Abstract
Background
Prostate cancer is a common cause of death in developed countries, yet the benefits of screening for prostate cancer still remain controversial. A prostate‐specific antigen (PSA) test result greater than 4 ng/mL (nanograms/millilitre) has commonly been used as the cut‐off level for seeking further tests to diagnose the presence (or absence) of prostate cancer. An increase in PSA levels may not necessarily be associated with an increased risk of prostate cancer, as PSA levels may also be increased in men with benign prostatic hyperplasia and prostatitis. Despite the uncertainty of the net benefit of early detection and treatment, safe and effective methods to prevent prostate cancer are of value. Consumers, seeking greater involvement in their healthcare, are increasingly turning to lifestyle modification and complementary and alternative medicines (CAMs) to maintain their health and prevent disease. Lycopene is a member of the carotenoid family, which is found abundantly in tomatoes, tomato‐based products, strawberries, and watermelon. It has been hypothesised that lycopene is a strong antioxidant, which may lower the risk of cancer (including prostate cancer) in people who have diets rich in lycopene.
Objectives
To determine whether lycopene reduces the incidence of prostate cancer and prostate cancer‐specific mortality. Secondary objectives include changes in PSA levels, prostate symptoms and the nature of adverse events associated with lycopene use.
Search methods
Electronic searches were conducted across MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) databases. No language or other limitations were imposed.
Selection criteria
Randomised controlled trials (RCTs) that investigated the use of lycopene for the prevention of prostate cancer were eligible for inclusion in this review.
Data collection and analysis
A search of electronic databases, performed in August 2011, identified 64 citations. All articles were selected for full‐text review. From these citations, three studies were identified as meeting the inclusion criteria. Handsearching did not provide any additional studies.
Main results
Three RCTs, with a total of 154 participants were included in this review. None of the studies reported data on prostate cancer mortality. All of the included studies differed with respect to design, participants included and allocation of lycopene. This clinical heterogeneity limits the value on the pooled estimated of the meta‐analyses. The methodological quality of two of the three included studies was assessed as posing a 'high' risk of bias. Meta‐analysis indicated no statistical difference in PSA levels between men randomised to receive lycopene and the comparison group (MD (mean difference) ‐0.34, 95% CI (confidence interval) ‐2.01 to 1.32). Only one study reported incidence of prostate cancer (10% in the lycopene group versus 30% in control group). The level of lycopene was also not statistically different in men randomised to receive lycopene and the comparison group (MD 0.39 µg/mL (micrograms/millilitre), 95% CI ‐0.19 to 0.98). No other meta‐analyses were possible since other outcomes assessed only had one study contributing data.
Authors' conclusions
Given that only three RCTs were included in this systematic review, and the high risk of bias in two of the three studies, there is insufficient evidence to either support, or refute, the use of lycopene for the prevention of prostate cancer. Similarly, there is no robust evidence from RCTs to identify the impact of lycopene consumption upon the incidence of prostate cancer, prostate symptoms, PSA levels or adverse events.
Plain language summary
Lycopene for the prevention of prostate cancer
Prostate cancer is a common form of cancer affecting men worldwide. Pharmaceutical interventions, such as 5‐alpha reductase inhibitors, have been identified as potentially preventing prostate cancer incidence in men. Many men modify lifestyle and consume complementary and alternative medicines to maintain better health and prevent disease. Lycopene is a supplement that has been suggested may assist in the prevention of prostate cancer due to its antioxidant effects. The objective of this systematic review was to identify the effectiveness of lycopene in the prevention of prostate cancer. This review identified 3 relevant studies, comprising 154 participants in total. Two of the studies were assessed to be of 'high' risk of bias. Meta‐analysis of two studies indicated no statistical difference in prostate specific antigen (PSA) levels between men randomised to receive lycopene and the comparison group (MD ‐0.34, 95% CI ‐2.01 to 1.32). None of the studies assessed prostate cancer mortality. No other meta‐analyses were possible since other outcomes assessed only had one study contributing data.
Background
Description of the condition
Prostate cancer is a common cause of death in developed countries with age standardised mortality rates ranging from 28 per 100,000 males in Norway and Sweden to 16 per 100,000 males in the US (AIHW 2007). A recent Cochrane systematic review concluded that screening for prostate cancer does not significantly decrease prostate cancer‐specific mortality (Ilic 2006; Ilic 2011). A variety of factors may contribute to the effectiveness of screening at the individual and population levels including; the accuracy of screening and diagnostic tests (Andriole 2009), uncertainty and variability in prostate cancer disease progression (Cordon‐Cordo 2007), and the impact of morbidities such as erectile dysfunction and incontinence that can occur as a consequence of common treatment methods (Stanford 2000). A prostate‐specific antigen (PSA) test result greater than 4 ng/mL has commonly been used as the cut‐off level for seeking further tests to diagnose the presence (or absence) of prostate cancer. An increase in PSA levels may not necessarily be associated with an increased risk of prostate cancer, as PSA levels may also be increased in men with benign prostatic hyperplasia and prostatitis (Hasui 1994). Despite the uncertainty of the net benefit of early detection and treatment, safe and effective methods to prevent prostate cancer are valued. Recent studies indicate that 5‐alpha reductase inhibitors, commonly used to treat uncomfortable lower urinary tract symptoms related to benign prostatic obstruction, may be beneficial in reducing prostate cancer incidence among men who undergo regular screening (Wilt 2008).
Consumers, seeking greater involvement in their healthcare, are increasingly turning to lifestyle‐based interventions and complementary and alternative medicines to maintain health and prevent disease, as evidenced through growth in the dietary supplements industry (Beebe‐Dimmer 2004). Motives range from an improvement in general health and well‐being, cures for cancer, improvement in quality of life and boosting of the immune system (Wilkinson 2008; Patterson 2002). CAMs are increasing being used by patients diagnosed with cancer. A 2005 study identified that up to 33% participants diagnosed with prostate cancer used some form of complementary and alternative medicine product or practice (Chan 2005). An overall rate of 25% was reported for ingested therapies, such as lycopene (Chan 2005).
Description of the intervention
Lycopene is a red pigment member of the carotenoid family found abundantly in tomatoes, tomato‐based products, strawberries, and watermelon (Najm 2008). Humans and other animals are unable to synthesise carotenoids and rely on an adequate consumption for their intake (Magri 2008). Consumption of lycopene through diet (including the 'Mediterranean diet) and supplements can contribute towards half of the carotenoids in the human serum (Ansari 2003).
In 2007 the World Cancer Research Fund reported that a high fruit and vegetable intake may be beneficial in reducing the risk of cancer, including lycopene for prostate cancer (WCRF 2007). Similarly, a study of women with a history of breast cancer concluded that a diet with increased vegetable and fruit intake was linked with a significantly reduced risk of cancer recurrence (Rock 2005).
How the intervention might work
Lycopene is an antioxidant whose actions prevent lipid oxidation in cells (Hwang 2005). It has been suggested that these antioxidant properties prevent carcinogenesis by protecting DNA, proteins, lipids and low density lipoproteins (Basu 2007). Molecular experiments have illustrated that the growth of human prostate cancer cells, which have been xenografted to immunosuppressed mice, is significantly suppressed when the diets of mice are supplemented with lycopene (Tang 2005).
Why it is important to do this review
Lycopene has been identified an antioxidant compound that potentially has a range of anti‐cancer properties, is abundant availability, is relatively low cost and has a lack of obvious side effects (Rackley 2006). A meta‐analysis of observational studies (including cohort, case‐control and nested‐case control) identified a 6% relative risk reduction in prostate cancer diagnosis in men consuming raw tomatoes and a 1% relative risk reduction in prostate cancer diagnosis in men consuming lycopene (Etminan 2004). The study authors concluded that tomato products may play a role in preventing the risk of prostate cancer (Etminan 2004). A recent Cochrane systematic review investigating selenium (which also has antioxidant properties) for preventing cancer identified a significant reduction in prostate cancer risk in men consuming selenium (OR (odds ratio) 0.78, 95% CI 0.66 to 0.92) (Dennert 2011). With increased consumer awareness of prostate cancer, many men may be attracted to consuming dietary supplements, such as lycopene, without the evidence to inform about its effectiveness about its cancer prevention properties and effectiveness.
Objectives
The primary objective of this systematic review is to determine whether lycopene reduces prostate cancer‐specific incidence and mortality. Secondary objectives included change in prostate specific antigen (PSA) levels, prostate symptoms and adverse events associated with lycopene use.
Methods
Criteria for considering studies for this review
Types of studies
All forms of randomised controlled trials (RCTs) were eligible for inclusion in this review. No language restrictions were placed on studies.
Types of participants
Adult (> 18 years) men of any ethnicity who had not previously been diagnosed with prostate cancer were eligible for inclusion in this review. Those with an increased risk of prostate cancer due to a family history of the disease or an elevated PSA level were included.
Types of interventions
Intervention included: dietary interventions aimed at increasing lycopene intake; lycopene supplements; and lycopene‐containing products used to prevent the development of prostate cancer. Studies employing any quantity of lycopene, taken over any duration of time and in combination with any other ingested supplements were included.
Types of outcome measures
Primary outcomes
The primary outcomes of this review were prostate cancer‐specific mortality and incidence of prostate cancer.
Secondary outcomes
Secondary outcomes measured included:
changes in PSA levels (doubling of PSA level from baseline for outcomes relating to increase in PSA levels, and halving of PSA levels compared to baseline for outcomes relating to decrease in PSA levels);
changes to prostate symptoms;
incidence of benign prostatic hyperplasia;
levels of lycopene; and
adverse events.
Search methods for identification of studies
Electronic searches
Electronic searches were conducted across MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) databases. No language or other limitations were imposed. The search strategy used for MEDLINE (and adopted for other databases) was:
exp Carotenoids/ or lycopene.mp. or exp Antioxidants/ or exp Lycopersicon esculentum/ or tomato*.mp.
prostat$ cancer.mp. or exp prostatic neoplasms/
exp Tertiary Prevention/ or exp Secondary Prevention/ or exp Primary Prevention/ or prevention.mp. or prevent*.mp.
1 and 2 and 3
limit 4 to humans
limit 5 to controlled trials
Searching other resources
Bibliographies of identified studies were searched for additional studies.
Data collection and analysis
Two reviewers (KF and DI) independently searched the identified studies for eligibility against a pre‐determined check list of inclusion criteria. A full text version of the article was obtained to assess if its title, or abstract, appears met the eligibility criteria. Studies were excluded if they failed to meet the inclusion criteria.
Selection of studies
Two reviewers (KF and DI) independently screened the titles and abstracts of all articles identified through the search strategy. Full papers of those that could not be excluded based on the title and abstract were retrieved and again screened based on the selection criteria. Articles that met the selection criteria were included.
Data extraction and management
Data extraction was conducted independently by two authors (KF and DI) using a data extraction form. The data extraction included information on the sample population (number of participants, demographic characteristics), method (intervention, setting, method of delivery, differences between intervention and control groups) and results.
Assessment of risk of bias in included studies
A risk of bias assessment was conducted independently by two authors (KF and DI) on all included trials, to appraise sequence generation, allocation concealment, blinding of participants and outcome assessors, outcome data, and selective‐outcome reporting. Each criterion was graded as 'met', 'unmet', 'unclear' or 'not appropriate'. A summary of the risk of bias assessment is presented in this review.
Measures of treatment effect
Statistical analysis was performed according to the statistical guidelines referenced in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Risk ratios (RR), with 95% confidence intervals (CI), were used to express dichotomous outcomes whilst continuous outcomes scores were expressed as mean differences with 95% confidence intervals.
Unit of analysis issues
No cluster RCTs were included in this systematic review.
Dealing with missing data
Missing data was dealt with by contacting the original study investigators to request the missing data, or provide further clarification on data. Analysis was performed on the available data in cases where the missing data was not available.
Assessment of heterogeneity
Heterogeneity was analysed by graphical interpretation of the forest plot and with the I2 statistic. An I2 value above 75% was considered to be an indicator of considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
Funnel plots were not graphed due to the small number of included studies.
Data synthesis
Pooled results of dichotomous outcomes were analysed using relative risk, utilising a fixed‐effects model. Continuous outcome measures were analysed using mean difference, utilising a fixed‐effects model. A random effects model was used where significant heterogeneity was indicated ('Analysis 1.7').
1.7. Analysis.
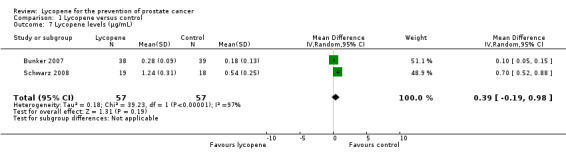
Comparison 1 Lycopene versus control, Outcome 7 Lycopene levels (µg/mL).
Subgroup analysis and investigation of heterogeneity
None of the planned subgroup analyses were performed as detailed in our protocol due to a lack of studies and data.
Sensitivity analysis
Sensitivity analysis was not performed to identify the robustness of results to trial quality since there were a small number of studies included in this review. Significant heterogeneity was identified in the analysis assessing lycopene levels ('Analysis 1.7'). This heterogeneity may be attributed to the differing doses used between the two studies (15 mg (milligrams) versus 30 mg).
Results
Description of studies
A total of three RCTs (n=154) (Bunker 2007;Mohanty 2005;Schwarz 2008) assessing the effectiveness of lycopene for the prevention of prostate cancer were identified as meeting the inclusion criteria for this systematic review. All of the included studies differed with respect to design, participants included and allocation of lycopene. Participants across the studies ranged from a total of 37 to 82 men. The studies included men from Germany, India and Trinidad and Tobago. Mean age was not provided for any of the studies; however ages ranged between 40 and 79 years. Length of follow up varied from 4 months, 6 months and 24 months in duration. Outcomes reported in the three studies included incidence of prostate cancer, change in PSA levels, change in prostate symptom score, incidence of benign prostatic hyperplasia and lycopene levels. For further detailed descriptive information about the studies refer to 'Characteristics of included studies' table.
Results of the search
A search of electronic databases was performed in August 2011. This search produced 64 citations, of which all were selected for full‐text review. Handsearching did not provide any additional studies. Of the 64 citations, three were eligible for inclusion in the review. The remaining 61 studies did not meet the eligibility criteria.
Included studies
Three RCTs were included in this review. See 'Characteristics of included studies' table for further details on the included studies.
Excluded studies
Studies were primarily excluded because they were not randomised controlled trials. Studies were also excluded because they did not meet other aspects of the eligibility criteria including not limiting participants to only men diagnosed with prostate cancer, and not having lycopene as the intervention. See table of 'Excluded studies' for further information.
Risk of bias in included studies
Assessment for risk of bias for each included study is described in the 'Characteristics of included studies' section. Risk of bias is also represented graphically in 'Figure 1' and 'Figure 2'. The risk of bias as determined for each included study is as follows.
1.
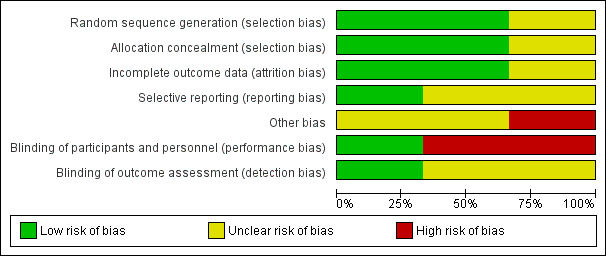
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
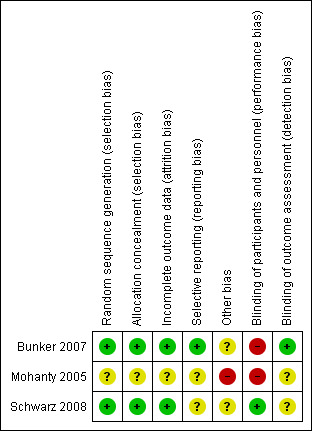
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Bunker 2007 ‐ 'high' risk of bias (no blinding of participants)
Mohanty 2005 ‐ 'high' risk of bias (no blinding of participants and lack of baseline demographic information)
Schwarz 2008 ‐ 'low' risk of bias (an 'unclear' risk of bias was given to blinding of outcome assessors and selective reporting of data)
In addition, none of the studies were sufficiently powered with respect to sample size to explore effects on primary and secondary outcomes. Both the Mohanty 2005 and Schwarz 2008 studies had less than 40 participants, whereas the Bunker 2007 study contained 82 participants. All studies also employed a highly specific eligibility criteria for recruiting participants, which affects the generalisability of results. The dose of lycopene across the three studies also differed significantly, ranging from 4 mg, 15 mg and 30 mg in dose. The composition of the lycopene intervention also differed across the studies, with some using a solely lycopene supplement, whilst other studies embedding the lycopene with other components within the pill. Participants also differed in terms of ingesting the pill ‐ with some studies requesting that participants take the pill twice a day (morning and night). The duration of ingesting the lycopene pills also differed significantly between the studies, ranging from 4 months, 6 months and 2 years.
Allocation
Sequence generation was clearly identified in the Bunker 2007 and Schwarz 2008 studies, whilst the Mohanty 2005 study did not provide any information on sequence generation. Only the Schwarz 2008 study described the method used for allocation concealment, whilst the study by other two studies did not provide sufficient information about methods used to account for allocation concealment. The authors of the Bunker 2007 study replied to correspondence about the trial and provided further information about the process used for randomisation and allocation concealment.
Blinding
Blinding of participants was only achieved in the Schwarz 2008 study, which was achieved by making the supplements identical in terms of taste, form, smell and appearance between the intervention and control groups. There was insufficient detail to determine whether blinding of outcome assessor was present. The Bunker 2007 study was an open trial, but did blind outcome assessors. The Mohanty 2005 study did not provide sufficient detail regarding blinding of participants or study personnel.
Incomplete outcome data
The Bunker 2007 and Schwarz 2008 studies provided complete data, with any withdrawal cited and explained. The Mohanty 2005 study did not address the issue of attrition bias.
Selective reporting
It was not possible to assess selective reporting for the three studies due to insufficient information. The authors of the Bunker 2007 trial reported no selective reporting of data.
Other potential sources of bias
No other potential sources of bias were identified.
Effects of interventions
Prostate cancer‐specific mortality
The impact of lycopene on prostate cancer‐specific mortality was not assessed by any of the three studies included in this systematic review.
Incidence of prostate cancer
Incidence of prostate cancer was only reported as an outcome in the Mohanty 2005 study. There was no significant difference between men randomised to the lycopene group and the comparison group (RR 0.33, 95% CI 0.08 to 1.46). The reported incidence of prostate cancer was 10% in the lycopene group versus 30% in control group.
PSA levels
PSA levels were reported in two of the included studies (Bunker 2007 and Schwarz 2008). Whilst there was a decrease in PSA levels between groups, the difference was not statistically significant (MD ‐0.34, 95% CI ‐2.01 to 1.32) ('Figure 3'). The Bunker 2007 study also reported decreases in PSA levels as an outcome. This study indicated no difference in the amount of men experiencing a decrease in PSA following their intervention, be it at one month post‐intervention or four months post‐intervention (RR 1.03, 95% CI 0.32 to 3.26). The Mohanty 2005 study reported increases in PSA levels as an outcome. This study indicated no difference in the amount of men experiencing an increase in PSA following their intervention (RR 0.67, 95% CI 0.29 to 1.52).
3.
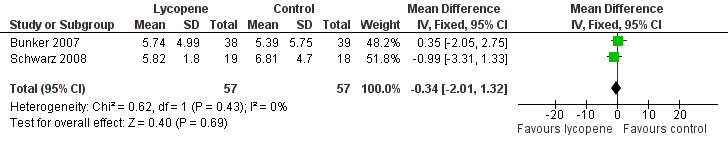
Forest plot of comparison: 1 Lycopene versus control, outcome: 1.2 PSA levels (ng/mL).
Adverse events
The study by Bunker 2007 reported no significant difference in adverse events experienced by participants in their study. One participant in the intervention group reported a heart attack. Nine men across both groups experienced indigestion/nausea throughout the trial, and seven men across both groups experienced diarrhoea. It was unknown whether these symptoms were attributable to the lycopene or multivitamin supplements in this study.
Lycopene levels
Lycopene levels were reported by two studies (Bunker 2007 and Schwarz 2008). Lycopene levels were significantly higher in the intervention groups (MD 0.39, 95% CI ‐0.19 to 0.98).
Prostate symptom score
The Schwarz 2008 study reported no difference between participants in prostate symptom score (as measured by the International Prostate Symptom Score Questionnaire) (MD 0.20, 95% CI ‐2.66 to 3.06).
Incidence of Benign Prostatic Hyperplasia
Incidence of benign prostatic hyperplasia (BPH) was recorded by the Mohanty 2005 study. It reported no significant difference in the incidence of BPH between study participants (RR 1.33, 95% CI 0.34 to 5.31).
Discussion
Summary of main results
Three RCTs, with a total of 154 participants were included in this review. None of the studies reported data on prostate cancer mortality. All of the included studies differed with respect to design, participants included and allocation of lycopene. This clinical heterogeneity limits the value on the pooled estimated of the meta‐analyses. The methodological quality of two of the three studies was assessed as posing a 'high' risk of bias. Meta‐analysis of two studies indicated no statistical difference in PSA levels between men randomised to receive lycopene and the comparison group (MD ‐0.34, 95% CI ‐2.01 to 1.32). The level of lycopene was also not statistically different in men randomised to receive lycopene and the comparison group (MD 0.39, 95% CI ‐0.19 to 0.98). No other meta‐analyses were possible since other outcomes assessed only had one study contributing data.
Overall completeness and applicability of evidence
There were several gaps in the reporting of criteria required for assessing the risk of bias of studies. All authors associated with studies that had an information gap were identified and contacted. Additional information about study information was only obtained from the authors of the Bunker 2007 study.
Quality of the evidence
The quality of the evidence was assessed using the approach outlined in 'Characteristics of included studies'. The body of evidence was classified as 'high', 'low', 'unclear' or 'not appropriate' risk of bias for each outcome. Risk of bias was assessed as 'high' for the majority of outcomes, as only the Schwarz 2008 study was classified to have a 'low' risk of bias. It is also noteworthy that the Schwarz 2008 study reported a positive effect of lycopene on selected outcomes.
Potential biases in the review process
This review primarily consisted of published data. The authors of the Mohanty 2005 study were contacted in order to obtain information regarding standard deviations for PSA and lycopene outcomes, but no reply was obtained. Future updated versions of the review will include more detailed analysis on primary and secondary outcomes as they become available.
Agreements and disagreements with other studies or reviews
A meta‐analysis of observational studies in 2004 identified 11 case‐control studies, five nested case‐control studies and five cohort studies, which examined the effectiveness of lycopene for the prevention of prostate cancer (Etminan 2004). The pooled relative risk for prostate cancer diagnosis across all studies was 0.99 (95% CI 0.93 to 1.06). The American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention suggest that eating five or more servings of vegetables and fruits each day (which may include lycopene) may protect against prostate cancer ‐ however, the effectiveness of which is still under investigation (Byers 2002). A systematic review of RCTs investigating lycopene supplementation in men with prostate cancer identified an inverse relationship between lycopene intake and PSA levels (Haseen 2009). The systematic review established that patients receiving lycopene reported lower cancer related symptoms, whilst also reporting no significant adverse events due to lycopene intake.
Authors' conclusions
Implications for practice.
The findings of this systematic review conclude that there is insufficient evidence to either support, or refute, the use of lycopene for the prevention of prostate cancer. Similarly, there is no robust evidence from RCTs to identify the impact of lycopene consumption upon the incidence of prostate cancer, prostate symptoms, PSA levels or adverse events. Given the lack of RCTs on this topic, clinicians and consumers may refer to the 2004 meta‐analysis of observational studies that identified a 1% relative risk reduction in the risk of prostate cancer diagnosis in men consuming lycopene (Etminan 2004).
It is also worth noting that the RCTs included in this systematic review relied on lycopene to be administered to men as supplements. Previous research has suggested that any beneficial effects from lycopene may be related to the antioxidants in the diet, rather than as supplements (Ahn 2005). Similarly, it may be the overall effect of a range of micronutrients rather than one which produces the benefit (Ahn 2005). Best estimates have suggested that the average daily intake ranges from 3.7 to 6.5 mg per day (Schweitzer 1999). It should be noted that the men who participated in the included studies received between 15 to 30 mg supplements of lycopene, without demonstrable improvement in primary and secondary outcomes.
Implications for research.
The increased number of men in the community consuming CAMs for the prevention of prostate cancer, and the current lack of high quality evidence, both support the call for a well designed, high methodological quality, randomised controlled trial to investigate the effectiveness lycopene for the prevention of prostate cancer. Such a trial should account for prostate cancer diagnosis, mortality, changes in PSA levels, adverse events, and cost‐effectiveness.
What's new
| Date | Event | Description |
|---|---|---|
| 25 October 2011 | Amended | A few slight edits. |
Acknowledgements
None.
Data and analyses
Comparison 1. Lycopene versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of prostate cancer | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.08, 1.46] |
| 2 PSA levels (ng/mL) | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐2.01, 1.32] |
| 3 Decrease in PSA levels | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.32, 3.26] |
| 4 Increase in PSA levels | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.29, 1.52] |
| 5 Prostate symptom score (IPSS) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐2.66, 3.06] |
| 6 Incidence of Benign Prostatic Hyperplasia | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.34, 5.21] |
| 7 Lycopene levels (µg/mL) | 2 | 114 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.19, 0.98] |
1.1. Analysis.
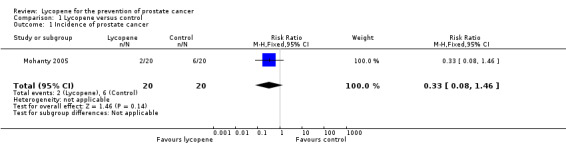
Comparison 1 Lycopene versus control, Outcome 1 Incidence of prostate cancer.
1.2. Analysis.
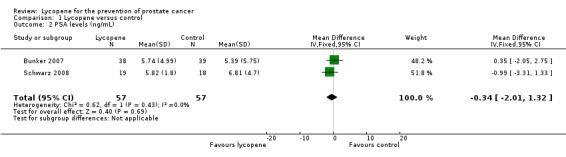
Comparison 1 Lycopene versus control, Outcome 2 PSA levels (ng/mL).
1.3. Analysis.
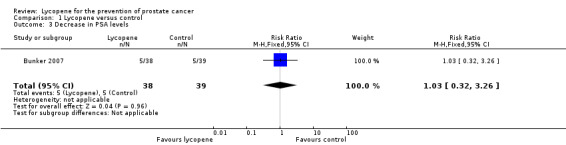
Comparison 1 Lycopene versus control, Outcome 3 Decrease in PSA levels.
1.4. Analysis.
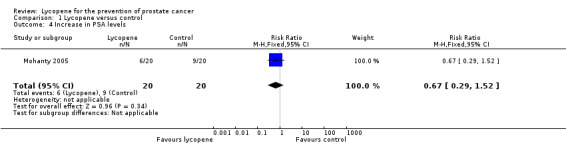
Comparison 1 Lycopene versus control, Outcome 4 Increase in PSA levels.
1.5. Analysis.
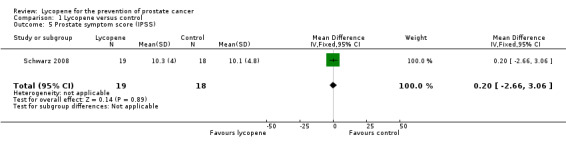
Comparison 1 Lycopene versus control, Outcome 5 Prostate symptom score (IPSS).
1.6. Analysis.
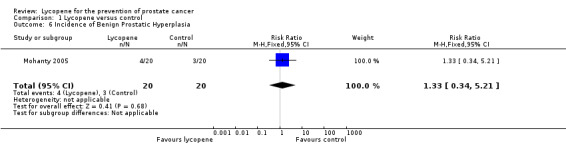
Comparison 1 Lycopene versus control, Outcome 6 Incidence of Benign Prostatic Hyperplasia.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bunker 2007.
| Methods | Randomised controlled trial in Tobago, Trinidad and Tobago. Individuals were randomised to receive either a multivitamin alone or the 30 mg/day lycopene plus multivitamin. This study reports on participants that were followed for a four month period from August to December 2003. | |
| Participants | Participants included men from the island of Tobago. The inclusion criteria for the trial were pathological evidence of HGPIN or atypical foci, or more than one non‐cancerous biopsy, no history of prostate cancer. Men were assigned sequential intervention study ID numbers at enrolment before randomisation. Numbers include:
|
|
| Interventions | All participants underwent a three week course of oral ciprofloxacin, 250 mg/day, prior to randomisation, to reduce the likelihood that serum PSA decline after lycopene administration might reflect an anti‐inflammatory response in men with subclinical prostatitis rather than cancer regression. Participants in the intervention received 'Lyc‐O‐Mato', which was 15 mg lycopene. The supplement was provided in two capsules (total 30 mg lycopene/day) with instructions to take one with breakfast, and one with the evening meal. A standard multivitamin with minerals was used daily in the multivitamin group (This multivitamin included vitamin A (vitamin A acetate and 40% as beta‐carotene), 5000 IU, Vitamin E (dl‐alpha tocopheryl acetate), 30 IU, vitamin C (as ascorbic acid), 60 milligrams (mg), and selenium (as sodium selenate), 20 micrograms (μg).) |
|
| Outcomes | PSA Serum samples were taken at baseline, 1 month and 4 months post‐randomisation. Patients were also assessed via the American Urological Association Benign Prostatic Hyperplasia (BPH) Scale and National Institutes of Health Chronic Prostatitis Symptom Index was assessed during these time points as well. | |
| Notes | Adverse events were also reported within the text. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a random number table and blocking by the trial statistician. "The randomization assignment for each ID was prepared in Pittsburgh by the Data Safety and Monitoring Board biostatistician (not a study investigator) using a random number table and blocking in groups of six to assign intervention group at randomization" |
| Allocation concealment (selection bias) | Low risk | Randomisation assignments were kept by a third party, but detail about how concealment was achieved was not provided. "the randomization assignments were sent to the study nurse in Tobago, who concealed the assignment until the randomization visit" Further contact with the authors of the study revealed that during the pre‐randomisation phase, the randomisation sequence prepared by the external biostatistician and was kept in the proverbial sealed envelope until the randomisation visit. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. "Eighty‐two participants were recruited to the study. One participant dropped out during the pre‐trial antibiotic run‐in. …one participant with pre‐randomization PSA of 64.8 ng/mL was excluded from the study…" |
| Selective reporting (reporting bias) | Low risk | Insufficient information to in paper to permit judgement of 'Yes' or 'No'. However, contact with made with the authors who stated that the study was free of selective reporting. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open label trial. "This study was a four‐month, randomized, open‐label, two‐arm clinical trial…" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This was an open label trial, however scientists analysing PSA serum were blind to randomisation. "The laboratory was blind to randomization group." Further contact with the authors identified that the serum tubes were labelled only by study ID and date of blood draw. There was no indication of intervention group on the serum tubes. The tubes were boxed in study ID numerical order and sent to the University of Pittsburgh Pathology lab for analysis of PSA by technicians with no connection to the study. |
Mohanty 2005.
| Methods | Randomised controlled trial at the Department of Urology in New Delhi, India. Individuals were randomised to receive either 4 mg lycopene, to be consumed twice a day for a year, or nothing (for the control participants). Both groups were followed for a two year period. | |
| Participants | A total of 40 patients with high‐grade prostatic intraepithelial neoplasia (HGPIN) were randomised into two groups. Both the intervention and control groups had equal number of participants with HGPIN, with grade II disease and HGPIN. Number include:
|
|
| Interventions | Participants randomised to the intervention group received 4 mg lycopene, twice a day for one year continuously. Participants in the control group were not given any interventions, but advised to reduce intake of tomato and melon. | |
| Outcomes | Outcomes assessed included changes in PSA levels, and incidence of BPH and prostate cancer. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of random sequence generation is provided. "There were 40 patients with HGPIN who were randomized into 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address the issue of attrition in the study. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'Yes' or 'No'. |
| Other bias | High risk | Insufficient information to assess whether an important risk of bias exists, however no baseline characteristics are provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of patients was not sufficiently described. The intervention group were given lycopene, whilst the control group were not given any medication (including a placebo). "All 20 patients in group A (study group) received 4 mg lycopene... None of the 20 patients in group B (control group) received any medication" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding outcome assessment personnel was not described. |
Schwarz 2008.
| Methods | Randomised controlled trial in Hohenheim, Stuttgart, Germany. Individuals were randomised to receive either a placebo or 15 mg/day lycopene. This study reports on participants that were followed for a 6‐month period from October 2004 to July 2005. | |
| Participants | Participants included men from Hohenheim. The inclusion criteria for the trial included a serum prostate‐specific antigen (PSA) concentration greater than 4.0 mg/L, histologically confirmed BPH, aged between 45 and 70 years and absence of acute illness. A total of 40 participants were initially recruited, with three participants dropping out during the course of the study. Two participants dropped out before commencing the supplement intake and one participant in the placebo group was excluded after 50 days due to an unexpected hospitalization due to factors not related to the trial (family member's death). Numbers include:
|
|
| Interventions | Lycopene supplements were provided as hard gelatin capsules containing 15 mg synthetic lycopene. A commercially available powder formulation containing 10% lycopene embedded in a matrix of gelatin and sucrose was used to fill the capsules. The placebo was a powder formulation without lycopene. | |
| Outcomes | The primary endpoint was defined as inhibition of the delta increase or decreased PSA levels in blood. Secondary endpoints were increases in the lycopene concentrations in blood and tissue (buccal mucosa cells (BMC)), reduced circulating insulin‐like growth factor (IGF‐1), and increases in IGF‐binding protein‐3 (IGF‐BP‐3) concentrations in blood. Additional variables measured were circulating concentrations of testosterone (free and bound), LDL cholesterol and total cholesterol, and blood glucose concentrations and routine hemograms. Additional examinations were digital rectal examination (DRE), trans‐rectal ultrasonography (TRUS) of the prostate and assessment of the International Prostate Symptom Score (IPSS). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved via computer generated randomisation. "Neither the study clinician nor any patients had access to the computer‐generated randomization plan." |
| Allocation concealment (selection bias) | Low risk | Allocation was based on a randomisation schedule, with sealed envelopes. "The study clinician was informed to allocate the supplements to the patients after inclusion into the study using increasing randomziation numbers. In case of an emergency, the treatment information was available at the center in sealed envelopes. The envelopes were returned after study termination and none of them had been opened." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. "A total of 40 patients entered the study. Two participants quit before beginning supplement intake. One participant in the placebo group was excluded after 50d due to an unexpected hospitalization resulting from a family member's death." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'Yes' or 'No'. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants was achieved. "The supplements were identical in form, taste, smell and appearance for lycopene and placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is insufficient information regarding blinding of investigators. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albanes 1995 | Not a lycopene intervention |
| Alkhenizan 2007 | Not a lycopene intervention |
| Allen 2009 | Not a lycopene intervention |
| Brawley 2001 | Not a RCT |
| Burri 2009 | Not exclusively a lycopene intervention and outcomes are not specific |
| Canby‐Hagino 2005 | Not a RCT |
| Chan 2000 | Not exclusively a lycopene intervention |
| Chlebowski 2010 | Not a RCT on lycopene use |
| Christen 2000 | Report of a study protocol |
| Cook 1999 | Not exclusively a lycopene intervention |
| Cook 2000 | Not exclusively a lycopene intervention |
| Costello 2001 | Not a RCT of a lycopene intervention |
| DeFrancesco 2001 | Descriptive report of prevention trial |
| Dennert 2011 | Systematic review on selenium |
| DePrimo 2001 | Not a RCT of a lycopene intervention |
| Druesne‐Pecollo 2010 | Systematic review on beta‐carotenes |
| Dunn 2010 | Relates to a selenium study |
| Ellinger 2006 | Descriptive report |
| Etminan 2005 | Systematic review on selenium |
| Fitzpatrick 2009 | Descriptive report |
| Frankel 2007 | Not related to lycopene |
| Gaziano 2009 | Not exclusively a lycopene intervention |
| Gey 1998 | Review |
| Gronberg 2003 | Descriptive report |
| Hatfield 2009 | Study relating to selenium |
| Heinonen 1994 | Does not include lycopene as an intervention |
| Heinonen 1998 | Not exclusively a lycopene intervention |
| Jiang 2010 | Meta‐analysis relating to selenium |
| Klein 2001 | RCT relating to selenium |
| Klein 2003 | RCT relating to selenium |
| Klein 2003b | RCT relating to selenium |
| Klein 2004 | Review article |
| Klein 2004b | Study relating to selenium |
| Lee 2006 | Report relating to Vitamin E |
| Li 2005 | Study not related to lycopene |
| Lin 2000 | Study not related to lycopene |
| Lippman 2005 | Study relating to selenium |
| Lippman 2009 | Not a single RCT |
| Marshall 2001 | RCT relating to selenium |
| Mayne 2005 | Report not related to lycopene |
| Meyer 2005 | Not exclusively a lycopene intervention |
| Nelson 2004 | Report not related to lycopene |
| Neuhouser 2009 | Does not include lycopene as an intervention |
| Pak 2002 | Study relating to selenium and Vitamin E |
| Pathak 2003 | Review article |
| Platz 2009 | Study relating to selenium and Vitamin E |
| Pryor 2000 | Study relating to Vitamin E |
| Rennert 2002 | Review article |
| Schröder 2005 | Does not meet inclusion criteria |
| Tan 2010 | Does not meet inclusion criteria |
| Thompson 2003 | Does not meet inclusion criteria |
| Trump 1994 | Does not meet inclusion criteria |
| Vaishampayan 2007 | Treatment group has existing prostate cancer |
| van Breemen 2005 | Treatment group has existing prostate cancer |
| Van Patten 2008 | Review article |
| Virtamo 2003 | Does not meet inclusion criteria |
| Watters 2009 | Does not meet inclusion criteria |
| Weinstein 2006 | Does not meet inclusion criteria |
| Wilkinson 2003 | Review article |
| Woodson 2002 | Does not meet inclusion criteria |
| Woodson 2003 | Does not meet inclusion criteria |
Differences between protocol and review
Change of contact author.
Contributions of authors
Dragan Ilic (DI), Kristian Forbes (KF) and Craig Hassed (CH) all initiated the review and wrote the protocol. DI and KF conducted the literature search, reviewed abstracts and full text studies for inclusion, performed quality assessment, data extraction, analysis and writing of the review. CH assisted with the inclusion of studies, quality assessment and contributed to the writing of the review.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bunker 2007 {published data only}
- Bunker C, McDonald A, Evans R, Rosa N, Boumosleh J, Patrick A. A randomized trial of lycopene supplementation in Tobago men with high prostate cancer risk. Nutrition and Cancer 2007;57:130‐7. [DOI] [PubMed] [Google Scholar]
Mohanty 2005 {published data only}
- Mohanty N, Saxena S, Singh U, Goyal N, Arora R. Lycopene as a chemopreventive agent in the treatment of high‐grade prostate intraepithelial neoplasia. Urologic Oncology: Seminars and Original Investigations 2005;23:383‐5. [DOI] [PubMed] [Google Scholar]
Schwarz 2008 {published data only}
- Schwarz S, Obermuller‐Jevic U, Hellmis E, Koch W, Jacobi G, Biesalski H. Lycopene inhibits disease progression in patients with Benign Prostate Hyperplasia. The Journal of Nutrition and Disease 2008;138:49‐53. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albanes 1995 {published data only}
- Albanes D, Heinonen OP, Huttunen JK, Taylor PR, Virtamo J, Edwards BK, et al. Effects of alpha‐tocopherol and beta‐carotene supplements on cancer incidence in the Alpha‐Tocopherol Beta‐Carotene Cancer Prevention Study. American Journal of Clinical Nutrition 1995;62:1427S‐30S. [DOI] [PubMed] [Google Scholar]
Alkhenizan 2007 {published data only}
- Alkhenizan A, Hafez K. The role of vitamin E in the prevention of cancer: a meta‐analysis of randomized controlled trials. Annals of Saudi Medicine 2007 2007;27:409‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2009 {published data only}
- Allen NE, Key TJ. Prostate cancer: neither vitamin E nor selenium prevents prostate cancer.. Nature Reviews Urology 2009;6:187‐188. [DOI] [PubMed] [Google Scholar]
Brawley 2001 {published data only}
- Brawley OW, Barnes S, Parnes H. The future of prostate cancer prevention. Annals of the New York Academy of Sciences 2001;952:145‐152. [DOI] [PubMed] [Google Scholar]
Burri 2009 {published data only}
- Burri BJ, Chapman MH, Neidlinger TR, Seo JS, Ishida BK. Tangerine tomatoes increase total and tetra‐cis‐lycopene isomer concentrations more than red tomatoes in healthy adult humans. International Journal of Food Sciences and Nutrition 2009;60:1‐16. [DOI] [PubMed] [Google Scholar]
Canby‐Hagino 2005 {published data only}
- Canby‐Hagino ED, Thompson IM. Mechanisms of disease: Prostate cancer‐a model for cancer chemoprevention in clinical practice. Nature Clinical Practice Oncology 2005;2:255‐261. [DOI] [PubMed] [Google Scholar]
Chan 2000 {published data only}
- Chan JM, Pietinen P, Virtanen M, Malila N, Tangrea J, Albanes D, Virtamo J. Diet and prostate cancer risk in a cohort of smokers, with a specific focus on calcium and phosphorus (Finland). Cancer Causes and Control 2000;11:859‐67. [DOI] [PubMed] [Google Scholar]
Chlebowski 2010 {published data only}
- Chlebowski RT, Menon R, Chaisanguanthum RM, Jackson DM. Prospective evaluation of two recruitment strategies for a randomized controlled cancer prevention trial. Clinical Trials 2010;7:744‐748. [DOI] [PubMed] [Google Scholar]
Christen 2000 {published data only}
- Christen WG, Gaziano JM, Hennekens CH. Design of Physicians' Health Study II‐a randomized trial of beta‐carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Annals of Epidemiology 2000;10:125‐34. [DOI] [PubMed] [Google Scholar]
Cook 1999 {published data only}
- Cook NR, Stampfer MJ, Ma J, Manson JE, Sacks FM, Buring JE, Hennekens CH. Beta‐carotene supplementation for patients with low baseline levels and decreased risks of total and prostate carcinoma. Cancer 1999;86:1783‐92. [PubMed] [Google Scholar]
Cook 2000 {published data only}
- Cook NR, Lee IM, Manson JE, Buring JE, Hennekens CH. Effects of beta‐carotene supplementation on cancer incidence by baseline characteristics in the Physicians' Health Study (United States). Cancer Causes and Control 2000;11:617‐26. [DOI] [PubMed] [Google Scholar]
Costello 2001 {published data only}
- Costello A. A randomized, controlled chemoprevention trial of selenium in familial prostate cancer: Rationale, recruitment, and design issues. Urology 2001;57:182‐184. [DOI] [PubMed] [Google Scholar]
DeFrancesco 2001 {published data only}
- DeFrancesco L. Prostate cancer prevention trial launched. Nature Medicine 2001;7:1076. [DOI] [PubMed] [Google Scholar]
Dennert 2011 {published data only}
- Dennert G, Zwahlen M, Brinkman M, Vinceti M, Zeegers MPA, Horneber M. Selenium for preventing cancer. Cochrane Database of Systematic Reviews 2011;5:CD005195. [DOI] [PMC free article] [PubMed] [Google Scholar]
DePrimo 2001 {published data only}
- DePrimo SE, Shinghal R, Vidanes G, Brooks J. Prevention of prostate cancer. Hematology ‐ Oncology Clinics of North America 2001;15:445‐457. [DOI] [PubMed] [Google Scholar]
Druesne‐Pecollo 2010 {published data only}
- Druesne‐Pecollo N, Latino‐Martel P, Norat T, Barrandon E, Bertrais S, Galan P, Hercberg S. Beta‐carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. International Journal of Cancer 2010;127:172‐184. [DOI] [PubMed] [Google Scholar]
Dunn 2010 {published data only}
- Dunn BK, Richmond ES, Minasian LM, Ryan AM, Ford L. A nutrient approach to prostate cancer prevention: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). Nutrition and Cancer 2010;62:896‐918. [DOI] [PubMed] [Google Scholar]
Ellinger 2006 {published data only}
- Ellinger S, Ellinger J, Stehle P. Tomatoes, tomato products and lycopene in the prevention and treatment of prostate cancer: do we have the evidence from intervention studies?. Current Opinion in Clinical Nutrition and Metabolic Care 2006;9:722‐727. [DOI] [PubMed] [Google Scholar]
Etminan 2005 {published data only}
- Etminan M, FitzGerald JM, Gleave M, Chambers K. Intake of selenium in the prevention of prostate cancer: a systematic review and meta‐analysis. Cancer Causes and Control 2005;16:1125‐1131. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 2009 {published data only}
- Fitzpatrick JM, Schulman C, Zlotta AR, Schroder F. Prostate cancer: a serious disease suitable for prevention. BJU International 2009;103:864‐870. [DOI] [PubMed] [Google Scholar]
Frankel 2007 {published data only}
- Frankel PH, Reid ME, Marshall J. A permutation test for a weighted Kaplan‐Meier estimator with application to the nutritional prevention of cancer trial. Contemporary Clinical Trials 2007;28:343‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaziano 2009 {published data only}
- Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA 2009;301:52‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gey 1998 {published data only}
- Gey K. Vitamins E plus C and interacting co‐nutrients required for optimal health. A critical and constructive review of epidemiology and supplementation data regarding cardiovascular disease and cancer.. Biofactors 1998;7:113‐174. [DOI] [PubMed] [Google Scholar]
Gronberg 2003 {published data only}
- Gronberg H. Prostate cancer epidemiology. Lancet 2003;361:859‐864. [DOI] [PubMed] [Google Scholar]
Hatfield 2009 {published data only}
- Hatfield DL, Gladyshev V. The Outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) reveals the need for better understanding of selenium biology.. Molecular Interventions 2009;9:18‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heinonen 1994 {published data only}
- Heinonen OP, Albanes D. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. New England Journal of Medicine 1994;330:1029‐35. [DOI] [PubMed] [Google Scholar]
Heinonen 1998 {published data only}
- Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, et al. Prostate cancer and supplementation with alpha‐tocopherol and beta‐carotene: incidence and mortality in a controlled trial. Journal of the National Cancer Institute 1998;90:440‐6. [DOI] [PubMed] [Google Scholar]
Jiang 2010 {published data only}
- Jiang L, Yang K‐h, Tian J‐h, Guan Q‐l, Yao N, Cao N, Mi D‐h, Wu J, Ma B, Yang S. Efficacy of antioxidant vitamins and selenium supplement in prostate cancer prevention: a meta‐analysis of randomized controlled trials.. Nutrition and Cancer 2010;62:719‐727. [DOI] [PubMed] [Google Scholar]
Klein 2001 {published data only}
- Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, Coltman C. SELECT: the next prostate cancer prevention trial. Selenum and Vitamin E Cancer Prevention Trial. Journal of Urology 2001;166:1311‐1315. [DOI] [PubMed] [Google Scholar]
Klein 2003 {published data only}
- Klein EA, Lippman SM, Thompson IM, Goodman PJ, Albanes D, Taylor PR, Coltman C. The selenium and vitamin E cancer prevention trial. World Journal of Urology 2003;21:21‐27. [DOI] [PubMed] [Google Scholar]
Klein 2003b {published data only}
- Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, Coltman C. SELECT: the selenium and vitamin E cancer prevention trial.. Urologic Oncology 2003;21:59‐65. [DOI] [PubMed] [Google Scholar]
Klein 2004 {published data only}
- Klein EA. Selenium and vitamin E cancer prevention trial. Annals of the New York Academy of Sciences 2004;1031:234‐41. [DOI: 10.1196/annals.1331.023] [DOI] [PubMed] [Google Scholar]
Klein 2004b {published data only}
- Klein EA, Thompson I. Update on chemoprevention of prostate cancer. Current Opinion in Urology 2004;14:143‐149. [DOI] [PubMed] [Google Scholar]
Lee 2006 {published data only}
- Lee IM, Gaziano JM, Buring JE. Vitamin E in the prevention of prostate cancer: where are we today?. Journal of the National Cancer Institute 2006;98:225‐227. [DOI] [PubMed] [Google Scholar]
Li 2005 {published data only}
- Li H, Kantoff PW, Giovannucci E, Leitzmann MF, Gaziano JM, Stampfer MJ, Ma J. Manganese superoxide dismutase polymorphism, prediagnostic antioxidant status, and risk of clinical significant prostate cancer. Cancer Research 2005;65:2498‐2504. [DOI] [PubMed] [Google Scholar]
Lin 2000 {published data only}
- Lin H, McCulloch CE, Turnbull BW, Slate EH, Clark L. A latent class mixed model for analysing biomarker trajectories with irregularly scheduled observations. Statistics in Medicine 2000;19:1303‐1318. [DOI] [PubMed] [Google Scholar]
Lippman 2005 {published data only}
- Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson IM, Jr, Kristal AR, Santella RM, Probstfield JL, Moinpour CM, Albanes D. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Journal of the National Cancer Institute 2005;97:94‐102. [DOI] [PubMed] [Google Scholar]
Lippman 2009 {published data only}
- Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009;301:39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2001 {published data only}
- Marshall J. Larry Clark's legacy: randomized controlled, selenium‐based prostate cancer chemoprevention trials. Nutrition and Cancer 2001;40:74‐77. [DOI] [PubMed] [Google Scholar]
Mayne 2005 {published data only}
- Mayne ST, Lippman S. Cigarettes: a smoking gun in cancer chemoprevention. Journal of the National Cancer Institute 2005;97:1319‐1321. [DOI] [PubMed] [Google Scholar]
Meyer 2005 {published data only}
- Meyer F, Galan P, Douville P, Bairati I, Kegle P, Bertrais S, et al. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. International Journal of Cancer 2005;116:182‐6. [DOI] [PubMed] [Google Scholar]
Nelson 2004 {published data only}
- Nelson WG, Marzo AM, DeWeese TL, Isaacs W. The role of inflammation in the pathogenesis of prostate cancer. Journal of Urology 2004;172:S6‐11. [DOI] [PubMed] [Google Scholar]
Neuhouser 2009 {published data only}
- Neuhouser ML, Barnett MJ, Kristal AR, Ambrosone CB, King IB, Thornquist M, et al. Dietary Supplement Use and Prostate Cancer Risk in the Carotene and Retinol Efficacy Trial. Cancer Epidemiology Biomarkers and Prevention 2009;18:2202‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pak 2002 {published data only}
- Pak RW, Lanteri VJ, Scheuch JR, Sawczuk I. Review of vitamin E and selenium in the prevention of prostate cancer: implications of the selenium and vitamin E chemoprevention trial. Integrative Cancer Therapies 2002;1:338‐344. [DOI] [PubMed] [Google Scholar]
Pathak 2003 {published data only}
- Pathak SK, Sharma RA, Mellon J. Chemoprevention of prostate cancer by diet‐derived antioxidant agents and hormonal manipulation (Review). International Journal of Oncology 2003;22:5‐13. [PubMed] [Google Scholar]
Platz 2009 {published data only}
- Platz E. Selenium, genetic variation, and prostate cancer risk: epidemiology reflects back on selenium and vitamin E cancer prevention trial. Journal of Clinical Oncology 2009;27:3569‐3572. [DOI] [PubMed] [Google Scholar]
Pryor 2000 {published data only}
- Pryor W. Vitamin E and heart disease: basic science to clinical intervention trials. Free Radical Biology and Medicine 2000;28:141‐164. [DOI] [PubMed] [Google Scholar]
Rennert 2002 {published data only}
- Rennert G. Dietary intervention studies and cancer prevention. European Journal of Cancer Prevention 2002;11:419‐25. [DOI] [PubMed] [Google Scholar]
Schröder 2005 {published data only}
- Schröder FH, Roobol MJ, Boevé ER, de Mutsert R, Zuijdgeest‐van Leeuwen SD, Kersten I, et al. Randomized, double‐blind, placebo‐controlled crossover study in men with prostate cancer and rising PSA: effectiveness of a dietary supplement. European Urology 2005;48:922‐31. [DOI] [PubMed] [Google Scholar]
Tan 2010 {published data only}
- Tan H‐L, Thomas‐Ahner JM, Grainger EM, Wan L, Francis DM, Schwartz SJ, Erdman JW, Jr, Clinton SK. Tomato‐based food products for prostate cancer prevention: what have we learned??. Cancer and Metastasis Reviews 2010;29:553‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2003 {published data only}
- Thompson IM, Basler J, Hensley D, Merveldt D, Jenkins CA, Higgins B, Leach R, Troyer D, Pollock B. Prostate cancer prevention: what do we know now and when will we know more?. Clinical Prostate Cancer 2003;1:215‐220. [DOI] [PubMed] [Google Scholar]
Trump 1994 {published data only}
- Trump D. Retinoids in bladder, testis and prostate cancer: epidemiologic, pre‐clinical and clinical observations. Leukemia 1994;8:S50‐54. [PubMed] [Google Scholar]
Vaishampayan 2007 {published data only}
- Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, Fontana J, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutrition and Cancer 2007;59:1‐7. [DOI] [PubMed] [Google Scholar]
van Breemen 2005 {published data only}
- Breemen RB. How do intermediate endpoint markers respond to lycopene in men with prostate cancer or benign prostate hyperplasia?. The Journal of Nutrition 2005;135:2062s‐4s. [DOI] [PubMed] [Google Scholar]
Van Patten 2008 {published data only}
- Patten CL, Boer JG, Tomlinson Guns E. Diet and dietary supplement intervention trials for the prevention of prostate cancer recurrence: a review of the randomized controlled trial evidence.. Journal of Urology 2008;180:2314‐2321. [DOI] [PubMed] [Google Scholar]
Virtamo 2003 {published data only}
- Virtamo J, Pietinen P, Huttunen JK, Korhonen P, Malila N, Virtanen MJ, et al. Incidence of cancer and mortality following alpha‐tocopherol and beta‐carotene supplementation: a postintervention follow‐up. JAMA 2003;290:476‐85. [DOI] [PubMed] [Google Scholar]
Watters 2009 {published data only}
- Watters JL, Gail MH, Weinstein SJ, Virtamo J, Albanes D. Associations between alpha‐tocopherol, beta‐carotene, and retinol and prostate cancer survival. Cancer Research 2009;69:3833‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinstein 2006 {published data only}
- Weinstein SJ, Stolzenberg‐Solomon R, Pietinen P, Taylor PR, Virtamo J, Albanes D. Dietary factors of one‐carbon metabolism and prostate cancer risk. American Journal of Clinical Nutrition 2006;84:929‐935. [DOI] [PubMed] [Google Scholar]
Wilkinson 2003 {published data only}
- Wilkinson S, Chodak G. Critical review of complementary therapies for prostate cancer. Journal of Clinical Oncology 2003;21:2199‐2210. [DOI] [PubMed] [Google Scholar]
Woodson 2002 {published data only}
- Woodson K, Triantos S, Hartman T, Taylor PR, Virtamo J, Albanes D. Long‐term alpha‐tocopherol supplementation is associated with lower serum vascular endothelial growth factor levels. Anticancer Research 2002;22:375‐378. [PubMed] [Google Scholar]
Woodson 2003 {published data only}
- Woodson K, Tangrea JA, Lehman TA, Modali R, Taylor KM, Snyder K, Taylor PR, Virtamo J, Albanes D. Manganese superoxide dismutase (MnSOD) polymorphism, alpha‐tocopherol supplementation and prostate cancer risk in the alpha‐tocopherol, beta‐carotene cancer prevention study (Finland). Cancer Causes and Control 2003;14:513‐518. [DOI] [PubMed] [Google Scholar]
Additional references
Ahn 2005
- Ahn J, Gammon M, Santella R, Gaudet M, Britton J, Teitelbaum S, et al. Associations between breast cancer risk and the catalase genotype, fruit and vegetable consumption and supplement use. American Journal of Epidemiology 2005;162:943‐52. [DOI] [PubMed] [Google Scholar]
AIHW 2007
- AIHW (Australian Institute of Health and Welfare) & AACR (Australasian Association of Cancer Registries). Cancer in Australia: an overview, 2006. Cancer series no. 37. cat. no. CAN 32. Canberra: AIHW 2007.
Andriole 2009
- Andriole GL, Grubb III RL, Buys SS, Chia D, Church TR, Fouad MN, et al. Mortality Results from a Randomized Prostate‐Cancer Screening Trial. New England Journal of Medicine 2009;360(13):1310‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ansari 2003
- Ansari M, Gupta N. A comparison of lycopene and orchidectomy versus orchidectomy alone in the management of advanced prostate cancer. BJU International 2003;92:375‐8. [DOI] [PubMed] [Google Scholar]
Basu 2007
- Basu A, Imrhan V. Tomatoes versus lycopene in oxidative stress and carcinogenesis: conclusions from clinical trials. European Journal of Clinical Nutrition 2007;61:295‐303. [DOI] [PubMed] [Google Scholar]
Beebe‐Dimmer 2004
- Beebe‐Dimmer JL, Wood DP, Gruber SB, Douglas JA, Bonner JD, Mohai C, et al. Use of complementary and alternative medicine in men with family history of prostate cancer: a pilot study. Urology 2004;63(2):282‐7. [DOI] [PubMed] [Google Scholar]
Byers 2002
- Byers T, Nestle M, McTiernan A, Doyle C, Currie‐Williams A, Gansler T, Thun M, American Cancer Society 2001 Nutrition and Physical Activity Guidelines Advisory Committee. American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention. CA: A Cancer Journal for Clinicians 2002;52:92‐119. [DOI] [PubMed] [Google Scholar]
Chan 2005
- Chan JM, Elkin EP, Silva SJ, Broering JM, Latini DM, Carroll PR. Total and specific complementary and alternative medicine use in a large cohort of men with prostate cancer. Urology 2005;66(6):1223‐8. [DOI] [PubMed] [Google Scholar]
Cordon‐Cordo 2007
- Cordon‐Cardo C, Kotsianti A, Verbel DA, Teverovskiy M, Capodieci P, Hamann S, et al. Improved prediction of prostate cancer recurrence through systems pathology. Journal of Clinical Investigation 2007;117(7):1876‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Etminan 2004
- Etminan M, Takkouche B, Caamano‐Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta‐analysis of observational studies. Cancer Epidemiology, Biomarkers & Prevention 2004;13:340‐5. [PubMed] [Google Scholar]
Haseen 2009
- Haseen F, Cantwell M, O'Sullivan J, Murray L. Is there a benefit from lycopene supplementation in men with prostate cancer? A systematic review. Prostate Cancer and Prostatic Diseases 2009;12:325‐32. [DOI] [PubMed] [Google Scholar]
Hasui 1994
- Hasui Y, Marutsuka K, Asada Y, Ide H, Nishi S, Osada Y. Relationship between serum prostate specific antigen and histological prostatitis in patients with benign prostatic hyperplasia. The Prostate 1994;25:91‐96. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Hwang 2005
- Hwang E, Bowen P. Effects of lycopene and tomato paste extracts on DNA and lipid oxidation in LNCaP human prostate cancer cells. Biofactors 2005;23:97‐105. [DOI] [PubMed] [Google Scholar]
Ilic 2006
- Ilic D, O'Connor D, Green S, Wilt TJ. Screening for prostate cancer. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004720.pub2] [DOI] [PubMed] [Google Scholar]
Ilic 2011
- Ilic D, O'Connor D, Green S, Wilt T. Screening for prostate cancer: an updated Cochrane systematic review. BJU International 2011;107:882‐91. [DOI] [PubMed] [Google Scholar]
Magri 2008
- Magri V, Trinchieri A, Perletti G, Marras E. Activity of Serenoa repens, lycopene and selenium on prostatic disease: evidences and hypotheses. Archives of Italian Urology and Andrology 2008;80(2):65‐78. [PubMed] [Google Scholar]
Najm 2008
- Najm W, Lie D. Dietary Supplements Commonly Used for Prevention. Primary Care 2008;35(4):749‐67. [DOI] [PubMed] [Google Scholar]
Patterson 2002
- Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ, et al. Types of alternative medicine used by patients with breast, colon, or prostate cancer: predictors, motives, and costs. Journal of Alternative and Complementary Medicine 2002;8(4):477‐85. [DOI] [PubMed] [Google Scholar]
Rackley 2006
- Rackley JD, Clark PE, Hall MC. Complementary and Alternative Medicine for Advanced Prostate Cancer. The Urologic Clinics of North America 2006;33(2):237‐46. [DOI] [PubMed] [Google Scholar]
Rock 2005
- Rock C, Flatt S, Nataraja L, THomson C, Bardwell W, Newman V, et al. Plasma carotenoids and recurrence‐free survival in women with a history of breast cancer. Journal of Clinical Oncology 2005;23:6631‐8. [DOI] [PubMed] [Google Scholar]
Stanford 2000
- Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. Journal of the American Medical Association 2000;283(3):354‐60. [DOI] [PubMed] [Google Scholar]
Tang 2005
- Tang L, Jin T, Zeng X, Wang J. Lycopene inhibits the growth of human androgen‐independent prostate cancer cells in vitro and in BALB/c nude mice. Journal of Nutrition 2005;135:287‐90. [DOI] [PubMed] [Google Scholar]
WCRF 2007
- World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity and the prevention of cancer: A global perspective. Washington, DC 2007.
Wilkinson 2008
- Wilkinson S, Farrelly S, J Low, A Chakraborty, R Williams, S Wilkinson. The use of complementary therapy by men with prostate cancer in the UK. European Journal of Cancer Care 2008;17(5):492‐9. [DOI] [PubMed] [Google Scholar]
Wilt 2008
- Wilt T, MacDonald R, Hagerty K, Schellhammer P, Kramer BS. 5‐alpha‐reductase inhibitors for prostate cancer prevention. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD007091] [DOI] [PMC free article] [PubMed] [Google Scholar]


