Background:
TP (thromboxane A2 receptor) plays an eminent role in the pathophysiology of endothelial dysfunction and cardiovascular disease. Moreover, its expression is reported to increase in the intimal layer of blood vessels of cardiovascular high-risk individuals. Yet it is unknown, whether TP upregulation per se has the potential to affect the homeostasis of the vascular endothelium.
Methods:
We combined global transcriptome analysis, lipid mediator profiling, functional cell analyses, and in vivo angiogenesis assays to study the effects of endothelial TP overexpression or knockdown/knockout on the angiogenic capacity of endothelial cells in vitro and in vivo.
Results:
Here we report that endothelial TP expression induces COX-2 (cyclooxygenase-2) in a Gi/o- and Gq/11-dependent manner, thereby promoting its own activation via the auto/paracrine release of TP agonists, such as PGH2 (prostaglandin H2) or prostaglandin F2 but not TxA2 (thromboxane A2). TP overexpression induces endothelial cell tension and aberrant cell morphology, affects focal adhesion dynamics, and inhibits the angiogenic capacity of human endothelial cells in vitro and in vivo, whereas TP knockdown or endothelial-specific TP knockout exerts opposing effects. Consequently, this TP-dependent feedback loop is disrupted by pharmacological TP or COX-2 inhibition and by genetic reconstitution of PGH2-metabolizing prostacyclin synthase even in the absence of functional prostacyclin receptor expression.
Conclusions:
Our work uncovers a TP-driven COX-2–dependent feedback loop and important effector mechanisms that directly link TP upregulation to angiostatic TP signaling in endothelial cells. By these previously unrecognized mechanisms, pathological endothelial upregulation of the TP could directly foster endothelial dysfunction, microvascular rarefaction, and systemic hypertension even in the absence of exogenous sources of TP agonists.
Keywords: angiogenesis, cyclooxygenase 2, endothelial cells, endothelial dysfunction, prostaglandin H2, thromboxan A2 receptor
Highlights.
The TP (thromboxane A2 receptor) positively regulates COX-2 (cyclooxygenase-2) expression in human endothelial cells.
Increased expression of the TP triggers an aberrant COX-2–dependent positive feedback loop in human endothelial cells that results in persistent, most likely PGH2 (prostaglandin H2)-mediated, auto/paracrine TP activation.
Increased TP expression induces endothelial cell tension and dysfunction and inhibits angiogenic endothelial cell functions in vitro and in vivo.
These TP-related effects are triggered by ROCK (Rho-associated coiled-coil containing protein kinase)-LIMK2 (LIM domain kinase 2)-myosin II–dependent signal transduction in human endothelial cells.
TxA2 (thromboxane A2) is a potent, yet very short-lived mediator of platelet activation and vasoconstriction and exerts its effects by activation of the heptahelical TP (TxA2 receptor).1 In humans, 2 different isoforms of the TP have been identified, namely TPα and TPβ, which (1) derive from alternative splicing, (2) are characterized by equal affinity to TxA2 but possess different C-terminal tails, and (3) may differ in their signal transducing properties.1 In contrast, in mice and rats, TP orthologues have been described that are similar to the human TPα isoform.1 In addition to TxA2, other eicosanoids, that is, the cyclooxygenase-derived prostaglandins PGH2 (prostaglandin H2) and PGF2α (prostaglandin F2α) and the F2-isoprostane 8-iso-PGF2α, act as affine agonists of the TP in vitro and in vivo.1–3 On the mechanistic level, both human TP isoforms couple to Gq/11, G12/13, and Gi/o proteins, thereby linking the TP to downstream effectors involved in cytoskeletal remodeling, cell tension, adhesion, and proliferation.1,4–6 The TP also represents a relevant effector in the vascular endothelium, where it influences neovascularization processes7–20 and promotes the development of endothelial dysfunction,21,22 as well as atherosclerotic vascular disease.21,23,24 In addition, TP expression is reported to increase in the intimal layer of blood vessels of cardiovascular high-risk patients and atherosclerotic murine blood vessels,25,26 suggesting an excess of harmful vascular endothelial TP signal transmission in cardiovascular disease. These observations also raise the question whether an increased TP expression per se negatively affects the homeostasis and angiogenic capacity of the vascular endothelium and thus contributes to the phenomena of endothelial dysfunction and microvascular rarefaction, which are observed, for example, in individuals with hypertension and cardiovascular disease.1,27–30
Here we report a previously unrecognized TP-driven positive feedback loop in endothelial cells, in which increased TP expression induces COX-2 (cyclooxygenase-2; PTGS2 [prostaglandin-endoperoxide synthase 2]) to promote its own activation via the auto/paracrine release of TP agonists, such as PGH2 or PGF2α but not TxA2. The resulting persistent TP activity then enhances endothelial cell tension, alters endothelial cell morphology and focal adhesion dynamics, affects the expression profile of important mediators of endothelial cell homeostasis, for example, VEGFR-1 (vascular endothelial growth factor receptor 1), VEGFR-2 (vascular endothelial growth factor receptor 2), and eNOS (endothelial NO synthase), and reduces the angiogenic capacity of endothelial cells in vitro and in vivo through the activation of a ROCK (Rho-associated coiled-coil containing protein kinase)-, LIMK2 (LIM domain kinase 2)-, and myosin II–dependent signal transduction pathway. This feedback loop that inhibits angiogenesis-associated endothelial cell functions can be disrupted by pharmacological TP or COX-2 inhibition, as well as by genetic reconstitution of PGH2-metabolizing prostacyclin synthase, interventions that reduce the synthesis, bioavailability, or actions of endogenous TP-activating prostanoids. Moreover, we reveal that shear stress during laminar flow and RhoA (Ras homolog gene family, member A) activity are stimuli that induce TP expression in human endothelial cells of arterial and venous origin.
Materials and Methods
A comprehensive description of Materials and Methods can be found in the Supplemental Materials and Methods.
Results
TP Inhibits the Angiogenic Capacity of Human Endothelial Cells
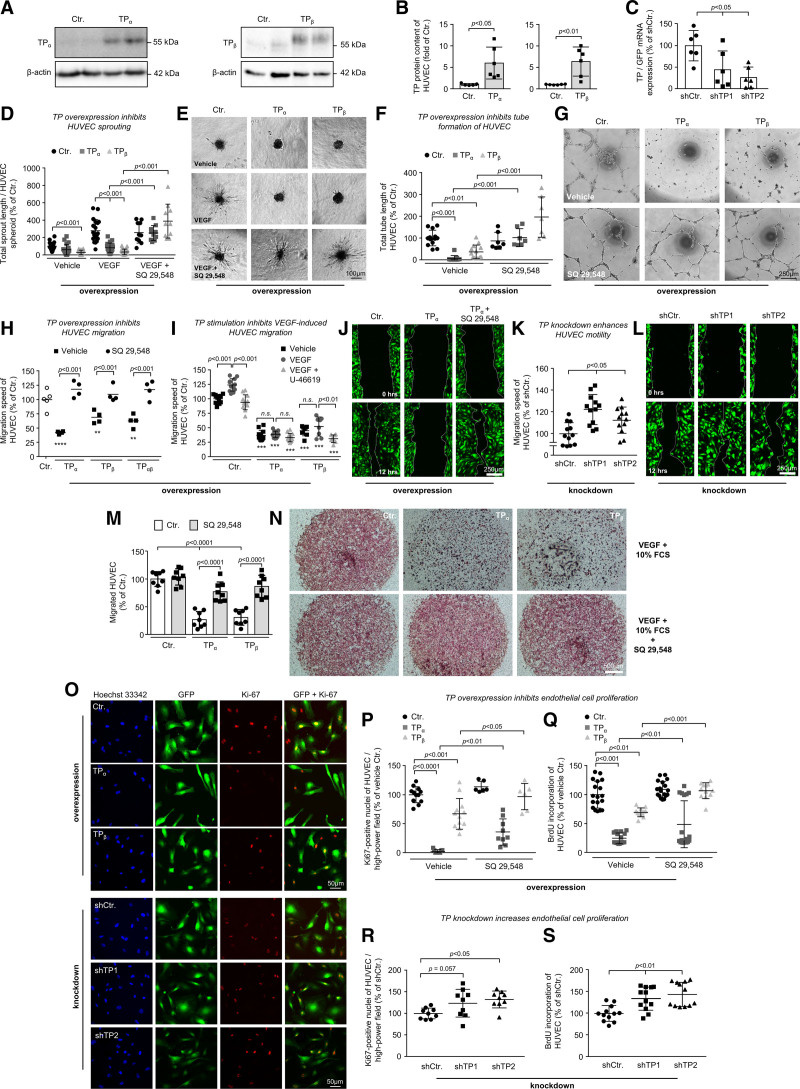
We used RNA interference–mediated knockdown or stable overexpression of the TP (lentiviral gene transfer; Figure 1A through 1C) to elucidate the impact of the receptor on the angiogenic capacity of human umbilical vein endothelial cells (HUVECs) and human coronary artery endothelial cells. First, overexpression of either TPα or TPβ, in the absence of exogenous agonists, strongly reduced HUVEC sprouting (Figure 1D and 1E), tube formation (Figure 1F and 1G), nondirected motility (Figure S1A and S1B), scratch-induced migration (Figure 1H through 1J), chemotactic trans-well migration (Figure 1M and 1N), and proliferation (Figure 1O through 1Q; Figure S2A and S2B)—effects that were blocked by concomitant pharmacological TP inhibition (Videos S1 through S3). The inhibitory efficacy of TP overexpression gradually increased with increasing TP gene doses (multiplicity of infection) as shown for scratch-induced HUVEC migration (Figure S1C and S1D) and was not (TPα) or only slightly (TPβ) enhanced by U-46619, a stable full TP agonist, suggesting that endogenous activation of the receptor already triggered an almost maximum biological response (Figure 1I). Similar results were obtained in human coronary artery endothelial cells, in which TP overexpression disturbed tube formation and scratch-induced migration—effects reversed by the pharmacological TP antagonist SQ-29548 (Figure S1E through S1G). Interestingly, the conditioned medium from TP-overexpressing HUVECs did not confer antimigratory effects on native HUVECs compared with the conditioned medium from corresponding control cells, suggesting that the stable components of the secretome of TP-overexpressing endothelial cells are probably not involved in the motility-reducing effect of the receptor (Figure S1H). In contrast to TP overexpression, TP knockdown significantly increased scratch-induced migration (Figure 1K and 1L) and proliferation (Figure 1O, 1R, and 1S; Figure S2C) of HUVECs.
Figure 1.
Increased TP (thromboxane A2 receptor) expression reduces the angiogenic capacity of human endothelial cells in vitro—an effect that is blocked by pharmacological TP inhibition. Overexpression of either the TPα or the TPβ isoform in human umbilical vein endothelial cells (HUVECs) was quantified using Western blot analyses (A and B), respectively, whereas the (nonspecific) knockdown of both isoforms was analyzed using qRT-PCR (quantitative real time PCR; C). The statistical analyses shown in B were performed using the unpaired 2-tailed Student t test, whereas all other statistical analyses were performed using 1-way ANOVA followed by the Sidak multiple comparisons test. Overexpression of the TP abolishes VEGF (vascular endothelial growth factor)-induced HUVEC sprouting (D and E; n=10–20) and tube formation of HUVECs (F and G; n=7–13) in vitro—an effect reversed by pharmacological TP inhibition (SQ-29548; 3×10−5 mol/L). Overexpression of TPα, TPβ, or both TPα and TPβ (H–J) inhibits scratch-induced migration of HUVECs—an effect that is reversed by pharmacological TP inhibition with SQ-29548 (3×10−5 mol/L). **P<0.01/****P<0.0001 vs control-transduced HUVECs (n=4–12). Pharmacological activation of the TP using the synthetic TP agonist U-46619 (3×10−5 mol/L) significantly inhibits migration of VEGF-stimulated control and TPβ-overexpressing HUVECs (I). ***P<0.001 vs equally treated control-transduced HUVECs (n=8–12). M and N, TPα and TPβ overexpression also reduced chemotactic HUVEC motility in trans-well migration assays—an effect reversed by pharmacological TP inhibition with SQ-29548 (3×10−5 mol/L). K and L, In contrast, knockdown of the TP using 2 different shRNAs (shTP1 and shTP2) enhances directional migration of HUVECs in the endothelial scratch assay in vitro as compared with nontargeting shRNA control (n=12). TP overexpression decreases BrdU incorporation of HUVECs (Q; n=12–18) and HUVEC expression of proliferation marker Ki-67 (O and P; n=6–12)—an effect that is partly reversed by pharmacological TP antagonism with SQ-29548 (3×10−5 mol/L). In contrast, TP knockdown in HUVECs increases the expression of proliferation marker Ki-67 (O and R) and BrdU incorporation (S) of these cells as compared with nontargeting shRNA control (n=9–12).
TP Inhibits the Angiogenic Capacity of Human Endothelial Cells In Vivo
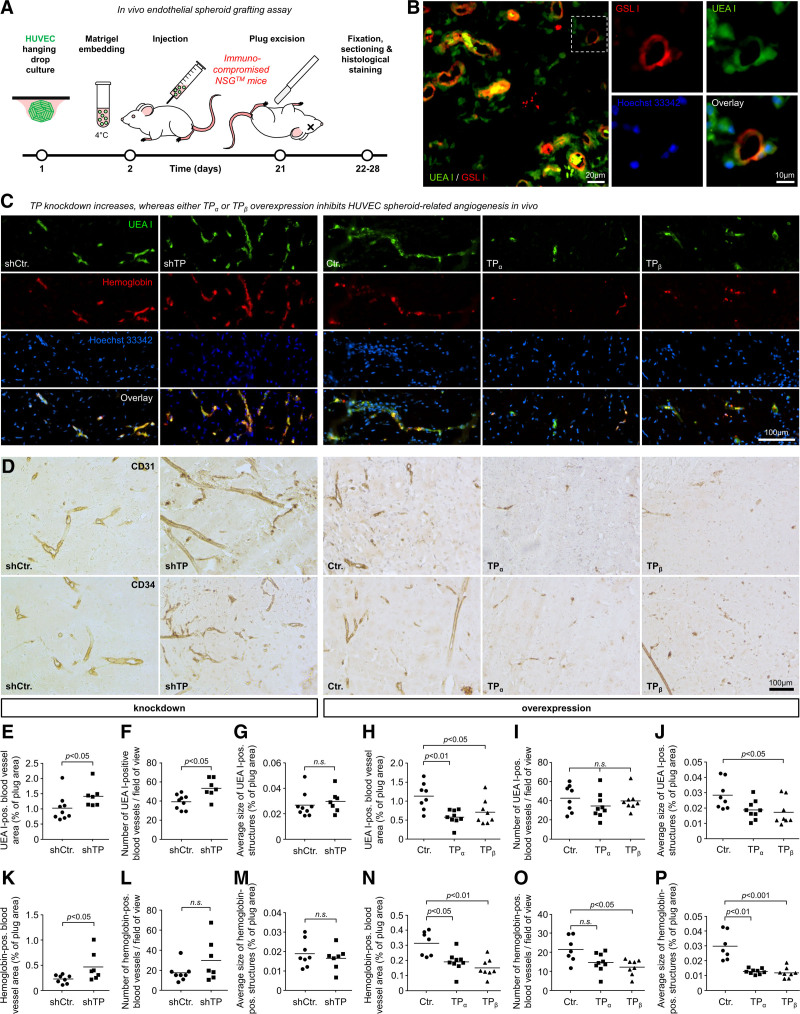
To assess the impact of the TP on the angiogenic capacity of human endothelial cells in vivo, we used the HUVEC spheroid grafting assay in immunocompromised NSG mice (Figure 2A).31,32 Interestingly and as described previously by Laib et al,31 costainings with Griffonia simplicifolia lectin I isolectin B4 (a specific marker for murine endothelial cells) and Ulex europaeus agglutinin I (a specific marker for human endothelial cells) indicated that hybrid blood vessels composed of HUVECs and murine host endothelial cells have formed in the implanted plugs (Figure 2B). These neovessels colocalized with murine hemoglobin (Figure 2C), indicating a connection to the endogenous blood circulation of the murine host, and were also positive for human endothelial cell markers CD31/34 (cluster of differentiation 31/34; Figure 2D). Stable shRNA-mediated knockdown of the TP (lentiviral gene transfer) increased the angiogenic capacity of implanted HUVEC in vivo as evidenced by an increase in the blood vessel area and by an increase in the average density of Ulex europaeus agglutinin I–stained (Figure 2C, 2E through 2G) and hemoglobin-positive (Figure 2K through 2M) blood vessels. In contrast, TPα or TPβ overexpression, respectively, caused significant reduction of the angiogenic capacity of HUVECs in vivo as evidenced by decreased density, size, and number of Ulex europaeus agglutinin I–stained (Figure 2C, 2H through 2J) and hemoglobin-positive (Figure 2C, 2N through 2P) neovessels in the xenograft plugs.
Figure 2.
The endothelial TP (thromboxane A2 receptor) inhibits humanoid neovessel formation in vivo. A, Schematic illustration of the experimental setup of the human umbilical vein endothelial cell (HUVEC) spheroid-based grafting assay in immunodeficient NSG mice. B, Microscopic image of xenograft plug sections stained with Hoechst 33342, FITC-labeled Ulex europaeus agglutinin I (UEA I), and DyLight 594-labeled Griffonia simplicifolia lectin I (GSL I) isolectin B4 for visualization of nuclei, implanted HUVECs, and murine endothelial cells, respectively. The magnification of the selected region on the left indicates that implanted human endothelial cells form hybrid blood vessels with endothelial cells from the murine host. C, Representative microscopic pictures of xenograft plug sections stained with UEA I (green)—an antibody directed against murine hemoglobin subunit alpha (red) and Hoechst 33342 (blue), respectively. Detection of hemoglobin served as a surrogate for the connection of intraplug blood vessels to the functional blood circulation of the murine host. UEA I–positive and hemoglobin-positive neovessel structures were determined by histomorphometric immunofluorescence analysis. D, Immunohistochemical analyses revealed that the vascular network derived from implanted HUVEC cells expressed typical human endothelial cell markers CD31 (cluster of differentiation 31) and CD34 (cluster of differentiation 34). E–G, shRNA-mediated knockdown of the TP in human endothelial cells (HUVECs) enhances VEGF (vascular endothelial growth factor)- and bFGF (basic fibroblast growth factor)-induced blood vessel formation of these cells in vivo as compared with control HUVECs expressing nontargeting shRNA (n=7–9). H–J, In contrast, overexpression of either TPα or TPβ in HUVECs reduces VEGF- and bFGF-induced blood vessel formation in vivo as compared with control-transduced HUVECs (n=8–9). Blood vessel growth was analyzed by quantification of the UEA I–positive blood vessel area (E and H). Moreover, the number of UEA I–positive blood vessels per high-power field (F and I) and the average size of these vessels (G and J) were determined. K–M, In addition, shRNA-mediated knockdown of the TP in HUVECs increases functional (hemoglobin positive) blood vessel formation of these cells in vivo as compared with control HUVECs expressing nontargeting shRNA (n=7–8). The statistical analyses in E–G and K–M were performed using the Mann-Whitney U test. N–P, In contrast, overexpression of either TPα or TPβ in HUVECs reduces functional blood vessel formation in vivo as compared with control-transduced HUVECs (n=7–9). Statistical analyses in H–J and N–P were performed using the Kruskal-Wallis test followed by the Dunn test for multiple comparisons. Functional blood vessels were analyzed by quantification of murine hemoglobin-positive blood vessel area (K and N). Moreover, the number of hemoglobin-positive blood vessels per high-power field (L and O) and the average size of these vessels (M and P) were determined.
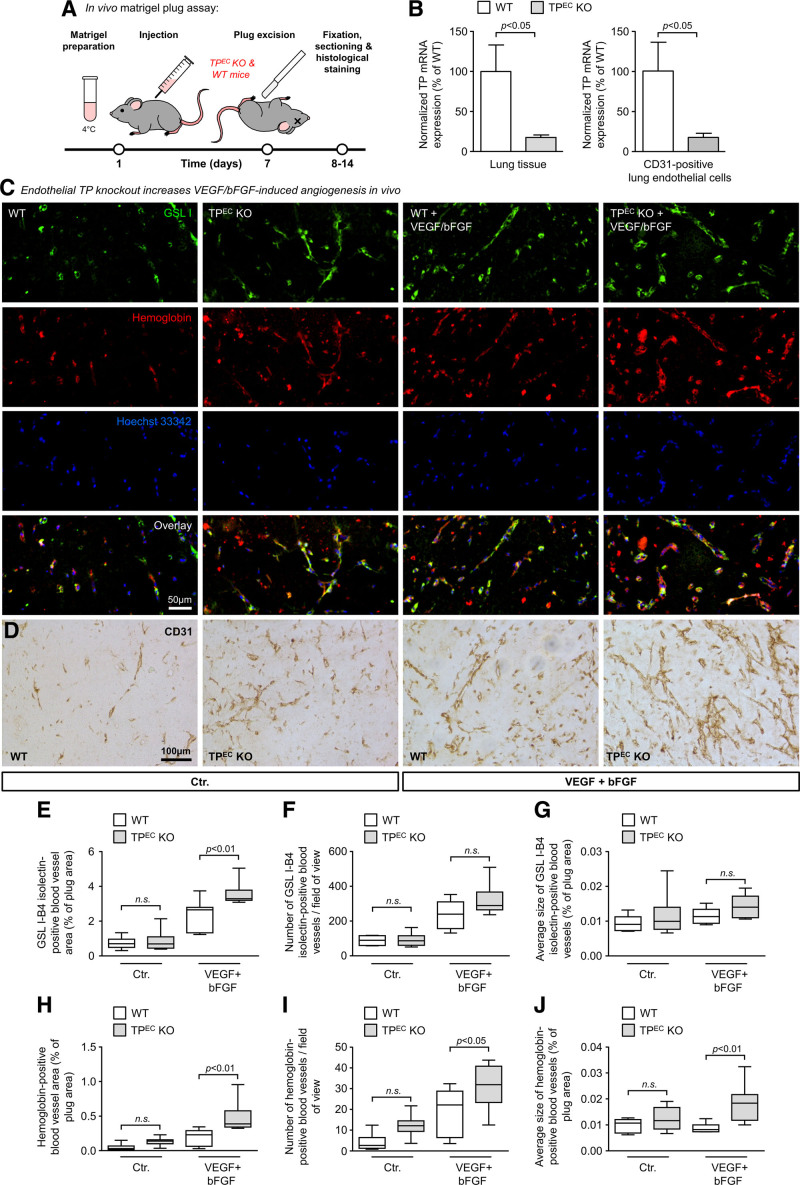
Endothelial TP Deletion Increases VEGF- and bFGF-Induced Angiogenesis In Vivo
Next, we elucidated in which way vascular endothelial-specific deletion of the TP in mice affects the angiogenic response in the matrigel plug assay of angiogenesis (Figure 3). We observed that vascular endothelial TP knockout mice (Figure 3A and 3B) as compared with sex-matched TP-expressing (wild type) littermates showed an increased angiogenic response to VEGF and bFGF (basic fibroblast growth factor). This was evidenced by an increase in the Griffonia simplicifolia lectin I isolectin B4–positive (Figure 3C and 3E) and hemoglobin-positive blood vessel area (Figure 3C and 3H) and an increase in the average number of hemoglobin-positive blood vessels per high-power field (Figure 3C and 3I).
Figure 3.
Endothelial-specific TP (thromboxane A2 receptor) knockout in mice induces VEGF (vascular endothelial growth factor)- and bFGF (basic fibroblast growth factor)-mediated neovessel formation in the matrigel plug assay in vivo. A, Schematic illustration of the experimental setup of the matrigel plug assay in endothelial TP knockout mice (TPEC KO) and wild-type sex-matched littermates (WT). B, Reduction of TP mRNA expression in total lung tissue (n=6–8) and in CD31 (cluster of differentiation 31)-positive lung endothelial cells isolated from adult TPEC KO mice and wild-type littermates (n=4–6). Data are shown as mean±SEM. The statistical analyses in B were performed using the unpaired 2-tailed Student t test (lung tissue) or the Mann-Whitney U test (CD31-positive lung endothelial cells). C, Representative microscopic pictures of matrigel plug sections stained with Griffonia simplicifolia lectin I (GSL I)—an antibody directed against murine hemoglobin and Hoechst 33342 for the visualization of murine endothelial cells, red blood cells, and nuclei, respectively. D, Representative microscopic pictures of matrigel plug sections stained with an antibody directed against murine CD31 (pecam-1)—a marker of endothelial cells. E–J, Selective deletion of the TP in the vascular endothelium of mice (TPEC KO) enhances VEGF- and bFGF-induced growth of blood vessels but does not significantly augment spontaneous matrigel-induced neovessel formation in the matrigel plug model of angiogenesis (n=6–8). The data are shown as minimum-to-maximum box and whisker plots (including median with 25% and 75% percentiles [IQR]) and were analyzed using 1-way ANOVA followed by the Sidak multiple comparisons test. Blood vessel growth was analyzed by quantification of the GSL I–positive blood vessel area (E), the number of GSL I–positive blood vessels per high-power field (F), as well as the average size of these vessels (G). Moreover, the number of functional blood vessels was analyzed by quantification of hemoglobin-positive blood vessel area (H), the number of hemoglobin-positive blood vessels per high-power field (I), as well as the average size of these vessels (J).
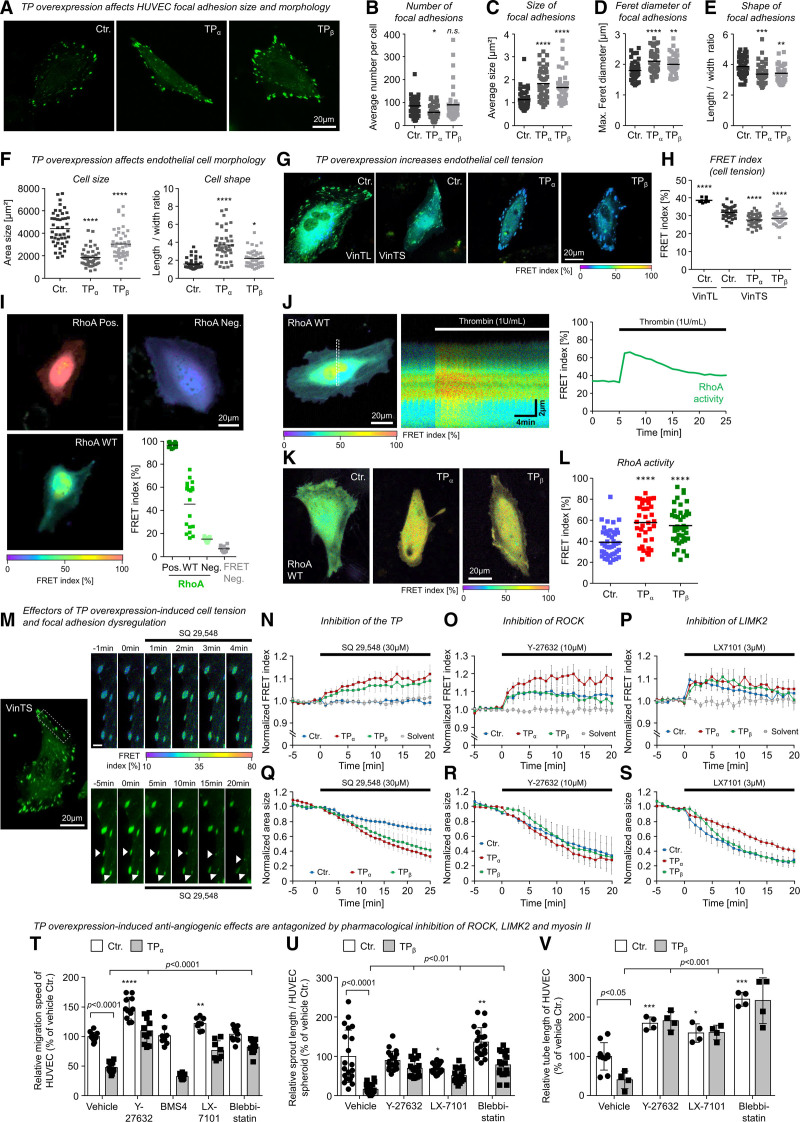
TP Overexpression Increases Cell Tension, Disturbs Focal Adhesion Dynamics, and Inhibits Angiogenic Endothelial Cell Functions via ROCK, LIMK2, and Myosin II Activation
Our microscopic live-cell analyses suggested that increasing TP expression per se generated cellular tension to induce contraction of human endothelial cells, the collapse of endothelial tubes, and endothelial spheroid sprout retraction. To substantiate these observations, we analyzed endothelial cell tension generated at focal adhesions, focal adhesion dynamics, and the morphology of HUVEC using a live-cell setup and a previously validated vinculin-based Förster resonance energy transfer biosensor.33 In these experiments, we observed that increased TP expression per se increased cell tension, reduced endothelial cell size, induced an elongated cellular phenotype (TPα), and favored the formation of large, irregularly shaped focal adhesions in particular at the cell margin of HUVEC (Figure 4A through 4H; Figure S3A). In this context, we observed that the vinculin tension sensor colocalized with the focal adhesion marker paxillin. In addition, analyses of live cells showed that vinculin tension sensor–positive focal adhesion complexes were associated with the actin cytoskeleton. In TP-overexpressing cells, a marked cortical localization of actin fibers was observed (Figure S3). In line with the observed changes in cell tension, as well as localization and morphology of focal adhesion complexes, the adhesion process of TP-overexpressing cells on differentially coated surfaces was retarded (Figure S4A through S4C). On the contrary, TP knockdown did not consistently alter the adhesiveness of HUVECs (Figure S4D through S4F) and had no effect on focal adhesion morphology (Figure S5). To investigate whether the increased cell tension in TP-overexpressing HUVECs might be related to increased RhoA activity, we measured the RhoA activity in live TP-overexpressing and corresponding control HUVECs using a validated Förster resonance energy transfer–based RhoA biosensor34–36 (Figure 4I through 4L). In these analyses, we observed significantly increased activity of RhoA in TP-overexpressing cells (Figure 4K and 4L). In agreement with these results, pharmacological TP inhibition rapidly reduced cell tension in TP-overexpressing HUVECs (Figure 4M and 4N; Video S4). Moreover, pharmacological inhibition of the RhoA effectors and actomyosin regulators ROCK1/2 and LIMK2 resulted in a comparable decrease in cell tension as observed with pharmacological TP inhibition (Figure 4O and 4P). Moreover, TP inhibition also induced a rapid regression of focal adhesion size, especially in TP-overexpressing HUVECs (Figure 4M and 4Q; Video S5), and it rapidly increased the cell size of TP-overexpressing HUVECs (Figure S6B). This regression of focal adhesion complexes was also mimicked by pharmacological inhibition of ROCK1/2 and LIMK2 (Figure 4R and 4S). In accordance with these observations, pharmacological inhibition of ROCK1/2, LIMK2 (but not LIMK1 [LIM domain kinase 1]), and myosin II all significantly diminished TP overexpression–induced inhibition of endothelial cell migration, tube formation, and angiogenic sprouting (Figure 4T through 4V). Taken together, these data suggest that the TP increases cell tension, disturbs focal adhesion dynamics, and inhibits the angiogenic capacity of human endothelial cells in vitro via activation of a ROCK1/2-, LIMK2-, and myosin II–dependent signal transduction pathway.
Figure 4.
The TP (thromboxane A2 receptor) induces morphological changes, cellular tension, and inhibition of angiogenesis-associated endothelial cell functions via a ROCK (Rho-associated coiled-coil containing protein kinase)-, LIMK2 (LIM domain kinase 2)-, and myosin II–dependent signal transduction pathway. A, Representative microscopic pictures of living control-transduced or TP-overexpressing human umbilical vein endothelial cells (HUVECs) in 2-dimensional culture additionally expressing the fluorescent vinculin-based tension biosensor (VinTS) for visualization of endothelial focal adhesions. B–E, TP overexpression reduces vascular endothelial cell size, induces an elongated cell shape (F), and favors the formation of large, irregularly shaped focal adhesions in particular at the cell margin of HUVECs (C–E). Statistical analysis of endothelial cell morphology parameters (n=43–45; *P<0.05/**P<0.01/***P<0.001/****P<0.0001 vs control-transduced HUVECs). G and H, TP overexpression increases vascular endothelial cell tension as indicated by a reduction of VinTS-associated Förster resonance energy transfer (FRET) in TP-overexpressing as compared with control-transduced HUVECs. In contrast, control HUVECs that express VinTL—a tailless tension-insensitive variant of the sensor—show a significantly higher FRET at focal adhesions as compared with control HUVECs expressing VinTS (n=9–45; ****P<0.0001 vs control-transduced HUVECs). I, Dynamic range of RhoA (Ras homolog gene family, member A) activity related FRET index of constitutive active (Pos.), wild-type, and dominant negative (Neg.) variants of the RhoA FRET biosensor. FRET Neg. represents separated donor/acceptor pair (mCerulean3/mVenus). J, Stimulation of HUVECs with 1 U/mL thrombin induces a transient activation of RhoA measured by the FRET biosensor. K and L, HUVECs transduced with lentiviral particles (Ctr., TPα, TPβ) were transfected with RhoA FRET biosensor. FRET index of TP-overexpressing cells was significantly higher than in Ctr. indicating a higher basal RhoA activity in these cells (Ctr., n=43; TPα, n=37; TPβ, n=42; ****P<0.0001). M, Representative microscopic pictures of a TPβ-overexpressing endothelial cell transiently expressing the VinTS biosensor treated with TP antagonist SQ-29548. Top shows an increasing FRET index within minutes after addition of SQ-29548, whereas bottom visualizes the SQ-29548–related regression of focal adhesions. Scale bar, 3 µm. N, Pharmacological inhibition of the TP using SQ-29548 (3×10−5 mol/L) reduces vascular endothelial cell tension in TP-overexpressing but not in control-transduced HUVECs. Q, SQ-29548 also induces a rapid regression of focal adhesion size in TP-overexpressing and control HUVECs. Similar effects on cell tension and regression of focal adhesions could be observed by inhibition of actomyosin regulators ROCK (O and R; 10 µM Y-27632) and LIMK2 (P and S; 3 µM LX-7101). Data are shown as mean±SEM (n=6–10). O–Q, Pharmacological inhibition of ROCK (Y-27632; 10 µmol/L), LIMK2 (LX-7101; 3 µmol/L), and myosin II (blebbistatin; 30 µmol/L) but not LIMK1 (BMS4; 0.5 µmol/L) significantly attenuates the inhibitory effect of TPα or TPβ overexpression on HUVEC migration (T; n=8–12), spheroid sprouting (U; n=17–20), and tube formation (V; n=4–10). Control-transduced HUVECs (Ctr.) served as appropriate comparative group. *P<0.05/**P<0.01/***P<0.001/****P<0.0001 vs vehicle-treated Ctr. All statistical analyses were performed using 1-way ANOVA followed by the Sidak multiple comparisons test.
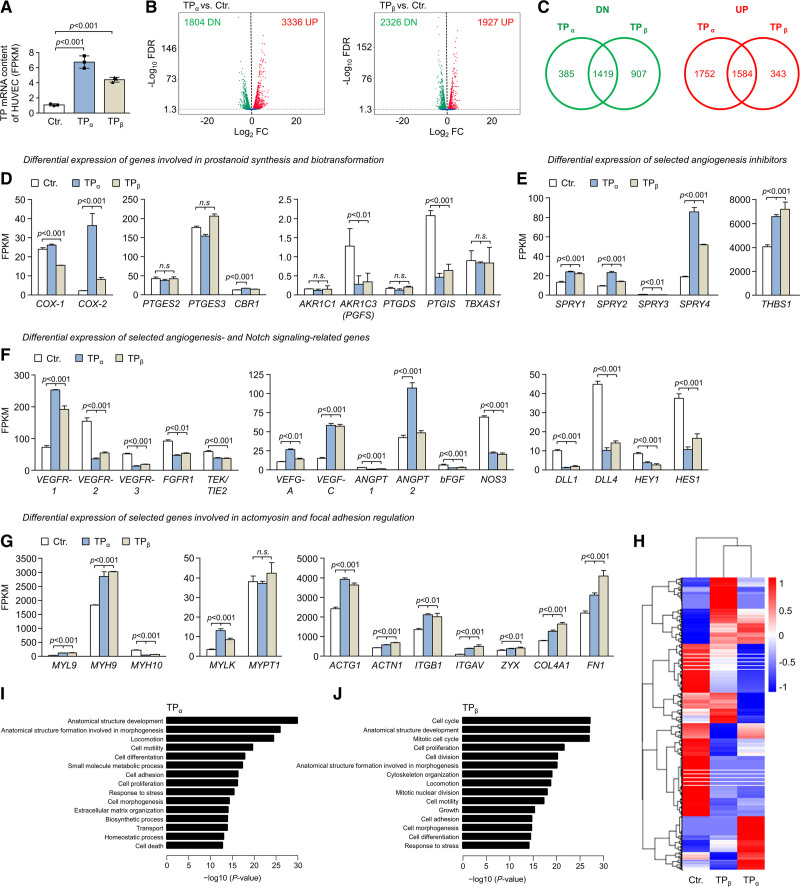
Increased TP Expression Induces Profound Changes in the Transcriptome of HUVECs
To further explore the angiostatic effects induced by TP overexpression, we performed global transcriptome profiling of TP-overexpressing and control HUVECs via RNA sequencing (Figure 5; Data Sets 1 and 2). TPα or TPβ overexpression, respectively, using lentiviral gene transfer significantly increased HUVEC TP mRNA levels as compared with control cells (Figure 5A). RNA sequencing analysis of 3 biological replicates showed that TP overexpression led to a profound change in HUVEC gene expression with a total of 5140 (TPα) or 4253 (TPβ) differentially expressed genes (DEGs; Figure 5B). A considerable overlap of TPα- and TPβ-regulated DEGs was observed with 1419 jointly downregulated and 1584 jointly upregulated genes, respectively (Figure 5C). Nevertheless, as illustrated by hierarchical clustering of DEGs, also gene clusters with opposing regulation by the TP isoforms were observed (Figure 5H; Data Set 3). Regarding prostanoid synthesis and biotransformation, TP overexpression strongly upregulated COX-2 (PTGS2), whereas it reduced the expression of PTGIS (prostacyclin synthase) and prostaglandin F synthase (PGFS [prostaglandin F synthase]/AKR1C3 [Aldo-keto reductase family 1 member C3]) and did not affect or only moderately affected the expression of prostaglandin E synthases, AKR1C1 (Aldo-keto reductase family 1 member C1), and CBR1 (carbonyl reductase 1; both involved in PGF2α formation) or the TBXAS1 (TxA2 synthase; Figure 5D). These data suggested that TP overexpression could trigger an enhanced biosynthesis of TP agonists (PGH2 and TxA2) due to increased COX-2 and reduced PTGIS and PGFS expression. In addition, TP overexpression significantly downregulated angiogenic mediators VEGFR-2, VEGFR-3 (vascular endothelial growth factor receptor 3), FGFR1 (fibroblast growth factor receptor 1), and TEK (TEK tyrosine kinase)/TIE2 (tyrosine kinase with Ig and EGF homology domains 2), while VEGFR-1, an endothelial decoy receptor for VEGF-A (vascular endothelial growth factor A),37 was upregulated (Figure 5F). Moreover, TP overexpression upregulated several antiangiogenic mediators, that is, THBS1 (thrombospondin 1), and induced elements or regulators of the endothelial actomyosin apparatus (Figure 5E and 5G). Gene ontology pathway enrichment analyses of TP-induced DEGs revealed that TP overexpression–related DEGs were enriched for gene ontology biological process terms associated with, for example, locomotion, cell motility, cell adhesion, cell proliferation, cell division, mitotic cell cycle, cell morphogenesis, cytoskeleton organization, and extracellular matrix organization (Figure 5I and 5J).
Figure 5.
TP (thromboxane A2 receptor) overexpression induces profound changes in the human umbilical vein endothelial cell (HUVEC) transcriptome. A, Expression levels of TPα or TPβ mRNA in lentiviral transduced HUVECs was analyzed by RNA-seq (n=3). B, Volcano plots showing differential gene expression in TPα- and TPβ-overexpressing HUVECs, respectively, as determined by RNA-seq. C, The overlap of mRNAs significantly upregulated (UP; red) or downregulated (DN; green) in TPα- and TPβ-overexpressing HUVECs is depicted by Venn diagrams. The numbers shown in the diagrams indicate the number of transcripts with significantly deregulated expression upon overexpression of TPα or TPβ in HUVECs. Fragments per kilobase of transcript per million mapped reads (FPKM) of each gene was calculated based on gene length and read counts mapped to the respective gene. Differential gene expression analysis was performed using the DESeq2 software package (v1.20.0). Resulting P values were adjusted using the Benjamini and Hochberg approach to control the false discovery rate (FDR). The expression level of selected mRNAs involved in cellular prostanoid formation and biotransformation (D), inhibition of angiogenesis (E), regulation of angiogenesis and Notch signaling (F), or actomyosin and focal adhesion regulation (G) were determined by RNA-seq in control-transduced HUVECs (Ctr.), as well as in HUVECs transduced to overexpress the TPα or TPβ isoform and are shown as FPKM. Data are displayed as mean+SD (n=3). Statistical analyses in D–G were performed using 1-way ANOVA followed by the Sidak multiple comparisons test. H, Hierarchical clustering of differentially expressed genes using FPKM as the input. Transcript expression profiles derived from HUVECs transduced to overexpress either TPα or TPβ, respectively and from control-transduced HUVECs (Ctr.) are shown. The heat map color range from red to blue represents the log10(FPKM+1) value in which red denotes genes with high expression levels and blue denotes genes with low expression levels. I and J, Top gene ontology (GO) biological process terms significantly enriched in differentially expressed genes of TPα-overexpressing or TPβ-overexpressing HUVECs. GO pathway enrichment analyzes were performed using the GOseq R software package (v1.34.1). GO terms with an adjusted P<0.05 were considered significantly enriched.
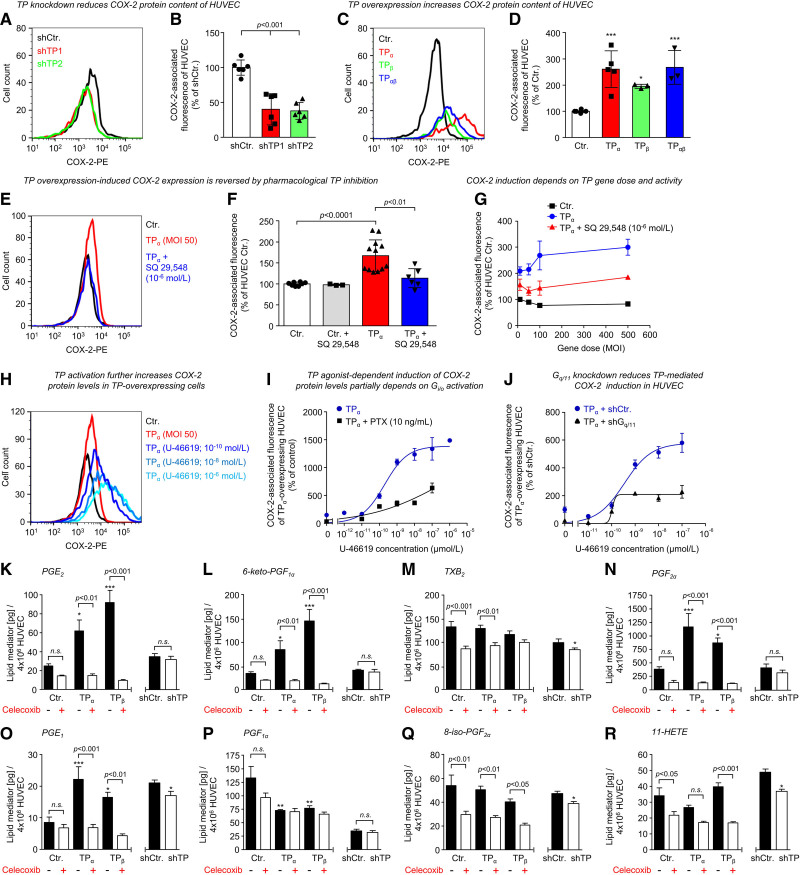
In further experiments, we validated the impact of the TP on COX-2 expression in human endothelial cells. These analyses demonstrated that knockdown of the TP significantly reduced, while overexpression of the TP elevated, COX-2 protein levels of HUVECs (Figure 6A through 6D; Figure S7). As depicted for TPα, a trend toward gene dose dependency was observed for TP overexpression–induced COX-2 levels in HUVECs (Figure 6G). Moreover, COX-2 levels were decreased by concomitant pharmacological TP inhibition, suggesting a TP-driven positive feedback loop (Figure 6E through 6G), whereas the TP agonist U-46619 further increased COX-2 levels depending on concentration (Figure 6H through 6J). Pharmacological inhibition of Gi/o proteins using pertussis toxin or knockdown of Gq/11 significantly attenuated TP overexpression–driven and U-46619–induced COX-2 expression in HUVECs, suggesting that both G proteins play a role in TP-related COX-2 induction (Figure 6I and 6J). Moreover, we validated further TP overexpression–induced DEGs of interest, for example, VEGFR-2, using qRT-PCR (quantitative real time PCR) and obtained similar results (Figure S8A through S8H). We then further explored the TP-downregulated genes VEGFR-2 and DLL4, which are known as important players in angiogenesis and affect key features of angiogenic sprouting, such as tip-cell-stalk-cell competition.37 In this context, DLL4 is a target gene of VEGFR-2, known to form a positive feedback loop with the VEGFR-2 that is essential for selection of tip cells during angiogenic sprouting of endothelial cells.38 The expression of DLL4 (delta-like 4) thus reflects VEGFR-2 activity in human endothelial cells.
Figure 6.
TP (thromboxane A2 receptor) levels regulate COX-2 (cyclooxygenase-2) expression and prostanoid synthesis of human endothelial cells. Flow cytometric analyses of TP-related regulation of COX-2 protein levels in human umbilical vein endothelial cells (HUVECs). shRNA-mediated knockdown of the TP using 2 different shRNAs (shTP1 and shTP2; A and B) significantly reduces, while overexpression (MOI 500) of the TP (either the TPα isoform, the TPβ isoform, or both TP isoforms [TPαβ]; C and D) induces COX-2 protein content of HUVECs as compared with appropriate control (shCtr.; Ctr.). A and C, Representative flow cytometric plots depicting TP-induced changes in COX-2–associated fluorescence. Data are shown as mean±SD (n=3–6). *P<0.05/***P<0.001 vs Ctr. All statistical analyses were performed using 1-way ANOVA followed by the Sidak multiple comparisons test. E and F, TP overexpression-induced COX-2 expression is reduced by pharmacological TP inhibition with SQ-29548 (10−6 mol/L) as shown for HUVECs overexpressing the TPα isoform (MOI 50). In contrast, SQ-29548 does not reduce COX-2 expression in control-transduced HUVECs (Ctr.). Data are shown as mean±SD (n=3–12). G, TPα overexpression MOI-dependently induces COX-2 protein levels in HUVECs. Pharmacological TP inhibition with SQ-29548 (10−6 mol/L) reduces TPα overexpression–induced COX-2 protein levels in HUVECs. Data are shown as mean±SD (n=3). H–J, Increasing concentrations of the TP agonist U-46619 further induce COX-2 protein levels with a half maximal effective concentration of 1.94×10−10 mol/L in TPα-overexpressing HUVECs. Data are shown as mean±SEM (n=3–9). E and I, Representative flow cytometric plots depicting SQ-29548–induced or U-46619–induced changes in COX-2–associated fluorescence. Pharmacological inhibition of Gi/o proteins using pertussis toxin (PTX; 10 ng/mL; I) and shRNA-mediated knockdown of Gq/11 (J) reduce U-46619–induced COX-2 protein levels in TPα-overexpressing HUVECs. Data are shown as mean±SD (n=3). K–R, Targeted liquid chromatography-tandem mass spectrometry–based lipid mediator profiling was used to analyze and quantify the impact of the TP on the formation of prostanoids and related lipid mediators in HUVECs. Overexpression of TPα or TPβ, respectively, increases the formation of PGE2 (prostaglandin E2; K), 6-keto-PGF1α (main metabolite of prostacyclin; L), PGF2α (prostaglandin F2α; N), and PGE1 (O), whereas it does not affect or reduce the formation of TxB2 (stable metabolite of TxA2 [thromboxane A2]; M), PGF1α (prostaglandin F1α; P), 8-iso-prostaglandin F2α (8-iso-PGF2α; Q), or 11-HETE (R). Moreover, celecoxib (100 nmol/L) abolishes the TP-induced formation of various prostanoids, indicating that an increase in COX-2 activity is responsible for TP-related shifts in the prostanoid profile of HUVECs. In contrast, shRNA-mediated TP knockdown significantly reduces the formation of TxB2, PGE1, 8-iso-PGF2α, and 11-HETE in HUVECs. Data are shown as mean+SEM (n=5). *P<0.05/**P<0.01/***P<0.001 vs Ctr. HUVECs.
Again, both TPα and TPβ overexpression reduced VEGFR-2 and DLL4 protein levels as detected by flow cytometry in HUVECs (Figure S9A and S9B). Furthermore, we observed a strong positive correlation between VEGFR-2 and DLL4 expression in HUVECs grown in VEGF-containing medium, supporting the mechanistic concept that DLL4 expression is strongly dependent on VEGFR-2 activity in human endothelial cells (Figure S9C). Interestingly, the COX-2 inhibitor celecoxib (100 nmol/L) significantly attenuated TP overexpression–induced downregulation of both VEGFR-2 and DLL4, whereas in control-transduced cells with physiological TP expression levels, it reduced VEGFR-2 and DLL4 expression (Figure S9A and S9B). In contrast to TP overexpression, TP knockdown increased both VEGFR-2 and DLL4 expression in HUVECs (Figure S9D). In this context, VEGF withdrawal from the medium had no impact on TP knockdown–induced VEGFR-2 upregulation but completely abolished DLL4 upregulation in TP-depleted cells (Figure S9E). Thus, these data substantiate the TP as a negative regulator of VEGFR-2 expression in human endothelial cells and the mechanistic interrelation between VEGFR-2 activity and DLL4 expression.
Prostanoid and Related Lipid Mediator Profiling in TP-Regulated HUVEC
To quantify the impact of the TP on the generation of prostanoids and related lipid mediators in HUVECs, we performed targeted liquid chromatography-tandem mass spectrometry–based lipid mediator profiling in TP knockdown, as well as TP-overexpressing HUVECs kept in the absence or presence of the selective COX-2 inhibitor celecoxib (Figure 6K through 6R). Interestingly, TP overexpression increased the formation of PGE2 (prostaglandin E2) and PGE1 (prostaglandin E1), while it did not significantly affect the formation of TxB2 (stable metabolite of the short-lived TxA2), 8-iso-PGF2α, 11-HETE, and PGF1α (prostaglandin F1α; Figure 6K, 6M, 6O, 6P through 6R). Unexpectedly, TP overexpression also induced the formation of 6-keto-PGF1α (main metabolite of unstable prostacyclin) and PGF2α (Figure 6L and 6N), despite the TP-mediated reduction in PTGIS and PGFS expression (Figure 5D). However, it has also been reported that PGF2α is synthesized from PGE2 by CBR1, AKR1C1, and AKR1C2 (Aldo-keto reductase family 1 member C2; the latter two enzymes being expressed in HUVEC at considerably lower levels than CBR1).39,40 Moreover, celecoxib prevented TP-induced formation of various prostanoids but did not, or rather moderately, reduce TxB2 formation in HUVECs, indicating that (1) in endothelial cells primarily COX-1 is functionally coupled to TBXAS1 and that (2) an increase in COX-2 activity is responsible for TP-related shifts in the prostanoid profile of HUVECs. In contrast, TP knockdown moderately reduced the formation of TxB2, PGE1, 8-iso-PGF2α, and 11-HETE in HUVECs (Figure 6M, 6O, 6Q, and 6R).
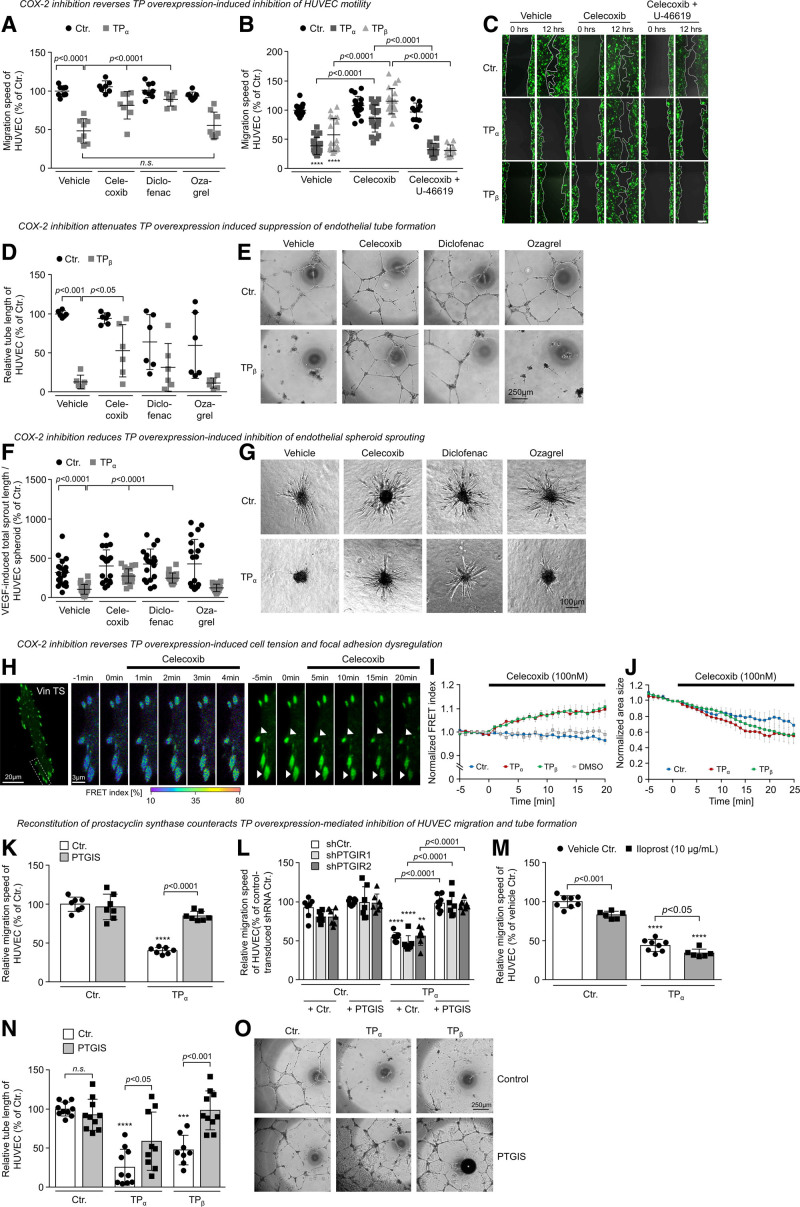
Role of COX-2 and PTGIS in TP Overexpression-Induced Inhibition of Angiogenic Endothelial Cell Functions
We next examined the role of COX-2 in TP overexpression–mediated inhibition of angiogenic endothelial cell functions. We observed that both the nonspecific COX-1 and COX-2 inhibitor diclofenac and the COX-2–specific inhibitor celecoxib but not the TBXAS1 inhibitor ozagrel reversed the inhibitory effect of TP overexpression on HUVEC migration, tube formation, and spheroid sprouting (Figure 7A, 7D, and 7F). Interestingly, U-46619 reestablished the antimigratory TP effect in celecoxib-treated TP-overexpressing HUVECs, indicating that celecoxib blocked the TP-driven endogenous formation of inhibitory TP agonists in HUVECs (Figure 7B and 7C). Celecoxib also reduced endothelial cell tension and induced the regression of focal adhesions in TP-overexpressing and control HUVECs (Figure 7H through 7J; Videos S4 and S5). In addition, celecoxib induced cell spreading of TP-overexpressing HUVECs after an approximate lag time of 5 minutes (Figure S6C), suggesting that celecoxib disrupts persistent TP activation via inhibition of the formation of short-lived alternative TP agonists, that is, PGH2 or PGF2α.41,42 As TP-induced downregulation of PGH2-metabolizing PTGIS and PGFS may contribute to an accumulation of COX-derived TP-agonistic PGH2 in endothelial cells, we chose to reconstitute PTGIS in TP-overexpressing HUVECs to explore the functional consequences of this intervention (Figure 7K through 7O). Interestingly, PTGIS reconstitution significantly reduced the inhibitory effect of TP overexpression on HUVEC migration and tube formation, whereas it had no significant effect on control-transduced HUVECs. The effect of PTGIS reconstitution was independent of PTGIR (prostacyclin receptor) activation, as TP-induced effects were also reversed in TP-overexpressing HUVECs engineered to overexpress PTGIS in the absence of PTGIR expression (Figure 7L). In addition, the stable prostacyclin analog iloprost did not antagonize antimigratory effects of the TP in TP-overexpressing HUVECs (Figure 7M).
Figure 7.
Pharmacological inhibition of COX-2 (cyclooxygenase-2) and reconstitution of PTGIS (prostacyclin synthase) disrupt the TP (thromboxane A2 receptor)-driven feedback loop in human umbilical vein endothelial cells (HUVECs). A, Pharmacological inhibition of COX-2 using the specific COX-2 inhibitor celecoxib (100 nmol/L) or the nonspecific COX-1 and COX-2 inhibitor diclofenac (10 µmol/L) but not inhibition of the thromboxane A2 synthase (ozagrel; 100 nmol/L) reduces the inhibitory effect of TP overexpression on HUVEC migration (n=7–8). B, Inhibitory effect of TPα and TPβ overexpression on HUVEC migration is reversed by celecoxib-mediated inhibition of COX-2. Additional application of U-46619 (3×10−5 mol/L) reinforces TP-mediated inhibition of HUVEC migration. ****P<0.0001 vs Ctr. (n=10–19). All statistical analyses were performed using 1-way ANOVA followed by the Sidak multiple comparisons test. C, Representative microscopic pictures of HUVEC scratches directly after and 12 h after wounding of the HUVEC monolayer. Celecoxib and diclofenac but not ozagrel reduce TPα- or TPβ-induced inhibition of HUVEC tube formation (D and E; n=6) or sprouting (F and G; n=19–30). Scale bar, 250 µm. H, Representative microscopic pictures of a TPβ-overexpressing endothelial cell transiently expressing the VinTS biosensor kept in the presence or absence of celecoxib (100 nmol/L). Left shows an increasing FRET index within minutes after addition of celecoxib, whereas right visualizes the celecoxib-related regression of focal adhesions. Data are shown as mean±SEM (n=7–10). I, Pharmacological inhibition of COX-2 using celecoxib reduces endothelial cell tension in TP-overexpressing but not in control-transduced HUVECs. Celecoxib also induces regression of focal adhesions in TP-overexpressing and control HUVECs (J). K, Reconstitution of prostacyclin synthase (PTGIS) reverses TPα overexpression–induced inhibition of HUVEC migration (n=7). L, This effect is independent of the PTGIR (prostacyclin receptor) as indicated by similar effects of PTGIS reconstitution in TPα-overexpressing control (shCtr.) and PTGIR knockdown (shPTGIR1 and shPTGIR2) cells (n=6–8). M, In contrast, the stable PTGIR agonist iloprost (10 µmol/L) reduces migration of control and TPα-overexpressing HUVECs, respectively (n=6–8). N and O, Moreover, PTGIS reconstitution significantly reduces the inhibitory effect of TP overexpression on tube formation of HUVECs (n=8–10). **P<0.01/***P<0.001/****P<0.0001 vs appropriate Ctr.
Taken together, our data suggest that increasing TP expression induces a primarily COX-2–dependent overproduction of TP-agonistic prostanoids, that is, PGH2 or PGF2α, in endothelial cells that serve as autocrine or paracrine activators of the TP. The resulting persistent TP activation drives changes in the endothelial transcriptome and triggers a ROCK1/2-, LIMK2-, and myosin II–dependent effector pathway that increases endothelial cell tension, alters cell morphology, impairs focal adhesion dynamics, and reduces the angiogenic capacity of endothelial cells (Figure S10).
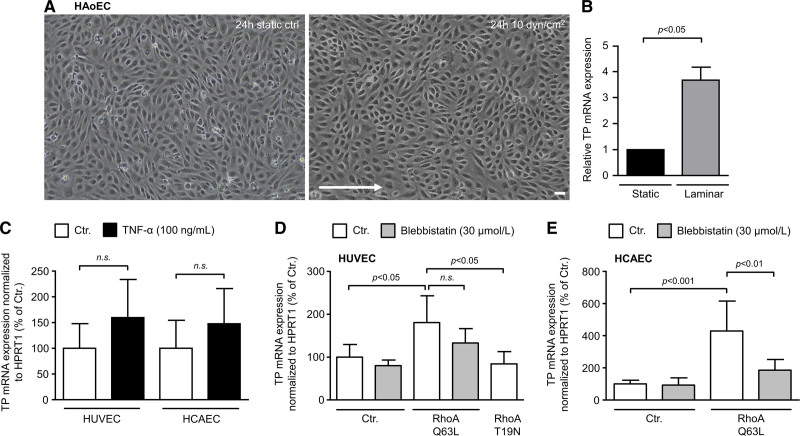
Impact of Shear Stress, Inflammatory Stimuli, and RhoA Activity on TP Expression Levels in Human Endothelial Cells
To date, it is largely unknown by which cellular signals TP expression is regulated in human endothelial cells. We, therefore, investigated potential mechanisms that might upregulate TP expression in human endothelial cells of arterial and venous origin. Interestingly, we found that TP mRNA expression increased in native human aortic endothelial cells under laminar flow conditions (Figure 8A and 8B), indicating physiological induction of TP expression via shear stress and mechanical cues in human aortic endothelial cell. In line with these results, constitutive activation of RhoA signaling (transduction with constitutively active RhoA Q63L mutant) significantly increased TP mRNA expression in HUVECs and human coronary artery endothelial cells (Figure 8D and 8E). Interestingly, RhoA-induced TP upregulation could be partly reversed by blebbistatin, a pharmacological inhibitor of nonmuscle myosin II ATPase activity, thus indicating that cell tension may be an important signal for TP upregulation. In contrast, the proinflammatory cytokine TNF-α (tumor necrosis factor alpha) did not significantly affect TP mRNA levels in HUVECs or human coronary artery endothelial cell (Figure 8C).
Figure 8.
Impact of shear stress, inflammatory signals, and RhoA (Ras homolog gene family, member A) activation on TP (thromboxane A2 receptor) expression in human aortic endothelial cells (HAoECs), human umbilical vein endothelial cells (HUVECs), and human coronary artery endothelial cells (HCAECs). A, Native HAoECs were exposed to shear stress (24-h laminar flow, 10 dyn/cm2 equals 0.1 mN/cm2) and compared with static control cells. Scale bar, 50 µm. B, Laminar flow increases TP mRNA expression indicating TP expression is regulated via mechanical cues. Statistical analyses in B were performed using the Wilcoxon test. C, HUVECs (n=3) and HCAECs (n=3) were treated with proinflammatory TNF-α (tumor necrosis factor alpha) for 24 h. A nonsignificant trend toward increased TP expression was observed. D and E, HUVECs (n=3–6) and HCAECs (n=6) were transduced with lentiviral vectors for the expression of a constitutively active RhoA (Q63L) mutant. In both cell types, constitutive activation of RhoA increased TP mRNA expression, whereas the dominant negative RhoA variant T19N had no significant effect in this context. Increased TP expression was partly reduced by blebbistatin—a pharmacological inhibitor of nonmuscle myosin II ATPase activity. The statistical analyses in C–E were performed using 1-way ANOVA followed by the Sidak multiple comparisons test.
Discussion
Besides its well-defined role in platelet aggregation and primary hemostasis, the TP fosters the pathogenesis of endothelial dysfunction21,22 and atherosclerotic vascular disease21,23,24 and affects neovascularization processes.7–20 In this context, previous expression analyses suggested elevated TP levels in the intimal layer of blood vessels of cardiovascular high-risk patients and murine atherosclerotic lesions,25,26 raising the possibility that an excess of deleterious endothelial TP signal transmission occurs under these circumstances. An important goal of this work was, therefore, to clarify in which way upregulation of the TP affects the functionality of vascular endothelial cells. In a comprehensive series of experiments, we demonstrated that an increase in endothelial TP expression (1) elevates endothelial cell tension, (2) disrupts focal adhesion dynamics, and (3) decreases the angiogenic capacity of vascular endothelial cells in vitro and in vivo, whereas TP knockdown exerts opposing effects. The discovery of an inverse relationship between endothelial TP levels and the angiogenic capacity of endothelial cells is of high scientific interest and potential clinical relevance because this finding reveals a novel mechanism by which pathological upregulation of both human TP isoforms could promote endothelial dysfunction, microvascular rarefaction, and systemic hypertension in the absence of exogenous sources of TP agonists. These findings are supported by global transcriptome analyses demonstrating that upregulation of both TP isoforms similarly affects the expression profile of important mediators of endothelial cell homeostasis, for example, VEGFR-1, VEGFR-2, or eNOS/NOS3 (NO synthase 3).37 Moreover, in global transcriptome analyses, TP overexpression–related DEGs were enriched for gene ontology biological process terms associated with, for example, cell motility, cell adhesion, cell proliferation, cell division, cell morphogenesis, cytoskeleton organization, and extracellular matrix organization, findings that support several functional observations made in our experimental approach. Worth noting is that the discovery of an antiangiogenic TP-dependent feedback loop, while novel, is consistent with previously published data that showed an inhibitory effect of the TP on angiogenesis.7–13,15,16 Nonetheless, other groups have suggested a supportive role of the TP in this process.17–20 For instance, global TP knockout was associated with a reduction in neovessel formation in the murine hindlimb ischemia model—a phenotype that was rescued by transplantation of TP-expressing (wild type) bone marrow and depended on a TP-related P-selectin expression on platelets.17 In contrast to the findings of Amano et al, Michel et al20 using the same model neither found an effect of pharmacological TP inhibition (S18886, terutroban) nor of aspirin on postischemic neovascularization, whereas both interventions reduced angiotensin II–related neovascularization in this context. The reasons for these discrepancies are unclear. However, there may exist fundamental differences between the effects of the TP on neovascularization processes in humans compared with mice. One explanation could be that the human TPβ isoform or an interplay of TPα and TPβ isoforms elicits different signal transduction in endothelial cells than the murine TP, which shares structural similarity with the human TPα isoform. Nonetheless, we show that endothelial-specific deletion of the TP in mice promotes VEGF- and bFGF-induced angiogenesis in the matrigel plug assay in vivo, thereby supporting our findings in human endothelial cells that the TP is a negative regulator of endothelial cell–related blood vessel formation. Also, pharmacological TP inhibition has been already shown to ameliorate endothelial dysfunction in cardiovascular high-risk individuals, supporting the notion that pharmacological disruption of the TxA2-TP axis comes with a strong potential to improve endothelial homeostasis in humans.22 On top of that, our data suggest that pharmacological TP inhibition could represent a novel therapeutic strategy to increase the angiogenic potential and reduce stiffness of vascular endothelial cells in disease states associated with increased endothelial TP expression.
An important novel finding of this work is that the TP drives an auto/paracrine positive feedback loop by which the receptor triggers persistent self-activation independent of exogenous sources of TP ligands. In this positive feedback loop, increasing endothelial TP expression induces COX-2 and downregulates PTGIS and PGFS—effects that most likely promote endothelial biosynthesis and accumulation of TP-agonistic PGH2. By this means, TP overexpression is directly linked to persistent TP activation in endothelial cells, although we cannot entirely exclude the possibility that ligand-independent, constitutive activity of the receptor also contributes to these effects. It has to be noted in this context that endothelial COX-2 and its primary biosynthetic downstream products prostacyclin (PGI2) and PGE2 are generally considered to be important mediators of endothelial integrity and neovascularization processes that additionally play a key role in inflammation and cancer progression.43,44 Despite these well-established COX-2 functions, our data uncovers a previously unrecognized feedback in which COX-2 could act as driver of aberrant TP activity in endothelial cells and thus may negatively affect endothelial function via this route when the endothelial TP is upregulated.
Interestingly, paradoxical PGH2-TP–related effects of COX-2 overexpression have previously been observed in preclinical analyses in diabetic rats, in which the expression of COX-2 is markedly increased in the renal cortex. In these animals, but not in healthy control animals, selective inhibition of COX-2 abolished arachidonic acid–mediated constriction of renal blood vessels.45 The authors also showed in prior studies that the renal vasoconstrictor response to arachidonic acid was inhibited by nonselective COX-1 and COX-2 inhibitor indomethacin and by a TP antagonist but not by a TxA2 synthase inhibitor, indicating that the response was mediated by an endoperoxide, such as PGH2.46 These data, therefore, suggest that aberrant COX-2/PGH2/TP–dependent signaling occurs in the pathophysiological context of vascular dysfunction in vivo. In this regard, our work also uncovers stimuli, such as laminar flow or constitutive RhoA activity, that upregulate the TP in human endothelial cells of arterial and venous origin and may thus be relevant inducers of endothelial TP expression and activity in vivo. Nevertheless, further studies in human individuals are required to identify pathophysiological settings associated with enhanced vascular TP expression.
Focusing on the impact of the TP on prostanoid biosynthesis in human endothelial cells, we performed lipid mediator profiling in TP-regulated HUVECs grown in the presence or absence of the COX-2 inhibitor celecoxib. These analyses revealed that TP overexpression induced the biosynthesis of PGE2, PGE1, PGF2α, and PGI2, while the amounts of TxA2 remained unaffected. Thus, TxA2 synthase appears to be functionally coupled to COX-1 rather than to COX-2, whereas formation of prostaglandins of the E2, F2, and I2 type depends more on COX-2 activity in endothelial cells. Indeed, pharmacological inhibition of COX-2 profoundly reduced endothelial PGE2, PGF2α, and PGI2 biosynthesis, whereas endothelial TxA2 synthesis was not or only moderately decreased, which is consistent with findings from other47,48 but not all groups.18 Therefore, we believe that the TP-driven COX-2–dependent positive feedback loop increases the endothelial bioavailability of PGH2—a potent TP agonist and substrate of various prostaglandin synthases—to induce formation of multiple prostaglandins despite downregulation of PTGIS and PGFS on the one hand and to trigger persistent TP activation on the other. In line with this hypothesis, reconstitution of downregulated PTGIS in TP-overexpressing endothelial cells—an enzyme that converts PGH2 to PGI2—attenuated the TP-related inhibition of proangiogenic endothelial cell functions, most likely because it enzymatically reduced the endothelial bioavailability of PGH2 and PGH2-induced TP activation. This conclusion is supported by the fact that the effect of PTGIS reconstitution did not depend on amplified prostacyclin synthesis as (1) PTGIS reconstitution also reversed TP-related effects in the absence of functional prostacyclin receptor expression and (2) the stable prostacyclin mimetic iloprost did not antagonize antimigratory TP effects. Thus, our data point to a TP-controlled positive feedback loop in human endothelial cells in which COX-2–derived TP-agonistic PGH2 and possibly PGF2α could serve as endogenous TP agonists.
In additional mechanistic analyses, we were able to reveal that the TP activates a ROCK-, LIMK2-, and myosin II–dependent signal transduction pathway that inhibits proangiogenic functions, increases the tension, and induces morphological changes of endothelial cells. Indeed, TP overexpression increased endothelial cell tension, cell contractions, and the formation of large, irregular focal adhesions—effects that have been observed together with functional defects as a consequence of constitutive Rho-ROCK activation in other cell types.5 Thus, our data suggest that TP upregulation persistently enhances actomyosin activity to increase cellular tension and affect the morphology and angiogenic capacity of vascular endothelial cells.
In conclusion, our work uncovers an auto/paracrine positive feedback loop and reveals important downstream effector mechanisms by which the endothelial TP triggers persistent self-activation independent of exogenous sources of TP ligands and affects endothelial cell homeostasis. Since pharmacological TP inhibition disrupts this feedback loop, our results suggest that pharmacological TP inhibition may represent a novel therapeutic strategy to improve vascular endothelial homeostasis in disease states associated with increased TP expression.
Data Availability
The data that support the findings of this study are available within the article and the Supplemental Material or from the corresponding author upon reasonable request. RNA sequencing data that support the findings of this study have been deposited in the US National Library of Medicine, National Center for Biotechnology Information Sequence Read Archive (Gene Expression Omnibus) under accession code GSE146888.
Article Information
Acknowledgments
We gratefully acknowledge Prof Hellmut Augustin for having contributed to technical expertise with 3-dimensional angiogenesis assays and Dorothea Frenzel for her expert technical assistance.
Sources of Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG INST 271/342-1, BE 3246/4-1, and BE 3246/6-1) and by the European Regional Development Fund of the European Commission (W21029490) to R.A. Benndorf, by DFG TR22-B04 SE/JG to S. Ergün, by DFG FR 4239/1-1 to M. Frye, and by DFG SFB1127 ChemBioSys and SFB1278 Polytarget project number 316213987 (projects A04 and C02) to O. Werz.
Disclosures
None.
Supplemental Material
Supplemental Materials and Methods
Figures S1–S10
Videos S1–S5
Data Set 1–3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AKR1C1/C2/C3
- aldo-keto reductase family 1 member C1/C2/C3
- bFGF
- basic fibroblast growth factor
- CBR1
- carbonyl reductase 1
- CD31/34
- cluster of differentiation 31/34
- COX-1/2
- cyclooxygenase-1/2
- DEG
- differentially expressed gene
- eNOS
- endothelial NO synthase
- FGFR1
- fibroblast growth factor receptor 1
- HUVEC
- human umbilical vein endothelial cell
- LIMK1/2
- LIM domain kinase 1/2
- NOS3
- NO synthase 3
- PGE1/2
- prostaglandin E1/2
- PGF1α
- prostaglandin F1α
- PGF2α
- prostaglandin F2α
- PGFS
- prostaglandin F synthase
- PGH2
- prostaglandin H2
- PTGIR
- prostacyclin receptor
- PTGIS
- prostacyclin synthase
- PTGS2
- prostaglandin-endoperoxide synthase 2
- RhoA
- Ras homolog gene family, member A
- ROCK
- Rho-associated coiled-coil containing protein kinase
- TBXAS1
- thromboxane A2 synthase
- TEK
- TEK tyrosine kinase
- THBS1
- thrombospondin 1
- TIE2
- tyrosine kinase with Ig and EGF homology domains 2
- TNF-α
- tumor necrosis factor alpha
- TP
- thromboxane A2 receptor
- TxA2
- thromboxane A2
- VEGF-A
- vascular endothelial growth factor A
- VEGFR-1/2/3
- vascular endothelial growth factor receptor 1/2/3
R. Eckenstaler and A. Ripperger contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.121.317380.
For Sources of Funding and Disclosures, see page 460.
Contributor Information
Robert Eckenstaler, Email: robert.eckenstaler@pharmazie.uni-halle.de.
Anne Ripperger, Email: anne.ripperger@pharmazie.uni-halle.de.
Michael Hauke, Email: michael.hauke@pharmazie.uni-halle.de.
Markus Petermann, Email: markus.petermann@pharmazie.uni-halle.de.
Sandra A. Hemkemeyer, Email: s.hemkemeyer@uke.de.
Edzard Schwedhelm, Email: schwedhelm@uke.de.
Maike Frye, Email: m.frye@uke.de.
Oliver Werz, Email: oliver.werz@uni-jena.de.
Andreas Koeberle, Email: Andreas.Koeberle@uibk.ac.at.
Heike Braun, Email: heike.braun@mail.de.
References
- 1.Bauer J, Ripperger A, Frantz S, Ergün S, Schwedhelm E, Benndorf RA. Pathophysiology of isoprostanes in the cardiovascular system: implications of isoprostane-mediated thromboxane A2 receptor activation. Br J Pharmacol. 2014; 171:3115–3131. doi: 10.1111/bph.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kent KC, Collins LJ, Schwerin FT, Raychowdhury MK, Ware JA. Identification of functional PGH2/TxA2 receptors on human endothelial cells. Circ Res. 1993; 72:958–965. doi: 10.1161/01.res.72.5.958 [DOI] [PubMed] [Google Scholar]
- 3.Audoly LP, Rocca B, Fabre JE, Koller BH, Thomas D, Loeb AL, Coffman TM, FitzGerald GA. Cardiovascular responses to the isoprostanes iPF(2alpha)-III and iPE(2)-III are mediated via the thromboxane A(2) receptor in vivo. Circulation. 2000; 101:2833–2840. doi: 10.1161/01.cir.101.24.2833 [DOI] [PubMed] [Google Scholar]
- 4.Kinsella BT. Thromboxane A2 signalling in humans: a ‘Tail’ of two receptors. Biochem Soc Trans. 2001; 29(pt 6):641–654. doi: 10.1042/0300-5127:0290641 [DOI] [PubMed] [Google Scholar]
- 5.Moers A, Nieswandt B, Massberg S, Wettschureck N, Grüner S, Konrad I, Schulte V, Aktas B, Gratacap MP, Simon MI, et al. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat Med. 2003; 9:1418–1422. doi: 10.1038/nm943 [DOI] [PubMed] [Google Scholar]
- 6.Hodge RG, Ridley AJ. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016; 17:496–510. doi: 10.1038/nrm.2016.67 [DOI] [PubMed] [Google Scholar]
- 7.Ashton AW, Cheng Y, Helisch A, Ware JA. Thromboxane A2 receptor agonists antagonize the proangiogenic effects of fibroblast growth factor-2: role of receptor internalization, thrombospondin-1, and alpha(v)beta3. Circ Res. 2004; 94:735–742. doi: 10.1161/01.RES.0000122043.11286.57 [DOI] [PubMed] [Google Scholar]
- 8.Ashton AW, Ware JA. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ Res. 2004; 95:372–379. doi: 10.1161/01.RES.0000138300.41642.15 [DOI] [PubMed] [Google Scholar]
- 9.Ashton AW, Yokota R, John G, Zhao S, Suadicani SO, Spray DC, Ware JA. Inhibition of endothelial cell migration, intercellular communication, and vascular tube formation by thromboxane A(2). J Biol Chem. 1999; 274:35562–35570. doi: 10.1074/jbc.274.50.35562 [DOI] [PubMed] [Google Scholar]
- 10.Battinelli EM, Markens BA, Italiano JE, Jr. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011; 118:1359–1369. doi: 10.1182/blood-2011-02-334524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benndorf RA, Schwedhelm E, Gnann A, Taheri R, Kom G, Didié M, Steenpass A, Ergün S, Böger RH. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A(2) receptor: a potential link between oxidative stress and impaired angiogenesis. Circ Res. 2008; 103:1037–1046. doi: 10.1161/CIRCRESAHA.108.184036 [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Yokota R, Tang S, Ashton AW, Ware JA. Reversal of angiogenesis in vitro, induction of apoptosis, and inhibition of AKT phosphorylation in endothelial cells by thromboxane A(2). Circ Res. 2000; 87:739–745. doi: 10.1161/01.res.87.9.739 [DOI] [PubMed] [Google Scholar]
- 13.Pal S, Wu J, Murray JK, Gellman SH, Wozniak MA, Keely PJ, Boyer ME, Gomez TM, Hasso SM, Fallon JF, et al. An antiangiogenic neurokinin-B/thromboxane A2 regulatory axis. J Cell Biol. 2006; 174:1047–1058. doi: 10.1083/jcb.200603152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocca B, Loeb AL, Strauss JF, 3rd, Vezza R, Habib A, Li H, FitzGerald GA. Directed vascular expression of the thromboxane A2 receptor results in intrauterine growth retardation. Nat Med. 2000; 6:219–221. doi: 10.1038/72334 [DOI] [PubMed] [Google Scholar]
- 15.Shen Y, Zuo S, Wang Y, Shi H, Yan S, Chen D, Xiao B, Zhang J, Gong Y, Shi M, et al. Thromboxane governs the differentiation of adipose-derived stromal cells toward endothelial cells in vitro and in vivo. Circ Res. 2016; 118:1194–1207. doi: 10.1161/CIRCRESAHA.115.307853 [DOI] [PubMed] [Google Scholar]
- 16.Tsou PS, Amin MA, Campbell PL, Zakhem G, Balogh B, Edhayan G, Ohara RA, Schiopu E, Khanna D, Koch AE, et al. Activation of the thromboxane A2 receptor by 8-isoprostane inhibits the pro-angiogenic effect of vascular endothelial growth factor in scleroderma. J Invest Dermatol. 2015; 135:3153–3162. doi: 10.1038/jid.2015.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amano H, Ito Y, Eshima K, Kato S, Ogawa F, Hosono K, Oba K, Tamaki H, Sakagami H, Shibuya M, et al. Thromboxane A2 induces blood flow recovery via platelet adhesion to ischaemic regions. Cardiovasc Res. 2015; 107:509–521. doi: 10.1093/cvr/cvv139 [DOI] [PubMed] [Google Scholar]
- 18.Daniel TO, Liu H, Morrow JD, Crews BC, Marnett LJ. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Res. 1999; 59:4574–4577 [PubMed] [Google Scholar]
- 19.Nie D, Lamberti M, Zacharek A, Li L, Szekeres K, Tang K, Chen Y, Honn KV. Thromboxane A(2) regulation of endothelial cell migration, angiogenesis, and tumor metastasis. Biochem Biophys Res Commun. 2000; 267:245–251. doi: 10.1006/bbrc.1999.1840 [DOI] [PubMed] [Google Scholar]
- 20.Michel F, Silvestre JS, Waeckel L, Corda S, Verbeuren T, Vilaine JP, Clergue M, Duriez M, Levy BI. Thromboxane A2/prostaglandin H2 receptor activation mediates angiotensin II-induced postischemic neovascularization. Arterioscler Thromb Vasc Biol. 2006; 26:488–493. doi: 10.1161/01.ATV.0000201969.93348.74 [DOI] [PubMed] [Google Scholar]
- 21.Félétou M, Cohen RA, Vanhoutte PM, Verbeuren TJ. TP receptors and oxidative stress hand in hand from endothelial dysfunction to atherosclerosis. Adv Pharmacol. 2010; 60:85–106. doi: 10.1016/B978-0-12-385061-4.00004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesault PF, Boyer L, Pelle G, Covali-Noroc A, Rideau D, Akakpo S, Teiger E, Dubois-Randé JL, Adnot S. Daily administration of the TP receptor antagonist terutroban improved endothelial function in high-cardiovascular-risk patients with atherosclerosis. Br J Clin Pharmacol. 2011; 71:844–851. doi: 10.1111/j.1365-2125.2010.03858.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, Arai H, Oida H, Yurugi-Kobayashi T, Yamashita JK, et al. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest. 2004; 114:784–794. doi: 10.1172/JCI21446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang M, Cyrus T, Yao Y, Vocun L, Praticò D. Involvement of thromboxane receptor in the proatherogenic effect of isoprostane F2alpha-III: evidence from apolipoprotein E- and LDL receptor-deficient mice. Circulation. 2005; 112:2867–2874. doi: 10.1161/CIRCULATIONAHA105.562223 [DOI] [PubMed] [Google Scholar]
- 25.Cyrus T, Ding T, Praticò D. Expression of thromboxane synthase, prostacyclin synthase and thromboxane receptor in atherosclerotic lesions: correlation with plaque composition. Atherosclerosis. 2010; 208:376–381. doi: 10.1016/j.atherosclerosis.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 26.Katugampola SD, Davenport AP. Thromboxane receptor density is increased in human cardiovascular disease with evidence for inhibition at therapeutic concentrations by the AT(1) receptor antagonist losartan. Br J Pharmacol. 2001; 134:1385–1392. doi: 10.1038/sj.bjp.0704416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011; 146:873–887. doi: 10.1016/j.cell.2011.08.039 [DOI] [PubMed] [Google Scholar]
- 28.Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev. 2013; 93:1317–1542. doi: 10.1152/physrev.00004.2012 [DOI] [PubMed] [Google Scholar]
- 29.Antonios TF, Kaski JC, Hasan KM, Brown SJ, Singer DR. Rarefaction of skin capillaries in patients with anginal chest pain and normal coronary arteriograms. Eur Heart J. 2001; 22:1144–1148. doi: 10.1053/euhj.2000.2442 [DOI] [PubMed] [Google Scholar]
- 30.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension. 1999; 34(4 pt 1):655–658. doi: 10.1161/01.hyp.34.4.655 [DOI] [PubMed] [Google Scholar]
- 31.Laib AM, Bartol A, Alajati A, Korff T, Weber H, Augustin HG. Spheroid-based human endothelial cell microvessel formation in vivo. Nat Protoc. 2009; 4:1202–1215. doi: 10.1038/nprot.2009.96 [DOI] [PubMed] [Google Scholar]
- 32.Braun H, Hauke M, Ripperger A, Ihling C, Fuszard M, Eckenstaler R, Benndorf RA. Impact of DICER1 and DROSHA on the angiogenic capacity of human endothelial cells. Int J Mol Sci. 2021; 22:9855. doi: 10.3390/ijms22189855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010; 466:263–266. doi: 10.1038/nature09198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin B, Yin T, Wu YI, Inoue T, Levchenko A. Interplay between chemotaxis and contact inhibition of locomotion determines exploratory cell migration. Nat Commun. 2015; 6:6619. doi: 10.1038/ncomms7619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Unen J, Reinhard NR, Yin T, Wu YI, Postma M, Gadella TW, Goedhart J. Plasma membrane restricted RhoGEF activity is sufficient for RhoA-mediated actin polymerization. Sci Rep. 2015; 5:14693. doi: 10.1038/srep14693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reinhard NR, van Helden SF, Anthony EC, Yin T, Wu YI, Goedhart J, Gadella TW, Hordijk PL. Spatiotemporal analysis of RhoA/B/C activation in primary human endothelial cells. Sci Rep. 2016; 6:25502. doi: 10.1038/srep25502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016; 17:611–625. doi: 10.1038/nrm.2016.87 [DOI] [PubMed] [Google Scholar]
- 38.Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013; 3:a006569. doi: 10.1101/cshperspect.a006569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dozier BL, Watanabe K, Duffy DM. Two pathways for prostaglandin F2 alpha synthesis by the primate periovulatory follicle. Reproduction. 2008; 136:53–63. doi: 10.1530/REP-07-0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo C, Wang W, Liu C, Myatt L, Sun K. Induction of PGF2α synthesis by cortisol through GR dependent induction of CBR1 in human amnion fibroblasts. Endocrinology. 2014; 155:3017–3024. doi: 10.1210/en.2013-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nugteren DH, Hazelhof E. Isolation and properties of intermediates in prostaglandin biosynthesis. Biochim Biophys Acta. 1973; 326:448–461. doi: 10.1016/0005-2760(73)90145-8 [DOI] [PubMed] [Google Scholar]
- 42.Félétou M, Huang Y, Vanhoutte PM. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol. 2011; 164:894–912. doi: 10.1111/j.1476-5381.2011.01276.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu L, Zhang Y, Guo Z, Wang M. Cardiovascular biology of prostanoids and drug discovery. Arterioscler Thromb Vasc Biol. 2020; 40:1454–1463. doi: 10.1161/ATVBAHA.119.313234 [DOI] [PubMed] [Google Scholar]
- 44.Mitchell JA, Kirkby NS. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br J Pharmacol. 2019; 176:1038–1050. doi: 10.1111/bph.14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quilley J, Chen YJ. Role of COX-2 in the enhanced vasoconstrictor effect of arachidonic acid in the diabetic rat kidney. Hypertension. 2003; 42:837–843. doi: 10.1161/01.HYP.0000085650.29823.F2 [DOI] [PubMed] [Google Scholar]
- 46.Quilley J, McGiff JC, Nasjletti A. Role of endoperoxides in arachidonic acid-induced vasoconstriction in the isolated perfused kidney of the rat. Br J Pharmacol. 1989; 96:111–116. doi: 10.1111/j.1476-5381.1989.tb11790.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, Lawson JA, FitzGerald GA. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002; 296:539–541. doi: 10.1126/science.1068711 [DOI] [PubMed] [Google Scholar]
- 48.Tang SY, Monslow J, Todd L, Lawson J, Puré E, FitzGerald GA. Cyclooxygenase-2 in endothelial and vascular smooth muscle cells restrains atherogenesis in hyperlipidemic mice. Circulation. 2014; 129:1761–1769. doi: 10.1161/CIRCULATIONAHA.113.007913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006; 1:241–245. doi: 10.1038/nprot.2006.37 [DOI] [PubMed] [Google Scholar]
- 50.Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc. 2009; 4:495–505. doi: 10.1038/nprot.2009.22 [DOI] [PubMed] [Google Scholar]
- 51.Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998; 143:1341–1352. doi: 10.1083/jcb.143.5.1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007; 2:329–333. doi: 10.1038/nprot.2007.30 [DOI] [PubMed] [Google Scholar]
- 53.Deppe S, Ripperger A, Weiss J, Ergün S, Benndorf RA. Impact of genetic variability in the ABCG2 gene on ABCG2 expression, function, and interaction with AT1 receptor antagonist telmisartan. Biochem Biophys Res Commun. 2014; 443:1211–1217. doi: 10.1016/j.bbrc.2013.12.119 [DOI] [PubMed] [Google Scholar]
- 54.Weil J, Benndorf R, Fredersdorf S, Griese DP, Eschenhagen T. Norepinephrine upregulates vascular endothelial growth factor in rat cardiac myocytes by a paracrine mechanism. Angiogenesis. 2003; 6:303–309. doi: 10.1023/B:AGEN.0000029411.76494.33 [DOI] [PubMed] [Google Scholar]
- 55.Weiss J, Sauer A, Herzog M, Böger RH, Haefeli WE, Benndorf RA. Interaction of thiazolidinediones (glitazones) with the ATP-binding cassette transporters P-glycoprotein and breast cancer resistance protein. Pharmacology. 2009; 84:264–270. doi: 10.1159/000241734 [DOI] [PubMed] [Google Scholar]
- 56.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001; 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 57.Ripperger A, Benndorf RA. The C421A (Q141K) polymorphism enhances the 3’-untranslated region (3’-UTR)-dependent regulation of ATP-binding cassette transporter ABCG2. Biochem Pharmacol. 2016; 104:139–147. doi: 10.1016/j.bcp.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 58.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015; 12:357–360. doi: 10.1038/nmeth.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yates A, Akanni W, Amode MR, Barrell D, Billis K, Carvalho-Silva D, Cummins C, Clapham P, Fitzgerald S, Gil L, et al. Ensembl 2016. Nucleic Acids Res. 2016; 44(D1):D710–D716. doi: 10.1093/nar/gkv1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010; 28:511–515. doi: 10.1038/nbt.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014; 15:550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010; 11:R14. doi: 10.1186/gb-2010-11-2-r14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Werner M, Jordan PM, Romp E, Czapka A, Rao Z, Kretzer C, Koeberle A, Garscha U, Pace S, Claesson HE, et al. Targeting biosynthetic networks of the proinflammatory and proresolving lipid metabolome. FASEB J. 2019; 33:6140–6153. doi: 10.1096/fj.201802509R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Werz O, Gerstmeier J, Libreros S, De la Rosa X, Werner M, Norris PC, Chiang N, Serhan CN. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat Commun. 2018; 9:59. doi: 10.1038/s41467-017-02538-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koeberle A, Muñoz E, Appendino GB, Minassi A, Pace S, Rossi A, Weinigel C, Barz D, Sautebin L, Caprioglio D, et al. SAR studies on curcumin’s pro-inflammatory targets: discovery of prenylated pyrazolocurcuminoids as potent and selective novel inhibitors of 5-lipoxygenase. J Med Chem. 2014; 57:5638–5648. doi: 10.1021/jm500308c [DOI] [PubMed] [Google Scholar]
- 66.Gehling UM, Willems M, Schlagner K, Benndorf RA, Dandri M, Petersen J, Sterneck M, Pollok JM, Hossfeld DK, Rogiers X. Mobilization of hematopoietic progenitor cells in patients with liver cirrhosis. World J Gastroenterol. 2010; 16:217–224. doi: 10.3748/wjg.v16.i2.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cyphert JM, Allen IC, Church RJ, Latour AM, Snouwaert JN, Coffman TM, Koller BH. Allergic inflammation induces a persistent mechanistic switch in thromboxane-mediated airway constriction in the mouse. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L140–L151. doi: 10.1152/ajplung.00152.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001; 193:741–754. doi: 10.1084/jem.193.6.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.King MA, Covassin L, Brehm MA, Racki W, Pearson T, Leif J, Laning J, Fodor W, Foreman O, Burzenski L, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009; 157:104–118. doi: 10.1111/j.1365-2249.2009.03933.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available within the article and the Supplemental Material or from the corresponding author upon reasonable request. RNA sequencing data that support the findings of this study have been deposited in the US National Library of Medicine, National Center for Biotechnology Information Sequence Read Archive (Gene Expression Omnibus) under accession code GSE146888.