Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Inhibitors of human Syk kinase suppress parasite egress.
Syk inhibitors prevent the tyrosine phosphorylation of band 3 in P falciparum parasitized red blood cells, reducing the release of microparticles.
Abstract
Band 3 (also known as the anion exchanger, SLCA1, AE1) constitutes the major attachment site of the spectrin-based cytoskeleton to the erythrocyte’s lipid bilayer and thereby contributes critically to the stability of the red cell membrane. During the intraerythrocytic stage of Plasmodium falciparum’s lifecycle, band 3 becomes tyrosine phosphorylated in response to oxidative stress, leading to a decrease in its affinity for the spectrin/actin cytoskeleton and causing global membrane destabilization. Because this membrane weakening is hypothesized to facilitate parasite egress and the consequent dissemination of released merozoites throughout the bloodstream, we decided to explore which tyrosine kinase inhibitors might block the kinase-induced membrane destabilization. We demonstrate here that multiple Syk kinase inhibitors both prevent parasite-induced band 3 tyrosine phosphorylation and inhibit parasite-promoted membrane destabilization. We also show that the same Syk kinase inhibitors suppress merozoite egress near the end of the parasite’s intraerythrocytic lifecycle. Because the entrapped merozoites die when prevented from escaping their host erythrocytes and because some Syk inhibitors have displayed long-term safety in human clinical trials, we suggest Syk kinase inhibitors constitute a promising class of antimalarial drugs that can suppress parasitemia by inhibiting a host target that cannot be mutated by the parasite to evolve drug resistance.
Introduction
The human malaria parasite, Plasmodium falciparum, currently infects >200 million people annually, causing ∼500 000 deaths per year and imposing considerable morbidity on the surviving population. 1,2 Because strains of P falciparum are rapidly emerging that are resistant to all known antimalarial drugs, including artemisinin, quinine, chloroquine, piperaquine, and mefloquine and their derivatives, considerable emphasis is now being focused on development of new therapies with novel mechanisms of action. 2 -6 Although reports of promising new antimalarials are now appearing in the literature, 7 -15 that vast majority of these novel drug candidates target parasite-encoded proteins/pathways, leaving the opportunity open for adventitious parasites to mutate resistance to the new therapeutics. As noted by others, the ideal solution to this problem would emerge if new antimalarials could be developed that would function by inhibiting a host-encoded protein/pathway that the parasite must coopt in order to survive and proliferate. 8,16 Following this guidance, the goal of this work was to identify an erythrocyte-encoded enzyme whose inhibition would prevent the parasite’s propagation and thereby terminate the parasitemia.
In our search for such a critical erythrocyte enzyme, we undertook to identify the parasite-induced changes in erythrocyte membrane biology that might require the integral participation of human red blood cell (RBC) components. 7,17 -25 Although the Plasmodium species is well known to export hundreds of proteins into the erythrocyte compartment, including lipid kinases, multiple structural proteins, and a tyrosine phosphatase-like enzyme that must be activated to facilitate parasite invasion, 26 -29 we noted that the steady increase in tyrosine phosphorylation of erythrocyte membrane band 3 during parasite maturation would likely involve an erythrocyte tyrosine kinase. 19,20,30 This line of investigation was further encouraged by the observation that tyrosine phosphorylation of band 3 had been previously shown to cause rupture of the band 3–ankyrin bridge connecting the erythrocyte membrane to its spectrin/actin cytoskeleton, and rupture of this bridge promotes severe membrane destabilization, vesiculation, and fragmentation. 31 Considering these observations together, we hypothesized that parasite-triggered activation of some RBC tyrosine kinase might cause the membrane weakening that enables egress of the parasite from its RBC host and thereby facilitate propagation of the parasitemia. To test this hypothesis, we elected to examine whether any RBC tyrosine kinase inhibitors might effectively prevent both RBC membrane weakening and the consequent escape of mature merozoites from their infected host erythrocytes. In this paper, we demonstrate that multiple inhibitors of erythrocyte Syk tyrosine kinase block the customary erythrocyte membrane vesiculation that occurs during parasite maturation. We further show that these same inhibitors suppress parasite egress at the end of its lifecycle and thereby prevent spread of the merozoites in cultures of fresh human erythrocytes.
Materials and methods
Unless otherwise stated, all materials were obtained from Sigma-Aldrich (St. Louis, MO).
Cultivation of P falciparum–infected RBCs
Freshly drawn blood (Rh+) from healthy adults of both sexes was used. Patients provided written, informed consent before entering the study. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki and was approved by Hue University Ethics Committee. Blood anticoagulated with heparin was stored in citrate-phosphate-dextrose with adenine prior to its use. RBCs were separated from plasma and leukocytes by 3 washings in RPMI 1640 medium. P falciparum laboratory strains Palo Alto, it-G, and Dd2 (mycoplasma-free) and fresh isolates from the Quang Tri province of Vietnam were cultivated in RPMI 1640 medium containing N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, supplemented with 20 mM glucose, 2 mM glutamine, 0.025 mM adenine, and 32 mg/L gentamycin at 2% hematocrit. 32,33 Parasite cultures were synchronized as described by Lambros and Vanderberg. 34 For the assessment of half-maximal drug concentration for inhibition of malaria survival (IC50) values, 35 synchronized P falciparum–infected RBCs cultures were used at ring stage (14 to 18 hours postinfection) or trophozoite stage (34 hours to 38 hours), at a parasitemia of ∼10%. Where indicated, cultures were treated with the Syk inhibitors: Syk inhibitor II, Syk inhibitor IV (from now on abbreviated as SYK II and SYK IV, respectively), and R406 (Calbiochem, Darmstadt, Germany), at concentrations ranging from 0.05 to 5 µM. Untreated controls were cultured in parallel under the same conditions and processed identically. To assess both total parasitemia and infected cell morphology, thin smears were prepared from cultures, collected at the indicated times, and stained with Diff-Quick stain (Medion Diagnostics) with at least 1000 cells being examined for each condition. For the studies on parasitized RBC (pRBC) membranes, parasites were cultured at high parasitemias (∼50%) until the ring stage and used to prepare the RBC membranes. Cultures were then diluted with washed RBCs to bring the total parasitemia to ∼10%. Trophozoites and schizonts pRBCs were separated by Percoll gradient and then diluted to 50% parasitemia with washed RBCs as previously described. 34
Membrane preparation and western blotting
Standard hypotonic membranes from both uninfected and malaria-infected RBC were prepared at 4°C in 1.5 mL hemolysis buffer (5 mmol/L sodium phosphate, 1 mmol/L EDTA, pH 8.0) containing protease and phosphatase inhibitor cocktails. 36 Parasitemia was at 50%, as described above. To minimize handling artifacts, the preparation of hypotonic membranes, including twice-repeated 2-minute washing steps, was performed in <10 minutes. The preparations were frozen for storage at −80°C until use. Membrane protein content was quantified using the DC Protein assay (Bio-Rad, Hercules, CA).
Membranes from both infected and uninfected RBCs were solubilized in Laemmli buffer, 37 and proteins were separated on 8% polyacrylamide. Nitrocellulose membranes were probed with mouse anti-band 3, mouse anti-phosphotyrosine, or rabbit anti-Syk (Cell Signaling Technology, Inc) diluted to 1:1000 as described previously. 38 Quantitative densitometry analysis was performed using Odyssey V3.0 software.
Analysis of microparticles in P falciparum–infected RBC cultures.
Microparticles (MPs) collected by centrifugation of P falciparum–infected RBC cultures were quantitated in the presence or absence of R406 performed using a FACS Calibur flow cytometer (Becton Dickinson Biosciences, San Jose, CA) as previously described. 39
Hemoglobin release quantification.
Following centrifugation at 1000g, hemoglobin concentration was measured in the culture supernatant as described. 40,41 To pellet the MPs, the supernatant was further centrifuged at 100 000g for 3 hours at 4°C. 39
Release of merozoites from their host red cell.
Merozoite egress from schizonts was quantified by flow cytometry. Aliquots from a synchronized parasite culture were taken before, during, and after reinfection and stained with ethidium bromide (EtBr) at 5 µg/mL (final concentration) for 20 minutes. This staining allows distinguishing schizont from merozoite during flow cytometry based on their physical properties forward (FSC) and side (SSC) light scattering and EtBr staining intensity at 564 to 606 nm with excitation at 488 nm, using a FACSCalibur flow cytometer (BD Biosciences, Sunnyvale, CA) and CellQuest software (BD Biosciences). CytoCount beads (DAKO, Glostrup, Denmark) were used as control. To determine whether the merozoites that remained trapped inside their host RBCs membrane were still viable, we applied a moderate shear stress to mature schizonts, passing them 20 times through a sterile BD ultrafine needle insulin syringe, 30 G × 12.7 mm, to disrupt the host cell membrane and to release the merozoites.
P falciparum DNA extraction and quantification by quantitative polymerase chain reaction.
Ten microliters of P falciparum culture, 2% of hematocrit, incubated in the presence or absence of SYK II, SYK IV, or R406, was spotted onto filter paper and dried at room temperature (RT) for 20 minutes or stored at −20°C until use. Spots were then transferred into a sterile microcentrifuge tube, and DNA extraction was performed according to manufacturer-suggested procedures (Nurex Srl, Italy). DNA amplification was conducted using primers and probe for the 18rRNA gene of P falciparum adapted from Kamau et al. 42 Amplification and detection were achieved using CFX96 Touch Real-Time PCR Detection System (Bio-Rad). Parasites were counted by a standard curve obtained from serial dilutions of parasites counted by microscopy.
Quantification of hemozoin generation.
Ring-stage infected RBCs (5-10 hours postinfection) were enriched from synchronized cultures to >95% parasitemia using a 90% Percoll-mannitol cushion. 43 Parasites were supplemented with 1 or 5 µM R406 or kept untreated as controls under standard culture conditions for 38 hours. Then, parasites (43-48 hours postinfection) were hypotonically lysed and washed in excess of lysis buffer (10 mM phosphate buffer, pH 8.0). Sedimented hemozoin was solubilized in 0.1 N NaOH/0.05% Triton X-100 (vol/vol) and quantified by heme-dependent luminol-enhanced luminescence as described. 44
IC50 measurement.
To calculate the IC50, we used ICEstimator software, 1.2 version. The program estimates IC50 through nonlinear regression using a standard function of the R software. 35
Fluorescence and DIC microscopy.
To stain merozoites and erythrocytes membrane, cells were harvested from a synchronized culture at 42 to 48 hours postinfection, washed, and incubated with the nuclear stain 4′,6-diamidino-2-phenylindole (300 nM) for 1 to 5 minutes at RT and with phycoerythrin-conjugated monoclonal mouse anti-glycophorin A antibody (DAKO; 1:50 final dilution) for 30 minutes at RT. Analysis of fluorescence was performed by fluorescence microscopy (Leica Microsystems) in wet smears. Fluorescent images were acquired with a fluorescence microscope (Leica DR IRB; Leica Microsystems, Germany) equipped with a Leica DFC 420C camera, a 100× oil planar apochromatic objective, and version 3.3.1 of the Leica DFC image software (Leica Microsystems).
Synchronized P falciparum (Palo Alto) cultures at 1.5% parasitemia and 2% hematocrit were treated at 12 hpi with 5 μM R406 or DMSO for controls. Starting at 44 hpi, aliquots of cultures were removed every 2 to 4 hours and diluted in warm complete media for monitoring of egress by differential interference contrast (DIC) microscopy. For imaging, samples were injected into chambers (HybriWell HBW20; Grace Bio-Labs, Bend, OR) and then sealed just prior to analysis in a 37°C heated chamber. DIC microscopy was performed using a confocal microscope (Nikon A1R MP Confocal) with a 100× oil objective. Untreated and treated infected erythrocytes with either SYK II or R406 were monitored throughout the timeframe of untreated parasite egress. In total, 10 egress events of untreated cultures were recorded, whereas 10 defective egress events for either SYK II or R406 treated cultures were characterized.
Scanning electron microscopy.
Asexual late trophozoites untreated or treated with R406 (2.5 µM and 5µM) were processed for scanning electron microscopy (SEM) according to Tiburcio et al. 45 All samples were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) overnight at 4°C. Then, parasites were let to adhere on poly-lysine–coated glass coverslips for 4 hours, washed in cacodylate buffer, and postfixed with 1% OsO4 in 0.1 M sodium cacodylate buffer for an additional 1 hour at RT. Samples were then washed and dehydrated through a graded series of ethanol solutions (30% to 100% ethanol), critical point dried and gold sputtered (thickness 30 nm), and examined by a field emission gun (INSPECT F, FEI) scanning electron microscope.
Results
Effects of Syk inhibition on phosphorylation and depletion of band 3 in parasitized erythrocytes
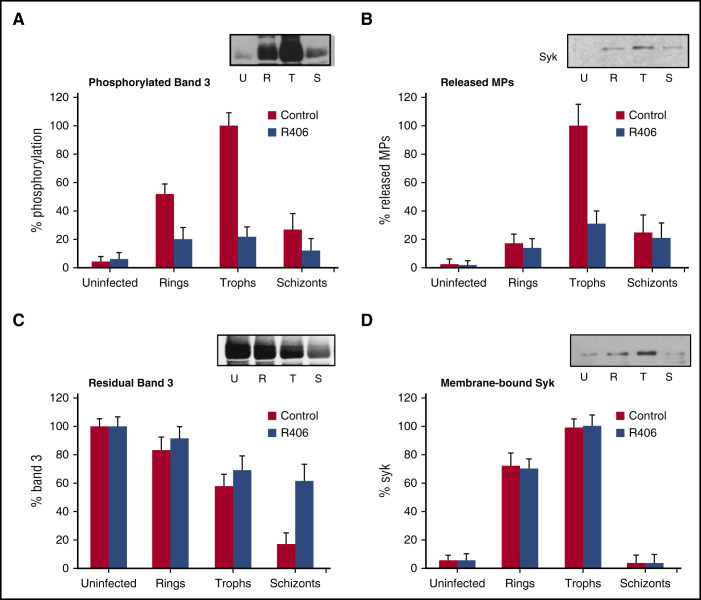
As shown in Figure 1A, the levels of band 3 tyrosine phosphorylation are very low in uninfected RBCs but increase progressively during P falciparum development through its ring and trophozoite stages before eventually declining at the schizont stage (Figure 1A). A Syk-specific inhibitor (R406) causes a substantial decrease of band 3 phosphorylation during all stages of pRBC development. In parallel to band 3 phosphorylation, the rate of MP released from pRBCs displays a maximum at the trophozoite stage and a subsequent decrease at the schizont stage (Figure 1B). The insert in Figure 1B shows an anti-Syk western blot performed on isolated MPs; it should be noticed that MPs contain Syk and that the amount of Syk is proportional to the rate of band 3 phosphorylation observed in Figure 1A. Importantly, R406 causes a reduction in MP release, but has no effect on the binding of Syk to the membranes. This observation is in accordance with previous results. 38,39 To evaluate if Syk inhibitors also cause a decrease in hemolysis, we measured the amount of hemoglobin released into the culture medium at the trophozoite stage (∼36 hours postinfection) and then ultracentrifuged the culture medium to assess whether the hemoglobin might be encapsulated in MPs or free in the medium. In accordance with the decreased release of MPs in treated cultures, we observed a 54% decline in the amount of released hemoglobin in the treated samples. Importantly, in both the control and the treated cultures, >80% of the hemoglobin present in the supernatant was sedimentable by ultracentrifugation, indicating that it is contained within the MPs.
Figure 1.
Abrogation of the P falciparum–induced erythrocyte membrane modifications by SYK inhibitors at different P falciparum lifecycle stages. Synchronized P falciparum cultures were supplemented with SYK inhibitor R406 (1.0 µM) at ring stage (24 hours after invasion). (A) Band 3 tyrosine phosphorylation levels are expressed as percentages of band 3 maximal phosphorylation measured at trophozoite stage and were normalized by the content of band 3 at each stage (% phosphorylation). The insert shows a representative western blot of tyrosine phosphorylated band 3 in the absence of inhibitors in uninfected RBCs (U); ring-infected RBCs (R); trophozoite-infected RBCs (T); and schizont-infected RBCs (S). (B) Number of MPs released from control and R406-treated cells. Values are expressed as a percentage of the maximal number of released MPs measured at trophozoite stage (% Released MPs). The insert shows a representative anti-Syk western blot of MPs released at different stages (U, R, T, S). (C) Band 3 content measured in control and R406-treated cells. Values are expressed as percentage of the band 3 measured at different stages using uninfected cells as reference (% band 3). The insert shows a representative western blot of band 3 at different stages (U, R, T, S). (D) Membrane-bound Syk measured in control and R406-treated cells. Values are expressed as a percentage of the maximal levels of bound Syk measured at trophozoite stage (% Syk). The insert shows a representative western blot of Syk at different stages (U, R, T, S). Data are the average of 6 independent experiments ± standard deviation (SD). Nitrocellulose membranes were stained with rabbit anti-Syk (Cell Signaling Technology, Inc., CA) diluted to 1:1000, and with goat anti-rabbit IRDye 800CW (LI-COR), diluted to 1:50 000. Quantitative densitometry analysis was performed using Odyssey V3.0 software. Photomicrographs were acquired using Odyssey from LI-COR, setting 300dpi as definition.
Consistent with the fact that band 3 comprises a major constituent of RBC MPs, 7,39 we observed a progressive depletion of band 3 from pRBCs during parasite maturation, declining to ∼20% of the initial content at the schizont stage (Figure 1C). It should be noticed that band 3 depletion will likely amplify the membrane destabilization triggered by band 3 phosphorylation. 31 Syk binding to oxidized band 3 and its subsequent translocation to the membrane has been shown to follow its activation. 36 Figure 1D shows that Syk binding to the RBC membrane increases with parasite development, showing a dramatic drop at the schizont stage. The decrease in membrane-bound Syk at the schizont stage may be explained by the progressive loss of both band 3 and Syk (Figure 1C-D) during the release of MPs (Figure 1B-C).
Similarly, the observed depletion of Syk from the host cell membrane may also account for the drop in band 3 phosphorylation observed at the schizont stage (Figure 1A), but additional mechanisms such as profound structural and metabolic changes occurring during the last phases of the parasite development could also explain the decrease of band 3 phosphorylation observed in schizont-infected RBCs. 46 To confirm that above effects of R406 indeed do derive from their inhibition of RBC Syk, we then tested the effect of other Syk-specific inhibitors (R406, Syk II, and Syk IV) on band 3 phosphorylation. Importantly, they all inhibited parasite-induced band 3 tyrosine phosphorylation, MP release, and band 3 depletion induced by P falciparum to a similar extent. In Figure 1, we show the effects of R406 as a representative compound. Taken together, these results show that Syk inhibitors efficiently suppress band 3 phosphorylation and the consequent MPs release from the host cell membrane. Because of their action, Syk inhibitors are expected to substantially reduce the host cell membrane weakening occurring at the end of parasite development.
Effect of Syk inhibition on the parasite egress
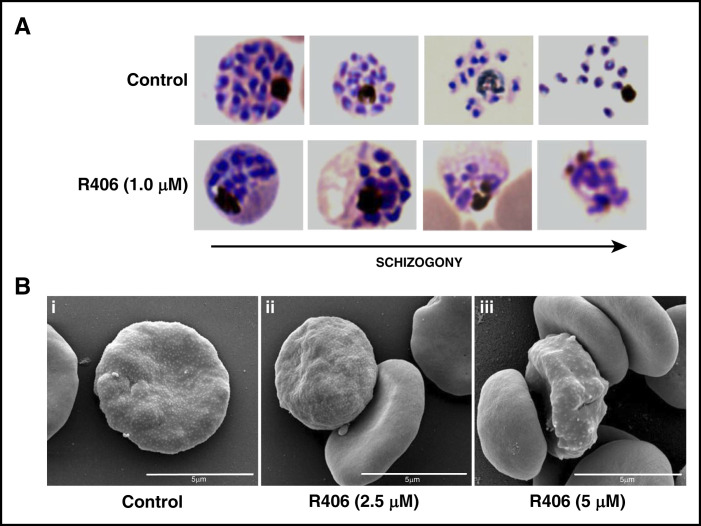
Because the predominant effect of Syk inhibitors on pRBC development occurs during the last stages of parasite maturation, we hypothesized that they may limit the destabilization of the host cell membrane required for the egress of the mature merozoites. To clarify this issue, the accumulation of merozoite aggregates was consistently observed in Syk inhibitor–treated cultures. Healthy merozoites had egressed from untreated pRBCs, suggesting as a consequence that degradative processes begin soon after merozoites are blocked in their attempt to escape their host RBCs (Figure 2A). The most striking morphological alteration observed in fixed blood smears of parasite culture, in comparison with control cultures, was the presence of merozoite aggregates. Thus, staining of R406-treated parasite cultures with anti–glycophorin A at the normal time of merozoite egress and afterward revealed that merozoites are entrapped by residual RBC membrane, as suggested by the glycophorin-containing membranous structures colocalized with the 4′,6-diamidino-2-phenylindole–staining merozoites. Untreated control cultures, however, never showed colocalization of merozoites and RBC membranes (see supplemental Figure 1, available on the Blood Web site).
Figure 2.
Morphological changes and knobs formation in late maturation stages induced by Syk inhibitors. Representative pictures of parasites at 48 hours postinfection in control and in R406-treated cultures selected (A) from Diff-Quik fix stained thin blood films and (B) representative picture of asexual late trophozoite (i) untreated or treated with R406 (ii) 2.5 μΜ and (iii) 5 μΜ obtained by SEM.
As Syk inhibition appears to affect host cell membrane changes occurring at the end of the parasite maturation, we investigated its effect on the formation of knobs. Figure 2B shows the results obtained by SEM in pRBCs treated with R406 at the concentrations of 2.5 µM and 5 µM. SEM analysis clearly shows that knobs are still formed on the surface pRBCs treated by R406.
DIC microscopy recording of living P falciparum cultures also revealed that Syk inhibitors quantitatively prevented merozoite egress from pRBCs. A representative egress of untreated and a failed egress in R406 (5 µM) treated pRBC is shown in the supplemental videos. Those observations were further substantiated by the lower number of countable merozoites released from isolated schizonts treated with relatively low concentrations of R406 (1.0 µM) during the course of 2 hours of culture (Table 1).
Table 1.
Infection and the egress rates of mature schizont isolated from cultures treated with R406 (1.0 and 2.5 μM)
| Control | [R406] (1 µM) | [R406] (2.5 µM) | ||||
|---|---|---|---|---|---|---|
| No shear stress | Shear stress | No shear stress | Shear stress | No shear stress | Shear Stress | |
| Infection rate | 3.48 ± 0.96 | 4.24 ± 1.21 | 0.63 ± 0.25 | 2.6 ± 0.83 | 0.13 ± 0.06 | 0.30 ± 0.39 |
| Egress rate | 6.60 ± 2.41 | 9.37 ± 3.10 | 1.12 ± 0.84 | 3.9 ± 1.11 | 0.27 ± 0.48 | 1.09 ± 1.16 |
A portion of the schizonts were subjected to a rapid shear stress treatment to test its effect on the infection and egress rates. Results are the mean of 3 independent experiments (± SD) and expressed as number of released merozoites (egress rate) or number of infected RBC (infection rate) per each schizont present in the culture (no shear stress/shear stress).
Infection rate, RBC infected per schizont; egress rate, merozoites released per schizont.
To determine whether the merozoites that are trapped in Syk inhibitor–treated pRBCs are still viable, we applied a moderate shear stress to mature schizonts using a standard method to disrupt the host cell membrane and to release the merozoites. Following shear stress, R406-treated cells were challenged with fresh RBCs to assess their capability to infect these RBCs (Table 1). Shear stress treatment of schizonts promoted a measurable improvement in both the egress rate and the infection rate, suggesting that many of the merozoites that become entrapped within the inhibitor-treated pRBCs are still viable, but incapable to egress. With higher concentrations (2.5 μM) of R406, the effect of shear stress on the infection rates markedly decreased (Table 1), suggesting a directly toxic effect of higher amounts of Syk inhibitors on the parasites. All of these experiments were also performed using SYK II and SYK IV with results appearing indistinguishable from the results obtained with R406.
Comprehensively, those results indicate that Syk inhibition affects the egress phase due to their entrapment in residual RBC membranes, although high concentrations of inhibitors can also hinder the production of viable merozoites.
Effect of Syk inhibition on the parasite growth
To further investigate the action of Syk inhibitors on P falciparum cultures, we measured their activity at different concentrations, at different durations of treatment, on different parasite stages, and in different strains.
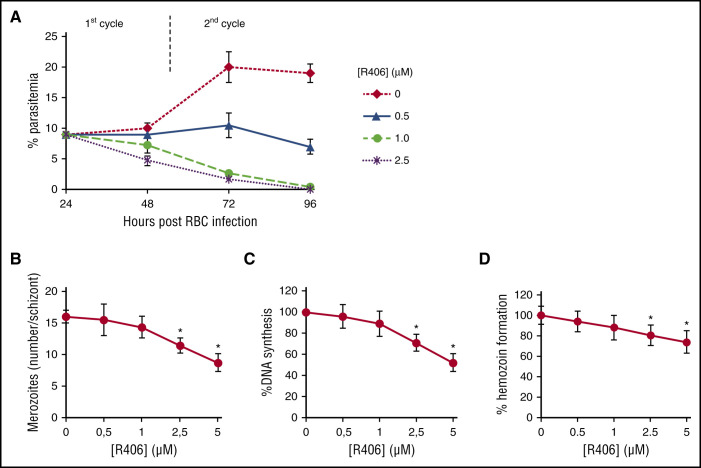
In a first set of experiments, parasitemia was monitored through 2 parasite cycles in the presence of different concentrations of R406 as representative Syk inhibitor, R406 was added 14 to 20 hours postinfection (Figure 3). At low concentrations <1 mM, we observed a modest decrease in parasitemia during the first cycle of growth and a substantial decrease in parasitemia during the second cycle (Figure 3A). This phenomenon was confirmed by a modest, nonsignificant decrease in the number of merozoites per mature schizont at the end of the first cycle of growth (Figure 3B), in DNA synthesis (Figure 3C) and also in hemozoin formation (Figure 3D). At higher concentrations of R406, a reduction of parasitemia was also apparent during the first cycle of growth, and a significant reduction of DNA, hemozoin synthesis, and merozoite production was observed (Figure 3A-D). As expected, there was good correlation among the number of countable merozoites and DNA and hemozoin synthetic activities.
Figure 3.
Effect of Syk inhibitors on P falciparum intraerythrocytic growth cycle. (A) Parasitemia during the course of 2 growth cycles; R406 was added at 14 to 20 hours postinfection. (B) Number of merozoites per schizont measured at 48 hours postinfection. Data are the average of 7 experiments ± SD. (C) DNA synthetic activity measured at 42 to 48 hours postinfection using the untreated cells as a reference (% DNA synthesis). (D) The amount of hemozoin produced by the parasite during maturation from ring to late trophozoite/schizont stage in the infected RBCs in the presence of R406 and in untreated parasites. Hemozoin was quantified in terms of heme content and is expressed as mean hemozoin-heme per infected RBCs. Data are the average ± SD of 4 independent experiments. * Significant differences to untreated control parasites at P < .05.
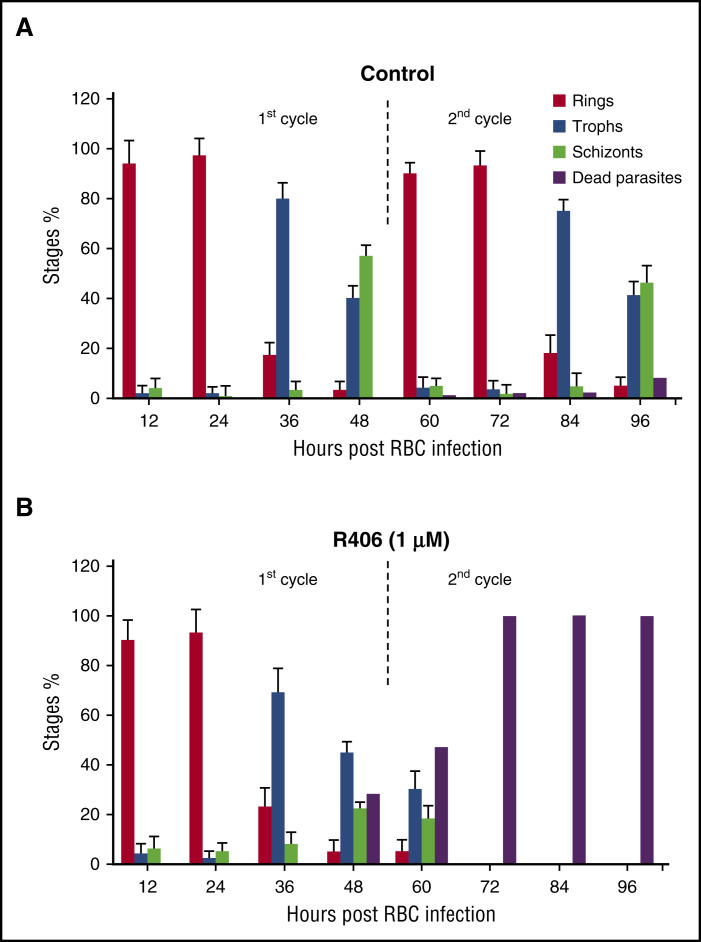
To further characterize the changes induced by Syk inhibition, we measured the relative abundance of each parasite stage (rings, trophozoites, schizonts) at different time points. In comparison with untreated cultures, R406 (1 µM) treatment caused few changes during the first lifecycle. However, during the second cycle of growth, R406 treatment resulted in a marked reduction in ring-stage parasites and an accumulation of dead parasites (Figure 4A-B). With still higher concentrations of Syk inhibitors, anomalous schizont forms were also observed at the end of the first cycle (see above). The initial number of parasites was 150 000/µL (12 hpi) in control and treated cultures. Parasitemia did not significantly change during the first cycle. At the beginning of the second cycle, the parasite number was 515 000/µL (60 hpi) in control cells and 45 000/μL in treated cells. The number of viable parasites remained constant in control cells along the second cycle (84 hpi), whereas it dropped to zero in the treated ones. In a second set of experiments, P falciparum synchronous cultures were treated with different Syk inhibitors at variable concentrations. IC50 was measured after 24 and 48 hours of treatment using 2 different parasite strains and fresh isolates from malaria patients living in an area of suspected artemisinin resistance in central Vietnam (Quang Tri province) (Table 2).
Figure 4.
Effect of Syk inhibitors on parasite stage differentiation. Percentage of the different parasite stages (Stages %) during the course of 2 growth cycles. R406 was added at 20 hours postinfection, measured in (A) control cells and (B) R406 (1.0 µM) cells. The percentage of severely altered parasites (dead parasites) is also shown. Data are means ± SD.
Table 2.
IC50 values of the Syk inhibitors SYK II, SYK IV, and R406 in 2 different P falciparum laboratory strains (Palo Alto and Dd2) and ex vivo grown isolates from Vietnamese malaria patients
| 24 h | 48 h | |
|---|---|---|
| Palo Alto strain | ||
| Syk inhibitors | IC50 (μΜ) | IC50 (μΜ) |
| SYK II | 5.01 ± 0.44 | 0.9 ± 0.16 |
| SYK IV | 7.28 ± 0.4 | 1.75 ± 0.31 |
| R406 | 2.62 ± 0.83 | 0.55 ± 0.19 |
| Dd2 strain | ||
| Syk inhibitors | IC50 (μΜ) | IC50 (μΜ) |
| SYK II | 4.62 ± 0.44 | 0.68 ± 0.15 |
| SYK IV | 6.39 ± 0.46 | 1.28 ± 0.28 |
| R406 | 3.58 ± 0.37 | 0.69 ± 0.26 |
| Ex vivo–grown isolates from Vietnam | ||
| SYK II (N = 23) | 4.48 ± 0.37 | 0.71 ± 0.9 |
| R406 (N= 23) | 3.02 ± 0.23 | 0.44 ± 0.4 |
Syk inhibitors were added to synchronized parasite cultures at 16 to 20 hours postinvasion. The IC50 was determined after 24 and 48 hours incubation of the treated cultures by counting the parasitemia during the 2 subsequent erythrocytic cycles (40-44 and 64-68 hours after first invasion).
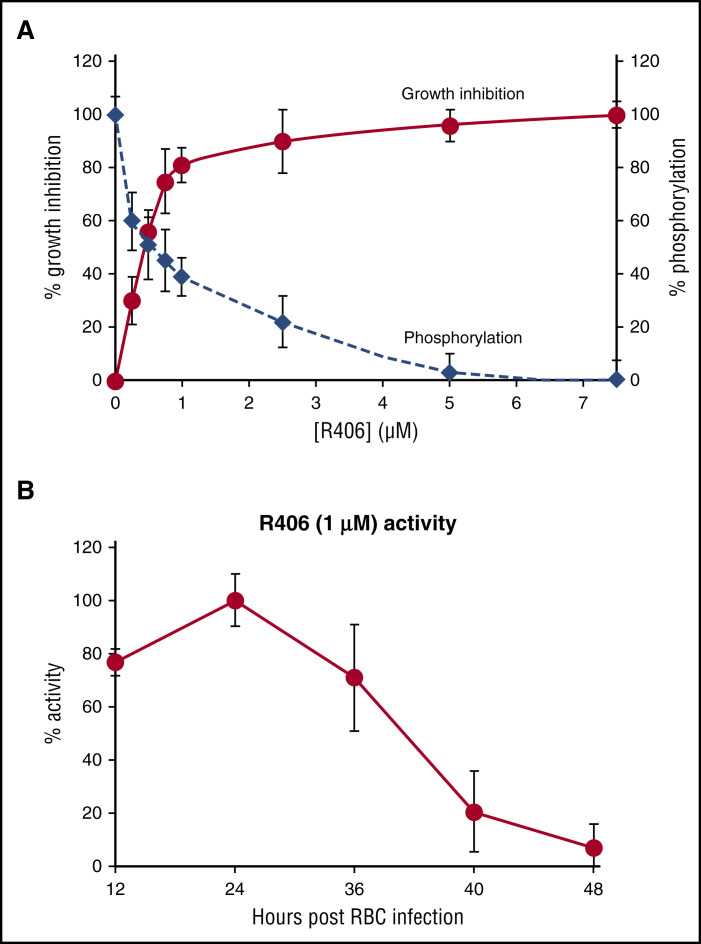
In agreement with the delayed effect of Syk inhibitors, their IC50 were much higher when measured during the first cycle of growth (24 hours after treatment) than at the second cycle (48 hours after treatment) (Table 2). The tested Syk inhibitors revealed similar IC50, although R406 showed consistently lower IC50. To further support the hypothesis that Syk inhibition interferes with the parasite growth, suppressing the phosphorylation of band 3, we measured phosphorylated band 3 levels in parallel to parasite growth inhibition at the different concentration of R406 used to measure the IC50. Figure 5A shows a good correlation between the inhibition of band 3 phosphorylation and parasite growth suppression.
Figure 5.
Effect of Syk inhibitors on growth inhibition and band 3 phosphorylation; stage-dependent sensitivity of parasite to R406 treatment. (A) Parasite growth inhibition (% growth inhibition) and the levels of band 3 phosphorylation at different concentrations of R406 expressed as a percentage of the band 3 maximal phosphorylation levels measured in untreated cultures (% phosphorylation). Parasitemia was measured at the second cycle of growth, and band 3 phosphorylation was measured 36 hours postinfection. (B) Relative activity of R406 (1.0 µM) added at 12, 24, 36, 40, and 48 hours postinfection. Values are expressed as percentage of the maximal R406 activity (treatment time 24 hours postinfection) measured as % activity. Data are the average of 5 experiments ± SD.
In a third set of experiments, we measured the stage dependency of Syk inhibitors activity. Figure 5B shows that adding R406 (1.0 µM) at different stages of parasite development, the maximal activity was observed between 12 and 36 hours postinfection corresponding to ring and trophozoite stages. We also performed experiments in which R406 was added 24 hours postinfection and then washed out after 8 and 12 hours. Under those conditions, R406 exhibited similar inhibitory effects on parasite growth as in experiments where it was not removed from the cultures (data not shown). This result indicates that a short-term inhibition of Syk at the ring and trophozoite stages markedly affects the capability of the parasite to complete its growth cycle. However, addition of R406 in the last 8 hours of parasite development (40 hours postinfection) was much less effective (Figure 4B). However, addition of R406 in the last 8 hours of parasite development (40 hours postinfection) was much less effective (Figure 4B) and it may be due to the low amount of residual Syk and band 3 observed at late parasite maturation stages (see Figure 1C-D).
Discussion
The erythrocyte anion exchanger, band 3, constitutes the major integral protein of the human erythrocyte membrane, where it comprises ∼25% of the total membrane protein. Band 3 serves not only to catalyze exchange of anions across the membrane 47,48 but also to nucleate a complex of glycolytic enzymes on the membrane, 49,50 provide a binding site for multiple kinases and phosphatases, 49 and anchor the spectrin-actin cytoskeleton to the bilayer. 51,52 Although all of these functions contribute critically to RBC biology, its role in linking the cytoskeleton to the membrane may be most critical, because rupture of either the band 3-ankyrin or the band 3-adducin bridge to the spectrin-based membrane skeleton leads to membrane destabilization and fragmentation. 53 -55 Interestingly, Syk inhibitors do not apparently play a role in the assembling of parasite proteins that ultimately lead to the formation of knobs in the host cell membrane. 56
Previous work has shown that phosphorylation of tyrosines 8 and 21 in the cytoplasmic domain of band 3 in fact causes dissociation of ankyrin from band 3, resulting in rupture of the major bridge linking band 3 to the cytoskeleton. 31,39,57 Although healthy RBCs show no obvious evidence of band 3 tyrosine phosphorylation, P falciparum–infected RBCs display a gradual increase in band 3 phosphorylation leading to the anticipated membrane destabilization and vesiculation. Band 3 phosphorylation is very plausibly due to oxidative stress exerted by the parasite growth because it is capable of activating the docking of Syk to band 3 and to inhibit Tyr phosphatases. 36,38 Because this process eventually culminates in the rupture of the host cell membrane and release of the newly developed merozoites into circulation, we wondered whether selective inhibition of the tyrosine phosphorylation of band 3 might inhibit the phosphorylation-induced membrane destabilization sufficiently to prevent parasite egress and thereby terminate parasitemia.
We have shown here that Syk tyrosine kinase inhibitors not only prevent the tyrosine phosphorylation of band 3 in P falciparum pRBCs but also reduce the subsequent release of membrane-derived MPs, mitigate the loss of band 3 and Syk from the infected cells, and block the egress of mature merozoites from the pRBCs. Because the impact of Syk inhibitors on these parasite-mediated processes is proportional to their inhibition of band 3 phosphorylation, we hypothesize that an appropriately designed inhibitor of erythrocyte Syk might prevent parasite egress and thereby constitute a treatment of P falciparum malaria.
Although the above observations might be interpreted to imply that the antimalarial properties of Syk inhibitors all derive from blockade of band 3 tyrosine phosphorylation, closer scrutiny of the data suggests that Syk kinase inhibitors suppress parasitemia by more than a single mechanism. Thus, at low inhibitor concentrations (>twofold below IC50), tyrosine phosphorylation of band 3 is reduced, loss of band 3 from the RBC membrane is mitigated, and mature merozoite egress is suppressed. That the entrapped parasites in such treated cells are neither dead nor inactivated can be discerned from the observation that parasites released from these RBCs by shear stress are fully capable of invading uninfected RBCs and propagating the parasitemia. In contrast, at higher Syk inhibitor concentrations, a decrease in parasite DNA synthesis, hemozoin formation, and number of merozoites per schizont all suggest that additional parasite processes have been perturbed and that P falciparum viability has been compromised.
Although we have emphasized the importance of tyrosine phosphorylation on the mechanism of merozoite egress from infected RBCs, it should be noted that the egress process is very complex, involving multiple mechanisms, including activation of proteases, phospholipases, and osmotic swelling. 13,58 -60 Indeed, our current understanding of the relative contributions of each of these mechanisms to parasite egress is too inadequate to allow prediction of whether blockade of any single mechanism might be sufficient to constitute a mutation-resistant therapy for malaria. However, the fact that the P falciparum genome encodes no classical protein tyrosine kinases 61,62 suggests that mutations in a parasite kinase that might compensate for an inhibited Syk kinase should be difficult to evolve. Syk inhibitors are currently undergoing human clinical trials for treatment of autoimmune, allergic, and neoplastic disorders. 63 -69 In the case of autoimmune diseases, Syk inhibitors must be administered on a regular basis in perpetuity, requiring that the therapy be very well tolerated. For instance, R406 has demonstrated a good safety profile in phase 3 clinical trials for the treatment of rheumatoid arthritis and idiopathic thrombocytopenic purpura, and Imatinib, a bcr-abl inhibitor with relatively potent action on Syk, 70,71 has been used for continuous treatment of chronic myeloid leukemia. 72 Importantly, the IC50 values of both inhibitors for treatment of malaria are within the plasma concentrations achieved following oral administration for treatment of the above pathologies. 73 -75 Because Syk inhibitors have a relatively long half-life and act at the end of the parasite cycle, they would be expected to gradually decrease parasitemia and lead to symptomatic resolution. These characteristics may be an advantage if administered in association with a fast-acting antimalarial drug such as an artemisinin derivative, allowing for both a rapid and prolonged antiplasmodial activity. In conclusion, considering their demonstrated tolerability, Syk inhibitors may represent a new class of antimalarial drugs that possess a unique mechanism of action on a nonparasite target, should not lead to the selection of resistant strains, and may therefore represent a strategic partner drug for counteracting artemisinin resistance.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Claudio Fozza and Pietro Fresu for their support. The authors also acknowledge the help of Gioacchino Greco, responsible for the blood transfusion center of Alghero, Italy, for his contribution to the malaria culture.
This study was supported by grants from Fondazione Banco di Sardegna Protocol U1056.2014/AI.938MGB Practice 2014.0040 and in part by research funding by a grant from Hulow Company.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.P. designed and executed experiments, interpreted data, and was a primary contributor to the text and figures and writing of the manuscript; K.R.K. executed experiments regarding the DIC video microscopy; M.C.P. and I.T. executed experiments, contributed to figures, and assisted with the studies in Vietnam; E.S. and O.A.S. executed experiments regarding the fluorescence microscopy and quantification of hemozoin; M.P. and L.B. executed experiments regarding SEM; H.D.C., P.S.L. and F.M.T. contributed to the writing and revising of the manuscript; and all authors have made significant contributions to improve the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonella Pantaleo, Department of Biomedical Sciences, University of Sassari, Sassari 07100, Italy; e-mail: apantaleo@uniss.it.
References
- 1. WHO. World Malaria Report 2016; Geneva: 2016. License: CC BY-NC-SA 3.0 IGO.
- 2. WHO. Eliminating Malaria; 2016. Geneva: 2016. http://apps.who.int/iris/bitstream/10665/205565/1/WHO_HTM_GMP_2016.3_eng.pdf. Accessed 30 April 2016.
- 3. WHO. Artemisinin and artemisinin-based combination therapy resistance October 2016. http://apps.who.int/iris/bitstream/10665/250294/1/WHO-HTM-GMP-2016.11-eng.pdf. Accessed 2 November 2016.
- 4. WHO. Artemisinin and artemisinin-based combination therapy resistance April 2016. http://apps.who.int/iris/bitstream/10665/208820/1/WHO_HTM_GMP_2016.5_eng.pdf. Accessed 28 June 2016.
- 5. Pantaleo A, Pau MC, Chien HD, Turrini F. Artemisinin resistance, some facts and opinions. J Infect Dev Ctries. 2015;9(6):597-599. [DOI] [PubMed] [Google Scholar]
- 6. Kaur H, Clarke S, Lalani M, et al. Fake anti-malarials: start with the facts. Malar J. 2016;15:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pantaleo A, Ferru E, Vono R, et al. New antimalarial indolone-N-oxides, generating radical species, destabilize the host cell membrane at early stages of Plasmodium falciparum growth: role of band 3 tyrosine phosphorylation. Free Radic Biol Med. 2012;52(2):527-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pathak V, Colah R, Ghosh K. Tyrosine kinase inhibitors: New class of antimalarials on the horizon? Blood Cells Mol Dis. 2015;55(2):119-126. [DOI] [PubMed] [Google Scholar]
- 9. Singh A, Maqbool M, Mobashir M, Hoda N. Dihydroorotate dehydrogenase: a drug target for the development of antimalarials [published correction appears in Eur J Med Chem. 2017;128:346-347]. Eur J Med Chem. 2017;125:640-651. [DOI] [PubMed] [Google Scholar]
- 10. Quiliano M, Mendoza A, Fong KY, et al. Exploring the scope of new arylamino alcohol derivatives: Synthesis, antimalarial evaluation, toxicological studies, and target exploration. Int J Parasitol Drugs Drug Resist. 2016;6(3):184-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pavadai E, El Mazouni F, Wittlin S, de Kock C, Phillips MA, Chibale K. Identification of new human malaria parasite plasmodium falciparum dihydroorotate dehydrogenase inhibitors by pharmacophore and structure-based virtual screening. J Chem Inf Model. 2016;56(3):548-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flannery EL, Chatterjee AK, Winzeler EA. Antimalarial drug discovery - approaches and progress towards new medicines. Nat Rev Microbiol. 2013;11(12):849-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kesely KR, Pantaleo A, Turrini FM, Olupot-Olupot P, Low PS. Inhibition of an erythrocyte tyrosine kinase with imatinib prevents plasmodium falciparum egress and terminates parasitemia. PLoS One. 2016;11(10):e0164895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parhizgar AR, Tahghighi A. Introducing new antimalarial analogues of chloroquine and amodiaquine: a narrative review. Iran J Med Sci. 2017;42(2):115-128. [PMC free article] [PubMed] [Google Scholar]
- 15. Burrows JN, Duparc S, Gutteridge WE, et al. New developments in anti-malarial target candidate and product profiles [published correction appears in Malar J. 2017;16(1):151]. Malar J. 2017;16(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lavogina D, Budu A, Enkvist E, et al. Targeting Plasmodium falciparum protein kinases with adenosine analogue-oligoarginine conjugates. Exp Parasitol. 2014;138:55-62. [DOI] [PubMed] [Google Scholar]
- 17. Cooke BM, Mohandas N, Coppel RL. Malaria and the red blood cell membrane. Semin Hematol. 2004;41(2):173-188. [DOI] [PubMed] [Google Scholar]
- 18. Tokumasu F, Ostera GR, Amaratunga C, Fairhurst RM. Modifications in erythrocyte membrane zeta potential by Plasmodium falciparum infection. Exp Parasitol. 2012;131(2):245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pantaleo A, Ferru E, Carta F, Valente E, Pippia P, Turrini F. Effect of heterozygous beta thalassemia on the phosphorylative response to Plasmodium falciparum infection. J Proteomics. 2012;76(Spec No):251-258. [DOI] [PubMed] [Google Scholar]
- 20. Pantaleo A, Ferru E, Carta F, et al. Analysis of changes in tyrosine and serine phosphorylation of red cell membrane proteins induced by P. falciparum growth. Proteomics. 2010;10(19):3469-3479. [DOI] [PubMed] [Google Scholar]
- 21. Proellocks NI, Coppel RL, Mohandas N, Cooke BM. Malaria parasite proteins and their role in alteration of the structure and function of red blood cells. Adv Parasitol. 2016;91:1-86. [DOI] [PubMed] [Google Scholar]
- 22. Maier AG, Cooke BM, Cowman AF, Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Microbiol. 2009;7(5):341-354. [DOI] [PubMed] [Google Scholar]
- 23. Haldar K, Mohandas N. Erythrocyte remodeling by malaria parasites. Curr Opin Hematol. 2007;14(3):203-209. [DOI] [PubMed] [Google Scholar]
- 24. Kats LM, Proellocks NI, Buckingham DW, et al. Interactions between Plasmodium falciparum skeleton-binding protein 1 and the membrane skeleton of malaria-infected red blood cells. Biochim Biophys Acta. 2015;1848(7):1619-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sherling ES, Knuepfer E, Brzostowski JA, Miller LH, Blackman MJ, van Ooij C. The Plasmodium falciparum rhoptry protein RhopH3 plays essential roles in host cell invasion and nutrient uptake. eLife. 2017;6:23239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang VM, Chavchich M, Waters NC. Targeting protein kinases in the malaria parasite: update of an antimalarial drug target. Curr Top Med Chem. 2012;12(5):456-472. [DOI] [PubMed] [Google Scholar]
- 27. Ward P, Equinet L, Packer J, Doerig C. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics. 2004;5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fernandez-Pol S, Slouka Z, Bhattacharjee S, et al. A bacterial phosphatase-like enzyme of the malaria parasite Plasmodium falciparum possesses tyrosine phosphatase activity and is implicated in the regulation of band 3 dynamics during parasite invasion. Eukaryot Cell. 2013;12(9):1179-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tewari R, Straschil U, Bateman A, et al. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe. 2010;8(4):377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pantaleo A, De Franceschi L, Ferru E, Vono R, Turrini F. Current knowledge about the functional roles of phosphorylative changes of membrane proteins in normal and diseased red cells. J Proteomics. 2010;73(3):445-455. [DOI] [PubMed] [Google Scholar]
- 31. Ferru E, Giger K, Pantaleo A, et al. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood. 2011;117(22):5998-6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193(4254):673-675. [DOI] [PubMed] [Google Scholar]
- 33. Trager W. The cultivation of Plasmodium falciparum: applications in basic and applied research on malaria. Ann Trop Med Parasitol. 1987;81(5):511-529. [DOI] [PubMed] [Google Scholar]
- 34. Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65(3):418-420. [PubMed] [Google Scholar]
- 35. Le Nagard H, Vincent C, Mentré F, Le Bras J. Online analysis of in vitro resistance to antimalarial drugs through nonlinear regression. Comput Methods Programs Biomed. 2011;104(1):10-18. [DOI] [PubMed] [Google Scholar]
- 36. Pantaleo A, Ferru E, Giribaldi G, et al. Oxidized and poorly glycosylated band 3 is selectively phosphorylated by Syk kinase to form large membrane clusters in normal and G6PD-deficient red blood cells. Biochem J. 2009;418(2):359-367. [DOI] [PubMed] [Google Scholar]
- 37. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680-685. [DOI] [PubMed] [Google Scholar]
- 38. Pantaleo A, Ferru E, Pau MC, et al. Band 3 erythrocyte membrane protein acts as redox stress sensor leading to its phosphorylation by p (72) Syk. Oxid Med Cell Longev. 2016;2016:6051093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferru E, Pantaleo A, Carta F, et al. Thalassemic erythrocytes release microparticles loaded with hemichromes by redox activation of p72Syk kinase. Haematologica. 2014;99(3):570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Antonini E, Brunori M. In: Surgenor DM, ed. “Hemoglobin and methemoglobin” in the red blood cell, vol. 2. New York, NY: Academic Press; 1975:753-797. [Google Scholar]
- 41. Pantaleo A, Ferru E, Carta F, et al. Irreversible AE1 tyrosine phosphorylation leads to membrane vesiculation in G6PD deficient red cells. PLoS One. 2011;6(1):e15847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kamau E, Alemayehu S, Feghali KC, Saunders D, Ockenhouse CF. Multiplex qPCR for detection and absolute quantification of malaria. PLoS One. 2013;8(8):e71539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Skorokhod OA, Davalos-Schafler D, Gallo V, et al. Oxidative stress-mediated antimalarial activity of plakortin, a natural endoperoxide from the tropical sponge Plakortis simplex. Free Radic Biol Med. 2015;89:624-637. [DOI] [PubMed] [Google Scholar]
- 44. Lin JW, Spaccapelo R, Schwarzer E, et al. Replication of Plasmodium in reticulocytes can occur without hemozoin formation, resulting in chloroquine resistance. J Exp Med. 2015;212(6):893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tiburcio MG, Anversa L, Kanunfre KA, Ferreira AW, Rodrigues Júnior V, Silva LA. Anti-leishmania infantum IgG antibody avidity in visceral leishmaniasis. Clin Vaccine Immunol. 2013;20(11):1697-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fang X, Reifman J, Wallqvist A. Modeling metabolism and stage-specific growth of Plasmodium falciparum HB3 during the intraerythrocytic developmental cycle. Mol Biosyst. 2014;10(10):2526-2537. [DOI] [PubMed] [Google Scholar]
- 47. Hamasaki N, Okubo K. Band 3 protein: physiology, function and structure. Cell Mol Biol. 1996;42(7):1025-1039. [PubMed] [Google Scholar]
- 48. Arakawa T, Kobayashi-Yurugi T, Alguel Y, et al. Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science. 2015;350(6261):680-684. [DOI] [PubMed] [Google Scholar]
- 49. Chu H, Low PS. Mapping of glycolytic enzyme-binding sites on human erythrocyte band 3. Biochem J. 2006;400(1):143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weber RE, Voelter W, Fago A, Echner H, Campanella E, Low PS. Modulation of red cell glycolysis: interactions between vertebrate hemoglobins and cytoplasmic domains of band 3 red cell membrane proteins. Am J Physiol Regul Integr Comp Physiol. 2004;287(2):R454-R464. [DOI] [PubMed] [Google Scholar]
- 51. Grey JL, Kodippili GC, Simon K, Low PS. Identification of contact sites between ankyrin and band 3 in the human erythrocyte membrane. Biochemistry. 2012;51(34):6838-6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bennett V, Stenbuck PJ. Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem. 1980;255(13):6424-6432. [PubMed] [Google Scholar]
- 53. Franco T, Chu H, Low PS. Identification of adducin-binding residues on the cytoplasmic domain of erythrocyte membrane protein, band 3. Biochem J. 2016;473(19):3147-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anong WA, Franco T, Chu H, et al. Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood. 2009;114(9):1904-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Anong WA, Weis TL, Low PS. Rate of rupture and reattachment of the band 3-ankyrin bridge on the human erythrocyte membrane. J Biol Chem. 2006;281(31):22360-22366. [DOI] [PubMed] [Google Scholar]
- 56. Cooke BM, Stuart J, Nash GB. The cellular and molecular rheology of malaria. Biorheology. 2014;51(2-3):99-119. [DOI] [PubMed] [Google Scholar]
- 57. Bordin L, Fiore C, Bragadin M, Brunati AM, Clari G. Regulation of membrane band 3 Tyr-phosphorylation by proteolysis of p72(Syk) and possible involvement in senescence process. Acta Biochim Biophys Sin (Shanghai). 2009;41(10):846-851. [DOI] [PubMed] [Google Scholar]
- 58. Das S, Hertrich N, Perrin AJ, et al. Processing of Plasmodium falciparum merozoite surface protein MSP1 activates a spectrin-binding function enabling parasite egress from RBCs. Cell Host Microbe. 2015;18(4):433-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Engelberg K, Paul AS, Prinz B, et al. Specific phosphorylation of the PfRh2b invasion ligand of Plasmodium falciparum. Biochem J. 2013;452(3):457-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Braun-Breton C, Abkarian M. Red blood cell spectrin skeleton in the spotlight. Trends Parasitol. 2016;32(2):90-92. [DOI] [PubMed] [Google Scholar]
- 61. Doerig C, Abdi A, Bland N, et al. Malaria: targeting parasite and host cell kinomes. Biochim Biophys Acta. 2010;1804(3):604-612. [DOI] [PubMed] [Google Scholar]
- 62. Doerig C, Meijer L. Antimalarial drug discovery: targeting protein kinases. Expert Opin Ther Targets. 2007;11(3):279-290. [DOI] [PubMed] [Google Scholar]
- 63. Kitai M, Fukuda N, Ueno T, et al. Effects of a spleen tyrosine kinase inhibitor on progression of the lupus nephritis in mice. J Pharmacol Sci. 2017;134(1):29-36. [DOI] [PubMed] [Google Scholar]
- 64. Deng GM, Kyttaris VC, Tsokos GC. Targeting Syk in autoimmune rheumatic diseases. Front Immunol. 2016;7:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kaur M, Singh M, Silakari O. Inhibitors of switch kinase ‘spleen tyrosine kinase’ in inflammation and immune-mediated disorders: a review. Eur J Med Chem. 2013;67:434-446. [DOI] [PubMed] [Google Scholar]
- 66. Ruzza P, Biondi B, Calderan A. Therapeutic prospect of Syk inhibitors. Expert Opin Ther Pat. 2009;19(10):1361-1376. [DOI] [PubMed] [Google Scholar]
- 67. Weinblatt ME, Kavanaugh A, Burgos-Vargas R, et al. Treatment of rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 2008;58(11):3309-3318. [DOI] [PubMed] [Google Scholar]
- 68. Genovese MC, Kavanaugh A, Weinblatt ME, et al. An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents. Arthritis Rheum. 2011;63(2):337-345. [DOI] [PubMed] [Google Scholar]
- 69. Patterson H, Nibbs R, McInnes I, Siebert S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin Exp Immunol. 2014;176(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee SJ, Wang JY. Exploiting the promiscuity of imatinib. J Biol. 2009;8(3):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Winger JA, Hantschel O, Superti-Furga G, Kuriyan J. The structure of the leukemia drug imatinib bound to human quinone reductase 2 (NQO2). BMC Struct Biol. 2009;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mughal A, Aslam HM, Khan AM, Saleem S, Umah R, Saleem M. Bcr-Abl tyrosine kinase inhibitors- current status. Infect Agent Cancer. 2013;8(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Baluom M, Grossbard EB, Mant T, Lau DT. Pharmacokinetics of fostamatinib, a spleen tyrosine kinase (SYK) inhibitor, in healthy human subjects following single and multiple oral dosing in three phase I studies. Br J Clin Pharmacol. 2013;76(1):78-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McAdoo SP, Tam FW. Fostamatinib disodium. Drugs Future. 2011;36(4):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Braselmann S, Taylor V, Zhao H, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319(3):998-1008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.