in the death, thromboembolic complications, or other serious adverse events in the arms involving aprotinin. So, evidence from well‐ designed randomised clinical trials (particularly those evaluating tranexamic acid, amino caproic acid, cell salvage, thromboelastography, and low central venous pressure) are necessary before any of the interventions can be advocated.
Quality of the evidence
The quality of evidence was generally poor as shown in the 'Summary of findings' tables (Table 1; Table 2; Table 3; Table 4).
There was inconsistency of results between direct meta‐analysis and network meta‐analysis probably due to bias and heterogeneity. The trial sequential analysis revealed that the number of patients included in the meta‐analyses for 60‐day mortality was very small compared with the optimal number of patients required to make any firm conclusions.
Potential biases in the review process
We imputed standard deviation when they were not available from trial reports or author replies. We imputed this as the maximum standard deviation from similar trials. The effect of such imputation is to decrease the weight of the study for calculation of mean differences and bias the effect estimate to no effect in case of standardised mean difference. We performed sensitivity analysis of using the average standard deviation, which did not alter the results. The alternative is to exclude these trials for these outcomes, which will make interpretation of the results from these trials even more difficult. We imputed the missing mean values from the median values. This might have introduced bias.
Agreements and disagreements with other studies or reviews
Previous reviews mainly assessed the role of antifibrinolytics and concluded that antifibrinolytics (mainly aprotinin) were safe and decreased blood loss although there was no reduction in the overall mortality or morbidity (Molenaar 2007; Liu 2008). By following a more rigorous assessment of the evidence, we conclude that aprotinin, tranexamic acid, recombinant factor VIIa, low central venous pressure, and thromboelastography may lower blood loss and transfusion requirements. A Cochrane review which assessed the role of antifibrinolytics in all surgeries concluded that there were no serious adverse events related to tranexamic acid and epsilon amino caproic acid and that aprotinin decreased the blood loss and transfusion requirements compared to the lysine analogues (tranexamic acid and epsilon amino caproic acid) but increased the risk of death (Henry 2011). However, the data related to death were obtained from the trial in which aprotinin increased risk of death due to cardiac causes in high‐risk cardiac procedures (Fergusson 2008).
Authors' conclusions
Implications for practice.
Aprotinin, recombinant factor VIIa, and thromboelastography groups may potentially lower blood loss and transfusion requirements. However, their benefit‐harms ratios are not sufficiently studied due to the risk of systematic errors (bias) and the risk of random errors (play of chance).
Implications for research.
Further well‐designed trials with low risk of systematic error and low risk of random errors are necessary. Such trials should report the findings according to the CONSORT Statement (http://www.consort‐statement.org/).
What's new
| Date | Event | Description |
|---|---|---|
| 20 March 2012 | Amended | Spelling mistake in the name of Luit Penninga, peer reviewer on the review, is corrected. |
Acknowledgements
To The Cochrane Hepato‐Biliary Group.
Peer Reviewers: Luit Penninga, Denmark; Christian Gluud, Denmark. Contact Editor: Frederik Keus, The Netherlands.
Appendices
Appendix 1. Search strategies for identification of studies
| Database | Period of Search | Search Strategy |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Wiley) | Issue 3, 2011. | #1 Blood loss OR bleeding OR hemorrhage OR haemorrhage OR hemorrhages OR haemorrhages OR hemostasis OR haemostasis OR transfusion #2 MeSH descriptor Hemorrhage explode all trees #3 MeSH descriptor Blood Transfusion explode all trees #4 (#1 OR #2 OR #3) #5 liver OR hepatic OR hepato #6 MeSH descriptor Liver explode all trees #7 (#5 OR #6) #8 resection OR segmentectomy OR graft* OR transplant* #9 (#7 AND #8) #10 hepatectomy #11 MeSH descriptor Hepatectomy explode all trees #12 MeSH descriptor Liver Transplantation explode all trees #13 (#9 OR #10 OR #11 OR #12) #14 (#4 AND #13) |
| MEDLINE (Pubmed) | 1990 to September 2011. | (Blood loss OR bleeding OR hemorrhage OR haemorrhage OR hemorrhages OR haemorrhages OR hemostasis OR haemostasis OR transfusion OR "Hemorrhage"[Mesh] OR "Blood Transfusion"[Mesh]) AND (((liver OR hepatic OR hepato OR “liver”[MeSH]) AND (resection OR segmentectomy OR graft* OR transplant*)) OR hepatectomy OR “hepatectomy”[MeSH] OR “Liver Transplantation”[MeSH]) AND (((randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp]) NOT (animals [mh] NOT human [mh])))) |
| EMBASE (OvidSP) | 1990 to September 2011. | 1 (blood loss or bleeding or hemorrhage or haemorrhage or hemorrhages or haemorrhages or hemostasis or haemostasis or transfusion).af. 2 exp bleeding/ or exp blood transfusion/ 3 1 or 2 4 (liver or hepatic or hepato).af. 5 (segmentectomy or resection or graft* or transplant*).af. 6 4 and 5 7 hepatectomy.af. 8 exp liver resection/ or exp liver transplantation/ 9 6 or 7 or 8 10 3 and 9 11 exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomised controlled trial/ or single‐blind procedure/ 12 (random* or factorial* or crossover* or placebo*).af. 13 11 or 12 14 10 and 13 |
| Science Citation Index Expanded (http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame) | 1990 to September 2011. | #1 TS=(Blood loss OR bleeding OR hemorrhage OR haemorrhage OR hemorrhages OR haemorrhages OR hemostasis OR haemostasis OR transfusion) #2 TS=(((liver OR hepatic OR hepato) AND (resection OR segmentectomy OR graft* OR transplant*)) OR hepatectomy) #3 TS=(random* OR blind* OR placebo* OR meta‐analysis) #4 #3 AND #2 AND #1 |
| metaRegister of Controlled Trials (Current Controlled Trials) | September 2011. | (transfusion or bleeding or hemorrhage or haemorrhage) AND (liver transplant* or liver graft*) |
Appendix 2. Sample SAS code for network meta‐analysis
| Sample code | Type of outcome |
| data NetworkMetaAnalysis; input trial x n arm1 arm3 arm6 arm7 arm9; datalines; 3 0 25 0 0 1 0 0 3 0 20 1 0 0 0 0 5 4 42 0 0 1 0 0 5 2 42 0 0 0 1 0 5 2 40 1 0 0 0 0 6 4 64 0 0 1 0 0 6 2 63 0 1 0 0 0 8 1 33 0 1 0 0 0 8 2 30 1 0 0 0 0 10 1 39 0 1 0 0 0 10 0 41 1 0 0 0 0 17 0 24 0 1 0 0 0 17 0 27 0 0 1 0 0 18 1 16 0 0 1 0 0 18 0 16 1 0 0 0 0 21 7 58 0 0 0 0 1 21 12 63 0 0 0 0 1 21 6 62 1 0 0 0 0 25 2 18 0 0 0 0 1 25 1 24 0 0 0 0 1 25 2 22 0 0 0 0 1 25 2 19 1 0 0 0 0 27 2 46 0 1 0 0 0 27 0 43 0 1 0 0 0 27 3 48 1 0 0 0 0 28 0 10 0 0 0 0 1 28 0 10 1 0 0 0 0 33 0 10 0 0 1 0 0 33 0 10 1 0 0 0 0 run; * the parameters for random effects model can be calculated from simple logistic regression; * code for simple logistic regression; proc logistic data=NetworkMetaAnalysis; class arm; model x/n = arm; run; * random effects for trial and treatment contrasts; proc nlmixed data=NetworkMetaAnalysis; parms base=‐1 s2trialr=1 s2het=1 i=‐2.9109 a=‐0.0194 c=‐0.7863 f=‐0.0567 g=‐0.0849; logitp = (base + trialr) + arm9*(i+het) + arm3*(c+het) + arm6*(f+het) + arm7*(g+het); P = exp(logitp)/(1+exp(logitp)); model x ˜ binomial(n,P); random trialr het ˜ normal([0,0],[s2trialr,0,s2het]) subject=trial; run |
Binary outcome |
| data NetworkMetaAnalysis;
/* paste header row after input*/ input trial mean n MaxSD AvgSD arm1 arm3 arm6 arm9 arm18 arm19; /*paste the data after the datalines; */ datalines; 3 28 25 27 27 0 0 1 0 0 0 3 24 20 39.2 39.2 1 0 0 0 0 0 7 35 43 23 23 0 0 0 0 1 0 7 35 43 60.8 60.8 1 0 0 0 0 0 8 9 33 6.8 6.8 0 1 0 0 0 0 8 9 30 12.2 12.2 1 0 0 0 0 0 17 26 24 7 7 0 1 0 0 0 0 17 31 27 19 19 0 0 1 0 0 0 21 22 56 38.2 18.4 0 0 0 1 0 0 21 19 62 33 15.9 0 0 0 1 0 0 21 17 61 29.5 14.2 1 0 0 0 0 0 26 7.8 33 4.96 4.96 0 0 0 0 0 1 26 8.8 32 3.98 3.98 1 0 0 0 0 0 run; title1 'Hospital stay ‐ Maximum standard deviation'; proc nlmixed data=NetworkMetaAnalysis; parms base=‐1 s2trialr=1 s2het=1; y = (base + trialr) + arm3*(c+het)+ arm6*(f+het)+ arm9*(i+het)+ arm18*(r+het)+ arm19*(s+het); se2 = MaxSD**2/n; model mean ˜ normal(y,se2); random trialr het ˜ normal([0,0],[s2trialr,0,s2het]) subject=trial; run |
Continuous outcome |
Data and analyses
Comparison 1. Intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 60‐day mortality | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Aprotinin versus control | 3 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.18, 1.45] |
| 1.2 Tranexamic acid versus control | 3 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.17, 1.76] |
| 1.3 Epsilon amino caproic acid (EACA) versus control | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.13, 3.94] |
| 1.4 Antithrombin III versus control | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.65] |
| 1.5 Recombinant factor VIIa (rFVIIa) versus control | 3 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.33, 6.95] |
| 1.6 Aprotinin: bolus versus continuous infusion | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Solvent detergent plasma versus standard fresh frozen plasma | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Tranexamic acid versus EACA | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.67] |
| 1.9 Tranexamic acid versus aprotinin | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.12 [0.71, 23.76] |
| 2 Mortality at maximal follow‐up | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Antithrombin III versus control | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 1.85] |
| 2.2 Thromboelastography versus control | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.40] |
| 3 Primary graft non‐function | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Aprotinin versus control | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.25] |
| 3.2 Tranexamic acid versus control | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.57] |
| 3.3 Recombinant factor VIIa (rFVIIa) versus control | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Retransplantation | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Aprotinin versus control | 2 | 217 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.02, 1.79] |
| 4.2 Tranexamic acid versus control | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.18, 3.48] |
| 4.3 Prostaglandin versus control | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Aprotinin: bolus versus continuous infusion | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.05, 10.85] |
| 4.5 Tranexamic acid versus aprotinin | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.12, 71.17] |
| 5 Thromboembolic episodes | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Aprotinin versus control | 3 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.18, 1.96] |
| 5.2 Tranexamic acid versus control | 5 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.38, 12.64] |
| 5.3 Epsilon amino caproic acid (EACA) versus control | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.09, 9.89] |
| 5.4 Recombinant factor VIIa (rFVIIa) versus control | 2 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.65, 2.91] |
| 5.5 Tranexamic acid versus EACA | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.39, 10.34] |
| 5.6 Tranexamic acid versus aprotinin | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.37, 10.37] |
| 6 Serious adverse events | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Aprotinin versus control | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.49, 2.11] |
| 6.2 Recombinant factor VIIa (rFVIIa) versus control | 2 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.94, 1.78] |
| 6.3 Low central venous pressure versus control | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.55, 0.92] |
| 7 Blood loss | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Aprotinin versus control | 3 | 195 | Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐3.39, 0.66] |
| 7.2 Tranexamic acid versus control | 2 | 65 | Mean Difference (IV, Random, 95% CI) | ‐4.98 [‐10.18, 0.23] |
| 7.3 Antithrombin III versus control | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 1.9 [‐4.86, 8.66] |
| 7.4 Thromboelastography versus control | 2 | 62 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.85, ‐0.41] |
| 7.5 Low central venous pressure versus control | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐1.88, ‐0.50] |
| 7.6 Tranexamic acid versus aprotinin | 2 | 71 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐2.31, 0.29] |
| 8 Red‐cell or whole blood transfusion | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Aprotinin versus control | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.40, 1.18] |
| 8.2 Tranexamic acid versus control | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.98] |
| 8.3 Epsilon amino caproic acid (EACA) versus control | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.06] |
| 8.4 Recombinant factor VIIa (rFVIIa) versus control | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.86, 0.97] |
| 8.5 Tranexamic acid versus EACA | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.02] |
| 8.6 Tranexamic acid versus aprotinin | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.23] |
| 9 Red cell or whole blood transfusion | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Aprotinin versus control | 8 | 375 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.61 [‐0.82, ‐0.40] |
| 9.2 Tranexamic acid versus control | 4 | 159 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.59, 0.06] |
| 9.3 Epsilon amino caproic acid (EACA) versus control | 1 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.71, 0.36] |
| 9.4 Antithrombin III versus control | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.77, 0.69] |
| 9.5 Recombinant factor VIIa (rFVIIa) versus control | 2 | 221 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.32, 0.23] |
| 9.6 Oestrogen versus control | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐1.32, 0.15] |
| 9.7 Prostaglandin versus control | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.92, 0.83] |
| 9.8 Norepinephrine versus control | 1 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.53, 0.44] |
| 9.9 Thromboelastography versus control | 2 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.25, ‐0.20] |
| 9.10 Low central venous pressure versus control | 1 | 86 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.17 [‐1.62, ‐0.71] |
| 9.11 Aprotinin: bolus versus continuous infusion | 1 | 23 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.86 [‐0.00, 1.73] |
| 9.12 Aprotinin: high dose versus medium dose | 1 | 189 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.12, 0.45] |
| 9.13 Aprotinin: high dose versus low dose | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐2.47, ‐0.45] |
| 9.14 Whole blood versus blood components | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.72, 0.65] |
| 9.15 Solvent detergent plasma versus standard fresh frozen plasma | 1 | 25 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.60, 0.98] |
| 9.16 Tranexamic acid versus EACA | 1 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.66, 0.20] |
| 9.17 Tranexamic acid versus aprotinin | 3 | 198 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.36, 0.19] |
| 10 Platelet transfusion | 21 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Aprotinin versus control | 5 | 185 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.73, ‐0.14] |
| 10.2 Tranexamic acid versus control | 4 | 159 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.49, 0.16] |
| 10.3 Epsilon amino caproic acid (EACA) versus control | 1 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.52, 0.55] |
| 10.4 Antithrombin III versus control | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.73, 0.73] |
| 10.5 Recombinant factor VIIa (rFVIIa) versus control | 2 | 216 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.31, 0.25] |
| 10.6 Oestrogen versus control | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.26, 0.20] |
| 10.7 Norepinephrine versus control | 1 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐0.15, 0.83] |
| 10.8 Thromboelastography versus control | 1 | 28 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.91, 0.58] |
| 10.9 Aprotinin: high dose versus medium dose | 1 | 189 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.77, ‐0.19] |
| 10.10 Whole blood versus blood components | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.81, 0.56] |
| 10.11 Solvent detergent plasma versus standard fresh frozen plasma | 1 | 25 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.71, 0.86] |
| 10.12 Tranexamic acid versus EACA | 1 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.37, 0.48] |
| 10.13 Tranexamic acid versus aprotinin | 3 | 198 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.36, 0.20] |
| 11 Plasma transfusion | 25 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Aprotinin versus control | 8 | 420 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.53, ‐0.13] |
| 11.2 Tranexamic acid versus control | 4 | 159 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.53, 0.11] |
| 11.3 Epsilon amino caproic acid (EACA) versus control | 1 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.66, 0.41] |
| 11.4 Antithrombin III versus control | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.69, 0.77] |
| 11.5 Recombinant factor VIIa (rFVIIa) versus control | 2 | 262 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.28, 0.25] |
| 11.6 Oestrogen versus control | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.41, 0.07] |
| 11.7 Prostaglandin versus control | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.88, 0.88] |
| 11.8 Norepinephrine versus control | 1 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.38, 0.60] |
| 11.9 Thromboelastography versus control | 1 | 28 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐1.60, ‐0.05] |
| 11.10 Aprotinin: bolus versus continuous infusion | 1 | 23 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.78, 0.87] |
| 11.11 Aprotinin: high dose versus medium dose | 1 | 189 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.22, 0.35] |
| 11.12 Aprotinin: high dose versus low dose | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.31 [‐2.29, ‐0.32] |
| 11.13 Whole blood versus blood components | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.53 [‐2.32, ‐0.74] |
| 11.14 Tranexamic acid versus EACA | 1 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.65, 0.21] |
| 11.15 Tranexamic acid versus aprotinin | 2 | 71 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.45, 0.48] |
| 12 Cryoprecipitate | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Aprotinin versus control | 3 | 147 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.82, ‐0.16] |
| 12.2 Tranexamic acid versus control | 4 | 181 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.57, 0.02] |
| 12.3 Epsilon amino caproic acid (EACA) versus control | 1 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.53, 0.54] |
| 12.4 Antithrombin III versus control | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.73, 0.73] |
| 12.5 Oestrogen versus control | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.72, 0.72] |
| 12.6 Thromboelastography versus control | 1 | 28 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [1.00, 0.49] |
| 12.7 Whole blood versus blood components | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.97, 0.41] |
| 12.8 Solvent detergent plasma versus standard fresh frozen plasma | 1 | 25 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.68, 0.89] |
| 12.9 Tranexamic acid versus EACA | 1 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.69, 0.38] |
| 13 Hospital stay | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 Aprotinin versus control | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐4.94, 4.94] |
| 13.2 Tranexamic acid versus control | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐16.18, 24.18] |
| 13.3 Recombinant factor VIIa (rFVIIa) versus control | 1 | 180 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [‐7.51, 14.31] |
| 13.4 Norepinephrine versus control | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐3.18, 1.18] |
| 13.5 Low central venous pressure versus control | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐19.43, 19.43] |
| 13.6 Tranexamic acid versus aprotinin | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐2.69, 12.69] |
| 14 Intensive therapy unit stay | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 Aprotinin versus control | 2 | 199 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.78, 0.36] |
| 14.2 Tranexamic acid versus control | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐4.75, 2.75] |
| 14.3 Recombinant factor VIIa (rFVIIa) versus control | 2 | 199 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.14, 0.58] |
| 14.4 Norepinephrine versus control | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐1.65, 1.39] |
| 14.5 Low central venous pressure versus control | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.71, 1.71] |
| 14.6 Aprotinin: bolus versus continuous infusion | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐3.99, 7.99] |
| 14.7 Tranexamic acid versus aprotinin | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.40, 2.40] |
1.2. Analysis.
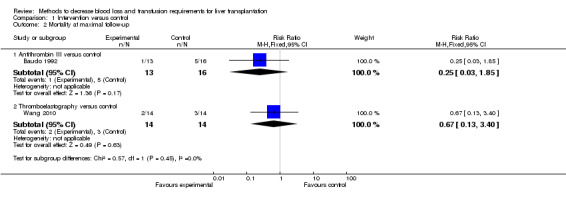
Comparison 1 Intervention versus control, Outcome 2 Mortality at maximal follow‐up.
1.8. Analysis.
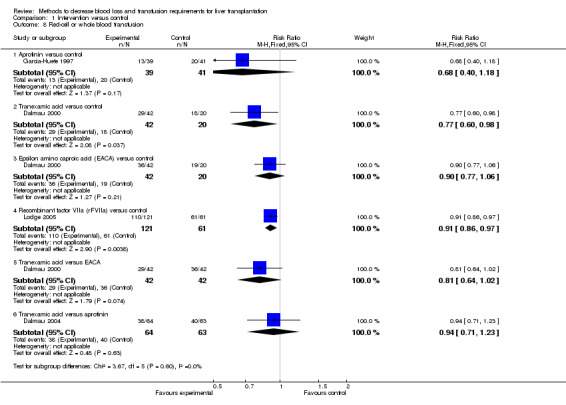
Comparison 1 Intervention versus control, Outcome 8 Red‐cell or whole blood transfusion.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahn 2008.
| Methods | Randomised clinical trial. | |
| Participants | Country: Korea. Sample size: 74. Post‐randomisation drop‐out(s): 8 (10.8%). Revised sample size: 66. Females: 12 (16.2%). Mean age: 51 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Adult patients undergoing primary orthotopic liver transplantation. Exclusion criteria: 1. Severe cardiovascular, cerebrovascular, pulmonary, or end‐stage renal diseases. 2. Taking medications likely to alter coagulation within two weeks of the study. 3. Allergic reaction to HES. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 31). Further details: high molecular weight hydroxy ethyl starch. Group 2: control (n = 35). Further details: low molecular weight hydroxy ethyl starch. | |
| Outcomes | The outcomes reported were thromboelastography parameters. None of the outcomes included in this review were reported. | |
| Notes | Attempts were made to contact the author in September 2011. Reason for post‐randomisation drop‐out(s): sampling error or transfusion with blood products. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Baudo 1992.
| Methods | Randomised clinical trial. | |
| Participants | Country: Italy. Sample size: 29. Post‐randomisation drop‐out(s): not stated. Revised sample size: 29. Females: 10 (34.5%). Mean age: 42.9 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: Patients undergoing orthotopic liver transplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 13). Further details: antithrombin III bolus on induction (100 ‐ plasma activity level) x kg body weight followed by continuous infusion of 1000 units per hour. Group 2: control (n = 16). Further details: control (no intervention). | |
| Outcomes | The outcomes reported were mortality, blood loss, and blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Boylan 1996.
| Methods | Randomised clinical trial. | |
| Participants | Country: Canada. Sample size: 45. Post‐randomisation drop‐out(s): not stated. Revised sample size: 45. Females: not stated. Mean age: 49.2 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Primary isolated orthotopic liver transplantation between 1992 and 1994. Exclusion criteria: 1. Patients with primary biliary cirrhosis or primary sclerosing cholangitis. 2. Predisposition to thrombotic tendency. 3. Fulminant liver failure. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 25). Further details: tranexamic acid 40 mg/kg/hour continuous infusion from induction of anaesthesia to unclamping of portal vein. maximum dose = 20 gram. Group 2: control (n = 20). Further details: normal saline. | |
| Outcomes | The outcomes reported were mortality, retransplantation, hospital stay, and transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Study agents were prepared by the hospital pharmacy using a randomisation schedule provided in sealed envelopes". |
| Allocation concealment (selection bias) | Low risk | Quote: "Study agents were prepared by the hospital pharmacy using a randomisation schedule provided in sealed envelopes; all other personnel were blinded to randomisation status". Comment: Probably adequate allocation concealment, although details of the sealed envelope such as whether they were opaque and consecutively numbered were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study agents were prepared by the hospital pharmacy using a randomisation schedule provided in sealed envelopes; all other personnel were blinded to randomisation status". Comment: Normal saline was used as placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Study agents were prepared by the hospital pharmacy using a randomisation schedule provided in sealed envelopes; all other personnel were blinded to randomisation status". Comment: Normal saline was used as placebo. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: In a preliminary report, the authors had excluded two patients out of 30 patients. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Cottam 1991.
| Methods | Randomised clinical trial. | |
| Participants | Country: UK. Sample size: 8. Post‐randomisation drop‐out(s): not stated. Revised sample size: 8. Females: not stated. Mean age: not stated. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: Patients undergoing liver transplantation | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 4). Further details: aprotinin 2 million KIU at induction followed by 500,000 KIU per hour and 50,000 KIU for every unit of blood transfused. Group 2: control (n = 4). Further details: no intervention. | |
| Outcomes | The outcomes reported were fibrinolysis parameters. None of the outcomes of interest for this review were included in the report. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Dalmau 2000.
| Methods | Randomised clinical trial. | |
| Participants | Country: Spain. Sample size: 153. Post‐randomisation drop‐out(s): 29 (19%). Revised sample size: 124. Females: 45 (29.4%). Mean age: 58 years. Piggyback: 124 (81%). Conventional: 0 (0%). Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing orthotopic liver transplantation. Exclusion criteria: 1. Patients with Budd Chiari syndrome. 2. Acute hepatic failure. 3. Retransplantation of less than 1 month. 4. Simultaneous liver and kidney transplantation. 5. Amyloidosis. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention1 (n = 42). Further details of intervention: tranexamic acid at 10 mg/kg/hour from the anaesthesia induction to graft reperfusion. Group 2: intervention2 (n = 42). Further details of intervention: epsilon aminocaproic acid at 16 mg/kg/hour from the anaesthesia induction to graft reperfusion. Group 3: control (n = 40). Further details: normal saline as placebo. | |
| Outcomes | The outcomes reported were mortality, thrombotic events, and transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. Reason for post‐randomisation drop‐out(s): acute hepatic failure (3), retransplantation (11), simultaneous kidney and liver transplantations (3), renal insufficiency with dialysis (1), primary amyloidotic neuropathy (3), incomplete data (5), and air embolism with cardiac arrest (3). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Although a placebo was used, the nature of the placebo was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Although a placebo was used, the nature of the placebo was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Dalmau 2004.
| Methods | Randomised clinical trial. | |
| Participants | Country: Spain. Sample size: 127. Post‐randomisation drop‐out(s): 0 (0%). Revised sample size: 127. Females: 38 (29.9%). Mean age: 53.5 years. Piggyback: 127 (100%). Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing orthotopic liver transplantation. Exclusion criteria: 1. Patients with Budd Chiari syndrome. 2. Acute hepatic failure. 3. Retransplantation of less than 1 month. 4. Simultaneous liver and kidney transplantation. 5. Amyloidosis. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 64). Further details: tranexamic acid bolus at induction (quantity not stated) followed by continuous infusion of 10 mg/kg/hour until 2 hours after unclamping of portal vein. Group 2: control (n = 63). Further details: aprotinin bolus of 2 million ki units at induction followed by continuous infusion of 500,000 KIU/hour until 2 hours after portal vein unclamping. | |
| Outcomes | The outcomes reported were mortality, retransplantation, reoperation, complications, and blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Drugs were prepared using a randomisation schedule provided in sealed envelopes". |
| Allocation concealment (selection bias) | Low risk | Quote: "Drugs were prepared using a randomisation schedule provided in sealed envelopes. The anesthesiologist, nurse, and surgeons were unaware of the details of the randomisation". Comment: Further details of sealed envelopes were not available. However, based on the steps taken to conceal the drug from the health providers, the sealed envelopes were probably adequate. . |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both drugs were diluted in normal saline in order to administer them at a rate of 100 ml/h after the bolus dose... The anesthesiologist, nurse, and surgeons were unaware of the details of the randomisation". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both drugs were diluted in normal saline in order to administer them at a rate of 100 ml/h after the bolus dose".. The anesthesiologist, nurse, and surgeons were unaware of the details of the randomisation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Low risk | Quote: "This study has not been the recipient of any grant or other financial support". |
Feng 2010.
| Methods | Randomised clinical trial. | |
| Participants | Country: China. Sample size: 86. Post‐randomisation drop‐out(s): not stated. Revised sample size: 86. Females: 14 (16.3%). Mean age: 47.5 years. Piggyback: 86 (100%). Conventional: 0 (0%). Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients scheduled to undergo primary liver transplantation. 2. Age above 18 years. Exclusion criteria: 1. Retransplantation. 2. Split liver transplantation. 3. Intraoperative demonstration of extended portal thrombosis. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 43). Further details: low central venous pressure (CVP kept below 5 mm hg or 40% below the baseline value) with mean arterial pressure at more than 60 mm Hg by limiting infusion volume, adjusting posture with a 5 to 10 degree head‐up tilt (reverse‐Trendelenberg patient position), and infusion of somatostatin and nitroglycerin (0.5–3.0 mcg/min/kg). Group 2: control (n = 43). Further details: standard management. | |
| Outcomes | The outcomes reported were complications, hospital stay, blood loss, and blood transfusion requirements. | |
| Notes | Author replied to questions related to bias in October 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 86 opaque sealed envelopes including 43 envelopes with the number ¡°1¡± and another 43 envelopes with the number ¡°2¡± were put into one locked box. Once the patient was chosen for protocol and informed consent was obtained, a staff took out one envelope from the locked box randomly. After opening the envelope, the number ¡°1¡± meant the patient was divided into ¡° the LCVP group¡±, while the number ¡°2¡± meant the patient was divided into ¡°the control group¡±. Comment: This method is similar to shuffling. This information was provided by author's replies to questions. |
| Allocation concealment (selection bias) | Low risk | Quote: 86 opaque sealed envelopes including 43 envelopes with the number ¡°1¡± and another 43 envelopes with the number ¡°2¡± were put into one locked box. Once the patient was chosen for protocol and informed consent was obtained, a staff took out one envelope from the locked box randomly. After opening the envelope, the number ¡°1¡± meant the patient was divided into ¡° the LCVP group¡±, while the number ¡°2¡± meant the patient was divided into ¡°the control group¡±. Comment: This method is similar to shuffling. This information was provided by author's replies to questions. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There were no post‐randomisation drop‐outs". Comment: This information was provided by author's replies to questions. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Low risk | Quote: "This study was supported by grants from the National Science and Technology (S & T) major project, China (2008ZX10002‐026), and the Science and Technology Bureau of Zhejiang Province, China (2009C33091)." Comment: This information was provided by author's replies to questions. |
Findlay 2001.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Sample size: 63. Post‐randomisation drop‐out(s): not stated. Revised sample size: 63. Females: 31 (49.2%). Mean age: 51 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing primary orthotopic liver transplantation. Exclusion criteria: 1. Pre‐OLT diagnosis of hepatorenal syndrome. 2. Established renal failure. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 30). Further details: aprotinin ‐ loading dose 1 million KIU over 30 minutes; followed by an infusion of 250,000 KIU/hour until skin closure. Group 2: control (n = 33). Further details: placebo (normal saline). | |
| Outcomes | The outcomes reported were mortality, serious adverse events, hospital stay, transfusion requirements. | |
| Notes | Author replied to questions related to risk of bias in October 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was by a stratified randomization scheme with 2 factors ‐ surgeon (one for each of 3 surgeons) and diagnosis (hepatitis or non‐hepatitis) for a total of 6 strata (3 surgeons x 2 diagnostic groups). For each stratum a blocked randomization list was produced by the Biostatistics group and maintained by the pharmacy. When a subject came to transplant pharmacy determined the stratum, selected the next envelope for that stratum and then issued the indicated trial medication (aprotinin or placebo) to the operating room". Comment: This information was provided by author's replies to questions. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was by a stratified randomization scheme with 2 factors ‐ surgeon (one for each of 3 surgeons) and diagnosis (hepatitis or non‐hepatitis) for a total of 6 strata (3 surgeons x 2 diagnostic groups). For each stratum a blocked randomization list was produced by the Biostatistics group and maintained by the pharmacy. When a subject came to transplant pharmacy determined the stratum, selected the next envelope for that stratum and then issued the indicated trial medication (aprotinin or placebo) to the operating room". Comment: This information was provided by author's replies to questions. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects were randomised to the administration of either aprotinin or placebo (an equivalent infusion of normal saline)". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Subjects were randomised to the administration of either aprotinin or placebo (an equivalent infusion of normal saline)". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: There were no post‐randomisation drop‐outs. Comment: This information was provided by author's replies to questions.. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | High risk | Quote: Funding was partially by a grant from Bayer Pharmaceuticals (as noted in the original publication), partially from internal Mayo departmental funding. Comment: This information was provided by author's replies to questions.. |
Frenette 1998.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Sample size: 30. Post‐randomisation drop‐out(s): not stated. Revised sample size: 30. Females: not stated. Mean age: 49.5 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing orthotopic liver transplantation. 2. Age more than 18 years. 3. Patients having a reaction time (R‐time) more than 15 minutes. Exclusion criteria: 1. Patients already taking oestrogen therapy. 2. Received blood products in preparation for surgery. 3. Patients undergoing retransplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 15). Further details: conjugated oestrogen 100 mg intravenous when the reaction time was longer than 15 minutes at the beginning of surgery and 30 minutes after reperfusion of the new graft. Group 2: control (n = 15). Further details: placebo (equal volume of normal saline). | |
| Outcomes | The outcomes reported were blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Study drugs were prepared by the hospital pharmacy using a randomisation schedule; all other personnel were blinded to the randomisation. The researchers were informed about the contents of the blinded syringes after the study". |
| Allocation concealment (selection bias) | Low risk | Quote: "Study drugs were prepared by the hospital pharmacy using a randomisation schedule; all other personnel were blinded to the randomisation. The researchers were informed about the contents of the blinded syringes after the study". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study drugs were prepared by the hospital pharmacy using a randomisation schedule; all other personnel were blinded to the randomisation. The researchers were informed about the contents of the blinded syringes after the study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Study drugs were prepared by the hospital pharmacy using a randomisation schedule; all other personnel were blinded to the randomisation. The researchers were informed about the contents of the blinded syringes after the study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Garcia‐Huete 1997.
| Methods | Randomised clinical trial. | |
| Participants | Country: Spain. Sample size: 80. Post‐randomisation drop‐out(s): not stated. Revised sample size: 80. Females: 29 (36.3%). Mean age: 50 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: Primary elective liver transplantation. Exclusion criteria: 1. Acute hepatic failure. 2. Retransplantation. 3. Multiorgan transplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 39). Further details: aprotinin 2 million KIU at induction followed by a continuous infusion of 500,000 KIU during procedure. Group 2: control (n = 41). Further details: placebo (normal saline). | |
| Outcomes | The outcomes reported were mortality, retransplantation, reoperation due to bleeding, and transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "prospective, randomised, double‐blind, and placebo‐controlled trial". Comment: The authors used placebo to achieve the blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "prospective, randomised, double‐blind, and placebo‐controlled trial". Comment: The authors used placebo to achieve the blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Groh 1993.
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany. Sample size: 20. Post‐randomisation drop‐out(s): 2 (10%). Revised sample size: 18. Females: not stated. Mean age: 50 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing primary elective orthotopic liver transplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 9). Further details: aprotinin 2 million KIU loading dose after induction of anaesthesia, followed by continuous infusion of 500,000 KIU/ hour until the end of procedure. Group 2: control (n = 9). Further details: placebo. | |
| Outcomes | The outcomes reported were blood transfusion requirements. | |
| Notes | Attempts were made to contact the authors in September 2011. Reason for post‐randomisation drop‐out(s): death, massive surgical haemorrhage (group not stated). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Although a placebo was used, the nature of the placebo was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Although a placebo was used, the nature of the placebo was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Two patients had to be excluded from statistical evaluation because of massive surgical haemorrhage and death during the study period". |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Hei 2005.
| Methods | Randomised clinical trial. | |
| Participants | Country: China. Sample size: 40. Post‐randomisation drop‐out(s): not stated. Revised sample size: 40. Females: not stated. Mean age: not stated. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: Patients undergoing liver transplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 20). Further details: aprotinin 40,000 KIU/kg/hour continuous infusion. Group 2: control (n = 20). Further details: control (no intervention). | |
| Outcomes | The outcomes reported were blood loss and blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Himmelreich 1992.
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany. Sample size: 23. Post‐randomisation drop‐out(s): not stated. Revised sample size: 23. Females: not stated. Mean age: not stated. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing primary orthotopic liver transplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 13). Further details: aprotinin intravenous bolus 0.5 million KIU thrice ‐ at the beginning of the operation, anhepatic phase, and at reperfusion. Group 2: control (n = 10). Further details: aprotinin intravenous continuously at 0.2 million KIU per hour from beginning of surgery, increased to 0.4 million KIU per hours during the anhepatic phase and at reperfusion; and decreased to 0.1 million KIU till skin closure. | |
| Outcomes | The outcomes reported were mortality, retransplantation, and transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomised by sealed envelopes with serial numbers containing the way of aprotinin administration". Comment: Further details of the sealed envelope method was not available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Himmelreich 1993.
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany. Sample size: 20. Post‐randomisation drop‐out(s): not stated. Revised sample size: 20. Females: not stated. Mean age: not stated. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing orthotopic liver transplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 10). Further details: prostaglandin (PGE1) at 10 mcg/hour increased to 40 mcg/hour in steps of 10 mcg/hour from the beginning of the operation; (infusion was temporarily stopped if the systolic pressure was less than 100 mm hg. Group 2: control (n = 10). Further details: control (no intervention). | |
| Outcomes | The outcomes reported were retransplantation and blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In a consecutive series, twenty patients with final liver disease underwent their first OLT at the University Hospital Rudolf Virchow, Berlin, between August and November 1990". |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Ickx 1993.
| Methods | Randomised clinical trial. | |
| Participants | Country: Belgium. Sample size: 19. Post‐randomisation drop‐out(s): not stated. Revised sample size: 19. Females: not stated. Mean age: not stated. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing orthotopic liver transplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 10). Further details: aprotinin 187,500 KIU/hour from the beginning of the procedure. Group 2: control (n = 9). Further details: control (placebo). | |
| Outcomes | The outcomes reported were blood loss and blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: A placebo was used. It was not clear what was used for placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: A placebo was used. It was not clear what was used for placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Ickx 1995.
| Methods | Randomised clinical trial. | |
| Participants | Country: Belgium. Sample size: 20. Post‐randomisation drop‐out(s): not stated. Revised sample size: 20. Females: not stated. Mean age: not stated. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing orthotopic liver transplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 10). Further details: tranexamic acid 80 mg/kg bolus followed by 40 mg/kg/hour. Group 2: control (n = 10). Further details: aprotinin 2 million KIU bolus followed by 0.5 million KIU/hour. | |
| Outcomes | The outcomes reported were blood loss and blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Ickx 2006.
| Methods | Randomised clinical trial. | |
| Participants | Country: Belgium. Sample size: 51. Post‐randomisation drop‐out(s): not stated. Revised sample size: 51. Females: not stated. Mean age: not stated. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing primary orthotopic liver transplantation. Exclusion criteria: 1. Fulminant hepatitis. 2. Past history of thromboembolic events. 3. Retransplantation. | |
| Interventions | The patients were randomised to the following groups.
Group 1: intervention (n = 27).
Further details: tranexamic acid 40 mg/kg as bolus initiated during the anhepatic phase and maintained at 40 mg/kg/hour as continuous infusion until 2 hours after reperfusion. Group 2: control (n = 24). Further details: aprotinin 2 million KIU as bolus initiated during the anhepatic phase and maintained at 0.5 million KIU as continuous infusion until 2 hours after reperfusion. |
|
| Outcomes | The outcomes reported were mortality, hospital stay, blood loss, and blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | High risk | Quote: "Drugs were provided by Choay, Bournonville, France". |
Kaspar 1997.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Sample size: 32. Post‐randomisation drop‐out(s): not stated. Revised sample size: 32. Females: not stated. Mean age: not stated. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing orthotopic liver transplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 16). Further details: tranexamic acid 2 mg/kg/hour after induction of anaesthesia until the end of surgery. Group 2: control (n = 16). Further details: control (placebo ‐ normal saline). | |
| Outcomes | The outcomes reported were mortality, primary non‐function, retransplantation, and blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The infusions were prepared by the hospital pharmacy using a computer generated randomisation schedule. All investigators were blinded to the composition of the solutions". |
| Allocation concealment (selection bias) | Low risk | Quote: "The infusions were prepared by the hospital pharmacy using a computer generated randomisation schedule. All investigators were blinded to the composition of the solutions". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The infusions were prepared by the hospital pharmacy using a computer generated randomisation schedule. All investigators were blinded to the composition of the solutions". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The infusions were prepared by the hospital pharmacy using a computer generated randomisation schedule. All investigators were blinded to the composition of the solutions". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "After institutional review board approval and informed consent, a prospective, placebo‐controlled, double‐blind study was performed on 32 consecutive patients undergoing OLT". |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Laine 2003.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Sample size: 33. Post‐randomisation drop‐out(s): not stated. Revised sample size: 33. Females: not stated. Mean age: not stated. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing liver transplantation. 2. Age more than 18 years. Exclusion criteria: 1. Patients with B or AB negative blood group. 2. Patients with serum alloantibodies. 3. ABO mismatched donor liver. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 18). Further details: whole blood. Group 2: control (n = 15). Further details: component therapy. | |
| Outcomes | The outcomes reported were volume of fluid transfused. None of the outcomes included in this review were reported in this trial. | |
| Notes | Attempts were made to contact the authors in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Lassale 1996.
| Methods | Randomised clinical trial. | |
| Participants | Country: France. Sample size: 20. Post‐randomisation drop‐out(s): not stated. Revised sample size: 20. Females: 7 (35%). Mean age: 47.5 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing primary liver transplantation for chronic indications. 2. Age more than 18 years. Exclusion criteria: 1. Retransplantation. 2. Allergies to aprotinin. 3. Prior acute pancreatitis. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 10). Further details: aprotinin 100,000 KIU/hour by continuous infusion. Group 2: control (n = 10). Further details: aprotinin 200,000 KIU/hour by continuous infusion. | |
| Outcomes | The outcomes reported were blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Le protocole d’administration a été attribué au hasard par une table de randomisation". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Lodge 2005.
| Methods | Randomised clinical trial. | |
| Participants | Country: Australia, Canada, Denmark, Germany, Spain, Sweden, United Kingdom. Sample size: 209. Post‐randomisation drop‐out(s): 26 (12.4%). Revised sample size: 183. Females: 72 (34.4%). Mean age: 52.7 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: 182 (87.1%). Live donor: 0 (0%). Inclusion criteria: 1. Patients scheduled to undergo orthotopic liver transplantation. 2. Age greater than 18 years. Exclusion criteria: 1. Previous liver transplantation. 2. Split liver transplantation. 3. Multiorgan transplantation. 4.Living related‐donor transplantation. 5. Renal insufficiency requiring dialysis. 6. Documented coagulation disorders. 7. Documented history or presence of portal vein thrombosis. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention 1 (n = 58). Further details of intervention: rFVIIa 120 mcg/Kg as bolus doses starting from within 10 minutes of skin incision; repeated every 2 hours until 30 minutes before expected reperfusion; and a final dose on skin closure. Group 2: intervention 2 (n = 63). Further details of intervention: rFVIIa 60 mcg/Kg as bolus doses starting from within 10 minutes of skin incision; repeated every 2 hours until 30 minutes before expected reperfusion; and a final dose on skin closure. Group 3: control (n = 61). Further details of intervention: placebo (not specified). | |
| Outcomes | The outcomes reported were mortality, adverse events, hospital stay and transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. Reason for post‐randomisation drop‐out(s): Did not meet the inclusion criteria on the day of operation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Although the authors state it is a double blinded placebo controlled randomised trial, the nature of the placebo was not known. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Although the authors state it is a double blinded placebo controlled randomised trial, the nature of the placebo was not known. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Marcel 1996.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Sample size: 44. Post‐randomisation drop‐out(s): not stated. Revised sample size: 44. Females: not stated. Mean age: not stated. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing orthotopic liver transplantation with venovenous bypass. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 21). Further details: aprotinin 200,000 KIU/hour immediately after induction (duration not stated). Group 2: control (n = 23). Further details: placebo (normal saline). | |
| Outcomes | The outcomes reported were blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised by a computer program to receive, via an infusion pump, identical volumes of aprotinin (n = 21) or normal saline solution (n = 23)". |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "After institutional review board approval and informed consent, a prospective, placebo‐controlled, double‐blind study was performed on 44 consecutive patients undergoing OLT with veno‐venous bypass". Comment: Normal saline was used as placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "After institutional review board approval and informed consent, a prospective, placebo‐controlled, double‐blind study was performed on 44 consecutive patients undergoing OLT with veno‐venous bypass". Comment: Normal saline was used as placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Merle 1997.
| Methods | Randomised clinical trial. | |
| Participants | Country: Netherlands. Sample size: 20. Post‐randomisation drop‐out(s): not stated. Revised sample size: 20. Females: not stated. Mean age: not stated. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Cirrhotic patients with severe liver failure undergoing liver transplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 10). Further details: aprotinin 2 million KIU prior to skin incision followed by continuous infusion of 0.5 million KIU until the end of surgery. Group 2: control (n = 10). Further details: placebo (nature not stated). | |
| Outcomes | The outcomes reported were blood transfusion requirements. | |
| Notes | Attempts were made to contact the authors in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Although the authors state that they used placebo, the nature of the placebo was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Although the authors state that they used placebo, the nature of the placebo was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Milroy 1995.
| Methods | Randomised clinical trial. | |
| Participants | Country: United Kingdom. Sample size: 55. Post‐randomisation drop‐out(s): 3 (5.5%). Revised sample size: 52. Females: 26 (47.3%). Mean age: 44.8 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients with chronic or fulminant liver failure undergoing orthotopic liver transplantation. 2. Age greater than 18 years. Exclusion criteria: 1. Previous exposure or allergy to aprotinin. 2. History of pancreatitis. 3. Oliguric renal failure. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 26). Further details: aprotinin 2 million KIU bolus after induction before laparotomy followed by continuous infusion at 0.5 million KIU/hour until the patient was transferred to ITU. Group 2: control (n = 26). Further details: placebo (normal saline). | |
| Outcomes | The outcomes reported were haemodynamic parameters. None of the outcomes of interest for this review were reported. | |
| Notes | Attempts were made to contact the authors in September 2011. Reason for post‐randomisation drop‐out(s): patient died because of heart failure (1); operation abandoned due to tissue oedema (1); inoperable liver tumour (1). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by a previously determined computer‐generated schedule using coded ampoules which were supplied by the manufacturer (Bayer plc) in identical case packs. The investigators were unaware of the coding schedule". |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by a previously determined computer‐generated schedule using coded ampoules which were supplied by the manufacturer (Bayer plc) in identical case packs. The investigators were unaware of the coding schedule". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Randomization was performed by a previously determined computer‐generated schedule using coded ampoules which were supplied by the manufacturer (Bayer plc) in identical case packs. The investigators were unaware of the coding schedule". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Randomization was performed by a previously determined computer‐generated schedule using coded ampoules which were supplied by the manufacturer (Bayer plc) in identical case packs. The investigators were unaware of the coding schedule". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | High risk | Quote: "We thank Bayer Pharmaceuticals for their support and sponsorship". |
Planinsic 2005.
| Methods | Randomised clinical trial. | |
| Participants | Country: Denmark, Finland, Germany, Spain, USA. Sample size: 87. Post‐randomisation drop‐out(s): 4 (4.6%). Revised sample size: 83. Females: 26 (29.9%). Mean age: 50.2 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients scheduled to undergo orthotopic liver transplantation. 2. Age more than 18 years. Exclusion criteria: 1. Previous liver transplantation. 2. Multiorgan transplantation. 3. Living related‐donor transplantation. 4. Renal insufficiency requiring dialysis. 5. Documented inherited coagulation disorders. 6. Documented history or presence of portal vein thrombosis. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention 1 (n = 22). Further details of intervention: rFVIIa 80 mcg/Kg as bolus dose within 10 minutes of skin incision. Group 2: intervention 2 (n = 24). Further details of intervention: rFVIIa 40 mcg/Kg as bolus dose within 10 minutes of skin incision. Group 3: intervention 2 (n = 18). Further details of intervention: rFVIIa 20 mcg/Kg as bolus dose within 10 minutes of skin incision. Group 3: control (n = 19). Further details of intervention: placebo (not specified). | |
| Outcomes | The outcomes reported were serious adverse events and blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. Reason for post‐randomisation drop‐out(s): liver transplantation cancelled. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Although the authors state placebo was used, the nature of the placebo was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Although the authors state placebo was used, the nature of the placebo was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Ponnudurai 2005.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA. Sample size: 65. Post‐randomisation drop‐out(s): not stated. Revised sample size: 65. Females: 21 (32.3%). Mean age: 53.4 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Adult patients about to undergo orthotopic liver transplantation for endstage liver disease. Exclusion criteria: 1. Patients undergoing combined liver and kidney transplantation. 2. Retransplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 33). Further details: norepinephrine 0.5 mcg/min increased in 1 mcg increments up to 6 mcg/min to maintain a mean arterial pressure and pulmonary capillary wedge pressures at > 80% of baseline values. Group 2: control (n = 32). Further details: placebo (normal saline). | |
| Outcomes | The outcomes reported were hospital stay and blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computer‐generated list of random numbers, patients were assigned to receive norepinephrine (vasopressor group) or IV fluids and isotonic saline (placebo group) by the research coordinator, who had no part in the clinical management of patients". |
| Allocation concealment (selection bias) | Low risk | Quote: "Using a computer‐generated list of random numbers, patients were assigned to receive norepinephrine (vasopressor group) or IV fluids and isotonic saline (placebo group) by the research coordinator, who had no part in the clinical management of patients". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The blinding of the investigators to isotonic saline or norepinephrine was done by the Department of Pharmacy at the UMDNJ. Blinding was maintained throughout the study period by pharmacists, who had no role in the clinical management of the study patients and data collection. The vasopressor and placebo were both colorless fluids". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The blinding of the investigators to isotonic saline or norepinephrine was done by the Department of Pharmacy at the UMDNJ. Blinding was maintained throughout the study period by pharmacists, who had no role in the clinical management of the study patients and data collection. The vasopressor and placebo were both colorless fluids". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Porte 2000.
| Methods | Randomised clinical trial. | |
| Participants | Country: Finland, Italy, Netherlands, Sweden. Sample size: 141. Post‐randomisation drop‐out(s): 5 (3.5%). Revised sample size: 136. Females: 33 (23.4%). Mean age: 51.4 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients who underwent primary orthotopic transplantation for acute or chronic liver disease. 2. Age at least 18 years. Exclusion criteria: 1. Retransplantation. 2. Known or suspected exposure to aprotinin. 3. Malignant disease. 4. pre‐existing thrombotic conditions. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention 1 (n = 45). Further details of intervention: 2 million KIU aprotinin as a loading dose given intravenously over 20 min before and during induction of anaesthesia, followed by a continuous infusion of 1 million KIU/hour until 2 hours after graft reperfusion. An additional dose of one million KIU was given half an hour before reperfusion. Group 2: intervention 2 (n = 43). Further details of intervention: 2 million KIU aprotinin as a loading dose given intravenously over 20 min before and during induction of anaesthesia, followed by a continuous infusion of 0.5 million KIU/hour until 2 hours after graft reperfusion. Group 3: control (n = 48). Further details: placebo (not stated). | |
| Outcomes | The outcomes reported were mortality, primary graft non‐function, retransplantation, reoperation, adverse effects, ITU stay, and blood transfusion requirements. | |
| Notes | Attempts were made to contact the authors in September 2011. Reason for post‐randomisation drop‐out(s): protocol violation (5); allergic reaction (1). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: From the other details available in this trial, it is highly likely that the authors used an appropriate method of random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "The trial drug was provided double blind by the manufacturer in blocks of 12 identical case packs. Each case pack contained all bottles for one patient, identifiable only by the sequence number". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The trial drug was provided double blind by the manufacturer in blocks of 12 identical case packs. Each case pack contained all bottles for one patient, identifiable only by the sequence number". Comment: The nature of placebo was not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The trial drug was provided double blind by the manufacturer in blocks of 12 identical case packs. Each case pack contained all bottles for one patient, identifiable only by the sequence number". Comment: The nature of placebo was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | High risk | Quote: "Aprotinin and placebo were donated by Bayer, Wuppertal, Germany". |
Pugliese 2007.
| Methods | Randomised clinical trial. | |
| Participants | Country: Italy. Sample size: 20. Post‐randomisation drop‐out(s): not stated. Revised sample size: 20. Females: not stated. Mean age: not stated. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing orthotopic liver transplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 10). Further details: rFVIIa 40 mcg/kg as bolus immediately before anaesthesia induction. Group 2: control (n = 10). Further details: placebo (details not known). | |
| Outcomes | The outcomes reported were mortality, primary graft non‐function, ITU stay, and blood transfusion requirements (the transfusion requirements during the entire operation and immediate post‐operative period was not reported and so this information could not be used). | |
| Notes | Attempts were made to contact the authors in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Although the authors state that a placebo was used, they do not state the nature of the placebo or the groups who knew whether the test drug was rFVIIa or placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Although the authors state that a placebo was used, they do not state the nature of the placebo or the groups who knew whether the test drug was rFVIIa or placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Rummo 2010.
| Methods | Randomised clinical trial. | |
| Participants | Country: Belarus. Sample size: 34. Post‐randomisation drop‐out(s): not stated. Revised sample size: 34. Females: not stated. Mean age: 43 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: 34 (100%). Live donor: 0 (0%). Inclusion criteria: 1. Patients undergoing orthotopic liver transplantation. 2. Cadaveric donors. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 17). Further details: thromboelastography to determine transfusion requirements. Group 2: control (n = 17). Further details: standard method to determine transfusion requirements. | |
| Outcomes | The outcomes reported were sepsis, blood loss, and blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Soilleux 1995.
| Methods | Randomised clinical trial. | |
| Participants | Country: France. Sample size: 189. Post‐randomisation drop‐out(s): not stated. Revised sample size: 189. Females: not stated. Mean age: 43.5 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Adult patients undergoing primary orthotopic liver transplantation. Exclusion criteria: 1. Retransplantation | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 94). Further details: aprotinin 2 million KIU bolus on induction followed by continuous infusion of 0.5 million KIU until transfer to ITU. Group 2: control (n = 95). Further details: aprotinin 0.5 million KIU bolus on induction followed by continuous infusion of 150,000 KIU until transfer to ITU. | |
| Outcomes | The outcomes reported were blood transfusion requirements. | |
| Notes | Attempts were made to contact the authors in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computer‐generated list of random numbers, patients were assigned to receive norepinephrine (vasopressor group) or IV fluids and isotonic saline (placebo group) by the research coordinator, who had no part in the clinical management of patients". |
| Allocation concealment (selection bias) | Low risk | Quote: "Using a computer‐generated list of random numbers, patients were assigned to receive norepinephrine (vasopressor group) or IV fluids and isotonic saline (placebo group) by the research coordinator, who had no part in the clinical management of patients". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The blinding of the investigators to isotonic saline or norepinephrine was done by the Department of Pharmacy at the UMDNJ. Blinding was maintained throughout the study period by pharmacists, who had no role in the clinical management of the study patients and data collection. The vasopressor and placebo were both colorless fluids". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The blinding of the investigators to isotonic saline or norepinephrine was done by the Department of Pharmacy at the UMDNJ. Blinding was maintained throughout the study period by pharmacists, who had no role in the clinical management of the study patients and data collection. The vasopressor and placebo were both colorless fluids". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Wang 2010.
| Methods | Randomised clinical trial. | |
| Participants | Country: Taiwan. Sample size: 28. Post‐randomisation drop‐out(s): not stated. Revised sample size: 28. Females: 10 (35.7%). Mean age: 55 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing orthotopic liver transplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 14). Further details: thromboelastography determined decision to transfuse. Group 2: control (n = 14). Further details: standard method to determine decision to transfuse. | |
| Outcomes | The outcomes reported were mortality, blood loss, and transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Williamson 1999.
| Methods | Randomised clinical trial. | |
| Participants | Country: United Kingdom. Sample size: 28. Post‐randomisation drop‐out(s): 3 (10.7%). Revised sample size: 25. Females: 13 (46.4%). Mean age: 49 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Adult patients with liver disease or undergoing liver transplantation (only liver transplantation patients were included in this review). | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 12). Further details: solvent detergent treated fresh frozen plasma. Group 2: control (n = 13). Further details: standard fresh frozen plasma. | |
| Outcomes | The outcomes reported were blood transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. Reason for post‐randomisation drop‐out(s): protocol violation (3). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Assignment was accomplished by the opening of a previously allocated, computer‐generated, randomly numbered envelope". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Assignment was accomplished by the opening of a previously allocated, computer‐generated, randomly numbered envelope". Comment: Further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | High risk | Quote: "Supported in part by Octapharma AG (Vienna, Austria)". |
Yassen 1993.
| Methods | Randomised clinical trial. | |
| Participants | Country: United Kingdom. Sample size: 20. Post‐randomisation drop‐out(s): not stated. Revised sample size: 20. Females: 11 (55%). Mean age: 47.2 years. Piggyback: not stated. Conventional: not stated. Cadaveric donor: not stated. Live donor: not stated. Inclusion criteria: 1. Patients undergoing orthotopic liver transplantation. | |
| Interventions | The patients were randomised to the following groups. Group 1: intervention (n = 10). Further details: tranexamic acid 10 mg/kg bolus dose at the start of anhepatic phase followed by 3 mg/kg/hour until the patient is transferred to ITU. Group 2: control (n = 10). | |
| Outcomes | The outcomes reported were serious adverse effects, blood loss, and transfusion requirements. | |
| Notes | Attempts were made to contact the author in September 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chapman 2006 | Does not include patients undergoing liver transplantation. |
| Grosse 1991 | Not a randomised clinical trial. |
| Grosse 1993 | Not a randomised clinical trial. |
| Ickx 1993a | Not a randomised clinical trial. |
| Kang 1993 | Review. |
| Kufner 2000 | Review. |
| Lentschener 1996 | Letter to editor with reference to included trial. |
| Levy 2008 | Review. |
| Marino 1988 | Not a randomised clinical trial. |
| Meijer 2003 | Not a randomised clinical trial. |
| Molenaar 2002 | Letter to editor with no data from RCT. |
| Neuhaus 1989 | Not a randomised clinical trial. |
| Palareti 1991 | Not a randomised clinical trial. |
| Porte 2001 | Editorial. |
| Porte 2004 | Editorial. |
| Porte 2004a | Review. |
| Porte 2005 | Editorial. |
| Rosea 2000 | Editorial. |
| Sankarankutty 2006 | Not a randomised clinical trial. |
| Segal 2000 | Letter to editor with no data from RCT. |
| Spohr 2002 | Review. |
| Suárez 1993 | Not a randomised clinical trial. |
| Takaya 1995 | Not a randomised clinical trial. |
RCT = randomised clinical trial.
Differences between protocol and review
The risk of bias domains were updated according to The Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Higgins 2011).
Network analysis was performed in addition to routine meta‐analysis.
Fisher's exact test was used when there was only one trial included in the comparison of a binary outcome between two groups.
In the protocol we mentioned that the proportion of patients who developed mortality in the control group will be based on the overall proportion of patients who died in this systematic review. However the trials included included those more than 15 years old and there has been significant decrease in the mortality over this period (ELTR 2011). So, we based our trial sequential calculations based on an approximation of the ELTR data (ELTR 2011).
Contributions of authors
KS Gurusamy wrote the review, assessed the trials for inclusion, extracted data, performed data analysis, and wrote the draft of the review. T Pissanou assessed the trials for inclusion and extracted data independently. H Pikhart helped with the analysis of data. J Vaughan improved the readability of the review and clarified any unclear issues. AK Burroughs and BR Davidson critically commented on the review. All the authors approve of the present review.
Sources of support
Internal sources
Department of Surgery, University College London, UK.
External sources
-
NIHR Cochrane Incentive Scheme, UK.
A grant of £5000 by the UK Government health research funding body (National Insititute of Health Research or NIHR) to perform this review.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Ahn 2008 {published data only}
- Ahn HJ, Yang M, Gwak MS, Koo MS, Bang SR, Kim GS, et al. Coagulation and biochemical effects of balanced salt‐based high molecular weight vs saline‐based low molecular weight hydroxyethyl starch solutions during the anhepatic period of liver transplantation. Anaesthesia 2008;63(3):235‐42. [DOI] [PubMed] [Google Scholar]
Baudo 1992 {published data only}
- Baudo F, DeGasperi A, deCataldo F, Caimi TM, Cattaneo D, Redaelli R, et al. Antithrombin III supplementation during orthotopic liver transplantation in cirrhotic patients: a randomized trial. Thrombosis Research 1992;68(4‐5):409‐16. [DOI] [PubMed] [Google Scholar]
Boylan 1996 {published data only}
- Boylan JF, Klinck JR, Sandler AN, Arellano R, Greig PD, Nierenberg H, et al. Tranexamic acid reduces blood loss, transfusion requirements, and coagulation factor use in primary orthotopic liver transplantation. Anesthesiology 1996;85(5):1043‐8. [DOI] [PubMed] [Google Scholar]
Cottam 1991 {published data only}
- Cottam S, Hunt B, Segal H, Ginsburg R, Potter D. Aprotinin inhibits tissue plasminogen activator‐mediated fibrinolysis during orthotopic liver transplantation. Transplantation Proceedings 1991;23(3):1933. [PubMed] [Google Scholar]
- Hunt B J, Cottam S, Segal H, Ginsburg R, Potter D. Inhibition by aprotinin of tPA‐mediated fibrinolysis during orthotopic liver transplantation. Lancet 1990;336(8711):381. [DOI] [PubMed] [Google Scholar]
Dalmau 2000 {published data only}
- Dalmau A, Sabate A, Acosta F, Garcia Huete L, Koo M, Sansano T, et al. Tranexamic acid reduces red cell transfusion better than epsilon‐ aminocaproic acid or placebo in liver transplantation. Anesthesia and Analgesia 2000;91(1):29‐34. [DOI] [PubMed] [Google Scholar]
- Dalmau A, Sabaté A, Acosta F, Garcia‐Huete L, Koo M, Reche M, et al. Comparative study of antifibrinolytic drugs in orthotopic liver transplantation. Transplantation proceedings 1999;31(6):2361‐2. [DOI] [PubMed] [Google Scholar]
Dalmau 2004 {published data only}
- Dalmau A, Sabaté A, Koo M, Bartolomé C, Rafecas A, Figueras J, et al. The prophylactic use of tranexamic acid and aprotinin in orthotopic liver transplantation: A comparative study. Liver Transplantation 2004;10(2):279‐84. [DOI] [PubMed] [Google Scholar]
Feng 2010 {published data only}
- Feng ZY, Xu X, Zhu SM, Bein B, Zheng SS. Effects of low central venous pressure during preanhepatic phase on blood loss and liver and renal function in liver transplantation. World Journal of Surgery 2010;34(8):1864‐73. [DOI] [PubMed] [Google Scholar]
Findlay 2001 {published data only}
- Findlay JY, Kufner RP. Aprotinin reduces vasoactive medication use during adult liver transplantation. Journal of Clinical Anesthesia 2003;15(1):19‐23. [DOI] [PubMed] [Google Scholar]
- Findlay JY, Rettke SR, Ereth MH, Plevak DJ, Krom RAF, Kufner RP. Aprotinin reduces red blood cell transfusion in orthotopic liver transplantation: A prospective, randomized, double‐blind study. Liver Transplantation 2001;7(9):802‐7. [DOI] [PubMed] [Google Scholar]
Frenette 1998 {published data only}
- Frenette L, Cox J, McArdle P, Eckhoff D, Bynon S. Conjugated estrogen reduces transfusion and coagulation factor requirements in orthotopic liver transplantation. Anesthesia and Analgesia 1998;86(6):1183‐6. [DOI] [PubMed] [Google Scholar]
Garcia‐Huete 1997 {published data only}
- Garcia‐Huete L, Domenech P, Sabate A, MartinezBrotons F, Jaurrieta E, Figueras J. The prophylactic effect of aprotinin on intraoperative bleeding in liver transplantation: A randomized clinical study. Hepatology 1997;26(5):1143‐8. [DOI] [PubMed] [Google Scholar]
- Garcia‐Huete L, Sabate‐Pes A. Aprotinine in orthotopic liver transplantation. Medicina Clinica 1997;108(11):439. [PubMed] [Google Scholar]
- García L, Sabaté A, Domenech P, Martinez Brotons F, Drudis R, Jaurrieta E. Aprotinin in orthotopic liver transplantation. Transplantation Proceedings 1995;27(4):2290‐1. [PubMed] [Google Scholar]
Groh 1993 {published data only}
- Azad SC, Groh J, Welte M, Pratschke E, Kratzer MAA. Effect of aprotinin on transfusion requirements and coagulation parameters in orthotopic liver transplantation (OLT). Annals of Hematology 1992;64(Supplement):A41. [Google Scholar]
- Groh J, Welte M, Azad S C, Anthuber M, Haller M, Kratzer MA. Does aprotinin really reduce blood loss in orthotopic liver transplantation?. Seminars in Thrombosis and Hemostasis 1993;19(3):306‐8. [DOI] [PubMed] [Google Scholar]
- Groh J, Welte M, Azad SC, Forst H, Pratschke E, Kratzer MAA. Does aprotinin affect blood loss in liver transplantation. Lancet 1992;340(8812):173. [DOI] [PubMed] [Google Scholar]
- Kratzer MA, Azad SC, Groh J, Welte M, Haller M, Pratschke E. [The effects of aprotinin. Blood loss and coagulation parameters in orthotopic liver transplantation: A clinical‐experimental, prospective and randomized double‐blind study]. Der Anaesthesist 1997;46(4):294‐302. [DOI] [PubMed] [Google Scholar]
- Welte M, Groh J, Azad S, Anbuther M, Haller M, Kratzer MAA. No beneficial effect of aprotinin on blood loss and coagulation in liver transplantation. Anesthesiology 1992;77(Supplement 3A):A225. [Google Scholar]
- Welte M, Groh J, Azad S, Anthuber M, Haller M, Kratzer MAA. Effect of aprotinin on coagulation parameters in liver transplantation. Seminars in Thrombosis and Hemostasis 1993;19(3):297‐9. [DOI] [PubMed] [Google Scholar]
Hei 2005 {published data only}
- Hei ZQ, Luo CF, Li SR, Ma WH, Luo GJ, Chi XJ. Effect of aprotinin on perioperative requirements of blood and liquid in severe hepatitis patients undergoing liver transplantation. Chinese Pharmaceutical Journal 2005;40(18):1432‐5. [Google Scholar]
Himmelreich 1992 {published data only}
- Bechstein WO, Riess H, Blumhardt G, Himmelreich G, Jochum M, Gerlach H, et al. Aprotinin in orthotopic liver transplantation. Seminars in Thrombosis and Hemostasis 1993;19(3):262‐7. [DOI] [PubMed] [Google Scholar]
- Himmelreich G, Bechstein WO, Jochum M, Gerlach H, Neuhaus P, Riess H. Effect of two different dosages of aprotinin on hemostasis and graft function in orthotopic liver transplantation (OLT). Annals of Hematology 1994;68(Suppl 1):A48. [Google Scholar]
- Himmelreich G, Muser M, Neuhaus P, Bechstein WO, Slama KJ, Jochum M, et al. Different aprotinin applications influencing hemostatic changes in orthotopic liver transplantation. Transplantation 1992;53(1):132‐6. [DOI] [PubMed] [Google Scholar]
Himmelreich 1993 {published data only}
- Himmelreich G, Hundt K, Bechstein WO, Rossaint R, Neuhaus P, Riess H. Influence of prostaglandin E1 infusion on hemostasis in orthotopic liver transplantation. Seminars in Thrombosis and Hemostasis 1993;19(3):273‐8. [DOI] [PubMed] [Google Scholar]
- Himmelreich G, Hundt K, Neuhaus P, Bechstein WO, Roissant R, Riess H. Evidence that intraoperative prostaglandin E1 infusion reduces impaired platelet aggregation after reperfusion in orthotopic liver transplantation. Transplantation 1993;55(4):819‐26. [DOI] [PubMed] [Google Scholar]
Ickx 1993 {published data only}
- Ickx B, Pradier O, Groote F, Hendrice C, Gelin M, Stadt TJ, et al. Does aprotinin really reduce blood loss during orthotopic liver transplantation [abstract]. Hepatology 1993;18(3):740. [Google Scholar]
Ickx 1995 {published data only}
- Ickx B, Pierre S, Pradier O, DeGroote F, Vandestadt J, Gelin M, et al. Comparison of the beneficial effect of aprotinin and tranexamic acid on perioperative blood loss during liver transplantation (abstract). British Journal of Anaesthesia 1995;74(Suppl 1):62. [Google Scholar]
Ickx 2006 {published data only}
- Ickx BE, Linden PJ, Melot C, Wijns W, Pauw L, Vandestadt J, et al. Comparison of the effects of aprotinin and tranexamic acid on blood loss and red blood cell transfusion requirements during the late stages of liver transplantation. Transfusion 2006;46(4):595‐605. [DOI] [PubMed] [Google Scholar]
Kaspar 1997 {published data only}
- Kaspar M, Ramsay MAE, Nguyen AT, Cogswell M, Hurst G, Ramsay KJ. Continuous small‐dose tranexamic acid reduces fibrinolysis but not transfusion requirements during orthotopic liver transplantation. Anesthesia and Analgesia 1997;85(2):281‐5. [DOI] [PubMed] [Google Scholar]
Laine 2003 {published data only}
- Laine E, Steadman R, Calhoun L, Blackall D, Levin P, Braunfeld M, et al. Comparison of RBCs and FFP with whole blood during liver transplant surgery. Transfusion 2003;43(3):322‐7. [DOI] [PubMed] [Google Scholar]
Lassale 1996 {published data only}
- Lassale B, Rathelot P, Angelini B, Bongrand MC, Timon David P. Aprotinin in orthotopic liver transplantation (OLT): Clinical and economical interest in blood requirements. Journal de Pharmacie Clinique 1996;15(3):187‐91. [Google Scholar]
Lodge 2005 {published data only}
- Lodge JP, Jonas S, Jones R M, Olausson M, Mir‐Pallardo J, Soefelt S, et al. Efficacy and safety of repeated perioperative doses of recombinant factor VIIa in liver transplantation. Liver Transplantation 2005;11(8):973‐9. [DOI] [PubMed] [Google Scholar]
- Lodge JPA, Jonas S, Jones RM, Olausson M, Mir‐Pallardo J, Soefelt S, et al. Efficcy and safety of recombinant factor VIIa (RFVIIa) on transfusion reduction in orthotopic liver transplantation (OLT) ‐ a randomised, double‐blind, placebo‐controlled trial. Transplantation 2004;78(2):93. [Google Scholar]
Marcel 1996 {published data only}
- Marcel RJ, Stegall WC, Suit CT, Arnold JC, Vera RL, Ramsay MAE, et al. Continuous small‐dose aprotinin controls fibrinolysis during orthotopic liver transplantation. Anesthesia and Analgesia 1996;82(6):1122‐5. [DOI] [PubMed] [Google Scholar]
Merle 1997 {published data only}
- Merle JC, Cherqui D, Lauzet JY, Malassagne B, Duvoux C, Fagniez PL, et al. Aprotinin significantly reduces blood transfusion requirements in liver transplantation for severe cirrhosis: a double‐blind randomized controlled trial. Anesthesiology 1997;87(3A):A83. [Google Scholar]
Milroy 1995 {published data only}
- Milroy SJ, Cottam S, Tan KC, Hilmi I, Oyesola B. Improved haemodynamic stability with administration of aprotinin during orthotopic liver transplantation. British Journal of Anaesthesia 1995;75(6):747‐51. [DOI] [PubMed] [Google Scholar]
- Segal HC, Hunt BJ, Cottam S, Beard C, Francis J L, Potter D, et al. Changes in the contact system during orthotopic liver transplantation with and without aprotinin. Transplantation 1995;59(3):366‐70. [PubMed] [Google Scholar]
- Segal HC, Hunt BJ, Cottam S, Downing A, Beard C, Francis JL, et al. Fibrinolytic activity during orthotopic liver transplantation with and without aprotinin. Transplantation 1994;58(12):1356‐60. [PubMed] [Google Scholar]
Planinsic 2005 {published data only}
- Planinsic RM, Meer J, Testa G, Grande L, Candela A, Porte RJ, et al. Safety and efficacy of a single bolus administration of recombinant factor VIIa in liver transplantation due to chronic liver disease. Liver Transplantation 2005;11(8):895‐900. [DOI] [PubMed] [Google Scholar]
Ponnudurai 2005 {published data only}
- Ponnudurai RN, Koneru B, Akhtar SA, Wachsberg RH, Fisher A, Wilson DJ, et al. Vasopressor administration during liver transplant surgery and its effect on endotracheal reintubation rate in the postoperative period: a prospective, randomized, double‐blind, placebo‐controlled trial. Clinical Therapeutics 2005;27(2):192‐8. [DOI] [PubMed] [Google Scholar]
Porte 2000 {published data only}
- Molenaar IQ, Begliomini B, Grazi GL, Ringers J, Terpstra OT, Porte RJ. The effect of aprotinin on renal function in orthotopic liver transplantation. Transplantation 2001;71(2):247‐52. [DOI] [PubMed] [Google Scholar]
- Molenaar IQ, Begliomini B, Martinelli G, Putter H, Terpstra OT, Porte RJ. Reduced need for vasopressors in patients receiving aprotinin during orthotopic liver transplantation. Anesthesiology 2001;94(3):433‐8. [DOI] [PubMed] [Google Scholar]
- Molenaar IQ, Legnani C, Groenland TH, Palareti G, Begliomini B, Terpstra OT, et al. Aprotinin in orthotopic liver transplantation: evidence for a prohemostatic, but not a prothrombotic, effect. Liver Transplantation 2001;7(10):896‐903. [DOI] [PubMed] [Google Scholar]
- Porte RJ, Molenaar IQ, Begliomini B, Groenland THN, Januszkiewicz A, Lindgren L, et al. Aprotinin and transfusion requirements in orthotopic liver transplantation: A multicentre randomised double‐blind study. Lancet 2000;355(9212):1303‐9. [DOI] [PubMed] [Google Scholar]
- Porte RJ, Molenaar IQ, Begliomini B, Groenland THN, Januszkiewicz A, Lindgren L, et al. Aprotinin reduces blood loss and transfusion requirements in orthotopic liver transplantation: A placebo‐controlled multicenter study. Transplantation 1999;67(7):S260. [Google Scholar]
Pugliese 2007 {published data only}
- Pugliese F, Ruberto F, Summonti D, Perrella S, Cappannoli A, Tosi A, et al. Activated recombinant factor VII in orthotopic liver transplantation. Transplantation Proceedings 2007;39(6):1883‐5. [DOI] [PubMed] [Google Scholar]
Rummo 2010 {published data only}
- Rummo OO, Shcherba AE, Minou AF, Dzyadzko AM, Avdey EL, Santotski EO, et al. Impact of thromboelastometry on requirement of blood transfusion and incidence of septic complications in deceased donor liver transplantation. Liver Transplantation 2010;16(Suppl 1):S219. [Google Scholar]
Soilleux 1995 {published data only}
- Soilleux H, Gillon MC, Mirand A, Daibes M, Leballe F, Ecoffey C. Comparative effects of small and large aprotinin doses on bleeding during orthotopic liver transplantation. Anesthesia and Analgesia 1995;80(2):349‐52. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang SC, Shieh JF, Chang KY, Chu YC, Liu CS, Loong CC, et al. Thromboelastography‐guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplantation Proceedings 2010;42(7):2590‐3. [DOI] [PubMed] [Google Scholar]
Williamson 1999 {published data only}
- Freeman JW, Williamson LM, Llewelyn C, Fisher N, Allain JP, Bellamy M, et al. A randomized trial of solvent/detergent and standard fresh frozen plasma in the treatment of the coagulopathy seen during orthotopic liver transplantation. Vox Sanguinis 1998;74(Supplement 1):225‐9. [DOI] [PubMed] [Google Scholar]
- Williamson LM, Llewelyn CA, Fisher NC, Allain JP, Bellamy MC, Baglin TP, et al. A randomized trial of solvent/detergent‐treated and standard fresh‐frozen plasma in the coagulopathy of liver disease and liver transplantation. Transfusion 1999;39(11‐12):1227‐34. [DOI] [PubMed] [Google Scholar]
Yassen 1993 {published data only}
- Yassen K, Bellamy MC, Sadek SA, Webster NR. Tranexamic acid reduces blood loss during orthotopic liver transplantation. Clinical Transplantation 1993;7(5):453‐8. [Google Scholar]
References to studies excluded from this review
Chapman 2006 {published data only}
- Chapman WC, Lockstadt H, Singla N, Kafie FE, Lawson J H. Phase 2, randomized, double‐blind, placebo‐controlled, multicenter clinical evaluation of recombinant human thrombin in multiple surgical indications. Journal of Thrombosis and Haemostasis 2006;4(9):2083‐5. [DOI] [PubMed] [Google Scholar]
Grosse 1991 {published data only}
- Grosse H, Lobbes W, Frambach M, Broen O, Ringe B, Barthels M. The use of high dose aprotinin in liver transplantation: the influence on fibrinolysis and blood loss. Thrombosis Research 1991;63(3):287‐97. [DOI] [PubMed] [Google Scholar]
Grosse 1993 {published data only}
- Grosse H, Lobbes W, Frambach M, Ringe B, Barthels M. Influence of high‐dose aprotinin on hemostasis and blood requirement in orthotopic liver transplantation. Seminars in Thrombosis and Hemostasis 1993;19(3):302‐5. [DOI] [PubMed] [Google Scholar]
Ickx 1993a {published data only}
- Ickx B, Pradier O, DeGroote F, Hendrice C, Toungouz M, Vandestadt J, et al. Effect of two different dosages of aprotonin on perioperative blood loss during liver transplantation. Seminars in Thrombosis and Hemostasis 1993;19(3):300‐1. [DOI] [PubMed] [Google Scholar]
Kang 1993 {published data only}
- Kang Y. Clinical use of synthetic antifibrinolytic agents during liver transplantation. Seminars in Thrombosis and Hemostasis 1993;19(3):258‐61. [DOI] [PubMed] [Google Scholar]
Kufner 2000 {published data only}
- Kufner R P. Use of antifibrinolytics in orthotopic liver transplantation. Transplantation Proceedings 2000;32(3):636‐7. [DOI] [PubMed] [Google Scholar]
Lentschener 1996 {published data only}
- Lentschener C, Benhamou D, Cottam S, Milroy SJ. Aprotinin during liver transplantation (3). British Journal of Anaesthesia 1996;76(6):882. [DOI] [PubMed] [Google Scholar]
Levy 2008 {published data only}
- Levy JH. Pharmacologic methods to reduce perioperative bleeding. Transfusion 2008;48(1):31S‐38S. [DOI] [PubMed] [Google Scholar]
Marino 1988 {published data only}
- Marino IR, Weber T, Esquivel CO, Kang YG, Starzl TE, Duquesnoy RJ. Intraoperative blood transfusion requirements and deficient hemostasis in highly alloimmunized patients undergoing liver transplantation. Transplantation Proceedings 1988;20(6):1087‐9. [PMC free article] [PubMed] [Google Scholar]
Meijer 2003 {published data only}
- Meijer K, Hendriks HG, Wolf JT, Klompmaker IJ, Lisman T, Hagenaars AA, et al. Recombinant factor VIIa in orthotopic liver transplantation: influence on parameters of coagulation and fibrinolysis. Blood Coagulation and Fibrinolysis 2003;14(2):169‐74. [DOI] [PubMed] [Google Scholar]
Molenaar 2002 {published data only}
- Molenaar IQ, Porte RJ. Aprotinin and thromboembolism in liver transplantation: Is there really a causal effect?. Anesthesia and Analgesia 2002;94(5):1367‐8. [DOI] [PubMed] [Google Scholar]
Neuhaus 1989 {published data only}
- Neuhaus P, Bechstein WO, Lefèbre B, Blumhardt G, Slama K. Effect of aprotinin on intraoperative bleeding and fibrinolysis in liver transplantation. Lancet 1989;335(8668):924‐5. [DOI] [PubMed] [Google Scholar]
Palareti 1991 {published data only}
- Palareti G, Legnani C, Maccaferri M, Gozzetti G, Mazziotti A, Martinelli G, et al. Coagulation and fibrinolysis in orthotopic liver transplantation: role of the recipient's disease and use of antithrombin III concentrates. S. Orsola Working Group on Liver Transplantation. Haemostasis 1991;21(2):68‐76. [DOI] [PubMed] [Google Scholar]
Porte 2001 {published data only}
- Porte RJ, Slooff MJH. Aprotinin: Safe and effective in all patients undergoing orthotopic liver transplantation?. Liver Transplantation 2001;7(9):808‐10. [DOI] [PubMed] [Google Scholar]
Porte 2004 {published data only}
- Porte RJ. Antifibrinolytics in liver transplantation: They are effective, but what about the risk‐benefit ratio?. Liver Transplantation 2004;10(2):285‐8. [DOI] [PubMed] [Google Scholar]
Porte 2004a {published data only}
- Porte RJ, Hendriks HG, Slooff MJ. Blood conservation in liver transplantation: The role of aprotinin. Journal of Cardiothoracic and Vascular Anesthesia 2004;18(4 Suppl):31S‐37S. [DOI] [PubMed] [Google Scholar]
Porte 2005 {published data only}
- Porte RJ, Caldwell SH. The role of recombinant factor VIIa in liver transplantation. Liver Transplantation 2005;11(8):872‐4. [DOI] [PubMed] [Google Scholar]
Rosea 2000 {published data only}
- Rosea E. Liver transplantation, the problem of peroperative bleeding. Presse Medicale 2000;29(23):1289‐90. [PubMed] [Google Scholar]
Sankarankutty 2006 {published data only}
- Sankarankutty AK, Teixeira AC, Souza FF, Mente ED, Oliveira GR, Almeida RC, et al. Impact of blood salvage during liver transplantation on reduction in transfusion requirements. Acta Cirurgica Brasileira 2006;21(Suppl 1):44‐7. [DOI] [PubMed] [Google Scholar]
Segal 2000 {published data only}
- Segal H, Hunt BJ. Aprotinin: pharmacological reduction of perioperative bleeding. Lancet 2000;355(9212):1289‐90. [DOI] [PubMed] [Google Scholar]
Spohr 2002 {published data only}
- Spohr F, Bottiger BW. Measures for reducing the use of blood transfusions. Anaesthesist 2002;51(3):221‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suárez 1993 {published data only}
- Suárez M, Sangro B, Herrero JI, Picardi A, Páramo JA, Quiroga J, et al. Effectiveness of aprotinin in orthotopic liver transplantation. Seminars in Thrombosis and Hemostasis 1993;19(3):292‐6. [DOI] [PubMed] [Google Scholar]
Takaya 1995 {published data only}
- Takaya S, Doyle H, Todo S, Irish W, Fung JJ, Starzl TE. Reduction of primary nonfunction with prostaglandin E1 after clinical liver transplantation. Transplantation Proceedings 1995;27(2):1862‐7. [PMC free article] [PubMed] [Google Scholar]
Additional references
Bismuth 1987
- Bismuth H, Castaing D, Ericzon BG, Otte JB, Rolles K, Ringe B, et al. Hepatic transplantation in Europe. First Report of the European Liver Transplant Registry. Lancet 1987;2(8560):674‐6. [DOI] [PubMed] [Google Scholar]
Boin 2008
- Boin IF, Leonardi MI, Luzo AC, Cardoso AR, Caruy CA, Leonardi LS. Intraoperative massive transfusion decreases survival after liver transplantation. Transplantation Proceedings 2008;40(3):789‐91. [DOI] [PubMed] [Google Scholar]
Boyd 2007
- Boyd SD, Stenard F, Lee DK, Goodnough LT, Esquivel CO, Fontaine MJ. Alloimmunization to red blood cell antigens affects clinical outcomes in liver transplant patients. Liver Transplantation 2007;13(12):1654‐61. [DOI] [PubMed] [Google Scholar]
Calne 1979
- Calne RY, Smith DP, McMaster P, Craddock GN, Rolles K, Farman JV, et al. Use of partial cardiopulmonary bypass during the anhepatic phase of orthotopic liver grafting. Lancet 1979;2(8143):612‐4. [DOI] [PubMed] [Google Scholar]
Cescon 2006
- Cescon M, Grazi GL, Grassi A, Ravaioli M, Vetrone G, Ercolani G, et al. Effect of ischemic preconditioning in whole liver transplantation from deceased donors. A pilot study. Liver Transplantation 2006;12(4):628‐35. [DOI] [PubMed] [Google Scholar]
Corno 2006
- Corno V, Colledan M, Dezza MC, Guizzetti M, Lucianetti A, Maldini G, et al. Extended right split liver graft for primary transplantation in children and adults. Transplantation International 2006;19(6):492‐9. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Diprose 2005
- Diprose P, Herbertson MJ, O'Shaughnessy D, Deakin CD, Gill RS. Reducing allogeneic transfusion in cardiac surgery: A randomized double‐blind placebo‐controlled trial of antifibrinolytic therapies used in addition to intra‐operative cell salvage. British Journal of Anaesthesia 2005;94(3):271‐8. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
ELTR 2011
- ELTR. European liver transplant registry. Evolution of 93,364 liver transplantations in Europe. http://www.eltr.org/spip.php?article152 2011 (accessed on 29th August 2011).
Fergusson 2008
- Fergusson DA, Hebert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, et al. A comparison of aprotinin and lysine analogues in high‐risk cardiac surgery. New England Journal of Medicine 2008;358(22):2319‐31. [DOI] [PubMed] [Google Scholar]
Gluud 2011
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2011, Issue 2. Art. No.: LIVER.
Gurusamy 2009a
- Gurusamy KS, Li J, Sharma D, Davidson BR. Cardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007338] [DOI] [PubMed] [Google Scholar]
Gurusamy 2009b
- Gurusamy KS, Li J, Sharma D, Davidson BR. Pharmacological interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008085] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2009c
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. The British Journal of Surgery 2009;96(4):342‐9. [DOI] [PubMed] [Google Scholar]
Gurusamy 2011a
- Gurusamy KS, Pamecha V, Davidson BR. Piggy‐back graft for liver transplantation. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008258] [DOI] [PubMed] [Google Scholar]
Gurusamy 2011b
- Gurusamy KS, Koti R, Pamecha V, Davidson BR. Veno‐venous bypass versus none for liver transplantation. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD007712] [DOI] [PubMed] [Google Scholar]
Hendriks 2005
- Hendriks HG, Meer J, Wolf JT, Peeters PM, Porte RJ, Jong K, et al. Intraoperative blood transfusion requirement is the main determinant of early surgical re‐intervention after orthotopic liver transplantation. Transplant International 2005;17(11):673‐9. [DOI] [PubMed] [Google Scholar]
Henry 2011
- Henry DA, Carless PA, Moxey AJ, O'Connell D, Stokes BJ, Fergusson DA, et al. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD001886.pub4] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Colloboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Jabbour 2005
- Jabbour N, Gagandeep S, Mateo R, Sher L, Genyk Y, Selby R. Transfusion free surgery: single institution experience of 27 consecutive liver transplants in Jehovah's Witnesses. Journal of the American College of Surgeons 2005;201(3):412‐7. [DOI] [PubMed] [Google Scholar]
Jeon 2010
- Jeon H, Lee SG. Living donor liver transplantation. Current Opinion in Organ Transplantation 2010;15(3):283‐7. [DOI] [PubMed] [Google Scholar]
Kamath 2001
- Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology 2001;33(2):464‐70. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Koneru 2005
- Koneru B, Fisher A, He Y, Klein KM, Skurnick J, Wilson DJ, et al. Ischemic preconditioning in deceased donor liver transplantation: a prospective randomized clinical trial of safety and efficacy. Liver Transplantation 2005;11(2):196‐202. [DOI] [PubMed] [Google Scholar]
Lim 2006
- Lim SG, Wai CT, Costa M, Sutedja DS, Lee YM, Lee KH, et al. Referral patterns and waiting times for liver transplantation in Singapore. Singapore Medical Journal 2006;47(7):599‐603. [PubMed] [Google Scholar]
Liu 2008
- Liu CM, Chen J, Wang XH. Requirements for transfusion and postoperative outcomes in orthotopic liver transplantation: a meta‐analysis on aprotinin. World Journal of Gastroenterology 2008;14(9):1425‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Massicotte 2005
- Massicotte L, Sassine MP, Lenis S, Seal RF, Roy A. Survival rate changes with transfusion of blood products during liver transplantation. Canadian Journal of Anaesthesia 2005;52(2):148‐55. [DOI] [PubMed] [Google Scholar]
Massicotte 2006
- Massicotte L, Lenis S, Thibeault L, Sassine MP, Seal RF, Roy A. Effect of low central venous pressure and phlebotomy on blood product transfusion requirements during liver transplantations. Liver Transplantation 2006;12(1):117‐23. [DOI] [PubMed] [Google Scholar]
MHRA 2007
- MHRA. Aprotinin (Trasylol): Suspension of UK marketing authorisations (licences). http://www.mhra.gov.uk/Safetyinformation/Safetywarningsalertsandrecalls/Safetywarningsandmessagesformedicines/CON2033201 2007 (accessed 3rd September 2011).
Mills 2011
- Mills EJ, Bansback N, Ghement I, Thorlund K, Kelly S, Puhan MA, et al. Multiple treatment comparison meta‐analyses: a step forward into complexity. Clinical Epidemiology 2011;3:193‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Molenaar 2007
- Molenaar IQ, Warnaar N, Groen H, Tenvergert EM, Slooff MJ, Porte RJ. Efficacy and safety of antifibrinolytic drugs in liver transplantation: a systematic review and meta‐analysis. American Journal of Transplantation 2007;7(1):185‐94. [DOI] [PubMed] [Google Scholar]
Murphy 2004
- Murphy GJ, Allen SM, Unsworth‐White J, Lewis CT, Dalrymple‐Hay MJ. Safety and efficacy of perioperative cell salvage and autotransfusion after coronary artery bypass grafting: A randomized trial. Annals of Thoracic Surgery 2004;77(5):1553‐9. [DOI] [PubMed] [Google Scholar]
Nardo 2005
- Nardo B, Bertelli R, Montalti R, Beltempo P, Puviani L, Pacile V, et al. Red blood cell transfusion in liver transplantation: a case‐control study. Transplantation Proceedings 2005;37(10):4389‐92. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
OPTN/SRTR 2009
- OPTN/SRTR. Welcome to the 2009 OPTN / SRTR annual report: Transplant data 1999‐2008. http://www.ustransplant.org/annual_reports/current/default.htm 2009 (accessed on 7th March 2011).
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pivalizza 2003
- Pivalizza EG, Warters RD, Gebhard R. Desmopressin before liver transplantation. Canadian Journal of Anaesthesia 2003;50(7):748‐9. [DOI] [PubMed] [Google Scholar]
Ramos 2003
- Ramos E, Dalmau A, Sabate A, Lama C, Llado L, Figueras J, et al. Intraoperative red blood cell transfusion in liver transplantation: influence on patient outcome, prediction of requirements, and measures to reduce them. Liver Transplantation 2003;9(12):1320‐7. [DOI] [PubMed] [Google Scholar]
Ray 2008
- Ray WA, Stein CM. The aprotinin story ‐ is BART the final chapter?. New England Journal of Medicine 2008;358(22):2398‐400. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Salanti 2008
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Statistical Methods in Medical Research 2008;17(3):279‐301. [DOI] [PubMed] [Google Scholar]
SAS 9.2 [Computer program]
- SAS Institute Inc. SAS 9.2. Cary, NC, USA.: SAS Institute Inc, 2008.
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Shiffman 2006
- Shiffman ML, Saab S, Feng S, Abecassis MI, Tzakis AG, Goodrich NP, et al. Liver and intestine transplantation in the United States, 1995‐2004. American Journal of Transplantation 2006;6(5 Pt 2):1170‐87. [DOI] [PubMed] [Google Scholar]
StatsDirect 2.7 [Computer program]
- StatsDirect Ltd. StatsDirect statistical software. Version 2.7.8. StatsDirect Ltd, 2010.
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. Trial sequential analysis (TSA). http://ctu.dk/tsa/downloads.aspx 2011.
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- Copenhagen Trial Unit. TSA viewer. Version 0.9 Beta. Copenhagen Trial Unit, 2011.
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


