Abstract
Aim.
Sympathetic overactivity, which predicts poor outcome in patients with heart failure, normalizes following cardiac transplantation. We tested the hypothesis that hemodynamic improvement following left ventricular assist device (LVAD) implantation is also associated with reductions in centrally generated sympathetic activity.
Methods and Results.
In eight patients with heart failure (2 women, 6 men, age 44-66 years), we continuously recorded ECG, beat-to-beat finger blood pressure, respiration, and muscle sympathetic nerve activity (MSNA) before and after implantation of the continuous-flow LVAD devices HeartWare HVAD (n=4) and HeartMate II (n=2), and the non-continuous-flow device HeartMate 3 (n=2).
LVAD implantation increased cardiac output by 1.29±0.88 L/min (p=0.060) and mean arterial pressure by 16.2±7.9 mmHg (p<0.001), while reducing pulse pressure by 25.3±9.8 mmHg (p<0.001). LVAD implantation did not change MSNA burst frequency (−1.3±7.5 bursts/min, p=0.636), total activity (+0.62±1.83 au, p=0.369), or normalized activity (+0.63±4.23, p=0.685). MSNA burst incidence was decreased (−7.8±9.3 bursts/100 heart beats, p=0.049). However, cardiac ectopy altered MSNA bursting patterns that could be mistaken for sympatholysis.
Conclusion.
Implantation of current design LVAD does not consistently normalize sympathetic activity in patients with end-stage heart failure despite hemodynamic improvement.
Keywords: Heart failure, Left ventricular assist device, Microneurography, Sympathetic nerve activity, Baroreflex
Internet abstract
In heart failure, sympathetic activity is typically elevated. Improved hemodynamics has been suggested to rapidly normalize sympathetic activity after cardiac transplantation. Left ventricular assist devices (LVAD) improve hemodynamic function and survival prospects in end-stage heart failure. It is uncertain whether LVAD implantation lowers centrally generated sympathetic vasoconstrictor activity. In eight patients with heart failure, we recorded hemodynamic parameters, respiration, and muscle sympathetic nerve activity (MSNA) before and after implantation of LVAD. Implantation improved hemodynamics but did not reduce MSNA substantially. Whether the remaining diseased native heart, reduced pulse pressure, or other mechanisms may account for the failure to normalize sympathetic activity deserves further study.
Introduction
Sympathetic vasomotor tone1 and plasma norepinephrine levels2 predict mortality in patients with heart failure. A cross-sectional comparison between heart failure patients before and after cardiac transplantation suggested that transplantation may normalize sympathetic hyperactivity.3 In a longitudinal study, cardiac transplantation lowered muscle sympathetic nerve activity (MSNA) which has been attributed to improved hemodynamics.4 This can be explained, along with other factors, by Ohm’s law of hydrodynamics, which implies that improved cardiac output via increased arterial pressure may reflexively lower sympathetic vasoconstrictor activity. In line with this, an inverse relationship between cardiac output and MSNA has been reported in healthy subjects5 as well as in heart failure patients.6 Left-ventricular assist devices (LVAD) also improve hemodynamic function7,8 and survival9 in patients with end-stage heart failure. In cross-sectional samples, sympathetic activity was within the normal range in pulsatile- and non-pulsatile-flow LVAD patients.10,11 Longitudinal studies suggesting decrease in sympathetic activity8,12 did not directly measure sympathetic nerve activity.
Aims
We tested the hypothesis that LVAD implantation decreases centrally generated sympathetic vasoconstrictor nerve activity in patients with end-stage heart failure in a longitudinal study design. To be able to assess the time course of sympatholysis, we sought to investigate patients twice, namely early and late, after LVAD implantation.
Methods
Of 22 patients with end-stage heart failure with reduced ejection fraction who had been considered for LVAD implantation, eight were available for MSNA measurements before and after implantation (2 women, 6 men, age 56±8 years, BMI 25.5±4.8 kg/m2, EF 10-25%). Four patients had been diagnosed with dilated and four patients with ischemic cardiomyopathy. Two patients featured obstructive and one patient central sleep apnoea. All patients were on diuretics and renin-angiotensin system inhibitors. Seven patients were on beta-blockers. Patients on inotropic medications, e.g. levosimendan, were excluded. The investigation conformed with the principles outlined in the Declaration of Helsinki. The Hannover Medical School Review Board approved the study (approval #5097) and written informed consent was obtained from the patients before study entry.
We conducted measurements during supine rest after an overnight fast in the morning hours before and again early and/or late following LVAD implantation. All measurements were conducted with the patients in a stable state, i. e. without left or right heart decompensation, bleeding, or infection. Without any physical exercise, aortic valves open only sporadically as in the majority of our post-implant measurements.13 As is common practice in post-operative care, LVAD speed adjustments in our patients ensure valve opening at least intermittently. Four patients were able to follow the pattern of inert gas rebreathing for non-invasive determination of cardiac output (Innocor, Innovision, Odense, Denmark). We continuously recorded electrocardiogram, beat-by-beat finger blood pressure (Finometer Midi, Finapres Medical Systems, Amsterdam, The Netherlands), and impedance cardiogram (Niccomo, Medis GmbH, Ilmenau, Germany) to assess O-wave amplitude, a non-invasive measure for left-ventricular dysfunction.14 We obtained muscle sympathetic nerve activity (MSNA) from the right peroneal nerve (Nerve Traffic Analyzer 662C-3, University of Iowa, Iowa City, IA, USA) using unipolar tungsten electrodes. Because arrhythmic events are numerous in these patients, we did not exclude event-related sympathetic bursts (see supplementary material). Data are expressed as mean±SD. Differences were compared by paired t-tests. P values <0.05 were considered statistically significant.
Results
Four patients were implanted with a HeartWare (Medtronic, Minneapolis, MN, USA), two with a HeartMate II, and two with a HeartMate 3 device (Abbott, Pleasanton, CA, USA). In all patients, we obtained good quality MSNA recordings before and after implantation. Early and late measurements took place 8-22 days post-implantation, when the patients were still hospitalized but in a steady state, and 185-725 days post-implantation during outpatient care, respectively. In two patients, we obtained both, early and late measurements following implantation (Patients #5 and #6 in Figure 1). In three patients, early measurements were not possible due to concomitant clinical issues. One patient had died and in one patient LVAD-generated electrical noise obscured nerve recordings. Table 1 reports individual medications and LVAD settings for all pertinent measurements. For instance, except for Patient #5, all patients were on beta-blocker therapy during all measurements.
Figure 1.
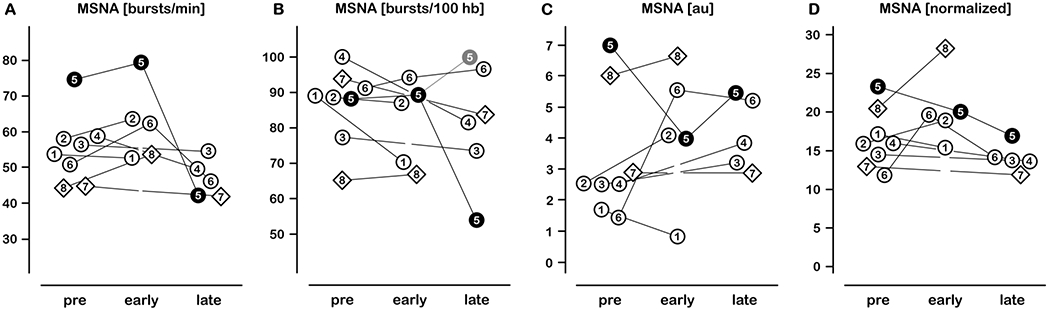
Individual sympathetic activities before, early, and late after LVAD implantation. Diamonds represent Patients #7 and #8 with pulsatile LVAD. Patient #5 data are depicted as filled circles: Note the misleading decrease in burst frequency and incidence during late post-implant measurement which is caused by bigeminal rhythm. If related to hemodynamically effective heartbeats, MSNA burst incidence was virtually 100 bursts/100 heart beats (Panel B, grey data point, Patient #5; see supplementary material for detailed information).
MSNA, muscle sympathetic nerve activity; hb, heart beats; au, arbitrary units.
Table 1.
Cardiovascular medication and LVAD parameters.
| Patient # Pre/Early/Late | 1 PEL | 2 PEL | 3 PEL | 4 PEL | 5 PEL | 6 PEL | 7 PEL | 8 PEL |
|---|---|---|---|---|---|---|---|---|
| Beta blocker | ●●□ | ●●□ | ●□● | ●□● | ○●● | ●●● | ●□● | ●●□ |
| AT1 blocker, ACE inhibitor | ●●□ | ●●□ | ●□○ | ●□● | ●○○ | ●●● | ●□● | ●●□ |
| Aldosterone antagonist | ●●□ | ●○□ | ●□○ | ●□● | ○○● | ○○○ | ●□● | ●●□ |
| Thiazide diuretic | ●●□ | ●○□ | ●□○ | ○□● | ●●○ | ○○○ | ●□● | ○○□ |
| Loop diuretic | ●●□ | ●○□ | ●□● | ●□● | ●●● | ●●● | ●□● | ●●□ |
| Antiarrhythmic | ●●□ | ●●□ | ●□○ | ●□○ | ●○○ | ○○○ | ○□○ | ○○□ |
| Statin | ○○□ | ○○□ | ●□● | ●□○ | ●○○ | ●●● | ○□○ | ●●□ |
| Platelet aggregation inhibitor | ○●□ | ○●□ | ●□● | ○□● | ●●● | ○●● | ●□● | ●●□ |
| Anticoagulant | ●●□ | ●●□ | ○□● | ●□● | ○●● | ○●● | ○□○ | ○●□ |
|
| ||||||||
| Speed [rpm] | 3000 | 2600 | 2840 | 2920 | 2480* | 2600* | 5700 | 5600 |
| Flow [L/min] | 5.3 | 4.9 | 6.3 | 6.9 | 4.8/4.4 | 4.3/3.1 | 4.1 | 4.1 |
| Power [W] | 5.8 | 3.7 | 5.0 | 5.6 | 3.2/3.1 | 3.5/3.3 | 4.4 | 4.4 |
| Pulsatility index | 5.6 | 5.6 | ||||||
P/E/L, pre-implant/early post-implant/late post-implant measurement.
●, intake; ○, no intake; □, no measurement.
, on both post-implant occasions.
Patients on inotropic medications, e.g. levosimendan, were excluded.
None of the aortic valves have been oversewn.
Hemodynamics and MSNA before and after LVAD implantation are presented in Table 2. Cardiac output, mean arterial pressure, and O-wave amplitudes were all improved following LVAD implantation while heart-generated pulse pressure significantly decreased. MSNA was substantially elevated in most patients before implantation. Yet, MSNA responses to LVAD implantation were heterogeneous (Figure 1). We did not observe major MSNA reductions regardless of LVAD type; diamonds in Figure 1 denote patients with pulsatile LVAD.
Table 2.
Comparison of hemodynamics and sympathetic activity before and after LVAD implantation (mean±SD).
| Parameter | Pre-implant N=8 | Early post-implant N=5 | Late post-implant N=5 | Mean post-implant | P value | |
|---|---|---|---|---|---|---|
| Hemodynamics | ||||||
| CO | [L/min] | 2.58 ± 0.67 | 4.56 ± 0.76 | 3.56 ± 1.53 | 3.87 ± 1.18 | 0.060 |
| SBP | [mmHg] | 79.5 ± 13.9 | 79.5 ± 8.9 | 78.6 ± 15.2 | 79.9 ± 11.1 | 0.905 |
| MBP | [mmHg] | 56.4 ± 8.5 | 74.8 ± 7.8 | 68.3 ± 11.8 | 72.6 ± 8.2 | <0.001 |
| DBP | [mmHg] | 44.9 ± 6.6 | 72.9 ± 7.4 | 66.0 ± 13.4 | 70.7 ± 9.5 | <0.001 |
| HR | [bpm] | 65.9 ± 10.8 | 77.2 ± 8.7 | 64.0 ± 14.9 | 70.3 ± 11.9 | 0.082 |
| PP | [mmHg] | 34.6 ± 9.6 | 6.7 ± 2.3 | 12.5 ± 5.5 | 9.3 ± 4.1 | <0.001 |
| PP7 | [mmHg] | 47.4 | 24.8 | 24.8 | ||
| PP8 | [mmHg] | 39.9 | 25.3 | 25.3 | ||
| O-wave | [mOhms/s] | 627 ± 211 | 273 ± 102 | 305 ± 203 | 264 ± 127 | 0.001 |
| MSNA (burst frequency, burst incidence, total activity, normalized total activity) | ||||||
| MSNA | [bursts/min] | 55.0 ± 9.6 | 62.2 ± 10.8 | 46.6 ± 5.5 | 53.7 ± 6.7 | 0.636 |
| MSNA | [bursts/100 hb] | 86.6 ± 10.7 | 81.6 ± 12.1 | 78.0 ± 15.7 | 78.8 ± 9.7 | 0.049/0.198* |
| MSNA | [au] | 3.34 ± 2.04 | 4.23 ± 2.20 | 4.13 ± 1.17 | 3.96 ± 1.75 | 0.369 |
| MSNA | [normalized] | 16.5 ± 3.8 | 20.4 ± 4.7 | 14.1 ± 1.8 | 17.1 ± 5.1 | 0.685 |
CO, Cardiac output (N=4); SBP/MBP/DBP, systolic/mean/diastolic blood pressure; PP, pulse pressure; PP7/8, artificial pulse pressures in Patients #7 and #8 with pulsatile LVAD; HR, heart rate; MSNA, muscle sympathetic nerve activity; hb, heart beats; au, arbitrary units; normalized, relative to largest burst; P values for mean post- vs pre-implant;
, P value for alternative burst incidence calculation of Patient #5 (100 bursts/100 pulses instead of 54 bursts/100 hb).
In the supplement, original recordings from Patient #5 illustrate in an exemplary way how clinical consequences of severe heart failure affect sympathetic bursting patterns. The patient showed high MSNA, Cheyne-Stokes breathing, and occasional ectopic beats before LVAD implantation. LVAD implantation increased cardiac output ~1.0 L/min and mean blood pressure ~19 mmHg, and Cheyne-Stokes respiration disappeared. Eleven months after implantation (late post-implant), the patient showed bigeminy with increased pulse pressure and reduced number of sympathetic bursts. However, this must be seen as spurious sympatholysis as these bursts were larger indicating an increased number of action potentials per sympathetic burst (see supplementary material and Figure 1, Patient #5).
Conclusion
The important finding of our longitudinal study is that hemodynamic improvements following LVAD implantation do not consistently translate to major reductions in central sympathetic outflow. The finding contrasts with cross-sectional data in patients implanted with continuous-flow LVAD, which showed increased10 or normal sympathetic activity.11 Normalization of sympathetic overactivity after cardiac transplantation has been attributed to restored hemodynamic function.4,15 LVAD-generated pulsatility may16 or may not be important for baroreflex-mediated sympathetic inhibition.11 HeartMate 3 incorporates a pulse-mode algorithm, which involves automated modulations in pump speed, but data are lacking to suggest that this imparts a physiologic pulse. The two patients who have been implanted with the device did not fare better.
Heart failure is characterized by heterogeneous changes in afferent, central, and efferent autonomic neuromodulation,17 rendering individual net results of LVAD implantation hard to predict. For instance, the proportional characteristic of baroreceptors inhibits sympathetic outflow with increased mean arterial pressure, while their differential property counteracts sympatholysis because of reduced pulse pressure (Table 2).18 Moreover, depending on the pre-implant activity of paradoxical sympathoexcitatory cardiopulmonary afferents, LVAD implantation may or may not mitigate sympathetic tone.19 Sympathoexcitation may also result from cardiac dysrhythmias and changes in respiratory control, which may persist after LVAD implantation. Furthermore, afferents from the remaining diseased hearts could maintain sympathetic activation.20 Heart failure is accompanied not only by autonomic but also by humoral disturbances. For instance, arginine vasopressin levels are significantly elevated,21 the vasoconstrictor action of which is most likely counteracted by some degree of sympathoinhibition.22 Cardiac transplantation or LVAD implantation lower AVP levels21 which can be expected to increase MSNA. Cardiac remodelling, deteriorated pump function, and sympathetic activation constitute a vicious cycle.23 In patients whose device could be explanted, myocardial recovery and catecholamine reduction were strongly correlated.24 Hence, timing of LVAD implantation may have a bearing on sympathetic activity and the outcome.25 It remains to be determined if LVAD designs, which lower sympathetic overactivity by generating a more physiological pulse, improve outcomes in patients with potentially reversible myocardial dysfunction or when LVAD implantation is considered as destination therapy.
Our study has limited statistical power because of the small sample size. As end-stage heart failure patients are difficult to study, we hope for a more complete picture when data from different laboratories can be taken into account. Large variability in sympathetic nerve activity is another weakness, which possibly arises from the heterogeneity of patients’ medical histories, responses to LVAD, implanted devices and their consequences for arterial baroreceptor stimulation and entrainment of outflow, and post-implant study dates. LVAD implantation decreased plasma norepinephrine levels in some studies12,24 but not in others.26 Because we did not measure plasma or myocardial catecholamine levels, we cannot extrapolate our findings to districts other than skeletal muscle which, however, may have prognostic value by itself.1 Furthermore, we cannot exclude that changes in medications may have confounded our analysis. Finally, we do not know whether sympathoexcitation would diminish in patients with myocardial recovery.
Supplementary Material
Acknowledgements
We thank patients and the staff of the Department of Cardiac, Thoracic, Transplantation, and Vascular Surgery, Hannover Medical School, Hannover, Germany for their support. We would like to express our special thanks to Alexandra Schoede for diligent coordination.
Funding
AD is supported by the National Heart, Lung, and Blood Institute of the National Health (Award Number NIH 1R01HL142583). KH and JT were supported by the German Federal Ministry of Economy and Technology (BMWi; 50WB1517).
Footnotes
Conflict of interest: none declared.
References
- 1.Barretto ACP, Santos AC, Munhoz R, Rondon MUPB, Franco FG, Trombetta IC, Roveda F, Matos LNJ de, Braga AMW, Middlekauff HR, Negrao CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 2009;135:302–307. [DOI] [PubMed] [Google Scholar]
- 2.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984;311:819–823. [DOI] [PubMed] [Google Scholar]
- 3.Rundqvist B, Elam M, Eisenhofer G, Friberg P. Normalization of total body and regional sympathetic hyperactivity in heart failure after heart transplantation. J Heart Lung Transplant 1996;15:516–526. [PubMed] [Google Scholar]
- 4.Rundqvist B, Casale R, Bergmann-Sverrisdottir Y, Friberg P, Mortara A, Elam M. Rapid fall in sympathetic nerve hyperactivity in patients with heart failure after cardiac transplantation. J Card Fail 1997;3:21–26. [DOI] [PubMed] [Google Scholar]
- 5.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol 2005;568:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Najem B, Preumont N, Unger P, Jansens J-L, Houssière A, Ciarka A, Stoupel E, Degaute J-P, Van De Borne P. Sympathetic nerve activity after thoracoscopic cardiac resynchronization therapy in congestive heart failure. J Card Fail 2005;11:529–533. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy PM, Savage RM, Fraser CD, Vargo R, James KB, Goormastic M, Hobbs RE. Hemodynamic and physiologic changes during support with an implantable left ventricular assist device. J Thorac Cardiovasc Surg 1995;109:409–417. [DOI] [PubMed] [Google Scholar]
- 8.Drakos SG, Athanasoulis T, Malliaras KG, Terrovitis JV, Diakos N, Koudoumas D, Ntalianis AS, Theodoropoulos SP, Yacoub MH, Nanas JN. Myocardial sympathetic innervation and long-term left ventricular mechanical unloading. JACC Cardiovasc Imaging 2010;3:64–70. [DOI] [PubMed] [Google Scholar]
- 9.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435–1443. [DOI] [PubMed] [Google Scholar]
- 10.Markham DW, Fu Q, Palmer MD, Drazner MH, Meyer DM, Bethea BT, Hastings JL, Fujimoto N, Shibata S, Levine BD. Sympathetic neural and hemodynamic responses to upright tilt in patients with pulsatile and nonpulsatile left ventricular assist devices. Circ Heart Fail 2013;6:293–299. [DOI] [PubMed] [Google Scholar]
- 11.Tank J, Heusser K, Malehsa D, Hegemann K, Haufe S, Brinkmann J, Tegtbur U, Diedrich A, Bara C, Jordan J, Strüber M. Patients with continuous-flow left ventricular assist devices provide insight in human baroreflex physiology. Hypertension 2012;60:849–855. [DOI] [PubMed] [Google Scholar]
- 12.Frazier OH, Benedict CR, Radovancevic B, Bick RJ, Capek P, Springer WE, Macris MP, Delgado R, Buja LM. Improved left ventricular function after chronic left ventricular unloading. Ann Thorac Surg 1996;62:675–681. [DOI] [PubMed] [Google Scholar]
- 13.Haufe S, Bara C, Eigendorf J, Chobanyan-Jurgens K, Rojas SV, Schmitto J, Tegtbur U, Jordan J, Tank J. Physical activity guided by pulse pressure in patients with continuous flow left ventricular assist devices: A pilot study. Circulation 2017;135:1567–1569. [DOI] [PubMed] [Google Scholar]
- 14.Woltjer H, Bogaard H, Vries P de. The technique of impedance cardiography. Eur Heart J 1997;18:1396–1403. [DOI] [PubMed] [Google Scholar]
- 15.Levine TB, Olivari MT, Cohn JN. Effects of orthotopic heart transplantation on sympathetic control mechanisms in congestive heart failure. Am J Cardiol 1986;58:1035–1040. [DOI] [PubMed] [Google Scholar]
- 16.Cornwell WK 3rd, Tarumi T, Stickford A, Lawley J, Roberts M, Parker R, Fitzsimmons C, Kibe J, Ayers C, Markham D, Drazner MH, Fu Q, Levine BD. Restoration of pulsatile flow reduces sympathetic nerve activity among individuals with continuous-flow left ventricular assist devices. Circulation 2015;132:2316–2322. [DOI] [PubMed] [Google Scholar]
- 17.Floras JS, Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J 2015;36:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James JEA, Daly MDB. Comparison of the reflex vasomotor responses to separate and combined stimulation of the carotid sinus and aortic arch baroreceptors by pulsatile and non-pulsatile pressures in the dog. J Physiol 1970;209:257–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millar PJ, Murai H, Floras JS. Paradoxical muscle sympathetic reflex activation in human heart failure. Circulation 2015;131:459–468. [DOI] [PubMed] [Google Scholar]
- 20.Wang H-J, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 2014;64:745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imamura T, Kinugawa K, Hatano M, Fujino T, Inaba T, Maki H, Kinoshita O, Nawata K, Kyo S, Ono M. Low cardiac output stimulates vasopressin release in patients with stage D heart failure–Its relevance to poor prognosis and reversal by surgical treatment. Circ J 2014;CJ-14-0368. [DOI] [PubMed] [Google Scholar]
- 22.Floras JS, Aylward PE, Abboud FM, Mark AL. Inhibition of muscle sympathetic nerve activity in humans by arginine vasopressin. Hypertension 1987;10:409–416. [DOI] [PubMed] [Google Scholar]
- 23.El-Armouche A, Eschenhagen T. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev 2009;14:225–241. [DOI] [PubMed] [Google Scholar]
- 24.George RS, Birks EJ, Cheetham A, Webb C, Smolenski RT, Khaghani A, Yacoub MH, Kelion A. The effect of long-term left ventricular assist device support on myocardial sympathetic activity in patients with non-ischaemic dilated cardiomyopathy. Eur J Heart Fail 2013;15:1035–1043. [DOI] [PubMed] [Google Scholar]
- 25.Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: patient selection and outcomes. Eur J Heart Fail 2017;19:595–602. [DOI] [PubMed] [Google Scholar]
- 26.Klotz S, Burkhoff D, Garrelds IM, Boomsma F, Danser AHJ. The impact of left ventricular assist device-induced left ventricular unloading on the myocardial renin-angiotensin-aldosterone system: therapeutic consequences? Eur Heart J 2009;30:805–812. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz-Cano MJ, Ramazyan L, Schramm R, Lauenroth V, Paluszkiewicz L, Rojas S, Gummert J, Morshuis M. Clinical implications of late-onset right ventricular failure after implantation of a continuous-flow left ventricular assist device as bridge to transplantation. Eur J Cardiothorac Surg 2021;60:177–185. [DOI] [PubMed] [Google Scholar]
- 28.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


