Abstract
Acyclovir (ACV) has shown efficacy in the prophylactic suppression of human cytomegalovirus (HCMV) reactivation in immunocompromised renal transplant patients without the toxicity associated with ganciclovir (GCV). The HCMV UL97 gene product, a protein kinase, is responsible for the phosphorylation of GCV in HCMV-infected cells. This report provides evidence for the phosphorylation of ACV by UL97. Anabolism studies with the HCMV wild-type strain AD169 and with recombinant mutants derived from marker transfer experiments performed by using mutant UL97 DNA from both clinical isolates and a laboratory-derived strain resistant to GCV showed that mutations in the UL97 gene cripple the ability of recombinant virus-infected cells to anabolize both GCV and ACV. These mutant UL97 recombinant viruses were less susceptible to both GCV and ACV than was the wild-type strain. A recombinant herpes simplex virus type 1 strain, in which the thymidine kinase gene is deleted and the UL13 gene is replaced with the HCMV UL97 gene, was able to induce the phosphorylation of ACV in infected cells. Finally, purified UL97 phosphorylated both GCV and ACV to their monophosphates. Our results indicate that UL97 promotes the selective activity of ACV against HCMV.
Human cytomegalovirus (HCMV)-associated disease is a major concern in the immunocompromised patient population. In AIDS patients, HCMV infections can lead to retinitis that can cause blindness (12). HCMV infection in bone marrow and solid organ transplant recipients can produce life-threatening infections, the most common being HCMV pneumonitis (40) and gastrointestinal hemorrhage (22), and is associated with 20% of renal graft failures that occur in the first 6 months after transplantation (33). Ganciclovir (GCV) is widely used to treat HCMV infections but has been associated with significant leukopenia and thrombocytopenia in some patients. Its closely related nucleoside analog, acyclovir (ACV), has been used intravenously for prophylaxis of HCMV infection. ACV is effective in suppression of both HCMV infection and HCMV-associated disease in bone marrow and renal transplant patients (3, 36, 39) as well as HCMV-associated disease in heart transplant patients (15). Suppression of HCMV-caused retinitis in some AIDS patients using ACV prophylaxis has also been demonstrated (41).
Valaciclovir, a valine ester prodrug of ACV, is currently approved for treatment of genital herpes and varicella-zoster virus (VZV) infections and is rapidly and almost completely converted to ACV after oral administration. Plasma ACV levels that are comparable to those achieved by intravenous administration of ACV are found after valaciclovir administration (28). Valaciclovir significantly reduces the risk of HCMV disease and has shown activity, both as a preemptive agent and as a prophylactic agent, for HCMV-associated disease in AIDS patients (17, 23). However, better-tolerated doses of valaciclovir for treatment of HCMV disease need to be determined, since significantly higher mortality rates were observed in AIDS patients administered 8 g of valaciclovir/day than in AIDS patients administered 3.2 or 0.8 g of ACV/day (17).
In herpes simplex virus (HSV)- and VZV-infected cells, the viral thymidine kinase (TK) is responsible for phosphorylating both ACV and GCV to their monophosphates (MPs) (14, 21). After monophosphorylation, host cellular kinases convert the drug MP to its triphosphate (TP), which then selectively inhibits the viral DNA polymerase activity (8, 14). Incorporation of ACV-MP into the nascent viral DNA chain results in premature chain termination (8, 34). GCV-MP is also incorporated into the growing viral DNA chain but is not an absolute chain terminator (24).
HCMV does not encode a TK, but phosphorylation of ACV and GCV is enhanced in cells infected with HCMV (4). The product of the HCMV UL97 open reading frame controls the phosphorylation of GCV in infected cells (46). HCMV mutants which contain UL97 mutations are deficient in their ability to induce the intracellular phosphorylation of GCV. This correlates with reduced GCV susceptibility of these viruses (1, 2, 9, 25, 46). UL97 induces GCV phosphorylation in heterologous systems, suggesting that no other HCMV proteins are required for GCV phosphorylation (27, 30, 35, 37).
The UL97 protein is a protein kinase that phosphorylates itself and certain histone proteins (26, 27). Homologues of UL97 exist in all of the human herpesviruses that have been sequenced to date. The UL97 protein shares partial amino acid homology to the HSV type 1 (HSV-1) UL13, particularly in conserved regions thought to be responsible for protein kinase activity (7). UL97 can partially compensate for some functions of the HSV UL13 and also mediate the phosphorylation of GCV in recombinant HSV-1-infected Vero cells (38).
In this study the role of HCMV UL97 in both the intracellular and extracellular activation of ACV was examined. Sensitivity to ACV and intracellular phosphorylation of ACV by UL97 mutant viruses and the wild-type laboratory strain AD169 were determined. The role of UL97 in the intracellular activation of ACV in recombinant HSV-1-infected cells was also examined. Finally, using a purified UL97 assay system, the phosphorylation of GCV and ACV was observed in the absence of contaminating cellular activity. To our knowledge, this is the first report indicating that purified UL97 directly phosphorylates either GCV or ACV.
MATERIALS AND METHODS
Virus strains.
Strain AD169 was obtained from the American Type Culture Collection (Manassas, Va.). The GCV-resistant mutant 759rD100 was derived by serial passage of strain AD169 in increasing concentrations of GCV (5). The laboratory recombinant strain, XbaF 4-3-1, was obtained in marker transfer experiments in which GCV resistance was transferred to AD169 by the XbaF fragment of the 759rD100 genome (46). Other recombinant strains were obtained by recombination of PCR-amplified UL97 DNA fragments from GCV-resistant clinical isolates and full-length AD169 DNA (1, 9, 25). These were plaque purified three times in MRC-5 cells by using cell-free virus. Construction of the HSV-1 recombinant strains, R4970 (UL13−, UL97+, TK−) and R7355 (UL13−, UL97−, TK−), was described previously (38). Construction of baculoviruses expressing glutathione S-transferase (GST)-UL97 fusion proteins was described previously (27). BVUL97 expresses wild-type UL97, while BVUL97K355Q expresses UL97 containing an amino acid substitution of lysine with glutamine at position 355.
Chemicals.
[8-3H]GCV (14.9 Ci/mmol) and [8-3H]ACV (16.9 Ci/mmol) were obtained from Moravek Biochemicals (Brea, Calif.). The MPs, diphosphates (DPs), and TPs of GCV and ACV were synthesized by methods described previously (16, 19). All other chemicals were from outside sources and were of reagent grade or better.
Anti-HCMV drug susceptibility assays.
Susceptibilities to antiviral compounds were determined by plaque reduction assays (4) in MRC-5 cells. Medium overlays containing seven drug concentrations and a drug-free control were tested in triplicate. Data for 50% inhibitory concentration (IC50) were analyzed by a linear regression analysis program (SAS Probit; SAS Institute, Cary, N.C.).
HSV-1 plaque reduction assays.
HSV-1 plaque reduction assays were performed as described previously (38). Briefly, Vero cells infected with wild-type HSV-1 strain F(F) or recombinant HSV-1 strains were overlaid with 199-O media containing 0, 5, 15, 40, or 60 μM of ACV. Infected cells were incubated at 37°C for 2 days and were then fixed and stained with Giemsa stain.
Analysis of intracellular metabolites (HPLC).
Confluent MRC-5 cells in 25-cm2 flasks were infected with AD169 or XbaF 4-3-1 at a multiplicity of infection (MOI) of 0.8 or were mock infected. Three days postinfection the cells were pulsed with either [8-3H]ACV (200 μM) or [8-3H]GCV (20 μM) for 18 h. Vero cells, plated in 60-mm-diameter dishes, were infected with HSV-1 (F), R4970 (UL13−, UL97+, TK−), or R7355 (UL13−, UL97−, TK−) at an MOI of 10 or were mock infected. At 8 h postinfection these cells were pulsed with [8-3H]ACV (200 μM) for 15 h. After pulse labeling, cells were washed with cold phosphate-buffered saline and extracted with ice-cold 80% acetonitrile. The cellular extracts were clarified by centrifugation (770 × g, 15 min, 4°C), and the resulting supernatants were evaporated to dryness and then reconstituted in high-performance liquid chromatography (HPLC)-grade water to an equivalent of 106 cells per 120 μl. Intracellular ACV and GCV phosphates were quantitated by an anion-exchange HPLC method described previously (38).
UL97 enzyme assay.
The expression and purification of the wild-type and the K355Q mutant GST-UL97 fusion proteins were described previously (27). UL97 phosphorylation reaction mixtures contained 5 mM ATP, 5 mM MgCl2, 50 mM sodium HEPES (pH 7.5), 1.2 μM [8-3H]ACV or 1.14 μM [8-3H]GCV (16 mCi/μmol), and UL97 at 0.028 mg/ml in a final volume of 0.5 ml. Blank reaction mixtures were identical but contained 0.1 mg of bovine serum albumin (BSA) per ml instead of UL97 protein. Reaction mixtures were incubated for up to 8 h at 37°C. The mixtures were placed on ice and carriers and/or internal standards were added as follows: 5 μl each of 2 mM AMP, GMP, ADP, ATP, and GTP, 5 μl of either 2 mM ACV or 2 mM GCV, 5 μl of 2 mM ACV-MP or GCV-MP, and 100 μl of 10 mM ammonium phosphate. Anion-exchange HPLC was performed on a Whatman Partisil 10 SAX column (100 by 4.6 mm) with a gradient of 10 mM ammonium phosphate (pH 5.5)–5% methanol to 800 mM ammonium phosphate (pH 5.5)–5% methanol. The flow rate was 1 ml/min, with 5-min flow of the 10 mM buffer, followed by 25-min flow of the linear gradient to 800 mM buffer. Fractions were collected, and radioactivity was determined by liquid scintillation counting in Wallac Optiphase Supermix scintillation cocktail in a Beckman LS6000TA counter. Rates of phosphorylation were based on percent conversion of protein to MP (up to approximately 2%).
RESULTS
Susceptibility of HCMV UL97 mutant recombinants to ACV.
To test the effects of UL97 mutations on ACV susceptibility, we examined a panel of recombinant viruses each contributing, by recombination with AD169, a UL97 mutation that confers resistance to GCV. The UL97 mutations present in the GCV-resistant, recombinant strains were representative of a cross section of UL97 mutations found in clinical and laboratory strains to date (Table 1). ACV susceptibilities of both clinical and laboratory-derived UL97 mutant HCMV strains were determined by plaque reduction assays as described in the Materials and Methods section. The plaque reduction assay measures the ability of the virus to form plaques in the presence of drug. As previously shown the UL97 mutant viruses showed GCV IC50s elevated from four- to sevenfold compared to AD169 IC50s (Table 1). The ACV IC50s for the UL97 mutant viruses ranged from 2.5- to 4-fold higher than the AD169 ACV IC50s.
TABLE 1.
In Vitro GCV and ACV susceptibility of HCMV recombinant viruses
| Virus | Amino acid change | Reference | Plaque reduction (IC50, μM)a
|
|
|---|---|---|---|---|
| GCV | ACV | |||
| AD169 | Wild type | 5.1 ± 0.1 | 77 ± 2.1 | |
| XbaF 4-3-1 | AACR590-593 deletion | 47 | 34 ± 1.4 | 270 ± 63 |
| 8702rec 2-1-2 | A594V | 9 | 36 ± 9.8 | 250 ± 43 |
| 8704rec 8-1-2 | L595S | 9 | 35 ± 3.5 | 220 ± 14 |
| 8805rec 1-1-1 | M460V | 9 | 21 ± 2.9 | 200 ± 23 |
| 9219rec 7-1-1 | L595 deletion | 1 | 34 ± 9.8 | 190 ± 15 |
| 9330rec 5-1-1 | H520Q | 26 | 28 ± 4.9 | 280 ± 4.0 |
Values are the means ± standard deviations of three separate experiments.
Anabolism of ACV in UL97 mutant HCMV-infected cells.
Stanat et al. (44) determined that there is a relationship between GCV resistance and reduced intracellular levels of GCV phosphates in HCMV-infected cells. To determine if the reduced ACV susceptibility of the UL97 mutant recombinant virus, XbaF 4-3-1, correlated with reduced intracellular levels of phosphorylated ACV in cells infected with XbaF 4-3-1, intracellular anabolism studies were performed.
Cells infected with XbaF 4-3-1 or AD169 or mock-infected cells were pulsed with either 20 μM [14C]GCV or 200 μM [14C]ACV and then analyzed by HPLC. This allowed us to determine the effect of the four-amino-acid deletion in UL97 on the intracellular phosphorylation of ACV. As shown previously, total GCV phosphate levels from XbaF 4-3-1-infected cells were ninefold lower than the levels found in AD169-infected cells but were sixfold higher than those in uninfected cells (Table 2). The levels of total ACV phosphates in XbaF 4-3-1-infected cells were 5-fold lower than the levels in AD169-infected cells but were 3.5-fold higher than those in mock-infected cells. The proportions of individual GCV and ACV anabolites (MP, DP, and TP) showed a similar pattern. The decreased levels of GCV and ACV phosphates in XbaF 4-3-1-infected cells, compared to the levels found in AD169-infected cells, correlated with the increased GCV and ACV IC50s for these viruses. Compared with AD169-infected cells XbaF 4-3-1-infected cells showed a ninefold decrease in the total GCV phosphate level which correlated with a sevenfold increase in the GCV IC50 and, similarly, a fivefold reduction in the total ACV phosphate level correlated with a fourfold rise in the ACV IC50. Extracts from cells infected with HCMV recombinants containing a variety of UL97 mutations were also analyzed for total GCV and ACV phosphate levels. Reduced levels of GCV and ACV total phosphates were also observed in these UL97 mutant-infected cells compared to levels seen in AD169-infected cells (data not shown).
TABLE 2.
Phosphorylation of GCV and ACV in HCMV-infected MRC-5 cellsa
| Virus or cell | Anabolites (pmol/106 cells)b of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| GCV
|
ACV
|
|||||||
| MP | DP | TP | Total | MP | DP | TP | Total | |
| AD169 | 0.7 ± 0.05 | 7.4 ± 0.6 | 80 ± 4.6 | 88 | 6.0 ± 0.5 | 2.7 ± 0.8 | 35 ± 6.3 | 44 |
| XbaF 4-3-1 | 0.2 ± 0.04 | 1.9 ± 0.2 | 7.6 ± 0.5 | 9.6 | 1.3 ± 0.1 | 0.7 ± 0.1 | 6.2 ± 1.1 | 8.2 |
| Mock-infected cells | 0.02 ± 0.01 | 0.2 ± 0.05 | 1.4 ± 0.1 | 1.6 | 0.2 ± 0.06 | 0.3 ± 0.03 | 1.9 ± 0.06 | 2.4 |
Three days postinfection, cells were pulsed with [8-3H]ACV (200 μM) or [8-3H]GCV (20 μM) for 18 h. Intracellular anabolites were measured by anion-exchange HPLC.
Values are the means ± standard deviations for three samples.
Susceptibility of recombinant HSV-1 strains to ACV.
Ng et al. (38) constructed an HSV-1 recombinant strain (R4970) in which the HSV UL13 gene was replaced by the HCMV UL97 gene, and in addition, the TK gene was deleted. To determine the role of UL97 in the ACV susceptibilities of wild-type HSV-1 (F), R4970 (TK−, UL13−, UL97+), and R7355 (TK−, UL13−, UL97−), plaque reduction assays were performed. As expected, HSV-1 (F) was susceptible to ACV, with an IC50 of <1 μM. R4970 had intermediate sensitivity to ACV, with an IC50 of 19 μM, and R7355 had an ACV IC50 of 51 μM (Table 3).
TABLE 3.
In vitro susceptibility and phosphorylation of ACV in recombinant HSV-1-infected Vero cellsa
| Virus (phenotype) or cell | IC50 (μM) | ACV anabolites (pmol/106 cells)b
|
|||
|---|---|---|---|---|---|
| ACV-MP | ACV-DP | ACV-TP | Total | ||
| HSV-1 (F) (TK+, UL13+, UL97+) | <1 | 7.8 ± 0.04 | 3.0 ± 0.01 | 46 ± 2.6 | 56.8 |
| R4970 (TK−, UL13−, UL97+) | 19 ± 0.6 | 2.0 ± 0.07 | 1.1 ± 0.5 | 18 ± 7.4 | 21.1 |
| R7355 (TK−, UL13−, UL97−) | 51 ± 4.2 | 0.9 ± 0.5 | 0.3 ± 0.05 | 9.8 ± 1.1 | 11.0 |
| Mock-infected Vero cells | 0.04 ± 0.05 | 0.07 ± 0.01 | 1.5 ± 0.2 | 1.6 | |
Eight hours postinfection, cells were pulsed with [8-3H]ACV (200 μM) for 15 h. Intracellular anabolites of ACV were measured by anion-exchange HPLC.
Values are the means ± standard deviations for two samples.
Anabolism of ACV in recombinant HSV-1-infected cells.
The UL97+ recombinant virus, R4970, has been reported to induce GCV phosphorylation in infected Vero cells (38). To determine if ACV is also phosphorylated in R4970-infected cells, Vero cells were infected with either R4970, HSV-1 (F) (wild-type), or R7355 (UL13−, UL97−, TK−) or mock infected and then pulsed with [8-3H]ACV. Levels of ACV phosphates were determined by HPLC analyses (Table 3). Cells infected with HSV-1 (F) efficiently induced ACV phosphorylation. Vero cells infected with R4970 contained ACV phosphate levels that were 2-fold higher than those found in R7355-infected cells and 13-fold higher than those in mock-infected cells. However, it should be noted that the ACV-TP levels may have been overestimated due to the presence of a small interfering peak detected in the Vero cell extracts (data not shown). Attempts to resolve the TP and interfering peaks by modification of the HPLC method were unsuccessful.
Purified UL97 phosphorylation of ACV and GCV.
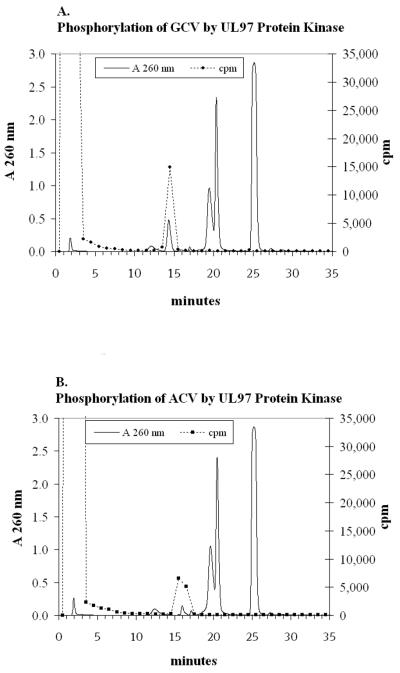
To show that the HCMV UL97 phosphotransferase is capable of directly phosphorylating ACV and GCV, substrate studies with purified UL97 were performed. Wild-type and K355Q mutant GST-UL97 fusion proteins expressed from baculovirus were purified and determined to be greater than 90% pure. Wild-type or K355Q mutant UL97 protein was added to a reaction mixture containing either approximately 1 μM [8-3H]GCV or approximately 1 μM [8-3H]ACV and allowed to react for 8 h at 37°C. Following addition of millimolar concentrations of unlabeled drug phosphates, the radioactive products were analyzed by HPLC. When wild-type GST-UL97 fusion protein was used, the peaks of radioactivity corresponded to the authentic standards of GCV-MP or ACV-MP (Fig. 1A and B). When mutant K355Q GST-UL97 protein or BSA was used, no GCV- or ACV-MPs were detected.
FIG. 1.
Anion-exchange HPLC profiles of wild-type GST-UL97 enzyme reactions at 8 h with 3H-labeled GCV (A) or 3H-labeled ACV (B), as described in the Materials and Methods section. Retention times of authentic carriers which appear in the UV profile are as follows: ACV, 1.8 min; GCV, 1.8 min; AMP, 12.2 min; GCV-MP, 14.3 min; ACV-MP, 15.9 min; GMP, 17.1 min; ADP, 20.4 min; ATP, 25.0 min; and GTP, 27.2 min. The UV peak at 19.5 min is an unknown peak generated from the UL97 preparation. Radioactive peaks were collected as 1-ml fractions. The scale for the counts per minute (cpm) was expanded to more clearly show the MP cpm; the cpm in fraction 2 were approximately 600,000, and those in fraction 3 were approximately 80,000.
Wild-type UL97 enzyme phosphorylated both GCV and ACV to their MPs at rates of 2.2 pmol/min/mg of protein and 1.2 pmol/min/mg of protein, respectively. The rate of formation of MP was linear over time for 8 h (time points were at 1.7, 4, and 8 h). The MP peak counts per minute obtained from wild-type UL97 were approximately 40-fold higher than background levels observed in a blank reaction mixture incubated with BSA.
DISCUSSION
The direct role of the HCMV UL97 protein in the phosphorylation of ACV in HCMV-infected cells was demonstrated in this report. We previously determined that ACV phosphorylation in cells was enhanced following HCMV infection, in the absence of a TK homologue encoded by the HCMV genome (4). Cytoplasmic 5′-nucleotidase, which is a mammalian cell enzyme that recognizes ACV as a substrate, likely accounts for the levels of ACV-MP formed in uninfected cells (29). However, neither cytoplasmic 5′-nucleotidase nor acid 5′-nucleotidase levels are induced by HCMV infection (5). Furthermore, ACV is not a substrate for mammalian deoxyguanosine kinase, which is induced after HCMV infection (5). As demonstrated here and as shown previously, even with the lack of a TK, AD169-infected cells induced the phosphorylation of ACV and AD169 virus showed some sensitivity to ACV in plaque reduction assays (4, 45).
Mutations present in UL97 are responsible for decreased GCV phosphorylation in infected cells and ultimately for GCV resistance (1, 9, 25, 46). These mutations also appear to be responsible for a decrease in the in vitro ACV susceptibility of these UL97 mutants. Elevated GCV and ACV IC50s were observed for the UL97 mutants compared to GCV and ACV IC50s for AD169. Lurain et al. (31) also reported that ACV sensitivity is decreased in a GCV-resistant HCMV strain containing a UL97 mutation different from those included in this study. Therefore, UL97 is important not only for GCV susceptibility but also for ACV susceptibility of HCMV in infected cells.
Reduced susceptibility of HCMV to GCV has been strongly correlated to reduced levels of GCV phosphates in infected cells (44). In this report, total ACV phosphate levels, as well as GCV phosphate levels, were reduced in UL97 mutant-infected cells compared to levels induced in AD169-infected cells. The reduced ACV and GCV phosphate levels in UL97 mutant-infected cells correlated with reductions in viral susceptibility to these antivirals. Therefore, mutations in UL97 that affect GCV phosphorylation also affect ACV phosphorylation.
A recombinant HSV-1 virus, R4970, in which the HSV-1 UL13 gene is replaced with the HCMV UL97 gene and the TK gene is deleted, has been constructed (38). In Vero cells infected with this recombinant virus, UL97 partially compensated for some of the functions of the HSV-1 UL13 and also contributed to GCV susceptibility and GCV phosphorylation (38). This virus allowed us to observe the role of UL97 in ACV susceptibility and phosphorylation outside the context of HCMV infection. In this study, the UL97-positive recombinant virus had intermediate susceptibility to ACV compared to ACV susceptibility levels of the wild-type HSV-1 strain and the UL97-negative recombinant virus. Therefore, UL97 plays a role in the in vitro susceptibility of ACV in R4970-infected cells.
Levels of ACV phosphates in cells infected with the UL97-positive recombinant HSV-1 were elevated compared to levels found in cells infected with R7355 (UL13−, UL97−, TK−). It was puzzling that R7355 induced ACV phosphorylation at a level approximately 6.5-fold over that by mock-infected cells since HSV-1 infection does not stimulate accumulation of ACV in infected cells or stimulate cellular kinases that may phosphorylate ACV in infected cells. Ng et al. also observed that the levels of GCV phosphates were threefold higher in R7355-infected Vero cells than in mock-infected cells. The higher degree of increase in ACV phosphates in R7355-infected cells than in mock-infected cells seen in this study is possibly due to the higher concentration of ACV (200 μM) used compared to the concentration of GCV (20 μM) used in the study of Ng et al. (38). The increase in ACV phosphate levels in R4970-infected cells over those in R7355-infected cells provides additional evidence that UL97 is important in the intracellular phosphorylation of ACV.
We demonstrated here that ACV and GCV were phosphorylated in vitro by wild-type UL97 enzyme. The K355Q mutant UL97 enzyme that contained the catalytic lysine substitution did not phosphorylate GCV or ACV. As was shown previously, this mutated enzyme was incapable of autophosphorylation (27). Thus, the catalytic lysine is likely required for both the protein kinase function and the nucleotide phosphorylation function of the enzyme. No contaminating kinases capable of phosphorylating ACV or GCV were present in our baculovirus preparations, as evidenced by the lack of ACV and GCV phosphorylation manifested by the K355Q mutant UL97 enzyme, which was purified in a manner identical to the wild-type UL97 enzyme.
ACV was phosphorylated less efficiently than GCV by UL97, which corresponds to the observation that HCMV is more susceptible to GCV than ACV in vitro (18, 32, 42). However, the relative ability of the HCMV UL97 to monophosphorylate ACV and GCV is not the sole determinant of the antiviral potencies of these two drugs. It is important to note that the intracellular half-life of ACV-TP is only 1 to 2 h whereas that of GCV-TP is 15 to 25 h (4, 20). Another factor significant to relative potency of these two drugs involves the second phosphorylation step in cells; cellular GMP kinase has Km for ACV-MP three- to fivefold higher than that for GCV-MP (6). Importantly, ACV-TP is a more potent alternative substrate inhibitor of the HCMV DNA polymerase than is GCV-TP (5- to 10-fold) (44). Furthermore, ACV is an obligate chain terminator (8, 34), whereas GCV does not completely block viral DNA synthesis (24).
The function of UL97 in phosphorylating naturally occurring nucleosides, as well as GCV, ACV, and penciclovir (PCV) has also been studied in cells infected with a vaccinia virus strain expressing UL97. No increase in nucleotide metabolism of naturally occurring nucleosides was seen in cells infected with vaccinia virus expressing UL97; therefore, UL97 is not believed to function as a nucleoside kinase (37). However, an increase in the rate of metabolism of the nucleoside analogues, GCV, ACV, and PCV, was observed in cells infected with a vaccinia virus strain expressing wild-type UL97. With this vaccinia virus system, ACV and PCV were phosphorylated to a lesser extent than GCV in these infected cells (47). When cells were infected with vaccinia virus expressing UL97 containing the same four-amino-acid deletion as that in XbaF 4-3-1, a markedly reduced, but not completely abolished, metabolism of the antivirals was detected (47). Therefore, this UL97 mutation may not completely block the phosphorylation of GCV and ACV by the UL97. However, this study did not examine the role of UL97 in ACV phosphorylation in herpesvirus-infected cells, nor was direct phosphorylation of ACV or GCV demonstrated by using purified UL97.
Prolonged treatment of patients with antivirals can heighten concern about the emergence of drug-resistant virus. Suboptimal dosing associated with GCV maintenance therapy may select for strains of HCMV that are resistant to GCV (13). Since ACV and GCV are similar structurally and in their mechanisms of action, could prophylactic ACV therapy select for GCV-resistant CMV? Drew et al. (11) reported that long-term exposure of human immunodeficiency virus-positive patients to high-dose ACV therapy did not induce the resistance of HCMV, isolated from these patients, to GCV. In another study, exposure to ACV or GCV for periods of 2 to 5 weeks did not alter the mean susceptibility of isolates obtained from immunocompromised patients (10). Additionally, ACV-resistant HCMV, selected in vitro, that contained DNA polymerase alterations did not exhibit cross-resistance to GCV in vitro, and vice versa (43). Three ACV-resistant HCMV strains selected in our laboratory did not contain UL97 mutations but rather contained DNA polymerase mutations (unpublished data). This is the opposite of the situation seen in clinical GCV-resistant viruses, in which mutations occur more frequently in the UL97 gene than in the DNA polymerase gene. Additional studies will be needed to determine whether ACV resistance of HCMV arises in the clinical setting and, if so, the mechanism of ACV resistance in clinical isolates.
Until now, the mechanism of action of ACV against HCMV was unknown. The observation of phosphorylation of ACV by the HCMV UL97 protein kinase in this study provides a rationale for the efficacy of ACV and valaciclovir therapy in preventing HCMV infection and disease in the immunocompromised host.
ACKNOWLEDGMENTS
The studies performed at the University of Chicago were aided by United States Public Health Service grants CA47451, CA71933, and CA78766 from the National Cancer Institute.
We thank Leslie Walton for assistance with preparing the figure for publication.
REFERENCES
- 1.Baldanti F, Silini E, Sarasini A, Talarico C L, Stanat S C, Biron K K, Furione M, Bono F, Palu G, Gerna G. A three-nucleotide deletion in the UL97 open reading frame is responsible for the ganciclovir resistance of a human cytomegalovirus clinical isolate. J Virol. 1995;69:796–800. doi: 10.1128/jvi.69.2.796-800.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldanti F, Underwood M R, Talarico C L, Simoncini L, Sarasini A, Biron K K, Gerna G. The Cys607→Tyr change in the UL97 phosphotransferase confers ganciclovir resistance to two human cytomegalovirus strains recovered from two immunocompromised patients. Antimicrob Agents Chemother. 1998;42:444–446. doi: 10.1128/aac.42.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balfour H H, Jr, Chace B A, Stapleton J T, Simmons R L, Fryd D S. A randomized, placebo-controlled trial of oral acyclovir for the prevention of cytomegalovirus disease in recipients of renal allographs. N Engl J Med. 1989;320:1381–1387. doi: 10.1056/NEJM198905253202105. [DOI] [PubMed] [Google Scholar]
- 4.Biron K K, Stanat S C, Sorrell J B, Fyfe J A, Keller P M, Lambe C U, Nelson D J. Metabolic activation of the nucleoside analog 9-{[2-hydroxyl-1-(hydroxymethyl)ethoxy]methyl} guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc Natl Acad Sci USA. 1985;82:2473–2477. doi: 10.1073/pnas.82.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biron K K, Fyfe J A, Stanat S C, Leslie L K, Sorrell J B, Lambe C U, Coen D M. A human cytomegalovirus mutant resistant to the nucleoside analog 9-{[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl} guanine (BW B759U) induces reduced levels of BW B759U triphosphate. Proc Natl Acad Sci USA. 1986;83:8769–8773. doi: 10.1073/pnas.83.22.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehme R. Phosphorylation of the antiviral precursor 9-(1,3-dihydroxy-2-propoxymethyl)guanine monophosphate by guanylate kinase isozymes. J Biol Chem. 1984;259:12346–12349. [PubMed] [Google Scholar]
- 7.Chee M S, Lawrence G L, Barrell B G. Alpha-, beta-, and gammaherpesviruses encode a putative phosphotransferase. J Gen Microbiol. 1989;70:1151–1160. doi: 10.1099/0022-1317-70-5-1151. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y C, Grill S P, Dutschman G E, Nakayama K, Bastow K F. Metabolism of 9-(1,3-dihydroxy-2-propoxymethyl)guanine, a new anti-herpes virus compound, in herpes simplex virus-infected cells. J Biol Chem. 1983;258:12460–12464. [PubMed] [Google Scholar]
- 9.Chou S C, Erice A, Jordan M C, Vercellotti G M, Michels K R, Talarico C L, Stanat S C, Biron K K. Analysis of the UL97 phosphotransferase coding sequence in clinical cytomegalovirus isolates and identification of mutations conferring ganciclovir resistance. J Infect Dis. 1995;171:576–583. doi: 10.1093/infdis/171.3.576. [DOI] [PubMed] [Google Scholar]
- 10.Cole N L, Balfour H H., Jr In vitro susceptibility of cytomegalovirus isolates from immunocompromised patients to acyclovir and ganciclovir. Diagn Microbiol Infect Dis. 1987;6:255–261. doi: 10.1016/0732-8893(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 11.Drew W L, Anderson R, Lang W, Miner R C, Davis G, Lalezari J. Failure of high-dose oral acyclovir to suppress CMV viruria or induce ganciclovir-resistant CMV in HIV antibody positive patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:289–291. doi: 10.1097/00042560-199503010-00010. [DOI] [PubMed] [Google Scholar]
- 12.Drew W L, Buhles W, Erlich K S. Herpesvirus infections (cytomegalovirus, herpes simplex virus, varicella zoster virus). How to use ganciclovir (DHPG) and acyclovir. Infect Dis Clin N Am. 1988;2:495–509. [PubMed] [Google Scholar]
- 13.Drew W L, Miner R C, Busch D F, Follanbee S E, Gullet J, Mahalko S G, Gordan A M, Owen W F, Jr, Matthews T R, Buhles W C, DeArmond B. Prevalence of CMV resistance in patients receiving ganciclovir for serious CMV infection. J Infect Dis. 1991;163:716–719. doi: 10.1093/infdis/163.4.716. [DOI] [PubMed] [Google Scholar]
- 14.Elion G B, Furman P A, Fyfe J A, de Miranda P, Beauchamp L, Schaeffer H J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl)guanine. Proc Natl Acad Sci USA. 1977;74:5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkins C C, Frist W H, Dummer J S, Stewart J R, Merrill W H, Carden K A, Bender H W. Cytomegalovirus disease after heart transplantation: is acyclovir prophylaxis indicated? Ann Thorac Surg. 1993;56:1267–1273. doi: 10.1016/0003-4975(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 16.Ertl P, Snowden W, Lowe D, Miller W, Collins P, Littler E. A comparative study of the in vitro and in vivo antiviral activities of acyclovir and penciclovir. Antivir Chem Chemother. 1995;6:89–97. [Google Scholar]
- 17.Feinberg J E, Hurwitz S, Cooper D, Sattler F R, MacGregor R R, Powderly W, Holland G N, Griffiths P D, Pollard R B, Youle M, Gill M J, Holland F J, Power M E, Owens S, Coakley D, Fry J, Jacobson M A. A randomized, double-blind trial of valaciclovir prophylaxis for cytomegalovirus disease in patients with advanced human immunodeficiency virus infection. J Infect Dis. 1998;177:48–56. doi: 10.1086/513804. [DOI] [PubMed] [Google Scholar]
- 18.Field A K, Davies M E, DeWitt D, Perry H C, Liou R, Germenshausen J, Karkas J D, Ashton W T, Johnston D B R, Tolman R L. 9-[(2-Hydroxy-1-(hydroxymethyl)ethoxy)methyl]guanine: a selective inhibitor of herpes group virus replication. Proc Natl Acad Sci USA. 1983;80:4139–4143. doi: 10.1073/pnas.80.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furman P A, St. Clair M H, Fyfe J A, Rideout J L, Keller P M, Elion G B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979;32:72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furman P A, de Miranda P, St. Clair M H, Elion G B. Metabolism of acyclovir in virus-infected and uninfected cells. Antimicrob Agents Chemother. 1981;20:518–524. doi: 10.1128/aac.20.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fyfe J A, Keller P M, Furman P A, Miller R L, Elion G B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978;253:8721–8727. [PubMed] [Google Scholar]
- 22.Glenn J. Cytomegalovirus infections following renal transplantation. Rev Infect Dis. 1981;3:1151–1178. doi: 10.1093/clinids/3.6.1151. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths P D, Feinberg J E, Fry J, Sabin C, Dix L, Gor D, Ansari A, Emery V C. The effect of valaciclovir on cytomegalovirus viremia and viruria detected by polymerase chain reaction in patients with advanced human immunodeficiency virus disease. J Infect Dis. 1998;177:57–64. doi: 10.1086/513806. [DOI] [PubMed] [Google Scholar]
- 24.Hamzeh F M, Lietman P S, Gibson W, Hayward G S. Identification of the lytic origin of DNA replication in human cytomegalovirus by a novel approach utilizing ganciclovir-induced chain termination. J Virol. 1990;64:6184–6195. doi: 10.1128/jvi.64.12.6184-6195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanson M N, Preheim L C, Chou S C, Talarico C L, Biron K K, Erice A. Novel mutation in the UL97 gene of a clinical cytomegalovirus strain conferring resistance to ganciclovir. Antimicrob Agents Chemother. 1995;39:1204–1205. doi: 10.1128/aac.39.5.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He, Z., and D. M. Coen. Personal communication.
- 27.He Z, He Y-S, Kim Y, Chu L, Ohmstede C, Biron K K, Coen D M. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J Virol. 1997;71:405–411. doi: 10.1128/jvi.71.1.405-411.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson M A, Gallant J, Wang L H, Coakley D, Weller S, Gary D, Squires L, Smiley M L, Blum M R, Feinberg J. Phase I trial of valaciclovir, the l-valyl ester of acyclovir, in patients with advanced human immunodeficiency virus disease. Antimicrob Agents Chemother. 1994;38:1534–1540. doi: 10.1128/aac.38.7.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keller P M, McKee S A, Fyfe J A. Cytoplasmic 5′-nucleotidase catalyzes acyclovir phosphorylation. J Biol Chem. 1985;260:8664–8667. [PubMed] [Google Scholar]
- 30.Littler E, Stuart A D, Chee M S. The human cytomegalovirus UL97 open reading frame encodes a protein which phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992;358:160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- 31.Lurain N S, Spafford L E, Thompson K D. Mutation in the UL97 open reading frame of human cytomegalovirus strains resistant to ganciclovir. J Virol. 1994;68:4427–4431. doi: 10.1128/jvi.68.7.4427-4431.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mar E-C, Cheng Y-C, Huang E-S. Effect of 9-(1,3-dihydroxy-2-proxymethyl) guanine on human cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 1983;24:518–521. doi: 10.1128/aac.24.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marker S C, Howard R J, Simmons R I, et al. Cytomegalovirus infection: a quantitative prospective study of three hundred twenty consecutive renal transplants. Surgery. 1981;89:660–671. [PubMed] [Google Scholar]
- 34.McQuirt P V, Furman P A. Acyclovir inhibition of viral DNA chain elongation in herpes simplex virus-infected cells. Am J Med. 1982;73(Suppl. 1A):67–71. doi: 10.1016/0002-9343(82)90066-3. [DOI] [PubMed] [Google Scholar]
- 35.Metzger C, Michel D, Schneider K, Lüske A, Schlicht H-J, Mertens T. Human cytomegalovirus UL97 kinase confers ganciclovir susceptibility to recombinant vaccinia virus. J Virol. 1994;68:8423–8427. doi: 10.1128/jvi.68.12.8423-8427.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyers J D, Reed E C, Shepp D H, et al. Acyclovir for prevention of cytomegalovirus infection and disease after allogeneic marrow transplantation. N Engl J Med. 1988;318:70–75. doi: 10.1056/NEJM198801143180202. [DOI] [PubMed] [Google Scholar]
- 37.Michel D, Pavic I, Zimmerman A, Haupt E, Wunderlich K, Heuschmid M, Mertens T. The UL97 gene product of human cytomegalovirus is an early-late protein with a nuclear localization but is not a nucleoside kinase. J Virol. 1996;70:6340–6346. doi: 10.1128/jvi.70.9.6340-6346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng T I, Talarico C, Burnette T C, Biron K, Roizman B. Partial substitution of the functions of the herpes simplex virus 1 UL13 gene by the human cytomegalovirus UL97 gene. Virology. 1996;225:347–358. doi: 10.1006/viro.1996.0609. [DOI] [PubMed] [Google Scholar]
- 39.Nunan T O, King M, Bull P, Banatvala J E, Jones N F, Hilton P J. Parenteral acyclovir therapy for cytomegalovirus infection after renal transplantation. Clin Nephrol. 1984;22:28–31. [PubMed] [Google Scholar]
- 40.Peterson P K, Balfour H H, Marker S C, Fryd D S, Howard R J, Simmons R L. Cytomegalovirus disease in renal allograft recipients: a prospective study of the clinical features, risk factors and impact on renal transplantation. Medicine. 1980;59:283–300. [PubMed] [Google Scholar]
- 41.Sha B E, Benson C A, Deutsch T A, Urbanski P A, Phair J P, Kessler H A. Suppression of cytomegalovirus retinitis in persons with AIDS with high-dose intravenous acyclovir. J Infect Dis. 1991;164:777–780. doi: 10.1093/infdis/164.4.777. [DOI] [PubMed] [Google Scholar]
- 42.Smee D F, Martin J C, Verheyden J P, Matthews T R. Anti-herpesvirus activity of the acyclic nucleoside 9-(1,3-dihydroxy-2-propoxymethyl)guanine. Antimicrob Agents Chemother. 1983;23:676–682. doi: 10.1128/aac.23.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snoeck R, Andrei G, De Clercq E. Patterns of resistance and sensitivity to antiviral compounds of drug-resistant strains of human cytomegalovirus selected in vitro. Eur J Clin Microbiol Infect Dis. 1996;15:574–579. doi: 10.1007/BF01709366. [DOI] [PubMed] [Google Scholar]
- 44.Stanat S C, Reardon J E, Erice A, Jordan M C, Drew W L, Biron K K. Ganciclovir-resistant cytomegalovirus clinical isolates: mode of resistance to ganciclovir. Antimicrob Agents Chemother. 1991;35:2191–2197. doi: 10.1128/aac.35.11.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan V, Coen D M. Isolation of foscarnet-resistant human cytomegalovirus: patterns of resistance and sensitivity to other antiviral drugs. J Infect Dis. 1991;164:781–784. doi: 10.1093/infdis/164.4.781. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan V, Talarico C L, Stanat S C, Davis M, Coen D M, Biron K K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992;358:162–164. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann A, Michel D, Pavic I, Hampl W, Luske A, Neyts J, De Clercq E, Mertens T. Phosphorylation of acyclovir, ganciclovir, penciclovir, and S2242 by the cytomegalovirus UL97 protein: a quantitative analysis using recombinant vaccinia viruses. Antivir Res. 1997;36:35–42. doi: 10.1016/s0166-3542(97)00034-x. [DOI] [PubMed] [Google Scholar]