Summary
Background
Coronavirus disease 2019 (COVID-19) has negatively affected access to healthcare systems and treatment timelines. This study was designed to explore the impact of the COVID-19 pandemic on patients who underwent percutaneous coronary intervention (PCI).
Methods
From January 2019 to December 2020, 489,001 patients from 1068 institutions were registered in the Japanese nationwide PCI (J-PCI) registry. We constructed generalized linear models to assess the difference in the daily number of patients and in-hospital outcomes between 2019 and 2020.
Findings
In total, 207 institutions (19·3%) had closed or restricted access during the first COVID-19 outbreak in May 2020; the number of closed or restricted institutions had plateaued at a median of 121 institutions (11·3%). The daily case volume of PCI significantly decreased in 2020 (by 6·7% compared with that in 2019; 95% confidence interval [CI], 6·2–7·2%; p < 0·001). Marked differences in the presentation of PCI patients were observed; more patients presented with ST-segment elevation myocardial infarction (18·3% vs. 17·5%; p < 0·001), acute heart failure (4·49% vs. 4·30%; p = 0·001), cardiogenic shock (3·79% vs. 3·45%; p < 0·001), and cardiopulmonary arrest (2·12% vs. 2·00%; p = 0·002) in 2020. The excess adjusted in-hospital mortality rate in patients treated in 2020 relative to those treated in 2019 was significant (adjusted odds ratio, 1·054; 95% CI, 1·004–1·107; p = 0·03).
Interpretation
While the number of patients who underwent PCI substantially decreased during the COVID-19 pandemic, more patients presented with high-risk characteristics and were associated with significantly higher adjusted in-hospital mortality.
Funding
The J-PCI registry is a registry led and supported by the Japanese Association of Cardiovascular Intervention and Therapeutics. The present study was supported by the Grant-in-Aid from the Ministry of Health and Labour (No. 20IA2002 and 21FA1015), the Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI; No. 21K08064), and the Japan Agency for Medical Research and Development (No. 17ek0210097h000).
Keywords: Coronavirus disease 2019, Percutaneous coronary intervention, ST-segment elevation myocardial infarction
Research in context.
Evidence before this study
Coronavirus disease 2019 (COVID-19) presents a global health threat and has caused substantial mortality. The number of hospital admissions for acute coronary syndrome decreased during the COVID-19 pandemic, while patients with STEMI did not undergo primary PCI in a timely manner. Excess deaths in 2020 substantially exceeded the reported deaths from COVID-19 worldwide.
Added value of this study
The most significant decrease in PCI volumes both for overall patients and those with ST-segment elevation myocardial infarction occurred during the first wave of COVID-19 outbreak and coincided with an increased in mortality. Yet, this was the least severe of the COVID-19 waves as judged by the number of cases.
Statistically significant differences in the presentation pattern of PCI patients were observed; higher proportion of patients presented with STEMI, acute heart failure, cardiogenic shock, and cardiopulmonary arrest.
Adjusted in-hospital mortality was significantly higher in patients treated in 2020 than those treated in 2019, while the adjusted in-hospital mortality for STEMI patients was similar between 2019 and 2020.
Implications of all the available evidence
We observed the substantial decrease in the number of patients who underwent PCI and corresponding increase in adjusted in-hospital mortality during the COVID-19 pandemic. Careful monitoring of cardiovascular mortality is warranted to assess the true impact of COVID-19.
Alt-text: Unlabelled box
Introduction
Coronavirus disease 2019 (COVID-19) presents a global health threat and has caused substantial mortality.1 Many countries have imposed restrictions based on social distancing and movement, with the aim of mitigating and managing the spread of COVID-19. In 2020, the Japanese government declared a ‘State of Emergency’ for major metropolitan areas on April 7 and subsequently expanded the declaration to a nationwide level on April 16. As the number of confirmed COVID-19 patients increased, some major cardiovascular centers were mandated to reserve their emergency and acute medical service capacity for an increasing number of critically ill patients with COVID-19. This has necessitated the need for restructuring of resources to meet those needs.
For patients with cardiovascular diseases, particularly those with acute coronary syndrome, timely treatment reduces mortality and related complications. The most common form of coronary revascularization is percutaneous coronary intervention (PCI) with approximately 250,000 procedures undertaken in Japan annually.2 Previous surveys have indicated that more than half of the members of the Japanese Association of Cardiovascular Intervention and Therapeutics (CVIT) reported postponing PCI procedures during the pandemic.3,4 Understanding the consequences of the COVID-19 pandemic on public health and future planning in terms of PCI is crucial because PCI requires accurate triaging and timely application. COVID-19 has influenced the ways in which patients with cardiovascular diseases interact with healthcare services; studies have reported decreases in admissions and diagnosis of health conditions.5, 6, 7, 8, 9, 10, 11 However, these studies that characterized cardiovascular practice patterns remain limited to a local or regional level; there is a lack of data on activity and outcomes for PCI surrounding the impact of COVID-19 from a national perspective. Furthermore, the reports are limited to demonstrating reductions in the procedural volume during the pandemic, and longer-term assessment (e.g., year-round) remains unknown.
This study was designed to investigate the impact of COVID-19 pandemic on activity and postprocedural outcomes of PCI on Japanese population and estimate the effects of various political and social restrictions on the PCI practice pattern.
Methods
Japanese percutaneous coronary intervention (J-PCI) registry
The J-PCI registry is an ongoing prospective multicenter nationwide PCI registry maintained by CVIT.12,13 It has been incorporated into the National Clinical Data (NCD), a Japanese nationwide prospective web-based registry linked to medical and surgical board certification. Inclusion criteria for the registration of J-PCI were any patients who underwent PCI, irrespective of their indications. The primary aim of this study is to assess descriptive analyses; and the primary clinical outcome measure was in-hospital mortality, which was defined as death either until discharge or within 30 days after PCI for patients with prolonged hospitalization. ST-segment elevation myocardial infarction (STEMI) was defined as acute myocardial infarction with ST-segment elevation on two or more contiguous leads (≥ 0·2 mV in a precordial lead at the J point or ≥ 0·1 mV in a limb lead), new left bundle branch block, or posterior myocardial infarction on a 12-lead electrocardiogram accompanied by elevated cardiac biomarkers. Elevated cardiac biomarkers were defined as elevated creatine kinase or creatine kinase myocardial band levels (two-fold higher than the normal values) or elevated troponin levels (≥ 99th percentile). Other detail definitions of patient background, clinical presentation, and angiographic and procedural details are available at the J-PCI registry website (http://cvit.jp/registry/jpci_definition.pdf). The J-PCI registry protocol conformed to the principles outlined in the Declaration of Helsinki and was approved by the Institutional Review Board of the Network for Promotion of Clinical Studies (a specialized nonprofit organization affiliated with Osaka University Graduate School of Medicine in Osaka, Japan). The requirement for written informed consent was waived due to the cross-sectional retrospective study design.
COVID-19
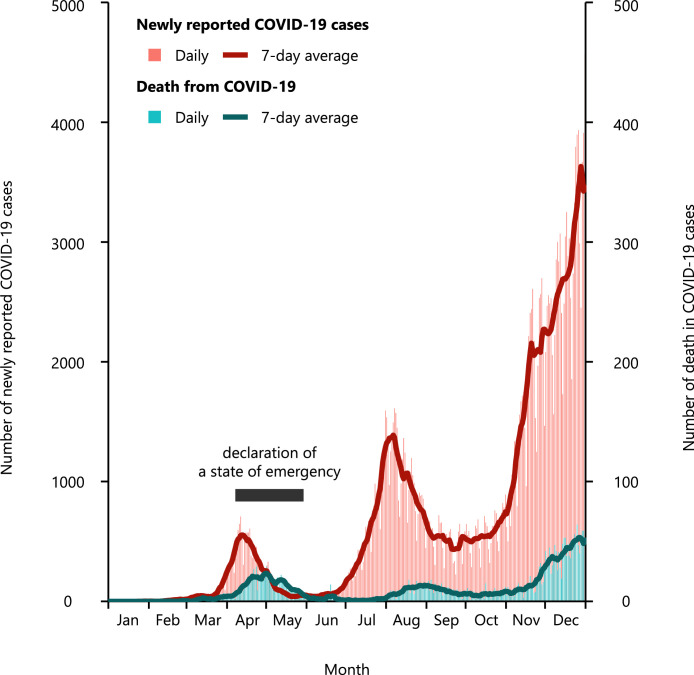
In Japan, the first COVID-19 patient was reported on January 15, 2020. As of December 31, 2020, 239,192 COVID-19 patients had been reported, and 3,501 COVID-19 patients died. In 2020, there were three waves of COVID-19 outbreak (Figure 1). In response to the first rapid spread of COVID-19 patients and their mortality, a ‘State of Emergency’ had been declared from April 07 to May 25. The details of a ‘State of Emergency’ are shown in Online Methods and Online Figure 1. On a per prefecture basis, the daily numbers of COVID-19 patients and deaths from COVID-19 were obtained from the COVID-19 portal website (https://covid19japan.com/ accessed on May 10, 2021). These data are based on national and prefectural government reports in Japan; original data sources included the Ministry of Health, Labour, and Welfare and reports from prefectural/city government offices. The data were used under the non-commercial public use clause under the Creative Commons by Attribution Non-Commercial 4·0 International License.
Figure 1.
The daily number of newly reported coronavirus disease 2019 (COVID-19) patients and the daily number of deaths from COVID-19.
The blue line indicates the 7-day average of daily numbers.
COVID-19, coronavirus disease 2019.
Status of participating institutions
Using the Gathering Medical Information System (G-MIS), the daily status of institutions was surveyed by the Ministry of Health, Labour, and Welfare, and was reported from May 18, 2020 as regular, restricted, closed, or not reported for outpatient care, emergency care, inpatient care, dialysis, and chemotherapy departments. We obtained the data on the daily status of institutions from the Cabinet Secretariat portal website (https://corona.go.jp/dashboard/ accessed on April 17, 2021). We classified institutions as closed when at least one of the departments among outpatient care, emergency care, and inpatient care departments was reported to be closed, and we classified institutions as restricted when any department among outpatient care, emergency care, and inpatient care departments was reported to be restricted.
Statistical analysis
Categorical variables, which were presented as numbers with relative percentages, were compared using the chi-square test, whereas continuous variables, which were expressed as mean ± standard deviation, were compared using Student's t-test. We developed generalized linear models to assess the difference in the daily number of patients between 2019 and 2020 under the assumption that the daily number of patients follows a Poisson distribution with log link function. Since off days (i.e., Saturday, Sunday, and national holidays) are associated with the daily number of patients and in-hospital mortality, we included a presentation on off days in all models as an explanatory variable. To assess the difference in the number of PCI procedures per institution and per prefecture between 2019 and 2020, we used univariate linear regression models in which intercepts were set to zero. We constructed logistic regression models to calculate odds ratios (ORs) and their 95% confidence intervals (95% CIs) for the risk of in-hospital mortality between patients treated in 2019 and those treated in 2020. To account for differences in patient characteristics, all clinical variables listed in Table 1 in addition to off days were simultaneously included in the multivariable models as explanatory variables. In case of missing values, we excluded the patients from the multivariable models. To clarify whether there were regional disparities of the impact of COVID-19, we divided prefectures into three groups according to the percent COVID-19 cases (total reported number of COVID-19 cases until December 31, 2021) per population as a sensitivity analysis (Online Figure 2). Two-sided p-values of less than 0·05 were used to denote statistical significance. All data were analyzed using the R statistical software version 4·0·2 (R Foundation for Statistical Computing, Vienna, Austria).
Table 1.
Patient characteristics in overall patients.
| 2020 (N = 236,807) |
2019 (N = 252,194) | P value (2020 versus 2019) | ||||
|---|---|---|---|---|---|---|
| Overall | During the declaration of a ‘State of Emergency’ (N = 19,603) | Not during the declaration of a ‘State of Emergency’ (N = 217,204) | P value (during versus not during) | |||
| Age, year* | 71·2 ± 11·3 | 71·0 ± 11·4 | 71·2 ± 11·3 | 0·02 | 71·0 ± 11·2 | <0·001 |
| Male* | 181,131 (76·5%) | 15,092 (77·0%) | 166,039 (76·4%) | 0·09 | 192,866 (76·5%) | 0·91 |
| Hypertension* | 179,265 (75·7%) | 14,802 (75·5%) | 164,463 (75·7%) | 0·52 | 189,538 (75·2%) | <0·001 |
| Hyperlipidemia* | 159,040 (67·2%) | 13,079 (66·7%) | 145,961 (67·2%) | 0·17 | 166,699 (66·1%) | <0·001 |
| Diabetes* | 107,180 (45·3%) | 8972 (45·8%) | 98,208 (45·2%) | 0·14 | 112,240 (44·5%) | <0·001 |
| Smoker* | 71,749 (30·3%) | 6188 (31·6%) | 65,561 (30·2%) | <0·001 | 75,744 (30·0%) | 0·04 |
| Renal failure* | 55,370 (23·4%) | 4529 (23·1%) | 50,841 (23·4%) | 0·34 | 53,675 (21·3%) | <0·001 |
| Dialysis* | 17,272 (7·29%) | 1,481 (7·55%) | 15,791 (7·27%) | 0·15 | 17,396 (6·90%) | <0·001 |
| Prior percutaneous coronary intervention* | 106,176 (45·6%) | 8,461 (44·1%) | 97,715 (45·7%) | <0·001 | 113,351 (45·6%) | 0·63 |
| Prior coronary artery bypass grafting* | 7,889 (3·39%) | 639 (3·33%) | 7,250 (3·39%) | 0·68 | 8,209 (3·30%) | 0·12 |
| Prior heart failure* | 37,340 (16·1%) | 3,260 (17·1%) | 34,080 (16·0%) | <0·001 | 37,424 (15·2%) | <0·001 |
| Prior myocardial infarction* | 53,067 (22·9%) | 4,269 (22·4%) | 48,798 (22·9%) | 0·10 | 55,322 (22·4%) | <0·001 |
| Chronic obstructive pulmonary disease* | 7,272 (3·07%) | 619 (3·16%) | 6653 (3·06%) | 0·48 | 6630 (2·63%) | <0·001 |
| Peripheral vascular disease* | 19,320 (8·16%) | 1679 (8·57%) | 17,641 (8·12%) | 0·03 | 19,632 (7·78%) | <0·001 |
| Cardiopulmonary arrest* | 4935 (2·12%) | 403 (2·11%) | 4532 (2·12%) | 0·91 | 4939 (2·00%) | 0·002 |
| Cardiogenic shock* | 8808 (3·79%) | 749 (3·92%) | 8059 (3·78%) | 0·33 | 8547 (3·45%) | <0·001 |
| Heart failure at presentation* | 10,435 (4·49%) | 931 (4·88%) | 9504 (4·46%) | 0·008 | 10,635 (4·30%) | 0·001 |
| Clinical presentation | <0·001 | <0·001 | ||||
| ST-segment elevation myocardial infarction | 43,314 (18·3%) | 3758 (19·2%) | 39,556 (18·2%) | 44,172 (17·5%) | ||
| Non-ST-segment elevation myocardial infarction | 15,320 (6·47%) | 1388 (7·08%) | 13,932 (6·41%) | 14,651 (5·81%) | ||
| Unstable angina pectoris | 32,787 (13·8%) | 2922 (14·9%) | 29,865 (13·7%) | 35,794 (14·2%) | ||
| Stable coronary artery disease | 145,386 (61·4%) | 11,535 (58·8%) | 133,851 (61·6%) | 157,577 (62·5%) | ||
| Extent of coronary artery disease | 0·21 | <0·001 | ||||
| Single-vessel disease | 147,629 (62·3%) | 12,092 (61·7%) | 135,537 (62·4%) | 159,193 (63·1%) | ||
| Double-vessel disease | 54,950 (23·2%) | 4613 (23·5%) | 50,337 (23·2%) | 57,503 (22·8%) | ||
| Triple-vessel disease | 24,668 (10·4%) | 2104 (10·7%) | 22,564 (10·4%) | 25,674 (10·2%) | ||
| Left main disease | 9560 (4·04%) | 794 (4·05%) | 8766 (4·04%) | 9824 (3·90%) | ||
| Lesion location | ||||||
| Right coronary artery | 78,121 (33·0%) | 6370 (32·5%) | 71,751 (33·0%) | 0·13 | 83,427 (33·1%) | 0·50 |
| Left anterior descending or left main coronary artery | 127,073 (53·7%) | 10,633 (54·2%) | 116,440 (53·6%) | 0·09 | 134,502 (53·3%) | 0·02 |
| Left circumflex coronary artery | 57,193 (24·2%) | 4813 (24·6%) | 52,380 (24·1%) | 0·17 | 61,512 (24·4%) | 0·052 |
| Bypass graft | 986 (0·416%) | 93 (0·474%) | 893 (0·411%) | 0·21 | 989 (0·392%) | 0·19 |
| In-hospital outcomes | ||||||
| Death | 4588 (1·94%) | 393 (2·00%) | 4195 (1·93%) | 0·49 | 4364 (1·73%) | <0·001 |
| Procedure related cardiac death | 341 (0·144%) | 32 (0·163%) | 309 (0·142%) | 0·52 | 324 (0·128%) | 0·15 |
| Non-procedure related cardiac death | 3370 (1·42%) | 285 (1·45%) | 3085 (1·42%) | 0·73 | 3178 (1·26%) | <0·001 |
| Non-cardiac death | 877 (0·370%) | 76 (0·388%) | 801 (0·369%) | 0·72 | 862 (0·342%) | 0·10 |
| Myocardial infarction | 1375 (0·581%) | 105 (0·536%) | 1270 (0·585%) | 0·41 | 1341 (0·532%) | 0·02 |
| Bleeding | 935 (0·395%) | 74 (0·377%) | 861 (0·396%) | 0·73 | 891 (0·353%) | 0·02 |
| Access site | 504 (0·213%) | 38 (0·194%) | 466 (0·215%) | 0·60 | 494 (0·196%) | 0·20 |
| Non-access site | 447 (0·189%) | 37 (0·189%) | 410 (0·189%) | 1·00 | 415 (0·165%) | 0·047 |
| Tamponade | 349 (0·147%) | 28 (0·143%) | 321 (0·148%) | 0·94 | 403 (0·160%) | 0·28 |
| Cardiogenic shock | 2287 (0·966%) | 178 (0·908%) | 2109 (0·971%) | 0·41 | 2166 (0·859%) | <0·001 |
| Stent thrombosis | 348 (0·147%) | 28 (0·143%) | 320 (0·147%) | 0·95 | 380 (0·151%) | 0·76 |
| Emergent surgery | 184 (0·0777%) | 15 (0·0765%) | 169 (0·0778%) | 1·00 | 245 (0·0971%) | 0·02 |
Categorical variables were compared using the chi-square test, whereas continuous variables were compared using Student's t-test.
Values were missing for prior percutaneous coronary intervention in 7451 patients; prior coronary artery bypass grafting in 7639 patients; prior heart failure in 10664 patients; prior myocardial infarction in 9885 patients; cardiopulmonary arrest in 9058 patients; cardiogenic shock in 9189 patients; and heart failure at presentation in 9334 patients.
variables included in the multivariable logistic regression models.
Role of the funding source
The funders had no role in study design, data collection, data analysis, interpretation, and writing of the report.
Results
Overall PCI volume
From January 2019 to December 2020, 492,717 consecutive patients undergoing PCI in 1159 institutions were registered in the J-PCI registry. Among them, we excluded 3716 patients from the institute in which PCI was registered only in one years in either 2019 or 2020 (2019: 1033 patients from 28 institutions; 2020: 2683 patients from 46 institutions). In the final analysis, we included 489,001 patients from 1068 institutions in which at least one patient was registered in both 2019 and 2020.
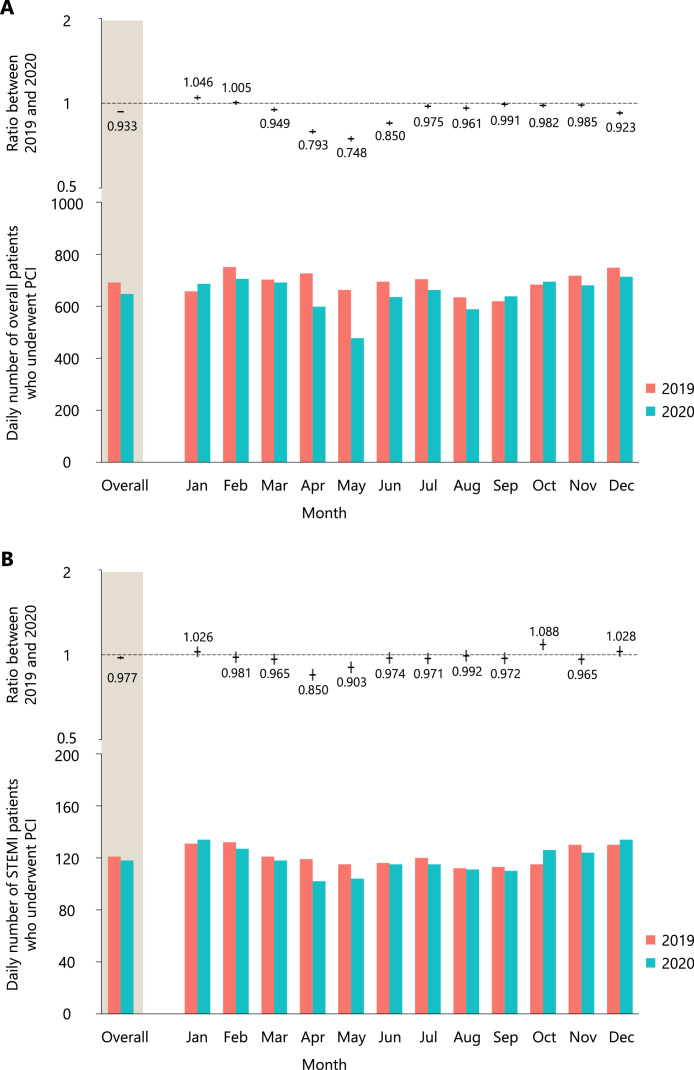
The total number of patients who underwent PCI decreased from 252,194 in 2019 to 236,807 in 2020. From 2019 to 2020, the daily number of patients decreased significantly by 6·7% (95% CI, 6·2–7·2%; p < 0·001), mainly during the first wave of COVID-19 outbreak (April: 20·7%, 95% CI 19·1–22·2%, p < 0·001; May: 25·2%, 95% CI 23·6–26·8%, p < 0·001) (Figure 2A). Moreover, the daily number of patients who underwent PCI for STEMI significantly decreased by 2·3% (95% CI, 1·0–3·6%; p < 0·001). We observed a significant decrease in the number of patients with STEMI who underwent PCI during the first wave of COVID-19 outbreak (April: 15·0%, 95% CI 10·8–19·0%, p < 0·001; May: 9·7%, 95% CI 5·3–13·9%, p < 0·001) (Figure 2B).
Figure 2.
Ratios between 2019 and 2020 for the daily number of patients who underwent percutaneous coronary intervention (PCI) in the overall patients (A) and patients with ST-segment elevation myocardial infarction (STEMI) (B).
In the upper panel, the horizontal lines indicate ratios, whereas the vertical lines indicate their 95% confidence intervals. In the lower panel, the daily numbers of overall patients who underwent PCI in 2019 and 2020 are shown. A presentation on off days (i.e., Saturday, Sunday, and national holidays) was included in the models as an explanatory variable.
PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Percent COVID-19 cases per population varied widely among prefectures from 0·015% (Akita prefecture) to 0·454% (Tokyo prefecture). We divided prefectures into three groups according to the percent COVID-19 cases per population (> 0·229%, 5 prefectures, 171,347 patients; 0·120% to 0·229%, 9 prefectures, 160,002 patients; and ≤0·120%, 33 prefectures, 157,652 patients) (Online Figure 2). The daily number of patients who underwent PCI during the first wave of COVID-19 outbreak decreased to a similar extent in each group (Online Figure 3).
Scattered plots for the number of overall patients and patients with STEMI between 2019 and 2020 showed that the PCI volume decreased, irrespective of institutional and prefectural volumes (Online Figure 4).
Institutional status
The daily status of 1068 institutions is shown in Online Figure 5 (since the survey was performed after May 18, the maximal number of closed or restricted institutions before or during the first wave of COVID-19 outbreak was not captured). We confirmed that at least 207 institutions (19·3%) were closed or restricted during the first wave of COVID-19 outbreak. The number of closed or restricted institutions decreased after the first wave of COVID-19 outbreak but plateaued after June (median: 121 institutions [11·3%]). Slight increases in the number of closed or restricted institutions were observed during the second and third waves of COVID-19 outbreak (second wave: 142 institutions [13·2%] on August 17; third wave: 146 institutions [13·6%] on December 17).
Patient characteristics
Patient characteristics differed significantly between patients treated in 2019 and those treated in 2020 (Table 1). While the observed difference was numerically minimal for most of the variables, patients treated in 2020 more often presented with STEMI (18·3% vs. 17·5%; p < 0·001) than those treated in 2019. Moreover, patients treated in 2020 more often presented with cardiopulmonary arrest (2·12% vs. 2·00%; p = 0·002), cardiogenic shock (3·79% vs. 3·45%; p < 0·001), and heart failure (4·49% vs. 4·30%; p = 0·001). The higher prevalence rates of acute myocardial infarction and heart failure were particularly profound during the declaration of a ‘State of Emergency’.
Patients who underwent PCI for STEMI in 2020 more often had comorbidities, such as hyperlipidemia, diabetes, and renal failure, and more often had a history of PCI, heart failure, and myocardial infarction than those treated in 2019 (Table 2). In patients who underwent PCI for STEMI, the prevalence of cardiogenic shock was significantly higher in 2020 than that in 2019 (13·6% vs. 12·7%; p < 0·001), whereas no significant differences in the presentation of cardiopulmonary arrest (6·97% vs. 6·77%; p = 0·26) and heart failure (13·5% vs. 13·2%; p = 0·21) were observed. In patients with STEMI, data on the door-to-balloon time were available in 35,319 patients (80·0%) in 2019 and 35,579 patients (82·1%) in 2020. No significant difference in the door-to-balloon time was observed between 2019 (83·2 ± 55·8 min) and 2020 (83·3 ± 53·6 min; p = 0·78). The door-to-balloon time was numerically longer, but comparable between patients who underwent PCI during the declaration of a ‘State of Emergency’ (84·6 ± 53·9 min) and those who underwent PCI not during the declaration of a ‘State of Emergency’ (83·2 ± 53·6 min; p = 0·15).
Table 2.
Patient characteristics in patients presented with ST-segment elevation myocardial infarction.
| 2020 (N = 43,314) |
2019 (N = 44,172) | P value (2020 versus 2019) | ||||
|---|---|---|---|---|---|---|
| Overall | During the declaration of a ‘State of Emergency’ (N = 3758) | Not during the declaration of a ‘State of Emergency’ (N = 39,556) | P value (during versus not during) | |||
| Age, year | 69·6 ± 13·0 | 69·4 ± 12·9 | 69·7 ± 13·0 | 0·20 | 69·5 ± 13·0 | 0·09 |
| Male | 32,649 (75·4%) | 2839 (75·5%) | 29,810 (75·4%) | 0·82 | 33,502 (75·8%) | 0·11 |
| Hypertension | 29,225 (67·5%) | 2573 (68·5%) | 26,652 (67·4%) | 0·18 | 29,741 (67·3%) | 0·66 |
| Hyperlipidemia | 25,312 (58·4%) | 2240 (59·6%) | 23,072 (58·3%) | 0·13 | 25,449 (57·6%) | 0·01 |
| Diabetes | 15,503 (35·8%) | 1371 (36·5%) | 14,132 (35·7%) | 0·37 | 15,486 (35·1%) | 0·02 |
| Smoker | 16,368 (37·8%) | 1478 (39·3%) | 14,890 (37·6%) | 0·04 | 16,653 (37·7%) | 0·79 |
| Renal failure | 7870 (18·2%) | 677 (18·0%) | 7193 (18·2%) | 0·81 | 7204 (16·3%) | <0·001 |
| Dialysis | 977 (2·26%) | 101 (2·69%) | 876 (2·21%) | 0·07 | 957 (2·17%) | 0·38 |
| Prior percutaneous coronary intervention | 5993 (13·9%) | 527 (14·1%) | 5466 (13·9%) | 0·76 | 5849 (13·3%) | 0·01 |
| Prior coronary artery bypass grafting | 427 (0·990%) | 37 (0·990%) | 390 (0·990%) | 1·00 | 383 (0·872%) | 0·07 |
| Prior heart failure | 2787 (6·52%) | 258 (6·97%) | 2529 (6·48%) | 0·27 | 2643 (6·07%) | 0·007 |
| Prior myocardial infarction | 4570 (10·6%) | 422 (11·3%) | 4148 (10·6%) | 0·16 | 4333 (9·92%) | <0·001 |
| Chronic obstructive pulmonary disease | 1146 (2·65%) | 107 (2·85%) | 1039 (2·63%) | 0·45 | 1016 (2·30%) | 0·001 |
| Peripheral vascular disease | 1432 (3·31%) | 136 (3·62%) | 1296 (3·28%) | 0·28 | 1327 (3·00%) | 0·01 |
| Cardiopulmonary arrest | 2997 (6·97%) | 245 (6·58%) | 2752 (7·00%) | 0·35 | 2969 (6·77%) | 0·26 |
| Cardiogenic shock | 5861 (13·6%) | 497 (13·3%) | 5364 (13·7%) | 0·61 | 5547 (12·7%) | <0·001 |
| Heart failure at presentation | 5798 (13·5%) | 507 (13·6%) | 5291 (13·5%) | 0·81 | 5783 (13·2%) | 0·21 |
| Extent of coronary artery disease | 0·60 | 0·01 | ||||
| Single-vessel disease | 26,535 (61·3%) | 2319 (61·7%) | 24,216 (61·2%) | 27,224 (61·6%) | ||
| Double-vessel disease | 10,044 (23·2%) | 879 (23·4%) | 9165 (23·2%) | 10,167 (23·0%) | ||
| Triple-vessel disease | 5242 (12·1%) | 443 (11·8%) | 4799 (12·1%) | 5168 (11·7%) | ||
| Left main disease | 1493 (3·45%) | 117 (3·11%) | 1376 (3·48%) | 1613 (3·65%) | ||
| Lesion location | ||||||
| Right coronary artery | 17,844 (41·2%) | 1541 (41·0%) | 16,303 (41·2%) | 0·82 | 18,127 (41·0%) | 0·64 |
| Left anterior descending or left main coronary artery | 23,431 (54·1%) | 2049 (54·5%) | 21,382 (54·1%) | 0·59 | 23,872 (54·0%) | 0·88 |
| Left circumflex coronary artery | 6394 (14·8%) | 533 (14·2%) | 5861 (14·8%) | 0·31 | 6596 (14·9%) | 0·48 |
| Bypass graft | 81 (0·187%) | 7 (0·186%) | 74 (0·187%) | 1·00 | 74 (0·168%) | 0·55 |
| In-hospital outcomes | ||||||
| Death | 2653 (6·13%) | 214 (5·69%) | 2439 (6·17%) | 0·26 | 2512 (5·69%) | 0·006 |
| Procedure related cardiac death | 167 (0·386%) | 12 (0·319%) | 155 (0·392%) | 0·58 | 160 (0·362%) | 0·61 |
| Non-procedure related cardiac death | 2076 (4·79%) | 165 (4·39%) | 1911 (4·83%) | 0·24 | 1983 (4·49%) | 0·03 |
| Non-cardiac death | 410 (0·947%) | 37 (0·985%) | 373 (0·943%) | 0·87 | 369 (0·835%) | 0·09 |
| Myocardial infarction | 84 (0·194%) | 6 (0·160%) | 78 (0·197%) | 0·76 | 100 (0·226%) | 0·33 |
| Bleeding | 343 (0·792%) | 31 (0·825%) | 312 (0·789%) | 0·89 | 319 (0·722%) | 0·25 |
| Access site | 155 (0·358%) | 15 (0·399%) | 140 (0·354%) | 0·76 | 161 (0·364%) | 0·91 |
| Non-access site | 195 (0·450%) | 17 (0·452%) | 178 (0·450%) | 1·00 | 168 (0·380%) | 0·12 |
| Tamponade | 120 (0·277%) | 11 (0·293%) | 109 (0·276%) | 0·98 | 113 (0·256%) | 0·59 |
| Cardiogenic shock | 1155 (2·67%) | 88 (2·34%) | 1067 (2·70%) | 0·21 | 1091 (2·47%) | 0·07 |
| Stent thrombosis | 176 (0·406%) | 13 (0·346%) | 163 (0·412%) | 0·63 | 191 (0·432%) | 0·59 |
| Emergent surgery | 80 (0·185%) | 7 (0·186%) | 73 (0·185%) | 1·00 | 104 (0·235%) | 0·12 |
Categorical variables were compared using the chi-square test, whereas continuous variables were compared using Student's t-test.
In-hospital outcomes
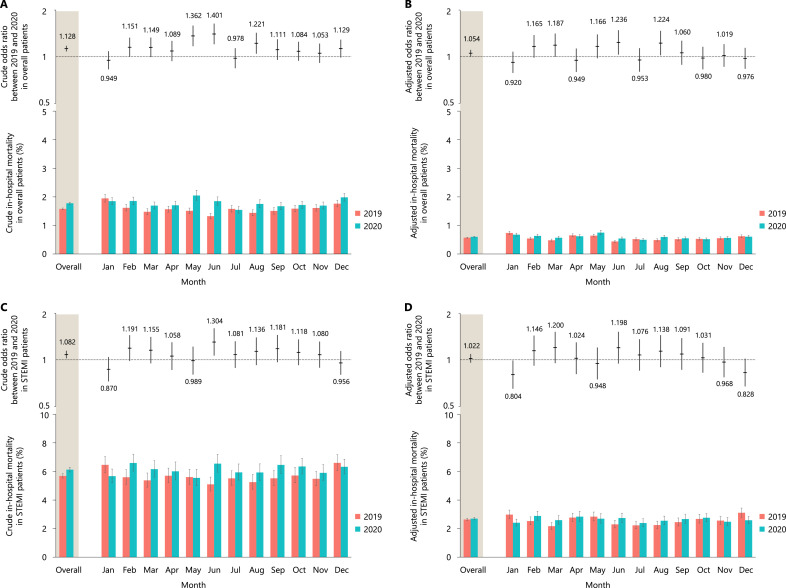
The crude in-hospital mortality rate was significantly higher in 2020 (4588 deaths, 1·78%; 95% CI, 1·74–1·81%) than in 2019 (4364 death, 1·57%; 95% CI, 1·55–1·61%; crude OR, 1·128; 95% CI, 1·081–1·176; p < 0·001), mainly driven by a significantly higher crude in-hospital mortality in May, June, and August (Figure 3A). There was no significant difference in causes of death between 2020 and 2019 (procedure related cardiac death: 7·1% versus 7·8%; non-procedure related cardiac death: 73·5% versus 72·8%; and non-cardiac death: 19·1% versus 19·8%; p = 0·75). After adjusting for patient characteristics, the excess adjusted in-hospital mortality rate in patients treated in 2020 (0·60%; 95% CI, 0·59–0·62%) relative to those treated in 2019 (0·57%; 95% CI, 0·56–0·58%) was significant (adjusted OR, 1·054; 95% CI, 1·004–1·107; p = 0·03) (Figure 3B). In patients with STEMI, the crude in-hospital mortality rate was also significantly higher in patients treated in 2020 (2653 deaths; 6·12%, 95% CI, 5·96–6·29%) than those treated in 2019 (2512 death, 5·68%; 95% CI, 5·54–5·84%; crude OR, 1·082; 95% CI, 1·023–1·145; p = 0·006) (Figure 3C), whereas the observed difference was no longer significant after adjusting for patient characteristics (2020: 2·69%, 95% CI 2·61–2·78% vs. 2019: 2·64%, 95% CI 2·56–2·72%; adjusted OR, 1·022, 95% CI 0·958–1·091; p = 0·51) (Figure 3D). There were no clear regional disparities in the crude and adjusted in-hospital mortality rates in the stratified analysis (Online Figure 6).
Figure 3.
The crude and adjusted odds ratios between 2019 and 2020 and the crude and adjusted in-hospital mortality rates in the overall patients (A and B) and patients with ST-segment elevation myocardial infarction (STEMI) (C and D).
In the upper panel, the horizontal lines indicate odds ratios, whereas the vertical lines indicate their 95% confidence intervals. In the lower panel, the crude and adjusted in-hospital mortality rates with 95% confidence intervals are shown. To calculate the adjusted odds ratios, variables listed in Table 1 were included in the multivariate models. A presentation on off days (i.e., Saturday, Sunday, and national holidays) was also included in all models as an explanatory variable.
STEMI, ST-segment elevation myocardial infarction.
Discussion
This analysis of a Japanese nationwide PCI registry describes insights regarding the impact of the COVID-19 pandemic on procedural volume, patient presentation, and adjusted in-hospital outcomes. The daily case volume of PCI decreased by 6·7% during the pandemic in 2020 with 207 of 1068 institutions closed or restricted during the first wave of COVID-19 outbreak in May 2020. Moreover, statistically significant differences in the presentation pattern of PCI patients were observed; higher proportion of patients presented with STEMI, acute heart failure, cardiogenic shock, and cardiopulmonary arrest. Adjusted in-hospital mortality was significantly higher in patients treated in 2020 than those treated in 2019, while the adjusted in-hospital mortality for STEMI patients was similar between 2019 and 2020.
Our observations are in agreement with those of previous reports from various countries in which the number of hospital admissions for acute coronary syndrome decreased during the COVID-19 pandemic.5, 6, 7, 8, 9, 10, 11 In our registry, the number of patients who underwent PCI decreased from 2019 to 2020, not only for those who presented with STEMI but also for patients with broader range of PCI indications. Although there was a substantial decrease in the number of patients who received PCI during the first wave of COVID-19 outbreak, no clear signs of decrease during the second and third waves of COVID-19 outbreak. At least more than 20% of J-PCI institutions were either closed or restricted under the declaration of a ‘State of Emergency’, whereas the percentage of closed or restricted institutions remained stable at approximately 10–15% during the second and third waves of COVID-19 outbreak. Owing to the first-ever outbreak of COVID-19 with an unparalleled pressure on the healthcare system, some institutions depleted their resources to accept patients with cardiovascular concerns who might be potentially transmissible of COVID-19, resulting in a substantial decrease in the number of patients who underwent PCI during the first wave of COVID-19 outbreak. Of note, the first wave was the least severe of the three COVID-19 waves as judged by the number of cases. Our findings suggest that restricting access to healthcare facilities, and not absolute COVID-19 case volume, was largely responsible for the decrease in the PCI volume and increased mortality. In retrospect, the second and third wave of the pandemic were managed more successfully despite significantly higher cases of COVID-19 compared to the first wave. Standardized guidance for COVID-19 precautions in the cardiovascular care system had been established during these periods.14 Polymerase chain reaction testing for COVID-19 became widely available in cardiovascular centers by the later part of 2020. These approaches in the healthcare system might have mitigated the depletion of resources after the first wave of COVID-19 outbreak. It should also be noted that the reduction in the number of patients who underwent PCI during the first wave of COVID-19 pandemic may very well be multifactorial. For instance, it is possible that patients refrained from visiting healthcare facilities due to fear of COVID-19 contagion in the setting of the first rapid spread of COVID-19. During the declaration of a ‘State of Emergency’, patients who underwent PCI more often had comorbidities and presented with more severe conditions, indicating that patients with less severe symptoms hesitated to undergo PCI under the stay-at-home mandate.
The previous reports from the New York State hospitals have indicated that decrease in STEMI-related PCIs were mainly confined to counties with high density of COVID-19 deaths.10 Rather than avoided PCIs or a reduction in the incidence of STEMI, this decrease in STEMI-related PCIs was primarily related to patients not presenting to hospitals or delay in their admissions. Further, in a study of 18 sites representing primary PCI hospitals and healthcare systems across the US, there was marked reduction in the number of activations for STEMI leading to the reductions in angiography and primary PCI volumes after initiation of pandemic mitigation measures with door-to-balloon time increasing on average by 20%.11 These studies, together with ours, drive the common message; the COVID-19 pandemic has adversely affected many aspects of STEMI care. However, reginal difference does exist, since STEMI hospitals and healthcare systems have different configurations, transfer, and time to treatment protocols. For instance, door-to-balloon time was not significantly affected in our study. Japan has a universal healthcare system and ambulance services are administered by a government‐based system, with minimum fee applied to the insurers. In addition, a greater number of operators per institution in Japan may have aided patients with STEMI undergo primary PCI in a timely manner.
The crude in-hospital mortality rate increased from 2019 to 2020 by 12·8% for the overall patients and by 8·2% for patients with STEMI. After adjusting for patient characteristics, the higher excess mortality risk in 2020 relative to that in 2019 was significant for overall patients, whereas it was attenuated for patients with STEMI. Our findings suggested that the more severe patient characteristics in 2020 than in 2019 were a main driver for the observed difference in crude in-hospital mortality. The COVID-19 pandemic might prevent patients from visiting healthcare facilities or prevent PCI-capable institutions from accepting patients; thus, patients presented with more severe clinical status that caused a higher crude in-hospital mortality rate. Not only the depletion of healthcare resources and patients’ hesitation of visiting healthcare facilities but also the direct cardiotoxicity and thrombogenicity of COVID-19 might have increased the in-hospital mortality.15
Excess deaths in 2020 substantially exceeded the reported deaths from COVID-19 worldwide.16,17 In our registry, although a significantly smaller number of patients underwent PCI in 2020 than in 2019, the absolute number of in-hospital deaths in 2020 was 224 more than that in 2019, which might at least in part contribute to the increase in excess deaths in 2020 on top of the 3501 reported deaths from COVID-19 in Japan. Several reports have shown that patients with STEMI did not undergo primary PCI in a timely manner during the COVID-19 pandemic,8,18,19 significantly increasing myocardial damage.20 Moreover, physical activity and dietary patterns may be adversely affected by the COVID-19 pandemic, which might have further increased the atherosclerotic disease burden. Recently, vaccines and novel antagonist therapies successfully reduced the number of COVID-19 patients and improved the clinical outcomes after COVID-19 infection21, 22, 23; however, careful monitoring of cardiovascular mortality is warranted to assess the true impact of COVID-19.
Limitations
This study has several important limitations. First, the study was observational, and thus, we cannot conclude that the reported associations are causative. Second, since the registry is based on patient-level data, a survivor bias cannot be excluded. Some patients might have longer symptom onset to balloon time because of COVID-19 outbreaks; and did not undergo primary PCI. While primary PCI was performed in approximately 95% of STEMI patients before COVID-19 outbreak in Japan,24,25 there might be a possibility that rate of primary PCI in STEMI patients decreased during the COVID-19 outbreak. Our analysis could have underestimated the true impact of COVID-19 on myocardial damage since data on those who died before cardiac catheterization or those who refrained from seeking emergent treatment cannot be analyzed. Although the J-PCI registry mandates consecutive registration, auditing of the data was limited during the COVID-19 pandemic, and there may be inconsistency in the collected data between 2019 and 2020. Although the pandemic had a negative impact on healthcare systems worldwide, it should be noted that they were affected differently (including differences in the timing and extent of restrictions imposed), which may limit the generalizability of our results.
Conclusions
During the COVID-19 pandemic, while the number of patients who underwent PCI substantially decreased, patients who underwent PCI in 2020 more often presented with high-risk characteristics than those treated in 2019 and were associated with significantly higher in-hospital mortality.
Data sharing statement
The individual participant data that underlie the results reported in this article, after de-identification (in the text, tables, figures, and appendices), will be shared. Individual participant data will be available immediately following publication; no end date. Researchers who provide a methodologically sound proposal with a designated form will be allowed access to the individual participant data. These proposals will be reviewed and approved by a scientific committee of CVIT.
Author contribution
Kyohei Yamaji: Data curation, formal analysis, investigation, methodology, validation, visualisation, writing – original draft of the manuscript.
Shun Kohsaka: Conceptualisation, investigation, methodology, supervision, writing – original draft, and writing – review & editing of the manuscript.
Taku Inohara: Formal analysis, investigation, methodology, writing – review & editing of the manuscript.
Yohei Numasawa: Supervision, resources, writing – review & editing of the manuscript.
Hirohiko Ando: Supervision, resources, writing – review & editing of the manuscript.
Hideki Wada: Supervision, resources, writing – review & editing of the manuscript.
Hideki Ishii: Supervision, resources, writing – review & editing of the manuscript.
Tetsuya Amano: Supervision, resources, writing – review & editing of the manuscript.
Hiroaki Miyata: Resources of the manuscript.
Yuji Ikari: Supervision, resources, project administration, writing – review & editing of the manuscript.
Declaration of interests
K.Y. reports investigator-initiated grant funding from Abbott. S.K. reports investigator-initiated grant funding from Bayer and Daiichi Sankyo and personal fees from Bristol-Myers Squibb. T.I. has a research grant from Boston Scientific. H.I. receives lecture fees from Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, MSD, Otsuka, and Pfizer. T.A. receives lecture fees from Astellas Pharma, AstraZeneca, Bayer, Daiichi Sankyo, and Bristol-Myers Squibb. The rest of the authors have no conflict of interest to report.
Acknowledgment
The authors are indebted to the participating patients and the members of CVIT for collecting data.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100434.
Appendix. Supplementary materials
References
- 1.Banerjee A., Pasea L., Harris S., et al. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet. 2020;395(10238):1715–1725. doi: 10.1016/S0140-6736(20)30854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inohara T., Kohsaka S., Spertus J.A., et al. Comparative trends in percutaneous coronary intervention in Japan and the United States, 2013 to 2017. J Am Coll Cardiol. 2020;76(11):1328–1340. doi: 10.1016/j.jacc.2020.07.037. [DOI] [PubMed] [Google Scholar]
- 3.Ishii H., Amano T., Yamaji K., Kohsaka S., Yokoi H., Ikari Y. Implementation of percutaneous coronary intervention during the COVID-19 pandemic in japan- nationwide survey report of the Japanese Association of Cardiovascular Intervention and therapeutics for cardiovascular disease. Circ J. 2020;84(12):2185–2189. doi: 10.1253/circj.CJ-20-0708. [DOI] [PubMed] [Google Scholar]
- 4.Ishii H., Amano T., Kohsaka S., Morino Y., Yokoi H., Ikari Y. National survey of percutaneous coronary intervention during the COVID-19 pandemic in Japan: second report of the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc Interv Ther. 2021 doi: 10.1007/s12928-021-00776-5. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mafham M.M., Spata E., Goldacre R., et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396(10248):381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Rosa S., Spaccarotella C., Basso C., et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020;41(22):2083–2088. doi: 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon M.D., McNulty E.J., Rana J.S., et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383(7):691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 8.De Luca G., Verdoia M., Cercek M., et al. Impact of COVID-19 pandemic on mechanical reperfusion for patients with STEMI. J Am Coll Cardiol. 2020;76(20):2321–2330. doi: 10.1016/j.jacc.2020.09.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morishita T., Takada D., Shin J.H., Higuchi T., Kunisawa S., Imanaka Y. Trends, treatment approaches, and in-hospital mortality for acute coronary syndrome in japan during the coronavirus disease 2019 pandemic. J Atheroscler Thromb. 2021 doi: 10.5551/jat.62746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannan E.L., Wu Y., Cozzens K., et al. Percutaneous coronary intervention for ST-elevation myocardial infarction before and during COVID in New York. Am J Cardiol. 2021;142:25–34. doi: 10.1016/j.amjcard.2020.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia S., Stanberry L., Schmidt C., et al. Impact of COVID-19 pandemic on STEMI care: an expanded analysis from the United States. Catheter Cardiovasc Interv. 2021;98(2):217–222. doi: 10.1002/ccd.29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakakura K., Inohara T., Kohsaka S., et al. Incidence and determinants of complications in rotational atherectomy: insights from the national clinical data (J-PCI Registry) Circ Cardiovasc Interv. 2016;9(11) doi: 10.1161/CIRCINTERVENTIONS.116.004278. [DOI] [PubMed] [Google Scholar]
- 13.Yamaji K., Kohsaka S., Morimoto T., et al. Relation of ST-segment elevation myocardial infarction to daily ambient temperature and air pollutant levels in a Japanese nationwide percutaneous coronary intervention registry. Am J Cardiol. 2017;119(6):872–880. doi: 10.1016/j.amjcard.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Kishi T., Mizuno A., Ishida M., et al. Recommendations for maintaining the cardiovascular care system under the conditions of the COVID-19 pandemic- 1st edition, April 2020. Circ J. 2020;84(11):2023–2026. doi: 10.1253/circj.CJ-20-0518. [DOI] [PubMed] [Google Scholar]
- 15.Katsoularis I., Fonseca-Rodríguez O., Farrington P., Lindmark K., Fors Connolly A.M. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398(10300):599–607. doi: 10.1016/S0140-6736(21)00896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brant L.C.C., Nascimento B.R., Teixeira R.A., et al. Excess of cardiovascular deaths during the COVID-19 pandemic in Brazilian capital cities. Heart. 2020;106(24):1898–1905. doi: 10.1136/heartjnl-2020-317663. [DOI] [PubMed] [Google Scholar]
- 17.Islam N., Shkolnikov V.M., Acosta R.J., et al. Excess deaths associated with covid-19 pandemic in 2020: age and sex disaggregated time series analysis in 29 high income countries. BMJ. 2021;373:n1137. doi: 10.1136/bmj.n1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gramegna M., Baldetti L., Beneduce A., et al. ST-segment-elevation myocardial infarction during COVID-19 pandemic: insights from a regional public service healthcare hub. Circ Cardiovasc Interv. 2020;13(8) doi: 10.1161/CIRCINTERVENTIONS.120.009413. [DOI] [PubMed] [Google Scholar]
- 19.Kitahara S., Fujino M., Honda S., et al. COVID-19 pandemic is associated with mechanical complications in patients with ST-elevation myocardial infarction. Open Heart. 2021;8(1) doi: 10.1136/openhrt-2020-001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechner I., Reindl M., Tiller C., et al. Impact of COVID-19 pandemic restrictions on ST-elevation myocardial infarction: a cardiac magnetic resonance imaging study. Eur Heart J. 2021 doi: 10.1093/eurheartj/ehab621. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon A.C., Mouncey P.R., Al-Beidh F., et al. Interleukin-6 receptor antagonists in critically Ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daida H., Miyauchi K., Ogawa H., et al. Management and two-year long-term clinical outcome of acute coronary syndrome in Japan: prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ J. 2013;77(4):934–943. doi: 10.1253/circj.cj-13-0174. [DOI] [PubMed] [Google Scholar]
- 25.Ishihara M., Fujino M., Ogawa H., et al. Clinical presentation, management and outcome of Japanese patients with acute myocardial infarction in the troponin era - Japanese Registry of acute myocardial infarction diagnosed by universal definition (J-MINUET) Circ J. 2015;79(6):1255–1262. doi: 10.1253/circj.CJ-15-0217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.