Abstract
Background and aims
Although the Internet has provided convenience and efficiency in many areas of everyday life, problems stemming from Internet use have also been identified, such as Internet gaming disorder (IGD). Internet addiction, which includes IGD, can be viewed as a behavioral addiction or impulse control disorder. This study investigated the altered functional and effective connectivity of the core brain networks in individuals with IGD compared to healthy controls (HCs).
Methods
Forty-five adults with IGD and 45 HCs were included in this study. To examine the brain networks related to personality traits that influence problematic online gaming, the left and right central executive network (CEN) and the salience network (SN) were included in the analysis. Also, to examine changes in major brain network topographies, we analyzed the default mode network (DMN).
Results
IGD participants showed lower functional connectivity between the dorsal lateral prefrontal cortex (DLPFC) and other regions in the CEN than HC participants during resting state. Also, IGD participants revealed reduced functional connectivity between the dorsal anterior cingulate cortex and other regions in the SN and lower functional connectivity in the medial prefrontal cortex of the anterior DMN. Notably, in IGD individuals but not HC individuals, there was a positive correlation between IGD severity and effective connectivity and a positive correlation between reward sensitivity and effective connectivity within the ventral striatum of the SN.
Conclusions
Problematic online gaming was associated with neurofunctional alterations, impairing the capacity of core brain networks.
Keywords: internet gaming disorder, central executive network, salience network, impulsivity, reward sensitivity
Introduction
Since the late 1990s, the rapid development of the Internet has provided convenience and efficiency in many areas of everyday life. However, problems with Internet use have also raised, including the risk of developing Internet gaming disorder (IGD). Internet addiction or pathological Internet use has been defined as compulsive, excessive Internet use, characterized by withdrawal symptoms, increased tolerance, and disability of daily living (Beard & Wolf, 2001; Kwon, Chung, & Lee, 2011). According to a previous study that defined the criteria for IGD, tolerance refers to feeling the need to play games longer than intended in order to experience excitement (Petry et al., 2014). In previous studies, the main behavior criterion for problematic online gaming has been loss of control over Internet use, which presents as a persistence in online gaming despite the awareness that it is directly harmful to one's psychosocial performance (Ko et al., 2009; Lee, 2005; Na, Park, & Kim, 2007). For example, problematic Internet use was associated with the risk of comorbidities, such as ADHD (Lemenager et al., 2018), or suicidal ideation (Guo et al., 2018). IGD was also associated with individuals' negative characteristics such as negative time perspective (Lukavská, 2018), high levels of social problems, low emotional intelligence, and dysfunctional family relationships (Torres-Rodríguez, Griffiths, Carbonell, & Oberst, 2018). IGD has been included in Section 3 of the research appendix of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) (American Psychiatric Association, 2013), suggesting that Internet gaming disorder is a form of behavioral addiction (Petry et al., 2014) and the further study confirmed the validity of the DSM standard (Müller, Beutel, Dreier, & Wölfling et al., 2019). Additionally, the World Health Organization has included gaming disorder in the ‘Disorders Due to Substance Use or Addictive Behaviors’ section of the 11th edition of the International Classification of Diseases (ICD-11) (Kircaburun, Griffiths, & Billieux, 2019; World Health Organization, 2018).
The consensus on IGD needs to develop through a re-evaluation of already existing data on IGD and studies in various fields (Griffiths et al., 2016). The gaming disorder (GD) classification was included in ICD-11 based on clinical evidence that has accumulated (King et al., 2019), and thus it could expect to facilitate treatment and prevention for those who need it (Rumpf et al., 2018). Similarly, IGD should also be considered in the field of mental disorders because of harm such as impaired control, functional impairment, negative intrapersonal and interpersonal effects (King & Delfabbro, 2018; King et al., 2018).
Excessive Internet gaming despite negative consequences is considered to be linked to a loss of control, characterized by impulsivity and reward sensitivity in gaming behavior. Internet addiction, including IGD, can be viewed as a behavioral addiction or impulse control disorder not otherwise specified, similar to compulsive shopping or compulsive gambling (Lyvers, Karantonis, Edwards, & Thorberg, 2016). Impulsivity means a tendency to act in a risky or situationally inappropriate manner despite negative consequences (Dickman, 1990). In previous studies, it has been reported that individuals with IGD showed impulsivity characteristics similar to other addictive disorders (Choi et al., 2014b; Ding et al., 2014; Kim et al., 2017; Wang et al., 2016). For example, individuals with IGD have shown higher levels of impulsivity compared with an alcohol dependence (AD) group (Choi et al., 2014b), and the severity of Internet addiction is related to impulsivity (Lee et al., 2012). Reward sensitivity is also related to Internet addiction (Lyvers et al., 2016), smartphone dependency (Kim et al., 2016), and substance abuse (Simons, Dvorak, & Lau-Barraco, 2009; Zisserson & Palfai, 2007). According to Gray, brain activation systems (BASs), including reward responsiveness, are theoretical biopsychological systems related to personality traits involving sensitivity toward stimuli that are associated with positive reinforcement and regulation of motivational behavior (Carver, 2004; Franken, Muris, & Georgieva, 2006; Gray & McNaughton, 2000). In previous studies related to reward responsiveness, it has reported that individuals with IGD showed short-sighted characteristic of paying that pays attention to immediate, resulting in losing money in the long term (Chang, Kim, & Kim, 2013), These dysfunctional behaviors are similar to substance dependence (Pawlikowski & Brand, 2011). Therefore, it can be assumed that impulsivity and reward sensitivity affect cognitive control failure in Internet game.
In terms of functional brain networks, three brain connectivity networks—the central executive, salience, and default mode networks—have been identified as central to the understanding of higher cognitive function (Menon, 2011). In brain networks, the connectivity refers to a pattern of anatomical or functional links between distinct brain areas, which controls how the brain processes information. These functional links are estimated via statistical dependencies and causal interactions, which are referred to as functional and effective connectivity, respectively. That is, whereas functional connectivity captures signal synchrony without any explicit reference to directional effects, effective connectivity describes the directional effects of one brain area over another. The central executive network (CEN) is a fronto-parietal network that is crucial to working memory and cognitive control of thought, emotion, and behavior, and includes the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), and posterior parietal cortex (PPC) (Cole et al., 2013; Cole, Repovs, & Anticevic, 2014; Menon, 2011). The salience network (SN) is involved in the detection of personally salient internal and external stimuli to direct behavior (Toga, 2015), with the goal of maintaining homeostasis. This network consists of the dorsal anterior cingulate cortex, the fronto-insular cortex (FIC), and the limbic area, including the ventral striatum (VS) (Cai, Chen, Szegletes, Supekar, & Menon, 2015; Seeley et al., 2007). Finally, the default mode network (DMN) plays an important role in self-related processes, emotion regulation, social cognition, autobiographical memory, and future-oriented thinking, and consists of the medial prefrontal cortex (MPFC), lateral parietal lobes (IPL), and the posterior cingulate cortex (PCC) (Di & Biswal, 2014; Menon, 2011; Sharaev, Zavyalova, Ushakov, Kartashov, & Velichkovsky, 2016).
IGD is different from substance dependency in that no chemical intoxication or substance intake is involved (Grant, Potenza, Weinstein, & Gorelick, 2010). However, excessive Internet gaming use may lead to dependence similar to that observed in substance dependency (Han et al., 2018). The previous studies have reported controversial results in brain networks related to cognitive function. For example, the IGD group showed increased functional connectivity within the cognitive network compared with both Internet gambling disorder and healthy controls (Bae et al., 2017). However, in the other study, it has been suggested that decreased functional connectivity in CEN might reflect impaired executive control across substance and behavioral addictions (Dong, Lin, & Potenza, 2015). It is known that both AD and IGD subjects show cognitive deficits in executive function, including problems with self-control and adaptive responses (Chang et al., 2013; Han et al., 2015). In addition, in a recent brain connectivity study, IGD individuals showed similar neurobiological underpinnings to individuals with substance use disorders (Ding et al., 2013), and a previous study on functional connectivity reported that impaired cognitive control in IGD individuals might be related to abnormal central executive and salience networks (Yuan et al., 2016). In particular, individuals with IGD showed similar DLPFC functional connectivity to the AD group, which indicates that IGD and AD may have similar deficits in executive function (Han et al., 2015). On the other hand, IGD individuals demonstrated decreased functional connectivity of the DLPFC with the striatal area, compared to AD individuals (Han et al., 2015), and had different neurophysiological patterns of brain connectivity in resting-state EEG activity (Park et al., 2017; Son et al., 2015). Therefore, it is important to investigate brain connectivity in the various network in order to identify distinguishable features of IGD.
Recent studies have been provided novel evidence for the role of a core network including the CEN, SN, and DMN in IGD (Wang et al., 2017; Zhang et al., 2017). In the previous study, Internet addiction including IGD was associated with imbalanced interactions among the CEN, SN, and DMN (Wang et al., 2017). Another study to assess alterations in the inter-network interactions of brain networks in IGD suggested that deficient modulation of the CEN versus the DMN by the SN might provide a better framework for understanding the neural basis of IGD (Zhang et al., 2017). The previous studies on interaction among brain networks have tended to focus on deficient modulation among brain networks, rather than the personality traits such as impulsivity and reward sensitivity that influence brain connectivity. We hypothesized impulse and compensatory responses affect the cognitive control failures in Internet gaming, and investigated the functional and effective changes in brain connectivity associated with these personality traits in IGD. The investigation of neuroimaging features related to personal traits may be helpful in understanding the characteristic of IGD and in developing treatment plans for IGD patients.
In summary, we investigated alterations in functional and effective connectivity on the CEN, SN, and DMN in IGD, based on the regions of interest (ROIs) of these core networks in relation to behavioral addiction. In addition, we investigated the relationships between brain connectivity and personality factors, such as impulsivity and reward sensitivity, and between behavioral aspects of IGD, such as the time spent Internet gaming per week and the severity of IGD. In terms of personality traits, we hypothesized that the severity of IGD might influence the correlation between impulsivity and brain connectivity of the CEN due to a loss of control in excessive gaming, and that reward sensitivity in IGD would show a positive correlation with the connectivity of the VS in the SN, based on excessive gaming.
Materials and methods
Participants
This study was conducted on adult men and women aged 19–35, who were recruited online. A total of 5,500 adults participated in the online survey on Internet gaming use and 838 people among online game users agreed to participate in the fMRI study. Of these, 182 people responded to the MRI safety screening questionnaire, and we recruited 97 adults who had passed the MRI safety screening questionnaire for the study. The participants were classified into two groups based on a clinician-administered interview and the IGD diagnostic criteria in the DSM-5, with a cut-off threshold of a score of 5 (Petry et al., 2014). In total, forty-eight adults with IGD (32 male and 16 female) and 49 healthy controls (HCs) (34 male and 15 female) were recruited for the fMRI study. All participants underwent the Mini-International Neuropsychiatric Interview by the clinician to screen out participants with a current psychiatric diagnosis, and intelligence testing was estimated using a (Burgess, Flint, & Adshead, 1992). We used the Korean version of the Alcohol Use Disorders Identification Test (AUDIT-K) (Kim et al., 1999) to screen for excessive drinking and alcohol use disorders and used the Fagerstrom Tolerance Questionnaire (FTQ) and Fagerstrom Test for Nicotine Dependence (FTND) (Ahn et al., 2002) to screen for nicotine dependence. Exclusion criteria included past or current major medical disorders (e.g., diabetes mellitus), neurological disorders (e.g., seizure disorders, head injuries), or psychiatric disorders (e.g., major depressive disorder, anxiety disorders). Three participants were excluded because of depressive disorder (two participants with IGD and one HC), and the data from four participants were excluded because of severe head motion during analysis (one participant with IGD and three HCs). Therefore, forty-five adults with IGD (31 male and 14 female, 27.76 ± 5.31 years) and 45 HCs (31 male and 14 female, 25.29 ± 4.07 years) were used in this study (Table 1). All participants were right-handed as assessed by the Edinburgh handedness inventory (Oldfield, 1971).
Table 1.
Regions of interest
|
Regions |
MNI coordinates | Network | Ref. | ||
| x | y | z | |||
| L. DLPFC | −45 | 19 | 30 | Central Executive Network | Cole et al., (2013) |
| R. DLPFC | 45 | 19 | 30 | ||
| L. VLPFC | 41 | 43 | 4 | ||
| R. VLPFC | −40 | 40 | 2 | ||
| L. PPC | 41 | −55 | 45 | ||
| R. PPC | −41 | −56 | 41 | ||
| R. dACC | 10 | 34 | 24 | Salience Network |
Cai et al., (2015), Seeley et al., (2007) |
| L. FI | −32 | 24 | −10 | ||
| R. FL | 38 | 36 | −10 | ||
| L. VS | −18 | 6 | 4 | ||
| R. VS | 18 | 6 | 4 | ||
| R. MPFC | 3 | 54 | −2 | Default Mode Network |
Sharaev et al., (2016), Di & Biswal, (2014) |
| PCC | 0 | −52 | 26 | ||
| L. IPL | −50 | −63 | 32 | ||
| R. IPL | 48 | −69 | 35 | ||
Abbreviations: DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; PPC, posterior parietal cortex; dACC, dorsal anterior cingulate cortex; FI, orbital frontoinsula; VS, ventral striatum; MPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; IPL, inferior parietal lobule.
Questionnaires
Severity of IGD
The severity of IGD was assessed by a clinician, based on the nine items described in the DSM-5: preoccupation, tolerance, withdrawal, persistence, escape, problems, deception, displacement, and conflict (Few et al., 2013). A reliability test for the scale yielded a Cronbach's alpha of 0.95 (Lemmens, Valkenburg, & Gentile, 2015).
Impulsivity
Dysfunctional impulsivity was measured using the 12 items on the respective subscale of Dickman's Impulsivity Inventory (DII) (Dickman, 1990). This tool evaluates dysfunctional and functional self-reported impulsivity, and it consists of 23 true or false items. We used the dysfunctional impulsivity scale (DFDII) in the Korean version of the DII to investigate the tendency to act with less forethought (Smillie & Jackson, 2006), which is associated with problematic online gaming (Cronbach's alpha 0.85).
Reward sensitivity
We used the Korean version of the brain inhibition system (BIS) and brain activation system (BAS) (Kim & Kim, 2001) translating the BIS/BAS inventory of Carver & White (Carver & White, 1994). It was reported that the reliability of the BIS/BAS inventory of Korean version was fairly high and factorial structures of the scale were consistent with those of Carver & White (Kim & Kim, 2001). We focused on BAS-RR for investigating the correlation between brain connectivity and reward sensitivity in IGD. The BIS scale consists of seven items (Cronbach's alpha 0.78), while the BAS scale includes three subscales: five items of Reward Responsiveness (BAS-RR) (Cronbach's alpha 0.85), four items of Drive (BAS-D) (Cronbach's alpha 0.87), and four items of Fun Seeking (BAS-FS) (Cronbach's alpha 0.78). Participants completed the BIS/BAS questionnaire using a 4-point Likert-type response scale (1: strongly disagree, 4: strongly agree). The BIS and BAS are general motivation systems that underlie behavior and affect (Steketee, Foa, & Grayson, 1982). The BIS is a conflict detection and monitoring system that supports exploratory behavior to resolve approach-avoidance conflict (Bunford, Roberts, Kennedy, & Klumpp, 2017; Corr, 2002), and the subscales of BAS are associated with strong and quick goal pursuit, receptivity to reward, and the desire for new and potentially rewarding experiences (Carver & Scheier, 1994).
Image acquisition
Functional and structural MRI data were acquired using a 3T MRI system (Siemens MAGNETOM Verio, Erlangen, Germany) equipped with a 16-channel head coil. Participants' heads were cushioned with attached earmuffs. The functional images were obtained using a T2*-weighted gradient echo-planar imaging sequence: repetition time (TR) = 2,000ms, echo time (TE) = 30 ms, flip angle = 90°, voxel size = 3.59 mm × 3.59 mm × 3.60 mm, image matrix = 64 × 64, field of view = 230 mm, slice number = 31, and scan duration = 6 min 40 sec. During scanning, the subjects were instructed to fixate their eyes on the crosshair and to remain as motionless as possible, while at rest. Structural images with a resolution of 1 mm × 1 mm × 1 mm were acquired using a 3D T1-weighted gradient echo sequence (176 slices, TR = 2,300 ms, TE = 2.22 ms, image matrix = 256 × 256).
fMRI preprocessing
All analyses of resting state fMRI (rsfMRI) data were performed in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/). Preprocessing consisted of spatial realignment to correct for head movement, normalization into the same coordinate frame as the template brain in the Montreal Neurological Institute space, and spatial smoothing with a Gaussian kernel of 8 mm full width at half maximum. A nonlinear deformation field for spatial normalization was derived from the segmentation of the structural MRI volume coregistered to the mean of the realigned rsfMRI volumes. For the time series of rsfMRI data, a general linear model (GLM) was constructed to adjust for the effect of head movement and non-neuronal fluctuations. The range of head movement was within the voxel size (2 mm) of the functional MRI data. Also, there were no significant differences in maximum excursion movement values in x and z of planes of translation between IGD (x = 0.04 ± 0.40 mm; y = 0.22 ± 0.21 mm; z = 0.05 ± 0.88 mm) and HC (x = 0.06 ± 0.30 mm; y = 0.10 ± 0.17 mm; z = 0.11 ± 0.50 mm) [t (88) = 0.17, P = 0.87; t (88) = 3.19, P = 0.002; t (88) = 0.46, P = 0.64]. There were no significant differences in maximum excursion movement values in each of planes of rotation between IGD (pitch= 0.19 ± 1.16°; roll = 0.03 ± 0.53°, and yaw = 0.04 ± 0.48°) and HC (pitch = 0.07 ± 0.43°; roll = 0.01 ± 0.28°, and yaw = −0.02 ± 0.18°) [t (88) = 0.69, P = 0.49; t (88) = 0.28, P = 0.78; t (88) = 0.78, P = 0.44]. The GLM contained nuisance regressors comprising six head movement parameters estimated during the preprocessing step, the mean cerebrospinal fluid signal, and the mean white matter signal, in addition to a discrete cosine transform basis set for high-pass filtering at 1/128 Hz.
ROIs selection
To examine the alteration of brain networks influencing problematic online gaming, the ROIs of the CEN, SN, and DMN were included in the analysis (Table 1). For the CEN, we created six seed ROIs centered in the bilateral DLPFC, VLPFC, and PPC, using MNI coordinate locations from a previous study that suggested a central role for fronto-parietal networks in cognitive control and adaptive implementation (Cole et al., 2013). For the SN network, five ROIs each were seeded in the dACC, bilateral FIC, and VS, based on previous studies which that focused on homeostatic regulation and reward processing (Cai et al., 2015; Seeley et al., 2007). Finally, four seed ROIs each were chosen in the MPFC, bilateral IPL, and PCC, as these regions are part of the DMN network that mediates internal modes of cognitive activity (Di & Biswal, 2014; Sharaev et al., 2016). Each ROI was defined as a 6 mm-radius sphere centered at the respective coordinates, and the representative signal of the ROI was extracted as the principal eigenvariate using a singular value decomposition of signals across all voxels within the ROI.
Functional connectivity and effective connectivity
The representative signal of the ROI was extracted as the principal eigenvariate for each core network, and the functional connectivity strengths were used in parametric inferences at the group level. The correlation coefficients were converted to z-scores via Fisher's z-transformation, and functional connectivity strengths, were used in parametric inferences at the group level. In the inferences, statistical significance was identified as a P-value of 0.05 or less, with a false discovery rate (FDR) estimation to correct for multiple comparisons.
Whereas functional connectivity refers to the dependency between distant brain regions, as an observable phenomenon, effective connectivity is based on a parametric model that aims to account for the observed dependency (Friston, 2011). The goal of dynamic causal modeling (DCM) is to identify effective connectivity among neuronal states that explains observed fMRI data. Stochastic DCM employs an extended model accounting for stochastic neuronal fluctuations, but it requires excessive computational complexity. Thus, for computational efficiency, we used spectral DCM (spDCM) that employs a deterministic model under a stationarity assumption. The spDCM has been developed as a way of modeling effective connectivity that predicts or generates observed functional connectivity (Friston, Kahan, Biswal, & Razi, 2014). In spDCM, effective connectivity is estimated for the spectral density of neuronal fluctuations, rather than the time-varying neuronal fluctuations themselves. Here, we had the opportunity to address effective connectivity between brain regions by employing spDCM as implemented in SPM12. For each core network, we were interested in the effective connectivity between specific pairs of ROIs that showed group differences in functional connectivity. We proposed 36 effective connectivity models in CEN, and 25 effective connectivity models in SN and 16 effective connectivity models in DMN; they were distinguished depending on whether individual extrinsic connections were on or off. To estimate network interactions at the neuronal level, we used the fully connected model that is, the network having all possible extrinsic connections, and the model was selected to be the best for the CEN, SN, and DMN. The effective connectivity model was inverted using spDCM to estimate model parameters. Effective connectivity strengths were entered into parametric inferences at the group level, and statistical significance was identified as a P-value of 0.05 or less with FDR multiple comparisons correction. Additionally, we conducted three separate Pearson correlations of brain networks with behavioral aspects of IGD and personality factors in each group. The brain networks included the prefrontal cortex of the CEN and the DMN, within VS of SN strength. For behavioral aspects, the time spent Internet gaming per week and the severity of IGD were considered, and for personality traits of IGD, impulsivity and reward sensitivity were considered.
Ethics
Each participant provided written informed consent in accordance with the Declaration of Helsinki, and the study protocol was approved by the institutional review board of Seoul St. Mary's Hospital. All experiments were performed in accordance with relevant guidelines and regulations.
Results
Demographics
Table 2 summarizes the demographic and clinical characteristics of the two groups. The groups did not differ in duration of education, K-WAIS, or duration of online gaming use. However, the time spent on online gaming per week [t (88) = 7.30, P < 0.001], the cost for gaming [t (88) = 4.35, P < 0.001], and the IGD scores [t (88) = 21.53, P < 0.001] were significantly different. According to impulsivity, IGD individuals had a higher score than HCs on the DFDII [t (88) = 5.07, P < 0.001]. The IGD individuals also showed higher scores on the BIS [t (88) = 3.25, P < 0.005] and BAS [t (88) = 3.36, P < 0.001], and particularly higher scores on the BAS-RR [t (88) = 2.60, P < 0.05], and BAS-FS [t (88) = 4.37, P < 0.001]. An Analysis of Covariance (ANCOVA) was used to control for differences in age between the groups for the personality traits and behavioral aspects of IGD (P < 0.05). The IGD individuals showed higher scores than HCs on the DFDII [F (2, 87) = 22.40, P < 0.001], BIS [F (2, 87) = 10.92, P < 0.005], BAS-RR [F (2, 87) = 5.42, P < 0.05], and BAS-FS [F (2, 87) = 8.07, P < 0.01]. IGD individuals also had higher scores than HCs on the IGD score [F (2, 87) = 427.11, P < 0.001], time spent online gaming per week [F (2, 87) = 48.69, P < 0.001], and cost for gaming [F (2, 87) = 19.19, P < 0.001].
Table 2.
Demographic characteristics of the IGD and NC
| IGD (n = 45) | HC (n = 45) | t score | |||
| mean | SD | mean | SD | ||
| Age | 27.76 | 5.31 | 25.29 | 4.07 | 2.47∗ |
| K-WAIS | 108.58 | 12.03 | 113.29 | 12.01 | −1.86 |
| Education duration (years) | 14.36 | 3.50 | 15.27 | 1.95 | −1.53 |
| Gender | |||||
| Male | 69% (n = 31) | 69% (n = 31) | x2 = 0.000 | ||
| Female | 31% (n = 14) | 31% (n = 14) | |||
| Duration of Internet gaming (year) | 13.4 | 6.58 | 12.4 | 4.16 | 0.86 |
| Time for Internet gaming per week (hours) | 23.82 | 12.02 | 9.66 | 4.96 | 7.30∗∗∗ |
| IGD score | 5.53 | 1.34 | 0.6 | 0.75 | 21.53∗∗∗ |
| SAPS | 41 | 9.67 | 27.07 | 5.62 | 8.35∗∗∗ |
| DFDII | 5.07∗∗∗ | ||||
| BIS scale | 19.98 | 3.07 | 18.02 | 2.62 | 3.25∗∗ |
| BAS scale | 36.04 | 6.31 | 32.18 | 4.44 | 3.36∗ |
| Reward responsiveness | 14.36 | 2.83 | 13.02 | 1.96 | 2.60∗ |
| Drive | 10.62 | 2.31 | 9.80 | 2.07 | 1.78 |
| Fun seeking | 11.07 | 2.02 | 9.36 | 1.68 | 4.37∗∗∗ |
| Economic status | |||||
| Upper | 16% | 13% | x2 = 4.63 | ||
| Upper-middle | 36% | 20% | |||
| Middle | 38% | 54% | |||
| Lower-middle | 10% | 13% | |||
| Lower | 0% | 0% | |||
Abbreviations: IGD, Internet Gaming Disorder group; HC, Healthy control group; IGDs, Internet Gaming Disorder scale; SAPS, Smartphone Addiction Proneness Scale; DFDII, dysfunctional impulsivity Inventory; BIS, Behavioral inhibition system; BAS, Behavioral activation system.
∗P < 0.05, ∗∗P < 0.005, ∗∗∗P < 0.001.
Functional and effective connectivity
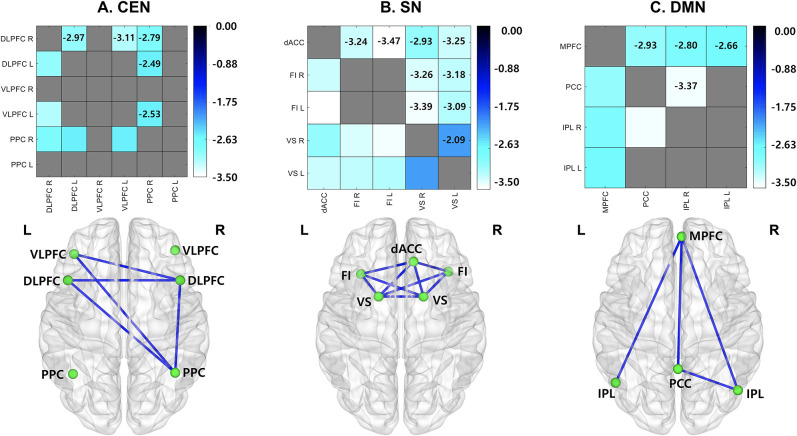
To explore potential factors underlying the effective connectivity results, we examined functional connectivity between the nodes that showed differential functional connectivity between IGD and HC individuals. The results from the functional connectivity analyses between groups are presented in Fig. 1. An Analysis of Covariance (ANCOVA) was used to control for differences in age between groups in the brain networks (P < 0.05). In the CEN, statistical analysis of group differences in strength of functional connectivity revealed that right DLPFC connectivity with bilateral VLPFC [L: F (2, 87) = 9.39, P < 0.005; R: F (2, 87) = 5.23, P < 0.05], and left DLPFC [F (2, 87) = 7.65, P < 0.01] was significantly less in IGD individuals compared to HC individuals, as was left DLPFC connectivity with bilateral VLPFC [L: F (2, 87) = 5.69, P < 0.05; R: F (2, 87) = 4.20, P < 0.05]. Additionally, the group differences of functional connectivity showed that right PPC connectivity with bilateral DLPFC [L: F (2, 87) = 5.95, P < 0.05; R: F (2, 87) = 6.94, P < 0.05] and bilateral VLPFC [L: F (2, 87) = 6.14, P < 0.05; R: F (2, 87) = 4.55, P < 0.05] was significantly less in IGD individuals compared to HCs, as was left PPC connectivity with right DLPFC [F (2, 87) = 5.70, P < 0.05], bilateral VLPFC [L: F (2, 87) = 4.02, P < 0.05; R: F (2, 87) = 4.37, P < 0.05], and right PPC [F (2, 87) = 4.01, P < 0.05] (Fig. 1A). In the SN, we found that dACC connectivity with bilateral FIC [L: F (2, 87) = 11.90, P < 0.005; R: F (2, 87) = 8.38, P < 0.01] and bilateral VS [L: F (2, 87) = 9.45, P < 0.005; R: F (2, 87) = 7.51, P < 0.01], was less in IGD individuals than in HCs, and that left FIC connectivity with bilateral VS [L: F (2, 87) = 8.75, P < 0.005; R: F (2, 87) = 10.30, P < 0.005], and right FIC connectivity with bilateral VS [L: F (2, 87) = 7.64, P < 0.01; R: F (2, 87) = 7.42, P < 0.01] were weaker in IGD individuals than in HCs (Fig. 1B). In the DMN, we found that the MPFC connectivity with bilateral IPL [L: F (2, 87) = 4.76, P < 0.05; R: F (2, 87) = 6.37, P < 0.05] and PCC [F (2, 87) = 6.10, P < 0.05] was less in IGD individuals than in HCs, and that PCC connectivity with the right IPL [F (2, 87) = 9.27, P < 0.01] was less in IGD individuals than in HCs. No ROIs showed greater functional connectivity in IGD individuals than in HCs (Fig. 1C).
Figure 1.
Group differences of functional connectivity. IGD individuals showed weaker functional connectivity in the CEN (A), SN (B), and DMN (C) compared to HCs
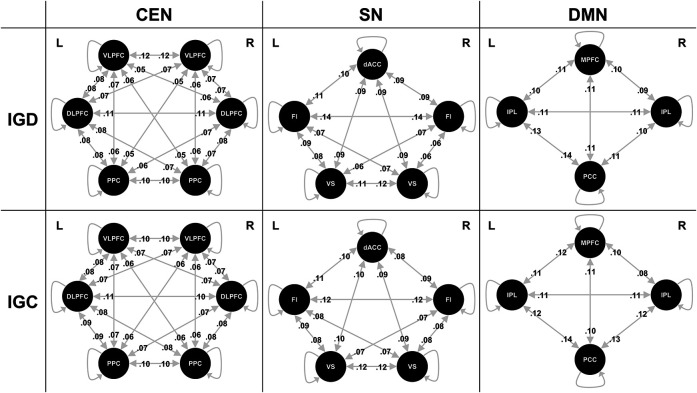
The probabilities for all models for each network analysis (within-CEN, within-SN, and within-DMN) are shown in Fig. 2. Each of the three core networks was specified for each participant, all with fully connected intrinsic models and no input entering the system in either IGD or HC individuals. However, no links showed significantly different effective connectivity between IGD and HC individuals in the CEN, SN, or DMN after FDR correction.
Figure 2.
Effective connectivity in the CEN, SN, and DMN. Each of the three core networks was specified for each participant, all with fully connected intrinsic models and no input entering the system
Relationship between the brain connectivity and behavioral aspects of IGD
We investigated the correlations between behavioral aspects of IGD and brain networks including the prefrontal cortex of the CEN, the DMN, and the VS of the SN, and compared the two groups using z scores. There were no significant correlations between functional connectivity in the prefrontal cortex of the CEN, DMN, or the VS of the SN and behavioral aspects of IGD. In the CEN, there were significant correlations between the time spent Internet gaming per week and effective connectivity from the right VLPFC to the left VLPFC (r = 0.44, P < 0.05), and effective connectivity from the left VLPFC to the right VLPFC (r = 0.42, P < 0.05) in the HC group; no significant results were identified in the IGD group. Also, the correlations of effective connectivity from the right VLPFC to the left VLPFC (z = 2.09, P < 0.05), and from the left VLPFC to the right VLPFC (z = 2.01, P < 0.05) were significantly different across groups. In the SN, there were no significant correlations with the IGD or HC groups. In the DMN, there were significant correlations between the time spent Internet gaming per week and the effective connectivity from the MPFC to the left IPL (r = 0.38, P < 0.05) for the HC group; no significant results were identified in the IGD group. Also, the correlation between the effective connectivity from the MPFC to the left IPL with severity of IGD was a significant difference across groups (z = 2.62, P < 0.01).
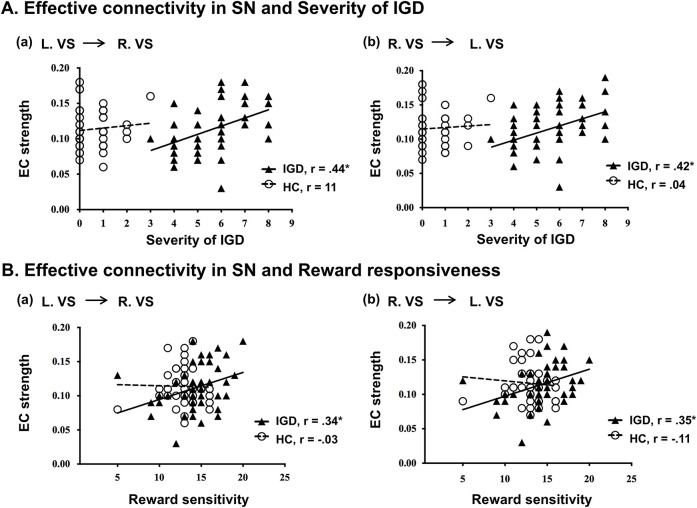
We found a correlation between IGD score and effective connectivity. Neither the IGD group nor the HC group showed any significant correlations between severity of IGD and the brain network in the prefrontal cortex of the CEN and DMN. In the SN, there were significant correlations between the severity of IGD and the effective connectivity from the right VS to the left VS (r = 0.42, P < 0.05), and effective connectivity from the left VS to the right VS (r = 0.44, P < 0.05) in the IGD group; no significant results were identified in the HC group (Fig. 3A).
Figure 3.
Effective connectivity in VS correlated with severity of IGD and reward sensitivity. Correlations between effective connectivity in VS and severity of IGD (A). IGD individuals showed positive correlations between effective connectivity from the left VS to the right VS and reward sensitivity (Aa), and between effective connectivity from the right VS to the left VS and reward sensitivity (Ab). Correlations between effective connectivity in the SN and reward sensitivity (B). IGD individuals showed positive correlations between effective connectivity from the left VS to the right VS and the reward sensitivity (Ba), and between effective connectivity from the right VS to the left VS and reward sensitivity (Bb)
The Relationship between the brain connectivity and personality factors
We investigated the correlations between behavioral aspects of IGD and brain networks including the prefrontal cortex of the CEN and DMN, within VS of SN, and compared the two groups using z scores. There were no significant correlations between functional connectivity in the prefrontal cortex of the CEN, the DMN, or the VS of the SN and personality traits. In addition, there were no significant correlations between impulsivity and brain connectivity within the CEN, SN, and DMN in either the IGD or HC groups after FDR correction. However, we found a moderating effect of IGD on impulsivity and brain connectivity within the CEN, SN, and DMN in both the IGD and HC groups after FDR correction. The moderating effect for the IGD score indicated that individuals with high IGD scores showed a positive relationship between impulsivity and effective connectivity from the left VLPFC to the right DLPFC [ΔR2 = 0.094, ΔF1,88 = 6.70, P = 0.01, b = 0.001, t (88) = 2.65, P < 0.05] and between impulsivity and effective connectivity from the right DLPFC to the left VLPFC [ΔR2 = 0.07, ΔF1,88 = 4.82, P = 0.031, b = 0.001, t (88) = 2.20, P < 0.05] under resting state.
In addition, there were significant correlations between reward sensitivity and effective connectivity in the SN; however, there were no significant correlations within the CEN and the DMN. In the SN of IGD individuals, there were significant correlations between the effective connectivity from the left VS to the right VS and reward sensitivity (r = 0.34, P < 0.05), and from the right VS to the left VS and reward sensitivity (r = 0.35, P < 0.05). No further significant results were identified in HC individuals (Fig. 3B). In particular, the correlation between effective connectivity from the right VS to the left VS and reward sensitivity was a significant difference across groups (z = 2.13, P < 0.05).
Discussion
Although the prevalence of IGD varies across countries and regions (range: 0.7%–15.6%) due to differences in sample characteristics and in the screening tool used, problematic online gaming has a high prevalence overall, and interest in Internet addiction is increasing worldwide (Yen et al., 2012). This study aimed to investigate the altered functional and effective connectivity of the core brain network in IGD individuals as compared to HCs. Furthermore, another important objective was to identify interactions between brain connectivity and behavioral aspects such as time spent Internet gaming per week or severity of IGD, and between brain connectivity and personality factors such as impulsivity and reward sensitivity in relation to addictive behavior in online gaming.
In this study, IGD participants showed lower functional connectivity during resting state between the VLPFC and DLPFC, and between the PPC and DLPFC in the CEN than did HC participants. These results are likely to reflect symptom-related neurofunctional alterations that compromise functional connectivity capacity. In previous studies related to IGD, decreased functional connectivity within the CEN was associated with impaired cognitive control (Yuan et al., 2016, 2017). Therefore, the present findings are consistent with other research, and suggests that decreased functional connectivity in the CEN may represent an important feature in indexing impaired executive control across substance and behavioral addictions (Dong et al., 2015). Regarding behavioral aspects, while HCs showed a positive correlation between effective connectivity in bilateral VLPFCs and time spent Internet gaming, there was no significant correlation in IGD individuals. It could be suggested that effective connectivity of the bilateral VLPFC in HCs influenced control over excessive Internet gaming. In a previous study, it was proposed that the CEN implements feedback control to regulate symptoms, and that an undamaged control system plays a protective role against a variety of mental illnesses (Cole et al., 2014).
We expected that high impulsivity in IGD individuals would result in more effort required to control the CEN during resting state. A previous study reported the role of impulsiveness in several psychiatric disorders associated with prefrontal dysfunctions and cognitive deficits, and the prefrontal cortex is recognized to play a major role in controlling impulses (Etkin & Wager, 2007). It is also thought that impulsivity plays an important role in the early stage of substance use disorder (SUD), and is a predisposing risk factor for SUDs (Choi et al., 2014a; Robbins, Curran, & de Wit, 2012). Loss of control in Internet gaming is an important criterion of IGD (Petry et al., 2014), and high impulsivity could make individuals succumb to the rewarding effects of gaming (Yen et al., 2017). Although we did not detect a significant correlation between impulsivity and brain connectivity in the CEN in this study, we did identify that the severity of IGD influenced a positive correlation between impulsivity and effective connectivity between the DLPFC and VLPFC. This suggests that the efficiency of this brain region in processing inhibitory control is related to the severity of IGD. In other words, more impulsive individuals with high IGD severity may need to recruit greater resources within the PFC to maintain a resting state. These results seem to be consistent with previous research, which has reported a positive correlation between right VLPFC abnormalities and measures of impulsivity related to cognitive dysfunction in schizophrenia (Chai et al., 2011; Kaladjian, Jeanningros, Azorin, Anton, & Mazzola-Pomietto, 2011), and suggested that VLPFC hyperactivity may reflect extension and compensatory recruitment of cortical activity due to inefficient DLPFC functioning (Minzenberg, Laird, Thelen, Carter, & Glahn, 2009; Tan, Choo, Fones, & Chee, 2005).
The present study found that IGD individuals had reduced functional connectivity between the dACC and other regions in the SN as compared to HCs. Given that the dACC plays an important role in response selection, guiding overt behavior, and modulating autonomic reactivity (Menon & Uddin, 2010), the decreased connectivity in the dACC in IGD individuals suggests that IGD individuals have a deficit in cognitive monitoring of external environment in order to maintain a suitable response. Interestingly, we identified that higher IGD severity increased effective connectivity between bilateral VSs. VS has been particularly associated with the anticipation or prediction of reward (Haruno et al., 2004; O'Doherty et al., 2004), and recently, it has been proposed that this striatum is involved in coding stimulus saliency. In a previous study, the volume of VS was correlated with Internet addiction scores (Yuan et al., 2017), and another previous study reported that the amplitude of low-frequency fluctuation (ALFF) in bilateral VS was correlated with Internet addiction scores (Kühn & Gallinat, 2015). In addition, IGD individuals showed positive correlations between reward sensitivity and effective connectivity within the bilateral VS. The personality trait of reward sensitivity is hypothesized to reflect the functioning of the brain's BAS (Lyvers et al., 2016), and high levels of BAS are associated with addictive behaviors (Yen et al., 2012). Recently, the BAS has been linked not only to Internet addiction (Dong, Hu, & Lin, 2013), but also to excessive smartphone use (Chun et al., 2017). In IGD individuals, the higher the reward sensitivity scores, the more effective the connectivity between bilateral VSs. These results suggest that the personality trait of reward sensitivity in IGD is associated with VS abnormalities. Therefore, it is possible that higher reward sensitivity in IGD evokes alterations in brain connectivity associated with reward processing and thus influences excessive Internet gaming.
Additionally, we identified that IGD individuals showed lower functional connectivity in the DMN than HCs did. These results are consistent with previous studies of alterations in the DMN of adolescents with IGD. Previous studies using seed-based correlations and ICA methods also reported decreased functional connectivity in the DMN (W. W. Li et al., 2015; Wang et al., 2017), a result consistent with findings in substance addiction research (Ma et al., 2011). These findings suggest that alteration of DMN may underlie the disturbance of self-referential processing and awareness for both substance and behavioral addictions (Volkow, Wang, Fowler, & Tomasi, 2012). In the previous study using cognitive tasks, it was reported that individuals with IGD showed altered modulation in the DMN and deficits in executive control function (Wang et al., 2016). Therefore, alterations in the DMN of IGD individuals might be related to the fact that individuals with IGD continue to play online games despite negative consequence. Also, we identified a positive correlation between effective connectivity from the MPFC to the IPC and time spent Internet gaming in HCs, while there were no significant correlations in IGD individuals. It is possible that effective connectivity from the MPFC to the IPC in HCs influenced control over excessive Internet gaming.
Finally, several important limitations need to be considered. First, although we controlled for major psychiatric disorders through clinical interviews, game-usage patterns, such as game genre, were not considered. In the previous study, the game genre has been reported to influence clinical characteristics that can predict IGD development. Second, although gender differences in IGD have been reported in previous studies (Dong et al., 2018; Sun et al., 2019), we did not consider gender differences. . In subsequent studies, it will be necessary to identify differences according to the gendered relationship between personality traits and brain network in IGD individuals, using the same gender distribution. Third, contrary to findings of functional connectivity, the results of effective connectivity did not show any significant differences between groups, which is not consistent with previous research. In a previous study using stochastic DCM, individuals with Internet addiction showed aberrant effective connectivity within the frontal-basal ganglia pathway engaged by response inhibition (B. Li et al., 2014). In another previous study using Granger causality analysis, altered effective connectivity within the SN in adolescents with IGD compared to HCs was detected (Yuan et al., 2016). In the current study, there were no significant differences between groups in the direction strength of the effective connectivity within each network, after FDR correction for multiple comparisons. An additional study focused on specific ROIs associated with IGD and pathways of intrinsic connectivity may address this issue.
Conclusion
Although this study has limitations, it enhances our understanding of brain connectivity related to personality traits of IGD. IGD individuals showed less functional connectivity in the CEN, SN, and DMN than HCs did. These results might be associated with cognitive deficits stemming from problematic online gaming, and might reflect neurofunctional alterations that impair the capacity of core brain networks. Additionally, we found that the severity of IGD and reward sensitivity were positively correlated with effective connectivity of the SN in IGD individuals. These results may provide clues that develop our understanding of the functionality of brain connectivity in relation to impulsivity and reward sensitivity in behavioral addiction.
Funding sources
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF–2014M3C7A1062893) and the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF–2017R1D1A1B03035471). This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Authors' contribution
D-J.K. and J-W.C. contributed to the conception and design of study. J-Y.K. and J.C. contributed to the acquisition of imaging data. H.C. and D.J.J undertook the clinical assessments. J-W.C. and C-H.P. performed imaging data analysis and wrote the manuscript including the figures and tables. D-J.K. assisted with the explanation of data and contributed to the final draft of the manuscript. D-J.K. and I.Y.C. contributed to the interpretation of data in terms of internet gaming disorder. H.C., K-J.A., J.-S.C., and D-J.K. contributed revising the manuscript logically for important theoretical content. All authors contributed to the manuscript and have approved the final manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- Ahn, H. K., Lee, H. J., Jung, D. S., Lee, S. Y., Kim, S. W., & Kang, J. H. (2002). The reliability and validity of Korean version of questionnaire for nicotine dependence. Journal of the Korean Academy of Family Medicine , 23(8), 999–1008. [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Bae, S., Han, D. H., Jung, J., Nam, K. C., & Renshaw, P. F. (2017). Comparison of brain connectivity between internet gambling disorder and internet gaming disorder: A preliminary study. Journal of Behavioral Addictions , 6(4), 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard, K. W., & Wolf, E. M. (2001). Modification in the proposed diagnostic criteria for Internet addiction. CyberPsychology and Behavior , 4(3), 377–383. 10.1089/109493101300210286. [DOI] [PubMed] [Google Scholar]
- Bunford, N., Roberts, J., Kennedy, A. E., & Klumpp, H. (2017). Neurofunctional correlates of behavioral inhibition system sensitivity during attentional control are modulated by perceptual load. Biological Psychology , 127, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, A., Flint, J., & Adshead, H. (1992). Factor structure of the wechsler adult intelligence scale–revised (WAIS–R): A clinical sample. British Journal of Clinical Psychology , 31(3), 336–338. 10.1111/j.2044-8260.1992.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Cai, W., Chen, T., Szegletes, L., Supekar, K., & Menon, V. (2015). Aberrant cross-brain network interaction in children with attention-deficit/hyperactivity disorder and its relation to attention deficits: A multisite and cross-site replication study. Biological Psychiatry. 10.1016/j.biopsych.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Carver, C. S. (2004). Negative affects deriving from the behavioral approach system. Emotion , 4(1), 3. [DOI] [PubMed] [Google Scholar]
- Carver, C. S., & Scheier, M. F. (1994). Situational coping and coping dispositions in a stressful transaction. Journal of Personality and Social Psychology , 66(1), 184. [DOI] [PubMed] [Google Scholar]
- Carver, C. S., & White, T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology , 67(2), 319. [Google Scholar]
- Chai, X. J., Whitfield-Gabrieli, S., Shinn, A. K., Gabrieli, J. D., Castanón, A. N., McCarthy, J. M., et al. (2011). Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology , 36(10), 2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, M. S., Kim, H. M., & Kim, S. Y. (2013). The effects of behavioral activation system/behavioral inhibition system (BAS/BIS) on decision-making in internet game addict. Korean Journal of Health Psychology , 18, 69–85. 10.17315/kjhp.2013.18.1.005. [DOI] [Google Scholar]
- Choi, S. W., Kim, H., Kim, G. Y., Jeon, Y., Park, S., Lee, J. Y., et al. (2014a). Similarities and differences among internet gaming disorder, gambling disorder and alcohol use disorder: A focus on impulsivity and compulsivity. Journal of Behavioral Addictions , 3(4), 246–253. 10.1556/JBA.3.2014.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. S., Park, S. M., Roh, M. S., Lee, J. Y., Park, C. B., Hwang, J. Y., et al. (2014b). Dysfunctional inhibitory control and impulsivity in Internet addiction. Psychiatry Research , 215(2), 424–428. 10.1016/j.psychres.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Chun, J. W., Choi, J., Kim, J. Y., Cho, H., Ahn, K. J., Nam, J. H., et al. (2017). Altered brain activity and the effect of personality traits in excessive smartphone use during facial emotion processing. Scientific Reports , 7(1), 1–13. 10.1038/s41598-017-08824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, M. W., Repovš, G., & Anticevic, A. (2014). The frontoparietal control system: A central role in mental health. The Neuroscientist , 20(6), 652–664. 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, M. W., Reynolds, J. R., Power, J. D., Repovs, G., Anticevic, A., & Braver, T. S. (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience , 16(9), 1348. 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr, P. J. (2002). JA Gray's reinforcement sensitivity theory: Tests of the joint subsystems hypothesis of anxiety and impulsivity. Personality and Individual Differences , 33(4), 511–532. 10.1016/S0191-8869(01)00170-2. [DOI] [Google Scholar]
- Di, X., & Biswal, B. B. (2014). Identifying the default mode network structure using dynamic causal modeling on resting-state functional magnetic resonance imaging. Neuroimage , 86, 53–59. 10.1016/j.neuroimage.2013.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman, S. J. (1990). Functional and dysfunctional impulsivity: Personality and cognitive correlates. Journal of Personality and Social Psychology , 58(1), 95. 10.1037/0022-3514.58.1.95. [DOI] [PubMed] [Google Scholar]
- Ding, W. N., Sun, J. H., Sun, Y. W., Chen, X., Zhou, Y., Zhuang, Z. G., et al. (2014). Trait impulsivity and impaired prefrontal impulse inhibition function in adolescents with internet gaming addiction revealed by a Go/No-Go fMRI study. Behavioral and Brain Functions , 10(1), 20. 10.1186/1744-9081-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, W. N., Sun, J. H., Sun, Y. W., Zhou, Y., Li, L., Xu, J. R., et al. (2013). Altered default network resting-state functional connectivity in adolescents with Internet gaming addiction. PloS One , 8(3). 10.1371/journal.pone.0059902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G., Hu, Y., & Lin, X. (2013). Reward/punishment sensitivities among internet addicts: Implications for their addictive behaviors. Progress in Neuro-Psychopharmacology and Biological Psychiatry , 46, 139–145. 10.1016/j.pnpbp.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Dong, G., Lin, X., & Potenza, M. N. (2015). Decreased functional connectivity in an executive control network is related to impaired executive function in Internet gaming disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry , 57, 76–85. 10.1016/j.pnpbp.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G., Zheng, H., Liu, X., Wang, Y., Du, X., & Potenza, M. N. (2018). Gender-related differences in cue-elicited cravings in Internet gaming disorder: The effects of deprivation. Journal of Behavioral Addictions , 7(4), 953–964. 10.1556/2006.7.2018.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A., & Wager, T. D. (2007). Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry , 164(10), 1476-1488. 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Few, L. R., Miller, J. D., Rothbaum, A. O., Meller, S., Maples, J., Terry, D. P., et al. (2013). Examination of the Section III DSM-5 diagnostic system for personality disorders in an outpatient clinical sample. Journal of Abnormal Psychology , 122(4), 1057. 10.1037/a0034878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken, I. H., Muris, P., & Georgieva, I. (2006). Gray's model of personality and addiction. Addictive Behaviors , 31(3), 399–403. 10.1016/j.addbeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Friston, K. J. (2011). Functional and effective connectivity: A review. Brain Connectivity , 1(1), 13–36. 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Friston, K. J., Kahan, J., Biswal, B., & Razi, A. (2014). A DCM for resting state fMRI. Neuroimage , 94, 396–407. 10.1016/j.neuroimage.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, J. E., Potenza, M. N., Weinstein, A., & Gorelick, D. A. (2010). Introduction to behavioral addictions. The American Journal of Drug and Alcohol Abuse , 36(5), 233–241. 10.3109/00952990.2010.491884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, J. A., & McNaughton, N. (2000). The neuropsychology of anxiety: An enquiry into the function of the septo-hippocampal system. Vol. 2 Oxford, UK: Oxford Univ. 10.1093/acprof:oso/9780198522713.001.0001. [DOI] [Google Scholar]
- Griffiths, M. D., Van Rooij, A. J., Kardefelt‐Winther, D., Starcevic, V., Király, O., Pallesen, S., et al. (2016). Working towards an international consensus on criteria for assessing internet gaming disorder: A critical commentary on petry et al.(2014). Addiction , 111(1), 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, L., Luo, M., Wang, W. X., Huang, G. L., Xu, Y., Gao, X., et al. (2018). Association between problematic Internet use, sleep disturbance, and suicidal behavior in Chinese adolescents. Journal of Behavioral Addictions , 7(4), 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. W., Han, D. H., Bolo, N., Kim, B., Kim, B. N., & Renshaw, P. F. (2015). Differences in functional connectivity between alcohol dependence and internet gaming disorder. Addictive Behaviors , 41, 12–19. 10.1016/j.addbeh.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, X., Wu, X., Wang, Y., Sun, Y., Ding, W., Cao, M., et al. (2018). Alterations of resting-state static and dynamic functional connectivity of the dorsolateral prefrontal cortex in subjects with internet gaming disorder. Frontiers in Human Neuroscience , 12, 41. 10.3389/fnhum.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno, M., Kuroda, T., Doya, K., Toyama, K., Kimura, M., Samejima, K., et al. (2004). A neural correlate of reward-based behavioral learning in caudate nucleus: A functional magnetic resonance imaging study of a stochastic decision task. Journal of Neuroscience , 24(7), 1660–1665. 10.1523/JNEUROSCI.3417-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaladjian, A., Jeanningros, R., Azorin, J. M., Anton, J. L., & Mazzola-Pomietto, P. (2011). Impulsivity and neural correlates of response inhibition in schizophrenia. Psychological Medicine , 41(2), 291–299. 10.1017/S0033291710000796. [DOI] [PubMed] [Google Scholar]
- Kim, Y., Jeong, J. E., Cho, H., Jung, D. J., Kwak, M., Rho, M. J., et al. (2016). Personality factors predicting smartphone addiction predisposition: Behavioral inhibition and activation systems, impulsivity, and self-control. PloS One , 11(8). 10.1371/journal.pone.0159788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K. & Kim, W. S. (2001). Korean-BAS/bis scale. Korean Journal of Health Psychology , 6(2), 19–37. [Google Scholar]
- Kim, Y. J., Lim, J. A., Lee, J. Y., Oh, S., Kim, S. N., Kim, D. J., et al. (2017). Impulsivity and compulsivity in internet gaming disorder: A comparison with obsessive–compulsive disorder and alcohol use disorder. Journal of Behavioral Addictions , 6(4), 545–553. 10.1556/2006.6.2017.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. S., Oh, M. K., Park, B. K., Lee, M. K., & Kim, G. J. (1999). Screening criteria of alcoholism by alcohol use disorders identification test (AUDIT) in Korea. Journal of the Korean Academy of Family Medicine , 20(9), 1152–1159. [Google Scholar]
- King, D. L., & Delfabbro, P. H. (2018). The concept of “harm” in Internet gaming disorder. Journal of Behavioral Addictions , 7(3), 562–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, D. L., Delfabbro, P. H., Potenza, M. N., Demetrovics, Z., Billieux, J., & Brand, M. (2018). Internet gaming disorder should qualify as a mental disorder. Australian and New Zealand Journal of Psychiatry , 52(7), 615–617. [DOI] [PubMed] [Google Scholar]
- King, D. L., Delfabbro, P. H., Potenza, M. N., Demetrovics, Z., Billieux, J., & Brand, M. (2019). Logic, evidence and consensus: Towards a more constructive debate on gaming disorder. Australian and New Zealand Journal of Psychiatry , 53(11), 1047–1049. [DOI] [PubMed] [Google Scholar]
- Kircaburun, K., Griffiths, M. D., & Billieux, J. (2019). Psychosocial factors mediating the relationship between childhood emotional trauma and internet gaming disorder: A pilot study. European Journal of Psychotraumatology , 10(1), 1565031. 10.1080/20008198.2018.1565031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, C. H., Liu, G. C., Hsiao, S., Yen, J. Y., Yang, M. J., Lin, W. C., et al. (2009). Brain activities associated with gaming urge of online gaming addiction. Journal of Psychiatric Research , 43(7), 739–747. 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Kühn, S., & Gallinat, J. (2015). Brains online: Structural and functional correlates of habitual internet use. Addiction Biology , 20(2), 415–422. 10.1111/adb.12128. [DOI] [PubMed] [Google Scholar]
- Kwon, J. H., Chung, C. S., & Lee, J. (2011). The effects of escape from self and interpersonal relationship on the pathological use of Internet games. Community Mental Health Journal , 47(1), 113–121. 10.1007/s10597-009-9236-1. [DOI] [PubMed] [Google Scholar]
- Lee, J. Y. (2005). Differences of psychological characteristics depending on the subtypes of Internet addiction. Research Adolescent , 12, 43–61. [Google Scholar]
- Lee, H. W., Choi, J. S., Shin, Y. C., Lee, J. Y., Jung, H. Y., & Kwon, J. S. (2012). Impulsivity in internet addiction: A comparison with pathological gambling. Cyberpsychology, Behavior, and Social Networking , 15(7), 373–377. 10.1089/cyber.2012.0063. [DOI] [PubMed] [Google Scholar]
- Lemenager, T., Hoffmann, S., Dieter, J., Reinhard, I., Mann, K., & Kiefer, F. (2018). The links between healthy, problematic, and addicted Internet use regarding comorbidities and self-concept-related characteristics. Journal of Behavioral Addictions , 7(1), 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens, J. S., Valkenburg, P. M., & Gentile, D. A. (2015). The Internet gaming disorder scale. Psychological Assessment , 27(2), 567. 10.1037/pas0000062. [DOI] [PubMed] [Google Scholar]
- Li, B., Friston, K. J., Liu, J., Liu, Y., Zhang, G., Cao, F., et al. (2014). Impaired frontal-basal ganglia connectivity in adolescents with internet addiction. Scientific Reports , 4, 5027. 10.1038/srep05027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Li, Y., Yang, W., Zhang, Q., Wei, D., Li, W., et al. (2015). Brain structures and functional connectivity associated with individual differences in Internet tendency in healthy young adults. Neuropsychologia , 70, 134–144. 10.1016/j.neuropsychologia.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Lukavská, K. (2018). The immediate and long-term effects of time perspective on Internet gaming disorder. Journal of Behavioral Addictions , 7(1), 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyvers, M., Karantonis, J., Edwards, M. S., & Thorberg, F. A. (2016). Traits associated with internet addiction in young adults: Potential risk factors. Addictive Behaviors Reports , 3, 56–60. 10.1016/j.abrep.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, N., Liu, Y., Fu, X. M., Li, N., Wang, C. X., Zhang, H., et al. (2011). Abnormal brain default-mode network functional connectivity in drug addicts. PloS One , 6(1). 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences , 15(10), 483–506. 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function , 214(5–6), 655–667 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg, M. J., Laird, A. R., Thelen, S., Carter, C. S., & Glahn, D. C. (2009). Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry , 66(8), 811–822. 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, K. W., Beutel, M. E., Dreier, M., & Wölfling, K. (2019). A clinical evaluation of the DSM-5 criteria for Internet Gaming Disorder and a pilot study on their applicability to further Internet-related disorders. Journal of behavioral addictions , 8(1), 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na, E. Y., Park, S. R., & Kim, E. M. (2007). Ways of media use and application in the subtypes of adolescents’ internet use. Korean Journal of Communication , 51, 392–427. [Google Scholar]
- O'Doherty, J., Dayan, P., Schultz, J., Deichmann, R., Friston, K., & Dolan, R. J. (2004). Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science , 304(5669), 452–454. 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia , 9(1), 97–113. 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park, S. M., Lee, J. Y., Kim, Y. J., Lee, J. Y., Jung, H. Y., Sohn, B. K., et al. (2017). Neural connectivity in internet gaming disorder and alcohol use disorder: A resting-state EEG coherence study. Scientific Reports , 7(1), 1–12. 10.1038/s41598-017-01419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlikowski, M., & Brand, M. (2011). Excessive internet gaming and decision making: Do excessive World of warcraft players have problems in decision making under risky conditions?. Psychiatry Research , 188(3), 428–433. 10.1016/j.psychres.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Petry, N. M., Rehbein, F., Gentile, D. A., Lemmens, J. S., Rumpf, H. J., Mößle, T., et al. (2014). An international consensus for assessing internet gaming disorder using the new DSM‐5 approach. Addiction , 109(9), 1399–1406. 10.1111/add.12457. [DOI] [PubMed] [Google Scholar]
- Robbins, T. W., Curran, H. V., & De Wit, H. (2012). Special issue on impulsivity and compulsivity. 10.1007/s00213-011-2584-x. [DOI] [PubMed] [Google Scholar]
- Rumpf, H. J., Achab, S., Billieux, J., Bowden-Jones, H., Carragher, N., Demetrovics, Z., et al. (2018). Including gaming disorder in the ICD-11: The need to do so from a clinical and public health perspective: Commentary on: A weak scientific basis for gaming disorder: Let us err on the side of caution (van Rooij et al., 2018). Journal of Behavioral Addictions , 7(3), 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience , 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharaev, M. G., Zavyalova, V. V., Ushakov, V. L., Kartashov, S. I., & Velichkovsky, B. M. (2016). Effective connectivity within the default mode network: Dynamic causal modeling of resting-state fMRI data. Frontiers in Human Neuroscience , 10, 14. 10.3389/fnhum.2016.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, J. S., Dvorak, R. D., & Lau-Barraco, C. (2009). Behavioral inhibition and activation systems: Differences in substance use expectancy organization and activation in memory. Psychology of Addictive Behaviors , 23(2), 315. 10.1037/a0015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie, L. D., & Jackson, C. J. (2006). Functional impulsivity and reinforcement sensitivity theory. Journal of Personality , 74(1), 47–84. 10.1111/j.1467-6494.2005.00369.x. [DOI] [PubMed] [Google Scholar]
- Son, K. L., Choi, J. S., Lee, J., Park, S. M., Lim, J. A., Lee, J. Y., et al. (2015). Neurophysiological features of internet gaming disorder and alcohol use disorder: A resting-state EEG study. Translational Psychiatry, 5(9), e628-e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee, G., Foa, E. B., & Grayson, J. B. (1982). Recent advances in the behavioral treatment of obsessive-compulsives. Archives of General Psychiatry , 39(12), 1365–1371. 10.1001/archpsyc.1982.04290120001001. [DOI] [PubMed] [Google Scholar]
- Sun, Y., Wang, Y., Han, X., Jiang, W., Ding, W., Cao, M., et al. (2019). Sex differences in resting-state cerebral activity alterations in internet gaming disorder. Brain Imaging and Behavior , 13(5), 1406–1417. 10.1007/s11682-018-9955-4. [DOI] [PubMed] [Google Scholar]
- Tan, H. Y., Choo, W. C., Fones, C. S., & Chee, M. W. (2005). fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. American Journal of Psychiatry , 162(10), 1849–1858. 10.1176/appi.ajp.162.10.1849. [DOI] [PubMed] [Google Scholar]
- Toga, A. W. (2015). Brain mapping: An encyclopedic reference. Academic Press. [Google Scholar]
- Torres-Rodríguez, A., Griffiths, M. D., Carbonell, X., & Oberst, U. (2018). Internet gaming disorder in adolescence: Psychological characteristics of a clinical sample. Journal of Behavioral Addictions , 7(3), 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow, N. D., Wang, G. J., Fowler, J. S., & Tomasi, D. (2012). Addiction circuitry in the human brain. Annual Review of Pharmacology and Toxicology , 52, 321–336. 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L., Shen, H., Lei, Y., Zeng, L. L., Cao, F., Su, L., et al. (2017). Altered default mode, fronto-parietal and salience networks in adolescents with Internet addiction. Addictive Behaviors , 70, 1–6. 10.1016/j.addbeh.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Wang, L., Wu, L., Lin, X., Zhang, Y., Zhou, H., Du, X., et al. (2016). Dysfunctional default mode network and executive control network in people with Internet gaming disorder: Independent component analysis under a probability discounting task. European Psychiatry , 34, 36–42. 10.1016/j.eurpsy.2016.01.2424. [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2018). International statistical classification of diseases and related health problems, 11th Revision (ICD-11). World Health Organization. https://icd.who.int/browse11/l-m/en. [Google Scholar]
- Yen, J. Y., Cheng-Fang, Y., Chen, C. S., Chang, Y. H., Yeh, Y. C., & Ko, C. H. (2012). The bidirectional interactions between addiction, behaviour approach and behaviour inhibition systems among adolescents in a prospective study. Psychiatry Research , 200(2–3), 588–592. 10.1016/j.psychres.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Yen, J. Y., Liu, T. L., Wang, P. W., Chen, C. S., Yen, C. F., & Ko, C. H. (2017). Association between Internet gaming disorder and adult attention deficit and hyperactivity disorder and their correlates: Impulsivity and hostility. Addictive Behaviors , 64, 308–313. 10.1016/j.addbeh.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Yuan, K., Qin, W., Yu, D., Bi, Y., Xing, L., Jin, C., et al. (2016). Core brain networks interactions and cognitive control in internet gaming disorder individuals in late adolescence/early adulthood. Brain Structure and Function , 221(3), 1427–1442. 10.1007/s00429-014-0982-7. [DOI] [PubMed] [Google Scholar]
- Yuan, K., Yu, D., Cai, C., Feng, D., Li, Y., Bi, Y., et al. (2017). Frontostriatal circuits, resting state functional connectivity and cognitive control in internet gaming disorder. Addiction Biology , 22(3), 813–822. 10.1111/adb.12348. [DOI] [PubMed] [Google Scholar]
- Zhang, J. T., Ma, S. S., Yan, C. G., Zhang, S., Liu, L., Wang, L. J., et al. (2017). Altered coupling of default-mode, executive-control and salience networks in Internet gaming disorder. European Psychiatry , 45, 114–120. 10.1016/j.eurpsy.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Zisserson, R. N., & Palfai, T. P. (2007). Behavioral Activation System (BAS) sensitivity and reactivity to alcohol cues among hazardous drinkers. Addictive Behaviors , 32(10), 2178–2186. 10.1016/j.addbeh.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]