Abstract
Herein we reported the synthesis and evaluation of novel analogues of UK-5099 both in vitro and in vivo for the development of mitochondrial pyruvate carrier (MPC) inhibitors to treat hair loss. A comprehensive understanding of the structure–activity relationship was obtained by varying four positions of the hit compound, namely, the alkyl group on the N1 position, substituents on the indole core, various aromatic and heteroaromatic core structures, and various Michael acceptors. The major discovery was that the inhibitors with a 3,5-bis(trifluoromethyl)benzyl group at the N1 position were shown to have much better activity than JXL001 (UK-5099) to increase cellular lactate production. Additionally, analogue JXL069, possessing a 7-azaindole heterocycle, was also shown to have significant MPC inhibition activity, which further increases the chemical space for drug design. Finally, more than ten analogues were tested on shaved mice by topical treatment and promoted obvious hair growth on mice.
Graphical Abstract
INTRODUCTION
Hair loss, also known as alopecia or baldness, is a very common health problem. Fifty percent of males and twenty-five percent of females by age 50 suffer pattern hair loss.1 Common types of hair loss include male-pattern hair loss, female-pattern hair loss, alopecia areata, and a thinning of hair known as telogen effluvium.1 Minoxidil (Rogaine) and finasteride (Propecia) are the two FDA-approved medicines to treat hair loss but only 30 to 40 percent of patients experience hair growth with the requirement of repeated treatment and serious side effects have been reported.2 Surgical procedures to treat baldness are expensive and can be painful. Therefore, a better therapy for hair loss is highly desired.
Hair follicle growth occurs in cycles. Each cycle consists of a long growing phase (anagen), a short transitional phase (catagen), and a short resting phase (telogen).3 The ability of the hair follicles to maintain this cycle depends on the presence of hair follicle stem cells (HFSCs).4 The HFSCs are typically inactive but can quickly “wake up” and actively divide when a new hair growth cycle is initiated.5,6 When HFSCs fail to activate, hair loss occurs. Our Collaborators, Lowry, Christofk and coworkers discovered that the activation of HFSCs can be achieved by stimulating the activity of lactate dehydrogenase (LDH), the enzyme catalyzing the reduction of pyruvate to lactate.7 Genetic deletion of MPC or pharmacological inhibition of MPC via treatment with UK-5099 resulted in increased LDH activity in HFSCs.7 When the MPC is knocked down or pharmacologically inhibited, pyruvate in the cytoplasm cannot enter the mitochondria and instead is converted to other metabolites, such as lactate by lactate dehydrogenase (LDH), which results in the increase of LDH activity and the activation of HFSCs. Overall, our collaborators discovered a novel mechanism to promote hair growth and MPC has been proven to be a promising therapeutic target for the treatment of hair loss.
MPC, located on the inner mitochondrial membrane, is a transporter protein that transports pyruvate from the cytoplasm to the mitochondria. Although great efforts have been taken to study MPC, its molecular structure is still not clear.8,9 The MPC protein family consists of three members, MPC1, MPC2, and MPC3. The MPC1-MPC2 complex, an oligomeric structure of about 150 kD, is an essential component of the mitochondrial pyruvate carrier in yeast, flies, and mammals.9 UK-5099 is a known covalent inhibitor of MPC by reversibly reacting with a cysteine on the protein with a Ki value of 0.05 μM.10 UK-5099 has been reported to promote lactate production rate as a result of MPC inhibition in various settings.11,12 Our collaborators, Lowry, Christofk and coworkers, demonstrated that topical treatment of mice in telogen phase with UK-5099 (20 μM) led to a robust acceleration of the hair cycle.7 Therefore, UK-5099 is a good hit compound for developing novel MPC inhibitors as a treatment of hair loss.
Herein we describe an extensive structure–activity relationship (SAR) study of a series of (E)-2-cyano-3-(1H-indol-3-yl)acrylic acids and their structural analogues. We have developed a general synthetic route to these compounds that allows one to prepare many analogues rapidly. All of these new compounds have been tested for their ability to promote cellular lactate production in vitro. The compounds that enhanced cellular lactate production rate were then tested on mice topically for their ability to accelerate hair growth. We have identified more than ten compounds with potent MPC inhibitory activity, and the ability to promote hair growth on mice.
RESULTS AND DISCUSSION
Chemistry.
The synthesis of the analogues, which are labeled JXL001–JXL096, was carried out as shown in the various schemes. The synthesis of JXL001 is shown in Scheme 1. Copper-catalyzed N-arylation of indole-3-carboxaldehyde 1 with iodobenzene followed by Knoevenagel condensation with ethyl 2-cyanoacetate provided the ester version of UK-5099, JXL004. Finally, aqueous LiOH mediated saponification delivered the desired compound JXL001. Following the same synthetic route with substituted indole-3-carboxaldehydes and substituted iodobenzenes, various analogues were synthesized rapidly (Table 1).
Scheme 1.
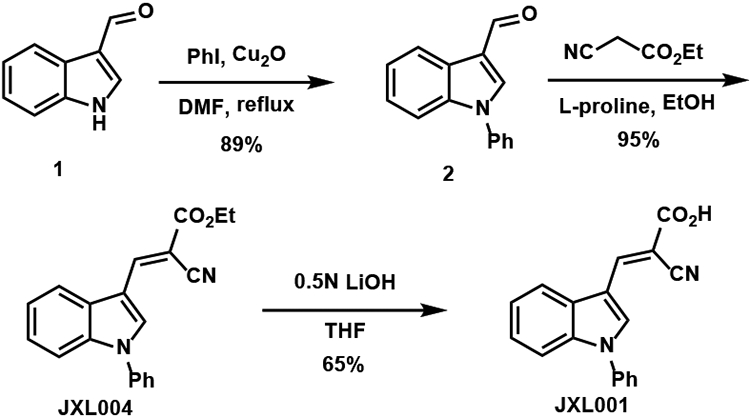
Synthesis of JXL001.
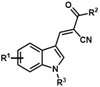
Table 1.
Analogues synthesized by following Scheme 1.
| |||
|---|---|---|---|
| Analogue | R1 | R2 | R3 |
| JXL001 | H | OH | Ph |
| JXL002 | H | OH | H |
| JXL003 | H | OEt | H |
| JXL004 | H | OEt | Ph |
| JXL005 | 6-Cl | OEt | Ph |
| JXL006 | H | OEt | 2-OMeC6H4 |
| JXL007 | H | OEt | 4-OMeC6H4 |
| JXL012 | H | OH | 4-OMeC6H4 |
| JXL013 | 6-Cl | OH | Ph |
| JXL014 | H | OH | 2-OMeC6H4 |
| JXL026 | 4-Cl | OH | Ph |
| JXL027 | 6-F | OH | Ph |
| JXL028 | 7-Cl | OH | Ph |
| JXL029 | 5-F | OH | Ph |
| JXL035 | 4-Cl | OH | Ph |
| JXL093 | 4-Cl | OEt | Ph |
Analogue JXL020 was synthesized by the synthetic route shown in Scheme 2. Benzylation of indole-3-carboxaldehyde 1 with 3,5-bis(trifluoromethyl)benzyl bromide generated compound 3 in good yield. Knoevenagel condensation with ethyl 2-cyanoacetate provided JXL011. Finally, aqueous LiOH mediated saponification afforded analogue JXL020. When substituted indole-3-carboxaldehydes and various benzyl bromides were used, various analogues were synthesized rapidly by following the same synthetic route as for JXL020. The analogues are listed in Table 2. Based on the result of our SAR study, JXL020, the analogues with the 3,5-bis(trifluoromethyl)benzyl group at the N1 position were shown to have much better activity than JXL001 in promoting cellular lactate production. A series of analogues with a 3,5-bis(trifluoromethyl)benzyl group at the N1 position were synthesized by following a similar synthetic route as shown for JXL020 (Table 3).
Scheme 2.
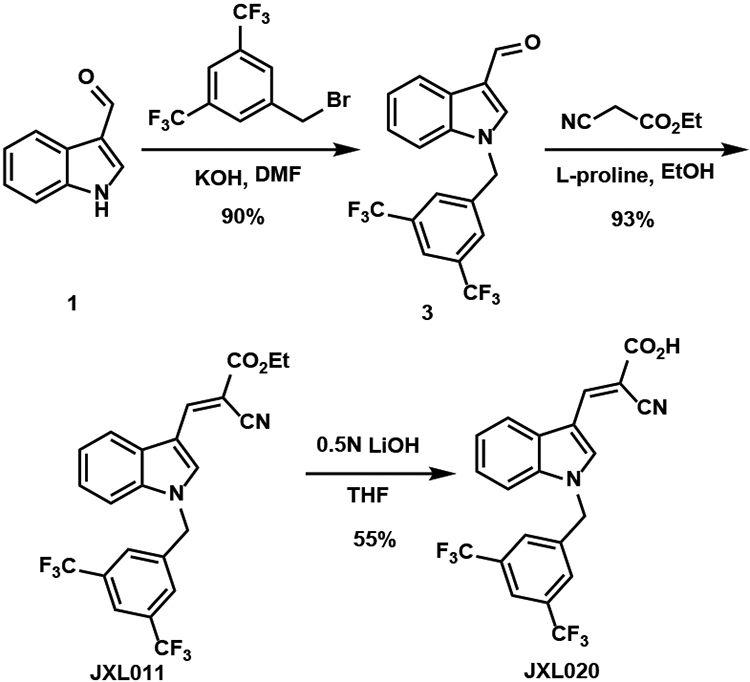
Synthesis of JXL020.
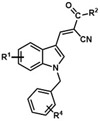
Table 2.
Analogues synthesized by following Scheme 2.
| |||
|---|---|---|---|
| Analogue | R1 | R2 | R4 |
| JXL008 | H | OEt | 4-F |
| JXL009 | H | OEt | 3,4-di-F |
| JXL010 | H | OEt | 3,5-di-F |
| JXL011 | H | OEt | 3,5-bis(CF3) |
| JXL015 | H | OH | 4-F |
| JXL016 | H | OH | 3,4-di-F |
| JXL017 | H | OH | 3,5-di-F |
| JXL018 | H | OEt | H |
| JXL019 | H | OH | H |
| JXL020 | H | OH | 3,5-bis(CF3) |
| JXL036 | 5-F | OH | 3,4-di-F |
| JXL037 | 5-F | OH | 3,5-di-F |
| JXL039 | 6-F | OH | 3,4-di-F |
| JXL040 | 6-F | OH | 3,5-di-F |
Table 3.
Analogues with 3,5-bis(trifluoromethyl)benzyl group at the N1 position.
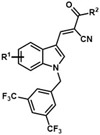
| |||||
|---|---|---|---|---|---|
| Analogue | R1 | R2 | Analogue | R1 | R2 |
| JXL038 | 5-F | OH | JXL062 | 6-Br | OH |
| JXL041 | 6-F | OH | JXL063 | 6-Cl | OH |
| JXL050 | 4-Cl | OH | JXL064 | 7-Cl | OH |
| JXL051 | 4-Br | OH | JXL065 | 4-Br | OEt |
| JXL052 | 4-F | OH | JXL066 | 4-F | OEt |
| JXL053 | 7-F | OH | JXL068 | H | Ot-Bu |
| JXL054 | 5-Cl | OH | JXL078 | H | OMe |
| JXL055 | 4-CN | OH | JXL081 | H | morpholine |
| JXL056 | 4-CO2H | OH | JXL089 | 5-Cl | OEt |
| JXL057 | 4-OBn | OH | JXL090 | 4-CN | OEt |
| JXL058 | 6-OBn | OH | JXL091 | 4-CO2Me | OEt |
| JXL059 | 7-OBn | OH | JXL092 | 4-CO2Me | OH |
| JXL060 | 4-OMe | OH | JXL094 | H | NH2 |
| JXL061 | 5-Br | OH | |||
The analogue JXL079 with a substituted homobenzyl group at the N1 position was synthesized as shown in Scheme 3a. The homobenzyl mesylate 7 was synthesized from the commercially available compound 3,5-bis(trifluoromethyl)benzyl bromide by nucleophilic addition of its derived Grignard reagent to carbon dioxide followed by DIBAL reduction and mesylation. Nucleophilic substitution of compound 7 with the indole 8 generated compound 9. Upon treatment with trifluoroacetic acid, the t-butyl group was cleaved to form the desired analogue JXL079. The amide analogue JXL080 was synthesized by a DCC coupling reaction of JXL003 and compound 6 (Scheme 3b).
Scheme 3.
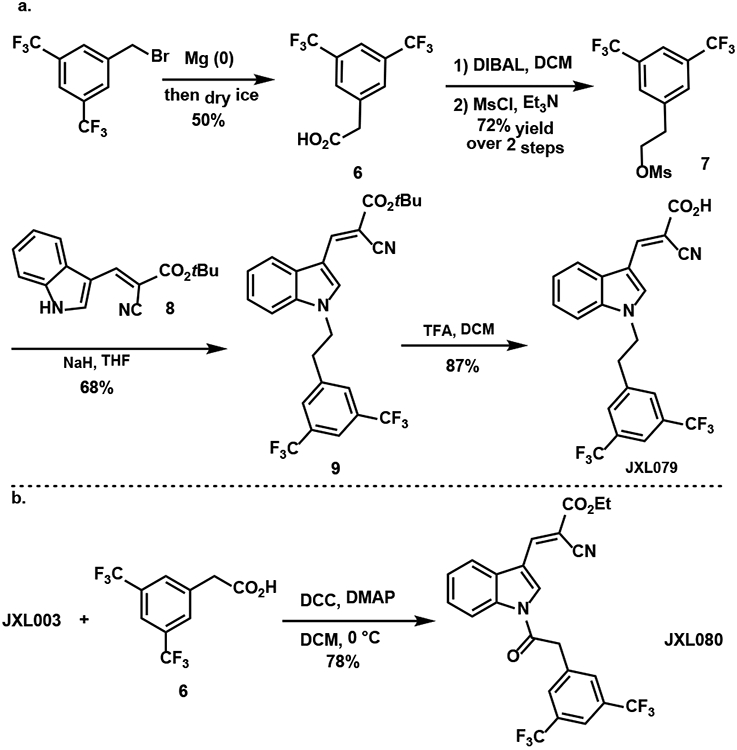
Synthesis of JXL079 and JXL080.
In addition to analogues with the indole core, analogues with other aromatic and heteroaromatic cores were also synthesized. 4-Pyridinecarboxaldehyde and substituted benzaldehydes were used to synthesize analogues following a synthetic route similar to that used for JXL001 (Scheme 1). The analogues are listed in Table 4. Some analogues with nitrogen-containing heterocycles were also synthesized using a synthetic route similar to that used for JXL069 in Scheme 4. Benzylation of 7-azaindole 4 with KOH as the base generated compound 5 with good yield and regioselectivity. Vilsmeier-Haack reaction followed by Knoevenagel condensation with ethyl 2-cyanoacetate provided JXL082. Finally, aqueous LiOH mediated saponification furnished the analogue JXL069. Analogues with a 4-azaindole, a 6-azaindole, and a substituted 7-azaindole core were successfully synthesized and are listed in Table 5. The Vilsmeier-Haack reaction failed to generate the 3-carboxyaldehydes of 4-azaindole and 6-azaindole, so the formylation was carried out using hexamethylenetetramine (HMTA) in acetic acid (Duff reaction).
Table 4.
Analogues with benzene core and JXL022.

|
|||
|---|---|---|---|
| Analogue | R5 | Analogue | R5 |
| JXL030 | 2-F | JXL045 | 3-CF3, 4-CF3 |
| JXL032 | 4-F | JXL046 | 2-Cl, 3-CF3 |
| JXL034 | 4-CF3 | JXL047 | 3-CF3, 4-F |
| JXL042 | 3-F 4-Me | JXL048 | H |
| JXL043 | 3-F, 4-F | JXL049 | 4-OH |
| JXL044 | 2-F, 4-F | ||
Scheme 4.
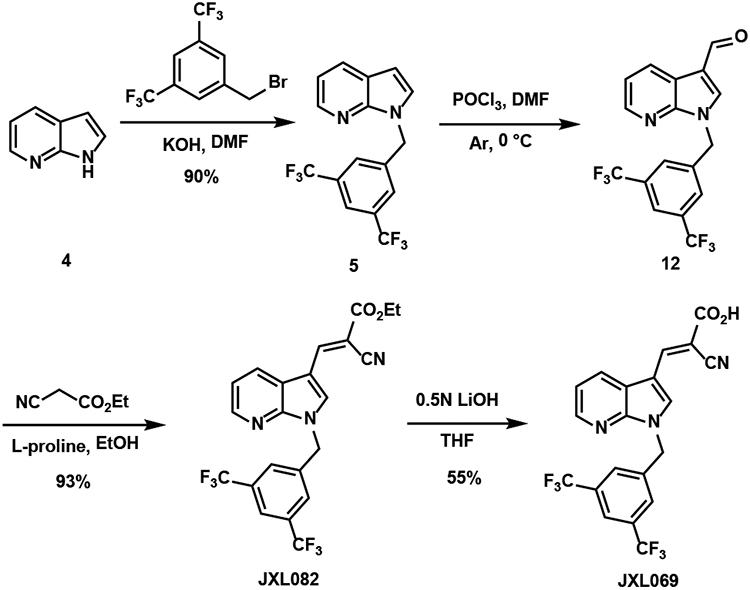
Synthesis of JXL069.
Table 5.
Analogues with azaindole cores.
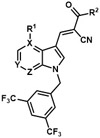
| |||||
|---|---|---|---|---|---|
| Analogue | X | Y | Z | R1 | R2 |
| JXL069 | C | CH | N | H | OH |
| JXL072 | N | CH | CH | N/A | OH |
| JXL073 | C | N | CH | H | OH |
| JXL076 | C | CH | N | Cl | OH |
| JXL077 | C | CH | N | Br | OH |
| JXL082 | C | CH | N | H | OEt |
| JXL087 | C | CH | N | Cl | OEt |
| JXL088 | C | CH | N | Br | OEt |
The analogues with substituents at the 3-position of the acrylates, JXL083, JXL084 and JXL085, were synthesized as shown in Scheme 5. The aldehyde 3 was oxidized by a Pinnick oxidation to the carboxylic acid 10, which was further converted to the acyl chloride 11. Deprotonation of ethyl 2-cyanoacetate by LDA and nucleophilic addition to compound 11 generated analogue JXL083. Analogue JXL084 was synthesized by acetylation of JXL083 and JXL085 was synthesized by chlorination of JXL083.
Scheme 5.
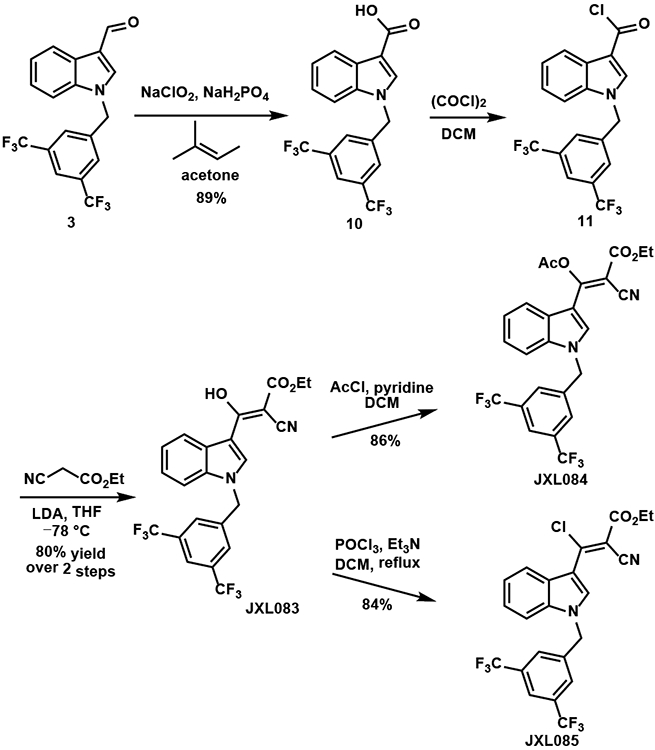
Synthesis of JXL083, JXL084 and JXL085.
Analogues with various Michael acceptors were synthesized by Knoevenagel condensations (Table 6). The bis-nitrile JXL021 was synthesized by the condensation with malononitrile and JXL025 was synthesized by the condensation with diethyl malonate followed by LiOH mediated mono-saponification. JXL086 and JXL095 were generated by the condensation of aldehydes 3 and 12, respectively, with diethyl (cyanomethyl)phosphonate. JXL096 was synthesized from JXL086 by trimethylsilyl bromide (TMSBr) mediated cleavage of the ethyl groups. Analogues with thiazolidinedione (TZD) structures were synthesized similarly as JXL024. Condensation of various aldehydes with either thiazolidine-2,4-dione or 2-iminothiazolidin-4-one in reflux acetic acid afforded the analogues listed in Table 7.
Biological Evaluation. SAR Analysis.
When the MPC is inhibited, pyruvate in the cytoplasm cannot enter the mitochondria and instead is converted to other metabolites, such as lactate by lactate dehydrogenase (LDH). Since our collaborators previously found that LDH activity correlated with hair follicle stem cell activation, and genetic deletion of LDH activity via LDHa knockout in hair follicle stem cells blocked their activation,7 we were particularly interested in using rerouting of pyruvate towards lactate production as a readout for MPC inhibition in this study. The use of cell-based lactate production as a readout of MPC inhibition was a practical first level read-out for screening new compounds, but that it is certainly not synonymous with MPC inhibition. Our hit compound, UK-5099, is known as a covalent inhibitor. In order to confirm its mechanism of action, the analogue of UK-5099 without the alkene double bond was synthesized and tested in the cell-based assay. the reduced UK-5099 showed no effect on lactate production rate, meaning that it did not inhibit MPC (data was not shown). This result supports that UK-5099 is a covalent inhibitor.
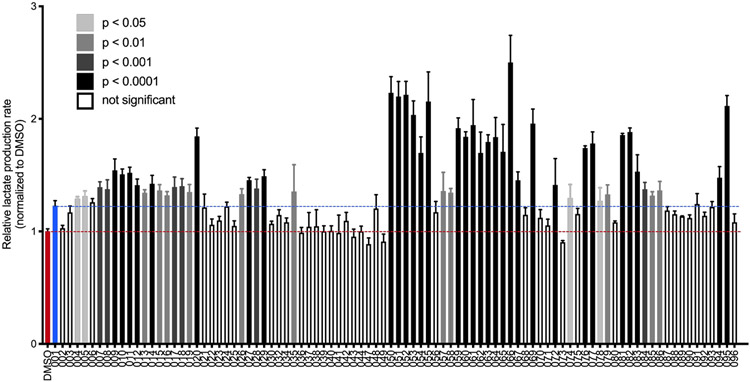
A dose-response experiment of the commercial UK-5099 and in house prepared JXL001 with MCF10A cells was conducted to confirm their inhibition of MPC by measuring the cells’ lactate production rate (data not shown). This result indicated that both samples promoted lactate production in a dose-dependent pattern. Because both samples provided the highest lactate production rate at 10 μM and the same concentration of UK5099 was used in the earlier in vivo study to detect an effect on hair growth7, a concentration of 10 μM was chosen for the evaluation of the activity of the analogues to promote cellular lactate production. The data of the in vitro test is shown in Figure 3.
Figure 3. In vitro evaluation of analogues of JXL001–JXL096.
Human breast epithelial (MCF10A) cells were treated with 10 μM analogue for 24-30 hours and cellular lactate production rate was measured, relative to vehicle (DMSO) treatment and normalized to cell number. Data were compiled from sequential batches of experiments, each with their own DMSO controls and JXL001 and JXL020 treatments for comparison. Error bars indicate +1 s.d. Representative experiments are shown, with most analogues tested at least twice. Shading indicates adjusted p-values calculated by performing ordinary one-way ANOVA followed by Dunnett's post-test, comparing each analogue to DMSO.
Here we used the (E)-2-cyano-3-(1-phenyl-1H-indol-3-yl)acrylic acid (JXL001) as a reference compound and de-signed new compounds in order to vary the four most easily altered positions, namely: (1) the alkyl group at N1, (2) the substituents on the indole core, (3) various aromatic and heteroaromatic cores, and (4) various Michael acceptors. Our goal was to identify candidates for the treatment of hair loss with significant improvement in potency.
Analogues at the N1 Position (R3).
We envisioned that modifying the N-phenyl group would be a good starting point for conducting an SAR by medicinal chemistry. Several N-aryl substituted analogues are listed in Table 1. Analogue JXL002 without the phenyl group showed little inhibitory effect on MPC, implying that the phenyl group is important for UK-5099’s biological activity. Similarly, compound JXL003 was worse than JXL004 in term of stimulating lactate production. Analogues with N-2-methoxyphenyl and N-4-methoxyphenyl groups (JXL006 and JXL007, esters; JXL014 and JXL012, carboxylic acids) showed similar or slightly better activity than JXL001 to promote the cellular lactate production rate. Due to the harsh reaction conditions and low reaction yields for the N-arylation step, we switched our attention to analogues with N-benzyl groups.
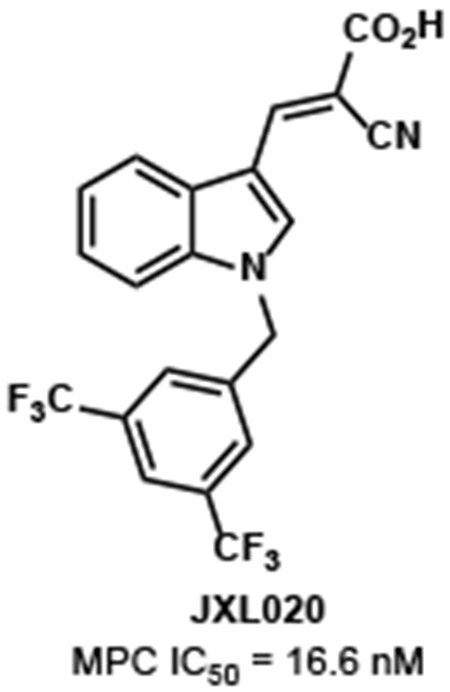
We then investigated the N-benzyl analogues, because the homologated analogues possess high structural similarity with JXL001. A series of analogues with different benzyl groups were synthesized and tested (Table 2). Although most of these analogues showed better activity in increasing lactate production rate, the analogue with the 3,5-bis(trifluoromethyl)benzyl group, JXL020, was shown to have the highest ability to inhibit MPC. JXL079, the homologue of JXL020, was shown to have lower potency than JXL020 in inhibition of MPC. Additionally, the amide JXL080 did not promote the cellular lactate production. Up to this point, the 3,5-bis(trifluoromethyl)benzyl group was the best moiety at the N1-position of the indole core.
Analogues with substituents on the indole ring.
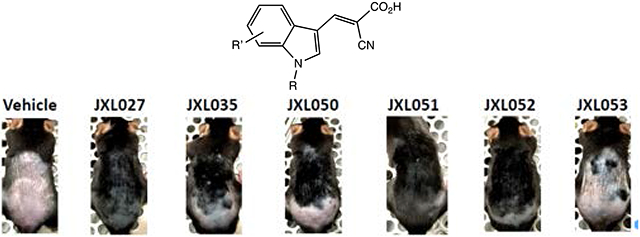
Taking JXL020 as the new hit compound, the influence of substituents on the indole core was explored (the analogues are listed in Table 3). Comparing the four analogues containing 4-F, 5-F, 6-F and 7-F (JXL052, JXL038, JXL041 and JXL053) with JXL020, JXL052 (the 4-F analogue) and JXL053 (the 7-F analogue) showed better activity than JXL020 to increase lactate production rate, while the other two analogues could hardly promote the lactate production. For chloro-substituted analogues (JXL 050, JXL054, JXL063 and JXL064), only the 4-Cl analogue (JXL050) showed better activity than JXL020 to increase the lactate production rate. Similarly, the analogue with 4-Br (JXL051) was better than JXL020, while analogues with 5-Br and 6-Br were worse than JXL020. Among the three benzyloxy-substituted indole analogues (JXL057, JXL058 and JXL059), only the 7-OBn analogue JXL059 showed better activity than JXL020 but the 4-OBn analogue failed to increase its biological activity in promoting lactate production rate, probably due to the steric effect of the large substituent. Based on the analysis, more analogues with small substituents at 4-position of indole were synthesized and evaluated. The analogue with 4-CN (JXL55) was better than JXL020 and the analogue with 4-OMe (JXL060) was similar to JXL020 in terms of promoting lactate production. The analogue with 4-COOH (JXL56) showed much worse activity than JXL020 in inhibition of MPC, perhaps due to its high polarity which may make it hard to penetrate the cell membrane. Based on this data, it seems that large substituents on the indole ring are harmful for the inhibitory activity for MPC and small substituents at the 4-position are beneficial for the desired activity.
Analogues with aromatic and heteroaromatic cores other than the indole core.
Analogues with 4-pyridine and many substituted benzene cores (Table 4) were shown to have poorer activity than JXL001 in promoting cellular lactate production, implying that the indole core structure is important for the biological activity.
We further evaluated other aromatic cores to identify novel MPC inhibitors (Table 5). Analogues containing azaindole cores were synthesized according to Scheme 4. To our delight, the 7-azaindole analogue (JXL069) was much more potent than the 4-azaindole analogue (JXL072), the 6-azaindole analogue (JXL073) and the hit compound JXL020 with indole core. The improved inhibitory activity may result from a new ligand-protein interaction or the electronic effect of the azaindole structure. The 4-Cl-7-azaindole and 4-Br-7-azaindole analogues (JXL076 and JXL077) showed similar activity as JXL020 in promoting lactate production rate. This new azaindole structure broadened the chemical space for further SAR study.
Analogues with various Michael acceptors.
We envisioned that manipulating the Michael acceptor moiety might be important for the potency and selectivity of the analogues. Modification of this group can change the molecule’s lipophilicity and electrophilicity. To test this idea, analogues with different alkyl groups were synthesized (Table 3). The methyl ester (JXL078) and the t-butyl ester analogues (JXL068) showed a dramatic loss of potency compared with the corresponding carboxylic acid analogue (JXL020), while the ethyl ester (JXL011) showed similar potency as JXL020. Based on this discovery, many other ethyl ester analogues were tested. To our delight, most of them were shown to have similar potency to their corresponding carboxylic acid analogues, such as JXL065 and JXL051, JXL066 and JXL052, and JXL082 and JXL069.
The analogues with malononitrile (JXL021) and dicarboxylate (JXL025) were shown to have poorer activity than JXL001 in increasing the lactate production rate (Figure 1). Analogues containing the TZD groups in Figure 2 failed to show promising activity in promoting lactate production. Amide analogues (JXL081 and JXL094) showed very strong ability to promote lactate production; however, substantial cell death was observed (data was not shown). The indole core-based diethyl phosphonate analogue (JXL086, Figure 1) promoted lactate production more than JXL001. However, the corresponding phosphonic acid analogue (JXL096) failed to promote lactate production rate, perhaps due to its poor cell permeability. The 7-azaindole core-based diethyl phosphonate analogue (JXL095) showed even stronger potency than JXL086 in promoting lactate production. Overall, adjusting the Michael acceptors showed a dramatic effect on the biological activity.
Figure 1.
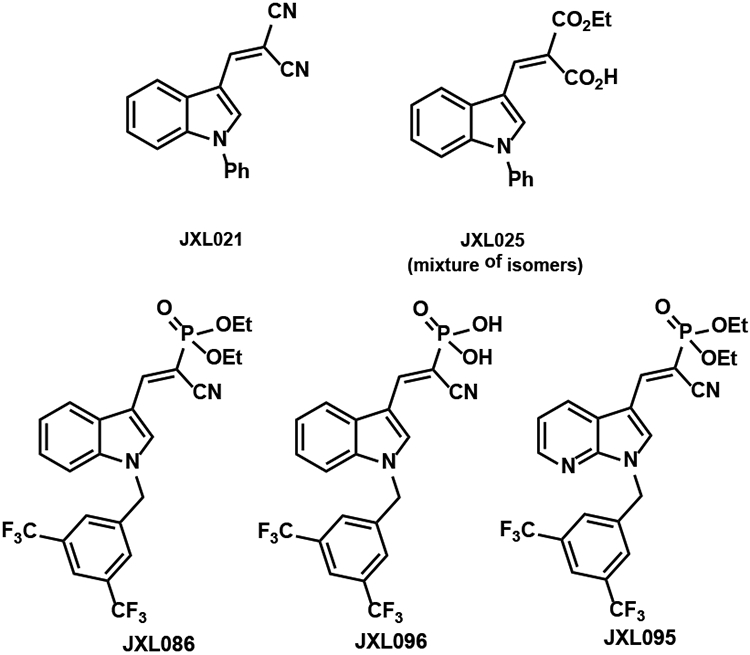
Analogues with various Michael acceptors.
Figure 2.
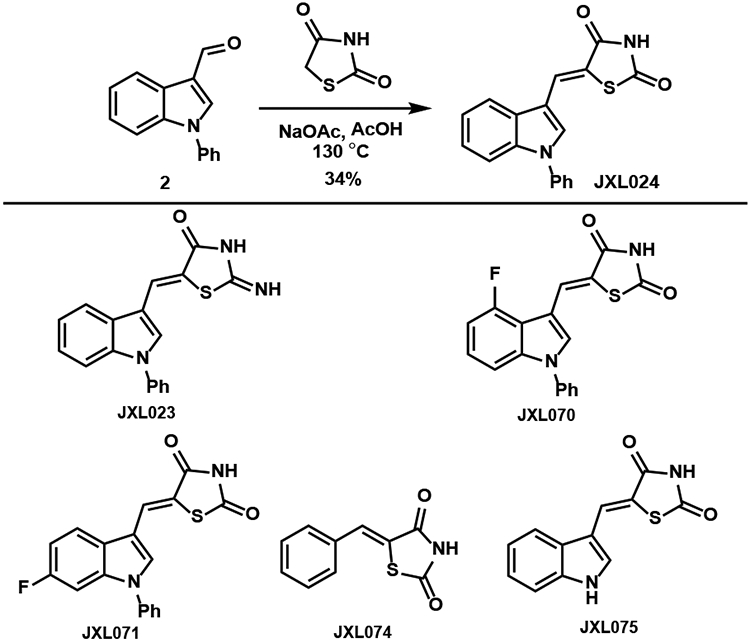
Analogues containing TZD group.
IC50 determination for several analogues.
The test of mitochondrial pyruvate uptake would directly confirm MPC inhibition. However, given the structural similarity between UK5099 (which doesn't inhibit pyruvate carboxylase (PC) or pyruvate dehydrogenase (PDH) activity) and the new analogs, it's reasonable that decreased pyruvate driven respiration is a result of MPC inhibition. Since the MPC enables pyruvate-fueled respiration, measuring how well the UK-5099 analogues block the oxygen consumption rate (OCR) can be an assay of MPC inhibition.13 The pyruvate-fueled OCR assay we used on permeabilized cells for measuring IC50 of our MPC inhibitors is the most direct functional assay available for measuring MPC inhibition. In this assay, the only substrate for mitochondrial respiration given to the permeabilized cells is pyruvate. The MPC inhibitors block the pyruvate-fueled OCRs in a dose-dependent manner. We were able to convince ourselves that these values were direct since our MPC inhibitors had no effect on succinate-fueled OCR, suggesting a direct effect on the MPC and not some other component of the electron transport chain. Analogues JXL001, JXL020 and JXL069 were tested for their ability to inhibit the pyruvate-fueled respiration of permeabilized cells, and IC50 values were determined (Figure 4). JXL001 showed an IC50 value around 140 nM. JXL020 and JXL069 showed much lower IC50 values (16.6 nM and 42.8 nM) than JXL001, which implies that they are more potent than JXL001 in inhibiting MPC.
Figure 4. IC50 measurement of JXL001, JXL020 and JXL069.
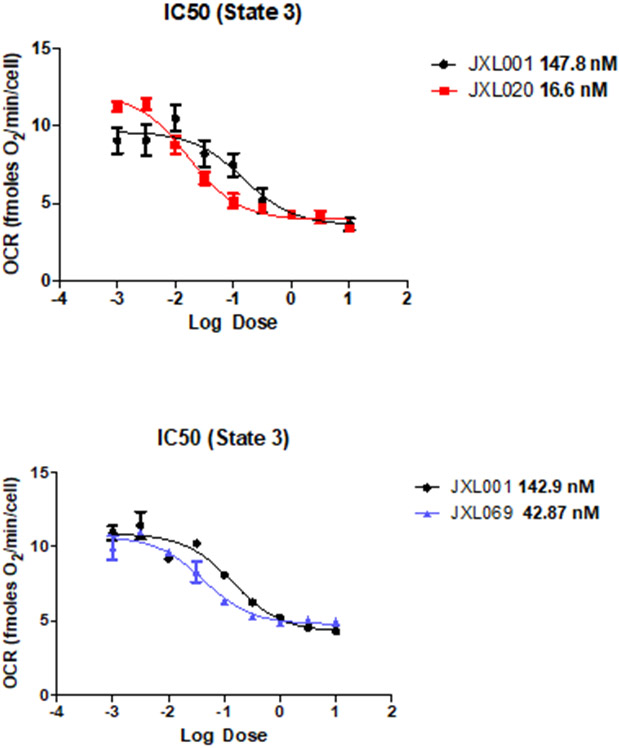
MCF10A cells were treated with analogues to measure oxygen consumption rate (OCR) under different conditions. State 3 is the measurement of respiration in permeabilized cells in the presence of pyruvate, malate, and ADP. IC50 values indicated in bold. Error bars denote +/− 1 SEM (n=2).
In vivo evaluation of MPC inhibitors.
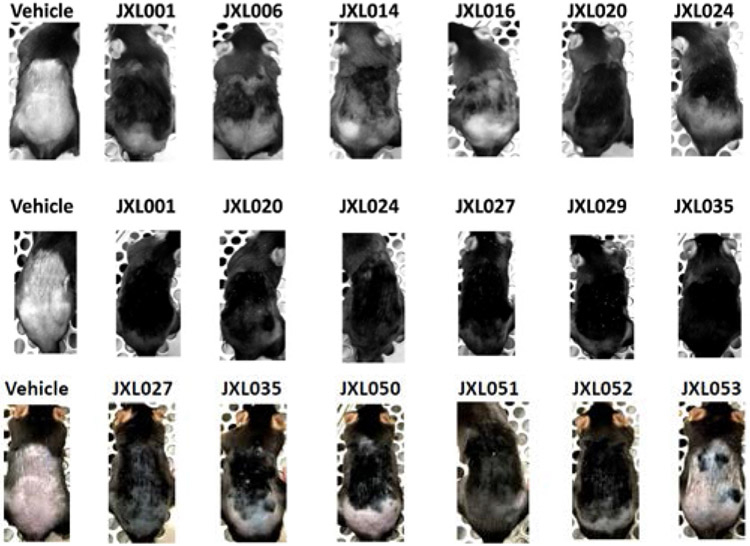
To determine the efficacy of the compounds on hair growth, C57BL/6J mice were shaved at postnatal day 50 during the telogen phase of the hair cycle. Compounds were suspended in lotion and applied topically to the shaved back skin of the mice every other day for 2 weeks. As seen in Figure 5, all the analogues that showed the ability to promote lactate production in the in vitro assay were also able to stimulate hair growth over the course of 2 weeks. JXL020, the analogue with 3,5-bis(trifluoromethyl)benzyl group at the N1 position strongly promoted hair growth. The 4-substituted analogues, JXL035, JXL050, JXL051 and JXL052 were also shown to have strong ability to accelerate hair growth on mice. This data showed that most of the MPC inhibitors active in vitro could promote hair growth on mice. We tried to compare the in vivo efficacy of the MPC inhibitors with different in vitro potency on MPC inhibition. However, the hair growth assay is not very quantitative with respect to timing. All the MPC inhibitors perform better than the controls, but it is difficult to run enough animals together to see statistically significant data to distinguish between the activities of the various MPC inhibitors.
Figure 5. Evaluation of analogues in vivo.
On each row, the mice were treated at the same time with the corresponding MPC inhibitors. Animals treated topically with the analogue (20 μM) show pigmentation and hair growth, indicative of entry into anagen, after 8 days of treatment. Full anagen, indicated by a full coat of hair, is achieved after 14 days of treatment. Mice treated topically with vehicle control do not show pigmentation nor hair growth even after 12 days of treatment.
CONCLUSIONS
This study reports the synthesis and evaluation of novel analogues of UK-5099 both in vitro and in vivo for the development of MPC inhibitors to treat hair loss. We modified four sites around the core structure and tested the activity of those analogues in promoting cellular lactate production to establish a structure-activity relationship for this system. The substituent at the N1 position is crucial for the inhibition of MPC. After screening various aryl and substituted benzyl groups, the analogue containing a 3,5-bis(trifluoromethyl)benzyl group at the N1 position, JXL020, was shown to have much better ability than JXL001 to promote cellular lactate production in vitro (IC50 = 16.6 nM) and accelerate hair growth on mice. Modification of substituents on the indole core structure revealed that small substituents, such as F, Cl, Br and CN, at the C4 position were beneficial for the biological activity. In addition, analogues with different aromatic and heteroaromatic core structures were synthesized and tested. Analogue JXL069, having a 7-azaindole heterocycle, was also shown to have significantly better activity than the analogues containing a 4-azaindole or a 6-azaindole core structure. This discovery further increases the chemical space for the development of MPC inhibitors. Furthermore, different types of Michael acceptors were synthesized and tested for their ability to increase lactate production rate. Analogues with the ethyl ester functional group were shown to have similar activity to inhibit MPC as their corresponding carboxylic acid analogues, such as ester JXL011 and acid JXL020. This finding might provide more opportunities in drug formulation, since the esters have different solubility than the carboxylic acids. Analogues containing amides (JXL081 and JXL094) and diethyl phosphonates (JXL086 and JXL095) showed potent MPC inhibition. Finally, more than ten analogues were tested on mice topically to promote hair growth. The analogues containing the 3,5-bis(trifluoromethyl)benzyl group at the N1 position (JXL020, JXL050, JXL051 and JXL052) stimulated hair growth dramatically. With the novel and potent MPC inhibitors in hand, the pharmacokinetic and safety tests of these compounds are ongoing with the final goal of developing drugs to treat hair growth.
EXPERIMENTAL SECTION
General.
Toluene was distilled from sodium under an argon atmosphere. Dichloromethane was distilled from calcium hydride under an argon atmosphere. All other solvents or reagents were purified according to literature procedures. 1H NMR spectra were recorded on Bruker spectrometers at 500 MHz and are reported relative to deuterated solvent signals. Data for 1H NMR spectra are reported as follows: chemical shift (δ ppm), multiplicity, coupling constant (Hz), and integration. Splitting patterns are designated as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad. 13C NMR spectra were recorded on Bruker spectrometers at 100 MHz. Data for 13C NMR spectra are reported as follows: chemical shift (δ ppm), multiplicity, and coupling constant (Hz). Splitting patterns are designated the same way as in 1H NMR. High resolution mass spectrometry was taken on a Waters LCT Premier with ACQUITY UPLC with Autosampler. Mass spectra were recorded from 70 to 2000 Da. Purity of compounds was analyzed with an Agilent 1200 HPLC system equipped a G1312A binary pump, a G1314A autosampler, a G1314A VWD and an Agilent SB-Aq C18 column (1.8μm, 4.6x50 mm). Mass spectra were recorded using an Agilent 6130 LC/MS system equipped with an ESI source. Method: solution A: H2O w/ 0.1% formic acid and solution B: ACN w/ 0.1% formic acid; increase of B from 10-99%, 0.5-2 min; 99%, 2-17 min; flow rate 1.0 mL/min; UV 254 nm. The purity of all final compounds was determined to be >95% by analytical HPLC analysis except the compounds JXL024, JXL025 and JXL056 with around 90% purity. All solvents were LC–MS/MS grade and purchased from Fisher Scientific.
Experimental detail for the synthesis of JXL001 and related analogues
To the solution of indole-3-carboxaldehyde (2.8 mmol, 411 mg) in dry DMF (6 mL) were added Cu2O (0.3 equiv, 0.84 mmol, 120 mg), K2CO3 (2.0 equiv, 5.6 mmol, 774 mg), and iodobenzene (2.0 equiv, 5.6 mmol, 624 μL) sequentially. The reaction was stirred and refluxed for 24 h, at which point TLC indicated that the reaction was completed. After it was cooled to 21 °C, the reaction mixture was filtrated through a Celite pad eluting with ethyl acetate. The filtrate was washed by saturated NaCl solution and organic phase was dried by sodium sulfate and concentrated. The residue was purified by flash column chromatography (hexanes:ethyl acetate = 8:1) to provide the desired product. yield: 89%, 550.7 mg.
To the solution of 1-phenyl-indole-3-carbaldehyde (1 mmol, 221 mg) in ethanol (1 mL) were added ethyl 2-cyanoacetate (1.3 equiv, 1.3 mmol, 140 μL) and L-proline (40 mol%, 0.4 mmol, 58 mg). The reaction was stirred at 21 °C for 12 h and yellow solid precipitated gradually. After completion of the reaction, ice-cold water (2 mL) was added into the reaction vial. The solid was separated by Buchner funnel filtration and washed with water (2 mL × 3) and dried to afford the desired product. yield: 95%, 300 mg.
To the solution of (E)-ethyl 2-cyano-3-(1-phenyl-1H-indol-3-yl)acrylate (0.32 mmol, 100 mg) in THF (2 mL) was added 0.5N LiOH solution (3 equiv, 0.6 mmol, 1.2 mL). The reaction mixture was stirred at 21 °C for 1 h. After reaction completion shown by TLC, THF was evaporated. Concentrated HCl was added dropwise to acidify the reaction mixture until the pH was lower than 1, meanwhile yellow solid precipitated. Ice-cold water (5 mL) was added to the reaction mixture and the solid was separated by Buchner funnel filtration and washed with water (5 mL × 3). After dried by vacuum, the solid was washed by 2 mL of solvent mixture (hexanes:ethyl acetate = 5:1) 5 to 10 times and monitored by TLC until non-polar impurities disappear (The non-polar compound was the retro-Aldol condensation product, which can be recovered from the filtrate). Finally, the purity of the product was checked by NMR. yield: 65%, 60 mg.
The following compounds were synthesized by a route similar to that described for JXL001: JXL002, JXL003, JXL004, JXL005, JXL006, JXL007, JXL012, JXL013, JXL014, JXL021, JXL025, JXL026, JXL027, JXL028, JXL029, JXL035, and JXL093.
Experimental detail for the synthesis of JXL020
To the solution of indole-3-carboxaldehyde (3 mmol, 435 mg) in dry DMF (6 mL) were added 3,5-bis(trifluoromethyl)benzyl bromide (1.2 equiv, 3.6 mmol, 660 μL) and KOH (1.2 equiv, 3.6 mmol, 200 mg) at 0 °C. The reaction mixture was stirred at 21 °C for 2 h. After the reaction completion shown by TLC, water (6 mL) was added to the reaction vial. The reaction mixture was extracted by dichloromethane (15 mL × 3). The combined organic layer was dried by sodium sulfate and concentrated. The residue was purified by flash column chromatography (hexanes:ethyl acetate = 8:1) to provide the desired product. yield: 90%, 1001.7 mg.
To the solution of 1-(3,5-bis(trifluoromethyl)benzyl)-1H-indole-3-carbaldehyde (1 mmol, 371 mg) in ethanol (1 mL) were added ethyl 2-cyanoacetate (1.3 equiv, 1.3 mmol, 140 μL) and L-proline (40 mol%, 0.4 mmol, 58 mg). The reaction was stirred at 21 °C for 12 h and yellow solid precipitated gradually. After completion of the reaction, ice-cold water (2 mL) was added into the reaction vial. The solid was separated by Buchner funnel filtration and washed with water (2 mL × 3) and dried to afford the desired product. yield: 93%, 433 mg.
To the solution of (E)-ethyl 3-(1-(3,5-bis(trifluoro-methyl)benzyl)-1H-indol-3-yl)-2-cyanoacrylate (0.21 mmol, 100 mg) in THF (2 mL) was added 0.5N LiOH solution (3 equiv, 0.4 mmol, 0.8 mL). The reaction mixture was stirred at 21 °C for 1 h. After reaction completion shown by TLC, THF was evaporated. Concentrated HCl was added dropwise to acidify the reaction mixture until pH was lower than 1, meanwhile yellow solid precipitated. Ice-cold water (5 mL) was added to the reaction mixture and the solid was separated by Buchner funnel filtration and washed with water (5 mL × 3). After dried by vacuum, the solid was washed by 2 mL of solvent mixture (hexanes:ethyl acetate = 5:1) 5 to 10 times and monitored by TLC until non-polar impurities disappear (The non-polar compound was the retro-aldol condensation product, which can be recovered from the filtrate). Finally, the purity of the product was checked by NMR. yield: 55%, 52 mg.
The following compounds were synthesized by a route similar to that described for JXL020: JXL008, JXL009, JXL010, JXL011, JXL015, JXL016, JXL017, JXL018, JXL019, JXL036, JXL037, JXL038, JXL039, JXL040, JXL041, JXL050, JXL051, JXL052, JXL053, JXL054, JXL055, JXL56, JXL057, JXL058, JXL059, JXL060, JXL061, JXL062, JXL063, JXL064, JXL065, JXL066, JXL068, JXL078, JXL081, JXL089, JXL090, and JXL091.
Experimental detail for the synthesis of JXL024
To the solution of 1-phenyl-1H-indole-3-carbaldehyde (0.4 mmol, 90 mg) in AcOH (3 mL) were added thiazolidine-2,4-dione (1 equiv, 0.4 mmol, 46.8 mg) and NaOAc (3 equiv, 98 mg). The reaction mixture was stirred at reflux for 24 hours. After it was cooled to 21 °C, the reaction mixture was filtered by vacuum filtration and washed by AcOH (3 mL × 3) and water (5 mL × 3). After drying by vacuum, the desired product was produced. yield: 34%, 44 mg.
The following compounds were synthesized by a route similar to that described for JXL024: JXL023, JXL067, JXL070, JXL072, JXL074, and JXL075.
Experimental detail for the synthesis of JXL022
To the solution of 4-pyridinecarboxaldehyde (1 mmol, 107 mg) in ethanol (1 mL) were added ethyl 2-cyanoacetate (1.3 equiv, 1.3 mmol, 140 μL) and L-proline (40 mol%, 0.4 mmol, 58 mg). The reaction was stirred at 21 °C for 12 h and yellow solid precipitated gradually. After completion of the reaction, ice-cold water (2 mL) was added into the reaction vial. The solid was separated by Buchner funnel filtration and washed with water (2 mL × 3) and dried to afford the desired product, ethyl (E)-2-cyano-3-(pyridin-4-yl)acrylate, which was used for the next step without further purification.
To the solution of (E)-2-cyano-3-(pyridin-4-yl)acrylate (0.21 mmol, 42.4 mg) in THF (2 mL) was added 0.5N LiOH solution (3 equiv, 0.4 mmol, 0.8 mL). The reaction mixture was stirred at 21 °C for 1 h. After reaction completion shown by TLC, THF was evaporated. Concentrated HCl was added dropwise to acidify the reaction mixture until pH was lower than 1, meanwhile yellow solid precipitated. Ice-cold water (5 mL) was added to the reaction mixture and the solid was separated by Buchner funnel filtration and washed with water (5 mL × 3). After dried by vacuum, the solid was washed by 2 mL of solvent mixture (hexanes:ethyl acetate = 5:1) 5 to 10 times and monitored by TLC until non-polar impurities disappear. Finally, the purity of the product was checked by NMR. yield: 64%, 23.4 mg.
The following compounds were synthesized by a route similar to that described for JXL022: JXL030, JXL031, JXL032, JXL033, JXL034, JXL042, JXL43, JXL044, JXL045, JXL046, JXL047, JXL048, and JXL049.
Experimental detail for the synthesis of JXL069
To the solution of 7-azaindole 4 (1 mmol, 118 mg) in dry DMF (2 mL) were added 3,5-bis(trifluoromethyl)benzyl bromide (1.2 equiv, 1.2 mmol, 220 μL) and KOH (1.2 equiv, 1.2 mmol, 67 mg) at 0 ˚C. The reaction mixture was stirred at 21 °C for 2 h. After the reaction completion shown by TLC, water (6 mL) was added to the reaction vial. The reaction mixture was extracted by dichloromethane (15 mL × 3). The combined organic layer was dried by sodium sulfate and concentrated. The residue was purified by flash column chromatography (hexanes:ethyl acetate = 12:1) to provide the desired benzylation product 5.
POCl3 (1 mmol, 90 μL) was added dropwise to DMF (2 mL) at 0 °C under argon. After stirring for 10 min, a solution of compound 2 (1 mmol, 334 mg) in DMF (2 mL) was added slowly with stirring. The mixture was kept at 21 °C overnight. The reaction was quenched by adding water (5 mL) at 0 °C, then extracted with dichloromethane (10 mL × 3). The combined organic layer was dried by sodium sulfate and concentrated. The residue was purified by flash column chromatography (hexanes:ethyl acetate = 4:1) to provide the desired aldehyde 12.
To the solution of aldehyde 3 (1 mmol, 372 mg) in ethanol (2 mL) was added ethyl 2-cyanoacetate (1.3 equiv, 1.3 mmol, 140 μL) and L-proline (40 mol%, 0.4 mmol, 58 mg). The reaction was stirred at 21 °C for 12 h and yellow solid precipitated gradually. After completion of the reaction, ice-cold water (2 mL) was added into the reaction vial. The solid was separated by Buchner funnel filtration and washed with water (2 mL × 3) and dried to afford the desired product JXL082.
To the solution of JXL082 (0.21 mmol, 100 mg) in THF (2 mL) was added 0.5N LiOH solution (3 equiv, 0.4 mmol, 0.8 mL). The reaction mixture was stirred at 21 °C for 1 h. After reaction completion shown by TLC, THF was evaporated. Concentrated HCl was added dropwise to acidify the reaction mixture until pH was lower than 1, meanwhile yellow solid precipitated. Ice-cold water (5 mL) was added to the reaction mixture and the solid was filtrated by PYREX™ Hirsch-Type Funnel with Fritted Disc and washed with water (5 mL × 3). After dried by vacuum, the solid was washed by 2 mL of solvent mixture (hexanes:ethyl acetate = 5:1) 5 to 10 times and monitored by TLC until non-polar impurities disappear (The non-polar compound was the retro-Aldol condensation product, which can be recovered from the filtrate). Finally, the purity of the product JXL069 was checked by NMR.
The following compounds were synthesized by a route similar to that described for JXL069: JXL072, JXL073, JXL076, JXL077, JXL082, JXL087, and JXL088.
Experimental detail for the synthesis of JXL079
A flask containing Mg powder (10 mmol, 240 mg) and a stir bar was sealed and vacuumed and refilled with argon three times. Anhydrous diethyl ether (32 mL) and bis(trifluoromethyl)benzyl bromide (8 mmol, 1.46 mL) was added to the reaction flask. The reaction mixture was stirred and refluxed for 30 min and then ground dry ice powder (5 g) was added into the reaction flask. After 1 h, the reaction was complete as shown by TLC. The extra Mg powder was filtered off and the solvent was evaporated under vacuum. 1N HCl (20 mL) was added to the residue and the precipitate was filtered and dried to provide the desired carboxylic acid.
A flask containing a stir bar was sealed, vacuumed and refilled with argon three times. Anhydrous dichloromethane (20 mL) and DIBAL (1 M in hexanes, 6 mmol, 6 mL) were added to the flask. The crude carboxylic acid (2 mmol, 544 mg) dissolved in dry dichloromethane (10 mL) was added to the reaction flask at −78 °C. After 2 h, the reaction was complete as shown by TLC and it was then quenched by adding sat. ammonium chloride (10 mL). The resulting mixture was extracted with dichloromethane (20 mL×3) and the organic phases were combined and evaporated on the rotary evaporator. The residue was purified by flash column chromatography (hexanes:ethyl acetate = 10:1) to afford the desired product 2-(3,5-bis(trifluoromethyl)phenyl)ethan-1-ol (yield: 90%, 464 mg).
To a solution of 2-(3,5-bis(trifluoromethyl)phenyl)ethan-1-ol (0.2 mmol, 51.6 mg) in dichloro-methane (2 mL) was added triethylamine (0.22 mmol, 31 μL) and mesyl chloride (MsCl, 0.2 mmol, 17 μL) at 0 °C. After stirring for 1 h, the reaction was complete as shown by TLC. The solvent was removed by flowing air over the open flask. The residue was purified by flash column chromatography (hexanes:ethyl acetate = 10:1) to afford the desired product 3,5-bis(trifluoromethyl)phenethyl methanesulfonate (yield: 82%, 55.1 mg).
A flask containing NaH (60%, 0.22 mmol, 8.8 mg) and a stir bar was sealed, vacuumed and refilled with argon three times. Anhydrous THF (3 mL) and a solution of tert-butyl (E)-2-cyano-3-(1H-indol-3-yl)acrylate (0.2 mmol, 53.6 mg) in THF (2 mL) were added into the reaction flask. The reaction mixture was stirred for 30 min and then 3,5-bis(trifluoromethyl)phenethyl methanesulfonate (0.164 mmol, 55.1 mg) in 2 mL THF was added. The reaction was stirred for 24 h and quenched by sat. NH4Cl solution. The resulting mixture was extracted with dichloromethane (4 mL×3) and the organic phases were combined and evaporated on the rotary evaporator. The residue was purified by flash column chromatography (hexanes:ethyl acetate = 10:1) to afford the desired product tert-butyl (E)-3-(1-(3,5-bis(trifluoromethyl)-phenethyl)-1H-indol-3-yl)-2-cyano-acrylate (yield: 68%, 69 mg).
To a solution of methyl tert-butyl (E)-3-(1-(3,5-bis(trifluoromethyl)phenethyl)-1H-indol-3-yl)-2-cyanoacrylate (0.1 mmol, 50.8 mg) in dichloromethane (2 mL) was added trifluoroacetic acid (3 equiv, 0.3 mmol, 34 μL). The reaction mixture was stirred at 21 °C for 30 min and a yellow solid precipitated. After the reaction was complete as shown by TLC, the reaction solvent was evaporated by flowing air over the open flask. The solid was washed by 2 mL of solvent mixture (hexanes:ethyl acetate = 5:1) 5 to 10 times and monitored by TLC until all the non-polar impurities disappeared. Finally, the purity of the product was checked by NMR. yield: 87%, 39 mg.
Experimental detail for the synthesis of JXL080
To a solution of ethyl (E)-2-cyano-3-(1H-indol-3-yl)acrylate (0.5 mmol, 112 mg) in dichloromethane (5 mL) were added 2-(3,5-bis(trifluoromethyl)phenyl)acetic acid (0.55 mmol, 150 mg), DMAP (catalytic amount, 6 mg), and DCC (0.5 mmol, 103 mg) at 0 °C. The mixture was allowed to reach 21 °C and stirred overnight. The white precipitate was filtered, and the resulting solution was concentrated in vacuum. The solid was purified by flash column chromatography (hexanes:ethyl acetate = 10:1) to afford the desired product (yield: 78%, 192.6 mg).
Experimental detail for the synthesis of JXL083
To a solution of 1-(3,5-bis(trifluoromethyl)benzyl)-1H-indole-3-carbaldehyde (10 mmol, 3.71 g) in acetone (60 mL) was added 2-methyl-2-butene (9 mL), NaH2PO4 (3 equiv, 4.4 g) and NaClO2 (6.6 mmol, 6 g) in 6 mL water. The reaction mixture was stirred at 21 °C for 24 h. After the reaction was complete as shown by TLC, the reaction solvent was evaporated on the rotary evaporator. The crude material was dissolved in ethyl acetate (30 mL) and water (30 mL) and extracted with ethyl acetate (30 mL × 3). The organic phase was combined, dried with sodium sulfate and evaporated on the rotary evaporator. The solid was purified by flash column chromatography (hexanes:ethyl acetate = 2:1) to afford the desired product 1-(3,5-bis(trifluoromethyl)benzyl)-1H-indole-3-carboxylic acid (yield: 89%, 3.44 g).
A 100 mL round bottom flask with a stir bar containing the carboxylic acid (5 mmol, 1935 mg) from the previous step was sealed, vacuumed and refilled with argon three times. To the flask was added 50 mL dichloromethane and oxalyl chloride (25 mmol, 2.1 mL) dropwise. The reaction mixture was stirred at 21 °C for 1.5 h. The reaction solvent was evaporated by vacuum and the resulting compound was used for the next step.
A 100 mL round bottom flask with a stir bar was sealed, vacuumed and refilled with argon three times. Diisopropylamine (5.5 mmol, 765 μL) and THF (10 mL) were added into the flask and it was cooled to −78 °C. n-BuLi (2.5 M in hexanes, 5 mmol, 2 mL) was added slowly to the flask. After the mixture had stirred for 30 min, a solution of ethyl 2-cyanoacetate (5 mmol, 590 μL) in THF (10 mL) was added slowly to the flask. After the mixture had stirred for 1 h, a solution of the acyl chloride (5 mmol from previous step) in THF (5 mL) was added slowly to the reaction mixture. After 1 h, the reaction was quenched by adding aqueous 1M HCl solution (10 mL) and extracted with ethyl acetate (10 mL × 3). The organic phase was combined, dried with sodium sulfate and evaporated by rotary evaporator. The solid was purified by flash column chromatography (hexanes:ethyl acetate = 10:1) to afford the desired product Ethyl (Z)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-2-cyano-3-hydroxyacrylate (JXL083) (yield: 80%, 1.93 g).
Experimental detail for the synthesis of JXL084
To a solution of JXL083 (0.5 mmol, 240 mg) in dichloromethane (10 mL) was added pyridine (0.5 mmol, 40 μL) and acetyl chloride (1.0 mmol, 84 μL). The reaction mixture was stirred for 1h and TLC indicated that the reaction was complete. The reaction solvent was evaporated by flowing air over the open flask. The residue was purified by flash column chromatography (hexanes:ethyl acetate = 10:1) to afford the desired product Ethyl (Z)-3-acetoxy-3-(1-(3,5-bis(trifluoro-methyl)benzyl)-1H-indol-3-yl)-2-cyanoacrylate (JXL084) (yield: 86%, 225 mg).
Experimental detail for the synthesis of JXL085
To a solution of JXL083 (0.5 mmol, 240 mg) in dichloromethane (10 mL) was added triethylamine (1.0 mmol, 139.5 μL) and phosphoryl chloride (0.55 mmol, 520 μL). The reaction mixture was stirred for 1h at reflux and TLC indicated that the reaction was complete. The reaction solvent was evaporated by flowing air over the open flask. The residue was purified by flash column chromatography (hexanes:ethyl acetate = 10:1) to afford the desired product Ethyl (Z)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-3-chloro-2-cyanoacrylate (JXL085) (yield: 84%, 210 mg).
Experimental detail for the synthesis of JXL086
To the solution of 1-(3,5-bis(trifluoromethyl)benzyl)-1H-indole-3-carboxaldehyde (1 mmol, 371 mg) in ethanol (3 mL) were added diethyl cyanomethylphosphate (1.3 equiv, 1.3 mmol, 204 μL) and L-proline (40 mol%, 0.4 mmol, 58 mg). The reaction was stirred at 50 °C for 24 h. After completion of the reaction as indicated by TLC, the reaction solvent was evaporated by flowing air over the open flask. The solid was purified by flash column chromatography (hexanes:ethyl acetate = 2:1) to afford the desired product JXL086 (yield: 90%, 477 mg).
Experimental detail for the synthesis of JXL096
A solution of JXL086 (30 mg, 0.057 mmol) in dichloromethane (2 mL) was cooled to 0 °C and bromotrimethylsilane (40 μL, 0.3 mmol) was added dropwise under argon. The mixture was warmed to 21 °C and stirred for 12 h. The solvent was evaporated under vacuum and the resulting residue was then dissolved in methanol (2 mL). The mixture was stirred at 21 °C for 2 h. Evaporation of all volatiles under vacuum gave the phosphoric acid JXL096 (yield: 92%, 25 mg).
Characterization Data for JXL001–JX096.
(E)-2-Cyano-3-(1-phenyl-1H-indol-3-yl)acrylic acid (JXL001/UK-5099)
1H NMR (500 MHz, DMSO-d6) δ 8.59 (s, 1H), 8.56 (s, 1H), 8.06 (m, 1H), 7.65 (m, 4H), 7.53 (m, 2H), 7.34 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.5, 145.6, 137.7, 136.3, 133.6, 130.5, 128.9, 128.0, 125.0, 124.9, 123.3, 119.9, 118.4, 111.9, 96.7. HRMS (ESI, m/z) calcd for C18H12N2O2 ([M − H]−): 287.0821. Found: 287.0826.
(E)-2-Cyano-3-(1H-indol-3-yl)acrylic acid (JXL002)
1H NMR (500 MHz, DMSO-d6) δ 12.48 (s, 1H), 8.51 (s, 1H), 8.49 (s, 1H), 7.91 (d, J = 6.5 Hz, 1H), 7.53 (d, J = 7.0 Hz, 1H), 7.23 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.0, 146.5, 136.5, 132.4, 127.3, 123.9, 122.4, 118.9, 118.8, 113.2, 110.2, 94.0. HRMS (ESI, m/z) calcd for C12H8N2O2 ([M − H]−): 211.0508. Found: 211.0511.
Ethyl (E)-2-cyano-3-(1H-indol-3-yl)acrylate (JXL003)
1H NMR (500 MHz, CDCl3) δ 12.55 (s, 1H), 8.53 (s, 1H), 8.52 (s, 1H), 7.92 (d, J = 7.6 Hz, 1H), 7.53 (d, J = 7.8 Hz, 1H), 7.26 (app. t, J = 7.4 Hz, 1H), 7.22 (app. t, J = 7.4 Hz, 1H), 4.24 (q, J = 7.0 Hz, 2H), 1.26 (t, J = 7.0 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.6, 147.0, 136.6, 133.0, 127.3, 124.0, 122.5, 118.9, 118.4, 113.3, 110.3, 92.6, 61.8, 14.5. HRMS (ESI, m/z) calcd for C14H12N2O2 ([M − H]−): 239.0820. Found: 239.0818.
Ethyl (E)-2-cyano-3-(1-phenyl-1H-indol-3-yl)acrylate (JXL004)
1H NMR (500 MHz, CDCl3) δ 8.71 (s, 1H), 8.66 (s, 1H), 7.90 (d, J = 7.2 Hz, 1H), 7.54 (m, 6H), 7.36 (m, 2H), 4.39 (q, J = 7.1 Hz, 2H), 1.42 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.6, 145.6, 137.8, 136.4, 133.2, 129.9, 128.5, 124.8, 124.4, 123.0, 118.5, 117.9, 111.6, 111.5, 95.4, 62.0, 14.3. HRMS (ESI, m/z) calcd for C20H16N2O2 ([M + H]+): 317.1290. Found: 317.1269.
Ethyl (E)-3-(6-chloro-1-phenyl-1H-indol-3-yl)-2-cyanoacrylate (JXL005)
1H NMR (500 MHz, CDCl3) δ 8.67 (s, 1H), 8.58 (s, 1H), 7.81 (d, J = 8.5 Hz, 1H), 7.60 (m, 2H), 7.52 (m, 4H), 7.34 (d, J = 8.4 Hz, 1H), 4.39 (q, J = 7.1 Hz, 2H), 1.41 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.4, 145.1, 137.3, 136.8, 133.5, 130.5, 130.0, 128.8, 126.8, 124.8, 123.6, 119.5, 117.5, 111.6, 111.4, 96.4, 62.1, 14.2. HRMS (ESI, m/z) calcd for C20H15ClN2O2 ([M + H]+): 351.0900. Found: 351.0907.
Ethyl (E)-2-cyano-3-(1-(2-methoxyphenyl)-1H-indol-3-yl)acrylate (JXL006)
1H NMR (500 MHz, CDCl3) δ 8.67 (s, 1H), 8.66 (s, 1H), 7.89 (d, J = 7.8 Hz, 1H), 7.49 (app. t, J = 8.6 Hz, 1H), 7.41 (d, J = 7.6 Hz, 1H), 7.35 (app. t, J = 7.3 Hz, 1H), 7.30 (t, J = 7.5 Hz, 1H), 7.23 (d, J = 8.1 Hz, 1H),7.13 (m, 2H), 4.39 (q, J = 7.1 Hz, 2H), 3.81 (s, 3H), 1.41 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.8, 154.2, 146.0, 137.2, 135.2, 130.3, 127.8, 126.0, 124.0, 122.6, 120.8, 118.2, 118.0, 112.3, 111.8, 111.0, 94.7, 61.8, 55.7, 14.3. HRMS (ESI, m/z) calcd for C21H18N2O3 ([M + H] +): 347.1396. Found: 347.1403.
Ethyl (E)-2-cyano-3-(1-(4-methoxyphenyl)-1H-indol-3-yl)acrylate (JXL007)
1H NMR (500 MHz, CDCl3) δ 8.65 (s, 1H), 8.64 (s, 1H), 7.89 (d, J = 7.2 Hz, 1H), 7.44 (m, 3H), 7.35 (m, 2H), 7.07 (d, J = 8.8 Hz, 2H), 4.38 (q, J = 7.1 Hz, 2H), 3.90 (s, 3H), 1.41 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.8, 159.5, 145.6, 136.8, 133.5, 130.6, 128.3, 126.2, 124.2, 122.9, 118.4, 118.0, 115.0, 111.5, 111.1, 94.9, 61.9, 55.6, 14.2. HRMS (ESI, m/z) calcd for C21H18N2O3 ([M + H]+): 347.1396. Found: 347.1401.
Ethyl (E)-2-cyano-3-(1-(4-fluorobenzyl)-1H-indol-3-yl)acrylate (JXL008)
1H NMR (500 MHz, CDCl3) δ 8.60 (app. s, 2H), 7.85 (d, J = 6.8 Hz, 1H), 7.32 (m, 3H), 7.15 (m, 2H), 7.03 (app. t, 2H), 5.39 (s, 2H), 4.37 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.7, 162.5 (d, Jc-f = 247.7 Hz), 145.7, 136.1, 133.8, 130.9, 128.6, 128.5, 124.0, 122.7, 118.6, 118.0, 116.0 (d, Jc-f = 21.9 Hz), 110.9, 110.4, 94.6, 61.9, 50.7, 14.2. HRMS (ESI, m/z) calcd for C21H17FN2O2 ([M + H]+): 347.1352. Found: 347.1351.
Ethyl (E)-2-cyano-3-(1-(3,4-difluorobenzyl)-1H-indol-3-yl)acrylate (JXL009)
1H NMR (500 MHz, CDCl3) δ 8.60 (s, 1H), 8.59 (s, 1H), 7.86 (d, J = 7.8 Hz, 1H), 7.33 (m, 2H), 7.28 (s, 1H), 7.13 (m, 1H), 6.95 (m, 1H), 6.89 (m, 1H), 5.39 (s, 2H), 4.38 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.7, 150.7 (dd, J = 251.2, 13.2 Hz), 150.2 (dd, J = 250.4, 12.6 Hz), 145.7, 136.1, 133.7, 132.3, 128.6, 124.3, 122.9, 122.7, 120.0, 118.8, 118.0 (d, J = 17.5 Hz), 115.9 (d, J = 18.0 Hz), 110.8, 110.6, 95.2, 62.1, 50.4, 14.4. HRMS (ESI, m/z) calcd for C21H16F2N2O2 ([M + H]+): 367.1258. Found: 367.1272.
Ethyl (E)-2-cyano-3-(1-(3,5-difluorobenzyl)-1H-indol-3-yl)acrylate (JXL010)
1H NMR (500 MHz, CDCl3) δ 8.61 (s, 1H), 8.59 (s, 1H), 7.87 (d, J = 7.1 Hz, 1H), 7.33 (m, 2H), 7.26 (d, J = 7.2 Hz, 1H), 6.75 (app. t, J = 8.7 Hz, 1H), 6.64 (app. d, J = 5.7 Hz, 2H), 5.41 (s, 2H), 4.38 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.6, 163.3 (dd, Jc-f = 251.0, 12.5 Hz), 145.5, 139.2, 136.0, 133.6, 128.4, 124.3, 122.9, 118.7, 118.0, 110.7, 110.6, 109.5 (dd, Jc-f = 19.9, 6.4 Hz), 103.8 (t, Jc-f = 25.2 Hz), 95.3, 62.0, 50.4, 14.2. HRMS (ESI, m/z) calcd for C21H16F2N2O2 ([M + H]+): 367.1258. Found: 367.1264.
Ethyl (E)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-2-cyanoacrylate (JXL011)
1H NMR (500 MHz, CDCl3) δ 8.62 (s, 1H), 8.61 (s, 1H), 7.90 (d, J = 7.7 Hz, 1H), 7.85 (s, 1H), 7.57 (s, 2H), 7.35 (m, 2H), 7.23 (d, J = 7.8 Hz, 1H), 5.56 (s, 2H), 4.38 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.6, 145.6, 138.1, 136.0, 133.4, 132.7 (q, J = 33.8 Hz), 128.6, 126.8, 124.7, 123.2, 122.9 (q, J = 273.4 Hz), 122.6, 119.0, 118.0, 111.2, 110.4, 95.9, 62.1, 50.5, 14.3. HRMS (ESI, m/z) calcd for C23H16F6N2O2 ([M + H]+): 467.1194. Found: 467.1206.
(E)-2-Cyano-3-(1-(4-methoxyphenyl)-1H-indol-3-yl)acrylic acid (JXL012)
1H NMR (500 MHz, DMSO-d6) δ 8.54 (s, 1H), 8.52 (s, 1H), 8.05 (d, J = 7.7 Hz, 1H), 7.58 (app. d, J = 8.7 Hz, 2H), 7.44 (m, 1H), 7.33 (m, 2H), 7.16 (app. d, J = 8.7 Hz, 2H), 3.82 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 164.7, 160.5, 136.7, 133.9, 130.5, 127.8, 126.6, 124.8, 123.2, 120.0, 119.5, 118.6, 115.6, 115.4, 111.9, 110.9, 55.9. HRMS (ESI, m/z) calcd for C19H14N2O3 ([M + H]+): 319.1083. Found: 319.1077.
(E)-3-(6-Chloro-1-phenyl-1H-indol-3-yl)-2-cyanoacrylic acid (JXL013)
1H NMR (500 MHz, DMSO-d6) δ 13.58 (br. s, 1H), 8.59 (s, 1H), 8.54 (s, 1H), 8.11 (d, J = 7.5 Hz, 1H), 7.67 (m, 4H), 7.53 (m, 2H), 7.35 (d, J = 7.5 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 164.3, 145.4, 137.3, 136.7, 134.4, 130.6, 129.6, 129.1, 126.7, 125.1, 123.5, 121.5, 118.2, 111.6, 111.3, 97.9. HRMS (ESI, m/z) calcd for C18H11ClN2O2 ([M + H]+): 323.0587. Found: 323.0587.
(E)-2-Cyano-3-(1-(2-methoxyphenyl)-1H-indol-3-yl)acrylic acid (JXL014)
1H NMR (500 MHz, DMSO-d6) δ 8.55 (s, 1H), 8.47 (s, 1H), 8.02 (d, J = 7.4 Hz, 1H), 7.54 (m, 2H), 7.31 (m, 3H), 7.15 (m, 2H), 3.74 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 164.6, 154.2, 145.8, 137.3, 135.1, 131.1, 128.2, 127.3, 125.7, 124.6, 123.0, 121.5, 119.3, 118.5, 113.6, 112.2, 110.8, 96.1, 56.2. HRMS (ESI, m/z) calcd for C19H14N2O3 ([M + H]+): 319.1083. Found: 319.1084.
(E)-2-Cyano-3-(1-(4-fluorobenzyl)-1H-indol-3-yl)acrylic acid (JXL015)
1H NMR (500 MHz, DMSO-d6) δ 8.64 (s, 1H), 8.46 (s, 1H), 7.93 (d, J = 7.1Hz, 1H), 7.61 (d, J = 7.3Hz, 1H), 7.33 (m, 2H), 7.26 (m, 2H), 7.16 (m, 2H), 5.60 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.8, 162.0 (d, Jc-f = 244.3 Hz), 145.6, 136.4, 134.6, 133.1, 130.0, 128.0, 124.1, 122.8, 119.2, 118.5, 116.0 (d, Jc-f = 21.7 Hz), 112.0, 109.8, 95.0, 49.6. HRMS (ESI, m/z) calcd for C19H13FN2O2 ([M + H]+): 321.1039. Found: 321.1046.
(E)-2-Cyano-3-(1-(3,4-difluorobenzyl)-1H-indol-3-yl)acrylic acid (JXL016)
1H NMR (500 MHz, DMSO-d6) δ 13.34 (br. s, 1H), 8.62 (s, 1H), 8.47 (s, 1H), 7.94 (d, J = 7.3 Hz, 1H), 7.61 (d, J = 7.6 Hz, 1H), 7.40 (m, 2H), 7.27 (m, 2H), 7.10 (br. s, 1H), 5.61 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.8, 149.7 (dd, Jc-f = 253.3, 13.6 Hz), 149.4 (dd, Jc-f = 246.3, 11.6 Hz), 145.7, 136.4, 134.7, 134.5, 128.0, 124.7 (dd, Jc-f = 5.9, 3.0 Hz), 124.2, 122.8, 119.2, 118.4, 118.3 (d, Jc-f = 17.0 Hz), 117.1 (d, Jc-f = 17.6 Hz), 112.0, 109.9, 95.0, 49.3. HRMS (ESI, m/z) calcd for C19H12F2N2O2 ([M + H]+): 339.0945. Found: 339.0946.
(E)-2-Cyano-3-(1-(3,5-difluorobenzyl)-1H-indol-3-yl)acrylic acid (JXL017)
1H NMR (500 MHz, DMSO-d6) δ 8.67 (s, 1H), 8.48 (s, 1H), 7.95 (d, J = 4.8 Hz, 1H), 7.59 (d, J = 4.4 Hz, 1H), 7.27 (m, 2H), 7.15 (s, 1H), 6.98 (br. s, 2H), 5.65 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.8, 162.9 (dd, Jc-f = 247.0, 12.8 Hz), 145.6, 141.4, 136.4, 134.8, 128.0, 124.2, 122.9, 119.3, 118.4, 111.9, 111.0 (d, Jc-f = 26.1 Hz), 110.8, 103.8 (t, Jc-f = 26.5 Hz), 95.5, 49.5. HRMS (ESI, m/z) calcd for C19H12F2N2O2 ([M + H]+): 339.0945. Found: 339.0944.
Ethyl (E)-3-(1-benzyl-1H-indol-3-yl)-2-cyanoacrylate (JXL018)
1H NMR (500 MHz, CDCl3) δ 8.61 (s, 1H), 8.60 (s, 1H), 7.85 (d, J = 7.9 Hz, 1H), 7.33 (m, 6H), 7.17 (m, 2H), 5.42 (s, 2H), 4.37 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.8, 145.7, 136.2, 135.1, 134.0, 129.0, 128.5, 128.2, 126.8, 124.0, 122.6, 118.5, 118.1, 111.0, 110.3, 94.3, 61.8, 51.4, 14.2. HRMS (ESI, m/z) calcd for C21H18N2O2 ([M + H]+): 331.1447. Found: 331.1442.
(E)-3-(1-Benzyl-1H-indol-3-yl)-2-cyanoacrylic acid (JXL019)
1H NMR (500 MHz, DMSO-d6) δ 13.34 (br. s, 1H), 8.65 (s, 1H), 8.48 (s, 1H), 7.93 (d, J = 6.9 Hz, 1H), 7.60 (d, J = 6.8 Hz, 1H), 7.25 (m, 7H), 5.62 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.8, 145.7, 136.8, 136.5, 134.7, 129.1, 128.2, 127.6, 124.0, 122.7, 120.0, 119.2, 118.5, 112.1, 109.7, 94.7, 50.4. HRMS (ESI, m/z) calcd for C19H14N2O2 ([M + H]+): 303.1133. Found: 303.1118.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-2-cyanoacrylic acid (JXL020)
1H NMR (500 MHz, DMSO-d6) δ 13.37 (br. s, 1H), 8.75 (s, 1H), 8.48 (s, 1H), 7.99 (m, 4H), 7.65 (s, 1H), 7.28 (m, 2H), 5.83 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.7, 145.7, 140.3, 136.3, 134.8, 131.1, 130.8 (q, J = 31.1 Hz), 128.9, 128.7, 127.9, 124.8, 124.3, 122.9 (q, J = 273.4 Hz), 122.2, 119.3, 118.3, 95.6, 49.2. HRMS (ESI, m/z) calcd for C21H12F6N2O2 ([M + H]+): 439.0881. Found: 439.0880.
2-((1-Phenyl-1H-indol-3-yl)methylene)malononitrile (JXL021)
1H NMR (500 MHz, CDCl3) δ 8.62 (s, 1H), 8.13 (s, 1H), 7.80 (d, J = 7.4 Hz, 1H), 7.61 (m, 2H), 7.53 (m, 4H), 7.40 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 149.8, 137.3, 136.5, 133.6, 130.1, 128.9, 127.7, 124.9, 124.8, 123.7, 118.2, 115.1, 115.0, 111.9, 111.8, 73.7. HRMS (ESI, m/z) calcd for C18H11N3 ([M + H]+): 321.0698. Found: 321.0695.
Ethyl (E)-2-cyano-3-(pyridin-4-yl)acrylate (JXL022)
1H NMR (500 MHz, CDCl3) δ 8.81 (d, J = 5.2 Hz, 2H), 8.18 (s, 1H), 7.74 (d, J = 5.2 Hz, 2H), 4.41 (q, J = 7.1 Hz, 2H), 1.41 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 161.2, 152.0, 151.0, 137.9, 123.2, 114.2, 108.2, 63.2, 14.0. HRMS (ESI, m/z) calcd for C11H10N2O2 ([M + H]+): 203.0820. Found: 203.0815.
(Z)-2-Imino-5-((1-phenyl-1H-indol-3-yl)methylene)thiazolidin-4-one (JXL023)
1H NMR (500 MHz, DMSO-d6) δ 11.94 (br. s, 1H), 9.33 (br. s, 1H), 8.97 (s, 1H), 7.64 (m, 10H). 13C NMR (126 MHz, DMSO-d6) δ 180.9, 174.8, 172.5, 138.6, 136.2, 130.5, 129.3, 128.1, 126.3, 124.8, 124.5, 122.2, 120.3, 119.7, 113.5, 111.5. HRMS (ESI, m/z) calcd for C18H13N3OS ([M + H]+): 320.0858. Found: 320.0862.
(Z)-5-((1-Phenyl-1H-indol-3-yl)methylene)thiazolidine-2,4-dione (JXL024)
1H NMR (500 MHz DMSO-d6) δ 7.98 (m, 2H), 7.79 (s, 1H), 7.66 (app. d, J = 7.7 Hz, 2H), 7.62 (app. t, J = 7.7 Hz, 2H), 7.54 (d, J = 8.0 Hz, 1H), 7.49 (t, J = 7.1 Hz, 1H), 7.30 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 172.5, 169.5, 138.4, 136.2, 130.5, 129.9, 128.3, 128.2, 125.0, 124.6, 122.4, 121.5, 121.4, 119.6, 113.0, 111.5. HRMS (ESI, m/z) calcd for C18H12N2O2S ([M + H]+): 321.0698. Found: 321.0695.
2-(Ethoxycarbonyl)-3-(1-phenyl-1H-indol-3-yl)acrylic acid (a mixture of E/Z isomers, 1:1 ratio) (JXL025)
1H NMR (500 MHz, DMSO-d6) δ 13.08 (br. s, 1H), 7.87 (m, 3H), 7.61 (m, 4H), 7.52 (m, 2H), 7.30 (m, 2H), 4.26 (m, 2H), 1.23 (m, 3H). 13C NMR (126 MHz, DMSO-d6) δ 168.9, 167.8, 166.1, 164.9, 138.3, 136.1, 132.9, 132.1, 131.1, 130.9, 130.8, 128.5, 124.9, 124.4, 122.6, 122.2, 119.5, 111.6, 111.0, 61.7, 61.3, 14.7, 14.4. HRMS (ESI, m/z) calcd for C20H17NO4 ([M + H]+): 336.1236. Found: 336.1239.
(E)-2-Cyano-3-(4-fluoro-1-phenyl-1H-indol-3-yl)acrylic acid (JXL026)
1H NMR (500 MHz, DMSO-d6) δ 8.65 (s, 1H), 8.61 (s, 1H), 7.67 (m, 4H), 7.57 (m, 1H), 7.36 (m, 2H), 7.19 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 164.4, 156.8 (d, Jc-f = 245.6 Hz), 146.4, 138.7, 137.6, 133.4, 130.7, 129.5, 125.9, 125.4, 118.2, 116.3, 116.2, 109.5 (d, Jc-f = 34.5 Hz), 109.2 (d, Jc-f = 23.2 Hz), 98.0. HRMS (ESI, m/z) calcd for C18H11FN2O2 ([M + H]+): 307.0880. Found: 307.0885.
(E)-2-Cyano-3-(6-fluoro-1-phenyl-1H-indol-3-yl)acrylic acid (JXL027)
1H NMR (500 MHz, DMSO-d6) δ 13.59 (br. s, 1H), 8.62 (s, 1H), 8.59 (s, 1H), 8.16 (m, 1H), 7.66 (m, 4H), 7.56 (m, 1H), 7.36 (d, J = 9.2 Hz, 1H), 7.25 m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 164.6, 160.8 (d, Jc-f = 240.0 Hz), 145.7, 137.6, 136.6, 134.4, 130.8, 129.1, 125.0, 124.7, 121.6, 118.4, 111.8 (d, Jc-f = 24.2 Hz), 111.5, 98.7 (d, Jc-f = 26.2 Hz), 97.7. HRMS (ESI, m/z) calcd for C18H11FN2O2 ([M + H]+): 307.0883. Found: 307.0886.
(E)-2-Cyano-3-(7-fluoro-1-phenyl-1H-indol-3-yl)acrylic acid (JXL028)
1H NMR (500 MHz, DMSO-d6) δ 13.62 (br. s, 1H), 8.56 (s, 1H), 8.47 (s, 1H), 7.89 (br. s, 1H), 7.61 (m, 5H), 7.30 (br. s, 1H), 7.17 (br. s, 1H). 13C NMR (126 MHz, DMSO-d6) δ 164.4, 149.7 (d, Jc-f = 247.5 Hz), 145.5, 139.0, 135.5, 131.9, 129.9, 129.3, 126.2, 124.3, 124.0, 118.3, 115.9, 111.8, 110.9 (d, Jc-f = 17.4 Hz), 98.1. HRMS (ESI, m/z) calcd for C18H11FN2O2 ([M + H]+): 307.0883. Found: 307.0870.
(E)-2-Cyano-3-(5-fluoro-1-phenyl-1H-indol-3-yl)acrylic acid (JXL029)
1H NMR (500 MHz, DMSO-d6) δ 13.57 (br. s, 1H), 8.67 (s, 1H), 8.60 (s, 1H), 8.01 (d, J = 9.0 Hz, 1H), 7.70 (m, 4H), 7.58 (m, 2H), 7.24 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 164.6, 159.4 (d, Jc-f = 237.8 Hz), 145.8, 137.7, 135.1, 133.1, 130.7, 129.2, 129.1, 125.2, 118.5, 113.7, 113.2 (d, Jc-f = 26.5 Hz), 111.4, 105.7 (d, Jc-f = 24.2 Hz), 97.2. HRMS (ESI, m/z) calcd for C18H11FN2O2 ([M + H]+): 307.0883. Found: 307.0872.
(E)-2-Cyano-3-(2-fluorophenyl)acrylic acid (JXL030)
1H NMR (500 MHz, DMSO-d6) δ 8.49 (s, 1H), 8.31 (app. t, J=7.2 Hz, 1H), 7.63 (m, 1H), 7.36 (app. t, J=7.4 Hz, 1H), 7.30 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 162.9, 161.5 (d, Jc-f = 256.2 Hz), 145.4 (d, Jc-f = 7.8 Hz), 135.0 (d, Jc-f = 9.2 Hz), 128.7, 124.7, 119.8 (d, Jc-f = 10.9 Hz), 115.8 (d, Jc-f = 21.9 Hz), 114.9, 105.9.HRMS (ESI, m/z) calcd for C10H6FNO2 ([M − H]−): 190.0304. Found: 190.0305.
(E)-2-Cyano-3-(4-fluorophenyl)acrylic acid (JXL032)
1H NMR (500 MHz, DMSO-d6) δ 8.30 (s, 1H), 8.10 (m, 2H), 7.29 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.2 (d, Jc-f = 255.2 Hz), 163.5, 153.1, 133.3 (d, Jc-f = 9.3 Hz), 128.3, 116.0 (d, Jc-f = 22.4 Hz), 115.3, 103.2. HRMS (ESI, m/z) calcd for C10H6FNO2 ([M − H]−): 190.0304. Found: 190.0303.
(E)-2-Cyano-3-(4-(trifluoromethyl)phenyl)acrylic acid (JXL034)
1H NMR (500 MHz, DMSO-d6) δ 8.39 (s, 1H), 8.17 (d, J = 7.7 Hz, 2H), 7.84 (d, J = 7.7 Hz, 2H). 13C NMR (126 MHz, DMSO-d6) δ 162.8, 152.6, 135.4, 133.1 (q, Jc-f = 32.9 Hz), 131.0, 125.7, 123.7 (q, Jc-f = 272.2 Hz), 114.8, 106.7. HRMS (ESI, m/z) calcd for C11H6F3NO2 ([M − H]−): 240.0272. Found: 240.0271.
(E)-3-(4-Chloro-1-phenyl-1H-indol-3-yl)-2-cyanoacrylic acid (JXL035)
1H NMR (500 MHz, DMSO-d6) δ 9.22 (s, 1H), 8.68 (s, 1H), 7.64 (m, 3H), 7.59 (m, 2H), 7.48 (d, J = 7.6 Hz, 1H), 7.38 (m, 1H), 7.32 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 164.5, 146.4, 138.0, 137.4, 134.5, 130.7, 130.5, 129.5, 125.7, 125.3, 124.9, 123.7, 118.4, 111.7, 110.9, 97.5. HRMS (ESI, m/z) calcd for C18H11ClN2O2 ([M + H]+): 323.0587. Found: 323.0587.
(E)-2-Cyano-3-(1-(3,4-difluorobenzyl)-5-fluoro-1H-indol-3-yl)acrylic acid (JXL036)
1H NMR (500 MHz, DMSO-d6) δ 8.69 (s, 1H), 8.47 (s, 1H), 7.84 (d, J = 9.6 Hz, 1H), 7.63 (dd, J = 8.9, 4.3 Hz, 1H), 7.41 (m, 2H), 7.14 (m, 2H), 5.61 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.9, 159.4 (d, Jc-f = 237.3 Hz), 149.8 (dd, Jc-f = 247.1, 12.7 Hz), 149.5 (dd, Jc-f = 246.6, 12.3 Hz), 146.0, 136.1, 134.5, 133.1, 129.1, 125.0, 118.5, 118.4, 117.4, 113.6, 112.5 (d, Jc-f = 26.2 Hz), 110.1, 105.2 (d, Jc-f = 25.1 Hz), 95.5, 49.7. HRMS (ESI, m/z) calcd for C19H11F3N2O2 ([M + H]+): 357.0851. Found: 357.0857.
(E)-2-Cyano-3-(1-(3,5-difluorobenzyl)-5-fluoro-1H-indol-3-yl)acrylic acid (JXL037)
1H NMR (500 MHz, DMSO-d6) δ 8.71 (s, 1H), 8.48 (s, 1H), 7.85 (dd, J = 9.6, 2.0 Hz, 1H), 7.62 (dd, J = 8.9, 4.3 Hz, 1H), 7.16 (m, 2H), 7.00 (app. d, J = 6.2 Hz, 2H), 5.65 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.9, 163.0 (d, Jc-f = 247.7 Hz), 159.3 (d, Jc-f = 237.6 Hz), 145.9, 141.4, 136.3, 133.2, 129.1, 118.5, 113.6, 112.6 (d, Jc-f = 26.3 Hz), 111.3, 110.2, 105.3 (d, Jc-f = 24.9 Hz), 104.0 (t, Jc-f = 25.2 Hz), 95.9, 49.8. HRMS (ESI, m/z) calcd for C19H11F3N2O2 ([M + H]+): 357.0851. Found: 357.0851.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-5-fluoro-1H-indol-3-yl)-2-cyanoacrylic acid (JXL038)
1H NMR (500 MHz, DMSO-d6) δ 8.80 (s, 1H), 8.49 (s, 1H), 8.06 (s, 1H), 8.04 (s, 2H), 7.86 (dd, J = 9.6, 2.1 Hz, 1H), 7.69 (dd, J = 8.9, 4.3 Hz, 1H), 7.17 (dt, J = 9.0, 2.2 Hz, 1H), 5.83 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.8, 159.3 (d, Jc-f = 237.5 Hz), 145.9, 140.3, 136.2, 133.1, 131.1 (q, Jc-f = 33.1 Hz), 129.1, 123.6 (q, Jc-f = 272.2 Hz), 118.4, 113.5, 112.7, 112.5, 110.4, 105.5, 105.3, 96.1, 49.6. HRMS (ESI, m/z) calcd for C21H11F7N2O2 ([M + H]+): 457.0787. Found: 457.0787.
(E)-2-Cyano-3-(1-(3,4-difluorobenzyl)-6-fluoro-1H-indol-3-yl)acrylic acid (JXL039)
1H NMR (500 MHz, DMSO-d6) δ 8.64 (s, 1H), 8.46 (s, 1H), 7.99 (dd, J = 8.7, 5.1 Hz, 1H), 7.58 (dd, J = 9.8, 1.8 Hz, 1H), 7.43 (m, 2H), 7.12 (m, 2H), 5.57 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.8, 160.3 (d, Jc-f = 239.3 Hz), 149.8 (dd, Jc-f = 239.3, 25.2 Hz), 149.6 (dd, Jc-f = 246.3, 25.2 Hz), 145.8, 136.8, 135.3, 134.5, 125.1, 125.0, 124.6, 121.1, 118.5, 117.5, 111.3 (d, Jc-f = 23.9 Hz), 110.2, 98.7 (d, Jc-f = 26.5 Hz), 96.2, 49.4. HRMS (ESI, m/z) calcd for C19H11F3N2O2 ([M + H]+): 357.0851. Found: 457.0835.
(E)-2-Cyano-3-(1-(3,5-difluorobenzyl)-6-fluoro-1H-indol-3-yl)acrylic acid (JXL040)
1H NMR (500 MHz, DMSO-d6) δ 8.65 (s, 1H), 8.46 (s, 1H), 8.00 (dd, J = 8.6, 5.2 Hz, 1H), 7.57 (d, J = 9.7 Hz, 1H), 7.15 (m, 2H), 7.03 (s, 1H), 7.02 (s, 1H), 5.62 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.8, 163.0 (d, Jc-f = 239.4 Hz), 162.9 (d, Jc-f = 248.6 Hz), 160.4 (d, Jc-f = 239.4 Hz), 145.5, 141.3, 136.8, 135.2, 124.6, 121.1, 118.4, 111.3, 111.1, 110.3, 104.0 (t, Jc-f = 25.2 Hz), 98.7 (d, Jc-f = 26.5 Hz), 96.9, 49.6. HRMS (ESI, m/z) calcd for C19H11F3N2O2 ([M + H]+): 357.0851. Found: 357.0847.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-6-fluoro-1H-indol-3-yl)-2-cyanoacrylic acid (JXL041)
1H NMR (500 MHz, DMSO-d6) δ 8.74 (s, 1H), 8.48 (s, 1H), 8.06 (app. s, 3H), 8.01 (dd, J = 8.7, 5.1 Hz, 1H), 7.66 (dd, J = 9.8, 2.0 Hz, 1H), 7.13 (dt, J = 9.3, 2.1 Hz, 1H), 5.78 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.7, 159.9 (d, Jc-f = 264.6 Hz), 145.8, 140.2, 136.8, 135.3, 131.1 (q, Jc-f = 33.3 Hz), 129.1, 124.5, 123.6 (q, Jc-f = 273.7 Hz), 122.5, 121.2, 118.4, 111.4 (d, Jc-f = 25.2 Hz), 110.4, 98.6 (d, Jc-f = 27.2 Hz), 96.7, 49.4. HRMS (ESI, m/z) calcd for C21H11F7N2O2 ([M + H]+): 457.0787. Found: 457.0783.
(E)-2-Cyano-3-(3-fluoro-4-methylphenyl)acrylic acid (JXL042)
1H NMR (500 MHz, DMSO-d6) δ 8.28 (s, 1H), 7.78 (m, 2H), 7.49 (t, J = 8.0 Hz, 1H), 2.30 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 163.6, 160.9 (d, Jc-f = 244.6 Hz), 153.5, 133.0, 131.6 (d, Jc-f = 7.5 Hz), 130.8 (d, Jc-f = 17.6 Hz), 127.2, 117.9 (d, Jc-f = 23.9 Hz), 116.4, 104.5, 15.0. HRMS (ESI, m/z) calcd for C11H8FNO2 ([M − H]−): 204.0461. Found: 204.0460.
(E)-2-Cyano-3-(3,4-difluorophenyl)acrylic acid (JXL043)
1H NMR (500 MHz, DMSO-d6) δ 8.32 (s, 1H), 8.09 (d, J = 8.0 Hz, 1H), 7.94 (br. s, 1H), 7.67 (d, J = 8.8 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 163.3, 152.3, 152.2 (dd, Jc-f = 255.4, 12.6 Hz), 149.9 (dd, Jc-f = 248.2, 12.6 Hz), 129.8, 128.8, 120.1 (d, Jc-f = 17.6 Hz), 119.1 (d, Jc-f = 17.6 Hz), 116.3, 105.8. HRMS (ESI, m/z) calcd for C10H5F2NO2 ([M − H]−): 208.0210. Found: 208.0212.
(E)-2-Cyano-3-(2,4-difluorophenyl)acrylic acid (JXL044)
1H NMR (500 MHz, DMSO-d6) δ 14.2 (br. s, 1H), 8.27 (s, 1H), 8.24 (m, 1H), 7.52 (m, 1H), 7.35 (dt, J = 8.6, 2.2 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 165.5 (dd, Jc-f = 255.8, 12.6 Hz), 163.1, 161.9 (dd, Jc-f = 270.5, 12.6 Hz), 145.1, 131.1, 117.0, 115.9, 113.5 (d, Jc-f = 22.7 Hz), 106.8, 105.6 (t, Jc-f = 26.5 Hz). HRMS (ESI, m/z) calcd for C10H5F2NO2 ([M − H]−): 208.0210. Found: 208.0208.
(E)-3-(3,5-bis(trifluoromethyl)phenyl)-2-cyanoacrylic acid (JXL045)
1H NMR (500 MHz, CD3OD) δ 8.59 (s, 2H), 8.48 (s, 1H), 8.19 (s, 1H). HRMS (ESI, m/z) calcd for C12H5F6NO2 ([M + H]+): 308.0146. Found: 308.0143.
(E)-2-Cyano-3-(4-fluoro-3-(trifluoromethyl)phenyl)acrylic acid (JXL047)
1H NMR (500 MHz, DMSO-d6) δ 8.46 (dd, J = 7.1, 1.8 Hz, 1H), 8.44 (s, 1H), 8.40 (m, 1H), 7.75 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 163.3, 161.1 (d, Jc-f = 262.1 Hz), 152.2, 137.6 (d, Jc-f = 10.1 Hz), 130.5, 129.3, 122.6 (q, Jc-f = 272.8 Hz), 118.9 (d, Jc-f = 21.2 Hz), 118.2 (qd, Jc-f = 32.9, 12.6 Hz), 116.2, 106.3. HRMS (ESI, m/z) calcd for C11H5F4NO2 ([M + H]+): 258.0178. Found: 258.0176.
(E)-2-Cyano-3-phenylacrylic acid (JXL048)
1H NMR (500 MHz, DMSO-d6) δ 8.33 (s, 1H), 8.02 (m, 2H), 7.59 (m, 3H). 13C NMR (126 MHz, DMSO-d6) δ 163.7, 154.9, 133.6, 132.0, 131.1, 129.8, 116.5, 104.3. HRMS (ESI, m/z) calcd for C10H7NO2 ([M − H]−): 172.0399. Found: 172.0396.
(E)-2-cyano-3-(4-hydroxyphenyl)acrylic acid (JXL049)
1H NMR (500 MHz, DMSO-d6) δ 8.16 (s, 1H), 7.95 (d, J = 8.8 Hz, 2H), 6.92 (d, J = 8.8 Hz, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.2, 163.0, 153.7, 133.6, 122.8, 117.2, 116.3, 99.2. HRMS (ESI, m/z) calcd for C10H7NO3 ([M − H]−): 188.0348. Found: 188.0346.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-4-chloro-1H-indol-3-yl)-2-cyanoacrylic acid (JXL050)
1H NMR (500 MHz, DMSO-d6) δ 9.20 (s, 1H), 8.90 (s, 1H), 8.06 (m, 3H), 7.72 (d, J = 7.5 Hz, 1H), 7.32 (m, 2H), 5.86 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.7, 146.7, 140.0, 138.0, 135.6, 131.1 (q, Jc-f = 33.1 Hz), 129.1, 125.6, 125.1, 124.7, 124.5, 123.7, 123.6 (q, Jc-f = 273.7 Hz), 122.5, 111.6, 109.9, 96.7, 49.6. HRMS (ESI, m/z) calcd for C21H11ClF6N2O2 ([M + H]+): 473.0492. Found: 473.0494.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-4-bromo-1H-indol-3-yl)-2-cyanoacrylic acid (JXL051)
1H NMR (500 MHz, DMSO-d6) δ 9.37 (s, 1H), 8.91 (s, 1H), 8.07 (app. s, 3H), 7.77 (d, J = 8.2 Hz, 1H), 7.51 (d, J = 7.6 Hz, 1H), 7.23 (t, J = 8.0 Hz, 1H), 5.85 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.7, 146.3, 140.0, 138.0, 135.8, 131.0 (q, Jc-f = 33.1 Hz), 129.1, 127.9, 125.4, 124.9, 123.7 (q, Jc-f = 273.2 Hz), 122.5, 118.2, 113.6, 122.1, 110.2, 96.6, 49.5. HRMS (ESI, m/z) calcd for C21H11BrF6N2O2 ([M + H]+): 518.9968. Found: 518.9948.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-4-fluoro-1H-indol-3-yl)-2-cyanoacrylic acid (JXL052)
1H NMR (500 MHz, DMSO-d6) δ 8.80 (s, 1H), 8.58 (s, 1H), 8.05 (app. s, 3H), 7.54 (d, J = 8.2 Hz, 1H), 7.29 (d, J = 12.9 Hz, 1H), 7.09 (dd, J = 11.1, 8.2 Hz, 1H), 5.85 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.5, 155.7 (d, Jc-f = 239.4 Hz), 146.5, 140.1, 138.8, 134.5, 131.1 (q, Jc-f = 33.0 Hz), 129.0, 125.1 (d, Jc-f = 7.6 Hz), 124.7, 123.6 (q, Jc-f = 273.5 Hz), 122.5, 118.1, 116.1 (d, Jc-f = 18.5 Hz), 108.8, 108.5, 97.2, 49.7. HRMS (ESI, m/z) calcd for C21H11F7N2O2 ([M + H]+): 457.0787. Found: 457.0786.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-7-fluoro-1H-indol-3-yl)-2-cyanoacrylic acid (JXL053)
1H NMR (500 MHz, DMSO-d6) δ 8.73 (br. s, 1H), 8.48 (br. s, 1H), 8.06 (br. s, 1H), 7.89 (br. s, 2H), 7.78 (br. s, J = 7.4 Hz, 1H), 7.22 (br. s, 1H), 7.10 (br. s, 1H), 5.89 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.6, 159.7 (d, Jc-f = 245.7 Hz), 145.6, 141.0, 136.2, 132.1, 130.0 (q, Jc-f = 33.0 Hz), 128.4, 123.9, 123.8, 123.6 (q, Jc-f = 273.3 Hz), 122.4 (d, Jc-f = 18.9 Hz), 118.1, 115.8, 110.6, 110.2 (d, Jc-f = 18.9 Hz), 97.3, 52.0. HRMS (ESI, m/z) calcd for C21H11F7N2O2 ([M + H]+): 457.0787. Found: 457.0792.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-5-chloro-1H-indol-3-yl)-2-cyanoacrylic acid (JXL054)
1H NMR (500 MHz, DMSO-d6) δ 8.78 (s, 1H), 8.51 (s, 1H), 8.12 (s, 1H), 8.06 (s, 1H), 8.03 (s, 2H), 7.70 (d, J = 7.4 Hz, 1H), 7.33 (d, J = 7.0 Hz, 1H), 5.83 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.7, 145.7, 140.2, 135.9, 135.1, 131.1 (q, Jc-f = 33.0 Hz), 129.3, 129.0, 127.9, 124.4, 123.6 (q, Jc-f = 274.0 Hz), 122.5, 119.5, 118.4, 113.6, 109.9, 96.9, 49.5. HRMS (ESI, m/z) calcd for C21H11ClF6N2O2 ([M + H]+): 473.0492. Found: 473.0498.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-4-cyano-1H-indol-3-yl)-2-cyanoacrylic acid (JXL055)
1H NMR (500 MHz, DMSO-d6) δ 9.03 (s, 1H), 9.00 (s, 1H), 8.09 (m, 4H), 7.80 (d, J = 7.3 Hz, 1H), 7.47 (t, J = 7.8 Hz, 1H), 5.90 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.4, 144.4, 139.9, 136.7, 131.1 (q, Jc-f = 33.0 Hz), 129.7, 129.2, 126.6, 124.3, 123.6 (q, Jc-f = 273.7 Hz), 122.7, 118.6, 117.9, 117.8, 109.3, 101.7, 98.1, 49.5. HRMS (ESI, m/z) calcd for C22H11F6N3O2 ([M + H]+): 464.0834. Found: 464.0832.
(E)-1-(3,5-Bis(trifluoromethyl)benzyl)-3-(2-carboxy-2-cyanovinyl)-1H-indole-4-carboxylic acid (JXL056)
1H NMR (500 MHz, DMSO-d6) δ 9.26 (br. s, 1H), 8.88 (br. s, 1H), 8.05 (app. s, 3H), 7.94 (br. s, 1H), 7.76 (br. s, 1H), 7.37 (br. s, 1H), 5.85 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 169.3, 165.0, 150.6, 140.2, 137.7, 136.0, 131.1 (q, Jc-f = 33.0 Hz), 129.0, 125.9, 125.2, 124.7, 123.6 (q, Jc-f = 273.7 Hz), 123.5, 122.5, 118.3, 116.1, 110.1, 96.4, 49.3. HRMS (ESI, m/z) calcd for C22H12F6N2O4 ([M + H]+): 483.0779. Found: 483.0778.
(E)-3-(4-(Benzyloxy)-1-(3,5-bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-2-cyanoacrylic acid (JXL057)
1H NMR (500 MHz, DMSO-d6) δ 9.15 (s, 1H), 8.72 (s, 1H), 8.04 (s, 1H), 7.99 (s, 2H), 7.54 (br. s, 3H), 7.37 (br. s, 2H), 7.28 (m, 2H), 6.95 (s, 1H), 5.81 (s, 2H), 5.28 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.0, 153.7, 148.6, 140.5, 137.9, 133.4, 131.2 (q, Jc-f = 32.8 Hz), 128.9, 128.8, 128.1, 127.5, 125.4, 124.7, 123.6 (q, Jc-f = 273.7 Hz), 122.3, 118.5, 117.0, 110.5, 105.6, 105.2, 95.5, 70.0, 49.5. HRMS (ESI, m/z) calcd for C28H18F6N2O3 ([M + H]+): 545.1300. Found: 545.1299.
(E)-3-(6-(Benzyloxy)-1-(3,5-bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-2-cyanoacrylic acid (JXL058)
1H NMR (500 MHz, DMSO-d6) δ 8.63 (s, 1H), 8.43 (s, 1H), 8.05 (s, 1H), 8.03 (s, 2H), 7.86 (d, J = 8.5 Hz, 1H), 7.32 (m, 6H), 6.97 (d, J = 8.3 Hz, 1H), 5.77 (s, 2H), 5.09 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.0, 156.7, 146.1, 140.5, 137.5, 137.3, 134.3, 131.0 (q, Jc-f = 32.8 Hz), 128.9, 128.8, 128.3, 128.2, 126.9, 123.6 (q, Jc-f = 273.4 Hz), 122.4, 121.9, 118.5, 113.3, 110.5, 96.8, 95.6, 70.2, 49.2. HRMS (ESI, m/z) calcd for C28H18F6N2O3 ([M + H]+): 545.1300. Found: 545.1292.
(E)-3-(7-(Benzyloxy)-1-(3,5-bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-2-cyanoacrylic acid (JXL059)
1H NMR (500 MHz, DMSO-d6) δ 8.61 (br. s, 1H), 8.45 (br. s, 1H), 7.97 (br. s, 1H), 7.60 (br. s, 2H), 7.51 (br. s, 1H), 7.25 (br. s, 2H), 7.16 (br. s, 2H), 6.91 (br. s, 1H), 5.94 (s, 2H), 5.13 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.8, 146.6, 145.7, 142.3, 136.6, 135.4, 130.7 (q, Jc-f = 32.8 Hz), 130.5, 128.8, 128.4, 128.0, 127.5, 125.7, 124.1, 123.6 (q, Jc-f = 273.7 Hz), 121.8, 118.3, 111.8, 110.3, 107.1, 96.2, 70.3, 52.4. HRMS (ESI, m/z) calcd for C28H18F6N2O3 ([M + H]+): 545.1300. Found: 545.1298.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-4-methoxy-1H-indol-3-yl)-2-cyanoacrylic acid (JXL060)
1H NMR (500 MHz, DMSO-d6) δ 8.99 (s, 1H), 8.71 (s, 1H), 8.05 (s, 1H), 8.00 (s, 2H), 7.24 (app. s, 2H), 6.82 (d, J = 6.0 Hz, 1H), 5.81 (s, 2H), 3.92 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.0, 154.8, 148.6, 140.5, 137.8, 133.3, 131.0 (q, Jc-f = 32.8 Hz), 128.9, 125.4, 123.6 (q, Jc-f = 273.7 Hz), 122.5, 118.5, 116.8, 110.5, 105.0, 104.3, 95.3, 56.2, 49.5. HRMS (ESI, m/z) calcd for C22H14F6N2O3 ([M + H]+): 469.0987. Found: 469.0987.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-5-bromo-1H-indol-3-yl)-2-cyanoacrylic acid (JXL061)
1H NMR (500 MHz, DMSO-d6) δ 8.75 (s, 1H), 8.49 (s, 1H), 8.25 (s, 1H), 8.06 (s, 1H), 8.02 (m, 3H), 7.64 (app. s, 1H), 7.44 (app. s, 1H), 5.82 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.7, 145.3, 140.3, 135.5, 135.3, 131.1 (q, Jc-f = 32.8 Hz), 129.8, 128.9, 127.0, 124.7, 123.6 (q, Jc-f = 274.0 Hz), 122.5, 122.3, 118.5, 113.9, 109.9, 97.7, 49.5. HRMS (ESI, m/z) calcd for C21H11BrF6N2O2 ([M + H]+): 516.9987. Found: 516.9983.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-6-bromo-1H-indol-3-yl)-2-cyanoacrylic acid (JXL062)
1H NMR (500 MHz, DMSO-d6) δ 8.72 (s, 1H), 8.47 (s, 1H), 8.06 (s, 2H), 8.03 (s, 1H), 7.94 (d, J = 8.2 Hz, 1H), 7.39 (d, J = 7.9 Hz, 1H), 5.81 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.7, 145.6, 140.2, 137.4, 135.3, 131.1 (q, Jc-f = 32.8 Hz), 129.1, 127.0, 125.9, 123.6 (q, Jc-f = 274.0 Hz), 122.5, 121.5, 118.2, 117.2, 114.8, 110.3, 96.9, 49.3. HRMS (ESI, m/z) calcd for C21H11BrF6N2O2 ([M + H]+): 516.9987. Found: 516.9965.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-6-chloro-1H-indol-3-yl)-2-cyanoacrylic acid (JXL063)
1H NMR (500 MHz, DMSO-d6) δ 8.75 (s, 1H), 8.47 (s, 1H), 8.05 (m, 3H), 8.00 (d, J = 7.4 Hz, 1H), 7.89 (s, 1H), 7.28 (d, J = 6.5 Hz, 1H), 5.81 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.7, 145.6, 140.2, 137.0, 135.4, 131.1 (q, Jc-f = 32.8 Hz),129.2, 129.0, 126.7, 123.6 (q, Jc-f = 274.0 Hz), 123.3, 122.5, 121.2, 118.2, 111.9, 110.3, 97.0, 49.3. HRMS (ESI, m/z) calcd for C21H11ClF6N2O2 ([M + H]+): 473.0492. Found: 473.0493.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-7-chloro-1H-indol-3-yl)-2-cyanoacrylic acid (JXL064)
1H NMR (500 MHz, DMSO-d6) δ 8.69 (s, 1H), 8.50 (s, 1H), 8.03 (s, 1H), 7.97 (d, J = 7.5 Hz, 1H), 7.73 (s, 2H), 7.32 (d, J = 7.1 Hz, 1H), 7.25 (m, 1H), 6.14 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.5, 145.1, 142.1, 136.9, 131.5, 131.2 (q, Jc-f = 32.8 Hz), 127.6, 126.1, 124.1, 123.6 (q, Jc-f = 274.0 Hz), 122.5, 122.0, 118.8, 118.0, 116.9, 110.3, 97.9, 51.8. HRMS (ESI, m/z) calcd for C21H11ClF6N2O2 ([M + H]+): 473.0492. Found: 473.0496.
Ethyl (E)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-4-bromo-1H-indol-3-yl)-2-cyanoacrylate (JXL065)
1H NMR (500 MHz, DMSO-d6) δ 9.67 (s, 1H), 8.71 (s, 1H), 7.86 (s, 1H), 7.56 (s, 2H), 7.52 (d, J = 7.4 Hz, 1H), 7.17 (m, 2H), 5.55 (s, 2H), 4.37 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 163.4, 146.9, 137.6, 137.4, 134.3, 132.7 (q, Jc-f = 33.9 Hz), 128.2, 126.7, 125.6, 125.1, 122.8 (q, Jc-f = 273.4 Hz), 122.7, 118.0, 114.9, 111.8, 109.9, 96.3, 62.2, 50.6, 14.3. HRMS (ESI, m/z) calcd for C23H15BrF6N2O2 ([M + H]+): 545.0299. Found: 545.0300.
Ethyl (E)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-4-fluoro-1H-indol-3-yl)-2-cyanoacrylate (JXL066)
1H NMR (500 MHz, DMSO-d6) δ 8.86 (s, 1H), 8.61 (s, 1H), 7.86 (s, 1H), 7.56 (s, 2H), 7.23 (m, 1H), 7.01 (m, 2H), 5.54 (s, 2H), 4.37 (q, J = 7.1 Hz, 2H), 1.39 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 163.1, 157.4 (d, J = 243.9 Hz), 147.4, 138.2 (d, J = 10.1 Hz), 137.8, 133.2, 132.7 (q, Jc-f = 33.9 Hz), 126.7, 125.3, 122.8 (q, Jc-f = 273.4 Hz), 122.7, 117.9, 116.9 (d, Jc-f = 17.6 Hz), 110.0, 109.0 (d, Jc-f = 19.5 Hz), 106.6, 97.1, 62.2, 50.7, 14.3. HRMS (ESI, m/z) calcd for C23H15F7N2O2 ([M + H]+): 485.1100. Found: 485.1100.
(Z)-5-((1-(3,5-Bis(trifluoromethyl)benzyl)-1H-indol-3-yl)methylene)-2-iminothiazolidin-4-one (JXL067)
1H NMR (500 MHz, DMSO-d6) δ 9.21 (s, 1H), 9.01 (s, 1H), 8.03 (s, 1H), 7.94 (br. s, 3H), 7.86 (d, J = 7.7 Hz, 1H), 7.81 (s, 1H), 7.58 (d, J = 7.9 Hz, 1H), 7.24 (m, 1H), 7.19 (m, 1H), 5.76 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 180.0, 174.9, 141.4, 136.5, 131.0 (q, Jc-f = 32.8 Hz), 130.4, 128.5, 127.9, 125.1, 123.8, 123.6 (q, Jc-f = 274.0 Hz), 122.1, 121.7, 120.8, 119.3, 111.8, 111.3, 48.9. HRMS (ESI, m/z) calcd for C21H13F6N3OS ([M + H]+): 470.0762. Found: 470.0761.
tert-Butyl (E)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-2-cyanoacrylate (JXL068)
1H NMR (500 MHz, CDCl3) δ 8.57 (s, 1H), 8.54 (s, 1H), 7.86 (d, J = 7.6 Hz, 1H), 7.84 (s, 1H), 7.56 (s, 2H), 7.33 (m, 2H), 7.22 (d, J = 7.4 Hz, 1H), 5.55 (s, 2H), 1.59 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 162.4, 144.7, 138.2, 135.9, 133.0, 132.7 (q, Jc-f = 32.8 Hz), 128.6, 126.7, 124.5, 123.0, 122.9 (q, Jc-f = 277.2 Hz), 122.6, 119.0, 118.2, 111.1, 110.4, 97.7, 82.9, 50.4, 28.1. HRMS (ESI, m/z) calcd for C25H20F6N2O2 ([M + H]+): 495.1507. Found: 495.1503.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-1H-pyrrolo[2,3-b]pyridin-3-yl)-2-cyanoacrylic acid (JXL069)
1H NMR (500 MHz, DMSO-d6) δ 8.85 (s, 1H), 8.47 (m, 3H), 8.09 (s, 2H), 8.04 (s, 1H), 7.35 (dd, J = 7.1, 4.6 Hz, 1H), 5.84 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.6, 147.8, 146.1, 145.5, 141.0, 135.2, 131.0 (q, Jc-f = 32.8 Hz), 129.4, 129.2, 123.6 (q, Jc-f = 274.0 Hz), 122.3, 120.1, 119.1, 118.2, 108.7, 97.1, 47.8. HRMS (ESI, m/z) calcd for C20H11F6N3O2 ([M + H]+): 440.0834. Found: 440.0834.
(Z)-5-((4-Fluoro-1-phenyl-1H-indol-3-yl)methylene)thiazolidine-2,4-dione (JXL070)
1H NMR (500 MHz, DMSO-d6) δ 12.45 (br. s, 1H), 8.11 (s, 1H), 7.83 (s, 1H), 7.65 (m, 4H), 7.52 (s, 1H), 7.32 (m, 2H), 7.12 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 168.0, 167.7, 156.9 (d, Jc-f = 245.7 Hz), 138.7 (d, Jc-f = 10.1 Hz), 137.9, 130.8, 130.5, 128.8, 125.5, 125.4, 124.3, 120.0, 116.2 (d, Jc-f = 18.9 Hz), 110.7, 108.5, 108.1 (d, Jc-f = 18.9 Hz). HRMS (ESI, m/z) calcd for C18H11FN2O2S ([M + H]+): 339.0640. Found: 339.0610.
(Z)-5-((6-Fluoro-1-phenyl-1H-indol-3-yl)methylene)thiazolidine-2,4-dione (JXL071)
1H NMR (500 MHz, DMSO-d6) δ 12.42 (s, 1H), 8.06 (app. s, 2H), 7.85 (s, 1H), 7.67 (m, 2H), 7.62 (m, 2H), 7.50 (m, 1H), 7.31 (d, J = 9.6 Hz, 1H), 7.16 (t, J = 8.5 Hz, 1H). 13C NMR (126 MHz, DMSO-d6) δ 168.0, 167.7, 160.6 (d, Jc-f = 245.7 Hz), 138.0, 136.3 (d, Jc-f = 12.6 Hz), 131.0, 130.6, 128.5, 125.0, 124.8, 123.5, 121.3 (d, Jc-f = 10.0 Hz), 120.0, 112.6, 111.1 (d, Jc-f = 18.9 Hz), 98.2 (d, Jc-f = 18.9 Hz). HRMS (ESI, m/z) calcd for C18H11FN2O2S ([M + H]+): 339.0604. Found: 339.0601.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-1H-pyrrolo[3,2-b]pyridin-3-yl)-2-cyanoacrylic acid (JXL072)
1H NMR (500 MHz, DMSO-d6) δ 8.94 (s, 1H), 8.68 (s, 1H), 8.54 (s, 1H), 8.11 (m, 4H), 7.35 (s, 1H), 5.87 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.5, 145.9, 144.2, 140.1, 135.9, 131.1 (q, Jc-f = 32.8 Hz), 129.6, 129.2, 124.7, 123.6 (q, Jc-f = 274.0 Hz), 122.5, 120.0, 119.5, 117.9, 110.1, 97.2, 49.8. HRMS (ESI, m/z) calcd for C20H11F6N3O2 ([M + H]+): 440.0834. Found: 440.0834.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-1H-pyrrolo[2,3-c]pyridin-3-yl)-2-cyanoacrylic acid (JXL073)
1H NMR (500 MHz, DMSO-d6) δ 9.14 (s, 1H), 8.96 (s, 1H), 8.55 (s, 1H), 8.40 (d, J = 5.1 Hz, 1H), 8.19 (s, 2H), 8.11 (app. s, 2H), 5.95 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.5, 145.5, 140.7, 139.9, 138.1, 134.5, 133.6, 131.1 (q, Jc-f = 32.8 Hz), 129.4, 127.2, 123.6 (q, Jc-f = 274.0 Hz), 122.7, 118.0, 114.6, 109.7, 97.9, 49.8. HRMS (ESI, m/z) calcd for C30H11F6N3O2 ([M + H]+): 440.0834. Found: 440.0837.
(Z)-5-Benzylidenethiazolidine-2,4-dione (JXL074)
1H NMR (500 MHz, DMSO-d6) δ 12.60 (br. s, 1H), 7.77 (s, 1H), 7.58 (app. d, J = 7.3 Hz, 2H), 7.51 (app. t, J = 7.4 Hz, 2H), 7.46 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 168.4, 167.8, 133.5, 132.3, 130.9, 130.5, 129.8, 124.0. HRMS (ESI, m/z) calcd for C10H7NO2S ([M − H]−): 204.0119. Found: 204.0122.
(Z)-5-((1H-Indol-3-yl)methylene)thiazolidine-2,4-dione (JXL075)
1H NMR (500 MHz, DMSO-d6) δ 12.28 (s, 1H), 12.11 (s, 1H), 8.03 (s, 1H), 7.87 (d, J = 7.3 Hz, 1H), 7.72 (s, 1H), 7.48 (d, J = 7.6 Hz, 1H), 7.23 (m, 1H), 7.18 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 168.2, 167.8, 136.7, 129.1, 127.3, 125.0, 123.5, 121.5, 118.8, 116.7, 112.9, 110.9. HRMS (ESI, m/z) calcd for C12H8N2O2S ([M + H]+): 243.0228. Found: 243.0230.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-4-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)-2-cyanoacrylic acid (JXL076)
1H NMR (500 MHz, DMSO-d6) δ 9.00 (s, 1H), 8.98 (s, 1H), 8.36 (s, 1H), 8.12 (s, 2H), 8.04 (br. s, 1H), 7.47 (br. s, 1H), 5.85 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.4, 148.7, 145.8, 145.7, 140.2, 136.1, 135.3, 131.0 (q, Jc-f = 32.8 Hz), 129.5, 123.6 (q, Jc-f = 274.0 Hz), 122.4, 120.4, 117.7, 116.8, 108.2, 98.1, 48.2. HRMS (ESI, m/z) calcd for C20H10ClF6N3O2 ([M + H]+): 474.0444. Found: 474.0448.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-4-bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)-2-cyanoacrylic acid (JXL077)
1H NMR (500 MHz, DMSO-d6) δ 9.17 (s, 1H), 9.02 (s, 1H), 8.27 (d, J = 5.0 Hz, 1H), 8.12 (s, 2H), 8.05 (s, 1H), 7.63 (d, J = 5.0 Hz, 1H), 5.85 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.4, 148.2, 145.5, 145.3, 140.2, 135.4, 131.0 (q, Jc-f = 32.8 Hz), 129.6, 124.9, 123.7, 123.6 (q, Jc-f = 274.0 Hz), 122.4, 118.4, 117.7, 108.6, 97.9, 48.2. HRMS (ESI, m/z) calcd for C20H10BrF6N3O2 ([M + H]+): 517.9939. Found: 517.9935.
Methyl (E)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-2-cyanoacrylate (JXL078)
1H NMR (500 MHz, CDCl3) δ 8.62 (s, 1H), 8.61 (s, 1H), 7.89 (d, J = 7.7 Hz, 1H), 7.84 (s, 1H), 7.57 (s, 2H), 7.35 (m, 2H), 7.24 (m, 1H), 5.55 (s, 2H), 3.92 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 164.5, 145.8, 138.1, 136.0, 133.5, 132.7 (q, Jc-f = 32.8 Hz), 128.6, 126.8, 124.7, 123.9, 123.6 (q, Jc-f = 274.0 Hz), 123.2, 119.1, 118.0, 111.2, 110.5, 95.4, 53.0, 50.5. HRMS (ESI, m/z) calcd for C22H14F6N2O2 ([M + H]+): 453.1038. Found: 453.1045.
(E)-3-(1-(3,5-Bis(trifluoromethyl)phenethyl)-1H-indol-3-yl)-2-cyanoacrylic acid (JXL079)
1H NMR (500 MHz, CD3OD) δ 8.51 (s, 1H), 8.14 (s, 1H), 7.83 (d, J = 7.3 Hz, 1H), 7.74 (s, 1H), 7.52 (m, 3H), 7.31 (m, 2H), 4.64 (t, J = 6.4 Hz, 2H), 3.35 (t, J = 6.3 Hz, 2H). 13C NMR (126 MHz, CD3OD) δ 165.3, 145.3, 141.1, 136.1, 133.6, 131.3 (q, Jc-f = 32.8 Hz), 129.3, 128.3, 123.7, 123.3 (q, Jc-f = 272.5 Hz), 122.3, 120.3, 118.1, 117.6, 110.6, 109.6, 94.1, 47.8, 35.0. HRMS (ESI, m/z) calcd for C22H14F6N2O2 ([M + H]+): 453.1038. Found: 453.1031.
Ethyl (E)-3-(1-(2-(3,5-bis(trifluoromethyl)phenyl)acetyl)-1H-indol-3-yl)-2-cyanoacrylate (JXL080)
1H NMR (500 MHz, CDCl3) δ 8.90 (s, 1H), 8.52 (s, 1H), 8.49 (d, J = 8.1 Hz, 1H), 7.88 (s, 1H), 7.86 (s, 2H), 7.78 (d, J = 7.5 Hz, 1H), 7.47 (m, 2H), 4.50 (s, 2H), 4.41 (q, J = 7.1 Hz, 2H), 1.43 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 168.0, 162.2, 144.2, 135.6, 134.4, 132.3 (q, Jc-f = 33.6 Hz), 130.1, 129.8, 128.8, 127.8, 127.3, 125.5, 123.1 (q, Jc-f = 273.3 Hz), 118.3, 117.1, 117.0, 115.9, 101.8, 62.8, 41.9, 14.3. HRMS (ESI, m/z) calcd for C24H16F6N2O3 ([M + H]+): 495.1143. Found: 495.1136.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-2-(morpholine-4-carbonyl)acrylonitrile (JXL081)
1H NMR (500 MHz, CDCl3) δ 8.48 (s, 1H), 8.34 (s, 1H), 7.84 (s, 2H), 7.56 (app. s, 2H), 7.32 (m, 2H), 7.22 (m, 1H), 5.54 (s, 2H), 3.77 (br. s, 8H). 13C NMR (126 MHz, CDCl3) δ 164.0, 145.2, 138.4, 135.8, 132.7 (q, Jc-f = 34.0 Hz), 131.8, 128.4, 126.8, 125.0, 124.5, 123.1 (q, Jc-f = 273.3 Hz), 122.8, 121.8, 119.0, 118.6, 111.4, 110.3, 98.1, 66.7, 50.3, 50.0. HRMS (ESI, m/z) calcd for C25H19F6N3O2 ([M + H]+): 508.1460. Found: 508.1459.
Ethyl (E)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-1H-pyrrolo[2,3-b]pyridin-3-yl)-2-cyanoacrylate (JXL082)
1H NMR (500 MHz, CDCl3) δ 8.64 (s, 1H), 8.51 (s, 1H), 8.48 (dd, J = 4.6, 1.2 Hz, 1H), 8.21 (dd, J = 7.9, 1.2 Hz, 1H), 7.83 (s, 1H), 7.77 (s, 2H), 7.34 (dd, J = 7.9, 4.6 Hz, 1H), 5.69 (s, 2H), 4.38 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.3, 147.6, 145.7, 145.0, 138.5, 132.7, 132.3 (q, Jc-f = 33.6 Hz), 127.9, 127.7, 123.1 (q, Jc-f = 273.3 Hz), 122.5, 120.3, 119.0, 117.6, 109.4, 97.0, 62.3, 48.3, 14.3. HRMS (ESI, m/z) calcd for C22H15F6N3O2 ([M + H]+): 468.1147. Found: 468.1146.
Ethyl (Z)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-2-cyano-3-hydroxyacrylate (JXL083)
1H NMR (500 MHz, CDCl3) δ 14.61 (s, 1H), 8.67 (s, 1H), 8.31 (dd, J = 7.0 1.3 Hz, 1H), 7.84 (s, 1H), 7.57 (s, 2H), 7.33 (m, 2H), 7.21 (d, J = 7.4 Hz, 1H), 5.53 (s, 2H), 4.40 (q, J = 7.1 Hz, 2H), 1.41 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 179.1, 172.2, 138.1, 136.0, 135.7, 132.7 (q, Jc-f = 33.6 Hz), 126.9, 124.5, 123.7, 123.6, 122.6, 122.9 (q, Jc-f = 273.4 Hz), 121.8, 118.3, 110.1, 109.6, 73.1, 62.3, 50.4, 14.3. HRMS (ESI, m/z) calcd for C23H16F6N2O3 ([M + H]+): 483.1143. Found: 483.1145.
Ethyl (Z)-3-acetoxy-3-(1-(3,5-bis(trifluoro-methyl)benzyl)-1H-indol-3-yl)-2-cyanoacrylate (JXL084)
1H NMR (500 MHz, CDCl3) δ 8.56 (s, 1H), 7.90 (m, 1H), 7.85 (s, 1H), 7.61 (s, 2H), 7.32 (m, 2H), 7.24 (m, 1H), 5.51 (s, 2H), 4.29 (q, J = 7.1 Hz, 2H), 2.48 (s, 3H), 1.36 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 166.9, 161.4, 137.7, 136.2, 135.5, 132.8 (q, Jc-f = 33.9 Hz), 127.0, 126.7, 124.6, 123.6, 122.8 (q, Jc-f = 273.4 Hz), 122.7, 121.8, 117.7, 110.7, 109.8, 89.3, 61.8, 50.5, 29.7, 21.3, 14.2. HRMS (ESI, m/z) calcd for C25H18F6N2O4 ([M + H]+): 525.1249. Found: 525.1233.
Ethyl (Z)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-3-chloro-2-cyanoacrylate (JXL085)
1H NMR (500 MHz, CDCl3) δ 7.91 (s, 1H), 7.85 (s, 1H), 7.77 (m, 1H), 7.65 (s, 2H), 7.63 (s, 1H), 7.30 (m, 2H), 5.48 (s, 2H), 4.18 (q, J = 7.1 Hz, 2H), 1.18 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 161.0, 155.6, 138.1, 136.2, 135.4, 133.4, 132.7 (q, Jc-f = 33.7 Hz), 127.1, 126.6, 124.5, 124.3, 122.8 (q, Jc-f = 273.4 Hz), 121.5, 115.8, 122.1, 110.4, 102.5, 62.4, 50.0, 13.9. HRMS (ESI, m/z) calcd for C23H15ClF6N2O2 ([M + H]+): 501.0804. Found: 501.0803.
Diethyl (E)-(2-(1-(3,5-bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-1-cyanovinyl)phosphonate (JXL086)
1H NMR (500 MHz, CDCl3) δ 8.55 (s, 1H), 8.33 (d, J = 19.7 Hz, 1H), 7.86 (d, J = 7.9 Hz, 1H), 7.83 (s, 1H), 7.58 (s, 2H), 7.31 (m, 2H), 7.22 (m, 1H), 5.54 (s, 2H), 4.21 (m, 4H), 1.39 (t, J = 7.0 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 149.8, 138.3, 135.8, 132.7 (q, Jc-f = 33.7 Hz), 132.6, 128.2, 126.8, 124.5, 123.0, 122.9 (q, Jc-f = 273.4 Hz), 122.5, 119.0, 117.8 (d, Jc-p = 11.3 Hz), 112.2 (d, Jc-p = 18.9 Hz), 110.3, 91.6 (d, Jc-p = 207.9 Hz), 63.2, 50.4, 16.3. HRMS (ESI, m/z) calcd for C24H21F6N2O3P ([M + H]+): 531.1272. Found: 531.1274.
Ethyl (E)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-4-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)-2-cyanoacrylate (JXL087)
1H NMR (500 MHz, CDCl3) δ 9.22 (s, 1H), 8.77 (s, 1H), 8.32 (d, J = 5.2 Hz, 1H), 7.83 (s, 1H), 7.77 (s, 2H), 7.31 (d, J = 5.2 Hz, 1H), 5.67 (s, 2H), 4.37 (q, J = 7.1 Hz, 2H), 1.39 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.0, 148.5, 146.2, 145.4, 138.1, 137.4, 133.1, 132.4 (q, Jc-f = 33.7 Hz), 128.0, 122.9 (q, Jc-f = 273.4 Hz), 122.6, 121.8, 117.6, 117.2, 109.7, 97.8, 62.4, 48.6, 14.3. HRMS (ESI, m/z) calcd for C22H14ClF6N3O2 ([M + H]+): 502.0757. Found: 502.0756.
Ethyl (E)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-4-bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)-2-cyanoacrylate (JXL088)
1H NMR (500 MHz, CDCl3) δ 9.40 (s, 1H), 8.78 (s, 1H), 8.22 (d, J = 5.1 Hz, 1H), 7.83 (s, 1H), 7.77 (s, 2H), 7.49 (d, J = 5.1 Hz, 1H), 5.67 (s, 2H), 4.37 (q, J = 7.1 Hz, 2H), 1.39 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.1, 148.1, 145.7, 145.1, 138.1, 137.4, 133.4, 132.4 (q, Jc-f = 33.7 Hz), 123.7, 122.9 (q, Jc-f = 273.4 Hz), 122.6, 119.7, 117.6, 110.0, 101.4, 97.4, 62.4, 48.6, 14.3. HRMS (ESI, m/z) calcd for C22H14BrF6N3O2 ([M + H]+): 546.0252. Found: 546.0238.
Ethyl (E)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-5-chloro-1H-indol-3-yl)-2-cyanoacrylate (JXL089)
1H NMR (500 MHz, CDCl3) δ 8.60 (s, 1H), 8.50 (s, 1H), 7.85 (s, 1H), 7.83 (s, 1H), 7.55 (s, 2H), 7.28 (dd, J = 8.7, 1.7 Hz, 1H), 7.14 (d, J = 8.7 Hz, 1H), 5.54 (s, 2H), 4.38 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.2, 144.8, 137.7, 134.3, 134.1, 132.8 (q, Jc-f = 33.7 Hz), 129.7, 129.3, 126.7, 125.1, 122.8 (q, Jc-f = 273.4 Hz), 122.7, 118.9, 117.7, 111.6, 110.6, 96.9, 62.3, 50.6, 14.3. HRMS (ESI, m/z) calcd for C23H15ClF6N2O2 ([M + H]+): 501.0804. Found: 501.0801.
Ethyl (E)-3-(1-(3,5-bis(trifluoromethyl)benzyl)-4-cyano-1H-indol-3-yl)-2-cyanoacrylate (JXL090)
1H NMR (500 MHz, CDCl3) δ 9.29 (s, 1H), 8.78 (s, 1H), 7.87 (s, 1H), 7.68 (dd, J = 7.4, 0.8 Hz, 1H), 7.56 (s, 2H), 7.49 (dd, J = 8.4, 0.8 Hz, 1H), 7.38 (dd, J = 8.4, 7.4 Hz, 1H), 5.62 (s, 2H), 4.38 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 162.6, 144.3, 137.3, 136.2, 135.1, 132.9 (q, Jc-f = 33.7 Hz), 129.4, 127.3, 126.7, 124.1, 123.0, 122.8 (q, Jc-f = 273.4 Hz), 117.9, 117.5, 115.4, 110.7, 103.5, 98.6, 62.4, 50.6, 14.3. HRMS (ESI, m/z) calcd for C24H15F6N3O2 ([M + H]+): 492.1147. Found: 492.1149.
Methyl (E)-1-(3,5-bis(trifluoromethyl)benzyl)-3-(2-cyano-3-ethoxy-3-oxoprop-1-en-1-yl)-1H-indole-4-carboxylate (JXL091)
1H NMR (500 MHz, CDCl3) δ 9.36 (s, 1H), 8.71 (s, 1H), 7.93 (dd, 1H, J = 7.4, 1.1 Hz, 1H), 7.85 (s, 1H), 7.55 (s, 2H), 7.40 (dd, J = 8.3, 1.1 Hz, 1H), 7.34 (dd, J = 8.3, 7.4 Hz, 1H), 5.58 (s, 2H), 4.37 (q, J = 7.1 Hz, 2H), 4.04 (s, 3H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 167.8, 163.5, 150.1, 137.8, 137.2, 134.9, 132.8 (q, Jc-f = 33.7 Hz), 126.7, 125.8, 125.1, 123.6, 122.8 (q, Jc-f = 273.4 Hz), 122.7, 121.7, 118.0, 114.6, 111.3, 96.4, 62.1, 52.6, 50.5, 14.3. HRMS (ESI, m/z) calcd for C25H18F6N2O4 ([M + H]+): 525.1249. Found: 525.1238.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-4-(methoxycarbonyl)-1H-indol-3-yl)-2-cyanoacrylic acid (JXL092)
1H NMR (500 MHz, CDCl3) δ 9.09 (s, 1H), 8.58 (s, 1H), 7.75 (d, J = 7.5 Hz, 1H), 7.68 (s, 1H), 7.47 (s, 2H), 7.34 (d, J = 8.2 Hz, 1H), 7.20 (app. t, J = 7.9 Hz, 1H), 5.51 (s, 2H), 3.87 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.8, 165.1, 149.5, 138.2, 137.2, 134.8, 132.3 (q, Jc-f = 33.7 Hz), 126.8, 126.2, 125.5, 124.8, 123.3, 122.8 (q, Jc-f = 273.4 Hz), 122.3 118.2, 114.7, 110.9, 97.2, 52.4, 50.2. HRMS (ESI, m/z) calcd for C23H14F6N2O4 ([M + H]+): 497.0936. Found: 497.0939.
Ethyl (E)-3-(4-chloro-1-phenyl-1H-indol-3-yl)-2-cyanoacrylate (JXL093)
1H NMR (500 MHz, CDCl3) δ 9.51 (s, 1H), 8.78 (s, 1H), 7.59 (m, 2H), 7.51 (m, 3H), 7.40 (dd, J = 8.3, 0.8 Hz, 1H), 7.32 (dd, J = 7.7, 0.8 Hz, 1H), 7.22 (app. t, J = 8.0 Hz, 1H), 4.38 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 163.6, 147.6, 138.1, 137.4, 134.2, 130.1, 129.0, 126.9, 125.3, 124.6, 124.5, 124.3, 118.0, 111.8, 110.6, 96.0, 62.1, 14.3. HRMS (ESI, m/z) calcd for C20H15ClN2O2 ([M + H]+): 351.0900. Found: 351.0900.
(E)-3-(1-(3,5-Bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-2-cyanoacrylamide (JXL094)
1H NMR (500 MHz, CDCl3) δ 8.70 (s, 1H), 8.47 (s, 1H), 7.92 (d, J = 7.1 Hz, 1H), 7.85 (s, 1H), 7.58 (s, 2H), 7.34 (m, 2H), 7.23 (m, 1H), 5.55 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 163.0, 144.8, 138.1, 136.0, 132.7 (q, Jc-f = 33.7 Hz), 132.5, 128.6, 126.8, 126.1, 124.6, 123.1, 122.9 (q, Jc-f = 273.4 Hz), 122.6, 119.3, 111.3, 110.3, 96.1, 50.4. HRMS (ESI, m/z) calcd for C21H13F6N3O ([M + H]+): 438.1041. Found: 438.1046.
Diethyl (E)-(2-(1-(3,5-bis(trifluoromethyl)benzyl)-1H-pyrrolo[2,3-b]pyridin-3-yl)-1-cyanovinyl)phosphonate (JXL095)
1H NMR (500 MHz, CDCl3) δ 8.58 (s, 1H), 8.46 (dd, J = 4.7, 1.4 Hz, 1H), 8.24 (d, J = 19.5 Hz 1H), 8.20 (dd, J = 8.0, 1.4 Hz, 1H), 7.82 (s, 1H), 7.77 (s, 2H), 7.32 (dd, J = 8.0, 4.7 Hz, 1H), 5.67 (s, 2H), 4.22 (m, 4H), 1.40 (t, J = 7.1 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 149.2 (d, Jc-p = 8.2 Hz), 147.4, 145.6, 138.6, 132.4 (q, Jc-f = 33.7 Hz), 132.0, 127.9, 127.8, 123.0 (q, Jc-f = 273.4 Hz), 122.4, 119.9, 118.9, 117.4 (d, Jc-p = 11.3 Hz), 110.5 (d, Jc-p = 19.5 Hz), 92.8 (d, Jc-p = 205.1 Hz), 63.4, 48.3, 16.3. HRMS (ESI, m/z) calcd for C23H20F6N3O3P ([M + H]+): 532.1225. Found: 532.1238.
(E)-(2-(1-(3,5-Bis(trifluoromethyl)benzyl)-1H-indol-3-yl)-1-cyanovinyl)phosphonic acid (JXL096)
1H NMR (500 MHz, CD3OD) δ 8.58 (s, 1H), 8.25 (d, J = 19.6 Hz, 1H), 7.90 (s, 1H), 7.87 (m, 1H), 7.76 (s, 2H), 7.45 (m, 1H), 7.31 (m, 2H), 5.75 (s, 2H). 13C NMR (126 MHz, CD3OD) δ 146.7 (Jc-p = 7.2 Hz), 140.2, 136.1, 132.1, 131.9 (q, Jc-f = 33.7 Hz), 128.0, 127.2, 123.8, 122.3, 123.2 (q, Jc-f = 273.4 Hz), 121.4, 118.2, 117.4 (d, Jc-p = 11.3 Hz), 111.5 (Jc-p = 18.4 Hz), 110.5, 94.6 (d, Jc-p = 201.2 Hz), 49.1. HRMS (ESI, m/z) calcd for C20H13F6N2O3P ([M + H]+): 475.0646. Found: 475.0642.
In vitro evaluation of the analogues’ activity in promoting lactate production rate.
Human breast epithelial (MCF10A) cells were obtained from the American Type Culture Collection and cultured in DMEM:F12 media supplemented with 5% horse serum, 50 U/mL penicillin-streptomycin, 10 μg/mL insulin, 0.5 μg/mL hydrocortisone, 20 ng/mL EGF, and 10 μg/mL cholera toxin. To determine whether the analogues could promote cellular lactate production, MCF10A cells were treated with analogues and lactate concentrations were measured using a BioProfile Basic Analyzer (Nova Biomedical). Briefly, MCF10A cells were seeded in 6-well plates in triplicate, treated the following day with 10 μM analogue, or equivalent volume of DMSO, for 24-30 hours. Culture media lactate concentrations were measured and normalized to cell number and duration of the experiment to calculate a cellular lactate production rate (nmol lactate per million cells per hour). Ordinary one-way ANOVA followed by Dunnett's post-test was performed using GraphPad Prism 8.3.0, comparing each analogue to DMSO.
A Seahorse XF Analyzer (Agilent) was used to measure oxygen consumption rate (OCR) to calculate IC50 values for several of the analogues. MCF10A cells were seeded in 96 well plates, treated with half-log doses of analogue, ranging from 3 nM to 10 μM, in the presence of 5 mM pyruvate and 0.5 mM malate. OCR (fmol O2 per cell per min) was measured in duplicate and normalized to cell number. IC50 values were calculated using Prism (GraphPad).
In vivo evaluation of the selected analogues’ activity in accelerating hair growth on mice.
To determine the efficacy of the compounds on the hair cycle, wildtype C57BL/6J mice were shaved at postnatal day 50, and topically treated with the compounds disclosed herein suspended in lotion (20μM) every other day for up to 3 weeks. The lotion used was Transderma Plo Ultramax gel. The mice were treated topically every other day with ~300ul at 20uM. Photographs were taken every week to monitor pigmentation and hair growth.
All mice were kept under defined pathogen-free conditions at the AAALAC-approved animal facility of the Division of Laboratory Animals (DLAM) at UCLA. All animal experiments were performed with the approval of the UCLA Office of Animal Resource Oversight (OARO); the approval number is ARC # 2016-055-11A.
Supplementary Material
ACKNOWLEDGMENT
Dr. Xiaoguang Liu is a postdoctoral fellow supported by the UCLA Tumor Cell Biology Training Program (USHHS Ruth L. Kirschstein Institutional National Research Service Award #T32 CA009056). We thank Linsey Stiles at the UCLA Mitochondria Core for help with the Seahorse assays.
ABBREVIATIONS
- MPC
mitochondrial pyruvate carrier
- LDH
lactate dehydrogenase
- TZD
thiazolidinedinone
- OCR
oxygen consumption rate
- DMSO
dimethyl sulfoxide
Footnotes
Supporting Information.
This material is available free of charge via the Internet at http://pubs.acs.org.
High field proton and carbon NMR spectra for compounds JXL001–JXL096 (PDF)
Molecular formula strings (CSV)
X. L., H. R. C., W. E. L. and M. E. J. are coauthors on a patent application that includes the molecules described in this manuscript. The authors declare no competing financial interest.
REFERENCES
- 1.Vary JC Jr. Selected Disorders of Skin Appendages-Acne, Alopecia, Hyperhidrosis. Med. Clin. North Am 2015, 99, 1195–1211. [DOI] [PubMed] [Google Scholar]
- 2.Rogers NE; Avram MR Medical Treatments for Male and Female Pattern Hair Loss. J. Am. Acad. Dermatol 2008, 59, 547–566. [DOI] [PubMed] [Google Scholar]
- 3.Stenn KS; Paus R Controls of Hair Follicle Cycling. Physiol. Rev 2001, 81, 449–494. [DOI] [PubMed] [Google Scholar]
- 4.Hsu YC; Pasolli HA; Fuchs E Dynamics Between Stem Cells, Niche, and Progeny in The Hair Follicle. Cell, 2011, 144, 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs E The Tortoise and The Hair: Slow-Cycling Cells in The Stem Cell Race. Cell, 2009, 137, 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs E; Merrill BJ; Jamora C; DasGupta R At The Roots of A Never-Ending Cycle. Dev. Cell 2001, 1, 13–25. [DOI] [PubMed] [Google Scholar]
- 7.Flores A; Schell J; Krall AS; Jelinek D; Miranda M; Grigorian M; Braas D; White AC; Zhou JL; Graham NA; Graeber T; Seth P; Evseenko D; Coller HA; Rutter J; Christofk HR; Lowry WE Lactate Dehydrogenase Activity Drives Hair Follicle Stem Cell Activation. Nat. Cell Biol 2017, 19, 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herzig S; Raemy E; Montessuit S; Veuthey JL; Zamboni N; Westermann B; Kunji ERS; Martinou JC Identification and Functional Expression of The Mitochondrial Pyruvate Carrier. Science 2012, 337, 93–96. [DOI] [PubMed] [Google Scholar]
- 9.Bricker DK; Taylor EB; Schell JC; Orsak T; Boutron A; Chen YC; Cox JE; Cardon CM; Van Vranken JG; Dephoure N; Redin C; Boudina S; Gygi SP; Brivet M; Thummel CS; Rutter J A Mitochondrial Pyruvate Carrier Required for Pyruvate Uptake in Yeast, Drosophila, and Humans. Science 2012, 337, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halestrap AP The Mechanism of the Inhibition of The Mitochondrial Pyruvate Transporter by α-Cyanocinnamate Derivatives. Biochem. J 1976, 156, 181–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shearman MS; Halestrap AP The Concentration of the Mitochondrial Pyruvate Carrier in Rat Liver and Heart Mitochondria Determined with α-cyano-β-(1-phenylindol-3-yl)acrylate. Biochem. J 1984, 223, 673–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong Y; Li X; Yu D; Li X; Li Y; Long Y; Yuan Y; Ji Z; Zhang M; Wen JG; Nesland JM; Suo Z Application of Mitochondrial Pyruvate Carrier Blocker UK-5099 Creates Metabolic Reprogram and Greater Stem-Like Properties in LnCap Prostate Cancer Cells in vitro. Oncotarget, 2015, 6, 37758–37769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, Wall EA, Yadava N, Heuck AP, Ferrick DA, Henry RR, McDonald WG, Colca JR, Simon MI, Ciaraldi TP, Murphy AN Thiazolidinediones Are Acute, Specific Inhibitors of The Mitochondrial Pyruvate Carrier. Proc. Natl. Acad. Sci. U.S.A, 2013, 110, 5422–5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.