ABSTRACT
The ubiquitous diazotrophic soil bacterium Azotobacter vinelandii has been extensively studied as a model organism for biological nitrogen fixation (BNF). In A. vinelandii, BNF is regulated by the NifL-NifA two-component system, where NifL acts as an antiactivator that tightly controls the activity of the nitrogen fixation-specific transcriptional activator NifA in response to redox, nitrogen, and carbon status. While several studies reported that mutations in A. vinelandii nifL resulted in the deregulation of nitrogenase expression and the release of large quantities of ammonium, knowledge about the specific determinants for this ammonium-excreting phenotype is lacking. In this work, we report that only specific disruptions of nifL lead to large quantities of ammonium accumulated in liquid culture (∼12 mM). The ammonium excretion phenotype is associated solely with deletions of NifL domains combined with the insertion of a promoter sequence in the orientation opposite that of nifLA transcription. We further demonstrated that the strength of the inserted promoter could influence the amounts of ammonium excreted by affecting rnf1 gene expression as an additional requirement for ammonium excretion. These ammonium-excreting nifL mutants significantly stimulate the transfer of fixed nitrogen to rice. This work defines discrete determinants that bring about A. vinelandii ammonium excretion and demonstrates that strains can be generated through simple gene editing to provide promising biofertilizers capable of transferring nitrogen to crops.
IMPORTANCE There is considerable interest in the engineering of ammonium-excreting bacteria for use in agriculture to promote the growth of plants under fixed-nitrogen-limiting conditions. This work defines discrete determinants that bring about A. vinelandii ammonium excretion and demonstrates that strains can be generated through simple gene editing to provide promising biofertilizers capable of transferring nitrogen to crops.
KEYWORDS: nitrogen fixation, regulation, nifL, ammonium excretion, A. vinelandii, transfer of fixed nitrogen, rice, biofertilizer
INTRODUCTION
Access to fixed or available forms of N often limits the productivity of crop plants and, thus, the production of food, feed, fiber, and fuel. Since the Green Revolution, N fertilizers provided by the Haber-Bosch process have become an essential part of modern agriculture, sustaining crop yields and replacing N removed from the system at harvest. However, with the increasing global population, problems caused by unintended N leaching and the production of greenhouse gases have led to a global “nitrogen problem” (1, 2). More sustainable ways of managing the N cycle in soil and utilizing biological nitrogen fixation (BNF) are now imperative. Inhibition of ammonium assimilation and interference with the mechanisms by which ammonium inhibits either nitrogenase synthesis or activity have both been considered strategies to increase the amount of fixed nitrogen transferred from bacteria to the plant partner in associative or symbiotic plant-diazotroph relationships (3, 4). The manipulation of soil diazotrophs can potentially provide a means to reduce the use of synthetic nitrogen fertilizers, thus providing a solution to the nitrogen problem.
In Azotobacter vinelandii, the transcription of the genes required for the biosynthesis of its Mo-dependent nitrogenase (nif genes) is regulated by the NifL-NifA system. NifL is an antiactivator that tightly controls the activity of its partner protein NifA, a member of the family of σ54 transcriptional activators (5, 6), in response to redox, carbon, and energy status. The domain architecture of A. vinelandii NifL is similar to that of histidine protein kinases (HPKs). It is comprised of at least three discrete domains, including (i) two N-terminal Per-Arnt-Sim domains (PAS1 and PAS2) (7–9), one of which (PAS1) contains a flavin adenine dinucleotide cofactor; (ii) a central domain predicted to have a secondary structure similar to that of the dimerization and phosphotransfer (DHp) domains of the HPKs (10–12), linked to the N terminus of the protein via a glutamine-rich hydrophilic sequence termed the Q linker; and (iii) a C-terminal ATP-binding domain similar to that of the Gyrase, Hsp90, Histidine Kinase, MutL (GHKL) superfamily of ATPases (13–18) (Fig. 1). Current evidence suggests that NifL controls the activity of NifA through protein-protein interactions that are modulated by redox changes, ligand binding, and interactions with the nitrogen-responsive signal transduction protein GlnK (10, 19–27).
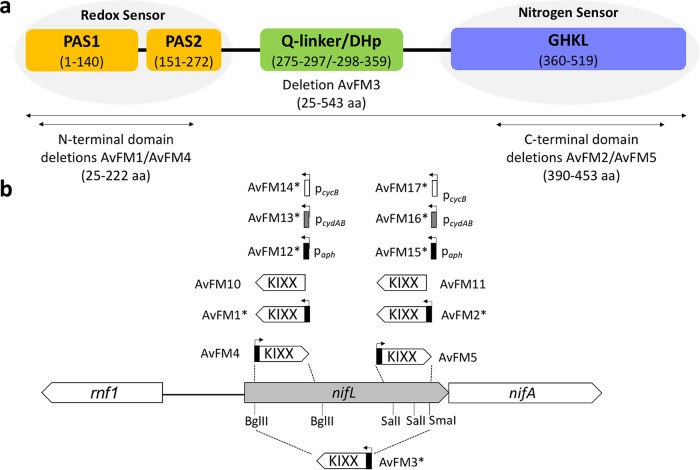
FIG 1.
Map of the nifLA region of A. vinelandii showing the restriction sites used for manipulations and the positions of KIXX and promoter inserts. (a) Domain structure of A. vinelandii NifL. The numbers refer to the primary amino acid (aa) sequence of the A. vinelandii NifL protein and mark the approximate boundaries of its N-terminal and C-terminal domains. The locations of the PAS domains (PAS1 and PAS2), the Q-linker/DHp domain, and the apparent ATP-binding-site GHKL domain are indicated. (b) Map of the nifL region of A. vinelandii showing the restriction sites used for manipulations and the positions of KIXX and promoter inserts. The arrows mark the directions of transcription of the aph, cydAB, and cycB promoters in the respective strains. AvFM1*, ΔnifL (PAS domains), with paph_KIXX in an orientation opposite that of nifLA; AvFM2*, ΔnifL (GHKL domain), with paph_KIXX in an orientation opposite that of nifLA; AvFM3*, ΔnifL (PAS, Q-linker/DHp, and GHKL domains), with paph_KIXX in an orientation opposite that of nifLA; AvFM4, ΔnifL (PAS domains), with paph_KIXX in the same orientation as that of nifLA; AvFM5, ΔnifL (GHKL domain), with paph_KIXX in the same orientation as that of nifLA; AvFM10, ΔnifL (PAS domains), with KIXX in an orientation opposite that of nifLA; AvFM11, ΔnifL (GHKL domain), with KIXX in an orientation opposite that of nifLA; AvFM12*, ΔnifL (PAS domains), with paph in an orientation opposite that of nifLA; AvFM13*, ΔnifL (PAS domains), with pcydAB in an orientation opposite that of nifLA; AvFM14*, ΔnifL (PAS domains), with pcycB in an orientation opposite that of nifLA; AvFM15*, ΔnifL (GHKL domain), with paph in an orientation opposite that of nifLA; AvFM16*, ΔnifL (GHKL domain), with pcydAB in an orientation opposite that of nifLA; AvFM17*, ΔnifL (GHKL domain), with pcycB in an orientation opposite that of nifLA. Ammonium-excreting strains are indicated with an asterisk.
Previous studies have reported specific deletion-insertion mutations of the nifL gene in A. vinelandii that result in the deregulation of nitrogenase expression and the release of significant quantities of ammonium during the late exponential and early stationary growth phases, yielding concentrations of about 10 mM in liquid growth medium (21, 28). These disruptions included deletions of the regions encoding the N-terminal sensor domain (MD371 strain) or the C-terminal nucleotide-binding domain (MV376 strain) of NifL associated with the insertion of a kanamycin resistance cassette (KIXX) in which the aph promoter directs transcription in the direction opposite that of nifLA (21, 28) (see Fig. S1 in the supplemental material). More recently, several laboratories have reported derivatives of this approach first described by Bali et al. (21) and Brewin et al. (28) with the construction of different A. vinelandii deregulated nitrogenase strains yielding different phenotypes (29, 30) (Fig. S1).
Over the past decades, speculations have been made to explain this ammonium-excreting phenotype. Ammonium release has been attributed to the passive loss of ammonium, which accumulates to high intracellular concentrations due to prolonged and enhanced nitrogenase expression, caused either by a loss of NifL function or by upsetting the NifL/NifA ratio through overexpressing the activator (28). A putative open reading frame overlapping A. vinelandii nifL could also be implicated in ammonium release (28). More recently, Mitra et al. (31) identified a positive cis-acting regulatory element of the nifLA operon within the coding region of the nifL gene of A. vinelandii. The deletion of this element resulted in the complete loss of promoter activity. Therefore, alternative mechanisms of nifLA operon regulation may exist in A. vinelandii, and the hypothesis is that the ammonium excretion phenotype could be associated with cis- or trans-acting regulatory elements. However, to date, a complete understanding of the molecular basis of ammonium release in nifL mutants has not been thoroughly investigated.
Given the ambiguity of the genetic determinants and the variations in the phenotypes of nifL mutants generated in previous work, we conducted a systematic study in which various constructs were generated with in-frame deletions of the individual NifL domains with and without an accompanying insertion of an antibiotic resistance marker. The results indicate that uncoupling nif gene expression in response to available fixed nitrogen through nifL deletion is insufficient to support ammonium production to affect excretion. Ammonium excretion occurs only when nifL deletions are accompanied by the increased expression of Rnf1, which potentially either increases the availability of reducing equivalents to support nitrogenase catalysis or controls the rate of maturation of the nitrogenase enzyme. There is growing interest in engineered inoculants capable of producing ammonium in excess that promote the growth of plants under fixed-nitrogen-limiting conditions. The ammonium-excreting nifL strains constructed in this work can significantly stimulate the transfer of fixed nitrogen to rice plants, suggesting that these strains could be used as effective biofertilizers.
RESULTS
Deletion of the PAS, Q-linker/DHp, and GHKL domains of NifL in A. vinelandii relieves ammonium repression of nitrogen fixation and could lead to ammonium excretion.
Various deletion-insertion mutations localized in the NifL functional domains were generated. In-frame deletions were introduced into the PAS, Q-linker/DHp, and GHKL domains (Fig. 1a) and accompanied by the insertion of a cassette cartridge in which a kanamycin resistance gene (KIXX) expressed from the aph promoter (paph_KIXX) was inserted in both possible orientations. The resulting strains in which the aph promoter within the KIXX cassette directed transcription away from nifLA transcription (AvFM1, AvFM2, and AvFM3) (Table 1 and Fig. 1b) were Nif+ and excreted large quantities of ammonium (around 12 mM at 48 h). The different strains grew at rates similar to those of the wild-type (WT) strain (Table 2) and reached comparable final growth maxima as observed by the optical density. The timing and extent of ammonium release were similar. The strains excreted ammonium toward the end of the exponential growth phase. The mean level of ammonium excreted in stationary-phase cultures was around 20 to 27 mol per kg of protein at 48 h (Fig. 2a). The ability of these nifL mutants to excrete ammonium was not related to defective ammonium assimilation since these mutants grew as well as the wild-type strain under diazotrophic and nondiazotrophic conditions (Table 2), as previously observed by Bali et al. (21) for the MV376 strain.
TABLE 1.
A. vinelandii bacterial strains used in this studya
| Strain | Genotype | Phenotype | Reference |
|---|---|---|---|
| DJ | Wild type | Nif+ | Setubal et al., 2009 (42) |
| DJ100 | ΔnifD | Nif− | Robinson et al., 1986 (50) |
| DJ1418 | ΔsrcX lacZ-Kan | Nif+ | This work |
| Δrnf1 | ΔrnfA1B1C1D1G1E1H1 | Nif+ | Curatti et al., 2005 (35) |
| AvFM1 | ΔnifL (PAS domains); paph_KIXX in an orientation opposite that of nifA | Nif+ NH4+ | This work |
| AvFM2 | ΔnifL (GHKL domain); paph_KIXX in an orientation opposite that of nifA | Nif+ NH4+ | This work |
| AvFM3 | ΔnifL (PAS, Q-linker, GHKL domains); paph_KIXX in an orientation opposite that of nifA | Nif+ NH4+ | This work |
| AvFM4 | ΔnifL (PAS domains); paph_KIXX in the same orientation as that of nifA | Lethal | This work |
| AvFM5 | ΔnifL (GHKL domain); paph_KIXX in the same orientation as that of nifA | Lethal | This work |
| AvFM6 | ΔnifD ΔnifL (PAS domains); paph_KIXX in the same orientation as that of nifA | Nif− | This work |
| AvFM7 | ΔnifD ΔnifL (GHKL domain); paph_KIXX in the same orientation as that of nifA | Nif− | This work |
| AvFM8 | ΔnifL (PAS domains); markerless | Nif+ | This work |
| AvFM9 | ΔnifL (GHKL domain); markerless | Nif+ | This work |
| AvFM10 | ΔnifL (PAS domains); KIXX in orientation opposite that of nifA | Nif+ | This work |
| AvFM11 | ΔnifL (GHKL domain); KIXX in an orientation opposite that of nifA | Nif+ | This work |
| AvFM12 | ΔnifL (PAS domains); paph in an orientation opposite that of nifA | Nif+ NH4+ | This work |
| AvFM13 | ΔnifL (PAS domains); pcydAB in an orientation opposite that of nifA | Nif+ NH4+ | This work |
| AvFM14 | ΔnifL (PAS domains); pcycB in an orientation opposite that of nifA | Nif+ NH4+ | This work |
| AvFM15 | ΔnifL (GHKL domain); paph in an orientation opposite that of nifA | Nif+ NH4+ | This work |
| AvFM16 | ΔnifL (GHKL domain); pcydAB in an orientation opposite that of nifA | Nif+ NH4+ | This work |
| AvFM17 | ΔnifL (GHKL domain); pcycB in an orientation opposite that of nifA | Nif+ NH4+ | This work |
| AvFM18 | ΔscrX paph_lacZ | Nif+ | This work |
| AvFM19 | ΔscrX pcydAB_lacZ | Nif+ | This work |
| AvFM20 | ΔscrX pcycB_lacZ | Nif+ | This work |
| AvFM21 | Δrnf1 ΔnifL (PAS domains); paph_KIXX in an orientation opposite that of nifA | Nif+ | This work |
| AvFM22 | Δrnf1 ΔnifL (GHKL domain); paph_KIXX in an orientation opposite that of nifA | Nif+ | This work |
The relevant information for each strain used in this study is compiled, including name, description of the genotype, description of the phenotype (Nif+ or Nif−, with NH3 for ammonium-excreting strains), and reference.
TABLE 2.
Growth rates of nifL mutant strains grown under diazotrophic and nondiazotrophic conditionsa
| Strain | Mean μ (OD600 units/h) ± SD |
|
|---|---|---|
| B medium | BN medium | |
| Wild type | 0.104 ± 0.004 | 0.205 ± 0.006 |
| AvFM1 | 0.103 ± 0.003 | 0.207 ± 0.009 |
| AvFM2 | 0.105 ± 0.001 | 0.222 ± 0.002 |
| AvFM3 | 0.108 ± 0.006 | |
| AvFM8 | 0.100 ± 0.001 | |
| AvFM9 | 0.104 ± 0.002 | |
| AvFM10 | 0.101 ± 0.004 | |
| AvFM11 | 0.108 ± 0.002 | |
| AvFM12 | 0.101 ± 0.001 | |
| AvFM15 | 0.108 ± 0.003 | |
| AvFM16 | 0.102 ± 0.001 | |
| AvFM17 | 0.105 ± 0.004 | |
| AvFM21 | 0.085 ± 0.002 | |
| AvFM22 | 0.085 ± 0.001 | |
| Δrnf1 | 0.088 ± 0.007 | |
The growth rates (μ) (in OD600 units per hour) and the genotypes of the different strains grown under diazotrophic conditions (WT, AvFM1, AvFM2, AvFM3, AvFM8, AvFM9, AvFM10, AvFM11, AvFM12, AvFM15, AvFM16, AvFM17, AvFM21, AvFM22, and Δrnf1) and under nondiazotrophic conditions (fixed-N source is readily available) (WT, AvFM1, and AvFM2) are summarized. Cells were grown aerobically in 6-well plates that contained 3 mL of Burk’s sucrose medium in the absence (B medium) or in the presence (BN medium) of 10 mM ammonium acetate and incubated at 30°C on a rotary shaker at 500 rpm with double orbital shaking (CLARIOstar; BMG Labtech). Three milliliters of growth medium was inoculated with 300 μL of the preculture at an optical density at 600 nm of 0.5. The results show the means and standard deviations for data from biological triplicates.
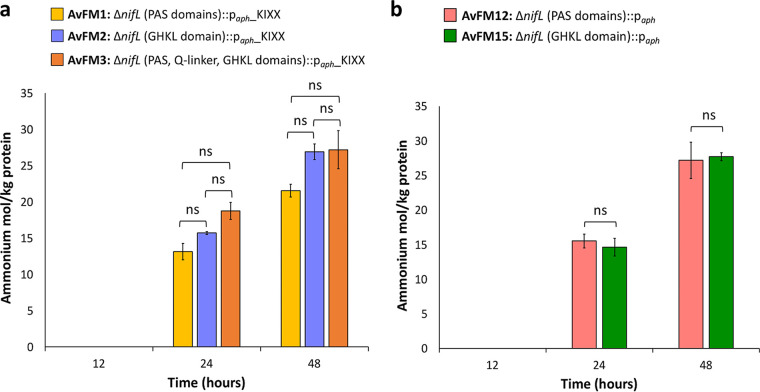
FIG 2.
Extracellular ammonium concentrations in cultures of nifL mutant strains generated with the KIXX cassette containing the aph promoter and the nifL mutant strains generated with the aph promoter under diazotrophic conditions. (a) Bar graph showing the quantification of ammonium present in the medium at the indicated time points for the AvFM1, AvFM2, and AvFM3 strains. (b) Bar graph showing the quantification of ammonium present in the medium at the indicated time points for the AvFM12 and AvFM15 strains. The changes in extracellular ammonium levels are presented as moles of ammonium excreted per kilogram of protein. The results show the means and standard deviations (error bars) for data from triplicate experiments. ns indicates not statistically significant (P > 0.05) according to an unpaired t test. Results of the unpaired t test for extracellular ammonium concentrations between the AvFM1 and AvFM2 strains are a P value of 0.14 at 24 h and a P value of 0.05 at 48 h, respectively; results between the AvFM1 and AvFM3 strains are a P value of 0.09 at 24 h and a P value of 0.36 at 48 h, respectively; and results between the AvFM2 and AvFM3 strains are a P value of 0.09 at 24 h and a P value of 0.05 at 48 h, respectively. Results of the unpaired t test for extracellular ammonium concentrations between AvFM12 and AvFM15 are a P value of 0.79 at 24 h and a P value of 0.89 at 48 h, respectively.
The nifL mutants with the KIXX cassette controlled by the strong aph promoter (paph_KIXX) inserted in the same orientation as that of nifLA transcription (AvFM4 and AvFM5) (Table 1 and Fig. 1b) could not be isolated free of wild-type nifL. Thus, no Kmr Amps transformants of the DJ strain were obtained with plasmid pFM1-1 or pFM2-1 (described in Table S2 in the supplemental material), confirming previous reports that KIXX insertions in this orientation could not be constructed (32). To test if the uncontrolled high-level expression of active nitrogenase was responsible for the lethal phenotype, the in-frame deletion of NifL with the insertion of the paph_KIXX cartridge in the same orientation as that of nifLA transcription was introduced into the background of a Nif− strain (DJ100) having a defined deletion within the nitrogenase structural gene nifD (MoFe protein α subunit) (33) (Table 1). These strains (AvFM6 and AvFM7) (Table 1) could be successfully generated when selected on ammonia-supplemented media, supporting the hypothesis that the apparent lethality of strains in which the strong aph promoter within the KIXX cassette is inserted in the same orientation as that of nifLA transcription (AvFM4 and AvFM5) is correlated with the expression of active nitrogenase.
Assessing the specific determinants of ammonium excretion within the antibiotic resistance cartridge.
To further assess the different phenotypes associated with the orientation of the antibiotic resistance marker, we generated markerless nifL deletion strains (AvFM8 and AvMF9) (Table 1). Surprisingly, the resulting strains with in-frame deletions but without insertions, AvFM8 and AvFM9, were Nif+ but did not release ammonium. These results indicate that a deletion of nifL alone was not sufficient to observe ammonium release and that the presence of the paph_KIXX cartridge was required for ammonium excretion. To delineate the specific determinants of the paph_KIXX cartridge responsible for ammonium excretion, we independently reinserted either the KIXX cassette open reading frame sequence (without the aph promoter) or just the aph promoter sequence itself into the chromosome in the same position and orientation as those shown for AvFM1 and AvFM2 (Fig. 1b). In-frame deletions of either the N-terminal or C-terminal domain of NifL in tandem with the insertion of the KIXX cassette open reading frame sequence (AvFM10 and AvFM11) resulted in a Nif+ phenotype, but the strains did not excrete ammonium (Table 1). However, the equivalent deletions in tandem with the insertion of the aph promoter sequence (AvFM12 and AvFM15) resulted in Nif+ strains that excrete ammonium (Table 1). The different strains grew at similar rates (Table 2) and reached comparable final growth maxima as observed by the optical density. The timing and extent of ammonium release were similar. The AvFM12 and AvFM15 strains excreted ammonium toward the end of the exponential growth phase. The mean level of ammonium excreted in stationary-phase cultures was around 12 mM and 24 to 27 mol per kg of protein at 48 h (Fig. 2b), similar to the levels observed in the parental strains (AvFM1 and AvFM2). These results indicate that the insertion of a strong promoter sequence in an orientation opposite from the direction of nifLA transcription was a required determinant for ammonium excretion.
Effect of the nifL mutations on the expression of genes located downstream and upstream of nifL.
To assess the potential effects of the deletion and/or insertion mutations on the expression of the downstream and upstream genes, which could be responsible for the ammonium excretion phenotype, we performed reverse transcriptase quantitative PCR (RT-qPCR) analyses.
We first examined nifA and nifH transcription abundances in the wild-type strain and the nifL mutants. At the mid-exponential phase (optical density at 600 nm [OD600] of 0.5), no significant difference in the nifA transcript levels was observed in the wild-type strain and the nifL mutants irrespective of the nitrogen status (Fig. 3a), confirming that neither the KIXX cassette nor the aph promoter altered the levels of the nifA transcript. This result excludes the possibility that ammonium release would result from NifA overexpression from unexpected promoter activity in the cassette or a promoter-like sequence generated by the KIXX insertion in the nifL region as previously suggested (21). In all nifL mutants tested (AvFM2, AvFM9, AvFM11, and AvFM15), nifH is constitutively expressed, and the levels of the nifH transcript remained unchanged in the absence and presence of 10 mM ammonium. This is in contrast to the large increase in the transcript level of nifH that was observed in the wild-type strain under diazotrophic growth conditions relative to growth under N-replete conditions (Fig. 3b and c).
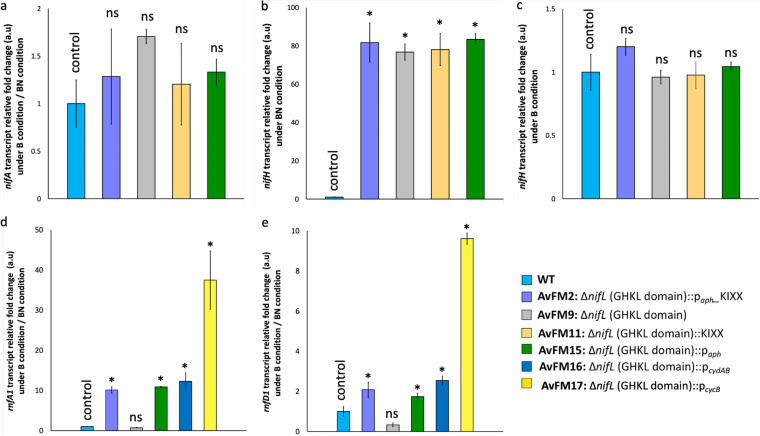
FIG 3.
Changes in nifA, nifH, rnfA1, and rnfD1 transcript levels. (a) Bar graph showing the n-fold changes in nifA transcripts under diazotrophic growth conditions (B) relative to nondiazotrophic growth conditions (BN) in the AvFM2, AvFM9, AvFM11, and AvFM15 strains compared to the wild-type (WT) strain (control). (b and c) Bar graphs showing the n-fold changes in nifH transcripts under nondiazotrophic (b) and diazotrophic (c) growth conditions in the AvFM2, AvFM9, AvFM11, and AvFM15 strains compared to the WT strain (control). (d and e) Bar graphs showing the n-fold changes in the rnfA1 (d) and rnfD1 (e) transcripts under diazotrophic growth conditions relative to nondiazotrophic growth conditions of the AvFM2, AvFM9, AvFM15, AvFM16, and AvFM17 strains compared to the WT strain (control). The results were normalized to gyrB transcript levels, which remained constant under the different experimental conditions tested. The results show the means and standard deviations (error bars) for data from biological triplicates. An asterisk indicates a significant difference (P < 0.05), and ns indicates not statistically significant (P > 0.05), according to an unpaired t test. For panel a, results of the unpaired t test for the n-fold changes in nifA transcripts between the WT strain (control) and the AvFM2, AvFM9, AvFM11, or AvFM15 strain under diazotrophic growth conditions relative to nondiazotrophic growth conditions are a P value of 0.5 (AvFM2), a P value of 0.09 (AvFM9), a P value of 0.66 (AvFM11), and a P value of 0.82 (AvFM15), respectively. For panel b, results of the unpaired t test for the n-fold changes in nifH transcripts between the WT strain and the AvFM2, AvFM9, AvFM11, or AvFM15 strain under nondiazotrophic growth conditions are a P value of 4.62E−07 (AvFM2), a P value of 8.31E−07 (AvFM9), a P value of 7.97E−07 (AvFM11), and a P value of 4.68E−07 (AvFM15), respectively. For panel c, results of the unpaired t test for the n-fold changes in nifH transcripts between the WT strain and the AvFM2, AvFM9, AvFM11, or AvFM15 strain under diazotrophic growth conditions are a P value of 0.31 (AvFM2), a P value of 0.22 (AvFM9), a P value of 0.13 (AvFM11), and a P value of 0.21 (AvFM15), respectively. For panel d, results of the unpaired t test for the n-fold changes in rnfA1 transcripts between the WT strain and the AvFM2, AvFM9, AvFM15, AvFM16, or AvFM17 strain under diazotrophic growth conditions relative to nondiazotrophic growth conditions are a P value of 3.25E−03 (AvFM2), a P value of 0.25 (AvFM9), a P value of 2.77E−03 (AvFM15), a P value of 2.61E−03 (AvFM16), and a P value of 5.39E−04 (AvFM17), respectively. For panel e, results of the unpaired t test for the n-fold changes in rnfD1 transcripts between the WT strain and the AvFM2, AvFM9, AvFM15, AvFM16, or AvFM17 strain under diazotrophic growth conditions relative to nondiazotrophic growth conditions are a P value of 2.18E−02 (AvFM2), a P value of 0.17 (AvFM9), a P value of 5.04E−02 (AvFM15), a P value of 2.42E−02 (AvFM16), and a P value of 8.72E−04 (AvFM17), respectively. a.u, arbitrary units.
Under diazotrophic conditions during the exponential growth phase, similar levels of the nifH transcript were observed in the wild-type and nifL mutant strains. We measured the whole-cell activity of nitrogenase using standard acetylene reduction assays during the exponential and stationary growth phases. The nitrogenase activity of the nifL mutants was comparable to the one observed with the wild-type strain during the exponential growth phase (4.47 ± 0.29 μmol ethylene/h/μg protein/mL of culture for the WT versus 7.21 ± 1.04 μmol ethylene/h/μg protein/mL of culture for the nifL mutant strains), which correlated with the nifH transcript abundances detected. During late log phase/stationary phase, the nitrogenase activity of the ammonium-excreting strains was observed to be 3-fold higher than that of the wild-type strain (0.14 ± 0.03 μmol ethylene/h/μg protein/mL of culture for the WT versus 0.43 ± 0.11 μmol ethylene/h/μg protein/mL of culture for the nifL mutant strains), consistent with our previous results (34). The nifL mutants typically excrete ammonium only when the nitrogenase is downregulated, e.g., when the wild-type strain is either not growing or growing with ammonium (21, 28). Therefore, nifL mutants excrete ammonium into the growth medium in late log and stationary growth phases but not during the exponential growth phase when nitrogen is limiting and nitrogenase activity is high (21, 28).
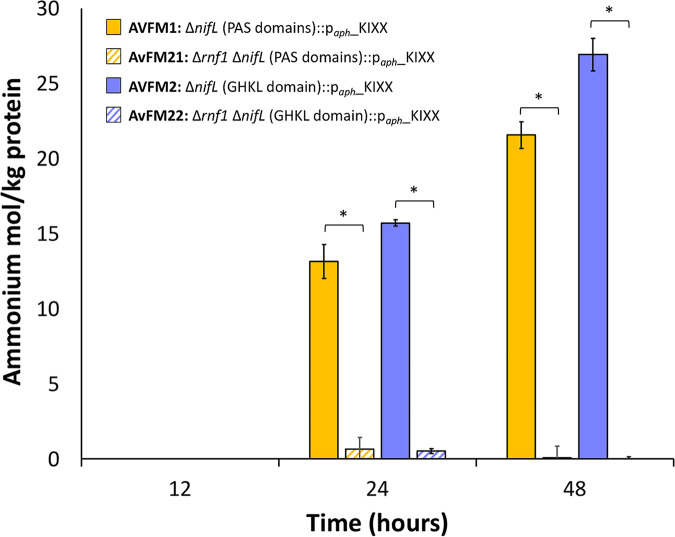
Since the insertion of the aph promoter in the opposite orientation in the nifL coding sequence was a requirement for ammonium excretion, we considered the possible implications of changes in the levels of expression of genes upstream of nifL as determinants of ammonium excretion. Adjacent to the nifL (Avin_50990) and nifA (Avin_51000) gene cluster is the rnf1 operon (rnfA1B1C1D1G1E1H1 [Avin_50920 to Avin_50980]). The transcription of nifLA and rnf1 proceeds divergently; however, the expression of rnf1 was shown to be NifA dependent (35). Rnf1 is a membrane-bound complex involved in generating low-potential reduced electron carriers to support nitrogen fixation through coupling the free energy of the proton motive force to drive the reduction of ferredoxin and/or flavodoxin by NADH (35). In addition, the A. vinelandii rnf1 gene cluster is required for the full expression of the nifHDK genes during the first hours of nitrogenase derepression and for the accumulation of nitrogenase Fe protein (35). RT-qPCRs showed that genes from the rnf1 operon (selected genes rnfA1 and rnfD1) were upregulated in the nifL mutant ammonium-excreting strains relative to the wild-type strain (Fig. 3d and e). This result suggests that the upregulation of the rnf1 genes upstream of nifL could be responsible for enabling ammonium release, presumably by providing sufficient reducing equivalents to enable high nitrogenase activity or by controlling the rate of maturation of nitrogenase. To confirm the role of the expression of the Rnf1 complex in the ammonium excretion phenotype, strains with modifications of nifL promoting ammonium excretion were generated in a background of a deletion of a portion of the rnf1 gene cluster (Δrnf1) (AvFM21 and AvFM22) (35) (Table 1). The resulting mutant strains exhibited growth rates similar to those of the Δrnf1 strain under diazotrophic growth conditions (Table 2) and did not exhibit the ammonium excretion phenotype (Fig. 4). These results imply that the increased expression of the Rnf1 complex is a key determinant for ammonium excretion in A. vinelandii nifL mutants.
FIG 4.
Extracellular ammonium concentrations in cultures of nifL mutant strains deficient for the Rnf1 complex under diazotrophic conditions. The bar graph shows the quantification of ammonium present in the medium at the indicated time points for the AvFM1, AvFM21, AvFM2, and AvFM22 strains. The changes in extracellular ammonium levels are presented as moles of ammonium excreted per kilogram of protein. The results show the means and standard deviations (error bars) for data from triplicate experiments. An asterisk indicates a significant difference (P < 0.05) according to an unpaired t test. Results of the unpaired t test for extracellular ammonium concentrations between the AvFM1 strain (control) and the AvFM21 strain are a P value of 3.37E−03 at 24 h and a P value of 5.72E−03 at 48 h, respectively. Results of the unpaired t test for extracellular ammonium concentrations between the AvFM2 strain (control) and the AvFM22 strain are a P value of 1.94E−05 at 24 h and a P value of 7.40E−06 at 48 h, respectively.
Promoter strength and ammonium excretion.
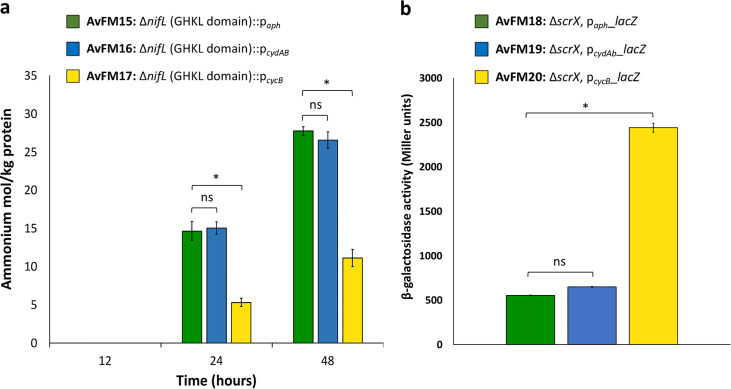
To further probe the link between the levels of rnf1 gene expression and the ammonium excretion phenotype, the deletion of the region encoding the GHKL domain of NifL was combined with the insertion of two A. vinelandii endogenous promoters of different strengths involved in the expression of the cydAB (36) and cycB (37) genes. The resulting strains (AvFM16 and AvFM17) (Table 1) were Nif+ and excreted ammonium. The strains grew at rates similar to those of the strain generated with the aph promoter (AvFM15) (Table 2) and reached comparable final growth maxima as observed by the optical density. The ammonium release levels varied relative to the promoter sequence used (Fig. 5a). As in the case of the above-described ammonium-excreting strains, the two strains (AvFM16 and AvFM17) excreted ammonium toward the end of the exponential growth phase, with the nifL mutant generated with the cydAB promoter (AvFM16) consistently producing more ammonium (around 27 mol per kg of protein at 48 h) than the nifL mutant generated with the cycB promoter (AvFM17) (around 11 mol per kg of protein at 48 h) (Fig. 5a). The amounts of ammonium excreted by the AvFM16 strain were, however, similar to the ones observed with the AvFM15 strain (aph promoter) (Fig. 5a). Similar observations were obtained with the deletion of the N-terminal domain of NifL combined with the promoter insertions (AvFM13 and AvFM14) (Fig. S2). This result indicates that the amount of ammonium excreted can be controlled and modulated using specific promoter sequences that presumably modulate the level of expression of Rnf1.
FIG 5.
Extracellular ammonium concentrations in cultures of the nifL mutant strains generated with the aph, cydAB, and cycB promoters and activities of paph_lacZ, pcydAB_lacZ, and pcydA_lacZ reporter genes in A. vinelandii under diazotrophic growth conditions. (a) Bar graph showing the quantification of ammonium present in the medium at the indicated time points for the AvFM15, AvFM16, and AvF17 strains. The changes in extracellular ammonium levels are presented as moles of ammonium excreted per kilogram of protein. The results show the means and standard deviations (error bars) for data from triplicate experiments. (b) Bar graph showing the β-galactosidase activities of the AvFM18, AvFM19, and AvF20 strains carrying the paph_lacZ, pcydAB_lacZ, and pcydA_lacZ reporters grown under diazotrophic growth conditions. The results show the means and standard deviations (error bars) for data from triplicate experiments. An asterisk indicates a significant difference (P < 0.05), and ns indicates not statistically significant (P > 0.05), according to an unpaired t test. For panel a, results of the unpaired t test for extracellular ammonium concentrations between the AvFM15 strain (control) and the AvFM16 or AvFM17 strain are a P value of 0.87 (AvFM16) and a P value of 4.36E−02 (AvFM17) at 24 h and a P value of 0.66 (AvFM16) and a P value of 2.36E−03 (AvFM17) at 48 h, respectively. For panel b, results of the unpaired t test for β-galactosidase activities between the AvFM20 strain (control) and the AvFM18 or AvFM19 strain are a P value of 1.23E−04 (AvFM18) and a P value of 1.26E−04 (AvFM19), respectively.
To examine further if the amounts of ammonium excreted were strongly correlated with the promoter sequences, the strengths of the three promoters paph, pcydAB, and pcycB in our strains were determined in vivo by measuring β-galactosidase activities in strains carrying the paph_lacZ, pcydAB_lacZ, and pcycB_lacZ fusions (AvFM18, AvFM19, and AvFM20) (Fig. 5b and Table 1). The lacZ gene from Escherichia coli placed under the transcriptional and translational control of the aph, cydAB, and cycB promoters was inserted into the A. vinelandii chromosome, replacing the scrX gene, which is located approximately 500 bp downstream of scrY and encodes a protein with sequence identity to alpha-glucosidases. The loss of scrX does not affect the ability of A. vinelandii to grow using sucrose as the sole carbon source, indicating that the scrX gene product is not essential for sucrose catabolism (38). The β-galactosidase activity of the strain harboring the pcycB_lacZ fusion (AvFM20) was significantly higher than those of the strains carrying the paph_lacZ (AvFM18) and pcydAB_lacZ (AvFM19) fusions. The cycB promoter was upregulated around 4-fold relative to the aph and cydAB promoters (Fig. 5b). However, the nifL mutant strains generated with the cycB promoter (AvFM14 or AvFM17) appeared to produce less ammonium than the nifL mutant strains generated with the aph and cydAB promoters (AvFM12 or AvFM15 and AvFM13 or AvFM16). These results suggest that the amounts of ammonium released into the growth medium did not directly proportionally correlate with the promoter strength. Furthermore, rnfA1 and rnfD1 appeared to be most upregulated in the nifL mutant generated with the cycB promoter (AvFM17) compared to the nifL mutants generated with the aph and cydAB promoters (AvFM15 and AvFM16). These strains having aph and cydAB promoter sequences exhibited similar upregulation levels for rnfA1 and rnfD1, consistent with the comparable strengths of the two promoters and in line with the similar levels of ammonium excretion. This indicates that the level of rnf1 gene expression played an essential role in the ability of nifL mutants to release ammonium and that fine-tuning of rnf1 gene expression was required for optimal ammonium excretion.
Ammonium-excreting nifL strains stimulate the transfer of fixed nitrogen to rice plants.
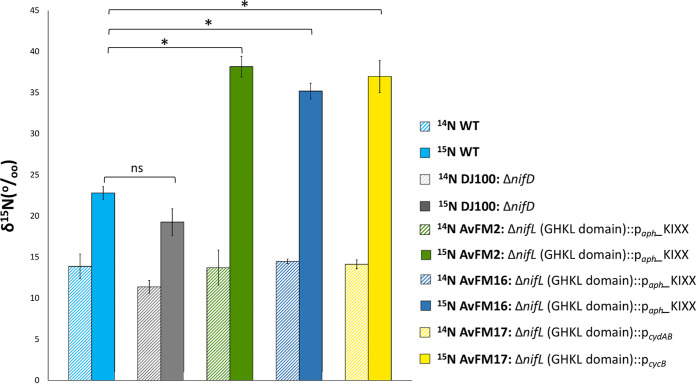
We investigated the ability of our ammonium-excreting strains to promote the transfer of fixed nitrogen to rice plant biomass by looking at direct 15N integration into plant tissues. 15N external labeling or enrichment techniques have been employed extensively to trace the direction and magnitude of N transfer between diazotrophic bacteria and plants. Significant differences were observed in 15N incorporation into rice plants between rice seedlings inoculated with the A. vinelandii wild-type strain and rice seedlings inoculated with ammonium-excreting nifL strains. Quantification of 15N incorporated into plant tissues demonstrated that the ammonium-excreting nifL strains could stimulate the transfer of fixed nitrogen to the plants (up to 67%), confirming the effect of a plant growth-promoting factor provided by these engineered nifL mutant strains (Fig. 6).
FIG 6.
15N incorporation experiments on rice plants (Oryza sativa) inoculated with A. vinelandii strains. Quantification of 15N incorporated into the rice plant tissues inoculated with the A. vinelandii wild-type (WT), DJ100 (ΔnifD), AvFM2, AvFM16, and AvFM17 strains was performed. The results show the means and standard deviations (error bars) for data from quintuplicate experiments. An asterisk indicates a significant difference (P < 0.05), and ns indicates not statistically significant (P > 0.05), according to an unpaired t test. Results of the unpaired t test for δ15N between the wild-type strain (control) and the DJ100, AvFM2, AvFM16, or AvFM17 strain are a P value of 0.24 (DJ100), a P value of 2.69E−02 (AvFM2), a P value of 4.21E−02 (AvFM16), and a P value of 1.85E−02 (AvFM17), respectively.
DISCUSSION
There is considerable interest in the engineering of ammonium-excreting bacteria for use as biofertilizers in agriculture. In addition to the strategy discussed here involving the manipulation of nifL, other genetic manipulations have been associated with an enhanced capacity for ammonium excretion in A. vinelandii and other diazotrophic bacteria: (i) engineering a NifA variant that prevents the nitrogen status signal from being conveyed to it by NifL (32), (ii) partial inhibition of glutamine synthetase (GS) or glutamate synthase (GOGAT) resulting in deficient ammonium assimilation (39, 40), and (iii) disruption of the ammonium/methylammonium transporter AmtB (29). A single-amino-acid substitution in NifA (NifA-E356K) disrupts the hierarchy of nif regulation in response to carbon and nitrogen status in A. vinelandii. The NifA-E356K substitution enabled the overexpression of nitrogenase in the presence of excess fixed nitrogen and the release of ammonium at millimolar levels outside the cell. However, both of these properties were conditional on the nature of the carbon source (32). A. vinelandii cells bearing a point mutation at the active site of GS (glnA-D49S) displayed a moderate mutant phenotype under diazotrophic growth conditions and excreted ammonium into the medium up to 1.7 mM. A double mutant strain (ΔnifL glnA-D49S) presented a stronger diazotrophic growth defect, and the maximum concentration of ammonium released into the medium was lower than that for the single mutant, at 1.0 mM (39). The deletion or disruption of the ammonium transport gene amtB in A. vinelandii resulted in the slow release of ammonium into the medium (29). The genetic manipulation of nifL developed here offers the advantage of high levels of ammonium release, which is not contingent upon the carbon sources provided (see Fig. S3 in the supplemental material). However, in terms of engineering a synthetic symbiosis, it has the disadvantage that nitrogenase expression is not regulated in these bacteria. On the other hand, the carbon-responsive control mechanism observed with the NifA-E356K variant may also require the engineering of the partner plant to provide a favorable carbon source (32). However, recent reports by Ortiz-Marquez et al. (39) and Barney et al. (29) suggest that the long-term stability of A. vinelandii ΔnifL strains may be problematic based on the potential for recombination events within the cell. Barney et al. (29) noticed that A. vinelandii strains AZBB158 and AZBB163 are prone to contamination by spontaneous cheaters, cells of A. vinelandii that evolve to quit fixing nitrogen and then prey upon the ammonium released by the remaining ammonium-excreting cells. While the potential for ammonium production far surpasses the needs of an individual cell under laboratory growth conditions, ammonium uptake by plants and other rhizosphere bacteria could decrease the amount of available ammonium produced by the ammonium-excreting nifL mutants in agronomic settings. Therefore, agronomic settings can differ from laboratory conditions and influence the occurrence of potential cheaters that might evolve to take advantage of the excess available ammonium.
In the model diazotroph Klebsiella pneumoniae, nitrogen fixation is also controlled at the transcriptional level by the regulatory proteins encoded by the nifLA operon. However, K. pneumoniae nifL mutants release comparatively little ammonium into the medium, around 100-fold less than that released by A. vinelandii nifL mutants (29). In work conducted by Setten et al. (41), the beneficial rhizobacterium Pseudomonas protegens Pf-5 was genetically modified to fix nitrogen using the genes encoding the nitrogenase of Pseudomonas stutzeri A1501. The engineered strain showed constitutive nitrogenase activity, released significant quantities of ammonium into the medium, and promoted the growth of Arabidopsis thaliana, Medicago sativa, Schenodorus arundinaceus, and Zea mays under nitrogen-deficient conditions. Similar manipulations in Pseudomonas putida, Pseudomonas veronii, and Pseudomonas taetrolens but not Pseudomonas balearica and Pseudomonas stutzeri resulted in high nitrogenase activity and high-level ammonium production, strongly suggesting that this phenotype depends on the genome context. In a recent study, Ryu et al. (33) transferred native and engineered nif clusters from diverse sources (Rhodobacter sphaeroides, Klebsiella oxytoca, P. stutzeri, or A. vinelandii) to two cereal endophytes (Azorhizobium caulinodans and Rhizobium sp.) and a well-characterized plant epiphyte (P. protegens Pf-5). Of these strains, the most promising candidates achieved high levels of inducible nitrogenase activity with reduced oxygen sensitivity, but none could excrete ammonium. The construction of ammonium-excreting mutants of any particular diazotroph through nif gene derepression via the manipulation of nifL may not be as straightforward, and our results with A. vinelandii imply that more than a single “target of regulation” is required. A better understanding of these genetic requirements can help in adopting the appropriate mutational strategies to construct ammonium-excreting mutants in other diazotrophs.
Our studies indicate that generating an ammonium excretion phenotype in A. vinelandii can be achieved by deregulating the nitrogen response when the rnf1 genes are upregulated, thereby highlighting the important role of energy homeostasis in nitrogen-fixing A. vinelandii. We present here supportive evidence using isotope labeling that atmospheric dinitrogen is fixed by the strains, released as ammonium, and transferred to the rice plant biomass, suggesting that these strains could be used as biofertilizers. We have generated ammonium-excreting derivatives of A. vinelandii in the absence of any transgenes through gene-editing approaches using native promoter sequences, allowing us to modulate and control the amount of ammonium produced. This feature is an important asset to be able to (i) match the specific fixed-nitrogen requirements for each crop and cultivar targeted, (ii) control the impact of the use of these biofertilizers on the influx of ammonium into the terrestrial biogeochemical nitrogen cycle, and (iii) minimize the metabolic load and fitness cost on the organism of the expression of multiple transgenes. The results presented in this study provide important new insights for developing a blueprint for engineering microorganisms for effective biofertilizers.
MATERIALS AND METHODS
Strains and media.
A. vinelandii strain DJ (wild-type strain; obtained from Dennis Dean, Virginia Tech, VA, USA) (42) and nifL mutants (this study) were grown aerobically at 30°C in Burk’s sucrose medium (B medium) (53) or Burk’s sucrose medium supplemented with 10 mM ammonium acetate (BN medium). Growth in B medium is referred to here as diazotrophic conditions, and growth in BN medium is referred to here as nondiazotrophic conditions. Two-hundred-milliliter liquid cultures, contained in 500-mL baffled Erlenmeyer flasks, were incubated on a rotary shaker at 180 rpm. The E. coli JM109 strain (Promega, Madison, WI) was used for cloning experiments. Ampicillin and kanamycin were used at 100 μg/mL and 50 μg/mL for E. coli and at 100 μg/mL and 5 μg/mL for A. vinelandii, respectively; rifampicin was used at 10 μg/mL for A. vinelandii.
Genetic constructs of Azotobacter vinelandii.
The A. vinelandii strains generated in this study were constructed as detailed in the supplemental material. The various plasmids and primers used to clone genes are listed in Table S1 in the supplemental material and Table 3, respectively. A graphical representation of the strains used in this study is shown in Fig. 1. Methods for the manipulation of A. vinelandii were described previously (28, 43, 44).
TABLE 3.
Sequences of the primers used in this study
| Primer | Sequencea |
|---|---|
| AvFM1-upstream-F-NdeI | 5′-GGAATTCCATATGGGCAGCAAGTGACCGAAGCATTGCCTTCGACGATCGCC-3′ |
| AvFM1-upstream-R-EcoRI | 5′-CCGGAATTCCGGGAAGCAGCTCGTCGCTCTCGGCGTGAG-3′ |
| AvFM1-downstream-F-EcoRI | 5′-CCGGAATTCGGCAGCAAGTGACCGAAGCATTGCCTTCGACGATCGCC-3′ |
| AvFM1-downstream-R-HindIII | 5′-CCCAAGCTTTCCTCGATGTTCACCACCAGGCGCACGGTCTGCG-3′ |
| AvFM2-upstream-F-NdeI | 5′-GGAATTCCATATGCGATTAAGGTGCGGCACAGGATTTGCTAATCTTCTCT-3′ |
| AvFM2-downstream-R-HindIII | 5′-CCCAAGCTTAACTTGCCCTTTTCCACCTCGCTTTCCAGGT-3′ |
| AvFM2-downstream-F-EcoRI | 5′-GGGGAATTCCATTCCGCCCGACCTGGTGCTGAAGGTGTTCGA-3′ |
| paph_KIXX-F-EcoRI | 5′-CCGGAATTCGGATCCGTCGAGCTCCCGGGAAGCT-3′ |
| paph_KIXX-R-EcoRI | 5′-CCGGAATTCTCAGAAGAACTCGTCAAGAAGGCGATAGAAGG-3′ |
| paph_KIXX-F-SmaI | 5′-TAACCCGGGGGATCCGTCGAGCTCCCGGGAAGCT-3′ |
| paph_KIXX-R-SalI | 5′-TGCGGTCGACGCGAAACGATCCTCATCCTGTCTCTTGATCAGATCTTGATCCC-3′ |
| paph_KIXX-F-SaII | 5′-ACGCGTCGACGGATCCGTCGAGCTCCCGGGAAGCT-3′ |
| paph_KIXX-F-R-SmaI | 5′-TCCCCCGGGTCAGAAGAACTCGTCAAGAAGGCGATAGAAGG-3′ |
| KIXX-F-EcoRI | 5′-CCGGAATTCATGATTGAACAAGATGGATTGCACGCAGGTTCTCCGGC-3′ |
| KIXX-R-EcoRI | 5′-GGGGAATTCTCAGAAGAACTCGTCAAGAAGGCGATAGAAGGCGATGC-3′ |
| KIXX-F-SmaI | 5′-TAACCCGGGATGATTGAACAAGATGGATTGCACGCAGGTTCTCCGGC-3′ |
| KIXX-R-SalI | 5′-TGCGGTCGACTCAGAAGAACTCGTCAAGAAGGCGATAGAAGGCGATGC-3′ |
| paph-F-EcoRI | 5′-CCGGAATTCGGATCCGTCGAGCTCCCGGGAAGCT-3′ |
| paph-R-EcoRI | 5′-CCGGAATTCGCGAAACGATCCTCATCCTGTCTCTTGATCAGATCTTGATCCC-3′ |
| paph-F-SmaI | 5′-TAACCCGGGGGATCCGTCGAGCTCCCGGGAAGCT-3′ |
| paph-R-SalI | 5′-TGCGGTCGACGCGAAACGATCCTCATCCTGTCTCTTGATCAGATCTTGATCCC-3′ |
| pcydAB-F-EcoRI | 5′-CCGGAATTCCTGCAGGTAGCCGAACACCTCCAGGTCCC-3′ |
| pcydAB-R-EcoRI | 5′-GGGGAATTCCAGGGACTCTCCTCGGTCGGGGATTGATCTTGCACTGCC-3′ |
| pcydAB-F-SmaI | 5′-TAACCCGGGCTGCAGGTAGCCGAACACCTCCAGGTCCC-3′ |
| pcydAB-R-SalI | 5′-TGCGGTCGACCAGGGACTCTCCTCGGTCGGGGATTGATCTTGCACTGCC-3′ |
| pcycB-F-EcoRI | 5′-CCGGAATTCACTCCGGCGCATTTCTAGCGGCCGCCGAAGTTCT-3′ |
| pcycB-R-EcoRI | 5′-GGGGAATTCCGTGGCTGATTACGTGCGCCCGCGGC-3′ |
| pcycB-F-SmaI | 5′-TAACCCGGGACTCCGGCGCATTTCTAGCGGCCGCCGAAGTTCT-3′ |
| pcycB-R-SalI | 5′-TGCGGTCGACCGTGGCTGATTACGTGCGCCCGCGGC-3′ |
| lacZ-F | 5′-ATGACCATGATTACGCCAAGCTTGCATGCCT-3′ |
| lacZ-R | 5′-GCGGATATCCTATGCGGCATCAGAGCAGATTGTACTGAGAGTGCACCA-3′ |
| lacZ-R-EcoRV | 5′-GCGGATATCCTATGCGGCATCAGAGCAGATTGTACTGAGAGTGCACCA-3′ |
| SOE-paph_lacZ-F1 | 5′-TGAGGATCGTTTCGCATGACCATGATTACGCCAAGCTTGCATGCCT-3′ |
| SOE-paph_lacZ-R1 | 5′-GGCGTAATCATGGTCATGCGAAACGATCCTCATCCTGTCTCTTGAT-3′ |
| SOE-paph_lacZ-F2 | 5′-ATCAAGAGACAGGATGAGGATCGTTTCGCATGACCATGATTACGCC-3′ |
| SOE-paph_lacZ-R2 | 5′-AGGCATGCAAGCTTGGCGTAATCATGGTCATGCGAAACGATCCTCA-3′ |
| SOE-pcydAB_lacZ-F1 | 5′-CCCGACCGAGGAGAGTCCCTGATGACCATGATTACGCCAAGCTTGCATGCCT-3′ |
| SOE-pcydAB_lacZ-R1 | 5′-CGTAATCATGGTCATCAGGGACTCTCCTCGGTCGGGGATTGATCTTGCACTGCC-3′ |
| SOE-pcydAB_lacZ-F2 | 5′-GGCAGTGCAAGATCAATCCCCGACCGAGGAGAGTCCCTGATGACCATGATTACG-3′ |
| SOE-pcydAB_lacZ-R2 | 5′-AGGCATGCAAGCTTGGCGTAATCATGGTCATCAGGGACTCTCCTCGGTCGG-3′ |
| SOE-pcycB_lacZ-F1 | 5′-GCACGTAATCAGCCACGATGACCATGATTACGCCAAGCTTGCATGCCT-3′ |
| SOE-pcycB_lacZ-R1 | 5′-CTTGGCGTAATCATGGTCATCGTGGCTGATTACGTGCGCCCGCGGC-3′ |
| SOE-pcycB_lacZ-F2 | 5′-GCCGCGGGCGCACGTAATCAGCCACGATGACCATGATTACGCCAAG-3′ |
| SOE-pcycB_lacZ-R2 | 5′-AGGCATGCAAGCTTGGCGTAATCATGGTCATCGTGGCTGATTACGTGC-3′ |
| paph-F-EcoRV | 5′-CGCGATATCCTGCAGGTAGCCGAACACCTCCAGGTCCC-3′ |
| pcydA-F-EcoRV | 5′-CGCGATATCACTCCGGCGCATTTCTAGCGGCCGCCGAAGTTCT-3′ |
| pcycB-F-EcoRV | 5′-CGCGATATCACTCCGGCGCATTTCTAGCGGCCGCCGAAGTTCT-3′ |
| paph-F | 5′-GGATCCGTCGAGCTCCCGGGAAGCT-3′ |
| paph-R | 5′-GCGAAACGATCCTCATCCTGTCTCTTGATCAGATCTTGATCC-3′ |
| pcydAB-F | 5′-ACTCCGGCGCATTTCTAGCGGCCGCCGAAGTTCT-3′ |
| pcydAB-R | 5′-CAGGGACTCTCCTCGGTCGGGGATTGATCTTGCACTGCC-3′ |
| pcycB-F | 5′-ACTCCGGCGCATTTCTAGCGGCCGCCGAAGTTCT-3′ |
| pcycB-R | 5′-CGTGGCTGATTACGTGCGCCCGCGGC-3′ |
| nifA-F | 5′-CGCGACGTGGTCTCGCTGAC-3′ |
| nifA-R | 5′-ATCACCCGTTCGCGGTCGTC-3′ |
| nifH-F | 5′-GTCTGGGCGGCCTGATCTGC-3′ |
| nifH-R | 5′-GTCACGCGGCACGAAGTGGA-3′ |
| rnfA1-F | 5′-CGAGATGATCATCCGCAAGGCCAGCCCGTCGCTGTA-3′ |
| rnfA1-R | 5′-GCCTTCGCGCACGCTGAGCAGCGGTACG-3′ |
| rnfD1-F | 5′-GCTCTATCAGCTGACCTCCGGCGGCCTGATACTGTGC-3′ |
| rnfD1-R | 5′-CGTAGATCAGCACGCCGCAGCCCACACCGAAGA-3′ |
| gyrB-F | 5′-ACGGCGTCGGTGTGGAGGTC-3′ |
| gyrB-R | 5′-CCAGATGGGTGCCACCGTCA-3′ |
Restriction sites are indicated in boldface type.
Ammonium quantification.
Samples of cultures were taken at different times and centrifuged (14,000 × g for 5 min). The cell pellets were used for protein quantification, and the filtered supernatants (through cellulose acetate membranes with a pore size of 0.25 μm) were used for ammonium quantification. Appropriate amounts of the filtered supernatant (filtration through cellulose acetate membranes with a pore size of 0.25 μm) were tested for the presence of ammonium by the indophenol method (45). This consisted of the addition, in order, of 0.5 mL of a phenol-sodium nitroprusside solution (50 g/L phenol, 0.25 g/L sodium nitroprusside), 0.5 mL of a sodium hypochlorite solution (0.1 M), and 0.1 mL of the sample. The mixture was incubated for 30 min at room temperature. The absorbance at 625 nm was measured, and the ammonium concentration was estimated from a standard curve obtained with ammonium chloride solutions at various concentrations assayed with the same reagent solutions. Moles of ammonium present in the supernatant fraction were reported in relation to the total amount of protein in the cell biomass.
Protein quantification.
Harvested cell pellets were disrupted by one cycle of sonication (7 W for 50 s) (ultrasonic homogenizer, model 3000; Biologics, Inc., Cary, NC, USA). Protein assays were performed on the same cell lysate for each time point and tested condition. Protein was quantified using the Coomassie protein assay from Thermo Scientific (Waltham, MA, USA). Thirty microliters of the sample was mixed with 1.5 mL of Thermo Scientific reagent and incubated at room temperature for 10 min. The absorbance at 595 nm was measured using a spectrophotometer (Thermo Spectronic BioMate 3; Thermo Scientific). The protein content of the sample was calculated using a standard curve (albumin standard used as described by the manufacturer).
β-Galactosidase assay.
β-Galactosidase activity was determined using an assay adapted from a method described previously by Miller (46). Cells were grown in sucrose-containing medium to mid-log or late log phase, and assays were conducted using the soluble fraction of crude extracts prepared by sonication and centrifugation. Relative β-galactosidase activities represent the specific rates of the absorbance change at 414 nm for the experimental samples divided by that for the control sample.
Nitrogenase activity.
To determine nitrogenase activity, acetylene reduction assays were conducted on freshly grown cultures by using a derivation of a previously described protocol (47). Cultured cells were transferred into a glass serum vial fitted with a rubber septum. Ten percent of the headspace gas was replaced with acetylene, and the culture was incubated for 60 min with shaking at 200 rpm at 30°C. An aliquot of the headspace was removed and analyzed for ethylene by using a GC Trace 1300 gas chromatograph with a flame ionization detector (Thermo Scientific). Gases were separated on a TG-Bond Q column (30 m by 0.32 mm by 10.0 μm). The injector, detector, and oven temperatures were 200°C, 230°C, and 30°C, respectively. Nitrogenase activity was quantified against a standard curve prepared with ethylene gas as a standard.
RNA extraction.
Total RNA was isolated from A. vinelandii cells using the RNeasy minikit (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. Genomic DNA was removed from RNA samples by DNase treatment (RNase-free DNase I; Ambion, Grand Island, NY, USA) for 30 min at 37°C. The Qiagen RNeasy MinElute kit (Qiagen) was used to purify DNase-treated total RNA from degraded DNA, DNase, contaminating proteins, and potential inhibitors of the reverse transcriptase reaction. The concentration of the eluted RNA was determined with a NanoDrop analyzer.
Reverse transcription reactions.
First-strand cDNA synthesis was primed from the purified total RNA template using specific reverse primers (Table 3). The reverse transcription reaction was performed using the reverse transcriptase SuperScript III kit (Invitrogen, Grand Island, NY, USA), as described by the manufacturer. The specific reverse primers were annealed to 250 ng of total RNA and extended for 1 h at 55°C using 200 U of SuperScript III reverse transcriptase.
Reverse transcriptase quantitative PCR.
Steady-state levels of specific mRNA transcripts from each sample were quantified by absolute reverse transcriptase quantitative PCR (RT-qPCR) using the engine Rotor-Gene Q system (Qiagen, Hilden, Germany). One microliter of single-stranded cDNA from the reverse transcriptase reaction mixture (see above) was used as the template for the RT-qPCR experiments. The RT-qPCR amplifications were performed using reagents from the DyNAmo SYBR green real-time PCR kit (Finnzymes, Lafayette, CO, USA). Specific primers were designed to amplify gene regions consisting of 90 to 110 nucleotides. The primers used for RT-qPCR (nifA-F, nifA-R, nifH-F, nifH-R, rnfA1-F, rnfA1-R, rnfD1-F, rnfD1-R, gyrB-F, and gyrB-R) (Table 3) were designed using Primer3 software (48). Amplification by RT-qPCR of single products of the expected sizes was verified on 2% (wt/vol) agarose gels, and the specificity of RT-qPCR products was confirmed by sequencing. Melting-curve analyses were performed on all RT-qPCR products to ensure that single DNA species were amplified. RT-qPCR amplifications were performed using the following cycling parameters: an initial single step at 95°C for 10 min (denaturation) was followed by 40 cycles of 94°C for 10 s (denaturation), 70°C for 20 s (primer annealing), and 72°C for 30 s (elongation). A final single step at 72°C for 1 min followed these 40 cycles. The relative expression ratio of a target gene was calculated based on the 2−ΔΔCT method (49), using the average cycle threshold (CT) calculated from triplicate measurements. Relative expression ratios from three independent experiments are reported. gyrB (Avin_00040) was used as a constitutive control gene for normalization. Relative abundances under each tested culture condition were then standardized to those under the BN medium control conditions.
Sterilization and germination of rice seeds.
For all plant experiments, the outer coat of rice seeds was removed prior to surface sterilization in 2% bleach for 15 min. Rice seeds were washed five times with sterile deionized water and then imbibed overnight at room temperature. After imbibition, the rice seeds were spread onto sterile wet Anchor 38# regular-weight seed germination paper in petri dishes and incubated at room temperature for 3 days. Germinated rice seeds were then transferred into germination pouches and kept in growth chambers for a week under 16 h of light and 8 h of darkness at 22°C. MilliQ water was added to the germination pouch buckets to keep some moisture to avoid drying the seedlings.
Coculture of rice seedlings and A. vinelandii strains.
After 1 week in growth chambers, rice seedlings were inoculated with bacterial strains (wild type, DJ100, AvFM2, AvFM16, and AvFM17) grown in Burk’s medium at 180 rpm at 30°C for 48 h and adjusted to an OD600 of 1.0 using fresh B medium. Pouches containing inoculated rice seedlings were then transferred in sealed Supelco push-pull gasbags, and 2% 15N2 or 14N2 gas was added to each bag. Each bag was placed into growth chambers for 1 week at 22°C (16 h of light and 8 h of darkness).
Sampling for isotope ratio mass spectrometry.
After a week of coculture, the shoots were harvested and dried at 65°C for 3 days. Dried shoots were powdered using metal balls and a bead beater (Mixer Mill MM 400; Retsch). The powdered samples were weighed prior to their submission to the mass spectrometry facility of the Department of Soil Science at the University of Wisconsin–Madison.
Data availability.
The genome sequence of Azotobacter vinelandii strain DJ has been deposited in the NCBI GenBank under accession number CP001157.1. A. vinelandii genes used in this study are as follows: nifL (Avin_50990), nifA (Avin_51000), nifH (Avin_01380), rnfA1 (Avin_50980), rnfD1 (Avin_50950), gyrB (Avin_00040; https://www.genome.jp/entry/avn:Avin_00040), cydAB (Avin_19910; https://www.genome.jp/entry/avn:Avin_19910), and cycB (Avin_47940; https://www.genome.jp/entry/avn:Avin_47940). Other relevant data supporting the findings of this study are available within this article and its associated supplemental material.
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation (grant number NSF-1331098). Partial salary support for J.W.P. was provided by the U.S. Department of Agriculture National Institute of Food and Agriculture, Hatch umbrella project number 1015621.
F.M. performed the experiments and analyzed the data. F.M. conceived the study and designed the experiments with input from all of the authors. D.K. performed the 15N incorporation experiments and analyzed the data. A.M.M. performed the acetylene reduction assays. E.R. performed the experiments. F.M. wrote the manuscript with input from all of the authors.
F.M., D.K., J.-M.A., and J.W.P. have filed a PCT application (number PCT/US2020/051368) and a U.S. utility application (number 17/024746) on this work.
Footnotes
Supplemental material is available online only.
Contributor Information
Florence Mus, Email: florence.mus@wsu.edu.
John W. Peters, Email: jw.peters@wsu.edu.
Jennifer B. Glass, Georgia Institute of Technology
REFERENCES
- 1.Borlaug NE. 1997. Feeding a world of 10 billion people: the miracle ahead. Biotechnol Dynamo Equip 11:3–13. 10.1080/13102818.1997.10818934. [DOI] [Google Scholar]
- 2.Erisman JW, Galloway JN, Dise NB, Sutton MA, Bleeker A, Grizzetti B, Leach AM, De Vries W. 2015. Nitrogen: too much of a vital resource. WWF science brief NL. WWF Netherlands, Zeist, The Netherlands. [Google Scholar]
- 3.Mus F, Crook MB, Garcia K, Garcia Costas A, Geddes BA, Kouri ED, Paramasivan P, Ryu M-H, Oldroyd GED, Poole PS, Udvardi MK, Voigt CA, Ané J-M, Peters JW. 2016. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl Environ Microbiol 82:3698–3710. 10.1128/AEM.01055-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colnaghi R, Green A, He L, Rudnick P, Kennedy C. 1997. Strategies for increased ammonium production in free-living or plant associated nitrogen fixing bacteria. Plant Soil 194:145–154. 10.1023/A:1004268526162. [DOI] [Google Scholar]
- 5.Morett E, Segovia L. 1993. The sigma 54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J Bacteriol 175:6067–6074. 10.1128/jb.175.19.6067-6074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Studholme DJ, Dixon R. 2003. Domain architectures of sigma 54-dependent transcriptional activators. J Bacteriol 185:1757–1767. 10.1128/JB.185.6.1757-1767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhulin IB, Taylor BL, Dixon R. 1997. PAS domain S-boxes in archaea, bacteria and sensors for oxygen and redox. Trends Biochem Sci 22:331–334. 10.1016/S0968-0004(97)01110-9. [DOI] [PubMed] [Google Scholar]
- 8.Taylor BL, Zhulin IB. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev 63:479–506. 10.1128/MMBR.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slavny P, Little R, Salinas P, Clarke TA, Dixon R. 2010. Quaternary structure changes in a second Per-Arnt-Sim domain mediate intramolecular redox signal relay in the NifL regulatory protein. Mol Microbiol 75:61–75. 10.1111/j.1365-2958.2009.06956.x. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Argudo I, Little R, Dixon R. 2004. Role of the amino‐terminal GAF domain of the NifA activator in controlling the response to the antiactivator protein NifL. Mol Microbiol 52:1731–1744. 10.1111/j.1365-2958.2004.04089.x. [DOI] [PubMed] [Google Scholar]
- 11.Wootton JC, Drummond MH. 1989. The Q-linker: a class of interdomain sequences found in bacterial multidomain regulatory proteins. Protein Eng 2:535–543. 10.1093/protein/2.7.535. [DOI] [PubMed] [Google Scholar]
- 12.Little R, Martinez-Argudo I, Perry S, Dixon R. 2007. Role of the H domain of the histidine kinase-like protein NifL in signal transmission. J Biol Chem 282:13429–13437. 10.1074/jbc.M610827200. [DOI] [PubMed] [Google Scholar]
- 13.Blanco G, Drummond M, Woodley P, Kennedy C. 1993. Sequence and molecular analysis of the nifL gene of Azotobacter vinelandii. Mol Microbiol 9:869–879. 10.1111/j.1365-2958.1993.tb01745.x. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, Saha SK, Tomomori C, Ishima R, Liu D, Tong KI, Park H, Dutta R, Qin L, Swindells MB, Yamazaki T, Ono AM, Kainosho M, Inouye M, Ikura M. 1998. NMR structure of the histidine kinase domain of the E. coli osmosensor EnvZ. Nature 396:88–92. 10.1038/23968. [DOI] [PubMed] [Google Scholar]
- 15.Dutta R, Inouye M. 2000. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem Sci 25:24–32. 10.1016/S0968-0004(99)01503-0. [DOI] [PubMed] [Google Scholar]
- 16.West AH, Stock AM. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem Sci 26:369–376. 10.1016/S0968-0004(01)01852-7. [DOI] [PubMed] [Google Scholar]
- 17.Wolanin PM, Thomason PA, Stock JB. 2002. Histidine protein kinases: key signal transducers outside the animal kingdom. Genome Biol 3:REVIEWS3013. 10.1186/gb-2002-3-10-reviews3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry S, Shearer N, Little R, Dixon R. 2005. Mutational analysis of the nucleotide-binding domain of the anti-activator NifL. J Mol Biol 346:935–949. 10.1016/j.jmb.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Little R, Reyes-Ramirez F, Zhang Y, van Heeswijk WC, Dixon R. 2000. Signal transduction to the Azotobacter vinelandii NIFL-NIFA regulatory system is influenced directly by interaction with 2-oxoglutarate and the PII regulatory protein. EMBO J 19:6041–6050. 10.1093/emboj/19.22.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill S, Austin S, Eydmann T, Jones T, Dixon R. 1996. Azotobacter vinelandii NIFL is a flavoprotein that modulates transcriptional activation of nitrogen-fixation genes via a redox-sensitive switch. Proc Natl Acad Sci USA 93:2143–2148. 10.1073/pnas.93.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bali A, Blanco G, Hill S, Kennedy C. 1992. Excretion of ammonium by a nifL mutant of Azotobacter vinelandii fixing nitrogen. Appl Environ Microbiol 58:1711–1718. 10.1128/aem.58.5.1711-1718.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eydmann T, Söderbäck E, Jones T, Hill S, Austin S, Dixon R. 1995. Transcriptional activation of the nitrogenase promoter in vitro: adenosine nucleosides are required for inhibition of NIFA activity by NIFL. J Bacteriol 177:1186–1195. 10.1128/jb.177.5.1186-1195.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narberhaus F, Lee HS, Schmitz RA, He L, Kustu S. 1995. The C-terminal domain of NifL is sufficient to inhibit NifA activity. J Bacteriol 177:5078–5087. 10.1128/jb.177.17.5078-5087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz RA. 2006. NifL of Klebsiella pneumoniae carries an N-terminally bound FAD cofactor, which is not directly required for the inhibitory function of NifL. FEMS Microbiol Lett 157:313–318. 10.1111/j.1574-6968.1997.tb12791.x. [DOI] [PubMed] [Google Scholar]
- 25.Söderbäck E, Reyes-Ramirez F, Eydmann T, Austin S, Hill S, Dixon R. 1998. The redox- and fixed nitrogen-responsive regulatory protein NIFL from Azotobacter vinelandii comprises discrete flavin and nucleotide-binding domains. Mol Microbiol 28:179–192. 10.1046/j.1365-2958.1998.00788.x. [DOI] [PubMed] [Google Scholar]
- 26.Toukdarian A, Kennedy C. 1986. Regulation of nitrogen metabolism in Azotobacter vinelandii: isolation of ntr and glnA genes and construction of ntr mutants. EMBO J 5:399–407. 10.1002/j.1460-2075.1986.tb04225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Little R, Colombo V, Leech A, Dixon R. 2002. Direct interaction of the NifL regulatory protein with the GlnK signal transducer enables the Azotobacter vinelandii NifL-NifA regulatory system to respond to conditions replete for nitrogen. J Biol Chem 277:15472–15481. 10.1074/jbc.M112262200. [DOI] [PubMed] [Google Scholar]
- 28.Brewin B, Woodley P, Drummond M. 1999. The basis of ammonium release in nifL mutants of Azotobacter vinelandii. J Bacteriol 181:7356–7362. 10.1128/JB.181.23.7356-7362.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barney BM, Eberhart LJ, Ohlert JM, Knutson CM, Plunkett MH. 2015. Gene deletions resulting in increased nitrogen release by Azotobacter vinelandii: application of a novel nitrogen biosensor. Appl Environ Microbiol 81:4316–4328. 10.1128/AEM.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortiz-Marquez JCF, Do Nascimento M, de los Angeles Dublan M, Curatti L. 2012. Association with an ammonium-excreting bacterium allows diazotrophic culture of oil-rich eukaryotic microalgae. Appl Environ Microbiol 78:2345–2352. 10.1128/AEM.06260-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitra R, Das HK, Dixit A. 2005. Identification of a positive transcription regulatory element within the coding region of the nifLA operon in Azotobacter vinelandii. Appl Environ Microbiol 71:3716–3724. 10.1128/AEM.71.7.3716-3724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batista MB, Brett P, Appia-Ayme C, Wang YP, Dixon R. 2021. Disrupting hierarchical control of nitrogen fixation enables carbon-dependent regulation of ammonia excretion in soil diazotrophs. PLoS Genet 17:e1009617. 10.1371/journal.pgen.1009617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryu MH, Zhang J, Toth T, Khokhani D, Geddes BA, Mus F, Garcia-Costas A, Peters JW, Poole PS, Ané JM, Voigt CA. 2020. Control of nitrogen fixation in bacteria that associate with cereals. Nat Microbiol 5:314–330. 10.1038/s41564-019-0631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barney BM, Plunkett MH, Natarajan V, Mus F, Knutson CM, Peters JW. 2017. Transcriptional analysis of an ammonium-excreting strain of Azotobacter vinelandii deregulated for nitrogen fixation. Appl Environ Microbiol 83:e01534-17. 10.1128/AEM.01534-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curatti L, Brown CS, Ludden PW, Rubio LM. 2005. Genes required for rapid expression of nitrogenase activity in Azotobacter vinelandii. Proc Natl Acad Sci USA 102:6291–6296. 10.1073/pnas.0501216102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moshiri F, Chawla A, Maier RJ. 1991. Cloning, characterization, and expression in Escherichia coli of the genes encoding the cytochrome d oxidase complex from Azotobacter vinelandii. J Bacteriol 173:6230–6241. 10.1128/jb.173.19.6230-6241.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rey L, Maier RJ. 1997. Cytochrome c terminal oxidase pathways of Azotobacter vinelandii: analysis of cytochrome c4 and c5 mutants and up-regulation of cytochrome c-dependent pathways with N2 fixation. J Bacteriol 179:7191–7196. 10.1128/jb.179.22.7191-7196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson DC, Unciuleac MC, Dean DR. 2006. Controlled expression and functional analysis of iron-sulfur cluster biosynthetic components within Azotobacter vinelandii. J Bacteriol 188:7551–7561. 10.1128/JB.00596-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortiz-Marquez JC, Do Nascimento M, Curatti L. 2014. Metabolic engineering of ammonium release for nitrogen-fixing multispecies microbial cell-factories. Metab Eng 23:154–164. 10.1016/j.ymben.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Ambrosio R, Ortiz-Marquez JCF, Curatti L. 2017. Metabolic engineering of a diazotrophic bacterium improves ammonium release and biofertilization of plants and microalgae. Metab Eng 40:59–68. 10.1016/j.ymben.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Setten L, Soto G, Mozzicafreddo M, Fox AR, Lisi C, Cuccioloni M, Angeletti M, Pagano E, Díaz-Paleo A, Ayub ND. 2013. Pseudomonas protegens Pf-5 for nitrogen fixation and its application to improve plant growth under nitrogen-deficient conditions. PLoS One 8:e63666. 10.1371/journal.pone.0063666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Setubal JC, dos Santos P, Goldman BS, Ertesvåg H, Espin G, Rubio LM, Valla S, Almeida NF, Balasubramanian D, Cromes L, Curatti L, Du Z, Godsy E, Goodner B, Hellner-Burris K, Hernandez JA, Houmiel K, Imperial J, Kennedy C, Larson TJ, Latreille P, Ligon LS, Lu J, Maerk M, Miller NM, Norton S, O’Carroll IP, Paulsen I, Raulfs EC, Roemer R, Rosser J, Segura D, Slater S, Stricklin SL, Studholme DJ, Sun J, Viana CJ, Wallin E, Wang B, Wheeler C, Zhu H, Dean DR, Dixon R, Wood D. 2009. Genome sequence of Azotobacter vinelandii, an obligate aerobe specialized to support diverse anaerobic metabolic processes. J Bacteriol 191:4534–4545. 10.1128/JB.00504-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabor S, Richardson CC. 1985. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA 82:1074–1078. 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Page WJ, von Tigerstrom M. 1979. Optimal conditions for transformation of Azotobacter vinelandii. J Bacteriol 139:1058–1061. 10.1128/jb.139.3.1058-1061.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergersen FJ (ed). 1980. Methods for evaluating biological nitrogen fixation. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 46.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 47.Klugkist J, Haaker H. 1984. Inhibition of nitrogenase activity by ammonium chloride in Azotobacter vinelandii. J Bacteriol 157:148–151. 10.1128/jb.157.1.148-151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. 2012. Primer3—new capabilities and interfaces. Nucleic Acids Res 40:e115. 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 50.Robinson AC, Burgess BK, Dean DR. 1986. Activity, reconstitution, and accumulation of nitrogenase components in Azotobacter vinelandii mutant strains containing defined deletions within the nitrogenase structural gene cluster. J Bacteriol 166:180–186. 10.1128/jb.166.1.180-186.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 and Fig. S1 to S3 and supplemental information. Download aem.01876-21-s0001.pdf, PDF file, 0.6 MB (572.8KB, pdf)
Data Availability Statement
The genome sequence of Azotobacter vinelandii strain DJ has been deposited in the NCBI GenBank under accession number CP001157.1. A. vinelandii genes used in this study are as follows: nifL (Avin_50990), nifA (Avin_51000), nifH (Avin_01380), rnfA1 (Avin_50980), rnfD1 (Avin_50950), gyrB (Avin_00040; https://www.genome.jp/entry/avn:Avin_00040), cydAB (Avin_19910; https://www.genome.jp/entry/avn:Avin_19910), and cycB (Avin_47940; https://www.genome.jp/entry/avn:Avin_47940). Other relevant data supporting the findings of this study are available within this article and its associated supplemental material.