Abstract
Coyotes (Canis latrans) are a highly adaptable canid species whose behavioral plasticity has allowed them to persist in a wide array of habitats throughout North America. As generalists, coyotes can alter movement patterns and change territorial strategies between residency (high site fidelity) and transiency (low site fidelity) to maximize fitness. Uncertainty remains about resident and transient coyote movement patterns and habitat use because research has reached conflicting conclusions regarding patterns of habitat use by both groups. We quantified effects of habitat on resident and transient coyote movement behavior using first passage time (FPT) analysis, which assesses recursive movement along an individual's movement path to delineate where they exhibit area‐restricted search (ARS) behaviors relative to habitat attributes. We quantified monthly movement rates for 171 coyotes (76 residents and 53 transients) and then used estimated FPT values in generalized linear mixed models to quantify monthly habitat use for resident and transient coyotes. Transients had greater movement rates than residents across all months except January. Resident FPT values were positively correlated with agricultural land cover during fall and winter, but negatively correlated with agriculture during spring. Resident FPT values were also negatively correlated with developed habitats during May–August, deciduous land cover during June–August, and wetlands during September–January except November. FPT values of transient coyotes were positively correlated with developed areas throughout much of the year and near wetlands during July–September. Transient FPT values were negatively correlated with agriculture during all months except June and July. High FPT values (ARS behavior) of residents and transients were generally correlated with greater densities of edge habitat. Although we observed high individual variation in space use, our study found substantive differences in habitat use between residents and transients, providing further evidence that complexity and plasticity of coyote habitat use is influenced by territorial strategy.
Keywords: Canis latrans, first passage time, space use, transiency
We utilized first passage time analysis to assess differences in movement patterns of resident and transient coyotes in the southeastern United States. Although we observed high individual variation in space use and habitat selection, our study found substantive differences in habitat selection between resident and transient coyotes, providing further evidence that complexity and plasticity of coyote habitat selection is influenced by social status.

1. INTRODUCTION
Coyotes (Canis latrans) are a highly adaptable canid whose behavioral plasticity has allowed them to persist in a wide array of habitats and climates, ranging from relatively undisturbed natural areas to highly developed urban environments (DeCandia et al., 2019; Gerht et al., 2009; Gompper, 2002). As opportunistic generalists, coyotes are able to switch among various food resources (Patterson et al., 1998; Randa et al., 2009), adjust their movement patterns to minimize conflicts with conspecifics, other predators, and humans (Berger & Gese, 2007; Fedriani et al., 2001), and change individual social strategies to maximize survival and reproduction (Macdonald, 1983). These characteristics have facilitated an extensive range expansion and growth of coyote populations over the past century, while other canid populations have declined (Hinton et al., 2019).
Range expansion of coyotes has had several impacts on newly colonized ecosystems, including altering prey population dynamics (Crimmins et al., 2012; Kilgo et al., 2010; Waser et al., 2014) and increasing interference competition for resources among established predator populations (Berger & Gese, 2007; Harrison et al., 1989; Johnson et al., 1996). Many of these observed trends are thought to be density dependent, with impacts becoming more pronounced as coyote populations increase and animals saturate the landscape (Gompper, 2002). As a result, managers and researchers recognize the need for a more comprehensive understanding of coyote spatial ecology, particularly territoriality and habitat selection, in recently colonized regions.
Adult coyotes typically exhibit one of two patterns of territorial space use: residency or transiency. Residents maintain small, mutually exclusive home ranges as breeding pairs, whereas transients typically move across landscapes without a social group and often overlap with other individuals’ home ranges (Gese, 2004; Hinton et al., 2015; Kamler & Gipson, 2000; Morin & Kelly, 2017). Territorial status has substantive implications for how coyotes interact with their surrounding environments, including habitat use and prey selection (Mills & Knowlton, 1991; Ward et al., 2018). Transient coyotes differ from residents because they are individuals who typically move alone, exhibit low site fidelity, and do not breed (Carmenzind, 1978; Hinton et al., 2015; Kamler & Gipson, 2000). Because they maintain territories with mates, residents have greater foraging success (Gese et al., 1996) and lower mortality rates (Knowlton et al., 1999) than do transients. Recent research on the spatial ecology of transient coyotes has focused on the space use (i.e., biding areas; Hinton et al., 2012, 2015) and behaviors (i.e., biding; Morin & Kelly, 2017) prior to transients establishing residency.
Several studies have investigated coyote space use and habitat selection, but relatively few have differentiated selection between resident and transient behaviors when conducting their analyses. Of those that made this differentiation, all noted that resident coyotes were found to select for open grassland, pasture, and agricultural habitats while avoiding developed habitats (Hinton et al., 2015; Kamler & Gipson, 2000). However, patterns of habitat selection for transient coyotes are more ambiguous. Kamler and Gipson (2000) found transients avoided grasslands and selected woodlands, whereas Hinton et al. (2015) found transient coyotes exhibited similar selection trends to residents by selecting open habitats, although transients were more likely to use roads than residents. Transient coyotes have also been documented using habitats associated with human development (Gerhrt et al., 2009; Mitchell et al., 2015). Notably, previous studies faced logistical and practical limitations in sample sizes or data resolution (VHF vs. GPS technology) that may have impacted observed trends (Hinton et al., 2015). Additionally, most previous research has quantified habitat selection by both residents and transients based on an individual's estimated home range (e.g., 3rd‐order resource selection functions [RSF]), an approach that may not be appropriate for transient coyotes who do not have stable home ranges over time (Morin & Kelly, 2017). For species who do not maintain stable home ranges, characterization of movement behaviors along an individual's movement path and association of those behaviors with the habitats in which they occur may be a more appropriate approach to determine habitat selection.
One such approach, first passage time (FPT) analyses (Fauchald & Tverra, 2003), allows for fine‐scale delineation of where an animal is spending time by estimating when an individual is exhibiting area‐restricted search behavior (ARS; i.e., slow travel speed and high tortuosity) along its movement path. Low FPT values are associated with faster linear movements (non‐ARS behavior), whereas higher FPT values indicate an animal's movements are slower and more sinuous (ARS behaviors). By using FPT analyses, researchers can assess residency time based on where an animal is engaging in ARS behaviors (e.g., foraging) vs. non‐ARS behavior (e.g., traveling), and these methodologies have successfully been used previously to investigate fine‐scale habitat selection of other mesocarnivores such as raccoons (Procyon lotor; Fauchald & Tverra, 2003; Byrne & Chamberlain, 2012). Additionally, FPT analyses do not rely on estimated home ranges required by traditional resource selection methodologies, ultimately reducing uncertainty in inferred patterns of correlation between ARS behaviors and environmental characteristics, especially for individuals that do not maintain home ranges.
Thus, our goal was to quantify the relationships between habitat characteristics and ARS behaviors of resident and transient coyotes across the southeastern United States using FPT analyses to distinguish spatiotemporal patterns of residency time for both groups. Because resident and transient coyotes are known to exhibit different preferences for land cover types (Hinton et al., 2015; Kamler & Gipson, 2000), we hypothesized that the differing territorial strategies and movement behaviors of resident and transient coyotes would influence space use and FPT values in relation to various land cover types. We predicted that transient coyote ARS behaviors (i.e., high FPT values) would be positively correlated with land cover types associated with travel corridors, such as human development and edge habitats, both of which have been found important to transient coyotes in previous studies (Gerhrt et al., 2009; Hinton et al., 2015). Contrarily, we expected ARS behaviors (i.e., high FPT values) of resident coyotes to be negatively correlated with human development but correlated with open land cover (e.g., agriculture) that were reported to be important land cover preferred by coyotes (Hinton et al., 2015; Kamler & Gipson, 2000). Finally, because resident coyotes form breeding pairs to defend territories and raise offspring whereas transient coyotes are solitary animals primarily dispersing from natal areas, we hypothesized that differences in resident and transient reproductive behaviors would affect spatiotemporal patterns in movement rates. We predicted that resident coyotes would exhibit reduced movement rates relative to transients during months when they were likely to be raising offspring.
2. METHODS
2.1. Study area
Our study area included regions of Alabama (Barbour, Macon, and Pike Counties), Georgia (Columbia, Jefferson, Lincoln, McDuffie, and Warren Counties), and South Carolina (Aiken, Barnwell, Edgefield, McCormick, and Saluda Counties) in the southeastern United States, totaling approximately 16,200 km2 (Figure 1). Coyotes captured in Georgia and South Carolina commonly moved between the respective study areas and likely represented one population, leaving two distinct study areas: the Alabama study area (ASA) and the Savannah River study area (SRA) of Georgia and South Carolina. Both study areas were comprised predominantly of privately owned land, but approximately 20% of the SRA was comprised of the Savannah River Site (SRS), an 803 km2 federal facility operated by the U.S. Department of Energy (DOE). Both study areas had mild subtropical climate throughout the year. Summers were generally hot and humid with an average high temperature of approximately 30°C, whereas winters were mild with an average low temperature of approximately 1°C (National Oceanic and Atmospheric Administration (NOAA), 2019). Habitats in both the ASA and the SRA were a mix of successional forest, agriculture, pastureland, pine plantations, and urban habitats. Agriculture in these regions included cotton (Gossypium spp.), corn (Zea mays), tobacco (Nicotiana tabacum), soybeans (Glycine max), and peanuts (Arachis hypogaea). For further details on our study areas see Ward et al. (2018).
FIGURE 1.
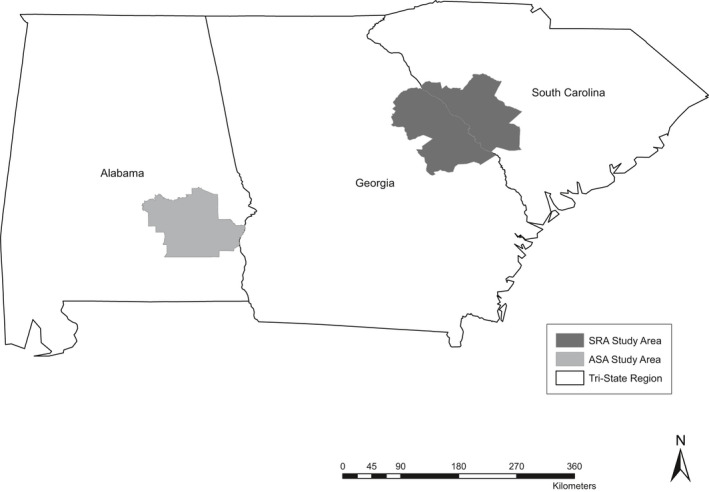
Alabama study area (ASA) and the Savannah River study area (SRA), located in Alabama, Georgia, and South Carolina, USA, where coyotes were captured and monitored with GPS collars during 2015–2017
2.2. Data collection
We deployed GPS collars on coyotes over three fall/winter seasons in 2015, 2016, and 2017. We captured animals with foothold traps (Victor #3 Softcatch, Woodstream Corporation, Lititz, Pennsylvania, USA; MB 550 or MB 450, Minnesota Trapline Products, Pennock, Minnesota, USA) with offset or padded jaws. During 2015–2016, animals were restrained with a catchpole, muzzle, and hobbles for processing. During 2017, we used chemical immobilization in addition to physical restraint because we collected biological samples (e.g., blood, feces, and parasites) in addition to fitting each animal with a collar. By using chemical immobilization when collecting these additional samples, we were able to minimize stress to the animal and reduce processing time. We anesthetized animals prior to processing using a ketamine/xylazine mixture administered at 0.8 ml/kg for ketamine and 0.1 ml/kg for xylazine. We then determined sex, weight, and age using tooth wear (Gipson et al., 2000). Coyotes >2 years old were considered adults, whereas 1–2‐year olds were considered juveniles, and animals <1 year old were classified as pups. We fitted each animal with a mortality‐sensitive satellite collar (either G2110E Iridium collar, Advanced Telemetry Systems, Isanti, Minnesota, USA or Litetrack Iridium collar, Lotek Wireless Inc., New Market, Ontario, Canada). Collars recorded locations at a 4‐h interval. Prior to release, we administered anesthetized animals yohimbine at 1.0 ml/kg. All animal handling procedures were approved by the University of Georgia Institutional Animal Care and Use Committee (protocols A2014 08‐025‐R2 and A2015 05‐004‐A5). To access lands to trap, state agencies and the DOE granted permission for publicly owned property while we obtained permission from landowners to access privately owned lands.
2.3. Movement data analysis
To determine territorial status of collared animals, we used a combination of ≥3 months of space use by coyotes (Hinton et al., 2015) and a rarefaction curve for each animal created by calculating monthly home ranges (Dellinger et al., 2013). Previous studies have found that resident coyotes in the southeastern U.S. maintain home ranges that range from approximately 5 to 45 km2 (Hinton et al., 2015, Mastro et al. 2019). Thus, we classified resident coyotes as animals that showed stable space use for ≥3 months and had home ranges smaller than 45 km2. Following Hinton et al. (2015), we classified transients as animals with ranges larger than 45 km2 who exhibited unstable space use over time. We estimated 95% home ranges and transient ranges and 50% core areas and biding areas using fixed kernel density with the reference (href) smoothing parameter (Worton, 1989). Using both methods for identifying territorial status allowed for confident classification of residents and transients, but also meant that we were unable to determine territorial status of animals with <3 months of movement data due to mortality or collar failure. If we were unable to determine territorial status for an individual, it was excluded from further analysis. For transient animals, we refer to space use patterns as biding areas because transients do not maintain territories (Hinton et al. 2012, 2015; Morin & Kelly, 2017).
Previous research has shown that coyote space use varies seasonally due to a variety of biological and ecological attributes (Hinton et al., 2015; Kamler & Gipson, 2000; Sasmal et al., 2019). However, the criteria researchers use to define ecologically or biologically relevant seasons typically varies among studies (e.g., seasons defined by environmental conditions vs. organism behaviors) depending upon the research question, data resolution, and study duration. Variation in season delineation can potentially bias results or mask important trends in spatial data (Basille et al., 2013; Thompson & McGarigal, 2002). To mitigate this issue, we decided to conduct all spatial analyses by month. Quantifying movement on a monthly basis allowed us to minimize potential bias due to misclassification of relevant seasons. We also quantified average movement rates for both resident and transient coyotes by dividing step length between two consecutive locations by the time interval (4 h) between those locations and compared movement rates per hour across months. Only locations with approximate 4‐hour time intervals (with a ~3‐min buffer allowed to account for occasional lags in satellite data transfer times) were included in analysis to minimize error associated with missing data. To determine whether movement behaviors between the two classes differed temporally, we used generalized linear mixed models (GLMMs) where movement rate was the response variable and territorial status (resident or transient) was the predictor variable. We included individual coyote as a random effect in all models and modeled each month separately. An alpha value of 0.05 was used to determine significance in all statistical tests.
We used FPT analyses following Fauchald and Tverra (2003) to quantify relationships between landscape features and monthly coyote space use and movement behaviors. FPT is the time required for an animal to cross a circle of a given radius (Johnson et al., 1992) and can be used to infer movement behaviors and inform residency times when FPT values are estimated along an individual's movement path. Low FPT values are associated with faster linear movements, whereas higher FPT values indicate an animal's movements are slower and more sinuous. More sinuous movements are inferred as ARS behavior, often associated with foraging or loafing behaviors. Thus, researchers are able to differentiate between different behavioral states (i.e., traveling vs. foraging/loafing) and quantify which habitats these behaviors occur within. We analyzed movement paths from resident and transient coyotes on a monthly basis by subsetting movement data by month and requiring an individual to have a minimum of 90 relocations within a month to be included in each monthly analysis. To determine the appropriate scale at which to estimate FPT values, we first interpolated locations every 20 m along movement paths and calculated FPT values at these locations for circles with radii ranging from 10 to 4000 m in 10 m increments. We then calculated the variance of log‐transformed FPT values for each trajectory and circle radius to determine at which radius the variance peaked, indicating the scale at which individuals were concentrating ARS behaviors (Fauchald & Tverra, 2003). This scale varied across individual movement paths, so we calculated an average scale across all individuals for each month for comparisons (Byrne & Chamberlain, 2012; Freitas et al., 2008). We then recalculated FPT values for all individuals using the averaged radius size for each month. By estimating FPT values at differing scales monthly and only including individuals which met robust data thresholds, we minimized bias introduced by seasonal and individual variation in movement patterns.
2.4. Habitat analyses
We assessed habitat composition of the study areas using a 30‐m resolution National Land Cover Database (NLCD) 2011 land cover raster layer. Using Spatial Analyst in ArcMap 10.3, we reclassified the NLCD raster layer into six primary land cover types: mixed deciduous forest, pine forest, wetland, agriculture, and developed. Because coyotes are known to use edge habitats (i.e., the boundary between two land cover types; Heske et al., 1999; Hinton et al., 2015; Tigas et al., 2002), we also calculated edge density within each habitat class using package “landscapemetrics” in Program R (Hesselbarth et al., 2019; R Core Team, 2018).
To determine which land cover characteristics were associated with ARS behaviors, we measured the distance of each location along an individual's movement path to each land cover type and quantified average edge density within a 100 m radius around each location. A distance‐based approach combined with a consistent measure of edge density at each location allowed for consistent quantification of an individual's spatial relationship to habitat covariates of interest (Benson, 2013) even as the scale at which FPT values were estimated varied across months. We then used a GLMM to determine whether areas with high FPT values (i.e., areas where individuals were engaging in ARS behaviors) were associated with particular land cover characteristics. We included FPT values as a continuous response variable in all models. Often, FPT values are reduced into two binary, categorical variables of high (ARS) and low (non‐ARS) values (Fauchald & Tverra, 2003). However, given the high level of individual variation we observed in sampled individuals, particularly among transient coyotes, creating a discrete threshold between FPT values in order to create a binary variable would likely introduce bias into our model interpretations. By quantifying FPT values as a continuous variable, we mitigated this potential bias and ultimately allowed for more nuanced interpretation of model outputs. We modeled resident and transient animals separately for each month, so the scale of FPT estimated values was consistent for all data included in a model. For both classes of coyote in each month, we ran a suite of six GLMMs with all land cover variables (mixed deciduous forest, pine forest, wetland, agriculture, developed, and edge habitat) and all biologically relevant subsets to test our predictions of resident and transient FPT values associated with various habitat types (Appendix 1). By including models with potentially biologically relevant variable subsets, we allowed for thorough analysis of the impacts of all six primary land cover types on coyote movement behaviors beyond those specifically identified in our predictions. Given the broad variation we observed among individuals, this conservative approach allowed us to be confident that the top‐ranked models were not only top ranked because a biologically important variable combination was excluded from analysis. In all models, we included individual coyote as an additive random effect to account for spatial and temporal autocorrelation between each individual's movement data. To avoid multicollinearity, we examined correlations among model variables by deriving a matrix of all possible Spearman correlation coefficient values. Any variables with a significant correlation (r 2 > .6; p < .05) were not simultaneously included in the same model in subsequent analysis. We also used variance inflation factor (VIF) to confirm variables were not displaying collinearity or instability (VIF > 5; Dormann et al., 2013; Kutner et al., 2004) and found no evidence of collinearity as all VIF was less than 2. We associated ARS behaviors with a particular land cover type when locations with high FPT values were significantly closer in distance (meters) to certain land cover types than locations with low FPT values. We then used Akaike's information criterion (AIC) to compare models and used the most parsimonious model to estimate model parameters, including beta coefficients (β), of correlation of habitat characteristics to ARS behaviors within the model. In the event that >1 model was within 2 AIC units of the top model, we model averaged to derive parameter estimates (Burnham & Anderson, 2002). We conducted all statistical analyses in Program R (R Core Team, 2018).
3. RESULTS
We deployed collars on 193 coyotes, 54 in the ASA and 139 in the SRA. We excluded 22 coyotes from analysis due to an insufficient number of relocations. Of the remaining 171 coyotes, 76 (44.4%) were residents and 53 (30.1%) were transients for the entire time they were monitored, whereas 42 (24.6%) exhibited both residency and transiency. We included individuals who were both residents and transients at different time periods during monitoring in analyses, but separated their movement paths into different paths during residency and transiency. Mean monthly 95% home range size for residents was 15.16 km2 (SD = 21.88 km2) and ranged from 11.18 to 30.51 km2, while mean 95% transient range size for transients was 368.81 km2 (SD = 799.80 km2) and ranged from 202.14 to 561.44 km2 (Figure 2; Appendix 2). Movement rates varied between residents and transients across all months except January and December, with transients generally having greater movement rates than residents (Figure 3; Appendix 3).
FIGURE 2.
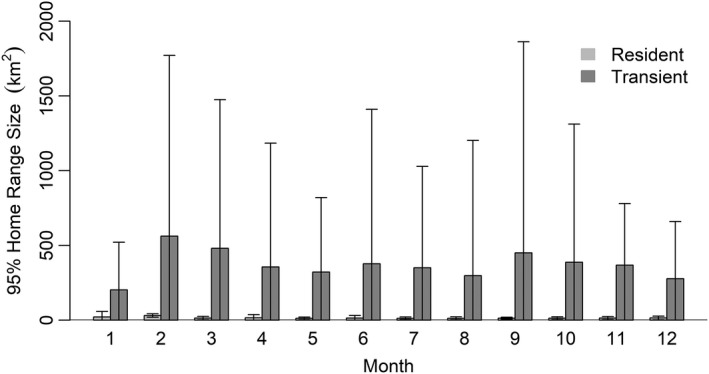
Mean monthly 95% home range or biding area estimates for resident and transient coyotes, respectively
FIGURE 3.
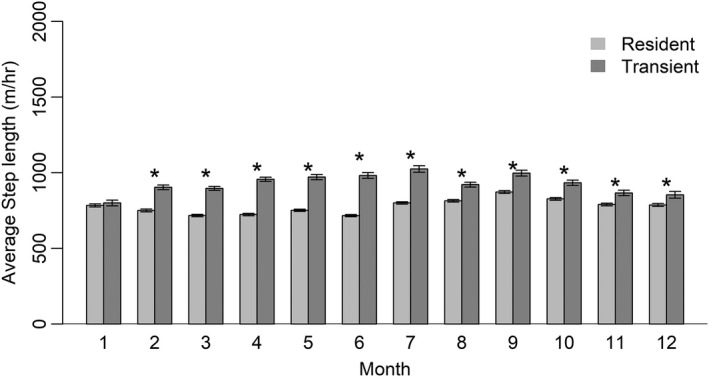
Average monthly movement rate for resident and transient coyotes monitored from January 2015 to June 2017 in the tristate region of Alabama, Georgia, and South Carolina. Error bars shown represent standard error, and asterisks denote significant differences between groups
We evaluated 1,501 monthly movement paths of individual coyotes (900 residents and 601 transients), with the number of individuals included in each month ranging from 52 to 74 individuals. We observed high FPT values (ARS behaviors) in all monthly movement datasets analyzed, and the average radius at which ARS behaviors occurred varied considerably across months (Figure 4). Modeling analyses revealed that all land cover variables affected ARS behaviors throughout the year; however, which variables were important and the direction of correlation (i.e., positive or negative) varied among months (Tables 1 and 2; Figure 5). Resident coyote FPT values were positively correlated with agriculture during fall and winter months, suggesting residents were more likely to engage in ARS behaviors near agricultural land cover during these months; however, FPT values were negatively correlated with agriculture during spring months. Resident FPT values were negatively correlated with developed habitats during May–August, deciduous land cover during June–August, and wetlands during September–January except during November (Figure 5). Edge density was positively correlated with resident FPT values in all months except April, June, July, and October (see Figure 5). Transient coyote FPT values were negatively correlated with developed areas throughout much of the year, suggesting transients were more likely to engage in ARS behavior near developed areas (Figure 5). Transient FPT values were also negatively correlated with wetlands during July–September. Transient FPT values were negatively correlated with agriculture across most months except June and July. Edge density was positively correlated with transient FPT values in all months except March, August, and November (Figure 5).
FIGURE 4.
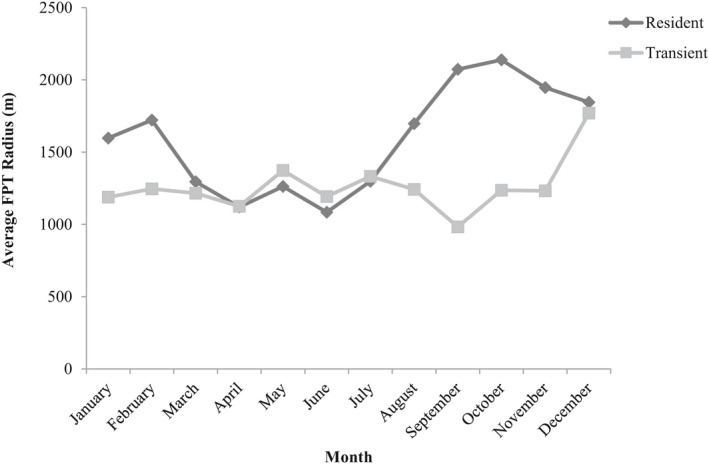
Average estimated radius at which first passage time (FPT) values were calculated each month for resident and transient coyotes in the tristate region of Alabama, Georgia, and South Carolina
TABLE 1.
Beta coefficient, standard error, t value, and p value estimates of the top‐ranked generalized linear mixed model (GLMM) estimating FPT values for resident coyotes monitored from January 2015 to June 2017 in Alabama, Georgia, and South Carolina
| Month | habitat type | Beta estimate | SE | t Value | p Value |
|---|---|---|---|---|---|
| January | Deciduous | −63.69 | 23.82 | −2.67 | <.001 |
| Wetland | 28.47 | 10.94 | 2.60 | <.001 | |
| Cropland | −19.35 | 7.79 | −2.48 | <.001 | |
| Develop | 60.12 | 10.73 | 5.60 | <.001 | |
| Pine Forest | 98.00 | 10.73 | 4.19 | <.001 | |
| Edge Density | 68.24 | 23.38 | 2.88 | <.001 | |
| February | Deciduous | 58.10 | 23.77 | 2.45 | .014 |
| Wetland | −28.47 | 11.39 | −2.49 | .014 | |
| Cropland | −20.92 | 7.25 | −2.89 | .003 | |
| Develop | −7.99 | 10.31 | −0.78 | .04 | |
| Pine Forest | −129.94 | 24.10 | −5.39 | <.001 | |
| Edge Density | 12.94 | 1.65 | 9.33 | <.001 | |
| March | Deciduous | −63.15 | 17.87 | −3.53 | <.001 |
| Wetland | −19.05 | 8.32 | −2.28 | .02 | |
| Cropland | −26.01 | 4.48 | −5.80 | <.001 | |
| Develop | 1.42 | 7.98 | 0.18 | .88 | |
| Pine Forest | 26.19 | 18.54 | 1.40 | .17 | |
| Edge Density | 14.26 | 6.25 | 2.34 | .02 | |
| April | Deciduous | 37.54 | 27.86 | 1.35 | .1 |
| Wetland | −39.42 | 11.68 | −3.38 | <.001 | |
| Cropland | 75.06 | 4.72 | 15.89 | <.001 | |
| Develop | 10.88 | 12.44 | 0.87 | .34 | |
| Pine Forest | −26.36 | 27.98 | −0.94 | .38 | |
| Edge Density | 24.25 | 18.77 | 1.01 | .27 | |
| May | Deciduous | 13.78 | 19.95 | 0.69 | .49 |
| Wetland | −17.23 | 8.37 | −2.06 | .04 | |
| Cropland | 11.18 | 3.25 | 3.44 | <.001 | |
| Develop | 32.69 | 9.41 | 3.47 | <.001 | |
| Pine Forest | −35.92 | 20.65 | −1.74 | .08 | |
| Edge Density | 19.16 | 2.12 | 1.24 | <.001 | |
| June | Deciduous | 72.92 | 21.56 | 3.38 | <.001 |
| Wetland | 15.33 | 9.30 | 1.65 | .09 | |
| Cropland | 70.38 | 3.51 | 20.07 | <.001 | |
| Develop | 31.91 | 10.28 | 3.10 | .001 | |
| Pine Forest | −1.16 | 23.77 | −0.05 | .9 | |
| Edge Density | 4.44 | 0.74 | 0.72 | .06 | |
| July | Deciduous | 42.52 | 21.69 | 1.96 | .04 |
| Wetland | −56.07 | 9.30 | −6.03 | <.001 | |
| Cropland | −2.39 | 3.41 | −0.70 | .48 | |
| Develop | 95.29 | 9.54 | 9.99 | <.001 | |
| Pine Forest | −26.90 | 23.06 | −1.17 | .24 | |
| Edge Density | 15.89 | 11.19 | 1.02 | .35 | |
| August | Deciduous | 38.17 | 26.92 | 1.42 | .04 |
| Wetland | −17.59 | 10.87 | −1.62 | <.001 | |
| Cropland | 3.74 | 3.42 | 1.09 | .48 | |
| Develop | 81.31 | 12.60 | 6.45 | <.001 | |
| Pine Forest | 49.00 | 29.28 | 1.67 | .24 | |
| Edge Density | 22.28 | 1.89 | 9.79 | <.001 | |
| September | Deciduous | 32.08 | 25.02 | 1.28 | .19 |
| Wetland | 50.70 | 10.23 | 4.95 | <.001 | |
| Cropland | 12.49 | 3.61 | 3.46 | <.001 | |
| Develop | −17.29 | 13.74 | −1.26 | .2 | |
| Pine Forest | −93.09 | 27.41 | −3.40 | <.001 | |
| Edge Density | 68.62 | 14.61 | 4.41 | <.001 | |
| October | Deciduous | −6.52 | 26.18 | −0.25 | .80 |
| Wetland | 30.49 | 11.35 | 2.69 | .007 | |
| Cropland | −12.89 | 4.04 | −3.19 | .001 | |
| Develop | 8.18 | 13.20 | 0.62 | .54 | |
| Pine Forest | −46.80 | 27.93 | −1.68 | .09 | |
| Edge Density | −1.24 | 6.77 | 0.23 | .66 | |
| November | Deciduous | 60.39 | 21.78 | 2.77 | .005 |
| Wetland | −3.27 | 9.29 | −0.35 | .73 | |
| Cropland | −33.12 | 5.17 | −6.40 | <.001 | |
| Develop | −45.65 | 10.34 | −4.10 | <.001 | |
| Pine Forest | 38.05 | 21.42 | 1.77 | <.001 | |
| Edge Density | 12.28 | 2.38 | 8.21 | <.001 | |
| December | Deciduous | 3.08 | 30.18 | 0.10 | .92 |
| Wetland | 44.39 | 12.53 | 3.54 | <.001 | |
| Cropland | −49.03 | 8.03 | −6.11 | <.001 | |
| Develop | 26.29 | 13.68 | 1.92 | .05 | |
| Pine Forest | 30.75 | 29.22 | 1.05 | .29 | |
| Edge Density | 52.97 | 6.05 | 5.78 | <.001 |
TABLE 2.
Beta coefficient, standard error, t value, and p value estimates of the top‐ranked generalized linear mixed model (GLMM) estimating the relationship between FPT values and land cover type for transient coyotes monitored from January 2015 to June 2017 in Alabama, Georgia, and South Carolina
| Month | Habitat type | Beta estimate | SE | t Value | p Value |
|---|---|---|---|---|---|
| January | Deciduous | −147.84 | 13.52 | −10.94 | <.001 |
| Wetland | 31.99 | 10.90 | 2.94 | .003 | |
| Cropland | 37.99 | 4.79 | 7.92 | <.001 | |
| Develop | 10.29 | 11.12 | 0.93 | .35 | |
| Pine Forest | 54.46 | 18.27 | 2.98 | .002 | |
| Edge Density | 21.28 | 5.68 | 4.67 | <.001 | |
| February | Deciduous | −78.59 | 12.97 | −6.06 | <.001 |
| Wetland | 15.16 | 5.67 | 2.67 | .007 | |
| Cropland | 42.24 | 2.25 | 18.79 | <.001 | |
| Develop | −20.49 | 6.80 | −3.01 | .002 | |
| Pine Forest | 22.53 | 10.36 | 2.18 | .02 | |
| Edge Density | 33.84 | 3.71 | 15.45 | <.001 | |
| March | Deciduous | −95.95 | 14.47 | −6.63 | <.001 |
| Wetland | 19.06 | 6.18 | 3.08 | .002 | |
| Cropland | 7.66 | 2.10 | 3.64 | <.001 | |
| Develop | −3.93 | 6.55 | −0.59 | .55 | |
| Pine Forest | −8.49 | 10.50 | 0.81 | .42 | |
| Edge Density | 5.58 | 2.72 | 1.72 | .06 | |
| April | Deciduous | −31.82 | 11.13 | −3.55 | 1.44 |
| Wetland | −6.96 | 5.73 | −1.41 | .16 | |
| Cropland | 10.33 | 2.14 | 6.31 | <.001 | |
| Develop | 15.48 | 6.06 | 2.87 | .004 | |
| Pine Forest | 96.24 | 12.36 | 10.44 | <.001 | |
| Edge Density | 19.51 | 2.63 | 16.35 | <.001 | |
| May | Deciduous | −5.53 | 8.39 | −1.66 | .62 |
| Wetland | 1.54 | 6.26 | −0.71 | .79 | |
| Cropland | 7.96 | 2.07 | −2.09 | <.001 | |
| Develop | 24.11 | 6.62 | −2.55 | <.001 | |
| Pine Forest | −8.70 | 13.38 | −6.22 | .48 | |
| Edge Density | 16.94 | 4.34 | 3.12 | <.001 | |
| June | Deciduous | −13.94 | 8.39 | −1.66 | .09 |
| Wetland | −4.45 | 6.26 | −1.71 | .48 | |
| Cropland | −4.32 | 2.07 | −2.09 | .04 | |
| Develop | −16.85 | 6.62 | −2.55 | .01 | |
| Pine Forest | −83.18 | 13.38 | −6.22 | <.001 | |
| Edge Density | 3.16 | 1.89 | 1.22 | .04 | |
| July | Deciduous | 13.84 | 6.25 | 2.22 | .02 |
| Wetland | −25.03 | 6.09 | −4.11 | <.001 | |
| Cropland | −5.38 | 2.14 | −2.52 | .012 | |
| Develop | −55.88 | 6.58 | −8.49 | <.001 | |
| Pine Forest | 49.43 | 10.71 | 4.61 | <.001 | |
| Edge Density | 24.37 | 8.81 | 2.69 | <.001 | |
| August | Deciduous | −15.30 | 10.35 | −1.48 | .14 |
| Wetland | −28.19 | 6.02 | −4.69 | <.001 | |
| Cropland | 4.82 | 2.01 | 2.40 | .01 | |
| Develop | −21.81 | 7.04 | −3.05 | .002 | |
| Pine Forest | −5.46 | 13.05 | −0.42 | .68 | |
| Edge Density | −0.23 | 6.92 | −0.02 | .95 | |
| September | Deciduous | −20.80 | 16.49 | −1.26 | .21 |
| Wetland | −15.09 | 7.88 | −1.91 | .05 | |
| Cropland | 44.99 | 2.85 | 15.76 | <.001 | |
| Develop | −69.98 | 9.48 | −7.38 | <.001 | |
| Pine Forest | 75.71 | 15.30 | 4.95 | <.001 | |
| Edge Density | 20.61 | 3.19 | 8.64 | <.001 | |
| October | Deciduous | −13.05 | 8.52 | −1.53 | .13 |
| Wetland | 2.18 | 4.56 | 0.48 | .63 | |
| Cropland | 2.79 | 1.64 | 1.69 | .09 | |
| Develop | −18.86 | 6.15 | −3.06 | .002 | |
| Pine Forest | 15.71 | 10.13 | 1.56 | .12 | |
| Edge Density | 9.48 | 1.83 | 6.34 | <.001 | |
| November | Deciduous | 5.27 | 12.83 | 4.11 | <.001 |
| Wetland | −4.22 | 7.68 | −0.05 | .96 | |
| Cropland | 3.51 | 3.23 | 10.72 | <.001 | |
| Develop | −2.10 | 9.83 | −2.13 | .033 | |
| Pine Forest | 2.53 | 15.43 | 1.64 | .11 | |
| Edge Density | 1.71 | 0.94 | 1.27 | .34 | |
| December | Deciduous | 148.06 | 21.68 | 6.83 | <.001 |
| Wetland | −94.08 | 11.21 | −8.39 | <.001 | |
| Cropland | 7.46 | 4.77 | 1.56 | .12 | |
| Develop | −19.25 | 13.15 | −1.46 | .14 | |
| Pine Forest | 57.99 | 18.77 | 3.09 | .002 | |
| Edge Density | 48.26 | 25.33 | 2.28 | .002 |
FIGURE 5.
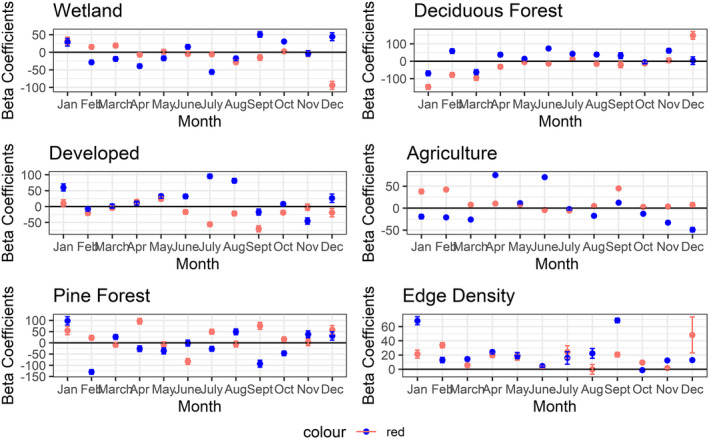
Beta coefficient estimates of habitat selection for resident and transient coyotes monitored from January 2015 to June 2017 in the tristate region of Alabama, Georgia, and South Carolina. Error bars shown represent standard error
4. DISCUSSION
We found that high FPT values (ARS behaviors) of coyotes correlated to specific land cover types across two large study areas in the Southeast, suggesting both resident and transient coyotes used particular habitats to engage in ARS behaviors such as foraging or loafing. Our results supported our first hypothesis that differences in territorial strategy (resident vs. transient) impacted space use and FPT values in relation to habitat characteristics. We found substantive variation in the direction and magnitude of correlations between high FPT values (ARS behaviors) and land cover type across months for both residents and transients, implying considerable temporal variation in individual behavior. This finding is not entirely surprising, as habitat selection by coyotes has been shown to be highly variable and context dependent, even for resident individuals (Gosselink et al., 2003; Harrison et al., 1991; Patterson & Messier, 2001). Additionally, contrary to traditional RSF approaches that rely solely on an animal's physical location to infer selection or use of particular habitats, FPT analysis accounts for the animal's movement path and associates physical locations with biological activities such as foraging or dinning (Fauchald & Tverra, 2003). Thus, although coyotes may be more likely to be near particular habitats throughout time, our findings suggest they are likely engaging in ARS behaviors in a diversity of habitats, reflecting their behavioral plasticity and generalist foraging strategy (Gosselink et al., 2003; Hinton et al., 2017; Ward et al., 2018).
4.1. Movement rates
We observed that movement rates varied across months for both residents and transients, although transient movement rates were greater than those of residents in all months except January. Previous work has found that transients typically have larger ranges (Hinton et al., 2015; Kamler & Gipson, 2000) and greater movement rates than residents (Sasmal et al., 2019). Our estimated monthly movement rates of coyotes were generally less than those previously reported in other studies for both residents (165.5–202.1 m/h vs. 295.3–449.8 m/h; Sasmal et al., 2019) and transients (183.4–229.7 m/h vs. 283.0–488.5 m/h; Sasmal et al., 2019). These differences likely arise from differences in classification criteria for residents and transients, as well as differences in the temporal scale at which movement rates were calculated between studies (differences in relocation fix rate and monthly vs. seasonal study periods) and our increased sample size, which would minimize the effect of outlier movement steps (i.e., long‐distance dispersal). Residents had lower movement rates during breeding and pup‐rearing season (March–August), with the lowest movement rates in June, a time when pups are likely emerging from the den yet still have limited mobility, thus indirectly limiting mobility of adults caring for pups (Andelt, 1985). Residents had the greatest movement rates during September, likely coinciding with dispersal of pups from their natal range (Andelt, 1985; Bekoff & Wells, 1986). Transients also had greatest movement rates during September, but exhibited relatively high movement rates throughout much of the year, with the lowest movement rates occurring in January (183.4 m/h; Figure 1b).
4.2. First passage time analysis
Previous research has found clear patterns of habitat selection in both resident and transient coyotes (Hinton et al., 2015; Holzman et al., 1992; Kamler & Gipson, 2000). Transient coyotes were previously found to be more likely to select for human‐disturbed habitats such as roads (Hinton et al., 2015) and urban development (Gerhrt et al., 2009). Similarly, we found that transient ARS behaviors were more likely to occur near developed areas during February and June–October, supporting our prediction that transient high FPT values (ARS behaviors) would be correlated with human developments. Conversely, resident FPT values were negatively correlated with developed areas during January and May–September, again supporting our predictions. Importantly, this time period overlaps with when individuals may be rearing pups (April–Sept; Bekoff & Wells, 1986; Kilgo et al., 2017), an activity only resident coyotes engage in (Gese, 2004; Mills & Knowlton, 1991). High FPT values associated with resident ARS behaviors during these months are likely a combination of denning/whelping (Mar–May), pup‐rearing (May–Sept), and foraging (year‐round) behaviors. Due to our large sample size and study extent, we did not attempt to empirically quantify whether resident animals successfully reproduced each year of monitoring, and thus we cannot differentiate between these behaviors. However, avoidance of developed areas by residents, and by proxy human activities known to increase mortality risk (Kitchen et al., 2000), during pup‐rearing may be a strategy to increase survival of both parents and pups (Figure 5).
We found resident high FPT values (ARS behaviors) were generally more likely to occur near wetlands from February–August (excluding June), which encompasses breeding (Jan–March) and pup‐rearing seasons (April–Sept) for coyotes. Residents with offspring are limited in their movements by the relatively reduced mobility of young pups (Andelt, 1985; Gese, 2004). Focusing foraging and pup‐rearing activities closer to wetlands and free water sources may decrease energetic costs associated with accessing water sources for both themselves and their offspring. Additionally, transient ARS behaviors were more likely to occur near wetlands from July to September. Resident and transient selection for wetlands overlaps with the warm summer months when the risk of heat stress for both is higher, and access to water for hydration and thermoregulation can mitigate this risk for both adults and (for residents) pups (Afik & Pinshow, 1993). Likewise, edge density was generally an important variable for both residents and transients, and the correlation between ARS behaviors and edge density was always positive when it is was significant. This finding supports previous work, indicating edge habitats provide important foraging opportunities for coyotes (Heske et al., 1999; Hinton et al., 2015; Ward et al., 2018).
Importantly, FPT analysis is known to be dependent on scale (Byrne & Chamberlain, 2012; Frair et al., 2005), with periods of ARS behavior potentially nested within larger periods of restricted movement behavior along an animal's movement path. The temporal scale of our movement data, where locations were collected every 4 h, allowed for extended monitoring of individuals by prolonging transmitter battery life but may have masked fine‐scale movement behaviors that could have influenced FPT estimation. Specifically, the 4‐hour fix rate interval has the potential to overestimate FPT by missing sinuous movements made during the interval between fixes. However, because the fix rate and FPT estimation methods (interpolation distance, range of scales at which FPT was estimated, etc.) was constant across all individuals, we are confident our methods allow for robust estimation of FPT at the temporal scale of our data and allow for accurate comparison among individuals. Additionally, the scale of ARS behaviors can be influenced by several different factors including habitat configuration and territoriality (Byrne & Chamberlain, 2012; Fauchald & Tverra, 2003; Frair et al., 2005). We quantified the spatial relationship between individual ARS behaviors and land cover types using a distance‐based approach to maintain consistency across individuals; however, this approach has limited ability to assess true habitat configuration relative to proportion‐based approaches (i.e., quantifying the proportion of each land cover type within a set area, such as the FPT radius used in each monthly analysis). However, the distance‐based approach allowed for consistent quantification among resident and transient individuals across months, making it the most appropriate approach for our study. Additionally, territoriality can influence the ability to delineate ARS behaviors because an individual may restrict its movements to within its home range due to territorial boundaries, and not necessarily because of ARS behaviors (e.g., foraging). All resident coyotes in our study could easily traverse their estimated home range during the month period at which we estimated FPT, allowing for the possibility that an animal may turn back on its path as it moves among portions of its home range in addition to foraging or resting behaviors. However, our research objectives were not to infer specific behaviors (i.e., foraging vs. denning vs. resting) within periods of ARS behavior, but rather to associate general ARS behavior associated with high FPT values with land cover characteristics for both resident and transient animals. Furthermore, the substantive variation in space use and movement rates among resident and transient animals and the temporal scale of our movement data (i.e., relocations every 4 h) likely indicate that patterns associated with inferred behavioral states may also vary widely among individuals. Regardless, we believe that the resolution of our analysis and spatial scale at which we inferred ARS behaviors were sufficient and appropriate to elucidate land cover characteristics associated with these behaviors.
The complex, variable patterns in space use and movement behaviors of both residents and transients make effective, continued management of coyotes difficult, especially at a landscape scale. Although we found clear evidence of spatiotemporal patterns associated with ARS behaviors for both resident and transient animals, the substantive variation among individual coyotes indicates that broad, generalized management actions (e.g., removal) may not be appropriate for targeting coyotes at a population level. Indeed, previous research has found that large‐scale management efforts in the Southeast are rarely successful at long‐term management of coyote populations (Kilgo et al., 2014; Kirepka et al., 2017). Rather, management actions are likely to be more effective at small scales when individual patterns of movement behavior are known.
CONFLICT OF INTEREST
The authors have no competing interests to declare.
AUTHOR CONTRIBUTIONS
Sarah C. Webster: Conceptualization (equal); Formal analysis (lead); Investigation (equal); Methodology (lead); Software (lead); Writing – original draft (lead); Writing – review & editing (equal). James C. Beasley: Writing – review & editing (equal). Joseph W. Hinton: Investigation (equal); Writing – review & editing (equal). Michael J. Chamberlain: Conceptualization (equal); Funding acquisition (lead); Writing – review & editing (equal).
ACKNOWLEDGMENTS
We appreciate assistance in trapping by C. Boyce, D. Eaton, and R. Johnson. We are grateful to many private landowners that granted us access to their properties. Funding was provided by a Federal Wildlife Restoration grant, Alabama Department of Conservation and Natural Resources, Georgia Department of Natural Resources—Wildlife Resources Division, South Carolina Department of Natural Resources, and Warnell School of Forestry and Natural Resources at the University of Georgia. Funding was also provided by the U.S. Department of Energy under Award No. DE‐EM0004391 to the University of Georgia Research Foundation. This manuscript was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of the authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Alabama Department of Conservation and Natural Resources, Georgia Department of Natural Resources—Wildlife Resources Division, and South Carolina Department of Natural Resources. We are grateful to the anonymous reviewers and editors who provided detailed reviews of this manuscript.
APPENDIX 1.
Akaike Information Criterion (AIC) model rankings for generalized linear mixed models (GLMMs) estimating the relationship between FPT values and landcover type for transient coyotes monitored from January 2015 – June 2017 in Alabama, Georgia, and South Carolina. K = number of parameters, AIC = Akaike Information Criterion, ΔAIC = Delta AIC, AICWt = AIC weight, LL = negative log likelihood, and Adj‐R 2 = adjusted R 2 value (provided only for top ranked models)
| Status | Month | Model | K | AIC | ΔAIC | AICWt | LL | Adj‐R 2 |
|---|---|---|---|---|---|---|---|---|
| Transient | January | Full Model a | 8 | 108978.1 | 0 | 1 | −54481.05 | .44 |
| Decid b + Pine c + Wet d | 6 | 109050 | 71.9 | 0 | −54519.01 | – | ||
| Decid + Pine | 5 | 109063.3 | 85.16 | 0 | −54526.65 | – | ||
| Crop e + Edge f + Dev g | 6 | 109111.2 | 133.03 | 0 | −54549.58 | – | ||
| Edge + Dev | 5 | 109117.8 | 139.69 | 0 | −54553.91 | – | ||
| Crop + Edge | 5 | 109175.1 | 196.95 | 0 | −54582.54 | – | ||
| February | Full Model | 8 | 248559 | 0 | 1 | −124271.5 | .56 | |
| Crop + Edge + Dev | 6 | 248608 | 49.05 | 0 | −124298 | – | ||
| Edge + Dev | 5 | 248615.2 | 56.24 | 0 | −124302.6 | – | ||
| Decid + Pine + Wet | 6 | 248916.8 | 357.87 | 0 | −124452.4 | – | ||
| Decid + Pine | 5 | 248933.5 | 374.55 | 0 | −124461.8 | – | ||
| Crop + Edge | 5 | 248976.2 | 417.28 | 0 | −124483.1 | – | ||
| March | Full Model | 8 | 287154.4 | 0 | 1 | −143569.2 | .47 | |
| Decid + Pine + Wet | 6 | 287173 | 18.57 | 0 | −143580.5 | – | ||
| Decid + Pine | 5 | 287186.2 | 31.76 | 0 | −143588.1 | – | ||
| Crop + Edge + Dev | 6 | 287208.1 | 53.7 | 0 | −143598.1 | – | ||
| Edge + Dev | 5 | 287216.6 | 62.15 | 0 | −143603.3 | – | ||
| Crop + Edge | 5 | 287226 | 71.63 | 0 | −143608 | – | ||
| April | Full Model | 8 | 256393 | 0 | 1 | −128188.5 | .23 | |
| Decid + Pine + Wet | 6 | 256446.4 | 53.47 | 0 | −128217.2 | – | ||
| Decid + Pine | 5 | 256452.9 | 59.97 | 0 | −128221.5 | – | ||
| Crop + Edge | 5 | 256458.5 | 65.5 | 0 | −128224.2 | – | ||
| Crop + Edge + Dev | 6 | 256514.7 | 121.7 | 0 | −128251.3 | – | ||
| May | Full Model | 8 | 208779.6 | 0 | 0.99 | −104381.8 | .66 | |
| Crop + Edge + Dev | 6 | 208790 | 10.35 | 0.01 | −104389 | – | ||
| Edge + Dev | 5 | 208793.3 | 13.72 | 0 | −104391.7 | – | ||
| Crop + Edge | 5 | 208803 | 23.38 | 0 | −104396.5 | – | ||
| Decid + Pine + Wet | 6 | 208815.5 | 35.88 | 0 | −104401.7 | – | ||
| Decid + Pine | 5 | 208819.1 | 39.47 | 0 | −104404.5 | – | ||
| June | Full Model | 8 | 180901.4 | 0 | 1 | −90442.71 | .38 | |
| Decid + Pine + Wet | 6 | 180918.1 | 16.67 | 0 | −90453.05 | – | ||
| Crop + Edge | 5 | 180918.6 | 17.14 | 0 | −90454.29 | – | ||
| Decid + Pine | 5 | 180921.6 | 20.21 | 0 | −90455.82 | – | ||
| Crop + Edge + Dev | 6 | 180953 | 51.52 | 0 | −90470.47 | – | ||
| Edge + Dev | 5 | 180957.2 | 55.75 | 0 | −90473.59 | – | ||
| July | Full Model | 8 | 161494.9 | 0 | 1 | −80739.45 | .35 | |
| Crop + Edge + Dev | 6 | 161529 | 34.11 | 0 | −80758.51 | – | ||
| Crop + Edge | 5 | 161529.5 | 34.52 | 0 | −80759.72 | – | ||
| Edge + Dev | 5 | 161546.3 | 51.39 | 0 | −80768.16 | – | ||
| Decid + Pine + Wet | 6 | 161582.5 | 87.63 | 0 | −80785.27 | – | ||
| Decid + Pine | 5 | 161595 | 100.04 | 0 | −80792.48 | – | ||
| August | Full Model | 8 | 159770.3 | 0 | 1 | −79877.13 | .27 | |
| Crop + Edge + Dev | 6 | 159782.3 | 12.03 | 0 | −79885.15 | – | ||
| Decid + Pine + Wet | 6 | 159789.3 | 19.03 | 0 | −79888.65 | – | ||
| Edge + Dev | 5 | 159809.9 | 39.61 | 0 | −79899.94 | – | ||
| Decid + Pine | 5 | 159810.1 | 39.79 | 0 | −79900.03 | – | ||
| Crop + Edge | 5 | 159811.8 | 41.49 | 0 | −79900.88 | – | ||
| September | Full Model | 8 | 137063.2 | 0 | 1 | −68523.57 | .30 | |
| Crop + Edge + Dev | 6 | 137098.5 | 35.31 | 0 | −68543.23 | – | ||
| Edge + Dev | 5 | 137107.3 | 44.16 | 0 | −68548.66 | – | ||
| Crop + Edge | 5 | 137323.2 | 260.05 | 0 | −68656.6 | – | ||
| Decid + Pine + Wet | 6 | 137348.2 | 285.02 | 0 | −68668.08 | – | ||
| Decid + Pine | 5 | 137352.2 | 289.08 | 0 | −68671.12 | – | ||
| October | Full Model | 8 | 133713.8 | 0 | 1 | −66848.86 | .11 | |
| Crop + Edge + Dev | 6 | 133726.4 | 12.64 | 0 | −66857.18 | – | ||
| Crop + Edge | 5 | 133727.1 | 13.37 | 0 | −66858.55 | – | ||
| Edge + Dev | 5 | 133729.3 | 15.54 | 0 | −66859.64 | – | ||
| Decid + Pine + Wet | 6 | 133730.2 | 16.42 | 0 | −66859.08 | – | ||
| Decid + Pine | 5 | 133734.2 | 20.44 | 0 | −66862.09 | – | ||
| November | Full Model | 8 | 122958.7 | 0 | 1 | −61471.35 | .24 | |
| Crop + Edge + Dev | 6 | 122992.8 | 34.05 | 0 | −61490.38 | – | ||
| Edge + Dev | 5 | 122996.8 | 38.03 | 0 | −61493.37 | – | ||
| Decid + Pine + Wet | 6 | 123080.9 | 122.2 | 0 | −61534.45 | – | ||
| Decid + Pine | 5 | 123085.5 | 126.75 | 0 | −61537.73 | – | ||
| Crop + Edge | 5 | 123100.4 | 141.71 | 0 | −61545.21 | – | ||
| December | Full Model | 8 | 100444 | 0 | 1 | −50213.97 | .48 | |
| Decid + Pine + Wet | 6 | 100456.2 | 12.2 | 0 | −50222.08 | – | ||
| Crop + Edge + Dev | 6 | 100509.8 | 65.82 | 0 | −50248.89 | – | ||
| Decid + Pine | 5 | 100528.1 | 84.07 | 0 | −50259.02 | – | ||
| Crop + Edge | 5 | 100561.2 | 117.22 | 0 | −50275.59 | – | ||
| Edge + Dev | 5 | 100574 | 130 | 0 | −50281.98 | – | ||
| Resident | January | Full Model | 8 | 172304 | 0 | 1 | −86143.98 | .53 |
| Crop + Edge + Dev | 6 | 172322.1 | 18.14 | 0 | −86155.05 | – | ||
| Edge + Dev | 5 | 172329.3 | 25.35 | 0 | −86159.65 | – | ||
| Decid + Pine + Wet | 6 | 172335.9 | 31.87 | 0 | −86161.92 | – | ||
| Decid + Pine | 5 | 172342.4 | 38.42 | 0 | −86166.19 | – | ||
| Crop + Edge | 5 | 172348.1 | 44.17 | 0 | −86169.07 | – | ||
| February | Full Model | 8 | 366361 | 0 | 1 | −183172.5 | .52 | |
| Crop + Edge + Dev | 6 | 366376.8 | 15.78 | 0 | −183182.4 | – | ||
| Edge + Dev | 5 | 366381.6 | 20.59 | 0 | −183185.8 | – | ||
| Decid + Pine + Wet | 6 | 366392.2 | 31.19 | 0 | −183190.1 | – | ||
| Decid + Pine | 5 | 366396.5 | 35.48 | 0 | −183193.2 | – | ||
| Crop + Edge | 5 | 366401.7 | 40.71 | 0 | −183195.9 | – | ||
| March | Full Model | 8 | 412732.1 | 0 | 1 | −206358 | .35 | |
| Crop + Edge + Dev | 6 | 412745.5 | 13.41 | 0 | −206366.8 | – | ||
| Edge + Dev | 5 | 412752.6 | 20.51 | 0 | −206371.3 | – | ||
| Decid + Pine + Wet | 6 | 412771.4 | 39.32 | 0 | −206379.7 | – | ||
| Crop + Edge | 5 | 412772.3 | 40.18 | 0 | −206381.1 | – | ||
| Decid + Pine | 5 | 412779.5 | 47.41 | 0 | −206384.8 | – | ||
| April | Full Model | 8 | 362301.7 | 0 | 1 | −181142.9 | .38 | |
| Crop + Edge + Dev | 6 | 362315.1 | 13.42 | 0 | −181151.6 | – | ||
| Edge + Dev | 5 | 362323.1 | 21.39 | 0 | −181156.5 | – | ||
| Decid + Pine + Wet | 6 | 362332.9 | 31.17 | 0 | −181160.4 | – | ||
| Crop + Edge | 5 | 362337.2 | 35.48 | 0 | −181163.6 | – | ||
| Decid + Pine | 5 | 362341.6 | 39.85 | 0 | −181165.8 | – | ||
| May | Full Model | 8 | 311526 | 0 | 1 | −155755 | .39 | |
| Crop + Edge + Dev | 6 | 311538.9 | 12.91 | 0 | −155763.5 | – | ||
| Edge + Dev | 5 | 311544.1 | 18.1 | 0 | −155767 | – | ||
| Decid + Pine + Wet | 6 | 311559.4 | 33.41 | 0 | −155773.7 | – | ||
| Crop + Edge | 5 | 311564.4 | 38.42 | 0 | −155777.2 | – | ||
| Decid + Pine | 5 | 311564.7 | 38.7 | 0 | −155777.3 | – | ||
| June | Full Model | 8 | 255054.8 | 0 | 1 | −127519.4 | .45 | |
| Crop + Edge + Dev | 6 | 255071.9 | 17.03 | 0 | −127529.9 | – | ||
| Edge + Dev | 5 | 255076.7 | 21.9 | 0 | −127533.4 | – | ||
| Decid + Pine + Wet | 6 | 255089.3 | 34.5 | 0 | −127538.7 | – | ||
| Decid + Pine | 5 | 255094.6 | 39.81 | 0 | −127542.3 | – | ||
| Crop + Edge | 5 | 255095.9 | 41.07 | 0 | −127542.9 | – | ||
| July | Full Model | 8 | 228091.6 | 0 | 1 | −114037.8 | .53 | |
| Crop + Edge + Dev | 6 | 228104.9 | 13.29 | 0 | −114046.4 | – | ||
| Edge + Dev | 5 | 228111.2 | 19.57 | 0 | −114050.6 | – | ||
| Decid + Pine + Wet | 6 | 228125.6 | 34.03 | 0 | −114056.8 | – | ||
| Decid + Pine | 5 | 228131.9 | 40.28 | 0 | −114060.9 | – | ||
| Crop + Edge | 5 | 228133.1 | 41.46 | 0 | −114061.5 | – | ||
| August | Full Model | 8 | 224832.4 | 0 | 1 | −112408.2 | .31 | |
| Crop + Edge + Dev | 6 | 224846 | 13.58 | 0 | −112417 | – | ||
| Edge + Dev | 5 | 224852.9 | 20.45 | 0 | −112421.4 | – | ||
| Decid + Pine + Wet | 6 | 224858 | 25.53 | 0 | −112423 | – | ||
| Crop + Edge | 5 | 224864 | 31.61 | 0 | −112427 | – | ||
| Decid + Pine | 5 | 224865 | 32.57 | 0 | −112427.5 | – | ||
| September | Full Model | 8 | 183486.7 | 0 | 1 | −91735.34 | .37 | |
| Crop + Edge + Dev | 6 | 183501.8 | 15.13 | 0 | −91744.9 | – | ||
| Decid + Pine + Wet | 6 | 183505 | 18.25 | 0 | −91746.47 | – | ||
| Edge + Dev | 5 | 183507.1 | 20.44 | 0 | −91748.56 | – | ||
| Decid + Pine | 5 | 183510.2 | 23.55 | 0 | −91750.12 | – | ||
| Crop + Edge | 5 | 183511.4 | 24.74 | 0 | −91750.71 | – | ||
| October | Full Model | 8 | 188601.5 | 0 | 1 | −94292.75 | .45 | |
| Crop + Edge + Dev | 6 | 188616.9 | 15.36 | 0 | −94302.43 | – | ||
| Edge + Dev | 5 | 188622.5 | 20.97 | 0 | −94306.24 | – | ||
| Crop + Edge | 5 | 188623.8 | 22.31 | 0 | −94306.91 | – | ||
| Decid + Pine + Wet | 6 | 188626.9 | 25.4 | 0 | −94307.45 | – | ||
| Decid + Pine | 5 | 188632.1 | 30.61 | 0 | −94311.06 | – | ||
| November | Full Model | 8 | 179386.9 | 0 | 1 | −89685.45 | .32 | |
| Crop + Edge + Dev | 6 | 179400.9 | 14.02 | 0 | −89694.46 | – | ||
| Edge + Dev | 5 | 179406 | 19.08 | 0 | −89697.99 | – | ||
| Decid + Pine + Wet | 6 | 179416.7 | 29.81 | 0 | −89702.36 | – | ||
| Crop + Edge | 5 | 179421.8 | 34.87 | 0 | −89705.89 | – | ||
| Decid + Pine | 5 | 188632.1 | 9245.21 | 0 | −94311.06 | – | ||
| December | Full Model | 8 | 143644.5 | 0 | 1 | −71814.25 | .38 | |
| Crop + Edge + Dev | 6 | 143658.8 | 14.29 | 0 | −71823.4 | – | ||
| Decid + Pine + Wet | 6 | 143663.7 | 19.21 | 0 | −71825.86 | – | ||
| Edge + Dev | 5 | 143664 | 19.5 | 0 | −71827.01 | – | ||
| Crop + Edge | 5 | 143667.5 | 23.02 | 0 | −71828.77 | – | ||
| Decid + Pine | 5 | 143669 | 24.49 | 0 | −71829.5 | – |
Global model with all variables.
Mixed Deciduous Forest.
Pine Forest.
Wetland.
Cropland.
Edge Density.
Developed.
APPENDIX 2.
Averaged estimates of monthly 95% ranges and 50% core areas for 171 resident and monthly 95% biding areas and 50% core biding areas for transient coyotes monitored from January 2015 – Jun 2017 in the tri‐state region of Alabama, Georgia, and South Carolina, USA
| Territorial Status | Month | 95% Ranges (km2) | SD | 50% Core Areas (km2) | SD |
|---|---|---|---|---|---|
| Resident | January | 21.28 | 36.07 | 4.51 | 7.54 |
| February | 30.51 | 11.70 | 4.99 | 15.20 | |
| March | 13.55 | 11.07 | 2.67 | 2.34 | |
| April | 14.98 | 21.68 | 2.67 | 2.67 | |
| May | 11.19 | 9.03 | 1.99 | 2.12 | |
| June | 13.55 | 17.24 | 2.64 | 4.01 | |
| July | 12.02 | 8.96 | 2.17 | 1.81 | |
| August | 11.97 | 9.41 | 2.51 | 1.92 | |
| September | 12.08 | 6.48 | 2.83 | 1.78 | |
| October | 12.59 | 8.87 | 2.97 | 2.31 | |
| November | 13.56 | 9.97 | 3.04 | 1.90 | |
| December | 14.66 | 12.03 | 3.42 | 2.68 | |
| Transient | January | 202.14 | 319.23 | 41.22 | 68.05 |
| February | 561.44 | 1209.62 | 128.21 | 296.28 | |
| March | 480.16 | 994.50 | 121.71 | 283.03 | |
| April | 355.87 | 827.62 | 83.52 | 226.82 | |
| May | 321.16 | 498.47 | 72.08 | 112.36 | |
| June | 377.04 | 1033.13 | 95.77 | 278.46 | |
| July | 349.52 | 678.56 | 82.90 | 219.06 | |
| August | 297.17 | 904.56 | 67.26 | 188.36 | |
| September | 449.67 | 1412.66 | 116.95 | 306.68 | |
| October | 387.23 | 924.21 | 95.88 | 279.92 | |
| November | 367.44 | 412.39 | 86.33 | 106.23 | |
| December | 276.90 | 382.69 | 57.25 | 89.60 |
APPENDIX 3.
Sample estimates for generalized linear mixed models comparing monthly movement rates between resident and transient coyotes monitored from January 2015 – June 2017 in the tri‐state region of Alabama, Georgia, and South Carolina, USA
| Month | Resident mean movement rate (m/h) | Resident standard error | Transient mean movement rate (m/h) | Transient standard error | t Statistic | Degrees of freedom | p Value |
|---|---|---|---|---|---|---|---|
| Jan | 183.01 | 2.47 | 183.43 | 4.25 | 0.61 | 13395 | .54 |
| Feb | 173.62 | 2.14 | 211.46 | 3.38 | 10.25 | 19748 | <.001 |
| Mar | 172.01 | 1.87 | 212.86 | 3.04 | 8.62 | 23708 | <.001 |
| Apr | 169.35 | 1.78 | 223.27 | 3.14 | 6.23 | 23089 | <.001 |
| May | 174.05 | 1.70 | 222.21 | 3.89 | 7.71 | 21954 | <.001 |
| Jun | 165.52 | 1.68 | 223.21 | 4.41 | 9.25 | 19347 | <.001 |
| Jul | 185.96 | 1.86 | 224.43 | 4.80 | 11.28 | 18570 | <.001 |
| Aug | 189.35 | 1.99 | 208.92 | 3.62 | 7.38 | 17207 | <.001 |
| Sep | 202.15 | 2.16 | 229.68 | 4.34 | 7.10 | 14662 | <.001 |
| Oct | 194.10 | 2.18 | 215.66 | 4.03 | 3.68 | 14910 | <.001 |
| Nov | 188.37 | 2.07 | 199.23 | 3.94 | 3.70 | 16089 | <.001 |
| Dec | 187.21 | 2.32 | 193.26 | 4.84 | 13.86 | 13583 | <.001 |
Webster, S. C. , Beasley, J. C. , Hinton, J. W. , & Chamberlain, M. J. (2022). Resident and transient coyotes exhibit differential patterns of movement behavior across heterogeneous landscapes in the southeastern United States. Ecology and Evolution, 12, e8725. 10.1002/ece3.8725
DATA AVAILABILITY STATEMENT
Animal movement data can be found online at MoveBank (MoveBank ID: 1966782762).
REFERENCES
- Afik, D. , & Pinshow, B. (1993). Temperature regulation and water economy in desert wolves. Journal of Arid Environments, 24, 197–209. 10.1006/jare.1993.1017 [DOI] [Google Scholar]
- Andelt, W. F. (1985). Behavioral ecology of coyotes in South Texas. Wildlife Monographs, 94, 3–45. [Google Scholar]
- Basille, M. , Fortin, D. , Dussault, C. , Ouellet, J. , & Courtois, R. (2013). Ecologically based definition of seasons clarifies predator‐prey interactions. Ecography, 36, 220–229. 10.1111/j.1600-0587.2011.07367.x [DOI] [Google Scholar]
- Bekoff, M. , & Wells, M. C. (1986). Social ecology and behavior of coyotes. Advanced Study of Behavior, 16, 251–338. [Google Scholar]
- Benson, J. F. (2013). Improving rigour and efficiency of use‐availability habitat selection analyses with systematic estimation of availability. Methods in Ecology and Evolution, 4, 244–251. 10.1111/2041-210x.12006 [DOI] [Google Scholar]
- Berger, K. M. , & Gese, E. M. (2007). Does interference competition with wolves limit the distribution and abundance of coyotes? Journal of Animal Ecology, 76(6), 1075–1085. 10.1111/j.1365-2656.2007.01287.x [DOI] [PubMed] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and multimodal inference: a practical information‐theoretic approach, 2nd ed. Springer. [Google Scholar]
- Byrne, M. E. , & Chamberlain, M. J. (2012). Using first‐passage time to link behavior and habitat in foraging paths of a terrestrial predator, the raccoon. Animal Behavior, 84, 593–601. [Google Scholar]
- Carmenzind, F. J. (1978). Behavioral ecology of coyotes on the National Elk Refuge. In Jackson W., & Bekoff M. (Eds.), Coyotes: Biology, behavior, and management (pp. 267–294). New York Academic Press. [Google Scholar]
- Crimmins, S. M. , Edwards, J. W. , & Houben, J. M. (2012). Canis latrans (Coyote) habitat use and feeding habits in central West Virginia. Northeastern Naturalist, 19, 411–420. [Google Scholar]
- DeCandia, A. L. , Henger, C. S. , Krause, A. , Gormezano, L. J. , Weckel, M. , Nagy, C. , Munshi‐South, J. , & vonHoldt, B. M. (2019). Genetics of urban colonization: Neutral and adpative variation in coyotes (Canis latrans) inhabiting the New York metropolitan area. Journal of Urban Ecology, 5(1), 1–12. 10.1093/jue/juz002 [DOI] [Google Scholar]
- Dellinger, J. A. , Proctor, C. , Steury, T. D. , Kelly, M. J. , & Vaughn, M. R. (2013). Habitat selection of a large carnivore, the red wolf, in a human‐altered landscape. Biological Conservation, 157, 324–330. 10.1016/j.biocon.2012.09.004 [DOI] [Google Scholar]
- Dormann, C. F. , Elith, J. , Bacher, S. , Buchmann, C. , Carl, G. , Carre, G. , Marquez, J. R. G. , Gruber, B. , Lafourcade, B. , Leitao, P. J. , Munkemuller, T. , Mcclean, C. , Osborne, P. E. , Reneking, B. , Schroder, B. , Skidmore, A. K. , Zurell, D. , & Lautenbach, S. (2013). Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography, 36, 27–26. 10.1111/j.1600-0587.2012.07348.x [DOI] [Google Scholar]
- Fauchald, P. , & Tverra, T. (2003). Using first‐passage time in the analysis of area‐restricted search and habitat selection. Ecology, 84, 282–288. [Google Scholar]
- Fedriani, J. M. , Fuller, T. K. , & Sauvajot, R. M. (2001). Does anthropogenic food enhance densities of omnivorous mammals? An example with coyotes in southern California. Ecography, 24, 325–331. [Google Scholar]
- Frair, J. L. , Merrill, E. H. , Visscher, D. R. , Fortin, D. , Beyer, H. L. , & Morales, J. M. (2005). Scales of movement by elk (Cervus elaphus) in response to heterogeneity in forage resources and predation risk. Landscape Ecology, 20, 273–287. 10.1007/s10980-005-2075-8 [DOI] [Google Scholar]
- Freitas, C. , Kovacs, K. M. , Lyderson, C. , & Ims, R. A. (2008). A novel method for quantifying habitat selection and predicting habitat use. Journal of Applied Ecology, 45, 1213–1220. 10.1111/j.1365-2664.2008.01505.x [DOI] [Google Scholar]
- Gerhrt, S. D. , Anchor, C. , & White, L. A. (2009). Home range and landscape use of coyotes in a metropolitan landscape: Conflict or coexistence? Journal of Mammalogy, 90, 1045–1057. 10.1644/08-MAMM-A-277.1 [DOI] [Google Scholar]
- Gese, E. M. (2004). Coyotes in Yellowstone National Park: the influence of dominance on foraging, territoriality, and fitness. In Macdonald D. W., & Sillero‐Zubiri C. (Eds.), The biology and conservation of wild canids (pp. 271–283). Oxford University Press. [Google Scholar]
- Gese, E. M. , Ruff, R. L. , & Crabtree, R. L. (1996). Social and nutritional factors influencing the dispersal of resident coyotes. Animal Behavior, 52, 1025–1043. 10.1006/anbe.1996.0250 [DOI] [Google Scholar]
- Gipson, P. S. , Ballard, W. B. , Nowak, R. M. , & Mech, L. D. (2000). Accuracy and precision of estimating age of gray wolves by tooth wear. Journal of Wildlife Management, 64, 752–758. 10.2307/3802745 [DOI] [Google Scholar]
- Gompper, M. E. (2002). Top carnivores in the suburbs? Ecological and conservation issues raised by colonization of north America by coyotes. BioScience, 52, 185–190. [Google Scholar]
- Gosselink, T. E. , VanDeelen, T. R. , Warner, R. E. , & Joselyn, M. G. (2003). Temporal habitat partitioning and spatial use of coyotes and red foxes in east‐central Illinois. Journal of Wildlife Management, 67, 90–103. 10.2307/3803065 [DOI] [Google Scholar]
- Harrison, D. J. , Bissionette, J. A. , & Sherburne, J. A. (1989). Spatial relationships between coyotes and red foxes in eastern Maine. Journal of Wildlife Management, 53, 181–185. 10.2307/3801327 [DOI] [Google Scholar]
- Harrison, D. J. , Harrison, J. A. , & O’Donoghue, M. (1991). Predispersal movements of coyote (Canis latrans) Pups in Eastern Maine. Journal of Mammalogy, 72(4), 756–763. [Google Scholar]
- Heske, E. J. , Robinson, S. K. , & Brawn, J. D. (1999). Predator activity and predation on songbird nests on forest‐field edge in east‐central Illinois. Landscape Ecology, 14, 345–354. [Google Scholar]
- Hesselbarth, M. H. K. , Sciaini, M. , With, K. A. , Wiegand, K. , & Nowosad, J. (2019). Landscapemetrics: An open source R tool to calculate landscape metrics. Ecography, 42, 1648–1657. [Google Scholar]
- Hinton, J. W. , Ashley, A. K. , Dellinger, J. A. , Gittleman, J. L. , van Manen, F. T. , & Chamberlain, M. J. (2017). Using diets of Canis breeding pairs to assess resource partitioning of sympatric red wolves and coyotes. Journal of Mammalogy, 98(2), 475–488. 10.1093/jmammal/gyw233 [DOI] [Google Scholar]
- Hinton, J. W. , Chamberlain, M. J. , & van Manen, F. T. (2012). Long‐distance movements of transient coyotes in eastern North Carolina. The American Midland Naturalist, 168(2), 281–288. 10.1674/0003-0031-168.2.281 [DOI] [Google Scholar]
- Hinton, J. W. , Heppenheimer, E. , West, K. M. , Caudill, D. , Karlin, M. L. , Kilgo, J. C. , Mayer, J. J. , Miller, K. V. , Walch, M. , vonHoldt, B. , & Chamberlain, M. J. (2019). Geographic patterns in morphometric and genetic variation for coyote populations with emphasis on southeastern coyotes. Ecology and Evolution, 9(6), 3389–3404. 10.1002/ece3.4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton, J. W. , van Manen, F. T. , & Chamberlain, M. J. (2015). Space use and habitat selection by resident and transient coyotes (Canis latrans). PLoS One, 10, e0132203. 10.1371/journal.pone.0132203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman, S. , Conroy, M. J. , & Pickering, J. (1992). Home range, movements, and habitat use of coyotes in southcentral Georgia. Journal of Wildlife Management, 56, 139–146. [Google Scholar]
- Johnson, A. R. , Wiens, J. A. , Milne, B. T. , & Crist, T. O. (1992). Animal movements and population dynamics in heterogeneous landscapes. Landscape Ecology, 7, 63–75. 10.1007/BF02573958 [DOI] [Google Scholar]
- Johnson, W. E. , Fuller, T. K. , & Franklin, W. L. (1996). Sympatry in canids: A review and assessment. In Gittleman J. L. (Ed.), Carnivore behavior, ecology, and evolution (pp. 189–218). Cornell University Press. [Google Scholar]
- Kamler, J. F. , & Gipson, P. S. (2000). Space and habitat use by resident and transient coyotes. Canadian Journal of Zoology, 78, 2106–2111. 10.1139/z00-153 [DOI] [Google Scholar]
- Kilgo, J. C. , Ray, H. S. , Ruth, C. , & Miller, K. V. (2010). Can coyotes affect deer populations in southeastern North America? Journal of Wildlife Management, 74, 929–933. [Google Scholar]
- Kilgo, J. C. , Shaw, C. E. , Vukovich, M. , Conroy, M. J. , & Ruth, C. (2017). Reproductive characteristics of a coyote population before and during exploitation. Journal of Wildlife Management, 81, 1386–1393. 10.1002/jwmg.21329 [DOI] [Google Scholar]
- Kilgo, J. C. , Vukovich, M. , Ray, H. S. , Shaw, C. E. , & Ruth, C. (2014). Coyote removal, understory cover, and survival of white‐tailed deer neonates. The Journal of Wildlife Management, 78, 1261–1271. 10.1002/jwmg.764 [DOI] [Google Scholar]
- Kirepka, E. M. , Kilgo, J. C. , & Rhodes, O. E. Jr (2017). Effect of compensatory immigration on the genetic structure of coyotes. The Journal of Wildlife Management, 81, 1394–1407. 10.1002/jwmg.21320 [DOI] [Google Scholar]
- Kitchen, A. M. , Gese, E. M. , & Shauster, E. R. (2000). Changes in coyote activity patterns due to reduced exposure to human persecution. Canadian Journal of Zoology, 78, 853–857. 10.1139/z00-003 [DOI] [Google Scholar]
- Knowlton, F. F. , Gese, E. M. , & Jaeger, M. M. (1999). Coyote depredation control: An interface between biology and management. Journal of Range Management, 52, 398–412. 10.2307/4003765 [DOI] [Google Scholar]
- Kutner, M. H. , Nachtsheim, C. J. , & Neter, J. (2004). Applied linear regression models, (Vol. 4, pp. 563–568). McGraw‐Hill. [Google Scholar]
- Macdonald, D. W. (1983). The ecology of carnivore social behavior. Nature, 301, 379–384. [Google Scholar]
- Mastro, L. L. , Morin, D. J. , & Gese, E. M. (2019). Home range and habitat use of West Virginia Canis latrans (Coyote). Northeastern Naturalist, 26(3), 616–628. 10.1656/045.026.0318 [DOI] [Google Scholar]
- Mills, L. S. , & Knowlton, F. F. (1991). Coyote space use in relation to prey abundance. Canadian Journal of Zoology, 69, 1516–1521. 10.1139/z91-212 [DOI] [Google Scholar]
- Mitchell, N. , Strohbach, M. W. , Pratt, R. , Finn, W. C. , & Strauss, E. G. (2015). Space use by resident and transient coyotes in an urban‐rural landscape mosaic. Wildlife Research, 42, 461–469. 10.1071/WR15020 [DOI] [Google Scholar]
- Morin, D. J. , & Kelly, M. J. (2017). The dynamic nature of territoriality, transience, and biding in an exploited coyote population. Wildlife Biology, 2017(1), 1–13. 10.2981/wlb.00335 [DOI] [Google Scholar]
- National Oceanic and Atmospheric Administration (NOAA) (2019). Climate at a glance: National time series [Internet]. Retrieved from https://www.ncdc.noaa.gov/cag/ [Google Scholar]
- Patterson, B. R. , Benjamin, L. K. , & Messier, F. (1998). Prey switching and feeding habits of eastern coyotes in relation to snowshoe hare and white‐tailed deer densities. Canadian Journal of Zoology, 76, 1885–1897. 10.1139/z98-135 [DOI] [Google Scholar]
- Patterson, B. R. , & Messier, F. (2001). Social organization and space use of coyotes in eastern Canada relative to prey distribution and abundance. Journal of Mammalogy, 82, 463–477. [DOI] [Google Scholar]
- R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Retrieved from http://www.R‐project.org/ [Google Scholar]
- Randa, L. A. , Cooper, D. M. , Meserve, P. L. , & Yunger, J. A. (2009). Prey switching of sympatric canids in response to variable prey abundance. Journal of Mammalogy, 90, 594–603. 10.1644/08-MAMM-A-092R1.1 [DOI] [Google Scholar]
- Sasmal, I. , Moorman, C. E. , Swingen, M. B. , Datta, S. , & DePerno, C. S. (2019). Seasonal space use of transient and resident coyotes (Canis latrans) in North Carolina, USA. Canadian Journal of Zoology, 97, 326–331. [Google Scholar]
- Thompson, C. M. , & McGarigal, K. (2002). The influence of research scale on bald eagle habitat selection along the lower Hudson River, New York (USA). Landscape Ecology, 17, 569–586. [Google Scholar]
- Tigas, L. A. , Van Vuren, D. H. , & Sauvajot, R. M. (2002). Behavioral responses of bobcats and coyotes to habitat fragmentation and corridors in an urban environment. Biological Conservation, 108, 299–306. 10.1016/S0006-3207(02)00120-9 [DOI] [Google Scholar]
- Ward, J. N. , Hinton, J. W. , Johannsen, K. L. , Karlin, M. L. , Miller, K. V. , & Chamberlain, M. J. (2018). Home range size, vegetation density, and season influences prey use by coyotes (Canis latrans). PLoS One, 13, e0203703. 10.1371/journal.pone.0203703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser, N. M. , Price, M. V. , Blumstein, D. T. , Arozqueta, S. R. , Escobar, B. D. C. , Pickens, R. , & Pistoia, A. (2014). Coyotes, deer, and wildflowers: diverse evidence points to a trophic cascade. Naturwissenschafften, 101, 427–436. 10.1007/s00114-014-1172-4 [DOI] [PubMed] [Google Scholar]
- Worton, B. J. (1989). Kernel methods for estimating the utilization distribution in home‐range studies. Ecology, 70, 164–168. 10.2307/1938423 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Animal movement data can be found online at MoveBank (MoveBank ID: 1966782762).


