Abstract
Background
Mucormycosis is a fulminant and rapidly progressing fungal infection associated with a high mortality rate. Mucormycosis is primarily seen in immunocompromised patients, especially those with uncontrolled diabetes mellitus (DM), and recently in coronavirus disease 2019 (COVID-19) patients.
Case Presentation
In this case report, we present a rare case of fatal mucormycosis in Palestine. A 34-year-old Palestinian female patient presented to the emergency department one-month post-COVID-19 infection with left facial pain. During her hospital stay, she deteriorated, with a random blood sugar level of 400 mg/dl and a hemoglobin A1c of 18% with metabolic acidosis and the appearance of swelling and black eschar on her left side of her face. Finally, she was diagnosed with mucormycosis and expired two days later.
Conclusion
In this unfortunate case report of mucormycosis, severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) infection, delayed diagnosis, misuse of corticosteroids, inappropriate use of antibiotics, and uncontrolled diabetes with ketoacidosis contributed to patient mortality and fatality. Therefore, appropriate patient assessment, rapid diagnosis, and selection of appropriate treatment are important and lifesaving.
Keywords: fungal infection, immunocompromised, acidosis, COVID-19, mucormycosis, diabetes
Introduction
Mucormycosis is a non-contagious angio-invasive fungal infection caused by mucormycetes, a mold belonging to the genus Rhizopus.1,2 It is a rare disease with a global incidence rate of 0.005–1.7 per million population.3 The main route of infection is the inhalation of sporangiospores, but it can also be acquired through the skin by burns, trauma, or any other type of skin injury.3,4 The disease may manifest in different forms depending on the level of immunosuppression and infection site. The most common type is rhinocerebral mucormycosis (30–50% of all cases).5 It is a life-threatening condition that commonly extends to the orbits and brain. Other types of mucormycosis include pulmonary, gastrointestinal, cutaneous, and central nervous system.4
There has been an increase in the reporting of COVID-19 associated mucormycotic infections during the COVID-19 pandemic.3 COVID-19 patients with DM, prolonged neutropenia, prolonged use of corticosteroids, and hematological malignancies are more likely to develop mucormycosis.6,7 SARS-CoV-2 infection causes deregulation of the immune system leading to decreased innate immunity response by disturbing ciliary clearance and lymphopenia, putting a patient at high risk for opportunistic fungal infection.8 76.3% of COVID-19 cases had received steroids in the 30 days preceding admission.3 Corticosteroids are recommended in patients diagnosed with COVID-19 that require oxygen supplements.9 However, corticosteroids are associated with many side effects, including hyperglycemia and immune suppression leading to opportunistic fungal infections. In addition, uncontrolled diabetic patients treated with corticosteroids are at higher risk of developing systemic fungal infection post-COVID-19.3 Diabetes was present in more than 80% of mucormycosis cases post COVID-19 infections, with concomitant diabetic ketoacidosis (DKA) in 14.9% of cases. This case report presents an interesting case of mucormycosis in a diabetic patient with DKA 30 days post SARS COV-2 infection and a recent history of steroid treatment.
Case Presentation
A 34-year-old Palestinian female patient with a past medical history of gestational diabetes 2 years ago and COVID-19 infections 30 days ago presented to the emergency department with a 3-week history of worsening severe pain on her left side of the face and loss of vision and movement of the left side. Her symptoms started immediately after recovery from COVID-19, which lasted for 10 days. One week prior to presentation, she was treated with ceftriaxone 2 g once daily and dexamethasone 4 mg three times daily for a presumed diagnosis of facial nerve palsy with no improvement. Past medical treatments include multiple steroid injections from an outpatient clinic for COVID-19 treatment. The patient had no history of follow-up or diabetes treatment.
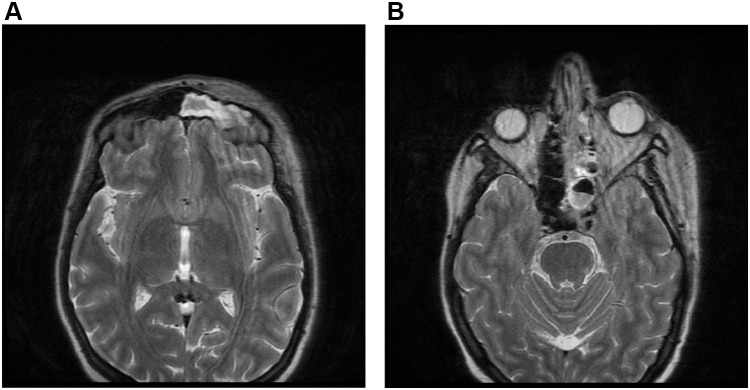
On admission, she was alert, oriented, and hemodynamically stable. Further evaluation by the medical team revealed periorbital oedema, complete left eye proptosis, dilated non-reactive pupil, left eye ophthalmoplegia, loss of vision, trigeminal involvement (v1.v2.v3), decreased hearing on the left side, and a vesicular rash on the left cheek. Laboratory results showed hemoglobin: 13.2 g/dl, white blood cell count (WBC): 14.5 K/uL (90% neutrophils), platelets count: 269 K/ul, creatinine: 0.54 mg/dl, serum potassium 4.9 mmol/l, random blood sugar (RBS): 277 mg/dl, erythrocyte sedimentation rate (ESR): 101 Mm/hr, C-reactive protein (CRP): 174 mg/dl. Clinical diagnosis of orbital cellulitis and suspected dural (cavernous) sinus thrombosis versus optic neuritis. The patient was empirically treated with vancomycin (1 g twice daily), piperacillin-tazobactam (4.5 g four times daily), enoxaparin (80 mg twice daily), and methylprednisolone (1 g once daily). On day 3, magnetic resonance imaging (MRI) with orbital view showed multifocal infarction (Figure 1A), a paranasal sinus extensive mucosal disease (Figure 1B), and cavernous sinus thrombosis (Figure 2). In addition, serial blood sugar measurements were 469m/dl, and the hemoglobin A1c level was 18%. Arterial blood gases (ABGs) and urine analysis were ordered and showed: pH 7.21, HCO3: 5, PCO2 9, PO2: 100, urine analysis revealed: traces of protein, glucose +2, and ketone bodies +3. The Methylprednisolone was discontinued, and the patient was transferred to the intensive care unit (ICU) for DKA. DKA management was initiated according to the local protocol with intravenous fluids, regular intravenous insulin infusion, and potassium. ABGs, serum electrolytes, and RBSs were monitored every 2 hours. Mucormycosis was suspected based on the patient’s clinical presentation, DKA, and MRI findings.
Figure 1.
MRI with orbital view showed multifocal infarction (A). MRI with orbital view showed left paranasal sinus extensive mucosal disease (B).
Figure 2.
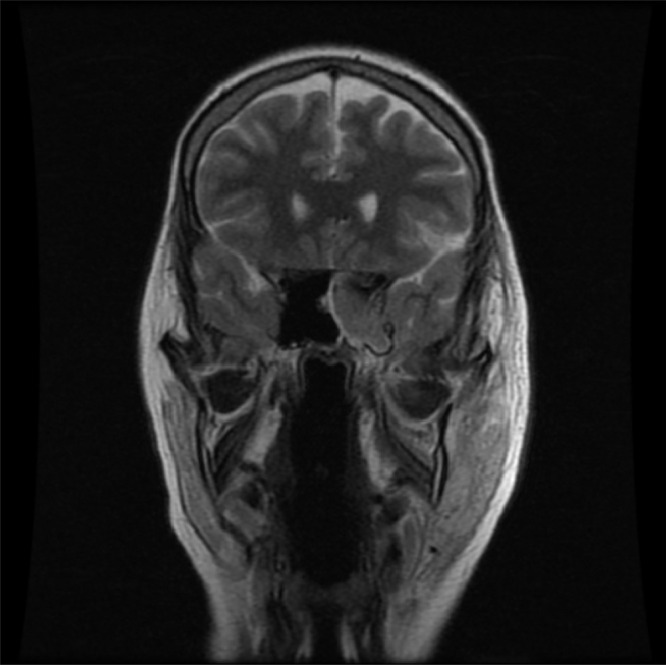
MRI showed cavernous sinus thrombosis.
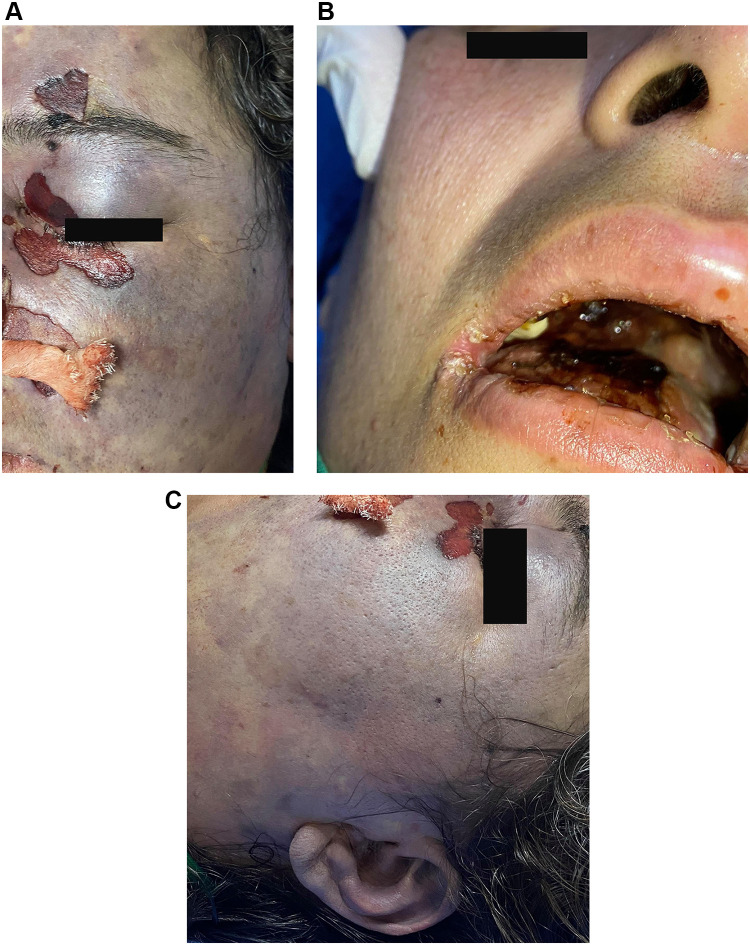
On the second day in the ICU, facial swelling, periorbital edema, and erythema which were more prominent on the left side as shown in (Figure 3A), and black eschar were visible in the nasal mucosa, palate, and skin overlying the orbit of her left side of the face (Figure 3B) with left auditory canal involvement as presented in (Figure 3C). Based on the clinical and radiological findings, the patient was diagnosed with rhino-orbital-cerebral mucormycosis. Conventional amphotericin was initiated, and the surgical team was informed of possible surgical debridement. Unfortunately, the patient rapidly deteriorated, became unconscious, and was hemodynamically unstable with refractory metabolic acidosis. She developed cardiac arrest and expired on day four of hospitalization.
Figure 3.
Facial swelling, periorbital edema, and erythema which are more prominent on the left side (A). Necrotic eschars on the palate (B). The black eschar extended to the left auditory side (C).
Discussion
Mucormycosis is a serious life-threatening fungal infection associated with significant mortality, which approaches 100% in some cases, despite the use of antifungals and surgical debridement.10
In rhinocerebral mucormycosis, patients usually present with symptoms of facial swelling, headache, sinus congestion, black lesions on different areas of the nose or mouth, and fever.11 As the infection disseminates to other organs, organ-specific symptoms start to appear. Pulmonary, cutaneous, and gastrointestinal symptoms have been described in many case reports.12,13
During the COVID-19 pandemic, emerging data from many studies and case reports have shown a suspected link between SARS-CoV-2 infection and mucormycosis. Covid-19 suppresses phagocytes allowing the fungal spores to spread.14 The RECOVERY collaborative group has recommended corticosteroids in COVID-19 patients that need supplementary oxygen.9 On the other hand, corticosteroid utilization in managing the covid-19 disease has immunosuppressant activity contributing to mucormycosis proliferation.15 Corticosteroids disrupt the migration, ingestion, and phagolysosome fusion macrophages, placing patients at higher risk of fungus coinfections. Prolonged use (> 3 weeks) of high-dose systemic corticosteroids (eg, prednisone or equivalent of > 1 mg/kg/d) increases the patient’s risk for mucormycosis.9 Even a short-term corticosteroid may cause mucormycosis, as reported by Veisi et al; In a mucormycosis case report, short-term corticosteroid therapy was the only factor predisposing the patient to rhino-orbitocerebral mucormycosis along with COVID-19.16 Corticosteroids were also found to increase DKA risk in high-risk diabetic patients.3 In addition, hyperglycemia was found to be associated with the release of reactive oxygen species that damage tissues and thus increase the risk of mucormycosis development.17 According to a systemic review by Singh et al, which included 101 cases of mucormycosis in patients presenting post COVID19, DM was the most important risk factor accounting for almost 80% of the cases with concomitant DKA in 15% of the cases. The acidosis associated with DKA contributes to mucormycosis as mucor spore germination increases in low pH media.3 Another study by Prakash et al showed that out of 388 cases of mucormycosis, 18%had DKA upon diagnosis.18 In a multicenter epidemiologic study conducted in India were 187 patients diagnosed with COVID-19 associated Mucormycosis (CAM) compared to 100 mucormycosis without COVID-19. The study revealed that inappropriate glucocorticoid use was independently associated with late CAM, and the COVID-19 pandemic has led to increased mucormycosis. Most patients in the CAM group had diabetes or received high doses of corticosteroids. In the Collaborative OPAI-IJO study on mucormycosis in COVID-19 (COSMIC), corticosteroids and DM were the most important predisposing factors in developing COVID-19-associated rhino-orbito-cerebral mucormycosis (ROCM).19
In this case, the main manifestation was (ROCM), commonly seen in diabetic patients.20 Differential diagnosis of mucormycosis can be challenging and is confirmed through a thorough examination of the orbit. In addition, the nasal cavity should be examined, and the hard palate should be checked for eschar. It should be noted that clinical, nasoendoscopic, and imaging findings may be subtle. Histopathological and microbiological investigations should be used to confirm the diagnosis.21
The cavernous sinuses are the most centrally located of the dural sinuses, positioned just lateral to the base of the sella turcica and the sphenoid paranasal sinuses. These irregularly shaped sinuses contain multiple trabeculae that act as sieves to trap pathogens, which explains the higher risk of infection of the cavernous sinuses compared to the other dural sinuses. In addition, several cranial nerves are located within a dural sleeve, lateral to the cavernous sinus: the oculomotor nerve (cranial nerve III), trochlear nerve (cranial nerve IV), ophthalmic (V1), and maxillary branches (V2) of the trigeminal nerve. In addition, the abducens nerve (cranial nerve VI) is located more medially within the cavernous sinus and abuts the cavernous segment of the internal carotid artery.15 Finally, conditions causing immunosuppression such as diabetes, chronic alcohol abuse, and long-term glucocorticoid therapy are risk factors for septic cavernous sinus thrombosis.16
The patient’s symptoms included periorbital oedema, complete left eye proptosis, dilated non-reactive pupil, left eye ophthalmoplegia, loss of vision, and hypoesthesia in the dermatomes served by the ophthalmic (V1) and maxillary (V2) branches of the fifth cranial nerve (trigeminal nerve). These findings suggest 3rd, 4th, and 6th cranial nerves involvement rather than 7th (facial nerve) involvement, excluding the possibility of Bell’s palsy and optic neuritis. Furthermore, suspicion of cavernous sinus involvement requires prompt imaging studies to confirm the diagnosis. In this case, the diagnosis of optic neuritis or Bell’s palsy should have been excluded based on the patient’s symptoms, cranial nerve anatomy, and involvement. Misdiagnosis and delayed treatment result in a higher risk of complications and unnecessary use of antimicrobials and corticosteroids, associated with higher morbidity and mortality.
Mucormycosis is rarely suspected, leading to delayed diagnosis and treatment. However, an appropriate diagnostic workup and early initiation of therapy are necessary because an increase in mortality from 35% to 66% is expected with a one-week delay in therapy initiation.4,20 In this case, the correct diagnosis was only suspected and confirmed after the appearance of swelling and black eschar on the left side.
The treatment for mucormycosis includes amphotericin B., and recently, some azoles have been effective. Immunotherapy has been reported as a new treatment modality for invasive fungal infections that do not respond to conventional therapy.22,23 In a case report of mucormycosis, the combination of nivolumab plus interferon-γ in the treatment of intractable mucormycosis showed promising results. In this case, the Initiation of conventional treatment with amphotericin B was delayed and ineffective. The mortality rate was very high due to highly uncontrolled diabetes with DKA, leading to an abysmal prognosis. Identifying the emerging risk factors associated with mucormycosis for this uncommon disease is essential.
Conclusion
In this unfortunate case of mucormycosis, the initial clinical presentation was not very specific; symptoms of the disease were identified when the optimum treatment became less effective and challenging. The SARS COV-2 infection, delay in diagnosis, overuse of corticosteroids, and uncontrolled diabetes with ketoacidosis contributed to this opportunistic fungal infection. Therefore, appropriate patient assessment, rapid diagnosis, and selection of the appropriate treatment are essential and lifesaving. Future research is warranted to develop rapid diagnostic techniques and immunotherapy to target this deadly pathogen.
Funding Statement
This research did not receive funding from funding agencies in the public, commercial, or not-for-profit sectors.
Informed Consent
This study was approved by the ethics committee of our institution. Informed consent to participate in the study was obtained from the patient family member. Her father gave informed consent to publish the case details, including images, and written consent was obtained.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Williams GH. Introduction to third edition. In: The Radical Reformation, 3rd Ed; 2020:1–21. doi: 10.5325/j.ctv1c9hns8.9 [DOI] [Google Scholar]
- 2.Martinez AJ. Medical Microbiology. Med Microbiol 4th ed.; 1996:1–9. Available from: https://www.ncbi.nlm.nih.gov/books/NBK7627/. Accessed November 12, 2021. [Google Scholar]
- 3.Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr Clin Res Rev. 2021;15(4):102146. doi: 10.1016/J.DSX.2021.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallis A, Mastronikolis SN, Naxakis SS, Papadas AT. Rhinocerebral mucormycosis: an update. Eur Rev Med Pharmacol Sci. 2010;14(11):987–992. [PubMed] [Google Scholar]
- 6.Song G, Liang G, Liu W. Fungal Co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;185(4):1. doi: 10.1007/S11046-020-00462-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(9). doi: 10.7759/CUREUS.10726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitaka H, Kuno T, Takagi H, Patrawalla P. Incidence and mortality of COVID-19-associated pulmonary aspergillosis: a systematic review and meta-analysis. Mycoses. 2021;64(9):993–1001. doi: 10.1111/MYC.13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.RECOVERY Collaborative Group, Horby P, Lim WS, et al Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/nejmoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of Mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S16. doi: 10.1093/CID/CIR865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhandari J, Thada PK, Nagalli S. Rhinocerebral Mucormycosis. StatPearls; July 7, 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559288/. Accessed December 3, 2021. [PubMed] [Google Scholar]
- 12.Novais AG, Capelo J, Costa M, et al. Pulmonary mucormycosis: a case report. IDCases. 2020;22:e00993. doi: 10.1016/j.idcr.2020.e00993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wotiye AB, Ks P, Ayele BA. Invasive intestinal mucormycosis in a 40-year old immunocompetent patient - A rarely reported clinical phenomenon: a case report. BMC Gastroenterol. 2020;20(1):1–6. doi: 10.1186/S12876-020-01202-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayaramayya K, Mahalaxmi I, Subramaniam MD, et al. Immunomodulatory effect of mesenchymal stem cells and mesenchymal stem-cell-derived exosomes for COVID-19 treatment. BMB Rep. 2020;53(8):400–412. doi: 10.5483/BMBRep.2020.53.8.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khatri A, Chang KM, Berlinrut I, Wallach F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient - case report and review of literature. J Mycol Med. 2021;31(2):101125. doi: 10.1016/J.MYCMED.2021.101125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veisi A, Bagheri A, Eshaghi M, Rikhtehgar MH, Rezaei Kanavi M, Farjad R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: a case report. Eur J Ophthalmol. 2021:112067212110094. doi: 10.1177/11206721211009450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for Mucormycosis. J Fungi (Basel, Switzerland). 2021;7(4). doi: 10.3390/JOF7040298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prakash H, Ghosh AK, Rudramurthy SM, et al. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol. 2019;57(4):395–402. doi: 10.1093/MMY/MYY060 [DOI] [PubMed] [Google Scholar]
- 19.Sen M, Honavar S, Sengupta S, et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India - Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69(7):1670–1692. doi: 10.4103/IJO.IJO_1565_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021;42:264.e5–264.e8. doi: 10.1016/J.AJEM.2020.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes TR, Hepschke JL, Jacobson I, Maloof A. Mucormycosis: early treatment is the key to survival. Med J Aust. 2021;215(9):401–403. doi: 10.5694/mja2.51290 [DOI] [PubMed] [Google Scholar]
- 22.Grimaldi D, Pradier O, Hotchkiss RS, Vincent JL. Nivolumab plus interferon-γ in the treatment of intractable mucormycosis. Lancet Infect Dis. 2017;17(1):18. doi: 10.1016/S1473-3099(16)30541-2 [DOI] [PubMed] [Google Scholar]
- 23.Scriven JE, Tenforde MW, Levitz SM, Jarvis JN. Modulating host immune responses to fight invasive fungal infections. Curr Opin Microbiol. 2017;40:95–103. doi: 10.1016/j.mib.2017.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]