Abstract
Objective:
The international prognostic index (IPI) and the revised IPI (R-IPI) are used to determine the prognosis in diffuse large B-cell lymphoma (DLBCL). However, these scoring systems are insufficient to identify very high-risk patients. Recently, the prognostic nutritional index (PNI) -calculated with lymphocyte count and albumin- has been used to determine the prognosis in DLBCL. This study aimed to evaluate the effect of PNI score on prognosis and survival in patients with high-risk DLBCL.
Methods:
Patients diagnosed with DLBCL and treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone were included. Pre-treatment IPI, R-IPI, and PNI scores and progression-free survival (PFS) and overall survival (OS) times were calculated. The cutoff value for PNI according to OS was determined by using the X-Tile program.
Results:
One hundred and ten patients were included, the median age was 63 years and the median follow-up period was 25 months. According to R-IPI, the median OS could not be reached for the very good risk group, and the median OS values were 83 and 17 months in the good and poor-risk groups, respectively (p=0.001). The cohort was divided into three groups according to the cut-off value for the PNI: patients with PNI <33 were classified as high-risk, 33-42 intermediate-risk, and ≥42 as low-risk. According to PNI, the median durations of PFS and OS were 2 months and 3 months in the high-risk group, 9 months and 19 months in the intermediate-risk group respectively, and in the low-risk group the median duration for PFS and OS could not be reached (p=0.001).
Conclusions:
The R-IPI is widely used to estimate the prognosis in DLBCL. But in our cohort, in the poor-risk patient group, the OS was 17 months according to R-IPI, while this period was 3 months according to PNI. This finding demonstrated that PNI might predict early mortality in DLBCL.
Keywords: Diffuse large B-cell lymphoma, prognostic nutritional index, survival
Abstract
Amaç:
Diffüz büyük B-hücreli lenfomada (DBBHL) prognozu belirlemede internasyonel prognostik indeks (IPI) ve revize edilmiş IPI (R-IPI) kullanılmaktadır. Ancak bu skorlama sistemleri çok yüksek riskli hastaları belirlemede yetersiz kalmaktadır. Son zamanlarda DBBHL’de prognozu belirlemede lenfosit ve albümin ile hesaplanan prognostik nutrisyonel indeks (PNİ) kullanılmaya başlanmıştır. Bu çalışmada amaç; DBBHL’de yüksek riskli hastalarda PNİ skorunun prognoz ve sağkalıma olan etkisini değerlendirmektir.
Yöntemler:
Çalışmaya DBBHL tanısı konmuş ve rituksimab, siklofosfamid, doksorubisin, vinkristin, prednizolon tedavisi almış hastalar alındı. Tedavi öncesi IPI, R-IPI ve PNİ skorları ile progresyonsuz sağkalım (PS) ve toplam sağkalım (TS) süreleri hesaplandı. TS’ye göre PNİ için sınır değer X-Tile programı ile belirlendi.
Bulgular:
Çalışmaya alınan 110 hastanın medyan yaşı 63, medyan takip süresi 25 aydı. R-IPI’ya göre çok iyi riskli grup için medyan TS süresine ulaşılamamışken, iyi ve kötü riskli gruplarda medyan TS sırası ile 83 ve 17 aydı (p=0,001). PNİ skoru için TS’de gruplar arasında fark yaratan sınır değere göre kohort üç gruba ayrıldı: PNİ<33 olan hastalar yüksek riskli, 33-42 arası orta riskli, PNİ≥42 olan hastalar iyi riskli olarak sınıflandırıldı. PNİ’ye göre medyan PS ve TS süreleri sırasıyla yüksek riskli grupta 2 ay ve 3 ay, orta riskli grupta 9 ay ve 19 aydı, iyi riskli grupta PS ve TS için medyan takip sürelerine ulaşılamadı (p=0,001).
Sonuçlar:
DBBHL’de prognozu belirlemede R-IPI kullanılmaktadır, fakat bizim kohortumuzda kötü riskli hasta grubunda R-IPI’ya göre hesaplanan TS 17 ay iken PNİ’ye göre bu sürenin 3 ay olduğu belirlenmiştir. Bu bulgu PNİ’nin erken mortaliteyi başarılı bir şekilde öngörebileceğini göstermektedir.
Keywords: Diffüz büyük B-hücreli lenfoma, prognostik nutrisyonel indeks, sağkalım
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common type of lymphoma in adults and accounts for 28% of all non-Hodgkin lymphomas1. Treatment was mainly composed of anthracycline-based combined chemotherapy regimens such as cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP) in the recent past but with the addition of rituximab to this combination (R-CHOP), 20% improvement in treatment responses were achieved2. Today, R-CHOP remains the gold standard treatment regimen in DLBCL3. However, DLBCL is a heterogeneous disease and the response to treatment may differ. A minority of patients may be unresponsive and have a worse outcome. To identify this patient group, -before the rituximab era- international prognostic index (IPI) has been the primary prognostic tool to determine the prognosis of DLBCL which is still the most commonly used scoring system4.
In the rituximab era, the revised-IPI (R-IPI) scoring system -that is plotted by the redistribution of the IPI elements- started to be widely used to make inferences about prognosis5. Yet IPI and R-IPI both could not strictly predict overall survival in high-risk patients, therefore a National Comprehensive Cancer Network IPI (NCCN-IPI) scoring system was created and the poorer survival in very high-risk patients was predicted better6.
Prognostic nutritional index (PNI) is a different prognostic parameter which is calculated by the formula of serum albumin (g/L) + 5 x absolute lymphocyte count (109/L) and has been substantially developed to determine preoperative immune nutrition status and the surgical risk in patients diagnosed as having gastrointestinal malignancies7. PNI has been also claimed to be a useful prognostic tool in hematological malignancies8.
The aim of this study was to evaluate whether the PNI score, which was a marker of nutrition and immune system, could predict progression-free survival (PFS) and overall survival (OS) in patients with high-risk DLBCL.
MATERIALS and METHODS
Patients diagnosed with DLBCL by pathological examination in our hematology clinic between 2010-2021 and treated with R-CHOP regimen were included in the study. Pre-treatment data were collected from patients’ files retrospectively. The laboratory values of serum albumin and absolute lymphocyte count were noted and PNI scores were calculated as described before7. According to the age, Eastern Cooperative Oncology Group (ECOG) performance score, Ann-Arbor stage of the disease, the presence of extranodal involvement, and serum lactate dehydrogenase (LDH) level at the time of diagnosis; the patients’ IPI and NCCN-IPI scores were calculated4,6. Pregnant women, patients under the age of 18, patients diagnosed with primary central nervous system lymphoma, acquired immunodeficiency syndrome related lymphoma, or with Richter’s transformation were excluded. Patients who had a history of solid organ malignancy or who were treated with a regimen other than R-CHOP were also excluded. PFS was defined as the time from diagnosis to progression or death, and OS was defined as the time from diagnosis to death from any cause. The primary end-point was the prediction of OS and the secondary end-point was the prediction of PFS. The study was approved by the Clinical Research Ethics Committee of Istanbul Medeniyet University Goztepe Training and Research Hospital (decision no: 2021/0495, date: 06.10.2021) and conducted per the Helsinki Declaration.
Statistical Analysis
The SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.) program was used for all statistical analyses and figure creation. Normality of the data was tested with the Kolmogorov-Smirnov test. If the normality requirement was satisfied for the descriptive statistics, the results were reported as mean +/- standard deviation, while if normality requirement was not satisfied the results were reported as median and range [minimum (min)-maximum (max)]. Chi-square test was used for categorical variables and Student's t-test for continuous variables for comparison between groups. The median survival time was calculated with the Kaplan-Meier method and to estimate the difference of survival between groups, log-rank test was performed. The X-tile 3.6.1 software (Yale University, New Haven, CT, USA) was used to determine the optimal cut-off value of PNI, which was identified from the min p-value according to the OS. All analyses were two-tailed and the type 1 error rate was determined as 5%.
RESULTS
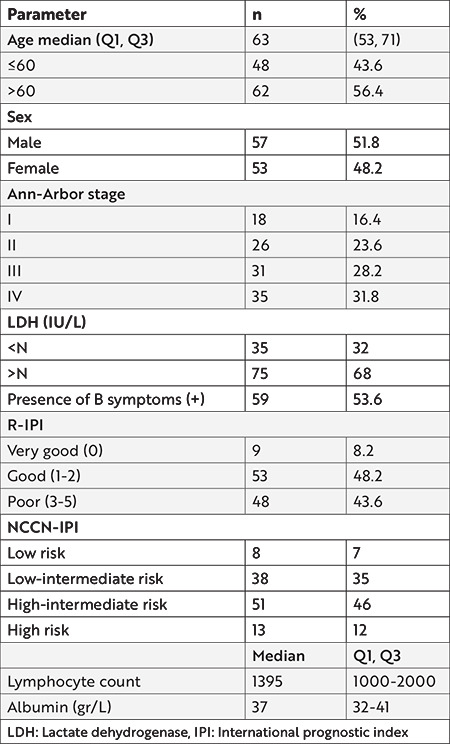
Of the 148 patients diagnosed with DLBCL, 38 patients who did not receive rituximab and anthracycline-based chemotherapy and whose data were missing were excluded. Therefore, 110 patients were included in the study cohort and evaluated retrospectively. Fifty-three patients (48%) were female and the median age was 63 years (range between 23-88). The characteristics of the patients at the time of diagnosis are summarized in Table 1. The median follow-up time was 25 months (min: 1-max: 97 months). The mean PNI score was calculated as 44.78±0.95 (range: 10.56-79.74).
Table 1. Patients’ characteristics at the time of diagnosis.
The patients were classified as very good, good, and poor-risk groups according to R-IPI. While the median PFS could not be reached for the very good risk disease group, the median PFS values for the good and poor-risk groups were 52 and 9 months, respectively (p=0.001) (Figure 1). Likewise, the median OS could not be reached for the very good risk group. The median OS values for the good and poor-risk disease groups were 83 and 17 months, respectively (p=0.001) (Figure 1).
Figure 1.
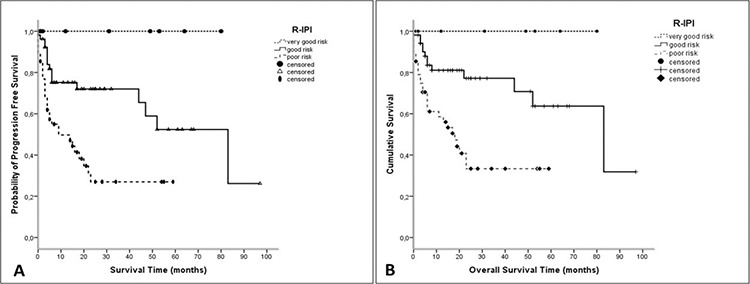
A, B) Progression-free survival and overall survival of patients according to R-IPI.
R-IPI: Revised international prognostic index
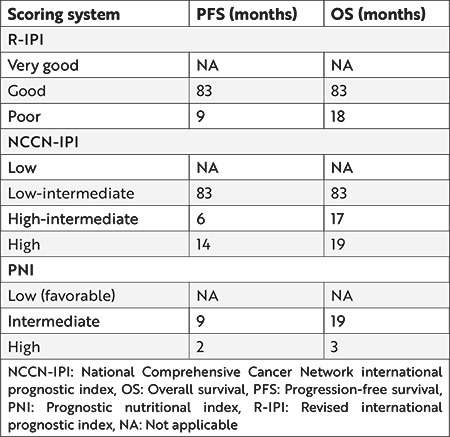
According to NCCN-IPI, the patients were classified into 4 groups: low, low-intermediate, high-intermediate, and high risk. While the median follow-up time could not be reached for PFS and OS in the low-risk group, they were 20 and 83 months in the low-intermediate group, respectively. The PFS ranks in the high-intermediate and high-risk groups were 23 and 17 months, and OS values were 17 and 19 months, respectively (Table 2).
Table 2. Whole cohort’s progression-free survival and overall survival times according to risk scores.
The cut-off value for PNI that made a difference in OS between groups was determined by using the X-Tile program and the cohort was divided into three groups as follows: patients with PNI<33 were classified as high-risk, 33-42 intermediate-risk, and ≥42 as low-risk (favorable). According to PNI, the median duration of PFS and OS were 2 months and 3 months in the high-risk group, 9 months and 19 months in the intermediate-risk group, respectively, and in the low-risk group the median duration of PFS and OS could not be reached (p=0.001) (Figure 2).
Figure 2.
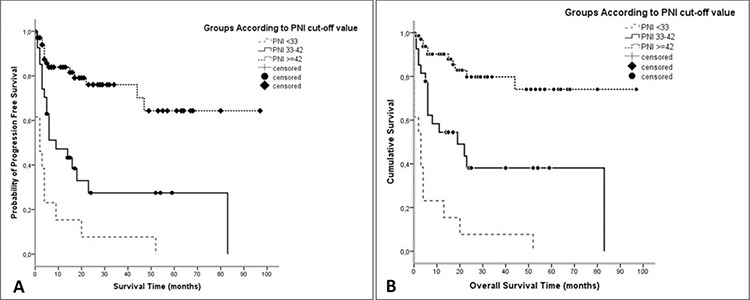
Progression-free survival and overall survival of patients according to PNI.
PNI: Prognostic nutritional index
The estimated five-year OS durations were 0%, 38.1%, and 73.8% for PNI high, intermediate, and low-risk groups, respectively (p=0.001).
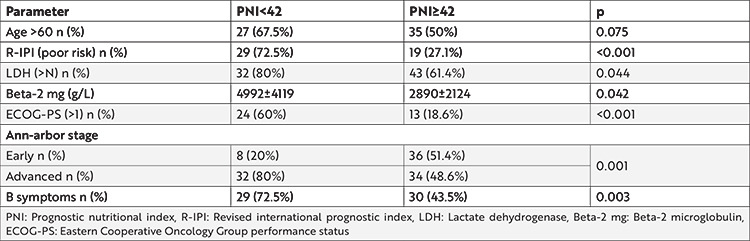
Comparison of parameters by dividing the entire cohort into 2 groups according to PNI as low-risk patients (PNI≥42) and intermediate plus high-risk patients (PNI<42) revealed that patients who were in the low-risk group had lower LDH and beta-2 microglobulin levels, better ECOG performance score, earlier stage, and fewer B symptoms. The age did not differ between these groups (Table 3).
Table 3. Comparison of parameters between low-risk patients and intermediate plus high-risk patients according to PNI.
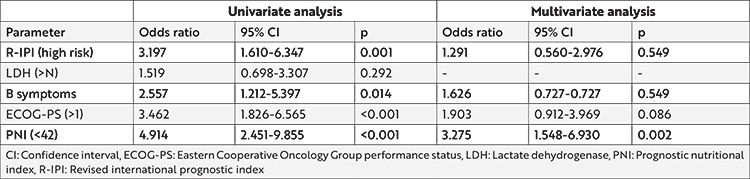
Univariate logistic regression analysis was performed to identify the risk factors for OS. IPI risk score, presence of B symptoms, poor performance score (ECOG>1), and low PNI were identified as poor risk factors for OS. High LDH was not associated with OS. Multivariate logistic regression analysis demonstrated a 3.27 times increased risk of death in patients with a low PNI score (p=0.002) (Table 4).
Table 4. Univariate and multivariate logistic regression analysis for overall survival (OS) in patients with diffuse large B-cell lymphoma.
DISCUSSION
Despite the improvements in survival with R-CHOP treatment in DLBCL, it remains an important cause of mortality among patients with hematological malignancies. While the incidence of DLBCL is growing, it is the 4th leading cause of death from cancer in people aged 20-40 years9. R-CHOP regimen remains the gold standard treatment but there is an emerging need for tailored therapies for high-risk patients. For this purpose, the identification of high-risk patients at the time of diagnosis is gaining greater importance. The most widely accepted risk scoring system, IPI, predicts a 55 percent 4-year OS for high-risk patients5. It is obvious that this modeling cannot distinct the group of patients with an OS probability of less than 50%. Hence, NCCN-IPI was developed with similar parameters and it was observed that it showed a better 5-year survival by 33% in the high-risk group6. To ameliorate these scoring systems, many different parameters such as hyperfibrinogenemia and albumin levels at diagnosis were examined in clinical trials and their effect on the survival probabilities were calculated10,11. Elevated serum C-reactive protein and free light chain levels, which were the markers of inflammation, were found to be independent prognostic factors12,13. After the notification of the low absolute lymphocyte count for being an independent poor risk factor in DLBCL, the idea of using the PNI score which was primarily developed for solitary malignancies came up to predict survival in DCBCL8,14.
In the present study, the predictive PNI score for OS was 42. Similar median score values were reported in solitary malignancies and DLBCL in previous studies, and similar cut-offs were reported to be predictive for OS (PNI score: 40-45)8,15. Although almost all of the studies on this topic were carried out in the Far East, the limited data obtained in studies conducted in Western countries also showed resemblance16. While the PNI score was found to favor OS in most studies, there were also a few studies suggesting that it could not impact OS17,18. When the cut-off value for OS was determined by using the X-tile program for the PNI score, which was divided into two prognostic groups in previous studies, the third group with a very poor prognosis was differentiated with a PNI<33 value. The reason why such a group was not defined in other studies might be that the patients who experienced early mortality were not included.
As the wide acceptance of R-IPI in determining the risk of DLBCL, our study demonstrated that the OS values were 17 months and 19 months in our high-risk patients according to R-IPI and NCCN-IPI, respectively, but OS was 3 months in the same patient population according to PNI with the cut-off value <33. This finding supports that PNI may predict early mortality in DLBCL better than both R-IPI and NCCN-IPI scores. In addition, our findings reveal that the PNI score also predicts the duration of PFS. Zhou et al.19 stated that a low PNI score could indicate short event-free survival (EFS) in patients receiving R-CHOP. Interestingly, it has been shown that the PNI score cannot separate risk groups for OS and EFS in patients receiving CHOP, unlike patients receiving R-CHOP19,20. While the PNI score is successful in demonstrating the prognosis in combination treatments with rituximab, it cannot sufficiently differentiate the high-risk group in patients who do not receive immunotherapy. The reason for this may be the already expected worse outcomes in patients treated with CHOP.
In this study, PNI was also found to have a positive or negative relationship with the known prognostic factors -as anticipated- such as Ann-Arbor stage, ECOG, LDH, IPI, presence of B symptoms, and beta-2 microglobulin. The absence of a direct relationship between PNI and age and the fact that the mean age did not differ significantly in the high-risk PNI group suggested that the poor prognosis in this patient group was due to the disease rather than the age-related fragility of the patients. Although the pathophysiology of the relationship between low PNI score and poor prognosis is not fully understood, hypoalbuminemia may be an explanatory factor as being an indicator of nutritional deficiency and inflammation21. In solitary organ malignancies, the inflammatory state is aggravated and the increase in tumor necrosis factor and IL-6 can deepen hypoalbuminemia. There is not enough knowledge yet on whether hypoalbuminemia is an outcome or a treatment target. Albumin has a greater effect on the calculation of the PNI score than the lymphocyte count. Lymphopenia has also been defined to be a poor prognostic factor in lymphoma22,23. Although lymphopenia in solitary malignancies is thought to be due to concomitant immune suppression, the relationship of lymphopenia with poor prognosis in lymphomas has not been fully elucidated. It is thought that early lymphocyte recovery after autologous stem cell transplantation gives better results in NHL and multiple myeloma, which is due to earlier immune restructuring24. In later studies it was reported that lymphocyte recovery depended on the number of NK lymphocytes25.
Several limitations of our study deserve to be mentioned. Since being a retrospective analysis, we were not able to evaluate the NK lymphocyte count of patients. Except for the scoring systems, it was not possible to evaluate the risk profile of patients per genetic risk factors and the cell of origin (germinal or nongerminal center), even though immune histochemically, which could not be performed in all patients. Although it is known that cytogenetically double/triple hit lymphoma and active B type lymphoma have a worse prognosis, designing studies in the framework of these parameters requires expensive and specialized procedures26,27.
CONCLUSIONS
PNI is a simple, inexpensive, and easily applicable risk profiling system and is especially successful in predicting early mortality. Prospective studies are needed to better investigate the causes of hypoalbuminemia and lymphopenia, which are components of the PNI score, in terms of targeted therapy alternatives in reducing early mortality.
Footnotes
Ethics
Ethics Committee Approval: The study was approved by the Clinical Research Ethics Committee of Istanbul Medeniyet University Goztepe Training and Research Hospital (decision no: 2021/0495, date: 06.10.2021) and conducted per the Helsinki Declaration.
Informed Consent: Retrospective study.
Peer-review: Externally and internally peer-reviewed.
Author Contributions
Surgical and Medical Practices: E.O., T.E., E.K., I.E.O., Concept: E.O., T.E., E.K., I.E.O., Design: E.O., Data Collection and/or Processing: E.O., T.E., E.K., B.K., I.E.O., Analysis and/or Interpretation: E.O., T.E., I.E.O., Literature Search: E.O., Writing: E.O., I.E.O.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–76. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 3.Sehn LH, Salles G. Diffuse Large B-Cell Lymphoma. N Engl J Med. 2021;384:842–58. doi: 10.1056/NEJMra2027612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 5.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–61. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837–42. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139:160–7. doi: 10.1016/0002-9610(80)90246-9. [DOI] [PubMed] [Google Scholar]
- 8.Luan C, Wang F, Wei N, Chen B. Prognostic nutritional index and the prognosis of diffuse large b-cell lymphoma: a meta-analysis. Cancer Cell Int. 2020;20:455. doi: 10.1186/s12935-020-01535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 10.Niu JY, Tian T, Zhu HY, et al. Hyperfibrinogenemia is a poor prognostic factor in diffuse large B cell lymphoma. Ann Hematol. 2018;97:1841–9. doi: 10.1007/s00277-018-3382-x. [DOI] [PubMed] [Google Scholar]
- 11.Bairey O, Shacham-Abulafia A, Shpilberg O, Gurion R. Serum albumin level at diagnosis of diffuse large B-cell lymphoma: an important simple prognostic factor. Hematol Oncol. 2016;34:184–92. doi: 10.1002/hon.2233. [DOI] [PubMed] [Google Scholar]
- 12.Maurer MJ, Micallef IN, Cerhan JR, et al. Elevated serum free light chains are associated with event-free and overall survival in two independent cohorts of patients with diffuse large B-cell lymphoma. J Clin Oncol. 2011;29:1620–6. doi: 10.1200/JCO.2010.29.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troppan KT, Schlick K, Deutsch A, et al. C-reactive protein level is a prognostic indicator for survival and improves the predictive ability of the R-IPI score in diffuse large B-cell lymphoma patients. Br J Cancer. 2014;111:55–60. doi: 10.1038/bjc.2014.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song MK, Chung JS, Seol YM, et al. Influence of low absolute lymphocyte count of patients with nongerminal center type diffuse large B-cell lymphoma with R-CHOP therapy. Ann Oncol. 2010;21:140–4. doi: 10.1093/annonc/mdp505. [DOI] [PubMed] [Google Scholar]
- 15.Luan CW, Tsai YT, Yang HY, et al. Pretreatment prognostic nutritional index as a prognostic marker in head and neck cancer: a systematic review and meta-analysis. Sci Rep. 2021;11:17117. doi: 10.1038/s41598-021-96598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Periša V, Zibar L, Knezović A, Periša I, Sinčić-Petričević J, Aurer I. Prognostic nutritional index as a predictor of prognosis in patients with diffuse large B cell lymphoma. Wien Klin Wochenschr. 2017;129:411–9. doi: 10.1007/s00508-016-1077-7. [DOI] [PubMed] [Google Scholar]
- 17.Yu W, Guo Q, Wang Z, et al. Clinical Significance of Prognostic Nutritional Index for Patients with Diffuse Large B-cell Lymphoma. Nutr Cancer. 2019;71:569–74. doi: 10.1080/01635581.2018.1540718. [DOI] [PubMed] [Google Scholar]
- 18.Hao X, Wei Y, Wei X, et al. Glasgow prognostic score is superior to other inflammation-based scores in predicting survival of diffuse large B-cell lymphoma. Oncotarget. 2017;8:76740–8. doi: 10.18632/oncotarget.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Q, Wei Y, Huang F, et al. Low prognostic nutritional index predicts poor outcome in diffuse large B-cell lymphoma treated with R-CHOP. Int J Hematol. 2016;104:485–90. doi: 10.1007/s12185-016-2052-9. [DOI] [PubMed] [Google Scholar]
- 20.Luan C, Wu X, Zhang J, Chen B. Low Prognostic Nutritional Index Indicates Dismal Prognosis in Patients with Diffuse Large B Cell Lymphoma. Clin Oncol Res. 2020;3:1–8. [Google Scholar]
- 21.Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J Parenter Enteral Nutr. 2019;43:181–93. doi: 10.1002/jpen.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talaulikar D, Choudhury A, Shadbolt B, Brown M. Lymphocytopenia as a prognostic marker for diffuse large B cell lymphomas. Leuk Lymphoma. 2008;49:959–64. doi: 10.1080/10428190801959026. [DOI] [PubMed] [Google Scholar]
- 23.Kim DH, Baek JH, Chae YS, et al. Absolute lymphocyte counts predicts response to chemotherapy and survival in diffuse large B-cell lymphoma. Leukemia. 2007;21:2227–30. doi: 10.1038/sj.leu.2404780. [DOI] [PubMed] [Google Scholar]
- 24.Porrata LF, Gertz MA, Inwards DJ, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98:579–85. doi: 10.1182/blood.v98.3.579. [DOI] [PubMed] [Google Scholar]
- 25.Porrata LF, Inwards DJ, Ansell SM, et al. Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: a prospective study. Biol Blood Marrow Transplant. 2008;14:807–16. doi: 10.1016/j.bbmt.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott DW. Cell-of-Origin in Diffuse Large B-Cell Lymphoma: Are the Assays Ready for the Clinic? Am Soc Clin Oncol Educ Book. 2015:458–66. doi: 10.14694/EdBook_AM.2015.35.e458. [DOI] [PubMed] [Google Scholar]
- 27.Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117:2319–31. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]


