Abstract
Objective:
Over 99% of the EMPA-REG OUTCOME trial participants had established cardiovascular disease (CVD). We aimed to investigate effectiveness and safety outcomes among patients with type 2 diabetes (T2D) initiating empagliflozin vs dipeptidyl peptidase-4 inhibitor (DPP-4i) across the broad spectrum of cardiovascular risk.
Methods:
In a population-based cohort study we identified 39,072 pairs of 1:1 propensity score-matched adult patients with T2D initiating empagliflozin or DPP-4i, using data from 2 U.S. commercial insurance databases and Medicare between 08/2014–09/2017. Primary outcomes were a composite of myocardial infarction (MI)/stroke, and hospitalization for heart failure (HHF). Safety outcomes were bone fractures, lower-limb amputations (LLA), diabetic ketoacidosis (DKA), and acute kidney injury (AKI). We estimated pooled hazard ratios (HR) and 95% CI adjusting for >140 baseline covariates.
Results:
Study participants had mean age of 60 years and only 28% had established CVD. Compared to DPP-4i, empagliflozin was associated with similar risk of MI/stroke [HR (95% CI), 0.99 (0.81–1.21)], and lower risk of HHF [0.48 (0.35–0.67) and 0.63 (0.54–0.74), based on a primary and any HF discharge diagnosis, respectively]. The HR was 0.52 (0.38–0.72) for all-cause mortality (ACM) and 0.83 (0.70–0.98) for a composite of MI/stroke/ACM. Empagliflozin was associated with a similar risk of LLA and fractures, an increased risk of DKA [1.71 (1.08–2.71)], and a decreased risk of AKI [0.60 (0.43–0.85)].
Conclusions:
In clinical practice, the initiation of empagliflozin vs DPP-4i was associated with a lower risk of HHF, ACM, and MI/stroke/ACM, a similar risk of MI/stroke, and a safety profile consistent with documented information.
Keywords: Empagliflozin, dipeptidyl peptidase-4 inhibitors, type 2 diabetes, cardiovascular outcomes, safety outcomes
Background
The EMPA-REG OUTCOME trial showed that empagliflozin, a sodium-glucose co-transporter-2 inhibitor (SGLT-2i), reduces the relative risk of cardiovascular death by 38%, all-cause mortality by 32%, and hospitalization for heart failure (HHF) by 35%, compared to placebo, when added to standard of care in patients with type 2 diabetes (T2D).1 More than 99% of patients included in the EMPA-REG OUTCOME trial had established cardiovascular disease. This evidence, together with evidence from other large randomized controlled trials (RCTs),2–4 prompted clinical guidelines to recommend the initiation of a SGLT-2i among patients with high cardiovascular risk or patients with established atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.5
The beneficial effects of empagliflozin are yet to be evaluated in routine clinical care in head-to-head comparisons with alternative glucose-lowering medications, particularly in patients without established cardiovascular disease. Furthermore, although safety data on empagliflozin and other SGLT-2i have been reported in large RCTs,1–3 evidence on safety of these agents is still accumulating,2,6–10 as RCTs are less reflective of routine care patients and treatment patterns, and as they are not typically powered to detect rare outcomes that may become evident in larger and more broadly defined populations.10 Specifically, the safety of empagliflozin has not been evaluated in a large real-world population for potential severe unintended adverse effects of SGLT-2i, such as bone fractures, lower-limb amputations (LLA), diabetic ketoacidosis (DKA), and acute kidney injury (AKI).
The EMPagliflozin compaRative effectIveness and SafEty (EMPRISE) study program is a multi-year monitoring program utilizing a new-user, active-comparator cohort study design with 1:1 propensity score (PS)-matching of patients initiating empagliflozin or a comparator to evaluate the effectiveness, safety and healthcare utilization of empagliflozin in routine care across the broad spectrum of baseline cardiovascular risk using real-world data from three U.S. healthcare utilization datasets.11 Within this program, we plan to collect accumulating data for a period of five years following the date of empagliflozin’s approval in the U.S., i.e., August 1, 2014 through September 30, 2019, and conduct four interim analyses after each twelve-month data update and a final analysis.
In this analysis, we aimed to assess the association between empagliflozin and several cardiovascular and safety outcomes compared to dipeptidyl peptidase-4 inhibitor (DPP-4i), using data through the third year of EMPRISE, i.e., August 2014 through September 2017.
Methods
Data source
Data were collected from one federal (Medicare fee-for-service) and two commercial (Optum Clinformatics Data Mart® Database and IBM MarketScan) data sources in the United States. Medicare is a federally funded program that provides health care coverage for nearly all legal residents of the U.S. aged ≥65 years and some disabled patients aged <65 years. Optum and MarketScan include a national commercially insured population in the U.S. and contain longitudinal medical, pharmacy claims from several different managed care plans, and results for outpatient laboratory tests for a subset of patients (approximately 45% in Optum and 5–10% in MarketScan) through linkage with national lab test provider chains. The study was approved by the Mass General Brigham Institutional Review Board, and signed data license agreements were in place for all data sources. The study was registered at EnCEPP (EUPAS20677) and on ClinicalTrials.gov (NCT03363464).
Study design and patient eligibility
EMPRISE is a sequentially built new-user, parallel-group, active-comparator retrospective cohort study, as previously described.11 For this analysis, we identified a cohort of T2D patients ≥18 years initiating empagliflozin or a DPP-4i from August 2014 through September 2017. As previously reported, DPP-4 inhibitors were chosen as the comparator group because they represent a comparable therapeutic alternative in a similar position in the T2D treatment pathway during the study period, have similar glycemic efficacy and hypoglycemia risk, and have demonstrated to be neutral on cardiovascular outcomes in clinical trials.11 Cohort entry date was the day of the first filled prescription of empagliflozin or a DPP-4i, with no use of SGLT-2i or DPP-4i in the preceding year. Patients were required to have continuous healthcare enrollment during the one year prior to and including the cohort entry date. We excluded patients who had a diagnosis of malignancy, end-stage kidney disease, human immunodeficiency virus, organ transplant, type 1 diabetes, secondary or gestational diabetes, or a nursing home admission during the baseline period (eTable 1, Figure 1 and eFigure 1).
Figure 1.
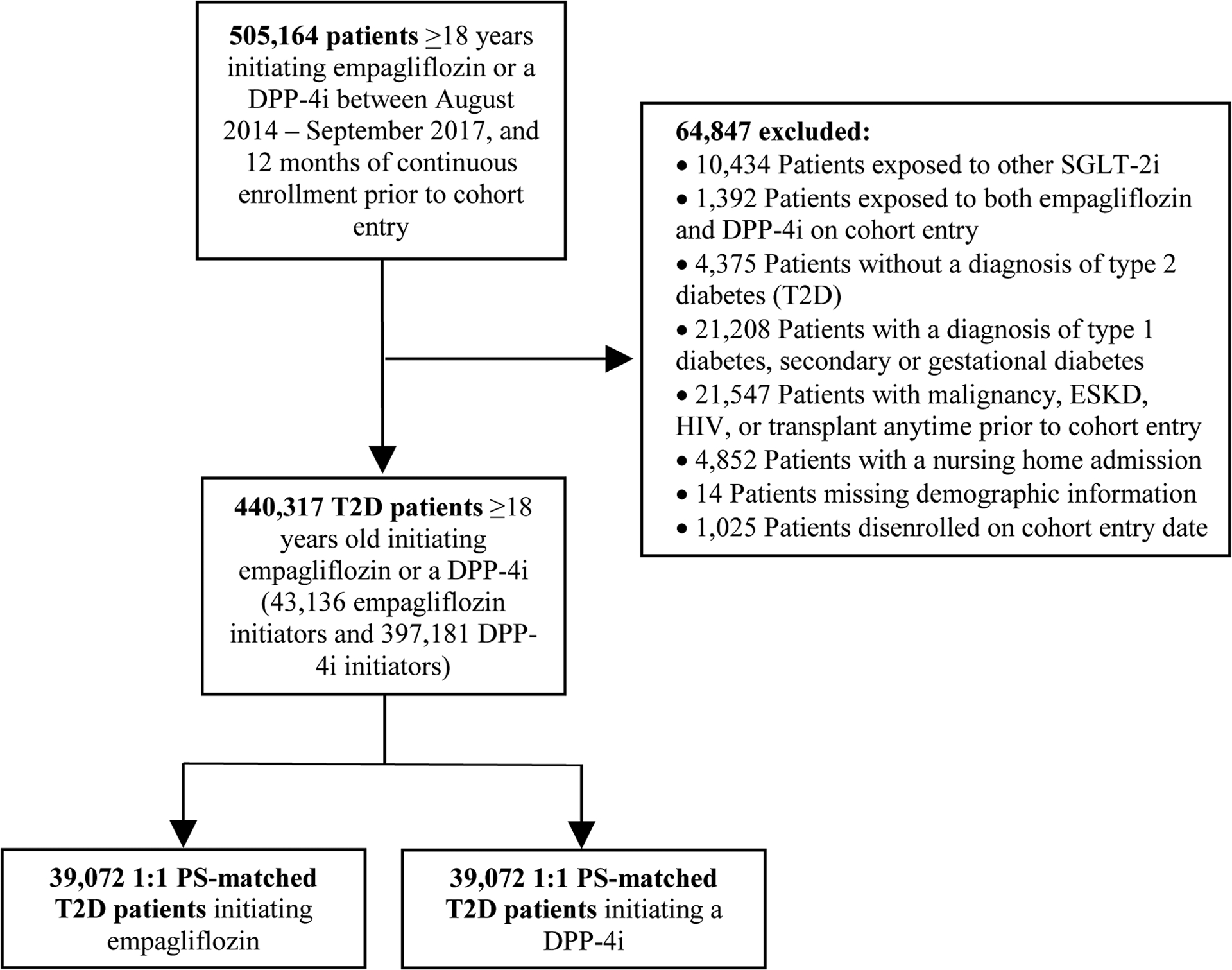
Study flowchart of overall study population of empagliflozin versus dipeptidyl peptidase-4 (DPP-4) inhibitor initiators. ESKD, end-stage kidney disease; HIV, human immunodeficiency virus; PS, propensity score; SGLT2, sodium-glucose cotransporter-2; T2D, type 2 diabetes
Outcomes and follow-up
The primary outcomes of interest were a composite of myocardial infarction or stroke and HHF, defined as a heart failure discharge diagnosis code in the primary position. The positive predictive values of the algorithms used to define these outcomes were 84% or higher in previous validation studies against medical records.12–15 We also assessed a broader definition of HHF, defined as a heart failure discharge diagnosis code in any position (HHF-broad; PPV = 79–96%).15 Secondary effectiveness outcomes included myocardial infarction and stroke assessed individually, all-cause mortality, and a composite of myocardial infarction, stroke and all-cause. Safety outcomes of interest included lower-limb amputations (LLA) requiring surgery, non-vertebral bone fractures (fracture of humerus, radius, ulna, or hip requiring intervention, or pelvis fracture), diabetic ketoacidosis (DKA) requiring hospitalization, and acute kidney injury (AKI) requiring hospitalization (investigated both as AKI discharge diagnosis in the primary or in any position). Safety outcomes were identified using algorithms validated against medical records.16–20 eTable 2 summarizes all outcome definitions. Follow-up began on the day after cohort entry and continued in an as-treated scheme until the first occurrence of treatment discontinuation or switch to a drug in the comparator class, the occurrence of a specific study outcome, a nursing home admission, death, plan disenrollment, or September 30, 2017. Treatment discontinuation was defined as not refilling a prescription within 30 days from the last day of expected coverage of the most recent prescription.
Patient characteristics
Patient characteristics were measured during the year prior to and including the cohort entry date (eFigure 1). These included demographic characteristics, calendar time (in quarters and days), comorbidities, diabetes-specific complications and other claims-based indicators of diabetes severity (e.g., number of hemoglobin A1c [HbA1c] or glucose tests ordered, endocrinologist visits, etc.), use of medications including diabetes medications, indicators of health care utilization as proxy for overall disease state, care intensity, and surveillance, and laboratory test results, which were available for a subset of 45–50% of patients in Optum and 5–10% in MarketScan through linkage with U.S. national lab test provider chains. We also measured a claims-based Combined Comorbidity Score21 and a frailty index22 to account for differences in overall comorbidity burden and frailty between treatment groups. The complete list of baseline patient characteristics is reported in eTable 3.
Statistical analysis
To account for the non-random allocation of patients to the treatment groups, we estimated a PS, which was calculated using a multivariable logistic regression that modeled the probability of initiating empagliflozin vs. DPP-4i as a function of over 140 pre-defined baseline characteristics.23 Except for laboratory test values, which were only available in a subset of the population, all prespecified variables were included in the PS model. Patients in both exposure groups were 1:1 PS-matched utilizing the nearest neighbor method without replacement with a maximum caliper of 0.01 of the PS.24 Standardized mean differences for each covariate were calculated to assess post-matching covariate balance between treatment groups, with values <0.1 interpreted as adequate balance between treatments.25 PS models were fitted and 1:1 matching performed separately within each database. Hazard ratios (HRs) and 95% confidence intervals (CI) were estimated using Cox proportional hazards models in each database and pooled across data sources using a fixed-effects meta-analysis, with quantification of the percentage of variation across databases due to heterogeneity through the I2 statistic. For the primary outcomes, we also produced Kaplan-Meier plots of cumulative incidence and compared incidence rates between treatment groups with log-rank tests.
To address potential informative censoring, in sensitivity analyses we re-defined treatment discontinuation as not refilling a prescription within 60 days (instead of 30 days) after the expiration of the last prescription’s supply, and we carried forward the exposure to the first-used medication for 365 days without considering drug discontinuation or switching, mimicking an “intention-to-treat” approach. To assess the presence of potential unmeasured confounding, we inspected the balance in laboratory test results, which were not included in the PS model as they were only available in a subset of the population,26 and evaluated the association of empagliflozin with a tracer outcome for which we expected a null finding, i.e., herpes zoster virus reactivation. Finally, as pre-exposure treatment with glucagon-like peptide-1 receptor agonists (GLP-1RA), which has demonstrated cardiovascular benefits in RCTs,27 is expected to be more frequently discontinued after initiation of a DPP-4i vs. empagliflozin, we excluded patients with GLP-1RA use at baseline, re-matched patients based on re-estimated PS, and censored at the time of GLP-1RA initiation, to assess the sensitivity of our primary findings to the potential time-varying confounding associated with the differential use of GLP-1RA during follow-up. We expected more DPP-4i than empagliflozin initiators to discontinue treatment with GLP-1RA as (1) the concomitant use of DPP-4i and GLP-1RA is not clinically recommended,5 due to their non-synergistic glucose-lowering action and the similarity in potential safety concerns, e.g., pancreatitis; and as (2) the combination of empagliflozin and GLP-1 RA may be considered in clinical practice, to further enhance the respective cardiovascular benefits demonstrated in clinical trials. We also conducted subgroup analyses for primary and selected secondary outcomes by history of established cardiovascular disease, defined as a diagnosis or procedure for myocardial infarction, unstable angina, coronary atherosclerosis or other forms of chronic ischemic heart disease, coronary procedure, congestive heart failure, ischemic or hemorrhagic stroke, transient ischemic attack, peripheral arterial disease or surgery, or lower extremity amputation, recorded during the baseline period. Within each subgroup, the PS was re-estimated, and PS-matching was re-performed.
All analyses were performed using Aetion platform version 4.10 with R version 3.2, which has been previously validated28, and SAS 9.4 Statistical Software (SAS Institute Inc., Cary, NC).
Results
We identified 440,317 eligible patients with T2D who initiated empagliflozin (43,136 overall, 10,768 in Optum, 20,188 in MarketScan, and 12,180 in Medicare) or DPP-4i (397,181 overall, 66,140 in Optum, 113,522 in MarketScan, and 217,519 in Medicare) across the three databases (Figure 1). Before PS-matching, empagliflozin initiators were younger, more likely male, with a lower burden of comorbidities overall, as measured by the Combined Comorbidity Score,21 and less frailty, as measured by the claims-based frailty index,22 compared to DPP-4i. Conversely, empagliflozin initiators tended to have higher prevalence of obesity, higher baseline use of insulin, and higher number of antidiabetic medications at cohort entry (Table 1 and eTable 3). After 1:1 PS-matching, we identified 39,072 pairs of empagliflozin or DPP-4i initiators (9,786 in Optum, 17,710 in MarketScan, and 11,576 in Medicare) with successful matching of 91% of empagliflozin patients. The distribution of all baseline characteristics was well balanced between the groups with standardized differences <0.1. Laboratory test results, including HbA1c and estimated glomerular filtration rate (eGFR), measured in a subset of the population, were also well balanced, despite not being included in the PS model. Study participants were 60 years old on average, 55% were male, 28% had history of established CVD, 62% were treated with metformin at cohort entry, 18% with insulin, and the mean HbA1c was 8.5% (Table 1 and eTable 3). Sitagliptin and linagliptin were the most frequently initiated agents within the DPP-4i class (62% and 18%, respectively) (eTable 4).
Table 1.
Selected baseline characteristics of patients initiating empagliflozin vs. DPP-4i before and after 1:1 PS matching
| BEFORE PS-MATCHING | AFTER PS-MATCHING | |||||
|---|---|---|---|---|---|---|
| Baseline characteristics | Empagliflozin (N = 43,136) |
DPP-4i (N = 397,181) |
St. Diff. | Empagliflozin (N = 39,072) |
DPP-4i (N = 39,072) |
St. Diff. |
| Demographics | ||||||
| Age, years; mean (sd) | 59.83 (8.99) | 67.33 (9.52) | −0.81 | 60.23 (9.05) | 60.32 (9.23) | −0.01 |
| Male; n (%) | 23,698 (54.9%) | 190,371 (47.9%) | 0.14 | 21,349 (54.6%) | 21,275 (54.5%) | 0.00 |
| White1; n (%) | 10,221 (83.9%) | 164,320 (75.5%) | 0.21 | 9,675 (83.6%) | 9,728 (84.0%) | −0.01 |
| Burden of comorbidities | ||||||
| Combined comorbidity score2; mean (sd) | 2.42 (1.70) | 3.04 (2.26) | −0.31 | 2.42 (1.72) | 2.42 (1.72) | 0.00 |
| Frailty index3, mean (sd) | 0.15 (0.04) | 0.17 (0.05) | −0.44 | 0.15 (0.04) | 0.15 (0.04) | 0.00 |
| Diabetes-related complications | ||||||
| Diabetic nephropathy; n (%) | 4,330 (10.0%) | 50,101 (12.6%) | −0.08 | 3,847 (9.8%) | 3,774 (9.7%) | 0.00 |
| Diabetic retinopathy; n (%) | 2,772 (6.4%) | 28,327 (7.1%) | −0.03 | 2,456 (6.3%) | 2,408 (6.2%) | 0.00 |
| Diabetic neuropathy; n (%) | 8,141 (18.9%) | 76,325 (19.2%) | −0.01 | 7,119 (18.2%) | 7,151 (18.3%) | 0.00 |
| Diabetes with peripheral circulatory disorders; n (%) | 2,341 (5.4%) | 27,420 (6.9%) | −0.06 | 2,091 (5.4%) | 2,108 (5.4%) | 0.00 |
| Diabetic Foot; n (%) | 864 (2.0%) | 9,961 (2.5%) | −0.03 | 758 (1.9%) | 773 (2.0%) | −0.01 |
| Lower extremity amputation; n (%) | 171 (0.4%) | 1,930 (0.5%) | −0.01 | 149 (0.4%) | 144 (0.4%) | 0.00 |
| Hypoglycemia; n (%) | 3,366 (7.8%) | 28,925 (7.3%) | 0.02 | 2,948 (7.5%) | 2,973 (7.6%) | 0.00 |
| Diabetic ketoacidosis; n (%) | 53 (0.1%) | 1,089 (0.3%) | −0.04 | 051 (0.1%) | 048 (0.1%) | 0.00 |
| Diabetes treatment | ||||||
| No. antidiabetic drugs at cohort entry; mean (sd) | 2.31 (0.93) | 2.14 (0.78) | 0.20 | 2.24 (0.90) | 2.22 (0.84) | 0.02 |
| No previous use of diabetes treatment4; n (%) | 3,011 (7.0%) | 53,723 (13.5%) | −0.22 | 2,989 (7.6%) | 2,914 (7.5%) | 0.00 |
| Long-term use of insulin5; n (%) | 5,539 (12.8%) | 28,233 (7.1%) | 0.19 | 4,389 (11.2%) | 4,294 (11.0%) | 0.01 |
| Diabetes drug on the day of cohort entry: | ||||||
| Metformin; n (%) | 26,342 (61.1%) | 250,135 (63.0%) | −0.04 | 24,169 (61.9%) | 24,028 (61.5%) | 0.01 |
| Sulfonylureas; n (%) | 11,357 (26.3%) | 129,684 (32.7%) | −0.14 | 10,569 (27.1%) | 10,574 (27.1%) | 0.00 |
| GLP-1RA; n (%) | 6,848 (15.9%) | 6,084 (1.5%) | 0.53 | 3,834 (9.8%) | 3,626 (9.3%) | 0.02 |
| Glitazones; n (%) | 2,815 (6.5%) | 20,965 (5.3%) | 0.05 | 2,474 (6.3%) | 2,439 (6.2%) | 0.00 |
| Insulin; n (%) | 8,576 (19.9%) | 41,648 (10.5%) | 0.26 | 6,905 (17.7%) | 6,825 (17.5%) | 0.01 |
| Lifestyle factors | ||||||
| Obesity; n (%) | 13,666 (31.7%) | 90,312 (22.7%) | 0.20 | 11,858 (30.3%) | 11,848 (30.3%) | 0.00 |
| Overweight; n (%) | 2,530 (5.9%) | 22,693 (5.7%) | 0.01 | 2,321 (5.9%) | 2,391 (6.1%) | −0.01 |
| Smoking; n (%) | 5,558 (12.9%) | 61,018 (15.4%) | −0.07 | 5,127 (13.1%) | 5,117 (13.1%) | 0.00 |
| Other comorbidities at baseline | ||||||
| Cardiovascular disease6; n (%) | 12,029 (27.9%) | 147,746 (37.2%) | −0.20 | 10,976 (28.1%) | 10,946 (28.0%) | 0.00 |
| Ischemic heart disease; n (%) | 9,078 (21.0%) | 102,729 (25.9%) | −0.12 | 8,232 (21.1%) | 8,322 (21.3%) | 0.00 |
| Acute myocardial infarction; n (%) | 707 (1.6%) | 8,755 (2.2%) | −0.04 | 643 (1.6%) | 651 (1.7%) | −0.01 |
| Coronary revascularization; n (%) | 501 (1.2%) | 5313 (1.3%) | −0.01 | 457 (1.2%) | 448 (1.1%) | 0.01 |
| Heart failure; n (%) | 2,643 (6.1%) | 44,237 (11.1%) | −0.18 | 2,453 (6.3%) | 2,437 (6.2%) | 0.00 |
| Atrial fibrillation; n (%) | 2,626 (6.1%) | 39,508 (9.9%) | −0.14 | 2,421 (6.2%) | 2,418 (6.2%) | 0.00 |
| Ischemic stroke; n (%) | 2,666 (6.2%) | 39,202 (9.9%) | −0.14 | 2,449 (6.3%) | 2,365 (6.1%) | 0.01 |
| Transient ischemic attack; n (%) | 644 (1.5%) | 9,777 (2.5%) | −0.07 | 609 (1.6%) | 605 (1.5%) | 0.01 |
| Other cerebrovascular disease; n (%) | 760 (1.8%) | 12,537 (3.2%) | −0.09 | 712 (1.8%) | 691 (1.8%) | 0.00 |
| Peripheral arterial disease or surgery; n (%) | 2,490 (5.8%) | 40,765 (10.3%) | −0.17 | 2,318 (5.9%) | 2,284 (5.8%) | 0.00 |
| Hypertension; n (%) | 33,686 (78.1%) | 329,328 (82.9%) | −0.12 | 30,429 (77.9%) | 30,474 (78.0%) | 0.00 |
| Hyperlipidemia; n (%) | 34,642 (80.3%) | 322,750 (81.3%) | −0.03 | 31,201 (79.9%) | 31,253 (80.0%) | 0.00 |
| Edema7; n (%) | 3,302 (7.7%) | 43,978 (11.1%) | −0.12 | 3,016 (7.7%) | 2,990 (7.7%) | 0.00 |
| Chronic kidney disease; n (%) | 3,507 (8.1%) | 72,844 (18.3%) | −0.30 | 3,318 (8.5%) | 3,335 (8.5%) | 0.00 |
| Chronic kidney disease stage 1–2; n (%) | 1,254 (2.9%) | 15,647 (3.9%) | −0.06 | 1,143 (2.9%) | 1,095 (2.8%) | 0.01 |
| Chronic kidney disease stage 3+; n (%) | 2,010 (4.7%) | 51,898 (13.1%) | −0.30 | 1,937 (5.0%) | 1,928 (4.9%) | 0.00 |
| Acute kidney injury; n (%) | 797 (1.8%) | 22,553 (5.7%) | −0.21 | 761 (1.9%) | 709 (1.8%) | 0.01 |
| Chronic obstructive pulmonary disease (COPD); n (%) | 2,743 (6.4%) | 43,969 (11.1%) | −0.17 | 2,582 (6.6%) | 2,617 (6.7%) | 0.00 |
| Fractures; n (%) | 1,477 (3.4%) | 18,041 (4.5%) | −0.06 | 1,332 (3.4%) | 1,395 (3.6%) | −0.01 |
| Falls; n (%) | 1,097 (2.5%) | 18,446 (4.6%) | −0.11 | 1,015 (2.6%) | 1,040 (2.7%) | −0.01 |
| Osteoporosis; n (%) | 1,484 (3.4%) | 29,916 (7.5%) | −0.18 | 1,414 (3.6%) | 1,404 (3.6%) | 0.00 |
| Dementia; n (%) | 794 (1.8%) | 22,883 (5.8%) | −0.21 | 760 (1.9%) | 727 (1.9%) | 0.00 |
| Other medication use | ||||||
| Angiotensin converting enzyme inhibitors; n (%) | 19,742 (45.8%) | 183,201 (46.1%) | −0.01 | 17,790 (45.5%) | 17,767 (45.5%) | 0.00 |
| Angiotensin II receptor blockers; n (%) | 13,333 (30.9%) | 124,786 (31.4%) | −0.01 | 11,984 (30.7%) | 12,082 (30.9%) | 0.00 |
| Beta-blockers; n (%) | 15,042 (34.9%) | 166,827 (42.0%) | −0.15 | 13,704 (35.1%) | 13,692 (35.0%) | 0.00 |
| Calcium-channel blockers; n (%) | 10,728 (24.9%) | 125,592 (31.6%) | −0.15 | 9,868 (25.3%) | 9,851 (25.2%) | 0.00 |
| Thiazides; n (%) | 5,457 (12.7%) | 57,288 (14.4%) | −0.05 | 4,939 (12.6%) | 4,863 (12.4%) | 0.01 |
| Loop diuretics; n (%) | 4,560 (10.6%) | 65,301 (16.4%) | −0.17 | 4,168 (10.7%) | 4,102 (10.5%) | 0.01 |
| Statins; n (%) | 30,358 (70.4%) | 280,291 (70.6%) | 0.00 | 27,259 (69.8%) | 27,280 (69.8%) | 0.00 |
| Other lipid-lowering drugs; n (%) | 6,260 (14.5%) | 53,027 (13.4%) | 0.03 | 5,556 (14.2%) | 5,498 (14.1%) | 0.00 |
| Antiplatelet agents; n (%) | 5,191 (12.0%) | 55,847 (14.1%) | −0.06 | 4,704 (12.0%) | 4,634 (11.9%) | 0.00 |
| Oral anticoagulants; n (%) | 2,387 (5.5%) | 32,584 (8.2%) | −0.11 | 2,198 (5.6%) | 2,239 (5.7%) | 0.00 |
| Oral corticosteroids; n (%) | 10,400 (24.1%) | 103,052 (25.9%) | −0.04 | 9,438 (24.2%) | 9,493 (24.3%) | 0.00 |
| Bisphosphonates; n (%) | 527 (1.2%) | 12,092 (3.0%) | −0.13 | 513 (1.3%) | 489 (1.3%) | 0.00 |
| Opioids; n (%) | 13,528 (31.4%) | 127,447 (32.1%) | −0.02 | 12,207 (31.2%) | 12,236 (31.3%) | 0.00 |
| Benzodiazepines; n (%) | 5,550 (12.9%) | 53,583 (13.5%) | −0.02 | 5,021 (12.9%) | 5,059 (12.9%) | 0.00 |
| Measures of healthcare utilization | ||||||
| Hospitalization within prior 30 days; n (%) | 459 (1.1%) | 17,557 (4.4%) | −0.20 | 449 (1.1%) | 453 (1.2%) | −0.01 |
| Hospitalization during prior 31–365 days; n (%) | 3,224 (7.5%) | 42,977 (10.8%) | −0.11 | 2,956 (7.6%) | 2,977 (7.6%) | 0.00 |
| No. hospital days; mean (sd) | 0.51 (2.48) | 1.14 (4.86) | −0.16 | 0.53 (2.52) | 0.53 (2.54) | 0.00 |
| No. emergency department visits; mean (sd) | 0.46 (1.61) | 0.78 (2.11) | −0.17 | 0.48 (1.64) | 0.48 (1.69) | 0.00 |
| No. office visits; mean (sd) | 9.08 (6.53) | 9.46 (7.15) | −0.06 | 8.96 (6.50) | 9.02 (6.75) | −0.01 |
| Endocrinologist visit within prior 30 days; n (%) | 8,404 (19.5%) | 30,912 (7.8%) | 0.35 | 6,157 (15.8%) | 5,916 (15.1%) | 0.02 |
| Endocrinologist visit during prior 31–365 days; n (%) | 8,551 (19.8%) | 35,308 (8.9%) | 0.31 | 6,392 (16.4%) | 6,167 (15.8%) | 0.02 |
| Cardiologist visit within prior 30 days; n (%) | 3,422 (7.9%) | 42,341 (10.7%) | −0.10 | 3,143 (8.0%) | 3,154 (8.1%) | 0.00 |
| Cardiologist visit during prior 31–365 days; n (%) | 11,794 (27.3%) | 129,083 (32.5%) | −0.11 | 10,697 (27.4%) | 10,844 (27.8%) | −0.01 |
| No. electrocardiograms, mean (sd) | 0.92 (1.76) | 1.18 (2.16) | −0.13 | 0.93 (1.77) | 0.93 (1.79) | 0.00 |
| Echocardiogram; n (%) | ||||||
| No. distinct prescriptions; mean (sd) | 12.48 (5.99) | 12.25 (5.99) | 0.04 | 12.24 (5.90) | 12.26 (6.06) | 0.00 |
| Use of glucose test strips; n (%) | 2,362 (5.5%) | 21,408 (5.4%) | 0.00 | 2,109 (5.4%) | 2,098 (5.4%) | 0.00 |
| No. HbA1c tests ordered; mean (sd) | 2.46 (1.32) | 2.33 (1.36) | 0.10 | 2.42 (1.31) | 2.42 (1.34) | 0.00 |
| Laboratory test results 8 | ||||||
| HbA1c (%); mean (sd) | 8.50 (1.77) | 8.37 (1.80) | 0.07 | 8.49 (1.78) | 8.55 (1.84) | −0.03 |
| Patients with HbA1c results available; n (%) | 6,734 (15.6%) | 43,174 (10.9%) | 0.14 | 6,083 (15.6%) | 6,610 (16.9%) | −0.04 |
| eGFR mL/min/1.73 m2; mean (sd) | 86.58 (19.04) | 78.60 (22.74) | 0.38 | 86.20 (19.16) | 84.63 (20.61) | 0.08 |
| Patients with creatinine results available; n (%) | 7,032 (16.3%) | 44,203 (11.1%) | 0.15 | 6,329 (16.2%) | 6,775 (17.3%) | −0.03 |
| Total cholesterol (mg/dl); mean (sd) | 176.23 (46.65) | 178.22 (46.97) | −0.04 | 176.79 (46.78) | 180.43 (49.09) | −0.08 |
| Patients with total cholesterol results available; n (%) | 6,419 (14.9%) | 39,654 (10%) | 0.15 | 5,795 (14.8%) | 6,211 (15.9%) | −0.03 |
| LDL level (mg/dl); mean (sd) | 87.45 (40.18) | 89.99 (43.17) | −0.06 | 87.96 (40.41) | 90.08 (41.07) | −0.05 |
| Patients with LDL results available; n (%) | 6,263 (14.5%) | 38,897 (9.8%) | 0.14 | 5,656 (14.5%) | 6,088 (15.6%) | −0.03 |
| HDL level (mg/dl); mean (sd) | 44.28 (13.02) | 46.07 (37.81) | −0.06 | 44.44 (13.09) | 44.83 (13.12) | −0.03 |
| Patients with HDL results available; n (%) | 6,312 (14.6%) | 39,177 (9.9%) | 0.14 | 5,701 (14.6%) | 6,123 (15.7%) | −0.03 |
| Triglyceride level (mg/dl); mean (sd) | 204.65 (208.79) | 193.37 (171.23) | 0.06 | 203.93 (207.44) | 201.92 (182.49) | 0.01 |
| Patients with triglyceride results available; n (%) | 6,344 (14.7%) | 39,193 (9.9%) | 0.15 | 5,728 (14.7%) | 6,154 (15.8%) | −0.03 |
PS: propensity-score; DPP-4i: dipeptidyl peptidase 4 inhibitors; St. Diff: standardized differences, i.e., the difference in means or proportions divided by the pooled standard deviation [Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in medicine 2009;28:3083-107]; sd: standard deviation; GLP-1RA: glucagon-like peptide-1 receptor agonists; HbA1c: hemoglobin A1c; eGFR: estimated glomerular filtration rate; LDL: low-density lipoproteins; HDL: high-density lipoproteins.
Only available in Medicare fee-for-service and Optum Clinformatics
Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. Journal of Clinical Epidemiology. 2011; 64 (7) 749–59.
Kim DH, Schneeweiss S, Glynn RJ. Comparing Approaches to Measure Frailty in Medicare Data: Deficit-Accumulation Frailty Index Versus Phenotypic Frailty. J Gerontol A Biol Sci Med Sci. 2018;73(7):989–990
Defined as patients without any use of glucose-lowering medications during the 12 months prior to cohort entry
Based on ICD coding
Defined as history of myocardial infarction, unstable angina, coronary atherosclerosis and other forms of chronic ischemic heart disease, coronary procedure, heart failure, ischemic or hemorrhagic stroke, transient ischemic attack, peripheral arterial disease or surgery, lower extremity amputation
Localized, generalized, or unspecified edema
Only available in Optum Clinformatics and IBM MarketScan
After PS-matching, the mean follow-up time on treatment was approximately 6 months in both exposure groups. Over 9,000 patients had follow-up time greater than 1 year. Most patients were censored due to treatment discontinuation (44%) or end of the study period, i.e. September 30, 2017 (40%) (eTable 5).
There were 2.8 vs. 6.1 events per 1,000 person years for the HHF outcome based on a heart failure discharge diagnosis in the primary position (HR 0.48, 95% CI 0.35–0.67), 14.1 vs. 23.0 events per 1,000 person years for the HHF-broad outcome (HR 0.63, 95% CI 0.54–0.74), and 10.6 vs. 10.7 events per 1,000 person years for the composite outcome of myocardial infarction or stroke (HR 0.99, 95% CI 0.81–1.21) among empagliflozin initiators vs. DPP-4i initiators, (Table 2). Database-specific estimates for these outcomes showed consistent results with I2 = 0% (eTable 6). Kaplan-Meier curves comparing the cumulative incidence of primary outcomes in empagliflozin vs. DPP-4i initiators were consistent with these findings (Figure 2). Kaplan-Meier curves for HHF outcomes separated early, within the first six months of treatment initiation. The proportional hazards assumption, which was assessed by testing the significance of the interaction term between exposure and time, was not violated.
Table 2.
Number of events, incidence rates, and hazard ratios for primary and secondary outcomes in 1:1 PS-matched initiators of empagliflozin vs. DPP-4i.
| Empagliflozin | DPP-4i | Empagliflozin vs. DPP4i |
|
|---|---|---|---|
| N = 39,072 | N = 39,072 | ||
| N events (IR/1000 PY) |
N events (IR/1000 PY) |
HR (95% CI) |
|
| Primary outcomes | |||
| HHF1 | 54 (2.83) | 111 (6.14) | 0.48 (0.35–0.67) |
| HHF-broad2 | 268 (14.07) | 413 (22.95) | 0.63 (0.54–0.74) |
| Myocardial infarction or stroke | 201 (10.55) | 193 (10.70) | 0.99 (0.81–1.21) |
| Secondary effectiveness outcomes | |||
| Myocardial infarction | 123 (6.45) | 121 (6.70) | 0.96 (0.75–1.24) |
| Stroke | 80 (4.19) | 73 (4.04) | 1.06 (0.77–1.45) |
| All-cause mortality | 58 (3.03) | 112 (6.19) | 0.52 (0.38–0.72) |
| Myocardial infarction, stroke, or all-cause mortality | 253 (13.28) | 295 (16.36) | 0.83 (0.70–0.98) |
| Safety outcomes | |||
| Lower-limb amputation | 53 (2.78) | 44 (2.43) | 1.12 (0.75–1.67) |
| Bone fracture3 | 56 (2.93) | 45 (2.49) | 1.18 (0.78–1.76) |
| Diabetic ketoacidosis | 52 (2.72) | 28 (1.55) | 1.71 (1.08–2.71) |
| Acute kidney injury1 | 51 (2.67) | 82 (4.54) | 0.60 (0.43–0.85) |
| Acute kidney injury - broad2 | 349 (18.34) | 536 (29.87) | 0.62 (0.55–0.71) |
PS: propensity score; DPP-4i: dipeptidyl peptidase 4 inhibitors; IR: Incidence rate; PY: person-years; HR: hazard ratio; CI: confidence intervals; HHF: hospitalization for heart failure
Defined as a discharge diagnosis code in the primary position
Defined as a discharge diagnosis code in any position
Fracture of humerus, radius, ulna, or hip requiring surgical repair, or pelvis fracture
Figure 2.
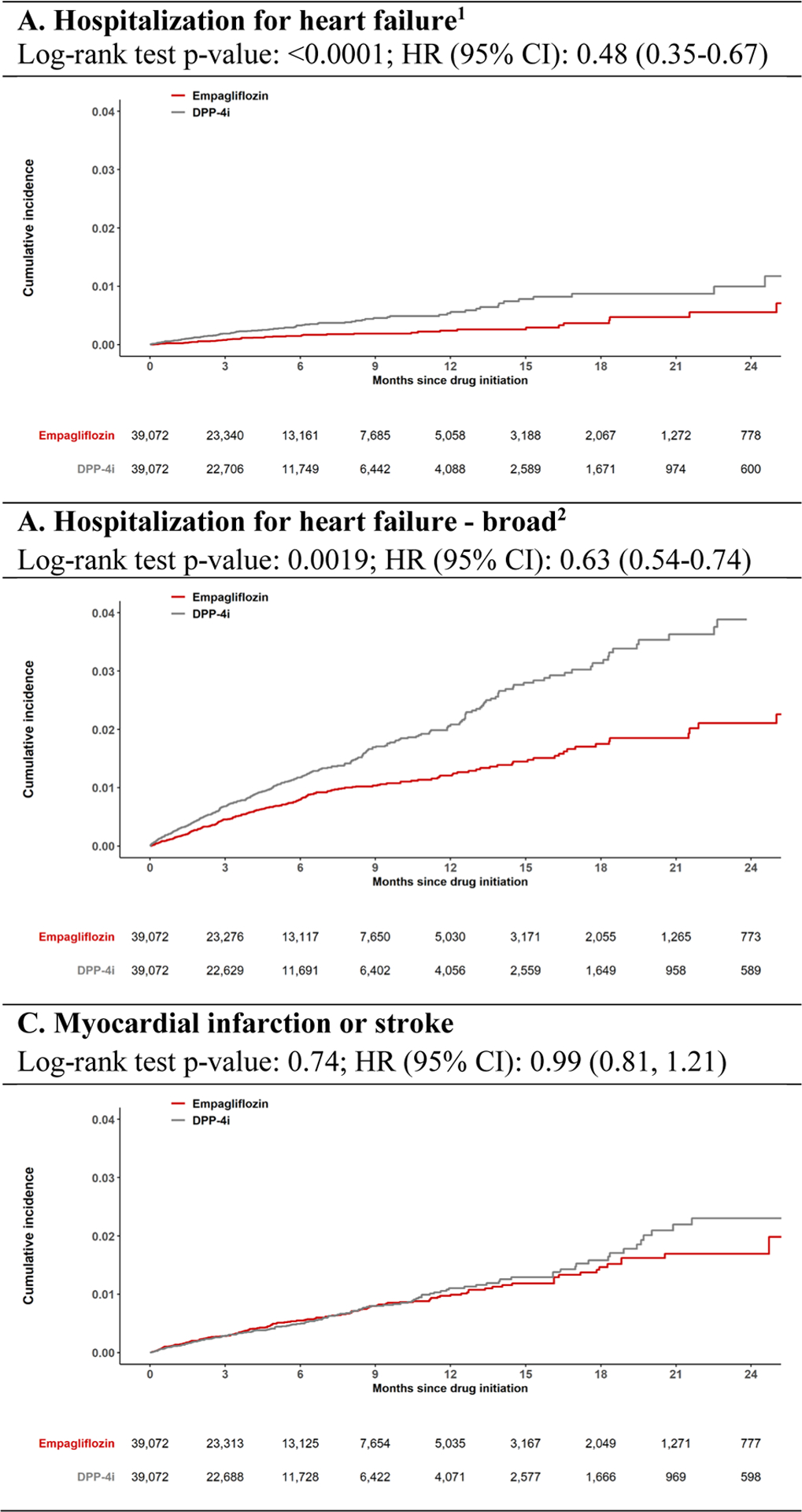
Cumulative incidence of primary outcomes comparing propensity score (PS)-matched empagliflozin versus dipeptidyl peptidase-4 (DPP-4) inhibitor initiators. CI, confidence interval; HR, hazard ratio. 1 Defined as a discharge diagnosis code in the primary position. 2 Defined as a discharge diagnosis code in any position
For the secondary effectiveness outcomes, no difference in the risk of myocardial infarction or stroke, considered as individual outcomes, was observed in empagliflozin vs. DPP-4i initiators (HR 0.96, 95% CI 0.75–1.24; and HR 1.06, 95% CI 0.77–1.45, respectively). However, empagliflozin initiation was associated with a lower risk of all-cause mortality (HR 0.52, 95% CI 0.38–0.72), and the composite outcome of myocardial infarction, stroke, or all-cause mortality (HR 0.83, 95% CI 0.70–0.98), compared with DPP-4i initiation. Overall findings were consistent with the results generated within the Medicare population, who had complete information on all-cause mortality (HR 0.54, 95% CI 0.38–0.78; and HR 0.77, 95% CI 0.61–0.97) (eTable 6).
For the safety outcomes, empagliflozin initiators had similar risk of LLA and bone fractures (HR 1.12, 95% CI 0.75–1.67; and HR 1.18, 95% CI 0.78–1.76), increased risk of DKA (HR 1.71, 95% CI 1.08–2.71), and lower risk of AKI (HR 0.60, 95% CI 0.43–0.85, based on an AKI discharge diagnosis in the primary position; and HR 0.62, 95% CI 0.55–0.71, based on an AKI discharge diagnosis in any position), compared with initiators of DPP-4i (Table 2).
Findings were consistent in sensitivity analyses, which re-defined treatment discontinuation as not refilling a prescription within 60 days (instead of 30 days) after the expiration of the last prescription’s supply, carried forward the exposure to the first-used medication for 365 days without considering drug discontinuation or switching, and excluded patients with baseline use of GLP-1RA (Table 3, eTables 7–8). As expected, the null hypothesis of no association between empagliflozin and the tracer outcome was not rejected (HR 0.97, 95% CI 0.81–1.17). Subgroup analyses by history of established cardiovascular disease produced results consistent with findings for primary and selected secondary outcomes (Table 3).
Table 3.
Sensitivity and subgroup analyses in 1:1 PS-matched initiators of empagliflozin vs. DPP-4i
| Empagliflozin | DPP-4i | Empagliflozin vs. DPP4i |
|
|---|---|---|---|
| N = 39,072 | N = 39,072 | ||
| N events (IR/1000 PY) |
N events (IR/1000 PY) |
HR (95% CI) |
|
| Primary analysis | |||
| HHF1 | 54 (2.83) | 111 (6.14) | 0.48 (0.35–0.67) |
| HHF-broad2 | 268 (14.07) | 413 (22.95) | 0.63 (0.54–0.74) |
| Myocardial infarction or stroke | 201 (10.55) | 193 (10.70) | 0.99 (0.81–1.21) |
| 60-day grace period analysis | |||
| HHF1 | 78 (3.497) | 136 (6.34) | 0.56 (0.42–0.74) |
| HHF-broad2 | 332 (14.81) | 495 (23.18) | 0.65 (0.57–0.75) |
| Myocardial infarction or stroke | 239 (10.65) | 234 (10.93) | 0.97 (0.81–1.17) |
| 1st exposure carried forward analysis | |||
| HHF1 | 98 (4.26) | 162 (7.09) | 0.60 (0.47–0.78) |
| HHF-broad2 | 394 (17.21) | 565 (24.85) | 0.70 (0.61–0.79) |
| Myocardial infarction or stroke | 260 (11.34) | 282 (12.36) | 0.92 (0.77–1.09) |
| No baseline use of GLP-1RA3 (32,497 patient pairs) | |||
| HHF1 | 47 (3.19) | 105 (7.27) | 0.47 (0.33–0.66) |
| HHF-broad2 | 226 (15.41) | 407 (28.32) | 0.57 (0.48–0.67) |
| Myocardial infarction or stroke | 167 (11.38) | 195 (13.53) | 0.86 (0.70–1.05) |
| Tracer outcome | |||
| Herpes zoster | 226 (11.89) | 216 (12.00) | 0.97 (0.81–1.17) |
| Subgroup analyses | |||
| History of CV disease4 (10,838 patient pairs) | |||
| HHF1 | 47 (9.58) | 78 (16.21) | 0.59 (0.41–0.86) |
| HHF-broad2 | 216 (44.42) | 216 (66.57) | 0.68 (0.57–0.80) |
| Myocardial infarction or stroke | 105 (21.46) | 124 (25.86) | 0.83 (0.64–1.08) |
| Secondary outcomes: | |||
| Myocardial infarction | 63 (12.86) | 73 (15.18) | 0.84 (0.60–1.18) |
| Stroke | 44 (8.96) | 55 (11.43) | 0.79 (0.53–1.17) |
| All-cause mortality | 37 (7.52) | 69 (14.29) | 0.53 (0.35–0.79) |
| Myocardial infarction, stroke, or all-cause mortality | 138 (28.20) | 185 (38.58) | 0.73 (0.58–0.91) |
| Lower-limb amputation | 26 (5.29) | 20 (4.14) | 1.24 (0.69–2.24) |
| No history of CV disease (28,092 patient pairs) | |||
| HHF1 | <11 | 14 (1.07) | 0.63 (0.27–1.47) |
| HHF-broad2 | 55 (3.89) | 84 (6.40) | 0.61 (0.43–0.85) |
| Myocardial infarction or stroke | 93 (6.59) | 88 (6.71) | 0.98 (0.73–1.32) |
| Secondary outcomes: | |||
| Myocardial infarction | 60 (4.25) | 47 (3.58) | 1.17 (0.80–1.72) |
| Stroke | 33 (2.34) | 42 (3.20) | 0.75 (0.47–1.18) |
| All-cause mortality | 20 (1.41) | 35 (2.66) | 0.53 (0.30–0.94) |
| Myocardial infarction, stroke, or all-cause mortality | 111 (7.87) | 120 (9.15) | 0.88 (0.68–1.14) |
| Lower-limb amputation | 25 (1.77) | 23 (1.75) | 0.98 (0.55–1.74) |
PS: propensity score; DPP-4i: dipeptidyl peptidase 4 inhibitors; IR: Incidence rate; PY: person-years; HR: hazard ratio; CI: confidence intervals; HHF: hospitalization for heart failure; GLP-1RA: glucagon-like peptide-1 receptor agonists; CV: cardiovascular
Defined as a discharge diagnosis code in the primary position
Defined as a discharge diagnosis code in any position
In this analysis, patients who started GLP-1RA during follow-up (4.5% among empagliflozin users and 3.1% among DPP-4i users) were censored at the time of GLP-1RA treatment initiation.
History of cardiovascular disease is defined as history of myocardial infarction, unstable angina, coronary atherosclerosis and other forms of chronic ischemic heart disease, coronary procedure, heart failure, ischemic or hemorrhagic stroke, transient ischemic attack, peripheral arterial disease or surgery, lower extremity amputation
Discussion
In this analysis from the multi-year EMPRISE study program including nearly 40,000 routine-care patients with T2D initiating empagliflozin 1:1 PS-matched to patients initiating DPP-4i in the U.S., we found that empagliflozin was associated with a 37–52% decreased risk of HHF compared with DPP-4i and a similar risk of myocardial infarction or stroke. The initiation of empagliflozin vs. DPP-4i was also associated with a reduction in the risk of all-cause mortality and a composite outcome of myocardial infarction, stroke, or all-cause mortality. Compared with initiation of DPP-4i, empagliflozin initiation was associated with a similar risk of LLA and fractures, a 71% increased risk of DKA, and a 38–40% decreased risk of AKI. Findings were consistent in sensitivity analyses and subgroup analyses of patients with and without history of established cardiovascular disease.
This analysis from the EMPRISE study program, which addressed the comparative effectiveness and safety of empagliflozin in routine care patients with mean age of 60 years across the broad spectrum of cardiovascular risk, and which included a population of empagliflozin users nearly ten times larger than the population enrolled in the EMPA-REG OUTCOME trial (N = 4,687), provides clinically relevant findings that complement available evidence from RCTs.1–3
When directly compared with an alternative glucose-lowering treatment, i.e., DPP-4i, empagliflozin was associated with substantial reduction in the risk of HHF. This finding is comparable in timing and magnitude to the effects of empagliflozin and other SGLT-2i on HHF in cardiovascular outcome trials in populations of patients with T2D1–3,29 or heart failure with reduced ejection fraction,30,31 and in real-world investigations that reported on the overall SGLT-2i class or on other SGLT-2i.32–34 The early occurrence of benefits with respect to heart failure endpoints has been observed as early as 12 weeks in the EMPA-REG OUTCOME trial, and the reduction in cardiac preload and afterload, caused by an osmotic diuresis, has been noted as one of the key mechanisms underlying the rapid reduction in systolic blood pressure, body weight, and subsequent risk of hospitalization for heart failure.35 Our results, which include information through the third year of EMPRISE, also confirm preliminary results from this study program with greater precision,36 including the observation of consistent reductions in HHF associated with the initiation of empagliflozin regardless of whether patients did or did not have history of established cardiovascular disease. In our study, empagliflozin and DPP-4i had comparable efficacy in prevention of myocardial infarction or stroke, independent of the presence of established cardiovascular disease at baseline. Similarly, neither empagliflozin nor other SGLT-2i produced meaningful reductions in atherosclerotic outcomes in placebo-controlled RCTs, though duration of follow-up was longer in clinical trials compared to our study.1–3 Conversely, we found that initiation of empagliflozin in clinical practice was associated with a decreased risk of all-cause mortality and of a composite outcome of myocardial infarction, stroke, or all-cause mortality. These findings are consistent with results from the EMPA-REG OUTCOME trial, which, in addition to HHF, showed substantial and early effects of empagliflozin on cardiovascular death and all-cause mortality.1,35 Through selective inhibition of SGLT2 in the proximal tubule of the kidney, empagliflozin leads to reduced renal glucose reabsorption and increased urinary glucose excretion, with reductions in volume and sodium load through its glucuretic, diuretic, and natriuretic properties. It has been suggested that hemodynamic changes in hematocrit and hemoglobin related to plasma volume contraction driven by empagliflozin, which manifested as early as 12 weeks after empagliflozin initiation in the EMPA-REG OUTCOME trial, may be an important mediator of the reduction in cardiovascular mortality risk observed with empagliflozin.37 This may ultimately also contribute to the reduction in the risk of overall mortality in patients with T2D.
In this analysis of EMPRISE, we report for the first time on selected safety outcomes associated with the initiation of empagliflozin in clinical practice. The CANVAS trial detected a nearly two-fold increased risk of LLA (6.3 v 3.4 per 1000 person years) and a 56% increased risk of bone fractures (HR 1.56, 95% CI: 1.18–2.06) among patients randomized to the SGLT-2i canagliflozin vs. placebo.2 Although no differences were seen in the risk of either outcome among patients randomized to canagliflozin vs. placebo in the subsequent CREDENCE trial,4 the label for canagliflozin was revised to include a warning for these outcomes6,8 and concerns were raised with respect to other SGLT-2i. In this study, we did not observe meaningful differences in the risk of either LLA or fractures among patients initiating empagliflozin compared with DPP-4i. These findings are consistent with results from the EMPA-REG OUTCOME trial, which found similar proportions of these outcomes in the empagliflozin and placebo arm.1,38 Real-world investigations to date have shown no increased risk of fractures associated with the use of SGLT-2i,9,39,40 though a potential increase in the risk of LLA has been detected, mostly for other SGLT-2i, among older patients and patients with cardiovascular disease.34,41 In our study, we did not observe large differences in the risk of LLA among patients initiating empagliflozin vs. DPP-4i in either Medicare patients or patients with cardiovascular disease, though point estimates for LLA remain imprecise in these subgroup analyses at this time of the EMPRISE program. In this analysis, we also investigated the association between the initiation of empagliflozin and DKA and found that empagliflozin was associated with a 71% increased risk, compared with DPP-4i. Although originally not detected in RCTs, cases of DKA, mostly characterized by euglycemia, started to appear in the post-marketing period among SGLT2i-treated patients with T2D, which resulted in a warning in 2015 to include DKA as an adverse effect of SGLT-2i.6 Subsequent RCTs and observational studies have confirmed the increase in DKA risk among SGLT-2i users,3,4,10 with a recent analyses suggesting a class effect.42 Finally, this study identified a decrease in the risk of AKI among patients initiating empagliflozin vs. DPP-4i. This is in contrast with previous concerns that SGLT-2i may lead to AKI due to an initial acute drop in eGFR after SGLT-2i initiation, and with early postmarketing reports of AKI cases with SGLT-2i that prompted a warning in 2016.43 The EMPA-REG OUTCOME trial and other RCTs showed that the risk of AKI is not increased and may even be reduced in patients randomized to SGLT-2i, compared to placebo,44 and a network meta-analysis of RCTs indicated a reduced risk of AKI with SGLT-2i vs. DPP-4i,45 possibly driven by a reduction in ischemia-reperfusion injury to the kidney and decreased tubular injury markers. Our results provide further reassurance on the safety of empagliflozin with respect to the risk of AKI.
This analysis from EMPRISE has limitations. First, although we increased comparability between treatment groups through extensive PS matching by many baseline characteristics, residual confounding by unmeasured or not well measured characteristics in claims (e.g., hemoglobin A1c level, diabetes duration, obesity) cannot be excluded. However, in a previous investigation based on claims data linked to electronic health records, we showed sufficient balance in many of these characteristics after using a new-user active comparator cohort design and adjusting for many claims-based proxies of diabetes severity.26 A similar balance in key laboratory test results was found in the current study after PS matching, despite not including this information in the PS models. Finally, we were able to replicate the expected null association with the tracer outcome of herpes zoster virus reactivation, which is unlikely to be associated with either empagliflozin or DPP-4i. Second, the mean study follow-up (i.e., time on treatment) was short, i.e., approximately six months, due to the short study period included in this analysis (August 2014 - September 2017), which was driven by the recency of empagliflozin availability on the U.S. market, and the substantially lower patient adherence to diabetes medications in clinical practice compared to RCTs. This limited our ability to address longer term benefit and harm of empagliflozin. However, the EMPA-REG OUTCOME trial showed substantial effects of empagliflozin on key outcomes, including HHF, cardiovascular death, and all-cause mortality, within the first six months of treatment.1,35 Third, even though primary and secondary outcomes were defined using previously validated claims-based algorithms, outcome misclassification cannot be ruled out. For example, as most published claims-based algorithms are still based on the ICD-9 codes, some misclassification associated to their translation into ICD-10 codes may exist. Fourth, we were unable to evaluate cardiovascular death or all-cause mortality as the primary outcome, as information on cause of death was not available and as the ascertainment completeness of all-cause mortality varied across the available administrative data, with complete information only available in the Medicare database. Fifth, there was moderate heterogeneity in some of the point estimates observed across the three databases. This is expected as the databases included different populations with different baseline risk for the outcomes. The small number of events observed in some of the databases, and the resulting imprecise point estimates also contributed to the observed heterogeneity across databases.
In conclusion, findings from EMPRISE showed that compared with DPP-4i, empagliflozin was associated with a similar risk of myocardial infarction or stroke, and a reduced risk of HHF and all-cause mortality in routine clinical care. Compared to patients initiating DPP-4i, empagliflozin initiators had a similar risk of LLA or fractures, an increased risk of DKA, and a decreased risk of AKI. These findings complement the EMPA-REG OUTCOME trial results observed in high risk patients by providing information on the comparative effectiveness and safety of empagliflozin vs. DPP-4i in routine care patients with T2D across the spectrum of cardiovascular disease and informing the risk-benefit balance of empagliflozin in clinical practice.
Supplementary Material
Funding Sources
This study was supported by a research grant to the Brigham and Women’s Hospital from Boehringer-Ingelheim. The authors had full control of the design and conduction of the study and interpretation of the study’s findings. The authors retained the right of publication and determined the final wording of the manuscript. Dr. Patorno was supported by a career development grant (K08AG055670) from the National Institute on Aging.
Conflict of Interest Disclosures
Dr. Pawar completed this work through his employment at Brigham and Women’s Hospital. He is now an employee of Sanofi.
Dr. Wexler reports serving on Data Monitoring Committees for Novo Nordisk not related to the topic of this work.
Dr. Glynn reports grants from Pfizer, Novartis, and Kowa outside the submitted work.
Kimberly G. Brodovicz: Employment (Boehringer-Ingelheim)
Anouk Déruaz-Luyet: Employment (Boehringer-Ingelheim)
Sebastian Schneeweiss is a consultant to Aetion, a software manufacturer of which he owns equity. His interests were declared, reviewed, and approved by the Brigham and Women’s Hospital and Partners HealthCare System in accordance with their institutional compliance policies.
Drs. Patorno, Paik, Najafzadeh, and Ms. Bessette have no conflicts of interest to disclose.
References
- 1.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. The New England journal of medicine. 2015;373(22):2117–2128. [DOI] [PubMed] [Google Scholar]
- 2.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. The New England journal of medicine. 2017;377(7):644–657. [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. The New England journal of medicine. 2019;380(4):347–357. [DOI] [PubMed] [Google Scholar]
- 4.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. The New England journal of medicine. 2019;380(24):2295–2306. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes care. 2021;44(Suppl 1):S111–S124. [DOI] [PubMed] [Google Scholar]
- 6.Administration USFaD. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about. Published 2015. Accessed.
- 7.Administration USFaD. FDA Drug Safety Communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-label-diabetes-drug-canagliflozin-invokana-invokamet. Published 2015. Accessed.
- 8.Administration USFaD. FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR). https://www.fda.gov/media/104870/download. Published 2017. Accessed.
- 9.Fralick M, Kim SC, Schneeweiss S, Kim D, Redelmeier DA, Patorno E. Fracture Risk After Initiation of Use of Canagliflozin. Annals of internal medicine. 2019;171(1):80. [DOI] [PubMed] [Google Scholar]
- 10.Fralick M, Schneeweiss S, Patorno E. Risk of Diabetic Ketoacidosis after Initiation of an SGLT2 Inhibitor. The New England journal of medicine. 2017;376(23):2300–2302. [DOI] [PubMed] [Google Scholar]
- 11.Patorno EPA, Franklin JM, Déruaz-Luyet A, Brodovicz KG, Bartels DB, Kulldorff M, Schneeweiss S. Baseline information from a post-marketing monitoring program on empagliflozin: Implications for study validity and exposure accrual. Pharmacoepidemiology and drug safety. 2018;27(Supplement 2):52–53.29152808 [Google Scholar]
- 12.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. American heart journal. 2004;148(1):99–104. [DOI] [PubMed] [Google Scholar]
- 13.Wahl PM, Rodgers K, Schneeweiss S, et al. Validation of claims-based diagnostic and procedure codes for cardiovascular and gastrointestinal serious adverse events in a commercially-insured population. Pharmacoepidemiol Drug Saf. 2010;19(6):596–603. [DOI] [PubMed] [Google Scholar]
- 14.Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33(10):2465–2470. [DOI] [PubMed] [Google Scholar]
- 15.Saczynski JS, Andrade SE, Harrold LR, et al. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21 Suppl 1:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bobo WV, Cooper WO, Epstein RA Jr., Arbogast PG, Mounsey J, Ray WA. Positive predictive value of automated database records for diabetic ketoacidosis (DKA) in children and youth exposed to antipsychotic drugs or control medications: a Tennessee Medicaid Study. BMC Med Res Methodol. 2011;11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton KM, Wagner EH, Ramsey SD, et al. The use of automated data to identify complications and comorbidities of diabetes: a validation study. J Clin Epidemiol. 1999;52(3):199–207. [DOI] [PubMed] [Google Scholar]
- 18.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45(7):703–714. [DOI] [PubMed] [Google Scholar]
- 19.Hudson M, Avina-Zubieta A, Lacaille D, Bernatsky S, Lix L, Jean S. The validity of administrative data to identify hip fractures is high--a systematic review. J Clin Epidemiol. 2013;66(3):278–285. [DOI] [PubMed] [Google Scholar]
- 20.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes for Acute Renal Failure. J Am Soc Nephrol. 2006;17(6):1688–1694. [DOI] [PubMed] [Google Scholar]
- 21.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH, Glynn RJ, Avorn J, et al. Validation of a Claims-Based Frailty Index Against Physical Performance and Adverse Health Outcomes in the Health and Retirement Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin DB. Estimating causal effects from large data sets using propensity scores. Annals of internal medicine. 1997;127(8 Pt 2):757–763. [DOI] [PubMed] [Google Scholar]
- 24.Ripollone JE, Huybrechts KF, Rothman KJ, Ferguson RE, Franklin JM. Implications of the Propensity Score Matching Paradox in Pharmacoepidemiology. American journal of epidemiology. 2018;187(9):1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin JM, Rassen JA, Ackermann D, Bartels DB, Schneeweiss S. Metrics for covariate balance in cohort studies of causal effects. Statistics in medicine. 2014;33(10):1685–1699. [DOI] [PubMed] [Google Scholar]
- 26.Patorno E, Gopalakrishnan C, Franklin JM, et al. Claims-based studies of oral glucose-lowering medications can achieve balance in critical clinical variables only observed in electronic health records. Diabetes, obesity & metabolism. 2018;20(4):974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2021. Diabetes care. 2021;44(Suppl 1):S125–S150. [DOI] [PubMed] [Google Scholar]
- 28.Wang SV, Verpillat P, Rassen JA, Patrick A, Garry EM, Bartels DB. Transparency and Reproducibility of Observational Cohort Studies Using Large Healthcare Databases. Clinical pharmacology and therapeutics. 2016;99(3):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. The New England journal of medicine. 2020. [DOI] [PubMed] [Google Scholar]
- 30.Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. The New England journal of medicine. 2020. [DOI] [PubMed] [Google Scholar]
- 31.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. The New England journal of medicine. 2019;381(21):1995–2008. [DOI] [PubMed] [Google Scholar]
- 32.Patorno E, Goldfine AB, Schneeweiss S, et al. Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study. Bmj. 2018;360:k119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasternak B, Ueda P, Eliasson B, et al. Use of sodium glucose cotransporter 2 inhibitors and risk of major cardiovascular events and heart failure: Scandinavian register based cohort study. Bmj. 2019;366:l4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patorno E, Pawar A, Bessette LG, et al. Comparative Effectiveness and Safety of Sodium-Glucose Cotransporter 2 Inhibitors Versus Glucagon-Like Peptide 1 Receptor Agonists in Older Adults. Diabetes care. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pellicori P, Ofstad AP, Fitchett D, et al. Early benefits of empagliflozin in patients with or without heart failure: findings from EMPA-REG OUTCOME. ESC Heart Fail. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patorno E, Pawar A, Franklin JM, et al. Empagliflozin and the Risk of Heart Failure Hospitalization in Routine Clinical Care. Circulation. 2019;139(25):2822–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inzucchi SE, Zinman B, Fitchett D, et al. How Does Empagliflozin Reduce Cardiovascular Mortality? Insights From a Mediation Analysis of the EMPA-REG OUTCOME Trial. Diabetes care. 2018;41(2):356–363. [DOI] [PubMed] [Google Scholar]
- 38.Inzucchi SE, Iliev H, Pfarr E, Zinman B. Empagliflozin and Assessment of Lower-Limb Amputations in the EMPA-REG OUTCOME Trial. Diabetes care. 2018;41(1):e4–e5. [DOI] [PubMed] [Google Scholar]
- 39.Yu OHY, Dell’Aniello S, Shah BR, et al. Sodium-Glucose Cotransporter 2 Inhibitors and the Risk of Below-Knee Amputation: A Multicenter Observational Study. Diabetes care. 2020;43(10):2444–2452. [DOI] [PubMed] [Google Scholar]
- 40.Abrahami D, Douros A, Yin H, Yu OHY, Azoulay L. Sodium-Glucose Cotransporter 2 Inhibitors and the Risk of Fractures Among Patients With Type 2 Diabetes. Diabetes care. 2019;42(9):e150–e152. [DOI] [PubMed] [Google Scholar]
- 41.Fralick M, Kim SC, Schneeweiss S, Everett BM, Glynn RJ, Patorno E. Risk of amputation with canagliflozin across categories of age and cardiovascular risk in three US nationwide databases: cohort study. Bmj. 2020;370:m2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douros A, Lix LM, Fralick M, et al. Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Diabetic Ketoacidosis : A Multicenter Cohort Study. Annals of internal medicine. 2020;173(6):417–425. [DOI] [PubMed] [Google Scholar]
- 43.Administration USFaD. FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-kidney-warnings-diabetes-medicines-canagliflozin. Published 2016. Accessed 2/16/2021, 2021.
- 44.Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. The lancet Diabetes & endocrinology. 2019;7(11):845–854. [DOI] [PubMed] [Google Scholar]
- 45.Zhao M, Sun S, Huang Z, Wang T, Tang H. Network Meta-Analysis of Novel Glucose-Lowering Drugs on Risk of Acute Kidney Injury. Clinical journal of the American Society of Nephrology : CJASN. 2020;16(1):70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


